Abstract
Introduction
The development of biotechnology has enabled the creation of various recombinant fusion proteins as a new class of biotherapeutics. The uniqueness of fusion proteins lies in their ability to fuse two or more protein domains, providing vast opportunities to generate novel combinations of functions. Pharmacokinetic (PK) studies, which are critical components in preclinical and clinical drug development, have not been fully explored for fusion proteins. The lack of general PK models and study guidelines has become a bottleneck for translation of fusion proteins from basic research to the clinic.
Areas covered
This article reviews the current status of PK studies for fusion proteins, covering the processes that affect PK. According to their PK properties, a classification of fusion proteins is suggested along with examples from the clinic or under development. Current limitations and future perspectives for PK of fusion proteins are also discussed.
Expert opinion
A PK model for bifunctional fusion proteins is presented to highlight the importance of mechanistic studies for a thorough understanding of the PK properties of fusion proteins. The model suggests investigating the receptor binding and subsequent intracellular disposition of individual domains, which can have dramatic impact on the PK of fusion proteins.
Keywords: Pharmacokinetics, fusion protein, receptor binding, disposition, target-mediated drug disposition
1. Introduction
Since the production of recombinant human insulin about 3 decades ago, recombinant proteins have become an important class of therapeutics with dramatically increased numbers and frequent use. The further development of recombinant technology allows for the production of not only natural proteins, but also novel proteins that do not occur in nature. The pharmacokinetic (PK) and pharmacodynamic (PD) properties are a challenge for the development of protein drugs, and have been extensively studied 1. However, the PK/PD properties of bifunctional fusion proteins are less well characterized than protein drugs with single domains such as hormones, growth factors, and monoclonal antibodies. Bifunctional fusion proteins, constructed by fusing the genes of two proteins together, combine the functions of the parent proteins in order to improve their PK and PD properties 2–5, or to introduce novel approaches in drug delivery or targeting 6. Six currently FDA approved fusion protein drugs including Enbrel® (TNF-R/Fc-IgG1), Ontak® (IL-2/diphtheria toxin), Orencia® (CTLA-4/Fc-IgG1), Amevive® (LFA-3/Fc-IgG1), Arcalyst® (IL-1R/Fc-IgG1), Nplate® (TPO/Fc-IgG1), Nulojix® (CTLA-4/Fc-IgG1) and Eylea® (VEGFR1&2/Fc-IgG1) have foreshown the advent of many more fusion protein drugs 7–9. Fusion protein drugs have significant clinical impact, belonging to the top four most lucrative biotech sectors. The combined sales of the first four fusion protein products has reached US$ 3.7 billion 10.
2. Current status of the PK study of fusion proteins
2.1. Absorption
Due to their relatively large molecular size (compared to small molecular drugs) and susceptibility to chemical and enzymatic degradation, many fusion protein drugs cannot be efficiently absorbed via non-invasive routes such as oral administration, and therefore are often administered invasively, e.g., intravenous or subcutaneous administration. Following subcutaneous administration, the absorption of drugs to the cardiovascular pool can either go through the blood capillary or through the lymphatics. It was suggested that compounds with molecular weight up to 1000 Dalton permeate the blood capillary very efficiently, and are hardly absorbed via the lymphatics 11. As macromolecules, the permeability of fusion proteins through the blood capillary is generally low, and their absorption to the blood mainly depends on the lymphatic vessels 11. The molecular weight of proteins has been suggested to have a linear relationship with the lymphatic absorption, where larger molecular weight results in increased lymphatic absorption 11. Proteins with molecular weight larger than 16,000 Dalton are mainly absorbed via the lymphatic draining, with more than 50% of the drug recovered from the lymphatics 11.
Although most fusion protein drugs are administered invasively, with the aid of functional domains which can be transported across delivery barriers, many fusion proteins can be absorbed via non-invasive means, such as oral and pulmonary routes 12. For instance, transferrin (Tf)-fusion proteins can be orally absorbed via the Tf-Tf receptor transcytosis across the intestinal epithelium cells 13–15. Another platform of Fc-fusion proteins enables pulmonary absorption of protein drugs via an immunoglobulin transport system (neonatal Fc receptor, FcRn, transcytosis) present in the upper and central airways. Protein drugs such as erythropoietin (Epo) or interferon beta (IFNβ) have been fused to the Fc fragment of IgG and successfully applied to noninvasively deliver bioactive proteins into the systemic circulation 16, 17. These fusion proteins greatly enhance the absorption of the protein drugs across delivery barrier, and will be further discussed in the Section 2.5.
2.2. Distribution
Similar to many protein and peptide drugs, the apparent volume of distribution for fusion proteins is usually small, and is limited to the volume of the extracellular space due to their low membrane permeability resulting from their large size and hydrophilicity 18. PK of fusion proteins may exhibit single-exponential, bi-exponential or multiple-exponential profile which can be well characterized by one, two or multiple compartment models comprised of central and peripheral compartments 19–21. The central compartment primarily represents the vascular space and the interstitial space of well-perfused organs, while the peripheral compartment represents the interstitial space of poorly perfused tissues. Thus, the volume of distribution of the central compartment(Vc) in which peptides and proteins initially distribute after intravenous administration is typically 3–8 L, approximately equal to or slightly higher than the plasma volume. Examples include interleukin 2 (IL-2)-diphtheria toxin fusion protein (an immunotoxin targeted toward IL-2 receptor bearing T-cells) and immunocytokine EMD 273066 huKS-IL2 (a fusion protein composed of two IL-2 molecules genetically fused to a humanized monoclonal antibody against adenocarcinoma-associated antigen), which display a Vc of 6 L 22, 23 and 3.9–7.1 L 24, respectively. The steady-state volume of distribution (Vss) frequently comprises with 14–20 L, generally not more than twice the volume of distribution of the central compartment 25. However, it should be noted that Vss is commonly calculated using non-compartmental analysis (NCA) methods, which assume first-order disposition processes with elimination occurring from the rapidly equilibrating or central compartment 26–28. This assumption is frequently not met for protein drugs that distribute to the peripheral tissues and undergo significant protease degradation, and therefore Vss by NCA needs to be carefully calculated to avoid potential over-or under -estimation 29, 30.
In many cases, when one or more of the protein domains in a fusion protein bind to intra- and extravascular proteins in the tissues, the biodistribution is not only affected by blood perfusion and permeability, but also by the biodistribution of the binding target, its expression level, turn-over rate, etc. Under these circumstances, active tissue uptake can be observed. For example, fusion proteins with targeting moieties (e.g. immunotoxins, immunocytokines) are designed to actively target the drug to disease sites with high target expression. Immunocytokine L19–IL-2 fusion protein, for example, intends to selectively deliver IL-2 to tumor vasculature using antibody fragment L19 to ED-B, a domain contained in the angiogenesis-associated isoform of fibronectin (B-FN). Twenty-four hours after injection, biodistribution studies in tumor-bearing animals suggested a tumor-to-blood ratio of 33 and a tumor-to-normal tissue ratio higher than 10 31.
On the other hand, fusion proteins with delivery moieties can distribute to tissues, such as the brain, that are not generally permeable to large molecules, thereby influencing the biodistribution. For instance, fusion proteins with transferrin (Tf) or anti-Tf antibody domain have been applied for active drug delivery across the blood-brain barrier (BBB). Tf is an iron-binding protein that transports iron for absorption, storage, and utilization by the body. Abundant Tf receptors (TfR) can be found on the brain capillary endothelium. After binding to TfRs on the BBB, the fusion proteins undergo transcytosis with the TfR and are able to across the barrier 32, 33.
2.3. Elimination
The elimination pathways for fusion proteins are very similar compared to protein and peptide drugs with a single protein domain. The major pathways include proteolysis, renal elimination, hepatic elimination, as well as receptor-mediated endocytosis. However, due to the different elimination mechanisms of each individual domain and their interactions, the elimination of fusion proteins can be much more complicated than single-domain protein drugs.
Proteolysis can be very substantial for peptides and small proteins, resulting in their short half-lives, ranging from several minutes to several hours 25. One approach in overcoming this challenge is to fuse the peptide/protein drug to a large carrier domain, making it less accessible to protease digestion. For example, Glucagon-Like Peptide-1 (GLP-1) is a ~4 kDa peptide with potent activity in stimulating insulin release in a glucose-dependent manner, and is a potential therapeutic drug for type II diabetes 34. However, the peptide is subject to rapid digestion by peptidases in vivo 35, limiting its clinical applications. In order to improve the stability for human use, several GLP-1 fusion proteins have been studied. Albiglutide, consisting of two repeats of modified GLP-1 fused to albumin, and LY2189265, consisting of IGG4-Fc fused to two modified GLP-1 peptides, both demonstrated a prolonged half-life of 4–5 days in human subjects (REF). In both examples, GLP-1 was modified to protect from dipeptidyl peptidase-IV cleavage 36, 37. Additionally, through the fusion of GLP-1 with Tf, the resultant GLP-1-Tf fusion protein was resistant to inactivation by peptidases, and had a half-life of approximately 2 days, as compared to 1–2 minutes for native GLP-1, in mice 38. This fusion protein, PF-04603629, has been clinically tested in 2008 but the results are not yet available.
