Background: The functional regulation of p63 Isoform γ (TAp63γ) by IκB Kinase β (IKKβ) remains unknown, although they were previously shown to bind to each other.
Results: IKKβ, but not its kinase dead mutant, suppressed the transcriptional activity of TAp63γ by disrupting its interaction with p300.
Conclusion: IKKβ negatively regulates the transcriptional activity of TAp63γ.
Significance: IKKβ may favor cell proliferation by inhibiting TAp63γ activity.
Keywords: p300, p63, Senescence, Signal transduction, Transcription, Tumor Necrosis Factor (TNF), IκB kinase-β
Abstract
Previously, we reported that IκB kinase-β(IKKβ) phosphorylates and stabilizes TAp63γ. However, the effect of this phosphorylation on TAp63γ transcriptional activity remains unclear. In this study, we showed that overexpression of IKKβ, but not its kinase dead mutant and IKKα, can surprisingly inhibit TAp63γ transcriptional activity as measured by luciferase assays and real-time PCR analyses of p63 target genes. This inhibition was impaired by ACHP, an IKKβ inhibitor, and enhanced by TNFα that activates IKKβ. Consistently, IKKβ inhibited the binding between TAp63γ and p300, a co-activator of TAp63γ, and consequently counteracted the positive effect of p300 on TAp63γ transcriptional activity. Through phosphorylation site prediction and mass spectrometry, we identified that Ser-4 and Ser-12 of p63 are IKKβ-targeting residues. As expected, IKKβ fails to suppress the transcriptional activity of the S4A/S12A double mutant p63. These results indicate that IKKβ can suppress TAp63γ activity by interfering with the interaction between TAp63γ and p300.
Introduction
The p63 transcriptional factor is a homolog of the p53 tumor suppressor and shares numerous target genes with the latter (1). It is considered the oldest ancestor among the p53 family members, including p63, p53, and p73 (2). While the transactivational (TA)4 domain and DNA-binding domain (DBD) of p63 display a high similarity with those of p53 in amino acid sequence, p63 possesses an extended C-terminal domain compared with p53. Because of alternative C-terminal splicing, p63 has at least three isoforms: TAp63α, TAp63β, and TAp63γ (1, 3). The size of the C terminus affects the function of p63. For example, TAp63γ with the shortest C terminus is the most transcriptionally active isoform among the three isoforms, whereas TAp63α is much less active due to the suppression by its own long C terminus (4). Also, an additional transcriptional start site was found in the p63 gene, and the product of this transcription is the TA-truncated isoform ΔNp63, which often functions as a dominant-negative regulator of TAp63 isoforms (1, 5–7).
While p63 is rarely mutated in cancers (8), mutations of p63 have been found to be associated with ectodermal dysplastic syndromes in humans (9–14). Consistent with this, animal studies have demonstrated that p63 plays a role in the development of squamous epithelia, as p63-deficient mice manifest severe limb truncations, absence of skin, hair, teeth, mammary, lachrymal, and salivary glands (15, 16). Interestingly, similar phenotypes were also shown in IKKα knock-out mice (10, 11). Recently, the IKKα gene was found to be a transcriptional target for p63 (17–20), suggesting a possibly functional link between p63 and IKKα in regulating ectodermal development, although IKKα has been shown to also form a kinase complex with IKKβ and IKKγ, which phosphorylates IκB and inactivates it, consequently activating NFκB in response to the pro-inflammatory cytokine TNFα (17–19). Previously, we also showed that IKKβ can phosphorylate and stabilize TAp63γ (20). However, the functional consequence of this phosphorylation remains unaddressed.
In an attempt to address this issue, we, surprisingly, found that IKKβ, but not its kinase dead mutant form, can suppress the transcriptional activity of TAp63γ in response to TNFα as measured by luciferase reporter assays and detection of endogenous p63 targets including p21 and miR-34. Our previous study showed that p300 could act as a coactivator of TAp63γ (6). Here, we further showed that IKKβ could interfere with the interaction between p63 and p300, consequently impairing the p300 activation of TAp63γ. Hence, these results suggest that IKKβ can inhibit the transcriptional activity of TA63γ possibly by disrupting the association of p300 with TAp63γ.
EXPERIMENTAL PROCEDURES
Cell Lines
Unless indicated specifically, all cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 50 units/ml penicillin, and 0.1 mg/ml streptomycin at 37 °C in 5% CO2.
Plasmids and Antibodies
Flag-IKKα, Flag-IKKβ, Flag-p300, CMV-empty, and CMV-β-gal plasmids were described previously (6, 20). The pGL4-miR-34a and mutant vectors were kindly given and described by Lin He et al. (21). Antibodies: Anti-Flag (Sigma), anti-p21 (M-19, Santa Cruz Biotechnology), anti-Myc (9E10, Santa Cruz Biotechnology) were purchased commercially.
Transient Transfection and Immunoblot
As described previously (22, 23), cells were transfected with indicated plasmids as shown in each figure by using TransFectin (Bio-Rad), following the manufacturer's instructions. 48 h post-transfection, cells were harvested and lysed in lysis buffer. The total protein concentration for each sample was determined, and an equal amount of total protein was then subjected to SDS-PAGE, followed by immunoblotting (IB). Immunoprecipitation (IP) was conducted by using antibodies as indicated in the figure legends and described previously (24). Beads were washed three times with lysis buffer. Bound proteins were detected by IB with antibodies as indicated in the figure legends.
Reverse Transcriptase-Polymerase Chain Reaction and Quantitative (Q) Real-time PCR Analysis
RT- and Q-PCR for mature miRNA were done using the methods described previously (25). RT- and Q-PCR for other genes were carried out as described previously (26). Briefly, Q-PCR was performed on an ABI 7300 real-time PCR system (Applied Biosystems) using SYBR Green Mix (Applied Biosystems). Relative gene expression was calculated following the manufacturer's instruction. All reactions were carried out in triplicate.
Luciferase Reporter Assays
Cells were transfected with pCMV-β-galactoside and indicated plasmids (total plasmid DNA 1 μg/well) as indicated in figures. Luciferase activity was determined and normalized by a factor of β-gal activity in the same assay, as described previously (27).
Chromatin Immunoprecipitation (ChIP)-PCR
ChIP analysis was performed as described previously (28) using anti-p63 and anti-p300 for endogenous proteins. Immunoprecipitated DNA fragments were analyzed by real-time PCR amplification using primers for p21 and control genes.
