Abstract
Salmonella is traditionally described as a facultative intracellular parasite, and host macrophages are regarded as the primary effector cells in both native and acquired immunity in mouse typhoid. This concept has not been unanimously accepted in the literature. Based on cell culture experiments and electron microscopic examinations of infected tissues, we observed that virulent Salmonella typhimurium is killed within polymorphs and macrophages of guinea pigs and mice. In a systemic disease, the organism propagates primarily in the extracellular locations of sinusoids and tissue lesions and within hepatocytes. Hence, it is more likely to be an extracellular pathogen and its virulence is directly related to its antiphagocytic property. The conspicuous absence of macrophages in the primary lesions of murine salmonellosis disputes the likelihood of their significant role in native resistance to the disease. Acquired cellular immunity is expressed as an enhanced antibacterial activity of macrophages facilitated by cytophilic antibodies rather than as an altered antibacterial action of immune macrophages. It is proposed that acquired immunity in murine salmonellosis is a synergistic manifestation of the innate capacity of polymorphs and macrophages to destroy ingested salmonellae, the activated antibacterial functions of macrophages mediated by cytophilic antibodies, the opsonic and agglutinating actions of antiserum, and the accelerated inflammation associated with delayed hypersensitivity to bacterial antigens. Unlike live attenuated vaccines, nonviable vaccines offer a significant, though not a solid, protection against subsequent challenges.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. W. Intracellular bacilli in intestinal and mesenteric lesions of typhoid fever. Am J Pathol. 1939 Sep;15(5):561–566.1. [PMC free article] [PubMed] [Google Scholar]
- Angerman C. R., Eisenstein T. K. Comparative efficacy and toxicity of a ribosomal vaccine, acetone-killed cells, lipopolysaccharide, and a live cell vaccine prepared from Salmonella typhhimurium. Infect Immun. 1978 Feb;19(2):575–582. doi: 10.1128/iai.19.2.575-582.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angerman C. R., Eisenstein T. K. Correlation of the duration and magnitude of protection against Salmonella infection afforded by various vaccines with antibody titers. Infect Immun. 1980 Feb;27(2):435–443. doi: 10.1128/iai.27.2.435-443.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAKKEN K., VOGELSANG T. M. The pathogenesis of Salmonella typhimurium infection in mice. Acta Pathol Microbiol Scand. 1950;27(1):41–50. doi: 10.1111/j.1699-0463.1950.tb05191.x. [DOI] [PubMed] [Google Scholar]
- Baron E. J., Proctor R. A. Inefficient in vitro killing of virulent or nonvirulent Salmonella typhimurium by murine polymorphonuclear neutrophils. Can J Microbiol. 1984 Oct;30(10):1264–1270. doi: 10.1139/m84-199. [DOI] [PubMed] [Google Scholar]
- Beckerdite S., Mooney C., Weiss J., Franson R., Elsbach P. Early and discrete changes in permeability of Escherichia coli and certain other gram-negative bacteria during killing by granulocytes. J Exp Med. 1974 Aug 1;140(2):396–409. doi: 10.1084/jem.140.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin W. H., Jr, Turnbough C. L., Jr, Posey B. S., Briles D. E. Salmonella typhimurium virulence genes necessary to exploit the Itys/s genotype of the mouse. Infect Immun. 1986 Mar;51(3):872–878. doi: 10.1128/iai.51.3.872-878.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V., Mackaness G. B., Collins F. M. Mechanisms of acquired resistance in mouse typhoid. J Exp Med. 1966 Oct 1;124(4):585–600. doi: 10.1084/jem.124.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonventre P. F., Imhoff J. G. Uptake of h-dihydrostreptomycin by macrophages in culture. Infect Immun. 1970 Jul;2(1):89–95. doi: 10.1128/iai.2.1.89-95.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briles D. E., Benjamin W., Jr, Posey B., Michalek S. M., McGhee J. R. Independence of macrophage activation and expression of the alleles of the Ity (immunity to typhimurium) locus. Microb Pathog. 1986 Feb;1(1):33–41. doi: 10.1016/0882-4010(86)90029-x. [DOI] [PubMed] [Google Scholar]
