Abstract
Emerging evidence suggests that epigenetic-based mechanisms contribute to various aspects of sex differences in brain and behavior. The major obstacle in establishing and fully understanding this linkage is identifying the traits that are most susceptible to epigenetic modification. We have proposed that sexual selection provides a conceptual framework for identifying such traits. These are traits involved in intrasexual competition for mates and intersexual choice of mating partners and generally entail a combination of male–male competition and female choice. These behaviors are programmed during early embryonic and postnatal development, particularly during the transition from the juvenile to adult periods, by exposure of the brain to steroid hormones, including estradiol and testosterone. We evaluate the evidence that endocrine-disrupting compounds, including bisphenol A, can interfere with the vital epigenetic and gene expression pathways and with the elaboration of sexually selected traits with epigenetic mechanisms presumably governing the expression of these traits. Finally, we review the evidence to suggest that these steroid hormones can induce a variety of epigenetic changes in the brain, including the extent of DNA methylation, histone protein alterations, and even alterations of noncoding RNA, and that many of the changes differ between males and females. Although much previous attention has focused on primary sex differences in reproductive behaviors, such as male mounting and female lordosis, we outline why secondary sex differences related to competition and mate choice might also trace their origins back to steroid-induced epigenetic programming in disparate regions of the brain.
Keywords: DNA methylation, histone proteins, neurodevelopment, sex dimorphism, steroid hormones
Introduction
Studies on epigenetics are changing how we interpret animal behavior and development (Bredy et al. 2010; Crews 2010; Curley and Mashoodh 2010; Meaney 2010; Miller 2010; Svrakic and Cloninger 2010; Weaver et al. 2004; Zhang and Meaney 2010). In the past, most behavioral differences were ascribed to polymorphism in either select groups of genes or physiochemical differences of unknown etiology (Bray 2008; Hamer 2002; Inoue and Lupski 2003; Jabbi et al. 2007; Kreek and LaForge 2007; Lee 2007; Mancama et al. 2003; Mantione et al. 2010; Proudnikov et al. 2008; Zhou et al. 2008; Zill et al. 2002). However, it is becoming apparent that at least some of these differences in behavior are related to epigenetic changes stemming from early social or developmental experience (Bredy et al. 2010; Crews 2010; Curley and Mashoodh 2010; Meaney 2010; Miller 2010; Svrakic and Cloninger 2010; Weaver et al. 2004; Zhang and Meaney 2010). Although there are varying definitions of epigenetics (Haig 2004), for the purpose of this review, we consider an epigenetic change to be a mitotically, postmitotically, or meiotically heritable change in gene expression that occurs independently of an alteration in DNA sequence (Youngson and Whitelaw 2008). Behavioral epigenetics will thus be broadly defined as any behavioral change that has an epigenetic basis and provides a means of understanding how environmental factors, in particular maternal care, early social experiences, and exposure to endocrine-disrupting compounds (EDCs[1]) and diet, lead to behavioral abnormalities not readily explained by conventional genetic mechanisms (Meaney 2010; Weaver et al. 2004; Zhang and Meaney 2010).
Genetically identical (monozygotic) twins have provided a means to study epigenetic effects on human behavior and development because marked differences can frequently be observed to distinguish such individuals (Alter et al. 2008; Kaminsky et al. 2008; Zwijnenburg et al. 2010). However, it is neither feasible nor ethical to control for the myriad environmental factors that might have contributed to individual differences between twins. Thus, the impact of epigenetics on behavior is best studied in the laboratory setting where the test animals can be maintained under uniform environmental conditions. Even with strong experiment controls, the contribution of these laboratory studies are often limited by the lack of a conceptual framework for identifying which behavioral traits are most susceptible to epigenetic changes and how such behavioral abnormalities might serve as useful barometers of environmental insults received during early development and manifested later in life as behavioral pathologies.
In this review, we propose that sexual selection provides the foundation of such a framework. Specifically, we will consider whether sexually selected traits, as opposed to naturally selected traits, are particularly vulnerable to environmentally induced epigenetic alterations. Sexual selection entails intrasexual competition for mates and intersexual choice of mating partners (Darwin 1871) and, for most mammals, involves a combination of intrasexual competition, usually among males, and intersexual choice, typically female choice of mates, although female–female competition and male choice are found in some species (Clutton-Brock 2007), including humans (Geary 2010). Unlike naturally selected traits, sexually selected traits, such as the long train of the male peacock, often reduce survival prospects. For example, these latter traits necessitate higher energy and foraging cost. They may also render the animal more susceptible to predation while searching for food or prospective mates (Andersson 1994). Although these traits are a handicap and impose fitness costs, they are also necessary for providing advantages over same-sex competitors and for attracting mates (Folstad and Karter 1992; Zahavi 1975). Moreover, the expression of sexually selected traits is more strongly dependent on development and current condition of the individual than naturally selected traits, and thus their expression can be highly variable across individuals and even in the same individual across breeding seasons (Pomiankowski and Møller 1995). When an animal is in poor health or is malnourished, sexually selected traits will likely be underexpressed, thereby reducing associated fitness costs and providing a greater likelihood of surviving to the next breeding cycle when the animal might be better poised to compete and reproduce (Mougent et al. 2005, 2006). The extreme variability in the expression of sexually selected traits suggests that the observed phenotype is strongly influenced by epigenetic processes and hence is able to respond rapidly to environmental fluctuations. Such plasticity would not be possible if the traits were under classic genetic control.
The outline of this review is as follows. First, we expand the discussion of sexually selected traits and their manifestation. We then consider the mechanisms whereby environmental changes, including exposure to EDCs such as bisphenol A (BPA1), during development can disrupt the expression of these traits. Last, we examine the evidence that sex steroid hormones underpin normal epigenetic changes that shape sex differences in morphologic development of the brain and later programming of adult behavioral responses. Most of the latter studies have focused on primary sex behaviors, but we suggest that consideration should be extended to include sexually selected traits. The potential for EDCs to affect sexually selected traits has not been extensively studied, but available research suggests these traits may be particularly vulnerable (Jašarević et al. 2011). By separating sexually selected traits from naturally selected traits, we may be able to account for some of the conflicting data on endocrine disruption of behavioral processes and provide useful barometers for endocrine disruption of neurobehavioral traits.
Sexual Selection
Darwin's (1871) theory of sexual selection identified the evolutionary mechanisms that account for the majority of sex differences in physical, behavioral, cognitive, and neural traits. These traits facilitate competition with members of the same sex over mates (intrasexual competition) and discriminative choice of mating partners (intersexual choice). Competition and mate choice can be observed in both sexes, but more typically these traits involve male–male competition, as manifested in access to mates or control of the resources males need to reproduce (e.g., nesting sites) and female choice of mating partners (Andersson 1994). We first consider why this pattern is so common and when variation from this pattern (i.e., male choice and female–female competition) is predicted to evolve. We then provide a brief overview and a few examples of intersexual choice and intrasexual competition, and finally we describe why steroidogenic hormone-induced epigenetic changes might be a critical aspect of the evolution and expression of these sexually selected traits.
Compete or Choose?
Darwin (1871) defined and described sexual selection but was not able to determine why males tend to compete and females tend to be choosy. Nearly 100 years later, Williams (1966) and Trivers (1972) proposed that the bias toward competition or choice is tightly linked to any sex difference in amount of parental investment. The sex that provides more than his or her share of parental investment is an important reproductive resource for members of the opposite sex (Trivers 1972). The result is competition among members of the lower-investing sex (typically males) over the parental investment of members of the higher-investing sex (typically females). Members of the higher-investment sex are thus in demand and, as a result, can be more quality minded when it comes to mates. The sex difference in parental investment provides potential inferences to be drawn about which sex will be more biased to compete for mates or engage in mate choice, but this behavioral pattern does not determine which specific traits will be involved in mediating same-sex competition or selection of breeder partners (Andersson 1994).
Sexually Selected Traits
Before describing how and why competition and mate choice influence the evolution and expression of sexually selected traits, we note there are several key aspects of these traits that suggest that they are strongly influenced by epigenetic processes. First, it is well established that prenatal exposure to gonadal sex steroid hormones, especially estradiol and testosterone, can shape the expression of several key developmental genes in the brain and other organ systems by epigenetic control mechanisms (reviewed in McCarthy et al. 2009). Hence, changes in exposure to such hormones during development could have a profound influence on the later manifestation of the traits in adulthood. In addition to setting up the basic framework of sexual development during early life, fluctuating postnatal exposures to sex steroid hormones, particularly at the time of sexual maturation or at the beginning of a new breeding period, program an animal for later reproductive competition, mate choice, and parenting (Adkins-Regan 2005). Clearly, the pursuit of a potential link between epigenetic control of gene expression and the phenomenon of sexual selection merits further consideration. Also as noted earlier, the highly variable and adaptive expression of these traits, their contribution to reproductive competition, and their associated fitness costs ensure that the traits have become highly responsive to environmental conditions, such as parasite load, availability of food, and climate (Geary 2010). One good example is the bidirection relation between immunocompetence and testosterone (Folstad and Karter 1992) (discussed further in the “Condition-Dependent Expression” section) whereby high parasite loads and accompanying heightened immune function appear to suppress adult testosterone production and expression of sexually selected traits, such as the bright plumage of males of many species of birds. Thus, improved health may come at the cost of a compromised, short-term ability to compete with other males for mates. On the other hand, these males will presumably have greater prospects of reproductive success in subsequent breeding seasons.
Intersexual Choice
Under most conditions, female parental investment is a valuable reproductive resource for males, and thus, female choice is more common than male choice (Andersson 1994; Darwin 1871; Trivers 1972). For species in which males parent and for species in which there are large individual differences in females’ ability to successfully rear offspring, male choice is predicted to evolve, although this prediction has not been as thoroughly tested as female choice (Amundsen 2000). In contrast, female choice has been well characterized in birds, fish, insects, reptiles, and mammals (Andersson 1994; Sargent et al. 1998). One critical evolutionary result is the exaggeration of male traits on which females base their choice of breeding partner(s). An example is man-o’-war birds, which include the greater and magnificent frigatebirds (Fregata minor and Fregata magnificens, respectively) (Figure 1), in which females choose mates, in part, based on the male's inflatable gular pouch. Such traits can also include elaborate and inexplicable behaviors, such as skypointing by male blue-footed boobies (Sula nebouxii) (Figure 2).
Figure 1.
The inflated gular pouch of the great and magnificent frigatebirds (Fregata minor and Fregata magnificens, respectively) is a sexually selected trait and likely to be a direct indicator of the male's health to a prospective female partner. Photo taken by Cheryl S. Rosenfeld on North Seymour Island in the Galápagos Archipelago.
Figure 2.

Example of skypointing with beak pointing upward and wings spread out by male blue-footed booby (Sula nebouxii) on right side of the photo, as female on left side observes this behavior, which is part of an elaborate courtship behavioral pattern that has evolved in this species. Photo taken by Cheryl S. Rosenfeld on North Seymour Island in the Galápagos Archipelago.
In many species, traits such as the gular pouch of frigatebirds (shown in Figure 1) are an indicator of the physical or genetic health of the male or serve as a direct marker of the male's ability (e.g., vigor in searching for food) to provide parental investment (Andersson 1994; Zahavi 1975). Male birds with large ornament structures, colorful plumage, or elaborate behaviors are chosen as mating partners more often than their less-flamboyant peers because these traits convey to females information on males’ immunocompetence and physical and developmental health (Hamilton and Zuk 1982). Immunocompetence has a heritable component, and thus offspring sired by healthy males appear to have lower mortality (Saino et al. 1995), as do grand-offspring in at least one species (Reid et al. 2005). However, there can be one potential negative effect (i.e., a cost) to the increases in testosterone needed for the development and elaboration of such traits, which is that the increase in testosterone may be accompanied by immunosuppression that might compromise the health and increase the mortality risks of less-fit males. Genetically fit males, in contrast, are able to maintain the high levels of testosterone needed for full elaboration of sexually selected traits and simultaneously possess a healthy immune system to ward off diseases (Folstad and Karter 1992).
