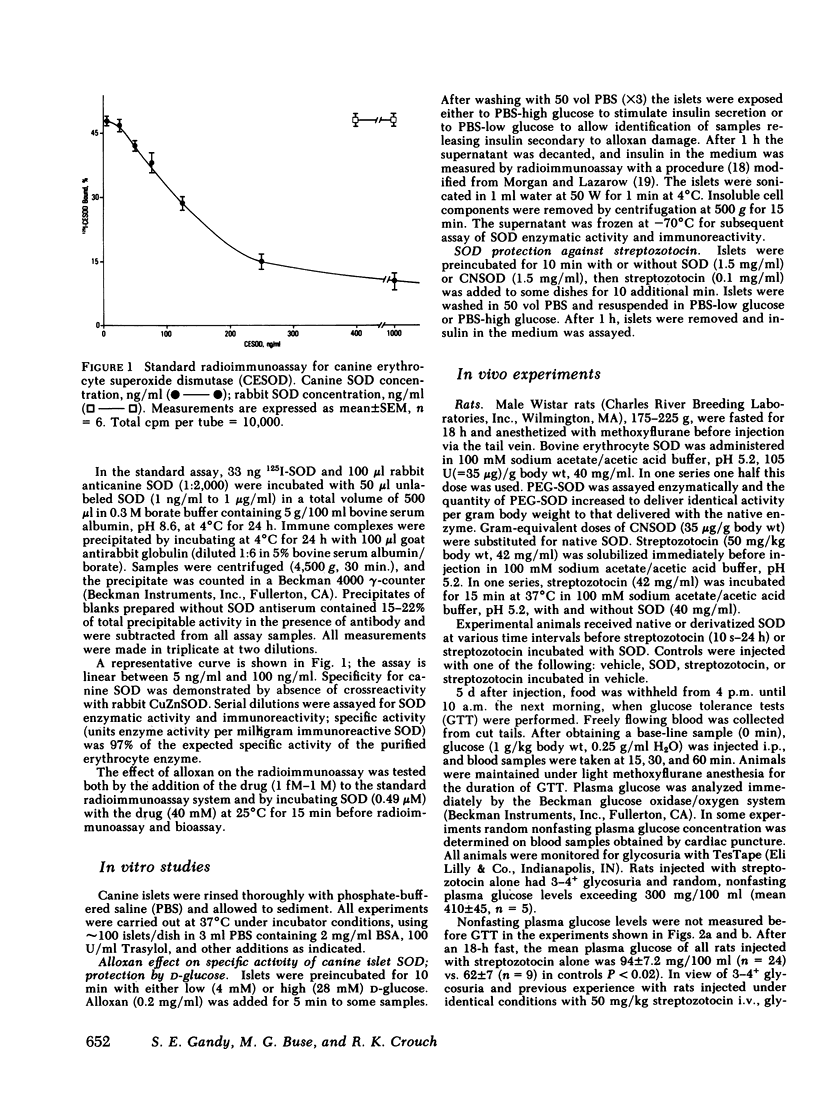
Abstract
Copper-zinc superoxide dismutase (SOD) is present in relatively high concentrations in the β-cells of human islets. The activity of the extracted enzyme is partially inhibited upon incubation with the diabetogenic drugs alloxan, streptozotocin, or Vacor. The role of this enzyme in protecting β-cells against chemically induced diabetes was further investigated.