- Briles D. E., Lehmeyer J., Forman C. Phagocytosis and killing of salmonella typhimurium by peritoneal exudate cells. Infect Immun. 1981 Aug;33(2):380–388. doi: 10.1128/iai.33.2.380-388.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmeier N. A., Heffron F. Intracellular survival of wild-type Salmonella typhimurium and macrophage-sensitive mutants in diverse populations of macrophages. Infect Immun. 1989 Jan;57(1):1–7. doi: 10.1128/iai.57.1.1-7.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell P. A. Immunocompetent cells in resistance to bacterial infections. Bacteriol Rev. 1976 Jun;40(2):284–313. doi: 10.1128/br.40.2.284-313.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin N. I., Svenson S. B., Lindberg A. A. Role of monoclonal O-antigen antibody epitope specificity and isotype in protection against experimental mouse typhoid. Microb Pathog. 1987 Mar;2(3):171–183. doi: 10.1016/0882-4010(87)90019-2. [DOI] [PubMed] [Google Scholar]
- Collins F. M., Campbell S. G. Immunity to intracellular bacteria. Vet Immunol Immunopathol. 1982 Jan;3(1-2):5–66. doi: 10.1016/0165-2427(82)90031-9. [DOI] [PubMed] [Google Scholar]
- Collins F. M. Effect of specific immune mouse serum on the growth of Salmonella enteritidis in mice preimmunized with living or ethyl alcohol-killed vaccines. J Bacteriol. 1969 Feb;97(2):676–683. doi: 10.1128/jb.97.2.676-683.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Mackaness G. B., Blanden R. V. Infection-immunity in experimental salmonellosis. J Exp Med. 1966 Oct 1;124(4):601–619. doi: 10.1084/jem.124.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Mackaness G. B. Delayed hypersensitivity and arthus reactivity in relation to host resistance in salmonella-infected mice. J Immunol. 1968 Nov;101(5):830–845. [PubMed] [Google Scholar]
- Collins F. M. Vaccines and cell-mediated immunity. Bacteriol Rev. 1974 Dec;38(4):371–402. doi: 10.1128/br.38.4.371-402.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diena B. B., Johnson E. M., Baron L. S., Wallace R., Greenberg L. Assay of typhoid vaccines with Salmonella typhosa-Salmonella typhimurium hybrids. Infect Immun. 1973 Jan;7(1):5–8. doi: 10.1128/iai.7.1.5-8.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. The binding of penicillin in relation to its cytotoxic action. III. The binding of penicillin by mammalian cells in tissue culture (HeLa and L strains). J Exp Med. 1954 Jul 1;100(1):117–124. doi: 10.1084/jem.100.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein T. K., Angerman C. R. Immunity to experimental Salmonella infection: studies on the protective capacity and immunogenicity of lipopolysaccharide, acetone-killed cells, and ribosome-rich extracts of Salmonella typhimurium in C3H/HeJ and CD-1 mice. J Immunol. 1978 Sep;121(3):1010–1014. [PubMed] [Google Scholar]
- Eisenstein T. K. Evidence for O antigens as the antigenic determinants in "ribosomal" vaccines prepared from Salmonella. Infect Immun. 1975 Aug;12(2):364–377. doi: 10.1128/iai.12.2.364-377.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein T. K., Killar L. M., Sultzer B. M. Immunity to infection with Salmonella typhimurium: mouse-strain differences in vaccine- and serum-mediated protection. J Infect Dis. 1984 Sep;150(3):425–435. doi: 10.1093/infdis/150.3.425. [DOI] [PubMed] [Google Scholar]
- Eisenstein T. K., Sultzer B. M. Immunity to Salmonella infection. Adv Exp Med Biol. 1983;162:261–296. doi: 10.1007/978-1-4684-4481-0_26. [DOI] [PubMed] [Google Scholar]
- FURNESS G. Interaction between Salmonella typhimurium and phagocytic cells in cell culture. J Infect Dis. 1958 Nov-Dec;103(3):272–277. doi: 10.1093/infdis/103.3.272. [DOI] [PubMed] [Google Scholar]
