Abstract
Salivary glands are highly susceptible to radiation, and patients with head and neck cancer treated with radiotherapy invariably suffer from its distressing side effect, salivary hypofunction. The reduction in saliva disrupts oral functions, and significantly impairs oral health. Previously, we demonstrated that adenoviral-mediated expression of Tousled-like kinase 1B (TLK1B) in rat submandibular glands preserves salivary function after single-dose ionizing radiation. To achieve long-term transgene expression for protection of salivary gland function against fractionated radiation, this study examines the usefulness of recombinant adeno-associated viral vector for TLK1B delivery. Lactated Ringers or AAV2/9 with either TLK1B or GFP expression cassette were retroductally delivered to rat submandibular salivary glands (1011 vg/gland), and animals were exposed, or not, to 20 Gy in eight fractions of 2.5 Gy/day. AAV2/9 transduced predominantly the ductal cells, including the convoluted granular tubules of the submandibular glands. Transgene expression after virus delivery could be detected within 5 weeks, and stable gene expression was observed till the end of study. Pilocarpine-stimulated saliva output measured at 8 weeks after completion of radiation demonstrated >10-fold reduction in salivary flow in saline- and AAV2/9-GFP-treated animals compared with the respective nonirradiated groups (90.8% and 92.5% reduction in salivary flow, respectively). Importantly, there was no decrease in stimulated salivary output after irradiation in animals that were pretreated with AAV2/9-TLK1B (121.5% increase in salivary flow; p<0.01). Salivary gland histology was better preserved after irradiation in TLK1B-treated group, though not significantly, compared with control groups. Single preemptive delivery of AAV2/9-TLK1B averts salivary dysfunction resulting from fractionated radiation. Although AAV2/9 transduces mostly the ductal cells of the gland, their protection against radiation assists in preserving submandibular gland function. AAV2/9-TLK1B treatment could prove beneficial in attenuating xerostomia in patients with head and neck cancer undergoing radiotherapy.
Shanmugam and colleagues demonstrate that a single instillation of recombinant AAV9 encoding Tousled-like kinase 1B (TLK1B) in rat submandibular glands leads to a complete amelioration of salivary dysfunction caused by fractionated radiation. They suggest that this treatment modality may be beneficial in attenuating xerostomia in headand-neck cancer patients undergoing radiotherapy.
Introduction
A majority of patients with head and neck cancer are treated with therapeutic radiation alone or in combination with other treatment modalities. Since radiation portals, most often, encompass a large part or whole of the major salivary glands, radiotherapy results in the unavoidable destruction of these tissues. Consequently, diminution of salivary gland function occurs in almost all patients undergoing regional radiotherapy and ∼80% of patients undergoing total body radiation before bone marrow transplantation (Sciubba and Goldenberg, 2006; Chambers et al., 2007a).
In clinical therapy, patients are exposed to small doses of radiation with the underlying premise of minimizing damage to normal tissues. However, salivary glands are highly sensitive to radiation, and salivary gland function is compromised despite fractionated radiotherapy. Within the first week of radiotherapy, salivary function drops significantly, and the chronic decline in function continues through therapy. Although some recovery of parotid gland function has been documented in the months and years after radiotherapy (Braam et al., 2005), the probability of gland dysfunction (a reduction to <25% preradiation levels) correlates well with the increase in the mean radiation dose (Roesink et al., 2001; Dijkema et al., 2010). Patients with head and neck cancer are generally treated with a total therapeutic dose of 65–70 Gy (Sciubba and Goldenberg, 2006), and nearly 95% patients have poor parotid function 1 year after radiation (Dijkema et al., 2010). Therefore, it is not surprising that the most common compliant in head and neck cancer survivors is the subjective perception of oral dryness, or xerostomia. Although the underlying radiobiological mechanism of salivary dysfunction remains unclear, the early reduction in salivary flow is argued to be caused by compromised membrane functioning of acinar cells (Konings et al., 2005), whereas the chronic and persistent decline in function is attributed to apoptosis of cells with latent DNA damage (Nagler, 2002).
Normal salivary flow facilitates key oral activities, and its reduction impairs functions of chewing, swallowing, speech, and taste. Further, hyposalivation accentuates radiation-induced oral mucositis, ulcerations, and odynophagia, and it increases the host's susceptibility to rampant dental decay, mucosal trauma, and infections (Sciubba and Goldenberg, 2006; Chambers et al., 2007a). The comorbidity associated with xerostomia severely limits patients' well-being and their prospect of continuing cancer treatment (Epstein et al., 2001; Bruce, 2004). Palliative relief of symptoms with artificial salivary substitutes and the use of prosecretory drugs, such as pilocarpine and cevimeline, have had limited success in treating xerostomia (Chambers et al., 2007b; Berk, 2008). The incorporation of salivary gland-sparing radiation techniques and the use of radioprotective drug, Amifostine, have, to some measure, diminished the severity of xerostomia (Rudat et al., 2008), but the chronic functional deterioration of the gland remains a major concern.
Human Tousled-like kinase 1 (TLK 1) is a 788 amino acid serine/threonine kinase that has important functions in DNA replication, chromatin assembly checkpoint arrest, and DNA repair (Sillje et al., 1999; Carrera et al., 2003; Groth et al., 2003; Hashimoto et al., 2008; Takayama et al., 2010). A spliced variant of TLK1, TLK1B, is identical to full-length protein except from the loss of 237 N-terminal amino acids (Li et al., 2001). TLK1 and TLK1B are known to phosphorylate the same substrates identified to date, namely, histone H3, anti-silencing factor 1, and Rad9 (Li et al., 2001; Sillje and Nigg, 2001; Sunavala-Dossabhoy et al., 2003; Sunavala-Dossabhoy and De Benedetti, 2009). We have shown that expression of TLK1B in mouse epithelial cells does not induce oncogenic transformation, but it does increase the cell's ability to overcome radiation injury (Li et al., 2001; Sunavala-Dossabhoy et al., 2003; Sunavala-Dossabhoy et al., 2005). This holds true for irradiated normal rat salivary acinar and ductal cells transduced with exogenous TLK1B as well (Palaniyandi et al., 2011; Sunavala-Dossabhoy et al., 2012). Although TLK1B facilitates repair of double-strand breaks, its precise role in this process is not completely elucidated. It is postulated that the coordinated chaperone and kinase functions of the protein facilitate repair through checkpoint arrest and through chromatin remodeling at break sites (Canfield et al., 2009; Sunavala-Dossabhoy and De Benedetti, 2009).
Using localized adenovirus gene delivery to rat submandibular glands, we recently demonstrated that TLK1B expression effectively attenuates salivary dysfunction after single-dose radiation (Palaniyandi et al., 2011). Nonetheless, short-lived gene expression and immunogenicity of the vector on repeated administration limit the prospect of translating adenovirus-TLK1B to head and neck cancer patient care where radiotherapy is delivered in small doses, daily, for 5–7 weeks. Adeno-associated viral vectors, alternately, provide lasting gene expression, and are relatively nonpathologic and nonimmunogenic. Naturally, they are in greater use in clinical trials for the treatment of a number of human diseases and genetic disorders (Gaudet et al., 2012; Gray, 2013; Weinberg et al., 2013). In preparation for a prospective clinical transition of TLK1B for radiotherapy-induced xerostomia, this study investigates the efficacy and safety of AAV-TLK1B gene transfer to submandibular salivary glands in mitigating the disorder.
