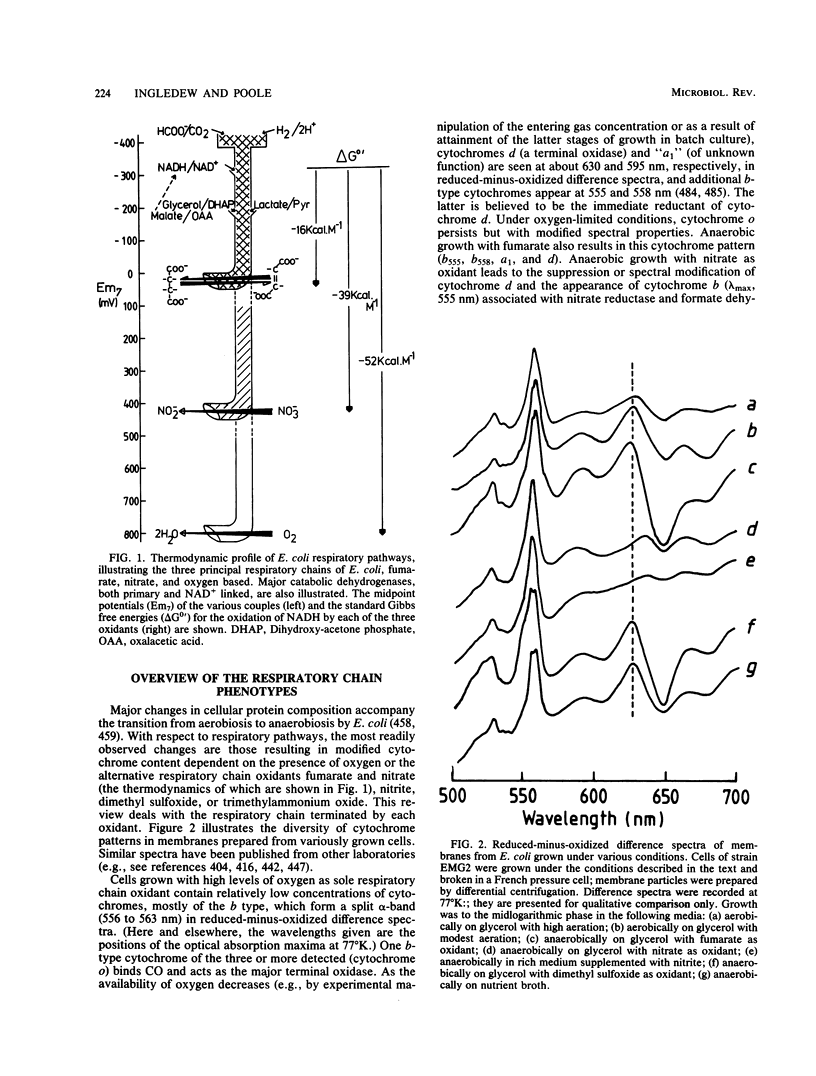
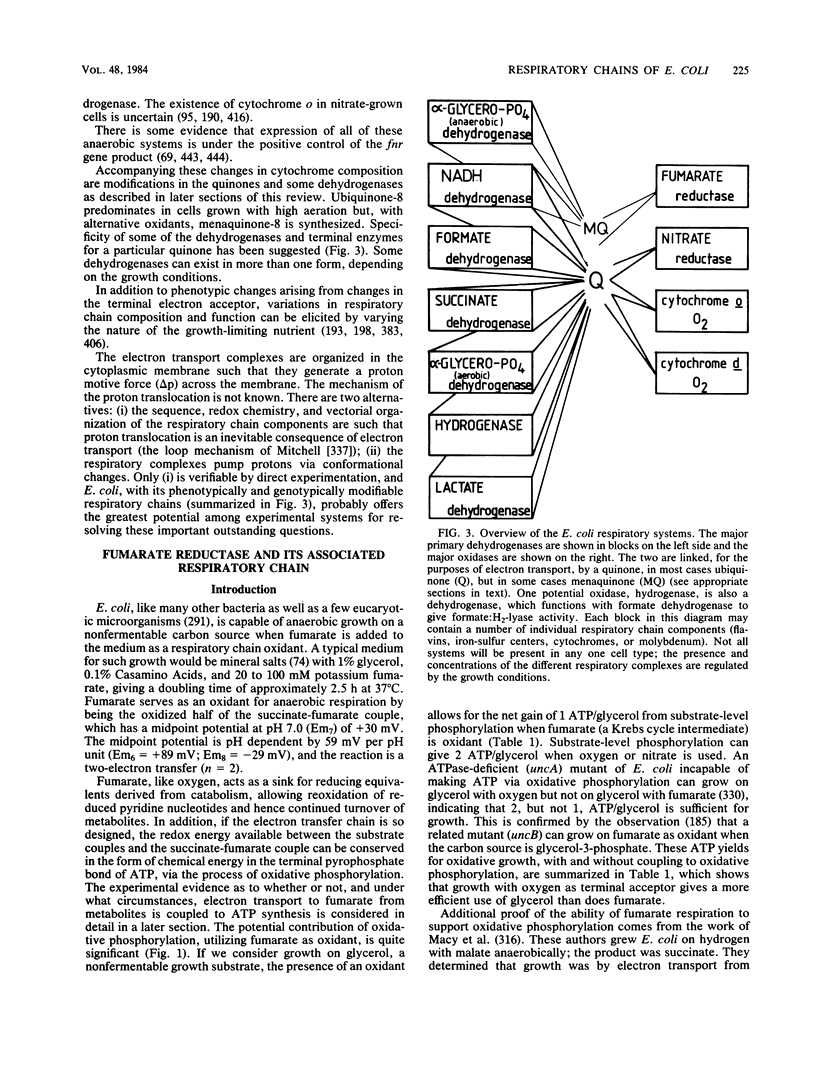
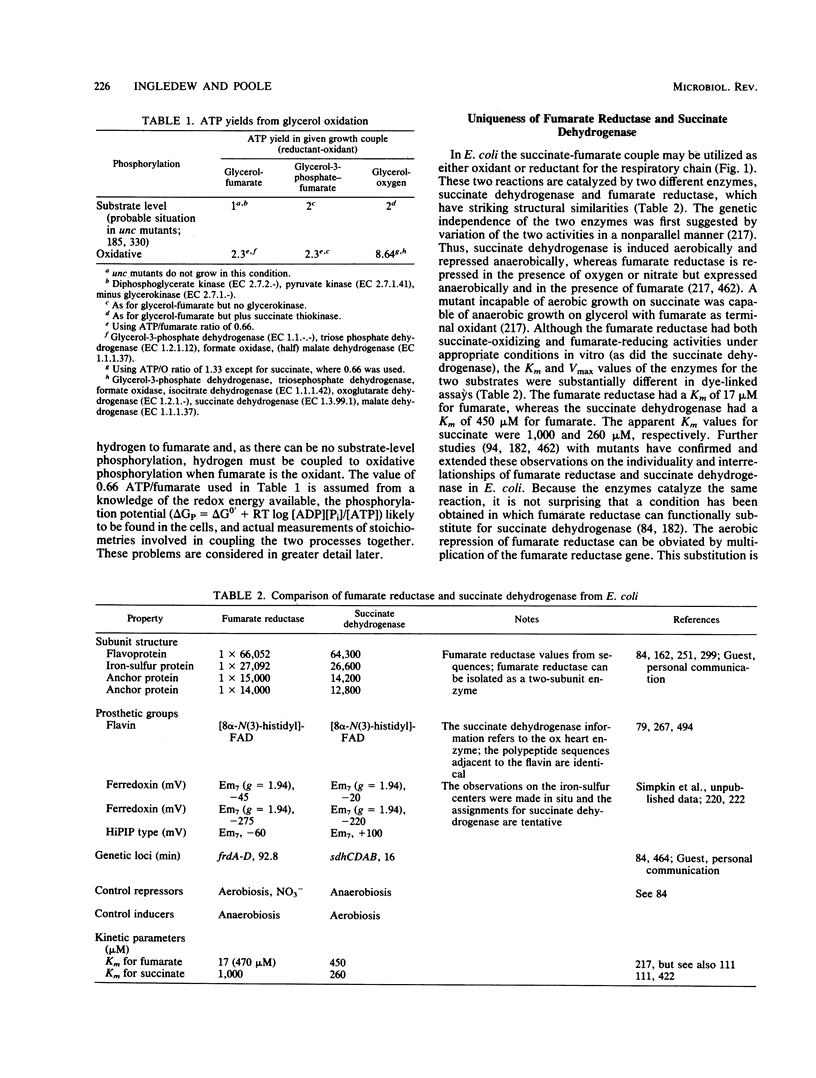
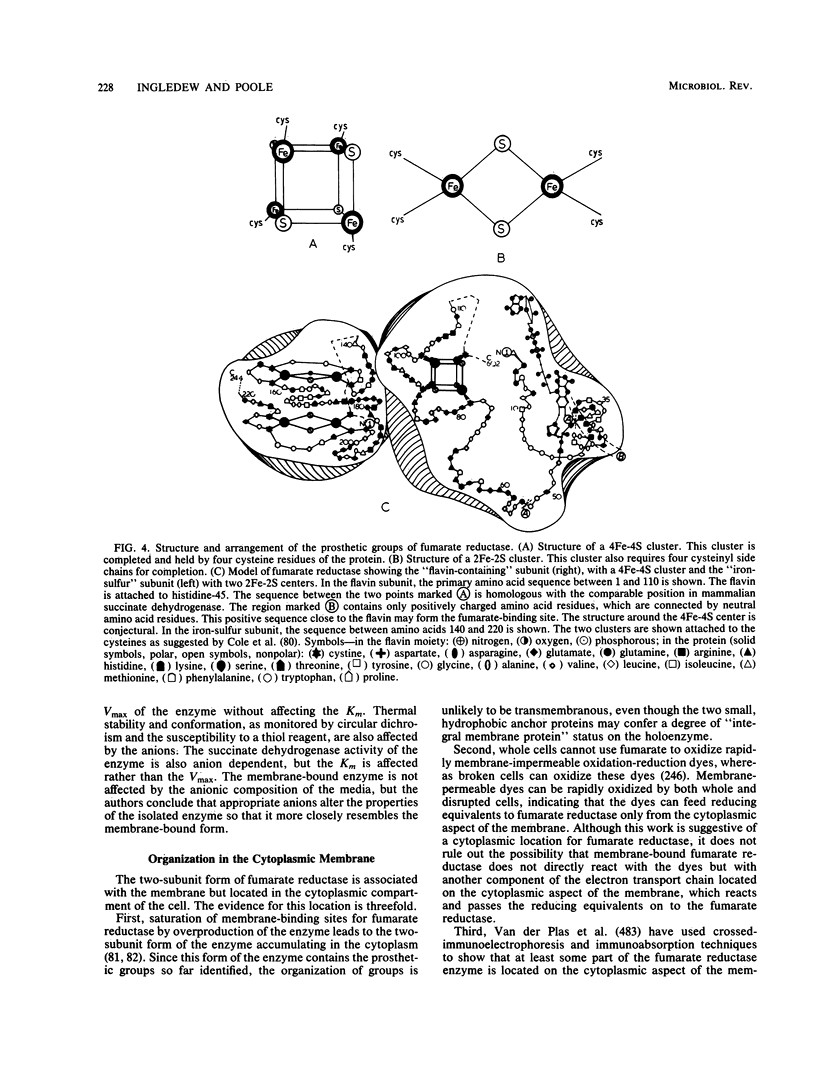
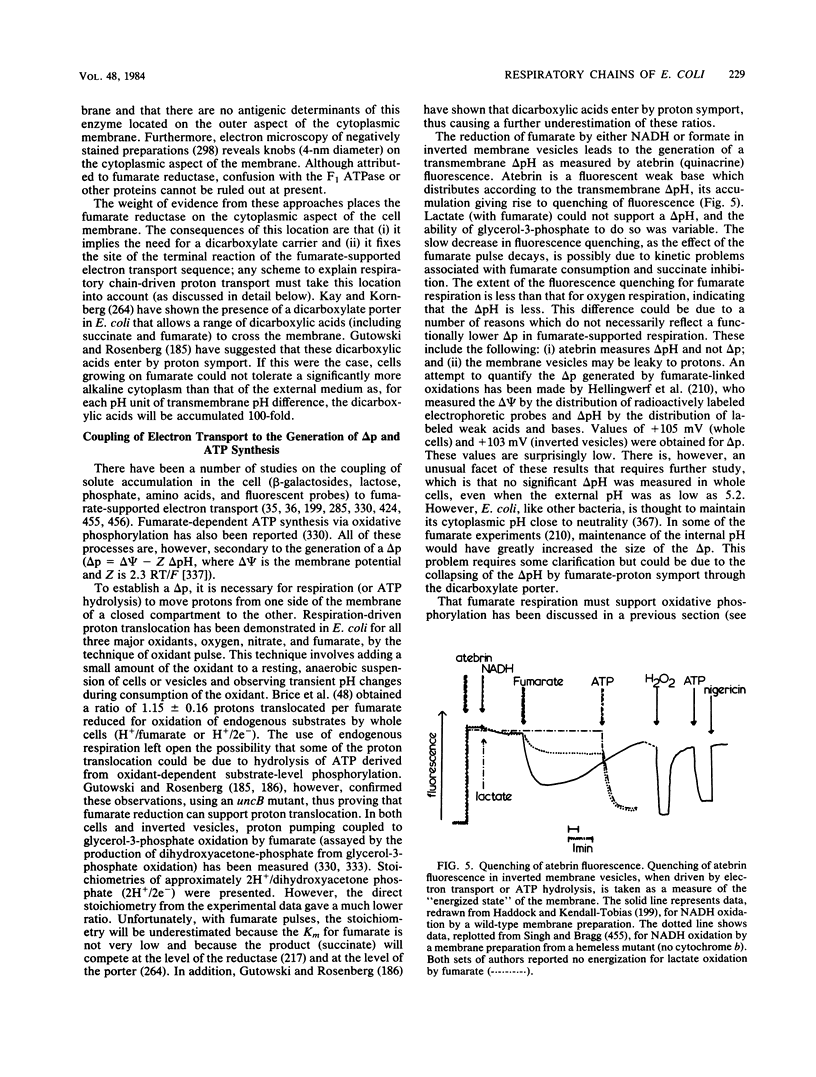
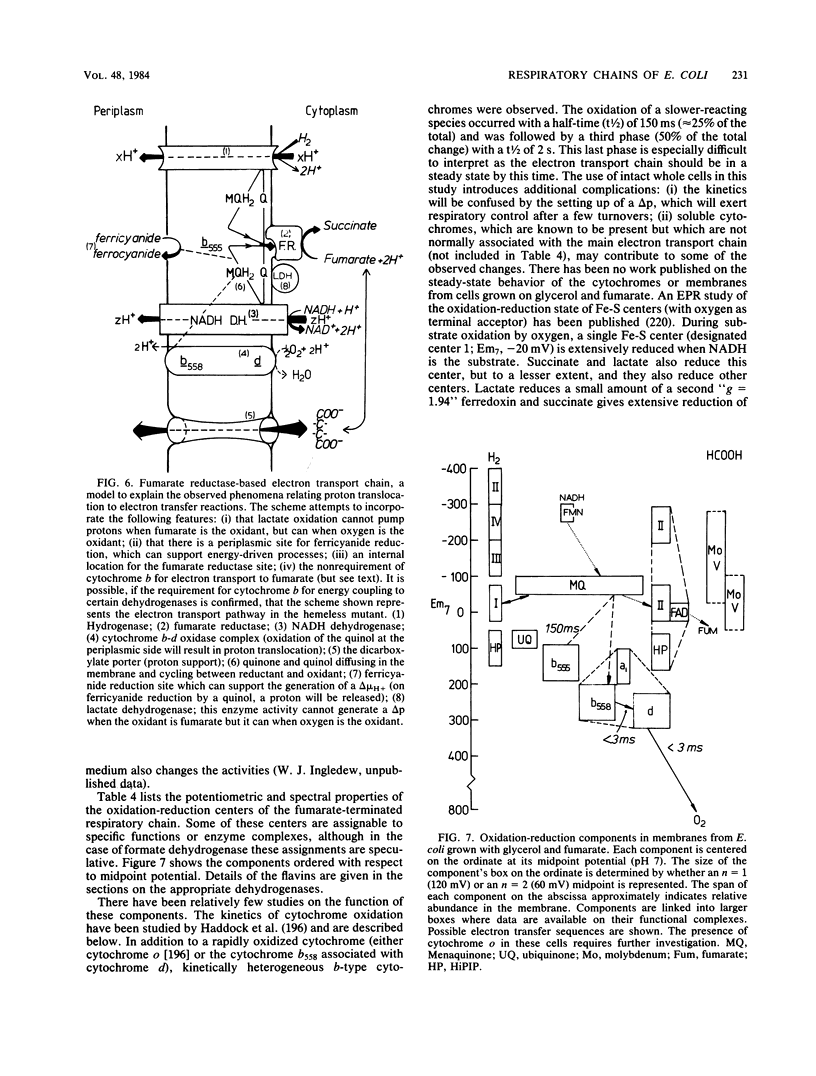
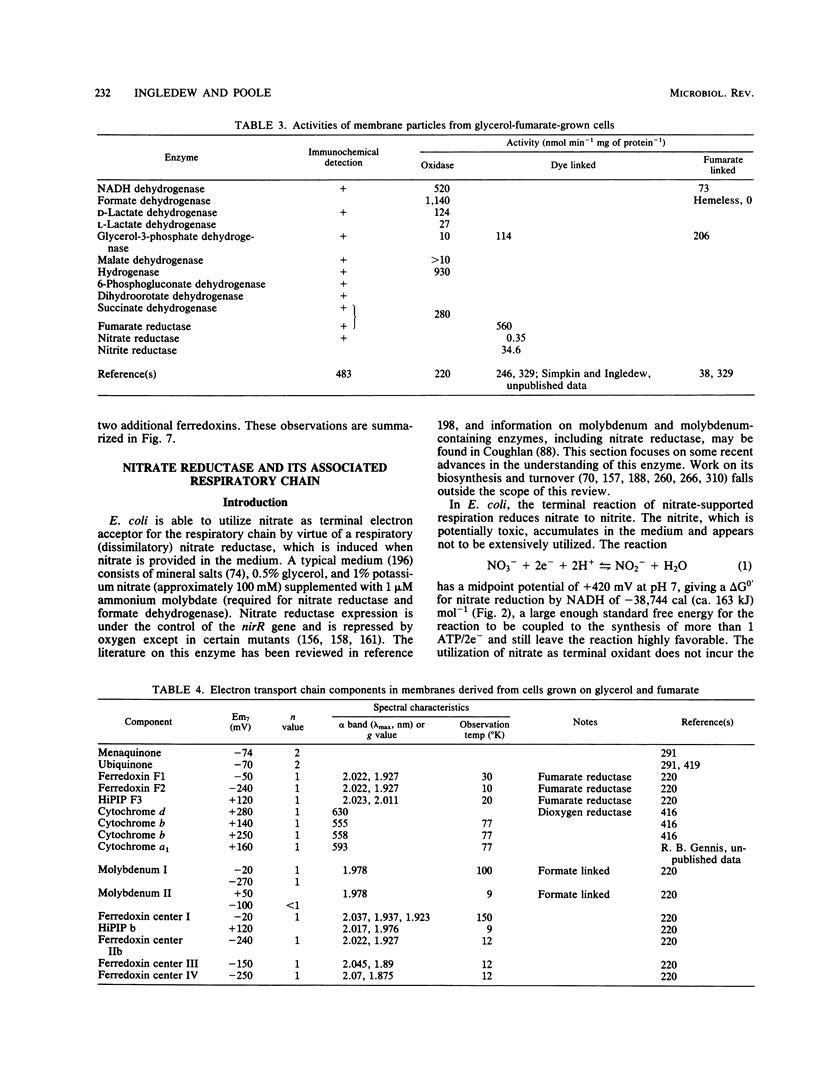
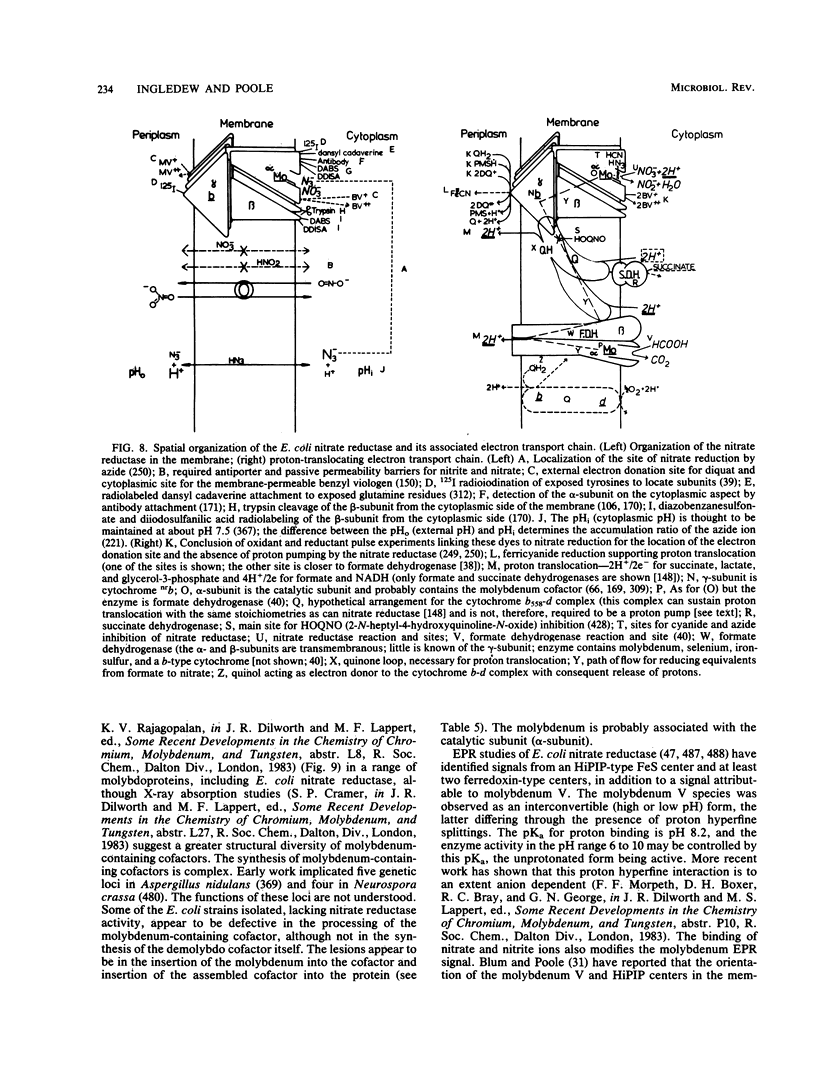
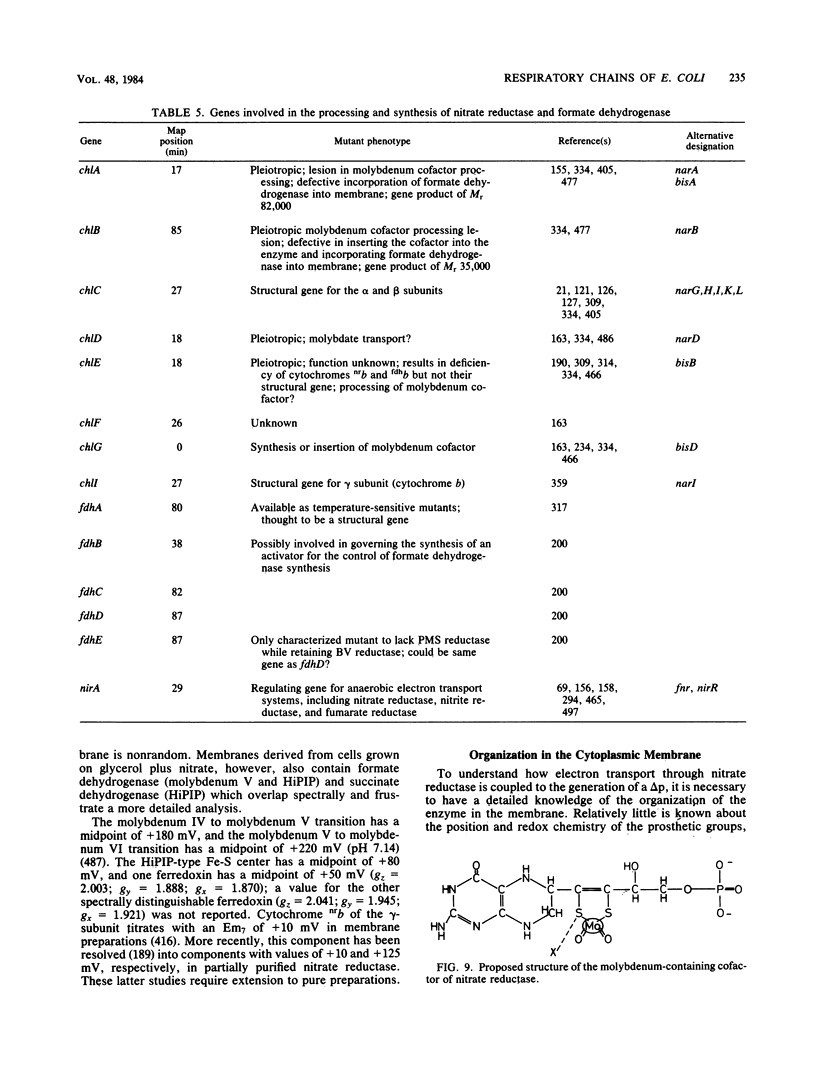
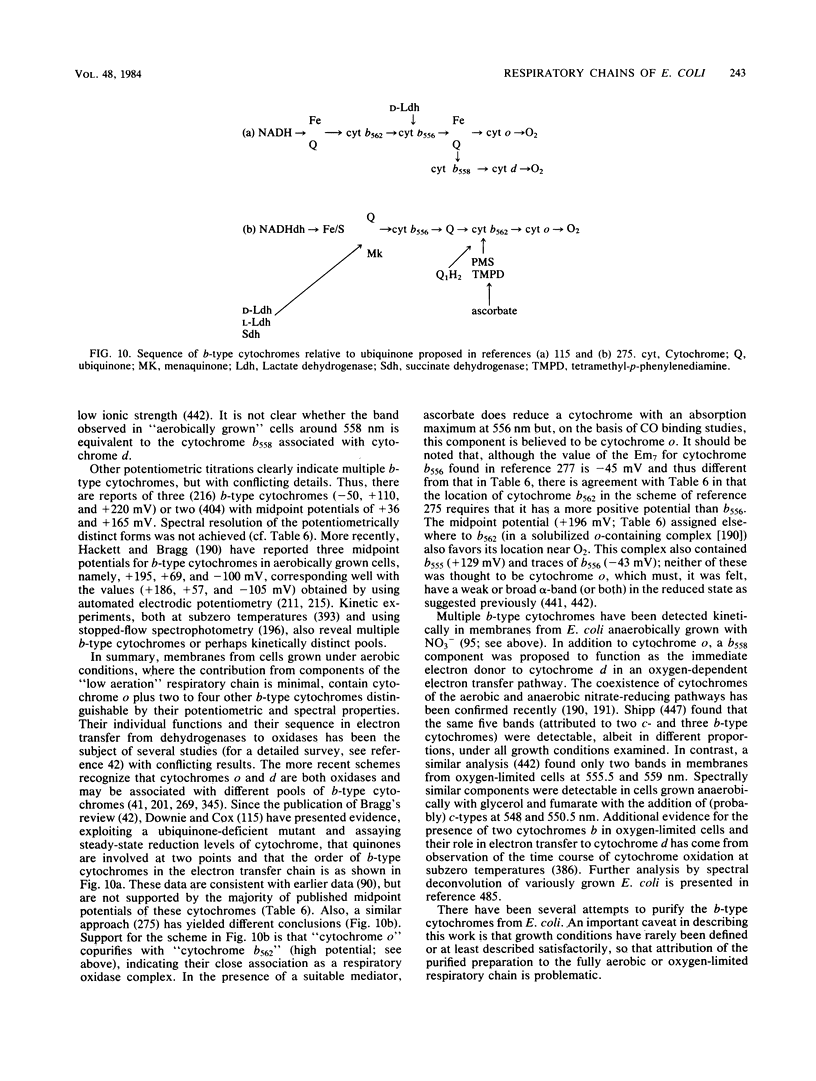
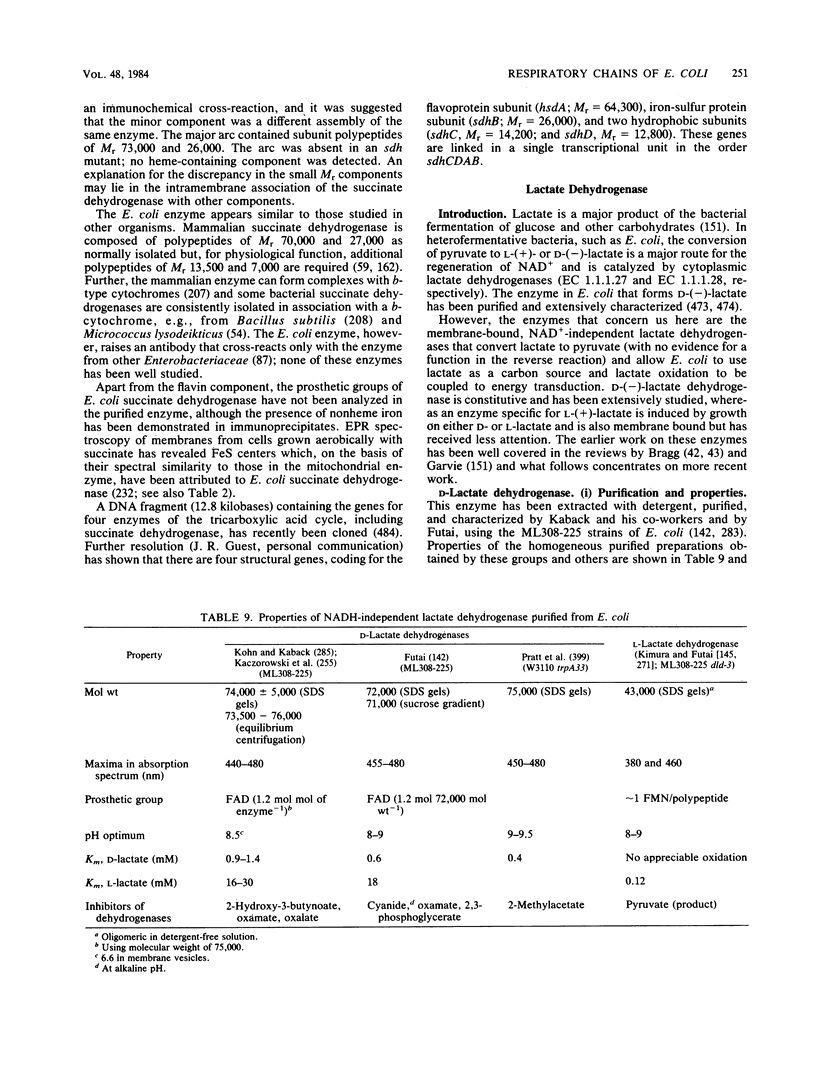
Full text
PDF




































Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou-Jaoudé A., Chippaux M., Pascal M. C. Formate-nitrite reduction in Escherchia coli K12. 1. Physiological study of the system. Eur J Biochem. 1979 Apr 2;95(2):309–314. doi: 10.1111/j.1432-1033.1979.tb12966.x. [DOI] [PubMed] [Google Scholar]
- Abou-Jaoudé A., Pascal M. C., Chippaux M. Formate-nitrite reduction in Escherichia coli K12. 2. Identification of components involved in the electron transfer. Eur J Biochem. 1979 Apr 2;95(2):315–321. doi: 10.1111/j.1432-1033.1979.tb12967.x. [DOI] [PubMed] [Google Scholar]
- Ackrell B. A., Asato R. N., Mower H. F. Multiple forms of bacterial hydrogenases. J Bacteriol. 1966 Oct;92(4):828–838. doi: 10.1128/jb.92.4.828-838.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams M. W., Hall D. O. Purification of the membrane-bound hydrogenase of Escherichia coli. Biochem J. 1979 Oct 1;183(1):11–22. doi: 10.1042/bj1830011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams M. W., Mortenson L. E., Chen J. S. Hydrogenase. Biochim Biophys Acta. 1980 Dec;594(2-3):105–176. doi: 10.1016/0304-4173(80)90007-5. [DOI] [PubMed] [Google Scholar]
- Adler L. W., Ichikawa T., Hasan S. M., Tsuchiya T., Rosen B. P. Orientation of the protonmotive force in membrane vesicles of Escherichia coli. J Supramol Struct. 1977;7(1):15–27. doi: 10.1002/jss.400070103. [DOI] [PubMed] [Google Scholar]
- Adler L. W., Rosen B. P. Functional mosaicism of membrane proteins in vesicles of Escherichia coli. J Bacteriol. 1977 Feb;129(2):959–966. doi: 10.1128/jb.129.2.959-966.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alben J. O., Moh P. P., Fiamingo F. G., Altschuld R. A. Cytochrome oxidase (a3) heme and copper observed by low-temperature Fourier transform infrared spectroscopy of the CO complex. Proc Natl Acad Sci U S A. 1981 Jan;78(1):234–237. doi: 10.1073/pnas.78.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander K., Young I. G. Alternative hydroxylases for the aerobic and anaerobic biosynthesis of ubiquinone in Escherichia coli. Biochemistry. 1978 Oct 31;17(22):4750–4755. doi: 10.1021/bi00615a024. [DOI] [PubMed] [Google Scholar]
- Alexander K., Young I. G. Three hydroxylations incorporating molecular oxygen in the aerobic biosynthesis of ubiquinone in Escherichia coli. Biochemistry. 1978 Oct 31;17(22):4745–4750. doi: 10.1021/bi00615a023. [DOI] [PubMed] [Google Scholar]
- Altendorf K. H., Staehelin L. A. Orientation of membrane vesicles from Escherichia coli as detected by freeze-cleave electron microscopy. J Bacteriol. 1974 Feb;117(2):888–899. doi: 10.1128/jb.117.2.888-899.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amy N. K. Identification of the molybdenum cofactor in chlorate-resistant mutants of Escherichia coli. J Bacteriol. 1981 Oct;148(1):274–282. doi: 10.1128/jb.148.1.274-282.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen K. B., von Meyenburg K. Are growth rates of Escherichia coli in batch cultures limited by respiration? J Bacteriol. 1980 Oct;144(1):114–123. doi: 10.1128/jb.144.1.114-123.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews S., Cox G. B., Gibson F. The anaerobic oxidation of dihydroorotate by Escherichia coli K-12. Biochim Biophys Acta. 1977 Oct 12;462(1):153–160. doi: 10.1016/0005-2728(77)90197-9. [DOI] [PubMed] [Google Scholar]
- Ashcroft J. R., Haddock B. A. Synthesis of alternative membrane-bound redox carriers during aerobic growth of Escherichia coli in the presence of potassium cyanide. Biochem J. 1975 May;148(2):349–352. doi: 10.1042/bj1480349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azoulay E., Rivière C., Giordano G., Pommier J. Participation of cytochrome b to the in-vitro reconstitution of the membrane-bound formate-nitrate reductase of Escherichia coli K 12 and the possible role of sulfhydryl groups and temperature in the reconstitution process. FEBS Lett. 1977 Jul 15;79(2):321–326. doi: 10.1016/0014-5793(77)80812-0. [DOI] [PubMed] [Google Scholar]
- BARRETT J. The prosthetic group of cytochrome a2. Biochem J. 1956 Dec;64(4):626–639. doi: 10.1042/bj0640626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAGG P. D., POLGLASE W. J. ELECTRON-TRANSPORT COMPONENTS OF STREPTOMYCIN-DEPENDENT ESCHERICHIA COLI. J Bacteriol. 1963 Sep;86:544–547. doi: 10.1128/jb.86.3.544-547.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar Tana J., Howlett B. J., Koshland D. E., Jr Flagellar formation in Escherichia coli electron transport mutants. J Bacteriol. 1977 May;130(2):787–792. doi: 10.1128/jb.130.2.787-792.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes E. M., Jr, Kaback H. R. Beta-galactoside transport in bacterial membrane preparations: energy coupling via membrane-bounded D-lactic dehydrogenase. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1190–1198. doi: 10.1073/pnas.66.4.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes E. M., Jr, Kaback H. R. Mechanisms of active transport in isolated membrane vesicles. I. The site of energy coupling between D-lactic dehydrogenase and beta-galactoside transport in Escherichia coli membrane vesicles. J Biol Chem. 1971 Sep 10;246(17):5518–5522. [PubMed] [Google Scholar]
- Barrett J., Sinclair P. The cytochrome c (552) of aerobically grown Escherichia coli str. McElroy and its function. Biochim Biophys Acta. 1967 Jul 5;143(1):279–281. doi: 10.1016/0005-2728(67)90133-8. [DOI] [PubMed] [Google Scholar]
- Beelen R. H., Feldmann A. M., Wijsman H. J. A regulatory gene and a structural gene for alaninase in Escherichia coli. Mol Gen Genet. 1973 Mar 19;121(4):369–374. doi: 10.1007/BF00433235. [DOI] [PubMed] [Google Scholar]
- Bennett R., Taylor D. R., Hurst A. D- and L-lactate dehydrogenases in Escherichia coli. Biochim Biophys Acta. 1966 Jun 15;118(3):512–521. doi: 10.1016/s0926-6593(66)80093-0. [DOI] [PubMed] [Google Scholar]
- Bernhard T., Gottschalk G. Cell yields of Escherichia coli during anaerobic growth on fumarate and molecular hydrogen. Arch Microbiol. 1978 Mar;116(3):235–238. doi: 10.1007/BF00417845. [DOI] [PubMed] [Google Scholar]
- Blake R., Hager L. P. Activation of pyruvate oxidase by monomeric and micellar amphiphiles. J Biol Chem. 1978 Mar 25;253(6):1963–1971. [PubMed] [Google Scholar]
- Blum H., Poole R. K., Ohnishi T. The orientation of iron-sulphur clusters in membrane multilayers prepared from aerobically-grown Escherichia coli K12 and a cytochrome-deficient mutant. Biochem J. 1980 Aug 15;190(2):385–393. doi: 10.1042/bj1900385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum H., Poole R. K. The molybdenum and iron-sulphur centres of Escherichia coli nitrate reductase are non-randomly oriented in the membrane. Biochem Biophys Res Commun. 1982 Aug;107(3):903–909. doi: 10.1016/0006-291x(82)90608-8. [DOI] [PubMed] [Google Scholar]
