Abstract
Cultivation of an obligate marine Streptomyces strain has provided the cytotoxic natural product chlorizidine. X-Ray crystallographic analysis revealed that the metabolite is composed of a chlorinated 2,3-dihydropyrrolizine ring attached to a chlorinated 5H-pyrrolo[2,1-a]isoindol-5-one. The carbon stereocenter in the dihydropyrrolizine is S-configured. Remarkably, the 5H-pyrrolo[2,1-a]isoindol-5-one moiety has no precedence in the field of natural products. The presence of this ring system, which was demonstrated to undergo facile nucleophilic substitution reactions at the activated carbonyl group, is essential to the molecule’s cytotoxicity against HCT-116 human colon cancer cells.
There are a substantial number of chemotherapeutic drugs on the market that are based on the scaffolds of actinomycete-derived natural products.1 For instance, chemical studies of terrestrial Streptomyces bacteria led to the discovery of actinomycin D,2a doxorubicin,2b bleomycin,2c carzinophilin,2d chromomycin A3,2e mithramycin,2f mitomycin C,2g sarkomycin,2h and streptozocin,2i and neocarzinostatin.2f No truly novel actinomycete-derived natural product structure has led to the development of a drug in recent years,1 which at least suggests that terrestrial actinomycetes are no longer a viable source for new lead compounds. Studies of marine actinomycete bacteria, however, continue to yield unique chemical structures with anticancer activity.3 For example, salinosporamide A from Salinispora tropica is poised to enter phase II clinical trials.4
In an effort to identify new chemotypes for therapeutic development, Streptomyces sp. strain CNH-287 was cultivated in a seawater-based medium (20 × 1 L).5 Notably, the strain required seawater for growth. Amberlite resin (XAD-18) was added after the first day of cultivation. The resin was filtered and extracted with acetone after seven days, and the crude material was then fractionated on silica gel. One fraction displayed significant cytotoxicity against HCT-116 human colon cancer. A cytotoxic metabolite with a prominent UV/vis profile was isolated from this fraction using C18 reversed-phase HPLC.
It proved difficult to obtain pure compound sufficient for full spectroscopic analysis. During concentration from either organic or aqueous solutions, extensive degradation occurred. Much less decomposition was observed when solutions were kept cool and dried under a stream of nitrogen. In addition, the metabolite could be stored in dilute solutions away from light and air.
A nonzero optical rotation, [α]D −35 (c 0.50, CH3CN), indicated that the natural product was optically active, and a strong IR stretch at 1721 cm−1 revealed the presence of a carbonyl group. Mass spectrometry data for the natural product [HRESI-FT-MS m/z (M+H)+ = 442.9511, 444.9481, 446.9452, 448.9422] showed a molecular ion cluster consistent with molecular formulae that include Cl2Br or Cl4. With 13 degrees of unsaturation, however, only C18H10Cl4N2O3 agreed with proton and carbon NMR data.
We initially attempted to solve the structure of the natural product using 1D and 2D NMR (COSY, HSQC, HMBC) experiments (Table 1). The numbering of the molecule is shown in Figure 1. Low-field signals at δH 6.55 (δC 101.9) and 6.42 (δC 108.2) were conspicuous, in addition to two overlapping signals at δH 5.80 (δC 99.3 and δC 53.0). A spin system including a proton at δH 5.80 and the remaining upfield methylene protons at δH 3.08, 2.90, 2.84, and 2.54 was apparent in the 1H-1H COSY spectrum. Interestingly, the upfield proton signals from δ 2.54-3.08 exhibited complex splitting patterns due to the flexibility in the molecule (vide infra). The paucity of hydrogen atoms and the plethora of quaternary carbon atoms made complete structural elucidation by NMR problematic.
Table 1.
