Abstract
Connexins are protein subunits that oligomerize into hexamers called connexons, gap junction hemichannels or just hemichannels. Because some gap junction channels are permeable to negatively and/or positively charged molecules up to ~ 1kDa in size, it was thought that hemichannels should not open to the extracellular space. A growing amount of evidence indicates that opening of hemichannels does occur under both physiological and pathological conditions in astrocytes and other cell types. Electrophysiological studies indicate that hemichannels have a low open probability under physiological conditions but may have a much higher open probability under certain pathological conditions. Some of the physiological behaviours of astrocytes that have been attributed to gap junctions may, in fact, be mediated by hemichannels. Hemichannels constituted of Cx43, the main connexin expressed by astrocytes, are permeable to small physiologically significant molecules, such as ATP, NAD+ and glutamate, and may mediate paracrine as well as autocrine signalling. Hemichannels tend to be closed by negative membrane potentials, high concentrations of extracellular Ca2+ and intracellular H+ ions, gap junction blockers and protein phosphorylation. Hemichannels tend to be opened by positive membrane potentials and low extracellular Ca2+, and possibly by as yet unidentified cytoplasmic signalling molecules. Exacerbated hemichannel opening occurs in metabolically inhibited cells, including cortical astrocytes, which contributes to the loss of chemical gradients across the plasma membrane and speeds cell death.
Keywords: astroglia, cell signalling, connexins, connexons, ischaemia
Introduction: general aspects of connexins and hemichannels
Connexins are transmembrane proteins that belong to a gene family of 19 and 20 members in mouse and human, respectively (Willecke et al. 2002). The nomenclature most widely used to refer to the different members of the connexin family uses Cx followed by the predicted molecular mass of the protein (in kDa, e.g. Cx43) (Beyer 1990). To indicate the species of origin a short prefix may be added (e.g. rCx43 for Cx43 from rat). Most connexin genes have similar organization in which the entire coding sequence is in a single exon; Cx36 is exceptional in that the coding sequence is contained in two exons. Alternative splicing of upstream elements may result in different promoters expressed in specific tissues (Willecke et al. 2002). Many vertebrate cells express more than one connexin type. A few connexins are expressed in only one tissue; others are expressed more widely. Newly synthesized connexins form hexamers, in particular intracellular compartments, which may differ among connexins (Martin et al. 2001, Sarma et al. 2002). In formation of gap junction channels, a hexamer or hemichannel (or connexon) is contributed by each cell. In this presentation, we consider the properties of hemichannels that are not apposed to a hemichannel in another cell, and we will refer to them simply as hemichannels.
The location at which connexins oligomerize into hemichannels is connexin type-dependent. Cx43 assembles in the trans Golgi apparatus, and Cx32 assembles in the ER (Musil & Goodenough 1993, Rahman et al. 1993, Díez et al. 1999, Martin et al. 2001, Sarma et al. 2002). Assembled hemichannels are transported to the plasma membrane through at least two different pathways, one resistant and the other sensitive to brefeldin A (George et al. 1999, Martin et al. 2001), a Golgi apparatus disruptor. Those transported through the brefeldin A sensitive pathway (e.g. Cx43 and presumably Cx32 and Cx50) travel to the plasma membrane via vesicles of 100–150 nm and those transported through the brefeldin A resistant pathway (e.g.Cx26) might directly integrate into the plasma membrane through a mechanism not yet understood (Zampighi et al. 1999, Ahmad & Evans 2002, Gaietta et al. 2002, Sarma et al. 2002). In an in vitro cell-free transcription/ translation system, Cx26 is integrated directly into plasma membranes post-translationally (Ahmad &; Evans 2002). Some cells expressing at least two connexins form heteromeric hemichannels (containing more than one type of connexin) (Jiang &; Goodenough 1996, Locke et al. 2000, Berthoud et al. 2001, Martínez et al. 2002), as well as homomeric hemichannels. Homomeric hemichannels of different connexin types in one cell may form junctions in distinct membrane domains (Spray et al. 1991, Guerrier et al. 1995). Junctions with a different type of hemichannel in each cell are termed heterotypic; not all possible connexin combinations will form heterotypic junctions.
The presence of hemichannels on the cell surface has been documented with several experimental approaches. Freeze-fracture replicas of the plasma membrane of Xenopus oocytes expressing Cx50 exhibit a new population of intramembrane particles (~9 nm in diameter) associated with a whole-cell current (Zampighi et al. 1999). Using an anti-Cx26 antibody that reacts with a region of the C-terminus of Cx26 and immunofluorescence, Cx26 has been localized at the membrane of the dendritic tips of horizontal cells of a teleost fish where gap junctions are absent, suggesting that Cx26 might form hemichannels (Janssen-Bienhold et al. 2001, Kamermans et al. 2001). Moreover, in human polymorphonuclear cells treated with proinflammatory agents, but not in resting cells, Cx43 hemichannels have been detected by immunofluorescence using an antibody directed to a region of extracellular loop 1 of Cx43 (Brañes et al. 2002). The relative levels of Cx43 hemichannels located in the surface of osteocytic MLO-Y4 and NRK–cells have been measured by Western blot analysis of biotinylated cell surface proteins (Musil & Goodenough 1993, Cooper & Lampe 2002, Plotkin et al. 2002). Functional studies using electrophysiological recording in solitary horizontal cells of catfish retina and cells of the urinary bladder epithelium of Necturus maculosus have identified membrane currents activated by lowering extracellular Ca2+ concentration and applying positive membrane potentials. Those currents showed pharmacological and permeability properties corresponding to hemichannels, but the connexin type expressed is unknown (DeVries & Schwartz 1992, Malchow et al. 1994, Vanoye et al. 1999). Definitive demonstrations of hemichannel-mediated currents have been obtained in coupling incompetent cell lines transfected with known connexins. Additional approaches to demonstrate the existence of functional hemichannels include comparison of wild type cells and cells from animals with a specific connexin gene knocked out or knocked down by antisense oligonucleotide treatment (Li et al. 1996, Contreras et al. 2002b). Gap junction blockers have also been used to inhibit hemichannel mediated currents and to decrease permeability to various gap junction permeable tracers (see below).
Exchange of ions and small molecules between the cytoplasm and extracellular space depends, in part, on mechanisms that control the number of hemichannels found at the cell surface and their open time. Under physiological conditions, hemichannel opening accounts for the release to the extracellular space of small molecules, some of which are likely to play a role in cell-cell signalling. It is likely that hemichannels serve as one pathway for uptake of small nutrients present in the extracellular milieu and they may have a role in several other physiological functions. In addition, placing cultured cells in conditions that mimic aspects of ischaemia, such as metabolic inhibition, induces opening of hemichannels, which accelerates the progression to cell death. The identification of diseases in which excessive hemichannel opening occurs and the discovery of pharmacological agents that selectively block these channels could provide new tools to reduce cell death from these diseases as well as from ischaemia.
Opening and closing hemichannels
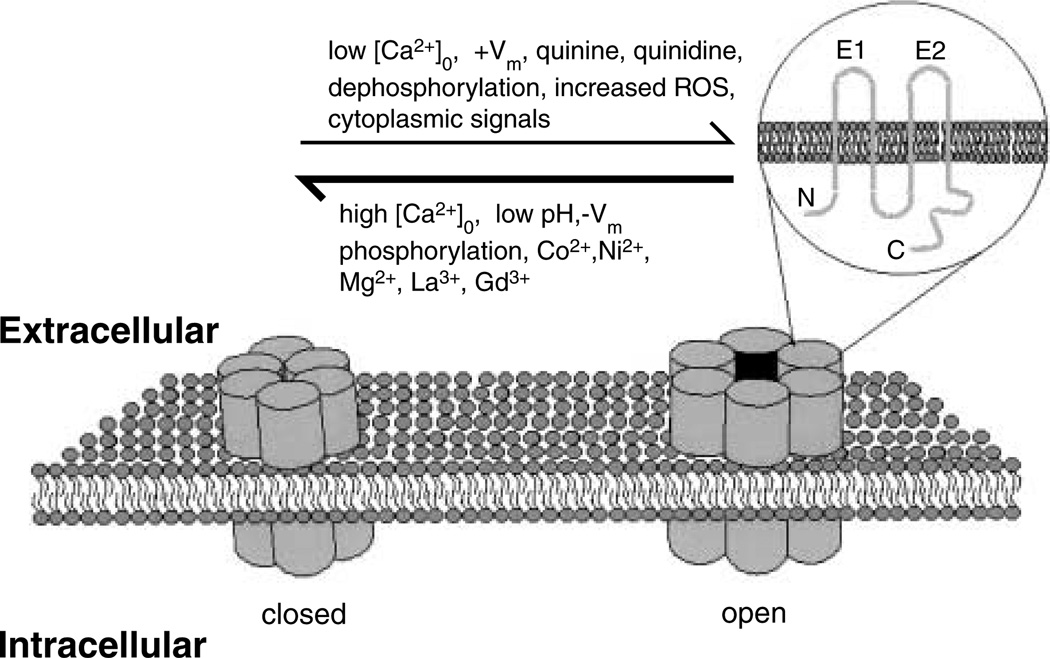
Because many gap junction channels exhibit a large non-specific permeability, hemichannels in the cell surface were expected to remain closed until appropriate pairing with a hemichannel in a contacting cell (e.g., Bennett et al. 1991). The earliest indication of hemichannel opening came from the finding that Cx46 expressed in Xenopus oocytes resulted in cell swelling and death (Paul et al. 1991). Shortly afterwards, the appearance of membrane currents with pharmacological properties similar to that of gap junction channels in retinal horizontal cells exposed to low extracellular Ca2+ concentrations were attributed to hemichannels (DeVries & Schwartz 1992). In subsequent years, macroscopic and microscopic membrane currents mediated by hemichannels formed by known connexin types have been recorded in heterologous expression systems, including Xenopus oocytes and several cell lines such as HeLa and RIN cells. Membrane currents mediated by hemichannels have also been recorded from primary cells, including horizontal neurons in fish retina, rat cardiac myocytes and rat cortical astrocytes (John et al. 1999, Kondo et al. 2000, Kamermans et al. 2001, Zhang & McMahon 2001, Contreras et al. 2002b, Stout et al. 2002). Agents favouring opening and closing of hemichannels are indicated in Fig. 1.
Figure 1.
Conditions that open or close hemichannels. Connexins are tetraspan proteins with both amino and carboxy terminals on the cytoplasmic side of the membrane (inset, upper right). Each hemichannel contains six connexin molecules and under physiological conditions are preferentially closed (left). Open probability of hemichannels is increased in low [Ca2+]O, at positive membrane potentials (+Vm), by such pharmacological agents as quinine and quinidine, and after metabolic inhibition leading to dephosphorylation and increase in reactive oxygen species (ROS) and generation of as yet unidentified cytoplasmic signals. Open probability is decreased by high [Ca2+]O, negative membrane potentials (−Vm), low intracellular pH, phosphorylation of cytoplasmic domains, polyvalent cations, including Co2+, Ni2+, Mg2+, La2+ and Gd3+, and low intracellular pH and other gap junction blockers.