Renal elimination can also constitute a significant portion of protein and peptide drug elimination, especially for those with small molecular weight (MW). The glomerulus in kidney has a sieving effect and can filter many protein drugs. Size, molecular conformation, and charge of the protein drug may affect the filtration rate of the glomerulus. Although the size selectivity is not well-established, proteins with a MW of <15 kDa are generally filtered freely in the glomeruli, while proteins up to 45 kDa are quite rapidly filtered, and proteins between 45 to 60 kDa filtered only restrictedly. Proteins larger than 60 kDa are generally not filtered through the kidney 39. Many protein drugs (e.g. IL-2,40 IL -11,41 growth hormone, 42 and insulin 43) are effectively eliminated through this route. By increasing the molecular weight, fusion proteins with large size may be able to minimize the renal elimination, and gain much longer plasma half-life. Therefore, fusing carrier proteins with large molecular size such as albumin, Fc of IgG, or Tf to small protein drugs can not only protect from proteolysis, but also decrease renal elimination to greatly prolong their half-life. A single-chain human insulin-human serum albumin fusion protein showed an elimination t1/2 of ~ 7 h in normoglycemic mice, with a predicted elimination half-life of 50 h in human 44, which is much longer than the half-life of insulin (4–6 minutes) 45. There are many other examples including interferon-α-albumin fusion protein 46, growth hormone-albumin fusion protein 47, factor VIII Fc fusion protein 48, 49, factor IX Fc fusion protein 5, 50, and growth hormone-Tf fusion proteins 51 that have displayed prolonged half -life and sustained activity in vivo.
Although the hepatic elimination for fusion proteins are not as important as it is for most small molecule drugs, some fusion proteins may be metabolized in the liver. The metabolism of fusion protein is generally not conducted via the same enzymes as small molecule drugs, such as cytochrome P450, but instead via proteolysis following endocytosis. Fusion proteins containing protein domains that are metabolized in the liver (e.g. insulin 52, tissue plasminogen 53), may have a significant hepatic elimination.
Receptor-mediated endocytosis and subsequent intracellular metabolism is a unique and critical elimination pathway for many protein drugs. Following the binding of protein drugs to receptors or targets expressed in target tissues, the endocytosis process of the complex usually leads to degradation of the protein drugs in the lysosome. The receptor/target binding exhibits high affinity (due to the specific binding) and low capacity (due to the limited receptor/target number). This saturable elimination process is also described as target-mediated drug disposition (TMDD) 54. Presumably, fusion proteins that are built from protein domains may also follow the similar elimination mechanism. Since the elimination of fusion proteins will be affected by two different domains, the PK of fusion protein can become much more complicated compared to single domain proteins, as will be discussed in more detail in this review.
2.4. Classification of fusion proteins according to their PK properties
When looking at the composition of fusion proteins, frequently, one domain conveys a specific function or biological activity such as target activation or inactivation (e.g. ligand for receptor), enzymatic activity(e.g. coagulation factors), or toxicity (e.g. diphtheria toxin), whereas the other domain supplies more general functionality such as improving stability and half-life, or providing novel targeting and delivery routes. The presence of two different functional domains increases the diversity and the complexity of their PK properties.
According to the binding properties of the specific functional domain, fusion proteins can be divided into two categories with distinct PK properties, where (i) binding to the biological target does not lead to altered distribution and/or elimination, and (ii) binding to the biological target is responsible for altered drug distribution and/or elimination leading to a loss of plasma concentration. Proteins in the first category include most drugs that bind soluble proteins (e.g. the receptor domain in Etanercept binds soluble tumor necrosis factor 55) or substrates (e.g. enzymes drugs such as Elspar and Alteplase), or protein drugs used for specific indications that do not require binding to any specific cell surface target (e.g. intravenous immunoglobulin to treat primary immunodeficiencies). Fusion proteins with protein drug domains in this category have relatively simple PK profiles, since they either have no target protein binding or their target binding does not lead to significant elimination. For proteins in the second category, a unique TMDD clearance mechanism 54 can constitute a major elimination pathway. TMDD refers to the process where a protein drug binds to its target with high affinity and to a significant extent (relative to the dose), resulting in alterations in the plasma drug concentration due to high tissue binding and/or elimination. This term is typically used to describe proteins that bind binding to cell-surface receptors, and are internalized and degraded through receptor-mediated endocytosis (RME) (e.g. interleukin-1, IL-1, domain in Rilonacept, which binds to IL-1 receptor on cell surface 56). When the magnitude of the drug target (i.e. receptor) levels is similar or larger than the plasma drug levels, drug elimination through RME can contribute a significant fraction. TMDD can also apply to mechanisms other than RME. For example, some monoclonal antibodies such as rituximab bind to surface antigens and are degraded via antibody dependent cellular cytotoxicity. Additionally, other monoclonal antibodies such as denosumab and omalizumab bind soluble IgE, but form trimer or hexamer immune complexes that are recognized and degraded by phagocytosis. Since the elimination processes are saturable, fusion proteins with protein drug domains affected by TMDD may display nonlinearity in their PK profiles, and exhibit a dose-dependent plasma half-life 57.
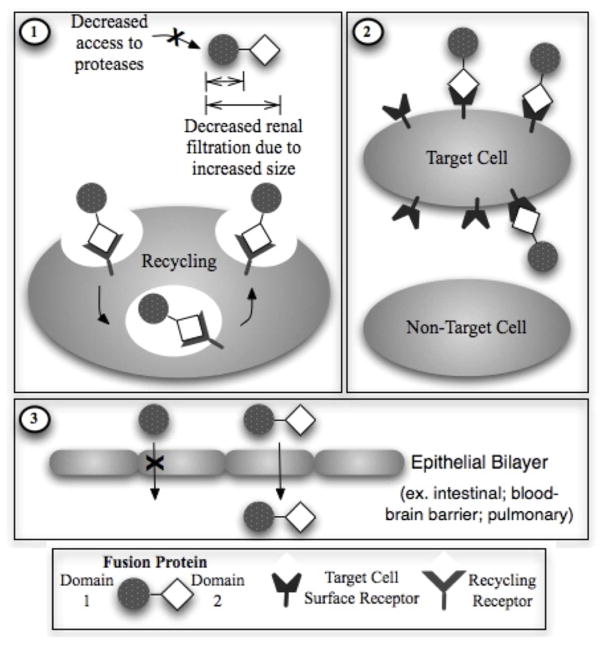
On the other hand, according to the impact of the second domain on the PK characteristics, fusion proteins can also be categorized into 3 classes (Figure 1). The first class contains a protein domain such as Fc domains of immunoglobulin, albumin or Tf to extend the plasma half-life of the fusion protein. In the second class, targeting moieties such as antibody or receptor ligand are utilized to direct the fusion protein to specific cells or tissues. The third class of fusion proteins utilizes the fusion partners to increase the absorption of the protein drug across various delivery barriers such as intestinal epithelium, pulmonary epithelium or BBB.
Figure 1.
Potential functions of Protein Domain 2 in a Fusion Protein. This domain usually serves a general function to improve pharmacokinetic and/or pharmacodynamic properties (i.e. Carrier domain). The possible functions may be to (1) increase in vivo stability/plasma half-life, (2) target specific tissues or cells, and/or (3) Facilitate transport or delivery to inaccessible sites.
PK of the first class of fusion proteins containing carrier protein domain (e.g. Fc-, albumin- or Tf- fusion proteins) is the most well-studied. The fusion of protein or peptide drugs with Fc domain, albumin or Tf has been demonstrated as a feasible approach to greatly enhance the plasma half-lives of protein and peptide drugs 38, 58, 59. The Fc, albumin and Tf proteins are suitable carrier proteins due to the following several reasons: First, they have molecular weights high enough (53 kDa for Fc domains, 67 kDa for albumin, and 80 kDa for Tf) to enable the fusion protein to evade the glomerular filtration, which is one of the most important clearance mechanisms for small proteins and peptides. Second, these proteins have endocytic recycling mechanisms that account for their long plasma half-lives (7–21 days for human IgG 60, 20 days for albumin, 7–10 days for Tf). After endocytosis, instead of being sorted to lysosome and degraded, IgG Fc-domain and albumin can bind tightly to the neonatal Fc receptors (FcRn) inside the acidic endosome, and are effectively recycled and released at the cell surface due to lower binding affinity at neutral pH 61–64. Similarly, the binding affinity of Tf to its receptor is high under acidic pH in the endosome, leading to effective recycling of Tf after iron delivery inside the endosome 13, 65, 66. Third, the endogenous counterparts of these proteins are largely abundant (endogenous concentration is around the several grams per liter range); therefore, the administration of exogenous fusion protein is unlikely to disturb the homeostasis of these proteins. Furthermore, as discussed in Section 2.3, fusion with a large carrier protein can decrease proteolytic degradation of a small peptide/protein drug. Examples for the first class of fusion proteins according to their second domain (including Fc-, albumin-, as well as Tf- fusion proteins) are summarized in Tables 1–3.
Table 1.