1H, 13C, and HMBC NMR spectral data for chlorizidine A (1) (CD3CN)
| C no. | δCa | δH, mult. (J, Hz)b | HMBCb |
|---|---|---|---|
| 2,3,5a,14,15c | |||
| 1 | 101.9 | 6.55, br s | |
| 5 | 163.0 | ||
| 6 | 163.9 | ||
| 7 | 113.3d | ||
| 8 | 157.5 | ||
| 9 | 108.2 | 6.42, s | 5,7,9a,9b |
| 9a | 135.5e | ||
| 9b | 132.7e | ||
| 10 | 53.0 | 5.80, ddf | 6-8,11 |
| 11 | 32.5 | 2.84, m | 7,12,12a |
| 2.54, m | 6-8,10,12,12a | ||
| 12 | 25.4 | 3.08, m | 10,11,12a,13 |
| 2.90, m | 10,11,12a,13 | ||
| 12a | 136.8 | ||
| 13 | 99.3 | 5.80, sf | 12a,14 |
75 MHz.
500 MHz.
δC = 116.9, 113.0d, 109.7, 106.0, 105.9.
Signals may be switched.
Overlapping signals.
Figure 1.
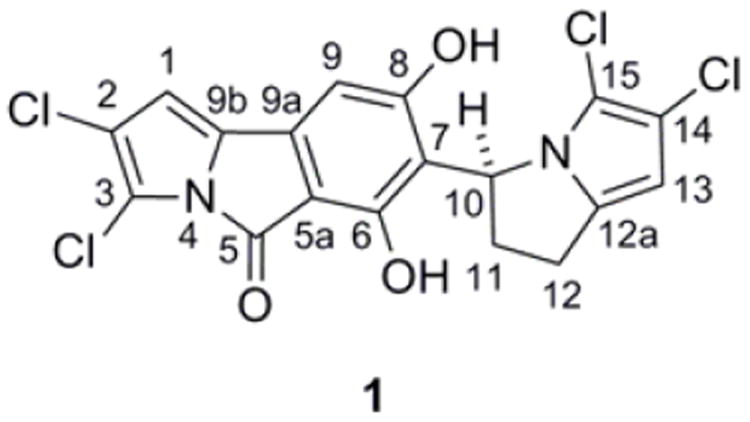
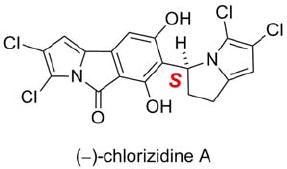
(−)-(S)-Chlorizidine A (1).
The structure of chlorizidine A (1) was finally determined using X-ray crystallographic techniques (Figure 2). Slow evaporation of a concentrated solution of 1 in benzene provided X-ray quality crystals. A molecule of benzene was incorporated into the crystal lattice.6 Additionally, the lone tertiary carbon stereocenter was assigned an S-configuration [Flack parameter −0.02(2)].
Figure 2.
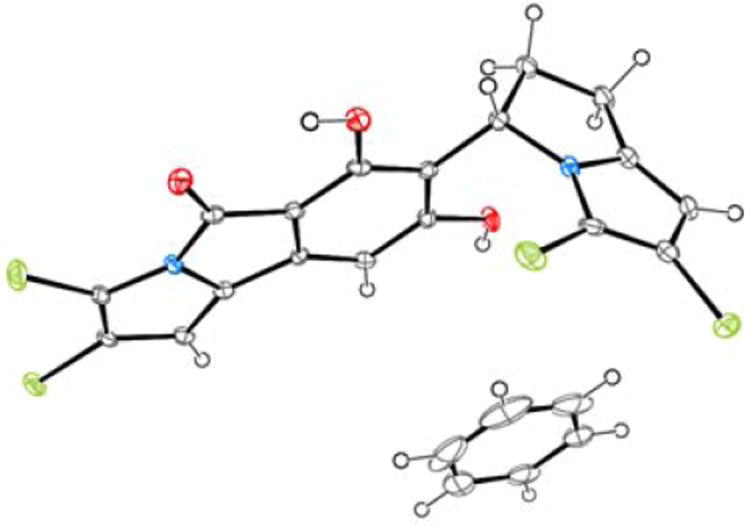
ORTEP plot of 1 with benzene (co-crystallized).
