Abstract
Transient gene expression is a useful approach for studying the functions of gene products. In the case of plants, Agrobacterium infiltration is a method of choice for transient introduction of genes for many species. However, this technique does not work efficiently in some species, such as Arabidopsis thaliana. Moreover, the infection of Agrobacterium is known to induce dynamic changes in gene expression patterns in the host plants, possibly affecting the function and localization of the proteins to be tested. These problems can be circumvented by biolistic delivery of the genes of interest.
Here, we present an optimized protocol for biolistic delivery of plasmid DNA into epidermal cells of plant leaves, which can be easily performed using the Bio-Rad Helios gene gun system. This protocol allows efficient and reproducible transient expression of diverse genes in Arabidopsis, Nicotiana benthamiana and N. tabacum, and is suitable for studies of the biological function and subcellular localization of the gene products directly in planta. The protocol also can be easily adapted to other species by optimizing the delivery gas pressure.
Keywords: Plant, Biolistic gene delivery, Transient expression, Bio-Rad Helios gene gun system, Leaf epidermis
1. Introduction
Ectopic gene expression in living organisms is an important mean for studying the function of the gene products. To this end, it would be ideal to obtain transgenic plants that stably express a gene of interest. However, production of such transgenic plants requires a significant time investment, and transgene loci are often silenced transcriptionally and/or post-transcriptionally. Moreover, the location of transgene insertions in the genome and the resulting gene expression variability may confound data interpretation (1). Thus, it is crucial to develop an efficient, reproducible, and relatively simple methodology for transient gene expression in plant tissues.
Currently, at least four types of basic approaches are available for transient gene expression in plants: polyethylene glycol (PEG)-mediated or electroporation-mediated transformation of protoplasts, infiltration of Agrobacterium tumefaciens (agroinfiltration), and biolistic bombardment. PEG-mediated and electroporation-mediated transformation of protoplasts work efficiently in some plant species (2, 3), but both are time-consuming and only allow for studies in isolated protoplasts, which notoriously do not reflect the biology of plant tissues. Transient gene expression by agroinfiltration represents a relatively non-invasive and cost-effective method that enables fine tuning of the transgene expression levels by changing the concentration of the Agrobacterium cell inoculum (4, 5), and is a favored technique for several plant species, such as tobacco (Nicotiana tabacum) or Nicotiana benthamiana. However, Agrobacterium infection induces changes in gene expression pattern of specific sets of genes, including defense-related genes (6–8), and also interferes with host RNA silencing pathways (9), introducing a potential bias into the experiments’ outcome and interpretation. Moreover, this technique does not work well in leaves of many plants, including Arabidopsis, which is the most widely used model species for plant biology research.
An alternative approach to DNA delivery for transient gene expression, which circumvents many shortcomings of agroinfiltration, is microbombardment (10–12). Here, we describe a protocol for delivery of plasmid DNA into the epidermis of plant leaves by microparticle bombardment, which can be easily achieved using the Bio-Rad Helios gene gun system. Our technique is characterized by its high efficiency, reproducibility, and suitability for transient expression of functional proteins with diverse biological activities and different patterns of subcellular localization in Arabidopsis, N. benthamiana, and N. tabacum. The technique can easily be adapted to other species by optimizing the delivery gas pressure.