In sum, male ornament structures and behavioral patterns are guiding signals that are strongly affected by the current and prior developmental condition of the male. Female mate choice reflects the evolution of the female's ability to interpret these species-specific traits and respond to these cues. Exaggerated male traits benefit the fittest males because males in poor health and body condition cannot express such traits without risking increased likelihood of further morbidity or mortality (Mougent et al. 2005, 2006; Zahavi 1975).
In species in which males assist in rearing the young or female reproductive success varies, females also may have evolved ornament structures, whose elaboration varies with fitness. In such species, males tend to be selective of their female breeding partners (Amundsen 2000). Roulin and colleagues, for example, determined that male barn swallows (Tyto alba) preferred colorful females as mates and that the offspring of highly ornamented females showed greater resistance to infection than did offspring of other females, suggesting that at least in this species ornamentation is indeed a direct indicator of female quality (Roulin 1999; Roulin et al. 2000).
Intrasexual Competition
Direct, physical, one-on-one, male–male competition is common across species of insects, fish, reptiles, and mammals and results in the evolution of sex differences in the physical traits that are used in this competition for mates (Andersson 1994). The typical result is that physically larger, healthier, and more aggressive males monopolize the reproductive potential of the majority of conspecific females, leading to extreme individual differences in reproductive success, with the dominant males siring many progeny and less-competitive ones fathering few, if any, offspring. Ultimately, this competition governs the evolution of extreme sex differences in physical size, armament and weaponry, and aggressiveness (Darwin 1871), as illustrated by the prominent horns used for direct male–male competition in the bighorn sheep ram (Ovis canadensis) (Figure 3).
Figure 3.
Picture of bighorn sheep ram (Ovis canadensis ). Two males compete by kneeling in front of each other and then trying to maneuver the points of their horns under the body of their competitor. Photo taken by Cheryl S. Rosenfeld in Yellowstone National Park, Wyoming.
However, the traits that facilitate intrasexual competition are not always physical or morphologic in nature. Sexual selection can operate on brain and cognitive traits in the same manner as on physical ones, particularly when the associated abilities and behavioral biases provide reproductive benefits. One well-studied example of cognitive sexual selection involves comparison of related species of voles (Microtus) (Gaulin 1992). In the polygynous meadow vole (M. pennsylvanicus), males compete by searching for and attempting to mate with females dispersed throughout the habitat. In contrast, males of monogamous prairie (M. ochrogaster) and pine voles (M. pinetorum) do not search for additional mates once paired. For meadow voles, intrasexual competition favors males that court the most females, which is possible only through expansion of the home range. This form of male–male competition (so-called scramble competition) should thus result in larger home ranges for male than for female meadow voles, but this home range expansion would not be predicted to be beneficial for monogamous prairie or pine vole males. Indeed, field studies indicate male meadow voles have home ranges that cover 4 to 5 times the area of females’ home ranges, but only during the breeding season (Gaulin 1992). On the other hand, the home ranges of male and female prairie and pine voles do not differ in size. Variation in size of the home range and ability to locate widely dispersed prospective breeding partners leads to the prediction of enhanced spatial abilities in male meadow voles compared with female meadow voles but no sex difference in monogamous prairie and pine voles, in which these traits are not advantageous. A series of laboratory and field studies has confirmed these predictions (Gaulin and Fitzgerald 1986, 1989). The use of molecular biological techniques to determine paternal lineages furthermore supports the finding that males with superior spatial abilities breed with more widely dispersed females and, on average, sire more offspring (Spritzer et al. 2005).
Enhanced brain development, particularly of the hippocampus region that supports spatial and other related cognitive abilities, is an essential component of this form of male–male competition (O’Keefe and Nadel 1978). Any associated sex differences might, therefore, be governed by prenatal or perinatal exposure to sex hormones and activated with adult-onset breeding season increases in testosterone that promote home range expansion (Galea et al. 1996; Perrot-Sinal et al. 1998). This same pattern of species-dependent differences is found for the overall volume of the hippocampus (Jacobs et al. 1990; Sherry et al. 1989). The hippocampus of male meadow voles is larger than that of male prairie and pine voles, but similar comparisons of male and female meadow voles have produced mixed results (Galea et al. 1999; Jacobs et al. 1990). Sex differences in mental tasks may not be related to total hippocampal volume, given that the hippocampus subserves many cognitive functions other than spatial abilities (e.g., working and reference memory). Rather, the sex differences might be more apparent in regions of the hippocampus related to the representation of large-scale space, including the Cornu Ammonis (CA1 and CA3) areas, and entorhinal cortex (Langston et al. 2010; Wills et al. 2010).
Parenting behavior may also be a reflection of a sexually selected trait to the extent it influences mate choice in select species. In monogamous pairs, such as California mice (Peromyscus californicus), males assist in rearing the offspring, including engaging in anogenital licking and grooming of the pups, as illustrated in Figure 4. In this species, the female may use other behavioral cues to assess the potential paternal capabilities of the males (Dudley 1974a, 1974b). Although there is clear evidence that maternal rearing and grooming might induce epigenetic changes in offspring that can persist for generations (Champagne 2008; Szyf et al. 2007), there is a paucity of comparable data on the effects of early paternal behavior on inducing analogous epigenetic changes. However, this field is rapidly evolving as alternative rodent models to the rat, such as the biparental California mouse, will undoubtedly shed light on paternal effects on offspring outcomes (Bredy et al. 2004; Gubernick and Teferi 2000). Paternal behavior assuredly leads to dramatic and long-lasting effects on offspring (Cantoni and Brown 1997), but the epigenetic alterations elicited remain to be determined.
Figure 4.

Parental behaviors of California mice (Peromyscus californicus). While the female nurses the pups, the male engages in anogenital licking and grooming of them. Photo taken by Cheryl S. Rosenfeld.
Effects of Gonad Steroid Hormones on Sex Differences in Behavior
The evolution and proximate expression of the described sex differences in physical, behavioral, and cognitive traits are programmed by prenatal and postnatal exposure of the brain and other organs to sex hormones (Arnold and Breedlove 1985; Morris et al. 2004; Phoenix et al. 1959). Currently, the distinction between steroid-controlled organization of the brain during prenatal and postnatal development is less rigid than originally proposed (as discussed in McCarthy and Arnold 2011). The relative contributions of organization and activation effects can vary from one species to the next, but the distinction between these effects still contributes to our understanding of the influence of hormones on many sex differences (Adkins-Regan 2005). For example, in adult meadow voles, testosterone increases significantly during the breeding season and contributes to the increased activity levels associated with home range expansion (Perrot-Sinal et al. 1998; Turner et al. 1983); castration prevents these season-dependent changes (Rowsemitt 1986). However, the situation is more complicated than simply an outcome of direct androgen action on a set of target genes. In select regions of the brain, the neurobehavioral effects associated with testosterone are secondary to estrogen effects and are an outcome of aromatization of testosterone into estradiol in specific brain regions (Bowers et al. 2010; Konkle and McCarthy 2011; Watson and Adins-Regan 1989a, 1989b). Moreover, as described below, it is increasingly clear that both testosterone and estradiol can influence the brain through epigenetic mechanisms. Testosterone, after being first converted to estradiol, is also necessary for development of the hippocampus itself (Bowers et al. 2010; Konkle and McCarthy 2011). Thus, depending on the brain region, both testosterone and estradiol are crucial for correct programming of normal neurobehavioral development.
Sex steroid hormones also play a key role in the timing of the transitions between prematuration stages of development, in the scheduling of reproduction, and in determining onset of senescence. Each developmental transition necessitates the ability to induce rapid, in some cases reversible but in other cases permanent, modifications in behavior and physiology. For example, prenatal exposure to estradiol is necessary for establishing sex differentiation of the central nervous system, and estradiol concentrations quickly plummet following the perinatal period and remain fairly low during the juvenile period. At the onset of adulthood, increase in aromatization of testosterone leads to greater estradiol concentration in some brain regions, which then coordinates the expression of sexually selected traits such as mating and parenting behaviors (Adkins-Regan 2005; McCarthy 2008). Thus, the functional role of a single hormone (e.g., estradiol in this example) is expected to differ across developmental stages, and, based on the potential rapid nature of these transitions, these steroid-induced behaviors are likely modulated by environmental epigenetic changes during the various life-stage transitions. Such mechanisms might be particularly important for the elaboration of sexually selected traits because their expression varies based on sex and development, breeding season, and current physical conditions.
Steroid hormones might trigger epigenetic changes during early and postnatal development and might also transmit important nongenomic information that leads to later vital reproductive behaviors at sex maturity. This complex relationship between life history, hormone signaling, and environmental cues lays the foundation for the epigenetic regulation of sex differences. Because males and females across various species possess contrasting life histories, there is presumably marked underlying sexual dimorphism in systemic, cellular, and molecular mechanisms driving these differences in all sexually reproducing species, including humans.
Condition-Dependent Expression
A critical feature of sexually selected traits is greater phenotypic variation than with naturally selected traits, partially because of dependence on more genetic loci and overall male condition (Pomiankowski and Møller 1995; Rowe and Houle 1996). Sexual selection also results in the evolution of greater trait complexity, such as more coloration or larger size, for one sex or the other. Greater complexity also suggests polygenic control, the need for prolonged developmental period, better conditions (e.g., higher quality food), or the combination of genetic and environmental factors for full expression (Pomiankowski and Møller 1995). Thus, the sex that has undergone intense intrasexual competition or intersexual choice is rendered more vulnerable to developmental, social, and ecologic disturbances and displays more phenotypic diversity (Pomiankowski and Møller 1995; Rowe and Houle 1996). The vulnerable and variable sex is typically the male, but there are presumably sexually selected traits in females that might also be susceptible to environmental insults, including hormonally induced epigenetic alterations.
This vulnerability is presumably the cost associated with the evolution of mechanisms that lead to exaggerated traits and account for the suppression of these costly behaviors in animals in poor body health and condition. For such animals, avoiding contemporary and potentially lethal competition provides the prospect survival to compete and breed in the future as they age.
Evidence for Disruption of Epigenetic Pathways and Sexually Selected Traits by EDCs
Because developmental exposure to steroid hormone governs many epigenetic processes in the brain responsible for later adult behaviors, including the expression of sexually selected traits (Arnold and Breedlove 1985; Morris et al. 2004; Phoenix et al. 1959), such traits might be rendered susceptible to EDCs, including chemicals such as BPA and vinclozolin (Crews et al. 2007; Jašarević et al. 2011). Figure 5 provides a working hypothetic model of how these EDCs and other environmental factors could modulate expression of sexually selected traits through epigenetic means. There is increasing evidence that EDCs can induce DNA methylation and histone protein changes in the brain (Bromer et al. 2010; Dolinoy et al. 2007; Doshi et al. 2011; Ho et al. 2006; Tang et al. 2012; Weinhouse et al. 2011; Weng et al. 2010; Yaoi et al. 2008). Additionally, there is mounting evidence that developmental exposure to EDCs can disrupt normal steroid-induced neurobehavioral responses (Cox et al. 2010; Della Seta et al. 2005; Gioiosa et al. 2007; Jašarević et al. 2011; Palanza et al. 1999, 2002; Patisaul and Bateman 2008; Xu et al. 2010, 2011). In the following section, we consider a few examples of these EDC-induced epigenetic and neurobehavioral changes.