Chlorizidine A (1) displays an unprecedented structure involving a nitrogen-containing carbon skeleton. The discovery of a naturally-occurring 5H-pyrrolo[2,1-a]isoindol-5-one ring system has not been previously described in the literature. Various synthetic accounts of the heterocycle, however, are well documented.7 The pyrroloisoindolone is connected to a dichlorinated 2,3-dihydro-1H-pyrrolizine at C-7.8 In the solid state, the congested region about the sp2-sp3 bond between the two ring systems forces the molecule to adopt a twisted conformation. In solution, though, 1 does not appear to exhibit atropisomerism.9
The phenolic substituents at C-6 and C-8 of chlorizidine A (1) could be readily functionalized. Treatment of 1 with acetic anhydride/triethylamine gave 2, and methylation with dimethyl sulfate provided 3 (Scheme 1). Acetate 2 was a stable chemical entity much less prone to degradation than 1. Interestingly, like the natural product, its proton NMR spectrum showed evidence of slow C-7/C-10 bond rotation relative to the NMR time scale. The well-resolved proton signals at C-1, C-9, C-10, and C-13 in 1 were now “doubled” in 2. The diacetate structure was confirmed using X-ray crystallography. The crystal was composed of two low-energy “twisted” conformers (see Supporting Information).
Scheme 1.
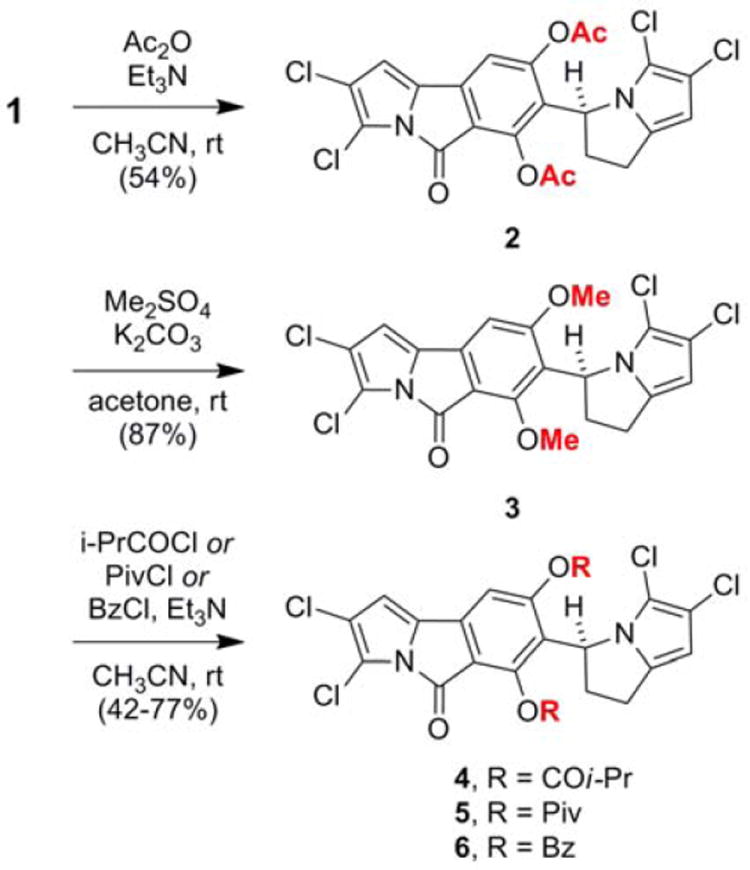
Acylation and methylation of the phenolic groups in chlorizidine A (1)
The semisynthesis of bulkier phenolic esters—isobutyrate 4, pivalate 5, and benzoate 6—was undertaken in an attempt to produce an atropiosmeric mixture, but this approach was not successful (see Scheme 1).6 Analysis of these derivatives was much more complex, as the ester functionalities revealed their own conformational preferences. The proton NMR spectra showed the simultaneous presence of several confomers with distinct chemical shifts (see Supporting Information).