Examples of FDA approved Fc-fusion proteins.*
| Name (trade name; sponsoring companies) | Effector protein | Impact of Fc domain on PK | Targets | Disease | Proposed mechanisms of action | Reference |
|---|---|---|---|---|---|---|
| Alefacept (Amevive; Astellas) | Human leukocyte function antigen-3 (LFA-3) | Prolong half-life | CD2 | Plaque psoriasis | Inhibition of CD2-dependent lymphocyte activation | 89 |
| Etanercept (Enbrel; Amgen/Pfizer) | p75 TNFαR | Prolong half-life | TNFα | RA, JIA, PA, AS and plaque psoriasis | Neutralization of TNFα activity | 55 |
| Abatacept (Orencia; Bristol-Myers Squibb) | Cytotoxic T-lymphocyte antigen-4 (CTLA-4) | Prolong half-life | CD80 and CD86 | RA and JIA | Inhibition of CD80/86-dependent T cell activation | 90 |
| Romiplostim (Nplate; Amgen) | Peptide | Prolong half-life | Thrombo-poietin receptor | Thrombocytopaenia in patients with idiopathic thrombocytopaenic purpura | Activation of thrombopoietin receptor | 91 |
| Rilonacept (Arcalyst; Regeneron) | Ligand-binding domains of IL-1 receptor and IL-1 receptor accessory protein (IL-1RAcP) | Prolong half-life | IL-1 | CAPS | Neutralization of IL-1 activity | 56 |
| Belatacept (Nulojix; tol-Myers Squibb) | Cytotoxic T-lymphocyte antigen-4 (CTLA-4) | Prolong half-life | CD80 and CD86 | prophylaxis of organ rejection in adult patients receiving a kidney transplant | Inhibition of CD80/86-dependent T cell activation | 8 |
| Aflibercept (Eylea; Regeneron) | Vascular endothelial growth factor receptor 1 and 2 (VEGFR 1 and 2) | Prolong half-life | VEGF-A, VEGF-B, PIGF | neovascular (Wet) age-related macular degeneration | Neutralization of VEGF activity | 9 |
Abbreviation: AS, ankylosing spondylitis; CAPS, cryopyrin-associated periodic syndrome; Fc, crystallizable fragment; IL, interleukin; JIA, juvenile idiopathic arthritis; PA, psoriatic arthritis; PIGF, placental growth factor; RA, rheumatoid arthritis; TNFα, tumor necrosis factor-α; VEGF, vascular endothelial growth factor.
Table 3.
Examples of investigational transferrin-fusion proteins
| Name | Effector protein | Impact on PK | Targets | Disease | Proposed mechanisms of action | Reference |
|---|---|---|---|---|---|---|
| GH-Tf | Growth hormone (GH) | Prolong half-life | GH receptor | GH deficiency | GH receptor agonist | 51 |
| G-CSF-Tf | Granulocyte colony stimulating factor (G-CSF) | Prolong half-life | G-CSF receptor | Neutropenia | G-CSF receptor agonist | 51 |
| GLP-1-Tf | Glucagon-like peptide-1 (GLP-1) | Prolong half-life | GLP-1 receptor | Type 2 diabetes mellitus | GLP-1 receptor agonist | 38 |
The second class of fusion proteins according to their second domain, such as immunotoxins 67 and cytokine fusions 68, specifically target the functional domain to the disease sites using the targeting moieties and display favorable biodistribution 69. In a biodistribution study conducted for Anti-Tac (Fv)-PE38, which is a single-chain recombinant immunotoxin, the fusion protein was actively taken up by the tumor xenograft in mice. At 6 hour after injection, over 6% of the injected dose/g was found in the ATAC-4 tumor expressing the Tac antigen 70. Uptake in the tumor was higher than in any other tissues 70. The specific targeting effect of these fusion proteins permits the use of highly toxic proteins to treat severe human diseases such as cancer. Examples of fusion proteins with targeting effect are summarized in Table 4.
Table 4.
Examples of investigational fusion proteins with targeting effect.
| Name | Effector protein domain | Targeting protein domain | Targets | Disease | Mechanism of action | Reference |
|---|---|---|---|---|---|---|
| Denileukin diftitox (Ontak) | Diphtheria toxin fusion protein | IL-2 | IL-2 receptor | Cutaneous T-cell lymphoma (CTCL) | Targeting diphtheria toxin to malignant cells that express those receptors | 94 |
| rGel-BLyS | Gelonin toxin | B lymphocyte stimulator (BLyS) | BLyS receptors | B cell malignancies | Targeting gelonin toxin to malignant cells that express those receptors | 95 |
| CAT-8015 (GCR-8015) | Pseudomonas exotoxin | dsFv, which targets the CD22 receptor | CD22 receptor on B-cells | Chronic lymphocytic hairy cell leukemia, non-Hodgkin’s lymphoma | CD22 antagonists, apoptosis stimulants | 96 |
| EMD 273063 (hu14.18-IL-2) | IL-2 | Humanized anti-GD2 monoclonal antibody | GD2 ganglioside on tumor cells | Neuroblastoma, melanoma | Immuno-stimulants | 97 |
| Tucotuzumab celmoleukin | IL-2 | Monoclonal antibody binds to EpCAM | EpCAM | Ovarian cancer, prostate cancer | Immuno-stimulants | 98 |
| L19-IL-2 fusion protein | IL-2 | L19 single chain antibody targeting the ED-B protein | ED-B | Colon cancer, teratocarcinoma, small-cell lung cancer and other solid tumors | Angiogenesis inhibitors, immuno-stimulants, IL-2 receptor agonists | 99 |
| L19-TNF | TNFα | L19 single chain antibody targeting the ED-B protein | ED-B | Cancer | Angiogenesis inhibitors, Immuno-stimulants, TNF agonists | 100 |
The last class of fusion proteins based on their second domain aim to facilitate novel delivery for protein drugs. The delivery moiety usually takes advantage of the active transport processes in vivo, such as the transcytosis of receptors across the delivery barriers. The delivery moiety binds to these receptors, and delivers the fused protein drugs across absorption barriers like Trojan horse 13, 71–73. For example, Tf receptors are expressed on the cell surface of various epithelium cells such as intestine epithelium, as well as BBB 13. Insulin receptor were also found to be expressed on BBB 74. FcRn is expressed on the intestine epithelium of human fetuses and adults 75. These receptors are found to undergo constitutive transcytosis 13, 71–73, 76. Thus, the ligands or antibodies against these receptors can serve as carrier proteins to bind to these receptors and actively deliver protein drugs across the absorption barriers. Examples of fusion proteins for drug delivery are summarized in Table 5.
Table 5.
Examples of investigational fusion proteins for drug delivery
| Name | Effector protein domain | Delivery protein domain | Delivery route | Impact of delivery protein on PK | Targets | Disease | Mechanism of action | Reference |
|---|---|---|---|---|---|---|---|---|
| FSH–Fc | Follicle-stimulating hormone (FSH) | Fc domain of IgG1 | Oral Pulmonary | Increase bioavailability through FcRn-mediated transcytosis | FSH receptor | male and female infertility | FSH receptor agonist | 101 |
| GH-Tf | Growth hormone (GH) | Transferrin (Tf) | Oral | Increase bioavailability through Tf receptor-mediated transcytosis | GH receptor | Growth hormone deficiency | GH receptor agonist | 15 |
| G-CSF-Tf | Granulocyte colony-stimulating factor (G-CSF) | Transferrin (Tf) | Oral | Increase bioavailability through Tf receptor-mediated transcytosis | G-CSF receptor | Neutropenia | G-CSF receptor agonist | 14 |
| Transferrin-antibody fusion proteins | antibody | Transferrin (Tf) | Intravenous | Increase delivery across BBB through Tf receptor-mediated transcytosis | not specified | not specified | not specified | 32 |
| HIRMAb-EPO fusion protein | Erythropoiet in (EPO) | monoclonal antibody to human insulin receptor (HIRMAb) | Intravenous | Increase delivery across BBB through insulin receptor-mediated transcytosis | EPO receptor | anemia | EPO receptor agonist | 77 |
The fusion of a protein drug with the delivery moiety could significantly improve the bioavailability of the protein drug in systemic circulation or tissue, which is otherwise close to zero. Many of these fusion proteins were found to be able to deliver protein drugs across the barriers at a pharmacologically active level. A recent example is the fusion of a human insulin receptor monoclonal antibody (HIRMAB) to erythropoietin (EPO) for EPO delivery across the BBB. The fusion protein showed a much higher brain uptake (6–10 fold) compared to EPO. The brain uptake was estimated to be 2.1% ID/100 g brain, indicating the peripheral injection of a very low dose of the fusion protein (1 μg/kg) in a 5-kg primate would produce a therapeutic concentration of EPO in the brain 77.
3. Current limitations
In contrast to the rapid development of fusion proteins, the understanding of determining factors that affect the PK of bifunctional fusion proteins is still very preliminary. Currently, most PK studies focus on empirical PK parameter determination, rather than the underlying mechanisms.
Due to the complexity of the bifunctional binding, there is no established guideline for studying and comparing the plasma half-lives of fusion proteins. Various functional and carrier domains possess inherent receptors with differences in tissue distribution, number of receptors, and nature of binding. Therefore, it is difficult to compare the PK parameters of two fusion proteins composed of different protein domains. In addition, when two protein domains are fused together, the impacts of one domain on the other (e.g. changes in receptor binding affinity, elimination mechanisms, biodistribution) are still largely unknown. It is difficult to predict the PK profile of the fusion protein, and to design fusion proteins for optimal PK characteristics.
4. Mechanistic PK study of bifunctional fusion proteins
To solve the above challenges, a molecular, mechanistic approach is taken in our laboratory 51. With the establishment of a mechanistic PK study for the fusion proteins, we would like to provide insight into the following questions: (1) what impacts do the two different functional domains have on the PK of the fusion protein? (2) How do the receptor binding and the subsequent intracellular processing affect the plasma half-life of the fusion protein? (3) After we identify the critical factors (e.g. receptor binding, intracellular processing) affecting the plasma half-life, how can we design fusion protein to achieve the optimal PK profiles?
