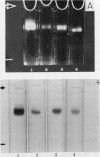
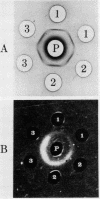
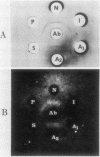
Abstract
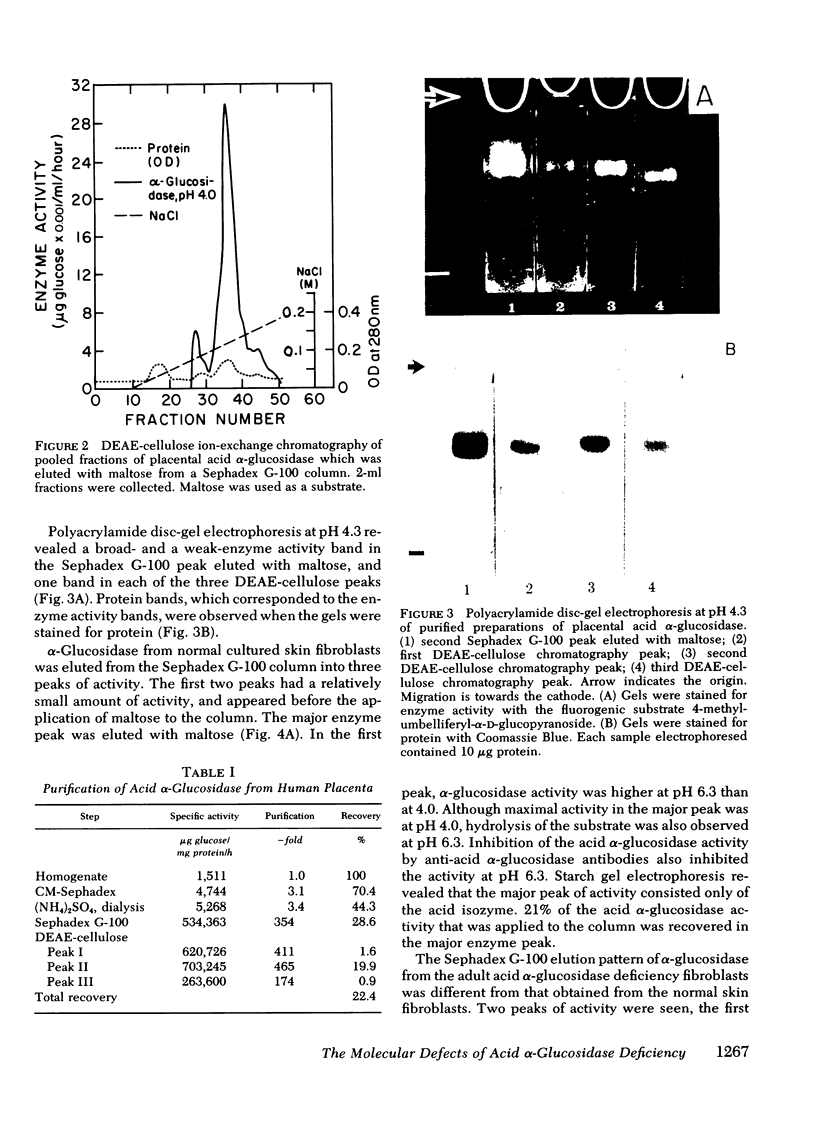
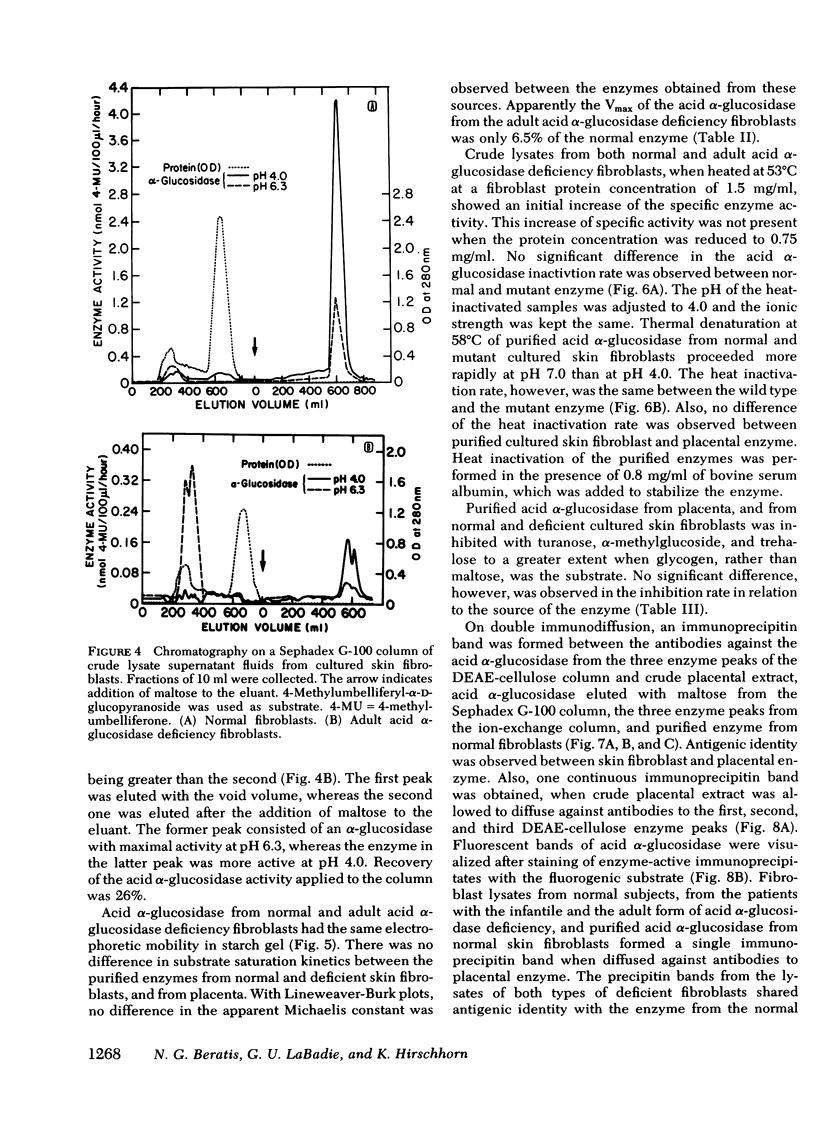
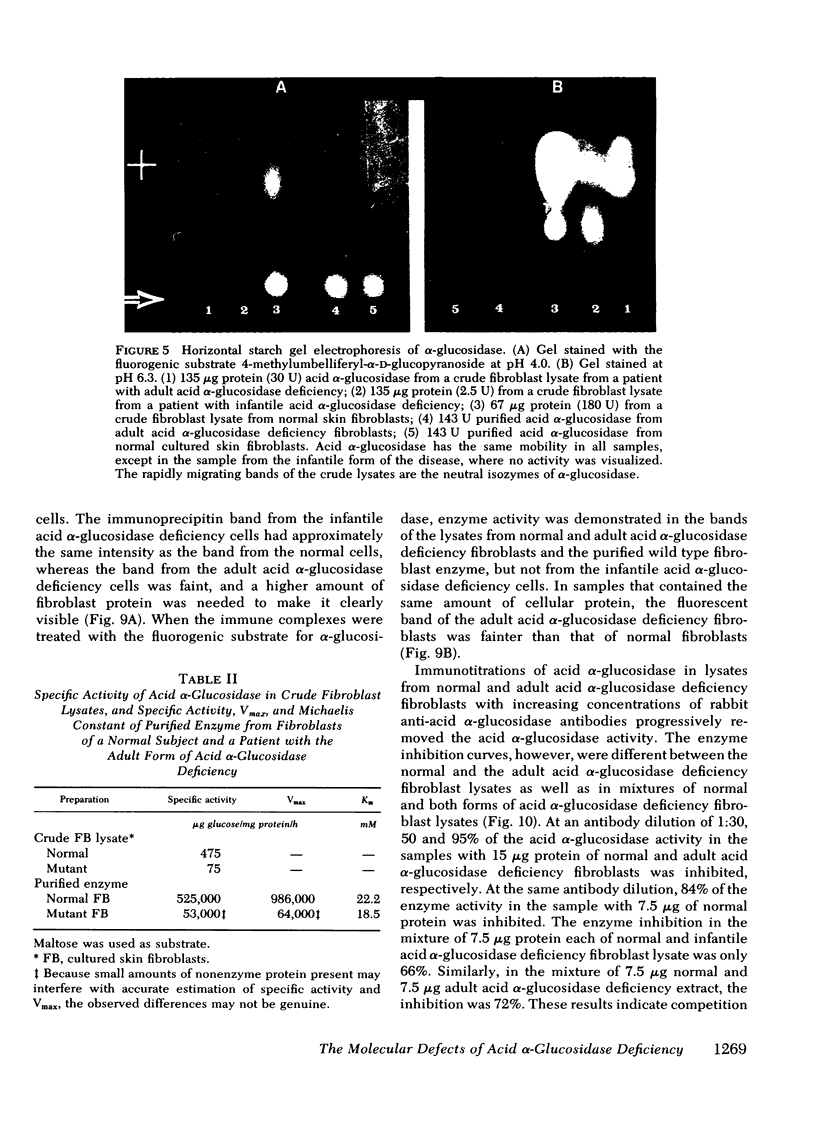
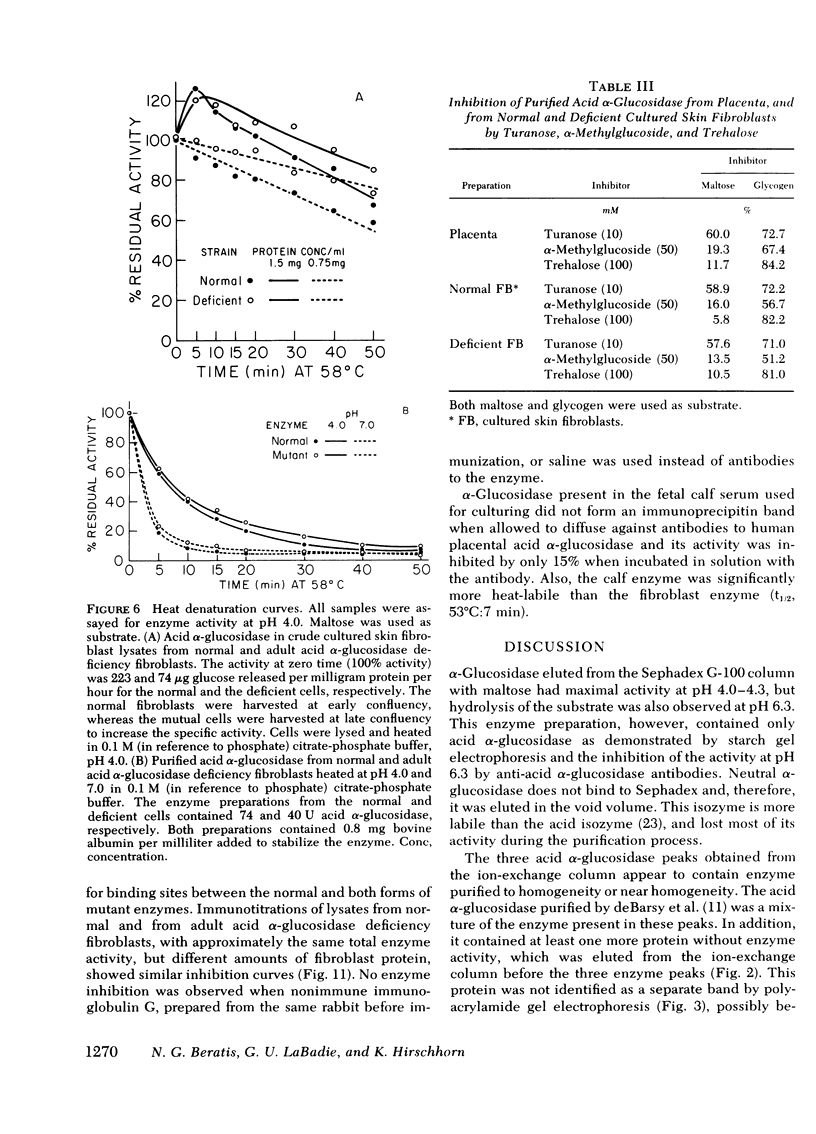
Different clinical expressions of acid alpha-glucosidase deficiency have been described. The present study was undertaken to investigate the basic metabolic defect in the infantile and adult forms of the disease. Acid alpha-glucosidase (EC 3.2.1.20) was purified from normal and from adult acid alpha-glucosidase deficiency fibroblasts. The pH optimum; Michaelis constant; electrophoretic mobility in starch; thermal denaturation at pH 4.0 and 7.0; and inhibition by turanose, alpha-methylglucoside and trehalose were the same in purified enzyme from normal and mutant cells. Placental acid alpha-glucosidase was purified to, or near, homogeneity. Monospecific antibodies raised against the enzyme in each of three enzyme peaks obtained from the last purification step were found to cross-react with the enzyme of all three peaks, and with purified, normal fibroblast enzyme. Cross-reacting material (CRM) also was identified in fibroblast lysates from normal subjects and from both forms of acid alpha-glucosidase deficiency. The amount of CRM in the adult form appeared to be significantly less than in normal cells or cells from the infantile form. Enzyme activity was demonstrated in the immune complexes of the normal and adult acid alpha-glucosidase deficiency fibroblasts, but not of the infantile form. Competition for antibody binding sites was observed between normal and both types of mutant enzymes. The findings indicate that this case of infantile acid alpha-glucosidase deficiency is the result of a structural gene mutation which causes the synthesis of a catalytically inactive (CRM-positive) enzyme protein. It appears that in the adult form, the mutation causes a reduction in the amount of the enzyme protein present in the cells.
