Abstract
Special mechanisms of mutation are induced during growth-limiting stress and can generate adaptive mutations that permit growth. These mechanisms may provide improved models for mutagenesis in antibiotic resistance, evolution of pathogens, cancer progression and chemotherapy resistance. Stress-induced reversion of an Escherichia coli episomal lac frameshift allele specifically requires DNA double-strand-break-repair (DSBR) proteins, the SOS DNA-damage response and its error-prone DNA polymerase, DinB. We distinguished two possible roles for the DSBR proteins. Each might act solely upstream of SOS, to create single-strand DNA that induces SOS. This could upregulate DinB and enhance mutation globally. Or any or all of them might function other than or in addition to SOS promotion, for example, directly in error-prone DSBR. We report that in cells with SOS genes derepressed constitutively, RecA, RuvA, RuvB, RuvC, RecF and TraI remain required for stress-induced mutation, demonstrating that these proteins act other than via SOS induction. RecA and TraI also act by promoting SOS. These and additional results with hyper-mutating recD and recG mutants support roles for these proteins via error-prone DSBR. Such mechanisms could localize stress-induced mutagenesis to small genomic regions, a potentially important strategy for adaptive evolution, both for reducing additional deleterious mutations in rare adaptive mutants and for concerted evolution of genes.
Keywords: Evolution, Genome instability, Genetic recombination, SOS response, Stress response
1. Introduction
Classical spontaneous mutations occur by a mode that had appeared to be universal [1,2,3, and many subsequent papers]. The mutations occur before cells are exposed to an environment in which the mutation might prove useful, in proliferating cells, and with a definable relationship to cell divisions [4, we call these generation-dependent mutations]. However, a growing body of work in bacteria and yeast has revealed that mutations also occur in response to stress-inducing, growth-limiting environments, by special mechanisms not seen in rapidly-growing cells [reviewed, Refs. 5,6–8]. These “stress-induced” or “stationary-phase” mutation mechanisms occasionally produce mutations that allow growth in the growth-limiting environment, called “adaptive mutations”. These were controversial initially because of the suggestion that the adaptive mutations might be formed preferentially [9], an idea that was rejected for the most well studied stress-induced mutation mechanism [10–13] and for others [e.g., Ref. 14]. A second round of controversy has concerned the idea of stress-induced increases in genome-wide mutation rate, which, though compatible with Darwinism, appears to challenge conservative neo-Darwinist ideals of constant and gradual evolutionary change [e.g., see Refs. 15,16–19]. In addition to and independently of these issues, the molecular mechanisms of stress-induced mutation are intriguing and important to understand because they may provide superior models for the origins of genetic changes that drive evolution of resistance of pathogens to antibiotics and to our immune systems [20–22], loss of growth control in oncogenesis [23], tumor progression and resistance to chemotherapy [24], aging [25], and perhaps much of evolution generally.
Stress-induced mutation occurs by multiple mutation mechanisms observed with different stress conditions, strains, and organisms, though with some similarities among the mechanisms described so far [6,7,26]. The mutation mechanisms operating in the Escherichia coli Lac frameshift reversion assay are the best characterized. In this assay, E. coli cells carrying a deletion of the chromosomal lac genes and a lac +1 frameshift allele on an F′ conjugative plasmid are plated onto solid medium with lactose as the sole carbon source [27]. For the next week or so, Lac+ revertant colonies accumulate. About half of the colonies observed on the second day of incubation (day two) result from generation-dependent mutations that occurred during growth of the cultures prior to plating; the remaining day-two colonies and colonies that appear on later days are stress-induced adaptive mutants, formed after exposure to the lactose medium [27–30] and consist of two kinds. Most colonies appearing from days 3–6 are Lac+ because of compensatory frameshift mutations [31,32] (“adaptive point mutants”). A smaller number carries amplification of the lac region as 20–100 tandem repeats, which provide sufficient activity from the leaky lac allele to allow growth [29]. The proportion of lac-amplified clones increases with time, constituting about 40% by day eight. Although amplification was proposed to be an intermediate in the point-mutation pathway [33], recent data indicate that point mutation and amplification are independent outcomes and mechanisms [19, discussed below], both induced by stress [34]. Also, point mutation can occur in the absence of selected amplification [19,35]. The stress-induced point-mutation mechanism is our main focus here.
Stress-induced point mutation requires E. coli double-strand-break-repair (DSBR) and homologous-recombination proteins RecA, RecBC, RecF, and RuvABC [27,36–39]. Conjugal transfer functions, though not actual transfer of the F′, are also required [40,41], specifically TraI, an F-encoded single-strand endonuclease and helicase ([35], below, and discussed in Ref. [41]). The SOS DNA damage response and DinB, an error-prone DNA polymerase that it upregulates, are required for point mutation but not lac-amplification [39,42]. The RpoS-controlled general/starvation-stress response, which also upregulates DinB [43], is required for both stress-induced point mutation and lac-amplification [34]. DNA polymerase I is required only for amplification, not point mutation ([19] and A. Slack, PC Thornton, DB Magner, SM Rosenberg and PJ Hastings, unpublished). Point mutation is increased in mutants lacking RecD DSBR-regulatory protein [36]. In cells lacking RecG DSBR protein, either point mutants, lac-amplified clones, or both are increased [37,38].
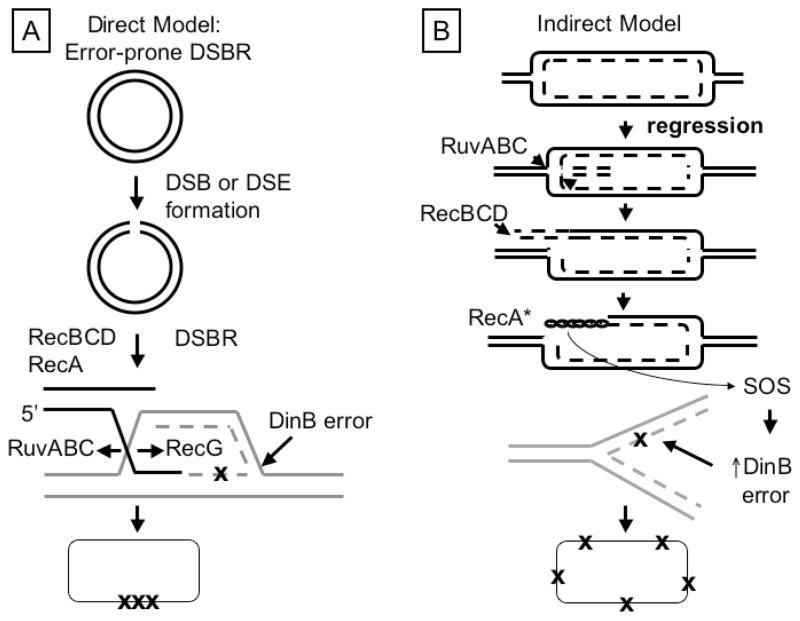
The involvement of DSBR proteins in the Lac system’s stress-induced point-mutation mechanism is potentially a feature with some generality, in that DSBR proteins are also required for an adaptive mutation mechanism in yeast [44, discussed below]. It is potentially also important both for the mutation mechanism and for its evolutionary consequences. Two equally plausible general models could have accounted for the roles of DSBR proteins in stress-induced point mutation [6]. In the first, the DSBR proteins play direct roles in mutation, which could occur, for example, via an error-prone-DSBR mechanism [Fig. 1A, Ref. 36, and several subsequent papers]. Recent results support such models [35]. Alternatively, because stress-induced point mutation also requires SOS induction [39,42], and some [45–47], or perhaps all [6] of the DSBR proteins might participate in SOS induction during stress-induced mutagenesis, the DSBR proteins might act indirectly, solely in DNA processing events that lead to SOS induction (e.g., Fig. 1B). This could promote mutation solely by increasing dinB expression, which might enhance mutation anywhere that new DNA is synthesized. Any of the proteins might participate via SOS-inducing, SOS-independent, or both mechanisms.
Fig. 1.
Models for the mechanism of stress-induced point mutation: possible roles of DSBR proteins. (A) Error-prone-DSBR model [modified from Ref. 36]. Mutation is proposed to result from error-prone synthesis during DSBR by homologous recombination. DSBs or ends (DSEs) could result from any of many mechanisms, spontaneously and otherwise [6]. The DSE is processed by RecBCD to reveal single-stranded (ss)DNA used by RecA for strand exchange with homologous DNA. The strand-exchange recombination intermediate is proposed to prime synthesis (dashed lines) and mutations could result from errors (X) made by DinB error-prone DNA polymerase, which is upregulated both by the SOS response and RpoS stationary-phase and general-stress response. Elsewhere, we suggest that DSBs form spontaneously but that their repair switches from high-fidelity to error-prone as part of the RpoS stress response [35]. Error-prone DSBR could lead to localized mutations (Xs) in genomes (discussed in Ref. [35]). (B) Indirect model: DSBR proteins promote SOS induction [modified from Ref. 6]. SOS induction results from accumulation of ssDNA, which is coated with RecA protein, activating RecA as a co-protease (RecA*) that facilitates the autoproteolytic cleavage of the LexA transcriptional repressor, upregulating >40 DNA-damage-response genes [51,88]. In this model, a replication fork stalls and is regressed to form a four-stranded Holliday-junction-like structure (a “chicken foot”). RuvABC could bind and cleave the structure producing DSEs, which RecBCD processes into ssDNA. RecA loads, becomes activated RecA*, inducing SOS and DinB up-regulation leading to DinB-generated errors and so mutations anywhere that new DNA is synthesized. This would disperse mutations (Xs) throughout each cell’s genome [35]. This model illustrates that all of the DSBR proteins could be required for point mutation even if DSBR itself were not. Their sole role could be promoting SOS induction.
We distinguish these general models and test the roles of several DSBR and other proteins here by showing that DSBR proteins are required for stress-induced mutagenesis in E. coli strains in which the SOS/LexA regulon is derepressed constitutively. Thus they act other than or in addition to via SOS induction. The results support and provide new details for possible error-prone-DSBR models for stress-induced point mutation. Error-prone DSBR models suggest the evolutionary consequence of preserving the integrity of most of the genome in any given cell while mutating small localized regions extensively.
2. Materials and Methods
2.1 Bacterial strains
E. coli strains (Table 1) were constructed using standard techniques [48]. Relevant genotypes were confirmed prior to each experiment by sensitivities to ultraviolet light, a phage lambda plaque-formation test, and by antibiotic resistances. Additionally, strains carrying the lexA(Def) sulA psiB mutations were verified as LexA-defective by transformation with plasmid pJP30 [19] carrying the gfp gene under the control of the LexA-regulated sulA promoter, and were shown to form green colonies (whereas the isogenic sulA psiB strains did not). A conjugation assay was used to confirm the presence of the ΔtraI mutation. Antibiotics and other additives were used at the following concentrations (μg/ml): chloramphenicol, 25; kanamycin, 50; tetracycline, 10; rifampicin, 100; trimethoprim, 100; X-gal, 40; threonine, 50; tryptophan, 50. The presence of recG and dinB in strains were confirmed by PCR with primers 5′-GTACCTGCGTGAAAACAGCA-3′ and 5′-TAACCTGAAATGGCGGTCTT-3′ and 5′-GCATTTCTCAAACCCTGAAATC-3′ and 5′-AATACCCGCATCCTTATTCCTT-3′, respectively. Stress-induced mutation phenotypes of strains were confirmed either by testing independent isolates of the same strain or by rescuing phenotypes with a wild-type copy of the gene via transduction, or both (see Table 3 “rescue” strains).