Senescence-associated β-Galactosidase (SA-β-Gal) Staining
To investigate senescence, the SA-β-gal activity in cultured H1299 cells was detected using the Senescence β-Galactosidase Staining kit (Cell Signaling) according to the manufacturer's recommendations. Briefly, H1299 cells were transfected with indicated plasmids. Four days after transfection, Senescence β-Galactosidase Staining was performed.
Site-directed Mutagenesis
Mutagenesis was carried out as described before (29). Briefly, the GFP-TAp63γ plasmid was used as a mutagenesis template. PCR amplification was carried out with Phusion high fidelity DNA polymerase as described in the manufacturer's manual (New England Biolabs) and verified by DNA sequencing.
Phosphorylation Analysis by Mass Spectrometry
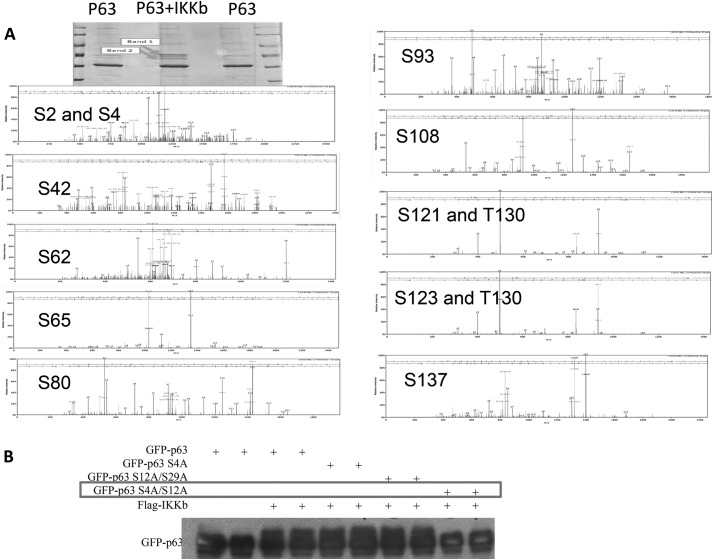
H1299 cells were transfected with TAp63γ or cotransfected with TAp63γ and Myc-IKKβ vector. Forty eight hrs after transfection, cells were harvested and subjected to SDS-PAGE. Putative bands of phosphorylation TAp63γ in Coomassie Blue-stained gel were collected and sent to the Proteomics Shared Resource at Oregon Health & Science University for Mass spectrometry analysis.
RESULTS
TAp63, but Not ΔNp63, Induces miR-34a Expression in Cells
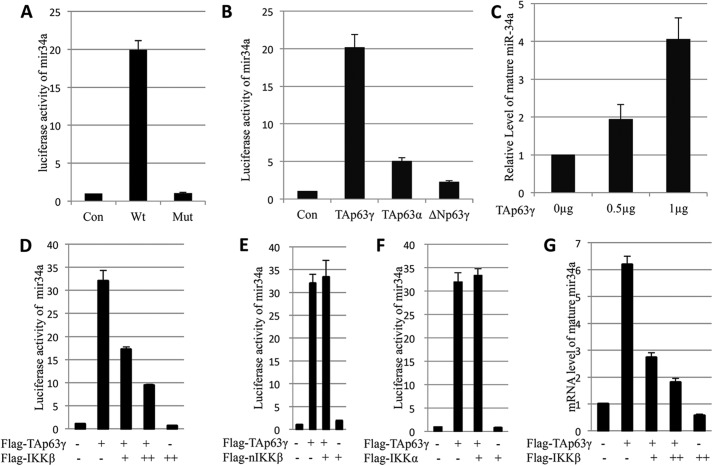
To study the effect of IKKβ on TAp63 activity, we initially wanted to employ the miR-34 promoter-driven luciferase reporter system. Although miR-34a was recently confirmed as a p63 transcriptional target (30), the effects of different p63 isoforms on miR-34a expression have not been characterized. To address this, we first checked the effects of different TAp63 isoforms on the miR-34a promoter-driving luciferase activity by co-transfecting p63 expression vectors with the miR-34a promoter-driven luciferase reporter vector in MEF p53−/− cells. As shown in Fig. 1A, overexpression of Myc-TAp63γ resulted in a 20-fold increase of the luciferase activity, but showed no significant effect on the empty and mutant promoter. Next, we tested which isoform of the p63 family members is more effective in induction of the luciferase activity by performing similar luciferase assays. As expected, TAp63γ was much more effective than was TAp63α or ΔNp63γ in activation of the luciferase activity (Fig. 1B). This result is in line with previous reports showing that TAp63α activity is suppressed by its C terminus (4) and that ΔNp63 cannot induce the expression of most of the p53 targets due to lack of the TA domain (1, 5–7). Consistently, TAp63γ induced the expression of endogenous miR-34a in a dose-dependent fashion (Fig. 1C). These results indicate that the TAp63γ is the most functional isoform for miR-34a transcription. Thus, we will use this isoform for the following experiments.
FIGURE 1.
IKKβ inhibits miR-34a expression induced by TAp63. A, Myc-TAp63γ was co-transfected with pGL4-mir34a promoter luciferase report vector, which contains wild type or mutated p53 binding site, into MEFp53−/− cells. 48 h after transfection, cells were harvested for luciferase assay. Luciferase activity was normalized by β-gal expression. B, human colon cancer cell line HCT116 p53−/− cells were transfected with different isoforms of p63. Luciferase activity was determined as above. C, TAp63γ induces mature hsa-mir34a expression in a dosage-dependent manner. H1299 cells were transfected with different dosage of Flag-TAp63γ. Cells were harvested 48 h post-transfection. Real-time PCR was used to detect mature hsa-mir34a expression level. GAPDH was used as an internal control. D, IKKβ represses TAp63γ-induced luciferase activity driven by the mir34a promoter. H1299 cells were transfected with indicated plasmids. 24 h after transfection, cells were harvested. β-Gal was used as internal control. E, IKKβ kinase dead mutant (nIKKβ) cannot repress TAp63γ transcriptional activity. Luciferase assay was performed as in panel D. F, IKKα cannot repress TAp63γ transcriptional activity. Luciferase assay was performed as in panel D. G, IKKβ represses induction of mir34a by TAp63γ in a dosage-dependent manner. H1299 cells were transfected with Flag-TAp63γ and titrated Flag-IKKβ. Cells were harvested for real-time PCR. Data are presented as means ± S.E., n = 3.
