Abstract
Adaptor protein (AP) complexes are the predominant coat proteins of membrane vesicles in post-Golgi trafficking of mammalian cells. Each AP complex contains a specific medium subunit, μ-adaptin, that selects cargo proteins bearing sequence-specific sorting motifs. Much less is known about the AP complexes and their μ subunits in plants. Because of uncertain homology, the μ-adaptins of Arabidopsis have been designated muA through muD [Happel et al. (2004) Plant J 37(5):678–693]. Furthermore, only muD has been assigned to a specific AP complex, AP-3, involved in Golgi-vacuolar trafficking [Niihama et al. (2009) Plant Cell Physiol 50(12):2057–2068, Zwiewka et al. (2011) Cell Res 21(12):1711–1722, and Wolfenstetter et al. (2012) Plant Cell 24(1):215–232]. In contrast, the μ subunit of neither the post-Golgi trafficking AP-1 complex nor the endocytic AP-2 complex has been identified. Here, we report the functional analysis of redundant AP-1 μ-adaptins AP1M1 (also known as muB1) and AP1M2 (also known as muB2). Coimmunoprecipitation revealed that both AP1M2 and its less strongly expressed isoform AP1M1 are complexed with the large subunit γ-adaptin of AP-1. In addition, AP1M2 was localized at or near the trans-Golgi network. Knockout mutations of AP1M2 impaired pollen function and arrested plant growth whereas the ap1m1 ap1m2 double mutant was nearly pollen-lethal. At the cellular level, the absence of AP1M2 entailed inhibition of multiple trafficking pathways from the trans-Golgi network to the vacuole and to the plasma membrane in interphase and to the plane of cell division in cytokinesis. Thus, AP-1 is crucial in post-Golgi trafficking in plant cells and required for cell division and plant growth.
Keywords: adaptor complex 1, membrane traffic, secretory pathway, development
In multicellular organisms, membrane traffic delivers cargo proteins such as receptors, ligands, and transporters to their subcellular destinations and, thus, enables cells to communicate with their neighbors and respond to the environment. The initial step of membrane traffic is vesicle formation at the donor compartment. In post-Golgi traffic, activated ADP ribosylation factor (ARF) GTPase drives efficient recruitment of one of several adaptor protein (AP) complexes to the membrane, which, in turn, recognizes sorting sequences of cargo molecules and often mediates clathrin assembly for clathrin-coated vesicle formation.
AP complexes are heterotetrameric, comprising two large subunits (α/γ/δ/ε and β), one medium subunit (μ) and one small subunit (σ). Large subunits α/γ/δ/ε mediate membrane recruitment; for example, α-adaptin of the AP-2 complex has a strong binding preference to phosphatidylinositol 4,5-biphosphate-enriched membranes (1). Whereas β-adaptin has a clathrin-binding motif and recognizes dileucine-based sorting sequences, µ-adaptin recognizes tyrosine- or dileucine-based sorting sequences (1). The precise function of the σ subunit is still unknown, but σ1B deficiency causes defects in long-term spatial memory in the mouse (2).
In general, the AP-1 complex (Apl4p/Apl2p/Apm1p/Aps1p or γ/β1/µ1/σ1) is involved in the trans-Golgi network (TGN)/late Golgi-to-lysosomal/vacuolar trafficking in concert with clathrin in eukaryotes (3, 4). In addition, AP-1 is involved in other trafficking pathways; in olfactory cilia of Caenorhabditis elegans and mammalian epithelial cells, it plays a role at the TGN or recycling endosomes in polar trafficking of certain plasma-membrane proteins to specific sites (5, 6), and in fission yeast, it is involved in secretion and cytokinesis (7). Besides anterograde transport, mammalian AP-1 complex is involved in retrograde transport of the mannose-6-phosphate receptor from endosomes to the TGN (8).
Mammalian AP-2 (α/β2/µ2/σ2), which has been best characterized, plays a pivotal role in clathrin-dependent endocytosis (9, 10). AP-2 depletion completely blocks endocytosis of transferrin receptor and is said to inhibit endocytosis of epidermal growth factor receptor, although the latter is rather controversial (11, 12). Whereas both AP-1 and AP-2 complexes are indispensable in embryonic development in higher eukaryotes (8, 13), AP-1 deficiency is synthetically lethal in yeast: with a temperature-sensitive clathrin heavy chain and a deletion of calcineurin, a regulator of Ca2+ signaling, in Saccharomyces cerevisiae and Schizosaccharomyces pombe, respectively (3, 7). Deficiency of AP-3 (δ/β3/µ3/σ3) causes a defect in vacuolar targeting of alkaline phosphatase (ALP), but not of carboxypeptidase Y (CPY), in yeast cells (14). In Drosophila, depletion of AP-3 results in defects in pigmentation and neurological activity (15), implying that AP-3 mediates sorting of cargoes destined for the lysosome or lysosome-related organelles. Occurrence of a fourth adaptor complex AP-4 (ε/β4/µ4/σ4) has been described or inferred in only a few organisms such as humans, mice, and Arabidopsis and is known to be involved in basolateral sorting in epithelial cells (1, 16). Very recently, an AP-5 complex has been found through a structure-prediction program and shown to be involved in clathrin-independent endosomal trafficking at the late endosome in mammals (17).
Post-Golgi trafficking is somewhat diverged in plants compared with animals, comprising the following major pathways in which AP complexes might be involved: (i) secretory traffic from the Golgi/TGN to the plasma membrane; (ii) vacuolar traffic delivering hydrolytic and proteolytic enzymes to the vacuole; and (iii) endocytosis from the plasma membrane to the TGN, which in plants also acts as an early endosome and, thus, is an intersection of secretory and endocytic traffic routes (18). The endocytosed cargo proteins can be passed on, via internalization into the multivesicular bodies (MVBs), to the vacuole for degradation or can be recycled, sometimes in a polar fashion, back to the plasma membrane (19).