Table 1.
Escherichia coli K-12 strains
| Strain | Relevant genotype | Reference or source |
|---|---|---|
| FC29 | Δ(lac-proB)XIII ara thi [F′Δ(lacI-lacZ)] | [27] |
| FC40 | Δ(lac-proB)XIII ara thi RifR [F′ lacI33ΩlacZ proAB+] | [27] |
| FS2055 | malB::Tn9 | FW Stahl (U Oregon) |
| GY8322 | Δ(srlR-recA)306::Tn10 [mini-F recA+] | S Sommers (Gif sur Yvette) |
| RDK2655 | recG258::Tn10miniKan | R Kolodner (San Diego) |
| SMR533 | FC40 malB::Tn9 | FC40 × P1(FS2055) |
| SMR624 | FC40 Δ(srlR-recA)306::Tn10 | [36] |
| SMR686 | FC40 recF332::Tn3 | [39] |
| SMR692 | FC40 recD6001::Tet::Kan | [36] |
| SMR701 | FC40 lexA3(Ind−) | [39] |
| SMR789 | FC40 ruvC53 eda-51::Tn10 | [38] |
| SMR868 | FC40 lexA3(Ind−) | [39] |
| SMR1549 | FC40 ruvA200 eda-51::Tn10 | [38] |
| SMR1552 | FC40 ruvB9 zea-3::Tn10 | [38] |
| SMR1982 | FC40 recG258::Tn10miniKan | [38] |
| SMR4562 | Δ(lac-proB)XIII ara thi RifR [F′ lacI33ΩlacZ proAB+] | Independent construction of FC40 [39] |
| SMR4582 | FC40 lexA3(Ind−) recG258::Tn10miniKan | SMR868 × P1(RDK2655) |
| SMR5400 | SMR4562 lexA51(Def) sulA211 psiB::cat | [39] |
| SMR5711 | SMR5400 recF332::Tn3 | SMR5400 × P1(SMR686) |
| SMR5830 | SMR4562 dinB10 [F′ dinB10] | [42] |
| SMR6002 | SMR4562 ΔtraI::dhfr | RG Ponder, NC Fonville, SM Rosenberg (unpublished) |
| SMR6488 | SMR5400 Δ(srlR-recA)306::Tn10 | SMR5400 × P1(GY8322) |
| SMR6575 | SMR5400 ruvC53 eda-51::Tn10 | SMR5400 × P1(SMR789) |
| SMR7627 | SMR5400 dinB10 [F′ dinB10] | This worka |
| SMR7727 | SMR5400 recD6001::mini-Tet::Kan | SMR5400 × P1(SMR692) |
| SMR7731 | SMR5400 traI::dhfr | SMR5400 × P1(SMR6002) |
| SMR8097 | SMR4562 ΔrecF1804::FRT::Kan::FRT | This work |
| SMR8129 | SMR5400 recG258::Tn10miniKan | SMR5400 × P1(SMR1982) |
| SMR8132 | SMR5400 ΔrecF1804::FRT::Kan::FRT | SMR5400 × P1(SMR8097) |
| SMR8134 | MG1655 zeb-2233::FRT::ble::FRT | This work |
| SMR8142 | SMR5400 ΔrecF1805::FRT | SMR8132 without Kan |
| SMR8160 | SMR4562 ΔrecF1805::FRT | SMR8097 without Kan |
| SMR8397 | SMR5400 Δ(srlR-recA)306::Tn10 | SMR5400 × P1(GY8322) |
| SMR8401 | SMR5400 traI::dhfr | SMR5400 × P1(SMR6002) |
| SMR8403 | SMR5400 recD6001::mini-Tet::Kan | SMR5400 × P1(SMR692) |
| SMR8406 | SMR5400 zib-636::Tn10 (recG+ rescue strain) | This workb |
| SMR8542 | SMR5400 ruvA200 eda-51::Tn10 | SMR5400 × P1(SMR1549) |
| SMR8543 | SMR5400 ruvB9 zea-3::Tn10 | SMR5400 × P1(SMR1552) |
| SMR8544 | SMR5400 ruvC53 eda-51::Tn10 | SMR5400 × P1(SMR789) |
| SMR8545 | FC40 lexA3(Ind−) recG258::Tn10miniKan | SMR1982 × P1(SMR533) × P1(SMR701) |
| SMR8546 | FC40 lexA3(Ind−) recG258::Tn10miniKan | SMR1982 × P1(SMR533) × P1(SMR701) |
| SMR8654 | SMR5400 zeb-2233::FRT::ble::FRT (ruvA+ rescue strain) | SMR8542 × P1(SMR8134) |
| SMR8656 | SMR5400 zeb-2233::FRT::ble::FRT (ruvB+ rescue strain) | SMR8543 × P1(SMR8134) |
| SMR8659 | FC40 lexA3(Ind−) recD6001::mini-Tet::Kan | SMR701 × P1(SMR692) |
| SMR8660 | FC40 lexA3(Ind−) recD6001::mini-Tet::Kan | SMR701 × P1(SMR692) |
| SMR8665 | SMR5400 zeb-2233::FRT::ble::FRT | SMR5400 × P1(SMR8134) |
| SMR8667 | SMR5400 zeb-2233::FRT::ble::FRT | SMR5400 × P1(SMR8134) |
Construction made by P1 transduction with sulA211, lexA51(Def), and psiB::cat alleles derived from DE274 (R. Woodgate, NIH), DE192 (D. Ennis, U. Southwestern Louisiana), and MC1061 (A. Bailone, Gif sur Yvette) strains, respectively.
Construction made by P1 transduction with recG+ linked to zib-636::Tn10 marker derived from RM4714 (R. Maurer).
Table 3.
Quantification of stress-induced-mutation phenotypes from multiple experiments
| Relevant genotypes compareda |
Temp.c | Number of Expts.d | Mean difference in mutation rate from control ± SEMe | ||
|---|---|---|---|---|---|
| Mutant (number of independent isolates)b | Isogenic Control | ||||
| lexA(Def) | Lex+ | 37 °C | 16 | 0.7 ± 0.1 | P = 0.065 |
| lexA(Def) | Lex+ | 32 °C | 14 | 0.5 ± 0.1 | P < 0.001 |
| ruvA | Lex+ | 32 °C | 5 | 0.1 ± 0.04 | P = 0.009 |
| ruvB | Lex+ | 32 °C | 5 | 0.09 ± 0.03 | P = 0.009 |
| ruvC | Lex+ | 32 °C | 6 | 0.05 ± 0.02 | P = 0.004 |
| recA | Lex+ | 37 °C | 7 | 0.01 ± 0.003 | P = 0.002 |
| traI | Lex+ | 37 °C | 5 | 0.01 ± 0.001 | P = 0.009 |
| recF::Tn3 | Lex+ | 37 °C | 7 | 0.3 ± 0.04 | P = 0.003 |
| ΔrecF::Kan | Lex+ | 37 °C | 4 | 0.3 ± 0.06 | P = 0.021 |
| ΔrecF | Lex+ | 37 °C | 3 | 0.4 ± 0.02 | P = 0.050 |
| recG | Lex+ | 32 °C | 12 | 22 ± 4 | P < 0.001 |
| lexA(Def) ruvA | lexA(Def) | 32 °C | 4 | 0.3 ± 0.1 | P = 0.021 |
| lexA(Def) ruvA+ rescue | lexA(Def) | 37 °C | 3 | 0.8 ± 0.2 | P = 0.827 |
| lexA(Def) ruvB | lexA(Def) | 32 °C | 4 | 0.2 ± 0.04 | P = 0.021 |
| lexA(Def) ruvB+ rescue | lexA(Def) | 37 °C | 3 | 0.6 ± 0.1 | P = 0.275 |
| lexA(Def) ruvC (2) | lexA(Def) | 32 °C | 5 | 0.4 ± 0.2 | P = 0.016 |
| lexA(Def) recA (2) | lexA(Def) | 37 °C | 10 | 0.1 ± 0.02 | P < 0.001 |
| lexA(Def) traI (2) | lexA(Def) | 37 °C | 8 | 0.2 ± 0.02 | P = 0.001 |
| lexA(Def) recF::Tn3 | lexA(Def) | 37 °C | 7 | 0.4 ± 0.05 | P = 0.003 |
| lexA(Def) ΔrecF::Kan | lexA(Def) | 37 °C | 7 | 0.5 ± 0.06 | P = 0.006 |
| lexA(Def) ΔrecF | lexA(Def) | 37 °C | 3 | 0.5 ± 0.06 | P = 0.050 |
| lexA(Def) recD (2) | lexA(Def) | 37 °C | 10 | 2 ± 0.3f | P = 0.002 |
| lexA(Def) recG (2) | lexA(Def) | 32 °C | 7 | 0.2 ± 0.06 | P = 0.002 |
| lexA(Def) recG+ rescue | lexA(Def) | 32 °C | 3 | 0.8 ± 0.09 | P = 0.275 |
| lexA(Ind−) | Lex+ | 37 °C | 8 | 0.3 ± 0.04 | P = 0.001 |
| lexA(Ind−) | Lex+ | 32 °C | 3 | 0.2 ± 0.06 | P = 0.050 |
| lexA(Ind−) recD (2) | lexA(Ind−) | 37 °C | 4 | 4 ± 1f | P = 0.019 |
| lexA(Ind−) recG (3) | lexA(Ind−) | 32 °C | 5 | 10 ± 1 | P = 0.008 |
Table 1 for full strain genotypes and information. “lexA(Def)” strains also carry sulA and psiB mutations (Results and Discussion).
The number of independent isolates of each genotype tested, when >1. For select strains, phenotypes were confirmed as not having been caused by additional mutations in the strains’ genomes (perhaps selected inadvertently as growth-defect-suppressing mutations) by reintroduction of the wild-type gene by transduction. These are designated “rescue”.
Temperature at which cultures were grown prior to plating at 37 °C (Materials and Methods).
Each independent experiment is one such as those shown in each of Fig. 3A–J.
Stress-induced mutation rates (mutants per day) are the change in colonies from day 4 to 5 [34]. The mean difference in rate between each mutant and its isogenic control was obtained by pair-wise comparison of the rates from mutant vs. respective control from experiments run in parallel (Table 2). P values were obtained by nonparametric Mann-Whitney Rank Sum test using the SYSTAT 11 statistics software by SYSTAT Software, Inc.