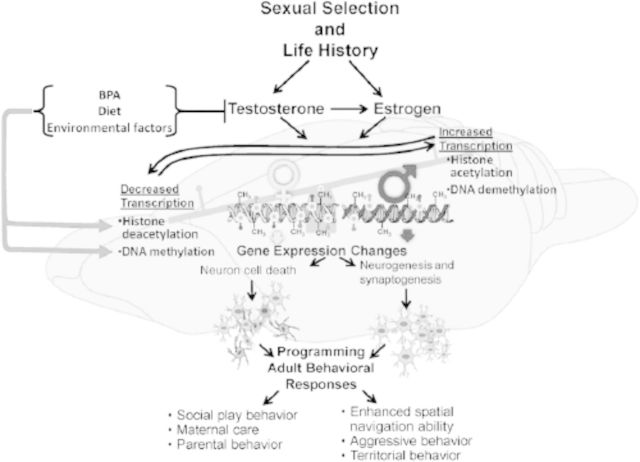
Figure 5.
Working hypothetical model of how endocrine disrupting compounds (EDC) such as bisphenol A (BPA) might disrupt sexually selected traits. Steroid hormones, including estrogen and testosterone, can underpin various epigenetic changes, including changes in DNA methylation patterns and histone protein modification. These epigenetic changes can lead to sex-dependent alteration in the neural architecture. Consequently, these epigenetic and morphologic differences underpin sexual differentiation of the brain and synchronization of adult behaviors. Given the sensitivity of these traits to developmental steroid hormone concentrations, exposure to EDCs that disrupt hormone signaling during these critical life history stages has the potential to disrupt the full expression of sexually selected traits. These traits thus might be employed as biomarkers for endocrine disruption and serve as the framework for experiments designed to test the effects of EDCs on brain, cognitive, and behavioral development in males and females.
BPA exposure can induce epigenetic changes in the DNA methylome (Bromer et al. 2010; Dolinoy et al. 2007; Doshi et al. 2011; Ho et al. 2006; Tang et al. 2012; Weinhouse et al. 2011; Weng et al. 2010; Yaoi et al. 2008). Exposure to BPA during the fetal period of mouse development has been proposed to lead to global DNA methylation changes in the developing forebrain, with some genes hypermethylated and others hypomethylated in exposed mice compared with control mice (Yaoi et al. 2008). However, this study did not try to correlate the epigenetic changes with later behavioral outcomes. A more recent experiment has indicated that developmental exposure to BPA induces persistent aberrant overexpression of certain genes, including ones encoding the DNA methyl transfersases (Dnmt3a/3b) and the putative demethylases, methyl-CpG binding domain proteins, Mbd2/4—changes that are correlated with later adult onset pathologies, including cancer (Tang et al. 2012).
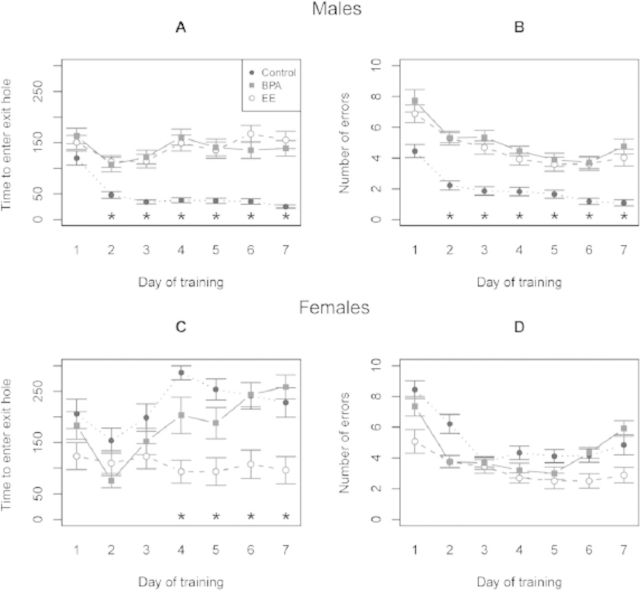
Additionally, there have been several reports that developmental exposure to BPA can disrupt later adult traits, including learning and memory, anxiety-like behaviors, and parental behavior, some of which are sexually dimorphic (Cox et al. 2010; Della Seta et al. 2005; Gioiosa et al. 2007; Jašarević et al. 2011; Palanza et al. 1999, 2002; Patisaul and Bateman 2008; Xu et al. 2010, 2011). Similar to meadow voles, male deer mice (Peromyscus maniculatus bairdii) expand their home range and exhibit enhanced spatial navigation ability upon reaching sexual maturity to locate prospective mates that are widely dispersed throughout the environment. We have recently demonstrated that developmental exposure of deer mouse males to either BPA or ethinyl estradiol through the maternal diet leads to disruption of these spatial navigation abilities when the animals become sexually mature, as assessed in the Barnes maze (Figure 6). These perturbed responses are likely caused by a failure among BPA- and ethinyl estradiol–exposed males to learn direction and position intra-maze cues that aid in navigating a large-scale space (Figure 7). In contrast with the poor responses observed in ethinyl estradiol–exposed males, female deer mice exposed developmentally to ethinyl estradiol became masculinized in their behavioral responses. These treated females quickly located the escape hole and adopted a search strategy similar to that observed in control males (Figures 6 and 7). No comparable response, however, was observed in female deer mice exposed to BPA, suggesting that BPA does not act solely as an estrogenic compound. BPA- and ethinyl estradiol–exposed males were also more anxious and demonstrated decreased exploratory behaviors compared with control, nonexposed males (Jašarević et al. 2011). It should be emphasized that in these experiments with deer mice there were no overt differences in general phenotype, including neuromuscular, olfactory, auditory, and visual senses between the animals exposed to BPA and those not exposed. The main distinguishing phenotype was in the behaviors that would contribute to reproductive success, which would likely pass unnoticed without behavioral testing.
Figure 6.
Effects of early developmental exposure to bisphenol A (BPA) and ethinyl estradiol (EE) on spatial learning and memory of adult male and female deer mice (Peromyscus maniculatus) in the Barnes maze. Latency (i.e., time required to escape the maze) across days of training for males (A) and females (C) (Mean ± SEM). Number of escape errors across days of training for males (B) and females (D) (Mean ± SEM, *p < 0.01). Adapted from Jašarević and colleagues (2011).
Figure 7.
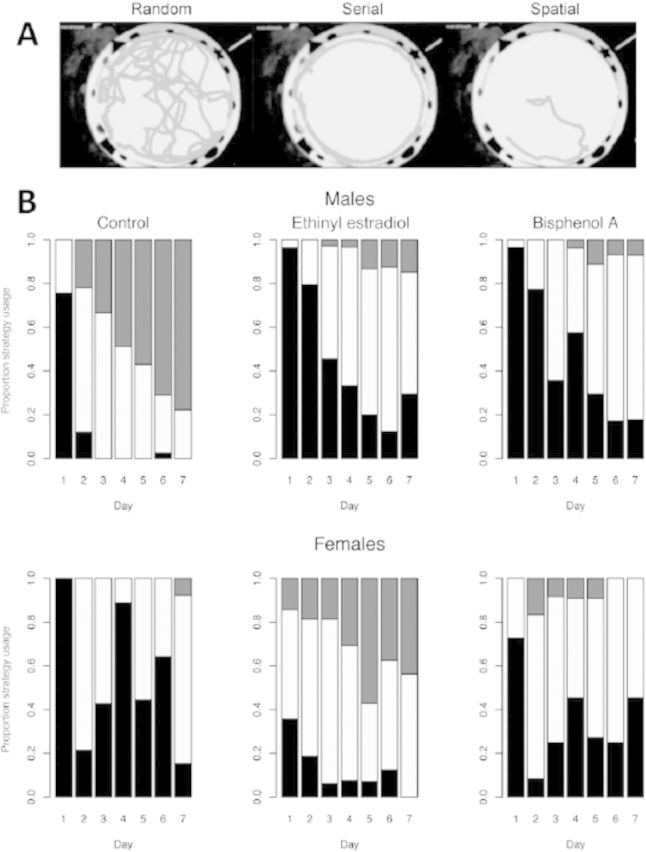
Effects of early developmental exposure to bisphenol A (BPA) and ethinyl estradiol on spatial search strategy of adult male and female deer mice (Peromyscus maniculatus) in the Barnes maze. (A) Examples of composite images from single animals tracked from entry to escape illustrating different spatial strategies used to exit the maze. (B) Distribution of different spatial strategies according to sex, diet exposure, and day of training. During the initial training period (day 1), most animals navigated by using a random strategy (black), followed by a serial search strategy (white). The most efficient spatial search strategy (gray) emerged when the animals began to use direction and position intra-maze cues. By day 3 of training, control males used more efficient strategies than control females and ethinyl estradiol– and BPA-exposed males, who in turn did not differ on any day (p < 0.0002). Ethinyl estradiol–exposed females used more efficient strategies than control and BPA-exposed females on all days except day 2. Adapted from Jašarević and colleagues (2011).
Although epigenetic alterations in the developing brain after developmental exposure to BPA have been demonstrated previously (Yaoi et al. 2008), it is not clear whether the mechanisms for disrupting sex differences in spatial abilities and anxiety behavior in Peromyscus are solely epigenetic in origin. It is unlikely that alterations in steroid hormone levels account for all of these differences, as we reported previously that developmental exposure to BPA had no major effects on adult serum steroid concentrations of testosterone and corticosterone in deer mouse males (Jašarević et al. 2011). Skinner and colleagues (2008) reported a similar finding with rats exposed to the antiandrogenic fungicide vinclozolin. Therefore, disruption of the adult endocrine system does not appear to be a factor in the deficits observed, although this does not exclude the possibility of permanent disorganization during the prenatal period. Nevertheless, disruption of sex-specific spatial navigation ability and anxiety-like behaviors presumably has epigenetic underpinnings, such as DNA methylation or chromatin remodeling alterations.
In deer mice, females have the final choice in their breeding partners. A mate choice experiment demonstrated that both control and BPA-exposed females preferred control males on an approximately 2:1 basis over BPA-exposed male deer mice (Figure 8). These findings indicate that developmental exposure of males to BPA and ethinyl estradiol not only disrupts their spatial navigational abilities but also compromises their attractiveness to females, presumably through olfactory cues (Penn and Potts 1999). Whether or not these effects of BPA and ethinyl estradiol on sexually selected traits have epigenetic underpinnings remains uncertain, but in some species of fish, ethinyl estradiol exposure is correlated with sex-dependent DNA methylation changes (Contractor et al. 2004; Stromqvist et al. 2010).
Figure 8.
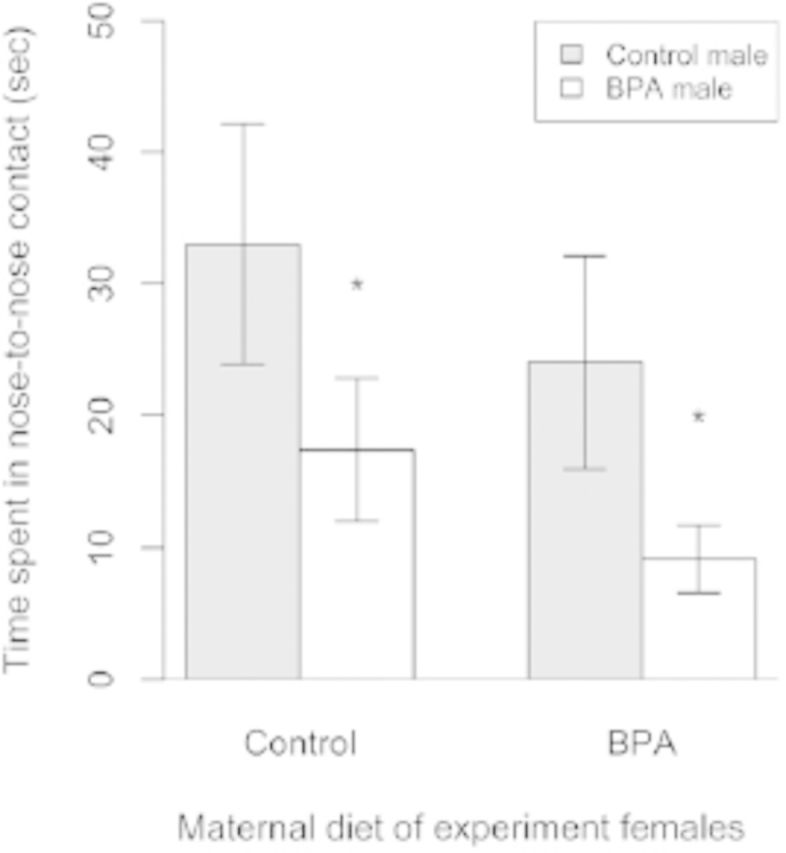
Female choice of control versus bisphenol A (BPA)–exposed males. Control and BPA-exposed females exhibited longer duration of nose-to-nose contact (Mean ± SEM) with control males than with BPA-exposed males. *p < 0.05. Adapted from Jašarević and colleagues (2011).
