Abstract
Many viruses express their genome, or part of their genome, initially as a polyprotein precursor that undergoes proteolytic processing. Molecular genetic analyses of viral gene expression have revealed that many of these processing events are mediated by virus-encoded proteinases. Biochemical activity studies and structural analyses of these viral enzymes reveal that they have remarkable similarities to cellular proteinases. However, the viral proteinases have evolved unique features that permit them to function in a cellular environment. In this article, the current status of plant and animal virus proteinases is described along with their role in the viral replication cycle. The reactions catalyzed by viral proteinases are not simple enzyme-substrate interactions; rather, the processing steps are highly regulated, are coordinated with other viral processes, and frequently involve the participation of other factors.
Full text
PDF


































Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akusjärvi G., Zabielski J., Perricaudet M., Pettersson U. The sequence of the 3' non-coding region of the hexon mRNA discloses a novel adenovirus gene. Nucleic Acids Res. 1981 Jan 10;9(1):1–17. doi: 10.1093/nar/9.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliperti G., Schlesinger M. J. Evidence for an autoprotease activity of sindbis virus capsid protein. Virology. 1978 Oct 15;90(2):366–369. doi: 10.1016/0042-6822(78)90321-5. [DOI] [PubMed] [Google Scholar]
- Alvey J. C., Wyckoff E. E., Yu S. F., Lloyd R., Ehrenfeld E. cis- and trans-cleavage activities of poliovirus 2A protease expressed in Escherichia coli. J Virol. 1991 Nov;65(11):6077–6083. doi: 10.1128/jvi.65.11.6077-6083.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson C. W. Expression and purification of the adenovirus proteinase polypeptide and of a synthetic proteinase substrate. Protein Expr Purif. 1993 Feb;4(1):8–15. doi: 10.1006/prep.1993.1002. [DOI] [PubMed] [Google Scholar]
- Anderson C. W. The proteinase polypeptide of adenovirus serotype 2 virions. Virology. 1990 Jul;177(1):259–272. doi: 10.1016/0042-6822(90)90479-b. [DOI] [PubMed] [Google Scholar]
- Andino R., Rieckhof G. E., Baltimore D. A functional ribonucleoprotein complex forms around the 5' end of poliovirus RNA. Cell. 1990 Oct 19;63(2):369–380. doi: 10.1016/0092-8674(90)90170-j. [DOI] [PubMed] [Google Scholar]
- Andreánsky M., Hrusková-Heidingsfeldová O., Sedlácek J., Konvalinka J., Bláha I., Jecmen P., Horejsí M., Strop P., Fábry M. High-level expression of enzymatically active bovine leukemia virus proteinase in E. coli. FEBS Lett. 1991 Aug 5;287(1-2):129–132. doi: 10.1016/0014-5793(91)80032-x. [DOI] [PubMed] [Google Scholar]
- Ansardi D. C., Porter D. C., Morrow C. D. Coinfection with recombinant vaccinia viruses expressing poliovirus P1 and P3 proteins results in polyprotein processing and formation of empty capsid structures. J Virol. 1991 Apr;65(4):2088–2092. doi: 10.1128/jvi.65.4.2088-2092.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argos P., Kamer G., Nicklin M. J., Wimmer E. Similarity in gene organization and homology between proteins of animal picornaviruses and a plant comovirus suggest common ancestry of these virus families. Nucleic Acids Res. 1984 Sep 25;12(18):7251–7267. doi: 10.1093/nar/12.18.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschauer B., Werner G., McCray J., Rosenwirth B., Bachmayer H. Biologically active protease 3C of human rhinovirus 1A is expressed from a cloned cDNA segment in Escherichia coli. Virology. 1991 Oct;184(2):587–594. doi: 10.1016/0042-6822(91)90429-f. [DOI] [PubMed] [Google Scholar]
- Ashorn P., McQuade T. J., Thaisrivongs S., Tomasselli A. G., Tarpley W. G., Moss B. An inhibitor of the protease blocks maturation of human and simian immunodeficiency viruses and spread of infection. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7472–7476. doi: 10.1073/pnas.87.19.7472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahner I., Lamb J., Mayo M. A., Hay R. T. Expression of the genome of potato leafroll virus: readthrough of the coat protein termination codon in vivo. J Gen Virol. 1990 Oct;71(Pt 10):2251–2256. doi: 10.1099/0022-1317-71-10-2251. [DOI] [PubMed] [Google Scholar]
- Batson S., Rundell K. Proteolysis at the 2A/2B junction in Theiler's murine encephalomyelitis virus. Virology. 1991 Apr;181(2):764–767. doi: 10.1016/0042-6822(91)90914-w. [DOI] [PubMed] [Google Scholar]
- Baum E. Z., Bebernitz G. A., Gluzman Y. Isolation of mutants of human immunodeficiency virus protease based on the toxicity of the enzyme in Escherichia coli. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5573–5577. doi: 10.1073/pnas.87.14.5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum E. Z., Bebernitz G. A., Palant O., Mueller T., Plotch S. J. Purification, properties, and mutagenesis of poliovirus 3C protease. Virology. 1991 Nov;185(1):140–150. doi: 10.1016/0042-6822(91)90762-z. [DOI] [PubMed] [Google Scholar]
- Bayliss C. D., Spies U., Shaw K., Peters R. W., Papageorgiou A., Müller H., Boursnell M. E. A comparison of the sequences of segment A of four infectious bursal disease virus strains and identification of a variable region in VP2. J Gen Virol. 1990 Jun;71(Pt 6):1303–1312. doi: 10.1099/0022-1317-71-6-1303. [DOI] [PubMed] [Google Scholar]
- Bazan J. F., Fletterick R. J. Detection of a trypsin-like serine protease domain in flaviviruses and pestiviruses. Virology. 1989 Aug;171(2):637–639. doi: 10.1016/0042-6822(89)90639-9. [DOI] [PubMed] [Google Scholar]
- Bazan J. F., Fletterick R. J. Viral cysteine proteases are homologous to the trypsin-like family of serine proteases: structural and functional implications. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7872–7876. doi: 10.1073/pnas.85.21.7872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont A., Le Moual H., Boileau G., Crine P., Roques B. P. Evidence that both arginine 102 and arginine 747 are involved in substrate binding to neutral endopeptidase (EC 3.4.24.11). J Biol Chem. 1991 Jan 5;266(1):214–220. [PubMed] [Google Scholar]
- Becker A. B., Roth R. A. An unusual active site identified in a family of zinc metalloendopeptidases. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3835–3839. doi: 10.1073/pnas.89.9.3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsham G. J., Brangwyn J. K., Ryan M. D., Abrams C. C., King A. M. Intracellular expression and processing of foot-and-mouth disease virus capsid precursors using vaccinia virus vectors: influence of the L protease. Virology. 1990 Jun;176(2):524–530. doi: 10.1016/0042-6822(90)90022-j. [DOI] [PubMed] [Google Scholar]
- Bernstein H. D., Sonenberg N., Baltimore D. Poliovirus mutant that does not selectively inhibit host cell protein synthesis. Mol Cell Biol. 1985 Nov;5(11):2913–2923. doi: 10.1128/mcb.5.11.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatti A. R., Weber J. Protease of adenovirus type 2. In vitro processing of core protein. Biochem Biophys Res Commun. 1978 Apr 14;81(3):973–979. doi: 10.1016/0006-291x(78)91446-8. [DOI] [PubMed] [Google Scholar]
- Bhatti A. R., Weber J. Protease of adenovirus type 2: partial characterization. Virology. 1979 Jul 30;96(2):478–485. doi: 10.1016/0042-6822(79)90105-3. [DOI] [PubMed] [Google Scholar]
- Billich S., Knoop M. T., Hansen J., Strop P., Sedlacek J., Mertz R., Moelling K. Synthetic peptides as substrates and inhibitors of human immune deficiency virus-1 protease. J Biol Chem. 1988 Dec 5;263(34):17905–17908. [PubMed] [Google Scholar]
- Bizub D., Weber I. T., Cameron C. E., Leis J. P., Skalka A. M. A range of catalytic efficiencies with avian retroviral protease subunits genetically linked to form single polypeptide chains. J Biol Chem. 1991 Mar 15;266(8):4951–4958. [PubMed] [Google Scholar]
- Björk I., Ylinenjärvi K., Olson S. T., Bock P. E. Conversion of antithrombin from an inhibitor of thrombin to a substrate with reduced heparin affinity and enhanced conformational stability by binding of a tetradecapeptide corresponding to the P1 to P14 region of the putative reactive bond loop of the inhibitor. J Biol Chem. 1992 Jan 25;267(3):1976–1982. [PubMed] [Google Scholar]
- Blair W. S., Semler B. L. Role for the P4 amino acid residue in substrate utilization by the poliovirus 3CD proteinase. J Virol. 1991 Nov;65(11):6111–6123. doi: 10.1128/jvi.65.11.6111-6123.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell T. L., Lapatto R., Wilderspin A. F., Hemmings A. M., Hobart P. M., Danley D. E., Whittle P. J. The 3-D structure of HIV-1 proteinase and the design of antiviral agents for the treatment of AIDS. Trends Biochem Sci. 1990 Nov;15(11):425–430. doi: 10.1016/0968-0004(90)90280-o. [DOI] [PubMed] [Google Scholar]
- Boege U., Wengler G., Wengler G., Wittmann-Liebold B. Primary structures of the core proteins of the alphaviruses Semliki Forest virus and Sindbis virus. Virology. 1981 Aug;113(1):293–303. doi: 10.1016/0042-6822(81)90156-2. [DOI] [PubMed] [Google Scholar]
- Bond J. S., Butler P. E. Intracellular proteases. Annu Rev Biochem. 1987;56:333–364. doi: 10.1146/annurev.bi.56.070187.002001. [DOI] [PubMed] [Google Scholar]
- Bone R., Silen J. L., Agard D. A. Structural plasticity broadens the specificity of an engineered protease. Nature. 1989 May 18;339(6221):191–195. doi: 10.1038/339191a0. [DOI] [PubMed] [Google Scholar]
- Bonneau A. M., Sonenberg N. Proteolysis of the p220 component of the cap-binding protein complex is not sufficient for complete inhibition of host cell protein synthesis after poliovirus infection. J Virol. 1987 Apr;61(4):986–991. doi: 10.1128/jvi.61.4.986-991.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boursnell M. E., Brown T. D., Foulds I. J., Green P. F., Tomley F. M., Binns M. M. Completion of the sequence of the genome of the coronavirus avian infectious bronchitis virus. J Gen Virol. 1987 Jan;68(Pt 1):57–77. doi: 10.1099/0022-1317-68-1-57. [DOI] [PubMed] [Google Scholar]
- Bozarth C. S., Weiland J. J., Dreher T. W. Expression of ORF-69 of turnip yellow mosaic virus is necessary for viral spread in plants. Virology. 1992 Mar;187(1):124–130. doi: 10.1016/0042-6822(92)90301-5. [DOI] [PubMed] [Google Scholar]
- Bransom K. L., Weiland J. J., Dreher T. W. Proteolytic maturation of the 206-kDa nonstructural protein encoded by turnip yellow mosaic virus RNA. Virology. 1991 Sep;184(1):351–358. doi: 10.1016/0042-6822(91)90851-2. [DOI] [PubMed] [Google Scholar]
- Brenner S. The molecular evolution of genes and proteins: a tale of two serines. Nature. 1988 Aug 11;334(6182):528–530. doi: 10.1038/334528a0. [DOI] [PubMed] [Google Scholar]
- Burley S. K., David P. R., Lipscomb W. N. Leucine aminopeptidase: bestatin inhibition and a model for enzyme-catalyzed peptide hydrolysis. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):6916–6920. doi: 10.1073/pnas.88.16.6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burley S. K., David P. R., Taylor A., Lipscomb W. N. Molecular structure of leucine aminopeptidase at 2.7-A resolution. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6878–6882. doi: 10.1073/pnas.87.17.6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein H., Bizub D., Skalka A. M. Assembly and processing of avian retroviral gag polyproteins containing linked protease dimers. J Virol. 1991 Nov;65(11):6165–6172. doi: 10.1128/jvi.65.11.6165-6172.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert J. G., Nagy E., Soler M., Dobos P. Characterization of the VPg-dsRNA linkage of infectious pancreatic necrosis virus. J Gen Virol. 1991 Oct;72(Pt 10):2563–2567. doi: 10.1099/0022-1317-72-10-2563. [DOI] [PubMed] [Google Scholar]
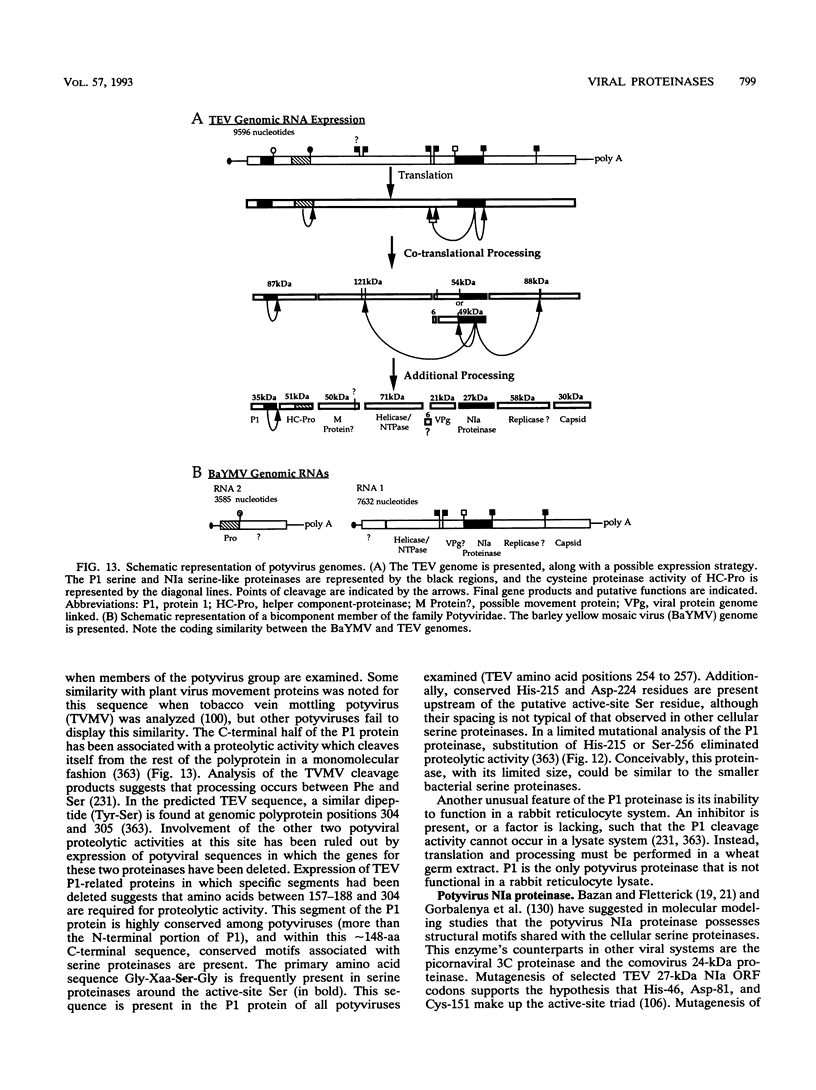
