Abstract
Smooth muscle differentiation and patterning is a fundamental process in urinary bladder development that involves a complex array of local environmental factors, epithelial-mesenchymal interaction, and signaling pathways. An epithelial signal is necessary to induce smooth muscle differentiation in the adjacent bladder mesenchyme. The bladder epithelium (urothelium) also influences the spatial organization of the bladder wall. Sonic hedgehog (Shh), which is expressed by the urothelium, promotes mesenchymal proliferation and induces differentiation of smooth muscle from embryonic bladder mesenchyme. Shh, whose signal is mediated through various transcription factors including Gli2 and BMP4, is likely also important in the patterning of bladder smooth muscle. However, it is not known to what extent early mediators of mesenchymal migration, other Shh-associated transcription factors, and crosstalk between the Shh signaling cascade and other pathways, are involved in the patterning of bladder smooth muscle. Here we review the role of epithelial-mesenchymal interaction and Shh signaling in smooth muscle differentiation and patterning in the bladder. We also discuss emerging signaling molecules, transcription factors, and mesenchyme properties that might be fruitful areas of future research in the process of smooth muscle formation in the bladder.
Keywords: bladder, smooth muscle, differentiation, patterning, sonic hedgehog
Introduction
The function of the adult human bladder is to store and empty urine at socially appropriate times. Despite this seemingly simple function, micturition is a complex anatomic and physiologic function that involves interaction between the urothelium, bladder smooth muscle, afferent and efferent nerves, spinal cord, and higher-level cerebral processing. The urinary bladder is a dynamic organ that is both compliant in order to allow storage of urine and contractile to allow for coordinated emptying. The detrusor, which is the muscle that contracts to expel urine, is comprised of randomly-oriented smooth muscle fibers and, under normal conditions, is under autonomic control. In conjunction with a normally functioning nervous system, the physiologic properties of the bladder are derived from the anatomic properties of the bladder smooth muscle. During filling, the normal adult bladder maintains a low intravesical pressure (< 10cm H2O) and has a capacity of 350-500cc. At a socially appropriate time, urethral pressure decreases and intravesical pressure increases as the bladder smooth muscle undergoes coordinated contraction, allowing for effective emptying. Concomitant with expelling urine through the urethra, detrusor contraction also closes the muscular tunnel through which the ureters traverse as they join the bladder. This prevents retrograde flow (vesico-ureteral reflux), which protects the kidneys from the pressure generated by detrusor contraction.
Over the last forty years, significant advances have been made in the understanding of how smooth muscle develops in the embryonic bladder. Observational studies of developing human embryos have shown that smooth muscle differentiates from embryonic mesenchyme by the twelfth week of gestation.[1] While such descriptive studies are useful in establishing the timeline of bladder organogenesis, they do not provide insight into the molecular mechanisms that underlie smooth muscle differentiation. Use of transgenic and knockout mice, tissue recombination experiments, and enhanced molecular biologic and immunohistochemical techniques have greatly increased our understanding of bladder smooth muscle development. The focus of this review is the complex array of local environmental factors, epithelial-mesenchymal interaction, and signaling pathways that are critical for smooth muscle differentiation and patterning in the urinary bladder.
Bladder Embryology
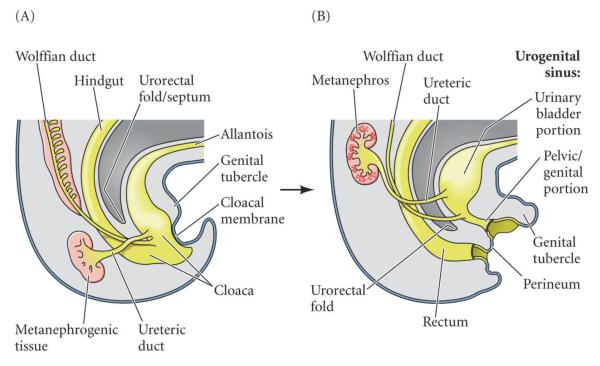
The bladder arises from the urogenital sinus (UGS) after the urorectal septum partitions the cloaca into the urogenital sinus ventrally and the rectum dorsally. The bladder then forms from the superior part of the UGS, which is of endodermal origin. (Figure 1) The adjacent mesenchyme, derived from splanchnopleural mesoderm, coalesces to surround the endodermal-derived epithelial lining of the lumen of the UGS and establishes the reciprocal epithelial-mesenchymal relationship that is critical for bladder development. There is emerging evidence that the epithelium of the bladder trigone, which classically is thought to be of mesodermal origin, is also derived from endoderm, as is the rest of bladder.[2-4] Similarly, recent studies have shown that the smooth muscle of the trigone arises primarily from the same primordial mesenchyme as the rest of the detrusor, which counters the previous hypothesis that the smooth muscle of the trigone was derived primarily from incorporation of ureteral smooth muscle.[5]
Figure 1. Bladder embryogenesis.
A) In humans, the division of the cloaca into the urogenital sinus (UGS) and the rectum occurs during the 6th week of gestation. The mesodermal-derived urorectal fold consists of two structures: the upper fold (Tourneux) divides the cloaca in a cranial-caudal direction and fuses in the midline with the lower fold (Rathke), which partitions the UGS and hindgut in a lateral-medial direction. B) The bladder then forms from the cranial aspect of the UGS. The allantois becomes the urachus and, in the adult, the median umbilical ligament. Reproduced with permission from Developmental Biology, Eighth Edition, 2006, Sinauer Associates Inc.
The full thickness of bladder mesenchyme, from the subepithelium to the sub-adventitial layers, has the potential to develop into smooth muscle. During normal development, bladder smooth muscle forms first in the outer zone (adventitial side) of peripheral mesenchyme, then proceeds towards the inner zone (luminal side). However, the region of the mesenchyme immediately adjacent to the urothelium, the subepithelium, remains mostly devoid of smooth muscle. The location of the urothelium on the luminal surface of the bladder is critical to this patterning of the bladder wall.[6]
Smooth Muscle
Embryology, Anatomy, and Physiology
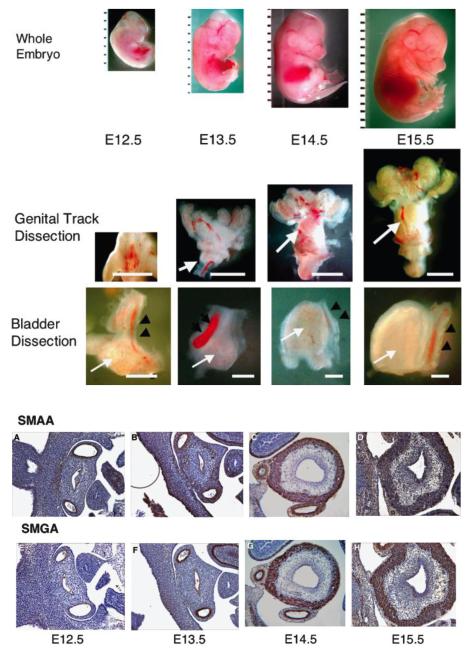
In humans, bladder smooth muscle has been reported to form between 7 – 12 weeks of gestation.[1, 7] The paucity of human tissue has obviously necessitated using animal models to investigate the molecular mechanisms of bladder differentiation. Although many models have been used, the mouse is most often used to study smooth muscle differentiation in the bladder. In the mouse, which has an approximate 20-day gestation, differentiation of smooth muscle in the mesenchyme begins at E13.5.[7] (Figure 2) The difference in timing of organogenesis between mice and humans is readily apparent; in mice, the bladder forms at the beginning of the third trimester while, in humans, the bladder forms in the first trimester. Such differences limit the generalizability of animal experiments to human development; however, they also have greatly increased our knowledge of bladder smooth muscle differentiation by allowing investigators to isolate and manipulate the mouse bladder prior to the onset of smooth muscle differentiation.
Figure 2. Time course of bladder organogenesis and smooth muscle differentiation in the mouse.
Gross dissections of mouse embryos: whole embryo (upper panels), genital tracts (middle panels), and bladder with umbilical arteries (lower panels). The white arrows indicate the urinary bladder. The black arrowheads indicate the umbilical arteries. Top panel: Ruler = 1 mm. Bars in the middle panels are 1 mm. Bars in the lower panels are 100 mm.
Smooth muscle (SM) actin staining of the embryonic bladder. (A–D) Smooth muscle α-actin (SMAA), (E–H) smooth muscle γ-actin (SMGA). E12.5 (A and E), E13.5 (B and F), E14.5 (C and G), and E15.5 (D and H). Note the positive staining of the umbilical arteries. Final magnifcation 100X A-H. Reproduced with permission from Shiroyanagi et al., Differentiation 2007. [7]
Although smooth muscle anatomy and physiology is beyond the scope of this review, a brief overview is warranted. Bladder smooth muscle fibers have a fusiform shape, are 300-400 μm in length, are of the single unit type, and have a greater ratio of actin to myosin and a larger length-tension curve than striated muscle.[8, 9] In single-unit smooth muscle, an autonomic nerve innervates a single cell within a bundle of muscle cells. The action potential is then is then propagated to neighboring cells through gap junctions, which causes a coordinated contraction. These properties allow bladder smooth muscle to stretch (necessary for bladder filling) but still maintain contractility (necessary for bladder emptying). As in striated muscle, the major structural components of smooth muscle are actin and myosin. However, in smooth muscle, contraction is regulated by calmodulin, which, after binding calcium, activates myosin light-chain kinase (MLCK). MLCK then phosphorylates the light chain component of the myosin head, which allows muscle fiber contraction. Caldesmon, a component of thin filaments in smooth muscle cells, binds calmodulin and inhibits actin-activated ATP hydrolysis by smooth muscle myosin.[10, 11] Calponin, after binding calcium, also tonically inhibits the ATPase activity of myosin.