A second metabolite that was prone to degradation, chlorizidine B (7), was isolated from culture extracts of CNH-287 (Scheme 2). A stable diacetate adduct 8 was constructed using acetic anhydride. An additional aromatic NMR signal corresponding to C-7 was noticeable. Presumably, pyrrole 7 arises from hydrolysis and decarboxylation of chlorizidine A (1). The notion that 7 is an artifact of the culturing process was substantiated by treating 1 with a pH 10 buffer composed of K2B4O7, K2CO3, and KOH.10 Under these conditions, the C-7 carboxylic acid was observed [LRESI-MS m/z (M–H)− = 459, 461, 463].11 Notably, many other compounds are formed from the degradation of chlorizidine (1) under basic conditions (see Supporting Information).
Scheme 2.
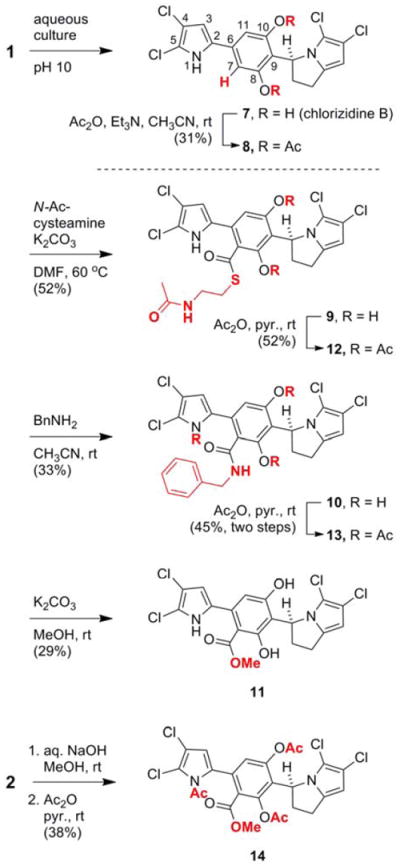
Reactivity of the 5H-pyrrolo[2,1-a]isoindol-5-one in chlorizidine A (1)
The central electrophilic carbonyl group of the 5H-pyrrolo[2,1-a]isoindol-5-one moiety engages sulfur-, oxygen-, and amine-containing nucleophiles in a substitution reaction, whereby the electron-poor dichloropyrrole functions as leaving group (see Scheme 2).5g When subjected to N-acetylcysteamine and potassium carbonate, thioester 9 was produced. Likewise, treatment of 1 with benzylamine gave amide 10 and treatment with potassium carbonate in methanol afforded ester 11. Unlike 9 and 10, ester 11 was not suitably stable for complete purification and analysis. Peracetylation of 9-11 furnished stable derivatives 12-14. A more efficient method for the synthesis of 14 was accomplished by treating diacetate 2 with aqueous NaOH in CH3OH followed by acetylation. Certainly, the isolation of pure chlorizidine A (1) is hindered, in part, by the lability of the pyrroloisoindolone moeity toward nucleophiles.
Chlorizidine A (1) and acylated derivatives 2, 4, and 5 exhibit noteworthy activity in a colon cancer cytotoxicity bioassay (Table 2).12 Against the HCT-116 adenocarcinoma cell line,13 1 showed an IC50 of 3.2-4.9 μM. Compounds 2, 4, and 5 showed similar activity. Irreversible methylation of the phenolic functionality in 1, yielding 3, rendered the compound completely inactive. Any of the series of derivatives lacking the key pyrroloisoindolone ring system (7-14) had no measurable activity, strongly suggesting that this moiety is a crucial part of the metabolite’s pharmacophore.
Table 2.
Cytotoxicity of 1-14 (μM)
| HCT116 (IC50)a | |
|---|---|
| 1 | 3.2-4.9 |
| 2 | 0.6-3.0 |
| 3 | NSA |
| 4 | 1.0-1.2 |
| 5 | 2.1-3.7 |
| 6-14 | NSA |
HCT-116 is a human colon cancer cell line. Positive control: etoposide (IC50 = 0.49-4.9 μM).
NSA = no significant activity.