- Carrington J. C., Cary S. M., Dougherty W. G. Mutational analysis of tobacco etch virus polyprotein processing: cis and trans proteolytic activities of polyproteins containing the 49-kilodalton proteinase. J Virol. 1988 Jul;62(7):2313–2320. doi: 10.1128/jvi.62.7.2313-2320.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington J. C., Cary S. M., Parks T. D., Dougherty W. G. A second proteinase encoded by a plant potyvirus genome. EMBO J. 1989 Feb;8(2):365–370. doi: 10.1002/j.1460-2075.1989.tb03386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington J. C., Dougherty W. G. A viral cleavage site cassette: identification of amino acid sequences required for tobacco etch virus polyprotein processing. Proc Natl Acad Sci U S A. 1988 May;85(10):3391–3395. doi: 10.1073/pnas.85.10.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington J. C., Dougherty W. G. Small nuclear inclusion protein encoded by a plant potyvirus genome is a protease. J Virol. 1987 Aug;61(8):2540–2548. doi: 10.1128/jvi.61.8.2540-2548.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington J. C., Freed D. D., Leinicke A. J. Bipartite signal sequence mediates nuclear translocation of the plant potyviral NIa protein. Plant Cell. 1991 Sep;3(9):953–962. doi: 10.1105/tpc.3.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington J. C., Freed D. D., Oh C. S. Expression of potyviral polyproteins in transgenic plants reveals three proteolytic activities required for complete processing. EMBO J. 1990 May;9(5):1347–1353. doi: 10.1002/j.1460-2075.1990.tb08249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington J. C., Freed D. D., Sanders T. C. Autocatalytic processing of the potyvirus helper component proteinase in Escherichia coli and in vitro. J Virol. 1989 Oct;63(10):4459–4463. doi: 10.1128/jvi.63.10.4459-4463.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington J. C., Herndon K. L. Characterization of the potyviral HC-pro autoproteolytic cleavage site. Virology. 1992 Mar;187(1):308–315. doi: 10.1016/0042-6822(92)90319-K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle E., Nowak T., Leidner U., Wengler G., Wengler G. Sequence analysis of the viral core protein and the membrane-associated proteins V1 and NV2 of the flavivirus West Nile virus and of the genome sequence for these proteins. Virology. 1985 Sep;145(2):227–236. doi: 10.1016/0042-6822(85)90156-4. [DOI] [PubMed] [Google Scholar]
- Chambers T. J., Grakoui A., Rice C. M. Processing of the yellow fever virus nonstructural polyprotein: a catalytically active NS3 proteinase domain and NS2B are required for cleavages at dibasic sites. J Virol. 1991 Nov;65(11):6042–6050. doi: 10.1128/jvi.65.11.6042-6050.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers T. J., Hahn C. S., Galler R., Rice C. M. Flavivirus genome organization, expression, and replication. Annu Rev Microbiol. 1990;44:649–688. doi: 10.1146/annurev.mi.44.100190.003245. [DOI] [PubMed] [Google Scholar]
- Chambers T. J., McCourt D. W., Rice C. M. Production of yellow fever virus proteins in infected cells: identification of discrete polyprotein species and analysis of cleavage kinetics using region-specific polyclonal antisera. Virology. 1990 Jul;177(1):159–174. doi: 10.1016/0042-6822(90)90470-c. [DOI] [PubMed] [Google Scholar]
- Chambers T. J., Weir R. C., Grakoui A., McCourt D. W., Bazan J. F., Fletterick R. J., Rice C. M. Evidence that the N-terminal domain of nonstructural protein NS3 from yellow fever virus is a serine protease responsible for site-specific cleavages in the viral polyprotein. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8898–8902. doi: 10.1073/pnas.87.22.8898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee P. K., Flint S. J. Adenovirus type 2 endopeptidase: an unusual phosphoprotein enzyme matured by autocatalysis. Proc Natl Acad Sci U S A. 1987 Feb;84(3):714–718. doi: 10.1073/pnas.84.3.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheah K. C., Leong L. E., Porter A. G. Site-directed mutagenesis suggests close functional relationship between a human rhinovirus 3C cysteine protease and cellular trypsin-like serine proteases. J Biol Chem. 1990 May 5;265(13):7180–7187. [PubMed] [Google Scholar]
- Cheng Y. S., Yin F. H., Foundling S., Blomstrom D., Kettner C. A. Stability and activity of human immunodeficiency virus protease: comparison of the natural dimer with a homologous, single-chain tethered dimer. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9660–9664. doi: 10.1073/pnas.87.24.9660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi G. H., Pawlyk D. M., Nuss D. L. The autocatalytic protease p29 encoded by a hypovirulence-associated virus of the chestnut blight fungus resembles the potyvirus-encoded protease HC-Pro. Virology. 1991 Aug;183(2):747–752. doi: 10.1016/0042-6822(91)91004-z. [DOI] [PubMed] [Google Scholar]
- Choi G. H., Shapira R., Nuss D. L. Cotranslational autoproteolysis involved in gene expression from a double-stranded RNA genetic element associated with hypovirulence of the chestnut blight fungus. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1167–1171. doi: 10.1073/pnas.88.4.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H. K., Tong L., Minor W., Dumas P., Boege U., Rossmann M. G., Wengler G. Structure of Sindbis virus core protein reveals a chymotrypsin-like serine proteinase and the organization of the virion. Nature. 1991 Nov 7;354(6348):37–43. doi: 10.1038/354037a0. [DOI] [PubMed] [Google Scholar]
- Choo Q. L., Richman K. H., Han J. H., Berger K., Lee C., Dong C., Gallegos C., Coit D., Medina-Selby R., Barr P. J. Genetic organization and diversity of the hepatitis C virus. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2451–2455. doi: 10.1073/pnas.88.6.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church F. C., Phillips J. E., Woods J. L. Chimeric antithrombin peptide. Characterization of an Arg-Gly-Asp (RGD)- and hirudin carboxyl terminus-containing synthetic peptides. J Biol Chem. 1991 Jun 25;266(18):11975–11979. [PubMed] [Google Scholar]
- Clark M. E., Dasgupta A. A transcriptionally active form of TFIIIC is modified in poliovirus-infected HeLa cells. Mol Cell Biol. 1990 Oct;10(10):5106–5113. doi: 10.1128/mcb.10.10.5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark M. E., Hämmerle T., Wimmer E., Dasgupta A. Poliovirus proteinase 3C converts an active form of transcription factor IIIC to an inactive form: a mechanism for inhibition of host cell polymerase III transcription by poliovirus. EMBO J. 1991 Oct;10(10):2941–2947. doi: 10.1002/j.1460-2075.1991.tb07844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke B. E., Sangar D. V. Processing and assembly of foot-and-mouth disease virus proteins using subgenomic RNA. J Gen Virol. 1988 Sep;69(Pt 9):2313–2325. doi: 10.1099/0022-1317-69-9-2313. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Anderson D. K., Retzel E. Comparisons of the pestivirus bovine viral diarrhoea virus with members of the flaviviridae. J Gen Virol. 1988 Oct;69(Pt 10):2637–2643. doi: 10.1099/0022-1317-69-10-2637. [DOI] [PubMed] [Google Scholar]
- Collett M. S. Molecular genetics of pestiviruses. Comp Immunol Microbiol Infect Dis. 1992 Jul;15(3):145–154. doi: 10.1016/0147-9571(92)90087-8. [DOI] [PubMed] [Google Scholar]
- Copeland T. D., Oroszlan S. Genetic locus, primary structure, and chemical synthesis of human immunodeficiency virus protease. Gene Anal Tech. 1988 Nov-Dec;5(6):109–115. doi: 10.1016/0735-0651(88)90010-6. [DOI] [PubMed] [Google Scholar]
- Cordingley M. G., Callahan P. L., Sardana V. V., Garsky V. M., Colonno R. J. Substrate requirements of human rhinovirus 3C protease for peptide cleavage in vitro. J Biol Chem. 1990 Jun 5;265(16):9062–9065. [PubMed] [Google Scholar]
- Cordingley M. G., Register R. B., Callahan P. L., Garsky V. M., Colonno R. J. Cleavage of small peptides in vitro by human rhinovirus 14 3C protease expressed in Escherichia coli. J Virol. 1989 Dec;63(12):5037–5045. doi: 10.1128/jvi.63.12.5037-5045.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik C. S., Largman C., Fletcher T., Roczniak S., Barr P. J., Fletterick R., Rutter W. J. Redesigning trypsin: alteration of substrate specificity. Science. 1985 Apr 19;228(4697):291–297. doi: 10.1126/science.3838593. [DOI] [PubMed] [Google Scholar]
- Craik C. S., Roczniak S., Largman C., Rutter W. J. The catalytic role of the active site aspartic acid in serine proteases. Science. 1987 Aug 21;237(4817):909–913. doi: 10.1126/science.3303334. [DOI] [PubMed] [Google Scholar]
- Craik C. S., Roczniak S., Sprang S., Fletterick R., Rutter W. Redesigning trypsin via genetic engineering. J Cell Biochem. 1987 Mar;33(3):199–211. doi: 10.1002/jcb.240330307. [DOI] [PubMed] [Google Scholar]
- Crawford N., Fire A., Samuels M., Sharp P. A., Baltimore D. Inhibition of transcription factor activity by poliovirus. Cell. 1981 Dec;27(3 Pt 2):555–561. doi: 10.1016/0092-8674(81)90397-4. [DOI] [PubMed] [Google Scholar]
- Darke P. L., Nutt R. F., Brady S. F., Garsky V. M., Ciccarone T. M., Leu C. T., Lumma P. K., Freidinger R. M., Veber D. F., Sigal I. S. HIV-1 protease specificity of peptide cleavage is sufficient for processing of gag and pol polyproteins. Biochem Biophys Res Commun. 1988 Oct 14;156(1):297–303. doi: 10.1016/s0006-291x(88)80839-8. [DOI] [PubMed] [Google Scholar]
- Dasmahapatra B., DiDomenico B., Dwyer S., Ma J., Sadowski I., Schwartz J. A genetic system for studying the activity of a proteolytic enzyme. Proc Natl Acad Sci U S A. 1992 May 1;89(9):4159–4162. doi: 10.1073/pnas.89.9.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debouck C., Gorniak J. G., Strickler J. E., Meek T. D., Metcalf B. W., Rosenberg M. Human immunodeficiency virus protease expressed in Escherichia coli exhibits autoprocessing and specific maturation of the gag precursor. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8903–8906. doi: 10.1073/pnas.84.24.8903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demangeat G., Greif C., Hemmer O., Fritsch C. Analysis of the in vitro cleavage products of the tomato black ring virus RNA-1-encoded 250K polyprotein. J Gen Virol. 1990 Aug;71(Pt 8):1649–1654. doi: 10.1099/0022-1317-71-8-1649. [DOI] [PubMed] [Google Scholar]
- DesJarlais R. L., Seibel G. L., Kuntz I. D., Furth P. S., Alvarez J. C., Ortiz de Montellano P. R., DeCamp D. L., Babé L. M., Craik C. S. Structure-based design of nonpeptide inhibitors specific for the human immunodeficiency virus 1 protease. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6644–6648. doi: 10.1073/pnas.87.17.6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessens J. T., Lomonossoff G. P. Mutational analysis of the putative catalytic triad of the cowpea mosaic virus 24K protease. Virology. 1991 Oct;184(2):738–746. doi: 10.1016/0042-6822(91)90444-g. [DOI] [PubMed] [Google Scholar]
- Devaney M. A., Vakharia V. N., Lloyd R. E., Ehrenfeld E., Grubman M. J. Leader protein of foot-and-mouth disease virus is required for cleavage of the p220 component of the cap-binding protein complex. J Virol. 1988 Nov;62(11):4407–4409. doi: 10.1128/jvi.62.11.4407-4409.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewalt P. G., Blair W. S., Semler B. L. A genetic locus in mutant poliovirus genomes involved in overproduction of RNA polymerase and 3C proteinase. Virology. 1990 Feb;174(2):504–514. doi: 10.1016/0042-6822(90)90104-y. [DOI] [PubMed] [Google Scholar]
- Dewalt P. G., Lawson M. A., Colonno R. J., Semler B. L. Chimeric picornavirus polyproteins demonstrate a common 3C proteinase substrate specificity. J Virol. 1989 Aug;63(8):3444–3452. doi: 10.1128/jvi.63.8.3444-3452.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewalt P. G., Semler B. L. Site-directed mutagenesis of proteinase 3C results in a poliovirus deficient in synthesis of viral RNA polymerase. J Virol. 1987 Jul;61(7):2162–2170. doi: 10.1128/jvi.61.7.2162-2170.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanaraj V., Dealwis C. G., Frazao C., Badasso M., Sibanda B. L., Tickle I. J., Cooper J. B., Driessen H. P., Newman M., Aguilar C. X-ray analyses of peptide-inhibitor complexes define the structural basis of specificity for human and mouse renins. Nature. 1992 Jun 11;357(6378):466–472. doi: 10.1038/357466a0. [DOI] [PubMed] [Google Scholar]
- DiIanni C. L., Davis L. J., Holloway M. K., Herber W. K., Darke P. L., Kohl N. E., Dixon R. A. Characterization of an active single polypeptide form of the human immunodeficiency virus type 1 protease. J Biol Chem. 1990 Oct 5;265(28):17348–17354. [PubMed] [Google Scholar]
- Ding M. X., Schlesinger M. J. Evidence that Sindbis virus NSP2 is an autoprotease which processes the virus nonstructural polyprotein. Virology. 1989 Jul;171(1):280–284. doi: 10.1016/0042-6822(89)90539-4. [DOI] [PubMed] [Google Scholar]
- Dittmar K. J., Moelling K. Biochemical properties of p15-associated protease in an avian RNA tumor virus. J Virol. 1978 Oct;28(1):106–118. doi: 10.1128/jvi.28.1.106-118.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon L. K., Hohn T. Initiation of translation of the cauliflower mosaic virus genome from a polycistronic mRNA: evidence from deletion mutagenesis. EMBO J. 1984 Dec 1;3(12):2731–2736. doi: 10.1002/j.1460-2075.1984.tb02203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty W. G., Carrington J. C., Cary S. M., Parks T. D. Biochemical and mutational analysis of a plant virus polyprotein cleavage site. EMBO J. 1988 May;7(5):1281–1287. doi: 10.1002/j.1460-2075.1988.tb02942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty W. G., Cary S. M., Parks T. D. Molecular genetic analysis of a plant virus polyprotein cleavage site: a model. Virology. 1989 Aug;171(2):356–364. doi: 10.1016/0042-6822(89)90603-x. [DOI] [PubMed] [Google Scholar]