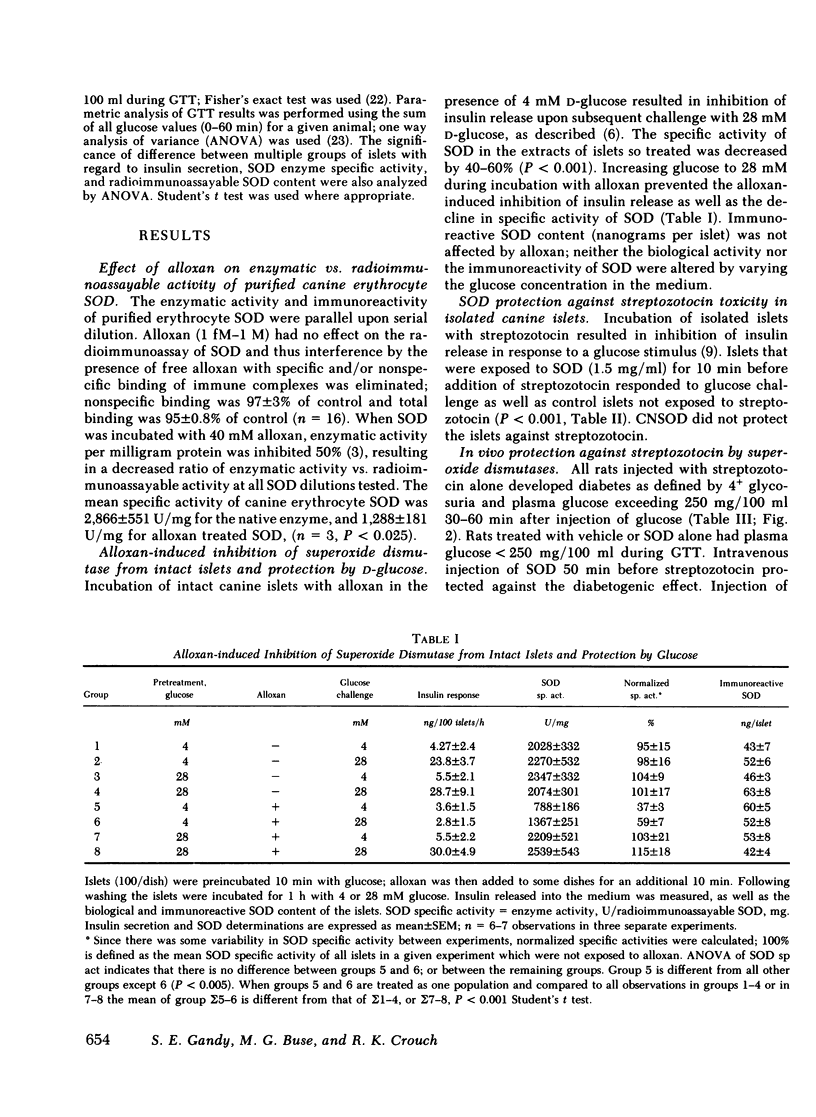
Incubation of intact canine islets with alloxan (0.2 mg/ml) and 4 mM glucose decreased the insulin secretory response by 87% during subsequent exposure to 28 mM glucose. Concomitantly the SOD-specific activity (units of enzyme activity per milligram immunoreactive SOD) decreased 50% in alloxan-exposed islets. When islets were protected from alloxan toxicity by including 28 mM glucose with alloxan, the insulin secretory response and SOD specific activity remained identical to controls. Thus, SOD specific activity correlates with maintenance of β-cell function.
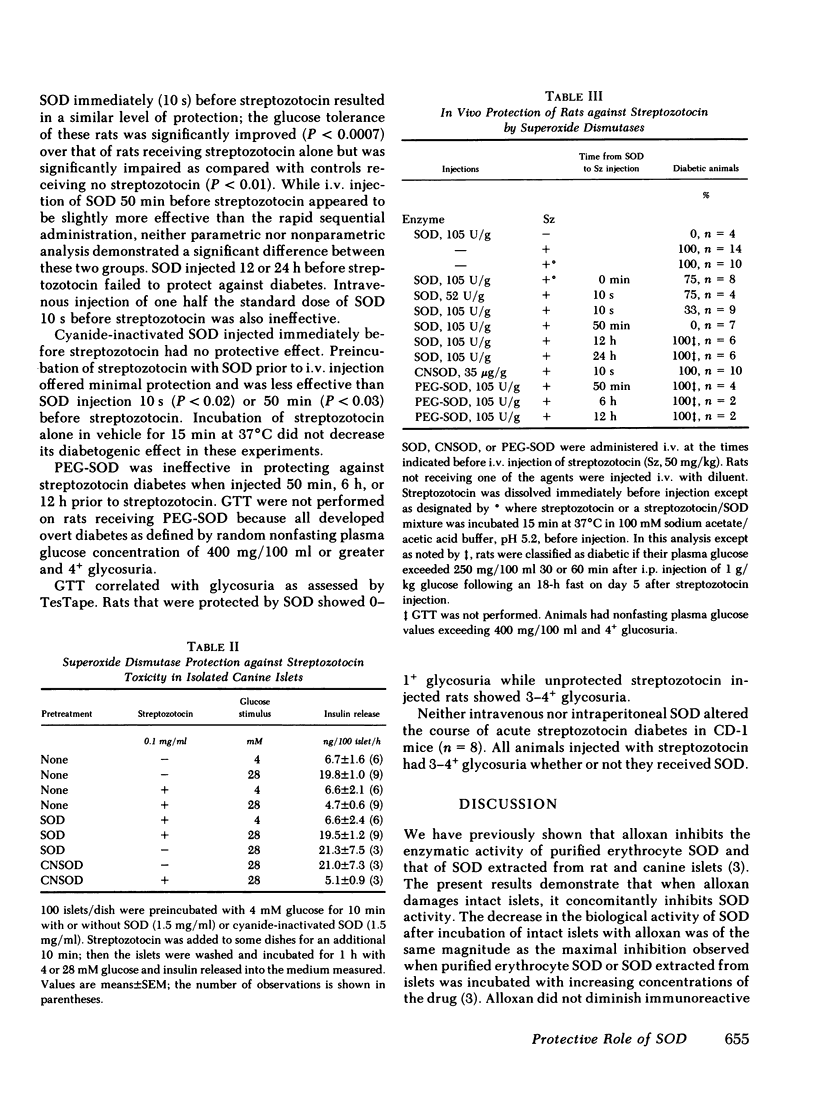
To test the effectiveness of SOD against streptozotocin in vitro, canine islets were incubated 10 min with or without streptozotocin (0.1 mg/ml) with 4 mM glucose; their functional integrity was tested subsequently as the insulin secretory response to 28 mM glucose. Exposure to streptozotocin alone decreased the response by 70%; inclusion of SOD (1.5 mg/ml) before and during exposure to streptozotocin completely prevented this effect. Cyanide-inactivated SOD was not effective.
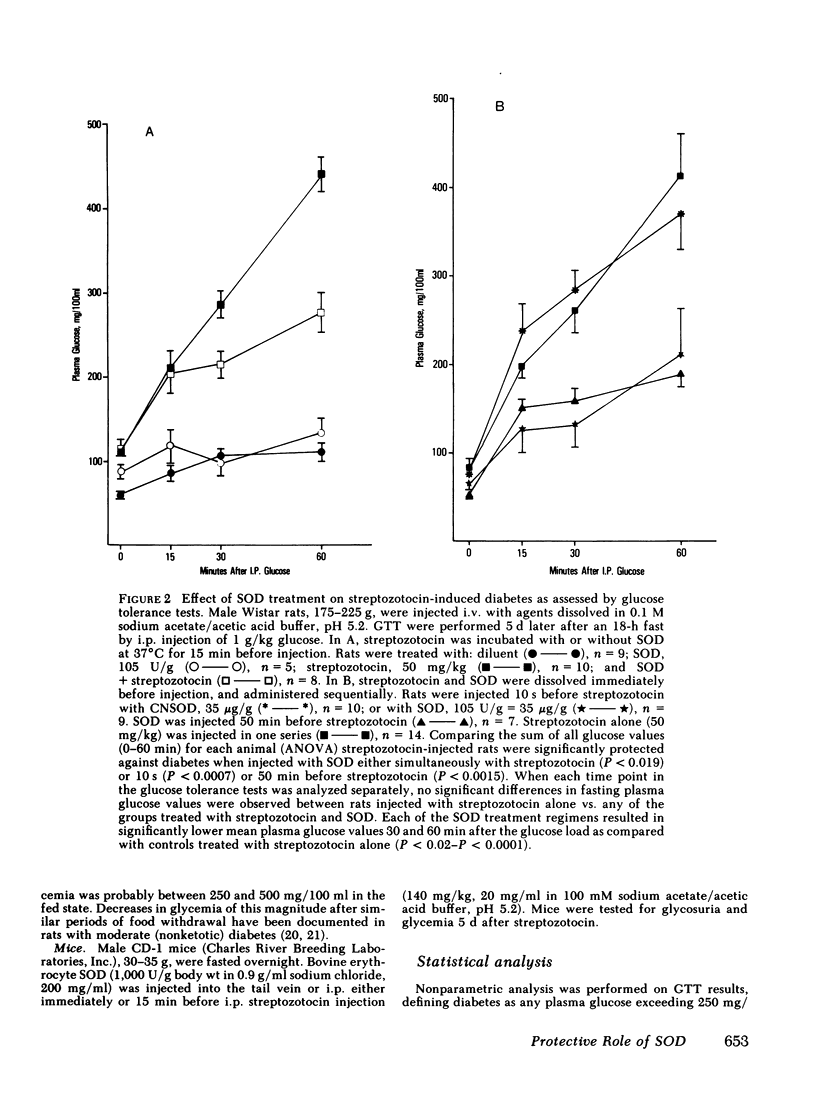
The potential of SOD to prevent streptozotocin-induced diabetes was tested in rats in vivo. SOD injected 10 s or 50 min before streptozotocin prevented or significantly attenuated diabetes. Injection of SOD and streptozotocin simultaneously was much less effective, and cyanide-inactivated SOD was ineffective. No protection was afforded by injection of SOD 12 or 24 h before streptozotocin.
