Abstract
Gap junctions are clusters of aqueous channels that connect the cytoplasm of adjoining cells. Each cell contributes a hemichannel, or connexon, to each cell–cell channel. The cell–cell channels are permeable to relatively large molecules, and it was thought that opening of hemichannels to the extracellular space would kill cells through loss of metabolites, collapse of ionic gradients and influx of Ca2+. Recent findings indicate that specific non-junctional hemichannels do open under both physiological and pathological conditions, and that opening is functional or deleterious depending on the situation. Most of these studies utilized cells in tissue culture that expressed a specific gap junction protein, connexin 43. Several such examples are reviewed here, with a particular focus on astrocytes.
Gap junctions mediate intercellular communication by providing ultrastructural cytoplasmic continuity, and they are integral to formation of the functional syncytium of astrocytes. Each of the joined cells contributes a hemichannel or connexon to each cell–cell channel (Figure 1; Box 1). Each hemichannel comprises a hexamer of connexins arranged around a central pore, and the cell–cell channels are gated by several stimuli, including transjunctional voltage, low pH and various pharmacological agents. Connexins are encoded by a gene family with at least 20 members in mammals [1].
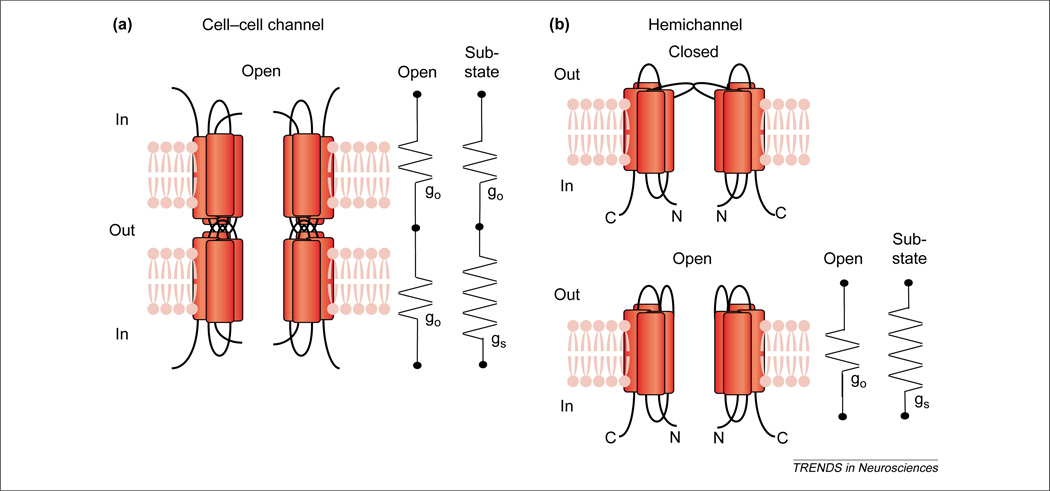
Figure 1.
Diagrams and equivalent circuits of cell–cell channels and hemichannels. (a) In the cell–cell channel, the conductances of the two hemichannels are in series; the circuits show both gates open at the fully open or main-state conductance, go (left) and one gate open at go and the other closed to the substate conductance, gs (right). (b) For the hemichannel, a single element represents each state: an open-state conductance (go) and a substate conductance (gs). Table 1 shows that the cell–cell open-state conductance is about half that of the hemichannel open state, consistent with series arrangement in the cell–cell channel. By contrast, the substate conductance of the hemichannel is larger than predicted from the cell–cell channel (and the substate conductance of the cell–cell channel is smaller than predicted from the hemichannel, except in Ref. [44]).
Box 1. Gap junctions – an overview.
Gap junctions provide high-conductance, high-permeability pathways between cells, and mediate electrical coupling and exchange of small molecules.
Gap-junction channels comprise two hemichannels or connexons, one contributed by each of the participating cells.
A hemichannel is generally closed before docking with another hemichannel; both hemichannels then open in a ligand-gating reaction where the ligand and gate are the same molecules.
Recent data indicate that unapposed, non-junctional hemichannels can open under some conditions.
Gap-junction pores are nominally 1.0–1.5 nm in diameter and are permeable to molecules of ~1 kDa [2]. Junctions formed from connexin 43 (Cx43), the predominant connexin in astrocytes, are permeable to Lucifer Yellow (443 Da, −2 charge) and propidium (420 Da, +2 charge). Other connexins appear to be more charge selective: Cx32 is more permeable to anions and Cx45 is more permeable to cations [2]. Single-channel conductance varies widely, from ~15 pS for Cx36 [3] and 110 pS for Cx43 [4] to ~375 pS for Cx37 [5]. The maximum size of permeant species also varies. Such diversity in selectivity and conductance are likely to account for the expansion of the connexin family.
Why shouldn’t hemichannels be open?
Given the permeability and conductance of gap junctions, and the expectation that an open hemichannel would have twice the conductance and permeability of a cell–cell channel (in terms of ion or molecular flux, rather than selectivity), it was thought unlikely that hemichannels in the non-junctional membrane would open [6]. Direct evidence for this was provided by measurements during formation of the first channel between a newly apposed pair of cells. Each cell was held at a different potential, and then when the first channel opened, increased current was seen in the cell held at a more positive potential and decreased (more negative) current was seen in the other cell, but the sum of the currents clamping the two cells remained constant [7]. Thus, there was no change in the current flowing into the extracellular space, and the hemichannels were closed before opening to form a cell– cell channel. When expression of Cx46 in Xenopus oocytes was found to be fatal and to cause a large non-specific increase in membrane conductance in low Ca2+ conditions, it seemed to be a rule that proved the exception: opening of (non-junctional) hemichannels is bad for cells, and something unusual about Cx46 expressed in oocytes allows hemichannel opening unless relatively high Ca2+ concentrations are added to prevent it [8,9].
So are these really hemichannel currents?