Chlorizidine A diacetate (2) was tested against the NCI’s panel of 60 tumor cell lines.14 It was modestly selective in terms of its cytotoxicity (See Supporting Information). However, against SK-MEL-5 and SK-MEL-2 melanoma cancer cells, 2 showed a pronounced LC50 of 3.6 μM and 11 μM, respectively. Against MDA-MB-231/ATCC breast cancer cells, an LC50 of 11 μM was also determined.
Chlorizidine A (1) has obvious structural similarity to marinopyrrole A (15), a secondary metabolite from marine-derived Streptomyces sp. CNQ-418 (Scheme 3).15 Biosynthetic precursor 16 is derived from a mixed NRPS-PKS pathway, whereby proline is loaded onto a peptidyl carrier protein, oxidized, chlorinated by an FADH2-dependent halogenase, and subsequently extended by the PKS machinery.16 Cyclization/aromatization provides monodeoxypyoluteorin (17).17 1,3’-Bipyrrole 15 is then formed via a novel atroposelective N,C-pyrrole coupling reaction.18 An alternative route using 16 that includes N-acylation and reduction could yield chlorizidine A (1). That 16, despite its simplicity, may be utilized to make two diverse, complex structure types is remarkable.
Scheme 3.
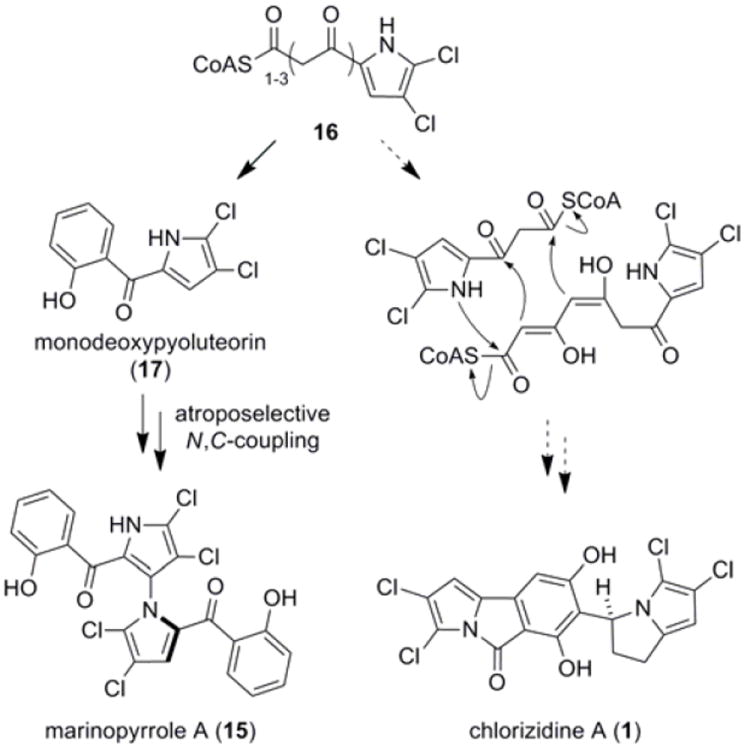
Proposed biosynthetic relationship of 1 to marinopyrrole A (15)
Derived from what appears to represent a new, obligate marine Streptomyces sp., chlorizidine is the first example of a natural product containing a 5H-pyrrolo[2,1-a]isoindol-5-one ring. Studies are now in progress to examine the biosynthesis and mechanism of action of these novel metabolites.
Supplementary Material
Acknowledgments
Financial support was provided by the NIH National Cancer Institute under grant R37 CA044848. X. A.-M. was supported by the commission for Universities and Research from the Department of Innovation, Universities and Enterprises of the Generalitat de Catalunya (fellowship 2007 BP-A 00042). We thank the NCI for performing the 60 cell line drug screen and Drs. Arnold L. Rheingold and Curtis Moore (UCSD) for providing X-ray diffraction structures. We also thank Kelley A. Gallagher (SIO) for the phylogenetic tree in the Supporting Information. In support of much of the work in this manuscript, Prof. Ted Molinski (UCSD) graciously provided laboratory space and equipment.