- Bonnefoy-Orth V., Lepelletier M., Pascal M. C., Chippaux M. Nitrate reductase and cytochrome bnitrate reductase structural genes as parts of the nitrate reductase operon. Mol Gen Genet. 1981;181(4):535–540. doi: 10.1007/BF00428749. [DOI] [PubMed] [Google Scholar]
- Boonstra J., Downie J. A., Konings W. N. Energy supply for active transport in anaerobically grown Escherichia coli. J Bacteriol. 1978 Dec;136(3):844–853. doi: 10.1128/jb.136.3.844-853.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonstra J., Huttunen M. T., Konings W. N. Anaerobic transport in Escherichia coli membrane vesicles. J Biol Chem. 1975 Sep 10;250(17):6792–6798. [PubMed] [Google Scholar]
- Boonstra J., Konings W. N. Generation of an electrochemical proton gradient by nitrate respiration in membrane vesicles from anaerobically grown Escherichia coli. Eur J Biochem. 1977 Sep;78(2):361–368. doi: 10.1111/j.1432-1033.1977.tb11748.x. [DOI] [PubMed] [Google Scholar]
- Boonstra J., Sips H. J., Konings W. N. Active transport by membrane vesicles from anaerobically grown Escherichia coli energized by electron transfer to ferricyanide and chlorate. Eur J Biochem. 1976 Oct 1;69(1):35–44. doi: 10.1111/j.1432-1033.1976.tb10855.x. [DOI] [PubMed] [Google Scholar]
- Boxer D. H., Clegg R. A. A transmembrane location for the proton-translocating reduced ubiquinone leads to nitrate reductase segment of the respiration chain of Escherichia coli. FEBS Lett. 1975 Dec 1;60(1):54–57. doi: 10.1016/0014-5793(75)80417-0. [DOI] [PubMed] [Google Scholar]
- Boxer D., Malcolm A., Graham A. Escherichia coli formate to nitrate respiratory pathway: structural analysis. Biochem Soc Trans. 1982 Dec;10(6):480–481. doi: 10.1042/bst0100480. [DOI] [PubMed] [Google Scholar]
- Bragg P. D. Effect of near-ultraviolet light on the respiratory chain of Escherichia coli. Can J Biochem. 1971 May;49(5):492–495. doi: 10.1139/o71-073. [DOI] [PubMed] [Google Scholar]
- Bragg P. D., Hou C. Reduced nicotinamide adenine dinucleotide oxidation in Escherichia coli particles. II. NADH dehydrogenases. Arch Biochem Biophys. 1967 Mar;119(1):202–208. doi: 10.1016/0003-9861(67)90447-x. [DOI] [PubMed] [Google Scholar]
- Bragg P. D., Rainnie D. J. The effect of silver ions on the respiratory chain of Escherichia coli. Can J Microbiol. 1974 Jun;20(6):883–889. doi: 10.1139/m74-135. [DOI] [PubMed] [Google Scholar]
- Bray R. C., Vincent S. P., Lowe D. J., Clegg R. A., Garland P. B. Electron-paramagnetic-resonance studies on the molybdenum of nitrate reductase from Escherichia coli K12. Biochem J. 1976 Apr 1;155(1):201–203. doi: 10.1042/bj1550201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock T. D. Microbial growth rates in nature. Bacteriol Rev. 1971 Mar;35(1):39–58. doi: 10.1128/br.35.1.39-58.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookman J. J., Downie J. A., Gibson F., Cox G. B., Rosenberg H. Proton translocation in cytochrome-deficient mutants of Escherichia coli. J Bacteriol. 1979 Feb;137(2):705–710. doi: 10.1128/jb.137.2.705-710.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock P. A., Myer Y. P. Circular dichroism and resonance Raman studies of cytochrome b562 from Escherichia coli. Biochemistry. 1978 Jul 25;17(15):3084–3091. doi: 10.1021/bi00608a022. [DOI] [PubMed] [Google Scholar]
- Burstein C., Tiankova L., Kepes A. Respiratory control in Escherichia coli K 12. Eur J Biochem. 1979 Mar;94(2):387–392. doi: 10.1111/j.1432-1033.1979.tb12905.x. [DOI] [PubMed] [Google Scholar]
- Butler W. L. Fourth derivative spectra. Methods Enzymol. 1979;56:501–515. doi: 10.1016/0076-6879(79)56048-0. [DOI] [PubMed] [Google Scholar]
- CASTOR L. N., CHANCE B. Photochemical action spectra of carbon monoxide-inhibited respiration. J Biol Chem. 1955 Nov;217(1):453–465. [PubMed] [Google Scholar]
- CASTOR L. N., CHANCE B. Photochemical determinations of the oxidases of bacteria. J Biol Chem. 1959 Jun;234(6):1587–1592. [PubMed] [Google Scholar]
- COHEN G. N., RICKENBERG H. V. Concentration spécifique réversible des amino acides chez Escherichia coli. Ann Inst Pasteur (Paris) 1956 Nov;91(5):693–720. [PubMed] [Google Scholar]
- Cammack R., Jackson R. H., Cornish-Bowden A., Cole J. A. Electron-spin-resonance studies of the NADH-dependent nitrite reductase from Escherichia coli K12. Biochem J. 1982 Nov 1;207(2):333–339. doi: 10.1042/bj2070333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldi R. A., Sweetland J., Merli A. Polypeptides in the succinate-coenzyme Q reductase segment of the respiratory chain. Biochemistry. 1977 Dec 27;16(26):5707–5710. doi: 10.1021/bi00645a009. [DOI] [PubMed] [Google Scholar]
- Chance B. Cytochrome kinetics at low temperatures: trapping and ligand exchange. Methods Enzymol. 1978;54:102–111. doi: 10.1016/s0076-6879(78)54012-3. [DOI] [PubMed] [Google Scholar]
- Chance B., Saronio C., Leigh J. S., Jr Compound C2, a product of the reaction of oxygen and the mixed-valence state of cytochrome oxidase. Optical evidence for a type-I copper. Biochem J. 1979 Mar 1;177(3):931–941. doi: 10.1042/bj1770931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B., Saronio C., Leigh J. S., Jr Functional intermediates in the reaction of membrane-bound cytochrome oxidase with oxygen. J Biol Chem. 1975 Dec 25;250(24):9226–9237. [PubMed] [Google Scholar]
- Chang Y. Y., Cronan J. E., Jr Genetic and biochemical analyses of Escherichia coli strains having a mutation in the structural gene (poxB) for pyruvate oxidase. J Bacteriol. 1983 May;154(2):756–762. doi: 10.1128/jb.154.2.756-762.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. Y., Cronan J. E., Jr Mapping nonselectable genes of Escherichia coli by using transposon Tn10: location of a gene affecting pyruvate oxidase. J Bacteriol. 1982 Sep;151(3):1279–1289. doi: 10.1128/jb.151.3.1279-1289.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry G. R., Chaiken I. M., MacGregor C. H. An activity from Escherichia coli membranes responsible for the modification of nitrate reductase to its precursor form. J Biol Chem. 1983 May 10;258(9):5828–5833. [PubMed] [Google Scholar]
- Chaudhry G. R., MacGregor C. H. Cytochrome b from Escherichia coli nitrate reductase. Its properties and association with the enzyme complex. J Biol Chem. 1983 May 10;258(9):5819–5827. [PubMed] [Google Scholar]
- Chaudhry G. R., MacGregor C. H. Escherichia coli nitrate reductase subunit A: its role as the catalytic site and evidence for its modification. J Bacteriol. 1983 Apr;154(1):387–394. doi: 10.1128/jb.154.1.387-394.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chippaux M., Bonnefoy-Orth V., Ratouchniak J., Pascal M. C. Operon fusions in the nitrate reductase operon and study of the control gene nir R in Escherichia coli. Mol Gen Genet. 1981;182(3):477–479. doi: 10.1007/BF00293938. [DOI] [PubMed] [Google Scholar]
- Clark D., Cronan J. E., Jr Escherichia coli mutants with altered control of alcohol dehydrogenase and nitrate reductase. J Bacteriol. 1980 Jan;141(1):177–183. doi: 10.1128/jb.141.1.177-183.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg R. A. Purification and some properties of nitrate reductase (EC 1.7.99.4) from Escherichia coli K12. Biochem J. 1976 Mar 1;153(3):533–541. doi: 10.1042/bj1530533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coakley W. T., Bater A. J., Lloyd D. Disruption of micro-organisms. Adv Microb Physiol. 1977;16:279–341. doi: 10.1016/s0065-2911(08)60050-8. [DOI] [PubMed] [Google Scholar]