- Finlay B. B., Falkow S. Common themes in microbial pathogenicity. Microbiol Rev. 1989 Jun;53(2):210–230. doi: 10.1128/mr.53.2.210-230.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GELZER J., SUTER E. The effect of antibody on intracellular parasitism of Salmonella typhimurium in mononuclear phagocytes in vitro: prolonged survival of infected monocytes in presence of antibody. J Exp Med. 1959 Nov 1;110:715–730. doi: 10.1084/jem.110.5.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germanier R. Immunity in experimental salmonellosis. 3. Comparative immunization with viable and heat-inactivated cells of Salmonella typhimurium. Infect Immun. 1972 May;5(5):792–797. doi: 10.1128/iai.5.5.792-797.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannella R. A., Washington O., Gemski P., Formal S. B. Invasion of HeLa cells by Salmonella typhimurium: a model for study of invasiveness of Salmonella. J Infect Dis. 1973 Jul;128(1):69–75. doi: 10.1093/infdis/128.1.69. [DOI] [PubMed] [Google Scholar]
- Goodpasture E. W. Concerning the Pathogenesis of Typhoid Fever. Am J Pathol. 1937 Mar;13(2):175–186.5. [PMC free article] [PubMed] [Google Scholar]
- Guo Y. N., Hsu H. S., Mumaw V. R., Nakoneczna I. Electronmicroscopy studies on the bactericidal action of inflammatory leukocytes in murine salmonellosis. J Med Microbiol. 1986 Mar;21(2):151–159. doi: 10.1099/00222615-21-2-151. [DOI] [PubMed] [Google Scholar]
- Guo Y. N., Hsu H. S., Mumaw V. R., Nakoneczna I. Electronmicroscopy studies on the opsonic role of antiserum and the subsequent destruction of Salmonellae within murine inflammatory leukocytes. J Med Microbiol. 1986 Dec;22(4):343–349. doi: 10.1099/00222615-22-4-343. [DOI] [PubMed] [Google Scholar]
- HIRSCH J. G. Immunity to infectious diseases: review of some concepts of Metchnikoff. Bacteriol Rev. 1959 Jun;23(2):48–60. doi: 10.1128/br.23.2.48-60.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOPPS H. E., SMADEL J. E., BERNHEIM B. C., DANAUSKAS J. X., JACKSON E. B. Effect of antibiotics on intracellular Salmonella typhosa. II. Elimination of infection by prolonged treatment. J Immunol. 1961 Aug;87:162–174. [PubMed] [Google Scholar]
- HSU H. S. IN VITRO STUDIES ON THE INTERACTIONS BETWEEN MACROPHAGES OF RABBITS AND TUBERCLE BACILLI. I. CELLULAR BASIS OF NATIVE RESISTANCE. Am Rev Respir Dis. 1965 Apr;91:488–498. doi: 10.1164/arrd.1965.91.4.488. [DOI] [PubMed] [Google Scholar]
- HSU H. S. IN VITRO STUDIES ON THE INTERACTIONS BETWEEN MACROPHAGES OF RABBITS AND TUBERCLE BACILLI. II. CELLULAR AND HUMORAL ASPECTS OF ACQUIRED RESISTANCE. Am Rev Respir Dis. 1965 Apr;91:499–509. doi: 10.1164/arrd.1965.91.4.499. [DOI] [PubMed] [Google Scholar]
- Hahn H., Kaufmann S. H. The role of cell-mediated immunity in bacterial infections. Rev Infect Dis. 1981 Nov-Dec;3(6):1221–1250. doi: 10.1093/clinids/3.6.1221. [DOI] [PubMed] [Google Scholar]
- Harrington K. A., Hormaeche C. E. Expression of the innate resistance gene Ity in mouse Kupffer cells infected with Salmonella typhimurium in vitro. Microb Pathog. 1986 Jun;1(3):269–274. doi: 10.1016/0882-4010(86)90051-3. [DOI] [PubMed] [Google Scholar]
- Herzberg M., Nash P., Hino S. Degree of immunity induced by killed vaccines to experimental salmonellosis in mice. Infect Immun. 1972 Jan;5(1):83–90. doi: 10.1128/iai.5.1.83-90.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochadel J. F., Keller K. F. Protective effects of passively transferred immune T- or B-lymphocytes in mice infected with Salmonella typhimurium. J Infect Dis. 1977 May;135(5):813–823. doi: 10.1093/infdis/135.5.813. [DOI] [PubMed] [Google Scholar]
- Hormaeche C. E., Harrington K. A., Joysey H. S. Natural resistance to salmonellae in mice: control by genes within the major histocompatibility complex. J Infect Dis. 1985 Nov;152(5):1050–1056. doi: 10.1093/infdis/152.5.1050. [DOI] [PubMed] [Google Scholar]
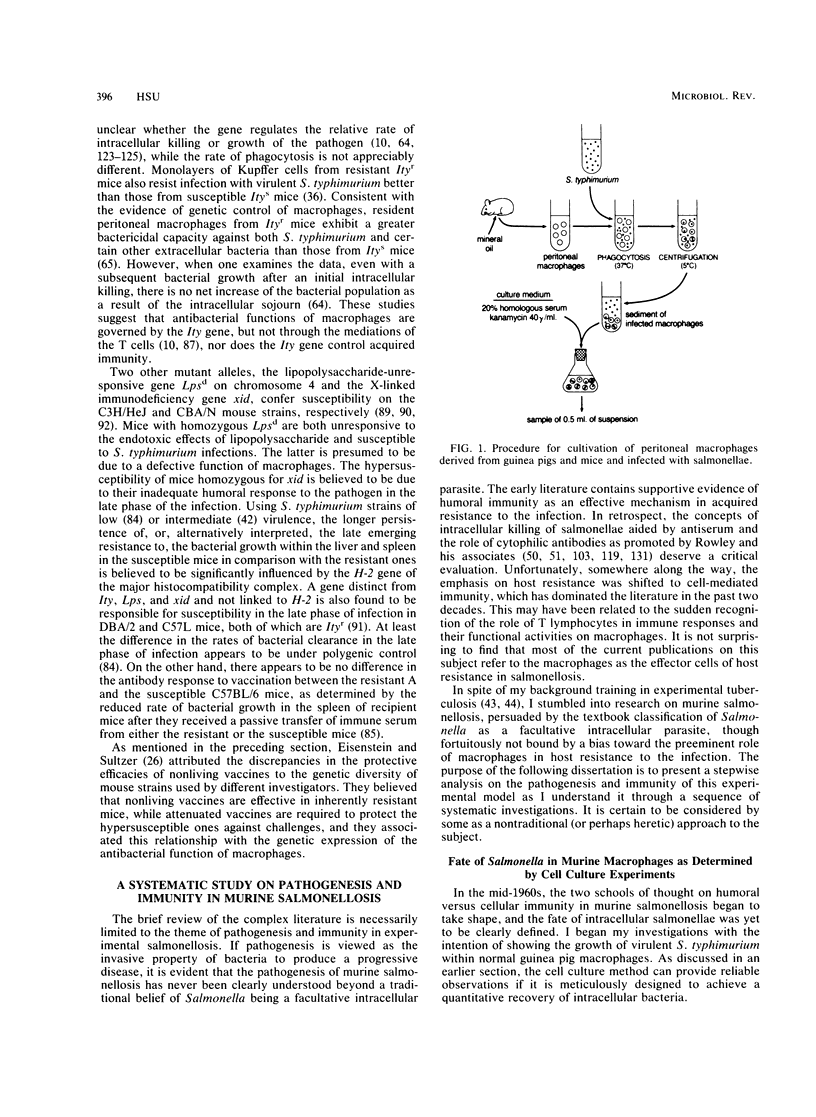
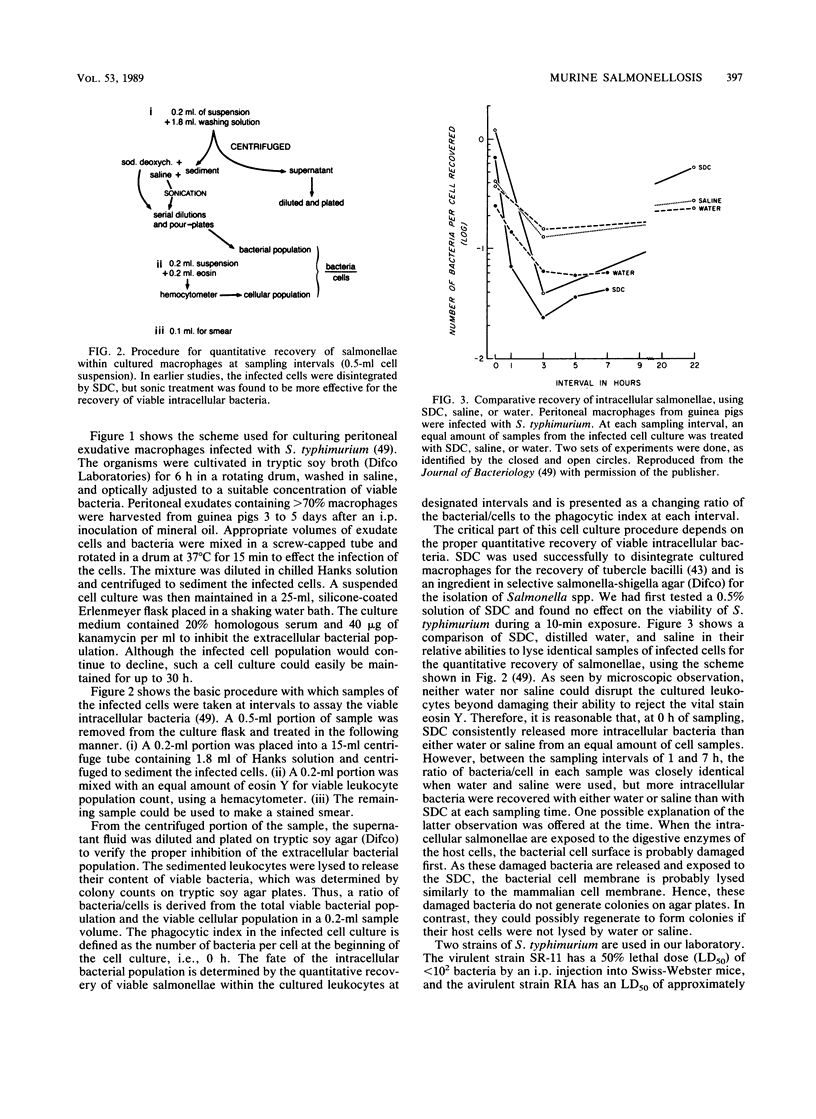