The ratio of lexA(Def) recD and lexA(Ind−) recD to their respective controls is not different, P =0.671
The Δ recF1804::FRT::Kan::FRT deletion allele was constructed by Jeanine M. Pennington [SM Rosenberg laboratory per Ref. 49]. This recF deletion lacks codons 1–342 of the 357-codon recF gene, but leaves the gyrB promoter in the terminal portion of recF gene intact, so as not to affect expression of the gyrase gene downstream. A derivative with the antibiotic marker removed was made as described [49]. The stress-induced mutation phenotypes (Table 3) of the ΔrecF1804::FRT::Kan::FRT and recF332::Tn3 alleles in Lex+ and in lexA(Def) cells are not different (P=0.571 and P=0.482, respectively, non-parametric Mann-Whitney rank sum test). The zeb-2233::FRT::ble::FRT allele was made by Matthew Blankschien [SM Rosenberg laboratory, per 49]. It contains the Sh ble gene from Streptoalloteichus hindustanus [from Ref. 50, strain AR30], which encodes resistance to zeocin. The marker is between chromosomal basepairs 1,937,178 and 1,937,179, between the pykA and msbB genes, at about 48 minutes in the chromosomal map.
2.2 DNA Sequencing
The lac region was amplified with primers 5′-AGGCTATTCTGGTGGCCGGA-3′ and 5′-GCCTCTTCGCTATTACGCCAGCT-3′ and sequenced (Lone Star Labs, Inc., Houston, TX) with primer 5′-ATATCCCGCCGTTAACCACC-3′.
2.3 Stress-induced mutation assays
Stress-induced mutation assays were as described [38]. All experiments presented had less than two-fold net population change during days 1–3 after plating. For all genotypes, controls were treated the same as the mutant strains during growth prior to plating on lactose. Strains containing recA, recF, recD, traI, and dinB mutations were grown to saturation at 37 °C for 48 hrs. Strains containing ruvA, ruvB, ruvC and recG mutations were grown at 32 °C for 48 hrs to reduce selection for rapidly-growing suppressor mutants [38], and in some experiments were grown at 37 °C and showed similar results. We noticed that incubation temperature prior to plating affected the stress-induced-mutation phenotype in Lex+ (“wild-type” control) cells, which produced more Lac+ revertants when grown at 32 °C than at 37 °C prior to plating (Table 3, see also [35]), but not in SOS/LexA-constitutive, lexA(Def) (Table 3), or SOS-uninducible, lexA3(Ind−) cells (Table 3). To control for potential temperature effects, controls and tester strains were grown at the same temperature prior to plating, and in some cases results were repeated with 37 °C pregrowth. For all experiments reported, values shown are mean ± one SEM for multiple independent cultures (usually 6) per strain and graphs are cumulative.
3. Results and Discussion
3.1 Strategies for examining effects on SOS/LexA regulon induction
The LexA transcriptional repressor controls the SOS response to DNA damage [51]. We used two kinds of lexA alleles [51,52] to study effects of LexA-regulon induction in vivo: lexA(Ind−) encodes a mutant LexA repressor that cannot undergo proteolytic cleavage making the LexA regulon (SOS genes) “uninducible”; and a lexA-defective [“lexA(Def)” or “lexA”] null allele which causes constitutive regulon derepression and constitutive overproduction of SOS proteins, including SulA, an inhibitor of cell division. lexA(Def) cells must therefore also carry a sulA-inactivating mutation to grow. Induction of LexA-regulon genes was shown to be required for stress-induced point mutation with the demonstration that lexA(Ind−) cells show reduced point mutation [27,39,42]. However, lexA(Def) sulA E. coli cells also showed reduced mutation, as if the constitutive overproduction of one or more LexA-controlled protein(s) inhibits stress-induced mutation [39]. We identified an F-encoded protein, PsiB, as (one of) the inhibitor(s), by showing that lexA(Def) sulA psiB cells have nearly normal levels of stress-induced point mutation, and have higher levels than seen for sulA psiB cells, showing that LexA-regulon de-repression promotes stress-induced mutation [39]. PsiB is an inhibitor/negative regulator of RecA [53]. An additional mutation also reactivated mutation in lexA(Def) sulA cells, suggesting that more than one SOS-upregulated gene might inhibit stress-induced mutation when LexA-controlled genes are overproduced constitutively [54]. We use the lexA(Def) sulA psiB genetic background as our SOS/LexA-constitutive background here and refer to it as “lexA(Def)” or “SOS/LexA-constitutive”.
3.2 Stress-induced point mutation in SOS/LexA-constitutive and LexA+ strains occurs by a similar mechanism
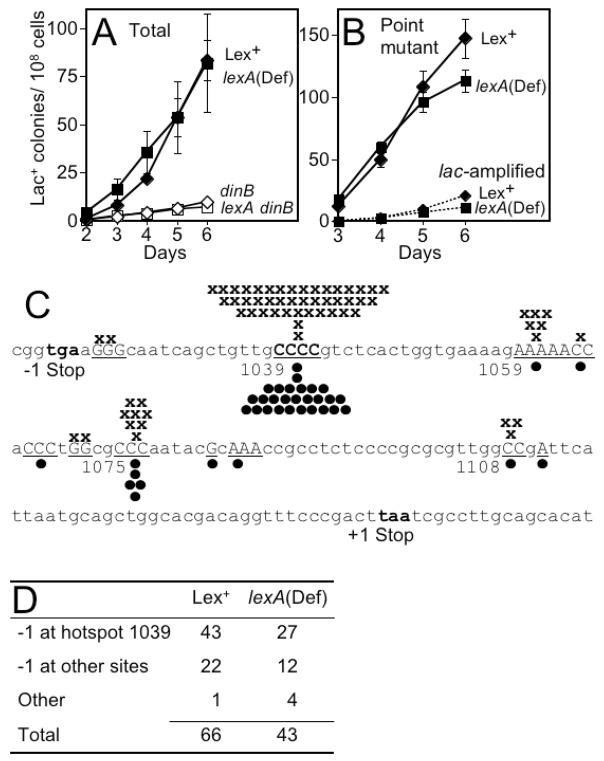
We wished to establish that stress-induced point mutation in SOS/LexA-constitutive cells occurs by a similar mechanism as in “wild-type” lexA+ sulA+ psiB+ cells (called “Lex+” here). First, we asked whether the DinB DNA polymerase is required for stress-induced point mutation in SOS/LexA-constitutive cells. SOS upregulates dinB expression [55,56]. If the stress-induced mutation mechanism in SOS/LexA-constitutive cells were similar to the usual mechanism in SOS-inducible cells, DinB should be required. We found that DinB is required similarly in Lex+ and SOS/LexA-constitutive cells (Fig. 2A).
Fig. 2.
Amounts and kinds of stress-induced mutations are similar in SOS/LexA-constitutive and Lex+ cells. (A) DinB is required in both Lex+ and SOS/LexA-constitutive cells. Total Lac+ colonies (point mutant and lac-amplified) are shown. Lex+, SMR4562 (◆); lexA(Def), SMR5400 (■); dinB, SMR5830 (◇); lexA(Def) dinB, SMR7627 (❑) (Table 1). (B) Similar stress-induced point mutation and amplification in Lex+ and SOS/LexA-constitutive strains. Point mutants (—); lac-amplified colonies (---), distinguished by colony morphology as per [19]. (C, D) Sequences of Lac+ adaptive point mutations in the Lex+ and SOS/LexA-constitutive strains are mostly −1 deletions at mononucleotide repeats: x, Lex+ [31,32]; ●, SOS/LexA constitutive. Base position numbers [32] indicate the base above the right end of the number. (D) Sequence summary. The four “other” mutations (not −1 deletions) in lexA(Def) are tandem duplications: of eleven bp (1029–1039); eleven bp (1044–1054); 86bp (1063–1148); and a 55-bp duplication (1081–1135) containing base substitutions at positions 1131 (A to T), 1137 (G to A), and 1145 (G to C). All four of these mutants grow more slowly than normal stress-induced point mutants, taking longer to form visible colonies under exact reconstructions of the selection conditions (data not shown). They are, therefore, likely to be slowly-growing, generation-dependent mutants that were present before lactose selection. They could be more prevalent in the SOS/LexA-constitutive background either because generation-dependent mutation might be altered/enhanced in these cells, or because stress-induced mutation is reduced slightly [Ref. 39, and Tables 2, 3] allowing more generation-dependent mutants in the total on later days.
Second, we sequenced adaptive point mutations generated in the SOS/LexA-constitutive cells. We compared 43 mutant sequences from SOS/LexA-constitutive cells with previously published mutant sequences for Lex+ cells (Fig. 2C, D). As in Lex+ cells, most of the Lac+ adaptive reversions are −1 deletions at the mononucleotide repeat hotspot at position 1039; the second largest class has −1 deletions mostly at other mononucleotide repeats (both unlike generation-dependent reversions which are heterogeneous [31,32]). Most of the stress-induced mutations in SOS/LexA-constitutive cells are similar to those formed in Lex+ cells (Fig. 2C, D).
Third, we find a similar proportion of lac-amplified versus point mutant colonies in Lex+ and SOS/LexA-constitutive cells (Fig. 2B). This is true even though the SOS/LexA-constitutive cells produced more variable numbers of Lac+ colonies in different experiments than we typically see for Lex+ cells, with the number of Lac+ colonies on average being ≤2-fold lower than Lex+ [Ref. 39, Tables 2 and 3]. The slightly lower number of total Lac+ colonies in SOS/LexA-constitutive than Lex+ cells could be caused by the sulA mutation, which also showed a small decrease relative to Lex+ [39], perhaps due to increased cell division in sulA mutants causing fewer sister molecules per cell, which might limit opportunities for error-prone DSBR [39]. Alternatively, it could be because another gene(s) besides psiB inhibits stress-induced mutagenesis somewhat when constitutively overexpressed in lexA(Def) cells (discussed, section 3.1). In SOS/LexA-constitutive strains the requirement for DinB, and very similar mutation sequences and proportions of amplified clones to those in Lex+ cells, indicate that stress-induced point mutation occurs by a similar or the same mechanism as in Lex+.
Table 2.