The evidence for open hemichannels can be briefly summarized (and criticized). Macroscopically, the existence of hemichannels was initially inferred from a non-selective conductance that depended on exogenous expression of Cx46 in Xenopus oocytes. Now, there are many other examples in which cells expressing connexins endogenously or by transfection develop permeability or macroscopic currents consistent with expression of hemichannels [10–14]. Conversely, cells from connexin knockout animals do not show these properties, even though wild-type cells do [12,14]. (However, connexin transfection can induce changes in the expression of many other genes [15], and connexins have binding partners and might transport other channel molecules to the cell surface [16–19].) The permeability and/or currents are inhibited by gap-junction blockers [10–14] (although most of the blockers are of dubious specificity). The pathway is permeable to larger molecules than is the ‘normal’ surface membrane, as are gap-junction channels [10–14] (but there are P2X7 receptors and mechanosensitive channels that let through larger molecules, including several of the gap-junction-permeant species [20,21]). The volume of this evidence is substantial, but it is still possible to doubt the existence of hemichannels.
Data from single channels resolves the debate. Cells transfected with connexins exhibit channels that are about twice the conductance of the cell–cell channels, as predicted from simple series arrangement of the two hemichannels [22] (Figure 1; Table 1). The channels are absent in the parental cells. The altered gene expression and transport-related explanations of putative hemichannel currents are made much less likely, because the single hemichannel conductance is consistent with the cell–cell channel conductance. The channels exhibit a substate identical to that induced in cell–cell channels by transjunctional voltage, Vj. (Although, as will be discussed, the substate conductances in hemichannels and cell–cell channels are inconsistent; the deviation is similar for five different connexins.) The hemichannels have fastgating transitions between the fully open state and the substate, and slow-gating transitions between the closed state and either the fully open state or substate, as is observed with cell–cell channels [22]. The hemichannels exhibit pharmacology like that of the cell–cell channels. The differences between hemichannels and cell–cell channels will be discussed in a later section, but the inescapable conclusion from the electrophysiological data is that hemichannels can open (at least, in the opinion of an electrophysiologist).
Table 1.
Comparison of main and substate conductances of cell–cell channels and hemichannels (pS)a
| Connexin | Cell–cell: open | Substate observed (calculated) | Refs | Hemichannel: open | Substate observed (calculated) | Refs |
|---|---|---|---|---|---|---|
| Cx30 | 146 | 25 (41) | [43] | 283 | 48 (30) | [44] |
| Cx43 | 115 | 30 (56) | [4] | 220 | 75 (40) | [22] |
| Cx45 | 30 | 4 (12) | [47] | 62 | 15 (4.6) | [46] |
| Cx46 | 150 | 28 (75) | [45] | 300 | 100 (33) | [45] |
| (32) | 250 | 37 (34) | [44] | |||
| Cx50 | 203 | 33 (60) | [44] | 357 | 77 (39) | [44] |
Substate conductances usually do not follow from simple series arrangement of hemichannels in cell–cell channels (Figure 1) (access resistance is neglected).
Where are hemichannels formed, and are they precursors of cell–cell channels?
Early studies of connexin biogenesis and trafficking indicated that Cx43 monomers were synthesized in the endoplasmic reticulum (ER), assembled into hemichannels in a post-Golgi compartment and transported to the cell surface before they became incorporated into junctional plaques [23]. Recent data indicate that Cx32 oligomerizes in the ER before reaching the trans-Golgi [24]. (No connexins are known to be glycosylated, and there are no consensus sequences for glycosylation in the extracellular domains of the connexins that have been examined in this regard; thus, one Golgi function is not required for connexin assembly [25,26].) Cx26 can be inserted into membranes post-translationally as well as co-translationally, and might never be found in the ER [27,28]. Brefeldin A, which disrupts the Golgi apparatus, prevents the trafficking of Cx32 and Cx43; however, Cx26 still reaches the cell surface.
Now, refined methods of labeling indicate that new channels are transported to the surface in an undirected manner and reach existing junctions by lateral diffusion, where they form new channels at their periphery [29,30]; channels being retired are removed from the interior of the junction [29]. This picture does not address the question of the initial formation of junctions [7]. Remarkably, gap junctions are internalized, piecemeal or in their entirety, with both junctional faces entering one of the cells. This mode of turnover requires the rupture and resealing of two membranes with no significant loss of cytoplasm. Moreover, a small amount of cytoplasm is transferred from one cell to the other (although this is membrane-bounded and presumably destroyed in lysosomes together with the junctions). The life cycle of hemichannels therefore involves a period when they are non-junctional and not opposed by another hemichannel.
What opens hemichannels?
Fibroblasts in vitro appear to have a few hemichannels open under basal conditions [31]. HeLa cells transfected with Cx43 show basal uptake of gap-junction-permeant molecules that is sensitive to pharmacological agents that block gap junctions [22]. However, calculations indicate that the observed uptake requires very little channel opening – a single channel per cell with a very low open probability would be sufficient. One can argue from these data that a cell can tolerate some hemichannel opening, but not very much. In several tissues, depolarization or polarization to positive potentials, particularly in low Ca2+ solutions, increases hemichannel opening measured by single-channel recording or tracer uptake. Low Ca2+ levels alone appear to cause hemichannel opening in astrocytes. One study indicates that significant opening can occur in astrocytes at low but not unreasonable external Ca2+ concentrations [32], whereas another study sees little effect at nominally zero Ca2+ [14]; the differences in these studies might arise from differences in culture conditions. Metabolic inhibition and ischemia can increase hemichannel opening. In cultured astrocytes, hemichannel opening induced by metabolic inhibition (measured by dye uptake) is reduced by nor-dihydroguaiaretic acid (NDGA), an inhibitor of lipoxygenase, suggesting control by a metabolite of arachidonic acid [14]. Although Cx43 is largely dephosphorylated by the time metabolic inhibition is inducing hemichannel opening, cell–cell coupling is not abolished, and dephosphorylation might not play a role in hemichannel opening. Protein kinase C (PKC)-mediated phosphorylation reduces hemichannel permeability to NAD+ [33]. Cx43 hemichannels are opened by alendronate, a bisphosphonate that inhibits dexamethasoneinduced apoptosis and is used to promote survival of osteoblasts in the prevention of osteoporosis [12]. (Opening was detected by dye uptake, which was prevented by gap-junction blockers.)