- Dougherty W. G., Parks T. D., Cary S. M., Bazan J. F., Fletterick R. J. Characterization of the catalytic residues of the tobacco etch virus 49-kDa proteinase. Virology. 1989 Sep;172(1):302–310. doi: 10.1016/0042-6822(89)90132-3. [DOI] [PubMed] [Google Scholar]
- Dougherty W. G., Parks T. D. Molecular genetic and biochemical evidence for the involvement of the heptapeptide cleavage sequence in determining the reaction profile at two tobacco etch virus cleavage sites in cell-free assays. Virology. 1989 Sep;172(1):145–155. doi: 10.1016/0042-6822(89)90116-5. [DOI] [PubMed] [Google Scholar]
- Dougherty W. G., Parks T. D. Post-translational processing of the tobacco etch virus 49-kDa small nuclear inclusion polyprotein: identification of an internal cleavage site and delimitation of VPg and proteinase domains. Virology. 1991 Aug;183(2):449–456. doi: 10.1016/0042-6822(91)90974-g. [DOI] [PubMed] [Google Scholar]
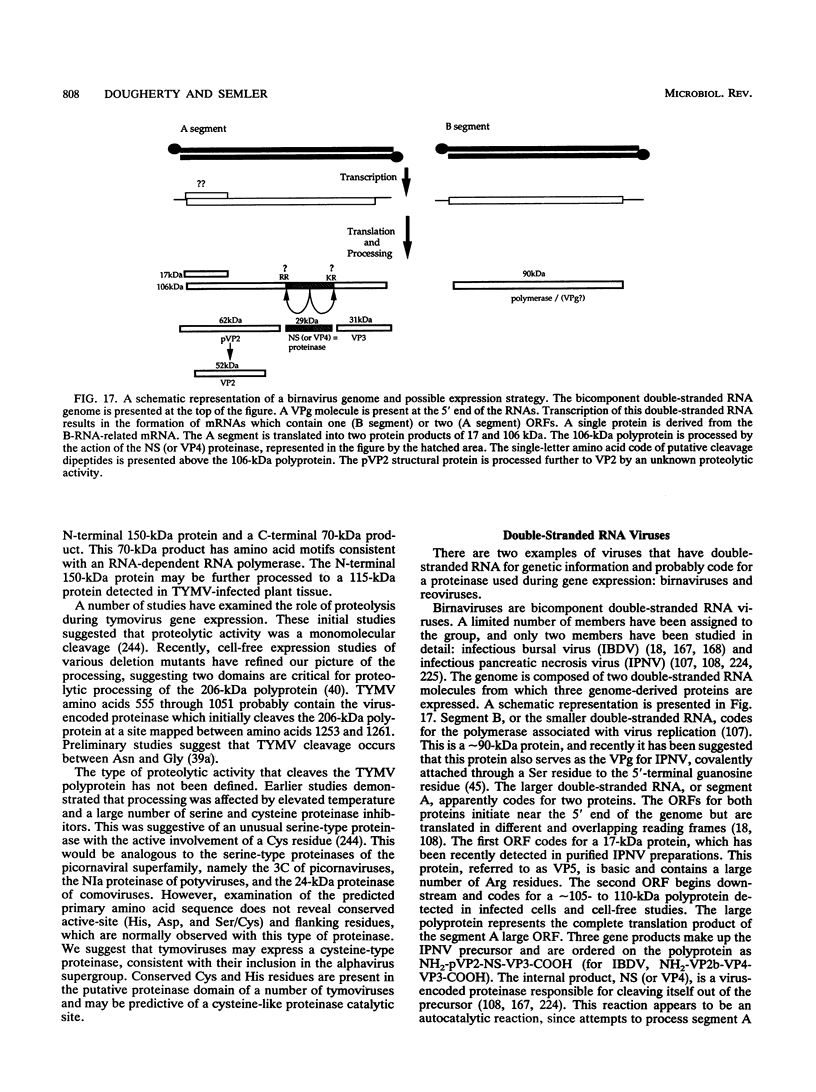
- Duncan R., Mason C. L., Nagy E., Leong J. A., Dobos P. Sequence analysis of infectious pancreatic necrosis virus genome segment B and its encoded VP1 protein: a putative RNA-dependent RNA polymerase lacking the Gly-Asp-Asp motif. Virology. 1991 Apr;181(2):541–552. doi: 10.1016/0042-6822(91)90887-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan R., Nagy E., Krell P. J., Dobos P. Synthesis of the infectious pancreatic necrosis virus polyprotein, detection of a virus-encoded protease, and fine structure mapping of genome segment A coding regions. J Virol. 1987 Dec;61(12):3655–3664. doi: 10.1128/jvi.61.12.3655-3664.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. J., Hemmingsen S. M. Molecular chaperones: proteins essential for the biogenesis of some macromolecular structures. Trends Biochem Sci. 1989 Aug;14(8):339–342. doi: 10.1016/0968-0004(89)90168-0. [DOI] [PubMed] [Google Scholar]
- Etchison D., Fout S. Human rhinovirus 14 infection of HeLa cells results in the proteolytic cleavage of the p220 cap-binding complex subunit and inactivates globin mRNA translation in vitro. J Virol. 1985 May;54(2):634–638. doi: 10.1128/jvi.54.2.634-638.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchison D., Milburn S. C., Edery I., Sonenberg N., Hershey J. W. Inhibition of HeLa cell protein synthesis following poliovirus infection correlates with the proteolysis of a 220,000-dalton polypeptide associated with eucaryotic initiation factor 3 and a cap binding protein complex. J Biol Chem. 1982 Dec 25;257(24):14806–14810. [PubMed] [Google Scholar]
- Evnin L. B., Vásquez J. R., Craik C. S. Substrate specificity of trypsin investigated by using a genetic selection. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6659–6663. doi: 10.1073/pnas.87.17.6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falgout B., Pethel M., Zhang Y. M., Lai C. J. Both nonstructural proteins NS2B and NS3 are required for the proteolytic processing of dengue virus nonstructural proteins. J Virol. 1991 May;65(5):2467–2475. doi: 10.1128/jvi.65.5.2467-2475.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk M. M., Grigera P. R., Bergmann I. E., Zibert A., Multhaup G., Beck E. Foot-and-mouth disease virus protease 3C induces specific proteolytic cleavage of host cell histone H3. J Virol. 1990 Feb;64(2):748–756. doi: 10.1128/jvi.64.2.748-756.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald P. M., McKeever B. M., VanMiddlesworth J. F., Springer J. P., Heimbach J. C., Leu C. T., Herber W. K., Dixon R. A., Darke P. L. Crystallographic analysis of a complex between human immunodeficiency virus type 1 protease and acetyl-pepstatin at 2.0-A resolution. J Biol Chem. 1990 Aug 25;265(24):14209–14219. [PubMed] [Google Scholar]
- Freimuth P., Anderson C. W. Human adenovirus serotype 12 virion precursors pMu and pVI are cleaved at amino-terminal and carboxy-terminal sites that conform to the adenovirus 2 endoproteinase cleavage consensus sequence. Virology. 1993 Mar;193(1):348–355. doi: 10.1006/viro.1993.1131. [DOI] [PubMed] [Google Scholar]
- Fütterer J., Gordon K., Sanfaçon H., Bonneville J. M., Hohn T. Positive and negative control of translation by the leader sequence of cauliflower mosaic virus pregenomic 35S RNA. EMBO J. 1990 Jun;9(6):1697–1707. doi: 10.1002/j.1460-2075.1990.tb08293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber E. A., Cross F. R., Hanafusa H. Processing of p60v-src to its myristylated membrane-bound form. Mol Cell Biol. 1985 Oct;5(10):2781–2788. doi: 10.1128/mcb.5.10.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
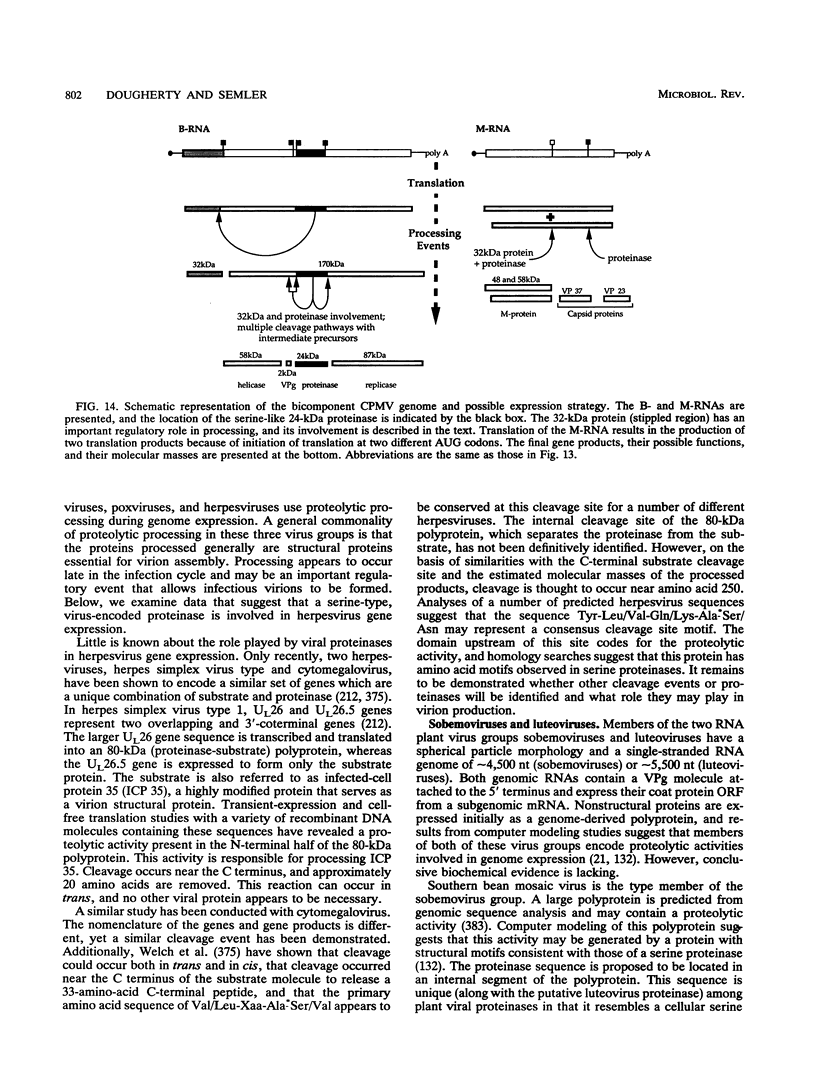
- Garcia J. A., Schrijvers L., Tan A., Vos P., Wellink J., Goldbach R. Proteolytic activity of the cowpea mosaic virus encoded 24K protein synthesized in Escherichia coli. Virology. 1987 Jul;159(1):67–75. doi: 10.1016/0042-6822(87)90348-5. [DOI] [PubMed] [Google Scholar]
- García J. A., Laín S., Cervera M. T., Riechmann J. L., Martín M. T. Mutational analysis of plum pox potyvirus polyprotein processing by the NIa protease in Escherichia coli. J Gen Virol. 1990 Dec;71(Pt 12):2773–2779. doi: 10.1099/0022-1317-71-12-2773. [DOI] [PubMed] [Google Scholar]
- García J. A., Riechmann J. L., Laín S. Proteolytic activity of the plum pox potyvirus NIa-like protein in Escherichia coli. Virology. 1989 Jun;170(2):362–369. doi: 10.1016/0042-6822(89)90426-1. [DOI] [PubMed] [Google Scholar]
- Gauss-Müller V., Jürgensen D., Deutzmann R. Autoproteolytic cleavage of recombinant 3C proteinase of hepatitis A virus. Virology. 1991 Jun;182(2):861–864. doi: 10.1016/0042-6822(91)90630-t. [DOI] [PubMed] [Google Scholar]
- Ghiso J., Saball E., Leoni J., Rostagno A., Frangione B. Binding of cystatin C to C4: the importance of sense-antisense peptides in their interaction. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1288–1291. doi: 10.1073/pnas.87.4.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giam C. Z., Boros I. In vivo and in vitro autoprocessing of human immunodeficiency virus protease expressed in Escherichia coli. J Biol Chem. 1988 Oct 15;263(29):14617–14620. [PubMed] [Google Scholar]
- Giantini M., Seliger L. S., Furuichi Y., Shatkin A. J. Reovirus type 3 genome segment S4: nucleotide sequence of the gene encoding a major virion surface protein. J Virol. 1984 Dec;52(3):984–987. doi: 10.1128/jvi.52.3.984-987.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldbach R. Genome similarities between plant and animal RNA viruses. Microbiol Sci. 1987 Jul;4(7):197–202. [PubMed] [Google Scholar]
- Gorbalenya A. E., Blinov V. M., Donchenko A. P. Poliovirus-encoded proteinase 3C: a possible evolutionary link between cellular serine and cysteine proteinase families. FEBS Lett. 1986 Jan 6;194(2):253–257. doi: 10.1016/0014-5793(86)80095-3. [DOI] [PubMed] [Google Scholar]
- Gorbalenya A. E., Donchenko A. P., Blinov V. M., Koonin E. V. Cysteine proteases of positive strand RNA viruses and chymotrypsin-like serine proteases. A distinct protein superfamily with a common structural fold. FEBS Lett. 1989 Jan 30;243(2):103–114. doi: 10.1016/0014-5793(89)80109-7. [DOI] [PubMed] [Google Scholar]
- Gorbalenya A. E., Donchenko A. P., Koonin E. V., Blinov V. M. N-terminal domains of putative helicases of flavi- and pestiviruses may be serine proteases. Nucleic Acids Res. 1989 May 25;17(10):3889–3897. doi: 10.1093/nar/17.10.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A. E., Koonin E. V., Blinov V. M., Donchenko A. P. Sobemovirus genome appears to encode a serine protease related to cysteine proteases of picornaviruses. FEBS Lett. 1988 Aug 29;236(2):287–290. doi: 10.1016/0014-5793(88)80039-5. [DOI] [PubMed] [Google Scholar]
- Gorbalenya A. E., Koonin E. V., Donchenko A. P., Blinov V. M. Coronavirus genome: prediction of putative functional domains in the non-structural polyprotein by comparative amino acid sequence analysis. Nucleic Acids Res. 1989 Jun 26;17(12):4847–4861. doi: 10.1093/nar/17.12.4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A. E., Koonin E. V., Lai M. M. Putative papain-related thiol proteases of positive-strand RNA viruses. Identification of rubi- and aphthovirus proteases and delineation of a novel conserved domain associated with proteases of rubi-, alpha- and coronaviruses. FEBS Lett. 1991 Aug 19;288(1-2):201–205. doi: 10.1016/0014-5793(91)81034-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon K., Pfeiffer P., Fütterer J., Hohn T. In vitro expression of cauliflower mosaic virus genes. EMBO J. 1988 Feb;7(2):309–317. doi: 10.1002/j.1460-2075.1988.tb02814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf L., Craik C. S., Patthy A., Roczniak S., Fletterick R. J., Rutter W. J. Selective alteration of substrate specificity by replacement of aspartic acid-189 with lysine in the binding pocket of trypsin. Biochemistry. 1987 May 5;26(9):2616–2623. doi: 10.1021/bi00383a031. [DOI] [PubMed] [Google Scholar]
- Graves M. C. Human immunodeficiency virus proteinase: now, then, what's next? Adv Exp Med Biol. 1991;306:395–405. doi: 10.1007/978-1-4684-6012-4_52. [DOI] [PubMed] [Google Scholar]
- Gráf L., Jancsó A., Szilágyi L., Hegyi G., Pintér K., Náray-Szabó G., Hepp J., Medzihradszky K., Rutter W. J. Electrostatic complementarity within the substrate-binding pocket of trypsin. Proc Natl Acad Sci U S A. 1988 Jul;85(14):4961–4965. doi: 10.1073/pnas.85.14.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn C. S., Strauss E. G., Strauss J. H. Sequence analysis of three Sindbis virus mutants temperature-sensitive in the capsid protein autoprotease. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4648–4652. doi: 10.1073/pnas.82.14.4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn C. S., Strauss J. H. Site-directed mutagenesis of the proposed catalytic amino acids of the Sindbis virus capsid protein autoprotease. J Virol. 1990 Jun;64(6):3069–3073. doi: 10.1128/jvi.64.6.3069-3073.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambidge S. J., Sarnow P. Translational enhancement of the poliovirus 5' noncoding region mediated by virus-encoded polypeptide 2A. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10272–10276. doi: 10.1073/pnas.89.21.10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanecak R., Semler B. L., Ariga H., Anderson C. W., Wimmer E. Expression of a cloned gene segment of poliovirus in E. coli: evidence for autocatalytic production of the viral proteinase. Cell. 1984 Jul;37(3):1063–1073. doi: 10.1016/0092-8674(84)90441-0. [DOI] [PubMed] [Google Scholar]
- Hardy W. R., Hahn Y. S., de Groot R. J., Strauss E. G., Strauss J. H. Synthesis and processing of the nonstructural polyproteins of several temperature-sensitive mutants of Sindbis virus. Virology. 1990 Jul;177(1):199–208. doi: 10.1016/0042-6822(90)90473-5. [DOI] [PubMed] [Google Scholar]
- Hardy W. R., Strauss J. H. Processing the nonstructural polyproteins of Sindbis virus: study of the kinetics in vivo by using monospecific antibodies. J Virol. 1988 Mar;62(3):998–1007. doi: 10.1128/jvi.62.3.998-1007.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon S. A., Updike W., Jia X. Y., Summers D. F., Ehrenfeld E. Polyprotein processing in cis and in trans by hepatitis A virus 3C protease cloned and expressed in Escherichia coli. J Virol. 1992 Sep;66(9):5242–5247. doi: 10.1128/jvi.66.9.5242-5247.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassell J. A., Weber J. Genetic analysis of adenovirus type 2. VIII. Physical locations of temperature-sensitive mutations. J Virol. 1978 Dec;28(3):671–678. doi: 10.1128/jvi.28.3.671-678.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedstrom L., Szilagyi L., Rutter W. J. Converting trypsin to chymotrypsin: the role of surface loops. Science. 1992 Mar 6;255(5049):1249–1253. doi: 10.1126/science.1546324. [DOI] [PubMed] [Google Scholar]
- Hellen C. U., Fäcke M., Kräusslich H. G., Lee C. K., Wimmer E. Characterization of poliovirus 2A proteinase by mutational analysis: residues required for autocatalytic activity are essential for induction of cleavage of eukaryotic initiation factor 4F polypeptide p220. J Virol. 1991 Aug;65(8):4226–4231. doi: 10.1128/jvi.65.8.4226-4231.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellen C. U., Lee C. K., Wimmer E. Determinants of substrate recognition by poliovirus 2A proteinase. J Virol. 1992 Jun;66(6):3330–3338. doi: 10.1128/jvi.66.6.3330-3338.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellen C. U., Wimmer E. Maturation of poliovirus capsid proteins. Virology. 1992 Apr;187(2):391–397. doi: 10.1016/0042-6822(92)90440-z. [DOI] [PubMed] [Google Scholar]