Stress-induced-mutation rates from multiple experiments
| Expt #a | Tempb | Mutation rates of relevant genotypes compared (mutants per day)c |
Difference in mutation rate from controld | |
|---|---|---|---|---|
| Mutant | Isogenic Control | |||
| lexA(Def) | Lex+ | |||
| 1 | 37 °C | 31 | 25 | 1.2 |
| 2 | 37 °C | 43 | 89 | 0.48 |
| 3 | 37 °C | 19 | 19 | 0.99 |
| 4 | 37 °C | 2.8 | 16 | 0.17 |
| 5 | 37 °C | 13 | 38 | 0.35 |
| 6 | 37 °C | 23 | 28 | 0.82 |
| 7 | 37 °C | 24 | 36 | 0.67 |
| 8 | 37 °C | 35 | 23 | 1.5 |
| 9 | 37 °C | 34 | 29 | 1.2 |
| 10 | 37 °C | 25 | 29 | 0.85 |
| 11 | 37 °C | 15 | 19 | 0.80 |
| 12 | 37 °C | 25 | 31 | 0.79 |
| 13 | 37 °C | 39 | 60 | 0.66 |
| 14 | 37 °C | 22 | 52 | 0.43 |
| 15 | 37 °C | 15 | 34 | 0.45 |
| 16 | 37 °C | 11 | 28 | 0.38 |
| lexA(Def) | Lex+ | |||
| 17 | 32° C | 40 | 48 | 0.85 |
| 18 | 32° C | 9.2 | 37 | 0.25 |
| 19 | 32° C | 16 | 34 | 0.47 |
| 20 | 32° C | 13 | 19 | 0.65 |
| 21 | 32° C | 40 | 28 | 1.4 |
| 22 | 32° C | 14 | 82 | 0.17 |
| 23 | 32° C | 22 | 33 | 0.68 |
| 24 | 32° C | 14 | 56 | 0.25 |
| 25 | 32° C | 15 | 42 | 0.37 |
| 26 | 32° C | 20 | 38 | 0.53 |
| 27 | 32° C | 19 | 63 | 0.30 |
| 28 | 32° C | 16 | 37 | 0.43 |
| 29 | 32° C | 19 | 110 | 0.17 |
| 30 | 32° C | 19 | 110 | 0.18 |
| ruvA | Lex+ | |||
| 18 | 32° C | 2.3 | 37 | 0.063 |
| 19 | 32° C | 8.3 | 34 | 0.25 |
| 20 | 32° C | 0.92 | 19 | 0.048 |
| 26 | 32° C | 4.2 | 38 | 0.11 |
| 27 | 32° C | 1.6 | 63 | 0.026 |
| ruvB | Lex+ | |||
| 18 | 32° C | 2.0 | 37 | 0.055 |
| 19 | 32° C | 6.8 | 34 | 0.20 |
| 20 | 32° C | 1.3 | 19 | 0.066 |
| 26 | 32° C | 4.2 | 38 | 0.11 |
| 27 | 32° C | 1.9 | 63 | 0.031 |
| ruvC | Lex+ | |||
| 17 | 32° C | 2.1 | 48 | 0.045 |
| 18 | 32° C | 1.3 | 37 | 0.034 |
| 19 | 32° C | 4.4 | 34 | 0.13 |
| 20 | 32° C | 0.94 | 19 | 0.049 |
| 26 | 32° C | 0.83 | 38 | 0.022 |
| 27 | 32° C | 0.96 | 63 | 0.015 |
| recA | Lex+ | |||
| 3 | 37 °C | 0.25 | 19 | 0.013 |
| 5 | 37 °C | 0.41 | 38 | 0.011 |
| 6 | 37 °C | 0.99 | 28 | 0.035 |
| 7 | 37 °C | 0.41 | 36 | 0.011 |
| 14 | 37 °C | 0.59 | 52 | 0.011 |
| 15 | 37 °C | 0.46 | 34 | 0.013 |
| 31 | 37 °C | 0.44 | 56 | 0.0078 |
| traI | Lex+ | |||
| 5 | 37 °C | 0.38 | 38 | 0.0099 |
| 6 | 37 °C | 0.51 | 28 | 0.018 |
| 7 | 37 °C | 0.44 | 36 | 0.012 |
| 14 | 37 °C | 0.53 | 52 | 0.010 |
| 15 | 37 °C | 0.40 | 34 | 0.012 |
| recF::Tn3 | Lex+ | |||
| 5 | 37 °C | 4.94 | 38 | 0.13 |
| 6 | 37 °C | 10 | 28 | 0.37 |
| 7 | 37 °C | 13 | 36 | 0.36 |
| 9 | 37 °C | 7.0 | 29 | 0.24 |
| 10 | 37 °C | 5.2 | 29 | 0.18 |
| 12 | 37 °C | 13 | 31 | 0.43 |
| 32 | 37 °C | 3.8 | 13 | 0.29 |
| ΔrecF::Kan | Lex+ | |||
| 9 | 37 °C | 13 | 29 | 0.46 |
| 10 | 37 °C | 8.8 | 29 | 0.30 |
| 12 | 37 °C | 10 | 31 | 0.34 |
| 14 | 37 °C | 9.8 | 52 | 0.19 |
| ΔrecF | Lex+ | |||
| 9 | 37 °C | 12 | 29 | 0.40 |
| 10 | 37 °C | 13 | 29 | 0.45 |
| 12 | 37 °C | 13 | 31 | 0.41 |
| recG | Lex+ | |||
| 21 | 32° C | 200 | 28 | 7.0 |
| 22 | 32° C | 680 | 82 | 8.2 |
| 23 | 32° C | 940 | 33 | 29 |
| 24 | 32° C | 450 | 56 | 8.0 |
| 25 | 32° C | 590 | 42 | 14 |
| 26 | 32° C | 1010 | 38 | 27 |
| 28 | 32° C | 760 | 37 | 21 |
| 33 | 32° C | 740 | 13 | 56 |
| 34 | 32° C | 620 | 20 | 31 |
| 35 | 32° C | 520 | 24 | 22 |
| 36 | 32° C | 1650 | 76 | 22 |
| 37 | 32° C | 1450 | 77 | 19 |
| lexA(Def) ruvA | lexA(Def) | |||
| 27 | 32° C | 1.9 | 19 | 0.10 |
| 29 | 32° C | 9.2 | 19 | 0.48 |
| 30 | 32° C | 7.1 | 19 | 0.37 |
| 38 | 32° C | 1.5 | 17 | 0.085 |
| lexA(Def) ruvA+ rescue | lexA(Def) | |||
| 39 | 37 °C | 12 | 19 | 0.61 |
| 40 | 37 °C | 66 | 90 | 0.73 |
| 41 | 37 °C | 66 | 57 | 1.16 |
| lexA(Def) ruvB | lexA(Def) | |||
| 27 | 32° C | 2.5 | 19 | 0.13 |
| 29 | 32° C | 3.6 | 19 | 0.19 |
| 30 | 32° C | 5.6 | 19 | 0.29 |
| 38 | 32° C | 5.3 | 17 | 0.31 |
| lexA(Def) ruvB+ rescue | lexA(Def) | |||
| 39 | 37 °C | 7.7 | 19 | 0.40 |
| 40 | 37 °C | 55 | 90 | 0.61 |
| 41 | 37 °C | 48 | 57 | 0.85 |
| lexA(Def) ruvC (2) | lexA(Def) | |||
| 17 | 32° C | 3.9 | 40 | 0.097 |
| 19 | 32° C | 17 | 16 | 1.1 |
| 26 | 32° C | 2.1 | 20 | 0.11 |
| 27 | 32° C | 4.3 | 19 | 0.23 |
| 27 | 32° C | 5.8 | 19 | 0.31 |
| lexA(Def) recA (2) | lexA(Def) | |||
| 1 | 37 °C | 4.0 | 31 | 0.13 |
| 2 | 37 °C | 3.0 | 43 | 0.070 |
| 3 | 37 °C | 1.5 | 19 | 0.080 |
| 5 | 37 °C | 2.3 | 13 | 0.17 |
| 6 | 37 °C | 7.3 | 23 | 0.32 |
| 7 | 37 °C | 4.3 | 24 | 0.17 |
| 14 | 37 °C | 2.7 | 22 | 0.12 |
| 15 | 37 °C | 1.4 | 15 | 0.090 |
| 14 | 37 °C | 2.6 | 22 | 0.12 |
| 15 | 37 °C | 1.6 | 15 | 0.10 |
| lexA(Def) traI (2) | lexA(Def) | |||
| 3 | 37 °C | 5.1 | 19 | 0.27 |
| 5 | 37 °C | 2.1 | 13 | 0.15 |
| 6 | 37 °C | 3.9 | 23 | 0.17 |
| 7 | 37 °C | 3.3 | 24 | 0.14 |
| 14 | 37 °C | 2.7 | 22 | 0.12 |
| 15 | 37 °C | 2.0 | 15 | 0.13 |
| 14 | 37 °C | 2.9 | 22 | 0.13 |
| 15 | 37 °C | 2.5 | 15 | 0.16 |
| lexA(Def) recF::Tn3 | lexA(Def) | |||
| 3 | 37 °C | 8.8 | 19 | 0.45 |
| 5 | 37 °C | 5.0 | 13 | 0.37 |
| 6 | 37 °C | 10 | 23 | 0.45 |
| 7 | 37 °C | 13 | 24 | 0.53 |
| 9 | 37 °C | 7.0 | 34 | 0.20 |
| 10 | 37 °C | 5.2 | 25 | 0.21 |
| 12 | 37 °C | 13 | 24 | 0.55 |
| lexA(Def) ΔrecF::Kan | lexA(Def) | |||
| 9 | 37 °C | 13 | 34 | 0.39 |
| 10 | 37 °C | 8.8 | 25 | 0.35 |
| 12 | 37 °C | 10 | 24 | 0.43 |
| 14 | 37 °C | 9.7 | 22 | 0.43 |
| 16 | 37 °C | 8.5 | 11 | 0.79 |
| 42 | 37 °C | 17 | 32 | 0.53 |
| 43 | 37 °C | 14 | 25 | 0.58 |
| lexA(Def) ΔrecF | lexA(Def) | |||
| 9 | 37 °C | 12 | 34 | 0.34 |
| 10 | 37 °C | 13 | 25 | 0.53 |
| 12 | 37 °C | 13 | 25 | 0.51 |
| lexA(Def) recD (2) | lexA(Def) | |||
| 3 | 37 °C | 53 | 19 | 2.7 |
| 4 | 37 °C | 7.4 | 2.8 | 2.7 |
| 6 | 37 °C | 37 | 23 | 1.6 |
| 7 | 37 °C | 61 | 24 | 2.5 |
| 8 | 37 °C | 32 | 35 | 0.92 |
| 11 | 37 °C | 42 | 15 | 2.8 |
| 14 | 37 °C | 44 | 22 | 2.0 |
| 15 | 37 °C | 44 | 15 | 2.8 |
| 14 | 37 °C | 39 | 22 | 1.8 |
| 15 | 37 °C | 59 | 15 | 3.8 |
| lexA(Def) recG (2) | lexA(Def) | |||
| 22 | 32 °C | 1.9 | 14 | 0.14 |
| 23 | 32 °C | 1.8 | 22 | 0.078 |
| 24 | 32 °C | 7.0 | 14 | 0.50 |
| 25 | 32 °C | 1.7 | 15 | 0.11 |
| 26 | 32 °C | 1.6 | 20 | 0.078 |
| 27 | 32 °C | 4.1 | 19 | 0.22 |
| 26 | 32 °C | 1.4 | 20 | 0.067 |
| lexA(Def) recG+ rescue | lexA(Def) | |||
| 24 | 32 °C | 13 | 14 | 0.91 |
| 25 | 32 °C | 10 | 15 | 0.65 |
| 27 | 32 °C | 17 | 19 | 0.91 |
| lexA(Ind−) | Lex+ | |||
| 16 | 37 °C | 8.9 | 28 | 0.31 |
| 33 | 37 °C | 6.7 | 13 | 0.51 |
| 34 | 37 °C | 4.7 | 20 | 0.23 |
| 35 | 37 °C | 9.3 | 24 | 0.38 |
| 44 | 37 °C | 7.7 | 33 | 0.24 |
| 45 | 37 °C | 13 | 51 | 0.24 |
| 46 | 37 °C | 10 | 52 | 0.20 |
| 47 | 37 °C | 5.4 | 19 | 0.29 |
| lexA(Ind−) | Lex+ | |||
| 28 | 32 °C | 10 | 37 | 0.28 |
| 36 | 32 °C | 8.4 | 76 | 0.11 |
| 37 | 32 °C | 8.9 | 77 | 0.12 |
| lexA(Ind−) recD (2) | lexA(Ind−) | |||
| 44 | 37 °C | 48 | 7.7 | 6.2 |
| 44 | 37 °C | 38 | 7.7 | 4.9 |
| 45 | 37 °C | 22 | 13 | 1.7 |
| 45 | 37 °C | 20 | 13 | 1.6 |
| lexA(Ind−) recG (3) | lexA(Ind−) | |||
| 36 | 32 °C | 88 | 8.4 | 10 |
| 36 | 32 °C | 46 | 8.4 | 5.4 |
| 36 | 32 °C | 63 | 8.4 | 7.5 |
| 37 | 32 °C | 110 | 8.9 | 12 |
| 37 | 32 °C | 112 | 8.9 | 13 |
Experiment number of each experiment done. Where the same number appears more than once for a genotype, these are for independent isolates of the same genotype.