Knowledge of these proteins is critical to the interpretation of many smooth muscle developmental biology experiments because the stage of smooth muscle differentiation can be characterized by its protein expression profile. Generally, smooth muscle alpha actin (SMAA) is considered a marker of early smooth muscle cell differentiation, calponin and SM22α, intermediate markers, and smooth muscle myosin heavy chain (SM-MHC) a marker of advanced differentiated smooth muscle cells.[12-15] SMAA was utilized as the marker of smooth muscle differentiation in the bladder for all of the experiments described in this review except in cases where use of other specific markers are noted.
Differentiation
Epithelium
An epithelial signal is necessary for induction of smooth muscle differentiation from bladder mesenchyme (BLM).[16, 17] Tissue recombination experiments have revealed the importance of epithelial-mesenchymal interaction during bladder development. Intact bladders harvested at embryonic day (E) 12, which is prior to the onset of smooth muscle formation, survive and undergo smooth muscle differentiation when grown as grafts beneath the kidney capsule of nude mice for two weeks whereas isolated E12 BLM does not develop and does not survive. However, E12 BLM onto which urothelium has been grafted survives and undergoes normal smooth muscle differentiation.[16] This epithelial signal necessary for mesenchymal survival and smooth muscle differentiation is neither restricted to embryonic urothelium, as fetal, newborn, and adult bladder epithelium (BLE) are all able to induce smooth muscle differentiation from BLM nor is it specific to BLE; ureteral, gastric, and corneal epithelium have been shown to induce smooth muscle differentiation from BLM, albeit to varying degrees.[17] The same has been shown in uterine smooth muscle (myometrium) development.[18] The resulting smooth muscle pattern also depends on epithelial-mesenchymal interaction. In the bladder, the pattern of smooth muscle appears to be governed by the mesenchyme (i.e. bladder mesenchyme plus gastric epithelium produces bladder-like smooth muscle pattern).[17] This mesenchymal-specific patterning of smooth muscle is somewhat different than the gut in which intestinal epithelium alone can induce differentiation and direct intestinal-like patterning of adjacent stroma.[19] However, for the bladder, it appears that most types of endodermal-derived epithelium are able to induce BLM to undergo smooth muscle differentiation and the pattern of smooth muscle organization is largely intrinsic to the mesenchyme.
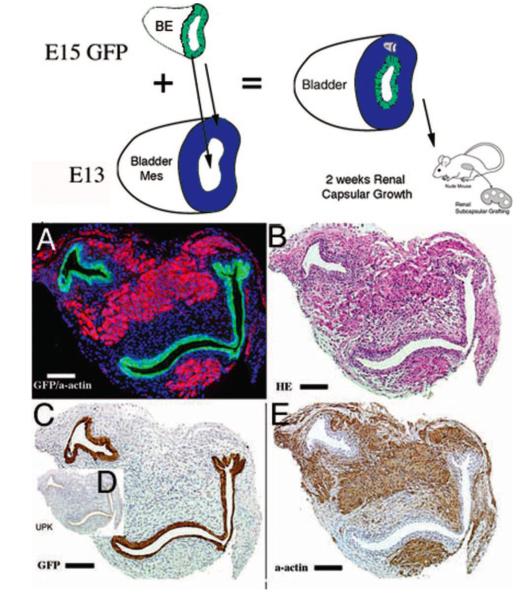
Although it appears that BLM dictates the spatial organization of smooth muscle layers, Cao et al. demonstrated that the location of the bladder epithelium significantly influences its ultimate pattern.[6] With the urothelium in its orthotopic (luminal) location, smooth muscle develops throughout the bladder wall except for a thin subepithelial layer. However, when urothelium was placed on both the luminal and adventitial aspects of the bladder, smooth muscle differentiation was inhibited immediately adjacent to both urothelia. (Figure 3) Epithelial-mesenchymal interaction has also been found to be important in patterning mesenchyme in other hollow-organ systems such as the intestine.[20]
Figure 3. Epithelium patterns bladder smooth muscle.
Schematic and results of urothelial recombination with bladder mesenchyme in the orthotopic location. Histologic serial sections: (A) Color triple florescent stain, GFP is green, alpha-smooth muscle actin is pink and Hoescht dye is blue representing the zone of smooth muscle inhibition or submucosa; (B) H&E dbff hematoxylin and eosin; immunohistochemistry: (C) GFP = green fluorescent protein; (D) UPK= uroplakin and (E) α-actin = smooth muscle alpha-actin. (magnification bar = 100 μm). Reproduced with permission Cao et al., Pediatric Research 2008. [6]
The robust epithelial-mesenchymal interaction that results in smooth muscle differentiation in embryonic BLM diminishes with age. Grafting E14 BLM recombined with embryonic BLE within the wall of adult bladders significantly decreased SMAA expression compared to the same recombinants grafted underneath the kidney capsule.[21] This indicates that the signals that induce smooth muscle differentiation in the embryonic bladder are inhibited by the local environment of the adult bladder. This finding may have important implications for development of cell or gene-based therapies for repair of the adult bladder, which likely does not have the same plasticity seen in the developing bladder.
Sonic Hedgehog
Signaling
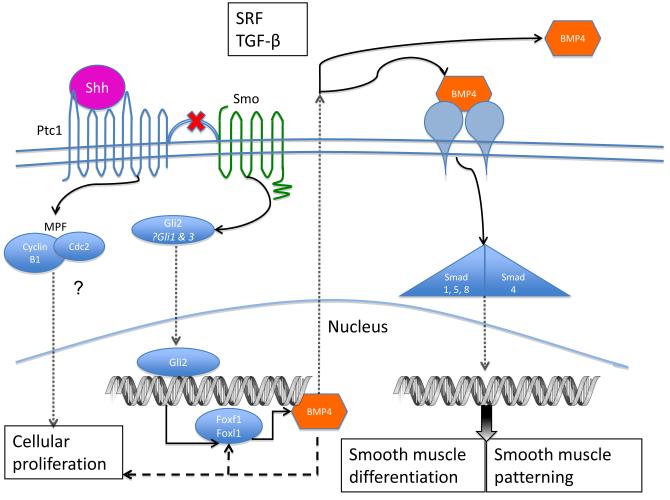
Sonic hedgehog (Shh) is a growth factor expressed by many types of epithelia that promotes mesenchymal proliferation [7, 22-25] and is necessary for the differentiation of smooth muscle from embryonic mesenchyme in diverse organ systems including the bladder, lung, and vasculature.[6, 7, 25-30] Shh is expressed by the bladder urothelium and is necessary for bladder development and smooth muscle differentiation.[7, 30, 31] Shh functions by binding to its membrane-bound receptor, Patched (Ptc1). In the unbound state, Ptc1 suppresses the activity of Smoothened (Smo); once Ptc1 binds Shh, the inhibitory activity on Smo is suppressed. The binding of Shh initiates an intracellular signal transduction cascade that activates three highly regulated Gli transcription factors: Gli1, Gli2 and Gli3. Both Gli1 and Gli2 are transcriptional activators that activate Shh target genes such as the Wnt family and the bone morphogenetic proteins (BMPs) of the TGFβ superfamily.
In the presence of Shh signaling, Gli2 is stabilized, and translocates to the nucleus to regulate numerous target genes involved in a number of different Shh-mediated cell processes including cell survival, cellular proliferation, and differentiation. (Figure 4) After nuclear translocation, Gli2 upregulates transcription of foxf1 and foxl1, which then go on to upregulate BMP4 transcription. In turn, BMP4 provides negative feedback on foxf1 transcription.[32, 33] Many experiments in the bladder have shown that BMP4 is found early in the developing bladder with highest levels in the subepithelial layer and significantly lower levels in the peripheral mesenchyme.
Figure 4. Proposed Shh signaling pathway in bladder mesenchymal cells.
Upon binding Shh, Ptc1 releases the inhibition of Smo. The effect of Shh on smooth muscle differentiation and patterning is mediated by Gli2, which, upon translocation into the nucleus, regulates downstream genes including BMP4. BMP4 may inhibit cellular proliferation and is a prime candidate for mediating the effect of Shh on smooth muscle patterning during bladder development. Other signaling molecules, such as TGF-β and SRF, likely have a role in bladder smooth muscle differentiation and may interact with the Shh pathway. The mechanism through which Shh affects cellular proliferation in the bladder is uncertain but MPF has been shown to be a Shh-responsive complex that regulates mitosis in other systems.