- Cole J. A., Coleman K. J., Compton B. E., Kavanagh B. M., Keevil C. W. Nitrite and ammonia assimilation by anaerobic continuous cultures of Escherichia coli. J Gen Microbiol. 1974 Nov;85(1):11–22. doi: 10.1099/00221287-85-1-11. [DOI] [PubMed] [Google Scholar]
- Cole J. A. Cytochrome c552 and nitrite reduction in Escherichia coli. Biochim Biophys Acta. 1968 Oct 1;162(3):356–368. doi: 10.1016/0005-2728(68)90122-9. [DOI] [PubMed] [Google Scholar]
- Cole J. A. Independent pathways for the anaerobic reduction of nitrite to ammonia by Escherichia coli. Biochem Soc Trans. 1982 Dec;10(6):476–478. doi: 10.1042/bst0100476. [DOI] [PubMed] [Google Scholar]
- Cole J. A., Newman B. M., White P. Biochemical and genetic characterization of nirB mutants of Escherichia coli K 12 pleiotropically defective in nitrite and sulphite reduction. J Gen Microbiol. 1980 Oct;120(2):475–483. doi: 10.1099/00221287-120-2-475. [DOI] [PubMed] [Google Scholar]
- Cole S. T., Grundström T., Jaurin B., Robinson J. J., Weiner J. H. Location and nucleotide sequence of frdB, the gene coding for the iron-sulphur protein subunit of the fumarate reductase of Escherichia coli. Eur J Biochem. 1982 Aug;126(1):211–216. doi: 10.1111/j.1432-1033.1982.tb06768.x. [DOI] [PubMed] [Google Scholar]
- Cole S. T., Guest J. R. Amplification of fumarate reductase synthesis with lambdafrdA transducing phages and orientation of frdA gene expression. Mol Gen Genet. 1980;179(2):377–385. doi: 10.1007/BF00425468. [DOI] [PubMed] [Google Scholar]
- Cole S. T., Guest J. R. Molecular genetic aspects of the succinate: fumarate oxidoreductases of Escherichia coli. Biochem Soc Trans. 1982 Dec;10(6):473–475. doi: 10.1042/bst0100473. [DOI] [PubMed] [Google Scholar]
- Cole S. T., Guest J. R. Production of a soluble form of fumarate reductase by multiple gene duplication in Escherichia coli K12. Eur J Biochem. 1979 Dec;102(1):65–71. doi: 10.1111/j.1432-1033.1979.tb06263.x. [DOI] [PubMed] [Google Scholar]
- Cole S. T. Nucleotide sequence coding for the flavoprotein subunit of the fumarate reductase of Escherichia coli. Eur J Biochem. 1982 Mar 1;122(3):479–484. doi: 10.1111/j.1432-1033.1982.tb06462.x. [DOI] [PubMed] [Google Scholar]
- Coleman K. J., Cornish-Bowden A., Cole J. A. Purification and properties of nitrite reductase from Escherichia coli K12. Biochem J. 1978 Nov 1;175(2):483–493. doi: 10.1042/bj1750483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Newton N. A., Gibson F., Snoswell A. M., Hamilton J. A. The function of ubiquinone in Escherichia coli. Biochem J. 1970 Apr;117(3):551–562. doi: 10.1042/bj1170551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Young I. G., McCann L. M., Gibson F. Biosynthesis of ubiquinone in Escherichia coli K-12: location of genes affecting the metabolism of 3-octaprenyl-4-hydroxybenzoic acid and 2-octaprenylphenol. J Bacteriol. 1969 Aug;99(2):450–458. doi: 10.1128/jb.99.2.450-458.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J. C., Edwards E. S., DeMoss J. A. Resolution of distinct selenium-containing formate dehydrogenases from Escherichia coli. J Bacteriol. 1981 Mar;145(3):1317–1324. doi: 10.1128/jb.145.3.1317-1324.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creaghan I. T., Guest J. R. Amber mutants of the -ketoglutarate dehydrogenase gene of Escherichia coli K12. J Gen Microbiol. 1972 Jul;71(2):207–220. doi: 10.1099/00221287-71-2-207. [DOI] [PubMed] [Google Scholar]
- Csonka L. N., Fraenkel D. G. Pathways of NADPH formation in Escherichia coli. J Biol Chem. 1977 May 25;252(10):3382–3391. [PubMed] [Google Scholar]
- Cunningham C. C., Hager L. P. Crystalline pyruvate oxidase from Escherichia coli. 3. Phospholipid as an allosteric effector for the enzyme. J Biol Chem. 1971 Mar 25;246(6):1583–1589. [PubMed] [Google Scholar]
- Cunningham C. C., Hager L. P. Reactivation of the lipid-depleted pyruvate oxidase system from Escherichia coli with cell envelope neutral lipids. J Biol Chem. 1975 Sep 25;250(18):7139–7146. [PubMed] [Google Scholar]
- Czerwinski E. W., Mathews F. S., Hollenberg P., Drickamer K., Hager L. P. Crystallographic study of cytochrome b 562 from Escherichia coli. J Mol Biol. 1972 Nov 28;71(3):819–821. doi: 10.1016/s0022-2836(72)80046-9. [DOI] [PubMed] [Google Scholar]
- Czerwinski E. W., Mathews F. S. Location of the iron atom and the non-crystallographic symmetry elements in cytochrome b562. J Mol Biol. 1974 Jun 15;86(1):49–57. doi: 10.1016/s0022-2836(74)80006-9. [DOI] [PubMed] [Google Scholar]
- DEEB S. S., HAGER L. P. CRYSTALLINE CYTOCHROME B1 FROM ESCHERICHIA COLI. J Biol Chem. 1964 Apr;239:1024–1031. [PubMed] [Google Scholar]
- Dancey G. F., Levine A. E., Shapiro B. M. The NADH dehydrogenase of the respiratory chain of Escherichia coli. I. Properties of the membrane-bound enzyme, its solubilization, and purification to near homogeneity. J Biol Chem. 1976 Oct 10;251(19):5911–5920. [PubMed] [Google Scholar]
- Dancey G. F., Shapiro B. M. The NADH dehydrogenase of the respiratory chain of Escherichia coli. II. Kinetics of the purified enzyme and the effects of antibodies elicited against it on membrane-bound and free enzyme. J Biol Chem. 1976 Oct 10;251(19):5921–5928. [PubMed] [Google Scholar]
- Daoud M. S., Haddock B. A. Electron transport in mutants of Escherichia coli deficient in their ability to synthesize adenosine 3':5'-cyclic monophosphate and the catabolite-gene activator protein. Biochem Soc Trans. 1976;4(4):711–714. doi: 10.1042/bst0040711. [DOI] [PubMed] [Google Scholar]
- DeMoss J. A. Limited proteolysis of nitrate reductase purified from membranes of Escherichia coli. J Biol Chem. 1977 Mar 10;252(5):1696–1701. [PubMed] [Google Scholar]
- Demoss J. A., Fan T. Y., Scott R. H. Characterization of subunit structural alterations which occur during purification of nitrate reductase from Escherichia coli. Arch Biochem Biophys. 1981 Jan;206(1):54–64. doi: 10.1016/0003-9861(81)90065-5. [DOI] [PubMed] [Google Scholar]
- Dervartanian D. V., Iburg L. K., Morgan T. V. EPR studies on phosphorylating particles from Azotobacter vinelandii. Biochim Biophys Acta. 1973 Apr 27;305(1):173–178. doi: 10.1016/0005-2728(73)90243-0. [DOI] [PubMed] [Google Scholar]
- Devor K. A., Schairer H. U., Renz D., Overath P. Active transport of beta-galactosides by a mutant of Escherichia coli defective in heme synthesis. Eur J Biochem. 1974 Jun 15;45(2):451–456. doi: 10.1111/j.1432-1033.1974.tb03569.x. [DOI] [PubMed] [Google Scholar]
- Dickie P., Weiner J. H. Purification and characterization of membrane-bound fumarate reductase from anaerobically grown Escherichia coli. Can J Biochem. 1979 Jun;57(6):813–821. doi: 10.1139/o79-101. [DOI] [PubMed] [Google Scholar]
- Doss M., Philipp-Dormston W. K. The effect of DL-lactate on regulation of porphyrin and heme biosynthesis in Escherichia coli and Achromobacter. FEBS Lett. 1974 Mar 15;40(1):173–175. doi: 10.1016/0014-5793(74)80920-8. [DOI] [PubMed] [Google Scholar]
- Douglas M. W., Ward F. B., Cole J. A. The formate hydrogenlyase activity of cytochrome c552-deficient mutants of Escherichia coli K12. J Gen Microbiol. 1974 Feb;80(2):557–560. doi: 10.1099/00221287-80-2-557. [DOI] [PubMed] [Google Scholar]