Materials and Methods
Vector constructs and AAV2/9 preparations
GFP or TLK1B sequence was inserted between the hybrid cytomegalovirus immediate early enhancer/chicken beta-actin promoter sequence and the bovine growth hormone polyadenylation sequence. The entire expression cassette was incorporated between AAV2 terminal repeats. Cross-packaged, recombinant AAV2/9 was generated as described previously (Klein et al., 2008). In brief, human embryonic kidney cells, 293T, were cotransfected using calcium phosphate with AAV2-terminal repeat–containing plasmid and packaging plasmids, pAd-deltaF6 and pAAV9, which provided adenoviral helper genes and the AAV9 capsid genes, respectively. Cells were collected 72 hr after transfections and lyzed by three freeze–thaw cycles. The crude lysates were treated with benzonase (Sigma) before applying them to iodixanol gradients (OptiPrep; Greiner Bio-One) and centrifuging at 70,000 g for 75 min at 20°C. The density gradient fraction containing AAV was collected, and the virus was washed and concentrated in lactated Ringer's solution (Baxter) using Amicon-Ultra 15 (100 kDa) filter units. The virus stock was titered for viral genome copies by qPCR assay (Bio-Rad) with primers targeted to GFP or TLK1B sequence. The viruses were aliquoted and stored at −80°C.
Cell culture and fluorescence microscopy
Rat salivary gland cell lines, A5 and ParC5, were kind gifts from Dr. Bruce Baum (National Institutes of Health [NIH] Clinical Center) and Dr. David Quissell (University of Colorado Health Sciences Center [UCHSC]), respectively. A5 cells are submandibular ductal cells, whereas ParC5 are parotid acinar cells. Cells were grown at 37°C in the presence of 5% CO2 in Dulbecco's modified Eagle's medium (DMEM; for A5 cells) or modified DMEM/F12 (for ParC5 cells) that was supplemented with 10% bovine growth serum (BGS; Hyclone), 2 mM L-Glutamine, and 1% antibiotic–antimycotic solution (Invitrogen). Cells were plated in six-well plates, and when 40–50% confluence was reached, AAV2/9-GFP (109 vg/well) was overlaid on cells in reduced-serum medium, OPTI-MEM (Life Technologies). After 4 hr of incubation, the cells were supplemented with equal volume of the complete medium containing 20% BGS. Native GFP fluorescence was evaluated on a fluorescence microscope using an fluorescein isothiocyanate filter daily from the day after transduction to day 7.
Salivary gland transduction in vivo
Male Sprague-Dawley rats (225–250 g) obtained from Harlan Laboratories, were anesthetized by intramuscular administration of ketamine chloride (42 mg/kg), xylazine (8 mg/kg), and acepromazine (1.4 mg/kg). Salivary secretions were suppressed by subcutaneous delivery of atropine (0.5 mg/kg). The submandibular salivary gland ducts were cannulated with thin polyethelene tubing, and virus (1011 vg/gland) or lactated Ringer's solution (vehicle) was instilled in the glands. The tubing was retained in place for 30 min before being removed. Animals were kept warm during recovery and placed in cages after they were ambulatory. All animals had access to food and water ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee and were in accordance with NIH guidelines.
Fractionated irradiation and collection of submandibular saliva
Five weeks after virus transduction, animals were anesthetized by intramuscular delivery of a mix of ketamine (33 mg/kg), xylazine (6 mg/kg), and acepromazine (1 mg/kg) for irradiation. They were placed in a supine position, and a 1 cm tissue-equivalent bolus was placed over the skin. Radiation collimated to the neck was delivered at 6 MV using the Elekla RTD with MLC Linear Accelerator (LSUHSC–Radiation Oncology). Animals were irradiated with 2.5 Gy/day, 4 times/week, for 2 weeks with a 2-day weekend interspersed in between. Animals received a cumulative dose of 20 Gy. After recovery from anesthesia, animals were returned to their cages and housed at the animal facility.
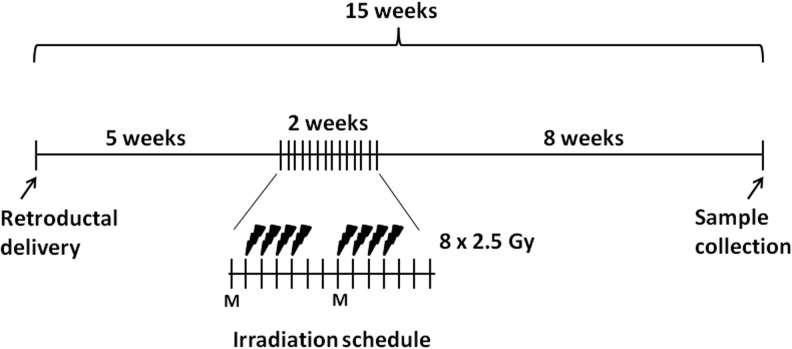
Stimulated submandibular salivary secretions were collected 8 weeks after completion of radiation. Pilocarpine (0.5 mg/kg) was subcutaneously administered in anesthetized animals, and collection started after saliva was observed at the ductal orifices. Submandibular/sublingual saliva that accumulated at the floor of the mouth was aspirated and collected in prechilled tubes over 20 min. To avoid collecting parotid secretions, absorbent cotton rolls were placed in the buccal pouches and at the rear of the mouth. Animals were later perfused with 4% paraformaldehyde/phosphate buffered saline (PBS), and submandibular glands and major organs were harvested for histological and immunohistochemical analysis. A schematic of the experiment schedule is shown in Fig. 1.
FIG. 1.
A schematic representation of an experiment schedule. Radiation commenced 5 weeks after delivery of virus (1011 vg/gland) or lactated Ringers to both submandibular glands. A radiation dose of 20 Gy was fractionated in eight doses of 2.5 Gy. Sample collection was done 8 weeks after irradiation. M, Monday.
Statistical analysis
Salivary flow rate data are expressed as mean±SEM. Statistical computing software, SAS, version 9.2, was used to analyze the data. A two-way analysis of variance was used to determine significant effects of treatment (lactated Ringers, AAV2/9-GFP, and AAV2/9-TLK1B), irradiation (nonexposed, exposed), and treatment–irradiation interaction on the salivary flow rate. The Bonferroni multiple comparison test was used to determine which pair-wise comparisons were significant.
In Vivo Imaging Systems imaging for GFP epifluorescence
To ascertain AAV9 transduction of submandibular salivary glands and associated vector spread, GFP gene expression was analyzed by In Vivo Imaging Systems (IVIS) imaging at 5 weeks after virus delivery. Animals were euthanized under anesthesia, and submandibular glands and major organs, including the heart, lung, liver, spleen, kidneys, and intestines, were evaluated by biophotonic GFP imaging using Xenon IVIS 100/XFO-12 apparatus. Images were acquired at the same settings: emission and excitation filters (GFP), exposure time (5 s), binning (8), and f/stop (1). Pseudocolored images showing a spatial distribution of photon counts were overlaid on photographs. Rats instilled with lactated Ringer's solution were negative controls. Images were analyzed using Living Image 4.0 (Caliper Life Sciences).