Downstream signaling from BMP4 activation is mediated by receptor-associated Smad proteins (R-Smad) 1, 5, and 8, which are distinct from the Smad proteins that mediate TGF-β signaling (R-Smad 2 and 3). Association of the R-Smad with Smad4, which is shared by both BMP4 and TGF-β signaling pathways, allows translocation of the Smad complex into the nucleus. Association of the R-Smad:Smad4 complex with different nuclear cofactors determines the ultimate transcriptional effect.[34] Further research is needed to elucidate the downstream mediators of BMP4/R-Smad/Smad4 in bladder development.
Mesenchymal proliferation
It is known that Shh acts as a mitogen during development. Shh expands and maintains cell subpopulations within the somite [35] and has been shown to promote mesenchymal proliferation in diverse tissues, including developing tooth, kidney, and ureter.[36, 37] Ptc1 mediates the effect of Shh on cellular proliferation. In its unbound state, Ptc1 sequesters M-phase promoting factor (MPF). MPF is a complex of cdc2 and cyclin B1, and has an important role in regulating the cell cycle. Ptc1, after binding Shh, releases MPF, which then translocates to the nucleus and drives the cell through the M-phase of mitosis.[38] It plausible that this mechanism is preserved in the bladder. Future research on the role of Shh, Ptc1, and MPF in regulating bladder mesenchyme proliferation might prove fruitful.
In a series of ex vivo experiments, progressively higher Shh concentrations up to 48nM increased the number of cells and organ size of E12 bladders after 72 hours in culture. These experiments demonstrated that increasing Shh concentrations increase bladder size and cell number and that there appears to be a threshold of the proliferative effect Shh has on bladder mesenchyme. It is known that urothelium expresses Shh and the adjacent mesenchyme, which responds to the Shh signal, co-express Ptc1 and BMP4 (Figure 5). Results from other studies raise BMP4 as a candidate gene that might mediate the effect of Shh on mesenchymal proliferation in the bladder. In the developing kidney, BMP4 inhibits mesenchymal proliferation; however, the overall mesenchymal response to the Shh signal is an increase in proliferation. This suggests that Shh promotes mesenchymal proliferation through BMP4-independent pathways and Shh can overcome the inhibitive effect of BMP4 on mesenchyme proliferation. [37] Although, to our knowledge, no studies have been performed in the fetal bladder, the similar expression pattern of Shh, Ptc1, and BMP4 in the embryonic kidney and bladder raise the possibility that a similar inhibitory feedback mechanism of BMP4 on the Shh-mediated promotion of mesenchymal proliferation might exist.[7] See Table 1 for organ systems in which Shh-BMP4 is known to be important for epithelial-mesenchymal interaction.
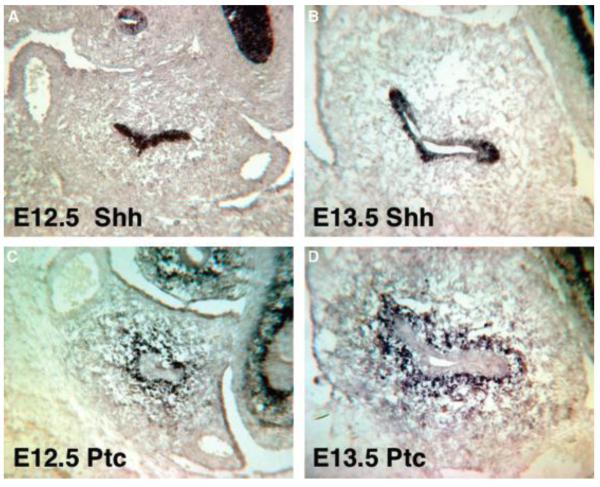
Figure 5. Shh and Ptc expression in the fetal bladder.
In situ hybridization of the embryonic bladder. Sonic hedgehog (Shh) (A and B), Patched (Ptc) (C and D). E12.5 bladder (A and C), E13.5 bladder (B and D). Final magnification 100x. Reproduced with permission from Shiroyanagi et al., Differentiation 2007. [7]
Table 1.
Role of Shh and BMP4 in different organs
| Organ | Shh expression in epithelium | BMP4 expression in mesenchyme |
Reference | |
|---|---|---|---|---|
| Important for mesenchymal growth |
Important for smooth muscle differentiation |
|||
| Bladder | Yes | Yes | Yes | [7, 26, 47, 69] |
| Ureter | Yes | Yes | Yes | [37] |
| Kidney | Yes | Yes | Yes | [26, 37] |
| Prostate | Yes | ? | Yesa | [70-74] |
| External genitalia | Yes | N/A | Yes | [25, 69, 75, 76] |
| Vagina | ? | ? | ? | |
| Lung | Yes | Yes | Yes | [77-79] |
| Gut | Yes | Yes | Yes | [20, 46, 78, 80-82] |
| Uterus | Yesb | No | No | [83] |
| Pancreas | Noc | N/A | Yesd | [84-86] |
| Tooth | Yes | N/A | Yes | [36, 87, 88] |
| Somite | Yes | N/A | Yes | [89, 90] |
| Mammary gland | Noe | N/A | Yes | [91-93] |
Unlike Shh, BMP4 inhibits prostatic duct budding and branching.
Progesterone induces Ihh expression in the adult mouse.
Shh suppression is necessary for the initiation of pancreas development but tightly regulated expression is necessary for later organogenesis.
BMP4 expression by the septum transversum mesenchyme is necessary to suppress pancreas differentiation from the endoderm.
Ihh, rather than Shh, appears to be the most important signal necessary for mammary gland development
Also in kidney organogenesis, deletion of smad4 results in disorganized nephrogenic mesenchyme, which illustrates the central role of Smad4 in mediating the cellular response to BMP4 and TGF-β signaling. In the same study, smad4 deletion did not produce a phenotypic change in the ureteric bud.[39] Given that BMP4 is necessary for ureteric bud morphogenesis, this suggests that an alternative pathway that does not involve the Smad complex can mediate the BMP4 signal, at least in the ureter.[40] The alternative pathway hypothesis is supported by the finding that diverse mesodermal patterning processes, which are regulated by TGF-β and BMP4 are unaffected by Smad4 deletion.[41] It is not known what effect smad4 deletion has in the bladder.
Smooth muscle differentiation and patterning
In addition to promoting mesenchymal proliferation, Shh regulates smooth muscle differentiation in the bladder. Smooth muscle differentiation occurs in the peripheral mesenchyme of intact bladders harvested at E12 when grown in organ culture without Shh, whereas isolated E12 BLM cultured without Shh does not undergo smooth muscle differentiation. However, E12 BLM cultured with exogenous Shh express markers of advanced smooth muscle differentiation, including SM-MHC, at 72 hours.[42] These results demonstrate that either Shh or an intact urothelium is necessary to support bladder growth and induce smooth muscle differentiation. (Figure 6) Given the localization of Shh to the urothelium, it is highly likely that the urothelium is the source of Shh.[7]
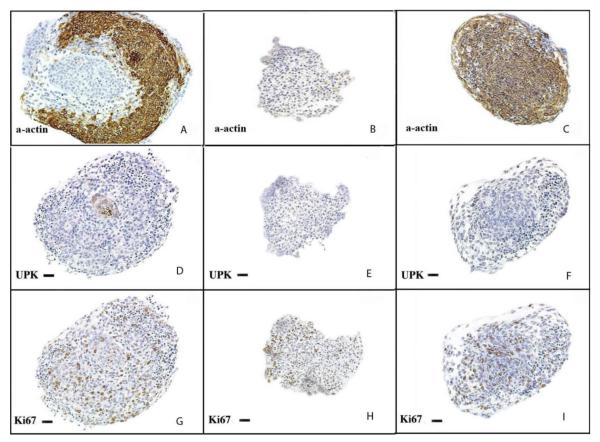
Figure 6. Urothelium and Shh regulates BLM smooth muscle differentiation.
Immunohistostaining of explants after 72 hours of incubation in serum free DMEM (20X). (A, D, G = intact bladders incubated without Shh) (A) Intact bladders incubated without Shh demonstrated strong α-actin staining of smooth muscle. (D) The urothelium, which stained for UPK, is seen at the center of the section (G) Diffuse expression of Ki67 was detected, indicating specimen viability. (B, E, H = BLM incubated in Shh-free media) (B) Neither smooth muscle nor (E) urothelium were detected. (H) K67 expression was detected, indicating viability. (C, F, I = BLM cultured with 480nM of Shh) (C) α-actin was expressed homogenously throughout the explant, indicating robust smooth muscle differentiation. (F) Uroplakin was not detected, demonstrating an absence of urothelial contamination of the specimen. (I) Ki67 expression was present. α-actin=smooth muscle α-actin, UPK=uroplakin, Ki67=nuclear proliferation marker. Final magnification 100x. Reproduced with permission from Cao et al., Differentiation 2010. [42]
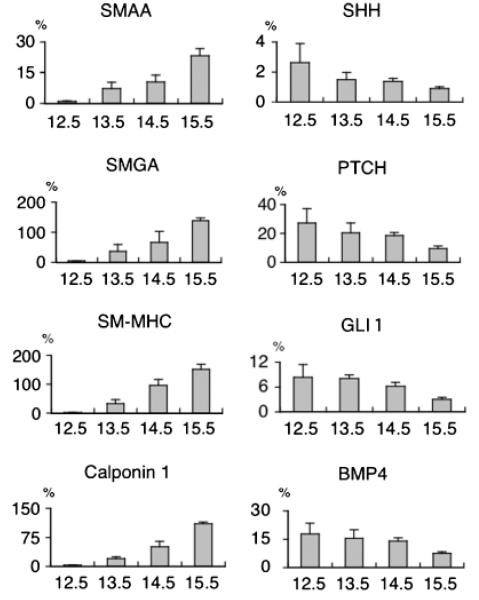
In vivo, Shh expression by the urothelium changes over time during bladder organogenesis. mRNA levels of Shh, Ptc1, Gli1, and BMP4 decrease linearly after peaking at E12.5. The converse is true for smooth muscle proteins. mRNA transcript levels for SMAA, smooth muscle gamma actin (SMGA), SM-MHC, and Calponin-1 are not detectable at E12.5 but increase in inverse proportion to the Shh pathway gene transcripts. (Figure 7) The temporal relationship between Shh signaling cascade proteins and smooth muscle marker expressions supports the hypothesis that Shh of epithelial origin induces and orchestrates smooth muscle differentiation in the bladder. As is the case with mesenchymal proliferation, it is likely that BMP4 is one of the prime mediators of the Shh signal in regulating smooth muscle differentiation. In vivo evidence is available in the ureter, where conditional bmp4 knockouts have significantly decreased and disorganized smooth muscle.[43]
Figure 7. mRNA expression levels of Shh-pathway and smooth muscle genes in the fetal bladder.