The Arabidopsis genome encodes subunits of four types of putative AP complexes (AP-1 to AP-4) including five medium subunits, muA, muB1, muB2, muC, and muD (20). There might be an additional AP complex because Arabidopsis appears to have orthologs of several subunits of a recently identified mammalian AP-5 complex; however, no matching sigma subunit has been identified (17). So far, the AP-3 complex comprising the subunits δ, β3, muD and σ3 is the only one that has been functionally characterized in Arabidopsis. Deprivation of β3-adaptin [protein affected trafficking 2 (PAT2)] or muD-adaptin (also known as AP-3 mu or PAT4) subunit of AP-3 affects vacuolar biogenesis (21, 22) and differentially inhibits vacuolar traffic in mesophyll protoplasts (23). Although the morphology of mutant plants appears normal, elimination of muD-adaptin or other subunits of AP-3 suppresses the knockout mutant phenotype of soluble N-ethylmaleimide-sensitive factor attachment receptor (Qb-SNARE) VPS ten interacting 11 (VTI11), which is involved in TGN-to-prevacuolar compartment (PVC) trafficking (24, 25). Information on other μ-adaptins is scarce. Taking both sequence similarity and evolutionary diversity of medium subunits of mammalian and plant AP complexes into account, we propose a nomenclature that relates the medium subunit to its respective complex. For example, muD adaptin (also known as At-muD-Ad, At1g56590) should, thus, be named AP3M. It is not clear which of the medium adaptins muA or muB1/muB2 is a subunit of the secretory AP-1 complex or the endocytic AP-2 complex. By sequence similarity, muA and muB might be more closely related to mammalian µ2 and µ1 subunits, respectively (1, 17). However, muA adaptin was localized to the trans-Golgi in Arabidopsis suspension cells and shown to interact with the cytosolic tail of pea vacuolar sorting receptor (VSR)1 and mammalian TGN38 in vitro (20). These observations suggest that muA adaptin might be the medium subunit of an AP complex acting on the Golgi-to-vacuole trafficking route rather than on endocytosis. Based on our analysis below, muB1 (also known as At-muB1-Ad, At1g10730) and muB2 (also known as At-muB2-Ad, At1g60780) are medium subunits of secretory AP-1 complexes and have been designated AP1M1 and AP1M2.
A knockout mutation in the Arabidopsis AP1M2 gene named hapless13 (hap13) was isolated as a male-gametophytic lethal (26), suggesting an essential role for AP1M2 adaptin in the haploid generation. In this report, we investigated two closely related AP1M adaptin variants, AP1M2 and the minor isoform AP1M1, to identify their cell-biological trafficking roles, their biological requirements during plant development, and their association with specific adaptor complexes. Our results suggest that AP1M1 and AP1M2 are functionally redundant medium subunits of the Arabidopsis AP-1 complex acting at the TGN to promote secretory and vacuolar trafficking and that this function is required for cell division and growth during both pollen and plant development.
Results
Transferred DNA Insertion AP1M2 Mutants ap1m2-1 and hap13 Display Growth-Retardation Phenotype.
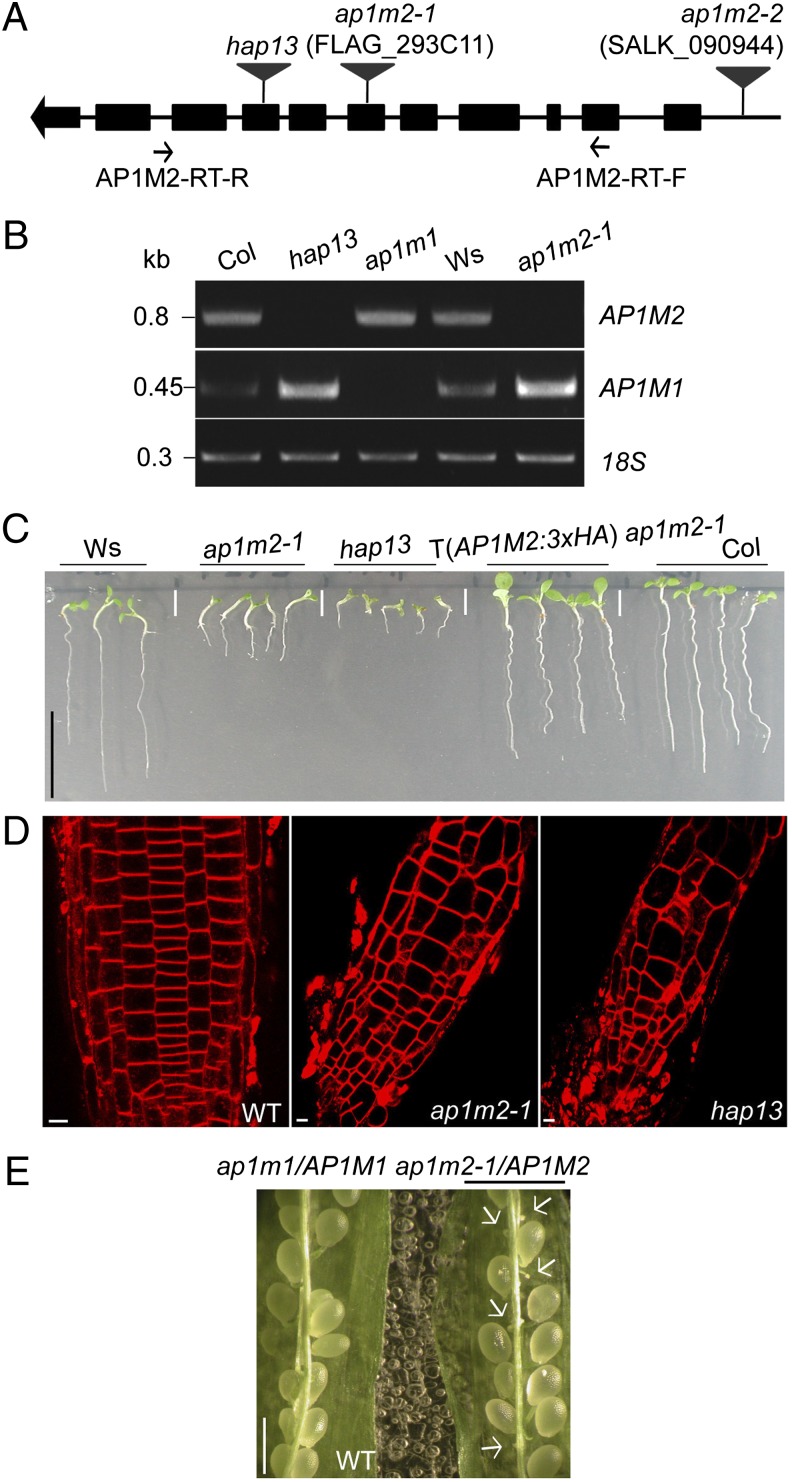
To gain insight into the physiological role of Arabidopsis AP1M2, we analyzed transferred DNA (T-DNA) insertion mutants, ap1m2-1 and hap13, which had the T-DNA inserted in different exons (Fig. 1A). RT-PCR analysis confirmed that these two ap1m2 mutants lacked AP1M2 transcript (Fig. 1B). These two knockout alleles of AP1M2 gave essentially a similar mutant phenotype of impaired seedling root growth. The hap13 mutant phenotype was slightly more pronounced, as judged by its earlier growth arrest (Fig. 1C). F1 seedlings from reciprocal crosses between ap1m2-1 and hap13 heterozygous plants displayed an ap1m2-1 mutant-like phenotype, suggesting modulation of the ap1m2 knockout phenotype by the genetic background of the parental ecotype. However, we cannot rule out other possible explanations such as a truncated hap13 protein having an unexpected effect on plant development. Surprisingly, the transcript level of the closest homolog of AP1M2, AP1M1 (Fig. S1), was slightly increased in the ap1m2-1 mutant, whereas the converse (i.e., up-regulation of AP1M2 in the ap1m1 mutant) was not the case (Fig. 1B and Fig. S2F). The significance of this observation is not clear at present. Homozygous mutant seedlings were vastly underrepresented among the offspring of ap1m2 heterozygous plants, suggesting requirement of AP1M2 in the haploid gametophytic generation (Fig. S2). Reciprocal crosses revealed that male transmission of the ap1m2-1 allele was reduced to less than 10% of the wild-type (WT) value (Fig. S2A). Although Johnson et al. had reported that the hap13 allele of AP1M2 was lethal to the male gametophyte (26), we recovered approximately 5% homozygous mutant seedlings from self-fertilized hap13 heterozygous plants, which matched the low frequency of hap13 transmission through the pollen in our growth conditions (Fig. S2B). Although hap13 and ap1m2-1 mutants still sustained normal root architecture, the shape, size, and file pattern of root cells were irregular and abnormal (Fig. 1D). Both ap1m2-1 and hap13 mutant plants were not able to develop to the flowering stage on soil. When grown in closed containers, however, ap1m2-1 mutant plants eventually flowered with defective and sterile floral organs (Fig. S2G). To analyze AP1M2 function in more detail, we generated transgenic plants that expressed AP1M2 tagged with an internal epitope between the two major domains of β-adaptin binding and cargo binding, as had been used for studying mammalian μ2-adaptin (27). The growth-retarded phenotype of the ap1m2-1 mutant was rescued by the overexpression of the epitope-tagged AP1M2 from either AP1M2 cis-acting expression elements or from the cassava vein mosaic virus (CsVMV) [AP1M2:3×HA (hemagglutinin) or AP1M2:myc (myelocytomatosis viral oncogene homolog); Fig. 1C and Fig. S2 C–F] (28).