- Hellen C. U., Wimmer E. The role of proteolytic processing in the morphogenesis of virus particles. Experientia. 1992 Feb 15;48(2):201–215. doi: 10.1007/BF01923512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmann G. M., Shaw J. G., Rhoads R. E. In vitro analysis of tobacco vein mottling virus NIa cistron: evidence for a virus-encoded protease. Virology. 1988 Apr;163(2):554–562. doi: 10.1016/0042-6822(88)90296-6. [DOI] [PubMed] [Google Scholar]
- Henderson L. E., Krutzsch H. C., Oroszlan S. Myristyl amino-terminal acylation of murine retrovirus proteins: an unusual post-translational proteins modification. Proc Natl Acad Sci U S A. 1983 Jan;80(2):339–343. doi: 10.1073/pnas.80.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higaki J. N., Gibson B. W., Craik C. S. Evolution of catalysis in the serine proteases. Cold Spring Harb Symp Quant Biol. 1987;52:615–621. doi: 10.1101/sqb.1987.052.01.070. [DOI] [PubMed] [Google Scholar]
- Himmler G., Frank S., Steinkellner H., Rüker F., Mattanovich D., Katinger H. W. Detection of the trans activity of the plum pox virus NIa-like protease in infected plants. J Gen Virol. 1990 Jul;71(Pt 7):1623–1625. doi: 10.1099/0022-1317-71-7-1623. [DOI] [PubMed] [Google Scholar]
- Hintermann E., Kuhn A. Bacteriophage T4 gene 21 encodes two proteins essential for phage maturation. Virology. 1992 Aug;189(2):474–482. doi: 10.1016/0042-6822(92)90571-6. [DOI] [PubMed] [Google Scholar]
- Hobman T. C., Lundstrom M. L., Gillam S. Processing and intracellular transport of rubella virus structural proteins in COS cells. Virology. 1990 Sep;178(1):122–133. doi: 10.1016/0042-6822(90)90385-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houde A., Weber J. M. Adenovirus type 2 precursor proteins are cleaved by proteinases of other adenoviruses. Virology. 1990 Nov;179(1):485–486. doi: 10.1016/0042-6822(90)90321-h. [DOI] [PubMed] [Google Scholar]
- Houde A., Weber J. M. The primary structure of human adenovirus type 12 protease. Nucleic Acids Res. 1988 Jul 25;16(14B):7195–7195. doi: 10.1093/nar/16.14.7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu I. N., Delbaere L. T., James M. N., Hofmann T. Penicillopepsin from Penicillium janthinellum crystal structure at 2.8 A and sequence homology with porcine pepsin. Nature. 1977 Mar 10;266(5598):140–145. doi: 10.1038/266140a0. [DOI] [PubMed] [Google Scholar]
- Hämmerle T., Hellen C. U., Wimmer E. Site-directed mutagenesis of the putative catalytic triad of poliovirus 3C proteinase. J Biol Chem. 1991 Mar 25;266(9):5412–5416. [PubMed] [Google Scholar]
- Håvarstein L. S., Kalland K. H., Christie K. E., Endresen C. Sequence of the large double-stranded RNA segment of the N1 strain of infectious pancreatic necrosis virus: a comparison with other Birnaviridae. J Gen Virol. 1990 Feb;71(Pt 2):299–308. doi: 10.1099/0022-1317-71-2-299. [DOI] [PubMed] [Google Scholar]
- Ivanoff L. A., Towatari T., Ray J., Korant B. D., Petteway S. R., Jr Expression and site-specific mutagenesis of the poliovirus 3C protease in Escherichia coli. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5392–5396. doi: 10.1073/pnas.83.15.5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks T. Translational suppression in gene expression in retroviruses and retrotransposons. Curr Top Microbiol Immunol. 1990;157:93–124. doi: 10.1007/978-3-642-75218-6_4. [DOI] [PubMed] [Google Scholar]
- Jackson R. J. A detailed kinetic analysis of the in vitro synthesis and processing of encephalomyocarditis virus products. Virology. 1986 Feb;149(1):114–127. doi: 10.1016/0042-6822(86)90092-9. [DOI] [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Polypeptide cleavages in the formation of poliovirus proteins. Proc Natl Acad Sci U S A. 1968 Sep;61(1):77–84. doi: 10.1073/pnas.61.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagadish M. N., Staton V. J., Hudson P. J., Azad A. A. Birnavirus precursor polyprotein is processed in Escherichia coli by its own virus-encoded polypeptide. J Virol. 1988 Mar;62(3):1084–1087. doi: 10.1128/jvi.62.3.1084-1087.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagadish M. N., Vaughan P. R., Irving R. A., Azad A. A., Macreadie I. G. Expression and characterization of infectious bursal disease virus polyprotein in yeast. Gene. 1990 Nov 15;95(2):179–186. doi: 10.1016/0378-1119(90)90360-4. [DOI] [PubMed] [Google Scholar]
- James M. N., Sielecki A. R. Structure and refinement of penicillopepsin at 1.8 A resolution. J Mol Biol. 1983 Jan 15;163(2):299–361. doi: 10.1016/0022-2836(83)90008-6. [DOI] [PubMed] [Google Scholar]
- Jayasuriya A. K., Nibert M. L., Fields B. N. Complete nucleotide sequence of the M2 gene segment of reovirus type 3 dearing and analysis of its protein product mu 1. Virology. 1988 Apr;163(2):591–602. doi: 10.1016/0042-6822(88)90300-5. [DOI] [PubMed] [Google Scholar]
- Jia X. Y., Ehrenfeld E., Summers D. F. Proteolytic activity of hepatitis A virus 3C protein. J Virol. 1991 May;65(5):2595–2600. doi: 10.1128/jvi.65.5.2595-2600.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jore J., De Geus B., Jackson R. J., Pouwels P. H., Enger-Valk B. E. Poliovirus protein 3CD is the active protease for processing of the precursor protein P1 in vitro. J Gen Virol. 1988 Jul;69(Pt 7):1627–1636. doi: 10.1099/0022-1317-69-7-1627. [DOI] [PubMed] [Google Scholar]
- Jupp R. A., Phylip L. H., Mills J. S., Le Grice S. F., Kay J. Mutating P2 and P1 residues at cleavage junctions in the HIV-1 pol polyprotein. Effects on hydrolysis by HIV-1 proteinase. FEBS Lett. 1991 Jun 3;283(2):180–184. doi: 10.1016/0014-5793(91)80583-o. [DOI] [PubMed] [Google Scholar]
- Jupp R. A., Richards A. D., Phylip L. H., Kay J., Konvalinka J., Strop P., Kostka V., Scarborough P. E., Farmerie W. G., Dunn B. M. Substrate cleavage by HIV-1 proteinase. Adv Exp Med Biol. 1991;306:461–467. doi: 10.1007/978-1-4684-6012-4_59. [DOI] [PubMed] [Google Scholar]
- Kamphuis I. G., Drenth J., Baker E. N. Thiol proteases. Comparative studies based on the high-resolution structures of papain and actinidin, and on amino acid sequence information for cathepsins B and H, and stem bromelain. J Mol Biol. 1985 Mar 20;182(2):317–329. doi: 10.1016/0022-2836(85)90348-1. [DOI] [PubMed] [Google Scholar]
- Kaplan A. H., Swanstrom R. Human immunodeficiency virus type 1 Gag proteins are processed in two cellular compartments. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4528–4532. doi: 10.1073/pnas.88.10.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlström A. R., Levine R. L. Copper inhibits the protease from human immunodeficiency virus 1 by both cysteine-dependent and cysteine-independent mechanisms. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5552–5556. doi: 10.1073/pnas.88.13.5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwazaki S., Minobe Y., Hibino H. Nucleotide sequence of barley yellow mosaic virus RNA 2. J Gen Virol. 1991 Apr;72(Pt 4):995–999. doi: 10.1099/0022-1317-72-4-995. [DOI] [PubMed] [Google Scholar]
- Kashiwazaki S., Minobe Y., Omura T., Hibino H. Nucleotide sequence of barley yellow mosaic virus RNA 1: a close evolutionary relationship with potyviruses. J Gen Virol. 1990 Dec;71(Pt 12):2781–2790. doi: 10.1099/0022-1317-71-12-2781. [DOI] [PubMed] [Google Scholar]
- Kay J., Dunn B. M. Viral proteinases: weakness in strength. Biochim Biophys Acta. 1990 Jan 30;1048(1):1–18. doi: 10.1016/0167-4781(90)90015-t. [DOI] [PubMed] [Google Scholar]
- Kean K. M., Agut H., Fichot O., Wimmer E., Girard M. A poliovirus mutant defective for self-cleavage at the COOH-terminus of the 3C protease exhibits secondary processing defects. Virology. 1988 Apr;163(2):330–340. doi: 10.1016/0042-6822(88)90273-5. [DOI] [PubMed] [Google Scholar]
- Kean K. M., Teterina N. L., Marc D., Girard M. Analysis of putative active site residues of the poliovirus 3C protease. Virology. 1991 Apr;181(2):609–619. doi: 10.1016/0042-6822(91)90894-h. [DOI] [PubMed] [Google Scholar]
- Kean K. M., Teterina N., Girard M. Cleavage specificity of the poliovirus 3C protease is not restricted to Gln-Gly at the 3C/3D junction. J Gen Virol. 1990 Nov;71(Pt 11):2553–2563. doi: 10.1099/0022-1317-71-11-2553. [DOI] [PubMed] [Google Scholar]
- Kibenge F. S., Jackwood D. J., Mercado C. C. Nucleotide sequence analysis of genome segment A of infectious bursal disease virus. J Gen Virol. 1990 Mar;71(Pt 3):569–577. doi: 10.1099/0022-1317-71-3-569. [DOI] [PubMed] [Google Scholar]
- Kibenge F. S., McKenna P. K., Dybing J. K. Genome cloning and analysis of the large RNA segment (segment A) of a naturally avirulent serotype 2 infectious bursal disease virus. Virology. 1991 Sep;184(1):437–440. doi: 10.1016/0042-6822(91)90865-9. [DOI] [PubMed] [Google Scholar]
- Kliewer S., Dasgupta A. An RNA polymerase II transcription factor inactivated in poliovirus-infected cells copurifies with transcription factor TFIID. Mol Cell Biol. 1988 Aug;8(8):3175–3182. doi: 10.1128/mcb.8.8.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump W., Marquardt O., Hofschneider P. H. Biologically active protease of foot and mouth disease virus is expressed from cloned viral cDNA in Escherichia coli. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3351–3355. doi: 10.1073/pnas.81.11.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl N. E., Emini E. A., Schleif W. A., Davis L. J., Heimbach J. C., Dixon R. A., Scolnick E. M., Sigal I. S. Active human immunodeficiency virus protease is required for viral infectivity. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4686–4690. doi: 10.1073/pnas.85.13.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konvalinka J., Horejsí M., Andreánsky M., Novek P., Pichová I., Bláha I., Fábry M., Sedlácek J., Foundling S., Strop P. An engineered retroviral proteinase from myeloblastosis associated virus acquires pH dependence and substrate specificity of the HIV-1 proteinase. EMBO J. 1992 Mar;11(3):1141–1144. doi: 10.1002/j.1460-2075.1992.tb05154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonin E. V., Choi G. H., Nuss D. L., Shapira R., Carrington J. C. Evidence for common ancestry of a chestnut blight hypovirulence-associated double-stranded RNA and a group of positive-strand RNA plant viruses. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10647–10651. doi: 10.1073/pnas.88.23.10647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korant B. D., Chow N. L., Lively M. O., Powers J. C. Proteolytic events in replication of animal viruses. Ann N Y Acad Sci. 1980;343:304–318. doi: 10.1111/j.1749-6632.1980.tb47260.x. [DOI] [PubMed] [Google Scholar]
- Korant B. D., Towatari T., Ivanoff L., Petteway S., Jr, Brzin J., Lenarcic B., Turk V. Viral therapy: prospects for protease inhibitors. J Cell Biochem. 1986;32(2):91–95. doi: 10.1002/jcb.240320202. [DOI] [PubMed] [Google Scholar]
- Kotler M., Danho W., Katz R. A., Leis J., Skalka A. M. Avian retroviral protease and cellular aspartic proteases are distinguished by activities on peptide substrates. J Biol Chem. 1989 Feb 25;264(6):3428–3435. [PubMed] [Google Scholar]
- Kotler M., Katz R. A., Danho W., Leis J., Skalka A. M. Synthetic peptides as substrates and inhibitors of a retroviral protease. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4185–4189. doi: 10.1073/pnas.85.12.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotler M., Katz R. A., Skalka A. M. Activity of avian retroviral protease expressed in Escherichia coli. J Virol. 1988 Aug;62(8):2696–2700. doi: 10.1128/jvi.62.8.2696-2700.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraut J. Serine proteases: structure and mechanism of catalysis. Annu Rev Biochem. 1977;46:331–358. doi: 10.1146/annurev.bi.46.070177.001555. [DOI] [PubMed] [Google Scholar]
- Kruijer W., van Schaik F. M., Sussenbach J. S. Nucleotide sequence analysis of a region of adenovirus 5 DNA encoding a hitherto unidentified gene. Nucleic Acids Res. 1980 Dec 20;8(24):6033–6042. doi: 10.1093/nar/8.24.6033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kräusslich H. G. Human immunodeficiency virus proteinase dimer as component of the viral polyprotein prevents particle assembly and viral infectivity. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3213–3217. doi: 10.1073/pnas.88.8.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kräusslich H. G., Ingraham R. H., Skoog M. T., Wimmer E., Pallai P. V., Carter C. A. Activity of purified biosynthetic proteinase of human immunodeficiency virus on natural substrates and synthetic peptides. Proc Natl Acad Sci U S A. 1989 Feb;86(3):807–811. doi: 10.1073/pnas.86.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kräusslich H. G., Nicklin M. J., Toyoda H., Etchison D., Wimmer E. Poliovirus proteinase 2A induces cleavage of eucaryotic initiation factor 4F polypeptide p220. J Virol. 1987 Sep;61(9):2711–2718. doi: 10.1128/jvi.61.9.2711-2718.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kräusslich H. G., Wimmer E. Viral proteinases. Annu Rev Biochem. 1988;57:701–754. doi: 10.1146/annurev.bi.57.070188.003413. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- König H., Rosenwirth B. Purification and partial characterization of poliovirus protease 2A by means of a functional assay. J Virol. 1988 Apr;62(4):1243–1250. doi: 10.1128/jvi.62.4.1243-1250.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson M. A., Dasmahapatra B., Semler B. L. Species-specific substrate interaction of picornavirus 3C proteinase suballelic exchange mutants. J Biol Chem. 1990 Sep 15;265(26):15920–15931. [PubMed] [Google Scholar]