The temperature at which each of the experiments were done. See Materials and Methods.
Stress-induced mutation rates (mutants per day) are the change in colonies from day 4 to 5 [34]. Table 1 for full strain genotypes and information. “lexA(Def)” strains also carry sulA and psiB mutations (Results and Discussion).
Fold effects are the mutation rate of the mutant strain divided by that of its isogenic control in the same experiment. The means ± SEMs for these are shown in Table 3.
3.3 RuvABC promote mutation other than or in addition to by promoting SOS
RuvA, RuvB and RuvC homologous recombination proteins function as a resolvasome complex that processes Holliday junctions, and all are required for stress-induced mutagenesis in the Lac system [Refs. 37,38, Fig. 3A]. RuvA recognizes and binds the four-stranded DNA junction; RuvB is a branch-migration motor protein (helicase) that moves the structure; and RuvC, is an endonuclease that cleaves Holliday junctions [57]. We find that RuvA, RuvB and RuvC are required for stress-induced mutagenesis in SOS/LexA-constitutive cells (Fig. 3B, Tables 2 and 3). By quantifying mutation rates (per day) derived from multiple independent experiments like those shown singly in Fig. 3 (Tables 2 and 3), we see that loss of RuvA, RuvB, and RuvC reduced stress-induced mutation in SOS/LexA-constitutive cells by about three-fold, five-fold, and three-fold, respectively, and in Lex+ cells by 10-fold, 10-fold and 20-fold respectively. (For all genotypes tested, see Table 3 for fold-comparisons and Table 2 for absolute mutation rates used to generate the fold-comparisons.) We conclude that RuvABC play roles in stress-induced mutation in addition to promoting SOS induction, supporting the idea that their well-defined functions in recombination contribute to mutation.
Fig. 3.
Representative examples of stress-induced mutation data. See Tables 2 and 3 for the mutation rates (per day) and their quantification from multiple experiments. In all graphs: Lex+, SMR4562 (◆); lexA(Def), SMR5400 (■). (A) RuvABC proteins are required in Lex+ cells [37,38]. ruvA, SMR1549 (•); ruvB, SMR1552 (▲); ruvC, SMR789 (—x—). (B) RuvABC proteins are also required in SOS/LexA-constitutive cells. lexA(Def) ruvA, SMR8542 (o); lexA(Def) ruvB, SMR8543 (△); lexA(Def) ruvC, SMR8544 (--x--). (C) Two independent constructs of lexA(Def) recA show decreased stress-induced mutation, though less than in Lex+. recA, SMR624 (▲); lexA(Def) recA isolate 1, SMR6488 (—△—), isolate 2, SMR8397 (--△--). (D) Two independent constructs of lexA(Def) traI show decreased stress-induced mutation, though less than in Lex+. traI, SMR6002 (•); lexA(Def) traI isolate 1, SMR7731 (—o—), isolate 2, SMR8401 (--o--). (E) RecF is required for stress-induced mutation in SOS/LexA-constitutive cells. Two alleles of recF show the same level of decrease. lexA(Def) ΔrecF::Kan, SMR8132 (•); lexA(Def) recF332::Tn3, SMR5711 (▲). (F) Loss of RecD increases stress-induced mutation in SOS/LexA-constitutive cells. lexA(Def) recD, SMR7727. (G) Loss of RecD in SOS-uninducible cells increases stress-induced mutation. lexA(Ind−), SMR701; lexA(Ind−) recD, SMR8660. (H) Loss of RecG in Lex+ cells increases stress-induced mutants. recG, SMR1982 (◇). (I) Loss of RecG in SOS/LexA-constitutive cells decreases stress-induced mutants. lexA(Def) recG, SMR8129 (o); lexA(Def) recG+ rescue strain (recG+ transduced into SMR8129) SMR8406 (❑). (J) Loss of RecG in SOS-uninducible cells increases in stress-induced mutants. lexA(Ind−), SMR701 (▲); lexA(Ind−) recG, SMR8546 (▽).
3.4 RecA promotes point mutation both via SOS-induction and SOS-induction-independent step(s)
E. coli RecA is a ssDNA binding protein required for recombination and SOS induction, and is conserved in most studied organisms [57]. In response to DNA damage, RecA becomes activated (RecA*) by loading onto ssDNA. RecA* both induces the SOS response, acting as a co-protease to facilitate the auto-proteolytic cleavage of LexA, and also catalyzes strand exchange with homologous DNA to initiate recombinational repair. We find that RecA is required for stress-induced point mutation in SOS/LexA-constitutive cells (Fig. 3C, Tables 2, 3). However, loss of recA caused only a ten-fold decrease in stress-induced mutation in the lexA(Def) cells, whereas loss of recA in Lex+ cells caused a 100-fold decrease (Table 3). This implies that in Lex+ cells RecA is required both for its role in inducing SOS and also for an additional function(s).
3.5 TraI stimulates SOS-induction and an additional step(s)
Previous work showed that one or more conjugative transfer (Tra) function(s), but not actual transfer, is required for stress-induced mutation [40,41]. TraI is an F-encoded endonuclease/helicase that makes single-strand nicks at the origin of transfer, oriT, on conjugal plasmids [58]. It has been shown recently to be more strongly required for stress-induced mutation ([35] and see Fig. 3D and Table 3) than other transfer genes [41], and also to be dispensable for stress-induced mutation if a DNA DSB is provided near lac by the I-SceI double-strand endonuclease [35]; also the I-SceI cuts promote mutagenesis more than ten times better than TraI. These data imply that TraI-generated single-strand nicks may become double-strand ends (DSEs) and so promote mutation. Given its role in producing ssDNA and possibly DSEs, TraI might promote mutation solely by promoting SOS induction, or those nicks might become DSEs which could be repaired mutagenically (suggested Refs. [37,59–61]). We find that TraI is required even when SOS is induced constitutively, but less in SOS/LexA-constitutive cells than in Lex+ (Fig. 3D, Table 3). Whereas ΔtraI decreases mutation about 100-fold in Lex+ cells, it decreases mutation about five-fold in SOS/LexA-constitutive cells. Therefore, TraI appears to promote mutation in Lex+ cells via stimulating SOS induction and by an additional route, such as leading to DSEs that are repaired mutagenically.
3.6 RecF promotes mutation other than by promoting SOS
RecF is thought to help RecA load onto ssDNA during recombinational DNA gap repair [62], to function in replication restart [63], and also participates in SOS induction by ssDNA lesions [45]. RecF is partially required for Lac+ adaptive mutation and loss of RecF does not decrease mutation further in SOS-uninducible strains carrying an uncleavable LexA repressor [39]. This could mean that the role of RecF is only in promoting SOS induction [39], but might also result from both SOS and RecF-facilitated recombination (or other) function acting in any linear pathway leading to mutation (not just the SOS component of the pathway). Supporting the latter possibility, we find that RecF is required even in SOS/LexA-constitutive cells (Fig. 3E, Table 3). This is true for the recF332::Tn3 allele used previously and also for two newly-constructed recF deletion alleles (Fig. 3E, Tables 2 and 3, Materials and Methods), and all show a similar decrease to that in Lex+ cells [39, Table 3]. Therefore the role of RecF in stress-induced mutation in Lex+ cells is other than or in addition to promoting SOS induction.
This suggests the possibility of a recombinational role for RecF in stress-induced mutation. This might seem surprising given the strong RecBC-dependence of stress-induced mutation [36] (implying involvement of DSEs) and the fact that most RecBC-dependent recombination is RecF-independent [57]. However there is a precedent: a form of DNA replication called inducible stable DNA replication (iSDR), which is thought to occur by priming replication during DSE-repair (similarly to Fig. 1A), strongly requires RecBC and partially requires RecF [64], as also observed for stress-induced mutation ([36,39], Fig. 3E, Table 3). Perhaps RecF facilitates specifically those DSBR reactions that lead to replication, making it appear specific to recombinational processes in which either synthesis [iSDR, see also 63], or errors generated during it (mutation), are the outcome assayed. Though possibly similar, iSDR is not identical to stress-induced mutation in that the former is RuvABC-independent [64].
3.7 RecD inhibits stress-induced mutation independently of effects on SOS induction
RecD is a regulatory subunit of the RecBCD DSBR/recombination enzyme and is required for the exonuclease activity of RecBCD [65–67]. Normally, RecBCD loads only onto DSEs and digests the DNA until it reaches a DNA sequence, Chi, at which the RecD-dependent exonuclease activity is diminished/altered, and ssDNA is produced allowing recombination and SOS induction [57]. In recD null mutants, creation of recombinagenic ssDNA appears to occur more efficiently, immediately upon RecBC(D−) loading onto DNA ends, and cells show hyperrecombination at DSEs [68,69]. RecB is required for stress-induced mutation and RecD inhibits it (recD− cells show increased mutation) [36]. The mechanism for this could be either that RecBC-dependent DSBR is required for mutation, or that SOS induction requires RecB [45] and is increased in recD mutants, or both. (It was also suggested that recD could increase stress-induced mutation by increasing F′ copy number relative to the bacterial chromosome [70], however, recD also inhibits chromosomal stress-induced mutation making this unlikely [71]). We could not test RecB directly, because we found that SOS/LexA-constitutive recB cells are nearly inviable (data not shown). However, we found that, as in Lex+ cells, loss of RecD increased stress-induced mutation in SOS/LexA-constitutive cells and in lexA(Ind−) uninducible [52] cells, in which point mutation is decreased about 85% [42]. In both of these strains, loss of RecD increases stress-induced mutation to a similar extent (Fig. 3F, G, Table 3), and to a similar extent as in Lex+ cells [36]. Therefore, RecD inhibits stress-induced mutation other than by inhibiting SOS-induction. This implies that RecBCD functions in stress-induced mutation independently of any additional effects on SOS induction.