Percent RNA gene expression normalized to GAPDH mRNA by real-time reverse transcription polymerase chain reaction (RT-PCR) in embryonic bladders from E12.5 to E15.5 mice. (Y-axis: % mRNA gene expression against GAPDH by real-time RT-PCR). Reproduced with permission from Shiroyanagi et al., Differentiation 2007. [7]
In the same set of ex vivo experiments that demonstrated that increasing Shh concentration increased bladder explant size and cell count, increasing Shh concentrations also increased SMAA expression. Similar to the effect seen on cellular proliferation, SMAA expression did not significantly change with an increase in Shh concentration from 48nM to 480nM, which may be due to receptor saturation.[42] One hypothesis is that all the observed effects of varying Shh concentrations on mesenchymal proliferation and smooth muscle differentiation are due to its graded proliferative effect on bladder mesenchyme. Smooth muscle differentiation occurs after mesenchymal expansion during bladder development. Consequently, the increase in α-actin expression by E12 bladder explants cultured in high concentrations of Shh may be a direct reflection of the population of mesenchymal cells available to undergo differentiation into smooth muscle. However, it is uncertain to what extent smooth muscle differentiation is dependent upon mesenchymal proliferation. Yu postulated that smooth muscle differentiation begins only when mesenchymal precursors reach a specific density.[37] However, there is also evidence that smooth muscle differentiation and patterning are distinct from mesenchymal proliferation and are mediated through different pathways.[44, 45] For example, while bladders of Gli2 null mice have a normal number of proliferating mesenchymal cells, these cells fail to pattern appropriately.[44]
Other signaling pathways are involved in bladder smooth muscle differentiation. Inhibition of Shh signaling with cyclopamine, which inhibits hedgehog signaling by binding to Smo, decreases or eliminates expression of Gli1 and Bmp4, as well as early markers of smooth muscle differentiation, such as SMAA. However, inhibition of Shh did not decrease expression of SM-MHC.[7] Therefore, while Shh and/or urothelium is necessary for the induction of smooth muscle differentiation, it is clear that other pathways are involved in the development of mature smooth muscle cells. Currently, it is not known what transcription factors, local environmental cues, or signaling cascades mediate these later phases of smooth muscle differentiation.
BLM is exquisitely sensitive to Shh and may have differential responses to Shh concentration. One hypothesis is that high levels of Shh inhibit smooth muscle differentiation. This can be inferred from urothelium recombination experiments in which smooth muscle differentiation was inhibited immediately adjacent to the urothelium where Shh concentrations are presumed to be highest, while smooth muscle differentiation was induced distant from the urothelium where Shh concentrations are presumed lowest.[6] Furthermore, Ptc1 is highly expressed in the mesenchyme immediately adjacent to the urothelium but has markedly decreased expression in the periphery of bladder where smooth muscle differentiation first occurs.[7] An alternative hypothesis is that the fate of cells at different positions within the bladder wall are determined by transcription factors active earlier in embryogenesis and Shh is simply the universal “on” switch that induces differentiation into previously determined cell types.
Shh has been shown in other organ systems to pattern tissue during embryogenesis; in the nervous system, cells from different areas in the developing brain differentiate into distinct cell types upon exposure to Shh-producing cells.[28, 29] In the gut, smooth muscle differentiation is inhibited in cells in closest proximity to the Shh source.[46] These findings are consistent with Shh acting as a paracrine factor that determines cell fate, possibly as a function of concentration gradient.[28, 29] Shiroyanagi proposed that Shh is necessary for the induction of smooth muscle differentiation but, after induction, smooth muscle differentiation can proceed throughout the mesenchyme only when Shh levels and its downstream mediators fall below the point at which smooth muscle differentiation is inhibited.
The temporal and spatial expression of Shh and its downstream mediators also indirectly support the hypothesis that Shh concentration affects patterning of bladder smooth muscle. At E12.5, Ptc1, Gli1, and Bmp4 mRNA is much more highly expressed in the sub-urothelial layer (which is devoid of smooth muscle) than the outer zone of the peripheral mesenchyme (where smooth muscle differentiation begins and is most robust). Gli1 expression is highly expressed in the subepithelial mesenchyme from E12.5-E13.5 but then decreases after E13.5. The converse is found in the peripheral mesenchyme: Gli1 expression increases from E12.5 to E15 but never reaches the level of expression seen in the subepithelial layer at E12.5. The expression profile of BMP4 in the peripheral mesenchyme follows the trend of Gli1 but is delayed by one day.[47] (Figure 8) These findings support the hypothesis that high levels of BMP4 inhibit smooth muscle formation in the subepithelial layer but permit and/or promote smooth muscle differentiation, as assessed by SMAA and SM-MHC, in the peripheral mesenchyme. Similar to the inhibitory effect it has on mesenchymal proliferation, high levels of BMP4 may inhibit smooth muscle differentiation. If this is confirmed, BMP4 would be a prime candidate for the mediator of Shh on smooth muscle differentiation and patterning in the bladder.[7]
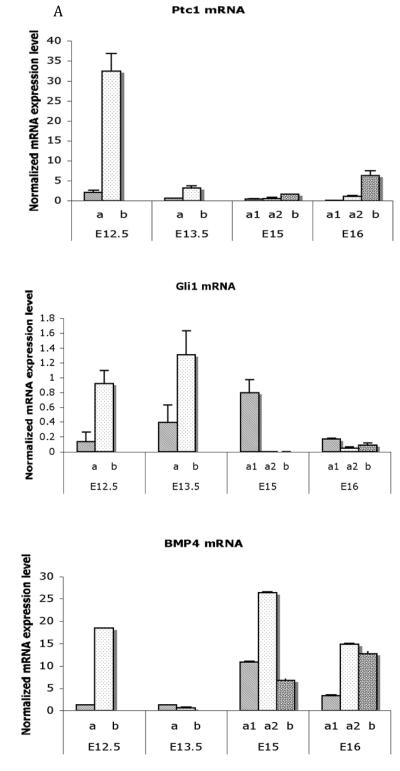
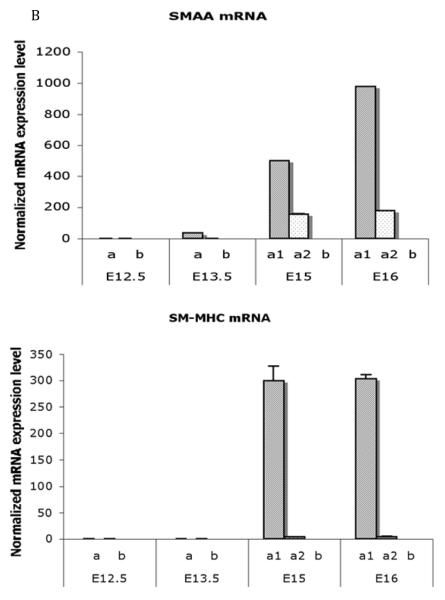
Figure 8. Differential spatial expression of Shh-pathway and smooth muscle genes in the fetal bladder.
A) Real time RT-PCR analysis of mRNA encoding Ptc1, Gli1 and Bmp4 in laser-captured mesenchymal components from each gestational stage. Expression of Ptc1, Gli1 and Bmp4 was restricted to a thin layer of mesenchymal cells in the submucosa immediately adjacent to the epithelium. Expression of both Ptc1 and Bmp4 genes was significantly decreased in all mesenchymal cells in the E13.5 bladder. Ptc1 remained at a low level in contrast to Bmp4 whose expression was increased at E15 and E16. Gli1 gene expression was up regulated in the submucosa at E13.5. At E15 the expression changed to the smooth muscle layer (Mann-Whitney test, p<0.001). a = serosal zone; a1 = smooth muscle layer; a2 = intermediate zone; b = submucosal zone.