Fig. 1.
T-DNA insertional AP1M2 mutants, ap1m2-1 and hap13, display growth-retardation phenotypes. (A) Diagram of the T-DNA insertions: ap1m2-1 (FLAG_293C11), sixth exon, ecotype Ws; ap1m2-2 (SALK_090944), promoter; hap13, eighth exon, ecotype Columbia (Col). (B) RT-PCR analysis with the primers indicated in A to detect AP1M2 and AP1M1 transcripts in ap1m2-1, hap13, and a T-DNA insertional AP1M1 mutant (see also Fig. S4). 18S rRNA (18S) was used as loading control. Kb, kilobase. (C and D) Phenotypes of ap1m2 mutants. (C) Seedling root growth is retarded in ap1m2 mutants. This mutant phenotype was fully complemented by expression of AP1M2::AP1M2:3×HA. T, transgene. (D) Seedling roots of WT and ap1m2 mutants stained with FM4-64. Note the irregular and swollen root cells in ap1m2-1 and hap13. (E) F1 offspring of self-fertilized ap1m1/AP1M1 ap1m2-1/AP1M2 (see also Tables S1 and S2 for genetic analysis). Arrows indicate unfertilized ovules. [Scale bars: 1 cm (C); 5 μm (D); 1 mm (E).]
AP1M2 and AP1M1 Are Functionally Redundant.
Loss of μ1A in higher eukaryotes brings about embryonic lethality (8). However, a single mutant of AP1M2 in Arabidopsis grew albeit poorly, which led to the hypothesis that there are still some functional AP-1 complex(es) in ap1m2 mutant plants. Of five medium adaptins in Arabidopsis, AP1M1 is ∼90% identical in amino acid sequence to AP1M2 (Fig. S1) and its expression is normally 10-fold lower than the AP1M2 expression (Fig. S3). Indeed, loss of ap1m1 did not cause any discernible morphological change, unlike ap1m2 mutants (Fig. S4). Furthermore, self-fertilization of ap1m2/AP1M2 ap1m1/AP1M1 double-mutant plants produced 20–30% unfertilized ovules, suggesting strong impairment of ap1m2 ap1m1 gametophytes (Fig. 1E and Table S1). Reciprocal crosses of doubly heterozygous plants with WT plants showed almost complete loss of ap1m2 ap1m1 transmission through the pollen (Table S2). These results support the conclusion that AP1M1 and AP1M2 are functionally redundant.
AP1M2 with an Internal Tag Preferentially Locates at the TGN.
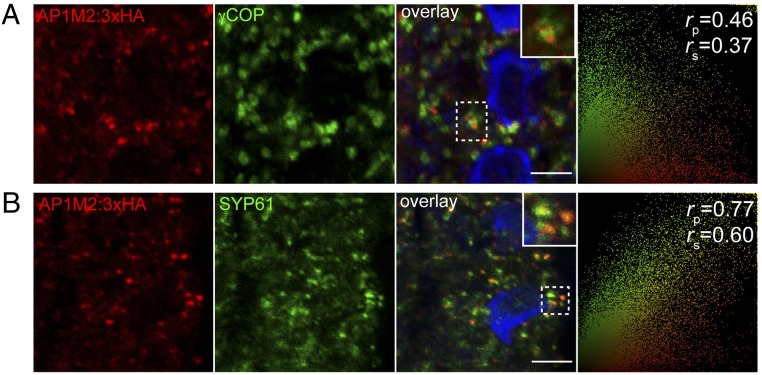
The subcellular localization of three times repeated HA-tagged AP1M2 was studied in seedling roots. To quantify the extent of colocalization of AP1M2 and compartment-specific markers, we used the linear Pearson correlation coefficient (rp) and the nonlinear Spearman correlation coefficient (rs) (29). No significant colocalization was detected with Golgi marker γ-subunit of coatomer complex (γ-COP) (Fig. 2A; rp/rs = 0.46/0.37) (30). In contrast, AP1M2 partially colocalized with TGN marker syntaxin of plants 61 (SYP61) (Fig. 2B, rp/rs = 0.77/0.60; see also Fig. S5B) (25). Brefeldin A (BFA) treatment resulted in colocalization of AP1M2 with ARF1 in endosomal BFA compartments, which are surrounded by γ-COP signals (Fig. S5A) (31). Taken together, these data suggest preferential localization of AP1M2 to the TGN or some closely related post-Golgi endomembrane compartment. Additional experiments rule out the association of AP1M2 with wortmannin-sensitive, ARA7/RAB-F2b–positive MVBs (Fig. S5D) (32, 33). Additionally, partial colocalization of AP1M2 with clathrin heavy chain (Fig. S5C; rp/rs = 0.71/0.53) was compatible with the idea that the AP1M2-containing AP complex might act in concert with clathrin in vesicle formation. In support of this conclusion, clathrin heavy chain was coimmunoprecipitated with purified recombinant GST:γ-adaptin protein in protein extract from seedlings (Fig. S5E). However, this result remains to be functionally validated.
Fig. 2.