The utility of sexual selection for identifying behaviors most susceptible to disruption by EDCs extends far beyond BPA and behavior in rodents. As discussed, sexually selected traits include the elaboration of physical ornaments (e.g., horn size or plumage), physical size, and adult behavior. Exposure to EDCs and other environmental toxins is therefore predicted to disrupt a full range of sexually selected traits across a myriad of mammalian and nonmammalian species. In support of this hypothesis, decreases in sexually dimorphic physical size of alligators (related to male–male competition) born in contaminated streams of Lake Apopka (Guillette et al. 1995) and reduced plumage coloration around the facial region of male American kestrels (Falco sparverius) exposed to polychlorinated biphenyls have been reported (Bortolotti et al. 2003). More recently, Holliday and Holliday (2011) observed growth retardation and deficits in bone remodeling in male diamondback terrapins (Malaclemys terrapin) developmentally exposed to PCBs; however the authors did not assess whether small body size in PCB-exposed males lessened their ability to compete with other males, although it would seem likely to do so. Nonetheless, bone remodeling and turnover are integral components of physiology in all vertebrate animals, so skeletal pathologies would have far-reaching impacts on feeding and locomotion, calcium deposition during egg shelling, and ultimately intrasexual competition and reproductive success (Holliday and Holliday 2011).
Disruption of sexually selected traits may also lead to a stable imprint across generations. Crews and colleagues (2007) have provided evidence for the transgenerational effects of exposure to the antiandrogenic fungicide vinclozolin on male quality and female choice. In particular, male rats whose great grandmothers had been treated with vinclozolin were less attractive to females. Moreover, exposure to vinclozolin led to inherited alterations in the DNA methylome of the brain (Anway and Skinner 2008; Guerrero-Bosagna et al. 2010; Skinner et al. 2008). In the grand-offspring (i.e., third generation [F3]) males and females of females that had been exposed to vinclozolin, overt gene expression changes in the hippocampus and amygdala were correlated with the behavioral differences (Skinner et al. 2008). Yet, males and females were not affected identically. Whereas F3 generation males demonstrated decreased anxiety-like behaviors compared with control males, F3 generation vinclozolin females were more anxiety prone than control females (Skinner et al. 2008). In order to grasp how EDCs might induce behavioral disturbances in exposed F1 generation offspring and their descendants, we provide a condensed overview of recent advances in the understanding of how sex steroid hormones induce epigenetic changes in the brain.
Epigenetic Effects of Sex Steroid Hormones in the Brain
Although steroids can induce a variety of effects by binding to their cognate receptors, which are themselves transcription factors that bind to the promoter regions of sets of target genes, it is increasingly being recognized that many steroid-induced changes in gene expression originate through alterations in the epigenome (Champagne et al. 2006). Much of the information has come from studies on primary sex differences (i.e. secondary sex characteristics, onset of sexual maturity, and differences in sexual behavior such as mounting and lordosis), but it is plausible that homologous mechanisms operate to influence the expression of those physical, behavioral, cognitive, and neural traits that are under strong sexual selection for the reasons noted above.
DNA Methylation
DNA methylation of CpG islands is now recognized as a driving force governing gene expression in various cell types, including neuron cells of the brain, and most likely contributes to the sexually dimorphic patterns in behaviors that characterize the reproductive strategies of species across a wide range of taxa (Imamura et al. 2001; Jones and Takai 2001; Lyko et al. 2010; McCarthy et al. 2009). One dramatic example occurs in honey bees, in which DNA methylation profoundly affects behavior patterns in the female bees (Lyko et al. 2010). Worker and queen female bees are genetically identical, but larvae destined to be queens are fed a royal jelly, which is known to contain the histone deacetylase, phenyl butyrate, as well as other uncharacterized nutrients, whereas those destined to become workers are fed a less-esoteric diet. As a result, there are marked differences in the DNA methylome of the brains of female queen bees versus worker castes, and these DNA methylation differences seemingly lead to alternative splicing and gene regulatory differences that guide how these very different kinds of females behave (Lyko et al. 2010).
In mammals, one particular way that sex steroid hormones might orchestrate sex-dependent epigenetic changes in the brain is by altering the activities of key DNA methyl transferases (DNMTs). For example, female rats express greater amounts of Dnmt3a messenger RNA (mRNA1) and its associated protein in the amygdala than males, although not within the preoptic and medial basal hypothalamus on postnatal day 1 (Kolodkin and Auger 2011). Moreover, when neonatal females are exposed to dihydrotestosterone and estradiol at concentrations sufficient to masculinize the male brain at birth, the expression of DNMT3a falls in the amygdala (Kolodkin and Auger 2011) and adult female behavior becomes masculinized.
DNMT3a and DNMT3b have been proposed to play an essential role in organizing the integration of contextual fear-induced memories (Miller and Sweatt 2007). Upon triggering fear conditioning, the mRNA expression pattern of these genes is increased in the CA1 region of the hippocampus, but silencing these enzymes with 5-azadeoxycytidine prevents this fear-induced memory consolidation (Miller and Sweatt 2007). Whether or not steroid hormones alter the expression of these DNMT isoforms in the hippocampus, thereby leading to sexually dimorphic neural phenotypes in terms of fear responses, is unclear, but this is a testable hypothesis.
Alterations in the expression of proteins that recognize and bind to methylated DNA, usually termed methyl-CpG binding proteins, such as methyl-CpG-binding protein 2 (MECP21) can provide behavioral phenotypes in humans and rodents (Defossez and Stancheva 2011). Once bound to methylated DNA, these proteins recruit additional corepressor proteins and histone deacetylase enzymes (HDACs1), and their combined actions ultimately lead to chromatin modification and decreased gene transcription (Nan et al. 1998). Humans with mutations in the gene MECP2, which is X linked, can develop Rett syndrome (Amir et al. 1999), a common cause of mental retardation (Hagberg 1985). At least in rodents, sexually dimorphic patterns in Mecp2 expression in the brain emerge as early as postnatal day 1, with females exhibiting higher expression of this transcript than males (Kurian et al. 2007). Juvenile male rodents that had been subjected during early neonatal life to small interfering RNA suppression of Mecp2 exhibit disturbed juvenile social play behavior, a behavioral phenotype that is more frequent in males than females (Olioff and Stewart 1978). Clearly, in this instance, a large-scale, genome-wide methylation change in the brain can be linked to behavioral abnormalities that are sexually dimorphic in their expression.
Sex steroids may induce masculinization and feminization in various regions of the rodent brain through the DNA methylation of the genes (Esr1 and Esr2) encoding the estrogen receptor #57441; (ESR1) and #57442; (ESR2). For example, the methylation status of the Esr1 gene in the rat brain can be altered by external stimulus, including maternal care (Champagne et al. 2006). Female pups reared by dams that engage in high-level licking and grooming demonstrate reduced Esr1 promoter methylation and increased expression of the gene in the preoptic area compared with females whose mothers were less attentive (Champagne et al. 2006).
Sexually dimorphic patterns of Esr1 expression occur in the medial preoptic area (mPOA1) of rats. The Esr1 promoter is hypermethylated, and expression is reduced in males relative to females (DonCarlos and Handa 1994; Kurian et al. 2010; Yokosuka et al. 1997). Treatment of neonatal female pups with estradiol leads to a masculinized pattern of Esr1 promoter methylation (Kurian et al. 2010), further supporting the idea that that hormones govern the sexually dimorphic differences in methylation status of the Esr1 promoter that, in turn, contribute to sex differences in behavior.
Esr1 expression in other regions of the brain is crucial for sculpting regional masculinization (Kudwa et al. 2006). Female rat pups treated with the ESR1 agonist propyl-pyrazole triol demonstrate increased cell death in the anteroventral periventricular nucleus but exhibit enhanced cell survival in the sexually dimorphic nucleus of the preoptic area, the net outcome of which is regional masculinization of the hypothalamus and a loss of female sex receptivity when the pups reach sexual maturity (Patchev et al. 2004). Further evidence that full Esr1 expression is necessary for providing correct neural phenotypes is provided by the behavioral abnormalities that result from suppression or knockdown of Esr1 expression with antisense oligonucleotides against this gene (McCarthy et al. 1993). In addition, Esr1 knockout mice exhibit both impaired male (Ogawa et al. 1997) and female sexual behavior (Ogawa et al. 1996).
In contrast, Esr2, which encodes the second estrogen receptor form, ESR2, has been postulated to mediate defeminization of the brain (Kudwa et al. 2005). In the developing ovary, neonatal exposure to estrogenic chemicals induces hypermethylation and subsequent decreased expression of Esr2 (Zama and Uzumcu 2009), but it remains to be determined whether similar effects occur in the brain. However, female mice treated with the ESR2 agonist compound diarylpropionitrile during the critical perinatal window do not demonstrate proper lordosis behavior when they reach sexual maturity (Kudwa et al. 2006). Diarylpropionitrile also blocks proper operation of the anteroventral periventricular gonadotropin surge generator (Bodo and Rissman 2006), resulting in persistent estrus (Patchev et al. 2004). By contrast, male mice that have targeted deletion of Esr2 are more prone to exhibit lordosis behavior than their wild-type male counterparts (Kudwa et al. 2005). Within the brain, Esr2 appears to be the dominant estrogen receptor form in the hippocampus (Shughrue et al. 1997) and may regulate anxiety-like and depression-associated behaviors (Krezel et al. 2001; Rocha et al. 2005). Whereas Esr1 is susceptible to DNA methylation during the neonatal period, steroid-induced methylation of Esr2 appears to occur after sexual differentiation of the brain (Kudwa et al. 2005) and is expressed in a sexually dimorphic manner. Estradiol treatment of females leads to even greater Esr2 methylation in the hippocampus than in control males and females (Kudwa et al. 2005). Another study has confirmed that Esr2 methylation status does not change at birth but shows later transient, site-specific, sex and estradiol treatment differences at weaning (postnatal day 20) and other site-specific DNA methylation differences emerging at adulthood (postnatal day 60) (Schwarz et al. 2010).
Some of the sexually dimorphic differences in the brain might be directly attributed to testosterone and to differential regional expression of Ar, the gene encoding its cognate receptor. In the cortical region of rats and mice, males express higher amounts of Ar mRNA than females, and the DNA methylation pattern of the Ar core promoter is increased in response to testosterone but decreased with estradiol exposure (Kumar and Thakur 2004). Although the brain cortex is not classically considered to demonstrate sexually dimorphic differences in function, the differential expression of Ar in this region suggests that there might be subtle differences that remain to be elucidated. Moreover, there may be other regions of the brain that demonstrate comparable differences in epigenetic and expression status of Ar between the sexes. Ar expression can also be effected by its coactivators (e.g., jumonji domain-containing 1c, JMJD1C, which is expressed in the brain) (Wolf et al. 2007). Moreover, JMJD1C is a potential demethylase (Wolf et al. 2007). Together, these data indicate that sex steroids influence the development of the brain by modulating the DNA methylation status of the control regions of key genes, including ones encoding their own receptors. Such changes presumably appear to yield effects, some of considerable magnitude, on both primary and secondary sex characteristics.