Histology and GFP immunohistochemistry
Submandibular glands and major organ tissues were collected and fixed in 4% paraformaldehyde overnight at 4°C before being processed for paraffin-embedding and sectioning. Sections of tissue samples were stained with hematoxylin and eosin, or processed for immunohistochemistry with rabbit anti-GFP (Invitrogen), or mouse anti–glutamate-glutamic acid-rich proteins (GRP) (an antibody to acinar cell-specific GRPs; a kind gift from Dr. Lawrence Tabak, NIDCR). Endogenous peroxidase activity was quenched in deparaffinized, dehydrated tissue sections with 0.3% H2O2/PBS for 30 min. Sections were permeabilized with 0.3% Triton X-100/PBS for 10 min, and blocked in 10% goat serum/5% bovine serum albumin with the avidin blocking solution (Vector Labs) to suppress nonspecific antibody binding. Primary antibody incubation (1:300) with the biotin blocking solution (Vector Labs) was done for 16 hr at 4°C followed by biotinylated anti-rabbit (1:200; Dako Cytomation) or biotinylated anti-mouse (1:50; BD Pharmigen) for 30 min. Sections were labeled with horseradish peroxidase (HRP)-conjugated Extravidin (1:2000; Sigma) for 30 min before proceeding to detect avidin–biotin peroxidase complexes with diaminobenzidine (DAB). Lactated Ringers-treated glands were negative controls for immunohistochemical staining.
Histopathological analysis of submandibular glands was performed using a focus score. Nested mononuclear cells that were >5 per 4 mm2 were scored. A minimum of four sections per animal were scored, and the total number of foci per animal were plotted. Scoring of GFP immunostaining was performed on the subjective assessment of stain intensity and percent area staining. Quantification of surface acinar cell area was done using ImageJ software (NIH). A minimum of three random microscope fields (200×) per gland were analyzed, and GRP-stained areas were summed.
Immunoblotting for TLK1B
Submandibular glands treated with lactated Ringers or AAV2/9-TLK1B were removed at 5 or 15 weeks after retroductal instillation. The tissues were minced and sonicated in the radio immunoprecipitation assay (RIPA) buffer with protease inhibitor cocktail (Roche), 5 mM sodium orthovanadate, and 1 mM phenylmethylsulfonyl fluoride (PMSF). The homogenates were centrifuged, and protein concentrations were determined using BCA Protein Assay (Pierce/Thermo Scientific). Equal amount of total protein (100 μg) from each sample was loaded on 10% sodium dodecyl sulfate polyacrylamide gel and transferred to nitrocellulose membranes. The membranes were blocked in 7% milk/PBS before incubating with rabbit anti-TLK1 (1:1000) followed by HRP-conjugated anti-rabbit (1:1000; Vector Laboratories), or HRP-conjugated anti-β actin (1:10,000; Sigma). The membrane was reacted with DAB for visualization of protein bands.
Results
AAV2/9 transduction in rat salivary gland cells in culture
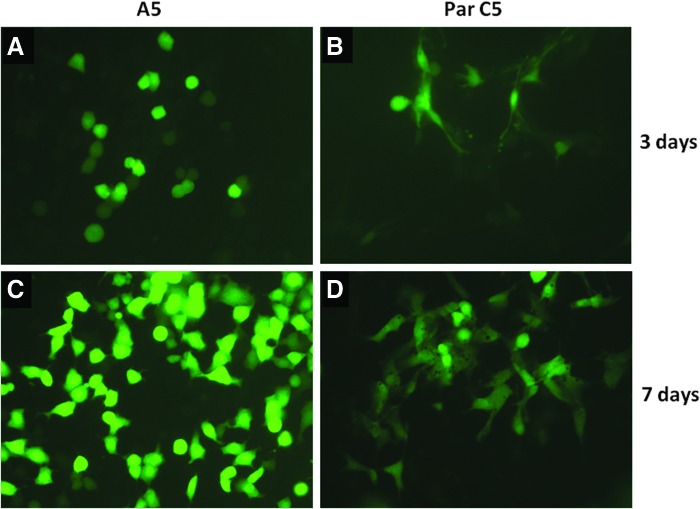
Before investigating AAV2/9 transduction in salivary glands in vivo, virus transduction was assessed in rat salivary cells in culture. A5, ductal cells of the submandibular gland, and ParC5, acinar cells of the parotid, were transduced with AAV2/9-GFP. As shown in Fig. 2, native GFP fluorescence was detected in 60–70% of each cell type, although the intensity of fluorescence was higher in A5, ductal cells, than in Par C5, acinar cells. GFP expression was observed starting at day 3 post-transduction (Fig. 2A and B), and fluorescence intensity increased with time. Peak fluorescence intensity was recorded at day 7 (Fig. 2C and D).
FIG. 2.
AAV2/9 transduction in rat salivary gland cells. AAV2/9-GFP (109 vg) transduction in rat submandibular ductal cells, A5 (A and C), and rat parotid acinar cells, ParC5 (B and D). Native GFP fluorescence recorded on a fluorescence microscope with fluorescein isothiocyanate filters at 3 (A and B) and 7 days (C and D) after virus transduction. Magnification, 400×.
AAV2/9-associated transgene expression in submandibular salivary glands in vivo
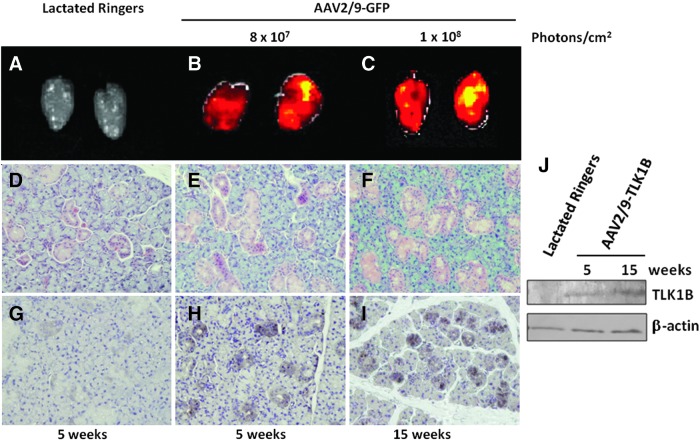
GFP gene expression ascertained after retroductal delivery of AAV2/9-GFP to submandibular glands revealed transgene expression in the tissue within 5 weeks. GFP epifluorescence measured at 5 and 15 weeks after virus transduction indicates that AAV2/9-mediated transgene expression in submandibular glands is long-term and stable (Fig. 3B and C). Immunogenicity of the vector was determined by evaluation of tissue sections, microscopically. No histological differences in gland architecture in virus-treated or lactated Ringers-treated animals were noted (Fig. 3D–F). To ascertain virus tropism for salivary cell type, immunohistochemical localization of GFP was performed. There was no GFP staining in lactated Ringers-treated glands, but in glands treated with AAV2/9-GFP, GFP expression was predominantly in the ductal epithelial cells with marginal number of acinar cells staining positive for it (Fig. 3H and I). Similar to results of virus transduction in salivary cells in culture, in vivo results confirm superior AAV2/9 transduction in the ductal cells. Immunohistochemical staining was observed in the intercalated and striated ducts and in convoluted granular tubules 5 weeks after virus delivery (Fig. 3H), and transgene expression was sustained up to the end of the study at 15 weeks (Fig. 3I). Submandibular glands treated with AAV2/9-TLK1B confirm longevity of transgene expression after virus delivery (Fig. 3J).