- Downie J. A., Cox G. B. Sequence of b cytochromes relative to ubiquinone in the electron transport chain of Escherichia coli. J Bacteriol. 1978 Feb;133(2):477–484. doi: 10.1128/jb.133.2.477-484.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downie J. A., Gibson F., Cox G. B. Membrane adenosine triphosphatases of prokaryotic cells. Annu Rev Biochem. 1979;48:103–131. doi: 10.1146/annurev.bi.48.070179.000535. [DOI] [PubMed] [Google Scholar]
- Edgar J. R., Bell R. M. Biosynthesis in Escherichia coli of sn-glycerol 3-phosphate, a precursor of phospholipid. Palmitoyl-CoA inhibition of the biosynthetic sn-glycerol-3-phosphate dehydrogenase. J Biol Chem. 1979 Feb 25;254(4):1016–1021. [PubMed] [Google Scholar]
- Edlund T., Grundström T., Normark S. Isolation and characterization of DNA repetitions carrying the chromosomal beta-lactamase gene of Escherichia coli K-12. Mol Gen Genet. 1979 Jun 7;173(2):115–125. doi: 10.1007/BF00330301. [DOI] [PubMed] [Google Scholar]
- Edwards C., Beer S., Siviram A., Chance B. Photochemical action spectra of bacterial a- and o-type oxidases using a dye laser. FEBS Lett. 1981 Jun 15;128(2):205–207. doi: 10.1016/0014-5793(81)80081-6. [DOI] [PubMed] [Google Scholar]
- Edwards E. S., Rondeau S. S., DeMoss J. A. chlC (nar) operon of Escherichia coli includes structural genes for alpha and beta subunits of nitrate reductase. J Bacteriol. 1983 Mar;153(3):1513–1520. doi: 10.1128/jb.153.3.1513-1520.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch H. G., Lester R. L. The purification and properties of formate dehydrogenase and nitrate reductase from Escherichia coli. J Biol Chem. 1975 Sep 10;250(17):6693–6705. [PubMed] [Google Scholar]
- Esfahani M., Rudkin B. B., Cutler C. J., Waldron P. E. Lipid-protein interactions in membranes: interaction of phospholipids with respiratory enzymes of Escherichia coli membrane. J Biol Chem. 1977 May 25;252(10):3194–3198. [PubMed] [Google Scholar]
- Evans J. B. Preparation of synchronous cultures of Escherichia coli by continuous-flow size selection. J Gen Microbiol. 1975 Nov;91(1):188–190. doi: 10.1099/00221287-91-1-188. [DOI] [PubMed] [Google Scholar]
- FRANK L., RANHAND B. PROLINE METABOLISM IN ESCHERICHIA COLI. 3. THE PROLINE CATABOLIC PATHWAY. Arch Biochem Biophys. 1964 Aug;107:325–331. doi: 10.1016/0003-9861(64)90338-8. [DOI] [PubMed] [Google Scholar]
- FRANK L., RYBICKI P. Studies of proline metabolism in Escherichia coli. I. The degradation of proline during growth of a proline-requiring auxotroph. Arch Biochem Biophys. 1961 Dec;95:441–449. doi: 10.1016/0003-9861(61)90174-6. [DOI] [PubMed] [Google Scholar]
- FUJITA T., ITAGAKI E., SATO R. Purification and properties of cytochrome bl from Escherichia coli. J Biochem. 1963 Apr;53:282–290. [PubMed] [Google Scholar]
- Farmer I. S., Jones C. W. The energetics of Escherichia coli during aerobic growth in continuous culture. Eur J Biochem. 1976 Aug 1;67(1):115–122. doi: 10.1111/j.1432-1033.1976.tb10639.x. [DOI] [PubMed] [Google Scholar]
- Fimmel A. L., Haddock B. A. Use of chlC-lac fusions to determine regulation of gene chlC in Escherichia coli K-12. J Bacteriol. 1979 Jun;138(3):726–730. doi: 10.1128/jb.138.3.726-730.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forget P., Dubourdieu M. Evidence for the presence of a small subunit as the principal component of the nitrate reductase of Escherichia Coli K 12. Biochem Biophys Res Commun. 1982 Mar 30;105(2):450–456. doi: 10.1016/0006-291x(82)91455-3. [DOI] [PubMed] [Google Scholar]
- Forget P. Evidence for the presence of carbohydrate units in the nitrate reductase A of Escherichia coli K12. FEBS Lett. 1977 May 15;77(2):182–186. doi: 10.1016/0014-5793(77)80230-5. [DOI] [PubMed] [Google Scholar]
- Forget P. The bacterial nitrate reductases. Solubilization, purification and properties of the enzyme A of Escherichia coli K 12. Eur J Biochem. 1974 Mar 1;42(2):325–332. doi: 10.1111/j.1432-1033.1974.tb03343.x. [DOI] [PubMed] [Google Scholar]
- Franklin F. C., Venables W. A. Biochemical, genetic, and regulatory studies of alanine catabolism in Escherichia coli K12. Mol Gen Genet. 1976 Dec 8;149(2):229–237. doi: 10.1007/BF00332894. [DOI] [PubMed] [Google Scholar]
- Franklin F. C., Venables W. A., Wijsman H. J. Genetic studies of D-alanine-dehydrogenase-less mutants of Escherichia coli K12. Genet Res. 1981 Oct;38(2):197–208. doi: 10.1017/s0016672300020528. [DOI] [PubMed] [Google Scholar]
- Fujita T., Sato R. Studies on soluble cytochromes in Enterobacteriaceae. IV. Possible involvement of cytochrome c-552 in anaerobic nitrite metabolism. J Biochem. 1966 Dec;60(6):691–700. doi: 10.1093/oxfordjournals.jbchem.a128495. [DOI] [PubMed] [Google Scholar]
- Fujita T., Sato Y. Studies on soluble cytochromes in Enterobacteriaceae. V. Nitrite-dependent gas evolution in cells containing cytochrome c-552. J Biochem. 1967 Aug;62(2):230–238. doi: 10.1093/oxfordjournals.jbchem.a128653. [DOI] [PubMed] [Google Scholar]
- Fujita T. Studies on soluble cytochromes in Enterobacteriaceae. II. Cytochromes b-562 and c-550. J Biochem. 1966 Sep;60(3):329–334. doi: 10.1093/oxfordjournals.jbchem.a128440. [DOI] [PubMed] [Google Scholar]
- Fukuyama T., Ordal E. J. Induced Biosynthesis of Formic Hydrogenlyase in Iron-Deficient Cells of Escherichia coli. J Bacteriol. 1965 Sep;90(3):673–680. doi: 10.1128/jb.90.3.673-680.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung L. W., Pratt E. A., Ho C. Biochemical and biophysical studies on the interaction of a membrane-bound enzyme, D-lactate dehydrogenase from Escherichia coli, with phospholipids. Biochemistry. 1979 Jan 23;18(2):317–324. doi: 10.1021/bi00569a014. [DOI] [PubMed] [Google Scholar]
- Futai M., Kimura H. Inducible membrane-bound L-lactate dehydrogenase from Escherichia coli. Purification and properties. J Biol Chem. 1977 Aug 25;252(16):5820–5827. [PubMed] [Google Scholar]
- Futai M. Membrane D-lactate dehydrogenase from Escherichia coli. Purification and properties. Biochemistry. 1973 Jun 19;12(13):2468–2474. doi: 10.1021/bi00737a016. [DOI] [PubMed] [Google Scholar]
- Futai M. Orientation of membrane vesicles from Escherichia coli prepared by different procedures. J Membr Biol. 1974;15(1):15–28. doi: 10.1007/BF01870079. [DOI] [PubMed] [Google Scholar]
- Futai M. Reconstitution of transport dependent on D-lactate or glycerol 3-phosphate in membrane vesicles of Escherichia coli deficient in the corresponding dehydrogenases. Biochemistry. 1974 May 21;13(11):2327–2333. doi: 10.1021/bi00708a014. [DOI] [PubMed] [Google Scholar]
- Futai M., Tanaka Y. Localization of D-lactate dehydrogenase in membrane vesicles prepared by using a french press or ethylenediaminetetraacetate-lysozyme from Escherichia coli. J Bacteriol. 1975 Oct;124(1):470–475. doi: 10.1128/jb.124.1.470-475.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAY C. T., GEST H. BIOLOGICAL FORMATION OF MOLECULAR HYDROGEN. Science. 1965 Apr 9;148(3667):186–192. doi: 10.1126/science.148.3667.186. [DOI] [PubMed] [Google Scholar]
- GRAY C. T., WIMPENNY J. W., HUGHES D. E., RANLETT M. A soluble c-type cytochrome from anaerobically grown Escherichia coli and various Enterobacteriaceae. Biochim Biophys Acta. 1963 Jan 8;67:157–160. doi: 10.1016/0006-3002(63)91809-2. [DOI] [PubMed] [Google Scholar]