Our results support hypotheses that (a) oxygen radicals mediate the β-cell toxicity of both alloxan and streptozotocin, and (b) β-cells may be particularly vulnerable to oxygen radical damage.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abuchowski A., van Es T., Palczuk N. C., Davis F. F. Alteration of immunological properties of bovine serum albumin by covalent attachment of polyethylene glycol. J Biol Chem. 1977 Jun 10;252(11):3578–3581. [PubMed] [Google Scholar]
- Bauer B. A., Younathan E. S. Gas chromatographic determination of the anomers of blood glucose in normal and diabetic rats. Biochem Med. 1980 Dec;24(3):293–299. doi: 10.1016/0006-2944(80)90023-x. [DOI] [PubMed] [Google Scholar]
- Boquist L. A new hypothesis for alloxan diabetes. Acta Pathol Microbiol Scand A. 1980 Jul;88(4):201–209. doi: 10.1111/j.1699-0463.1980.tb02487.x. [DOI] [PubMed] [Google Scholar]
- Cahill G. F., Jr, McDevitt H. O. Insulin-dependent diabetes mellitus: the initial lesion. N Engl J Med. 1981 Jun 11;304(24):1454–1465. doi: 10.1056/NEJM198106113042403. [DOI] [PubMed] [Google Scholar]
- Cohen G., Heikkila R. E. The generation of hydrogen peroxide, superoxide radical, and hydroxyl radical by 6-hydroxydopamine, dialuric acid, and related cytotoxic agents. J Biol Chem. 1974 Apr 25;249(8):2447–2452. [PubMed] [Google Scholar]
- Crouch R. K., Gandy S. E., Kimsey G., Galbraith R. A., Galbraith G. M., Buse M. G. The inhibition of islet superoxide dismutase by diabetogenic drugs. Diabetes. 1981 Mar;30(3):235–241. doi: 10.2337/diab.30.3.235. [DOI] [PubMed] [Google Scholar]
- Crouch R., Kimsey G., Priest D. G., Sarda A., Buse M. G. Effect of streptozotocin on erythrocyte and retinal superoxide dismutase. Diabetologia. 1978 Jul;15(1):53–57. doi: 10.1007/BF01219329. [DOI] [PubMed] [Google Scholar]
- Crouch R., Priest D. G., Duke E. J. Superoxide dismutase activities of bovine ocular tissues. Exp Eye Res. 1978 Nov;27(5):503–509. doi: 10.1016/0014-4835(78)90135-5. [DOI] [PubMed] [Google Scholar]
- Fischer L. J., Hamburger S. A. Inhibition of alloxan action in isolated pancreatic islets by superoxide dismutase, catalase, and a metal chelator. Diabetes. 1980 Mar;29(3):213–216. doi: 10.2337/diab.29.3.213. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Annu Rev Biochem. 1975;44:147–159. doi: 10.1146/annurev.bi.44.070175.001051. [DOI] [PubMed] [Google Scholar]
- Gandy S. E., 3rd, Galbraith R. A., Crouch R. K., Buse M. G., Galbraith G. M. Superoxide dismutase in human islets of Langerhans. N Engl J Med. 1981 Jun 18;304(25):1547–1548. doi: 10.1056/NEJM198106183042518. [DOI] [PubMed] [Google Scholar]
- Gold G., Manning M., Heldt A., Nowlain R., Pettit J. R., Grodsky G. M. Diabetes induced with multiple subdiabetogenic doses of streptozotocin: lack of protection by exogenous superoxide dismutase. Diabetes. 1981 Aug;30(8):634–638. doi: 10.2337/diab.30.8.634. [DOI] [PubMed] [Google Scholar]
- Grankvist K., Marklund S., Sehlin J., Täljedal I. B. Superoxide dismutase, catalase and scavengers of hydroxyl radical protect against the toxic action of alloxan on pancreatic islet cells in vitro. Biochem J. 1979 Jul 15;182(1):17–25. doi: 10.1042/bj1820017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grankvist K., Marklund S., Täljedal I. B. Superoxide dismutase is a prophylactic against alloxan diabetes. Nature. 1981 Nov 12;294(5837):158–160. doi: 10.1038/294158a0. [DOI] [PubMed] [Google Scholar]