- Lawson M. A., Semler B. L. Picornavirus protein processing--enzymes, substrates, and genetic regulation. Curr Top Microbiol Immunol. 1990;161:49–87. [PubMed] [Google Scholar]
- Lawson M. A., Semler B. L. Poliovirus thiol proteinase 3C can utilize a serine nucleophile within the putative catalytic triad. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):9919–9923. doi: 10.1073/pnas.88.22.9919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson T. G., Smith L. L., Palmenberg A. C., Thach R. E. Inducible expression of encephalomyocarditis virus 3C protease activity in stably transformed mouse cell lines. J Virol. 1989 Dec;63(12):5013–5022. doi: 10.1128/jvi.63.12.5013-5022.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar J. B., Winant R. C., Johnson P. H. Hirudin: amino-terminal residues play a major role in the interaction with thrombin. J Biol Chem. 1991 Jan 15;266(2):685–688. [PubMed] [Google Scholar]
- Lee C. K., Wimmer E. Proteolytic processing of poliovirus polyprotein: elimination of 2Apro-mediated, alternative cleavage of polypeptide 3CD by in vitro mutagenesis. Virology. 1988 Oct;166(2):405–414. doi: 10.1016/0042-6822(88)90511-9. [DOI] [PubMed] [Google Scholar]
- Lee H. J., Shieh C. K., Gorbalenya A. E., Koonin E. V., La Monica N., Tuler J., Bagdzhadzhyan A., Lai M. M. The complete sequence (22 kilobases) of murine coronavirus gene 1 encoding the putative proteases and RNA polymerase. Virology. 1991 Feb;180(2):567–582. doi: 10.1016/0042-6822(91)90071-I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P. W., Hayes E. C., Joklik W. K. Characterization of anti-reovirus immunoglobulins secreted by cloned hybridoma cell lines. Virology. 1981 Jan 15;108(1):134–146. doi: 10.1016/0042-6822(81)90533-x. [DOI] [PubMed] [Google Scholar]
- Libby R. T., Cosman D., Cooney M. K., Merriam J. E., March C. J., Hopp T. P. Human rhinovirus 3C protease: cloning and expression of an active form in Escherichia coli. Biochemistry. 1988 Aug 23;27(17):6262–6268. doi: 10.1021/bi00417a010. [DOI] [PubMed] [Google Scholar]
- Liebig H. D., Skern T., Luderer M., Sommergruber W., Blaas D., Kuechler E. Proteinase trapping: screening for viral proteinase mutants by alpha complementation. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):5979–5983. doi: 10.1073/pnas.88.14.5979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F. Y., Roizman B. The herpes simplex virus 1 gene encoding a protease also contains within its coding domain the gene encoding the more abundant substrate. J Virol. 1991 Oct;65(10):5149–5156. doi: 10.1128/jvi.65.10.5149-5156.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd R. E., Grubman M. J., Ehrenfeld E. Relationship of p220 cleavage during picornavirus infection to 2A proteinase sequencing. J Virol. 1988 Nov;62(11):4216–4223. doi: 10.1128/jvi.62.11.4216-4223.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd R. E., Jense H. G., Ehrenfeld E. Restriction of translation of capped mRNA in vitro as a model for poliovirus-induced inhibition of host cell protein synthesis: relationship to p220 cleavage. J Virol. 1987 Aug;61(8):2480–2488. doi: 10.1128/jvi.61.8.2480-2488.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd R. E., Toyoda H., Etchison D., Wimmer E., Ehrenfeld E. Cleavage of the cap binding protein complex polypeptide p220 is not effected by the second poliovirus protease 2A. Virology. 1986 Apr 15;150(1):299–303. doi: 10.1016/0042-6822(86)90291-6. [DOI] [PubMed] [Google Scholar]
- Loeb D. D., Hutchison C. A., 3rd, Edgell M. H., Farmerie W. G., Swanstrom R. Mutational analysis of human immunodeficiency virus type 1 protease suggests functional homology with aspartic proteinases. J Virol. 1989 Jan;63(1):111–121. doi: 10.1128/jvi.63.1.111-121.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb D. D., Swanstrom R., Everitt L., Manchester M., Stamper S. E., Hutchison C. A., 3rd Complete mutagenesis of the HIV-1 protease. Nature. 1989 Aug 3;340(6232):397–400. doi: 10.1038/340397a0. [DOI] [PubMed] [Google Scholar]
- Long A. C., Orr D. C., Cameron J. M., Dunn B. M., Kay J. A consensus sequence for substrate hydrolysis by rhinovirus 3C proteinase. FEBS Lett. 1989 Nov 20;258(1):75–78. doi: 10.1016/0014-5793(89)81619-9. [DOI] [PubMed] [Google Scholar]
- Louis J. M., Oroszlan S., Mora P. T. Studies of the autoprocessing of the HIV-1 protease using cleavage site mutants. Adv Exp Med Biol. 1991;306:499–502. doi: 10.1007/978-1-4684-6012-4_64. [DOI] [PubMed] [Google Scholar]
- Malcolm B. A., Chin S. M., Jewell D. A., Stratton-Thomas J. R., Thudium K. B., Ralston R., Rosenberg S. Expression and characterization of recombinant hepatitis A virus 3C proteinase. Biochemistry. 1992 Apr 7;31(13):3358–3363. doi: 10.1021/bi00128a008. [DOI] [PubMed] [Google Scholar]
- Mangel W. F., McGrath W. J., Toledo D. L., Anderson C. W. Viral DNA and a viral peptide can act as cofactors of adenovirus virion proteinase activity. Nature. 1993 Jan 21;361(6409):274–275. doi: 10.1038/361274a0. [DOI] [PubMed] [Google Scholar]
- Manning D. S., Leong J. C. Expression in Escherichia coli of the large genomic segment of infectious pancreatic necrosis virus. Virology. 1990 Nov;179(1):16–25. doi: 10.1016/0042-6822(90)90268-v. [DOI] [PubMed] [Google Scholar]
- Manning D. S., Mason C. L., Leong J. C. Cell-free translational analysis of the processing of infectious pancreatic necrosis virus polyprotein. Virology. 1990 Nov;179(1):9–15. doi: 10.1016/0042-6822(90)90267-u. [DOI] [PubMed] [Google Scholar]
- Marcus A., Luginbill B., Feeley J. Polysome formation with tobacco mosaic virus RNA. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1243–1250. doi: 10.1073/pnas.59.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margis R., Pinck L. Effects of site-directed mutagenesis on the presumed catalytic triad and substrate-binding pocket of grapevine fanleaf nepovirus 24-kDa proteinase. Virology. 1992 Oct;190(2):884–888. doi: 10.1016/0042-6822(92)90931-e. [DOI] [PubMed] [Google Scholar]
- Margis R., Viry M., Pinck M., Pinck L. Cloning and in vitro characterization of the grapevine fanleaf virus proteinase cistron. Virology. 1991 Dec;185(2):779–787. doi: 10.1016/0042-6822(91)90549-q. [DOI] [PubMed] [Google Scholar]
- Marr L. D., Sanchez A., Frey T. K. Efficient in vitro translation and processing of the rubella virus structural proteins in the presence of microsomes. Virology. 1991 Jan;180(1):400–405. doi: 10.1016/0042-6822(91)90046-e. [DOI] [PubMed] [Google Scholar]
- Martinez-Izquierdo J., Hohn T. Cauliflower mosaic virus coat protein is phosphorylated in vitro by a virion-associated protein kinase. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1824–1828. doi: 10.1073/pnas.84.7.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheson N. R., van Halbeek H., Travis J. Evidence for a tetrahedral intermediate complex during serpin-proteinase interactions. J Biol Chem. 1991 Jul 25;266(21):13489–13491. [PubMed] [Google Scholar]
- Mavankal G., Rhoads R. E. In vitro cleavage at or near the N-terminus of the helper component protein in the tobacco vein mottling virus polyprotein. Virology. 1991 Dec;185(2):721–731. doi: 10.1016/0042-6822(91)90543-k. [DOI] [PubMed] [Google Scholar]
- Mayo M. A., Robinson D. J., Jolly C. A., Hyman L. Nucleotide sequence of potato leafroll luteovirus RNA. J Gen Virol. 1989 May;70(Pt 5):1037–1051. doi: 10.1099/0022-1317-70-5-1037. [DOI] [PubMed] [Google Scholar]
- McDonald H., Hobman T. C., Gillam S. The influence of capsid protein cleavage on the processing of E2 and E1 glycoproteins of rubella virus. Virology. 1991 Jul;183(1):52–60. doi: 10.1016/0042-6822(91)90117-t. [DOI] [PubMed] [Google Scholar]
- McQuade T. J., Tomasselli A. G., Liu L., Karacostas V., Moss B., Sawyer T. K., Heinrikson R. L., Tarpley W. G. A synthetic HIV-1 protease inhibitor with antiviral activity arrests HIV-like particle maturation. Science. 1990 Jan 26;247(4941):454–456. doi: 10.1126/science.2405486. [DOI] [PubMed] [Google Scholar]
- Medberry S. L., Lockhart B. E., Olszewski N. E. Properties of Commelina yellow mottle virus's complete DNA sequence, genomic discontinuities and transcript suggest that it is a pararetrovirus. Nucleic Acids Res. 1990 Sep 25;18(18):5505–5513. doi: 10.1093/nar/18.18.5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek T. D., Lambert D. M., Dreyer G. B., Carr T. J., Tomaszek T. A., Jr, Moore M. L., Strickler J. E., Debouck C., Hyland L. J., Matthews T. J. Inhibition of HIV-1 protease in infected T-lymphocytes by synthetic peptide analogues. Nature. 1990 Jan 4;343(6253):90–92. doi: 10.1038/343090a0. [DOI] [PubMed] [Google Scholar]
- Melancon P., Garoff H. Processing of the Semliki Forest virus structural polyprotein: role of the capsid protease. J Virol. 1987 May;61(5):1301–1309. doi: 10.1128/jvi.61.5.1301-1309.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M., Jaskólski M., Rao J. K., Leis J., Wlodawer A. Crystal structure of a retroviral protease proves relationship to aspartic protease family. Nature. 1989 Feb 9;337(6207):576–579. doi: 10.1038/337576a0. [DOI] [PubMed] [Google Scholar]
- Miller M., Schneider J., Sathyanarayana B. K., Toth M. V., Marshall G. R., Clawson L., Selk L., Kent S. B., Wlodawer A. Structure of complex of synthetic HIV-1 protease with a substrate-based inhibitor at 2.3 A resolution. Science. 1989 Dec 1;246(4934):1149–1152. doi: 10.1126/science.2686029. [DOI] [PubMed] [Google Scholar]
- Miller R. H., Purcell R. H. Hepatitis C virus shares amino acid sequence similarity with pestiviruses and flaviviruses as well as members of two plant virus supergroups. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2057–2061. doi: 10.1073/pnas.87.6.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller W. A., Waterhouse P. M., Gerlach W. L. Sequence and organization of barley yellow dwarf virus genomic RNA. Nucleic Acids Res. 1988 Jul 11;16(13):6097–6111. doi: 10.1093/nar/16.13.6097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzayan C., Ingraham R., Wimmer E. Specificity of the polioviral proteinase 3C towards genetically engineered cleavage sites in the viral capsid. J Gen Virol. 1991 May;72(Pt 5):1159–1163. doi: 10.1099/0022-1317-72-5-1159. [DOI] [PubMed] [Google Scholar]
- Morch M. D., Drugeon G., Szafranski P., Haenni A. L. Proteolytic origin of the 150-kilodalton protein encoded by turnip yellow mosaic virus genomic RNA. J Virol. 1989 Dec;63(12):5153–5158. doi: 10.1128/jvi.63.12.5153-5158.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morch M. D., Zagórski W., Haenni A. L. Proteolytic maturation of the turnip-yellow-mosaic-virus polyprotein coded in vitro occurs by internal catalysis. Eur J Biochem. 1982 Oct;127(2):259–265. doi: 10.1111/j.1432-1033.1982.tb06864.x. [DOI] [PubMed] [Google Scholar]
- Musil D., Zucic D., Turk D., Engh R. A., Mayr I., Huber R., Popovic T., Turk V., Towatari T., Katunuma N. The refined 2.15 A X-ray crystal structure of human liver cathepsin B: the structural basis for its specificity. EMBO J. 1991 Sep;10(9):2321–2330. doi: 10.1002/j.1460-2075.1991.tb07771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navia M. A., Fitzgerald P. M., McKeever B. M., Leu C. T., Heimbach J. C., Herber W. K., Sigal I. S., Darke P. L., Springer J. P. Three-dimensional structure of aspartyl protease from human immunodeficiency virus HIV-1. Nature. 1989 Feb 16;337(6208):615–620. doi: 10.1038/337615a0. [DOI] [PubMed] [Google Scholar]
- Navia M. A., McKeever B. M. A role for the aspartyl protease from the human immunodeficiency virus type 1 (HIV-1) in the orchestration of virus assembly. Ann N Y Acad Sci. 1990;616:73–85. doi: 10.1111/j.1749-6632.1990.tb17829.x. [DOI] [PubMed] [Google Scholar]