Subcellular localization of internally tagged AP1M2 at or near the TGN. (A and B) Confocal images of seedling roots expressing AP1M2::AP1M2:3×HA in ap1m2-1 homozygous background counterstained with anti-γCOP (Golgi) (A) and anti-SYP61 antiserum (TGN) (B). The extent of colocalization was quantified using the linear Pearson correlation coefficient (rp) and the nonlinear Spearman rank correlation coefficient (rs); the resulting scatterplot images are shown on the right. A value of 1.0 means complete colocalization of two fluorescent signals. Note that AP1M2:3×HA preferentially colocalized with endosomes labeled with the TGN marker anti-SYP6 compared with the Golgi stacks labeled with γCOP. See also Fig. S5 A–D for further localization analysis. Blue, DAPI-stained chromatin. Insets show boxed areas at higher magnification. (Scale bars: 5 μm.)
AP1M2 and AP1M1 Biochemically Interact with Endogenous γ-Adaptin but Not with α-Adaptin.
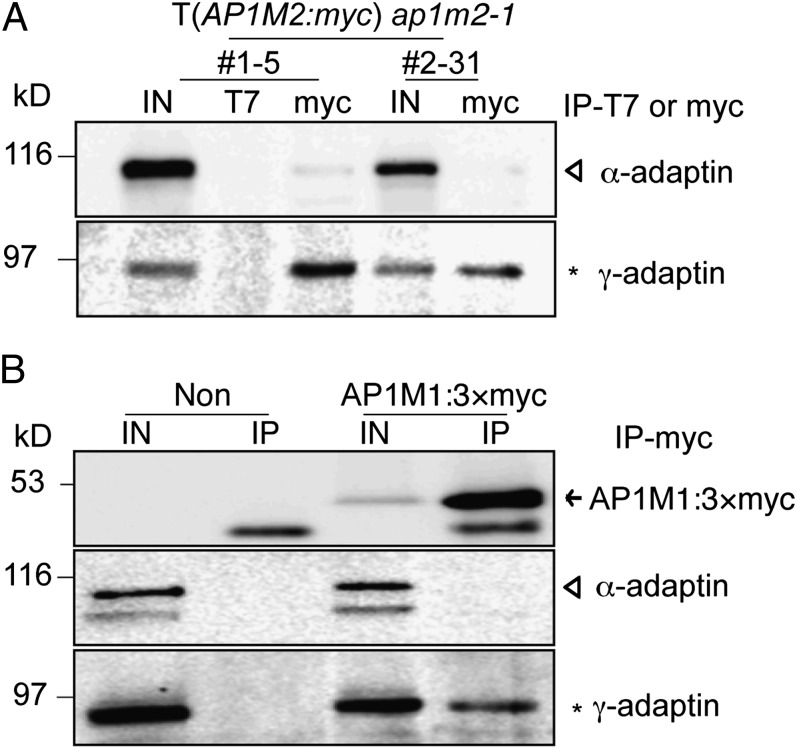
The localization data led us to hypothesize that AP1M2 is a medium adaptin of the AP-1 complex in Arabidopsis. One of the two large subunits is unique to each heterotetrameric AP complex. For example, α-adaptin is part of AP-2 involved in endocytosis whereas γ-adaptin is unique to secretory AP-1 (1). Thus, we raised antiserum against γ-adaptin (Fig. S6), enabling us to perform coimmunoprecipitation experiments to identify the large adaptin subunit with which AP1M2 would associate. Protein extracts from transgenic ap1m2-1 plants expressing myc-tagged AP1M2 were immunoprecipitated with anti-myc beads, and both total protein extract and immunoprecipitate were probed for the presence of α-adaptin (34) and γ-adaptin (Fig. 3A). In contrast to α-adaptin, γ-adaptin was detected in the immunoprecipitate, suggesting that AP1M2 is the medium-sized subunit of AP-1 in Arabidopsis (Fig. 3A). In addition, a similar coimmunoprecipitation experiment revealed that myc-tagged AP1M1 also interacts with γ-adaptin but not with α-adaptin (Fig. 3B). Other results supported the conclusion that AP1M2 is a subunit of the AP-1 complex. Specifically, the total protein level of γ-adaptin, but not of α-adaptin, was reduced and γ-adaptin was less detected in the membrane fraction of ap1m2-1 mutant seedlings (Fig. S6B). Similar alterations had been reported for γ-adaptin in μ1A-deficient mice (8). Transiently expressed HA:δ-adaptin, which is a large adaptin of the AP-3 complex (35), was not coimmunoprecipitated with AP1M2, thus confirming the specific association of AP1M2 with the AP-1 complex (Fig. S6C).
Fig. 3.
AP1M2 and AP1M1 interact with endogenous γ-adaptin. (A) Interaction of AP1M2 with γ-adaptin. Protein extracts of ap1m2-1 homozygous plants expressing CsVMV::AP1M2:myc were subjected to immunoprecipitation (IP) with anti-T7 or anti-myc antibody, and the immunoprecipitates were analyzed by immunoblotting using anti–α-adaptin or anti–γ-adaptin antiserum. Anti-T7 beads were used as control for nonspecific immunoprecipitation. (B) Interaction of AP1M1 with γ-adaptin. Protein extracts of protoplasts transformed with 35S::AP1M1:3×myc were subjected to immunoprecipitation with anti-myc antibody, and the immunoprecipitates were analyzed by immunoblotting using anti-myc, anti–α-adaptin, or anti–γ-adaptin antiserum. IN, input (total extract); Non, nontransformed protoplasts. Open triangles, α-adaptin; asterisks, γ-adaptin; arrow, AP1M1:3×myc.
Secretory and Vacuolar Pathways Are Impaired in ap1m2-1.