IKKβ, but Not IKKα and IKKβ Mutant, Inhibits TAp63γ-induced Expression of miR-34a
Previously, we showed that IKKβ phosphorylates and stabilizes TAp63γ (20). However, the functional outcome of this phosphorylation remains unknown. To address this remaining issue, we carried out the above mentioned miR-34a promoter luciferase assay in the presence of IKKβ. Unexpectedly, IKKβ inhibited the TAp63γ-induced luciferase activity in a dose-dependent manner (Fig. 1D). This inhibition was dependent on the IKKβ kinase activity, because the IKKβ kinase dead mutant K44A failed to inhibit this luciferase activity (Fig. 1E). Next, the specificity of this IKKβ-induced inhibition was checked with IKKα, another important component of the IKK complex. As shown in Fig. 1F, no significant inhibition was observed when Flag-IKKα was co-transfected with TAp63γ, indicating that the roles of IKKβ and IKKα in p63 transcriptional activity are different. To further verify the inhibitory effect of IKKβ on p63 transcriptional activity, the endogenous miR-34a level was assessed by Q-RT-PCR. The results showed that IKKβ also inhibits the TAp63γ-induced endogenous miR-34a transcription (Fig. 1G). These findings indicate that IKKβ inhibits both exogenous and endogenous miR-34a transcription induced by TAp63γ.
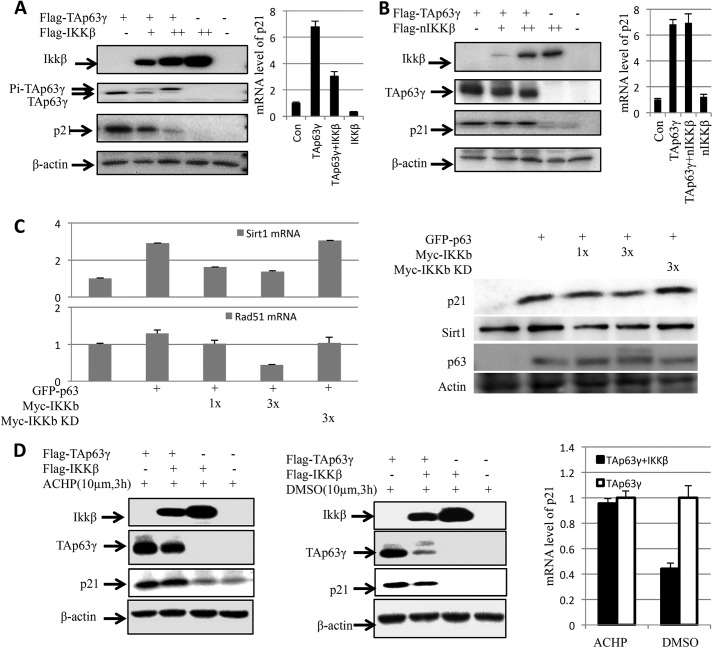
IKKβ Inhibits TAp63γ-induced p21 Expression
To determine whether the inhibition of IKKβ on TAp63γ transcriptional activity is only specific to miR-34a, we also tested if IKKβ affects the expression of p21, a well-known p53 target gene encoding a protein that inhibits cyclin-dependent kinases (31). When Flag-IKKβ was co-expressed with Flag-TAp63γ, it led to TAp63γ phosphorylation in a dose-dependent fashion, as expected (20). Overexpression of TAp63γ drastically induced both of the p21 protein and mRNA levels (Fig. 2A). However, this induction was markedly inhibited by overexpression of IKKβ in a dose-dependent fashion (Fig. 2A), which was consistent with the results in Fig. 1. This inhibition appeared to be phosphorylation dependent, as the kinase dead mutant form of IKKβ, which failed to phosphorylate TAp63γ (20), was unable to inhibit its transcriptional activity toward p21 (Fig. 2B). Similar to the IKKβ mutant, IKKα also showed no effect on the p21 expression that was induced by TAp63γ (data not shown). Next, we tested if IKKβ could also inhibit induction of p63 specific target genes, such as Rad51 (32) and sirt1 (33). As expected, IKKβ, but not IKKβ kinase dead mutant, inhibited p63-induced Rad51 and sirt1 expression in a dose-dependent fashion (Fig. 2C). Together with the results in Fig. 1, these results indicate that IKKβ, but not IKKβ kinase dead mutant and IKKα, can inhibit the activation of p21 and miR-34a expression by TAp63γ as well as the expression of at least two p63-specific target genes.
FIGURE 2.
IKKβ represses transcriptional activity of TAp63γ by phosphorylation. A, IKKβ represses p21 induction by TAp63γ. H1299 cells were transfected with indicated plasmids. Western blot was used to detect p21 protein level. Real-time PCR was used to detect mRNA level of p21. B, kinase dead mutated IKKβ (nIKKβ) cannot inhibit p21 expression by TAp63γ. Experiments were carried out as described in panel A. C, IKKβ inhibits Rad51 and Sirt1 expression induced by TAp63. H1299 cells were transfected with the GFP-TAp63γ, Myc-IKKβ, or Myc-IKKβ KD vector as indicated. 48 h after transfection, qPCR (left) and WB (right) were carried out to detect mRNA and protein levels of p21, Rad51, and Sirt1. Data are presented as means ± S.E., n = 3. D, IKKβ inhibitor abrogates the effect of IKKβ on transcriptional activity of TAp63γ. H1299 cells were transfected with plasmids as indicated for 36 h. Before harvesting, cells were treated with ACHP (IKKβ inhibitor) or DMSO (control) for 3 h. WB and qPCR were carried out to detect p21 protein and mRNA. Data are presented as means ± S.E., n = 3.
IKKβ Inhibitor Affects TAp63γ Transcriptional Activity
To further confirm the negative effect of IKKβ on TAp63γ activity, we next determined if IKKβ inhibitor could affect the transcriptional activity of TAp63γ by using an IKKβ inhibitor, called ACHP (34). As shown in Fig. 2D, in the presence of ACHP, compared with DMSO, the supershifted band of phosphorylated TAp63γ disappeared, and the p21 level was not affected by the overexpression of IKKβ. Also, the Q-RT-PCR results showed that ACHP at least partially rescues the expressions of p21 mRNA (Fig. 2D), which indicates that ACHP abrogates the inhibitory effect of IKKβ on TAp63γ transcriptional activity.