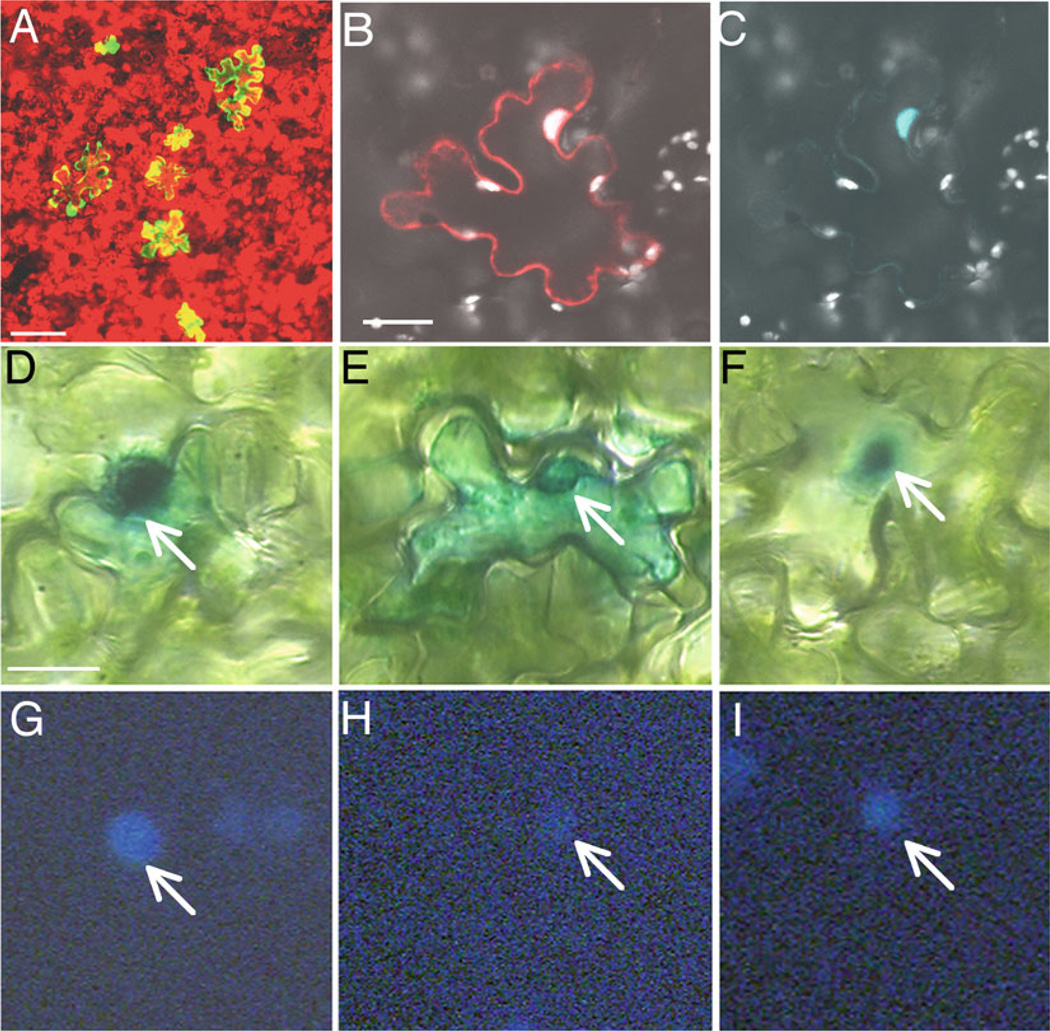
Figure 1 illustrates expression levels and localization patterns of different proteins expressed by this procedure. Using microbom-bardment with 0.6-µm gold particles prepared with the protocol described here, on average 4 ~ 8 cells expressing the unfused YFP are observed under 10× objective lens in 600 µm × 600 µm area (panel A), exemplifying the transformation efficiency of the technique. We also demonstrated the application of this technique to study protein localization in planta. Agrobacterium VirE2, VirE3, and VirF are previously demonstrated to localize to cell nucleus, and the Arabidopsis protein VirE2-interacting protein 1 (VIP1) is shown to be required for the targeting of VirE2 (13, 14). The biolistic bombardment technique was successfully utilized to analyze the localization of CFP-VirF fusion protein (panels B, C). Furthermore, a functional assay allowed us to show that β-glucuronidase (GUS)-tagged VirE2, which accumulates in the cell nucleus in wild-type tobacco (panels D, G), is localized in the cytoplasm in a vip 1 antisense background (panels E, H), and that co-expression of VirE3 restores the GUS-VirE2 nuclear localization (panels F, I) (14). In addition, this method was used with Arabidopsis thaliana to demonstrate cell-to-cell movement of the Tobacco mosaic virus movement protein (MP) tagged with YFP (11, 12). These data indicate that the protocol described in this article can be efficiently utilized to demonstrate intracellular localization and function of different proteins in plant tissues.