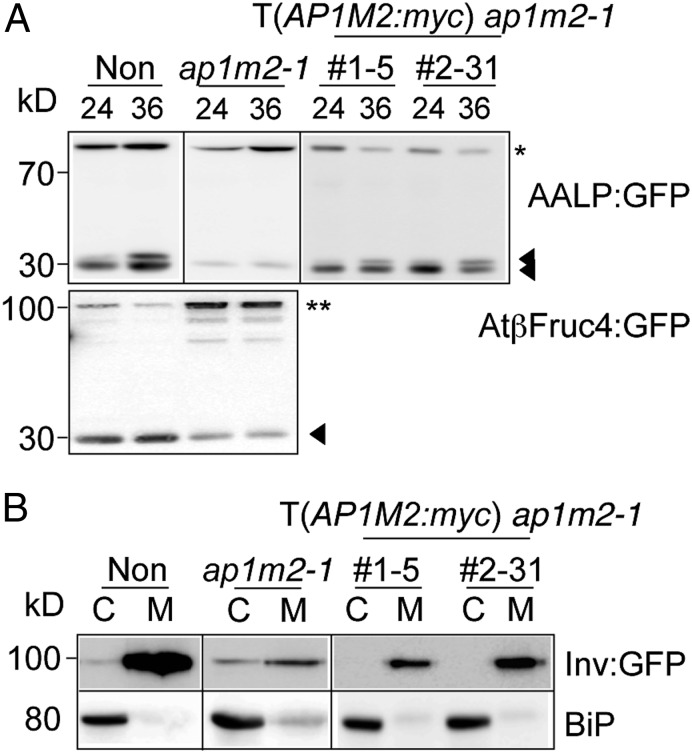
To identify trafficking pathways involving AP1M2, we examined the effect of the ap1m2-1 mutation on various post-Golgi trafficking pathways. Soluble cargo proteins sporamin and Arabidopsis aleurain-like protease (AALP) and the membrane cargo protein AtβFructosidase4 were used as GFP-fusion proteins to analyze vacuolar trafficking in ap1m2-1 protoplasts (36, 37). Unlike the situation in WT, these proteins were not processed in the ap1m2-1 mutant, as seen by the increase in intensity of the precursor band, and this defect was suppressed in protoplasts from ap1m2-1 plants expressing AP1M2:myc (Fig. 4A and Fig. S7A). Next, we examined trafficking of secretory invertase as a GFP-fusion protein and engineered secretory (sec) GFP. Compared with WT protoplasts, a substantial fraction of these proteins was retained inside ap1m2-1 mutant protoplasts (Fig. 4B and Fig. S7B) (36, 38). Purified recombinant maltose-binding protein (MBP):AP1M2 interacted with an epitope-tagged Arabidopsis VSR1, VSR1:HA, that was transiently expressed in protoplasts (Fig. S5E) (39). This result supports the notion that an AP1M2-containing AP complex might act in vesicle formation destined for the vacuole by binding directly with the VSR, although the place for this cargo-receptor interaction is still controversial (40). However, their physical interaction remains to be validated in vivo. Both AALP:GFP and invertase:GFP appeared to locate at the endoplasmic reticulum (ER) in ap1m2-1 mutant protoplasts (Fig. S7D). It is not yet clear what causes the accumulation of soluble cargo proteins in the ER, rather than the TGN, of mutant protoplasts. At present, it is poorly understood how a defect in post-Golgi trafficking might affect the ER exit of post-Golgi cargoes.
Fig. 4.
Secretory and vacuolar pathways are impaired in ap1m2-1. Transient-trafficking assays of vacuolar (A) or secretory (B) cargo proteins. Protoplasts isolated from nontransformed (Non), ap1m2-1, and two independent rescued plants (CsVMV::AP1M2:myc in ap1m2-1) were transformed with AALP:GFP or AtβFructosidase4:GFP (AtβFruc4:GFP). At 24 h after transformation, total protein extracts from protoplasts were subjected to immunoblot analysis with an anti-GFP antibody. Asterisk, full-length AALP:GFP; double closed triangles, processed bands of AALP:GFP; double asterisk, full-length AtβFruc4:GFP; closed triangle, processed band of AtβFruc4:GFP. (B) Invertase:GFP (Inv:GFP) was transformed into protoplasts prepared from the indicated plants. At 24 h after transformation, total protein that had been extracted from protoplasts (C) and medium (M) was subjected to immunoblot analysis with an anti-GFP antibody. ER-localized binding protein (BiP) was used as control for nonspecific leaking of cellular proteins.
To gain insight into the defect of vacuolar and secretory trafficking pathways, ultrastructural analysis of ap1m2-1 mutants was performed. The mutants exhibited abnormal morphology of the Golgi apparatus (Fig. 5 A and B; compare with Fig. 5C). Concanamycin A (ConcA) treatment also causes abnormal morphology of Golgi stacks and TGN and results in defective vacuolar and secretory trafficking, thus mimicking the ap1m2 mutant phenotype (41). Upon ConcA treatment, however, trafficking of AtβFructosidase4:GFP to the vacuole appeared not to be inhibited, although sporamin:GFP (Spo:GFP) was (Fig. S7C), suggesting that the ConcA-induced abnormal morphology of Golgi per se might not be the cause of defective vacuolar trafficking in the ap1m2 mutant. In contrast to the vacuolar and secretory pathways, endocytosis occurred normally in ap1m2-1 seedling roots, as evidenced by the localization of FM4-64, a common endocytic tracer, to BFA compartments and the tonoplast (Fig. S8 A, B, and G). In addition, PIN-FORMED 2 (PIN2), an auxin efflux carrier, showed polar localization in the plasma membrane of root tissues after BFA washout as in WT (Fig. S8C) (42). However, delivery of PIN2:GFP to the vacuole was severely impaired in ap1m2-1 seedling roots, because PIN2:GFP in ap1m2-1 accumulated in BFA bodies, whereas in WT, PIN2:GFP was observed in both the vacuole and BFA bodies after sequential treatments of translational inhibitor cycloheximide, darkness, and BFA (Fig. S8F). These results indicated that AP1M2 participates neither in endocytosis nor in recycling of PIN2 to the plasma membrane, whereas delivery of PIN2 to the vacuole was inhibited in the ap1m2 mutants. In contrast to mutant seedlings that lack the AP3M subunit of the AP-3 complex (22), ap1m2 mutants displayed vacuolar membranes of normal appearance (Fig. S8G). This observation also indicates that the defects of vacuolar trafficking in ap1m2-1 mutant seedlings did not result in structural defects of the vacuole. Altogether, AP1M2 appears to be essential in both secretion and vacuolar trafficking of some, but not all, proteins.
Fig. 5.
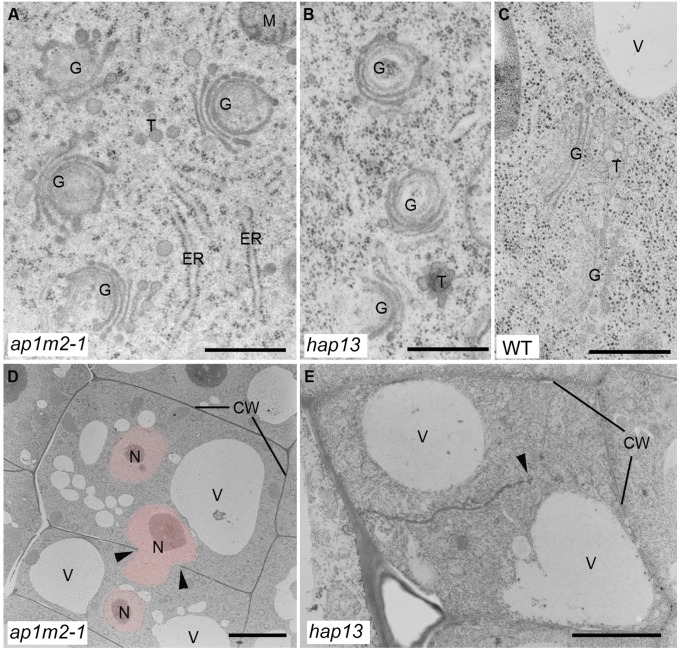
Ultrastructural analysis of ap1m2 mutants. Ultrathin sections of cryofixed and freeze-substituted root tips of 5-d-old ap1m2-1 and hap13 seedlings were examined by transmission electron microscopy. ap1m2 mutants displayed bent Golgi stacks (A and B) (compare with WT (C)] and cell wall stubs/incomplete cell walls (arrow heads) (D and E). Note also the presence of several nuclei (pink-colored) per cell in the ap1m2-1 mutant (D). CW, cell wall; G, Golgi stack; M, mitochondrion; N, nucleus; T, trans-Golgi network; V, vacuole. [Scale bars: 500 nm (A–C); 6 µm (D); 3 µm (E).]