- Nibert M. L., Fields B. N. A carboxy-terminal fragment of protein mu 1/mu 1C is present in infectious subvirion particles of mammalian reoviruses and is proposed to have a role in penetration. J Virol. 1992 Nov;66(11):6408–6418. doi: 10.1128/jvi.66.11.6408-6418.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibert M. L., Schiff L. A., Fields B. N. Mammalian reoviruses contain a myristoylated structural protein. J Virol. 1991 Apr;65(4):1960–1967. doi: 10.1128/jvi.65.4.1960-1967.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklin M. J., Harris K. S., Pallai P. V., Wimmer E. Poliovirus proteinase 3C: large-scale expression, purification, and specific cleavage activity on natural and synthetic substrates in vitro. J Virol. 1988 Dec;62(12):4586–4593. doi: 10.1128/jvi.62.12.4586-4593.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt R. F., Brady S. F., Darke P. L., Ciccarone T. M., Colton C. D., Nutt E. M., Rodkey J. A., Bennett C. D., Waxman L. H., Sigal I. S. Chemical synthesis and enzymatic activity of a 99-residue peptide with a sequence proposed for the human immunodeficiency virus protease. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7129–7133. doi: 10.1073/pnas.85.19.7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh C. S., Carrington J. C. Identification of essential residues in potyvirus proteinase HC-Pro by site-directed mutagenesis. Virology. 1989 Dec;173(2):692–699. doi: 10.1016/0042-6822(89)90582-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlendorf D. H., Foundling S. I., Wendoloski J. J., Sedlacek J., Strop P., Salemme F. R. Structural studies of the retroviral proteinase from avian myeloblastosis associated virus. Proteins. 1992 Nov;14(3):382–391. doi: 10.1002/prot.340140307. [DOI] [PubMed] [Google Scholar]
- Oroszlan S., Copeland T. D. Primary structure and processing of gag and env gene products of human T-cell leukemia viruses HTLV-ICR and HTLV-IATK. Curr Top Microbiol Immunol. 1985;115:221–233. doi: 10.1007/978-3-642-70113-9_14. [DOI] [PubMed] [Google Scholar]
- Oroszlan S., Luftig R. B. Retroviral proteinases. Curr Top Microbiol Immunol. 1990;157:153–185. doi: 10.1007/978-3-642-75218-6_6. [DOI] [PubMed] [Google Scholar]
- Orr D. C., Long A. C., Kay J., Dunn B. M., Cameron J. M. Hydrolysis of a series of synthetic peptide substrates by the human rhinovirus 14 3C proteinase, cloned and expressed in Escherichia coli. J Gen Virol. 1989 Nov;70(Pt 11):2931–2942. doi: 10.1099/0022-1317-70-11-2931. [DOI] [PubMed] [Google Scholar]
- Pallai P. V., Burkhardt F., Skoog M., Schreiner K., Bax P., Cohen K. A., Hansen G., Palladino D. E., Harris K. S., Nicklin M. J. Cleavage of synthetic peptides by purified poliovirus 3C proteinase. J Biol Chem. 1989 Jun 15;264(17):9738–9741. [PubMed] [Google Scholar]
- Palmenberg A. C., Parks G. D., Hall D. J., Ingraham R. H., Seng T. W., Pallai P. V. Proteolytic processing of the cardioviral P2 region: primary 2A/2B cleavage in clone-derived precursors. Virology. 1992 Oct;190(2):754–762. doi: 10.1016/0042-6822(92)90913-a. [DOI] [PubMed] [Google Scholar]
- Palmenberg A. C. Proteolytic processing of picornaviral polyprotein. Annu Rev Microbiol. 1990;44:603–623. doi: 10.1146/annurev.mi.44.100190.003131. [DOI] [PubMed] [Google Scholar]
- Palmenberg A. C., Rueckert R. R. Evidence for intramolecular self-cleavage of picornaviral replicase precursors. J Virol. 1982 Jan;41(1):244–249. doi: 10.1128/jvi.41.1.244-249.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Gagnon J., Vogt V. M., Ripley S., Eisenman R. N. The NH2-terminal sequence of the avian oncovirus gag precursor polyprotein (Pr76gag). Virology. 1978 Dec;91(2):423–433. doi: 10.1016/0042-6822(78)90388-4. [DOI] [PubMed] [Google Scholar]
- Parks G. D., Baker J. C., Palmenberg A. C. Proteolytic cleavage of encephalomyocarditis virus capsid region substrates by precursors to the 3C enzyme. J Virol. 1989 Mar;63(3):1054–1058. doi: 10.1128/jvi.63.3.1054-1058.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks G. D., Duke G. M., Palmenberg A. C. Encephalomyocarditis virus 3C protease: efficient cell-free expression from clones which link viral 5' noncoding sequences to the P3 region. J Virol. 1986 Nov;60(2):376–384. doi: 10.1128/jvi.60.2.376-384.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks T. D., Dougherty W. G. Substrate recognition by the NIa proteinase of two potyviruses involves multiple domains: characterization using genetically engineered hybrid proteinase molecules. Virology. 1991 May;182(1):17–27. doi: 10.1016/0042-6822(91)90643-p. [DOI] [PubMed] [Google Scholar]
- Parks T. D., Smith H. A., Dougherty W. G. Cleavage profiles of tobacco etch virus (TEV)-derived substrates mediated by precursor and processed forms of the TEV NIa proteinase. J Gen Virol. 1992 Jan;73(Pt 1):149–155. doi: 10.1099/0022-1317-73-1-149. [DOI] [PubMed] [Google Scholar]
- Partin K., Kräusslich H. G., Ehrlich L., Wimmer E., Carter C. Mutational analysis of a native substrate of the human immunodeficiency virus type 1 proteinase. J Virol. 1990 Aug;64(8):3938–3947. doi: 10.1128/jvi.64.8.3938-3947.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl L. H., Taylor W. R. A structural model for the retroviral proteases. Nature. 1987 Sep 24;329(6137):351–354. doi: 10.1038/329351a0. [DOI] [PubMed] [Google Scholar]
- Pearl L. H., Taylor W. R. Sequence specificity of retroviral proteases. Nature. 1987 Aug 6;328(6130):482–482. doi: 10.1038/328482b0. [DOI] [PubMed] [Google Scholar]
- Pearl L., Blundell T. The active site of aspartic proteinases. FEBS Lett. 1984 Aug 20;174(1):96–101. doi: 10.1016/0014-5793(84)81085-6. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Synthesis and proteolytic processing of cowpea mosaic virus proteins in reticulocyte lysates. Virology. 1979 Jul 30;96(2):463–477. doi: 10.1016/0042-6822(79)90104-1. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Translation of encephalomyocarditis virus RNA in vitro yields an active proteolytic processing enzyme. Eur J Biochem. 1978 Apr 17;85(2):457–462. doi: 10.1111/j.1432-1033.1978.tb12260.x. [DOI] [PubMed] [Google Scholar]
- Peng X. X., Shih D. S. Proteolytic processing of the proteins translated from the bottom component RNA of cowpea mosaic virus. The primary and secondary cleavage reactions. J Biol Chem. 1984 Mar 10;259(5):3197–3201. [PubMed] [Google Scholar]
- Peters S. A., Voorhorst W. G., Wellink J., van Kammen A. Processing of VPg-containing polyproteins encoded by the B-RNA from cowpea mosaic virus. Virology. 1992 Nov;191(1):90–97. doi: 10.1016/0042-6822(92)90169-p. [DOI] [PubMed] [Google Scholar]
- Peters S. A., Voorhorst W. G., Wery J., Wellink J., van Kammen A. A regulatory role for the 32K protein in proteolytic processing of cowpea mosaic virus polyproteins. Virology. 1992 Nov;191(1):81–89. doi: 10.1016/0042-6822(92)90168-o. [DOI] [PubMed] [Google Scholar]
- Petithory J. R., Masiarz F. R., Kirsch J. F., Santi D. V., Malcolm B. A. A rapid method for determination of endoproteinase substrate specificity: specificity of the 3C proteinase from hepatitis A virus. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11510–11514. doi: 10.1073/pnas.88.24.11510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer P., Hohn T. Involvement of reverse transcription in the replication of cauliflower mosaic virus: a detailed model and test of some aspects. Cell. 1983 Jul;33(3):781–789. doi: 10.1016/0092-8674(83)90020-x. [DOI] [PubMed] [Google Scholar]
- Pinck M., Reinbolt J., Loudes A. M., Le Ret M., Pinck L. Primary structure and location of the genome-linked protein (VPg) of grapevine fanleaf nepovirus. FEBS Lett. 1991 Jun 17;284(1):117–119. doi: 10.1016/0014-5793(91)80775-x. [DOI] [PubMed] [Google Scholar]
- Pletnev A. G., Yamshchikov V. F., Blinov V. M. Nucleotide sequence of the genome and complete amino acid sequence of the polyprotein of tick-borne encephalitis virus. Virology. 1990 Jan;174(1):250–263. doi: 10.1016/0042-6822(90)90073-z. [DOI] [PubMed] [Google Scholar]
- Power M. D., Marx P. A., Bryant M. L., Gardner M. B., Barr P. J., Luciw P. A. Nucleotide sequence of SRV-1, a type D simian acquired immune deficiency syndrome retrovirus. Science. 1986 Mar 28;231(4745):1567–1572. doi: 10.1126/science.3006247. [DOI] [PubMed] [Google Scholar]
- Preugschat F., Lenches E. M., Strauss J. H. Flavivirus enzyme-substrate interactions studied with chimeric proteinases: identification of an intragenic locus important for substrate recognition. J Virol. 1991 Sep;65(9):4749–4758. doi: 10.1128/jvi.65.9.4749-4758.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preugschat F., Yao C. W., Strauss J. H. In vitro processing of dengue virus type 2 nonstructural proteins NS2A, NS2B, and NS3. J Virol. 1990 Sep;64(9):4364–4374. doi: 10.1128/jvi.64.9.4364-4374.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prüfer D., Tacke E., Schmitz J., Kull B., Kaufmann A., Rohde W. Ribosomal frameshifting in plants: a novel signal directs the -1 frameshift in the synthesis of the putative viral replicase of potato leafroll luteovirus. EMBO J. 1992 Mar;11(3):1111–1117. doi: 10.1002/j.1460-2075.1992.tb05151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez L., Carrasco L. Lack of direct correlation between p220 cleavage and the shut-off of host translation after poliovirus infection. Virology. 1992 Jul;189(1):178–186. doi: 10.1016/0042-6822(92)90693-j. [DOI] [PubMed] [Google Scholar]
- Qu R. D., Bhattacharyya M., Laco G. S., De Kochko A., Rao B. L., Kaniewska M. B., Elmer J. S., Rochester D. E., Smith C. E., Beachy R. N. Characterization of the genome of rice tungro bacilliform virus: comparison with Commelina yellow mottle virus and caulimoviruses. Virology. 1991 Nov;185(1):354–364. doi: 10.1016/0042-6822(91)90783-8. [DOI] [PubMed] [Google Scholar]
- Rao J. K., Erickson J. W., Wlodawer A. Structural and evolutionary relationships between retroviral and eucaryotic aspartic proteinases. Biochemistry. 1991 May 14;30(19):4663–4671. doi: 10.1021/bi00233a005. [DOI] [PubMed] [Google Scholar]
- Reddy M. R., Viswanadhan V. N., Weinstein J. N. Relative differences in the binding free energies of human immunodeficiency virus 1 protease inhibitors: a thermodynamic cycle-perturbation approach. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10287–10291. doi: 10.1073/pnas.88.22.10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein A., McClure M. R., Rice N. R., Luftig R. B., Schultz A. M. Myristylation site in Pr65gag is essential for virus particle formation by Moloney murine leukemia virus. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7246–7250. doi: 10.1073/pnas.83.19.7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restrepo-Hartwig M. A., Carrington J. C. Regulation of nuclear transport of a plant potyvirus protein by autoproteolysis. J Virol. 1992 Sep;66(9):5662–5666. doi: 10.1128/jvi.66.9.5662-5666.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice C. M., Lenches E. M., Eddy S. R., Shin S. J., Sheets R. L., Strauss J. H. Nucleotide sequence of yellow fever virus: implications for flavivirus gene expression and evolution. Science. 1985 Aug 23;229(4715):726–733. doi: 10.1126/science.4023707. [DOI] [PubMed] [Google Scholar]
- Rice C. M., Levis R., Strauss J. H., Huang H. V. Production of infectious RNA transcripts from Sindbis virus cDNA clones: mapping of lethal mutations, rescue of a temperature-sensitive marker, and in vitro mutagenesis to generate defined mutants. J Virol. 1987 Dec;61(12):3809–3819. doi: 10.1128/jvi.61.12.3809-3819.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards A. D., Phylip L. H., Farmerie W. G., Scarborough P. E., Alvarez A., Dunn B. M., Hirel P. H., Konvalinka J., Strop P., Pavlickova L. Sensitive, soluble chromogenic substrates for HIV-1 proteinase. J Biol Chem. 1990 May 15;265(14):7733–7736. [PubMed] [Google Scholar]
- Richards A. D., Roberts R., Dunn B. M., Graves M. C., Kay J. Effective blocking of HIV-1 proteinase activity by characteristic inhibitors of aspartic proteinases. FEBS Lett. 1989 Apr 10;247(1):113–117. doi: 10.1016/0014-5793(89)81251-7. [DOI] [PubMed] [Google Scholar]
- Riechmann J. L., Laín S., García J. A. Highlights and prospects of potyvirus molecular biology. J Gen Virol. 1992 Jan;73(Pt 1):1–16. doi: 10.1099/0022-1317-73-1-1. [DOI] [PubMed] [Google Scholar]
- Ritzenthaler C., Viry M., Pinck M., Margis R., Fuchs M., Pinck L. Complete nucleotide sequence and genetic organization of grapevine fanleaf nepovirus RNA1. J Gen Virol. 1991 Oct;72(Pt 10):2357–2365. doi: 10.1099/0022-1317-72-10-2357. [DOI] [PubMed] [Google Scholar]
- Roberts N. A., Martin J. A., Kinchington D., Broadhurst A. V., Craig J. C., Duncan I. B., Galpin S. A., Handa B. K., Kay J., Kröhn A. Rational design of peptide-based HIV proteinase inhibitors. Science. 1990 Apr 20;248(4953):358–361. doi: 10.1126/science.2183354. [DOI] [PubMed] [Google Scholar]
- Rubinstein S. J., Hammerle T., Wimmer E., Dasgupta A. Infection of HeLa cells with poliovirus results in modification of a complex that binds to the rRNA promoter. J Virol. 1992 May;66(5):3062–3068. doi: 10.1128/jvi.66.5.3062-3068.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan M. D., King A. M., Thomas G. P. Cleavage of foot-and-mouth disease virus polyprotein is mediated by residues located within a 19 amino acid sequence. J Gen Virol. 1991 Nov;72(Pt 11):2727–2732. doi: 10.1099/0022-1317-72-11-2727. [DOI] [PubMed] [Google Scholar]