- Garland P. B., Davison M. T., Moore C. H. Rotational mobility of membrane-bound cytochrome o of Escherichia coli and cytochrome a1 of Thiobacillus ferro-oxidans [proceedings]. Biochem Soc Trans. 1979 Oct;7(5):1112–1114. doi: 10.1042/bst0071112. [DOI] [PubMed] [Google Scholar]
- Garland P. B., Downie J. A., Haddock B. A. Proton translocation and the respiratory nitrate reductase of Escherichia coli. Biochem J. 1975 Dec;152(3):547–559. doi: 10.1042/bj1520547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland P. B., Johnson K., Reid G. R. The diffusional mobility of proteins in the cytoplasmic membrane of Escherichia coli. Biochem Soc Trans. 1982 Dec;10(6):484–485. doi: 10.1042/bst0100484. [DOI] [PubMed] [Google Scholar]
- Garvie E. I. Bacterial lactate dehydrogenases. Microbiol Rev. 1980 Mar;44(1):106–139. doi: 10.1128/mr.44.1.106-139.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George-Nascimento C., Wakil S. J., Short S. A., Kaback H. R. Effect of lipids on the reconstitution of D-lactate oxidase in Escherichia coli membrane vesicles. J Biol Chem. 1976 Nov 10;251(21):6662–6666. [PubMed] [Google Scholar]
- Gerolimatos B., Hanson R. L. Repression of Escherichia coli pyridine nucleotide transhydrogenase by leucine. J Bacteriol. 1978 May;134(2):394–400. doi: 10.1128/jb.134.2.394-400.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert P., Beveridge E. G., Crone P. B. Inhibition of some respiration and dehydrogenase enzyme systems in Escherichia coli NCTC 5933 by phenoxyethanol. Microbios. 1977;20(79):29–37. [PubMed] [Google Scholar]
- Giordano G., Graham A., Boxer D. H., Haddock B. A., Azoulay E. Characterization of the membrane-bound nitrate reductase activity of aerobically grown chlorate-sensitive mutants of Escherichia coli K12. FEBS Lett. 1978 Nov 15;95(2):290–294. doi: 10.1016/0014-5793(78)81013-8. [DOI] [PubMed] [Google Scholar]
- Giordano G., Grillet L., Pommier J., Terriere C., Haddock B. A., Azoulay E. Precursor forms of the subunits of nitrate reductase in chlA and chlB mutants of Escherichia coli K12. Eur J Biochem. 1980 Apr;105(2):297–306. doi: 10.1111/j.1432-1033.1980.tb04501.x. [DOI] [PubMed] [Google Scholar]
- Giordano G., Grillet L., Rosset R., Dou J. H., Azoulay E., Haddock B. A. Characterization of an Escherichia coli K12 mutant that is sensitive to chlorate when grown aerobically. Biochem J. 1978 Nov 15;176(2):553–561. doi: 10.1042/bj1760553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano G., Rivière C., Azoulay E. Oxidative phosphorylation in intact chl-r mutants of Escherichia coli K 12. Biochimie. 1977;59(4):403–409. doi: 10.1016/s0300-9084(77)80316-7. [DOI] [PubMed] [Google Scholar]
- Girdlestone J., Bisson R., Capaldi R. A. Interaction of succinate--ubiquinone reductase (complex II) with (arylazido)phospholipids. Biochemistry. 1981 Jan 6;20(1):152–156. doi: 10.1021/bi00504a025. [DOI] [PubMed] [Google Scholar]
- Glaser J. H., DeMoss J. A. Phenotypic restoration by molybdate of nitrate reductase activity in chlD mutants of Escherichia coli. J Bacteriol. 1971 Nov;108(2):854–860. doi: 10.1128/jb.108.2.854-860.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick B. R., Wang P. Y., Schneider H., Martin W. G. Identification and partial characterization of an Escherichia coli mutant with altered hydrogenase activity. Can J Biochem. 1980 Apr;58(4):361–367. doi: 10.1139/o80-047. [DOI] [PubMed] [Google Scholar]
- Goldberg I., Lonberg-Holm K., Bagley E. A., Stieglitz B. Improved conversion of fumarate to succinate by Escherichia coli strains amplified for fumarate reductase. Appl Environ Microbiol. 1983 Jun;45(6):1838–1847. doi: 10.1128/aem.45.6.1838-1847.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A., Boxer D. H. Arrangement of respiratory nitrate reductase in the cytoplasmic membrane of Escherichia coli. Location of beta subunit. FEBS Lett. 1980 Apr 21;113(1):15–20. doi: 10.1016/0014-5793(80)80484-4. [DOI] [PubMed] [Google Scholar]
- Graham A., Boxer D. H., Haddock B. A., Mandrand-Berthelot A. M., Jones R. W. Immunochemical analysis of the membrane-bound hydrogenase of Escherichia coli. FEBS Lett. 1980 May 5;113(2):167–172. doi: 10.1016/0014-5793(80)80584-9. [DOI] [PubMed] [Google Scholar]
- Graham A., Boxer D. H. Immunochemical localization of nitrate reductase in Escherichia coli [proceedings]. Biochem Soc Trans. 1978;6(6):1210–1211. doi: 10.1042/bst0061210. [DOI] [PubMed] [Google Scholar]
- Graham A., Boxer D. H. Implication of alpha-subunit of Escherichia coli nitrate reductase in catalytic activity [proceedings]. Biochem Soc Trans. 1980 Jun;8(3):329–330. doi: 10.1042/bst0080329a. [DOI] [PubMed] [Google Scholar]
- Graham A., Boxer D. H. The membrane location of the beta-subunit of nitrate reductase from Escherichia coli [proceedings]. Biochem Soc Trans. 1980 Jun;8(3):331–331. doi: 10.1042/bst0080331. [DOI] [PubMed] [Google Scholar]
- Graham A., Boxer D. H. The organization of formate dehydrogenase in the cytoplasmic membrane of Escherichia coli. Biochem J. 1981 Jun 1;195(3):627–637. doi: 10.1042/bj1950627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A. The organization of hydrogenase in the cytoplasmic membrane of Escherichia coli. Biochem J. 1981 Aug 1;197(2):283–291. doi: 10.1042/bj1970283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray C. T., Wimpenny J. W., Hughes D. E., Mossman M. R. Regulation of metabolism in facultative bacteria. I. Structural and functional changes in Escherichia coli associated with shifts between the aerobic and anaerobic states. Biochim Biophys Acta. 1966 Mar 28;117(1):22–32. doi: 10.1016/0304-4165(66)90148-6. [DOI] [PubMed] [Google Scholar]
- Green G. N., Gennis R. B. Isolation and characterization of an Escherichia coli mutant lacking cytochrome d terminal oxidase. J Bacteriol. 1983 Jun;154(3):1269–1275. doi: 10.1128/jb.154.3.1269-1275.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundström T., Jaurin B. Overlap between ampC and frd operons on the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1111–1115. doi: 10.1073/pnas.79.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest J. R. Menaquinone biosynthesis: mutants of Escherichia coli K-12 requiring 2-succinylbenzoate. J Bacteriol. 1977 Jun;130(3):1038–1046. doi: 10.1128/jb.130.3.1038-1046.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest J. R. Partial replacement of succinate dehydrogenase function by phage- and plasmid-specified fumarate reductase in Escherichia coli. J Gen Microbiol. 1981 Feb;122(2):171–179. doi: 10.1099/00221287-122-2-171. [DOI] [PubMed] [Google Scholar]
- Guest J. R., Shaw D. J. Molecular cloning of menaquinone biosynthetic genes of Escherichia coli K12. Mol Gen Genet. 1981;181(3):379–383. doi: 10.1007/BF00425615. [DOI] [PubMed] [Google Scholar]
- Gutman M., Schejter A., Avi-Dor Y. The preparation and properties of the membranal DPNH dehydrogenase from Escherichia coli. Biochim Biophys Acta. 1968 Nov 26;162(4):506–517. doi: 10.1016/0005-2728(68)90057-1. [DOI] [PubMed] [Google Scholar]