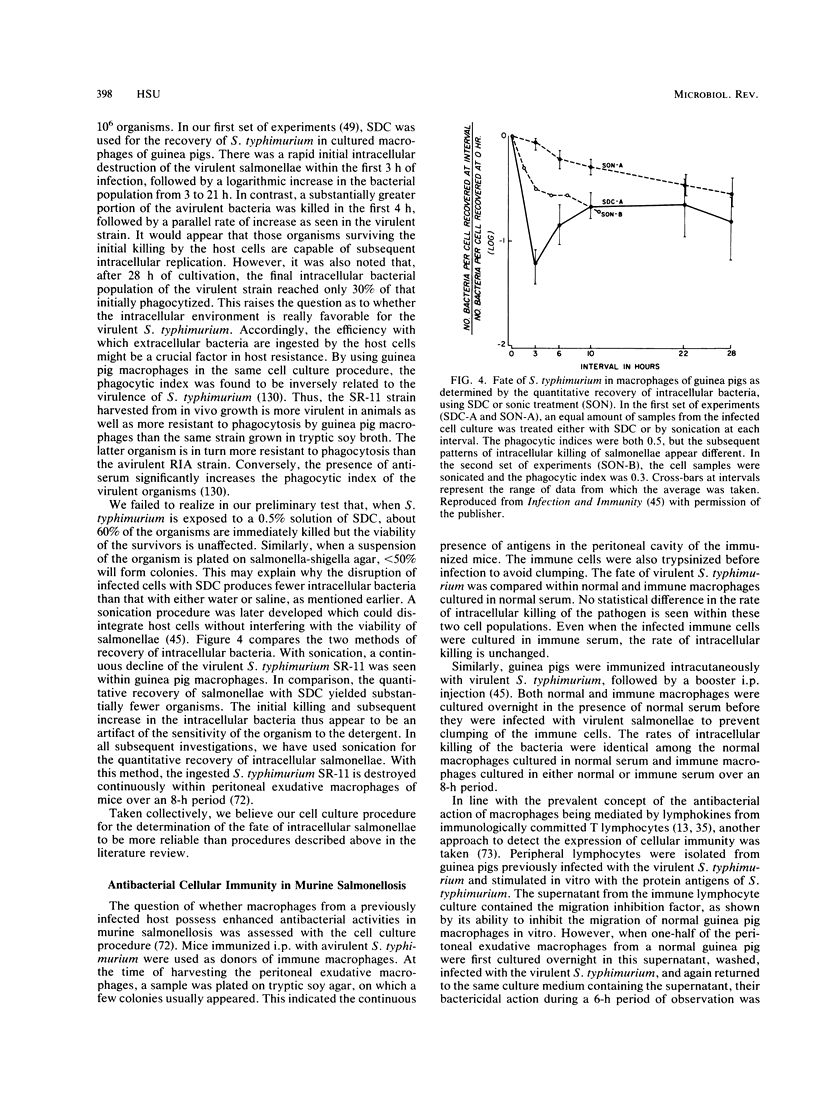
- Hsu H. S., Mayo D. R. Interactions between macrophages of guinea pigs and salmonellae. 3. Bactericidal action and cytophilic antibodies of macrophages of infected guinea pigs. Infect Immun. 1973 Aug;8(2):165–172. doi: 10.1128/iai.8.2.165-172.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu H. S., Miller K. B., Nakoneczna I. The role of delayed hypersensitivity in the enhancement of host resistance to infection. Can J Microbiol. 1980 Dec;26(12):1438–1442. doi: 10.1139/m80-239. [DOI] [PubMed] [Google Scholar]
- Hsu H. S., Nakoneczna I., Guo Y. Histopathological evidence for protective immunity induced by sonicated Salmonella vaccine. Can J Microbiol. 1985 Jan;31(1):54–61. doi: 10.1139/m85-012. [DOI] [PubMed] [Google Scholar]
- Hsu H. S., Piper V. M. Acquired resistance to and comparative virulence of Salmonella typhimurium demonstrated by cutaneous lesions in guinea pigs. J Reticuloendothel Soc. 1972 Apr;11(4):343–357. [PubMed] [Google Scholar]
- Hsu H. S., Radcliffe A. S. Interactions between macrophages of guinea pigs and Salmonellae. I. Fate of Salmonella typhimurium within macrophages of normal guinea pigs. J Bacteriol. 1968 Jul;96(1):191–197. doi: 10.1128/jb.96.1.191-197.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKIN C. R., ROWLEY D., AUZINS I. THE BASIS FOR IMMUNITY TO MOUSE TYPHOID. I. THE CARRIER STATE. Aust J Exp Biol Med Sci. 1964 Apr;42:215–228. doi: 10.1038/icb.1964.23. [DOI] [PubMed] [Google Scholar]
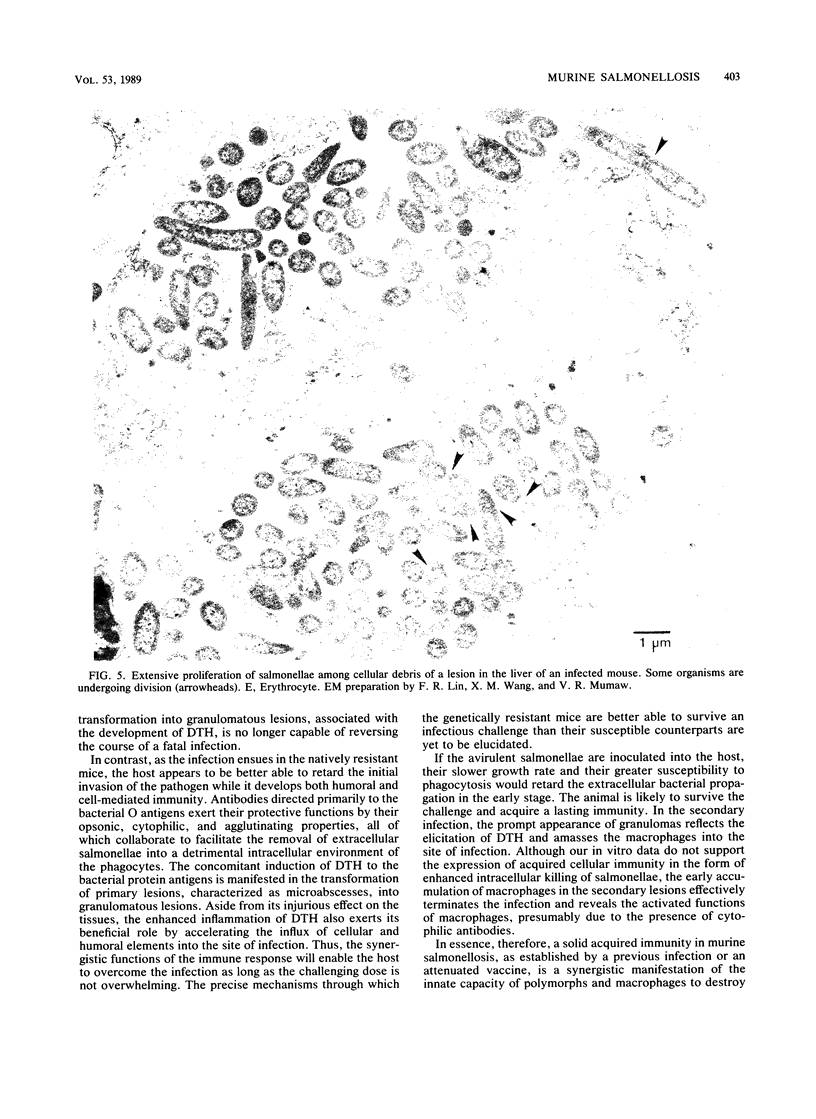
- JENKIN C. R., ROWLEY D. BASIS FOR IMMUNITY TO TYPHOID IN MICE AND THE QUESTION OF "CELLULAR IMMUNITY". Bacteriol Rev. 1963 Dec;27:391–404. doi: 10.1128/br.27.4.391-404.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jörbeck H. J., Svenson S. B., Lindberg A. A. Artificial Salmonella vaccines: Salmonella typhimurium O-antigen-specific oligosaccharide-protein conjugates elicit opsonizing antibodies that enhance phagocytosis. Infect Immun. 1981 May;32(2):497–502. doi: 10.1128/iai.32.2.497-502.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAPRAL F. A., SHAYEGANI M. G. Intracellular survival of staphylococci. J Exp Med. 1959 Jul 1;110(1):123–138. doi: 10.1084/jem.110.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killar L. M., Eisenstein T. K. Delayed-type hypersensitivity and immunity to Salmonella typhimurium. Infect Immun. 1986 May;52(2):504–508. doi: 10.1128/iai.52.2.504-508.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita E., Matsuda Y., Matsuda K., Kashiba S. Separate transfer of mouse protection and delayed-type hypersensitivity with Salmonella typhimurium transfer factor. Cell Immunol. 1984 Sep;87(2):528–537. doi: 10.1016/0008-8749(84)90021-2. [DOI] [PubMed] [Google Scholar]
- Kourany M., Kendrick P. L. Interaction between a human monocytic cell line and Salmonella typhosa. J Infect Dis. 1966 Oct;116(4):495–513. doi: 10.1093/infdis/116.4.495. [DOI] [PubMed] [Google Scholar]
- Krahenbuhl J. L., Remington J. S. In vitro induction of nonspecific resistance in macrophages by specifically sensitized lymphocytes. Infect Immun. 1971 Oct;4(4):337–343. doi: 10.1128/iai.4.4.337-343.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuusi N., Nurminen M., Saxen H., Valtonen M., Mäkelä P. H. Immunization with major outer membrane proteins in experimental salmonellosis of mice. Infect Immun. 1979 Sep;25(3):857–862. doi: 10.1128/iai.25.3.857-862.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang-Takasaki C. J., Saxén H., Mäkelä P. H., Leive L. Complement activation by polysaccharide of lipopolysaccharide: an important virulence determinant of salmonellae. Infect Immun. 1983 Aug;41(2):563–569. doi: 10.1128/iai.41.2.563-569.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F. R., Hsu H. S., Mumaw V. R., Moncure C. W. Confirmation of destruction of salmonellae within murine peritoneal exudate cells by immunocytochemical technique. Immunology. 1989 Jul;67(3):394–400. [PMC free article] [PubMed] [Google Scholar]
