Abstract
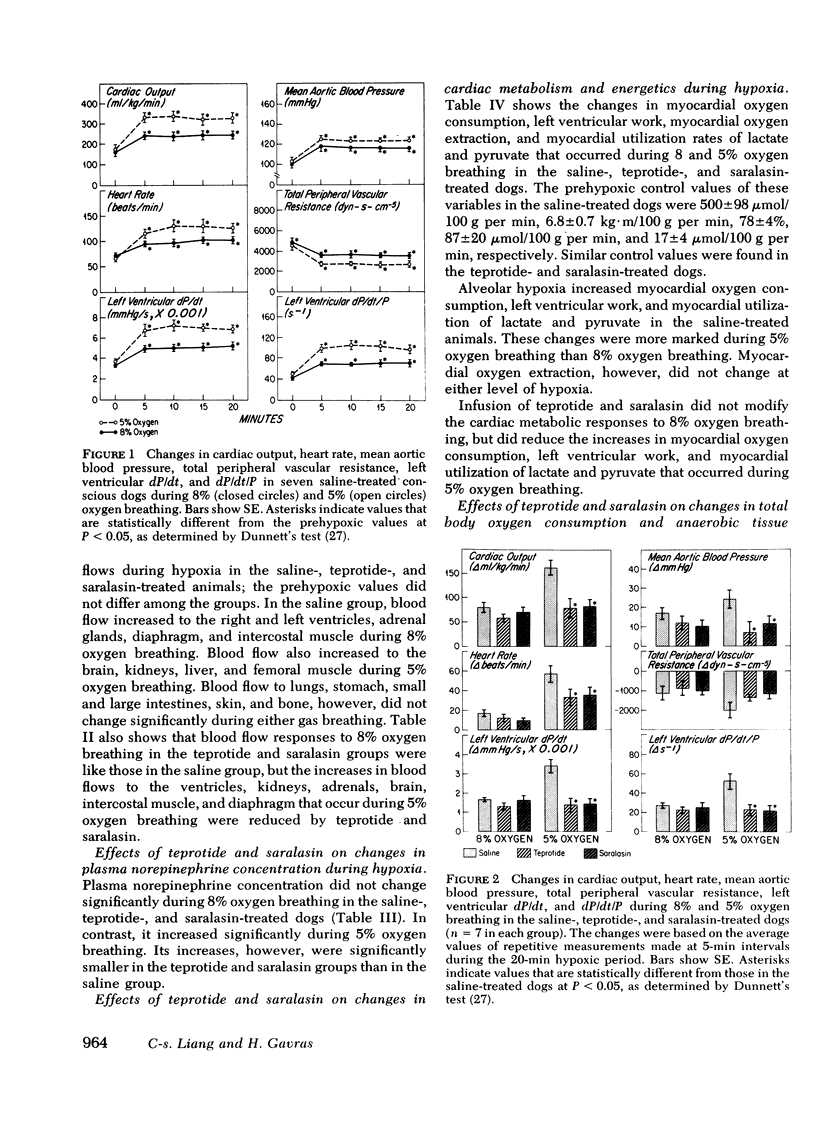
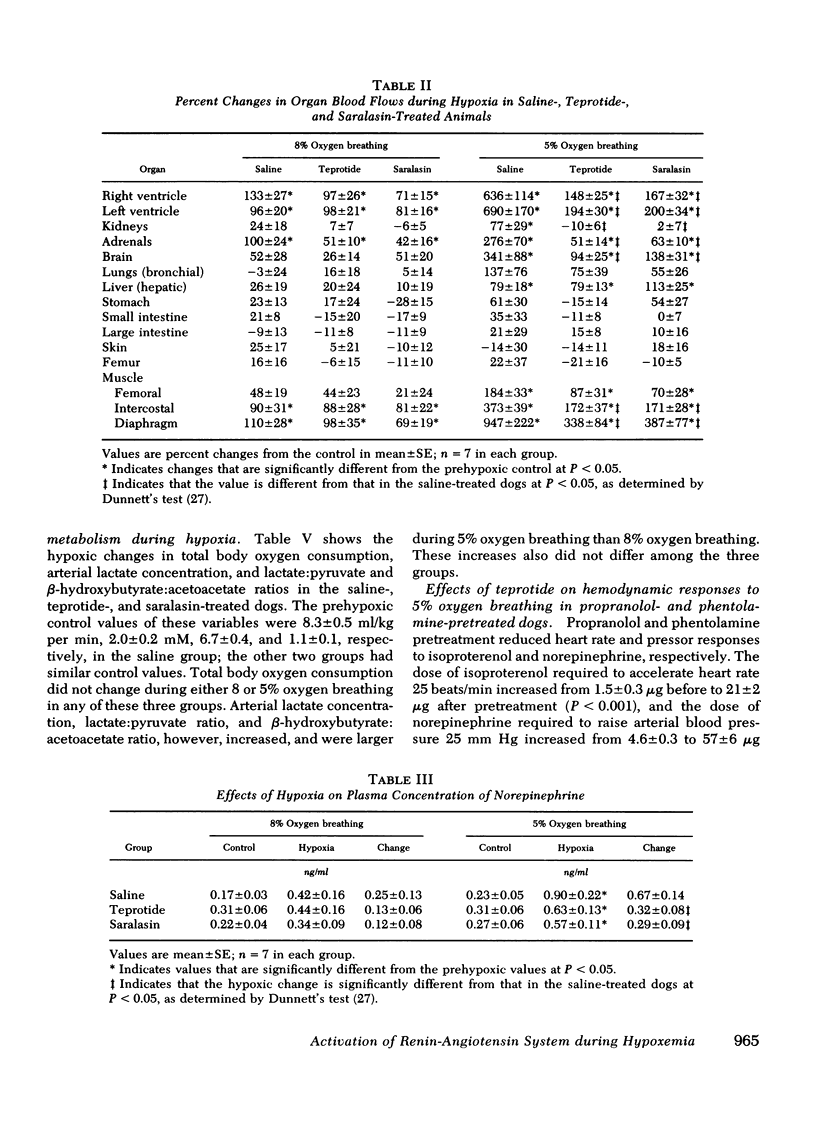
The role of the renin-angiotensin system in mediating the circulatory and metabolic responses to hypoxia was studied in three groups of conscious dogs that were infused continuously with normal saline, teprotide (10 μg/kg per min), and saralasin (1 μg/kg per min), respectively. Hypoxia was produced by switching from breathing room air to 5 or 8% oxygen-nitrogen mixture. Plasma renin activity increased from 2.3±0.4 to 4.9±0.8 ng/ml per h during 8% oxygen breathing, and from 2.8±0.4 to 8.4±1.8 ng/ml per h during 5% oxygen breathing. As expected, cardiac output, heart rate, mean aortic blood pressure, and left ventricular dP/dt and dP/dt/P increased during both 5 and 8% oxygen breathing in the saline-treated dogs; greater increases occurred during the more severe hypoxia. Teprotide and saralasin infusion diminished the hemodynamic responses to 5% oxygen breathing, but did not affect the responses to 8% oxygen breathing significantly. In addition, the increased blood flows to the myocardium, kidneys, adrenals, brain, intercostal muscle, and diaphragm that usually occur during 5% oxygen breathing were reduced by both agents. These agents also reduced the increases in plasma norepinephrine concentration during 5% oxygen breathing, but had no effects on tissue aerobic or anaerobic metabolism.