- Gutowski S. J., Rosenberg H. Effects of dicyclohexylcarbodi-imide on proton translocation coupled to fumarate reduction in anaerobically grown cells of Escherichia coli K-12. Biochem J. 1976 Dec 15;160(3):813–816. doi: 10.1042/bj1600813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutowski S. J., Rosenberg H. Proton translocation coupled to electron flow from endogenous substrates to fumarate in anaerobically grown Escherichia coli K12. Biochem J. 1977 Apr 15;164(1):265–267. doi: 10.1042/bj1640265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guynn R. W., Veech R. L. The equilibrium constants of the adenosine triphosphate hydrolysis and the adenosine triphosphate-citrate lyase reactions. J Biol Chem. 1973 Oct 25;248(20):6966–6972. [PubMed] [Google Scholar]
- HIRSCH C. A., RASMINSKY M., DAVIS B. D., LIN E. C. A FUMARATE REDUCTASE IN ESCHERICHIA COLI DISTINCT FROM SUCCINATE DEHYDROGENASE. J Biol Chem. 1963 Nov;238:3770–3774. [PubMed] [Google Scholar]
- Hackett C. S., MacGregor C. H. Synthesis and degradation of nitrate reductase in Escherichia coli. J Bacteriol. 1981 Apr;146(1):352–359. doi: 10.1128/jb.146.1.352-359.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett N. R., Bragg P. D. Membrane cytochromes of Escherichia coli chl mutants. J Bacteriol. 1983 May;154(2):719–727. doi: 10.1128/jb.154.2.719-727.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett N. R., Bragg P. D. Membrane cytochromes of Escherichia coli grown aerobically and anaerobically with nitrate. J Bacteriol. 1983 May;154(2):708–718. doi: 10.1128/jb.154.2.708-718.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock B. A., Downie J. A., Garland P. B. Kinetic characterization of the membrane-bound cytochromes of Escherichia coli grown under a variety of conditions by using a stopped-flow dual-wavelength spectrophotometer. Biochem J. 1976 Feb 15;154(2):285–294. doi: 10.1042/bj1540285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock B. A., Downie J. A. The reconstitution of functional respiratory chains in membranes from electron-transport-deficient mutants of Escherichia coli as demonstrated by quenching of atebrin fluorescence. Biochem J. 1974 Sep;142(3):703–706. doi: 10.1042/bj1420703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock B. A., Jones C. W. Bacterial respiration. Bacteriol Rev. 1977 Mar;41(1):47–99. doi: 10.1128/br.41.1.47-99.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock B. A., Kendall-Tobias M. W. Functional anaerobic electron transport linked to the reduction of nitrate and fumarate in membranes from Escherichia coli as demonstrated by quenching of atebrin fluorescence. Biochem J. 1975 Dec;152(3):655–659. doi: 10.1042/bj1520655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock B. A., Mandrand-Berthelot M. A. Escherichia coli formate-to-nitrate respiratory chain: genetic analysis. Biochem Soc Trans. 1982 Dec;10(6):478–480. doi: 10.1042/bst0100478. [DOI] [PubMed] [Google Scholar]
- Haddock B. A., Schairer H. U. Electron-transport chains of Escherichia coli. Reconstitution of respiration in a 5-aminolaevulinic acid-requiring mutant. Eur J Biochem. 1973 May;35(1):34–45. doi: 10.1111/j.1432-1033.1973.tb02806.x. [DOI] [PubMed] [Google Scholar]
- Haddock B. A. The reconstitution of oxidase activity in membranes derived from a 5-aminolaevulinic acid-requiring mutant of Escherichia coli. Biochem J. 1973 Dec;136(4):877–884. doi: 10.1042/bj1360877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldar K., Olsiewski P. J., Walsh C., Kaczorowski G. J., Bhaduri A., Kaback H. R. Simultaneous reconstitution of Escherichia coli membrane vesicles with D-lactate and D-amino acid dehydrogenases. Biochemistry. 1982 Sep 14;21(19):4590–4596. doi: 10.1021/bi00262a012. [DOI] [PubMed] [Google Scholar]
- Hamilton J. A., Cox G. B., Looney F. D., Gibson F. Ubisemiquinone in membranes from Escherichia coli. Biochem J. 1970 Jan;116(2):319–320. doi: 10.1042/bj1160319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson R. L., Rose C. Effects of an insertion mutation in a locus affecting pyridine nucleotide transhydrogenase (pnt::Tn5) on the growth of Escherichia coli. J Bacteriol. 1980 Jan;141(1):401–404. doi: 10.1128/jb.141.1.401-404.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison D. E. The regulation of respiration rate in growing bacteria. Adv Microb Physiol. 1976;14(11):243–313. doi: 10.1016/s0065-2911(08)60229-5. [DOI] [PubMed] [Google Scholar]
- Hatefi Y., Galante Y. M. Isolation of cytochrome b560 from complex II (succinateùbiquinone oxidoreductase) and its reconstitution with succinate dehydrogenase. J Biol Chem. 1980 Jun 25;255(12):5530–5537. [PubMed] [Google Scholar]
- Hederstedt L., Holmgren E., Rutberg L. Characterization of a succinate dehydrogenase complex solubilized from the cytoplasmic membrane of Bacillus subtilis with the nonionic detergent Triton X-100. J Bacteriol. 1979 May;138(2):370–376. doi: 10.1128/jb.138.2.370-376.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hederstedt L., Rutberg L. Succinate dehydrogenase--a comparative review. Microbiol Rev. 1981 Dec;45(4):542–555. doi: 10.1128/mr.45.4.542-555.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellingwerf K. J., Bolscher J. G., Konings W. N. The electrochemical proton gradient generated by the fumarate-reductase system in Escherichia coli and its bioenergetic implications. Eur J Biochem. 1981 Jan;113(2):369–374. doi: 10.1111/j.1432-1033.1981.tb05075.x. [DOI] [PubMed] [Google Scholar]
- Hendler R. W. Automated electrodic potentiometry of potassium ferricyanide and respiratory components. Anal Chem. 1977 Nov;49(13):1914–1918. doi: 10.1021/ac50021a011. [DOI] [PubMed] [Google Scholar]
- Hendler R. W., Burgess A. H. Fractionation of the electron-transport chain of Escherichia coli. Biochim Biophys Acta. 1974 Aug 23;357(2):215–230. doi: 10.1016/0005-2728(74)90062-0. [DOI] [PubMed] [Google Scholar]
- Hendler R. W., Burgess A. H. Respiration and protein synthesis in Escherichia coli membrane-envelope fragments. VI. Solubilization and characterization of the electron transport chain. J Cell Biol. 1972 Nov;55(2):266–281. doi: 10.1083/jcb.55.2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendler R. W., Nanninga N. Respiration and protein synthesis in Escherichia coli membrane-envelope fragments. 3. Electron microscopy and analysis of the cytochromes. J Cell Biol. 1970 Jul;46(1):114–129. doi: 10.1083/jcb.46.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendler R. W., Shrager R. I. Potentiometric analysis of Escherichia coli cytochromes in the optical absorbance range of 500 nm to 700 nm. J Biol Chem. 1979 Nov 25;254(22):11288–11299. [PubMed] [Google Scholar]
- Hendler R. W., Towne D. W., Shrager R. I. Redox properties of beta-type cytochromes in Escherichia coli and rat liver mitochondria and techniques for their analysis. Biochim Biophys Acta. 1975 Jan 31;376(1):42–62. doi: 10.1016/0005-2728(75)90203-0. [DOI] [PubMed] [Google Scholar]
- Hong J. S., Kaback H. R. Mutants of Salmonella typhimurium and Escherichia coli pleiotropically defective in active transport. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3336–3340. doi: 10.1073/pnas.69.11.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingledew W. J., Reid G. A., Poole R. K., Blum H., Ohnishi T. The iron-sulphur centres of aerobically-grown Escherichia coli K12. FEBS Lett. 1980 Feb 25;111(1):223–227. doi: 10.1016/0014-5793(80)80798-8. [DOI] [PubMed] [Google Scholar]
- Ingledew W. J. The electron transport chain of Escherichia coli grown anaerobically with fumarate as terminal electron acceptor: an electron paramagnetic resonance study. J Gen Microbiol. 1983 Jun;129(6):1651–1659. doi: 10.1099/00221287-129-6-1651. [DOI] [PubMed] [Google Scholar]