Hemichannels in teleost and elasmobranch horizontal cells are opened by quinine and by depolarization in low-Ca2+ solution; dopamine-receptor and cAMP agonists reduce hemichannel and cell–cell channel conductance in these systems [34–38]. The connexins in these cases are paralogs of the (largely) neuron-specific mammalian connexin Cx36. Hemichannels in horizontal cells of carp are implicated in an electrical feedback mechanism that acts on release of glutamate from the receptor cell [39]. The putative connexin is a paralog of mammalian Cx26, which is not known to form functional hemichannels and is not expressed in mammalian horizontal cells [40]. If the same mechanism is present in mammalian retina, it is likely to involve a different connexin.
A somewhat controversial gating mechanism is mechanical stimulation [13,41]: increased connexin expression and low Ca2+ solution increase sensitivity but the specificity of this is not well established.
Are cell–cell channels just two hemichannels in series?
For connexins that form functional hemichannels, one can ask how the hemichannel conductance relates to the cell–cell channel conductance. In the five connexins for which such data are available, the open hemichannel conductance is close to twice the open cell–cell channel conductance, as would follow from simple series connection [4,22,42–47] (Table 1, Figure 1). The same conclusion has been reached for several connexins that form heterotypic junctions: the heterotypic channel conductance was the series sum of the inferred hemichannel conductances (twice the single channel conductance of the homotypic junctions [47–49]). Moreover, in Cx43–Cx45 junctions, the single channel conductance was as predicted, but the sensitivity of the two hemichannels to Vj was also altered, consistent with a greater or lesser fraction of Vj falling across the lower-conductance and higher-conductance hemichannels, respectively [47].
It is typical of gap-junction channels to have a main or fully open state and a substate with a conductance of 10–30% of the main state; hemichannels exhibit similar main states and substates. In cell–cell channels, transitions between these states are induced by application of different magnitudes of Vj. Transitions between the fully open state and substate are rapid (<1 ms) and unresolved. However, the rate constants tend to be long (tenths of seconds) compared with those of excitable membrane channels. Voltage sensitivities depend greatly on the connexin, and the most sensitive are comparable in this parameter with the voltage-sensitive channels of excitable membranes [2]. The substates of cell–cell channels and hemichannels generally differ consistently from the prediction of simple series arrangement in cell–cell channels (Table 1). When the substate conductance of the cell–cell channel is calculated as the series sum of the open-state and substate conductances of the hemichannel, the result is too large by a factor of about two, although one published value for Cx46 does match the prediction [44]. Note that one side of a cell–cell channel is closed to the substate by one polarity of Vj, which tends to open the Vj gate on the other side; however, closing of both Vj gates at the same time must be a very rare event. (Calculating in the opposite direction, from the substate conductance of the cell– cell channel, which is a hemichannel in the substate in series with a hemichannel in the open state, generally produces a hemichannel substate conductance that is too small by a factor of about two.)
How can we explain these data? Not without handwaving. Most simply, the extracellular region of the hemichannel when it is at the substate conductance could have a very different configuration to that of the same region in the cell–cell channel. Vj or fast gating to the substate in cell–cell channels is thought to involve the cytoplasmic end of the channel, and charges implicated in the voltage sensor are located there; however, other charged residues affecting gating are found at the beginning of the first extracellular loop [45,50]. Although not yet adequately characterized, there are differences in singlechannel rectification in hemichannels. An open hemichannel could well exhibit asymmetry in fixed charges that causes rectification, whereas the asymmetry is neutralized in the cell–cell channel [45,51].
There are, at most, only small differences in permeability between hemichannels and cell–cell channels. Part of the evidence for opening of hemichannels is permeation of the surface membrane by relatively large molecules to which gap junctions, but not the surface membranes, are permeable. In quantifying permeability, one needs to know the number of open channels, and (for cell–cell channels) junctional conductance, which is essentially a measure of K+ permeability and allows comparison between cells expressing the same connexin. As a first approximation, permeability normalized to conductance should be the same for cell–cell channels and hemichannels – the hemichannel has twice the conductance and should have twice the permeability (in so far as series hemichannels make up cell–cell channels without change in properties).
Hemichannel open probability is small
Although there is no direct measure of the number of hemichannels inserted in the membrane using connexins labeled with enhanced green-fluorescent protein (eGFP) [22], reaction with antibodies to the extracellular loop indicates substantial and readily visualized accessibility of extracellular epitopes of Cx43 hemichannels in astrocytes [52]. Thus, the observations that only a few hemichannels open in Cx43-expressing cells indicate that open probability, Po, is very low. This conclusion holds, even in comparison with the perhaps surprisingly small fraction of gap-junction channels that are open: ~0.1 for Cx43 gap junctions [53]. Moreover, opening of cell–cell channels is not observed in plaques containing up to several hundred channels (i.e. Po = 0). This result suggests that cooperative interactions are necessary for cell–cell channel opening. A requirement for cooperativity could also explain the low open probability of hemichannels: a threshold concentration of hemichannels might be necessary for opening, which would be reached only infrequently in the random diffusion of hemichannels in the surface membrane [54]. The single-channel recordings suggest that when a hemichannel is opening, it is not that different from cell–cell channels in terms of open time and stability in the membrane.