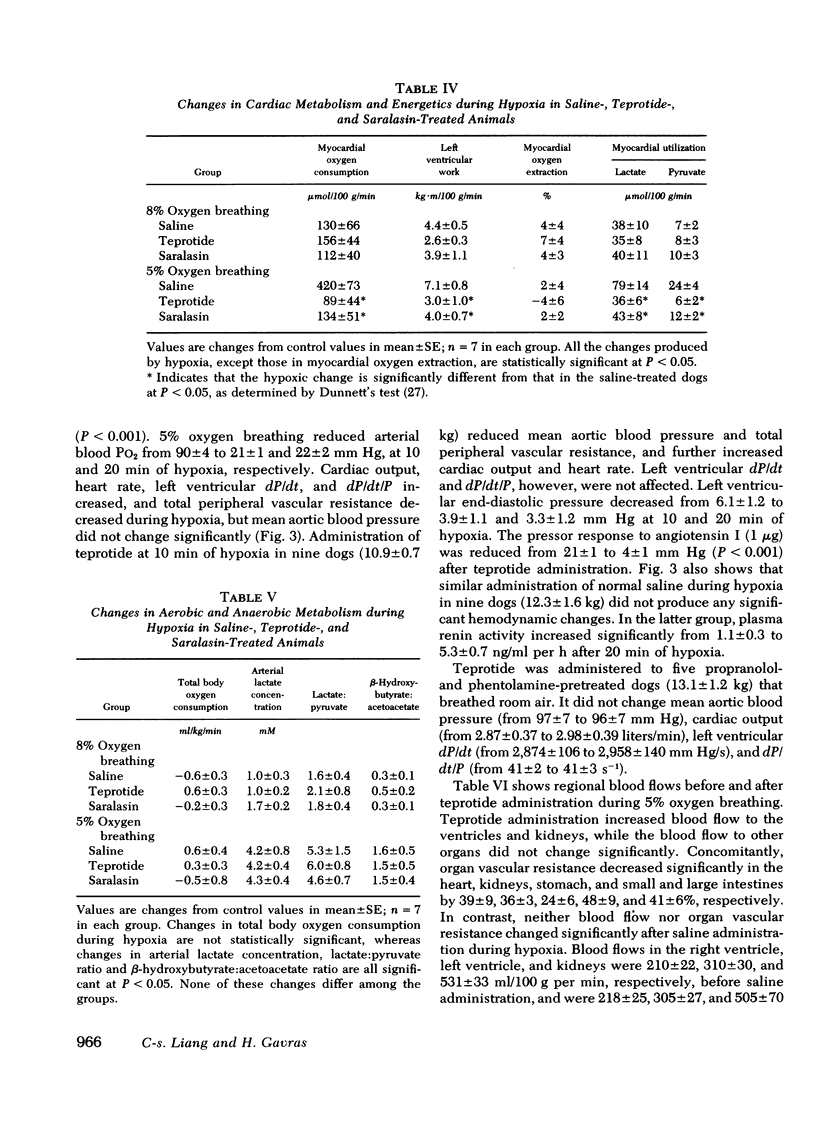
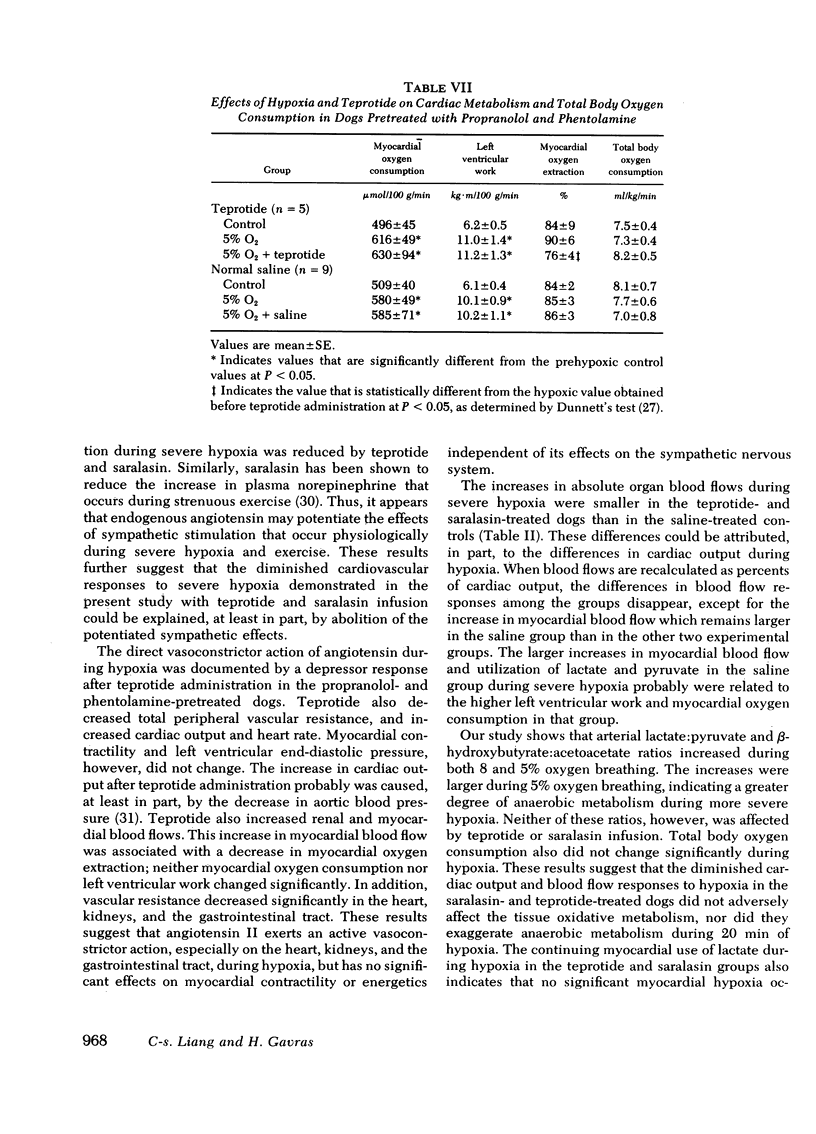
In dogs pretreated with propranolol and phentolamine, administration of teprotide (0.5 mg/kg) during 5% oxygen breathing reduced mean aortic blood pressure and total peripheral vascular resistance, and increased cardiac output and heart rate, but did not affect left ventricular dP/dt, dP/dt/P, and end-diastolic pressure. Simultaneously, renal and myocardial blood flows increased and myocardial oxygen extraction decreased, while myocardial oxygen consumption did not change significantly.
These results suggest that the renin-angiotensin system plays an important role in the hemodynamic responses to severe hypoxia. It appears that angiotensin not only exerts a direct vasoconstrictor action, especially upon the coronary and renal circulations, but also potentiates the cardiovascular effects of sympathetic stimulation that occur during severe hypoxia.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cain S. M. Relative rates of arterial lactate and oxygen-deficit accumulation in hypoxic dogs. Am J Physiol. 1973 May;224(5):1190–1194. doi: 10.1152/ajplegacy.1973.224.5.1190. [DOI] [PubMed] [Google Scholar]
- DEMOPOULOS H. B., HIGHMAN B., ALTLAND P. D., GERVING M. A., KALEY G. EFFECT OF HIGH ALTITUDE ON GRANULAR JUXTAGLOMERULAR CELLS AND THEIR POSSIBLE ROLE IN ERYTHROPOIETIN PRODUCTION. Am J Pathol. 1965 Mar;46:497–509. [PMC free article] [PubMed] [Google Scholar]
- Davidson D. M., Covell J. W., Malloch C. I., Ross J., Jr Factors influencing indices of left ventricle contractility in the conscious dog. Cardiovasc Res. 1974 May;8(3):299–312. doi: 10.1093/cvr/8.3.299. [DOI] [PubMed] [Google Scholar]
- Davis J. O., Freeman R. H. Mechanisms regulating renin release. Physiol Rev. 1976 Jan;56(1):1–56. doi: 10.1152/physrev.1976.56.1.1. [DOI] [PubMed] [Google Scholar]
- FRIEDLAND I. M., DIETRICH L. S. A rapid enzymic determination of L-lactic acid. Anal Biochem. 1961 Aug;2:390–392. doi: 10.1016/0003-2697(61)90014-8. [DOI] [PubMed] [Google Scholar]