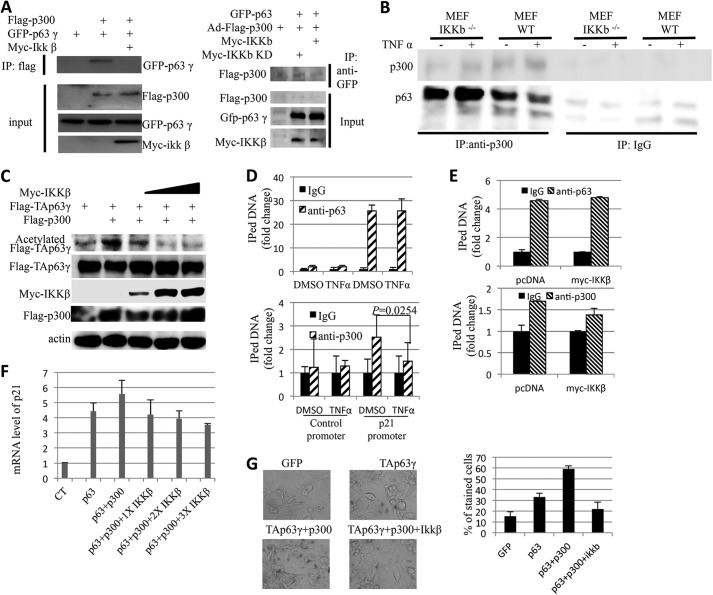
IKKβ Inhibits the Binding between p300 and TAp63γ and Suppresses Acetylation of TAp63γ by p300
Our previous work showed that p300 can bind to the N terminus of p63 and positively regulates p63 transcriptional activity by acetylating p63 (6). Interestingly, IKKβ phosphorylates the same domain of p63 (20). Therefore we speculate that the phosphorylation of p63 by IKKβ might affect the binding between p300 and p63 and thus regulate p63 acetylation by p300. To this end, we carried out a co-immunoprecipitation assay for the p300-TAp63γ complex in the presence or absence of IKKβ. In line with previous works, GFP-TAp63γ was only pulled down by the anti-Flag antibody in the cells overexpressed Flag-p300, but not in the cells without Flag-p300. By contrast, anti-Flag-p300 antibodies pulled down much less GFP-TAp63γ in the presence of IKKβ, indicating that IKKβ may inhibit the binding between p300 and TAp63γ (Fig. 3A, left panel). Consistently, reciprocal co-IP with anti-GFP-p63 antibodies showed a similar result (Fig. 3A, right panel). To further confirm the effect of IKKβ on endogenous p300 and p63 binding, we checked the effect of TNFα, an IKKβ activator, on this binding in MEFIKKβ−/− cells. Interestingly, endogenous p300 was co-immunoprecipitated with more p63 in MEFIKKβ−/− cells than that in wild type MEF cells regardless of TNFα treatment (Fig. 3B), supporting the idea that IKKβ plays a negative roles in p300 and p63 binding. Also, TNFα only impaired p300 and p63 binding in MEF cells, but not in MEFIKKβ−/− cells, indicating IKKβ is indispensible for suppression of p300 and p63 binding by TNFα (Fig. 3B). These results demonstrate that IKKβ inhibits the binding of p300 to TAp63γ. Next, we checked the effect of IKKβ on TAp63γ acetylation, for it was previously shown that p300 acetylates p63 (6). As expected, overexpressed p300 promoted acetylation of TAp63γ. Consistent with the result of Fig. 3A, IKKβ suppressed this TAp63γ acetylation in a dose-dependent fashion (Fig. 3C).
FIGURE 3.
IKKβ inhibits the interaction between p300 and p63γ, and impairs transcriptional activity of p63γ. A, H1299 cells were transfected with indicated plasmids, 48 h after transfection, cells were harvested. Flag-p300 (left panel) or GFP-TAp63γ (right panel) was pulled down by anti-Flag antibodies or 4A4 antibodies, and then the co-IPed proteins were probed with indicated antibodies. The input is shown in the lower panel. B, TNFα impairs the binding of p300 to p63 in MEF cells, but not in MEFIKKβ−/− cells. MEF and MEFIKKβ−/− cells were treated with or without TNFα and harvested for co-IP with anti-p300 antibodies or IgG. p300 and 4A4 antibodies were used for IB as indicated. C, IKKβ suppresses acetylation of p63 by p300. H1299 cells were transfected with indicated plasmids. Cell lysates were analyzed by WB with indicated antibodies. D, p300 is excluded from the p21 promoter by IKKβ activator, TNFα. Hacat cells were treated with DMSO or TNFα. ChIP was performed by indicated antibodies. The DNA pulled down by indicated antibodies was determined by qPCR. E, p300 is excluded from the p21 promoter by IKKβ. Hacat cells were transfected with the pCDNA or Myc-IKKβ plasmid. ChIP was performed with indicated antibodies. The DNA pulled down by indicated antibodies was determined by qPCR. F, H1299 cells were transfected with indicated plasmids, 48 h later, the cells were harvested, and the p21 mRNA level was detected by q-RT-PCR. The data were normalized with GAPDH mRNA. G, IKKβ inhibits senescence induced by p63 and p300. H1299 cells were transfected with indicated plasmids. Four days after transfection, Senescence β-Galactosidase Staining was performed. Quantification was shown in the lower panel. Data are presented as means ± S.E. *, p ≤ 0.05.
Because p300 enhances p63 activity on p21 transcription, it is logical to assume that IKKβ would impair this enhancement. To test this idea, we first carried out ChIP analyses to check the binding of p300 to the p21 promoter. As shown in Fig. 3D, both p63 and p300 bound to the p21 promoter, but not an un-related control sequence. Intriguingly, activation of IKKβ by TNFα abrogated the binding of p300 to the p21 promoter, but had no effect on the binding of p63 to the p21 promoter. In addition, overexpression of IKKβ also excluded p300, but not p63, from the p21 promoter (Fig. 3E). These results suggest that IKKβ could inhibit p63-activated p21 transcription by interrupting the p63-p300 binding at the promoter. This regulation was further confirmed in Fig. 3F, as this result showed that IKKβ inhibits the effect of p300 on TAp63γ transcriptional activity in a dose-dependent fashion. Since p21 can induce cellular senescence (35, 36), we then checked the effect of IKKβ on p63/p300 induced senescence. As shown in Fig. 3G, p300-enhanced senescence that was induced by TAp63γ, and this promotion was not observed when IKKβ was co-expressed with these two transcriptional regulators in cells. Altogether, these results show that IKKβ-induced phosphorylation of TAp63γ leads to the disruption of the p300-TAp63γ binding, and consequently inhibits TAp63γ transcriptional activity.