An extracellular signal released through hemichannels
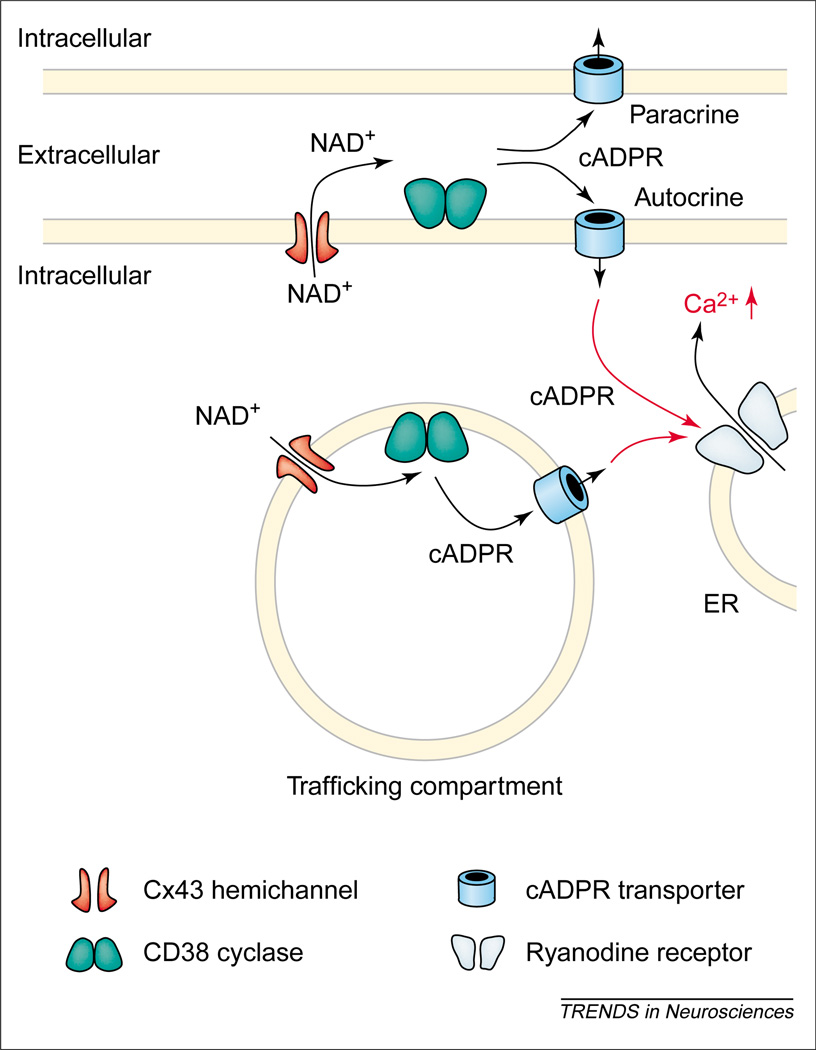
Cyclic ADP-ribose (cADPR) is a signaling molecule that causes release of Ca2+ from intracellular stores. Surprisingly, it is synthesized by the ectoenzyme CD38, an integral membrane protein with an extracellular active site that cyclizes NAD+ to form cADPR (Figure 2). cADPR, which does not permeate Cx43 hemichannels [31,33], reaches its site of action on ryanodine receptors by both active and passive transport across the surface membrane [55]. It can also have a paracrine action, which might be why an ectoenzyme is used. The precursor NAD+ is released from the cytoplasm into the medium through Cx43 hemichannels, as indicated by: (i) dependence on level of Cx43 expression in transfected or antisensetreated cells; (ii) sensitivity to gap-junction blockers; and (iii) influx into and efflux from liposomes incorporating isolated Cx43. cADPR is also synthesized in intracellular vesicles transporting CD38 to or from the surface; NAD+ enters the vesicles through Cx43 hemichannels and cADPR moves to the cytoplasm by action of the same nucleotide transporters that operate in the surface membrane [33,55]. This pathway of synthesis would prevent loss of NAD+ and cADPR by diffusion away from an ectoenzyme, but also prevent paracrine action. A negative-feedback loop exists in which rises in intracellular Ca2+ concentration activate PKC, which phosphorylates Cx43, thereby reducing permeability of or blocking hemichannels. A proposed mechanism is that access to substrate for vesicular or extracellular CD38 is regulated negatively by intracellular Ca2+, thus preventing excessive loss of NAD+, rise of cADPR, emptying of intracellular Ca2+ stores and possible cytotoxicity.
Figure 2.
Connexin 43 (Cx43) hemichannels mediate cyclic-ADP-ribose (cADPR) signaling by allowing outward passage of NAD+. Cells, including astrocytes, express CD38, an ectoenzyme that cyclizes NAD+ to form cADPR. cADPR then has to cross the surface membrane to reach ryanodine receptors on the endoplasmic reticulum (ER) to trigger release of Ca2+ into the cytoplasm; this action can be autocrine or paracrine. A pathway using intracellular vesicles can largely restrict action to the single cell.
CD38 is expressed by astrocytes, which in culture respond to extracellular cADPR with elevations in Ca2+ levels [56]. Rises in intracellular Ca2+ concentration in astrocytes can cause glutamate release and rises in Ca2+ levels in nearby neurons. The rises in neurons are not completely blocked by glutamate-receptor antagonists, indicating the presence of other mediators, which could include cADPR. This pathway of glial–neuronal signaling might be important in the normal operation of the nervous system [57].
What about Ca2+ waves?
Rises in intracellular Ca2+ concentration that spread in an epithelium [58] or a monolayer of cells in culture are termed Ca2+ waves. In general, the waves proceed in all directions from a stimulated cell and are limited in extent. It is arguable that they spread passively without regeneration (although, as will be discussed, this point is controversial and probably not always true). Ca2+ waves are observed in many cell types, including in cultured astrocytes [59–60], in white matter slices, where mediation is glial [61], and in gray matter, where neurons are involved and mediation might be more complex [62–64].