Full text
PDF

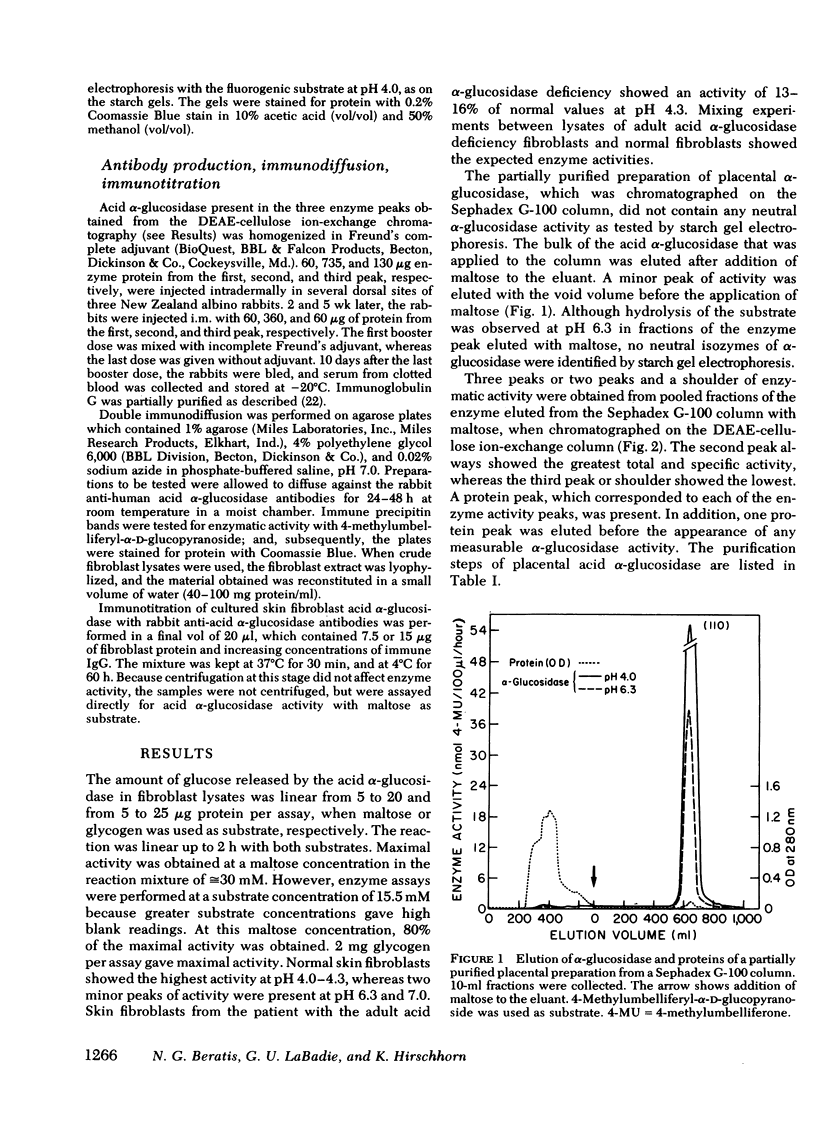
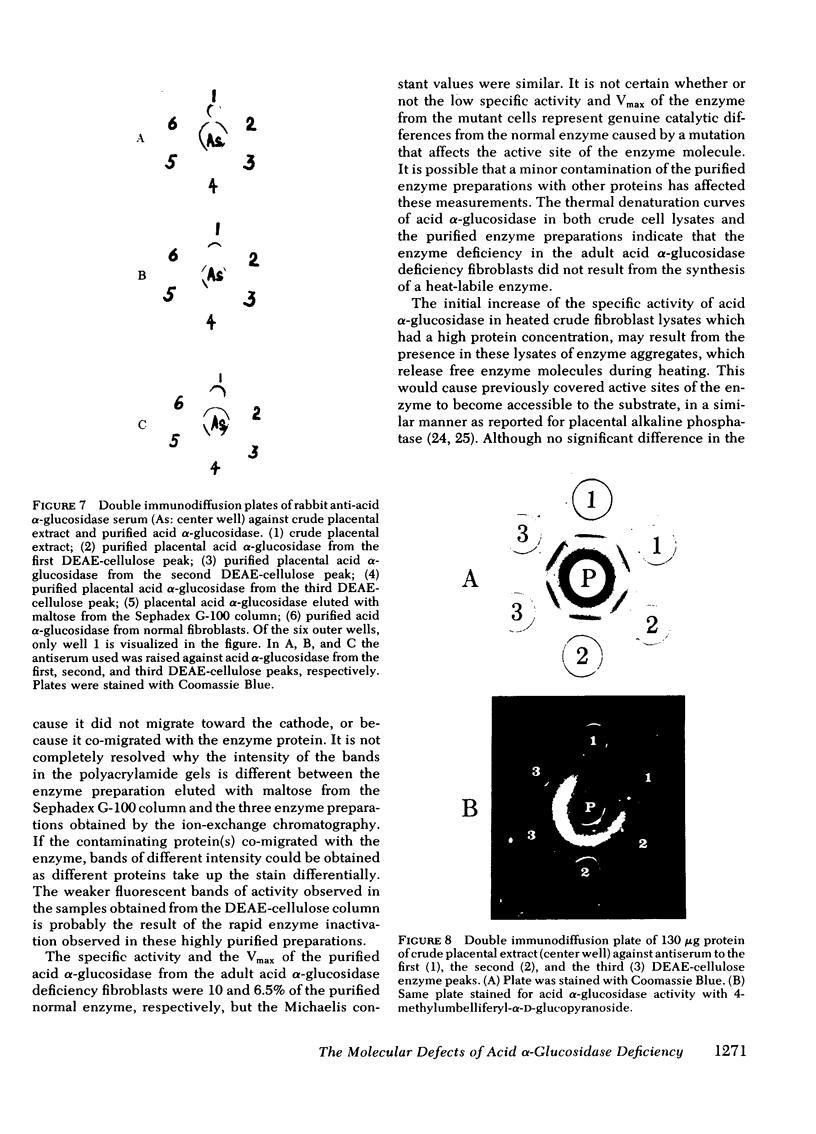
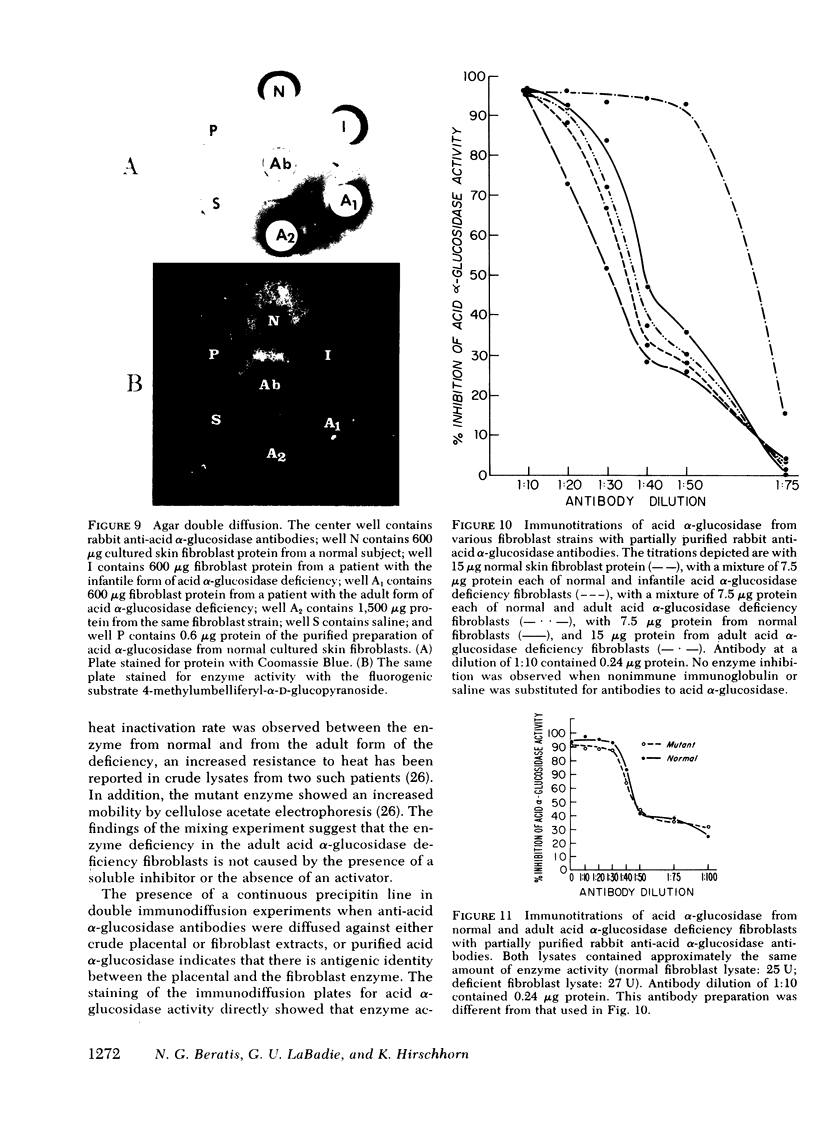


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angelini C., Engel A. G., Titus J. L. Adult acid maltase deficiency. Abnormalities in fibroblasts cultured from patients. N Engl J Med. 1972 Nov 9;287(19):948–951. doi: 10.1056/NEJM197211092871902. [DOI] [PubMed] [Google Scholar]
- Arnon R., Shapira E. Antibodies to papain. A selective fractionation according to inhibitory capacity. Biochemistry. 1967 Dec;6(12):3942–3950. doi: 10.1021/bi00864a041. [DOI] [PubMed] [Google Scholar]
- Auricchio F., Bruni C. B., Sica V. Further purification and characterization of the acid alpha-glucosidase. Biochem J. 1968 Jun;108(2):161–167. doi: 10.1042/bj1080161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beratis N. G., Hirschhorn K. Properties of placental alkaline phosphatase. 3. Thermostability and urea inhibition of isolated components of the three common phenotypes. Biochem Genet. 1972 Feb;6(1):1–8. doi: 10.1007/BF00485959. [DOI] [PubMed] [Google Scholar]
- Beratis N. G., Seegers W., Hirschhorn K. Properties of placental alkaline phosphatase. II. Interactions of fast- and slow-migrating components. Biochem Genet. 1971 Aug;5(4):367–377. doi: 10.1007/BF00485862. [DOI] [PubMed] [Google Scholar]