- Lin F. R., Hsu H. S., Mumaw V. R., Nakoneczna I. Intracellular destruction of salmonellae in genetically resistant mice. J Med Microbiol. 1989 Sep;30(1):79–87. doi: 10.1099/00222615-30-1-79. [DOI] [PubMed] [Google Scholar]
- Lin F. R., Wang X. M., Hsu H. S., Mumaw V. R., Nakoneczna I. Electron microscopic studies on the location of bacterial proliferation in the liver in murine salmonellosis. Br J Exp Pathol. 1987 Aug;68(4):539–550. [PMC free article] [PubMed] [Google Scholar]
- Lin J. H., Berry L. J. The use of strain LT2-Ml in identifying the protective antigens in a Salmonella typhimurium-derived ribosomal vaccine. J Reticuloendothel Soc. 1978 Feb;23(2):135–143. [PubMed] [Google Scholar]
- Lindberg A. A., Rosenberg L. T., Ljunggren A., Garegg P. J., Svensson S., Wallin N. H. Effect of synthetic disaccharide-protein conjugate as an immunogen in Salmonella infection in mice. Infect Immun. 1974 Sep;10(3):541–545. doi: 10.1128/iai.10.3.541-545.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissner C. R., Swanson R. N., O'Brien A. D. Genetic control of the innate resistance of mice to Salmonella typhimurium: expression of the Ity gene in peritoneal and splenic macrophages isolated in vitro. J Immunol. 1983 Dec;131(6):3006–3013. [PubMed] [Google Scholar]
- Lissner C. R., Weinstein D. L., O'Brien A. D. Mouse chromosome 1 Ity locus regulates microbicidal activity of isolated peritoneal macrophages against a diverse group of intracellular and extracellular bacteria. J Immunol. 1985 Jul;135(1):544–547. [PubMed] [Google Scholar]
- MITSUHASHI S., SATO I., TANAKA T. Experimental salmonellosis. Intracellular growth of Salmonella enteritidis ingested in mononuclear phagocytes of mice, and cellular basis of immunity. J Bacteriol. 1961 Jun;81:863–868. doi: 10.1128/jb.81.6.863-868.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORELLO J. A., BAKER E. E. INTERACTION OF SALMONELLA WITH PHAGOCYTES IN VITRO. J Infect Dis. 1965 Apr;115:131–141. doi: 10.1093/infdis/115.2.131. [DOI] [PubMed] [Google Scholar]
- Mackaness G. B., Blanden R. V. Cellular immunity. Prog Allergy. 1967;11:89–140. [PubMed] [Google Scholar]
- Mackaness G. B., Blanden R. V., Collins F. M. Host-parasite relations in mouse typhoid. J Exp Med. 1966 Oct 1;124(4):573–583. doi: 10.1084/jem.124.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. Resistance to intracellular infection. J Infect Dis. 1971 Apr;123(4):439–445. doi: 10.1093/infdis/123.4.439. [DOI] [PubMed] [Google Scholar]
- Mackaness G. B. The monocyte in cellular immunity. Semin Hematol. 1970 Apr;7(2):172–184. [PubMed] [Google Scholar]
- Marecki N. M., Hsu H. S., Mayo D. R. Cellular and humoral aspects of host resistance in murine salmonellosis. Br J Exp Pathol. 1975 Jun;56(3):231–243. [PMC free article] [PubMed] [Google Scholar]
- Mayo D. R., Hsu H. S., Lim F. Interactions between salmonellae and macrophages of guinea pigs. IV. Relationship between migration inhibition and antibacterial action of macrophages. Infect Immun. 1977 Oct;18(1):52–59. doi: 10.1128/iai.18.1.52-59.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misfeldt M. L., Johnson W. Identification of protective cell surface proteins in ribosomal fractions from Salmonella typhimurium. Infect Immun. 1979 Jun;24(3):808–816. doi: 10.1128/iai.24.3.808-816.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misfeldt M. L., Johnson W. Protective ability of Salmonella ribosomal protein and RNA in inbred mice. Infect Immun. 1978 Jul;21(1):286–291. doi: 10.1128/iai.21.1.286-291.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misfeldt M. L., Johnson W. Role of endotoxin contamination in ribiosomal vaccines prepared from Salmonella typhimurium. Infect Immun. 1977 Jul;17(1):98–104. doi: 10.1128/iai.17.1.98-104.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. A., Wray C., Sojka W. J. The effect of T and B lymphocyte depletion on the protection of mice vaccinated with a Gal E mutant of Salmonella typhimurium. Br J Exp Pathol. 1976 Jun;57(3):354–360. [PMC free article] [PubMed] [Google Scholar]