- Schechter I., Berger A. On the size of the active site in proteases. I. Papain. Biochem Biophys Res Commun. 1967 Apr 20;27(2):157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- Schiff L. A., Nibert M. L., Co M. S., Brown E. G., Fields B. N. Distinct binding sites for zinc and double-stranded RNA in the reovirus outer capsid protein sigma 3. Mol Cell Biol. 1988 Jan;8(1):273–283. doi: 10.1128/mcb.8.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider J., Kent S. B. Enzymatic activity of a synthetic 99 residue protein corresponding to the putative HIV-1 protease. Cell. 1988 Jul 29;54(3):363–368. doi: 10.1016/0092-8674(88)90199-7. [DOI] [PubMed] [Google Scholar]
- Schultz A. M., Oroszlan S. In vivo modification of retroviral gag gene-encoded polyproteins by myristic acid. J Virol. 1983 May;46(2):355–361. doi: 10.1128/jvi.46.2.355-361.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz A. M., Rein A. Unmyristylated Moloney murine leukemia virus Pr65gag is excluded from virus assembly and maturation events. J Virol. 1989 May;63(5):2370–2373. doi: 10.1128/jvi.63.5.2370-2373.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupham R. K., Jones K. J., Sagik B. P., Bose H. R., Jr Virus-directed post-translational cleavage in Sindbus virus-infected cells. J Virol. 1977 May;22(2):568–571. doi: 10.1128/jvi.22.2.568-571.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelmeier S., Schmidt H., Turk V., von der Helm K. Human immunodeficiency virus has an aspartic-type protease that can be inhibited by pepstatin A. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6612–6616. doi: 10.1073/pnas.85.18.6612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serghini M. A., Fuchs M., Pinck M., Reinbolt J., Walter B., Pinck L. RNA2 of grapevine fanleaf virus: sequence analysis and coat protein cistron location. J Gen Virol. 1990 Jul;71(Pt 7):1433–1441. doi: 10.1099/0022-1317-71-7-1433. [DOI] [PubMed] [Google Scholar]
- Shanks M., Lomonossoff G. P. The primary structure of the 24K protease from red clover mottle virus: implications for the mode of action of comovirus proteases. J Gen Virol. 1990 Mar;71(Pt 3):735–738. doi: 10.1099/0022-1317-71-3-735. [DOI] [PubMed] [Google Scholar]
- Shapira R., Choi G. H., Hillman B. I., Nuss D. L. The contribution of defective RNAs to the complexity of viral-encoded double-stranded RNA populations present in hypovirulent strains of the chestnut blight fungus Cryphonectria parasitica. EMBO J. 1991 Apr;10(4):741–746. doi: 10.1002/j.1460-2075.1991.tb08005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira R., Choi G. H., Nuss D. L. Virus-like genetic organization and expression strategy for a double-stranded RNA genetic element associated with biological control of chestnut blight. EMBO J. 1991 Apr;10(4):731–739. doi: 10.1002/j.1460-2075.1991.tb08004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira R., Nuss D. L. Gene expression by a hypovirulence-associated virus of the chestnut blight fungus involves two papain-like protease activities. Essential residues and cleavage site requirements for p48 autoproteolysis. J Biol Chem. 1991 Oct 15;266(29):19419–19425. [PubMed] [Google Scholar]
- Shih D. S., Bu M., Price M. A., Shih C. Y. Inhibition of cleavage of a plant viral polyprotein by an inhibitor activity present in wheat germ and cowpea embryos. J Virol. 1987 Mar;61(3):912–915. doi: 10.1128/jvi.61.3.912-915.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih D. S., Kaesberg P. Translation of brome mosaic viral ribonucleic acid in a cell-free system derived from wheat embryo. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1799–1803. doi: 10.1073/pnas.70.6.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih D. S., Shih C. T., Zimmern D., Rueckert R. R., Kaesberg P. Translation of encephalomyocarditis virus RNA in reticulocyte lysates: kinetic analysis of the formation of virion proteins and a protein required for processing. J Virol. 1979 May;30(2):472–480. doi: 10.1128/jvi.30.2.472-480.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirako Y., Strauss J. H. Cleavage between nsP1 and nsP2 initiates the processing pathway of Sindbis virus nonstructural polyprotein P123. Virology. 1990 Jul;177(1):54–64. doi: 10.1016/0042-6822(90)90459-5. [DOI] [PubMed] [Google Scholar]
- Showe M. K., Black L. W. Assembly core of bacteriophage T4: an intermediate in head formation. Nat New Biol. 1973 Mar 21;242(116):70–75. doi: 10.1038/newbio242070a0. [DOI] [PubMed] [Google Scholar]
- Showe M. K., Isobe E., Onorato L. Bacteriophage T4 prehead proteinase. I. Purification and properties of a bacteriophage enzyme which cleaves the capsid precursor proteins. J Mol Biol. 1976 Oct 15;107(1):35–54. doi: 10.1016/s0022-2836(76)80016-2. [DOI] [PubMed] [Google Scholar]
- Showe M. K., Isobe E., Onorato L. Bacteriophage T4 prehead proteinase. II. Its cleavage from the product of gene 21 and regulation in phage-infected cells. J Mol Biol. 1976 Oct 15;107(1):55–69. doi: 10.1016/s0022-2836(76)80017-4. [DOI] [PubMed] [Google Scholar]
- Showe M. K., Onorato L. Kinetic factors and form determination of the head of bacteriophage T4. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4165–4169. doi: 10.1073/pnas.75.9.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sielecki A. R., Fujinaga M., Read R. J., James M. N. Refined structure of porcine pepsinogen at 1.8 A resolution. J Mol Biol. 1991 Jun 20;219(4):671–692. doi: 10.1016/0022-2836(91)90664-r. [DOI] [PubMed] [Google Scholar]
- Silen J. L., Agard D. A. The alpha-lytic protease pro-region does not require a physical linkage to activate the protease domain in vivo. Nature. 1989 Oct 5;341(6241):462–464. doi: 10.1038/341462a0. [DOI] [PubMed] [Google Scholar]
- Simmons D. T., Strauss J. H. Translation of Sindbis virus 26 S RNA and 49 S RNA in lysates of rabbit reticulocytes. J Mol Biol. 1974 Jun 25;86(2):397–409. doi: 10.1016/0022-2836(74)90027-8. [DOI] [PubMed] [Google Scholar]
- Skalka A. M. Retroviral proteases: first glimpses at the anatomy of a processing machine. Cell. 1989 Mar 24;56(6):911–913. doi: 10.1016/0092-8674(89)90621-1. [DOI] [PubMed] [Google Scholar]
- Skern T., Sommergruber W., Auer H., Volkmann P., Zorn M., Liebig H. D., Fessl F., Blaas D., Kuechler E. Substrate requirements of a human rhinoviral 2A proteinase. Virology. 1991 Mar;181(1):46–54. doi: 10.1016/0042-6822(91)90468-q. [DOI] [PubMed] [Google Scholar]
- Smith S. O., Farr-Jones S., Griffin R. G., Bachovchin W. W. Crystal versus solution structures of enzymes: NMR spectroscopy of a crystalline serine protease. Science. 1989 May 26;244(4907):961–964. doi: 10.1126/science.2499045. [DOI] [PubMed] [Google Scholar]
- Smith T. A., Kohorn B. D. Direct selection for sequences encoding proteases of known specificity. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5159–5162. doi: 10.1073/pnas.88.12.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommergruber W., Zorn M., Blaas D., Fessl F., Volkmann P., Maurer-Fogy I., Pallai P., Merluzzi V., Matteo M., Skern T. Polypeptide 2A of human rhinovirus type 2: identification as a protease and characterization by mutational analysis. Virology. 1989 Mar;169(1):68–77. doi: 10.1016/0042-6822(89)90042-1. [DOI] [PubMed] [Google Scholar]
- Sonenberg N. Poliovirus translation. Curr Top Microbiol Immunol. 1990;161:23–47. doi: 10.1007/978-3-642-75602-3_2. [DOI] [PubMed] [Google Scholar]
- Sprang S., Standing T., Fletterick R. J., Stroud R. M., Finer-Moore J., Xuong N. H., Hamlin R., Rutter W. J., Craik C. S. The three-dimensional structure of Asn102 mutant of trypsin: role of Asp102 in serine protease catalysis. Science. 1987 Aug 21;237(4817):905–909. doi: 10.1126/science.3112942. [DOI] [PubMed] [Google Scholar]
- Steitz T. A., Shulman R. G. Crystallographic and NMR studies of the serine proteases. Annu Rev Biophys Bioeng. 1982;11:419–444. doi: 10.1146/annurev.bb.11.060182.002223. [DOI] [PubMed] [Google Scholar]
- Strauss E. G., De Groot R. J., Levinson R., Strauss J. H. Identification of the active site residues in the nsP2 proteinase of Sindbis virus. Virology. 1992 Dec;191(2):932–940. doi: 10.1016/0042-6822(92)90268-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strebel K., Beck E. A second protease of foot-and-mouth disease virus. J Virol. 1986 Jun;58(3):893–899. doi: 10.1128/jvi.58.3.893-899.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr Evidence for large precursor proteins in poliovirus synthesis. Proc Natl Acad Sci U S A. 1968 Mar;59(3):966–971. doi: 10.1073/pnas.59.3.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers D. F., Shaw E. N., Stewart M. L., Maizel J. V., Jr Inhibition of cleavage of large poliovirus-specific precursor proteins in infected HeLa cells by inhibitors of proteolytic enzymes. J Virol. 1972 Oct;10(4):880–884. doi: 10.1128/jvi.10.4.880-884.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X. H., Baltimore D. Human immunodeficiency virus tat-activated expression of poliovirus protein 2A inhibits mRNA translation. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2143–2146. doi: 10.1073/pnas.86.7.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain A. L., Miller M. M., Green J., Rich D. H., Schneider J., Kent S. B., Wlodawer A. X-ray crystallographic structure of a complex between a synthetic protease of human immunodeficiency virus 1 and a substrate-based hydroxyethylamine inhibitor. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8805–8809. doi: 10.1073/pnas.87.22.8805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacke E., Prüfer D., Salamini F., Rohde W. Characterization of a potato leafroll luteovirus subgenomic RNA: differential expression by internal translation initiation and UAG suppression. J Gen Virol. 1990 Oct;71(Pt 10):2265–2272. doi: 10.1099/0022-1317-71-10-2265. [DOI] [PubMed] [Google Scholar]
- Takahara Y., Ando N., Kohara M., Hagino-Yamagishi K., Nomoto A., Itoh H., Numao N., Kondo K. Purification of enzymatically active poliovirus proteinase 3C produced in Escherichia coli. Gene. 1989 Jul 15;79(2):249–258. doi: 10.1016/0378-1119(89)90207-2. [DOI] [PubMed] [Google Scholar]
- Takkinen K. Complete nucleotide sequence of the nonstructural protein genes of Semliki Forest virus. Nucleic Acids Res. 1986 Jul 25;14(14):5667–5682. doi: 10.1093/nar/14.14.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takkinen K., Peränen J., Keränen S., Söderlund H., Käriäinen L. The Semliki-Forest-virus-specific nonstructural protein nsP4 is an autoproteinase. Eur J Biochem. 1990 Apr 20;189(1):33–38. doi: 10.1111/j.1432-1033.1990.tb15456.x. [DOI] [PubMed] [Google Scholar]
- Takkinen K., Peränen J., Käriäinen L. Proteolytic processing of Semliki Forest virus-specific non-structural polyprotein. J Gen Virol. 1991 Jul;72(Pt 7):1627–1633. doi: 10.1099/0022-1317-72-7-1627. [DOI] [PubMed] [Google Scholar]
- Tang J., James M. N., Hsu I. N., Jenkins J. A., Blundell T. L. Structural evidence for gene duplication in the evolution of the acid proteases. Nature. 1978 Feb 16;271(5646):618–621. doi: 10.1038/271618a0. [DOI] [PubMed] [Google Scholar]
- Tang J., Wong R. N. Evolution in the structure and function of aspartic proteases. J Cell Biochem. 1987 Jan;33(1):53–63. doi: 10.1002/jcb.240330106. [DOI] [PubMed] [Google Scholar]
- Taylor J. W., Ott J., Eckstein F. The rapid generation of oligonucleotide-directed mutations at high frequency using phosphorothioate-modified DNA. Nucleic Acids Res. 1985 Dec 20;13(24):8765–8785. doi: 10.1093/nar/13.24.8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesar M., Marquardt O. Foot-and-mouth disease virus protease 3C inhibits cellular transcription and mediates cleavage of histone H3. Virology. 1990 Feb;174(2):364–374. doi: 10.1016/0042-6822(90)90090-e. [DOI] [PubMed] [Google Scholar]
- Thach R. E. Cap recap: the involvement of eIF-4F in regulating gene expression. Cell. 1992 Jan 24;68(2):177–180. doi: 10.1016/0092-8674(92)90461-k. [DOI] [PubMed] [Google Scholar]
- Thayer M. M., Flaherty K. M., McKay D. B. Three-dimensional structure of the elastase of Pseudomonas aeruginosa at 1.5-A resolution. J Biol Chem. 1991 Feb 15;266(5):2864–2871. doi: 10.2210/pdb1ezm/pdb. [DOI] [PubMed] [Google Scholar]