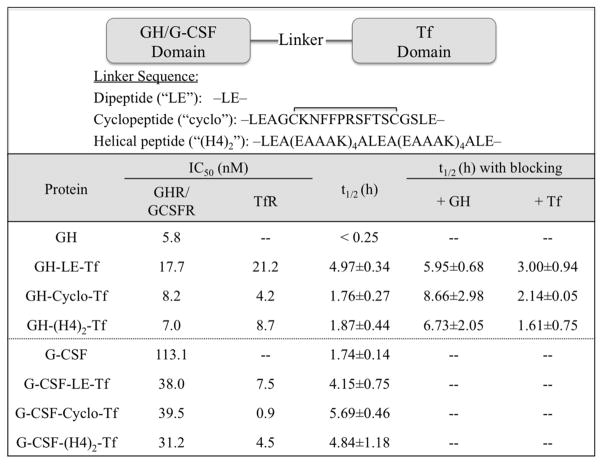
Our laboratory took a unique approach to investigate the molecular mechanisms that affect the PK of bifunctional fusion proteins. Bifunctional fusion proteins consisting of domain 1 (growth hormone (GH) or granulocyte colony-stimulating factor (G-CSF)) and domain 2 (Tf) were constructed with 3 linker peptides inserted between the 2 domains (Figure 2) 51. This approach enabled us to construct two series of fusion proteins containing the same protein domains, with different linker peptides between functional domains. Linker peptides have been applied to alter the receptor binding affinities of fusion proteins due to their capability to control the distance between functional domains 78. With this design, we aimed to generate fusion proteins that bind to the same receptors, but with different receptor binding affinities.
Figure 2.
Receptor binding and pharmacokinetic properties of various transferrin-fusion proteins. Fusion proteins with different linkers were recombinantly produced inHEK293 cells. IC50 values from competitive receptor binding assays were performed in IM-9 cells for GH receptor (GHR), NSF-60 cells for G-CSF receptor (G-CSFR), and Caco-2 cells for Tf receptor (TfR) using 125I-labeled proteins. The half-life values were determined following intravenous administration of fusion proteins to CF1 mice in the absence and presence of co-administered GH or Tf. The values represent mean ± standard deviation (n=3 to 4).
By comparing their PK profiles, we could elucidate the molecular mechanisms that affect the plasma half-life of bifunctional fusion proteins without the confounding factors (e.g. different binding receptors, tissue distributions). Since the Tf-fusion proteins resemble many fusion proteins with a carrier protein domain (e.g. Fc- or albumin- fusion proteins), the mechanistic PK study can potentially be applied to other fusion proteins currently under development for therapeutic use.
The results from our studies showed that insertion of linkers greatly affects the receptor binding affinities in both GH-Tf and G-CSF-Tffusion proteins. The dipeptide-linked GH-LE-Tf, which has the shortest linker, exhibited the weakest binding capacities for both GH receptor (GHR) and Tf receptor (Tf R). On the other hand, with two other longer and rigid linkers (cyclopeptide and helical peptide), GH-Tf fusion proteins exhibited stronger binding capacities to both receptors. In the context of G-CSF-Tf fusion proteins, the G-CSF receptor (G-CSFR) binding affinities were similar among the 3 fusion proteins, while the TfR binding affinities were significantly different (Figure 2).
The generation of fusion proteins with different receptor binding affinities enabled us to compare their PK profiles, and to elucidate the impact of receptor binding on the plasma half-life of fusion proteins. When the PK of Tf-fusion proteins was assessed in mice, 3 GH-Tf fusion proteins exhibited significantly different half-lives from each other (Figure 2). Similarly, the plasma half-lives of 3 G-CSF-Tf fusion proteins were different. Despite having the same component domains, change in receptor binding affinity dramatically altered the half-life of the Tf-fusion proteins, suggesting the importance of receptor binding in determining the half-life.
As discussed in Section 2.3, receptor binding and subsequent intracellular processing (e.g. endocytosis and lysosomal degradation) have been suggested as a major elimination pathway for many protein drugs 25. TMDD effect is one example 54. However, in the context of fusion proteins, the two protein domains may play different roles in the elimination pathway, and their effects can be cooperative or contradictive.
To evaluate the different roles of the 2 domains in affecting the half-life of the fusion proteins, the impact of receptor binding in GH-Tf was first investigated through the blockage of GHR binding by co-administration of excess free GH with GH-Tf (Figure 2). The blockage of GHR binding significantly prolonged the plasma half-life of 3 GH-Tf fusion proteins to a similar level (6 to 8 hours, not statistically significantly different from each other). This result suggests that binding of fusion protein to GHR likely leads to endocytosis and lysosomal degradation of the fusion proteins as reported for free GH 79. For many protein drugs, the binding to their receptors will lead to the classic pathway of endocytosis and lysosomal degradation 54. This process is a crucial factor in determining the plasma half-life, especially for biotechnology pharmaceuticals. Our result indicates that, similar to protein drugs, the receptor binding of a protein drug domain in a bifunctional fusion protein can also cause the degradation, and constitute a major elimination pathway of the fusion proteins.
Similarly, the effect of TfR binding on PK of GH-Tf was investigated through the co-administration of excess free Tf with GH -Tf (Figure 2). With the blockage of TfR binding, the half-life of GH-LE-Tf was significantly shortened, from 4.97 to 3.00 h, suggesting that the TfR binding may help recycle the fusion proteins through the classic Tf-TfR recycling pathway 80, and protect the Tf-fusion protein from intracellular degradation. The recycling pathway for Tf has been widely reported, and accounts for the long plasma half-life of serum Tf 65. Other supporting evidence is that with excess free GH competition, the half-lives of the 3 GH-Tf fusion proteins correlated very well with their TfR binding affinities. When GHR binding was blocked, GH-cyclo-Tf, which has the strongest TfR binding affinity, exhibited the longest half-life (Figure 2). This result is presumably due to the effect of TfR binding in recycling of the fusion protein. These findings from GH-Tf suggest that the two domains in fusion proteins can play completely opposite roles in regulating the intracellular processing, and affect the plasma half-life differently. The receptor binding of one domain (e.g. GH) may lead to degradation while the other (e.g. Tf) can salvage the fusion protein. This feature is unique to the fusion proteins as opposed to protein drugs with a single domain, and adds complexity into the PK studies of fusion proteins.
Not only can the two protein domains have distinct impacts on the plasma half-life, their relative strength of impacts can also be different. For Tf-fusion proteins, our data indicates that the effect of TfR binding is minor compared to GHR binding, since TfR binding only prolongs half-life of the fusion protein with the weakest GHR binding affinity (i.e. GH-LE-Tf). In addition, taking the fact that the half-lives of 3 GH-Tf fusion proteins correlate with their GHR binding affinities, but not TfR binding affinities, our study suggests that GHR binding is the primary binding site which overrides TfR binding in determining the plasma half-life.
The PK study of G-CSF-Tf further supports our hypothesis about receptor binding and intracellular processing of bifunctional fusion protein. The 3 fusion proteins with different linkers possess similar G-CSFR binding affinity, but display significantly different TfR binding affinity, indicating the difference in plasma half-life is mainly determined by TfR binding (Figure 2). Since stronger TfR binding affinity of G-CSF-Tf correlates with longer plasma half-life, this finding further confirms our conclusions from GH-Tf, that TfR binding leads to the recycling of the fusion protein and prolongs their plasma half-lives.
Taken together, our study of Tf-fusion proteins suggests a novel mechanistic PK scheme for bifunctional fusion proteins, focusing on the impact of receptor binding and intracellular processing. The scheme highlights the concept that two domains in a fusion protein can play different roles in affecting the plasma half-life. The GH/G-CSF domain in Tf-fusion proteins, as a representative of the protein drug domain in many fusion proteins, binds to target receptors on the cell surface, leading to the subsequent endosomal degradation of the fusion protein. On the other hand, the Tf domain, which resembles Fc and albumin domain in other fusion proteins, retains the fusion protein in the recycling endosome, and protects the fusion protein from degradation.
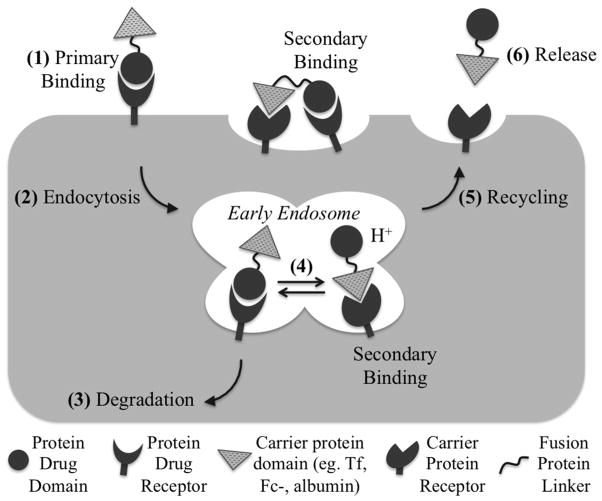
To the best of our knowledge, the PK model for G-CSF and GH Tf-fusion proteins was the first PK study for fusion proteins focusing on molecular mechanisms such as receptor binding and intracellular processing. This type of study establishes the mechanistic linkage between the biochemical properties of the fusion protein (e.g. receptor binding affinity) to the PK processes (e.g. distribution and metabolism), and can conceivably be applied to other fusion proteins consisting of a protein drug domain and a recycling protein domain. Based on the results, we proposed a mechanistic PK scheme for bifunctional fusion proteins, which is illustrated in Figure 3. In the presence of abundant endogenous levels of the recycling protein domain (i.e. Tf, albumin, Fc-domain), the fusion proteins first bind to the protein drug receptor on the target cell membrane. This binding is considered the primary binding, which enriches the fusion proteins onto the target cells. The binding of the fusion protein to the protein recycling domain receptor is indicated as secondary binding, since it occurs after the protein drug receptor binding, either at the cell surface (bivalent binding) or within the acidified endosome following endocytosis. The relative strength of the two receptor binding inside the endosome will determine the impact of each receptor on the plasma half-life of the fusion proteins.