Trafficking from the TGN to the Cell Plate Is Impaired in Dividing Cells of ap1m2 Mutants.
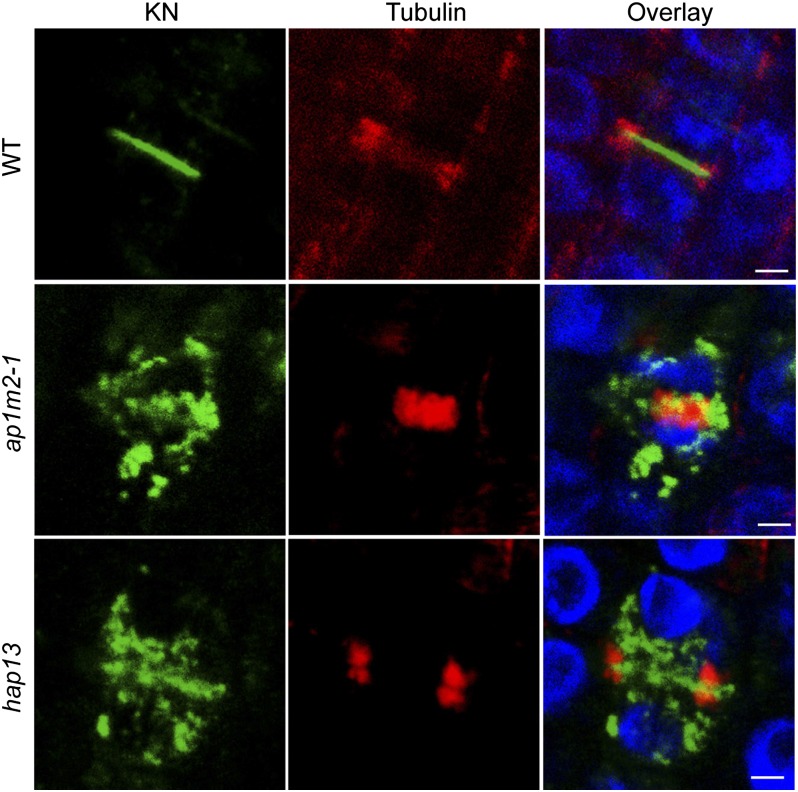
The ap1m2 mutants seedling roots were severely impaired in elongation (Fig. 1 C and D). Detailed analysis of root tissues of mutants seedlings revealed multinucleate cells and cell-wall stubs (Fig. 5 D and E), which might explain the growth-retarded seedling phenotype. These results raised the possibility that AP1M2 plays a pivotal role in cell plate formation during cytokinesis. The trafficking pathway from the TGN to the division plane is absolutely required for the formation of the cell plate, as revealed by the analysis of cytokinesis-specific syntaxin KNOLLE (KN) (43). As shown in Fig. 6, KN trafficking to the plane of cell division was blocked or strongly impaired in ap1m2 mutants. This result indicates that AP-1 plays an essential role in trafficking of KN to the plane of cell division during cytokinesis. However, because KN traffic to the division plane occurs by default, it is not clear at present whether KN traffic is affected directly or indirectly by impairment of AP-1.
Fig. 6.
Impaired KN trafficking to the plane of cell division in ap1m2 mutants. WT and ap1m2 homozygous mutant seedlings (ap1m2-1 and hap13) were fixed and immunostained with anti-KN (green) and anti-tubulin (red) antisera. Note that KN does not traffic to the plane of cell division marked by the phragmoplast microtubules in the ap1m2 mutants, in contrast to WT. Blue, DAPI-stained chromatin. (Scale bars: 5 μm.)
Discussion
Because of the vast evolutionary distance between animals and plants, as well as the lack of experimental studies in plants, the phylogenetic relationship between plant and animal μ-adaptins was uncertain (20). Our data now provide evidence that of the five types of μ-adaptins in Arabidopsis, AP1M2 and its functionally redundant variant AP1M1 represent the medium subunit of the AP-1 complex. Moreover, our functional data indicate that AP1M2 plays an essential role in multiple post-Golgi trafficking pathways leading from the TGN to the vacuole, the plasma membrane, and the cell-division plane. Thus, the role of AP-1 appears to be similarly complex in plants as in other organisms. In vacuolar trafficking, the role of Arabidopsis AP-1 resembles that of animal AP-1 in lysosomal/vacuolar trafficking (4). In contrast, loss of Apm1p, a medium adaptin of a yeast AP-1, causes a defect in vacuolar fusion but not in vacuolar transport of CPY (7). However, the role of AP1M2 in exocytosis of invertase:GFP and secGFP to the apoplast in plant cells differs from that in animal cells but is similar to that in fission yeast (7). In animal cells, AP-1 complexes are also involved in trafficking of membrane proteins to the plasma membrane [e.g., in polar trafficking to the basolateral plasma membrane from the TGN or common recycling endosomes in epithelial cells (6) and cilia of C. elegans (5)]. However, AP-1 in plant cells appeared not to play a role in polar trafficking of PIN2 in root cells. Another unique feature of plant AP-1 is its role in the trafficking from the TGN to the plane of cell division, as suggested by the impairment of KN traffic to the division plane. We observed both ultrastructural changes in Golgi stacks and retention of the secretory marker secGFP in the cells. At present, it is unclear whether a secretory defect has a secondary consequence on compartment structure or, conversely, whether the alteration of the Golgi stacks impacts secretory traffic. Nonetheless, these data suggest that AP1M is essential, directly or indirectly, for secretory traffic.
In summary, our data suggest a role for AP-1 in post-Golgi trafficking from the TGN to the vacuole and apoplast in interphase and to the plane of cell division in cytokinesis. These functions are essential, as evidenced by the growth and cell division defects of ap1m2 mutants seedlings. Whereas the role of AP-1 in secretory and vacuolar/lysosomal trafficking appears evolutionarily conserved in all eukaryotes, its role in cytokinesis appears to be shared only between specific organisms such as plants and fission yeast. This might reflect that secretory traffic makes the predominant contribution to cytokinesis in plants and possibly also in fission yeast [“multiple septation” phenotype of apm1 mutant (7)], whereas endosomal recycling appears to play a more prominent role in animal cytokinesis (43, 44).