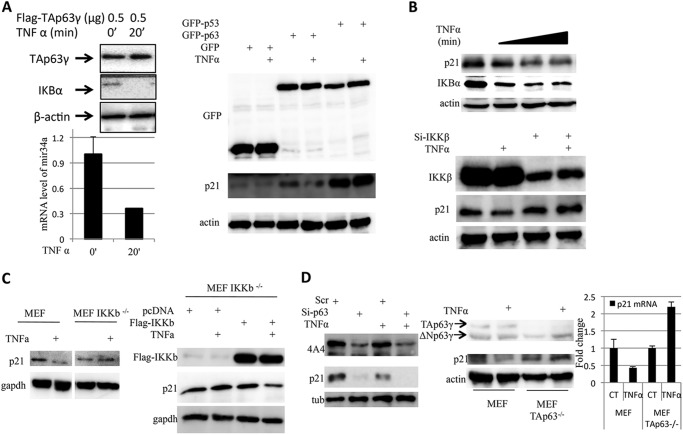
TNFα, an IKKβ Activator, Decreases p21 Level in an IKKβ- and TAp63-dependent Manner
To verify the effect of endogenous IKKβ on TAp63γ transcriptional activity, H1299 cells was treated with TNFα to induce endogenous IKKβ (17–19). Twenty minutes after TNFα treatment, the protein level of IκBα, a target of IKKβ, decreased dramatically, indicating that the endogenous IKKβ is stimulated as expected. The total RNAs from the same samples were extracted, and the miR-34a level was determined by Q-RT-PCR. As shown in Fig. 4A, the expression of miR-34a was dramatically inhibited by TNFα, which indicates that the endogenous IKKβ also inhibits TAp63γ transcriptional activity. These findings imply that IKKβ does inhibit TAp63γ transcriptional activity in certain physiological conditions. To check the specificity of this inhibition, H1299 cells were transfected with GFP, GFP-TAp63γ, or GFP-p53 plasmids and treated with or without TNFα to induce endogenous IKKβ. p21 expression was then determined by WB. As shown in the right panel of Fig. 4A, the induction of endogenous IKKβ by TNFα only suppressed TAp63γ activity, but not p53 activity. This result suggests that IKKβ specifically targets TAp63γ, but not p53. Next, we checked the existence of this TNFα-IKKβ-TAp63γ-p21 pathway in MCF7 cells. MCF7 cells were treated with TNFα and harvested at different time points. Endogenous IKKβ was activated as indicated by reduced IKBα (Fig. 4B). As expected, TNFα treatment decreased p21 level in an IKKβ-dependent manner, for knocking down IKKβ abrogated the reduction of p21 by TNFα (lower panel in Fig. 4B). To further confirm if IKKβ is dispensable for TNFα to inhibit p63 transcriptional activity, we checked the effect of TNFα on p21 level in MEFIKKβ−/− cells. As shown in Fig. 4C, TNFα suppressed p21 expression in MEF cells, but not in MEFIKKβ−/− cells. Interestingly, after introducing ectopic Flag-IKKβ into MEFIKKβ−/− cells, inhibition of p21 expression by TNFα was restored. These results suggest that TNFα suppresses p21 expression by activating IKKβ. We assumed that this reduction of p21 by TNFα also depends on TAp63γ activity, and the TNFα-IKKβ-TAp63γ-p21 pathway may exit in MCF7 cells. To this end, MCF7 cells were treated with TNFα in the presence or absence of p63 siRNA. As shown in the left panel of Fig. 4D, the effect of TNFα on reduction of p21 was impaired by knocking down p63. To further confirm the dependence of TAp63γ, p21 expression in MEF TAp63−/− cells after TNFα treatment was determined by Western blot. The results showed that TNFα can only decrease p21 level in wild type MEF cells by not in MEF TAp63−/− cells (Fig. 4D, right panel). These findings indicate that reduction of p21 by TNFα requires both IKKβ and TAp63, and also suggest that this TNFα-IKKβ-TAp63γ-p21 pathway exit in cells.
FIGURE 4.
Induction of IKKβ by TNFα represses transcriptional activity of TAp63γ. A, induction of IKKβ by TNFα represses TAp63γ induced miR-34a and p21 expression. H1299 cells were transfected with indicated plasmids for 36 h. Before harvesting, cells were treated with TNFα. WB and qPCR were carried out to determine miR-34a and p21 levels. B, TNFα inhibits p21 expression in an IKKβ-dependent fashion in MCF7 cells. MCF7 cells were transfected with indicated siRNA and then treated with TNFα. WB was carried out to check endogenous p21 levels. C, inhibition of p21 expression by TNFα depends on IKKβ activity. In the left panel, MEF and MEFIKKβ−/− cells were treated with or without TNFα. WB analyses were carried out to check endogenous p21 levels. In the right panel, the effect of TNFα on p21 expression was restored in MEFIKKβ−/− cells by ectopic Flag-IKKβ. D, inhibition of p21 expression by TNFα depends on TAp63 activity. In the left panel, MCF7 cells were transfected with indicated siRNA and then treated with TNFα. WB was carried out to check endogenous p21 levels. In the right panel, the effect of TNFα on p21 expression was only observed in MEF wild type cells, but not in MEFTAp63−/− cells.