- Fagard R., Amery A., Reybrouck T., Lijnen P., Moerman E., Bogaert M., De Schaepdryver A. Effects of angiotensin antagonism on hemodynamics, renin, and catecholamines during exercise. J Appl Physiol Respir Environ Exerc Physiol. 1977 Sep;43(3):440–444. doi: 10.1152/jappl.1977.43.3.440. [DOI] [PubMed] [Google Scholar]
- Gould A. B., Goodman S. A. The effect of hypoxia on the renin-angiotensinogen system. Lab Invest. 1970 May;22(5):443–447. [PubMed] [Google Scholar]
- HUCKABEE W. E. Control of concentration gradients of pyruvate and lactate across cell membranes in blood. J Appl Physiol. 1956 Sep;9(2):163–170. doi: 10.1152/jappl.1956.9.2.163. [DOI] [PubMed] [Google Scholar]
- Henry D. P., Starman B. J., Johnson D. G., Williams R. H. A sensitive radioenzymatic assay for norepinephrine in tissues and plasma. Life Sci. 1975 Feb 1;16(3):375–384. doi: 10.1016/0024-3205(75)90258-1. [DOI] [PubMed] [Google Scholar]
- Liang C. S., Gavras H., Hood W. B., Jr Renin-angiotensin system inhibition in conscious sodium-depleted dogs. Effects on systemic and coronary hemodynamics. J Clin Invest. 1978 Apr;61(4):874–883. doi: 10.1172/JCI109013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C. S. Metabolic control of circulation. Effects of iodoacetate and fluoroacetate. J Clin Invest. 1977 Jul;60(1):61–69. doi: 10.1172/JCI108769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molteni A., Zakheim R. M., Mullis K. B., Mattioli L. The effect of chronic alveolar hypoxia on lung and serum angiotensin I converting enzyme activity. Proc Soc Exp Biol Med. 1974 Oct;147(1):263–265. doi: 10.3181/00379727-147-38323. [DOI] [PubMed] [Google Scholar]
- OLIVER W. J., BRODY G. L. EFFECT OF PROLONGED HYPOXIA UPON GRANULARITY OF RENAL JUXTAGLOMERULAR CELLS. Circ Res. 1965 Jan;16:83–88. doi: 10.1161/01.res.16.1.83. [DOI] [PubMed] [Google Scholar]