- Ishida A. A carbon monoxide-binding hemoprotein formed by heme accumulation in Escherichia coli. J Biochem. 1977 Jun;81(6):1869–1878. doi: 10.1093/oxfordjournals.jbchem.a131649. [DOI] [PubMed] [Google Scholar]
- Ishimoto M., Shimokawa O. Reduction of trimethylamine N-oxide by Escherichia coli as anaerobic respiration. Z Allg Mikrobiol. 1978;18(3):173–181. doi: 10.1002/jobm.3630180304. [DOI] [PubMed] [Google Scholar]
- Itagaki E., Hager L. P. Studies on cytochrome b-562 of Escherichia coli. I. Purification and crystallization of cytochrome b-562. J Biol Chem. 1966 Aug 25;241(16):3687–3695. [PubMed] [Google Scholar]
- Itagaki E., Hager L. P. The amino acid sequence of cytochrome b562 of Escherichia coli. Biochem Biophys Res Commun. 1968 Sep 30;32(6):1013–1019. doi: 10.1016/0006-291x(68)90130-7. [DOI] [PubMed] [Google Scholar]
- Jackson R. H., Cole J. A., Cornish-Bowden A. The steady state kinetics of the NADH-dependent nitrite reductase from Escherichia coli K12. The reduction of single-electron acceptors. Biochem J. 1982 May 1;203(2):505–510. doi: 10.1042/bj2030505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. H., Cole J. A., Cornish-Bowden A. The steady-state kinetics of the NADH-dependent nitrite reductase from Escherichia coli K 12. Nitrite and hydroxylamine reduction. Biochem J. 1981 Oct 1;199(1):171–178. doi: 10.1042/bj1990171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. H., Cornish-Bowden A., Cole J. A. Prosthetic groups of the NADH-dependent nitrite reductase from Escherichia coli K12. Biochem J. 1981 Mar 1;193(3):861–867. doi: 10.1042/bj1930861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworowski A., Campbell H. D., Poulis M. I., Young I. G. Genetic identification and purification of the respiratory NADH dehydrogenase of Escherichia coli. Biochemistry. 1981 Mar 31;20(7):2041–2047. doi: 10.1021/bi00510a047. [DOI] [PubMed] [Google Scholar]
- Jaworowski A., Mayo G., Shaw D. C., Campbell H. D., Young I. G. Characterization of the respiratory NADH dehydrogenase of Escherichia coli and reconstitution of NADH oxidase in ndh mutant membrane vesicles. Biochemistry. 1981 Jun 9;20(12):3621–3628. doi: 10.1021/bi00515a049. [DOI] [PubMed] [Google Scholar]
- Jones C. W., Brice J. M., Edwards C. The effect of respiratory chain composition on the growth efficiencies of aerobic bacteria. Arch Microbiol. 1977 Oct 24;115(1):85–93. doi: 10.1007/BF00427850. [DOI] [PubMed] [Google Scholar]
- Jones C. W. Cytochrome patterns in classification and identification including their relevance to the oxidase test. Soc Appl Bacteriol Symp Ser. 1980;8:127–138. [PubMed] [Google Scholar]
- Jones C. W. Microbial oxidative phosphorylation. Biochem Soc Trans. 1978;6(2):361–363. doi: 10.1042/bst0060361. [DOI] [PubMed] [Google Scholar]
- Jones H., Venables W. A. Effects of solubilisation on some properties of the membrane-bound respiratory enzyme D-amino acid dehydrogenase of Escherichia coli. FEBS Lett. 1983 Jan 24;151(2):189–192. doi: 10.1016/0014-5793(83)80066-0. [DOI] [PubMed] [Google Scholar]
- Jones H., Venables W. A. Solubilisation of D-amino acid dehydrogenase of Escherichia coli K12 and its re-binding to envelope preparations. Biochimie. 1983 Mar;65(3):177–183. doi: 10.1016/s0300-9084(83)80082-0. [DOI] [PubMed] [Google Scholar]
- Jones R. G. Ubiquinone deficiency in an auxotroph of Escherichia coli requiring 4-hydroxybenzoic acid. Biochem J. 1967 Jun;103(3):714–719. doi: 10.1042/bj1030714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. W., Garland P. B. Sites and specificity of the reaction of bipyridylium compounds with anaerobic respiratory enzymes of Escherichia coli. Effects of permeability barriers imposed by the cytoplasmic membrane. Biochem J. 1977 Apr 15;164(1):199–211. doi: 10.1042/bj1640199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. W., Garland P. B. The proton-consuming site of the respiratory nitrate reductase of Escherichia coli is on the cytoplasmic aspect of the cytoplasmic membrane [proceedings]. Biochem Soc Trans. 1978;6(2):416–418. doi: 10.1042/bst0060416. [DOI] [PubMed] [Google Scholar]
- Jones R. W. Hydrogen-dependent proton translocation by membrane vesicles from Escherichia coli [proceedings]. Biochem Soc Trans. 1979 Oct;7(5):1136–1137. doi: 10.1042/bst0071136. [DOI] [PubMed] [Google Scholar]
- Jones R. W., Ingledew W. J., Graham A., Garland P. B. Topography of nitrate reductase of the cytoplasmic membrane of Escherichia coli: the nitrate-reducing site [proceedings]. Biochem Soc Trans. 1978;6(6):1287–1289. doi: 10.1042/bst0061287. [DOI] [PubMed] [Google Scholar]
- Jones R. W., Kranz R. G., Gennis R. B. Immunochemical analysis of the membrane-bound succinate dehydrogenase of Escherichia coli. FEBS Lett. 1982 Jun 1;142(1):81–87. doi: 10.1016/0014-5793(82)80224-x. [DOI] [PubMed] [Google Scholar]
- Jones R. W., Lamont A., Garland P. B. The mechanism of proton translocation driven by the respiratory nitrate reductase complex of Escherichia coli. Biochem J. 1980 Jul 15;190(1):79–94. doi: 10.1042/bj1900079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. W. The role of the membrane-bound hydrogenase in the energy-conserving oxidation of molecular hydrogen by Escherichia coli. Biochem J. 1980 May 15;188(2):345–350. doi: 10.1042/bj1880345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. W. The topography of the membrane-bound hydrogenase of Escherichia coli explored by non-physiological electron acceptors [proceedings]. Biochem Soc Trans. 1979 Aug;7(4):724–725. doi: 10.1042/bst0070724. [DOI] [PubMed] [Google Scholar]
- Jurtshuk P., Jr, Mueller T. J., Acord W. C. Bacterial terminal oxidases. CRC Crit Rev Microbiol. 1975 May;3(4):399–468. doi: 10.3109/10408417509108757. [DOI] [PubMed] [Google Scholar]
- Kaback H. R. Transport studies in bacterial membrane vesicles. Science. 1974 Dec 6;186(4167):882–892. doi: 10.1126/science.186.4167.882. [DOI] [PubMed] [Google Scholar]
- Kaczorowski G., Kohn L. D., Kaback H. R. Purification and properties of D-lactate dehydrogenase from Escherichia coli ML 308-225. Methods Enzymol. 1978;53:519–527. doi: 10.1016/s0076-6879(78)53054-1. [DOI] [PubMed] [Google Scholar]
- Kaczorowski G., Shaw L., F-entes M., Walsh C. Coupling of alanine racemase and D-alanine dehydrogenase to active transport of amino acids in Escherichia coli B membrane vesicles. J Biol Chem. 1975 Apr 25;250(8):2855–2865. [PubMed] [Google Scholar]
- Kaczorowski G., Shaw L., Laura R., Walsh C. Active transport in Escherichia coli B membrane vesicles. Differential inactivating effects from the enzymatic oxidation of beta-chloro-L-alanine and beta-chloro-D-alanine. J Biol Chem. 1975 Dec 10;250(23):8921–8930. [PubMed] [Google Scholar]
- Kaczorowski G., Walsh C. Active transport in Excherichia coli B membrane vesicles. Irreversible uncoupling by chloropyruvate. J Biol Chem. 1975 Dec 10;250(23):8931–8937. [PubMed] [Google Scholar]
- Kamitakahara J. R., Polglase W. J. The 503nm pigment of Escherichia coli. Biochem J. 1970 Dec;120(4):771–775. doi: 10.1042/bj1200771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karibian D., Couchoud P. Dihydro-orotate oxidase of Escherichia coli K12: purification, properties, and relation to the cytoplasmic membrane. Biochim Biophys Acta. 1974 Oct 17;364(2):218–232. doi: 10.1016/0005-2744(74)90007-2. [DOI] [PubMed] [Google Scholar]