Ser-4 and Ser-12 of TAp63 Are the Targeting Residues of IKKβ
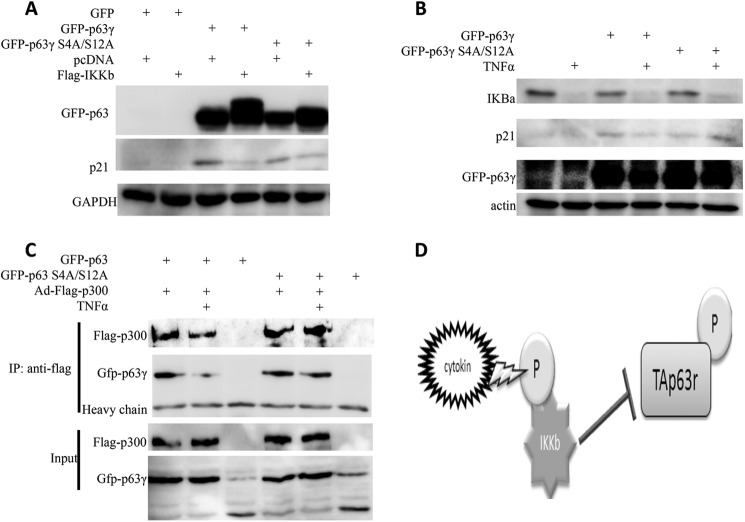
To identify the phosphorylation sites of TAp63 by IKKβ, we carried out both bioinformatics analysis and mass spectrometry analysis. By bioinformatics analysis, we identified a number of IKKβ consensus phosphorylation sites in p63 sequence (data not shown). To confirm these results, H1299 cells were cotransfected with IKKβ and TAp63, and subjected to SDS-PAGE. Compared with cells with TAp63 only in Coomassie staining gel, cells cotransfected with IKKβ and TAp63 had two more bands that could be phosphorylated TAp63 (Fig. 5A). These two bands were then collected and subjected to mass spectrometry (MS). Indeed, this MS analysis showed that these two bands are TAp63, and a number of serines and threonines were identified as potential IKKβ phosphorylation sites (Fig. 5A). We then generated more than a dozen of TAp63 mutants, including S4A, S4A/S12A, S12A/S29A, to further confirm these results and found that IKKβ fails to phosphorylate the TAp63 S4A/S12A mutant, indicating that S4 and S12 of TAp63 are phosphorylation sites of IKKβ (Fig. 5B). As TAp63 S4AS12A mutant displayed a similar transcriptional activity to that of TAp63 (Fig. 6, A and B), we checked the effect of IKKβ on the activity of this TAp63 S4A/S12A mutant. As expected, the p21 level was induced by both wild type and mutant TAp63. However, the dual S4A/S12A mutations of p63 impaired the effect of IKKβ on p21 expression (Fig. 6A), indicating that phosphorylation of TAp63 is required for the inhibition of TAp63-dependent p21 expression by IKKβ. Similarly, TNFα inhibited the transcriptional activity of TAp63, but not the TAp63 S4A/S12A mutant (Fig. 6B). Furthermore, TNFα fails to impair the binding of p300 to the TAp63 S4A/S12A mutant (Fig. 6C), suggesting S4 and S12 are indeed the IKKβ-targeted phosphorylation sites that are indispensable for the effect of IKKβ on TAp63 transcriptional activity.
FIGURE 5.
IKKβ phosphorylates p63 at S4 and S12. A, H1299 cells were transfected with TAp63γ or cotransfected with the TAp63γ and Myc-IKKβ vector as indicated. 48 h after transfection, cells were harvested and subjected to SDS-PAGE. The bands as indicated by arrows were cut and subjected to mass spectrometry. Mass spectrum of potential phosphorylation sites was shown here. B, H1299 cells were transfected with indicated plasmids. WB was carried out to detect TAp63γ and its supershifted/phosphorylated forms.
FIGURE 6.
IKKβ fails to affect the activity of TAp63γ S4A/S12A mutant. A, IKKβ fails to phosphorylate p63 S4A/S12A and to affect its transcriptional activity. H1299 cells were transfected with indicated plasmids for 36 h. WB was carried out to determine p21 levels and p63 phosphorylation. B, TNFα fails to affect p63 S4A/S12A transcriptional activity. H1299 cells were transfected with indicated plasmids for 36 h. Before harvesting, cells were treated with TNFα. WB was carried out to determine p21 levels. C, TNFα fails to affect the binding of p300 and p63 S4S12A. H1299 cells were transfected with indicated plasmids, and 48 h after transfection, cells were harvested. Flag-p300 was pulled down by anti-Flag antibodies, and then the co-IP proteins were probed with the indicated antibodies. The input is shown in the lower panel. D, model for the regulation of TAp63 by IKKβ.
DISCUSSION
In this study, we demonstrate that IKKβ can inhibit TAp63γ-dependent miR-34a promoter-driven luciferase activity and endogenous miR-34a expression. This negative effect was true to the p21 expression as well. We also showed that the kinase activity of IKKβ is critical for this inhibition, as IKKβ kinase dead mutant K44A showed no effect, and the IKKβ kinase inhibitor, ACHP, blocks this inhibition. Phosphorylation has been shown to induce conformational changes in a number of proteins, some of which are critical for protein-protein binding (37). In the case of IKKβ phosphorylation of TAp63γ, this phosphorylation led to the inhibition of the p300-TAp63γ binding and thus impaired the TAp63γ transcriptional activity because p300 bound to the same domain of TAp63γ as that for IKKβ-binding and mediated phosphorylation.
In cells, IKKβ is often activated through various pathways, including the pro-inflammatory cytokine TNFα (17–19). Interestingly, in addition to inducing pro-survival factor NFκB, TNFα also has anti-survival functions. IKKβ had been surprisingly shown to compromise these anti-survival functions in cells (38–40), indicating that IKKβ not only activates NFκB via degradation of IKBα, but could also counteract anti-survival signals introduced by various stresses or cytokines to keep NFκB active. Consistent with this notion, our study as described here shows that IKKβ inhibits p63 transcriptional activity. Also, the TNFα responsive inhibition of TAp63γ transcriptional activity suggests that IKKβ may regulate TAp63γ activity in response to this physiological signal.
Previously, we showed that IKKβ phosphorylates and stabilizes TAp63γ (20). Therefore, we previously expected that this kinase might promote TAp63γ activity. However, surprisingly, we found out in this study that IKKβ actually inhibits the activity of TAp63γ. In our initial attempt to identify IKKβ-targeted phosphorylation sites within TAp63γ, we learnt that there are multiple IKKβ sites in the N terminus of this transcriptional factor. Our future mutagenesis study of these individual phosphorylation amino acids, such as serines or threonines, would help us further elucidate the mechanisms underlying the negative regulation of TAp63γ activity by this cytokine responsive kinase. Indeed, our further analysis identified Ser-4 and Ser-12 as two main target residues within the N terminus of Tap63 by IKKβ in response to TNFα, as the double mutations at these residues impaired the inhibitory effect of this kinase on the transcriptional activity of TAp63. The development of antibodies specific to these phosphorylated sites in the near future would further validate this conclusion.
IKKβ is also a major activator of another transcriptional factor NFκB, which plays a key role in regulating the immune response to different stresses (17–19). A crosstalk of NFκB with the p53 pathway has recently been noted (41). These two pathways are rarely activated simultaneously in the same cells. This is largely because the activation of NFκB promotes cell proliferation and growth, while the activation of p53 induces apoptosis and cell cycle arrest, although both of the p53 and NFκB pathways can respond to the same type of stresses, like UV irradiation (42, 43). Our findings as presented in this study thereby unveil one new mechanism for how IKKβ inactivates TAp63γ that disfavors cell growth and proliferation, similar to p53, whereas it activates NFκB that favors cell growth and proliferation, in response to TNFα (Fig. 6D).
Acknowledgments
We thank Lin He, Xingyue He, and Gregory J. Hannon for generously offering plasmids, John Klimek and Larry David for mass spectrometry, Xiang Zhou for proofreading, and the members of the Lu laboratory for active discussion.