- Mäkelä P. H., Valtonen V. V., Valtonen M. Role of O-antigen (lipopolysaccharide) factors in the virulence of Salmonella. J Infect Dis. 1973 Jul;128(Suppl):81–85. doi: 10.1093/infdis/128.supplement_1.s81. [DOI] [PubMed] [Google Scholar]
- Nakoneczna I., Hsu H. S. Histopathological study of protective immunity against murine salmonellosis induced by killed vaccine. Infect Immun. 1983 Jan;39(1):423–430. doi: 10.1128/iai.39.1.423-430.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakoneczna I., Hsu H. S. The comparative histopathology of primary and secondary lesions in murine salmonellosis. Br J Exp Pathol. 1980 Feb;61(1):76–84. [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Karnovsky M. L., David J. R. Alterations of macrophage functions by mediators from lymphocytes. J Exp Med. 1971 Jun 1;133(6):1356–1376. doi: 10.1084/jem.133.6.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauciel C., Vilde F., Ronco E. Host response to infection with a temperature-sensitive mutant of Salmonella typhimurium in a susceptible and a resistant strain of mice. Infect Immun. 1985 Sep;49(3):523–527. doi: 10.1128/iai.49.3.523-527.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien A. D., Metcalf E. S. Control of early Salmonella typhimurium growth in innately Salmonella-resistant mice does not require functional T lymphocytes. J Immunol. 1982 Oct;129(4):1349–1351. [PubMed] [Google Scholar]
- O'Brien A. D., Metcalf E. S., Rosenstreich D. L. Defect in macrophage effector function confers Salmonella typhimurium susceptibility on C3H/HeJ mice. Cell Immunol. 1982 Mar 1;67(2):325–333. doi: 10.1016/0008-8749(82)90224-6. [DOI] [PubMed] [Google Scholar]
- O'Brien A. D., Rosenstreich D. L., Scher I., Campbell G. H., MacDermott R. P., Formal S. B. Genetic control of susceptibility to Salmonella typhimurium in mice: role of the LPS gene. J Immunol. 1980 Jan;124(1):20–24. [PubMed] [Google Scholar]
- O'Brien A. D., Scher I., Campbell G. H., MacDermott R. P., Formal S. B. Susceptibility of CBA/N mice to infection with Salmonella typhimurium: influence of the X-linked gene controlling B lymphocyte function. J Immunol. 1979 Aug;123(2):720–724. [PubMed] [Google Scholar]
- O'Brien A. D., Taylor B. A., Rosenstreich D. L. Genetic control of natural resistance to Salmonella typhimurium in mice during the late phase of infection. J Immunol. 1984 Dec;133(6):3313–3318. [PubMed] [Google Scholar]
- O'Brien A. D., Weinstein D. A., Soliman M. Y., Rosenstreich D. L. Additional evidence that the Lps gene locus regulates natural resistance to S. typhimurium in mice. J Immunol. 1985 May;134(5):2820–2823. [PubMed] [Google Scholar]
- Ornellas E. P., Roantree R. J., Steward J. P. The specificity and importance of humoral antibody in the protection of mice against intraperitoneal challenge with complement-sensitive and complement-resistant Salmonella. J Infect Dis. 1970 Feb;121(2):113–123. doi: 10.1093/infdis/121.2.113. [DOI] [PubMed] [Google Scholar]
- Patterson R. J., Youmans G. P. Demonstration in tissue culture of lymphocyte-mediated immunity to tuberculosis. Infect Immun. 1970 Jun;1(6):600–603. doi: 10.1128/iai.1.6.600-603.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson R. J., Youmans G. P. Multiplication of Mycobacterium tuberculosis Within Normal and "Immune" Mouse Macrophages Cultivated With and Without Streptomycin. Infect Immun. 1970 Jan;1(1):30–40. doi: 10.1128/iai.1.1.30-40.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul C., Shalala K., Warren R., Smith R. Adoptive transfer of murine host protection to salmonellosis with T-cell growth factor-dependent, Salmonella-specific T-cell lines. Infect Immun. 1985 Apr;48(1):40–43. doi: 10.1128/iai.48.1.40-43.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant J., Glynn A. A. Genetics of resistance to infection with Salmonella typhimurium in mice. J Infect Dis. 1976 Jan;133(1):72–78. doi: 10.1093/infdis/133.1.72. [DOI] [PubMed] [Google Scholar]