FIG. 3.
AAV2/9-associated stable transgene expression in rat salivary glands in vivo. (A–C) Biphotonic imaging of GFP in salivary glands at an expression interval of 5 weeks (n=3 each) or 15 weeks (n=4 each) after instillation of lactated Ringer's solution or AAV2/9-GFP (1011 vg/gland). (D–F) Histological evaluation of hematoxylin and eosin-stained salivary gland sections, and (G–I) immunohistochemical localization of GFP at 5 and 15 weeks after delivery of lactated Ringers or AAV2/9-GFP (1011 vg/gland). Nearly 25–50% of ductal cells were immunostained at moderate (score 2) intensity. Magnification, 200×. (J) Western blotting of submandibular gland tissue lysates with antibodies to TLK1 and β-actin at 5 and 15 weeks after retroductal delivery of lactated Ringers or AAV2/9-TLK1B (1011 vg/gland).
Vector spread after submandibular gland delivery in vivo
To evaluate the spread of vector after localized delivery of AAV2/9-GFP, submandibular glands and major organ tissues were excised and studied using IVIS biphotonic GFP imaging at 5 weeks after virus delivery. Apart from the submandibular salivary glands, the heart, lung, liver, kidneys, spleen, and intestine were evaluated for GFP expression by IVIS imaging 5 weeks after virus delivery. All major organs, except the intestine of virus-treated animals, demonstrated signal at background levels similar to no vector-treated controls (Supplementary Fig. S1A–E and G–K; Supplementary Data are available online at www.liebertonline.com/hum). GFP signal could be detected in intestines of treated animals (Supplementary Fig. S1L), but not in those of lactated Ringers-treated controls (Supplementary Fig. S1F). Similar to submandibular salivary glands, transgene expression in the intestines was long-lived, and it could be detected immunohistochemically within the simple columnar epithelial cells that line the crypts and the lamina propria at 15 weeks post-virus delivery (Supplementary Fig. S2).
TLK1B ameliorates fractionated radiation-induced hyposalivation
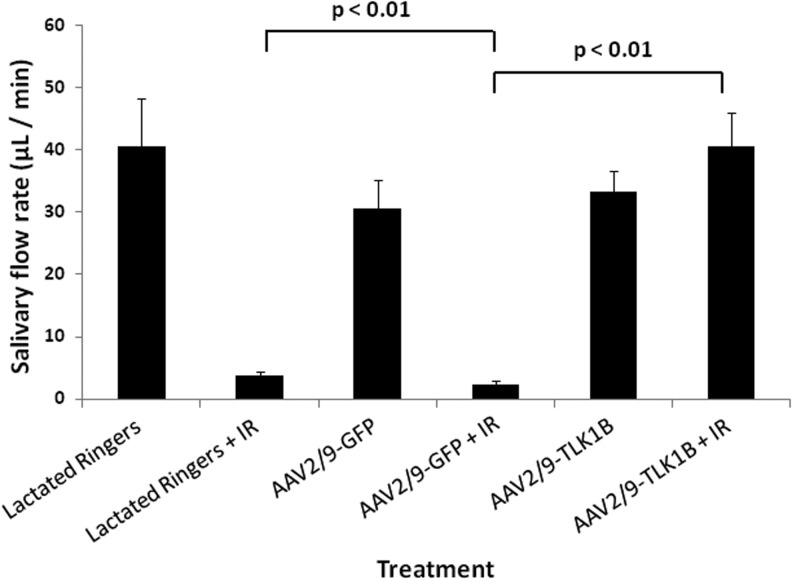
Previously, we had shown that a single-dose of 16 Gy compromises rat submandibular gland function, and pretreatment with adenovirus-TLK1B effectively salvaged salivary flow (Palaniyandi et al., 2011). To assess the effect of TLK1B on clinically relevant radiation dosing, animals were irradiated in fractions of 2.5 Gy daily, up to a total of 20 Gy (Fig. 1). Nonirradiated animals pretreated with lactated Ringers, AAV2/9-GFP, or AAV2/9-TLK1B showed average salivary flow rates of 40.5±7.9 μl/min, 30.6±4.5 μl/min, and 33.4±3.2 μl/min, respectively. Radiation treatment resulted in a significant reduction in salivary flow in animals that were pretreated with lactated Ringers or AAV2/9-GFP (3.7±0.7 μl/min and 2.3±0.6 μl/min, respectively). Unlike control animals, animals treated with AAV2/9-TLK1B demonstrated no drop in salivary flow after radiation (40.54±5.4 μl/min; Fig. 4). When compared with the corresponding nonirradiated groups, the lactated Ringers- and AAV2/9-GFP-treated animals exhibited a >90% reduction in salivary flow after radiation, but more importantly, AAV2/9-TLK1B groups showed no decline in salivary flow after radiation (121% increase); salivary flow was not significantly different in irradiated and nonirradiated animals treated similarly with TLK1B (Fig. 4).
FIG. 4.
Effects of fractionated radiation and AAV2/9-TLK1B on submandibular gland function. Five weeks after instillation of lactated Ringers, AAV2/9-GFP (1011 vg/gland), or AAV2/9-TLK1B (1011 vg/gland), rat submandibular glands were exposed, or not, to 2.5 Gy/day for a total of 8 days. Data (mean±SEM) shown are stimulated submandibular/sublingual salivary flow rates 8 weeks after completion of radiation. Lactated Ringers treated, n=4/each group; AAV9-GFP treated and AAV9-TLK1B treated, n=7/each group. The difference between irradiated lactated Ringers or AAV2/9-GFP and AAV2/9-TLK1B are significant by two-way analysis of variance (p<0.01).
Statistical analyses of salivary flow rates showed that treatment, irradiation, and interaction effects were highly significant (each with p<0.01). Pair-wise comparisons between AAV2/9-TLK1B and lactated Ringers groups, and between AAV2/9-TLK1B and AAV2/9-GFP groups were significant (p<0.01), but there was no significant difference between lactated Ringers and AAV2/9-GFP groups. A significant effect of radiation–treatment interaction (p<0.01) suggested that the effect of irradiation on salivary flow rate was different among the three treatment groups. Radiation had a significant effect on the AAV2/9-GFP-treated and lactated Ringers-treated animals (with significantly lower mean salivary flow rates than the corresponding nonirradiated animals), but radiation had no effect on the AAV2/9-TLK1B-treated animals.