Figure 3.
Proposed endocytic pathway and intracellular metabolism of fusion proteins. (1) Binding of a protein drug to its receptor is considered primary binding. Due to the high presence of endogenous carrier protein (i.e. albumin, Tf) in the blood, binding to the carrier domain is considered secondary binding. (2) The fusion protein will be internalized via RME, where (3) proteins that remain boind to the protein drug receptor will be degraded in the lysosome. (4) The relative strength of binding to the two receptors inside the endosome will determine the impact of each receptor on the plasma half-life of the fusion proteins, where (5) proteins that bind to the carrier protein receptor will be recycled and released from the cell.
5. Expert opinion
5.1. Implications of the mechanistic PK study
Our study on the evaluation of GH-Tf and G-CSF-Tf fusion proteins provides several implications for PK studies as well as the design of bifunctional fusion proteins. A critical finding from the GH-Tf studies was that fusion proteins are exhibiting TMDD through the primary GHR binding site, which affects plasma half-life in a dose-dependent manner (Figure 2). Although the half-lives of GH-Tf (~1.8–5 h) and GCSF-Tf (~4–6 h) were prolonged compared to the free protein drug (<15 min and 1.74 h for GH and G-CSF, respectively) (Figure 2) 51, they were substantially shorter than free human Tf in mouse (~25 h) (unpublished data). Therefore, depending on the properties of the primary binding site of the fusion protein, TMDD may be a critical determinant for the plasma half-life. TMDD is less of a concern for secondary binding of the carrier Tf protein, and also for other carrier proteins including Fc-and albumin, due to the high endogenous concentrations of these proteins. However, the secondary binding may become significant when either the primary binding is approaching saturation (e.g., GH-Tf results), or fusion proteins exhibit comparable primary binding affinities (e.g., G-CSF-Tf results). The findings also suggest that, in order to achieve optimal PK profile of fusion proteins, a good balance needs to be maintained between the binding affinities of the two domains, considering their different roles in regulating the disposition. For instance, a design that favors recycling over degradation may greatly enhance the plasma half-life. Due to the strong impacts of receptor binding and intracellular processing on PK of bifunctional fusion proteins, the determination of receptor binding affinities will be useful in predicting the plasma half-life. Additionally, when translating the half-life between species, the differences in receptor binding affinity, receptor abundance, turnover rate, and intracellular receptor routing are important considerations. A feasible tool to fine-turn the receptor binding affinity, as applied in our study, is linker technology. Linkers with various length and confirmation can greatly change the receptor binding affinity, while maintaining the binding receptors constant between constructs.
5.2. Research to be done
Although several fusion proteins are on the market and many more are under development, the systematic, mechanistic PK study of fusion proteins seems to lag behind. Currently, most PK studies for fusion proteins are limited to basic PK profile determination (e.g. half-life, Cmax, CL, AUC), or focus on half-life extension for fusion protein compared with the parent protein drug. Without dissecting the fusion protein into individual domains, and looking at their function separately, it is hard to catch the factors in fusion protein design that may have critical impacts on the PK profile.
Considering the importance of mechanistic knowledge in assessing the PK of single domain protein drugs (e.g. TMDD effect 54), bifunctional fusion proteins will benefit greatly from mechanistic studies due to their high complexity. For instance, fusion proteins which undergo recycling endocytosis (e.g. Tf, Fc- or albumin fusion proteins) may display different underlying disposition mechanisms comparing to those with targeting effects (e.g. antibody fusion proteins). Similarly, fusion proteins composed of different protein drugs (e.g. toxin versus ligand for cell-surface receptor) may display distinct elimination pathways. The molecular mechanisms affecting the PK may be generalized according to their classifications as suggested in Section 2.4.
In order to advance the understanding of PK/PD of fusion proteins, it is important to integrate the understanding of the molecular mechanisms that affect the disposition (absorption, distribution, metabolism and elimination)with the PK/PD mathematical modeling systems. Additionally, based on the knowledge of molecular mechanisms of each domain in a fusion protein, mathematical PK/PD models can be adapted from simple protein drugs with similar functional domains. There have been many studies focusing on modeling the TMDD of therapeutic proteins with fully mechanistic, or simplified PK models (e.g. quasi-equilibrium, or Michaelis–Menten approximations) 57, 81–83. Particularly, Gibiansky et al. extended the TMDD model from one binding target to two or more binding targets 84. This proposed model is applicable to many bifunctional fusion proteins which bind to two target proteins independently. However, caution should be taken for fusion proteins, such as Tf-fusion proteins that exhibit sequential binding processes. The model by Gibiansky’s group does not consider the dependence of the secondary target binding on the primary binding. In these cases, detailed mechanistic models of the intracellular disposition that utilize the TMDD framework should be applied instead. Krippendorff et. al. reported the general modeling of the receptor -mediated endocytosis, and explicitly took into account receptor binding and trafficking inside the cell 85. These models should be adapted to fusion proteins by adding the complexity of two functional domains with individual receptor binding and intracellular processing. Eventually, development of general mathematical models and study guidelines for different classes of fusion proteins will provide a comparable platform to enhance the overall understanding of PK properties and facilitate the design and development of bifunctional fusion proteins with optimal PK and PD properties.
Another important area to explore is the immunogenicity, which may have great impact on PK of fusion proteins. With chronic dosing, anti-drug antibody (ADA) formation is frequently observed with biotech drugs, especially for unnatural proteins or those derived from animals 86–88. Fusion proteins are constructed via the fusion of two or more proteins, and therefore are foreign to the human body. Presumably, the protein fusion may create novel structures and sequences that are potentially immunogenic. The presence of ADA can alter the PK profile, and may also obliterate the biological activity of a fusion protein. Because mechanisms underlying immunogenicity for fusion proteins and single domain proteins are similar, the immunogenicity assessment strategy for fusion proteins may follow the experiences from single domain protein drugs.
5.3. Ultimate goal
The advance of biotechnology has been accelerating, and the number of new biomolecules under drug development has been growing exponentially. Fusion proteins, as a unique class of biotherapeutics, provide vast opportunities for treating various human diseases, but present many challenges in their development as well. We envision that the PK study of fusion proteins will take more mechanistic, systematic approach, similar to the trend for other protein drugs.
Table 2.
Examples of investigational albumin-fusion proteins.
| Name | Effector protein | Impact of albumin domain on PK | Targets | Disease | Proposed mechanisms of action | Reference |
|---|---|---|---|---|---|---|
| Albulin-G | Insulin | Prolong half-life | Insulin receptor | Type 1 and 2 diabetes mellitus | Insulin receptor agonist | 44 |
| Cardeva | B-type natriuretic peptide | Prolong half-life | Atrial natriuretic factor receptors | Chronic heart failure | Diuretics | 92 |
| Albugranin (Neugranin) | Granulocyte colony stimulating factor (G-CSF) | Prolong half-life | G-CSF receptor | Neutropenia | G-CSF receptor agonist | 93 |
| Albiglutide (Syncria) | Glucagon-like peptide-1 (GLP-1) | Prolong half-life | GLP-1 receptor | Type 2 diabetes mellitus | GLP-1 receptor agonist | 36 |
| Albuferon-alpha | Interferon-α (INF-α) | Prolong half-life | INF-α receptor | Cancer, chronic myeloid leukaemia, hepatitis C | INF-α receptor agonist | 46 |
Highlights.
PK of fusion proteins can be much more complicated than that of single domain proteins. Their distribution is usually limited, but can be improved by the use of targeting domain to facilitate active tissue uptake, or by delivery domain to access to areas that are not generally permeable to large molecules. Elimination pathways include proteolysis, renal elimination, hepatic elimination, and receptor-mediated endocytosis, and can be complex due to different elimination mechanisms of each individual domain.
According to the binding properties of effector protein domain, fusion proteins can be grouped as: (i) possess no specific binding target, (ii) bind to soluble ligands or (iii) bind to cell-surface receptors. According to the PK properties of the other domain, fusion proteins can be grouped as: (i) possess carrier protein domain, (ii) possess targeting domain, (iii) possess delivery domain.
A mechanistic PK study of bifunctional fusion proteins suggests the strong impacts of receptor binding and intracellular processing on PK of fusion proteins, highlighting the determination of receptor binding affinities will be useful in predicting the plasma half-life.
The establishment of general empirical and mechanistic PK models for bifunctional fusion proteins can better characterize the contributions of each domain to the absorption, distribution, metabolism and elimination.