- Plant J., Glynn A. A. Locating salmonella resistance gene on mouse chromosome 1. Clin Exp Immunol. 1979 Jul;37(1):1–6. [PMC free article] [PubMed] [Google Scholar]
- ROWLEY D., TURNER K. J., JENKIN C. R. THE BASIS FOR IMMUNITY TO MOUSE TYPHOID. 3. CELL-BOUND ANTIBODY. Aust J Exp Biol Med Sci. 1964 Apr;42:237–248. doi: 10.1038/icb.1964.25. [DOI] [PubMed] [Google Scholar]
- ROWLEY D., WHITBY J. L. The bactericidal activity of mouse macrophages in vitro. Br J Exp Pathol. 1959 Oct;40:507–515. [PMC free article] [PubMed] [Google Scholar]
- Rhodes M. W., Hsu H. S. Effect of kanamycin on the fate of Salmonella enteritidis within cultured macrophages of guinea pigs. J Reticuloendothel Soc. 1974 Jan;15(1):1–12. [PubMed] [Google Scholar]
- Roantree R. J. Salmonella O antigens and virulence. Annu Rev Microbiol. 1967;21:443–466. doi: 10.1146/annurev.mi.21.100167.002303. [DOI] [PubMed] [Google Scholar]
- Robson H. G., Vas S. I. Resistance of inbred mice to Salmonella typhimurium. J Infect Dis. 1972 Oct;126(4):378–386. doi: 10.1093/infdis/126.4.378. [DOI] [PubMed] [Google Scholar]
- Ruitenberg E. J., Guinée P. A., Kruyt B. C., Berkvens J. M. Salmonella pathogenesis in germ-free mice. A bacteriological and histological study. Br J Exp Pathol. 1971 Apr;52(2):192–197. [PMC free article] [PubMed] [Google Scholar]
- SATO I., TANAKA T., SAITO K., MITSUHASHI S. Inhibition of Salmonella enteritidis ingested in mononuclear phagocytes from liver and subcutaneous tissue of mice immunized with live vaccine. J Bacteriol. 1962 Jun;83:1306–1312. doi: 10.1128/jb.83.6.1306-1312.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUTER E. Interaction between phagocytes and pathogenic microorganisms. Bacteriol Rev. 1956 Jun;20(2):94–132. doi: 10.1128/br.20.2.94-132.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUTER E., RAMSEIAR H. CELLULAR REACTIONS IN INFECTION. Adv Immunol. 1964;27:117–173. doi: 10.1016/s0065-2776(08)60707-5. [DOI] [PubMed] [Google Scholar]
- Saxen H., Mäkelä O. The protective capacity of immune sera in experimental mouse salmonellosis is mainly due to IgM antibodies. Immunol Lett. 1982 Nov;5(5):267–272. doi: 10.1016/0165-2478(82)90110-9. [DOI] [PubMed] [Google Scholar]
- Saxén H. Mechanism of the protective action of anti-Salmonella IgM in experimental mouse salmonellosis. J Gen Microbiol. 1984 Sep;130(9):2277–2283. doi: 10.1099/00221287-130-9-2277. [DOI] [PubMed] [Google Scholar]
- Saxén H., Reima I., Mäkelä P. H. Alternative complement pathway activation by Salmonella O polysaccharide as a virulence determinant in the mouse. Microb Pathog. 1987 Jan;2(1):15–28. doi: 10.1016/0882-4010(87)90111-2. [DOI] [PubMed] [Google Scholar]
- Simon H. B., Sheagren J. N. Cellular immunity in vitro. I. Immunologically mediated enhancement of macrophage bactericidal capacity. J Exp Med. 1971 Jun 1;133(6):1377–1389. doi: 10.1084/jem.133.6.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. A., Bigley N. J. Detection of delayed hypersensitivity in mice injected with ribonucleic acid-protein fractions of Salmonella typhimurium. Infect Immun. 1972 Sep;6(3):384–389. doi: 10.1128/iai.6.3.384-389.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. A., Bigley N. J. Ribonucleic acid-protein fractions of virulent Salmonella typhimurium as protective immunogens. Infect Immun. 1972 Sep;6(3):377–383. doi: 10.1128/iai.6.3.377-383.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenson S. B., Lindberg A. A. Artificial Salmonella vaccines: Salmonella typhimurium O-antigen-specific oligosaccharide-protein conjugates elicit protective antibodies in rabbits and mice. Infect Immun. 1981 May;32(2):490–496. doi: 10.1128/iai.32.2.490-496.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenson S. B., Nurminen M., Lindberg A. A. Artificial Salmonella vaccines: O-antigenic oligosaccharide-protein conjugates induce protection against infection with Salmonella typhimurium. Infect Immun. 1979 Sep;25(3):863–872. doi: 10.1128/iai.25.3.863-872.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson R. N., O'Brien A. D. Genetic control of the innate resistance of mice to Salmonella typhimurium: Ity gene is expressed in vivo by 24 hours after infection. J Immunol. 1983 Dec;131(6):3014–3020. [PubMed] [Google Scholar]