Histone and Corepressor Proteins
Methylation of DNA is not the sole means whereby the read-out status of genes can be programmed in a heritable manner without altering the DNA sequence. Another example is through modification of the histone proteins that bind DNA. Three of the best-characterized modifications to histone proteins are acetylation, deacetylation, and methylation (reviewed in Mehler 2008). Histone acetylase transferases are, for example, able to introduce acetyl groups onto the side chains of lysine residues on histones and mechanistically alter their net charge and shape in such a manner that transcription factors are able to gain easier access to the control sequences. Conversely, HDACs remove acetyl groups, restore the basic charge of epsilon amino groups on lysine residues, and generally promote binding of histones to DNA (reviewed in Mehler 2008). Similarly, methylation of arginine residues adds bulk and influences packing of histones into the nucleosome structure. Determination of the transcription state of histones is largely determined by the histone code, a combination of acetyl, methyl, and other marks on histone tail residues, and these alterations contribute to chromatin status, such as transcriptionally active (euchromatin) and inactive chromatin (heterochromatin) (reviewed in Mehler 2008). How these marks are maintained as the cells undergo mitosis is unclear, but additional factors presumably play a role in stabilizing the complexes and maintaining the transcriptional regulation of genes over time.
There are a number of examples of sex steroids directly and indirectly altering histone proteins and accompanying chromatin remodeling in neurons of various brain regions (reviewed in McCarthy et al. 2009; Qureshi and Mehler 2010). One such example is in the bed nucleus of the stria terminalis (BNST1). The BNST is part of the limbic forebrain, where several structural differences distinguish males and females, the most obvious being in the principal nucleus (BNSTp) (Forger et al. 2004; Guillamon et al. 1988; Hines et al. 1985, 1992). Males from all species examined (mice, rats, guinea pigs, and humans) possess a larger BNSTp than females (Forger et al. 2004; Guillamon et al. 1988; Hines et al. 1985, 1992). In rodents at least, this size difference is due to increased apoptosis in the female in the immediate postnatal period, which has been attributed to differences in testosterone exposure during parturition (Chung et al. 2000; Gotsiridze et al. 2007). It has been postulated that the ultimate cell number in the BNSTp is related to the extent of histone protein acetylation occurring after the postnatal testosterone exposure (Murray et al. 2009).
To test this hypothesis, the HDAC inhibitor valproic acid was administered to neonatal mice during this critical window of time. This treatment increased histone protein 3 acetylation in the brain (Murray et al. 2009), and, by 3 weeks of age, males treated with valproic acid and females cotreated with both valproic acid and testosterone (i.e., androgenized females) had volume and cell numbers in the BNSTp comparable with that of control females. These studies thus demonstrate that inhibition of HDAC activity prevented the normal increase in cell numbers and tissue volume that occurs in males, which is believed to be necessary to support masculine sexual behaviors regulated by this region. Additionally, androgenized females treated with valproic acid also demonstrated a reduction in cell numbers in this region of the brain; thereby further buttressing the notion that testosterone is essential in inducing epigenetic changes that masculinize the morphology of the BNSTp.
Examination of the genes whose expression pattern were altered by valproic acid exposure revealed that they were largely involved in controlling either the cell cycle or apoptosis and included the proapoptotic factor Bax (Glaser et al. 2003; Menegola et al. 2006). Hence, testosterone is believed to increase HDAC activity, leading to binding of histone proteins to DNA-encoding proapototic transcripts and suppression of such genes in males. However, in normal females, the absence of or very low levels of testosterone result in low levels of HDAC in the BNSTp, with the net effect that the histone proteins remain in an acetylated state and separated from the DNA encoding these proapoptotic transcripts, allowing for upregulation of the proapoptotic genes in females and subsequent cell death in this region. Furthermore, a recent report with mice lacking Esr1, Esr2, or Cyp19A1 demonstrated that the masculinization of BNSTp is dependent upon aromatization of testosterone to estrogen and binding of this hormone to ESR1 (Tsukahara et al. 2011).
Histone deacetylation during brain development has recently been linked to the permanent masculinization of primary sexual behavioral traits in rats, such as mounting, intromission, and ejaculation (Matsuda et al. 2011). During the critical period of sexual differentiation, histones associated with two key masculinization regulatory genes, Esr1 and Cyp19A1 (P450 aromatase), in the mPOA, which regulates male sexual behavior, underwent acetylation, a change that was accompanied by correspondingly increased sexual activity of the male rats. This change appeared to be the result of more HDAC 2 and 4 selectively binding to the promoters of the two genes in males than in females. Additionally, newborn male rats treated with either the HDAC inhibitor trichostatin A or antisense oligonucleotides directed against Hdac2 and 4 mRNA, had reduced amounts of ESR1 and CYP19A1 in the mPOA and suppressed key male sexual behaviors in adulthood (Matsuda et al. 2011).
Nuclear receptor corepressor proteins, such as NCOR1 (Horlein et al. 1995; Lavinsky et al. 1998; Yoon and Wong 2006), which suppresses the expression of androgen and estrogen receptors, might also play a role in masculinization of the brain (Lavinsky et al. 1998; Yoon and Wong 2006). NCOR1 binds to HDAC, thereby modulating histone deacetylation, and it also binds to methyl-binding proteins, including ZBTB33 (formerly called KAISO) (Yoon et al. 2003) and MECP2 (Cukier et al. 2008; Kokura et al. 2001). In the developing amygdala, estradiol partially leads to a sexually dimorphic pattern of expression for NCOR1 (Jessen et al. 2010). In the developing rat amygdala, small interfering RNA targeted against Ncor1 blunts sex differences in juvenile social play behavior in males, as measured by the reduced time the treated males engaged in wrestling and boxing, biting, pinning, or pouncing compared with control males (Jessen et al. 2010). Additionally, suppression of Ncor1 expression within this brain region increased anxiety-like behavior in juvenile males and females (Jessen et al. 2010). These findings might thus have relevance to male–male competition and overall mental health.
Short Noncoding RNA
The human genome consists of many noncoding RNAs (Mehler and Mattick 2006) that have a multiplicity of roles, including ones involved in regulating DNA methylation, chromatin organization, transcription, posttranscriptional RNA processing, and translation (Amaral et al. 2008; Mattick et al. 2009). Researchers are at the nascence of understanding the roles of noncoding RNA in sexually dimorphic regulation of the brain. However, there have been some preliminary assessments in mice examining specific microRNA, the largest group of noncoding RNA, in various regions of the brain, including the hippocampus, frontal cortex, and cerebellum, of males and females (Koturbash et al. 2011). There were clear differences in the expression of particular microRNAs in males versus females. For example, microRNA329, which is required for activity-dependent dendritic outgrowth of hippocampus neurons (Khudayberdiev et al. 2009), was selectively upregulated in the hippocampus of males. The role of microRNA in governing dendritic morphogenesis during development, synaptic transmission, long-term memory retrieval (Bredy et al. 2011), and other aspects of neural plasticity and memory might also explain sex differences in behavior and cognition.
Conclusions
We propose that sexually selected traits might be useful for examining the evolution of sex steroid–induced epigenetic mechanisms in the brain and other organs because these traits are dependent on pre- and postnatal sex steroid hormone exposure and, unlike naturally selected traits, their expression can be highly dynamic within and across individuals. The mechanisms underlying the dynamic expression of sexually selected traits are not well understood, but the fact that these traits are transiently expressed and highly responsive to environmental conditions suggests that these behaviors have epigenetic foundations. In support of this hypothesis, there is mounting evidence that sex steroid hormones induce a wide range of epigenetic effects in the brain (Imamura et al. 2001; Jones and Takai 2001; Lyko et al. 2010; McCarthy et al. 2009) and influence the expression of sexually selected traits (Adkins-Regan 2005; Geary 2010). Although elevated hormones, including testosterone and estradiol, govern the expression of such traits, excess of any of these hormones may also compromise the overall health of a given animal (Folstad and Karter 1992). Health status of an animal can vary across breeding seasons, and thus suppression of sexually selected traits during one season might increase the likelihood of survival and, correspondingly, the opportunity to compete in the next season (e.g., Mougent et al. 2005, 2006). In essence, there are potentially increased risks for breeding males exhibiting the high testosterone concentrations needed to express various sexually selected traits, including those considered anatomical, such as brightened plumage in birds and antlers in ruminant species, as well as behaviors, such as male–male aggression or increased spatial navigation ability that might increase the risk for predation. For these reasons, serum testosterone concentrations in such males declines after the breeding season, and these traits are thus confined only prior to and at the time the animals seek out mates, suggesting again that sexually selected traits are tightly regulated by environmental cues, including daylight and other factors, operating through the endocrine system,
Epigenetic mechanisms that lead to stable, irreversible marks for some genes and plasticity for others, might thus regulate the initial programming of sexually selected traits and account for their dynamic expression across breeding seasons. One problem is that most of the studies that have examined epigenetic regulation of neurobehavioral responses have tested these phenotypes in mouse and rat models, in which sexually selected traits are not as well defined or as exaggerated as in some other species that might provide superior models. Thus, one important issue is the determination of the degree and specificity of sexually dimorphic differences in the epigenomes of model species possessing clearly defined sexually selected aspects of phenotype. Although prior epigenetic studies with mice and rats can provide a useful framework to address this important issue, its resolution will not be easily achieved until the genomes of the emerging model species are sequenced and annotated, especially in the gene control regions where sexually dimorphic differences in DNA methylation status are presumed to have evolved. An attendant question is the extent to which modifications of the genome accompany the fluxes in phenotype associated with the onset and termination of the breeding period.
Finally, genera such as Microtus and Peromyscus, in which closely related species display very different courtship patterns and parental behaviors in rearing young, offer the opportunity to examine the brain changes accompanying these contrasting forms of behaviors, which are vital to the reproductive success and survival of the respective species. For example, are the highly evolved, sexually selected, spatial navigation abilities of the male deer mouse, P. maniculatus, which allow them to breed successfully in the wild, and the characteristic territorial aggression and male parenting behaviors of the monogamous male California mouse, P. californicus, accompanied by sexually dimorphic, gene-specific, epigenetic modifications in relevant regions of the brain? In the case of male P. californicus, it might be predicted that, at the time of parenting, areas of the brain that regulate parenting behavior, especially the mPOA might be especially affected (Champagne et al. 2006; Weaver 2007). In support of this notion, California mouse males are known to exhibit increased oxytocin immunoreactivity in the mPOA and hypothalamus at the time they are assisting in rearing their young (Lambert et al. 2011). It will be essential to determine not only whether EDCs disturb such activities of the male mice but also whether such chemicals intervene in any correlative patterning of brain gene expression.
Another final issue is whether or not adult exposure to EDCs interferes with the expression of genes governing sexually selected traits that have been programmed epigenetically during early development (e.g., in utero) but still require an adult-enhancing surge in sex steroid hormones for full manifestation in the adult animal? It is known, for example, that Esr2 methylation status is sex dependent and affected by later adult hormonal exposure (Schwarz et al. 2010), whereas castration of adult male rats leads to increased DNA methylation of the vasopressin (Avp) gene within the BNST but decreased methylation of the Esr1 gene in the same brain region (Auger et al. 2011). Along these same lines, DNA methylation of Esr1 in the brain cortex is regulated by estradiol during early development and at adulthood in females but not males (Wilson et al. 2011). Collectively, these data suggest that sexually selected traits might be vulnerable to adult as well as early developmental exposure to EDCs. We suggest that sexually selected traits can offer finely tuned sensors for judging whether or not environmental chemicals are having insidious, previously unsuspected effects on the health and long-term ability of a species to reproduce, provided that appropriate animal models are chosen for investigation.
Acknowledgments
Paizlee T. Sieli, Erin E. Twellman, Todd R. Schachtman, Thomas H. Welsh Jr., Lisa Caldwell, Denise A. Warzak, Joseph Reddy, and Scott Williams contributed to work described herein. We thank Dr. R. Michael Roberts for his critical review of the manuscript and Donald L. Connor for his assistance creating the working hypothetical model diagram of how EDCs might affect sexually selected traits (Figure 5). This work was supported by a Mizzou Advantage grant (to C.S. Rosenfeld and D.C. Geary) and a National Institutes of Health Challenge Grant (RC1 ES018195 to C.S. Rosenfeld).