TLK1B better preserves submandibular gland structure after fractionated ionizing radiation
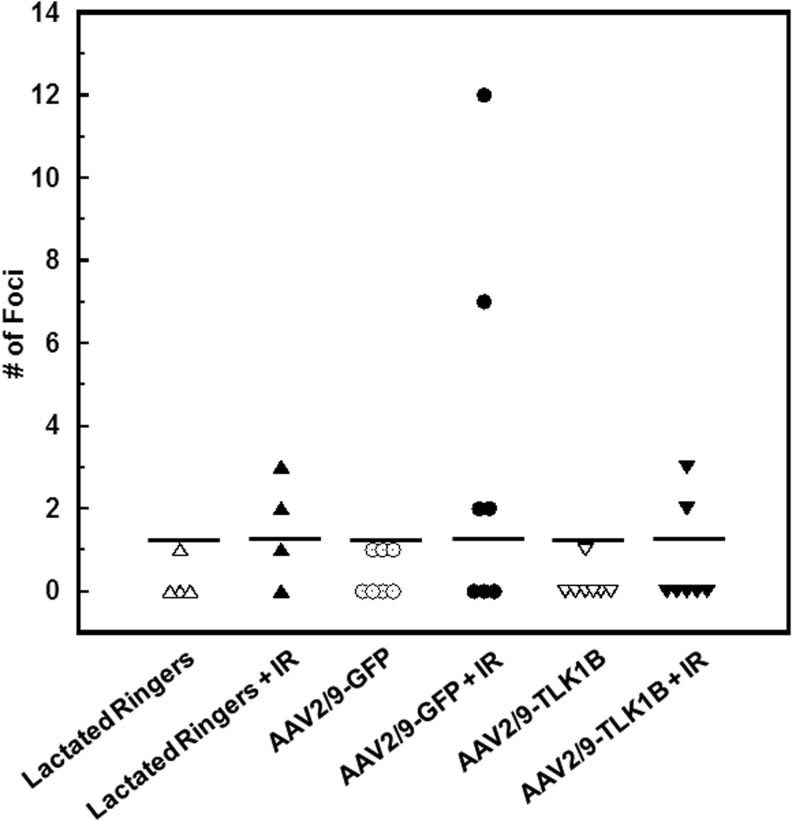
Histological examination of submandibular gland tissue revealed the presence of mucinous, signet-ring cells irrespective of treatment type or radiation. Although we do not understand the nature of these vacuoles, our results suggest that their presence does not affect salivary gland function. Submandibular glands extirpated 8 weeks after fractionated radiation (15 weeks after virus infusion; Fig. 1) demonstrated focal structural abnormalities such as inflammatory infiltrate and fibrosis, which were more pronounced in irradiated animals pretreated with lactated Ringers or GFP than in TLK1B-treated animals (Supplementary Fig. S3). Histopathological scoring of nested mononuclear cells demonstrated an increase in number of inflammatory cell foci after irradiation. Two or more foci of mononuclear cell infiltrates were observed after irradiation in 50% of lactated Ringers- (2/4 animals), 57% of AAV2/9-GFP- (4/7 animals), and 29% of AAV2/9-TLK1B-treated (2/7 animals) groups (Fig. 5).
FIG. 5.
Histopathological score of inflammatory foci in rat submandibular glands. Submandibular salivary glands of animals pretreated with lactated Ringers, AAV2/9-GFP, and AAV2/9-TLK1B were microscopically analyzed at the end of the experiment. A minimum of four sections per animal were analyzed for aggregates of >5 inflammatory cells. Nonirradiated animals demonstrated 0–1 inflammatory cell focus/animal. The total number of inflammatory foci per animal is presented.
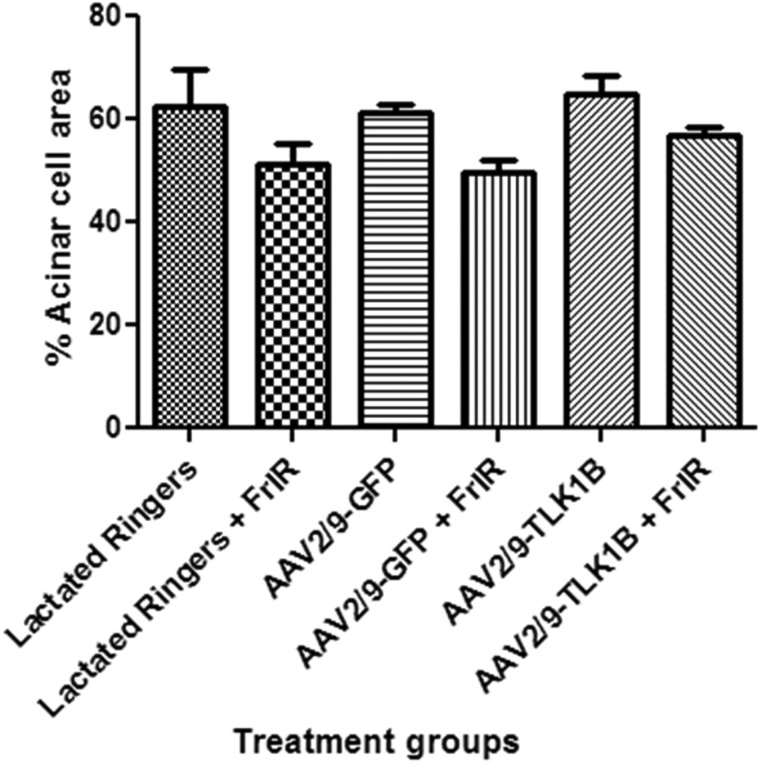
Acinar cells are the fluid-producing cells of the salivary glands. GRPs are exclusively expressed by acinar cells of rat salivary glands. To ascertain the effect of fractionated radiation on the viability of acinar cells, submandibular salivary glands were reacted with anti-GRP. The results indicate that the acinar component of the glands is similar in nonirradiated groups irrespective of treatment. Irradiation results in atrophy of acinar cells and a comparative decrease in acinar cell number in control gland treated with lactated Ringers or AAV2/9-GFP compared with TLK1B glands (Supplementary Fig. S4). The quantification of acinar surface area showed a modest decrease in surviving acinar cells after irradiation in all treatment groups. The average acinar cell area in nonirradiated groups was lactated Ringers, 62.5%±2.6%; AAV2/9-GFP, 61.3%±1.8%; and AAV2/9-TLK1B, 65.0%±3.4%. A decrease of 11.2% in irradiated lactated Ringers (mean 51.3%±4.0%), 11.5% in irradiated AAV2/9-GFP (mean 49.8%±2.2%), and 8.3% in irradiated AAV2/9-TLK1B (mean 56.7%±1.8%) was noted. Although the decrease in viable acinar cells was least pronounced in irradiated AAV2/9-TLK1B animals, it was not significant (Fig. 6).
FIG. 6.
Quantification of acinar cell surface area in rat submandibular gland tissues. Rat submandibular gland tissue sections reacted with glutamate–glutamic acid-rich proteins antibody were used for analysis. Three random microscopic fields per gland, at 200×magnification, were evaluated using ImageJ software (NIH), and the surface area occupied by acinar cells was measured. The data shown are mean±SD.