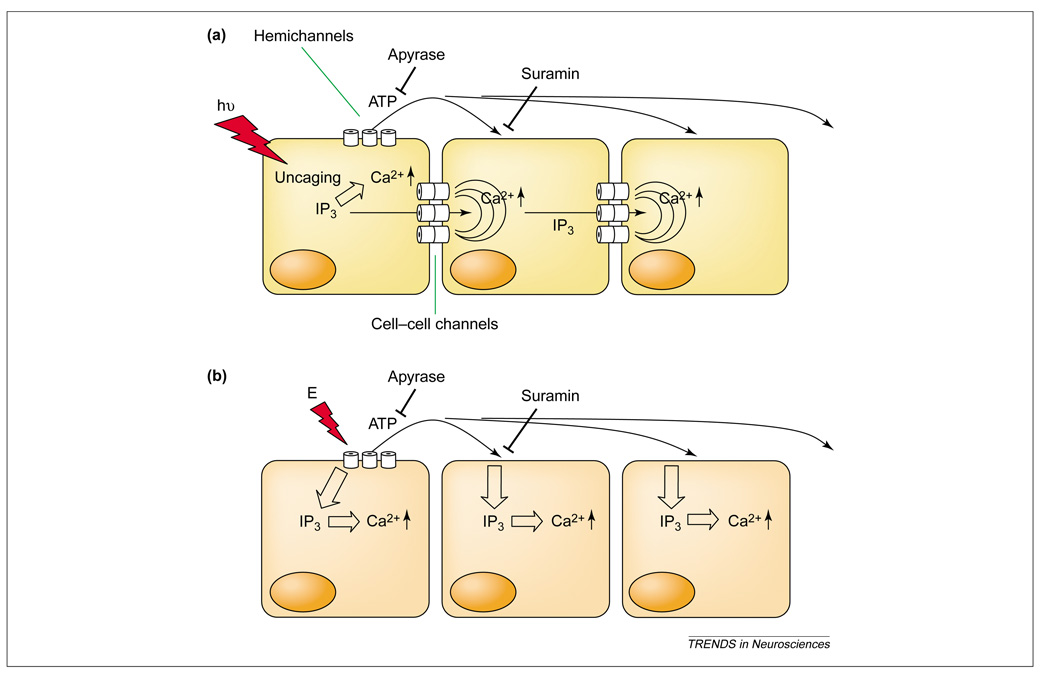
In the initial descriptions of Ca2+ waves, gap junctions were implicated in spread of inositol (1,4,5)-trisphosphate [Ins(1,4,5)P3] generated in a stimulated cell to neighboring cells, where it caused release of Ca2+ from intracellular stores [58] (Figure 3a). (Detection of Ins(1,4,5)P3 would be non-linear because of Ca2+-evoked Ca2+ release.) This explanation of Ca2+ waves was challenged by observations that Ca2+ waves could cross cell-free lanes and thus were mediated by an extracellular signal [60,65] (Figure 3b). Unfortunately, gap-junction blockers are ambiguous in distinguishing the two pathways, as hemichannels might be the source of extracellular signals (of which ATP is certainly one), and coupling between cells might contribute to the extracellular signal by allowing diffusion of the signal from neighboring cells into the releasing cell. After doubts were raised about gap-junction mediation, the situation was clarified by experiments showing that both mechanisms of wave propagation could be seen in the same cells, in this case HeLa cells transfected (or not) with eGFP-labeled connexins [66]. Where connexin hemichannels mediate ATP release, dependence of waves on connexin expression holds for both mechanisms. Waves mediated by an extracellular signal could be blocked by apyrase (which hydrolyzes ATP) and purine-receptor antagonists (e.g. suramin), and altered in extent by perfusion of medium across a monolayer [65] (Figure 3). Waves mediated by an intracellular signal required connexin expression and were not blocked by extracellular apyrase or purine-receptor antagonists. Moreover, the somewhat problematic initiation by mechanical stimulation was replaced by photorelease of Ins(1,4,5)P3, which is repeatable and non-damaging [66]. Rises in Ca2+ levels were seen to spread through a cell and then to initiate rises in an adjoining cell at an eGFP-tagged gap junction localized by its fluorescence. During extracellularly mediated waves, Ca2+ levels first rose at the ER owing to the action of an intracellular second messenger activated by extracellular ATP. To make the parallel system a little more complicated, intracellular Ins(1,4,5)P3 can induce release of ATP by a mechanism inhibited by a bath-applied peptide with the sequence of an extracellular region of Cx43, an agent likely to be a highly specific blocker of hemichannels [67]. These data suggest that ATP can induce ATP release, which would confer regenerative properties on Ca2+ waves. The extent of Ca2+ propagation in slices of corpus callosum is also indicative of regenerative propagation [61]. The possibility of regenerative release of ATP with the prospect of indefinitely propagating waves was raised early in the investigation of the extracellular pathway [68]. However, it was contraindicated by Arcuino et al. [60], who saw no difference in extent between a finite wave propagating across a cell-free area and one propagating across confluent cells. Thus, the presence of active propagation depends on cell type and specific conditions.
Figure 3.
Two models for conduction of Ca2+ waves. (a) Ca2+ waves are mediated by diffusion of cytoplasmic inositol (1,4,5)-trisphosphate (IP3) through gap junctions between cells. Evidence for this mechanism includes: (i) the waves are dependent on gap junctions, and Ca2+ increases in a downstream cell begin at junctions; (ii) the waves are not blocked by extracellular apyrase, an ATPase; (iii) the waves are not blocked by purine-receptor antagonists such as suramin; and (iv) the waves do not jump a gap between cells. The red lightning bolt represents a photo-uncaging stimulus (hν). (b) Ca2+ waves are mediated by ATP released through hemichannels. Evidence for this mechanism includes: (i) the waves require connexin expression; (ii) gap junction and hemichannel blockers prevent the waves; (iii) ATP is released by the initiator cell, and the Ca2+ wave extends as far as the ATP diffuses; (iv) the waves are blocked by extracellular apyrase; (v) the waves are blocked by suramin; and (vi) the waves jump cell-free gaps and are deflected by flow of medium. The red lightning bolt represents an electrical stimulus (E) that causes release of ATP by unclear and perhaps nonspecific mechanisms.
Connexin expression and ATP-mediated Ca2+ wave propagation have been correlated in a transformed astrocyte line [13,59]. However, connexin transfection markedly increased basal conductance of isolated cells to a degree that would require opening of many hemichannels (~50); yet the cells were healthy and not depolarized. A subsequent study showed ATP release (visualized by luciferase-mediated light emission) from single cells within a confluent monolayer in zero-Ca2+ solution [60]. ATP release was prevented by gap-junction blockers and the release ‘channel’ was permeable to molecules that would permeate gap junctions. However, the current when the release channel opened was not appropriate for hemichannels; it was too big for one hemichannel and did not appear to consist of multiple openings of Cx43 hemichannels. The data might result from cooperative opening of several hemichannels at the same time and from the rather unpredictable channel behavior sometimes observed with hemichannels under conditions of slow gating [22].
Hemichannels in the CNS
Astrocytes contain millimolar levels of free glutamate in the cytoplasm; they also express hemichannels. Under some culture conditions, low extracellular Ca2+ levels cause release of glutamate from astrocytes, evidently by opening of hemichannels (as evidenced by sensitivity to gap-junction blockers and dye uptake [32]). What is striking about these observations is that significant release was observed at Ca2+ levels that might well occur under conditions of ischemia, seizures or spreading depression. It will be of interest to evaluate Cx43-deficient astrocytes for glutamate release, but the other obvious suspects, reverse operation of the uptake system and P2X7 receptors, have been excluded pharmacologically. A low-Ca2+ solution was shown to induce release of glutamate from optic nerve (which contains astrocytes and axons but no neuronal cell bodies or synaptic terminals). Extending these observations to gray matter is likely to be difficult given all the other possible sources of glutamate.