This work was supported in part by NCI, National Institutes of Health (NIH) Grants 095441, CA079721, CA129828, and CA172468 (to H. L.); NIH Grant CA134796 and CPRIT (RP120124) (to E. R. F.); and NSFC Grant 81101966 (to Y. Z.).
- TA
- transactivational
- DBD
- DNA-binding domain
- SA-β-Gal
- senescence-associated β-galactosidase
- IKKβ
- IκB kinase β.
REFERENCES
- 1. Yang A., Kaghad M., Wang Y., Gillett E., Fleming M. D., Dötsch V., Andrews N. C., Caput D., McKeon F. (1998) p63, a p53 homolog at 3q27–29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol. Cell 2, 305–316 [DOI] [PubMed] [Google Scholar]
- 2. Dötsch V., Bernassola F., Coutandin D., Candi E., Melino G. (2010) p63 and p73, the ancestors of p53. Cold Spring Harbor Persp. Biol. 2, a004887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Osada M., Ohba M., Kawahara C., Ishioka C., Kanamaru R., Katoh I., Ikawa Y., Nimura Y., Nakagawara A., Obinata M., Ikawa S. (1998) Cloning and functional analysis of human p51, which structurally and functionally resembles p53. Nat. Med. 4, 839–843 [DOI] [PubMed] [Google Scholar]
- 4. Serber Z., Lai H. C., Yang A., Ou H. D., Sigal M. S., Kelly A. E., Darimont B. D., Duijf P. H., Van Bokhoven H., McKeon F., Dötsch V. (2002) A C-terminal inhibitory domain controls the activity of p63 by an intramolecular mechanism. Mol. Cell. Biol. 22, 8601–8611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Koster M. I., Kim S., Mills A. A., DeMayo F. J., Roop D. R. (2004) p63 is the molecular switch for initiation of an epithelial stratification program. Genes Dev. 18, 126–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. MacPartlin M., Zeng S., Lee H., Stauffer D., Jin Y., Thayer M., Lu H. (2005) p300 regulates p63 transcriptional activity. J. Biol. Chem. 280, 30604–30610 [DOI] [PubMed] [Google Scholar]
- 7. Koster M. I., Kim S., Huang J., Williams T., Roop D. R. (2006) TAp63α induces AP-2γ as an early event in epidermal morphogenesis. Dev. Biol. 289, 253–261 [DOI] [PubMed] [Google Scholar]
- 8. Michael D., Oren M. (2002) The p53 and Mdm2 families in cancer. Curr. Opin. Genet. Dev. 12, 53–59 [DOI] [PubMed] [Google Scholar]
- 9. Celli J., Duijf P., Hamel B. C., Bamshad M., Kramer B., Smits A. P., Newbury-Ecob R., Hennekam R. C., Van Buggenhout G., van Haeringen A., Woods C. G., van Essen A. J., de Waal R., Vriend G., Haber D. A., Yang A., McKeon F., Brunner H. G., van Bokhoven H. (1999) Heterozygous germline mutations in the p53 homolog p63 are the cause of EEC syndrome. Cell 99, 143–153 [DOI] [PubMed] [Google Scholar]
- 10. Hu Y., Baud V., Delhase M., Zhang P., Deerinck T., Ellisman M., Johnson R., Karin M. (1999) Abnormal morphogenesis but intact IKK activation in mice lacking the IKKα subunit of IκB kinase. Science 284, 316–320 [DOI] [PubMed] [Google Scholar]
- 11. Takeda K., Takeuchi O., Tsujimura T., Itami S., Adachi O., Kawai T., Sanjo H., Yoshikawa K., Terada N., Akira S. (1999) Limb and skin abnormalities in mice lacking IKKα. Science 284, 313–316 [DOI] [PubMed] [Google Scholar]
- 12. Ianakiev P., Kilpatrick M. W., Toudjarska I., Basel D., Beighton P., Tsipouras P. (2000) Split-hand/split-foot malformation is caused by mutations in the p63 gene on 3q27. Am. J. Hum. Genet. 67, 59–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McGrath J. A., Duijf P. H., Doetsch V., Irvine A. D., de Waal R., Vanmolkot K. R., Wessagowit V., Kelly A., Atherton D. J., Griffiths W. A., Orlow S. J., van Haeringen A., Ausems M. G., Yang A., McKeon F., Bamshad M. A., Brunner H. G., Hamel B. C., van Bokhoven H. (2001) Hay-Wells syndrome is caused by heterozygous missense mutations in the SAM domain of p63. Hum. Mol. Genet. 10, 221–229 [DOI] [PubMed] [Google Scholar]
- 14. van Bokhoven H., Hamel B. C., Bamshad M., Sangiorgi E., Gurrieri F., Duijf P. H., Vanmolkot K. R., van Beusekom E., van Beersum S. E., Celli J., Merkx G. F., Tenconi R., Fryns J. P., Verloes A., Newbury-Ecob R. A., Raas-Rotschild A., Majewski F., Beemer F. A., Janecke A., Chitayat D., Crisponi G., Kayserili H., Yates J. R., Neri G., Brunner H. G. (2001) p63 Gene mutations in eec syndrome, limb-mammary syndrome, and isolated split hand-split foot malformation suggest a genotype-phenotype correlation. Am. J. Hum. Genet. 69, 481–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mills A. A., Zheng B., Wang X. J., Vogel H., Roop D. R., Bradley A. (1999) p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature 398, 708–713 [DOI] [PubMed] [Google Scholar]
- 16. Yang A., Schweitzer R., Sun D., Kaghad M., Walker N., Bronson R. T., Tabin C., Sharpe A., Caput D., Crum C., McKeon F. (1999) p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature 398, 714–718 [DOI] [PubMed] [Google Scholar]
- 17. Israël A., Le Bail O., Hatat D., Piette J., Kieran M., Logeat F., Wallach D., Fellous M., Kourilsky P. (1989) TNF stimulates expression of mouse MHC class I genes by inducing an NFκB-like enhancer binding activity which displaces constitutive factors. EMBO J. 8, 3793–3800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. DiDonato J. A., Hayakawa M., Rothwarf D. M., Zandi E., Karin M. (1997) A cytokine-responsive IκB kinase that activates the transcription factor NF-κB. Nature 388, 548–554 [DOI] [PubMed] [Google Scholar]
- 19. Mercurio F., Zhu H., Murray B. W., Shevchenko A., Bennett B. L., Li J., Young D. B., Barbosa M., Mann M., Manning A., Rao A. (1997) IKK-1 and IKK-2: cytokine-activated IκB kinases essential for NF-κB activation. Science 278, 860–866 [DOI] [PubMed] [Google Scholar]