Discussion
The morbidity associated with radiotherapy-induced loss of salivary function severely compromises a patient's comfort, and treatment of the condition continues to be a challenge. Previously, we showed that exogenous TLK1B in rat submandibular salivary glands prevents salivary dysfunction caused by single-dose radiation. Using clinically relevant fractions of 2.5 Gy delivered on consecutive days with a 2-day break interspersed in between, severe salivary hypofunction could be recapitulated in the animal model at a cumulative dose of 20 Gy. We then demonstrate that lasting expression of TLK1B using AAV2/9-mediated submandibular gland gene transfer effectively preserves gland function against fractionated irradiation.
Although AAV has a broad host range, different serotypes have preferred transduction affinities for distinct tissues. Vector tropism is dictated by the interaction of the virus capsid proteins with target proteins on the cell surface. Unlike AAV2, which interacts with heparin sulfate proteoglycans, and AAV4 and 5 that interact with O- and N-linked sialic acids, respectively (Kaludov et al., 2001), AAV9 uses galactose as a receptor for transduction into cells (Bell et al., 2012). Our preliminary observations showed that AAV serotype 9 transduces rat submandibular salivary glands (Sunavala-Dossabhoy and Klein, 2009), and therefore we proceeded to evaluate it for salivary gland gene therapy. In mouse submandibular glands, AAV2 and 5 preferentially target striated and secretory duct cells, whereas AAV4 transduces cells of the convoluted granular tubules as well (Katano et al., 2006). Akin to AAV4 treatment in mice (Katano et al., 2006), AAV9 targeted ductal cells, including the convoluted granular tubules of rat submandibular glands. Likewise, expression of the transgene after AAV9 delivery to rat submandibular glands was detected within 5 weeks, and it persisted for a long period—through to 15 weeks (Voutetakis et al., 2004; Katano et al., 2006; Wang et al., 2006). Recombinant AAV vectors are gutless vectors that only possess the inverted terminal repeats of the viral genome. They depend on the cellular machinery for replication, and therefore the time lag between virus delivery and transgene expression could be associated with the delayed conversion of single-stranded rAAV DNA to a double-stranded functional molecule in slow-replicating cells of the salivary gland. The narrow interval between virus transduction and gene expression in rapidly proliferating salivary cells in culture lends credence to this supposition. Although wild-type AAV2 integrates into the human genome at a site-specific location on chromosome 19, the integration of rAAV is relatively infrequent and inefficient (McCarty et al., 2004). Therefore, long-term transgene expression in salivary glands suggests a preponderance of rAAV9 duplex DNA as episomal genomes or concatamers in submandibular salivary glands (Voutetakis et al., 2010). Stable expression without chromosomal integration averts the risk of deleterious mutagenesis in the host. rAAV has not been associated with any malignancies in humans and is, therefore, regarded as a relatively nonpathogenic gene delivery vehicle.
Vector biodistribution evaluated at the macroscopic and microscopic levels confirmed GFP expression in the target organ, submandibular salivary glands, but unexpectedly, in the intestines too. Systemic administration of AAV9 is known to effectively transduce the heart, liver, and skeletal muscles (Inagaki et al., 2006; Pacak et al., 2006). However, the lack of transgene expression in the heart and liver in our study suggests that vector spread to the intestines was likely a result of ingestion of residual virus that was expelled in oral cavity after the weardown of atropine effects rather than virus spread through systemic circulation. The prospect of intestinal expression after virus ingestion is supported by a previous study that demonstrated gene transfer to gut epithelium and lamina propria after oral administration of AAV (During et al., 1998). Prolonged expression of the transgene in the rapidly proliferating cells of the gut suggests the potential transduction of progenitor cells within the intestinal crypts (During et al., 1998).
Salivary glands are composed of three main epithelial cell types—the fluid-producing acinar cells, the myoepithelial cells, and the ductal cells that form conduits for saliva to flow to the oral cavity. The ductal tree plays an important role in modulating the composition of saliva, and in rodents, it is comprised of the intercalated ducts, the convoluted granular tubules, and the secretory and excretory ducts. Ductal cells have gained importance in salivary gland regeneration, since they were found to harbor putative progenitor stem cells (Takahashi et al., 1998; Man et al., 2001). Stem cells within the intercalated ducts can differentiate into amylase-producing acinar cells to aid recovery of gland function after radiation injury and after ductal ligation-induced gland atrophy (Lombaert et al., 2008; Cotroneo et al., 2010). Additionally, ductal cells of rodent salivary glands also serve as reservoirs of growth hormones, known to play a physiological role during development (Barka, 1980; Mori et al., 1983; Kato et al., 1991) Growth hormones stimulate cells to proliferate and may play a role in tissue regeneration. Therefore, apart from fluid-producing acinar elements, the protection of ductal elements can also favor the restoration of salivary function after injury in rodents.
Multiple plausible reasons have emerged to explain the loss of salivary gland function after radiation. The drop in salivary flow within a few days after radiation does not correlate with loss of acinar cell viability, and the loss in secretory function has been attributed to molecular damage-induced cellular dysfunction and to brief inanition caused by oropharayngeal mucositis (Nagler, 2002). Delayed death in cells with irreparable damage can occur over weeks or months, and progenitors cells of the gland divide to repopulate cells lost to radiation (Konings et al., 2005). Previous studies have shown an increase in proliferative activity in salivary glands within days after single-dose radiation (Ballagh et al., 1994; Peter et al., 1994). Increase in proliferative activity after radiation results in a more devastating effect of fractionated radiation on salivary gland function, since proliferating cells will be more susceptible to damage during subsequent doses of radiation. Therefore, our observations of a >90% decrease in salivary function after repeated small doses of 2.5 Gy is not surprising. However, with no widespread structural damage to the glands after radiation, histological manifestations alone appear to fall short in explaining the severity of gland malfunction in irradiated animals. A likely explanation for this observation is that the small doses of radiation were not sufficient to cause cell death, but they were severe enough to cause irreparable molecular changes and/or interfere with the proliferative activity of progenitors to incapacitate the function of the glands. Additionally, radiation damage to the gland vasculature could also play a role in compromising the secretory capacity of the glands (Cotrim et al., 2007).
High-dose radiation used to treat patients with head and neck cancer is associated with irreversible xerostomia, and clinical measures to protect salivary glands and preserve its function have shown benefit (Scrimger, 2011). Nonetheless, radiation-resultant compromised salivary gland function continues to be a challenge. Tousled-like kinase 1B and its larger variant TLK1 are cellular proteins that play a role in cell cycle arrest, chromatin remodeling, and DNA repair (Groth et al., 2003; Sunavala-Dossabhoy et al., 2005; Canfield et al., 2009; Sunavala-Dossabhoy and De Bendetti, 2009; De Benedetti, 2010). Their role in facilitating DNA repair is emphasized by observations that overexpression of the protein protects cells against death by radiation (Li et al., 2001; Sunavala-Dossabhoy et al., 2005), whereas suppression of its kinase activity or knockdown of the protein enhances the cell's susceptibility to DNA damage-induced cell death (Sunavala-Dossabhoy et al., 2003; Takayama et al., 2010). We have shown before that exogenous TLK1B in rat submandibular salivary glands effectively attenuates xerostomia associated with a single large-dose radiation (Palaniyandi et al., 2011; Sunavala-Dossabhoy et al., 2012). Now, we demonstrate that sustained expression of TLK1B associated with a single instillation of rAAV9 to rat submandibular glands is sufficient to completely ameliorate salivary dysfunction caused by fractioned radiation. Our results on TLK1B efficacy in ameliorating fractionated radiation-inflicted xerostomia generate enthusiasm for analyzing, in depth, the safety of the therapeutic with a prospect of furthering AAV2/9-TLK1B to cancer patient care.