- Beratis N. G., Turner B. M., Weiss R., Hirschhorn K. Arylsulfatase B deficiency in Maroteaux-Lamy syndrome: Cellular studies and carrier identification. Pediatr Res. 1975 May;9(5):475–480. doi: 10.1203/00006450-197505000-00003. [DOI] [PubMed] [Google Scholar]
- Brown B. I., Brown D. H., Jeffrey P. L. Simultaneous absence of alpha-1,4-glucosidase and alpha-1,6-glucosidase activities (pH 4) in tissues of children with type II glycogen storage disease. Biochemistry. 1970 Mar 17;9(6):1423–1428. doi: 10.1021/bi00808a017. [DOI] [PubMed] [Google Scholar]
- DI SANT'AGNESE P. A., ANDERSEN D. H., MASON H. H., BAUMAN W. A. Glycogen storage disease of the heart. I. Report of 2 cases in siblings with chemical and pathologic studies. Pediatrics. 1950 Sep;6(3):402–424. [PubMed] [Google Scholar]
- Dreyfus J. C., Proux D., Alexandre Y. Molecular studies on glycogen storage diseases. Enzyme. 1974;18(1):60–72. doi: 10.1159/000459414. [DOI] [PubMed] [Google Scholar]
- Engel A. G. Acid maltase deficiency in adults: studies in four cases of a syndrome which may mimic muscular dystrophy or other myopathies. Brain. 1970;93(3):599–616. doi: 10.1093/brain/93.3.599. [DOI] [PubMed] [Google Scholar]
- Fujimoto A., Fluharty A. L., Stevens R. L., Kihara H., Wilson M. G. Two alpha-glucosidases in cultured amniotic fluid cells and their differentiation in the prenatal diagnosis of Pompe's disease. Clin Chim Acta. 1976 Apr 15;68(2):177–186. doi: 10.1016/0009-8981(76)90417-4. [DOI] [PubMed] [Google Scholar]