- Harman D. The aging process. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7124–7128. doi: 10.1073/pnas.78.11.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson E. K., Fridovich I. The interaction of bovine erythrocyte superoxide dismutase with hydrogen peroxide: inactivation of the enzyme. Biochemistry. 1975 Dec 2;14(24):5294–5299. doi: 10.1021/bi00695a010. [DOI] [PubMed] [Google Scholar]
- Kansal P. C., Buse M. G. The effect of adrenergic blocking agents on plasma insulin and blood glucose during urethan or epinephrine induced hyperglycemia. Metabolism. 1967 Jun;16(6):548–556. doi: 10.1016/0026-0495(67)90085-6. [DOI] [PubMed] [Google Scholar]
- Kazumi T., Utsumi M., Yoshino G., Ishihara K., Hirose Y., Makimura H., Baba S. Somatostatin concentration responds to arginine in portal plasma: effects of fasting, streptozotocin diabetes, and insulin administration in diabetic rats. Diabetes. 1980 Jan;29(1):71–73. doi: 10.2337/diab.29.1.71. [DOI] [PubMed] [Google Scholar]
- Kelly K., Barefoot C., Sehon A., Petkau A. Bovine superoxide dismutase: a radioimmunoassay. Arch Biochem Biophys. 1978 Oct;190(2):531–538. doi: 10.1016/0003-9861(78)90307-7. [DOI] [PubMed] [Google Scholar]
- Marchalonis J. J. An enzymic method for the trace iodination of immunoglobulins and other proteins. Biochem J. 1969 Jun;113(2):299–305. doi: 10.1042/bj1130299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund S., Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974 Sep 16;47(3):469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- Matas A. J., Sutherland D. E., Steffes M. W., Najarian J. S. Short-term culture of adult pancreatic fragments for purification and transplantation of islets of Langerhans. Surgery. 1976 Aug;80(2):183–191. [PubMed] [Google Scholar]
- Petkau A., Kelly K., Chelack W. S., Pleskach S. D., Barefoot C., Meeker B. E. Radioprotection of bone marrow stem cells by superoxide dismutase. Biochem Biophys Res Commun. 1975 Dec 1;67(3):1167–1174. doi: 10.1016/0006-291x(75)90796-2. [DOI] [PubMed] [Google Scholar]
- Pottathil R., Chandrabose K. A., Cuatrecasas P., Lang D. J. Establishment of the interferon-mediated antiviral state: possible role of superoxide dismutase. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3343–3347. doi: 10.1073/pnas.78.6.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitch A., Fuller L., Mintz D., Severyn W., Noel J., Flaa C., Kyriakides G., Miller J. Responses of canine lymphocytes to allogeneic and autologous Islets of Langerhans in mixed cell cultures. J Clin Invest. 1981 May;67(5):1507–1516. doi: 10.1172/JCI110181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins M. J., Sharp R. A., Slonim A. E., Burr I. M. Protection against streptozotocin-induced diabetes by superoxide dismutase. Diabetologia. 1980 Jan;18(1):55–58. doi: 10.1007/BF01228303. [DOI] [PubMed] [Google Scholar]
- Scharp D. W., Downing R., Merrell R. C., Greider M. Isolating the elusive islet. Diabetes. 1980;29 (Suppl 1):19–30. doi: 10.2337/diab.29.1.s19. [DOI] [PubMed] [Google Scholar]
- Weaver D. C., McDaniel M. L., Lacy P. E. Alloxan uptake by isolated rat islets of Langerhans. Endocrinology. 1978 Jun;102(6):1847–1855. doi: 10.1210/endo-102-6-1847. [DOI] [PubMed] [Google Scholar]
- Weaver D. C., McDaniel M. L., Naber S. P., Barry C. D., Lacy P. E. Alloxan stimulation and inhibition of insulin release from isolated rat islets of Langerhans. Diabetes. 1978 Dec;27(12):1205–1214. doi: 10.2337/diab.27.12.1205. [DOI] [PubMed] [Google Scholar]