While under resting conditions opening of Cx46 expressed in Xenopus oocytes might be explained by the lack of endogenous regulatory factors present in the lens (see below), hemichannels formed by other connexins are opened by application of positive membrane potentials and/or low extracellular [Ca2+] solutions. Cx46 hemichannels exhibit two voltage gating mechanisms (Trexler et al. 1996), a fast form (<1 ms transition time) gating between the fully open state and a substate and a slow form (>5 ms transition time) gating between the open state and the fully closed state, possible transiting through several intermediate states. Thus, hemichannel gating has properties similar to gating of cell-cell channels as described earlier (Bukauskas &; Peracchia 1997, Bukauskas et al. 2001). Low extracellular [Ca2+] promotes opening of hemichannels in the exogenous expression systems mentioned above and in several mammalian cells, including Novikoff cells (Li et al. 1996), cardiac myocytes (John et al. 1999, Kondo et al. 2000), astrocytes (Hofer &; Dermietzel 1998, Stout et al. 2002, Contreras et al. 2002b), and human osteoblast-like cells (Romanello &; D’Andrea 2001). Cx43 hemichannel opening can be induced in cultured astrocytes by mechanical stimulation (Stout et al. 2002) and in osteocytic MLO-Y4 cells by treatment with alendronate, a bisphophonate used in the treatment of bone diseases (Plotkin et al. 2001). How alendronate induces opening of hemichannels remains unknown. Opening of hemichannels can be enhanced with quinine or quinidine in addition to the application of positive membrane potentials or low extracellular [Ca2+] (Malchow et al. 1994, White et al. 1999, Stout et al. 2002, Ripps et al. 2002) (Fig. 1), suggesting the existence of the hemichannel activator. Quinine does not affect the functional state of Cx43 and Cx46 gap junction channels expressed in Xenopus oocytes (White et al. 1999), although it blocks Cx36 channels with high potency (Srinivas et al. 2001); it is possible that it interacts with connexin domains exposed in hemichannels but not in cell–cell channels. The quinine effect on hemichannels cannot be explained solely by the quinineinduced intracellular alkalinization, because the action is still observed using 80 mM HEPES into the recording patch pipette (Dixon et al. 1996). The membrane current mediated by Cx38 hemichannels endogenously expressed by Xenopus oocytes is enhanced by quinine in a concentration-dependent fashion. The Hill coefficient of 1.9 suggests that the binding of at least two molecules of quinine is required to produce the effect (Ripps et al. 2002).
Membrane currents mediated by hemichannels are greatly reduced at negative membrane potentials or when the extracellular[Ca2+] is >1 mM (DeVries & Schwartz 1992, Ebihara & Steiner 1993, Pfahnl & Dahl 1999, Zampighi et al. 1999, Jedamzik et al. 2000, Ripps et al. 2002). Cx46 hemichannels are also blocked by extracellular application of Ni2+, Co2+ or Mg2+ (Ebihara & Steiner 1993, Ebihara et al. 2003) (Fig. 1). Closure of Cx43 hemichannels has been observed after extracellular application of La 3+ (Kim et al. 1999, Kondo et al. 2000, Contreras et al. 2002a,b) or Gd3+ (Kondo et al. 2000, Stout et al. 2002) (Fig. 1). Several gap junction channel blockers, including octanol, heptanol, carbenoxolone, oleamide, halothane, 18-α-glycyrrhetinic acid and 18-β-glycyrrhetinic acid block hemichannels (Li et al. 1996, 2001, John et al. 1999, Zampighi et al. 1999, Quist et al. 2000, Bruzzone et al. 2001a, Franco et al. 2001, Kamermans et al. 2001, Contreras et al. 2002a, Eskandari et al. 2002, Ripps et al. 2002, Stout et al. 2002). Nevertheless, pharmacological sensitivities of hemichannels formed by different connexin types differ. While Cx50 hemichannels expressed in Xenopus oocytes are blocked by niflumic acid, diphenyl-2-carb-oxylate and octanol, Cx46 hemichannels are resistant to these agents (Eskandari et al. 2002). The inhibitory effect of octanol on Cx50 hemichannels is not altered by the extracellular [Ca2+] and it is only observed when octanol is applied on the extracellular side of the hemichannels (Eskandari et al. 2002). These differences in pharmacological sensitivities suggest that in the future it will be possible to control the number of functional hemichannels in a connexin specific manner. These findings encourage the development of pharmacological tools that could be useful to demonstrate the physiological role of hemichannels formed by different connexins and in the treatment of diseases in which hemichannel gating mechanisms are implicated. For example, several Cx32 mutations causing X-linked Charcot-Marie-Tooth neuropathy increase Cx32 hemichannel opening (Castro et al. 1999, Abrams et al. 2002).
In teleost horizontal cells bathed in Ca2+-free Ringer’s solution and exposed to positive potentials, Cx35 hemichannels can be closed by retinoic acid (Zhang & McMahon 2001). The degree of inhibition of horizontal cell hemichannels by co-application of Ca2+ and retinoic acid is less than the sum of inhibition by Ca2+ or retinoic acid alone, suggesting that their actions are not independent (Zhang 8c McMahon 2001). Low intracellular pH also induces closure of hemichannels formed by various connexins (Trexler et al. 1999, Zampighi et al. 1999, Beahm & Hall 2002, Ripps et al. 2002). A short exposure of Cx46 hemichannels to low pH induces a rapid and reversible closure termed pH gating and a longer exposure induces a poorly reversible or irreversible closure termed pH inactivation (Trexler et al. 1999).
In mammalian cell lines, opening of Cx43 hemichannels induced by low extracellular [Ca2+] is blocked by activation of a protein kinase C-dependent pathway (Li et al. 1996, Liu et al. 1997). Similarly, the membrane current mediated by Cx46 hemichannels expressed in Xenopus oocytes is greatly reduced by activation of protein kinase C (Ngezahayo et al. 1998, Jedamzik et al. 2000). Therefore, protein phosphorylation may induce gating that keeps hemichannels closed. Moreover, Cx43 is substrate for mitogen activated protein (MAP) kinases (Warn-Cramer et al. 1998), also termed extracellular signal regulated kinases (ERKs). MAP kinases can be activated by tyrosine kinase receptors, such as the epidermal growth factor receptor, or through transduction pathways that activate protein kinase C via a cross-talk mechanism (Rivedal & Opsahl 2001, Ruch et al. 2001), leading to closure of Cx43 gap junction channels (Berthoud et al. 1993, Oh et al. 1993). A similar mechanism might operate on Cx43 hemichannels. In support of this notion, reconstituted Cx43 hemichannels remain preferentially closed after phosphorylation with purified MAP kinase, and phosphatase treated Cx43 forms functional hemichannels (Kim et al. 1999) (Fig. 1). It is surprising that MAP kinase keeps hemichannels closed without affecting gap junctional communication. Subcellular compartmentalization of MAP kinase may permit access to hemichannels but not to gap junction channels. Alternatively, protein phosphatases may have less access to phosphorylated hemichannels than to phosphorylated gap junction channels, thus increasing the level of hemichannel phosphorylation induced by basal MAP kinase activity.
Perch Cx35, but not skate Cx35, presents a consensus site for protein kinase A phoshorylation. Accordingly, perch but not skate Cx35 hemichannels are closed by activation of protein kinase A with 8Br-cAMP, a membrane permeant derivative of cAMP (Mitropoulou & Bruzzone 2003). Lens connexins (Cx44, Cx46 and Cx56) are phosphoproteins and are detected as multiple bands in immunoblots of lens homogenates (Paul et al. 1991, Berthoud et al. 1994, Gupta et al. 1994), but in oocytes they show the same electrophoretic mobilities as in vitro translated proteins (Paul et al. 1991, Gupta et al. 1994, Ebihara et al. 1995). In sheep lens fibres treated with a casein kinase I inhibitor, an increase in dye coupling has been observed, suggesting that phosphorylation of the lens fibre connexins by casein kinase leads to closure of gap junction channels (Cheng & Louis 2001). Cx46 can be phosphorylated by the endogenous casein kinase I activity of the lens (Cheng & Louis 1999). A similar mechanism might keep lens fibre hemichannels closed to maintain the high input resistance of these cells. Deficient regulation of phosphorylation of these connexins in Xenopus oocytes might explain the hemichannel opening at physiological voltages. In addition, the relatively negative potential at which these hemichannels open (−20 mV) (Ebihara & Steiner 1993) is closer to the resting membrane potential of oocytes (−30 to −60 mV) (Purnick et al. 2000) than to that of rat lens fibres (−60 to −75 mV) (Takeshita et al. 1993, Cheng et al. 2000).
Enhanced or reduced opening has also been observed in hemichannels formed by mutated and chimeric connexins. Two mutations linked to congenital cataract, Asn63Ser and frame shift 380, reduce opening of Cx46 hemichannels and formation of cell-cell channels (Pal et al. 2000). In Cx50, which like Cx46 forms hemichannels in oocytes, the mutation Cx50Hisl61Asn blocks hemichannels activity, but not gap junction formation (Beahm & Hall 2002). Several C-terminal mutations of Cx32, including some of those identified in X-linked Charcot–Marie–Tooth disease, prevent the formation of functional hemichannels (Castro et al. 1999). A chimeric connexin consisting of Cx32 with the first extracellular loop replaced by the corresponding Cx43 sequence forms hemichannels in Xenopus oocytes and has been useful in studying gating mechanisms (Pfahnl et al. 1997, Oh et al. 2000).
Conductance and permeability of hemichannels
Unitary activity of hemichannels has been recorded for Cx30, Cx32, Cx43, Cx45, Cx46 and Cx50 (Trexler et al. 1996, Ebihara et al. 1999, Valiunas & Weingart 2000, Abrams et al. 2002, Contreras et al. 2002b, Eskandari et al. 2002, Valiunas 2002). In Cx30, Cx43, Cx45 and Cx46, the unitary conductance is about twice that of the corresponding cell–cell channels, as would be predicted from simple series arrangement of two hemichannels in forming a single cell–cell channel. The discrepancies may be accounted for by differences in experimental conditions and solutions. All these hemichannels open at positive membrane potentials and low extracellular [Ca2+], but lens connexins of different species (i.e. rCx46, bCx44 and chCx56) also open at physiological voltages (Paul et al. 1991, Ebihara & Steiner 1993, Gupta et al. 1994, Trexler et al. 1996). Lens fibres have a high input resistance (Ebihara et al. 1995), indicating the absence of open hemichannels under physiological conditions. As discussed above, this apparent contradiction may result from differences in post-transcriptional modification of these connexins in oocytes and in lens fibres. The biophysical properties of the macroscopic current generated in Xenopus oocytes that coexpress Cx45.6 and Cx50 differ from those induced by the expression of either Cx45.6 or Cx50 alone, indicating the formation of heteromeric hemichannels with distinct properties (Ebihara et al. 1999).