- TURNER K. J., JENKIN C. R., ROWLEY D. THE BASIS FOR IMMUNITY TO MOUSE TYPHOID. 2. ANTIBODY FORMATION DURING THE CARRIER STATE. Aust J Exp Biol Med Sci. 1964 Apr;42:229–236. doi: 10.1038/icb.1964.24. [DOI] [PubMed] [Google Scholar]
- Valtonen M. V., Häyry P. O antigen as virulence factor in mouse typhoid: effect of B-cell suppression. Infect Immun. 1978 Jan;19(1):26–28. doi: 10.1128/iai.19.1.26-28.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valtonen M. V. Role of phagocytosis in mouse virulence of Salmonella typhimurium recombinants with O antigen 6,7 or 4,12. Infect Immun. 1977 Dec;18(3):574–582. doi: 10.1128/iai.18.3.574-582.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valtonen V. V. Mouse virulence of Salmonella strains: the effect of different smooth-type O side-chains. J Gen Microbiol. 1970 Dec;64(3):255–268. doi: 10.1099/00221287-64-3-255. [DOI] [PubMed] [Google Scholar]
- Venneman M. R., Berry L. J. Experimental salmonellosis: differential passive transfer of immunity with serum and cells obtained from ribosomal and ribonucleic acid-immunized mice. J Reticuloendothel Soc. 1971 May;9(5):491–502. [PubMed] [Google Scholar]
- Venneman M. R. Purification of immunogenically active ribonucleic acid preparations of Salmonella typhimurium: molecular-sieve and anion-exchange chromatography. Infect Immun. 1972 Mar;5(3):269–282. doi: 10.1128/iai.5.3.269-282.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITBY J. L., ROWLEY D. The role of macrophages in the elimination of bacteria from the mouse peritoneum. Br J Exp Pathol. 1959 Aug;40:358–370. [PMC free article] [PubMed] [Google Scholar]
- Wang X. M., Lin F. R., Hsu H. S., Mumaw V. R., Nakoneczna I. Electronmicroscopic studies on the location of salmonella proliferation in the murine spleen. J Med Microbiol. 1988 Jan;25(1):41–47. doi: 10.1099/00222615-25-1-41. [DOI] [PubMed] [Google Scholar]
- Wells P. S., Hsu H. S. Interactions Between Macrophages of Guinea Pigs and Salmonellae II. Phagocytosis of Salmonella typhimurium by Macrophages of Normal Guinea Pigs. Infect Immun. 1970 Aug;2(2):145–149. doi: 10.1128/iai.2.2.145-149.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youmans G. P. Relation between delayed hypersensitivity and immunity in tuberculosis. Am Rev Respir Dis. 1975 Feb;111(2):109–118. doi: 10.1164/arrd.1975.111.2.109. [DOI] [PubMed] [Google Scholar]
- Zwet T. L., Thompson J., Furth R. Effect of glucocorticosteroids on the phagocytosis and intracellular killing by peritoneal macrophages. Infect Immun. 1975 Oct;12(4):699–705. doi: 10.1128/iai.12.4.699-705.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dissel J. T., Leijh P. C., van Furth R. Differences in initial rate of intracellular killing of Salmonella typhimurium by resident peritoneal macrophages from various mouse strains. J Immunol. 1985 May;134(5):3404–3410. [PubMed] [Google Scholar]
- van Dissel J. T., Stikkelbroeck J. J., Michel B. C., Leijh P. C., van Furth R. Salmonella-typhimurium-specific difference in rate of intracellular killing by resident peritoneal macrophages from salmonella-resistant CBA and salmonella-susceptible C57BL/10 mice. J Immunol. 1987 Jun 15;138(12):4428–4434. [PubMed] [Google Scholar]
- van Dissel J. T., Stikkelbroeck J. J., Sluiter W., Leijh P. C., van Furth R. Differences in initial rate of intracellular killing of Salmonella typhimurium by granulocytes of Salmonella-susceptible C57BL/10 mice and Salmonella-resistant CBA mice. J Immunol. 1986 Feb 1;136(3):1074–1080. [PubMed] [Google Scholar]