- 20. MacPartlin M., Zeng S. X., Lu H. (2008) Phosphorylation and stabilization of TAp63γ by IκB kinase-β. J. Biol. Chem. 283, 15754–15761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. He L., He X., Lim L. P., de Stanchina E., Xuan Z., Liang Y., Xue W., Zender L., Magnus J., Ridzon D., Jackson A. L., Linsley P. S., Chen C., Lowe S. W., Cleary M. A., Hannon G. J. (2007) A microRNA component of the p53 tumour suppressor network. Nature 447, 1130–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jin Y., Zeng S. X., Sun X. X., Lee H., Blattner C., Xiao Z., Lu H. (2008) MDMX promotes proteasomal turnover of p21 at G1 and early S phases independently of, but in cooperation with, MDM2. Mol. Cell. Biol. 28, 1218–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang Y., Liao J. M., Zeng S. X., Lu H. (2011) p53 downregulates Down syndrome-associated DYRK1A through miR-1246. EMBO Reports 12, 811–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zeng S. X., Dai M. S., Keller D. M., Lu H. (2002) SSRP1 functions as a co-activator of the transcriptional activator p63. EMBO J. 21, 5487–5497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tang F., Hajkova P., Barton S. C., Lao K., Surani M. A. (2006) MicroRNA expression profiling of single whole embryonic stem cells. Nucleic Acids Res. 34, e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dai M. S., Sun X. X., Lu H. (2008) Aberrant expression of nucleostemin activates p53 and induces cell cycle arrest via inhibition of MDM2. Mol. Cell. Biol. 28, 4365–4376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jin Y., Dai M. S., Lu S. Z., Xu Y., Luo Z., Zhao Y., Lu H. (2006) 14-3-3γ binds to MDMX that is phosphorylated by UV-activated Chk1, resulting in p53 activation. EMBO J. 25, 1207–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liao J. M., Lu H. (2011) Autoregulatory suppression of c-Myc by miR-185–3p. J. Biol. Chem. 286, 33901–33909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang Q., Xiao H., Chai S. C., Hoang Q. Q., Lu H. (2011) Hydrophilic residues are crucial for ribosomal protein L11 (RPL11) interaction with zinc finger domain of MDM2 and p53 protein activation. J. Biol. Chem. 286, 38264–38274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Antonini D., Russo M. T., De Rosa L., Gorrese M., Del Vecchio L., Missero C. (2010) Transcriptional repression of miR-34 family contributes to p63-mediated cell cycle progression in epidermal cells. J. Investig. Dermatol. 130, 1249–1257 [DOI] [PubMed] [Google Scholar]
- 31. el-Deiry W. S., Tokino T., Velculescu V. E., Levy D. B., Parsons R., Trent J. M., Lin D., Mercer W. E., Kinzler K. W., Vogelstein B. (1993) WAF1, a potential mediator of p53 tumor suppression. Cell 75, 817–825 [DOI] [PubMed] [Google Scholar]
- 32. Lin Y. L., Sengupta S., Gurdziel K., Bell G. W., Jacks T., Flores E. R. (2009) p63 and p73 transcriptionally regulate genes involved in DNA repair. PLoS Genetics 5, e1000680. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33. Su X., Gi Y. J., Chakravarti D., Chan I. L., Zhang A., Xia X., Tsai K. Y., Flores E. R. (2012) TAp63 is a master transcriptional regulator of lipid and glucose metabolism. Cell Metab. 16, 511–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Murata T., Shimada M., Sakakibara S., Yoshino T., Kadono H., Masuda T., Shimazaki M., Shintani T., Fuchikami K., Sakai K., Inbe H., Takeshita K., Niki T., Umeda M., Bacon K. B., Ziegelbauer K. B., Lowinger T. B. (2003) Discovery of novel and selective IKK-β serine-threonine protein kinase inhibitors. Part 1. Bioorganic Medicinal Chemistry Letters 13, 913–918 [DOI] [PubMed] [Google Scholar]
- 35. Han Z., Wei W., Dunaway S., Darnowski J. W., Calabresi P., Sedivy J., Hendrickson E. A., Balan K. V., Pantazis P., Wyche J. H. (2002) Role of p21 in apoptosis and senescence of human colon cancer cells treated with camptothecin. J. Biol. Chem. 277, 17154–17160 [DOI] [PubMed] [Google Scholar]
- 36. Romanov V. S., Abramova M. V., Svetlikova S. B., Bykova T. V., Zubova S. G., Aksenov N. D., Fornace A. J., Jr., Pospelova T. V., Pospelov V. A. (2010) p21(Waf1) is required for cellular senescence but not for cell cycle arrest induced by the HDAC inhibitor sodium butyrate. Cell Cycle 9, 3945–3955 [DOI] [PubMed] [Google Scholar]
- 37. Hwang I., Thorgeirsson T., Lee J., Kustu S., Shin Y. K. (1999) Physical evidence for a phosphorylation-dependent conformational change in the enhancer-binding protein NtrC. Proc. Natl. Acad. Sci. U.S.A. 96, 4880–4885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tang G., Yang J., Minemoto Y., Lin A. (2001) Blocking caspase-3-mediated proteolysis of IKKβ suppresses TNF-α-induced apoptosis. Mol. Cell 8, 1005–1016 [DOI] [PubMed] [Google Scholar]
- 39. Maeda S., Chang L., Li Z. W., Luo J. L., Leffert H., Karin M. (2003) IKKβ is required for prevention of apoptosis mediated by cell-bound but not by circulating TNFα. Immunity 19, 725–737 [DOI] [PubMed] [Google Scholar]
- 40. Senftleben U., Li Z. W., Baud V., Karin M. (2001) IKKβ is essential for protecting T cells from TNFα-induced apoptosis. Immunity 14, 217–230 [DOI] [PubMed] [Google Scholar]
- 41. Ak P., Levine A. J. (2010) p53 and NF-κB: different strategies for responding to stress lead to a functional antagonism. Faseb. J. 24, 3643–3652 [DOI] [PubMed] [Google Scholar]
- 42. Maltzman W., Czyzyk L. (1984) UV irradiation stimulates levels of p53 cellular tumor antigen in nontransformed mouse cells. Mol. Cell. Biol. 4, 1689–1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. O'Dea E. L., Kearns J. D., Hoffmann A. (2008) UV as an amplifier rather than inducer of NF-κB activity. Mol. Cell 30, 632–641 [DOI] [PMC free article] [PubMed] [Google Scholar]