Plant cells have two variants of AP-1 complexes that contain different AP1M isoforms and are seemingly functionally redundant. However, we cannot rule out completely that AP1M1 and AP1M2 function in distinct pathways (e.g., in relation to environmental clues or specialized developmental processes).
Materials and Methods
Plant Material.
The ap1m2-1 T-DNA insertional mutant line [FLAG_293C11, Wassilewskija (Ws)] was purchased from the Versailles Arabidopsis Stock Center (http://dbsgap.versailles.inra.fr). See SI Materials and Methods for details.
Anti–γ-Adaptin Antibody Generation.
To generate anti–γ-adaptin antibody, recombinant MBP:γ-adaptin was used to immunize rabbits and antibody was affinity-purified using recombinant proteins. See SI Materials and Methods for details.
Molecular biology (including primer sequences, Table S3), genetic analysis, transient expression and in vivo targeting of reporter cargo proteins, western blot analysis and coimmunoprecipitation, fractionation, pull-down analysis, and semiquantitative RT-PCR analysis were performed as described in SI Materials and Methods.
See SI Materials and Methods for details on immunohistochemistry, chemical treatment, ultrastructure analysis, and software analysis.
Supplementary Material
Acknowledgments
We thank Farid El Kasmi and Marc Werner for generating the AP1M1:3×myc clone, Mark A. Johnson for the hapless13 mutant, and Natasha Raikhel for anti-SYP61 antiserum. This work was supported by the Advanced Biomass Research and Development Center, funded by Ministry of Education, Science and Technology Grant ABC-2012055057, and Ministry of Food, Agriculture, Forestry and Fisheries, Korea Grant 609004–05–4–SB240 (to K.S., H.K., and I.H.) and Deutsche Forschungsgemeinschaft Grant Ju179/15-1 (to M.P., I.R., Y.-D.S., U.M., and G.J.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1300460110/-/DCSupplemental.
References
- 1.Boehm M, Bonifacino JS. Adaptins: The final recount. Mol Biol Cell. 2001;12(10):2907–2920. doi: 10.1091/mbc.12.10.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glyvuk N, et al. AP-1/sigma1B-adaptin mediates endosomal synaptic vesicle recycling, learning and memory. EMBO J. 2010;29(8):1318–1330. doi: 10.1038/emboj.2010.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stepp JD, Pellicena-Palle A, Hamilton S, Kirchhausen T, Lemmon SK. A late Golgi sorting function for Saccharomyces cerevisiae Apm1p, but not for Apm2p, a second yeast clathrin AP medium chain-related protein. Mol Biol Cell. 1995;6(1):41–58. doi: 10.1091/mbc.6.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klumperman J, Kuliawat R, Griffith JM, Geuze HJ, Arvan P. Mannose 6-phosphate receptors are sorted from immature secretory granules via adaptor protein AP-1, clathrin, and syntaxin 6-positive vesicles. J Cell Biol. 1998;141(2):359–371. doi: 10.1083/jcb.141.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dwyer ND, Adler CE, Crump JG, L’Etoile ND, Bargmann CI. Polarized dendritic transport and the AP-1 mu1 clathrin adaptor UNC-101 localize odorant receptors to olfactory cilia. Neuron. 2001;31(2):277–287. doi: 10.1016/s0896-6273(01)00361-0. [DOI] [PubMed] [Google Scholar]
- 6.Fölsch H, Ohno H, Bonifacino JS, Mellman I. A novel clathrin adaptor complex mediates basolateral targeting in polarized epithelial cells. Cell. 1999;99(2):189–198. doi: 10.1016/s0092-8674(00)81650-5. [DOI] [PubMed] [Google Scholar]
- 7.Kita A, et al. Loss of Apm1, the micro1 subunit of the clathrin-associated adaptor-protein-1 complex, causes distinct phenotypes and synthetic lethality with calcineurin deletion in fission yeast. Mol Biol Cell. 2004;15(6):2920–2931. doi: 10.1091/mbc.E03-09-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyer C, et al. mu1A-adaptin-deficient mice: Lethality, loss of AP-1 binding and rerouting of mannose 6-phosphate receptors. EMBO J. 2000;19(10):2193–2203. doi: 10.1093/emboj/19.10.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aguilar RC, Ohno H, Roche KW, Bonifacino JS. Functional domain mapping of the clathrin-associated adaptor medium chains mu1 and mu2. J Biol Chem. 1997;272(43):27160–27166. doi: 10.1074/jbc.272.43.27160. [DOI] [PubMed] [Google Scholar]
- 10.Owen DJ, Evans PR. A structural explanation for the recognition of tyrosine-based endocytotic signals. Science. 1998;282(5392):1327–1332. doi: 10.1126/science.282.5392.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Motley A, Bright NA, Seaman MN, Robinson MS. Clathrin-mediated endocytosis in AP-2-depleted cells. J Cell Biol. 2003;162(5):909–918. doi: 10.1083/jcb.200305145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang F, Khvorova A, Marshall W, Sorkin A. Analysis of clathrin-mediated endocytosis of epidermal growth factor receptor by RNA interference. J Biol Chem. 2004;279(16):16657–16661. doi: 10.1074/jbc.C400046200. [DOI] [PubMed] [Google Scholar]
- 13.Zizioli D, et al. Early embryonic death of mice deficient in gamma-adaptin. J Biol Chem. 1999;274(9):5385–5390. doi: 10.1074/jbc.274.9.5385. [DOI] [PubMed] [Google Scholar]
- 14.Cowles CR, Odorizzi G, Payne GS, Emr SD. The AP-3 adaptor complex is essential for cargo-selective transport to the yeast vacuole. Cell. 1997;91(1):109–118. doi: 10.1016/s0092-8674(01)80013-1. [DOI] [PubMed] [Google Scholar]
- 15.Mullins C, Hartnell LM, Bonifacino JS. Distinct requirements for the AP-3 adaptor complex in pigment granule and synaptic vesicle biogenesis in Drosophila melanogaster. Mol Gen Genet. 2000;263(6):1003–1014. doi: 10.1007/pl00008688. [DOI] [PubMed] [Google Scholar]
- 16.Simmen T, Höning S, Icking A, Tikkanen R, Hunziker W. AP-4 binds basolateral signals and participates in basolateral sorting in epithelial MDCK cells. Nat Cell Biol. 2002;4(2):154–159. doi: 10.1038/ncb745. [DOI] [PubMed] [Google Scholar]