B Real time RT-PCR analysis of mRNA encoding SMAA and SM-MHC in laser captured mesenchymal components from each gestational stage. Expression of SMAA mRNA was detectable in the peripheral cellular population at E13.5 and increased to significantly high levels in smooth muscle cells at E15 and E16. SM-MHC was detectable temporal slightly later than SMAA and increasing in expression at E15 and E16 (Mann-Whitney test, p<0.001). a = serosal zone. a1 = smooth muscle layer; a2 = intermediate zone; b = submucosal zone. Reproduced with permission from Liu et al., Int J Dev Biol., 2010. [47]
Cheng et al. has provided further insight into the Shh-Ptc1-Gli2-BMP4 pathway’s role in patterning of bladder smooth muscle by demonstrating that Gli2 mediates the inductive effect of Shh on mesenchymal proliferation, as well as the radial patterning of smooth muscle seen in the bladder, possibly through regulation of BMP4.[44] Cell proliferation, especially in the subepithelial mesenchyme of the bladder, was significantly reduced in Gli2−/− mice. In WT mice, BMP4 co-localized with Gli2 and was highly expressed in the subepithelial mesenchyme of the bladder. In Gli2 knockouts, BMP4 levels were decreased and smooth muscle formed in the subepithelial mesenchyme where smooth muscle formation is inhibited in WT mice. Some caution must be exercised: while Gli1 and Gli2 generally mediate the Shh signal and Gli3 represses the Shh signal, the interaction between the different Gli signaling molecules and other components of the Shh cascade make interpretation of single knockout mutants difficult.[48, 49] However, the role of Gli2, BMP4, and the Smad pathway, which is activated by Shh, is most likely central to the process of smooth muscle differentiation and patterning in the bladder.
Despite these advances, it is not known what early mediators of mesenchymal migration, other Shh-associated transcription factors, and crosstalk between the Shh signaling cascade and other pathways, are involved in the patterning of bladder smooth muscle. Looking at different organ systems such as the nervous system in which the role of the Shh pathway is better understood may provide areas of investigation that could advance our understanding of how smooth muscle differentiation and patterning is regulated in the bladder (for review of Shh in brain development, see Ruiz I Altaba).[50]
TGF-β
TGF-β is known to induce smooth muscle differentiation in prostatic stromal cells
[51] TGF-β is also expressed in the developing bladder, with the highest expression levels found at E13.5 in the peripheral mesenchyme.[47] The temporal and spatial similarities of TGF-β expression in the bladder with known mediators of smooth muscle differentiation make TGF-β an attractive candidate for mediating smooth muscle differentiation. TGF-β signaling is primarily transduced by R-Smad 2 and 3; however, members of the TGF-β family have been shown to promote intestinal development and terminal smooth muscle differentiation in 10T1/2 cells through the hedgehog pathway.[13, 52-55]. Lien et al demonstrated that TGF-β stimulation of this cell line increased SMAA and SM22α through the PI3K/Akt pathway.[54] Furthermore, it has recently been shown that TGF-β induces expression of Gli1 and Gli2 through the Smad pathway, which could serve as possible points of cross-talk with the Shh pathway.[56]
SRF
Serum response factor (SRF) is a DNA-binding phosphoprotein that is a key controller of muscle-specific gene expression. Although it is expressed in a number of organs, SRF is important in muscle cell-specific gene expression and smooth muscle differentiation.[57-59] SRF-mediated transcription of smooth muscle genes depends on the interaction of SRF with appropriate cofactors, the specific combination of which determines the transcriptional target.[60] Myocardin, which is expressed abundantly in smooth muscle cells, is one of these cofactors and has been shown to be important in SRF-dependent gene transcription during smooth muscle differentiation.[61, 62]
SRF is present in E12.5 bladders and localizes to the peripheral mesenchyme.[63] It appears to be required for the initial differentiation of smooth muscle cells and has been proposed to have a role in the expression of SMAA during early smooth muscle differentiation.[63] The SMAA and SM22-α promoters both have binding sites for SRF.[64] SRF localizes to the peripheral bladder mesenchyme prior to SMAA expression.[63] Additionally, SRF is an attractive candidate gene for a downstream mediator of Shh on terminal smooth muscle differentiation as it is expressed most highly in the peripheral mesenchyme of E15 bladders, where SMAA expression has already begun and SM-MHC expression is peaking.[14, 47]. Shiroyanagi et al. have proposed that SRF is upregulated in the peripheral mesenchyme where Shh, Ptc1, and BMP4 levels are low, which seems to be necessary for smooth muscle differentiation.[7] (Table 2)
Table 2.
Smooth muscle development-related gene expression profiles in the mouse embryonic mesenchyme
| Bladder mesenchyme |
E12.5 | E13.5 | E15 | E16 | |
|---|---|---|---|---|---|
| Peripheral zone |
SMAA TGF-β1 Gli1 |
Outer zone | Gli1 Bmp4 SRF TGF-β1 SRF SMAA SM-MHC |
SMAA Bmp4 |
|
| Inner zone | SMAA Bmp4 |
SMAA Bmp4 |
|||
| Subepithelium | Ptc1 Gli1 Bmp4 |
Gli1 | TGF-β1 Bmp4 |
Ptc1 Bmp4 |
Reproduced with permission from Liu et al., Int J Dev Biol., 2010. [47]
Dlgh1
Discs-large homolog 1 (Dlgh1), which is highly expressed in urothelium but not in smooth muscle, is important for patterning smooth muscle in the ureter. Dlgh1−/− mice have a normal urothelium but lack a subepithelial layer and have misaligned ureteral smooth muscle.[65] It is possible that Dlgh1 regulates epithelial-mesenchymal signaling in the developing ureter and/or is involved in forming cytoskeletal architecture. Although, to our knowledge, the role of Dlgh1 in the bladder has not been examined, the similarities between mesenchymal proliferation and smooth muscle differentiation in the bladder, kidney, and ureter make Dlgh1 an attractive candidate gene in bladder development.
DNA-binding proteins
Investigations into mesenchymal development and differentiation in the genitourinary system and other organs have revealed that DNA-binding proteins such as T-box transcription factors are important in early mesenchymal migration and patterning.[66-68] In the ureters of Tbx18−/− mice, Ptc1 is down-regulated and mesenchymal BMP4 expression is lost. However, unlike Dlgh mutants, the epithelium of Tbx18 knockouts fails to differentiate into a functional urothelium. This results in decreased cell migration from the metanephric mesenchyme to the ureter; the mesenchymal cells that do migrate fail to differentiate into smooth muscle. Although Tbx18 is not expressed elsewhere in the urogenital system, it is likely that similar gene(s) are active in initial mesenchymal-epithelial interaction in the bladder; however, they have yet to be identified.
Other DNA-binding proteins appear to act later in smooth muscle differentiation. The zinc finger DNA binding protein Teashirst3 (Tshz3) is required for smooth muscle differentiation from mesenchyme in the cranial ureter but is not necessary for the earlier event of mesenchymal proliferation.[45] Unlike Tbx18, Tshz3 is expressed in the bladder. At E15.5, Tshz3 is expressed in the peripheral mesenchyme, which has already differentiated into smooth muscle. At E18.5, Tshz3 is more highly expressed in the subepithelial layer than in smooth muscle cells.[45] Tshz3 mediates the Shh signal downstream from Ptc1 and BMP4, and is expressed in both undifferentiated peripheral mesenchyme and differentiating smooth muscle cells. Tbx18 mutants fail to express myocardin and have been shown to activate non-BMP4 mediated pathways involved in smooth muscle differentiation. This raises the possibility that Tshz3 is involved in SRF-mediated smooth muscle cell differentiation.
Summary and Future Directions
The research conducted over the past 30 years has greatly advanced our understanding of the developmental biology of smooth muscle in the urinary bladder. In the bladder, as is true in most epithelialized organs, the interaction between the epithelium and mesenchyme is critical to the differentiation and organization of smooth muscle. The urothelium, which secretes Shh, acts in a paracrine fashion on the adjacent mesenchyme. It is likely that Shh is the factor that links mesenchymal proliferation and smooth muscle differentiation during normal bladder development.