- Hudgson P., Gardner-Medwin D., Worsfold M., Pennington R. J., Walton J. N. Adult myopathy from glycogen storage disease due to acid maltase deficiency. Brain. 1968 Sep;91(3):435–462. doi: 10.1093/brain/91.3.435. [DOI] [PubMed] [Google Scholar]
- Koster J. F., Slee R. G. Some properties of human liver acid alpha-glucosidase. Biochim Biophys Acta. 1977 May 12;482(1):89–97. doi: 10.1016/0005-2744(77)90357-6. [DOI] [PubMed] [Google Scholar]
- Koster J. F., Slee R. G., Van der Klei-Van Moorsel J. M., Rietra P. J., Lucas C. J. Physico-chemical and immunological properties of acid alpha-glucosidase from various human tissues in relation to glycogenosis type II (Pompe's disease). Clin Chim Acta. 1976 Apr 1;68(1):49–58. doi: 10.1016/0009-8981(76)90287-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mehler M., DiMauro S. Late-onset acid maltase deficiency. Detection of patients and heterozygotes by urinary enzyme assay. Arch Neurol. 1976 Oct;33(10):692–695. doi: 10.1001/archneur.1976.00500100026011. [DOI] [PubMed] [Google Scholar]
- Murray A. K., Brown B. I., Brown D. H. The molecular heterogeneity of purified human liver lysosomal alpha-glucosidase (acid alpha-glucosidase). Arch Biochem Biophys. 1978 Jan 30;185(2):511–524. doi: 10.1016/0003-9861(78)90196-0. [DOI] [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- Reuser A. J., Koster J. F., Hoogeveen A., Galjaard H. Biochemical, immunological, and cell genetic studies in glycogenosis type II. Am J Hum Genet. 1978 Mar;30(2):132–143. [PMC free article] [PubMed] [Google Scholar]