Biography
Eldin Jašarević, BA, is a PhD candidate in the Department of Psychological Sciences, the Interdisciplinary Neuroscience Program, and the Bond Life Sciences Center; David C. Geary, PhD, is a Curators’ Professor in the Department of Psychological Sciences and the Interdisciplinary Neuroscience Program; and Cheryl S. Rosenfeld, DVM, PhD, is an associate professor in the Bond Life Sciences Center and the Department of Biomedical Sciences, all at the University of Missouri, Columbia.
Footnotes
Abbreviations that appear ≥3x throughout this article: BNST, bed nucleus of the stria terminalis; BPA, bisphenol A; EDC, endocrine-disrupting compound; HDAC, histone deacetylase enzymes; MECP2, methyl-CpG-binding protein 2; mPOA, medial preoptic area; mRNA, messenger RNA.
References
- Adkins-Regan E. 2005. Hormones and Animal Social Behavior. Princeton NJ: Princeton University Press [Google Scholar]
- Alter MD, Rubin DB, Ramsey K, Halpern R, Stephan DA, Abbott LF, Hen R. 2008. Variation in the large-scale organization of gene expression levels in the hippocampus relates to stable epigenetic variability in behavior. PLoS One 3:e3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral PP, Dinger ME, Mercer TR, Mattick JS. 2008. The eukaryotic genome as an RNA machine. Science 319:1787–1789. [DOI] [PubMed] [Google Scholar]
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. 1999. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet 23:185–188. [DOI] [PubMed] [Google Scholar]
- Amundsen T. 2000. Why are female birds ornamented? Trends Ecol Evol 15:149–155. [DOI] [PubMed] [Google Scholar]
- Andersson M. 1994. Sexual Selection. Princeton NJ: Princeton University Press [Google Scholar]
- Anway MD, Skinner MK. 2008. Epigenetic programming of the germ line: Effects of endocrine disruptors on the development of transgenerational disease. Reprod Biomed Online 16:23–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, Breedlove SM. 1985. Organizational and activational effects of sex steroids on brain and behavior: A reanalysis. Horm Behav 19:469–498. [DOI] [PubMed] [Google Scholar]
- Auger CJ, Coss D, Auger AP, Forbes-Lorman RM. 2011. Epigenetic control of vasopressin expression is maintained by steroid hormones in the adult male rat brain. Proc Natl Acad Sci U S A 108:4242–4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodo C, Rissman EF. 2006. New roles for estrogen receptor beta in behavior and neuroendocrinology. Front Neuroendocrinol 27:217–232. [DOI] [PubMed] [Google Scholar]
- Bortolotti G, Fernie K, Smits J. 2003. Carotenoid concentration and coloration of American kestrels (Falco sparverius) disrupted by experimental exposure to PCBs. Funct Ecol 17:651–657. [Google Scholar]
- Bowers JM, Waddell J, McCarthy MM. 2010. A developmental sex difference in hippocampal neurogenesis is mediated by endogenous oestradiol. Biol Sex Differ 1:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray NJ. 2008. Gene expression in the etiology of schizophrenia. Schizophr Bull 34:412–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredy TW, Lee AW, Meaney MJ, Brown RE. 2004. Effect of neonatal handling and paternal care on offspring cognitive development in the monogamous California mouse (Peromyscus californicus). Horm Behav 46:30–38. [DOI] [PubMed] [Google Scholar]
- Bredy TW, Lin Q, Wei W, Baker-Andresen D, Mattick JS. 2011. MicroRNA regulation of neural plasticity and memory. Neurobiol Learn Mem 96:89–94. [DOI] [PubMed] [Google Scholar]
- Bredy TW, Sun YE, Kobor MS. 2010. How the epigenome contributes to the development of psychiatric disorders. Dev Psychobiol 52:331–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromer JG, Zhou Y, Taylor MB, Doherty L, Taylor HS. 2010. Bisphenol-A exposure in utero leads to epigenetic alterations in the developmental programming of uterine estrogen response. FASEB J 24:2273–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantoni D, Brown RE. 1997. Paternal investment and reproductive success in the California mouse, Peromyscus californicus. Anim Behav 54:377–386. [DOI] [PubMed] [Google Scholar]
- Champagne FA. 2008. Epigenetic mechanisms and the transgenerational effects of maternal care. Front Neuroendocrinol 29:386–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, Weaver IC, Diorio J, Dymov S, Szyf M, Meaney MJ. 2006. Maternal care associated with methylation of the estrogen receptor-alpha1b promoter and estrogen receptor-alpha expression in the medial preoptic area of female offspring. Endocrinology 147:2909–2915. [DOI] [PubMed] [Google Scholar]
- Chung WC, Swaab DF, De Vries GJ. 2000. Apoptosis during sexual differentiation of the bed nucleus of the stria terminalis in the rat brain. J Neurobiol 43:234–243. [PubMed] [Google Scholar]
- Clutton-Brock T. 2007. Sexual selection in males and females. Science 318:1882–1885. [DOI] [PubMed] [Google Scholar]
- Contractor RG, Foran CM, Li S, Willett KL. 2004. Evidence of gender-and tissue-specific promoter methylation and the potential for ethinylestradiol-induced changes in Japanese medaka (Oryzias latipes) estrogen receptor and aromatase genes. J Toxicol Environ Health A 67:1–22. [DOI] [PubMed] [Google Scholar]
- Cox KH, Gatewood JD, Howeth C, Rissman EF. 2010. Gestational exposure to bisphenol A and cross-fostering affect behaviors in juvenile mice Horm Behav 58:754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews D. 2010. Epigenetic modifications of brain and behavior: Theory and practice. Horm Behav 59:393–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews D, Gore AC, Hsu TS, Dangleben NL, Spinetta M, Schallert T, Anway MD, Skinner MK. 2007. Transgenerational epigenetic imprints on mate preference. Proc Natl Acad Sci U S A 104:5942–5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukier HN, Perez AM, Collins AL, Zhou Z, Zoghbi HY, Botas J. 2008. Genetic modifiers of MeCP2 function in Drosophila. PLoS Genet 4:e1000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curley JP, Mashoodh R. 2010. Parent-of-origin and trans-generational germline influences on behavioral development: The interacting roles of mothers, fathers, and grandparents. Dev Psychobiol 52:312–330. [DOI] [PubMed] [Google Scholar]
- Darwin C. 1871. The Descent of Man, and Selection in Relation to Sex. London: John Murray [Google Scholar]
- Defossez PA, Stancheva I. 2011. Biological functions of methyl-CpG-binding proteins. Prog Mol Biol Transl Sci 101:377–398. [DOI] [PubMed] [Google Scholar]
- Della Seta D, Minder I, Dessi-Fulgheri F, Farabollini F. 2005. Bisphenol-A exposure during pregnancy and lactation affects maternal behavior in rats. Brain Res Bull 65:255–260. [DOI] [PubMed] [Google Scholar]
- Dolinoy DC, Huang D, Jirtle RL. 2007. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci U S A 104:13056–13061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DonCarlos LL, Handa RJ. 1994. Developmental profile of estrogen receptor mRNA in the preoptic area of male and female neonatal rats. Brain Res Dev Brain Res 79:283–289. [DOI] [PubMed] [Google Scholar]
- Doshi T, Mehta SS, Dighe V, Balasinor N, Vanage G. 2011. Hypermethylation of estrogen receptor promoter region in adult testis of rats exposed neonatally to bisphenol A. Toxicology 289:74–82. [DOI] [PubMed] [Google Scholar]
- Dudley D. 1974a. Contributions of paternal care to the growth and development of the young in Peromyscus californicus. Behav Biol 1:155–166. [DOI] [PubMed] [Google Scholar]
- Dudley D. 1974b. Paternal behavior in the California mouse, Peromyscus californicus. Behav Biol 11:247–252. [DOI] [PubMed] [Google Scholar]
- Folstad I, Karter A. 1992. Parasites, bright male, and the immunocompetence handicap. Am Nat 139:603–622. [Google Scholar]
- Forger NG, Rosen GJ, Waters EM, Jacob D, Simerly RB, de Vries GJ. 2004. Deletion of Bax eliminates sex differences in the mouse forebrain. Proc Natl Acad Sci U S A 101:13666–13671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea L, Perrot-Sinal T, Kavaliers M, Ossenkopp K-P. 1999. Relations of hippocampal volume and dentate gyrus width to gonadal hormone levels in male and female meadow voles. Brain Res 821:381–391. [DOI] [PubMed] [Google Scholar]
- Galea LA, Kavaliers M, Ossenkopp KP. 1996. Sexually dimorphic spatial learning in meadow voles Microtus pennsylvanicus and deer mice Peromyscus maniculatus. J Exp Biol 199:195–200. [DOI] [PubMed] [Google Scholar]
- Gaulin SJ, Fitzgerald RW. 1986. Sex differences in spatial ability: An evolutionary hypothesis and test. Am Nat 127:74–88. [Google Scholar]
- Gaulin SJ, Fitzgerald RW. 1989. Sexual selection for spatial learning ability. Anim Behav 37:322–331. [Google Scholar]
- Gaulin SJC. 1992. Evolution of sex differences in spatial ability. Yearb Phys Anthropol 35:125–151. [Google Scholar]
- Geary DC. 2010. Male, Female: The Evolution of Human Sex Differences (2nd ed). Washington: American Psychological Association [Google Scholar]
- Gioiosa L, Fissore E, Ghirardelli G, Parmigiani S, Palanza P. 2007. Developmental exposure to low-dose estrogenic endocrine disruptors alters sex differences in exploration and emotional responses in mice. Horm Behav 52:307–316. [DOI] [PubMed] [Google Scholar]
- Glaser KB, Staver MJ, Waring JF, Stender J, Ulrich RG, Davidsen SK. 2003. Gene expression profiling of multiple histone deacetylase (HDAC) inhibitors: Defining a common gene set produced by HDAC inhibition in T24 and MDA carcinoma cell lines. Mol Cancer Ther 2:151–163. [PubMed] [Google Scholar]
- Gotsiridze T, Kang N, Jacob D, Forger NG. 2007. Development of sex differences in the principal nucleus of the bed nucleus of the stria terminalis of mice: Role of Bax-dependent cell death. Dev Neurobiol 67:355–362. [DOI] [PubMed] [Google Scholar]
- Gubernick DJ, Teferi T. 2000. Adaptive significance of male parental care in a monogamous mammal. Proc Biol Sci 267:147–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Bosagna C, Settles M, Lucker B, Skinner MK. 2010. Epigenetic transgenerational actions of vinclozolin on promoter regions of the sperm epigenome. PLoS One 55(9). pii: e13100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillamon A, Segovia S, del Abril A. 1988. Early effects of gonadal steroids on the neuron number in the medial posterior region and the lateral division of the bed nucleus of the stria terminalis in the rat. Brain Res Dev Brain Res 44:281–290. [DOI] [PubMed] [Google Scholar]
- Guillette LJ, Jr, Crain DA, Rooney AA, Pickford DB. 1995. Organization versus activation: The role of endocrine-disrupting contaminants (EDC) during embryonic development in wildlife. Environ Health Perspect 103:157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg B. 1985. Rett's syndrome: Prevalence and impact on progressive severe mental retardation in girls. Acta Paediatr Scand 74:405–408. [DOI] [PubMed] [Google Scholar]
- Haig D. 2004. The (dual) origin of epigenetics. Cold Spring Harb Symp Quant Biol 69:67–70. [DOI] [PubMed] [Google Scholar]
- Hamer D. 2002. Genetics. Rethinking behavior genetics. Science 298:71–72. [DOI] [PubMed] [Google Scholar]
- Hamilton W, Zuk M. 1982. Heritable true fitness and bright birds: A role for parasites? Science 218:384–387. [DOI] [PubMed] [Google Scholar]