In astrocytes in culture, lanthanum ions (La3+) applied extracellularly block hemichannels but not gap junctions [14]. Metabolic inhibition causes opening of hemichannels, and La3+ application delays cell death caused by metabolic inhibition; thus, hemichannel opening is deleterious under these conditions. Gap-junctional communication between healthy and unhealthy cells can increase survival of unhealthy cells or death of healthy cells depending on the relative numbers of cells and degree of coupling between the two groups. When the balance of unhealthy to healthy cells is unfavorable for cell survival, gap-junction blockers reduce cell death [69]; where gap junctions are bad, hemichannels are likely to only make things worse.
Concluding remarks
There can be little doubt that opening of gap-junction hemichannels is part of the cell repertoire. Hemichannels do provide a ‘leaky’ pathway, and quantification of permeation and electrical conductance needs to be extended. The predominant connexin in astrocytes is Cx43, but other connexins are expressed in astrocytes and neurons. Where hemichannels are carrying out physiological signaling, their value is clear. As part of a signaling pathway, they fail to discriminate between transmitter and ‘postsynaptic’ target when compared with the classical chemical synapses. When hemichannels open under conditions of metabolic inhibition or low Ca2+ levels, there is less reason to think that the response is a useful one, although limited cell death is not always bad for the organism. Untangling the many different forms of chemical communication in the CNS to identify those mediated by hemichannels will not be simple (Questions for Future Research); additional pharmacological and genetic tools will probably be required.
Questions for Future Research.
What stimuli cause hemichannel opening?
Why is the open probability of hemichannels so low?
Do open hemichannels account for movement across non-junctional surface membranes of gap-junction-permeant species?
Do all connexins form hemichannels that can open?
How important is hemichannel opening in cell physiology?
Acknowledgements
Our research is partially funded by grants from the National Institute for Health (NS45837 to M.V.L.B. and NS36706 to F.F.B.), from the F.M. Kirby Foundation Program in Neuroprotection and Repair, and from Fondo Nacional para el Desarrollo de Ciencia y Tecnología (FONDECYT 1030945 to J.C.S.). M.V.L.B. is the Sylvie and Robert S. Olnick Professor of Neuroscience.
References
- 1.Willecke K, et al. Structural and functional diversity of connexin genes in the mouse and human genome. Biol. Chem. 2002;383:725–737. doi: 10.1515/BC.2002.076. [DOI] [PubMed] [Google Scholar]
- 2.Harris AL. Emerging issues of connexin channels: biophysics fills the gap. Q. Rev. Biophys. 2001;34:325–472. doi: 10.1017/s0033583501003705. [DOI] [PubMed] [Google Scholar]
- 3.Teubner B, et al. Functional expression of the murine connexin 36 gene coding for a neuron-specific gap junctional protein. J. Membr. Biol. 2000;176:249–262. doi: 10.1007/s00232001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bukauskas FF, et al. Gating properties of gap junction channels assembled from connexin 43 and connexin 43 fused with green fluorescent protein. Biophys. J. 2001;81:137–152. doi: 10.1016/S0006-3495(01)75687-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumari SS, et al. Site-directed mutations in the transmembrane domain M3 of human connexin 37 alter channel conductance and gating. Biochem. Biophys. Res. Commun. 2001;280:440–447. doi: 10.1006/bbrc.2000.4121. [DOI] [PubMed] [Google Scholar]
- 6.Bennett MVL, et al. Gap junctions: new tools, new answers, new questions. Neuron. 1991;6:305–320. doi: 10.1016/0896-6273(91)90241-q. [DOI] [PubMed] [Google Scholar]
- 7.Bukauskas FF, et al. Biophysical properties of gap junction channels formed by mouse connexin 40 in induced pairs of transfected human HeLa cells. Biophys. J. 1995;68:2289–2298. doi: 10.1016/S0006-3495(95)80411-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paul DL, et al. Connexin 46, a novel lens gap junction protein, induces voltage-gated currents in nonjunctional plasma membrane of Xenopus oocytes. J. Cell Biol. 1991;115:1077–1089. doi: 10.1083/jcb.115.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ebihara L, Steiner E. Properties of a nonjunctional current expressed from a rat connexin 46 cDNA in Xenopus oocytes. J. Gen. Physiol. 1993;102:59–74. doi: 10.1085/jgp.102.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li H, et al. Properties and regulation of gap junctional hemichannels in the plasma membranes of cultured cells. J. Cell Biol. 1996;134:1019–1030. doi: 10.1083/jcb.134.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.John SA, et al. Connexin-43 hemichannels opened by metabolic inhibition. J. Biol. Chem. 1999;274:236–240. doi: 10.1074/jbc.274.1.236. [DOI] [PubMed] [Google Scholar]
- 12.Plotkin LI, et al. Transduction of cell survival signals by connexin-43 hemichannels. J. Biol. Chem. 2002;277:8648–8657. doi: 10.1074/jbc.M108625200. [DOI] [PubMed] [Google Scholar]
- 13.Stout CE, et al. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J. Biol. Chem. 2002;277:10482–10488. doi: 10.1074/jbc.M109902200. [DOI] [PubMed] [Google Scholar]
- 14.Contreras JE, et al. Metabolic inhibition induces opening of unapposed connexin 43 gap junction hemichannels and reduces gap junctional communication in cortical astrocytes in culture. Proc. Natl. Acad. Sci. U. S. A. 2002;99:495–500. doi: 10.1073/pnas.012589799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naus CC, et al. Identification of genes differentially expressed in C6 glioma cells transfected with connexin 43. Brain Res. Rev. 2000;32:259–266. doi: 10.1016/s0165-0173(99)00087-9. [DOI] [PubMed] [Google Scholar]