Supplementary Material
Acknowledgments
We wish to thank Dr. Chang and the staff at the Department of Radiation Oncology, Health Sciences Center–Shreveport, Louisiana State University, for their help with the use of the linear accelerator. We also thank Natalia Aladyshkina for her help with IVIS imaging and calculations. This work was supported by grant funding from the American Cancer Society (Award ID: 116945-RSG-09-038-01-CCE).
Author Disclosure Statement
No competing financial interests exist.
References
- Ballagh R.H. Kudryk K.G. Lampe H.B., et al. The pathobiology of salivary gland. III. PCNA-localization of cycling cells induced in rat salivary gland by low-dose x-radiation. Oral Surg. Oral Med. Oral Pathol. 1994;77:27–35. doi: 10.1016/s0030-4220(06)80103-9. [DOI] [PubMed] [Google Scholar]
- Barka T. Biologically active polypeptided in submandibular glands. J. Histoch. Cytochem. 1980;28:836–859. doi: 10.1177/28.8.7003006. [DOI] [PubMed] [Google Scholar]
- Bell C.L. Gurda B.L. Van Vliet K., et al. Identification of the galactose binding domain of the AAV9 capsid. J. Virol. 2012;86:7326–7333. doi: 10.1128/JVI.00448-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk L. Systemic pilocarpine for treatment of xerostomia. Expert Opin. Drug Metab. Toxicol. 2008;4:1333–1340. doi: 10.1517/17425255.4.10.1333. [DOI] [PubMed] [Google Scholar]
- Braam P.M. Roesink J.M. Moerland M.A, et al. Long-term parotid gland function after radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2005;62:659–664. doi: 10.1016/j.ijrobp.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Bruce S.D. Radiation-induced xerostomia: how dry is your patient? Clin. J. Oncol. Nurs. 2004;8:61–67. doi: 10.1188/04.CJON.61-67. [DOI] [PubMed] [Google Scholar]
- Canfield C. Rains J. De Benedetti A. TLK1B promotes repair of DSBs via its interaction with Rad9 and Asf1. BMC Mol. Biol. 2009;10:110. doi: 10.1186/1471-2199-10-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrera P. Moshkin Y.M. Gronke S., et al. Tousled-like kinase functions with the chromatin assembly pathway regulating nuclear divisions. Genes Dev. 2003;17:2578–2590. doi: 10.1101/gad.276703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers M.S. Rosenthal D.I. Weber R.S. Radiation-induced xerostomia. Head Neck. 2007a;29:58–63. doi: 10.1002/hed.20456. [DOI] [PubMed] [Google Scholar]
- Chambers M.S. Jones C.U. Biel M.A., et al. Open-label, long-term safety study of cevimeline in the treatment of postirradiation xerostomia. Int. J. Radiat. Oncol. Biol. Phys. 2007b;69:1369–1376. doi: 10.1016/j.ijrobp.2007.05.024. [DOI] [PubMed] [Google Scholar]
- Cotrim A.P. Sowers A. Mitchell J.B., et al. Prevention of irradiation-induced salivary hypofunction by microvessel protection in mouse salivary glands. Mol. Ther. 2007;15:2101–2106. doi: 10.1038/sj.mt.6300296. [DOI] [PubMed] [Google Scholar]
- Cotroneo E. Proctor G.B. Carpenter G.H. Regeneration of acinar cells following ligation of rat submandibular gland retraces the embryonic-perinatal pathway of cytodifferentiation. Differentiation. 2010;79:120–130. doi: 10.1016/j.diff.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Benedetti A. Tousled kinase TLK1B mediates chromatin assembly in conjunction with Asf1 regardless of its kinase activity. BMC Res. Notes. 2010;3:68. doi: 10.1186/1756-0500-3-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkema T. Raaijmakers C.P. Ten Haken R.K., et al. Parotid gland function after radiotherapy: the combined Michigan and Utrecht experience. Int. J. Radiat. Oncol. Biol. Phys. 2010;78:449–453. doi: 10.1016/j.ijrobp.2009.07.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- During M.J. Xu R. Young D., et al. Peroral gene therapy of lactose intolerance using an adeno-associated virus vector. Nat. Med. 1998;4:1131–1135. doi: 10.1038/2625. [DOI] [PubMed] [Google Scholar]
- Epstein J.B. Robertson M. Emerton S., et al. Quality of life and oral function in patients treated with radiation therapy for head and neck cancer. Head Neck. 2001;23:389–398. doi: 10.1002/hed.1049. [DOI] [PubMed] [Google Scholar]
- Gaudet D. Méthot J. Kastelein J. Gene therapy for lipoprotein lipase deficiency. Curr. Opin. Lipidol. 2012;23:310–320. doi: 10.1097/MOL.0b013e3283555a7e. [DOI] [PubMed] [Google Scholar]
- Gray S.J. Gene therapy and neurodevelopmental disorders. Neuropharmacology. 2013;68:136–142. doi: 10.1016/j.neuropharm.2012.06.024. [DOI] [PubMed] [Google Scholar]
- Groth A. Lukas J. Nigg E.A., et al. Human Tousled like kinases are targeted by an ATM- and Chk1-dependent DNA damage checkpoint. EMBO J. 2003;22:1676–1687. doi: 10.1093/emboj/cdg151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M. Matsui T. Iwabuchi K., et al. PKU-B/TLK1 regulates myosin II activities, and is required for accurate equaled chromosome segregation. Mutat. Res. 2008;657:63–67. doi: 10.1016/j.mrgentox.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Inagaki K. Fuess S. Storm T.A., et al. Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8. Mol. Ther. 2006;14:45–53. doi: 10.1016/j.ymthe.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaludov N. Brown K.E. Walters R.W., et al. Adeno-associated virus serotype 4 (AAV4) and AAV5 both require sialic acid binding for hemagglutination and efficient transduction but differ in sialic acid linkage specificity. J. Virol. 2001;75:6884–6893. doi: 10.1128/JVI.75.15.6884-6893.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katano H. Kok M.R. Cotrim A.P., et al. Enhanced transduction of mouse salivary glands with AAV5-based vectors. Gene Ther. 2006;13:594–601. doi: 10.1038/sj.gt.3302691. [DOI] [PubMed] [Google Scholar]
- Kato K. Yokose S. Tajima Y. Use of silver enhancement technique for immunohistochemical detection of EGF in rat submandibular gland. Biotech. Histochem. 1991;1:87–88. doi: 10.3109/10520299109110556. [DOI] [PubMed] [Google Scholar]
- Klein R.L. Dayton R.D. Tatom J.B., et al. AAV8, 9, Rh10, Rh43 vector gene transfer in the rat brain: effects of serotype, promoter and purification method. Mol. Ther. 2008;16:89–96. doi: 10.1038/sj.mt.6300331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konings A.W.T. Coppes R.P. Vissink A. On the mechanism of salivary gland radiosensitivity. Int. J. Rad. Oncol. Biol. Phys. 2005;62:1187–1194. doi: 10.1016/j.ijrobp.2004.12.051. [DOI] [PubMed] [Google Scholar]