- Ondetti M. A., Williams N. J., Sabo E. F., Pluscec J., Weaver E. R., Kocy O. Angiotensin-converting enzyme inhibitors from the venom of Bothrops jararaca. Isolation, elucidation of structure, and synthesis. Biochemistry. 1971 Oct 26;10(22):4033–4039. doi: 10.1021/bi00798a004. [DOI] [PubMed] [Google Scholar]
- Pals D. T., Masucci F. D., Sipos F., Denning G. S., Jr A specific competitive antagonist of the vascular action of angiotensin. II. Circ Res. 1971 Dec;29(6):664–672. doi: 10.1161/01.res.29.6.664. [DOI] [PubMed] [Google Scholar]
- RAMSEY L. H. Analysis of gas in biological fluids by gas chromatography. Science. 1959 Apr 3;129(3353):900–901. doi: 10.1126/science.129.3353.900. [DOI] [PubMed] [Google Scholar]
- Robertson A. L., Jr, Smeby R. R., Bumpus F. M., Page I. H. Production of renin by human juxtaglomercular cells in vitro. Circ Res. 1966 Jun;18(1 Suppl 1):131–142. doi: 10.1161/01.res.18.s6.i-131. [DOI] [PubMed] [Google Scholar]
- Rudolph A. M., Heymann M. A. The circulation of the fetus in utero. Methods for studying distribution of blood flow, cardiac output and organ blood flow. Circ Res. 1967 Aug;21(2):163–184. doi: 10.1161/01.res.21.2.163. [DOI] [PubMed] [Google Scholar]
- Sealey J. E., Gerten-Banes J., Laragh J. H. The renin system: Variations in man measured by radioimmunoassay or bioassay. Kidney Int. 1972 Apr;1(4):240–253. doi: 10.1038/ki.1972.34. [DOI] [PubMed] [Google Scholar]
- Tuffley R. E., Rubenstein D., Slater J. D., Williams E. S. Serum renin activity during exposure to hypoxia. J Endocrinol. 1970 Dec;48(4):497–510. doi: 10.1677/joe.0.0480497. [DOI] [PubMed] [Google Scholar]
- WILLIAMSON D. H., MELLANBY J., KREBS H. A. Enzymic determination of D(-)-beta-hydroxybutyric acid and acetoacetic acid in blood. Biochem J. 1962 Jan;82:90–96. doi: 10.1042/bj0820090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakheim R. M., Molteni A., Mattioli L., Park M. Plasma angiotensin II levels in hypoxic and hypovolemic stress in unanesthetized rabbits. J Appl Physiol. 1976 Oct;41(4):462–465. doi: 10.1152/jappl.1976.41.4.462. [DOI] [PubMed] [Google Scholar]