- Hines M, Allen LS, Gorski RA. 1992. Sex differences in subregions of the medial nucleus of the amygdala and the bed nucleus of the stria terminalis of the rat. Brain Res 579:321–326. [DOI] [PubMed] [Google Scholar]
- Hines M, Davis FC, Coquelin A, Goy RW, Gorski RA. 1985. Sexually dimorphic regions in the medial preoptic area and the bed nucleus of the stria terminalis of the guinea pig brain: A description and an investigation of their relationship to gonadal steroids in adulthood. J Neurosci 5:40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho S-M, Tang W-Y, Belmonte de Frausto J, Prins GS. 2006. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res 66:5624–5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliday DK, Holliday CM. 2011. The effects of the organopollutant PCB 126 on bone density in juvenile diamondback terrapins (Malaclemys terrapin). Aquat Toxicol 109:228–233. [DOI] [PubMed] [Google Scholar]
- Horlein AJ, Naar AM, Heinzel T, Torchia J, Gloss B, Kurokawa R, Ryan A, Kamei Y, Soderstrom M, Glass CK, Rosenfeld MG. 1995. Ligand-independent repression by the thyroid hormone receptor mediated by a nuclear receptor co-repressor. Nature 377:397–404. [DOI] [PubMed] [Google Scholar]
- Imamura T, Ohgane J, Ito S, Ogawa T, Hattori N, Tanaka S, Shiota K. 2001. CpG island of rat sphingosine kinase-1 gene: Tissue-dependent DNA methylation status and multiple alternative first exons. Genomics 76:117–125. [DOI] [PubMed] [Google Scholar]
- Inoue K, Lupski JR. 2003. Genetics and genomics of behavioral and psychiatric disorders. Curr Opin Genet Dev 13:303–309. [DOI] [PubMed] [Google Scholar]
- Jabbi M, Kema IP, van der Pompe G, te Meerman GJ, Ormel J, den Boer JA. 2007. Catechol-o-methyltransferase polymorphism and susceptibility to major depressive disorder modulates psychological stress response. Psychiatr Genet 17:183–193. [DOI] [PubMed] [Google Scholar]
- Jacobs LF, Gaulin SJ, Sherry DF, Hoffman GE. 1990. Evolution of spatial cognition: Sex-specific patterns of spatial behavior predict hippocampal size. Proc Natl Acad Sci U S A 87:6349–6352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jašarević E, Sieli PT, Twellman EE, Welsh TH, Jr, Schachtman TR, Roberts RM, Geary DC, Rosenfeld CS. 2011. Disruption of adult expression of sexually selected traits by developmental exposure to bisphenol A. Proc Natl Acad Sci U S A 108:11715–11720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen HM, Kolodkin MH, Bychowski ME, Auger CJ, Auger AP. 2010. The nuclear receptor corepressor has organizational effects within the developing amygdala on juvenile social play and anxiety-like behavior. Endocrinology 151:1212–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PA, Takai D. 2001. The role of DNA methylation in mammalian epigenetics. Science 293:1068–1070. [DOI] [PubMed] [Google Scholar]
- Kaminsky Z, Petronis A, Wang SC, Levine B, Ghaffar O, Floden D, Feinstein A. 2008. Epigenetics of personality traits: An illustrative study of identical twins discordant for risk-taking behavior. Twin Res Hum Genet 11:1–11. [DOI] [PubMed] [Google Scholar]
- Khudayberdiev S, Fiore R, Schratt G. 2009. MicroRNA as modulators of neuronal responses. Commun Integr Biol 2:411–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokura K, Kaul SC, Wadhwa R, Nomura T, Khan MM, Shinagawa T, Yasukawa T, Colmenares C, Ishii S. 2001. The Ski protein family is required for MeCP2-mediated transcriptional repression. J Biol Chem 276:34115–34121. [DOI] [PubMed] [Google Scholar]
- Kolodkin MH, Auger AP. 2011. Sex difference in the expression of DNA methyltransferase 3a in the rat amygdala during development. J Neuroendocrinol 23:577–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkle AT, McCarthy MM. 2011. Developmental time course of estradiol, testosterone, and dihydrotestosterone levels in discrete regions of male and female rat brain. Endocrinology 152:223–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koturbash I, Zemp F, Kolb B, Kovalchuk O. 2011. Sex-specific radiation-induced microRNAome responses in the hippocampus, cerebellum and frontal cortex in a mouse model. Mutat Res 722:114–118. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, LaForge KS. 2007. Stress responsivity, addiction, and a functional variant of the human mu-opioid receptor gene. Mol Interv 7:74–78. [DOI] [PubMed] [Google Scholar]
- Krezel W, Dupont S, Krust A, Chambon P, Chapman PF. 2001. Increased anxiety and synaptic plasticity in estrogen receptor beta-deficient mice. Proc Natl Acad Sci U S A 98:12278–12282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudwa AE, Bodo C, Gustafsson JA, Rissman EF. 2005. A previously uncharacterized role for estrogen receptor beta: Defeminization of male brain and behavior. Proc Natl Acad Sci U S A 102:4608–4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudwa AE, Michopoulos V, Gatewood JD, Rissman EF. 2006. Roles of estrogen receptors alpha and beta in differentiation of mouse sexual behavior. Neuroscience 138:921–928. [DOI] [PubMed] [Google Scholar]
- Kumar RC, Thakur MK. 2004. Androgen receptor mRNA is inversely regulated by testosterone and estradiol in adult mouse brain. Neurobiol Aging 25:925–933. [DOI] [PubMed] [Google Scholar]
- Kurian JR, Forbes-Lorman RM, Auger AP. 2007. Sex difference in mecp2 expression during a critical period of rat brain development. Epigenetics 2:173–178. [DOI] [PubMed] [Google Scholar]
- Kurian JR, Olesen KM, Auger AP. 2010. Sex differences in epigenetic regulation of the estrogen receptor-alpha promoter within the developing preoptic area. Endocrinology 151:2297–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert KG, Franssen CL, Bardi M, Hampton JE, Hainley L, Karsner S, Tu EB, Hyer MM, Crockett A, Baranova A, Ferguson T, Kinsley CH. 2011. Characteristic neurobiological patterns differentiate paternal responsiveness in two Peromyscus species. Brain Behav Evol 77:159–175. [DOI] [PubMed] [Google Scholar]
- Langston RF, Ainge JA, Couey JJ, Canto CB, Bjerknes TL, Witter MP, Moser EI, Moser MB. 2010. Development of the spatial representation system in the rat. Science 328:1576–1580. [DOI] [PubMed] [Google Scholar]
- Lavinsky RM, Jepsen K, Heinzel T, Torchia J, Mullen TM, Schiff R, Del-Rio AL, Ricote M, Ngo S, Gemsch J, Hilsenbeck SG, Osborne CK, Glass CK, Rosenfeld MG, Rose DW. 1998. Diverse signaling pathways modulate nuclear receptor recruitment of N-CoR and SMRT complexes. Proc Natl Acad Sci U S A 95:2920–2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MS. 2007. Role of genetic polymorphisms related to neurotransmitters and cytochrome P-450 enzymes in response to antidepressants. Drugs Today (Barc) 43:569–581. [DOI] [PubMed] [Google Scholar]
- Lyko F, Foret S, Kucharski R, Wolf S, Falckenhayn C, Maleszka R. 2010. The honey bee epigenomes: Differential methylation of brain DNA in queens and workers. PLoS Biol 8:e1000506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancama D, Arranz MJ, Landau S, Kerwin R. 2003. Reduced expression of the muscarinic 1 receptor cortical subtype in schizophrenia. Am J Med Genet B Neuropsychiatr Genet 119B:2–6 [DOI] [PubMed] [Google Scholar]
- Mantione K, Kream RM, Stefano GB. 2010. Variations in critical morphine biosynthesis genes and their potential to influence human health. Neuro Endocrinol Lett 31:11–18. [PubMed] [Google Scholar]
- Matsuda KI, Mori H, Nugent BM, Pfaff DW, McCarthy MM, Kawata M. 2011. Histone deacetylation during brain development is essential for permanent masculinization of sexual behavior. Endocrinology 152:2760–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattick JS, Amaral PP, Dinger ME, Mercer TR, Mehler MF. 2009. RNA regulation of epigenetic processes. Bioessays 31:51–59. [DOI] [PubMed] [Google Scholar]
- McCarthy MM. 2008. Estradiol and the developing brain. Physiol Rev 88:91–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Arnold AP. 2011. Reframing sexual differentiation of the brain. Nat Neurosci 14:677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Auger AP, Bale TL, De Vries GJ, Dunn GA, Forger NG, Murray EK, Nugent BM, Schwarz JM, Wilson ME. 2009. The epigenetics of sex differences in the brain. J Neurosci 29:12815–12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Schlenker EH, Pfaff DW. 1993. Enduring consequences of neonatal treatment with antisense oligodeoxynucleotides to estrogen receptor messenger ribonucleic acid on sexual differentiation of rat brain. Endocrinology 133:433–439. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. 2010. Epigenetics and the biological definition of gene x environment interactions. Child Dev 81:41–79. [DOI] [PubMed] [Google Scholar]
- Mehler MF. 2008. Epigenetic principles and mechanisms underlying nervous system functions in health and disease. Prog Neurobiol 86:305–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehler MF, Mattick JS. 2006. Non-coding RNAs in the nervous system. J Physiol 575:333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menegola E, Di Renzo F, Broccia ML, Giavini E. 2006. Inhibition of histone deacetylase as a new mechanism of teratogenesis. Birth Defects Res C Embryo Today 78:345–353. [DOI] [PubMed] [Google Scholar]
- Miller CA, Sweatt JD. 2007. Covalent modification of DNA regulates memory formation. Neuron 53:857–869. [DOI] [PubMed] [Google Scholar]
- Miller G. 2010. Epigenetics. The seductive allure of behavioral epigenetics. Science 329:24–27. [DOI] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, Breedlove SM. 2004. Sexual differentiation of the vertebrate nervous system. Nat Neurosci 7:1034–1039. [DOI] [PubMed] [Google Scholar]
- Mougent F, Reader BF, Piertney SB. 2005. Separating behavioral and physiological mechanisms in tstosterone-mediated trade-offs. Am Nat 166:158–168. [DOI] [PubMed] [Google Scholar]
- Mougent F, Redpath SM, Piertney SB. 2006. Elevated spring testosterone increases parasite intensity in male red grouse. Behavioral Ecology 17:17–125. [Google Scholar]
- Murray EK, Hien A, de Vries GJ, Forger NG. 2009. Epigenetic control of sexual differentiation of the bed nucleus of the stria terminalis. Endocrinology 150:4241–4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nan X, Ng HH, Johnson CA, Laherty CD, Turner BM, Eisenman RN, Bird A. 1998. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature 393:386–389. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L.1978. The Hippocampus as a Cognitive Map. Oxford: Clarendon Press [Google Scholar]
- Ogawa S, Lubahn DB, Korach KS, Pfaff DW. 1997. Behavioral effects of estrogen receptor gene disruption in male mice. Proc Natl Acad Sci U S A 94:1476–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Taylor JA, Lubahn DB, Korach KS, Pfaff DW. 1996. Reversal of sex roles in genetic female mice by disruption of estrogen receptor gene. Neuroendocrinology 64:467–470. [DOI] [PubMed] [Google Scholar]
- Olioff M, Stewart J. 1978. Sex differences in the play behavior of prepubescent rats. Physiol Behav 20:113–115. [DOI] [PubMed] [Google Scholar]
- Palanza P, Morellini F, Parmigiani S, vom Saal FS. 1999. Prenatal exposure to endocrine disrupting chemicals: Effects on behavioral development. Neurosci Biobehav Rev 23:1011–1027. [DOI] [PubMed] [Google Scholar]