- 17.Hirst J, et al. The fifth adaptor protein complex. PLoS Biol. 2011;9(10):e1001170. doi: 10.1371/journal.pbio.1001170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dettmer J, Hong-Hermesdorf A, Stierhof YD, Schumacher K. Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis. Plant Cell. 2006;18(3):715–730. doi: 10.1105/tpc.105.037978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park M, Jürgens G. Membrane traffic and fusion at post-Golgi compartments. Front Plant Sci. 2011;2:111. doi: 10.3389/fpls.2011.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Happel N, et al. Arabidopsis mu A-adaptin interacts with the tyrosine motif of the vacuolar sorting receptor VSR-PS1. Plant J. 2004;37(5):678–693. doi: 10.1111/j.1365-313x.2003.01995.x. [DOI] [PubMed] [Google Scholar]
- 21.Feraru E, et al. The AP-3 β adaptin mediates the biogenesis and function of lytic vacuoles in Arabidopsis. Plant Cell. 2010;22(8):2812–2824. doi: 10.1105/tpc.110.075424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zwiewka M, et al. The AP-3 adaptor complex is required for vacuolar function in Arabidopsis. Cell Res. 2011;21(12):1711–1722. doi: 10.1038/cr.2011.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolfenstetter S, Wirsching P, Dotzauer D, Schneider S, Sauer N. Routes to the tonoplast: The sorting of tonoplast transporters in Arabidopsis mesophyll protoplasts. Plant Cell. 2012;24(1):215–232. doi: 10.1105/tpc.111.090415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niihama M, Takemoto N, Hashiguchi Y, Tasaka M, Morita MT. ZIP genes encode proteins involved in membrane trafficking of the TGN-PVC/vacuoles. Plant Cell Physiol. 2009;50(12):2057–2068. doi: 10.1093/pcp/pcp137. [DOI] [PubMed] [Google Scholar]
- 25.Sanderfoot AA, Kovaleva V, Bassham DC, Raikhel NV. Interactions between syntaxins identify at least five SNARE complexes within the Golgi/prevacuolar system of the Arabidopsis cell. Mol Biol Cell. 2001;12(12):3733–3743. doi: 10.1091/mbc.12.12.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson MA, et al. Arabidopsis hapless mutations define essential gametophytic functions. Genetics. 2004;168(2):971–982. doi: 10.1534/genetics.104.029447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nesterov A, Carter RE, Sorkina T, Gill GN, Sorkin A. Inhibition of the receptor-binding function of clathrin adaptor protein AP-2 by dominant-negative mutant mu2 subunit and its effects on endocytosis. EMBO J. 1999;18(9):2489–2499. doi: 10.1093/emboj/18.9.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verdaguer B, de Kochko A, Beachy RN, Fauquet C. Isolation and expression in transgenic tobacco and rice plants, of the cassava vein mosaic virus (CVMV) promoter. Plant Mol Biol. 1996;31(6):1129–1139. doi: 10.1007/BF00040830. [DOI] [PubMed] [Google Scholar]
- 29.French AP, Mills S, Swarup R, Bennett MJ, Pridmore TP. Colocalization of fluorescent markers in confocal microscope images of plant cells. Nat Protoc. 2008;3(4):619–628. doi: 10.1038/nprot.2008.31. [DOI] [PubMed] [Google Scholar]
- 30.Stierhof YD, El Kasmi F. Strategies to improve the antigenicity, ultrastructure preservation and visibility of trafficking compartments in Arabidopsis tissue. Eur J Cell Biol. 2010;89(2–3):285–297. doi: 10.1016/j.ejcb.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 31.Geldner N, et al. The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth. Cell. 2003;112(2):219–230. doi: 10.1016/s0092-8674(03)00003-5. [DOI] [PubMed] [Google Scholar]
- 32.Lee GJ, Sohn EJ, Lee MH, Hwang I. The Arabidopsis rab5 homologs rha1 and ara7 localize to the prevacuolar compartment. Plant Cell Physiol. 2004;45(9):1211–1220. doi: 10.1093/pcp/pch142. [DOI] [PubMed] [Google Scholar]
- 33.Tse YC, et al. Identification of multivesicular bodies as prevacuolar compartments in Nicotiana tabacum BY-2 cells. Plant Cell. 2004;16(3):672–693. doi: 10.1105/tpc.019703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song K, et al. An A/ENTH domain-containing protein functions as an adaptor for clathrin-coated vesicles on the growing cell plate in Arabidopsis root cells. Plant Physiol. 2012;159(3):1013–1025. doi: 10.1104/pp.112.199380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee GJ, et al. EpsinR2 interacts with clathrin, adaptor protein-3, AtVTI12, and phosphatidylinositol-3-phosphate. Implications for EpsinR2 function in protein trafficking in plant cells. Plant Physiol. 2007;143(4):1561–1575. doi: 10.1104/pp.106.095349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim H, Park M, Kim SJ, Hwang I. Actin filaments play a critical role in vacuolar trafficking at the Golgi complex in plant cells. Plant Cell. 2005;17(3):888–902. doi: 10.1105/tpc.104.028829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jung C, et al. Identification of sorting motifs of AtβFruct4 for trafficking from the ER to the vacuole through the Golgi and PVC. Traffic. 2011;12(12):1774–1792. doi: 10.1111/j.1600-0854.2011.01276.x. [DOI] [PubMed] [Google Scholar]
- 38.Batoko H, Zheng HQ, Hawes C, Moore I. A rab1 GTPase is required for transport between the endoplasmic reticulum and golgi apparatus and for normal golgi movement in plants. Plant Cell. 2000;12(11):2201–2218. doi: 10.1105/tpc.12.11.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahmed SU, et al. The plant vacuolar sorting receptor AtELP is involved in transport of NH(2)-terminal propeptide-containing vacuolar proteins in Arabidopsis thaliana. J Cell Biol. 2000;149(7):1335–1344. doi: 10.1083/jcb.149.7.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Niemes S, et al. Sorting of plant vacuolar proteins is initiated in the ER. Plant J. 2010;62(4):601–614. doi: 10.1111/j.1365-313X.2010.04171.x. [DOI] [PubMed] [Google Scholar]
- 41.Robinson DG, Albrecht S, Moriysu Y. The V-ATPase inhibitors concanamycin A and bafilomycin A lead to Golgi swelling in tobacco BY-2 cells. Protoplasma. 2004;224(3-4):255–260. doi: 10.1007/s00709-004-0070-6. [DOI] [PubMed] [Google Scholar]
- 42.Abas L, et al. Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nat Cell Biol. 2006;8(3):249–256. doi: 10.1038/ncb1369. [DOI] [PubMed] [Google Scholar]
- 43.Reichardt I, et al. Plant cytokinesis requires de novo secretory trafficking but not endocytosis. Curr Biol. 2007;17(23):2047–2053. doi: 10.1016/j.cub.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 44.Albertson R, Riggs B, Sullivan W. Membrane traffic: A driving force in cytokinesis. Trends Cell Biol. 2005;15(2):92–101. doi: 10.1016/j.tcb.2004.12.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.