The permeability of hemichannels has been evaluated using a number of fluorescent tracers. Cx43 hemichannels are permeable to Lucifer yellow, ethidium bromide, carboxyfluorescein, 7-hydroxycoumarin-3-carboxylic acid and fura-2 (Li et al. 1996, Liu et al. 1997, Kondo et al. 2000, Contreras et al. 2002a, Plotkin et al. 2002, Stout et al. 2002) (Fig. 2). Interestingly, Cx43 hemichannels appear to be impermeable to propidium iodide, unlike Cx43 cell-cell channels (Elfgang et al. 1995). Cx46 hemichannels show a strong preference for cations and display marked inward rectification in symmetric solutions (Trexler et al. 1996). However, oocytes expressing Cx46 hemichannels take up negatively charged tracers, such as carboxyfluorescein (MW 376) (Hu & Dahl 1999) Cx46 hemichannels are only slightly permeable to Lucifer yellow, cadaverin, and biotin-X (MW 873), suggesting a molecular weight cutoff of about 1 kDa (Hu & Dahl 1999). Cx32 hemichannels are anion selective, and substitution of the first extracellular loop (E1) of Cx32 into Cx46 makes the chimeric Cx46 channels anion preferring, indicating that fixed charges in E1, positive in Cx32 and negative in Cx46, influence charge selectivity of the hemichannels (Trexler et al. 2000). Cx45 hemichannels are permeable to Lucifer yellow and propidium iodide and comparison of Lucifer yellow diffusion through Cx45 hemichannels and gap junction channels reveals that the latter are less permeable (Valiunas 2002).
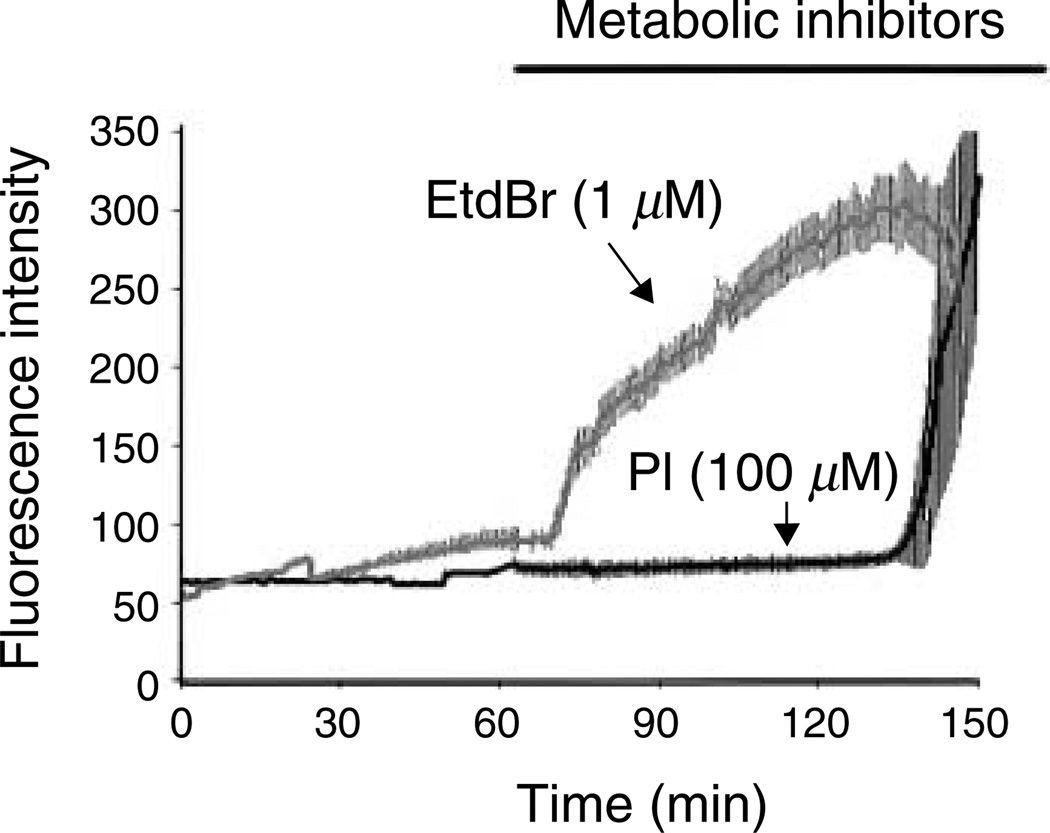
Figure 2.
Time course of ethidium bromide (EtdBr) and propidium iodide (PI) uptake in metabolically inhibited astrocytes. Confluent cultures of rat cortical astrocytes were treated with iodoacetic acid and antimycin A and the time course of EtdBr and IP uptake was measured (in separate experiments). EtdBr uptake began a few minutes after application of metabolic inhibitors (at 60 min). PI uptake was minimal until ~ 130 min of metabolic inhibition at which time uptake increased abruptly. Other experiments indicate that this delayed uptake is because of the loss of membrane integrity.
Cx43 hemichannels are permeable to small molecules, including NAD+, ATP and IP3 (Bruzzone et al. 2001a,b, Romanello & D’Andrea 2001, Arcuino et al. 2002, Stout et al. 2002). Similarly, cells expressing Cx32 release ATP, suggesting that it can permeate Cx32 hemichannels (Arcuino et al. 2002). Part of the evidence for permeation through hemichannels is that fluorescently labelled dextrans of somewhat higher molecular weight are not taken up by cells expressing Cx43 (Arcuino et al. 2002, Contreras et al. 2002a, Stout et al. 2002). Similarly, stachiose fluorescein (MW 1146) is not taken up by oocytes expressing Cx46 (Hu &; Dahl 1999).
In addition to gating, the number of hemichannels in the surface membrane will affect exchange of substances between the intracellular and extracellular milieu. As the half-life of several connexins (not including those in the lens) is known to be a few hours (Fallon & Goodenough 1981, Traub et al. 1987, 1989, Musil et al. 1990, Beardslee et al. 1998, Gaietta et al. 2002), it is likely that the number of hemichannels in the plasma membrane is affected by conditions that alter the turnover of these proteins. Inhibition of casein kinase 1 increases the number of surface hemichannels, suggesting a role of this kinase in assembly of hemichannels into cell-cell channels (Cooper &; Lampe 2002). Thus, total movement through hemichannels could be regulated over periods of seconds to hours by gating and by synthesis, insertion and retrieval of hemichannels from the plasma membrane.
Hemichannels in astrocytes under physiological conditions
Different cell types of the adult CNS, including neurons, astrocytes, oligodendrocytes and microglia, express different sets of connexins (Shiosaka et al. 1989, Dermietzel et al. 1997, 2000, Nadarajah et al. 1997, Nagy et al. 1997, 1999, Rash & Yasumura 1999, Sohl et al. 2000, Teubner et al. 2000, Venance et al. 2000, Eugenín et al. 2001, Oguro et al. 2001, Rash et al. 2001a,b, Altevogt et al. 2002, Long et al. 2002, Meier et al. 2002, Parenti et al. 2002), and for neurons and astrocytes at least the expression pattern changes during development (Dermietzel et al. 1991, Leung et al. 2002).
In vivo, astrocytes express Cx43 and low levels of Cx26, Cx30, and possibly Cx40, and Cx45 (Nagy et al. 1997, 1999, Dermietzel et al. 2000). In cultured rat astrocytes, unitary conductances of gap junction channels are similar to those observed in Cx43 transfectants (Dermietzel et al. 1991, Giaume et al. 1991, Moreno et al. 1994, Kwak et al. 1995, Bukauskas et al. 2001). Moreover, astrocytes cultured from Cx43-deficient mice exhibit very little gap junctional communication (Naus et al. 1997, Scemes et al. 1998, Dermietzel et al. 2000).
As most gap junction channel blockers also block hemichannels (see above), suppression of Ca2+ waves by gap junction blockers does not differentiate between two distinct possible mechanisms of propagation: communication through gap junctions and mediation by ATP released from hemichannels diffusing in extracellular space (Cotrina et al. 1998). Other data confirm the extracellular nature of the signalling, including propagation across cell free spaces, dependence on flow of extracellular fluid, sensitivity to purinergic antagonists and visualization of released ATP in the extracellular space (Hassinger et al. 1996, Cotrina et al. 1998, Guthrie et al. 1999, Arcuino et al. 2002). Moreover, low extracellular Ca2+ concentrations or application of quinine, two conditions known to open hemichannels, elicit local and propagating Ca2+ signals (Stout et al. 2002). Mechanical stimulation triggers ATP release apparently through Cx43 hemichannels and thus initiates propagation of Ca2+ waves in astrocytes and other electrically inexcitable cells (Arcuino et al. 2002, Stout et al. 2002). Astrocytes bathed in low [Ca2+] medium or mechanically stimulated show membrane currents and uptake of small fluorescent dyes inhibited by flufenamic acid or Gd3+ (Stout et al. 2002, Arcuino et al. 2002). It remains uncertain whether ATP release evokes ATP release in neighbouring cells (and whether propagation is passive or active and regenerative). Astrocytes in low extracellular [Ca2+] show an increased intracellular [Ca2+] response to ATP, whereas astrocytes in high extracellular [Ca2+] show a decreased intracellular [Ca2+] response to ATP. These results suggest that astrocytes possess a mechanism for coupling between extracellular [Ca2+] and the release of Ca2+ from intracellular stores, which may be important in pathological conditions associated with low extracellular Ca2+ such as seizures or ischaemia (Zanotti &; Charles 1997). Opening of Cx43 hemichannels in HOBIT osteoblastic cells is likely to participate in Ca2+ waves evoked by mechanical stimulation, and IP3 uptake evokes Ca2+ transients, suggesting a possible contribution of IP3 to wave propagation (Romanello &; D’Andrea 2001).
How do hemichannels open? To answer this question, Braet et al. (2003) used caged IP3 or Ca2+ and found that photoliberating IP3 in a single cell within a confluent culture of endothelial cells triggers an intercellular Ca2+ wave that is prevented by 18-α-glycyrrhetinic acid, the connexin mimetic peptide gap 26 (a 13-residue peptide with the same sequence as a region of Cx43 E1, suramin (a purinergic receptor blocker), apyrase (an ATPase) or purinergic receptor desensitization. The ATP release induced by uncaging IP3 was inhibited by buffering the intracellular [Ca2+] with BAPTA. The effect induced by gap 26 with its extracellular application, was reversible in 10–20 min, which is too rapid to be an action on junction formation, and did not block intercellular dye coupling; these properties suggest that gap 26 acts by blocking hemichannels (Braet et al. 2003). The block of IP3 induced hemi-channel opening by BAPTA suggests that IP3 acts by an indirect mechanism, such as dephosphorylation of the connexin or generation of another intracellular signal.