3.8 RecD inhibits point mutation
The increased Lac+ colonies in recD cells ([36], Fig. 3F,G, Table 3) could occur either by an increase in point mutation or lac-amplification or both. In Lex+ recD cells, most Lac+ colonies are point mutants [32]. However, given the SOS-independence of the recD phenotype (Fig. 3F, G, Table 3), and the SOS-independence of lac-amplification [42], it was possible that only lac-amplification increased in lexA(Def) recD and/or lexA(Ind−) recD cells. We determined the fraction of day-6 Lac+ colonies with point mutations versus lac-amplifications [per Ref. 29] and find that point mutation increased greatly in recD cells. In the lexA(Def) background, point mutants were ~90% (109/119 and 85/96 from two experiments) and ~99% (141/143 and 99/100) of the total day-6 Lac+ colonies in recD+ and recD, respectively. In the lexA(Ind−) background as well, in which point mutation but not amplification decreases [42], the remaining point mutation also increased in recD cells: recD+ and recD derivatives showed ~50% (63/120 and 45/93) and 70–90% (18/26 and 93/100) point mutants among day-6 colonies, respectively. Therefore, we conclude that RecD inhibits point mutation (this section), and that this inhibition occurs independently of effects on SOS induction (previous section).
3.9 RecG inhibits point mutation
RecG is a structure-specific DNA helicase that can branch-migrate Holliday (four-way) junctions and three-way junctions [72], possibly in an opposite direction from RuvAB branch-migration helicase [37,38,73], and has been proposed to regress stalled replication forks [73]. recG mutants are hyper-SOS-induced [46,47], and also show 10–25-fold increased stress-induced mutation ([37,38], Fig. 3H, Tables 2 and 3). First, we show that most or all of that increase is point mutation. As above for recD mutants, we see that point mutation is increased by loss of recG in the Lex+ background. Point mutants were ~50–60% (81/137 and 73/134 from two experiments) and ~97% (126/130 and 118/121) of the total day-8 Lac+ colonies in recG+ and recG strains, respectively, indicating that point mutation was greatly stimulated by loss of RecG.
3.10 RecG inhibits SOS induction and an SOS-induction-independent step(s) in point mutation
RecG could inhibit stress-induced point mutation either by unwinding (aborting) a DNA intermediate that otherwise leads to mutation, we suggested a strand-exchange intermediate that primes error-prone synthesis ([38] e.g., Fig. 1A), or by inhibiting SOS induction [16], or both.
Surprisingly, we find that RecG becomes required for mutation in SOS/LexA-constitutive cells (Fig. 3I, Table 3) in contrast to its antimutagenic role in Lex+ cells (e.g., Fig. 3H). We verified this result by testing an additional independent construct of the SOS/LexA-constitutive recG strain and by rescuing the phenotype with wild-type recG+ introduced (by P1 transduction) back into these cells to rule out the possibility of accumulation of suppressor mutations in the SOS/LexA-constitutive recG background [Fig. 3I, the strain “LexA(Def)(2)” is the rescued strain, Tables 2 and 3]. We hypothesized that RecG might be required in SOS/LexA-constitutive cells to counteract an effect of a LexA-controlled protein(s) that becomes antimutagenic only at abnormally high levels when constitutively overproduced, for example RuvAB (discussed below). In support of this idea, we see that RecG again inhibits stress-induced mutation in SOS/LexA-uninducible lexA(Ind−) cells (Fig. 3J, Table 3). This is also a strong inhibition of point mutation, rather than amplification, in that in the lex(Ind−) background, recG+ and recG derivatives showed ~30% (32/123 and 46/137) and 60–80% (82/105 and 62/112) point mutants among day-8 colonies, respectively. Therefore, RecG inhibits point mutation strongly in Lex+ and lexA(Ind−) cells, but is required in lexA(Def) cells. That is, RecG is required for mutation only in the circumstance of constitutive overproduction of LexA-regulated functions.
RecG inhibits stress-induced mutation somewhat less in lexA(Ind−) than in Lex+ cells (Tables 2 and 3), implying that RecG inhibits stress-induced point mutation in Lex+ cells both by a small inhibition of SOS-induction, and a larger inhibition of an SOS-independent step. These data support error-prone DSBR models for point mutation in which RecG inhibits a mutation intermediate directly (e.g., Fig. 1), and also inhibits SOS induction somewhat.
One model for how RecG inhibits point mutation, but promotes it only during LexA-regulon constitutive overexpression, is that RecG competes with the branch migration activity of RuvAB and branch-migrates Holliday junctions in the opposite polarity [37,38,73]. In Fig. 1A, RuvAB moves the junction leftward and RecG rightward. When RuvAB levels are low (normally), RecG rightward movement would inhibit mutation by unwinding/destabilizing the intermediate that primes the DNA synthesis. When RuvAB levels are higher than in a normal, induced SOS response, as in the SOS/LexA-constitutive strain, RuvAB could inhibit mutation by excessive leftwards migration of the junction, which might impede progression of the replication bubble rightward (because of supercoiling constraints). RecG might then facilitate mutation by branch migration rightward, allowing the replication bubble to progress.
Regardless of the specific model, the fact that one of the ways that RecG inhibits point mutation is independent of action on SOS induction, and that six other DSBR proteins play roles in mutation other than via inducing SOS (above), supports the idea that their roles in homologous recombinational DSBR promote stress-induced point mutation.
4. Further Discussion
We have shown that seven different DNA metabolism proteins that have in common their known roles in DSBR and homologous recombination all affect stress-induced point mutation in the E. coli Lac system independently of affects on SOS induction. The proteins are RecA, RecBCD, RuvA, RuvB, RuvC, RecF, and RecG. Whereas any one of these might have an unknown third function that might affect point mutation, in addition to recombination and (known or possible) SOS modulation, it is unlikely that all seven do. This supports error-prone DSBR models for point mutation [e.g., Fig. 1A, Ref. 36, and several subsequent papers]. A recent report also shows directly that DSBs made in cells are mutagenic in stationary phase via a switch to error-prone homologous recombinational repair in stationary phase, and that these induced DSBs promotes Lac+ stress-induced point mutation 6000-fold [35]. The results presented here clarify the roles of each of several DSBR and other proteins in stress-induced mutagenesis, showing that all tested act independently of SOS, and that TraI and RecA also act via promoting SOS, as apparently does RecG.
We note that although mutagenic-DSBR models had seemed to be the only possibility when RecBC-dependence of stress-induced Lac+ mutation was discovered [36], and for the next few years [37,38], this was because it had been thought initially that induction of genes of the SOS regulon (other than RecA) was not required for point mutation [27]. Later work showed that, contrary to that early report, SOS induction [39] and the SOS-controlled DinB error-prone DNA polymerase [42] were required for stress-induced mutagenesis. This made possible, and even likely, indirect models in which all of the DSBR proteins function solely in steps leading to SOS induction, DinB overproduction, and consequent generalized mutagenesis [Ref. 6, and Fig. 1B]. These models are ruled out by the data presented here, and see [35].
4.1 Regulation of point mutagenesis by SOS and RpoS stress responses restricts mutagenesis in time
In the Lac system, the stress-induced mutations are specific to stationary phase, and are regulated positively by at least two stress responses: the SOS DNA damage response [27,39]; and the RpoS starvation- and general-stress response [34], both of which upregulate the error-prone DNA polymerase, DinB [43,55,56]. Clearly these mutations are stress-induced, and stress-induction is conferred by coupling mutagenesis to known stress responses. Thus mutagenesis is limited in time. One does not know whether stress-induced mutagenesis by this or other mutation mechanisms evolved by selection for mutagenesis per se, or whether increased mutation is an unavoidable byproduct of some other stress-induced process. However, it is possible that increasing mutation rate during stress does confer a selective advantage [14]. Although most mutations are deleterious, failure to change genetically when poorly adapted to an environment could be fatal. With large enough populations of cells, promotion of rare adaptive mutations could be advantageous for a cell clone, even though many members of the clone would be harmed by increasing mutation rate. Coupling to stress-responses restricts the risky experiment of heightened mutagenesis to desperate times. Control of mutation by known stress responses is common to many different stress-induced mutation mechanisms [14,34,74–78].
4.2 Mechanism and regulation: DSBR restricts mutagenesis to localized genomic regions
Coupling of mutagenesis to DSBR (Fig. 1A) could also restrict mutagenesis even further, to small genome segments in any given cell: those segments in which DSBs are being repaired [35]. If the DSBs provoking mutagenesis were spontaneous, they might be relatively infrequent per cell and perhaps randomly distributed (in the genome) in populations. If so, all genomic regions might be mutated in a population [as appears to be the case, 10,11,12], but only small segments in any given cell would undergo mutation (Fig. 1A, bottom). Localizing mutagenesis in the genome (Fig. 1A, bottom) could have two important evolutionary consequences: reducing deleterious mutations in rare adaptive mutant cells; and facilitating concerted evolution of genes. For example, rare adaptive mutants could be crippled by deleterious mutations formed simultaneously and inherited in the same cell as the adaptive mutation. In the Lac system, increased mutation is induced throughout the genome [13], and chromosomal mutations (unlinked to lac) occur detectably in Lac+ cells [10–12]. If coupling of mutation to DSBR localizes mutagenesis to one or a few genomic regions per cell (Fig. 1A), this could reduce deleterious coincident mutations [35].
Similarly, a possible advantage of stress-induced mutagenesis could be the ability to alter functions of gene products (RNAs and proteins). Even in the Lac system, in which frameshifting deletions are assayed, which inactivate or reactivate genes, this could occur because the DinB DNA polymerase also makes base substitutions [56,79,80]. Evolution of new functions of RNAs, proteins and protein machines, often requires more than one mutation in a relatively small genomic area (a gene or gene cluster) [e.g., 81]. Localization of increased mutagenesis to small genomic regions might accomplish this. Thus, coupling of stress-induced mutagenesis to DSBR may confer at least two selective advantages [35].
4.3 Coupling mutagenesis to DSBR/recombination appears to have evolved more than once
A stress-induced mutation mechanism studied in budding yeast generates adaptive reversions of a lys frameshift gene during starvation for lysine, and does so using proteins required for non-homologous end-joining (NHEJ), one of two DSBR mechanisms operative in yeast [44]. NHEJ is a fundamentally different mechanism of DSBR from the homologous recombinational repair discussed here. This implies that this yeast stress-induced mutation mechanism evolved independently of the E. coli mechanism studied here. How the yeast NHEJ proteins promote mutagenesis is not yet known, but the convergence of the DSBR-protein dependence of this stress-induced mutation mechanism argues for independent evolution of DSBR-coupled mutagenesis.
Another DSBR-coupled mutation mechanism occurs in yeast. DSBR via homologous recombination targets mutations to the repaired region [82]. Though possibly related to the E. coli mechanism, the error-prone DNA polymerase used is not a member of the DinB superfamily [83]. Though not known to be “stress-induced”, this coupling to DSBR might imply stress-responsiveness in that damage responses to DSBs can be considered stress responses. Association of mutagenesis with homologous recombination was noted also previously in yeast [84,85] and in Salmonella [86]. Finally, several other bacterial mutation mechanisms are RecA-dependent, and not all use RecA in its capacity for SOS induction [14,26,87]. Some of these could represent examples of coupling of recombinational DNA repair to mutation.