- Salafsky I. S., Nadler H. L. A fluorometric assay of alpha-glucosidase and its application in the study of Pompe's disease. J Lab Clin Med. 1973 Mar;81(3):450–454. [PubMed] [Google Scholar]
- Seiler D., Kelleter R., Kölmel H. W., Heene R. Alpha-1,4-glucosidase activity in leucocytes and lymphocytes of 2 adult patients with glycogen-storage disease type II, (Pompe's disease). Experientia. 1973 Aug 15;29(8):972–973. doi: 10.1007/BF01930409. [DOI] [PubMed] [Google Scholar]
- Smith J., Zellweger H., Afifi A. K. Muscular form of glycogenosis, type II (Pompe). Neurology. 1967 Jun;17(6):537–549. doi: 10.1212/wnl.17.6.537. [DOI] [PubMed] [Google Scholar]
- Swaiman K. F., Kennedy W. R., Sauls H. S. Late infantile acid maltase deficiency. Arch Neurol. 1968 Jun;18(6):642–648. doi: 10.1001/archneur.1968.00470360064006. [DOI] [PubMed] [Google Scholar]
- Swallow D. M., Corney G., Harris H., Hirschhorn R. Acid alpha-glucosidase: a new polymorphism in man demonstrable by 'affinity' electrophoresis. Ann Hum Genet. 1975 May;38(4):391–406. doi: 10.1111/j.1469-1809.1975.tb00629.x. [DOI] [PubMed] [Google Scholar]
- de Barsy T., Jacquemin P., Devos P., Hers H. G. Rodent and human acid -glucosidase. Purification, properties and inhibition by antibodies. Investigation in type II glycogenosis. Eur J Biochem. 1972 Nov 21;31(1):156–165. doi: 10.1111/j.1432-1033.1972.tb02514.x. [DOI] [PubMed] [Google Scholar]