Fig. 1.
Examples of transient gene expression in leaf tissues following microbombardment. (a) Expression of a tandem repeat of the YFP gene in N. tabacum cv. Turk leaf (bar = 100 µ m), YFP expressing cells in yellow and chlorophyll auto fluorescence in red. (b, c) Expression of free DsRed and CFP-VirF fusion in a leaf cell of N. benthamiana (bar = 20 µ m), showing merged image with DsRed in red, CFP-VirF in blue, and chlorophyll autofluorescence in white (b) and CFP-VirF alone (c). For (a–c), observations were performed under a confocal microscope (Zeiss, LSM5 Pa), 24 h after microbombardment. (d–i) Localization of β-glucuronidase (GUS)-VirE2 fusion in A. thaliana leaves (bar = 10 β m). GUS-VirE2 is targeted to the nucleus in wild-type N. tabacum (d, g), whereas it is essentially cytoplasmic in vip1-antisense N. tabacum (e, h); in double transgenic vip1-antisense plants expressing VirE3, GUS-VirE2 nuclear localization is restored (f, i). Panels D–F represent GUS staining, and panels G–I represent DAPI staining. Arrows indicate cell nuclei. Histochemical GUS assay was done 24 h after microbombardment, and observation were performed after 3 h staining (as described in (13)).
2. Materials
Eppendorf tubes.
Microcentrifuge.
Ultrasonic cleaner (see Note 1).
Vortex mixer with adjustable speed.
Bio-Rad Helios Gene.
Bio-Rad Helios cartridge preparatory station (see Note 2).
Bio-Rad Tubing cutter.
Tank with Helium gas with Bio-Rad Helium gas regulator.
Tank with dry N2 gas.
Window screen mesh, cut into 10 × 10 cm squares.
Flat Styrofoam surface (e.g., a lid of a Styrofoam box).
Epifluorescence or confocal microscope.
Plant growth chamber.
Pro-Mix BX.
A. thaliana plants (4–6-week old) N. benthamiana, or N. tabacum (7–10 weeks) plants.
Plasmid DNA for expression of the gene of interest (at >0.5 µg/ µL in H2O, up to 50 µg total), (see Note 3).
Gold microparticles, 0.6 or 1.0 µm diameter (Bio-Rad or other brand).
Absolute ethanol (see Note 4).
Bio-Rad Tefzel tubing.
Bio-Rad Polyvinylpyrolidone (PVP), MW 360,000 (included in the Tefzel tubing kit).
5 mL Syringe (without needle).
Double-distilled water (ddH2O), autoclaved.
Spermidine stock solution: 3.0 M spermidine in ddH2O, stored at −20°C.
PVP stock solution: PVP in absolute ethanol at 20 mg/mL, stored at −20°C.
CaCl 2 solution: 1 M CaCl 2. Autoclave and store at room temperature.
Cotton balls.
Scintillation vials.
Drierite.
Whatmann filter paper.
Petri dishes.
Parafilm.
3. Methods
3.1. Plant Growth
Grow plants in environmental chamber with appropriate photoperiod cycle and humidity.
For Arabidopsis, grow one or two plants on Pro-Mix BX in a pot (10 × 10 × 10 cm) in an environment-controlled chamber with a short photoperiod (8 h of 130–150 µE/m 2 s light at 23°C/16 h dark at 20°C), and 40–65% relative humidity for 6–8 weeks (15) (see Note 5).
For N. benthamiana and N. tabacum, grow one plant on Pro-Mix BX in a pot (20 cm × 20 cm × 20 cm) in an environment controlled chamber with a long photoperiod (16 h of 130–150 µE/m2 s light at 23°C/8 h dark at 20°C) and 40–65% relative humidity for 7–10 weeks.