- Tillotson L., Shatkin A. J. Reovirus polypeptide sigma 3 and N-terminal myristoylation of polypeptide mu 1 are required for site-specific cleavage to mu 1C in transfected cells. J Virol. 1992 Apr;66(4):2180–2186. doi: 10.1128/jvi.66.4.2180-2186.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh H., Kikuno R., Hayashida H., Miyata T., Kugimiya W., Inouye S., Yuki S., Saigo K. Close structural resemblance between putative polymerase of a Drosophila transposable genetic element 17.6 and pol gene product of Moloney murine leukaemia virus. EMBO J. 1985 May;4(5):1267–1272. doi: 10.1002/j.1460-2075.1985.tb03771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasselli A. G., Hui J. O., Sawyer T. K., Staples D. J., Bannow C., Reardon I. M., Howe W. J., DeCamp D. L., Craik C. S., Heinrikson R. L. Specificity and inhibition of proteases from human immunodeficiency viruses 1 and 2. J Biol Chem. 1990 Aug 25;265(24):14675–14683. [PubMed] [Google Scholar]
- Torruella M., Gordon K., Hohn T. Cauliflower mosaic virus produces an aspartic proteinase to cleave its polyproteins. EMBO J. 1989 Oct;8(10):2819–2825. doi: 10.1002/j.1460-2075.1989.tb08428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda H., Nicklin M. J., Murray M. G., Anderson C. W., Dunn J. J., Studier F. W., Wimmer E. A second virus-encoded proteinase involved in proteolytic processing of poliovirus polyprotein. Cell. 1986 Jun 6;45(5):761–770. doi: 10.1016/0092-8674(86)90790-7. [DOI] [PubMed] [Google Scholar]
- Tremblay M. L., Déry C. V., Talbot B. G., Weber J. In vitro cleavage specificity of the adenovirus type 2 proteinase. Biochim Biophys Acta. 1983 Mar 16;743(2):239–245. doi: 10.1016/0167-4838(83)90220-0. [DOI] [PubMed] [Google Scholar]
- Tritch R. J., Cheng Y. E., Yin F. H., Erickson-Viitanen S. Mutagenesis of protease cleavage sites in the human immunodeficiency virus type 1 gag polyprotein. J Virol. 1991 Feb;65(2):922–930. doi: 10.1128/jvi.65.2.922-930.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakharia V. N., Devaney M. A., Moore D. M., Dunn J. J., Grubman M. J. Proteolytic processing of foot-and-mouth disease virus polyproteins expressed in a cell-free system from clone-derived transcripts. J Virol. 1987 Oct;61(10):3199–3207. doi: 10.1128/jvi.61.10.3199-3207.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanSlyke J. K., Franke C. A., Hruby D. E. Proteolytic maturation of vaccinia virus core proteins: identification of a conserved motif at the N termini of the 4b and 25K virion proteins. J Gen Virol. 1991 Feb;72(Pt 2):411–416. doi: 10.1099/0022-1317-72-2-411. [DOI] [PubMed] [Google Scholar]
- Vanslyke J. K., Whitehead S. S., Wilson E. M., Hruby D. E. The multistep proteolytic maturation pathway utilized by vaccinia virus P4a protein: a degenerate conserved cleavage motif within core proteins. Virology. 1991 Aug;183(2):467–478. doi: 10.1016/0042-6822(91)90976-i. [DOI] [PubMed] [Google Scholar]
- Veidt I., Bouzoubaa S. E., Leiser R. M., Ziegler-Graff V., Guilley H., Richards K., Jonard G. Synthesis of full-length transcripts of beet western yellows virus RNA: messenger properties and biological activity in protoplasts. Virology. 1992 Jan;186(1):192–200. doi: 10.1016/0042-6822(92)90073-x. [DOI] [PubMed] [Google Scholar]
- Veidt I., Lot H., Leiser M., Scheidecker D., Guilley H., Richards K., Jonard G. Nucleotide sequence of beet western yellows virus RNA. Nucleic Acids Res. 1988 Nov 11;16(21):9917–9932. doi: 10.1093/nar/16.21.9917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verchot J., Koonin E. V., Carrington J. C. The 35-kDa protein from the N-terminus of the potyviral polyprotein functions as a third virus-encoded proteinase. Virology. 1991 Dec;185(2):527–535. doi: 10.1016/0042-6822(91)90522-d. [DOI] [PubMed] [Google Scholar]
- Verver J., Goldbach R., Garcia J. A., Vos P. In vitro expression of a full-length DNA copy of cowpea mosaic virus B RNA: identification of the B RNA encoded 24-kd protein as a viral protease. EMBO J. 1987 Mar;6(3):549–554. doi: 10.1002/j.1460-2075.1987.tb04789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt V. M., Eisenman R., Diggelmann H. Generation of avian myeloblastosis virus structural proteins by proteolytic cleavage of a precursor polypeptide. J Mol Biol. 1975 Aug 15;96(3):471–493. doi: 10.1016/0022-2836(75)90174-6. [DOI] [PubMed] [Google Scholar]
- Vos H. L., van der Lee F. M., Reemst A. M., van Loon A. E., Sussenbach J. S. The genes encoding the DNA binding protein and the 23K protease of adenovirus types 40 and 41. Virology. 1988 Mar;163(1):1–10. doi: 10.1016/0042-6822(88)90227-9. [DOI] [PubMed] [Google Scholar]
- Vos P., Verver J., Jaegle M., Wellink J., van Kammen A., Goldbach R. Two viral proteins involved in the proteolytic processing of the cowpea mosaic virus polyproteins. Nucleic Acids Res. 1988 Mar 25;16(5):1967–1985. doi: 10.1093/nar/16.5.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warshel A., Naray-Szabo G., Sussman F., Hwang J. K. How do serine proteases really work? Biochemistry. 1989 May 2;28(9):3629–3637. doi: 10.1021/bi00435a001. [DOI] [PubMed] [Google Scholar]
- Weber I. T., Miller M., Jaskólski M., Leis J., Skalka A. M., Wlodawer A. Molecular modeling of the HIV-1 protease and its substrate binding site. Science. 1989 Feb 17;243(4893):928–931. doi: 10.1126/science.2537531. [DOI] [PubMed] [Google Scholar]
- Weber J. M., Anderson C. W. Identification of the gene coding for the precursor of adenovirus core protein X. J Virol. 1988 May;62(5):1741–1745. doi: 10.1128/jvi.62.5.1741-1745.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster A., Russell S., Talbot P., Russell W. C., Kemp G. D. Characterization of the adenovirus proteinase: substrate specificity. J Gen Virol. 1989 Dec;70(Pt 12):3225–3234. doi: 10.1099/0022-1317-70-12-3225. [DOI] [PubMed] [Google Scholar]
- Webster A., Russell W. C., Kemp G. D. Characterization of the adenovirus proteinase: development and use of a specific peptide assay. J Gen Virol. 1989 Dec;70(Pt 12):3215–3223. doi: 10.1099/0022-1317-70-12-3215. [DOI] [PubMed] [Google Scholar]
- Welch A. R., Woods A. S., McNally L. M., Cotter R. J., Gibson W. A herpesvirus maturational proteinase, assemblin: identification of its gene, putative active site domain, and cleavage site. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10792–10796. doi: 10.1073/pnas.88.23.10792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellink J., van Kammen A. Proteases involved in the processing of viral polyproteins. Brief review. Arch Virol. 1988;98(1-2):1–26. doi: 10.1007/BF01321002. [DOI] [PubMed] [Google Scholar]
- Wengler G., Czaya G., Färber P. M., Hegemann J. H. In vitro synthesis of West Nile virus proteins indicates that the amino-terminal segment of the NS3 protein contains the active centre of the protease which cleaves the viral polyprotein after multiple basic amino acids. J Gen Virol. 1991 Apr;72(Pt 4):851–858. doi: 10.1099/0022-1317-72-4-851. [DOI] [PubMed] [Google Scholar]
- Wiener J. R., Joklik W. K. Evolution of reovirus genes: a comparison of serotype 1, 2, and 3 M2 genome segments, which encode the major structural capsid protein mu 1C. Virology. 1988 Apr;163(2):603–613. doi: 10.1016/0042-6822(88)90301-7. [DOI] [PubMed] [Google Scholar]
- Windheuser M. G., Dwyer S., Dasmahapatra B. Expression of functional beta-galactosidase containing the coxsackievirus 3C protease as an internal fusion. Biochem Biophys Res Commun. 1991 May 31;177(1):243–251. doi: 10.1016/0006-291x(91)91974-h. [DOI] [PubMed] [Google Scholar]
- Wiskerchen M., Belzer S. K., Collett M. S. Pestivirus gene expression: the first protein product of the bovine viral diarrhea virus large open reading frame, p20, possesses proteolytic activity. J Virol. 1991 Aug;65(8):4508–4514. doi: 10.1128/jvi.65.8.4508-4514.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiskerchen M., Collett M. S. Pestivirus gene expression: protein p80 of bovine viral diarrhea virus is a proteinase involved in polyprotein processing. Virology. 1991 Sep;184(1):341–350. doi: 10.1016/0042-6822(91)90850-b. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Baltimore D. Relationship of retrovirus polyprotein cleavages to virion maturation studied with temperature-sensitive murine leukemia virus mutants. J Virol. 1978 Jun;26(3):750–761. doi: 10.1128/jvi.26.3.750-761.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlodawer A., Miller M., Jaskólski M., Sathyanarayana B. K., Baldwin E., Weber I. T., Selk L. M., Clawson L., Schneider J., Kent S. B. Conserved folding in retroviral proteases: crystal structure of a synthetic HIV-1 protease. Science. 1989 Aug 11;245(4918):616–621. doi: 10.1126/science.2548279. [DOI] [PubMed] [Google Scholar]
- Wu S. X., Rinehart C. A., Kaesberg P. Sequence and organization of southern bean mosaic virus genomic RNA. Virology. 1987 Nov;161(1):73–80. doi: 10.1016/0042-6822(87)90172-3. [DOI] [PubMed] [Google Scholar]
- Wyckoff E. E., Hershey J. W., Ehrenfeld E. Eukaryotic initiation factor 3 is required for poliovirus 2A protease-induced cleavage of the p220 component of eukaryotic initiation factor 4F. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9529–9533. doi: 10.1073/pnas.87.24.9529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyckoff E. E., Lloyd R. E., Ehrenfeld E. Relationship of eukaryotic initiation factor 3 to poliovirus-induced p220 cleavage activity. J Virol. 1992 May;66(5):2943–2951. doi: 10.1128/jvi.66.5.2943-2951.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto D., Matsumoto K., Ohishi H., Ishida T., Inoue M., Kitamura K., Mizuno H. Refined x-ray structure of papain.E-64-c complex at 2.1-A resolution. J Biol Chem. 1991 Aug 5;266(22):14771–14777. doi: 10.2210/pdb1pe6/pdb. [DOI] [PubMed] [Google Scholar]
- Yeh-Kai L., Akusjärvi G., Aleström P., Pettersson U., Tremblay M., Weber J. Genetic identification of an endoproteinase encoded by the adenovirus genome. J Mol Biol. 1983 Jun 15;167(1):217–222. doi: 10.1016/s0022-2836(83)80044-8. [DOI] [PubMed] [Google Scholar]
- Yoshinaka Y., Katoh I., Copeland T. D., Oroszlan S. Translational readthrough of an amber termination codon during synthesis of feline leukemia virus protease. J Virol. 1985 Sep;55(3):870–873. doi: 10.1128/jvi.55.3.870-873.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaka Y., Luftig R. B. Murine leukemia virus morphogenesis: cleavage of P70 in vitro can be accompanied by a shift from a concentrically coiled internal strand ("immature") to a collapsed ("mature") form of the virus core. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3446–3450. doi: 10.1073/pnas.74.8.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaka Y., Luftig R. B. Properties of a P70 proteolytic factor of murine leukemia viruses. Cell. 1977 Nov;12(3):709–719. doi: 10.1016/0092-8674(77)90271-9. [DOI] [PubMed] [Google Scholar]
- Ypma-Wong M. F., Dewalt P. G., Johnson V. H., Lamb J. G., Semler B. L. Protein 3CD is the major poliovirus proteinase responsible for cleavage of the P1 capsid precursor. Virology. 1988 Sep;166(1):265–270. doi: 10.1016/0042-6822(88)90172-9. [DOI] [PubMed] [Google Scholar]
- Ypma-Wong M. F., Filman D. J., Hogle J. M., Semler B. L. Structural domains of the poliovirus polyprotein are major determinants for proteolytic cleavage at Gln-Gly pairs. J Biol Chem. 1988 Nov 25;263(33):17846–17856. [PubMed] [Google Scholar]
- Ypma-Wong M. F., Semler B. L. In vitro molecular genetics as a tool for determining the differential cleavage specificities of the poliovirus 3C proteinase. Nucleic Acids Res. 1987 Mar 11;15(5):2069–2088. doi: 10.1093/nar/15.5.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S. F., Lloyd R. E. Characterization of the roles of conserved cysteine and histidine residues in poliovirus 2A protease. Virology. 1992 Feb;186(2):725–735. doi: 10.1016/0042-6822(92)90039-r. [DOI] [PubMed] [Google Scholar]
- Yu S. F., Lloyd R. E. Identification of essential amino acid residues in the functional activity of poliovirus 2A protease. Virology. 1991 Jun;182(2):615–625. doi: 10.1016/0042-6822(91)90602-8. [DOI] [PubMed] [Google Scholar]
- Zweerink H. J., Joklik W. K. Studies on the intracellular synthesis of reovirus-specified proteins. Virology. 1970 Jul;41(3):501–518. doi: 10.1016/0042-6822(70)90171-6. [DOI] [PubMed] [Google Scholar]
- de Groot R. J., Hardy W. R., Shirako Y., Strauss J. H. Cleavage-site preferences of Sindbis virus polyproteins containing the non-structural proteinase. Evidence for temporal regulation of polyprotein processing in vivo. EMBO J. 1990 Aug;9(8):2631–2638. doi: 10.1002/j.1460-2075.1990.tb07445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot R. J., Rümenapf T., Kuhn R. J., Strauss E. G., Strauss J. H. Sindbis virus RNA polymerase is degraded by the N-end rule pathway. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):8967–8971. doi: 10.1073/pnas.88.20.8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Helm K. Cleavage of Rous sarcoma viral polypeptide precursor into internal structural proteins in vitro involves viral protein p15. Proc Natl Acad Sci U S A. 1977 Mar;74(3):911–915. doi: 10.1073/pnas.74.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]