The role of hemichannels in ATP release is not entirely clear. In HOBIT cells overexpressing Cx43, the amount of ATP released under basal and mechanically stimulated conditions is similar to the non-transfected cells, ruling out a major involvement of connexin hemichannels in ATP release in these cells (Romanello et al. 2001). Moreover, Ca2+ waves between cells transfected with Cx43 were not blocked by 18-α-glycyrrhetinic acid or octanol (Cotrina et al. 1998). In addition, oleamide blocks gap junctions and propagation of Ca2+ waves (Guan et al. 1997), but does not block the permeability of Cx43 hemichannels to NAD+ (Bruzzone et al. 2001b). The discrepancies in published data may result from the existence of multiple pathways of ATP release and from differences in mechanism in different cell types and under different physiological conditions. The ionotropic ATP receptor P2X is permeable to small fluorescent dyes, either positively or negatively charged, including Lucifer yellow, ethidium bromide and YOPRO-1 (Ralevic & Brunstock 1998, Virginio et al. 1999, Ferrari et al. 2000, Bisaggio et al. 2001, Hubscher et al. 2001), and could be permeable to ATP. In addition, there is evidence of vesicular release of ATP through a pathway resistant to flufenamic acid and anandamide, two gap junction blockers (Coco et al. 2003).
NAD+ is another signalling molecule proposed to permeate hemichannels. Fibroblasts deficient in Cx43 expression do not release NAD+, but those transfected with Cx43 release NAD+ by passive diffusion (Bruzzone et al. 2001a). These data provided an explanation of the old paradox of intracellular NAD+ being the substrate for an ectoenzyme, CD38, that catalyses its conversion to cADPR. CD38 also mediates uptake of cADPR, which is a potent activator of rhyanodine receptors. Autocrine/paracrine Ca2+ signalling mediated by cADPR generated from NAD+ released through Cx43 hemichannels has been proposed to enhance cell proliferation and shorten the S phase of 3T3 fibroblasts (Franco et al. 2001). The same autocrine/paracrine mechanism may cause glutamate release from astrocytes leading to delayed Ca2+ transients in neurons in coculture (Verderio et al. 2001). In addition, Cx43 hemichannels mediate glutamate release from astrocytes under low calcium conditions that might be reached during strong stimulation or ischaemia (Ye et al. 2003). From data of this kind, hemichannels can be considered a new component in the tripartite interaction of pre-and post-synaptic elements and astrocytes proposed elsewhere (Araque et al. 1999). Quist et al. (2000), by using atomic force microscopy and dye uptake assay, examined extracellular Ca2+-dependent modulation of cell volume. They found that Cx43 hemichannels participate in regulation of cell volume in response to small changes in extracellular [Ca2+] (from 1.8 ≤ 1.6 mM) under isosmotic conditions. A similar mechanism might operate in astrocytes in pathological as well as physiological conditions.
Role of hemichannels in pathological conditions
Under normal conditions, astrocytes support nervous system function by supplying neurons with lactic acid as metabolic fuel and glutamine for generation of glutamate, and by maintaining the extracellular milieu particularly with respect to neurotransmitters and K+ (e.g. Chen &; Nicholson 2000). In numerous pathological conditions, including stroke and head trauma, tissue perfusion is deficient and ischaemia is a common factor. Ischaemia involves hypoxia, deficiency of nutrients and accumulation of metabolic products that can be toxic. After short periods of ischaemia followed by reperfusion, neurons are the main cell type affected. Focal ischaemia can induce necrosis of glial cells as well as neurons in the core of the lesion, and delayed neuronal death can occur days later in the ‘penumbra’, the region adjacent to the core.
In response to lack of oxygen, astrocytes switch to anaerobic metabolism (Peuchen et al. 1996), a property that contributes to their surviving periods of ischaemia that are lethal to neurons. The enhanced generation of lactic acid by anaerobic astrocytes in conjunction with the deficient tissue perfusion raises the concentration of this organic acid reducing neuronal viability (Goldman et al. 1989, Nedergaard et al. 1991). Thus, injuries that astrocytes tolerate but that alter their metabolism could indirectly affect neuronal susceptibility to the same injury. As the intensity and/or the duration of the ischemic episode increases the functions of astrocytes supportive of neurons (and themselves) are likely to become less and less efficient. The precise steps leading to astrocyte death are not yet established, but death is preceded by progressive membrane depolarization, cellular acidosis, decreased glucose utilization and ATP depletion. Studies in primary astrocyte cultures treated with metabolic inhibitors or oxygen/glucose deprivation have been useful in identifying the time course and mechanism of various astrocyte responses induced by ischaemia. Simultaneous inhibition of the glycolysis and mitochondrial respiration causes a drastic ATP depletion paralleled by a progressive reduction of electrochemical gradients across the plasma membrane (Harold &; Walz 1992). As membrane depolarization takes several minutes, it is plausible that it results, at least in part, from Cx43 hemichannel opening (Fig 2 and Fig 3; Contreras et al. 2002a). In support of this possibility, increases in macroscopic membrane current and unitary events ascribable to hemichannels induced by metabolic inhibition in HEK293 cells are reduced to near control levels by halothane, a hemichannel blocker (John et al. 1999). Moreover, in metabolically inhibited rabbit ventricular myocytes halothane or heptanol prevents the TTX and nifedipine resistant increase in intracellular [Na+] and [Ca2+] (Li et al. 2001). Both in HEK293 cells and rabbit ventricular myocytes the membrane conductance increased after metabolic inhibition, and the increase has a reversal potential close to zero, suggesting that the increase is mediated by a nonselective membrane channel (John et al. 1999, Kondo et al. 2000). Accordingly, metabolically inhibited astrocytes take up positive (ethidium, +1 as the Br− salt) and negative (Lucifer yellow, −2) fluorescent dyes from the extracellular medium (Fig. 2; Contreras et al. 2002a). The dye uptake is blocked by La 3+ or 18-α-glycyrrhetinic acid, both hemichannel blockers, and precedes plasma membrane break down as evidenced by lactate dehydrogenase release to the extracellular medium or cell uptake of dextran-LY (10 kDa) too large to pass through hemichannels (Contreras et al. 2002a). More convincing, dye uptake induced by metabolic inhibition is not seen in mouse astrocytes lacking Cx43 for atleast 3 h (Contreras et al. 2002a).
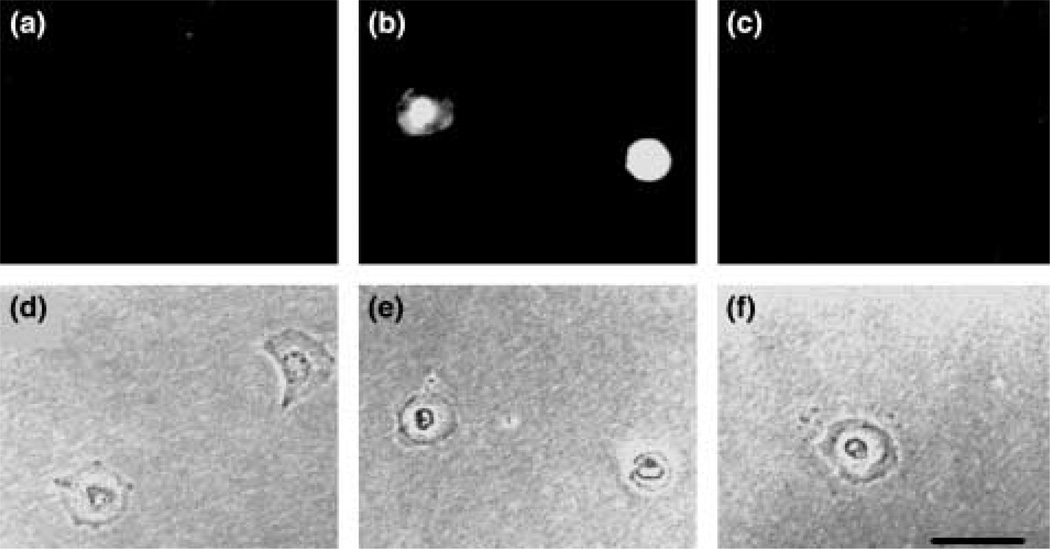
Figure 3.
Ethidium bromide uptake by astrocytes treated with metabolic inhibitors is blocked by 18-glycyrrhetinic acid. Four hours after plating at a very low density (200 cells/60 mm diameter culture dish) cortical rat astrocytes were exposed to iodoacetic acid plus antimycin A for 75 min or kept in control medium. Ethidium bromide (50 µm) was then applied for 2 min to cells in the control medium (a), to metabolically inhibited cells (b), and to metabolically inhibited cells treated with 75 µm 18β-glycyrrhetinic acid for 5 min before the dye application (c). Cells treated only with metabolic inhibitors were stained (b), and cells under control conditions or treated with metabolic inhibitors plus the gap junction blocker remained unstained; (d), (e) and (f) are phase contrast views of the fluorescent views showed in (a), (b) and (c), respectively. Bar 20 µm.
The mechanism that induces Cx43 hemichannel opening during metabolic inhibition remains elusive. The possibility that Cx43 dephosphorylation is necessary for opening of hemichannels in astrocytes is supported by the following findings: (i) reconstituted Cx43 hemichannels previously treated with phosphatase, but not those phosphorylated by MAP kinase, are active, i.e. they open and close (Kim et al. 1999); (ii) confluent cultures of metabolically inhibited astrocytes contain mostly dephosphorylated Cx43 (Li &; Nagy 2000, Martínez & Sáez 2000, Contreras et al. 2002a). To establish that dephosphorylation is necessary for opening of Cx43 hemichannels during metabolic inhibition will require direct evaluation of the state of phosphorylation of the Cx43 forming hemichannels. Gating of hemichannels may be mediated by generation of free radicals, which is increased in metabolically inhibited astrocytes (Taylor et al. 1996), and free radical scanvengers, such as trolox and melatonin, reduce opening of hemichannels by metabolic inhibition (Contreras et al. 2002a). Direct evaluation of the redox state of Cx43 should further extend our knowledge of this putative gating mechanism. A further indication of specific signalling pathways is that inhibitors of lipoxygenase, but not of cyclooxygenase, prevent opening of hemichannels (Contreras et al. 2002a).
The extent of injury following focal ischaemia (transient or permanent) is not established immediately after arterial occlusion; infarct volume expands over time. Episodes of spreading depression have been linked to this secondary increase in size of the infarct. Tissue bordering the infarction fails to repolarize following spreading depression and is incorporated into the lesion implying that infarcts may expand after each episode of spreading depression (Rawanduzy et al. 1997). Passage of potentially harmful cytosolic molecules between cells in the infarct and surrounding cells might cause amplification of injury in focal ischaemia. Octanol, a gap junction blocker, markedly reduces the size of necrotic foci in a rat model of stroke (Rawanduzy et al. 1997). However, most gap junction blockers are unspecific; halothane and octanol also reduce potency of chemical synapses (Puil & El-Beheiry 1990, Puil et al. 1994), and gap junction blockers inhibit spreading depression (e.g. Martins-Ferreira & Ribeiro 1995, Nedergaard et al. 1995, Rawanduzy et al. 1997).