Abbreviations
- BBB
Blood-brain barrier
- GLP-1
glucagon-like peptide-1
- G-CSF
granulocyte-colony stimulating factor
- GH
growth hormone
- NCA
Non-compartmental analysis
- PD
pharmacodynamics
- PK
pharmacokinetics
- RME
Receptor mediated endocytosis
- TMDD
target-mediated drug disposition
- Tf
transferrin
- TfR
transferrin receptor
- Vc
volume of distribution in the central compartment
- Vss
steady-state volume of distribution
References
- 1.Meibohm B. Pharmacokinetics and Pharmacodynamics of Biotech Drugs: Principles and Case Studies in Drug Development. Weinheim, FRG: WILEY-VCH Verlag GmbH & Co. KGaA; 2006. [Google Scholar]
- 2.Duttaroy A, Kanakaraj P, Osborn B, et al. Development of a long-acting insulin analog using albumin fusion technology. Diabetes. 2005;54(1):251–8. doi: 10.2337/diabetes.54.1.251. [DOI] [PubMed] [Google Scholar]
- 3.Osborn B, Olsen H, Nardelli B, et al. Pharmacokinetic and pharmacodynamic studies of a human serum albumin-interferon-alpha fusion protein in cynomolgus monkeys. J Pharmacol Exp Ther. 2002;303(2):540–8. doi: 10.1124/jpet.102.037002. [DOI] [PubMed] [Google Scholar]
- 4.Müller N, Schneider B, Pfizenmaier K, et al. Superior serum half life of albumin tagged TNF ligands. Biochem Biophys Res Commun. 2010;396(4):793–9. doi: 10.1016/j.bbrc.2010.04.134. [DOI] [PubMed] [Google Scholar]
- 5.Peters R, Low S, Kamphaus G, et al. Prolonged activity of factor IX as a monomeric Fc fusion protein. Blood. 2010;115(10):2057–64. doi: 10.1182/blood-2009-08-239665. [DOI] [PubMed] [Google Scholar]
- 6•.Schmidt SR. Fusion-proteins as biopharmaceuticals--applications and challenges. Curr Opin Drug Discov Devel. 2009;12(2):284–95. Review article summarizing fusion proteins approved and in clinical trials. [PubMed] [Google Scholar]
- 7•.Leader B, Baca Q, Golan D. Protein therapeutics: a summary and pharmacological classification. Nat Rev Drug Discov. 2008;7(1):21–39. doi: 10.1038/nrd2399. Comprehensive review article giving an overview of many different categories of protein therapeutics. [DOI] [PubMed] [Google Scholar]
- 8.Martin ST, Tichy EM, Gabardi S. Belatacept: a novel biologic for maintenance immunosuppression after renal transplantation. Pharmacotherapy. 2011;31(4):394–407. doi: 10.1592/phco.31.4.394. [DOI] [PubMed] [Google Scholar]
- 9.Teng LS, Jin KT, He KF, et al. Clinical applications of VEGF-trap (aflibercept) in cancer treatment. J Chin Med Assoc. 2010;73(9):449–56. doi: 10.1016/S1726-4901(10)70097-6. [DOI] [PubMed] [Google Scholar]
- 10.Aggarwal S. What’s fueling the biotech engine-2007. Nat Biotechnol. 2008;26(11):1227–33. doi: 10.1038/nbt1108-1227. [DOI] [PubMed] [Google Scholar]
- 11.Supersaxo A, Hein WR, Steffen H. Effect of molecular weight on the lymphatic absorption of water-soluble compounds following subcutaneous administration. Pharm Res. 1990;7(2):167–9. doi: 10.1023/a:1015880819328. [DOI] [PubMed] [Google Scholar]
- 12.Brown LR. Commercial challenges of protein drug delivery. Expert Opin Drug Deliv. 2005;2(1):29–42. doi: 10.1517/17425247.2.1.29. [DOI] [PubMed] [Google Scholar]
- 13.Widera A, Norouziyan F, Shen WC. Mechanisms of TfR-mediated transcytosis and sorting in epithelial cells and applications toward drug delivery. Adv Drug Deliv Rev. 2003;55(11):1439–66. doi: 10.1016/j.addr.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Bai Y, Ann DK, Shen WC. Recombinant granulocyte colony-stimulating factor-transferrin fusion protein as an oral myelopoietic agent. Proc Natl Acad Sci U S A. 2005;102(20):7292–6. doi: 10.1073/pnas.0500062102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amet N, Wang W, Shen WC. Human growth hormone-transferrin fusion protein for oral delivery in hypophysectomized rats. J Control Release. 2010;141(2):177–82. doi: 10.1016/j.jconrel.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bitonti AJ, Dumont JA, Low SC, et al. Pulmonary delivery of an erythropoietin Fc fusion protein in non-human primates through an immunoglobulin transport pathway. Proc Natl Acad Sci U S A. 2004;101(26):9763–8. doi: 10.1073/pnas.0403235101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vallee S, Rakhe S, Reidy T, et al. Pulmonary Administration of Interferon Beta-1a-Fc Fusion Protein in Non-Human Primates Using an Immunoglobulin Transport Pathway. J Interferon Cytokine Res. 2011;32:1–7. doi: 10.1089/jir.2011.0048. [DOI] [PubMed] [Google Scholar]
- 18.Reilly RM, Sandhu J, Alvarez-Diez TM, et al. Problems of delivery of monoclonal antibodies. Pharmaceutical and pharmacokinetic solutions. Clin Pharmacokinet. 1995;28(2):126–42. doi: 10.2165/00003088-199528020-00004. [DOI] [PubMed] [Google Scholar]
- 19.Forero A, Weiden PL, Vose JM, et al. Phase 1 trial of a novel anti-CD20 fusion protein in pretargeted radioimmunotherapy for B-cell non-Hodgkin lymphoma. Blood. 2004;104(1):227–36. doi: 10.1182/blood-2003-09-3284. [DOI] [PubMed] [Google Scholar]
- 20.Better M, Bernhard SL, Williams RE, et al. T cell-targeted immunofusion proteins from Escherichia coli. J Biol Chem. 1995;270(25):14951–7. doi: 10.1074/jbc.270.25.14951. [DOI] [PubMed] [Google Scholar]
- 21.Syed S, Schuyler PD, Kulczycky M, et al. Potent antithrombin activity and delayed clearance from the circulation characterize recombinant hirudin genetically fused to albumin. Blood. 1997;89(9):3243–52. [PubMed] [Google Scholar]
- 22.Hesketh P, Caguioa P, Koh H, et al. Clinical activity of a cytotoxic fusion protein in the treatment of cutaneous T-cell lymphoma. J Clin Oncol. 1993;11(9):1682–90. doi: 10.1200/JCO.1993.11.9.1682. [DOI] [PubMed] [Google Scholar]
- 23.Kelley VE, Bacha P, Pankewycz O, et al. Interleukin 2-diphtheria toxin fusion protein can abolish cell-mediated immunity in vivo. Proc Natl Acad Sci U S A. 1988;85(11):3980–4. doi: 10.1073/pnas.85.11.3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ko YJ, Bubley GJ, Weber R, et al. Safety, pharmacokinetics, and biological pharmacodynamics of the immunocytokine EMD 273066 (huKS-IL2): results of a phase I trial in patients with prostate cancer. J Immunother. 2004;27(3):232–9. doi: 10.1097/00002371-200405000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Tang L, Persky AM, Hochhaus G, et al. Pharmacokinetic aspects of biotechnology products. J Pharm Sci. 2004;93(9):2184–204. doi: 10.1002/jps.20125. [DOI] [PubMed] [Google Scholar]
- 26.Veng-Pedersen P, Gillespie W. Mean residence time in peripheral tissue: a linear disposition parameter useful for evaluating a drug’s tissue distribution. J Pharmacokinet Biopharm. 1984;12(5):535–43. doi: 10.1007/BF01060131. [DOI] [PubMed] [Google Scholar]
- 27.Straughn AB. Model-independent steady-state volume of distribution. J Pharm Sci. 1982;71(5):597–8. doi: 10.1002/jps.2600710532. [DOI] [PubMed] [Google Scholar]
- 28.Perrier D, Mayersohn M. Noncompartmental determination of the steady-state volume of distribution for any mode of administration. J Pharm Sci. 1982;71(3):372–3. doi: 10.1002/jps.2600710332. [DOI] [PubMed] [Google Scholar]
- 29•.Richter WF, Grimm HP, Theil FP. Potential errors in the volume of distribution estimation of therapeutic proteins composed of differently cleared components. J Pharmacokinet Pharmacodyn. 2011;38(5):581–93. doi: 10.1007/s10928-011-9209-1. Explores the potential sources of error in estimating volume of distribution for therapeutic proteins. [DOI] [PubMed] [Google Scholar]
- 30.Yates JW, Arundel PA. On the volume of distribution at steady state and its relationship with two-compartmental models. J Pharm Sci. 2008;97(1):111–22. doi: 10.1002/jps.21089. [DOI] [PubMed] [Google Scholar]
- 31.Carnemolla B, Borsi L, Balza E, et al. Enhancement of the antitumor properties of interleukin-2 by its targeted delivery to the tumor blood vessel extracellular matrix. Blood. 2002;99(5):1659–65. doi: 10.1182/blood.v99.5.1659. [DOI] [PubMed] [Google Scholar]