First, Shh induces cellular proliferation, possibly by regulating the mitotic cycle through the interaction between Ptc1 and cell cycle regulatory proteins as well as through the Gli pathway. It is possible that BMP4 may indirectly inhibit mesenchymal proliferation through as yet unidentified mechanisms. Then, once a critical mass of mesenchymal cells has been achieved, Shh acts along the well-described pathway through the Gli proteins and BMP4 to regulate smooth muscle differentiation and patterning. Further work should be done to elucidate what is assuredly a complex interaction between genes that orchestrate early mesenchymal migration and patterning, Shh and its known downstream mediators, and as yet unidentified proteins, during the fluid process of bladder development.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- [1].Newman J, Antonakopoulos GN. The fine structure of the human fetal urinary bladder. Development and maturation. A light, transmission and scanning electron microscopic study. J Anat. 1989;166:135–150. [PMC free article] [PubMed] [Google Scholar]
- [2].Tanaka ST, Ishii K, Demarco RT, Pope J.C.t., Brock JW, 3rd, Hayward SW. Endodermal origin of bladder trigone inferred from mesenchymal-epithelial interaction. J Urol. 2010;183:386–391. doi: 10.1016/j.juro.2009.08.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Tanagho EA, Pugh RC. The anatomy and function of the ureterovesical junction. Br J Urol. 1963;35:151–165. doi: 10.1111/j.1464-410x.1963.tb02610.x. [DOI] [PubMed] [Google Scholar]
- [4].Tejedo-Mateu A, Vilanova-Trias J, Ruano-Gil D. Contribution to the study of the development of the terminal portion of the Wolffian duct and the ureter. Eur Urol. 1975;1:41–45. [PubMed] [Google Scholar]
- [5].Viana R, Batourina E, Huang H, Dressler GR, Kobayashi A, Behringer RR, Shapiro E, Hensle T, Lambert S, Mendelsohn C. The development of the bladder trigone, the center of the anti-reflux mechanism. Development. 2007;134:3763–3769. doi: 10.1242/dev.011270. [DOI] [PubMed] [Google Scholar]
- [6].Cao M, Liu B, Cunha G, Baskin L. Urothelium patterns bladder smooth muscle location. Pediatr Res. 2008;64:352–357. doi: 10.1203/PDR.0b013e318180e4c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Shiroyanagi Y, Liu B, Cao M, Agras K, Li J, Hsieh MH, Willingham EJ, Baskin LS. Urothelial sonic hedgehog signaling plays an important role in bladder smooth muscle formation. Differentiation. 2007;75:968–977. doi: 10.1111/j.1432-0436.2007.00187.x. [DOI] [PubMed] [Google Scholar]
- [8].Uvelius B, Gabella G. Relation between cell length and force production in urinary bladder smooth muscle. Acta Physiol Scand. 1980;110:357–365. doi: 10.1111/j.1748-1716.1980.tb06681.x. [DOI] [PubMed] [Google Scholar]
- [9].Uvelius B. Relation between mechanical and morphological characteristics in urinary bladder smooth muscle. Acta Physiol Scand Suppl. 1980;483:1–51. [PubMed] [Google Scholar]
- [10].Deng M, Mohanan S, Polyak E, Chacko S. Caldesmon is necessary for maintaining the actin and intermediate filaments in cultured bladder smooth muscle cells. Cell Motil Cytoskeleton. 2007;64:951–965. doi: 10.1002/cm.20236. [DOI] [PubMed] [Google Scholar]
- [11].Yamboliev IA, Hedges JC, Mutnick JL, Adam LP, Gerthoffer WT. Evidence for modulation of smooth muscle force by the p38 MAP kinase/HSP27 pathway. Am J Physiol Heart Circ Physiol. 2000;278:H1899–1907. doi: 10.1152/ajpheart.2000.278.6.H1899. [DOI] [PubMed] [Google Scholar]
- [12].Duband JL, Gimona M, Scatena M, Sartore S, Small JV. Calponin and SM 22 as differentiation markers of smooth muscle: spatiotemporal distribution during avian embryonic development. Differentiation. 1993;55:1–11. doi: 10.1111/j.1432-0436.1993.tb00027.x. [DOI] [PubMed] [Google Scholar]
- [13].Bostrom K, Tintut Y, Kao SC, Stanford WP, Demer LL. HOXB7 overexpression promotes differentiation of C3H10T1/2 cells to smooth muscle cells. J Cell Biochem. 2000;78:210–221. doi: 10.1002/(sici)1097-4644(20000801)78:2<210::aid-jcb4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- [14].Miano JM, Cserjesi P, Ligon KL, Periasamy M, Olson EN. Smooth muscle myosin heavy chain exclusively marks the smooth muscle lineage during mouse embryogenesis. Circ Res. 1994;75:803–812. doi: 10.1161/01.res.75.5.803. [DOI] [PubMed] [Google Scholar]
- [15].Rodriguez LV, Alfonso Z, Zhang R, Leung J, Wu B, Ignarro LJ. Clonogenic multipotent stem cells in human adipose tissue differentiate into functional smooth muscle cells. Proc Natl Acad Sci U S A. 2006;103:12167–12172. doi: 10.1073/pnas.0604850103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Baskin LS, Hayward SW, Young P, Cunha GR. Role of mesenchymal-epithelial interactions in normal bladder development. J Urol. 1996;156:1820–1827. [PubMed] [Google Scholar]
- [17].DiSandro MJ, Li Y, Baskin LS, Hayward S, Cunha G. Mesenchymal-epithelial interactions in bladder smooth muscle development: epithelial specificity. J Urol. 1998;160:1040–1046. doi: 10.1097/00005392-199809020-00022. discussion 1079. [DOI] [PubMed] [Google Scholar]
- [18].Cunha GR, Battle E, Young P, Brody J, Donjacour A, Hayashi N, Kinbara H. Role of epithelial-mesenchymal interactions in the differentiation and spatial organization of visceral smooth muscle. Epithelial Cell Biol. 1992;1:76–83. [PubMed] [Google Scholar]
- [19].Del Buono R, Fleming KA, Morey AL, Hall PA, Wright NA. A nude mouse xenograft model of fetal intestine development and differentiation. Development. 1992;114:67–73. doi: 10.1242/dev.114.1.67. [DOI] [PubMed] [Google Scholar]
- [20].Madison BB, Braunstein K, Kuizon E, Portman K, Qiao XT, Gumucio DL. Epithelial hedgehog signals pattern the intestinal crypt-villus axis. Development. 2005;132:279–289. doi: 10.1242/dev.01576. [DOI] [PubMed] [Google Scholar]
- [21].Baskin L, DiSandro M, Li Y, Li W, Hayward S, Cunha G. Mesenchymal-epithelial interactions in bladder smooth muscle development: effects of the local tissue environment. J Urol. 2001;165:1283–1288. [PubMed] [Google Scholar]
- [22].Yang L, Wang Y, Mao H, Fleig S, Omenetti A, Brown KD, Sicklick JK, Li YX, Diehl AM. Sonic hedgehog is an autocrine viability factor for myofibroblastic hepatic stellate cells. J Hepatol. 2008;48:98–106. doi: 10.1016/j.jhep.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Koleva M, Kappler R, Vogler M, Herwig A, Fulda S, Hahn H. Pleiotropic effects of sonic hedgehog on muscle satellite cells. Cell Mol Life Sci. 2005;62:1863–1870. doi: 10.1007/s00018-005-5072-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mimeault M, Batra SK. Interplay of distinct growth factors during epithelial mesenchymal transition of cancer progenitor cells and molecular targeting as novel cancer therapies. Ann Oncol. 2007;18:1605–1619. doi: 10.1093/annonc/mdm070. [DOI] [PubMed] [Google Scholar]
- [25].Haraguchi R, Motoyama J, Sasaki H, Satoh Y, Miyagawa S, Nakagata N, Moon A, Yamada G. Molecular analysis of coordinated bladder and urogenital organ formation by Hedgehog signaling. Development. 2007;134:525–533. doi: 10.1242/dev.02736. [DOI] [PubMed] [Google Scholar]
- [26].Jenkins D, Winyard PJ, Woolf AS. Immunohistochemical analysis of Sonic hedgehog signalling in normal human urinary tract development. J Anat. 2007;211:620–629. doi: 10.1111/j.1469-7580.2007.00808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Passman JN, Dong XR, Wu SP, Maguire CT, Hogan KA, Bautch VL, Majesky MW. A sonic hedgehog signaling domain in the arterial adventitia supports resident Sca1+ smooth muscle progenitor cells. Proc Natl Acad Sci U S A. 2008;105:9349–9354. doi: 10.1073/pnas.0711382105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ericson J, Muhr J, Jessell TM, Edlund T. Sonic hedgehog: a common signal for ventral patterning along the rostrocaudal axis of the neural tube. Int J Dev Biol. 1995;39:809–816. [PubMed] [Google Scholar]
- [29].Ericson J, Muhr J, Placzek M, Lints T, Jessell TM, Edlund T. Sonic hedgehog induces the differentiation of ventral forebrain neurons: a common signal for ventral patterning within the neural tube. Cell. 1995;81:747–756. doi: 10.1016/0092-8674(95)90536-7. [DOI] [PubMed] [Google Scholar]