Specific approaches with gene knockout or antisense have revealed important neuroprotective actions of connexins during tissue injury. In heterozygous Cx43 KO mice, which exhibit reduced Cx43 expression, infarct size after stroke is greater than in wild type mice (Siushansian et al. 2001). In mixed astrocyte neuron cultures, inhibition of astrocyte coupling with gap junction blockers increases neuronal vulnerability to oxidative stress (Blanc et al. 1998) or glutamic acid toxicity (Ozog et al. 2002). In addition, FGF-2 upregu-lates Cx43 in rat embryonic day 14 midbrain cultures and promotes survival of dopaminergic neurons; oleamideinduced cell uncoupling abolishes this survival promoting effect (SiuYi Leung et al. 2001). In contrast, neuronal cell death in hippocampal slice cultures 48 h after oxygen/ glucose deprivation is less in slices from Cx43 KO mice than in slices from wild type mice (Frantseva et al. 2002a). Similarly, treatment with antisense oligodeoxy-nucleotides for Cx26 and Cx32 or for Cx43 reduces cell death caused by oxygen/glucose deprivation, and carbe-noxolone is neuroprotective (Frantseva et al. 2002a). Similar results were obtained in a model of traumatic brain injury (Frantseva et al. 2002b). In contrast, Cx32 KO mice show enhanced sensitivity to cerebral ischaemia (Oguro et al. 2001), suggesting gap junction mediated neuroprotection. In astrocytes and glioma cell lines subjected to metabolic inhibition, oxidative stress, or Ca2+ overload, propagation of damage from dying to resistant cells is mediated by gap junctions (Lin et al. 1998). Several other cell lines subjected to various insults show bystander killing mediated by Cx43 gap junctions (Azzam et al. 2001, Huang et al. 2001, Burrows et al. 2002, Sanson et al. 2002).
In summary, opposite roles for gap junctional communication in cell death have been demonstrated in different paradigms. Gap junctions can mediate metabolic cooperation and be neuroprotective. Alternatively, gap junctions can contribute to the propagation of injury. Relative numbers can affect the outcome; many resistant or healthy cells can rescue a few badly injured cells; a large number of dying cells can overcome the capabilities of a cell that would otherwise survive. In studies to date using gap junction blockers and KO animals, a contribution of hemichannels cannot be excluded. In a medium rich in nutrients and protective agents, such as vitamins that can scavenge free radicals, enhanced hemichannel opening could facilitate uptake of those extracellular compounds favouring cell survival. Conversely, prolonged hemichannel opening would lead to rise in intracellular Ca2+ and Na+, loss of K+ and intracellular metabolites, and finally cell death.
Acknowledgments
This work was partially financed by a grant of Fondo Nacional para el Desarrollo de Ciencia y Tecnología (FONDECYT 8890008 to J.C.S.), grants of the Nationallnstitute for Health(NS36706 to F.F.B.; NS07512 and NS45837 to M.V.L.B.) and grant from the F.M. Kirby Foundation (to M.V.L.B.).
References
- Abrams CK, Bennett MVL, Verselis VK, Bargiello TA. Voltage opens unopposed gap junction hemi-channels formed by a connexin 32 mutant associated with X-linked Charcot-Marie-Tooth disease. Proc Natl Acad Sci USA. 2002;99:3980–3984. doi: 10.1073/pnas.261713499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad S, Evans WH. Post-translational integration and oligomerisation of connexin 26 plasmam membranes and evidence for formation of membrena pores. Implications for the assembly of gap junctions. Biochem J. 2002;365:693–699. doi: 10.1042/BJ20011572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altevogt BM, Kleopa KA, Postma FR, Scherer SS, Paul DL. Connexin29 is uniquely distributed within myelinating glial cells of the central and peripheral nervous systems. J Neurosci. 2002;22:6458–6470. doi: 10.1523/JNEUROSCI.22-15-06458.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- Arcuino G, Lin JH, Takano T, et al. Intercellular calcium signalling mediated by point-source burst release of ATP. Proc Natl Acad Sci USA. 2002;99:9840–9845. doi: 10.1073/pnas.152588599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzam EI, De Toledo SM, Little JB. Direct evidence for the participation of gap junction-mediated intercellular communication in the transmission of damage signals from alpha-particle irradiated to nonirradiated cells. Proc Natl Acad Sci USA. 2001;98:473–478. doi: 10.1073/pnas.011417098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beahm DL, Hall JE. Hemichannel and gap junctional properties of connexin 50. Biophys J. 2002;82:2016–2031. doi: 10.1016/S0006-3495(02)75550-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardslee MA, Laing JG, Beyer EC, Saffitz JE. Rapid turnover of connexin43 in the adult rat heart. Circ Res. 1998;83:629–635. doi: 10.1161/01.res.83.6.629. [DOI] [PubMed] [Google Scholar]
- Bennett MVL, Barrio LC, Bargiello TA, Spray DC, Hertzberg E, Sáez JC. Gap junctions: new tools, new answers, new questions. Neuron. 1991;6:305–320. doi: 10.1016/0896-6273(91)90241-q. [DOI] [PubMed] [Google Scholar]
- Berthoud VM, Rook MB, Traub O, Hertzberg EL, Sáez JC. On the mechanisms of cell uncoupling induced by a tumor promoter phorbol ester in clone 9 cells, a rat liver epithelial cell line. Eur J Cell Biol. 1993;62:384–396. [PubMed] [Google Scholar]
- Berthoud VM, Cook AJ, Beyer EC. Characterization of the gap junction protein connexin56 in the chicken lens by immunofluorescence and immunoblotting. Invest Ophthalmol Vis Sci. 1994;35:4109–4117. [PubMed] [Google Scholar]
- Berthoud VM, Montegna EA, Atal N, Aithal NH, Brink PR, Beyer EC. Heteromeric connexons formed by the lens connexins, connexin43 and connexin56. EurJ Cell Biol. 2001;80:11–19. doi: 10.1078/0171-9335-00132. [DOI] [PubMed] [Google Scholar]
- Beyer EC. Molecular cloning and developmental expression of two chick embryo gap junction proteins. J Biol Chem. 1990;265:14439–14443. [PubMed] [Google Scholar]
- Bisaggio RD, Nihei OK, Persechini PM, Savino W, Alves LA. Characterization of P2 receptors in thymic epithelial cells. Cell Mol Biol. 2001;47:19–31. [PubMed] [Google Scholar]
- Blanc EM, Bruce-Keller AJ, Mattson MP. Astrocytic gap junctional communication decreases neuronal vulnerability to oxidative stress-induced disruption of Ca2+ homeostasis and cell death. J Neurochem. 1998;70:958–970. doi: 10.1046/j.1471-4159.1998.70030958.x. [DOI] [PubMed] [Google Scholar]
- Braet K, Vandame W, Martin PEM, Evans WH, Leybaert L. Photoliberating inositol-l,4,5-trispho-sphate triggers ATP release that is blocked by the connexin minetic peptide gap 26. Cell Calcium. 2003;33:37–48. doi: 10.1016/s0143-4160(02)00180-x. [DOI] [PubMed] [Google Scholar]
- Brañes MC, Contreras JE, Sáez JC. Activation of human polymorphonuclear cells induces formation of functional gap junctions and expression of connexins. Med Sci Monit. 2002;8:BR313–BR323. [PubMed] [Google Scholar]
- Bruzzone S, Franco L, Guida L, et al. A self-restricted CD38-connexin 43 cross-talk affects NAD+ and cyclic ADP-ribose metabolism and regulates intracellular calcium in 3T3 fibroblasts. J Biol Chem. 2001a;276:48300–48308. doi: 10.1074/jbc.M107308200. [DOI] [PubMed] [Google Scholar]
- Bruzzone S, Guida L, Zocchi E, Franco L, De Flora A. Connexin 43 hemi channels mediate Ca2+-regulated transmembrane NAD+ fluxes in intact cells. FASEB J. 2001b;15:10–12. doi: 10.1096/fj.00-0566fje. [DOI] [PubMed] [Google Scholar]
- Bukauskas FF, Bukauskiene A, Bennett MVL, Verselis VK. Gating properties of gap junction channels assembled from connexin43 and connexin43 fused with green fluorescent protein. Biophys J. 2001;81:137–152. doi: 10.1016/S0006-3495(01)75687-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukauskas FF, Peracchia C. Two distinct gating mechanisms in gap junction channels: CO2-sensitive and voltage-sensitive. Biophys J. 1997;72:2137–2142. doi: 10.1016/S0006-3495(97)78856-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows FJ, Gore M, Smiley WR, et al. Purified herpes simplex virus thymidine kinase retroviral particles: III. Characterization of bystander killing mechanisms in transfected tumor cells. Cancer Gene Ther. 2002;9:87–95. doi: 10.1038/sj.cgt.7700401. [DOI] [PubMed] [Google Scholar]
- Castro C, Gómez-Hernández JM, Silander K, Barrio LC. Altered formation of hemichannels and gap junction channels caused by C-terminal connexin-32 mutations. J Neurosci. 1999;19:3752–3760. doi: 10.1523/JNEUROSCI.19-10-03752.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KC, Nicholson C. Spatial buffering of potassium ions in brain extracellular space. Biophys J. 2000;78:2776–2797. doi: 10.1016/S0006-3495(00)76822-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng HL, Louis CF. Endogenous casein kinase I catalyzes the phosphorylation of the lens fiber cell con-nexin49. Eur J Biochem. 1999;263:276–286. doi: 10.1046/j.1432-1327.1999.00502.x. [DOI] [PubMed] [Google Scholar]
- Cheng HL, Louis CF. Functional effects of casein kinase I-catalyzed phosphorylation on lens cell-to-cell coupling. J Membr Biol. 2001;181:21–30. doi: 10.1007/s0023200100055. [DOI] [PubMed] [Google Scholar]
- Cheng Q, Lichtstein D, Russell P, Zigler JS., Jr. Use of a lipophilic cation to monitor electrical membrane potential in the intact rat lens. Invest Ophthalmol Vis Sci. 2000;41:482–487. [PubMed] [Google Scholar]
- Coco S, Calegari F, Pravettoni E, et al. Storage and release of ATP from astrocytes in culture. J Biol Chem. 2003;278:1354–1362. doi: 10.1074/jbc.M209454200. [DOI] [PubMed] [Google Scholar]
- Contreras JE, Sánchez HA, Eugenín EA, et al. Metabolic inhibition induces opening of unopposed connexin 43 gap junction hemichannels and reduces gap junctional communication in cortical astrocytes in culture. Proc Natl Acad Sci USA. 2002a;99:495–500. doi: 10.1073/pnas.012589799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras JE, Bukauskiene A, Sáez JC, Bukauskas FF, Bennett MVL. Gating and regulation of connexin 43 hemichannels. Mol Biol Cell. 2002b;13:351a. [Google Scholar]