- Palanza PL, Howdeshell KL, Parmigiani S, vom Saal FS. 2002. Exposure to a low dose of bisphenol A during fetal life or in adulthood alters maternal behavior in mice. Environ Health Perspect 110:415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patchev AV, Gotz F, Rohde W. 2004. Differential role of estrogen receptor isoforms in sex-specific brain organization. FASEB J 18:1568–1570. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Bateman HL. 2008. Neonatal exposure to endocrine active compounds or an ERbeta agonist increases adult anxiety and aggression in gonadally intact male rats. Horm Behav 53:580–588. [DOI] [PubMed] [Google Scholar]
- Penn DJ, Potts WK. 1999. The evolution of mating preferences and major histocompatibility complex genes. Am Nat 153:145–164. [DOI] [PubMed] [Google Scholar]
- Perrot-Sinal TS, Kavaliers M, Ossenkopp KP. 1998. Spatial learning and hippocampal volume in male deer mice: Relations to age, testosterone and adrenal gland weight. Neuroscience 86:1089–1099. [DOI] [PubMed] [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA, Young WC. 1959. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology 65:369–382. [DOI] [PubMed] [Google Scholar]
- Pomiankowski A, Møller A. 1995. A resolution of the lek paradox. Proc Natl Acad Sci U S A 260:21–19. [Google Scholar]
- Proudnikov D, Hamon S, Ott J, Kreek MJ. 2008. Association of polymorphisms in the melanocortin receptor type 2 (MC2R, ACTH receptor) gene with heroin addiction. Neurosci Lett 435:234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi IA, Mehler MF. 2010. Genetic and epigenetic underpinnings of sex differences in the brain and in neurological and psychiatric disease susceptibility. Prog Brain Res 186:77–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid J, Arcese P, Cassidy A, Hiebert S, Smith J, Stoddard P, Marr A, Keller L. 2005. Fitness correlates of song repertoire size in free-living song sparrows (Melospiza melodia). Am Nat 165:299–310. [DOI] [PubMed] [Google Scholar]
- Rocha BA, Fleischer R, Schaeffer JM, Rohrer SP, Hickey GJ. 2005. 17 Beta-estradiol-induced antidepressant-like effect in the forced swim test is absent in estrogen receptor-beta knockout (BERKO) mice. Psychopharmacology (Berl) 179:637–643. [DOI] [PubMed] [Google Scholar]
- Roulin A. 1999. Nonrandom pairing by male barn owls (Tyto alba) with respect to a female plumage trait. Behav Ecol 10:688–695. [Google Scholar]
- Roulin A, Jungi T, Pfister H, Dijkstra C. 2000. Female barn owls (Tyto alba) advertise good genes. Proc Royal Soc Lond Ser B Biolog Sci 267:937–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe L, Houle D. 1996. The lek paradox and the capture of genetic variance by condition dependent traits. Proc Natl Acad Sci U S A 263:1415–1421. [Google Scholar]
- Rowsemitt C. 1986. Seasonal variation in activity rhythms of male voles: Mediation by gonadal hormones. Physiol Behav 37:797–803. [DOI] [PubMed] [Google Scholar]
- Saino N, Møller A, Bolzern A. 1995. Testosterone effects on the immune system and parasite infestations in the barn swallow (Hirundo rustica): An experimental test of the immunocompetence hypothesis. Behav Ecol 6:397–404. [Google Scholar]
- Sargent R, Rush V, Wisenden B, Yan H. 1998. Courtship and mate choice in fishes: Integrating behavioral and sensory ecology. Am Zool 38:82–96. [Google Scholar]
- Schwarz JM, Nugent BM, McCarthy MM. 2010. Developmental and hormone-induced epigenetic changes to estrogen and progesterone receptor genes in brain are dynamic across the life span. Endocrinology 151:4871–4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry DF, Vaccarino A, Buckenham K, Herz R. 1989. The hippocampal complex of food- storing birds. Brain Behav Evol 34:308–317. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. 1997. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol 388:507–525. [DOI] [PubMed] [Google Scholar]
- Skinner MK, Anway MD, Savenkova MI, Gore AC, Crews D. 2008. Transgenerational epigenetic programming of the brain transcriptome and anxiety behavior. PLoS One 3:e3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spritzer MD, Solomon NG, Meikle DB. 2005. Influence of scramble competition for mates upon the spatial ability of male meadow voles. Anim Behav 69:425–436. [Google Scholar]
- Stromqvist M, Tooke N, Brunstrom B. 2010. DNA methylation levels in the 5’ flanking region of the vitellogenin I gene in liver and brain of adult zebrafish (Danio rerio): Sex and tissue differences and effects of 17alpha-ethinylestradiol exposure. Aquat Toxicol 98:275–281. [DOI] [PubMed] [Google Scholar]
- Svrakic DM, Cloninger RC. 2010. Epigenetic perspective on behavior development, personality, and personality disorders. Psychiatr Danub 22:153–166. [PubMed] [Google Scholar]
- Szyf M, Weaver I, Meaney M. 2007. Maternal care, the epigenome and phenotypic differences in behavior. Reprod Toxicol 24:9–19. [DOI] [PubMed] [Google Scholar]
- Tang WY, Morey LM, Cheung YY, Birch L, Prins GS, Ho SM. 2012. Neonatal exposure to estradiol/bisphenol A alters promoter methylation and expression of Nsbp1 and Hpcal1 genes and transcriptional programs of Dnmt3a/b and Mbd2/4 in the rat prostate gland throughout life. Endocrinology 153:42–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivers RL. 1972. Parental investment and sexual selection. In: Campbell B, ed. Sexual Selection and the Descent of Man, 1871–1971. Chicago: Aldine; p 136–179 [Google Scholar]
- Tsukahara S, Tsuda MC, Kurihara R, Kato Y, Kuroda Y, Nakata M, Xiao K, Nagata K, Toda K, Ogawa S. 2011. Effects of aromatase or estrogen receptor gene deletion on masculinization of the principal nucleus of the bed nucleus of the stria terminalis of mice. Neuroendocrinology 94:137–147. [DOI] [PubMed] [Google Scholar]
- Turner B, Iverson S, Severson K. 1983. Seasonal changes in open-field behavior in wild male meadow voles (Microtus pennsylvanicus). Behavi Neural Biol 39:60–77. [DOI] [PubMed] [Google Scholar]
- Watson J, Adkins-Regan E. 1989a. Activation of sexual behavior by implantation of testosterone propionate and estradiol benzoate into the preoptic area of the male Japanese quail (Coturnix japonica). Horm Behav 23:251–268. [DOI] [PubMed] [Google Scholar]
- Watson J, Adkins-Regan E. 1989b. Testosterone implanted in the preoptic area of male Japanese quail must be aromatized to activate copulation. Horm Behav 23:432–447. [DOI] [PubMed] [Google Scholar]
- Weaver IC. 2007. Epigenetic programming by maternal behavior and pharmacological intervention. Nature versus nurture: Let's call the whole thing off. Epigenetics 2:22–28. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. 2004. Epigenetic programming by maternal behavior. Nat Neurosci 7:847–854. [DOI] [PubMed] [Google Scholar]
- Weinhouse C, Anderson OS, Jones TR, Kim J, Liberman SA, Nahar MS, Rozek LS, Jirtle RL, Dolinoy DC. 2011. An expression microarray approach for the identification of metastable epialleles in the mouse genome. Epigenetics 6:1105–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng YI, Hsu PY, Liyanarachchi S, Liu J, Deatherage DE, Huang YW, Zuo T, Rodriguez B, Lin CH, Cheng AL, Huang TH. 2010. Epigenetic influences of low-dose bisphenol A in primary human breast epithelial cells. Toxicol Appl Pharmacol 248:111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GC. 1966. Adaptation and Natural Selection. Princeton NJ: Princeton University Press [Google Scholar]
- Wills TJ, Cacucci F, Burgess N, O’Keefe J. 2010. Development of the hippocampal cognitive map in preweanling rats. Science 328:1573–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson ME, Westberry JM, Trout AL. 2011. Estrogen receptor-alpha gene expression in the cortex: Sex differences during development and in adulthood. Horm Behav 59(3):353–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SS, Patchev VK, Obendorf M. 2007. A novel variant of the putative demethylase gene, s-JMJD1C, is a coactivator of the AR. Arch Biochem Biophys 460:56–66. [DOI] [PubMed] [Google Scholar]
- Xu X, Tian D, Hong X, Chen L, Xie L. 2011. Sex-specific influence of exposure to bisphenol-A between adolescence and young adulthood on mouse behaviors. Neuropharmacology 61:565–573. [DOI] [PubMed] [Google Scholar]
- Xu XH, Zhang J, Wang YM, Ye YP, Luo QQ. 2010. Perinatal exposure to bisphenol-A impairs learning-memory by concomitant down-regulation of N-methyl-D-aspartate receptors of hippocampus in male offspring mice. Horm Behav 58:326–333. [DOI] [PubMed] [Google Scholar]
- Yaoi T, Itoh K, Nakamura K, Ogi H, Fujiwara Y, Fushiki S. 2008. Genome-wide analysis of epigenomic alterations in fetal mouse forebrain after exposure to low doses of bisphenol A. Biochem Biophys Res Comm 376:563–567. [DOI] [PubMed] [Google Scholar]
- Yokosuka M, Okamura H, Hayashi S. 1997. Postnatal development and sex difference in neurons containing estrogen receptor-alpha immunoreactivity in the preoptic brain, the diencephalon, and the amygdala in the rat. J Comp Neurol 389:81–93. [DOI] [PubMed] [Google Scholar]
- Yoon HG, Chan DW, Reynolds AB, Qin J, Wong J. 2003. N-CoR mediates DNA methylation-dependent repression through a methyl CpG binding protein Kaiso. Mol Cell 12:723–734. [DOI] [PubMed] [Google Scholar]
- Yoon HG, Wong J. 2006. The corepressors silencing mediator of retinoid and thyroid hormone receptor and nuclear receptor corepressor are involved in agonist- and antagonist-regulated transcription by androgen receptor. Mol Endocrinol 20:1048–1060. [DOI] [PubMed] [Google Scholar]
- Youngson NA, Whitelaw E. 2008. Transgenerational epigenetic effects. Annu Rev Genomics Hum Genet 9:233–257. [DOI] [PubMed] [Google Scholar]
- Zahavi A. 1975. Mate selection: A selection for a handicap. J Theoret Biol 53:205–214. [DOI] [PubMed] [Google Scholar]
- Zama AM, Uzumcu M. 2009. Fetal and neonatal exposure to the endocrine disruptor methoxychlor causes epigenetic alterations in adult ovarian genes. Endocrinology 150:4681–4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang TY, Meaney MJ. 2010. Epigenetics and the environmental regulation of the genome and its function. Annu Rev Psychol 61:439–466., C431-433. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Zhu G, Hariri AR, Enoch MA, Scott D, Sinha R, Virkkunen M, Mash DC, Lipsky RH, Hu XZ, Hodgkinson CA, Xu K, Buzas B, Yuan Q, Shen PH, Ferrell RE, Manuck SB, Brown SM, Hauger RL, Stohler CS, Zubieta JK, Goldman D. 2008. Genetic variation in human NPY expression affects stress response and emotion. Nature 452:997–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zill P, Engel R, Baghai TC, Juckel G, Frodl T, Muller-Siecheneder F, Zwanzger P, Schule C, Minov C, Behrens S, Rupprecht R, Hegerl U, Moller HJ, Bondy B. 2002. Identification of a naturally occurring polymorphism in the promoter region of the norepinephrine transporter and analysis in major depression. Neuropsychopharmacology 26:489–493. [DOI] [PubMed] [Google Scholar]
- Zwijnenburg PJ, Meijers-Heijboer H, Boomsma DI. 2010. Identical but not the same: The value of discordant monozygotic twins in genetic research. Am J Med Genet B Neuropsychiatr Genet 153B:1134–1149 [DOI] [PubMed] [Google Scholar]