- [30].Yucel S, Liu W, Cordero D, Donjacour A, Cunha G, Baskin LS. Anatomical studies of the fibroblast growth factor-10 mutant, Sonic Hedge Hog mutant and androgen receptor mutant mouse genital tubercle. Adv Exp Med Biol. 2004;545:123–148. doi: 10.1007/978-1-4419-8995-6_8. [DOI] [PubMed] [Google Scholar]
- [31].Liu W, Li Y, Cunha S, Hayward G, Baskin L. Diffusable growth factors induce bladder smooth muscle differentiation. In Vitro Cell Dev Biol Anim. 2000;36:476–484. doi: 10.1290/1071-2690(2000)036<0476:dgfibs>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- [32].Mahlapuu M, Enerback S, Carlsson P. Haploinsufficiency of the forkhead gene Foxf1, a target for sonic hedgehog signaling, causes lung and foregut malformations. Development. 2001;128:2397–2406. doi: 10.1242/dev.128.12.2397. [DOI] [PubMed] [Google Scholar]
- [33].Madison BB, McKenna LB, Dolson D, Epstein DJ, Kaestner KH. FoxF1 and FoxL1 link hedgehog signaling and the control of epithelial proliferation in the developing stomach and intestine. J Biol Chem. 2009;284:5936–5944. doi: 10.1074/jbc.M808103200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Massague J. How cells read TGF-beta signals. Nat Rev Mol Cell Biol. 2000;1:169–178. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- [35].Marcelle C, Ahlgren S, Bronner-Fraser M. In vivo regulation of somite differentiation and proliferation by Sonic Hedgehog. Dev Biol. 1999;214:277–287. doi: 10.1006/dbio.1999.9389. [DOI] [PubMed] [Google Scholar]
- [36].Wu C, Shimo T, Liu M, Pacifici M, Koyama E. Sonic hedgehog functions as a mitogen during bell stage of odontogenesis. Connect Tissue Res. 2003;44(Suppl 1):92–96. [PubMed] [Google Scholar]
- [37].Yu J, Carroll TJ, McMahon AP. Sonic hedgehog regulates proliferation and differentiation of mesenchymal cells in the mouse metanephric kidney. Development. 2002;129:5301–5312. doi: 10.1242/dev.129.22.5301. [DOI] [PubMed] [Google Scholar]
- [38].Barnes EA, Kong M, Ollendorff V, Donoghue DJ. Patched1 interacts with cyclin B1 to regulate cell cycle progression. EMBO J. 2001;20:2214–2223. doi: 10.1093/emboj/20.9.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Oxburgh L, Chu GC, Michael SK, Robertson EJ. TGFbeta superfamily signals are required for morphogenesis of the kidney mesenchyme progenitor population. Development. 2004;131:4593–4605. doi: 10.1242/dev.01324. [DOI] [PubMed] [Google Scholar]
- [40].Brenner-Anantharam A, Cebrian C, Guillaume R, Hurtado R, Sun TT, Herzlinger D. Tailbud-derived mesenchyme promotes urinary tract segmentation via BMP4 signaling. Development. 2007;134:1967–1975. doi: 10.1242/dev.004234. [DOI] [PubMed] [Google Scholar]
- [41].Chu GC, Dunn NR, Anderson DC, Oxburgh L, Robertson EJ. Differential requirements for Smad4 in TGFbeta-dependent patterning of the early mouse embryo. Development. 2004;131:3501–3512. doi: 10.1242/dev.01248. [DOI] [PubMed] [Google Scholar]
- [42].Cao M, Tasian G, Wang MH, Liu B, Cunha G, Baskin L. Urothelium-derived Sonic hedgehog promotes mesenchymal proliferation and induces bladder smooth muscle differentiation. Differentiation. 2010 doi: 10.1016/j.diff.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wang GJ, Brenner-Anantharam A, Vaughan ED, Herzlinger D. Antagonism of BMP4 signaling disrupts smooth muscle investment of the ureter and ureteropelvic junction. J Urol. 2009;181:401–407. doi: 10.1016/j.juro.2008.08.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Cheng W, Yeung CK, Ng YK, Zhang JR, Hui CC, Kim PC. Sonic Hedgehog mediator Gli2 regulates bladder mesenchymal patterning. J Urol. 2008;180:1543–1550. doi: 10.1016/j.juro.2008.06.003. [DOI] [PubMed] [Google Scholar]
- [45].Caubit X, Lye CM, Martin E, Core N, Long DA, Vola C, Jenkins D, Garratt AN, Skaer H, Woolf AS, Fasano L. Teashirt 3 is necessary for ureteral smooth muscle differentiation downstream of SHH and BMP4. Development. 2008;135:3301–3310. doi: 10.1242/dev.022442. [DOI] [PubMed] [Google Scholar]
- [46].Sukegawa A, Narita T, Kameda T, Saitoh K, Nohno T, Iba H, Yasugi S, Fukuda K. The concentric structure of the developing gut is regulated by Sonic hedgehog derived from endodermal epithelium. Development. 2000;127:1971–1980. doi: 10.1242/dev.127.9.1971. [DOI] [PubMed] [Google Scholar]
- [47].Liu B, Feng D, Lin G, Cao M, Kan YW, Cunha GR, Baskin LS. Signalling molecules involved in mouse bladder smooth muscle cellular differentiation. Int J Dev Biol. 2010;54:175–180. doi: 10.1387/ijdb.082610bl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ruiz i Altaba A. Gli proteins encode context-dependent positive and negative functions: implications for development and disease. Development. 1999;126:3205–3216. doi: 10.1242/dev.126.14.3205. [DOI] [PubMed] [Google Scholar]
- [49].Ruiz i Altaba A. Combinatorial Gli gene function in floor plate and neuronal inductions by Sonic hedgehog. Development. 1998;125:2203–2212. doi: 10.1242/dev.125.12.2203. [DOI] [PubMed] [Google Scholar]
- [50].Ruiz i Altaba A, Palma V, Dahmane N. Hedgehog-Gli signalling and the growth of the brain. Nat Rev Neurosci. 2002;3:24–33. doi: 10.1038/nrn704. [DOI] [PubMed] [Google Scholar]
- [51].Peehl DM, Sellers RG. Induction of smooth muscle cell phenotype in cultured human prostatic stromal cells. Exp Cell Res. 1997;232:208–215. doi: 10.1006/excr.1997.3525. [DOI] [PubMed] [Google Scholar]
- [52].Dunn NR, Vincent SD, Oxburgh L, Robertson EJ, Bikoff EK. Combinatorial activities of Smad2 and Smad3 regulate mesoderm formation and patterning in the mouse embryo. Development. 2004;131:1717–1728. doi: 10.1242/dev.01072. [DOI] [PubMed] [Google Scholar]
- [53].van Eyll JM, Pierreux CE, Lemaigre FP, Rousseau GG. Shh-dependent differentiation of intestinal tissue from embryonic pancreas by activin A. J Cell Sci. 2004;117:2077–2086. doi: 10.1242/jcs.01067. [DOI] [PubMed] [Google Scholar]
- [54].Lien SC, Usami S, Chien S, Chiu JJ. Phosphatidylinositol 3-kinase/Akt pathway is involved in transforming growth factor-beta1-induced phenotypic modulation of 10T1/2 cells to smooth muscle cells. Cell Signal. 2006;18:1270–1278. doi: 10.1016/j.cellsig.2005.10.013. [DOI] [PubMed] [Google Scholar]
- [55].Ingram WJ, Wicking CA, Grimmond SM, Forrest AR, Wainwright BJ. Novel genes regulated by Sonic Hedgehog in pluripotent mesenchymal cells. Oncogene. 2002;21:8196–8205. doi: 10.1038/sj.onc.1205975. [DOI] [PubMed] [Google Scholar]
- [56].Dennler S, Andre J, Alexaki I, Li A, Magnaldo T, ten Dijke P, Wang XJ, Verrecchia F, Mauviel A. Induction of sonic hedgehog mediators by transforming growth factor-beta: Smad3-dependent activation of Gli2 and Gli1 expression in vitro and in vivo. Cancer Res. 2007;67:6981–6986. doi: 10.1158/0008-5472.CAN-07-0491. [DOI] [PubMed] [Google Scholar]
- [57].Belaguli NS, Schildmeyer LA, Schwartz RJ. Organization and myogenic restricted expression of the murine serum response factor gene. A role for autoregulation. J Biol Chem. 1997;272:18222–18231. doi: 10.1074/jbc.272.29.18222. [DOI] [PubMed] [Google Scholar]
- [58].Chen CY, Schwartz RJ. Identification of novel DNA binding targets and regulatory domains of a murine tinman homeodomain factor, nkx-2.5. J Biol Chem. 1995;270:15628–15633. doi: 10.1074/jbc.270.26.15628. [DOI] [PubMed] [Google Scholar]
- [59].Browning CL, Culberson DE, Aragon IV, Fillmore RA, Croissant JD, Schwartz RJ, Zimmer WE. The developmentally regulated expression of serum response factor plays a key role in the control of smooth muscle-specific genes. Dev Biol. 1998;194:18–37. doi: 10.1006/dbio.1997.8808. [DOI] [PubMed] [Google Scholar]
- [60].Wang DZ, Li S, Hockemeyer D, Sutherland L, Wang Z, Schratt G, Richardson JA, Nordheim A, Olson EN. Potentiation of serum response factor activity by a family of myocardin-related transcription factors. Proc Natl Acad Sci U S A. 2002;99:14855–14860. doi: 10.1073/pnas.222561499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Wang D, Chang PS, Wang Z, Sutherland L, Richardson JA, Small E, Krieg PA, Olson EN. Activation of cardiac gene expression by myocardin, a transcriptional cofactor for serum response factor. Cell. 2001;105:851–862. doi: 10.1016/s0092-8674(01)00404-4. [DOI] [PubMed] [Google Scholar]