- Cooper CD, Lampe PD. Casein kinase 1 regulates connexin-43 gap junction assembly. J Biol Chem. 2002;277:44962–44968. doi: 10.1074/jbc.M209427200. [DOI] [PubMed] [Google Scholar]
- Cotrina ML, Lin JHC, Alves-Rodrigues A, et al. Connexins regulate calcium signalling by controlling ATP release. Proc Natl Acad Sci USA. 1998;95:15735–15740. doi: 10.1073/pnas.95.26.15735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dermietzel R, Hertzberg EL, Kessler JA, Spray DC. Gap junctions between cultured astrocytes: immunocytochemical, molecular, and electrophysiological analysis. J Neurosci. 1991;11:1421–1432. doi: 10.1523/JNEUROSCI.11-05-01421.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dermietzel R, Farooq M, Kessler JA, Althaus H, Hertzberg EL, Spray DC. Oligodendrocytes express gap junction proteins connexin32 and connexin45. Glia. 1997;20:101–114. [PubMed] [Google Scholar]
- Dermietzel R, Gao Y, Scemes E, et al. Connexin43 null mice reveal that astrocytes express multiple ‘connexins. Brain Res Brain Res Rev. 2000;32:45–56. doi: 10.1016/s0165-0173(99)00067-3. [DOI] [PubMed] [Google Scholar]
- DeVries SH, Schwartz EA. Hemi-gap-junction channels in solitary horizontal cells of the catfish retina. J Physiol. 1992;445:201–230. doi: 10.1113/jphysiol.1992.sp018920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díez JA, Ahmad S, Evans WH. Assembly of heteromeric connexons in guinea-pig liver en route to the Golgi apparatus, plasma membrane and gap junctions. Eur J Biochem. 1999;262:142–148. doi: 10.1046/j.1432-1327.1999.00343.x. [DOI] [PubMed] [Google Scholar]
- Dixon DB, Takahashi K, Bieda M, Copenhagen DR. Quinine, intracellular pH and modulation of hemi-gap junctions in catfish horizontal cells. Vision Res. 1996;36:3925–3931. doi: 10.1016/s0042-6989(96)00129-0. [DOI] [PubMed] [Google Scholar]
- Ebihara L, Steiner E. Properties of a nonjunctional current expressed from a rat connexin46 cDNA in Xenopus oocytes. J Gen Physiol. 1993;102:59–74. doi: 10.1085/jgp.102.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara L, Berthoud VM, Beyer EC. Distinct behaviour of connexin56 and connexin46 gap junctional channels can be predicted from the behaviour of their hemi-gap-junctional channels. Biophys J. 1995;68:1796–1803. doi: 10.1016/S0006-3495(95)80356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara L, Xu X, Oberti C, Beyer EC, Berthoud VM. Co-expression of lens fiber connexins modifies hemi-gap-junctional channel behaviour. Biophys J. 1999;76:198–206. doi: 10.1016/S0006-3495(99)77189-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara L, Liu X, Pal JD. Effect of external magnesium and calcium on human connexin46 hemichannels. Biophys J. 2003;84:277–286. doi: 10.1016/S0006-3495(03)74848-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfgang L, Eckert R, Lichtenberg-Frate H, et al. Specific permeability and selective formation of gap junction channels in connexin-transfected HeLa cells. J Cell Biol. 1995;129:805–817. doi: 10.1083/jcb.129.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskandari S, Zampighi GA, Leung DW, Wright EM, Loo DD. Inhibition of gap junction hemichannels by chloride channel blockers. J Membr Biol. 2002;185:93–102. doi: 10.1007/s00232-001-0115-0. [DOI] [PubMed] [Google Scholar]
- Eugenín EA, Eckardt D, Theis M, Willecke K, Bennett MVL, Sáez JC. Microglia at brain stab wounds express connexin 43 and in vitro form functional gap junctions after treatment with interferon-gamma and tumor necrosis factor-alpha. Proc Natl Acad Sci USA. 2001;98:4190–4195. doi: 10.1073/pnas.051634298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon RF, Goodenough DA. Five-hour half life of mouse liver gap junction protein. J Cell Biol. 1981;90:521–526. doi: 10.1083/jcb.90.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari D, La Sala A, Chiozzi P, et al. The P2 purinergic receptors of human dendritic cells: identification and coupling to cytokine release. FASEB J. 2000;14:2466–2476. doi: 10.1096/fj.00-0031com. [DOI] [PubMed] [Google Scholar]
- Franco L, Zocchi E, Usai C, et al. Paracrine roles of NAD+ and cyclic ADP-ribose in increasing intracellular calcium and enhancing cell proliferation of 3T3 fibroblasts. J Biol Chem. 2001;276:21642–21648. doi: 10.1074/jbc.M010536200. [DOI] [PubMed] [Google Scholar]
- Frantseva MV, Kokarovtseva L, Perez Velásquez JL. Ischemia-induced brain damage depends on specific gap-junctional coupling. J Cereb Blood Flow Metab. 2002a;22:453–462. doi: 10.1097/00004647-200204000-00009. [DOI] [PubMed] [Google Scholar]
- Frantseva MV, Kokarovtseva L, Naus CG, et al. Specific gap junctions enhance the neuronal vulnerability to brain traumatic injury. J Neurosci. 2002b;22:644–653. doi: 10.1523/JNEUROSCI.22-03-00644.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaietta G, Deerinck TJ, Adams SR, et al. Multicolour and electron microscopic imaging of connexin trafficking. Science. 2002;269:503–507. doi: 10.1126/science.1068793. [DOI] [PubMed] [Google Scholar]
- George CH, Kendall JM, Evans WH. Intracellular trafficking pathways in the assembly of connexins into gap junctions. J Biol Chem. 1999;274:8678–8685. doi: 10.1074/jbc.274.13.8678. [DOI] [PubMed] [Google Scholar]
- Giaume C, Marin P, Codier J, Glowinski J, Premont J. Adrenergic regulation of intercellular communications between cultured striatal astrocytes from the mouse. Proc Natl Acad Sci USA. 1991;88:5577–5581. doi: 10.1073/pnas.88.13.5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman SA, Pulsinelli WA, Clarke WY, Kraig RP, Plum F. The effects of extracellular acidosis on neurons and glia in vitro. J Cereb Blood Flow Metab. 1989;9:471–477. doi: 10.1038/jcbfm.1989.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan X, Cravatt BF, Ehring GR, et al. The sleep-inducing lipid oleamide deconvolutes gap junction communication and calcium wave transmission in glial cells. J Cell Biol. 1997;139:1785–1792. doi: 10.1083/jcb.139.7.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrier A, Fonlupt P, Rabilloud I, et al. Gap junctions and cell polarity: connexin32 and connexin43 expressed in polarized thyroid epithelial cells assemble into separate gap junctions, which are located in distinct regions of the lateral plasma membrane domain. J Cell Sci. 1995;108:2609–2617. doi: 10.1242/jcs.108.7.2609. [DOI] [PubMed] [Google Scholar]
- Gupta VK, Berthoud VM, Atal N, Jarillo JA, Barrio LC, Beyer EC. Bovine connexin44, a lens gap junction protein: molecular cloning, immunological characterization, and functional expression. Invest Ophthalmol Vis Sci. 1994;35:3747–3758. [PubMed] [Google Scholar]
- Guthrie PB, Knappenberger J, Segal M, Bennett MVL, Charles AC, Kater SBI. ATP released from astrocytes mediates glial calcium waves. J Neurosci. 1999;19:520–528. doi: 10.1523/JNEUROSCI.19-02-00520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold DE, Walz W. Metabolic inhibition and electrical properties of type-1-like cortical astrocytes. Neuroscience. 1992;47:203–211. doi: 10.1016/0306-4522(92)90133-m. [DOI] [PubMed] [Google Scholar]
- Hassinger TD, Guthrie PB, Atkinson PB, Bennett MVL, Kater SB. An extracellular signalling component in propagation of astrocytic calcium waves. Proc Natl Acad Sci USA. 1996;93:13268–13273. doi: 10.1073/pnas.93.23.13268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer A, Dermietzel R. Visualization and functional blocking of gap junction hemichannels (connexons) with antibodies against external loop domains in astrocytes. Glia. 1998;24:141–154. doi: 10.1002/(sici)1098-1136(199809)24:1<141::aid-glia13>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Hu X, Dahl G. Exchange of conductance and gating properties between gap junction hemichannels. FEBS Lett. 1999;451:113–117. doi: 10.1016/s0014-5793(99)00558-x. [DOI] [PubMed] [Google Scholar]
- Huang R, Liu YG, Lin Y, et al. Enhanced apoptosis under low serum conditions in human glioblastoma cells by connexin 43 (Cx43) Mol Carcinog. 2001;32:128–138. doi: 10.1002/mc.1072. [DOI] [PubMed] [Google Scholar]
- Hubscher CH, Petruska JC, Rau KK, Johnson RD. Co-expression of P2X receptor subunits on rat nodose neurons that bind the isolectin GS-I-B4. Neuroreport. 2001;12:2995–2997. doi: 10.1097/00001756-200109170-00048. [DOI] [PubMed] [Google Scholar]
- Janssen-Bienhold U, Schultz K, Gellhaus A, Schmidt P, Ammermuller J, Weiler R. Identification and localization of connexin26 within the photoreceptor-horizontal cell synaptic complex. Vis Neurosci. 2001;18:169–178. doi: 10.1017/s0952523801182015. [DOI] [PubMed] [Google Scholar]
- Jedamzik B, Marten I, Ngezahayo A, Ernst A, Kolb HA. Regulation of lens rCx46-formed hemichannels by activation of protein kinase C, external Ca2+ and protons. J Membr Biol. 2000;173:39–46. doi: 10.1007/s002320001005. [DOI] [PubMed] [Google Scholar]
- Jiang JX, Goodenough DA. Heteromeric connexons in lens gap junction channels. Proc Natl Acad Sci USA. 1996;93:1287–1291. doi: 10.1073/pnas.93.3.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John SA, Kondo R, Wang SY, Goldhaber JI, Weiss JN. Connexin-43 hemichannels opened by metabolic inhibition. J Biol Chem. 1999;274:236–240. doi: 10.1074/jbc.274.1.236. [DOI] [PubMed] [Google Scholar]
- Kamermans M, Fahrenfort I, Schultz K, Janssen-Bienhold U, Sjoerdsma T, Weiler R. Hemichannel-mediated inhibition in the outer retina. Science. 2001;292:1178–1180. doi: 10.1126/science.1060101. [DOI] [PubMed] [Google Scholar]
- Kim DY, Kam Y, Koo SK, Joe CO. Gating connexin 43 channels reconstituted in lipid vesicles by mitogen-activated protein kinase phosphorylation. J Biol Chem. 1999;274:5581–5587. doi: 10.1074/jbc.274.9.5581. [DOI] [PubMed] [Google Scholar]
- Kondo RP, Wang SY, John SA, Weiss JN, Goldhaber JI. Metabolic inhibition activates a nonselective current through connexin hemichannels in isolated ventricular myocytes. J Mol Cel Cardiol. 2000;32:1859–1872. doi: 10.1006/jmcc.2000.1220. [DOI] [PubMed] [Google Scholar]