- 16.Giepmans BN, Moolenaar WH. The gap junction protein connexin 43 interacts with the second PDZ domain of the zona occludens-1 protein. Curr. Biol. 1998;8:931–934. doi: 10.1016/s0960-9822(07)00375-2. [DOI] [PubMed] [Google Scholar]
- 17.Kausalya PJ, et al. Connexin 45 directly binds to ZO-1 and localizes to the tight junction region in epithelial MDCK cells. FEBS Lett. 2001;505:92–96. doi: 10.1016/s0014-5793(01)02786-7. [DOI] [PubMed] [Google Scholar]
- 18.Giepmans BN, et al. Gap junction protein connexin-43 interacts directly with microtubules. Curr. Biol. 2001;11:1364–1368. doi: 10.1016/s0960-9822(01)00424-9. [DOI] [PubMed] [Google Scholar]
- 19.Schubert AL, et al. Connexin family members target to lipid raft domains and interact with caveolin-1. Biochemistry. 2002;41:5754–5764. doi: 10.1021/bi0121656. [DOI] [PubMed] [Google Scholar]
- 20.Di Virgilio F, et al. Cytolytic P2X purinoceptors. Cell Death Differ. 1998;5:191–199. doi: 10.1038/sj.cdd.4400341. [DOI] [PubMed] [Google Scholar]
- 21.Meyers JR, et al. Lighting up the senses: FM1-43 loading of sensory cells through nonselective ion channels. J. Neurosci. 2003;23:4054–4065. doi: 10.1523/JNEUROSCI.23-10-04054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Contreras JE, et al. Gating and regulation of Cx43 hemichannels. Proc. Natl. Acad. Sci. U. S. A. doi: 10.1073/pnas.1434298100. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Musil LS, Goodenough DA. Biochemical analysis of connexin 43 intracellular transport, phosphorylation, and assembly into gap junctional plaques. J. Cell Biol. 1991;115:1357–1374. doi: 10.1083/jcb.115.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Das Sarma J, et al. Multimeric connexin interactions prior to the trans-Golgi network. J. Cell Sci. 2001;114:4013–4024. doi: 10.1242/jcs.114.22.4013. [DOI] [PubMed] [Google Scholar]
- 25.Hertzberg EL, Gilula NB. Isolation and characterization of gap junctions from rat liver. J. Biol. Chem. 1979;254:2138–2147. [PubMed] [Google Scholar]
- 26.Henderson D, et al. Structure and biochemistry of mouse hepatic gap junctions. J. Mol. Biol. 1979;132:193–218. doi: 10.1016/0022-2836(79)90391-7. [DOI] [PubMed] [Google Scholar]
- 27.Martin PE, et al. Multiple pathways in the trafficking and assembly of connexin 26, 32 and 43 into gap junction intercellular communication channels. J. Cell Sci. 2001;114:3845–3855. doi: 10.1242/jcs.114.21.3845. [DOI] [PubMed] [Google Scholar]
- 28.Zhang JT, et al. Membrane integration of in vitro-translated gap junctional proteins: co- and post-translational mechanisms. Mol. Biol. Cell. 1996;7:471–482. doi: 10.1091/mbc.7.3.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaietta G, et al. Multicolor and electron microscopic imaging of connexin trafficking. Science. 2002;296:503–507. doi: 10.1126/science.1068793. [DOI] [PubMed] [Google Scholar]
- 30.Lauf U, et al. Dynamic trafficking and delivery of connexons to the plasma membrane and accretion to gap junctions in living cells. Proc. Natl. Acad. Sci. U. S. A. 2002;99:10446–10451. doi: 10.1073/pnas.162055899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bruzzone S, et al. Connexin 43 hemi channels mediate Ca2+-regulated transmembrane NAD+ fluxes in intact cells. FASEB J. 2001;15:10–12. doi: 10.1096/fj.00-0566fje. [DOI] [PubMed] [Google Scholar]
- 32.Ye ZC, et al. Functional hemichannels in astrocytes: a novel mechanism of glutamate release. J. Neurosci. 2003;23:3588–3596. doi: 10.1523/JNEUROSCI.23-09-03588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bruzzone S, et al. A self-restricted CD38-connexin 43 cross-talk affects NAD+ and cyclic ADP-ribose metabolism and regulates intracellular calcium in 3T3 fibroblasts. J. Biol. Chem. 2001;276:48300–48308. doi: 10.1074/jbc.M107308200. [DOI] [PubMed] [Google Scholar]
- 34.DeVries SH, Schwartz EA. Hemi-gap-junction channels in solitary horizontal cells of the catfish retina. J. Physiol. 1992;445:201–230. doi: 10.1113/jphysiol.1992.sp018920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malchow RP, et al. A novel action of quinine and quinidine on the membrane conductance of neurons from the vertebrate retina. J. Gen. Physiol. 1994;104:1039–1055. doi: 10.1085/jgp.104.6.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dixon DB, et al. Quinine, intracellular pH and modulation of hemi-gap junctions in catfish horizontal cells. Vis. Res. 1996;36:3925–3931. doi: 10.1016/s0042-6989(96)00129-0. [DOI] [PubMed] [Google Scholar]
- 37.White TW, et al. Functional characteristics of skate connexin 35, a member of the gamma subfamily of connexins expressed in the vertebrate retina. Eur. J. Neurosci. 1999;11:1883–1890. doi: 10.1046/j.1460-9568.1999.00607.x. [DOI] [PubMed] [Google Scholar]
- 38.Mitropoulou G, Bruzzone R. Modulation of perch connexin 35 hemi-channels by cyclic AMP requires a protein kinase A phosphorylation site. J. Neurosci. Res. 2003;72:147–157. doi: 10.1002/jnr.10572. [DOI] [PubMed] [Google Scholar]
- 39.Kamermans M, et al. Hemichannel-mediated inhibition in the outer retina. Science. 2001;292:1178–1180. doi: 10.1126/science.1060101. [DOI] [PubMed] [Google Scholar]
- 40.Deans MR, Paul DL. Mouse horizontal cells do not express connexin 26 or connexin 36. Cell Adhes. Commun. 2001;8:361–366. doi: 10.3109/15419060109080754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boucher S, Bennett SA. Differential connexin expression, gap junction intercellular coupling, and hemichannel formation in NT2/D1 human neural progenitors and terminally differentiated hNT neurons. J. Neurosci. Res. 2003;72:393–404. doi: 10.1002/jnr.10575. [DOI] [PubMed] [Google Scholar]