Supplement plants occasionally with commercially available fertilizers following manufacturers’ instructions (see Note 6).
3.2. Preparation of Working Solutions
Prepare spermidine working solution (50 mM in ddH2 O) and PVP working solution (50 µg/mL in ethanol) from stock solutions. These solutions need to be prepared fresh just prior to experiments.
3.3. DNA Precipitation Onto the Surface of Gold Microparticles
Weigh 12 mg of gold microparticles and transfer into a 1.5 mL microcentrifuge tube.
Add 100 µL of spermidine working solution.
Sonicate the mixture for 10 s, then vortex the tube vigorously for 10 s in order to disperse gold particles (see Note 7).
Add 25–50 µg of plasmid DNA, in a maximal volume of 100 µL (ideally 50 µL), to the gold microparticle suspension (when more than one plasmid are used, mix them thoroughly before adding plasmid DNA to the gold particles).
Sonicate the tube for 10 s, then vortex vigorously for 5 s at full speed (see Note 8). Lower the speed of vortex.
Open the tube, while continuing to mix the gold microparticle suspension (you must vortex with the lid open without spilling the suspension from the tube), add 100 µL of 1.0 M CaCl2 slowly, drop by drop, waiting 5 s between each drop (see Note 9).
Allow the suspension to settle at room temperature for about 10 min.
Meanwhile, connect the Tefzel tubing to N2 flow and dry the inner wall of the tubing for at least 5 min.
Centrifuge at 10,000 rpm for 30 s in a microcentrifuge to collect the gold microparticles. Remove the supernatant without disturbing the pellet.
Add 1 mL of absolute ethanol to the gold microparticles, resuspend, and centrifuge at 10,000 rpm for 30 s to wash the particles.
Repeat the wash twice more (a total of three washes) and remove the supernatant completely.
Resuspend the gold microparticles in 0.5 mL PVP-ethanol solution and transfer the microparticle suspension to a 15-mL conical tube. Wash the microcentrifuge tube with 0.5 mL PVP-ethanol solution to collect the microparticles as much as possible and add them to the conical tube.
Adjust the total volume of microparticle suspension in the conical tube to 3.0 mL with PVP-ethanol.
Sonicate the resulting mixture for 10 s to disperse the gold microparticles before proceeding to next step (see Note 10).
3.4. Cartridge Preparation with Tubing Prep Station
Close the N2 flow after drying the Tefzel tubing.
Remove the tubing from the Tubing Prep Station, and load it with the DNA-coated gold microparticle suspension using a 5-mL syringe connected to the Tefzel tubing via a short segment of flexible Tygon tubing (see Note 11).
Place the Tefzel tubing horizontally in the Tubing Prep Station immediately after loading.
Allow the Tefzel tubing with gold microparticle suspension to lie for 5 min for 1-µm gold or for 15 min for 0.6-µm gold, to settle the microparticles on the inner surface of the Tefzel tubing (see Note 12).
Remove ethanol from the tubing, using the 5-mL syringe. After ethanol removal, the gold microparticles must remain on the inner surface of the Tefzel tubing.
Turn the tubing 180°, wait for 5 s, then rotate the tubing at a speed of 60 rpm for 30 s.
Open the N2 flow for 10 min to dry the tubing.
Cut the microparticle-loaded Tefzel tubing into 1-cm-long segments (cartridges) using the Tubing Cutter supplied with the Tubing Prep Station. A 70-cm-long Tefzel tubing loaded with gold microparticle prepared using this protocol should yield approximately 50 cartridges.
The cartridges can be kept at −20°C in a scintillation vial containing drying agent, such as silica gel or Drierite. Overlay the drying agents with a cotton ball to secure the drying agent particles to the bottom of the container, place the prepared cartridges on top of the cotton ball, and tightly close the vial with its lid. The cartridges can be stored for several months in dry environment at −20°C.