- 32.Shin SU, Friden P, Moran M, et al. Transferrin-antibody fusion proteins are effective in brain targeting. Proc Natl Acad Sci U S A. 1995;92(7):2820–4. doi: 10.1073/pnas.92.7.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou QH, Boado RJ, Lu JZ, et al. Monoclonal antibody-glial-derived neurotrophic factor fusion protein penetrates the blood-brain barrier in the mouse. Drug Metab Dispos. 2010;38(4):566–72. doi: 10.1124/dmd.109.031534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gautier JF, Fetita S, Sobngwi E, et al. Biological actions of the incretins GIP and GLP-1 and therapeutic perspectives in patients with type 2 diabetes. Diabetes Metab. 2005;31(3 Pt 1):233–42. doi: 10.1016/s1262-3636(07)70190-8. [DOI] [PubMed] [Google Scholar]
- 35.Pasta P, Vecchio G, Carrea G. Conformation and proteolysis of glucagon and insulin in surfactant and lipid solutions. Biochim Biophys Acta. 1988;953(3):314–20. doi: 10.1016/0167-4838(88)90040-4. [DOI] [PubMed] [Google Scholar]
- 36.Baggio LL, Huang Q, Brown TJ, et al. A recombinant human glucagon-like peptide (GLP)-1-albumin protein (albugon) mimics peptidergic activation of GLP-1 receptor-dependent pathways coupled with satiety, gastrointestinal motility, and glucose homeostasis. Diabetes. 2004;53(9):2492–500. doi: 10.2337/diabetes.53.9.2492. [DOI] [PubMed] [Google Scholar]
- 37.Barrington P, Chien JY, Tibaldi F, et al. LY2189265, a long-acting glucagon-like peptide-1 analogue, showed a dose-dependent effect on insulin secretion in healthy subjects. Diabetes Obes Metab. 2011;13(5):434–8. doi: 10.1111/j.1463-1326.2011.01365.x. [DOI] [PubMed] [Google Scholar]
- 38.Kim BJ, Zhou J, Martin B, et al. Transferrin fusion technology: a novel approach to prolonging biological half-life of insulinotropic peptides. J Pharmacol Exp Ther. 2010;334(3):682–92. doi: 10.1124/jpet.110.166470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schenk S, Schoenhals GJ, de Souza G, et al. A high confidence, manually validated human blood plasma protein reference set. BMC Med Genomics. 2008;1:41. doi: 10.1186/1755-8794-1-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anderson PM, Sorenson MA. Effects of route and formulation on clinical pharmacokinetics of interleukin-2. Clin Pharmacokinet. 1994;27(1):19–31. doi: 10.2165/00003088-199427010-00003. [DOI] [PubMed] [Google Scholar]
- 41.Takagi A, Masuda H, Takakura Y, et al. Disposition characteristics of recombinant human interleukin-11 after a bolus intravenous administration in mice. J Pharmacol Exp Ther. 1995;275(2):537–43. [PubMed] [Google Scholar]
- 42.Johnson V, Maack T. Renal extraction, filtration, absorption, and catabolism of growth hormone. Am J Physiol. 1977;233(3):F185–96. doi: 10.1152/ajprenal.1977.233.3.F185. [DOI] [PubMed] [Google Scholar]
- 43.Rabkin R, Ryan MP, Duckworth WC. The renal metabolism of insulin. Diabetologia. 1984;27(3):351–7. doi: 10.1007/BF00304849. [DOI] [PubMed] [Google Scholar]
- 44.Duttaroy A, Kanakaraj P, Osborn BL, et al. Development of a long-acting insulin analog using albumin fusion technology. Diabetes. 2005;54(1):251–8. doi: 10.2337/diabetes.54.1.251. [DOI] [PubMed] [Google Scholar]
- 45.Duckworth WC, Bennett RG, Hamel FG. Insulin degradation: progress and potential. Endocr Rev. 1998;19(5):608–24. doi: 10.1210/edrv.19.5.0349. [DOI] [PubMed] [Google Scholar]
- 46.Subramanian GM, Fiscella M, Lamouse-Smith A, et al. Albinterferon alpha-2b: a genetic fusion protein for the treatment of chronic hepatitis C. Nat Biotechnol. 2007;25(12):1411–9. doi: 10.1038/nbt1364. [DOI] [PubMed] [Google Scholar]
- 47.Osborn BL, Sekut L, Corcoran M, et al. Albutropin: a growth hormone-albumin fusion with improved pharmacokinetics and pharmacodynamics in rats and monkeys. Eur J Pharmacol. 2002;456(1–3):149–58. doi: 10.1016/s0014-2999(02)02644-4. [DOI] [PubMed] [Google Scholar]
- 48.Powell JS, Josephson NC, Quon D, et al. Safety and prolonged activity of recombinant factor VIII Fc fusion protein in hemophilia A patients. Blood. 2012 doi: 10.1182/blood-2011-09-382846. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dumont JA, Liu T, Low SC, et al. Prolonged activity of a recombinant factor VIII-Fc fusion protein in hemophilia A mice and dogs. Blood. 2012 doi: 10.1182/blood-2011-08-367813. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Valentino LA. Recombinant FIXFc: a novel therapy for the royal disease? Expert Opin Biol Ther. 2011;11(10):1361–8. doi: 10.1517/14712598.2011.603304. [DOI] [PubMed] [Google Scholar]
- 51.Chen X, Lee HF, Zaro JL, et al. Effects of receptor binding on plasma half-life of bifunctional transferrin fusion proteins. Mol Pharm. 2011;8(2):457–65. doi: 10.1021/mp1003064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Authier F, Danielsen GM, Kouach M, et al. Identification of insulin domains important for binding to and degradation by endosomal acidic insulinase. Endocrinology. 2001;142(1):276–89. doi: 10.1210/endo.142.1.7916. [DOI] [PubMed] [Google Scholar]
- 53.Smedsrod B, Einarsson M. Clearance of tissue plasminogen activator by mannose and galactose receptors in the liver. Thromb Haemost. 1990;63(1):60–6. [PubMed] [Google Scholar]
- 54.Mager DE. Target-mediated drug disposition and dynamics. Biochem Pharmacol. 2006;72(1):1–10. doi: 10.1016/j.bcp.2005.12.041. [DOI] [PubMed] [Google Scholar]
- 55.Ducharme E, Weinberg JM. Etanercept. Expert Opin Biol Ther. 2008;8(4):491–502. doi: 10.1517/14712598.8.4.491. [DOI] [PubMed] [Google Scholar]
- 56.Hoffman HM, Throne ML, Amar NJ, et al. Efficacy and safety of rilonacept (interleukin-1 Trap) in patients with cryopyrin-associated periodic syndromes: results from two sequential placebo-controlled studies. Arthritis Rheum. 2008;58(8):2443–52. doi: 10.1002/art.23687. [DOI] [PubMed] [Google Scholar]
- 57•.Mager DE, Jusko WJ. General pharmacokinetic model for drugs exhibiting target-mediated drug disposition. J Pharmacokinet Pharmacodyn. 2001;28(6):507–32. doi: 10.1023/a:1014414520282. One of the first papers describing a mechanistic model for drugs with target-mediated drug disposition. [DOI] [PubMed] [Google Scholar]
- 58.Huang C. Receptor-Fc fusion therapeutics, traps, and MIMETIBODY technology. Curr Opin Biotechnol. 2009;20(6):692–9. doi: 10.1016/j.copbio.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 59.Schulte S. Half-life extension through albumin fusion technologies. Thromb Res. 2009;124 (Suppl 2):S6–8. doi: 10.1016/S0049-3848(09)70157-4. [DOI] [PubMed] [Google Scholar]
- 60.Spiegelberg HL, Fishkin BG. The catabolism of human G immunoglobulins of different heavy chain subclasses. 3. The catabolism of heavy chain disease proteins and of Fc fragments of myeloma proteins. Clin Exp Immunol. 1972;10(4):599–607. [PMC free article] [PubMed] [Google Scholar]
- 61.Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7(9):715–25. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- 62.Israel EJ, Wilsker DF, Hayes KC, et al. Increased clearance of IgG in mice that lack beta 2-microglobulin: possible protective role of FcRn. Immunology. 1996;89(4):573–8. doi: 10.1046/j.1365-2567.1996.d01-775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Anderson CL, Chaudhury C, Kim J, et al. Perspective-- FcRn transports albumin: relevance to immunology and medicine. Trends Immunol. 2006;27(7):343–8. doi: 10.1016/j.it.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 64.Chaudhury C, Mehnaz S, Robinson JM, et al. The major histocompatibility complex-related Fc receptor for IgG (FcRn) binds albumin and prolongs its lifespan. J Exp Med. 2003;197(3):315–22. doi: 10.1084/jem.20021829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dautry-Varsat A, Ciechanover A, Lodish H. pH and the recycling of transferrin during receptor-mediated endocytosis. Proc Natl Acad Sci U S A. 1983;80(8):2258–62. doi: 10.1073/pnas.80.8.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morgan E. Effect of pH and iron content of transferrin on its binding to reticulocyte receptors. Biochim Biophys Acta. 1983;762(4):498–502. doi: 10.1016/0167-4889(83)90052-6. [DOI] [PubMed] [Google Scholar]