- 42.Trexler EB, et al. Voltage gating and permeation in a gap junction hemichannel. Proc. Natl. Acad. Sci. U. S. A. 1996;93:5836–5841. doi: 10.1073/pnas.93.12.5836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valiunas V, et al. Biophysical properties of mouse connexin 30 gap junction channels studied in transfected human HeLa cells. J. Physiol. 1999;519:631–644. doi: 10.1111/j.1469-7793.1999.0631n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valiunas V, Weingart R. Electrical properties of gap junction hemichannels identified in transfected HeLa cells. Pflügers Arch. 2000;440:366–379. doi: 10.1007/s004240000294. [DOI] [PubMed] [Google Scholar]
- 45.Trexler EB, et al. The first extracellular loop domain is a major determinant of charge selectivity in connexin 46 channels. Biophys. J. 2000;79:3036–3051. doi: 10.1016/S0006-3495(00)76539-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Valiunas V. Biophysical properties of connexin-45 gap junction hemichannels studied in vertebrate cells. J. Gen. Physiol. 2002;119:147–164. doi: 10.1085/jgp.119.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bukauskas FF, et al. Coupling asymmetry of heterotypic connexin 45/connexin 43-EGFP gap junctions: properties of fast and slow gating mechanisms. Proc. Natl. Acad. Sci. U. S. A. 2002;99:7113–7118. doi: 10.1073/pnas.032062099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Valiunas V, et al. Formation of heterotypic gap junction channels by connexins 40 and 43. Circ. Res. 2000;86:E42–E49. doi: 10.1161/01.res.86.2.e42. [DOI] [PubMed] [Google Scholar]
- 49.Elenes S, et al. Heterotypic docking of Cx43 and Cx45 connexons blocks fast voltage gating of Cx43. Biophys. J. 2001;81:1406–1418. doi: 10.1016/S0006-3495(01)75796-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Verselis VK, et al. Opposite voltage gating polarities of two closely related connexins. Nature. 1994;368:348–351. doi: 10.1038/368348a0. [DOI] [PubMed] [Google Scholar]
- 51.Verselis VK, et al. Connexin hemichannels and cell–cell channels: comparison of properties. Braz. J. Med. Biol. Res. 2000;33:379–389. doi: 10.1590/s0100-879x2000000400003. [DOI] [PubMed] [Google Scholar]
- 52.Hofer A, Dermietzel R. Visualization and functional blocking of gap junction hemichannels (connexons) with antibodies against external loop domains in astrocytes. Glia. 1998;24:141–154. doi: 10.1002/(sici)1098-1136(199809)24:1<141::aid-glia13>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 53.Bukauskas FF, et al. Clustering of connexin 43-enhanced green fluorescent protein gap junction channels and functional coupling in living cells. Proc. Natl. Acad. Sci. U. S. A. 2000;97:2556–2561. doi: 10.1073/pnas.050588497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Verselis VK, Bukauskas FF. Connexin-GFPs shed light on regulation of cell–cell communication by gap junctions. Curr. Drug Targets. 2002;3:483–499. doi: 10.2174/1389450023347272. [DOI] [PubMed] [Google Scholar]
- 55.Guida L, et al. Equilibrative and concentrative nucleoside transporters mediate influx of extracellular cyclic ADP-ribose into 3T3 murine fibroblasts. J. Biol. Chem. 2002;277:47097–47105. doi: 10.1074/jbc.M207793200. [DOI] [PubMed] [Google Scholar]
- 56.Verderio C, et al. Evidence of a role for cyclic ADP-ribose in calcium signalling and neurotransmitter release in cultured astrocytes. J. Neurochem. 2001;78:646–657. doi: 10.1046/j.1471-4159.2001.00455.x. [DOI] [PubMed] [Google Scholar]
- 57.Haydon PG. Glia: listening and talking to the synapse. Nat. Rev. Neurosci. 2001;2:185–193. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- 58.Sanderson MJ, et al. Mechanical stimulation and intercellular communication increases intracellular Ca2+ in epithelial cells. Cell Regul. 1990;1:585–596. doi: 10.1091/mbc.1.8.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cotrina ML, et al. Connexins regulate calcium signaling by controlling ATP release. Proc. Natl. Acad. Sci. U. S. A. 1998;95:15735–15740. doi: 10.1073/pnas.95.26.15735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arcuino G, et al. Intercellular calcium signaling mediated by point-source burst release of ATP. Proc. Natl. Acad. Sci. U. S. A. 2002;99:9840–9845. doi: 10.1073/pnas.152588599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schipke CG, et al. Astrocyte Ca2+ waves trigger responses in microglial cells in brain slices. FASEB J. 2002;16:255–257. doi: 10.1096/fj.01-0514fje. [DOI] [PubMed] [Google Scholar]
- 62.Shatz CJ. Emergence of order in visual system development. Proc. Natl. Acad. Sci. U. S. A. 1996;93:602–608. doi: 10.1073/pnas.93.2.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Somjen GG. Mechanisms of spreading depression and hypoxic spreading depression-like depolarization. Physiol. Rev. 2001;81:1065–1096. doi: 10.1152/physrev.2001.81.3.1065. [DOI] [PubMed] [Google Scholar]
- 64.Peinado A. Immature neocortical neurons exist as extensive syncitial networks linked by dendrodendritic electrical connections. J. Neurophysiol. 2001;85:620–629. doi: 10.1152/jn.2001.85.2.620. [DOI] [PubMed] [Google Scholar]
- 65.Hassinger TD, et al. An extracellular signaling component in propagation of astrocytic calcium waves. Proc. Natl. Acad. Sci U. S. A. 1996;93:13268–13273. doi: 10.1073/pnas.93.23.13268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Paemeleire K, et al. Intercellular calcium waves in HeLa cells expressing GFP-labeled connexin 43, 32, or 26. Mol. Biol. Cell. 2000;11:1815–1827. doi: 10.1091/mbc.11.5.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Braet K, et al. Photoliberating inositol-1,4,5-trisphosphate triggers ATP release that is blocked by the connexin mimetic peptide gap 26. Cell Calcium. 2003;33:37–48. doi: 10.1016/s0143-4160(02)00180-x. [DOI] [PubMed] [Google Scholar]
- 68.Guthrie PB, et al. ATP released from astrocytes mediates glial calcium waves. J. Neurosci. 1999;19:520–528. doi: 10.1523/JNEUROSCI.19-02-00520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lin JH, et al. Gap-junction-mediated propagation and amplification of cell injury. Nat. Neurosci. 1998;1:494–500. doi: 10.1038/2210. [DOI] [PubMed] [Google Scholar]