Acknowledgments
We thank M. Berlyn and the E. coli Genetic Stock Center for providing strains, A. Campbell, A. Ellington, P.J. Hastings, J. Halliday, C. Herman, M.-J. Lombardo, G. McKenzie, J. Petrosino, J. Sawitzke, and A. Slack and for helpful discussions and/or comments on the manuscript, S. Leal for help with statistical analyses, R. Ponder, D. Magner, J. Pennington, M. Blankschien, L. Gumbiner-Russo and M. Price for reagents and discussions, and D. Ritter, R. Do, G. Olanrewaju, and K. Tran for preparation of media. Supported by a Department of Defense Breast Cancer Research Program postdoctoral fellowship (MNH) and National Institutes of Health grant R01-GM53158.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Luria SE, Delbrück M. Mutations of bacteria from virus sensitivity to virus resistance. Genetics. 1943;28:491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Newcomb HB. Origin of bacterial variants. Nature. 1949;164:150. doi: 10.1038/164150a0. [DOI] [PubMed] [Google Scholar]
- 3.Lederberg J, Lederberg EM. Replica plating and indirect selection of bacterial mutants. J Bacteriol. 1952;63:399–406. doi: 10.1128/jb.63.3.399-406.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lea DE, Coulson CA. The distribution of the numbers of mutants in bacterial populations. J Genet. 1949;49:264–285. doi: 10.1007/BF02986080. [DOI] [PubMed] [Google Scholar]
- 5.Foster PL. Mechanisms of stationary phase mutation: a decade of adaptive mutation. Annu Rev Genet. 1999;33:57–88. doi: 10.1146/annurev.genet.33.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenberg SM. Evolving responsively: adaptive mutation. Nat Rev Genet. 2001;2:504–515. doi: 10.1038/35080556. [DOI] [PubMed] [Google Scholar]
- 7.Hersh MN, Ponder RG, Hastings PJ, Rosenberg SM. Adaptive mutation and amplification in Escherichia coli: two pathways of genome adaptation under stress. Res Microbiol. 2004;155:352–359. doi: 10.1016/j.resmic.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 8.Matic I, Taddei F, Radman M. Survival versus maintenance of genetic stability: a conflict of priorities during stress. Res Microbiol. 2004;155:337–341. doi: 10.1016/j.resmic.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Cairns J, Overbaugh J, Miller S. The origin of mutants. Nature. 1988;335:142–145. doi: 10.1038/335142a0. [DOI] [PubMed] [Google Scholar]
- 10.Torkelson J, Harris RS, Lombardo MJ, Nagendran J, Thulin C, Rosenberg SM. Genome-wide hypermutation in a subpopulation of stationary-phase cells underlies recombination-dependent adaptive mutation. EMBO J. 1997;16:3303–3311. doi: 10.1093/emboj/16.11.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosche WA, Foster PL. The role of transient hypermutators in adaptive mutation in Escherichia coli. Proc Natl Acad Sci USA. 1999;96:6862–6867. doi: 10.1073/pnas.96.12.6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Godoy VG, Gizatullin FS, Fox MS. Some features of the mutability of bacteria during nonlethal selection. Genetics. 2000;154:49–59. doi: 10.1093/genetics/154.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bull HJ, Lombardo MJ, Rosenberg SM. Stationary-phase mutation in the bacterial chromosome: recombination protein and DNA polymerase IV dependence. Proc Natl Acad Sci USA. 2001;98:8334–8341. doi: 10.1073/pnas.151009798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bjedov I, Tenaillon O, Gérard B, Souza V, Denamur E, Radman M, Taddei F, Matic I. Stress-induced mutagenesis in bacteria. Science. 2003;300:1404–1409. doi: 10.1126/science.1082240. [DOI] [PubMed] [Google Scholar]
- 15.Chicurel M. Can organisms speed their own evolution? Science. 2001;292:1824–1827. doi: 10.1126/science.292.5523.1824. [DOI] [PubMed] [Google Scholar]
- 16.Foster PL. Adaptive mutation in Escherichia coli. J Bacteriol. 2004;186:4845, 4846–4852, 4861. doi: 10.1128/JB.186.15.4846-4852.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenberg SM, Hastings PJ. Adaptive point mutation and adaptive amplification pathways in the E. coli Lac system: stress responses producing genetic change. J Bacteriol. 2004;186:4838–4843. 4853, 4862–4863. doi: 10.1128/JB.186.15.4838-4843.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roth JR, Andersson DI. Adaptive mutation: how growth under selection stimulates Lac(+) reversion by increasing target copy number. J Bacteriol. 2004;186:4844, 4854, 4855–4860. doi: 10.1128/JB.186.15.4855-4860.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hastings PJ, Slack A, Petrosino JF, Rosenberg SM. Adaptive amplification and point mutation are independent mechanisms: evidence for various stress-inducible mutation mechanisms. PLoS Biol. 2004;2:e399. doi: 10.1371/journal.pbio.0020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez JL, Baquero F. Mutation frequencies and antibiotic resistance. Antimicrob Agents Chemother. 2000;44:1771–1777. doi: 10.1128/aac.44.7.1771-1777.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cirz RT, Chin JK, Andes DR, Crecy-Lagard VD, Craig WA, Romesberg FE. Inhibition of mutation and combating the evolution of antibiotic resistance. PLoS Biol. 2005;3:e176. doi: 10.1371/journal.pbio.0030176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKenzie GJ, Rosenberg SM. Adaptive mutations, mutator DNA polymerases and genetic change strategies of pathogens. Curr Opin Microbiol. 2001;4:586–594. doi: 10.1016/s1369-5274(00)00255-1. [DOI] [PubMed] [Google Scholar]
- 23.Strauss BS. The origin of point mutations in human tumor cells. Cancer Res. 1992;52:249–253. [PubMed] [Google Scholar]
- 24.Yuan J, Narayanan L, Rockwell S, Glazer PM. Diminished DNA repair and elevated mutagenesis in mammalian cells exposed to hypoxia and low pH. Cancer Res. 2000;60:4372–4376. [PubMed] [Google Scholar]
- 25.Finch CE, Goodman MF. Relevance of ‘adaptive’ mutations arising in non-dividing cells of microorganisms to age-related changes in mutant phenotypes of neurons. Trends Neurosci. 1997;20:501–507. doi: 10.1016/s0166-2236(97)01143-0. [DOI] [PubMed] [Google Scholar]
- 26.Rosenberg SM, Hastings PJ. Modulating mutation rates in the wild. Science. 2003;300:1382–1383. doi: 10.1126/science.1085691. [DOI] [PubMed] [Google Scholar]
- 27.Cairns J, Foster PL. Adaptive reversion of a frameshift mutation in Escherichia coli. Genetics. 1991;128:695–701. doi: 10.1093/genetics/128.4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKenzie GJ, Lombardo MJ, Rosenberg SM. Recombination-dependent mutation in Escherichia coli occurs in stationary phase. Genetics. 1998;149:1163–1165. doi: 10.1093/genetics/149.2.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hastings PJ, Bull HJ, Klump JR, Rosenberg SM. Adaptive amplification: an inducible chromosomal instability mechanism. Cell. 2000;103:723–731. doi: 10.1016/s0092-8674(00)00176-8. [DOI] [PubMed] [Google Scholar]
- 30.McKenzie GJ, Magner DB, Lee PL, Rosenberg SM. The dinB operon and spontaneous mutation in Escherichia coli. J Bacteriol. 2003;185:3972–3977. doi: 10.1128/JB.185.13.3972-3977.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foster PL, Trimarchi JM. Adaptive reversion of a frameshift mutation in Escherichia coli by simple base deletions in homopolymeric runs. Science. 1994;265:407–409. doi: 10.1126/science.8023164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenberg SM, Longerich S, Gee P, Harris RS. Adaptive mutation by deletions in small mononucleotide repeats. Science. 1994;265:405–407. doi: 10.1126/science.8023163. [DOI] [PubMed] [Google Scholar]
- 33.Hendrickson H, Slechta ES, Bergthorsson U, Andersson DI, Roth JR. Amplification-mutagenesis: evidence that “directed” adaptive mutation and general hypermutability result from growth with a selected gene amplification. Proc Natl Acad Sci USA. 2002;99:2164–2169. doi: 10.1073/pnas.032680899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lombardo MJ, Aponyi I, Rosenberg SM. General stress response regulator RpoS in adaptive mutation and amplification in Escherichia coli. Genetics. 2004;166:669–680. doi: 10.1093/genetics/166.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ponder RG, Fonville NC, Rosenberg SM. A switch from high-fidelity to error-prone DNA double-strand-break repair under stress-induced mutation. Mol Cell. 2005 doi: 10.1016/j.molcel.2005.07.025. in press. [DOI] [PubMed] [Google Scholar]
- 36.Harris RS, Longerich S, Rosenberg SM. Recombination in adaptive mutation. Science. 1994;264:258–260. doi: 10.1126/science.8146657. [DOI] [PubMed] [Google Scholar]
- 37.Foster PL, Trimarchi JM, Maurer RA. Two enzymes, both of which process recombination intermediates, have opposite effects on adaptive mutation in Escherichia coli. Genetics. 1996;142:25–37. doi: 10.1093/genetics/142.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harris RS, Ross KJ, Rosenberg SM. Opposing roles of the Holliday junction processing systems of Escherichia coli in recombination-dependent adaptive mutation. Genetics. 1996;142:681–691. doi: 10.1093/genetics/142.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McKenzie GJ, Harris RS, Lee PL, Rosenberg SM. The SOS response regulates adaptive mutation. Proc Natl Acad Sci USA. 2000;97:6646–6651. doi: 10.1073/pnas.120161797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Galitski T, Roth JR. Evidence that F′ transfer replication underlies apparent adaptive mutation. Science. 1995;268:421–423. doi: 10.1126/science.7716546. [DOI] [PubMed] [Google Scholar]
- 41.Foster PL, Trimarchi JM. Adaptive reversion of an episomal frameshift mutation in Escherichia coli requires conjugal functions but not actual conjugation. Proc Natl Acad Sci USA. 1995;92:5487–5490. doi: 10.1073/pnas.92.12.5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McKenzie GJ, Lee PL, Lombardo MJ, Hastings PJ, Rosenberg SM. SOS mutator DNA polymerase IV functions in adaptive mutation and not adaptive amplification. Mol Cell. 2001;7:571–579. doi: 10.1016/s1097-2765(01)00204-0. [DOI] [PubMed] [Google Scholar]
- 43.Layton JC, Foster PL. Error-prone DNA polymerase IV is controlled by the stress-response sigma factor, RpoS, in Escherichia coli. Mol Microbiol. 2003;50:549–561. doi: 10.1046/j.1365-2958.2003.03704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heidenreich E, Novotny R, Kneidinger B, Holzmann V, Wintersberger U. Non-homologous end joining as an important mutagenic process in cell cycle-arrested cells. EMBO J. 2003;22:2274–2283. doi: 10.1093/emboj/cdg203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McPartland A, Green L, Echols H. Control of recA gene RNA in E. coli: regulatory and signal genes. Cell. 1980;20:731–737. doi: 10.1016/0092-8674(80)90319-0. [DOI] [PubMed] [Google Scholar]