3.5. Microbombardment
Select well-expanded leaves from plants. Leaves with size larger than 50 mm × 70 mm for N. benthamiana, 100 mm × 125 mm for N. tabacum (these length measurements do not include petiole)or 15 × 35 mm for A. thaliana (the length measurement includes petiole) work well.
Remove the selected leaves with a sharp razor blade and immediately place them with the abaxial sides facing up onto a flat Styrofoam surface. The abaxial side of the leaf represents a better substrate for bombardment because of its lower trichome density and thinner cuticle (see Note 13).
Adjust the pressure of the gene gun at 100–120 psi for 1-µm gold microparticles, and 160–180 psi for 0.6-µm gold for N. benthamiana and N. tabacum, and at 90–110 psi for 1-µm gold and 140–160 psi for 0.6-µm gold for Arabidopsis (see Note 14).
Load the cartridge into the gun and shoot. Hold the tip of the barrel liner of the gene gun as close as possible to the leaf tissue, and aim to the center of the leaf mid-rib. Note that this procedure is aimed at transformation of the epidermal cell layers of the leaf (see Note 15).
Place the leaves into a Petri dish over three layers of wet Whatman filter paper, seal the Petri dish with Parafilm, and leave it in the dark at room temperature for 16–48 h to allow expression of the delivered transgene (see Note 16).
Analyze the transformed tissue under a confocal microscope (Fig. 1).
Acknowledgements
We apologize to colleagues whose original works have not been cited due to the lack of space. The work in our laboratory is supported by grants from NIH, NSF, USDA NIFA, and BARD to VC.
Footnotes
Ultrasonic cleaner for jewelry or glasses, such as Misonix ultrasonic cleaner, frequency 40 Hz.
Helios Gene Gun system and Tubing Prep Station are set up and used according to the manufacturer’s instructions.
Use plasmid purified using common commercial kits, such as Qiagen.
The ethanol bottle has to be opened freshly before each experiment, since ethanol that has absorbed moisture from air tends to give poor results.
The short photoperiod is required to obtain larger leaves from Arabidopsis plants; growing plants under long photoperiod conditions will result in much smaller leaves, which are less convenient for the experiments.
Ensure that the plants are healthy and well maintained. Leaves harvested from plants grown under inappropriate conditions yield poor transformation efficiency.
Dispersing the gold microparticles by sonication and vortexing is required for uniform DNA coating of the particle surface.
Poor quality of DNA-coated microparticles leads to low expression level. To achieve high transformation efficiency, DNA solution with a concentration higher than 0.5 mg/mL should be used.
The 1.0 M CaCl2 solution has to be added slowly, while the microparticle suspension is constantly mixed, for an even binding of DNA on the surface of the gold particles.
Sonication at this step is required for efficient particle loading into the Tefzel tubing in step 9.
Minimize the handling time for Subheading 3.3, 3.4, 3.5, and 4.
Insufficient amount of microparticles loaded into the cartridge may lead to low expression levels. The Tefzel tubing must remain stationary after the microparticle loading precisely as described in this Step.
For easier handling of small leaves (e.g., leaves from Arabidopsis), cover the leaves with a piece of window screen mesh and secure the mesh with pushpins to the Styrofoam surface. Maintaining leaves flat using the window screen mesh increases the efficiency of the particle delivery and minimizes the damage to the tissue during the bombardment.
The weight of the 0.6-µm microparticle is as ~22% of 1-µm microparticles, so the same pressure using the microparticle with different size results in different speed of the particles reaching the epidermis. Therefore, for transformation of other plant species with thin leaves, using 0.6-µm microparticle is preferable.
Even well-prepared cartridges could give low expression levels because of inappropriate bombardment conditions. Use the pressure values indicated in the text for N. benthamiana and Arabidopsis. For other plants, the pressures should be determined empirically. Too low pressure will give poor transformation whereas too high pressure will damage the cells.