Footnotes
Supporting Information Available Phylogenetic analysis of strain CNH-287. Isolation procedures for 1 and 7. Procedures for the synthesis of 2-6, 8-14 including HRMS data, proton and carbon NMR spectra, and UV/vis spectra. Crystallographic data for 1 (CCDC 900052) and 2 (CCDC 900051) in CIF format. The material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Newman DJ, Cragg GM. J Nat Prod. 2012;75:311–335. doi: 10.1021/np200906s.. Butler MS. Nat Prod Rep. 2005;22:162–195. doi: 10.1039/b402985m.. For a microbial perspective, see: Berdy J. J Antibiot. 2005;58:1–26. doi: 10.1038/ja.2005.1.. Demain AL, Sanchez S. J Antibiot. 2009;62:5–16. doi: 10.1038/ja.2008.16.
- 2.(a) Waksman SA, Woodruff HB. Proc Soc Exper Biol Med. 1940;45:609–614. [Google Scholar]; (b) Arcamone F, Cassinelli G, Fantini G, Grein A, Orezzi P, Pol C, Spalla C. Biotechnol Bioeng. 1969;11:1101–1110. doi: 10.1002/bit.260110607. [DOI] [PubMed] [Google Scholar]; (c) Umezawa H, Maeda K, Takeuchi T, Okami Y. J Antibiot. 1966;19:200–209. [PubMed] [Google Scholar]; (d) Hata T, Koga F, Sano Y, Kanamori K, Matsumae A, Sugawara R, Hoshi T, Shima T, Ito S, Tomizawa S. J Antibiot. 1954;7:107–112. [PubMed] [Google Scholar]; (e) Sato K, Okamura N, Utagawa K, Ito Y, Watanabe M. Sci Rep Res Inst Tohoku Univ Med. 1960;9:224–232. [PubMed] [Google Scholar]; (f) Julian PE, Schenck JR. Antibiotics and Chemotherapy. 1953;3:1218–1220. [PubMed] [Google Scholar]; (g) Hata T, Hoshi T, Kanamori K, Matsumae A, Sano Y, Shima T, Sugawara R. J Antibiot. 1956;9:141–146. [PubMed] [Google Scholar]; (h) Umezawa H, Takeuchi T, Nitta K, Yamamoto T, Yamaoka S. J Antibiot. 1953;6:101. [PubMed] [Google Scholar]; (i) Vavra JJ, Deboer C, Dietz A, Hanka LJ, Sokolski WT. Antibiot Annu. 1959;7:230–235. [PubMed] [Google Scholar]; (f) Ishida N, Miyazaki K, Kumagai K, Rikimaru M. J Antibiot. 1965;18:68–76. [PubMed] [Google Scholar]
- 3.(a) Blunt JW, Copp BR, Keyzers RA, Munro MHG, Prinsep MR. Nat Prod Rep. 2013;30:237–323. doi: 10.1039/c2np20112g. [DOI] [PubMed] [Google Scholar]; (b) Blunt JW, Copp BR, Keyzers RA, Munro MH, Prinsep MR. Nat Prod Rep. 2012;29:144–222. doi: 10.1039/c2np00090c. [DOI] [PubMed] [Google Scholar]; (c) Fenical W, Jensen PR. Nat Chem Biol. 2006;2:666–673. doi: 10.1038/nchembio841. [DOI] [PubMed] [Google Scholar]
- 4.Feling RH, Buchanan GO, Mincer TJ, Kauffman CA, Jensen PR, Fenical W. Angew Chem Int Ed. 2003;42:355–357. doi: 10.1002/anie.200390115. [DOI] [PubMed] [Google Scholar]
- 5.Strain CNH-287 (GenBank accession no. bankit JX680598) was obtained from a marine sediment sample collected at the intertidal zone near San Clemente, CA.
- 6.On the inclusion of solvent molecules in the crystal structures of organic molecules, see: Görbitz CH, Hersleth H-P. Acta Cryst. 2000;B56:526–534. doi: 10.1107/s0108768100000501.