- Li Y. DeFatta R. Anthony C., et al. A translationally regulated Tousled kinase phosphorylates histone H3 and confers radioresistance when overexpressed. Oncogene. 2001;20:726–738. doi: 10.1038/sj.onc.1204147. [DOI] [PubMed] [Google Scholar]
- Lombaert I.M.A. Brunsting J.F. Wierenga P.K., et al. Rescue of salivary gland function after stem cells transplantation in irradiated glands. PLoS One. 2008;3:e2063. doi: 10.1371/journal.pone.0002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man Y.G. Ball W.D. Marchetti L., et al. Contributions of intercalalted duct cells to the normal parenchyma of submandibular glands of adult rats. Anat. Rec. 2001;263:202–214. doi: 10.1002/ar.1098. [DOI] [PubMed] [Google Scholar]
- McCarty D.M. Young S.M., Jr. Samulski R.J. Integration of adeno-associated virus (AAV) and recombinant AAV vectors. Annu. Rev. Genet. 2004;38:819–845. doi: 10.1146/annurev.genet.37.110801.143717. [DOI] [PubMed] [Google Scholar]
- Mori M. Hamada K. Naito R., et al. Immunohistochemical localization of epidermal growth factor in rodent submandibular glands. Acta Histochem. Cytochem. 1983;16:536–547. [Google Scholar]
- Nagler R.M. The enigmatic mechanism of irradiation-induced damage to the major salivary glands. Oral Dis. 2002;8:141–146. doi: 10.1034/j.1601-0825.2002.02838.x. [DOI] [PubMed] [Google Scholar]
- Pacak C.A. Mah C.S. Thattaliyath B.D., et al. Recombinant adeno-associated virus serotype 9 leads to preferential cardiac transduction in vivo. Circ. Res. 2006;99:e3–e9. doi: 10.1161/01.RES.0000237661.18885.f6. [DOI] [PubMed] [Google Scholar]
- Palaniyandi S. Odaka Y. Green W., et al. Adenoviral delivery of Tousled kinase for the protection of salivary glands against ionizing radiation damage. Gene Ther. 2011;18:275–282. doi: 10.1038/gt.2010.142. [DOI] [PubMed] [Google Scholar]
- Peter B. Van Waarde M.A. Vissink A., et al. Radiation-induced cell proliferation in the parotid and submandibular glands of the rat. Radiat. Res. 1994;140:257–265. [PubMed] [Google Scholar]
- Roesink J.M. Moerland M.A. Battermann J.J., et al. Quantitative dose-volume response analysis of changes in parotid gland function after radiotherapy in the head-and-neck region. Int. J. Radiat. Oncol. Biol. Phys. 2001;51:938–946. doi: 10.1016/s0360-3016(01)01717-5. [DOI] [PubMed] [Google Scholar]
- Rudat V. Münter M. Rades D., et al. The effect of amifostine or IMRT to preserve the parotid function after radiotherapy of the head and neck region measured by quantitative salivary gland scintigraphy. Radiother. Oncol. 2008;89:71–80. doi: 10.1016/j.radonc.2008.07.016. [DOI] [PubMed] [Google Scholar]
- Sciubba J.J. Goldenberg D. Oral complications of radiotherapy. Lancet Oncol. 2006;7:175–183. doi: 10.1016/S1470-2045(06)70580-0. [DOI] [PubMed] [Google Scholar]
- Scrimger R. Salivary gland sparing in the treatment of head and neck cancer. Expert Rev. Anticancer Ther. 2011;11:1437–1448. doi: 10.1586/era.11.101. [DOI] [PubMed] [Google Scholar]
- Sillje H.H. Nigg E.A. Identification of human Asf1 chromatin assembly factors as substrates of Tousled-like kinases. Curr. Biol. 2001;11:1068–1073. doi: 10.1016/s0960-9822(01)00298-6. [DOI] [PubMed] [Google Scholar]
- Sillje H.H. Takahashi K. Tanaka K., et al. Mammalian homologues of the plant Tousled gene code for cell-cycle-regulated kinases with maximal activities linked to ongoing DNA replication. EMBO J. 1999;18:5691–5702. doi: 10.1093/emboj/18.20.5691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunavala-Dossabhoy G. De Benedetti A. Tousled homolog, TLK1, binds and phosphorylates Rad9; TLK1 acts as a molecular chaperone in DNA repair. DNA Repair (Amst.) 2009;8:87–102. doi: 10.1016/j.dnarep.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Sunavala-Dossabhoy G. Klein R. Gene Therapy Symposium; New Orleans, LA: 2009. Adeno-associated virus-mediated gene transfer in rat salivary glands. [Google Scholar]
- Sunavala-Dossabhoy G. Li Y. Williams B., et al. A dominant negative mutant of TLK1 causes chromosome missegregation and aneuploidy in normal breast epithelial cells. BMC Cell. Biol. 2003;4:16. doi: 10.1186/1471-2121-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunavala-Dossabhoy G. Balakrishnan S.K. Sen S., et al. The radioresistance kinase TLK1B protects the cells by promoting repair of double strand breaks. BMC Mol. Biol. 2005;6:19. doi: 10.1186/1471-2199-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunavala-Dossabhoy G. Palaniyandi S. Richardson C., et al. TAT-mediated delivery of Tousled protein to salivary glands protects against radiation-induced hypofunction. Int. J. Radiat. Oncol. Biol. Phys. 2012;84:257–265. doi: 10.1016/j.ijrobp.2011.10.064. [DOI] [PubMed] [Google Scholar]
- Takahashi S. Schoch E. Walker N.I. Origin of acinar cell regeneration after atrophy of rat parotid induced by duct obstruction. Int. J. Exp. Pathol. 1998;79:293–301. doi: 10.1046/j.1365-2613.1998.710405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama Y. Kokuryo T. Yokoyama Y., et al. Silencing of Tousled-like kinase 1 sensitizes cholangiocarcinoma cells to cisplatin-induced apoptosis. Cancer Lett. 2010;296:27–34. doi: 10.1016/j.canlet.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Voutetakis A. Kok M.R. Zheng C., et al. Reengineered salivary glands are stable endogenous bioreactors for systemic gene therapeutics. Proc. Natl. Acad. Sci. U. S. A. 2004;101:3053–3058. doi: 10.1073/pnas.0400136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voutetakis A. Zheng C. Cotrim A.P., et al. AAV5-mediated gene transfer to the parotid glands of non-human primates. Gene Ther. 2010;17:50–60. doi: 10.1038/gt.2009.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. Voutetakis A. Papa M., et al. Rapamycin control of transgene expression from a single AAV vector in mouse salivary glands. Gene Ther. 2006;13:187–190. doi: 10.1038/sj.gt.3302647. [DOI] [PubMed] [Google Scholar]
- Weinberg M.S. Samulski R.J. McCown T.J. Adeno-associated virus (AAV) gene therapy for neurological disease. Neuropharmacology. 2013;69:82–88. doi: 10.1016/j.neuropharm.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.