- Kwak BR, Sáez JC, Wilders R, et al. Effects of cGMP-dependent phosphorylation on rat and human connexin43 gap junction channels. Pflügers Arch. 1995;430:770–778. doi: 10.1007/BF00386175. [DOI] [PubMed] [Google Scholar]
- Leung DS, Unsicker K, Reuss B. Expression and developmental regulation of gap junction connexins cx26, cx32, cx43 and cx45 in the rat midbrain-floor. Int J Dev Neurosci. 2002;20:63–75. doi: 10.1016/s0736-5748(01)00056-9. [DOI] [PubMed] [Google Scholar]
- Li F, Sugishita K, Su Z, Ueda I, Barry WH. Activation of connexin-43 hemichannels can elevate [Ca(2+)]i and [Na(+)]i in rabbit ventricular myocytes during metabolic inhibition. J Mol Cell Cardiol. 2001;33:2145–2155. doi: 10.1006/jmcc.2001.1477. [DOI] [PubMed] [Google Scholar]
- Li H, Liu TF, Lazrak A, Peracchia C, et al. Properties and regulation of gap junctional hemichannels in the plasma membranes of cultured cells. J Cell Biol. 1996;134:1019–1030. doi: 10.1083/jcb.134.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WE, Nagy JI. Connexin43 phosphorylation state and intercellular communication in cultured astrocytes following hypoxia and protein phosphatase inhibition. Eur J Neurosci. 2000;12:2644–2650. doi: 10.1046/j.1460-9568.2000.00162.x. [DOI] [PubMed] [Google Scholar]
- Lin JH, Weigel H, Cotrina ML, et al. Gap-junction-mediated propagation and amplification of cell injury. Nat Neurosci. 1998;1:494–500. doi: 10.1038/2210. [DOI] [PubMed] [Google Scholar]
- Liu TF, Paulson AF, Li HY, Atkinson MM, Johnson RG. Inhibitory effects of 12-O-tetradecanoylphorbol-13-acetate on dye leakage from single Novikoff cells and on dye transfer between reaggregated cell pairs. Methods Find Exp Clin Pharmacol. 1997;19:573–577. [PubMed] [Google Scholar]
- Locke D, Perusinghe N, Newman T, Jayatilake H, Evans WH, Monaghan P. Developmental expression and assembly of connexins into homomeric and heteromeric gap junction hemichannels in the mouse mammary gland. J Cell Physiol. 2000;183:228–237. doi: 10.1002/(SICI)1097-4652(200005)183:2<228::AID-JCP9>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Long MA, Deans MR, Paul DL, Connors BW. Rhythmicity without synchrony in the electrically uncoupled inferior olive. J Neurosci. 2002;22:10898–10905. doi: 10.1523/JNEUROSCI.22-24-10898.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malchow RP, Qian H, Ripps HA. Novel action of quinine and quinidine on the membrane conductance of neurons from the vertebrate retina. J Gen Physiol. 1994;104:1039–1055. doi: 10.1085/jgp.104.6.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PE, Blundell G, Ahmad S, Errington RJ, Evans WH. Multiple pathways in the trafficking and assembly of connexin 26, 32 and 43 into gap junction intercellular communication channels. J Cell Sci. 2001;114:3845–3855. doi: 10.1242/jcs.114.21.3845. [DOI] [PubMed] [Google Scholar]
- Martínez AD, Hayrapetyan V, Moreno AP, Beyer EC. Connexin43 and connexin45 form heteromeric gap junction channels in which individual components determine permeability and regulation. Circ Res. 2002;90:1100–1107. doi: 10.1161/01.res.0000019580.64013.31. [DOI] [PubMed] [Google Scholar]
- Martínez AD, Sáez JC. Regulation of astrocyte gap junctions by hypoxia-reoxygenation. Brain Res Brain Res Rev. 2000;32:250–258. doi: 10.1016/s0165-0173(99)00086-7. [DOI] [PubMed] [Google Scholar]
- Martins-Ferreira H, Ribeiro LJ. Biphasic effects of gap junctional uncoupling agents on the propagation of retinal spreading depression. Braz J Med Biol Res. 1995;28:991–994. [PubMed] [Google Scholar]
- Meier C, Petrasch-Parwez E, Habbes HW, et al. Immunohistochemical detection of the neuronal connexin36 in the mouse central nervous system in comparison to connexin36-deficient tissues. Histochem Cell Biol. 2002;117:461–471. doi: 10.1007/s00418-002-0417-z. [DOI] [PubMed] [Google Scholar]
- Mitropoulou G, Bruzzone R. Modulation of perch connexin35 hemi-channels by cyclic AMP requires a protein kinase A phosphorylation site. J Neurosci Res. 2003;72:147–157. doi: 10.1002/jnr.10572. [DOI] [PubMed] [Google Scholar]
- Moreno AP, Sáez JC, Fishman GI, Spray DC. Human connexin43 gap junction channels. Regulation of unitary conductances by phosphorylation. Circ Res. 1994;74:1050–1057. doi: 10.1161/01.res.74.6.1050. [DOI] [PubMed] [Google Scholar]
- Musil LS, Cunningham BA, Edelman GM, Good-enough DA. Differential phosphorylation of the gap junction protein connexin43 in junctional communication-competent and -deficient cell lines. J Cell Biol. 1990;111:2077–2088. doi: 10.1083/jcb.111.5.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musil LS, Goodenough DA. Multisubunit assembly of an integral plasma membrane channel protein, gap junction connexin43, occurs after exit from the ER. Cell. 1993;74:1065–1077. doi: 10.1016/0092-8674(93)90728-9. [DOI] [PubMed] [Google Scholar]
- Nadarajah B, Jones AM, Evans WH, Parnavelas JG. Differential expression of connexins during neocortical development and neuronal circuit formation. J Neurosci. 1997;17:3096–3111. doi: 10.1523/JNEUROSCI.17-09-03096.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy JI, Ochalski PA, Li J, Hertzberg EL. Evidence for the co-localization of another connexin with connexin-43 at astrocytic gap junctions in rat brain. Neuroscience. 1997;78:533–548. doi: 10.1016/s0306-4522(96)00584-2. [DOI] [PubMed] [Google Scholar]
- Nagy JI, Patel D, Ochalski PA, Stelmack GL. Connexin30 in rodent, cat and human brain: selective expression in gray matter astrocytes, co-localization with connexin43 at gap junctions and late developmental appearance. Neuroscience. 1999;88:447–468. doi: 10.1016/s0306-4522(98)00191-2. [DOI] [PubMed] [Google Scholar]
- Naus CC, Bechberger JF, Zhang Y, et al. Altered gap junctional communication, intercellular signalling, and growth in cultured astrocytes deficient in connexin43. J Neurosci Res. 1997;49:528–540. doi: 10.1002/(SICI)1097-4547(19970901)49:5<528::AID-JNR3>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Goldman SA, Desai S, Pulsinelli WA. Acid-induced death in neurons and glia. J Neurosci. 1991;11:2489–2497. doi: 10.1523/JNEUROSCI.11-08-02489.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard M, Cooper AJ, Goldman SA. Gap junctions are required for the propagation of spreading depression. J Neurobiol. 1995;28:433–444. doi: 10.1002/neu.480280404. [DOI] [PubMed] [Google Scholar]
- Ngezahayo A, Zeilinger C, Todt II, Marten II, Kolb H. Inactivation of expressed and conducting rCx46 hemichannels by phosphorylation. Pflügers Arch. 1998;436:627–629. doi: 10.1007/s004240050681. [DOI] [PubMed] [Google Scholar]
- Oh SY, Schmidt SA, Murray AW. Epidermal growth factor inhibits gap junctional communication and stimulates serine-phosphorylation of connexin43 in WB cells by a protein kinase C-independent mechanism. Cell Adhes Commun. 1993;1:143–149. doi: 10.3109/15419069309095690. [DOI] [PubMed] [Google Scholar]
- Oh S, Abrams CK, Verselis VK, Bargiello TA. Stoichiometry of transjunctional voltage-gating polarity reversal by a negative charge substitution in the amino terminus of a connexin32 chimera. J Gen Physiol. 2000;116:13–31. doi: 10.1085/jgp.116.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguro K, Jover T, Tanaka H, et al. Global ischemia-induced increases in gap junctional proteins 32 (Cx32) and Cx36 in hippocampus and enhanced vulnerability of Cx32 knock-out mice. J Neurosci. 2001;21:7534–7542. doi: 10.1523/JNEUROSCI.21-19-07534.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozog MA, Siushansian R, Naus CC. Blocked gap junctional coupling increases glutamate-induced neurotoxicity in neuron-astrocyte co-cultures. J Neuropathol Exp Neurol. 2002;61:132–141. doi: 10.1093/jnen/61.2.132. [DOI] [PubMed] [Google Scholar]
- Pal JD, Liu X, Mackay D, et al. Connexin46 mutations linked to congenital cataract show loss of gap junction channel function. Am J Physiol Cell Physiol. 2000;279:C596–C602. doi: 10.1152/ajpcell.2000.279.3.C596. [DOI] [PubMed] [Google Scholar]
- Parenti R, Campisi A, Vanella A, Cicirata F. Immunocytochemical and RT-PCR analysis of connexin36 in cultures of mammalian glial cells. Arch Ital Biol. 2002;140:101–108. [PubMed] [Google Scholar]
- Paul DL, Ebihara L, Takemoto LJ, Swenson KI, Goodenough DA. Connexin46, a novel lens gap junction protein, induces voltage-gated currents in non-junctional plasma membrane of Xenopus oocytes. J Cell Biol. 1991;115:1077–1089. doi: 10.1083/jcb.115.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peuchen S, Clark JB, Duchen MR. Mechanisms of intracellular calcium regulation in adult astrocytes. Neuroscience. 1996;71:871–883. doi: 10.1016/0306-4522(95)00515-3. [DOI] [PubMed] [Google Scholar]
- Pfahnl A, Dahl G. Gating of cx46 gap junction hemichannels by calcium and voltage. Pflügers Arch. 1999;437:345–353. doi: 10.1007/s004240050788. [DOI] [PubMed] [Google Scholar]
- Pfahnl A, Zhou XW, Werner R, Dahl G. A chimeric connexin forming gap junction hemichannels. Pflügers Arch. 1997;433:773–779. doi: 10.1007/s004240050344. [DOI] [PubMed] [Google Scholar]
- Plotkin LI, Manolagas SC, Bellido T. Transduction of cell survival signals by connexin 43 hemichannels. J Biol Chem. 2002;277:8648–8657. doi: 10.1074/jbc.M108625200. [DOI] [PubMed] [Google Scholar]
- Puil E, El-Beheiry H. Anaesthetic suppression of transmitter actions in neocortex. Br J Pharmacol. 1990;101:61–66. doi: 10.1111/j.1476-5381.1990.tb12089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puil E, Meiri H, Yarom Y. Resonant behavior and frequency preferences of thalamic neurons. J Neurophysiol. 1994;71:575–582. doi: 10.1152/jn.1994.71.2.575. [DOI] [PubMed] [Google Scholar]