The time period between the bombardment and the microscopy/ activity assay should be determined empirically. For example, for imaging GFP and its different spectral variants, 24–36 h is usually sufficient, whereas for imaging of DsRed2, expression/protein maturation time of 48 h may be required.
References
- 1.Janssen BJ, Gardner RC. Localized transient expression of GUS in leaf discs following cocultivation with Agrobacterium. Plant Mol Biol. 1990;14:61–72. doi: 10.1007/BF00015655. [DOI] [PubMed] [Google Scholar]
- 2.Abel S, Theologis A. Transient transformation of Arabidopsis leaf protoplasts: a versatile experimental system to study gene expression. Plant J. 1994;5:421–427. doi: 10.1111/j.1365-313x.1994.00421.x. [DOI] [PubMed] [Google Scholar]
- 3.Yoo SD, Cho YH, Sheen J. Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc. 2007;2:1565–1572. doi: 10.1038/nprot.2007.199. [DOI] [PubMed] [Google Scholar]
- 4.Yang B, et al. The virulence factor AvrXa7 of Xanthomonas oryzae pv oryzae is a type III secretion pathway-dependent nuclear-localized double-stranded DNA-binding protein. Proc Natl Acad Sci USA. 2000;97:9807–9812. doi: 10.1073/pnas.170286897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodin MM, et al. pGD vectors: versatile tools for the expression of green and red fluorescent protein fusions in agroinfiltrated plant leaves. Plant J. 2002;31:375–383. doi: 10.1046/j.1365-313x.2002.01360.x. [DOI] [PubMed] [Google Scholar]
- 6.Ditt RF, Nester EW, Comai L. Plant gene expression response to Agrobacterium tumefaciens. Proc Natl Acad Sci USA. 2001;98:10954–10959. doi: 10.1073/pnas.191383498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ditt RF, et al. The Arabidopsis thaliana transcriptome in response to Agrobacterium tumefaciens. Mol Plant Microbe Interact. 2006;19:665–681. doi: 10.1094/MPMI-19-0665. [DOI] [PubMed] [Google Scholar]
- 8.Zaltsman A, et al. Agrobacterium induces expression of a host F-box protein required for tumorigenicity. Cell Host Microbe. 2010;7:197–209. doi: 10.1016/j.chom.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunoyer P, Himber C, Voinnet O. Induction, suppression and requirement of RNA silencing pathways in virulent Agrobacterium tumefaciens infections. Nat Genet. 2006;38:258–263. doi: 10.1038/ng1722. [DOI] [PubMed] [Google Scholar]
- 10.Taylor NJ, Fauquet CM. Microparticle bombardment as a tool in plant science and agricultural biotechnology. DNA Cell Biol. 2002;21:963–977. doi: 10.1089/104454902762053891. [DOI] [PubMed] [Google Scholar]
- 11.Ueki S, et al. Functional transient genetic transformation of Arabidopsis leaves by biolistic bombardment. Nat Protoc. 2009;4:71–77. doi: 10.1038/nprot.2008.217. [DOI] [PubMed] [Google Scholar]
- 12.Ueki S, et al. A cell-to-cell macromolecular transport assay in planta utilizing biolistic bombardment. J Vis Exp. 2010;42:2208. doi: 10.3791/2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tzfira T, Vaidya M, Citovsky V. Involvement of targeted proteolysis in plant genetic transformation by Agrobacterium. Nature. 2004;431:87–92. doi: 10.1038/nature02857. [DOI] [PubMed] [Google Scholar]
- 14.Lacroix B, et al. The VirE3 protein of Agrobacterium mimics a host cell function required for plant genetic transformation. EMBO J. 2005;24:428–437. doi: 10.1038/sj.emboj.7600524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rivero-Lepinckas L, Crist D, Scholl R. Growth of plants and preservation of seeds. Methods Mol Biol. 2006;323:3–12. doi: 10.1385/1-59745-003-0:3. [DOI] [PubMed] [Google Scholar]