- 46.Lloyd R, Buckman C. Genetic analysis of the recG locus of Escherichia coli K-12 and its role in recombination and DNA repair. J Bacteriol. 1991;173:1004–1011. doi: 10.1128/jb.173.3.1004-1011.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCool JD, Long E, Petrosino JF, Sandler HA, Rosenberg SM, Sandler SJ. Measurement of SOS expression in individual Escherichia coli K-12 cells using fluorescence microscopy. Mol Microbiol. 2004;53:1343–1357. doi: 10.1111/j.1365-2958.2004.04225.x. [DOI] [PubMed] [Google Scholar]
- 48.Miller JH. A Short Course in Bacterial Genetics. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1992. [Google Scholar]
- 49.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Borden A, O’Grady PI, Vandewiele D, Fernandez de Henestrosa AR, Lawrence CW, Woodgate R. Escherichia coli DNA polymerase III can replicate efficiently past a T-T cis-syn cyclobutane dimer if DNA polymerase V and the 3′ to 5′ exonuclease proofreading function encoded by dnaQ are inactivated. J Bacteriol. 2002;184:2674–2681. doi: 10.1128/JB.184.10.2674-2681.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Friedberg EC, Walker GC, Siede W. DNA Repair and Mutagenesis. ASM Press; Washington, D. C: 1995. [Google Scholar]
- 52.Mount DW, Low KB, Edmiston SJ. Dominant mutations (lex) in Escherichia coli K-12 which affect radiation sensitivity and frequency of ultraviolet light-induced mutations. J Bacteriol. 1972;112:886–893. doi: 10.1128/jb.112.2.886-893.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bagdasarian M, Bailone A, Angulo JF, Scholz P, Devoret R. PsiB, an anti-SOS protein, is transiently expressed by the F sex factor during its transmission to an Escherichia coli K-12 recipient. Mol Microbiol. 1992;6:885–893. doi: 10.1111/j.1365-2958.1992.tb01539.x. [DOI] [PubMed] [Google Scholar]
- 54.Layton JC, Foster PL. Error-prone DNA polymerase IV is regulated by the heat shock chaperone GroE in Escherichia coli. J Bacteriol. 2005;187:449–457. doi: 10.1128/JB.187.2.449-457.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kenyon CJ, Walker GC. DNA-damaging agents stimulate gene expression at specific loci in Escherichia coli. Proc Natl Acad Sci USA. 1980;77:2819–2823. doi: 10.1073/pnas.77.5.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim SR, Maenhaut-Michel G, Yamada M, Yamamoto Y, Matsui K, Sofuni T, Nohmi T, Ohmori H. Multiple pathways for SOS-induced mutagenesis in Escherichia coli: an SOS gene product (DinB/P) enhances frameshift mutations in the absence of any exogenous agents that damage DNA. Proc Natl Acad Sci USA. 1997;94:13792–13797. doi: 10.1073/pnas.94.25.13792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuzminov A. Recombinational repair of DNA damage in Escherichia coli and bacteriophage lambda. Microbiol Mol Biol Rev. 1999;63:751–813. doi: 10.1128/mmbr.63.4.751-813.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Byrd DR, Sampson JK, Ragonese HM, Matson SW. Structure-function analysis of Escherichia coli DNA helicase I reveals non-overlapping transesterase and helicase domains. J Biol Chem. 2002;277:42645–42653. doi: 10.1074/jbc.M205984200. [DOI] [PubMed] [Google Scholar]
- 59.Kuzminov A. Collapse and repair of replication forks in Escherichia coli. Mol Microbiol. 1995;16:373–384. doi: 10.1111/j.1365-2958.1995.tb02403.x. [DOI] [PubMed] [Google Scholar]
- 60.Rosenberg SM, Harris RS, Torkelson J. Molecular handles on adaptive mutation. Mol Microbiol. 1995;18:185–189. doi: 10.1111/j.1365-2958.1995.mmi_18020185.x. [DOI] [PubMed] [Google Scholar]
- 61.Rodriguez C, Tompkin J, Hazel J, Foster PL. Induction of a DNA nickase in the presence of its target site stimulates adaptive mutation in Escherichia coli. J Bacteriol. 2002;184:5599–5608. doi: 10.1128/JB.184.20.5599-5608.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morimatsu K, Kowalczykowski SC. RecFOR proteins load RecA protein onto gapped DNA to accelerate DNA strand exchange: a universal step of recombinational repair. Mol Cell. 2003;11:1337–1347. doi: 10.1016/s1097-2765(03)00188-6. [DOI] [PubMed] [Google Scholar]
- 63.Courcelle J, Carswell-Crumpton C, Hanawalt PC. RecF and RecR are required for resumption of replication at DNA replication forks in Escherichia coli. Proc Natl Acad Sci USA. 1997;94:3714–3719. doi: 10.1073/pnas.94.8.3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kogoma T. Stable DNA replication: interplay between DNA replication, homologous recombination, and transcription. Microbiol Mol Biol Rev. 1997;61:212–238. doi: 10.1128/mmbr.61.2.212-238.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jockovich ME, Myers RS. Nuclease activity is essential for RecBCD recombination in Escherichia coli. Mol Microbiol. 2001;41:949–962. doi: 10.1046/j.1365-2958.2001.02573.x. [DOI] [PubMed] [Google Scholar]
- 66.Taylor AF, Smith GR. RecBCD enzyme is a DNA helicase with fast and slow motors of opposite polarity. Nature. 2003;423:889–893. doi: 10.1038/nature01674. [DOI] [PubMed] [Google Scholar]
- 67.Dillingham MS, Spies M, Kowalczykowski SC. RecBCD enzyme is a bipolar DNA helicase. Nature. 2003;423:893–897. doi: 10.1038/nature01673. [DOI] [PubMed] [Google Scholar]
- 68.Thaler DS, Sampson E, Siddiqi I, Rosenberg SM, Thomason LC, Stahl FW, Stahl MM. Recombination of bacteriophage λ in recD mutants of Escherichia coli. Genome. 1989;31:53–67. doi: 10.1139/g89-013. [DOI] [PubMed] [Google Scholar]
- 69.Dabert PS, Ehrlich SD, Gruss A. Chi sequence protects against RecBCD degradation of DNA in vivo. Proc Natl Acad Sci USA. 1992;89:12073–12077. doi: 10.1073/pnas.89.24.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Foster PL, Rosche WA. Increased episomal replication accounts for the high rate of adaptive mutation in recD mutants of Escherichia coli. Genetics. 1999;152:15–30. doi: 10.1093/genetics/152.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bull HJ, McKenzie GJ, Hastings PJ, Rosenberg SM. Evidence that stationary-phase hypermutation in the Escherichia coli chromosome is promoted by recombination. Genetics. 2000;154:1427–1437. doi: 10.1093/genetics/154.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Whitby MC, Lloyd RG. Targeting Holliday junctions by the RecG branch migration protein of Escherichia coli. J Biol Chem. 1998;273:19729–19739. doi: 10.1074/jbc.273.31.19729. [DOI] [PubMed] [Google Scholar]
- 73.McGlynn P, Lloyd RG. Rescue of stalled replication forks by RecG: simultaneous translocation on the leading and lagging strand templates supports an active DNA unwinding model of fork reversal and Holliday junction formation. Proc Natl Acad Sci USA. 2001;98:8227–8234. doi: 10.1073/pnas.111008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gomez-Gomez JM, Blazquez J, Baquero F, Martinez JL. H-NS and RpoS regulate emergence of Lac Ara+ mutants of Escherichia coli MCS2. J Bacteriol. 1997;179:4620–4622. doi: 10.1128/jb.179.14.4620-4622.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ilves H, Horak R, Krvisaar M. Involvement of σS in starvation-induced transposition of Pseudomonas putida transposon Tn4652. J Bacteriol. 2001;183:5445–5448. doi: 10.1128/JB.183.18.5445-5448.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Saumaa S, Tover A, Kasak L, Kivisaar M. Different spectra of stationary-phase mutations in early-arising versus late-arising mutants of Pseudomonas putida: involvement of the DNA repair enzyme MutY and the stationary-phase sigma factor RpoS. J Bacteriol. 2002;84:6957–6965. doi: 10.1128/JB.184.24.6957-6965.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sung HM, Yasbin RE. Adaptive, or stationary-phase, mutagenesis, a component of bacterial differentiation in Bacillus subtilis. J Bacteriol. 2002;184:5641–5653. doi: 10.1128/JB.184.20.5641-5653.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Perez-Capilla T, Baquero MR, Gomez-Gomez JM, Ionel A, Martin S, Blazquez J. SOS-independent induction of dinB transcription by β-lactam-mediated inhibition of cell wall synthesis in Escherichia coli. J Bacteriol. 2005;187:1515–1518. doi: 10.1128/JB.187.4.1515-1518.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wagner J, Gruz P, Kim SR, Yamada M, Matsui K, Fuchs RPP, Nohmi T. The dinB gene encodes a novel E. coli DNA polymerase, DNA pol IV, involved in mutagenesis. Mol Cell. 1999;4:281–286. doi: 10.1016/s1097-2765(00)80376-7. [DOI] [PubMed] [Google Scholar]
- 80.Wagner J, Nohmi T. Escherichia coli DNA polymerase IV mutator activity: genetic requirements and mutational specificity. J Bacteriol. 2000;182:4587–4595. doi: 10.1128/jb.182.16.4587-4595.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Camps M, Naukkarinen J, Johnson BP, Loeb LA. Targeted gene evolution in Escherichia coli using a highly error-prone DNA polymerase I. Proc Natl Acad Sci USA. 2004;100:9727–9732. doi: 10.1073/pnas.1333928100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McGill CB, Holbeck SL, Strathern JN. The chromosome bias of misincorporations during double-strand break repair is not altered in mismatch repair-defective strains of Saccharomyces cerevisiae. Genetics. 1998;148:1525–1533. doi: 10.1093/genetics/148.4.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Holbeck SL, Strathern JN. A role for REV3 in mutagenesis during double-strand break repair in Saccharomyces cerevisiae. Genetics. 1997;147:1017–1024. doi: 10.1093/genetics/147.3.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Magni GE, von Borstel RC. Different rates of spontaneous mutation during mitosis and meiosis in yeast. Genetics. 1962;47:1097–1108. doi: 10.1093/genetics/47.8.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Esposito MS, Bruschi CV. Diploid yeast cells yield homozygous spontaneous mutations. Curr Genet. 1993;23:430–434. doi: 10.1007/BF00312630. [DOI] [PubMed] [Google Scholar]
- 86.Demerec M. Selfer mutants of Salmonella typhimurium. Genetics. 1963;48:1519–1531. doi: 10.1093/genetics/48.11.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shapiro JA. Genome organization, natural genetic engineering and adaptive mutation. Trends Genet. 1997;13:98–104. doi: 10.1016/s0168-9525(97)01058-5. [DOI] [PubMed] [Google Scholar]
- 88.Courcelle J, Khodursky A, Peter B, Brown P, Hanawalt PC. Comparative gene expression profiles following UV exposure in wild-type and SOS-deficient Escherichia coli. Genetics. 2001;158:41–64. doi: 10.1093/genetics/158.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]