- Purnick PE, Oh S, Abrams CK, Verselis VK, Bargiello TA. Reversal of the gating polarity of gap junctions by negative charge substitutions in the N-terminus of connexin 32. Biophys J. 2000;79:2403–2415. doi: 10.1016/S0006-3495(00)76485-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quist AP, Rhee SK, Lin H, Lal R. Physiological role of gap-junctional hemichannels. Extracellular calcium-dependent isosmotic volume regulation. J Cell Biol. 2000;148:1063–1074. doi: 10.1083/jcb.148.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman S, Carlile G, Evans WH. Assembly of hepatic gap junctions. Topography and distribution of connexin 32 in intracellular and plasma membranes determined using sequence-specific antibodies. J Biol Chem. 1993;268:1260–1265. [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Rash JE, Yasumura T. Direct immunogold labelling of connexins and aquaporin-4 in freeze-fracture replicas of liver, brain, and spinal cord: factors limiting quantitative analysis. Cell Tissue Res. 1999;296:307–321. doi: 10.1007/s004410051291. [DOI] [PubMed] [Google Scholar]
- Rash JE, Yasumura T, Davidson KG, Furman CS, Dudek FE, Nagy JI. Identification of cells expressing Cx43, Cx30, Cx26, Cx32 and Cx36 in gap junctions of rat brain and spinal cord. Cell Commun Adhes. 2001a;8:315–320. doi: 10.3109/15419060109080745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash JE, Yasumura T, Dudek FE, Nagy JI. Cell-specific expression of connexins and evidence of restricted gap junctional coupling between glial cells and between neurons. J Neurosci. 2001b;21:1983–2000. doi: 10.1523/JNEUROSCI.21-06-01983.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawanduzy A, Hansen A, Hansen TW, Nedergaard M. Effective reduction of infarct volume by gap junction blockade in a rodent model of stroke. J Neurosurg. 1997;87:916–920. doi: 10.3171/jns.1997.87.6.0916. [DOI] [PubMed] [Google Scholar]
- Ripps H, Qian H, Zakevicius J. Pharmacological enhancement of hemi-gap-junctional currents in Xenopus oocytes. J Neurosci Methods. 2002;121:81–92. doi: 10.1016/s0165-0270(02)00243-1. [DOI] [PubMed] [Google Scholar]
- Rivedal E, Opsahl H. Role of PKC and MAP kinase in EGF- and TPA-induced connexin43 phosphorylation and inhibition of gap junction intercellular communication in rat liver epithelial cells. Carcinogenesis. 2001;22:1543–1550. doi: 10.1093/carcin/22.9.1543. [DOI] [PubMed] [Google Scholar]
- Romanello M, D’Andrea P. Dual mechanism of intercellular communication in HOBIT osteoblastic cells: a role for gap-junctional hemichannels. J Bone Miner Res. 2001;16:1465–1476. doi: 10.1359/jbmr.2001.16.8.1465. [DOI] [PubMed] [Google Scholar]
- Romanello M, Pani B, Bicego M, D’Andrea P. Mechanically induced ATP release from human osteoblastic cells. Biochem Biophys Res Commun. 2001;289:1275–1281. doi: 10.1006/bbrc.2001.6124. [DOI] [PubMed] [Google Scholar]
- Ruch RJ, Trosko JE, Madhukar BV. Inhibition of connexin43 gap junctional intercellular communication by TPA requires ERK activation. J Cell Biochem. 2001;83:163–169. doi: 10.1002/jcb.1227. [DOI] [PubMed] [Google Scholar]
- Sanson M, Marcaud V, Robin E, Valery C, Sturtz F, Zalc B. Connexin 43-mediated bystander effect in two rat glioma cell models. Cancer Gene Ther. 2002;9:149–155. doi: 10.1038/sj.cgt.7700411. [DOI] [PubMed] [Google Scholar]
- Sarma JD, Wang F, Koval M. Targeted gap junction protein constructs reveal connexin-specific differences in oligomerization. J Biol Chem. 2002;211:20911–20918. doi: 10.1074/jbc.M111498200. [DOI] [PubMed] [Google Scholar]
- Scemes E, Dermietzel R, Spray DC. Calcium waves between astrocytes from Cx43 knockout mice. Glia. 1998;24:65–73. doi: 10.1002/(sici)1098-1136(199809)24:1<65::aid-glia7>3.0.co;2-#. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiosaka S, Yamamoto T, Hertzberg EL, Nagy JI. Gap junction protein in rat hippocampus: correlative light and electron microscope immunohistochemical localization. J Comp Neurol. 1989;281:282–297. doi: 10.1002/cne.902810210. [DOI] [PubMed] [Google Scholar]
- Siushansian R, Bechberger JF, Cechetto DF, Hachinski VC, Naus CC. Connexin43 null mutation increases infarct size after stroke. J Comp Neurol. 2001;440:387–394. doi: 10.1002/cne.1392. [DOI] [PubMed] [Google Scholar]
- SiuYi Leung D, Unsicker K, Reuss B. Gap junctions modulate survival-promoting effects of fibroblast growth factor-2 on cultured midbrain dopaminergic neurons. Mol Cell Neurosci. 2001;18:44–55. doi: 10.1006/mcne.2001.1002. [DOI] [PubMed] [Google Scholar]
- Sohl G, Guldenagel M, Beck H, et al. Expression of connexin genes in hippocampus of kainate-treated and kindled rats under conditions of experimental epilepsy. Brain Res Mol Brain Res. 2000;83:44–51. doi: 10.1016/s0169-328x(00)00195-9. [DOI] [PubMed] [Google Scholar]
- Spray DC, Moreno AP, Kessler JA, Dermietzel R. Characterization of gap junctions between cultured leptomeningeal cells. Brain Res. 1991;568:1–14. doi: 10.1016/0006-8993(91)91373-9. [DOI] [PubMed] [Google Scholar]
- Srinivas M, Hopperstad MG, Spray DC. Quinine blocks specific gap junction channel subtypes. Proc Natl Acad Sci USA. 2001;98:10942–10947. doi: 10.1073/pnas.191206198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout CE, Costantin JL, Naus CC, Charles AC. Intercellular calcium signalling in astrocytes via ATP release through connexin hemichannels. J Biol Chem. 2002;211:10482–10488. doi: 10.1074/jbc.M109902200. [DOI] [PubMed] [Google Scholar]
- Takeshita T, Okamura R, Nishi K. Effects of external high K+ on Na+ and H+ activities at the posterior cortical layers of rat lens in vitro. Ophthalmic Res. 1993;25:36–45. doi: 10.1159/000267219. [DOI] [PubMed] [Google Scholar]
- Taylor BM, Fleming WE, Benjamin CW, Wu Y, Mathews WR, Sun FF. The mechanism of cytoprotective action of lazaroids I: Inhibition of reactive oxygen species formation and lethal cell injury during periods of energy depletion. J Pharmacol Exp Ther. 1996;276:1224–1231. [PubMed] [Google Scholar]
- Teubner B, Degen J, Sohl G, et al. Functional expression of the murine connexin 36 gene coding for a neuron-specific gap junctional protein. J Membr Biol. 2000;116:249–262. doi: 10.1007/s00232001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub O, Look J, Paul D, Willecke K. Cyclic adenosine monophosphate stimulates biosynthesis and phosphorylation of the 26 kDa gap junction protein in cultured mouse hepatocytes. Eur J Cell Biol. 1987;43:48–54. [PubMed] [Google Scholar]
- Traub O, Look J, Dermietzel R, Brummer F, Hulser D, Willecke K. Comparative characterization of the 21-kD and 26-kD gap junction proteins in murine liver and cultured hepatocytes. J Cell Biol. 1989;108:1039–1051. doi: 10.1083/jcb.108.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trexler EB, Bennett MVL, Bargiello TA, Verselis VK. Voltage gating and permeation in a gap junction hemichannel. Proc Natl Acad Sci USA. 1996;93:5836–5841. doi: 10.1073/pnas.93.12.5836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trexler EB, Bukauskas FF, Bennett MVL, Bargiello TA, Verselis VK. Rapid and direct effects of pH on connexins revealed by the connexin46 hemichannel preparation. J Gen Physiol. 1999;113:721–742. doi: 10.1085/jgp.113.5.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trexler EB, Bukauskas FF, Kronengold J, Bargiello TA, Verselis VK. The first extracellular loop domain is a major determinant of charge selectivity in connexin46 channels. Biophys J. 2000;79:3036–3051. doi: 10.1016/S0006-3495(00)76539-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiunas V. Biophysical properties of connexin-45 gap junction hemichannels studied in vertebrate cells. J Gen Physiol. 2002;119:147–164. doi: 10.1085/jgp.119.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiunas V, Weingart R. Electrical properties of gap junction hemichannels identified in transfected HeLa cells. Pflugers Arch. 2000;440:366–379. doi: 10.1007/s004240000294. [DOI] [PubMed] [Google Scholar]
- Vanoye CG, Vergara LA, Reuss L. Isolated epithelial cells from amphibian urinary bladder express functional gap junctional hemichannels. Am J Physiol. 1999;276:C279–C284. doi: 10.1152/ajpcell.1999.276.1.C279. [DOI] [PubMed] [Google Scholar]
- Venance L, Rozov A, Blatow M, Burnashev N, Feldme-yer D, Monyer H. Connexin expression in electrically coupled postnatal rat brain neurons. Proc Natl Acad Sci USA. 2000;97:10260–10265. doi: 10.1073/pnas.160037097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verderio C, Bruzzone S, Zocchi E, et al. Evidence of a role for cyclic ADP-ribose in calcium signalling and neurotransmitter release in cultured astrocytes. J Neurochem. 2001;78:646–657. doi: 10.1046/j.1471-4159.2001.00455.x. [DOI] [PubMed] [Google Scholar]
- Virginio C, MacKenzie A, Rassendren FA, North RA, Surprenant A. Pore dilation of neuronal P2X receptor channels. Nat Neurosci. 1999;2:315–321. doi: 10.1038/7225. [DOI] [PubMed] [Google Scholar]
- Warn-Cramer BJ, Cottrell GT, Burt JM, Lau AF. Regulation of connexin-43 gap junctional intercellular communication by mitogen-activated kinase. J Biol Chem. 1998;273:9188–9196. doi: 10.1074/jbc.273.15.9188. [DOI] [PubMed] [Google Scholar]
- White TW, Deans MR, O’Brien J, et al. Functional characteristics of skate connexin35, a member of the gamma subfamily of connexins expressed in the vertebrate retina. Eur J Neurosci. 1999;11:1883–1890. doi: 10.1046/j.1460-9568.1999.00607.x. [DOI] [PubMed] [Google Scholar]
- Willecke K, Eiberger J, Degen J, et al. Structural and functional diversity of connexin genes in the mouse and human genome. Biol Chem. 2002;383:725–737. doi: 10.1515/BC.2002.076. [DOI] [PubMed] [Google Scholar]
- Ye Z, Wyeth MS, Baltam-Tekkok S, Ransom BR. Gap junction hemichannels in astrocytes mediate glutamate release upon removal of extracellular divalent cations. J Neurosci. 2002;23:3588–3596. doi: 10.1523/JNEUROSCI.23-09-03588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DQ, McMahon DG. Gating of retinal horizontal cell hemi gap junction channels by voltage, Ca2+, and retinoic acid. Mol Vis. 2001;7:247–252. [PubMed] [Google Scholar]
- Zampighi GA, Loo DD, Kreman M, Eskandari S, Wright EM. Functional and morphological correlates of connexin50 expressed in Xenopus laevis oocytes. J Gen Physiol. 1999;113:507–524. doi: 10.1085/jgp.113.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanotti S, Charles A. Extracellular calcium sensing by glial cells: low extracellular calcium induces intracellular calcium release and intercellular signalling. J Neurochem. 1997;69:594–602. doi: 10.1046/j.1471-4159.1997.69020594.x. [DOI] [PubMed] [Google Scholar]