- [62].Du KL, Ip HS, Li J, Chen M, Dandre F, Yu W, Lu MM, Owens GK, Parmacek MS. Myocardin is a critical serum response factor cofactor in the transcriptional program regulating smooth muscle cell differentiation. Mol Cell Biol. 2003;23:2425–2437. doi: 10.1128/MCB.23.7.2425-2437.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Li J, Shiroyanagi Y, Lin G, Haqq C, Lin CS, Lue TF, Willingham E, Baskin LS. Serum response factor, its cofactors, and epithelial-mesenchymal signaling in urinary bladder smooth muscle formation. Differentiation. 2006;74:30–39. doi: 10.1111/j.1432-0436.2006.00057.x. [DOI] [PubMed] [Google Scholar]
- [64].Chang PS, Li L, McAnally J, Olson EN. Muscle specificity encoded by specific serum response factor-binding sites. J Biol Chem. 2001;276:17206–17212. doi: 10.1074/jbc.M010983200. [DOI] [PubMed] [Google Scholar]
- [65].Mahoney ZX, Sammut B, Xavier RJ, Cunningham J, Go G, Brim KL, Stappenbeck TS, Miner JH, Swat W. Discs-large homolog 1 regulates smooth muscle orientation in the mouse ureter. Proc Natl Acad Sci U S A. 2006;103:19872–19877. doi: 10.1073/pnas.0609326103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Airik R, Bussen M, Singh MK, Petry M, Kispert A. Tbx18 regulates the development of the ureteral mesenchyme. J Clin Invest. 2006;116:663–674. doi: 10.1172/JCI26027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Bussen M, Petry M, Schuster-Gossler K, Leitges M, Gossler A, Kispert A. The T-box transcription factor Tbx18 maintains the separation of anterior and posterior somite compartments. Genes Dev. 2004;18:1209–1221. doi: 10.1101/gad.300104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Ludtke TH, Christoffels VM, Petry M, Kispert A. Tbx3 promotes liver bud expansion during mouse development by suppression of cholangiocyte differentiation. Hepatology. 2009;49:969–978. doi: 10.1002/hep.22700. [DOI] [PubMed] [Google Scholar]
- [69].Haraguchi R, Mo R, Hui C, Motoyama J, Makino S, Shiroishi T, Gaffield W, Yamada G. Unique functions of Sonic hedgehog signaling during external genitalia development. Development. 2001;128:4241–4250. doi: 10.1242/dev.128.21.4241. [DOI] [PubMed] [Google Scholar]
- [70].Freestone SH, Marker P, Grace OC, Tomlinson DC, Cunha GR, Harnden P, Thomson AA. Sonic hedgehog regulates prostatic growth and epithelial differentiation. Dev Biol. 2003;264:352–362. doi: 10.1016/j.ydbio.2003.08.018. [DOI] [PubMed] [Google Scholar]
- [71].Yu M, Gipp J, Yoon JW, Iannaccone P, Walterhouse D, Bushman W. Sonic hedgehog-responsive genes in the fetal prostate. J Biol Chem. 2009;284:5620–5629. doi: 10.1074/jbc.M809172200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Vezina CM, Allgeier SH, Fritz WA, Moore RW, Strerath M, Bushman W, Peterson RE. Retinoic acid induces prostatic bud formation. Dev Dyn. 2008;237:1321–1333. doi: 10.1002/dvdy.21526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Zhu G, Zhau HE, He H, Zhang L, Shehata B, Wang X, Cerwinka WH, Elmore J, He D. Sonic and desert hedgehog signaling in human fetal prostate development. Prostate. 2007;67:674–684. doi: 10.1002/pros.20563. [DOI] [PubMed] [Google Scholar]
- [74].Lamm ML, Catbagan WS, Laciak RJ, Barnett DH, Hebner CM, Gaffield W, Walterhouse D, Iannaccone P, Bushman W. Sonic hedgehog activates mesenchymal Gli1 expression during prostate ductal bud formation. Dev Biol. 2002;249:349–366. doi: 10.1006/dbio.2002.0774. [DOI] [PubMed] [Google Scholar]
- [75].Lin C, Yin Y, Veith GM, Fisher AV, Long F, Ma L. Temporal and spatial dissection of Shh signaling in genital tubercle development. Development. 2009;136:3959–3967. doi: 10.1242/dev.039768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Suzuki K, Bachiller D, Chen YP, Kamikawa M, Ogi H, Haraguchi R, Ogino Y, Minami Y, Mishina Y, Ahn K, Crenshaw EB, 3rd, Yamada G. Regulation of outgrowth and apoptosis for the terminal appendage: external genitalia development by concerted actions of BMP signaling [corrected] Development. 2003;130:6209–6220. doi: 10.1242/dev.00846. [DOI] [PubMed] [Google Scholar]
- [77].Li C, Hu L, Xiao J, Chen H, Li JT, Bellusci S, Delanghe S, Minoo P. Wnt5a regulates Shh and Fgf10 signaling during lung development. Dev Biol. 2005;287:86–97. doi: 10.1016/j.ydbio.2005.08.035. [DOI] [PubMed] [Google Scholar]
- [78].Sato H, Murphy P, Giles S, Bannigan J, Takayasu H, Puri P. Visualizing expression patterns of Shh and Foxf1 genes in the foregut and lung buds by optical projection tomography. Pediatr Surg Int. 2008;24:3–11. doi: 10.1007/s00383-007-2036-1. [DOI] [PubMed] [Google Scholar]
- [79].Miller LA, Wert SE, Clark JC, Xu Y, Perl AK, Whitsett JA. Role of Sonic hedgehog in patterning of tracheal-bronchial cartilage and the peripheral lung. Dev Dyn. 2004;231:57–71. doi: 10.1002/dvdy.20105. [DOI] [PubMed] [Google Scholar]
- [80].Ramalho-Santos M, Melton DA, McMahon AP. Hedgehog signals regulate multiple aspects of gastrointestinal development. Development. 2000;127:2763–2772. doi: 10.1242/dev.127.12.2763. [DOI] [PubMed] [Google Scholar]
- [81].Sasaki Y, Iwai N, Tsuda T, Kimura O. Sonic hedgehog and bone morphogenetic protein 4 expressions in the hindgut region of murine embryos with anorectal malformations. J Pediatr Surg. 2004;39:170–173. doi: 10.1016/j.jpedsurg.2003.10.009. discussion 170-173. [DOI] [PubMed] [Google Scholar]
- [82].Weaver M, Batts L, Hogan BL. Tissue interactions pattern the mesenchyme of the embryonic mouse lung. Dev Biol. 2003;258:169–184. doi: 10.1016/s0012-1606(03)00117-9. [DOI] [PubMed] [Google Scholar]
- [83].Takamoto N, Zhao B, Tsai SY, DeMayo FJ. Identification of Indian hedgehog as a progesterone-responsive gene in the murine uterus. Mol Endocrinol. 2002;16:2338–2348. doi: 10.1210/me.2001-0154. [DOI] [PubMed] [Google Scholar]
- [84].Hebrok M, Kim SK, St Jacques B, McMahon AP, Melton DA. Regulation of pancreas development by hedgehog signaling. Development. 2000;127:4905–4913. doi: 10.1242/dev.127.22.4905. [DOI] [PubMed] [Google Scholar]
- [85].Kawahira H, Scheel DW, Smith SB, German MS, Hebrok M. Hedgehog signaling regulates expansion of pancreatic epithelial cells. Dev Biol. 2005;280:111–121. doi: 10.1016/j.ydbio.2005.01.008. [DOI] [PubMed] [Google Scholar]
- [86].Rossi JM, Dunn NR, Hogan BL, Zaret KS. Distinct mesodermal signals, including BMPs from the septum transversum mesenchyme, are required in combination for hepatogenesis from the endoderm. Genes Dev. 2001;15:1998–2009. doi: 10.1101/gad.904601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Gritli-Linde A, Bei M, Maas R, Zhang XM, Linde A, McMahon AP. Shh signaling within the dental epithelium is necessary for cell proliferation, growth and polarization. Development. 2002;129:5323–5337. doi: 10.1242/dev.00100. [DOI] [PubMed] [Google Scholar]
- [88].Lin D, Huang Y, He F, Gu S, Zhang G, Chen Y, Zhang Y. Expression survey of genes critical for tooth development in the human embryonic tooth germ. Dev Dyn. 2007;236:1307–1312. doi: 10.1002/dvdy.21127. [DOI] [PubMed] [Google Scholar]
- [89].Cairns DM, Sato ME, Lee PG, Lassar AB, Zeng L. A gradient of Shh establishes mutually repressing somitic cell fates induced by Nkx3.2 and Pax3. Dev Biol. 2008;323:152–165. doi: 10.1016/j.ydbio.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Danesh SM, Villasenor A, Chong D, Soukup C, Cleaver O. BMP and BMP receptor expression during murine organogenesis. Gene Expr Patterns. 2009;9:255–265. doi: 10.1016/j.gep.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Michno K, Boras-Granic K, Mill P, Hui CC, Hamel PA. Shh expression is required for embryonic hair follicle but not mammary gland development. Dev Biol. 2003;264:153–165. doi: 10.1016/s0012-1606(03)00401-9. [DOI] [PubMed] [Google Scholar]
- [92].Cho KW, Kim JY, Song SJ, Farrell E, Eblaghie MC, Kim HJ, Tickle C, Jung HS. Molecular interactions between Tbx3 and Bmp4 and a model for dorsoventral positioning of mammary gland development. Proc Natl Acad Sci U S A. 2006;103:16788–16793. doi: 10.1073/pnas.0604645103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Hens JR, Dann P, Zhang JP, Harris S, Robinson GW, Wysolmerski J. BMP4 and PTHrP interact to stimulate ductal outgrowth during embryonic mammary development and to inhibit hair follicle induction. Development. 2007;134:1221–1230. doi: 10.1242/dev.000182. [DOI] [PubMed] [Google Scholar]