- 7.For the synthesis of 5H-pyrrolo[2, 1-a]isoindol-5-ones, see: Marsili A, Scartoni V, Morelli I, Pierangeli P. J Chem Soc, Perkin Trans. 1977;1:959–965.. Itahara T. J Chem Soc Chem Comm. 1981:254–255.. Maruyama K, Kubo Y. J Org Chem. 1981;46:3612–3622.. Crabb TA, Patel A, Newton RF, Price BJ, Tucker MJ. J Chem Soc, Perkin Trans. 1982;1:2783–2786.. Danheiser RL, Kwasigroch CA, Tsai Y-M. J Am Chem Soc. 1985;107:7233–7235.. Grigg R, Sridharan V, Stevenson P, Sukirthalingam S, Worakun T. Tetrahedron. 1990;46:4003–4018.. Yavari I, Islami MR. J Chem Research (S) 1998:166–167.. Kaden S, Reissig H-U, Brüdgam I, Hartl H. Synthesis. 2006:1351–1359.. McNab H, Tyas RG. J Org Chem. 2007;72:8760–8769. doi: 10.1021/jo0712502.
- 8.For related plant-derived pyrrolizidine alkaloids, see: Smith LW, Culvenor CCJ. J Nat Prod. 1981;44:129–152. doi: 10.1021/np50014a001.
- 9.The conformers could not be resolved using either achiral or chiral column chromatography.
- 10.Toward the end of the seven-day cultivation, the pH of the culture broth normally reaches 10.
- 11.Since the carboxylic acid compound could not be isolated, the decarboxylation event was not clearly demonstrated in vitro. For the facile decarboxylation of 2 4-dihydroxybenzoic acid derivatives, see: Horper W, Marner F-J. Phytochemistry. 1996;41:451–456.
- 12.The therapeutic properties of other 5H-pyrrolo[2, 1-a]isoindol-5-one-containing compounds have been described. For dihydropyridine-substituted 5H-pyrrolo[2, 1-a]isoindol-5-ones and their use, see: Kuhl A. 10,034,265. D E Patent. 2005. For 5H-pyrrolo[2, 1-a]isoindol-5-ones that function as Cdk4 inhibitors, see: Honma T, Hayashi K, Aoyama T, Hashimoto N, Machida T, Fukasawa K, Iwama T, Ikeura C, Ikuta M, Suzuki-Takahashi I, Iwasawa Y, Hayama T, Nishimura S, Morishima H. J Med Chem. 2001;44:4615–4627. doi: 10.1021/jm0103256.
- 13.Brattain MG, Fine WD, Khaled FM, Thompson J, Brittain DE. Cancer Res. 1981;41:1751–1756. [PubMed] [Google Scholar]
- 14.Shoemaker RH. Nat Rev Cancer. 2006;6:813–823. doi: 10.1038/nrc1951. [DOI] [PubMed] [Google Scholar]
- 15.(a) Hughes CC, Prieto-Davo A, Jensen PR, Fenical W. Org Lett. 2008;10:629–631. doi: 10.1021/ol702952n. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hughes CC, Kauffman CA, Jensen PR, Fenical W. J Org Chem. 2010;75:3240–3250. doi: 10.1021/jo1002054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.(a) Nowak-Thompson B, Chaney N, Wing JS, Gould SJ, Loper JE. J Bacteriol. 1999;181:2166–2174. doi: 10.1128/jb.181.7.2166-2174.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Thomas MG, Burkart MD, Walsh CT. Chemistry and Biology. 2002;9:171–184. doi: 10.1016/s1074-5521(02)00100-x. [DOI] [PubMed] [Google Scholar]; (c) Dorrestein PC, Yeh E, Garneau-Tsodikova S, Kelleher NL, Walsh CT. Proc Natl Acad Sci. 2005;102:13843–13848. doi: 10.1073/pnas.0506964102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takeda RJ. J Am Chem Soc. 1958;80:4749–4750. [Google Scholar]; (b) Takeda RJ. Bull Agr Chem Soc Jpn. 1959;23:126–130. [Google Scholar]
- 18.Yamanaka K, Ryan KS, Gulder TAM, Hughes CC, Moore BS. J Am Chem Soc. 2012;134:12434–12437. doi: 10.1021/ja305670f. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