- 67.De Lorenzo C, D’Alessio G. From immunotoxins to immunoRNases. Curr Pharm Biotechnol. 2008;9(3):210–4. doi: 10.2174/138920108784567254. [DOI] [PubMed] [Google Scholar]
- 68.Khawli LA, Hu P, Epstein AL. Cytokine, chemokine, and co-stimulatory fusion proteins for the immunotherapy of solid tumors. Handb Exp Pharmacol. 2008;(181):291–328. doi: 10.1007/978-3-540-73259-4_13. [DOI] [PubMed] [Google Scholar]
- 69.Pastan II, Kreitman RJ. Immunotoxins for targeted cancer therapy. Adv Drug Deliv Rev. 1998;31(1–2):53–88. doi: 10.1016/s0169-409x(97)00094-x. [DOI] [PubMed] [Google Scholar]
- 70.Kreitman RJ, Pastan I. Accumulation of a recombinant immunotoxin in a tumor in vivo: fewer than 1000 molecules per cell are sufficient for complete responses. Cancer Res. 1998;58(5):968–75. [PubMed] [Google Scholar]
- 71.Dumont JA, Low SC, Peters RT, et al. Monomeric Fc fusions: impact on pharmacokinetic and biological activity of protein therapeutics. BioDrugs. 2006;20(3):151–60. doi: 10.2165/00063030-200620030-00002. [DOI] [PubMed] [Google Scholar]
- 72.Wang YY, Lui PC, Li JY. Receptor-mediated therapeutic transport across the blood-brain barrier. Immunotherapy. 2009;1(6):983–93. doi: 10.2217/imt.09.75. [DOI] [PubMed] [Google Scholar]
- 73.Jones AR, Shusta EV. Blood-brain barrier transport of therapeutics via receptor-mediation. Pharm Res. 2007;24(9):1759–71. doi: 10.1007/s11095-007-9379-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pardridge WM, Eisenberg J, Yang J. Human blood-brain barrier insulin receptor. J Neurochem. 1985;44(6):1771–8. doi: 10.1111/j.1471-4159.1985.tb07167.x. [DOI] [PubMed] [Google Scholar]
- 75.Israel EJ, Taylor S, Wu Z, et al. Expression of the neonatal Fc receptor, FcRn, on human intestinal epithelial cells. Immunology. 1997;92(1):69–74. doi: 10.1046/j.1365-2567.1997.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hui EK, Boado RJ, Pardridge WM. Tumor necrosis factor receptor-IgG fusion protein for targeted drug delivery across the human blood-brain barrier. Mol Pharm. 2009;6(5):1536–43. doi: 10.1021/mp900103n. [DOI] [PubMed] [Google Scholar]
- 77.Boado RJ, Hui EK, Lu JZ, et al. Drug targeting of erythropoietin across the primate blood-brain barrier with an IgG molecular Trojan horse. J Pharmacol Exp Ther. 2010;333(3):961–9. doi: 10.1124/jpet.109.165092. [DOI] [PubMed] [Google Scholar]
- 78.Arai R, Ueda H, Kitayama A, et al. Design of the linkers which effectively separate domains of a bifunctional fusion protein. Protein Eng. 2001;14(8):529–32. doi: 10.1093/protein/14.8.529. [DOI] [PubMed] [Google Scholar]
- 79.van Kerkhof P, Strous G. The ubiquitin-proteasome pathway regulates lysosomal degradation of the growth hormone receptor and its ligand. Biochem Soc Trans. 2001;29(Pt 4):488–93. doi: 10.1042/bst0290488. [DOI] [PubMed] [Google Scholar]
- 80.Grant B, Donaldson J. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol. 2009;10(9):597–608. doi: 10.1038/nrm2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Levy G. Pharmacologic target-mediated drug disposition. Clin Pharmacol Ther. 1994;56(3):248–52. doi: 10.1038/clpt.1994.134. [DOI] [PubMed] [Google Scholar]
- 82.Mager DE, Krzyzanski W. Quasi-equilibrium pharmacokinetic model for drugs exhibiting target-mediated drug disposition. Pharm Res. 2005;22(10):1589–96. doi: 10.1007/s11095-005-6650-0. [DOI] [PubMed] [Google Scholar]
- 83.Yan X, Mager DE, Krzyzanski W. Selection between Michaelis-Menten and target-mediated drug disposition pharmacokinetic models. J Pharmacokinet Pharmacodyn. 2010;37(1):25–47. doi: 10.1007/s10928-009-9142-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gibiansky L, Gibiansky E. Target-mediated drug disposition model for drugs that bind to more than one target. J Pharmacokinet Pharmacodyn. 2010;37(4):323–46. doi: 10.1007/s10928-010-9163-3. [DOI] [PubMed] [Google Scholar]
- 85.Krippendorff BF, Kuester K, Kloft C, et al. Nonlinear pharmacokinetics of therapeutic proteins resulting from receptor mediated endocytosis. J Pharmacokinet Pharmacodyn. 2009;36(3):239–60. doi: 10.1007/s10928-009-9120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wolbink GJ, Aarden LA, Dijkmans BA. Dealing with immunogenicity of biologicals: assessment and clinical relevance. Curr Opin Rheumatol. 2009;21(3):211–5. doi: 10.1097/bor.0b013e328329ed8b. [DOI] [PubMed] [Google Scholar]
- 87.Barbosa MD. Immunogenicity of biotherapeutics in the context of developing biosimilars and biobetters. Drug Discov Today. 2011;16(7–8):345–53. doi: 10.1016/j.drudis.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 88.Putnam WS, Prabhu S, Zheng Y, et al. Pharmacokinetic, pharmacodynamic and immunogenicity comparability assessment strategies for monoclonal antibodies. Trends Biotechnol. 2010;28(10):509–16. doi: 10.1016/j.tibtech.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 89.Strober BE, Menon K. Alefacept for the treatment of psoriasis and other dermatologic diseases. Dermatol Ther. 2007;20(4):270–6. doi: 10.1111/j.1529-8019.2007.00140.x. [DOI] [PubMed] [Google Scholar]
- 90.Lundquist LM. Abatacept: a novel treatment for rheumatoid arthritis. Expert Opin Pharmacother. 2007;8(14):2371–9. doi: 10.1517/14656566.8.14.2371. [DOI] [PubMed] [Google Scholar]
- 91.Cines DB, Yasothan U, Kirkpatrick P. Romiplostim. Nat Rev Drug Discov. 2008;7(11):887–8. doi: 10.1038/nrd2741. [DOI] [PubMed] [Google Scholar]
- 92.Wang W, Ou Y, Shi Y. AlbuBNP, a recombinant B-type natriuretic peptide and human serum albumin fusion hormone, as a long-term therapy of congestive heart failure. Pharm Res. 2004;21(11):2105–11. doi: 10.1023/b:pham.0000048203.30568.81. [DOI] [PubMed] [Google Scholar]
- 93.Halpern W, Riccobene TA, Agostini H, et al. Albugranin, a recombinant human granulocyte colony stimulating factor (G-CSF) genetically fused to recombinant human albumin induces prolonged myelopoietic effects in mice and monkeys. Pharm Res. 2002;19(11):1720–9. doi: 10.1023/a:1020917732218. [DOI] [PubMed] [Google Scholar]
- 94.Turturro F. Denileukin diftitox: a biotherapeutic paradigm shift in the treatment of lymphoid-derived disorders. Expert Rev Anticancer Ther. 2007;7(1):11–7. doi: 10.1586/14737140.7.1.11. [DOI] [PubMed] [Google Scholar]
- 95.Wen X, Lyu MA, Zhang R, et al. Biodistribution, pharmacokinetics, and nuclear imaging studies of 111In-labeled rGel/BLyS fusion toxin in SCID mice bearing B cell lymphoma. Mol Imaging Biol. 13(4):721–9. doi: 10.1007/s11307-010-0391-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bang S, Nagata S, Onda M, et al. HA22 (R490A) is a recombinant immunotoxin with increased antitumor activity without an increase in animal toxicity. Clin Cancer Res. 2005;11(4):1545–50. doi: 10.1158/1078-0432.CCR-04-1939. [DOI] [PubMed] [Google Scholar]
- 97.Niculescu-Duvaz I. Technology evaluation: EMD-273063, EMD Lexigen. Curr Opin Mol Ther. 2004;6(5):559–66. [PubMed] [Google Scholar]
- 98.Johnson EE, Lum HD, Rakhmilevich AL, et al. Intratumoral immunocytokine treatment results in enhanced antitumor effects. Cancer Immunol Immunother. 2008;57(12):1891–902. doi: 10.1007/s00262-008-0519-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wagner K, Schulz P, Scholz A, et al. The targeted immunocytokine L19-IL2 efficiently inhibits the growth of orthotopic pancreatic cancer. Clin Cancer Res. 2008;14(15):4951–60. doi: 10.1158/1078-0432.CCR-08-0157. [DOI] [PubMed] [Google Scholar]
- 100.Halin C, Gafner V, Villani ME, et al. Synergistic therapeutic effects of a tumor targeting antibody fragment, fused to interleukin 12 and to tumor necrosis factor alpha. Cancer Res. 2003;63(12):3202–10. [PubMed] [Google Scholar]
- 101.Low SC, Nunes SL, Bitonti AJ, et al. Oral and pulmonary delivery of FSH-Fc fusion proteins via neonatal Fc receptor-mediated transcytosis. Hum Reprod. 2005;20(7):1805–13. doi: 10.1093/humrep/deh896. [DOI] [PubMed] [Google Scholar]