- Kauffman H. F., van Gelder B. F., DerVartanian D. V. Effect of ligands on cytochrome d from Azotobacter vinelandii. J Bioenerg Biomembr. 1980 Aug;12(3-4):265–276. doi: 10.1007/BF00744688. [DOI] [PubMed] [Google Scholar]
- Kauffman H. F., van Gelder B. F. The respiratory chain of Azotobacter vinelandii. I. Spectral properites of cytochrome d. Biochim Biophys Acta. 1973 May 30;305(2):260–267. doi: 10.1016/0005-2728(73)90174-6. [DOI] [PubMed] [Google Scholar]
- Kay W. W., Kornberg H. L. The uptake of C4-dicarboxylic acids by Escherichia coli. Eur J Biochem. 1971 Jan;18(2):274–281. doi: 10.1111/j.1432-1033.1971.tb01240.x. [DOI] [PubMed] [Google Scholar]
- Kenney W. C., Walker W. H., Singer T. P. Studies on succinate dehydrogenase. XX. Amino acid sequence around the flavin site. J Biol Chem. 1972 Jul 25;247(14):4510–4513. [PubMed] [Google Scholar]
- Kerr C. T., Miller R. W. Dihydroorotate-ubiquinone reductase complex of Escherichia coli B. J Biol Chem. 1968 Jun 10;243(11):2963–2968. [PubMed] [Google Scholar]
- Kim I. C., Bragg P. D. Properties of nonheme iron in a cell envelope fraction from Escherichia coli. J Bacteriol. 1971 Sep;107(3):664–670. doi: 10.1128/jb.107.3.664-670.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I. C., Bragg P. D. Some properties of the succinate dehydrogenase of Escherichia coli. Can J Biochem. 1971 Oct;49(10):1098–1104. doi: 10.1139/o71-159. [DOI] [PubMed] [Google Scholar]
- Kimura H., Futai M. Effects of phospholipids on L-lactate dehydrogenase from membranes of Escherichia coli. Activation and stabilization of the enzyme with phospholipids. J Biol Chem. 1978 Feb 25;253(4):1095–1110. [PubMed] [Google Scholar]
- Kistler W. S., Hirsch C. A., Cozzarelli N. R., Lin E. C. Second pyridine nucleotide-independent 1-alpha-glycerophosphate dehydrogenase in Escherichia coli K-12. J Bacteriol. 1969 Nov;100(2):1133–1135. doi: 10.1128/jb.100.2.1133-1135.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kistler W. S., Lin E. C. Anaerobic L- -glycerophosphate dehydrogenase of Escherichia coli: its genetic locus and its physiological role. J Bacteriol. 1971 Dec;108(3):1224–1234. doi: 10.1128/jb.108.3.1224-1234.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kistler W. S., Lin E. C. Purification and properties of the flavine-stimulated anaerobic L- -glycerophosphate dehydrogenase of Escherichia coli. J Bacteriol. 1972 Oct;112(1):539–547. doi: 10.1128/jb.112.1.539-547.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita K., Kasahara M., Anraku Y. Formation of a membrane potential by reconstructed liposomes made with cytochrome b562-o complex, a terminal oxidase of Escherichia coli K12. J Biol Chem. 1982 Jul 25;257(14):7933–7935. [PubMed] [Google Scholar]
- Kita K., Yamato I., Anraku Y. Purification and properties of cytochrome b556 in the respiratory chain of aerobically grown Escherichia coli K12. J Biol Chem. 1978 Dec 25;253(24):8910–8915. [PubMed] [Google Scholar]
- Kito M., Pizer L. I. Purification and regulatory properties of the biosynthetic L-glycerol 3-phosphate dehydrogenase from Escherichia coli. J Biol Chem. 1969 Jun 25;244(12):3316–3323. [PubMed] [Google Scholar]
- Kline E. S., Mahler H. R. The lactic dehydrogenases of E. coli. Ann N Y Acad Sci. 1965 Jul 31;119(3):905–919. doi: 10.1111/j.1749-6632.1965.tb47451.x. [DOI] [PubMed] [Google Scholar]
- Koch A. L. How bacteria face depression, recession, and depression. Perspect Biol Med. 1976 Autumn;20(1):44–63. doi: 10.1353/pbm.1976.0045. [DOI] [PubMed] [Google Scholar]
- Koch A. L., Wang C. H. How close to the theoretical diffusion limit do bacterial uptake systems function? Arch Microbiol. 1982 Feb;131(1):36–42. doi: 10.1007/BF00451496. [DOI] [PubMed] [Google Scholar]
- Kohn L. D., Kaback H. R. Mechanisms of active transport in isolated bacterial membrane vesicles. XV. Purification and properties of the membrane-bound D-lactate dehydrogenase from Escherichia coli. J Biol Chem. 1973 Oct 25;248(20):7012–7017. [PubMed] [Google Scholar]
- Konings W. N., Kaback H. R. Anaerobic transport in Escherichia coli membrane vesicles. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3376–3381. doi: 10.1073/pnas.70.12.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovatchev S., Vaz W. L., Eibl H. Lipid dependence of the membrane-bound D-lactate dehydrogenase of Escherichia coli. J Biol Chem. 1981 Oct 25;256(20):10369–10374. [PubMed] [Google Scholar]
- Kranz R. G., Gennis R. B. Immunological characterization of the cytochrome o terminal oxidase from Escherichia coli. J Biol Chem. 1983 Sep 10;258(17):10614–10621. [PubMed] [Google Scholar]
- Kranz R. G., Gennis R. B. Isoelectric focusing and crossed immunoelectrophoresis of heme proteins in the Escherichia coli cytoplasmic membrane. J Bacteriol. 1982 Apr;150(1):36–45. doi: 10.1128/jb.150.1.36-45.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristjansson J. K., Hollocher T. C. Substrate binding site for nitrate reductase of Escherichia coli is on the inner aspect of the membrane. J Bacteriol. 1979 Mar;137(3):1227–1233. doi: 10.1128/jb.137.3.1227-1233.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kröger A. Fumarate as terminal acceptor of phosphorylative electron transport. Biochim Biophys Acta. 1978 Oct 23;505(2):129–145. doi: 10.1016/0304-4173(78)90010-1. [DOI] [PubMed] [Google Scholar]
- Kung H. F., Henning U. Limiting availability of binding sites for dehydrogenases on the cell membrane of Escherichia coli. Proc Natl Acad Sci U S A. 1972 Apr;69(4):925–929. doi: 10.1073/pnas.69.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakchaura B. D., Fossum T., Jagger J. Inactivation of adenosine 5'-triphosphate synthesis and reduced-form nicotinamide adenine dinucleotide dehydrogenase activity in Escherichia coli by near-ultraviolet and violet radiations. J Bacteriol. 1976 Jan;125(1):111–118. doi: 10.1128/jb.125.1.111-118.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambden P. R., Guest J. R. Mutants of Escherichia coli K12 unable to use fumarate as an anaerobic electron acceptor. J Gen Microbiol. 1976 Dec;97(2):145–160. doi: 10.1099/00221287-97-2-145. [DOI] [PubMed] [Google Scholar]
- Larson T. J., Ehrmann M., Boos W. Periplasmic glycerophosphodiester phosphodiesterase of Escherichia coli, a new enzyme of the glp regulon. J Biol Chem. 1983 May 10;258(9):5428–5432. [PubMed] [Google Scholar]
- Lawford H. G., Haddock B. A. Respiration-driven proton translocation in Escherichia coli. Biochem J. 1973 Sep;136(1):217–220. doi: 10.1042/bj1360217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemire B. D., Robinson J. J., Bradley R. D., Scraba D. G., Weiner J. H. Structure of fumarate reductase on the cytoplasmic membrane of Escherichia coli. J Bacteriol. 1983 Jul;155(1):391–397. doi: 10.1128/jb.155.1.391-397.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemire B. D., Robinson J. J., Weiner J. H. Identification of membrane anchor polypeptides of Escherichia coli fumarate reductase. J Bacteriol. 1982 Dec;152(3):1126–1131. doi: 10.1128/jb.152.3.1126-1131.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang A., Houghton R. L. Coregulation of oxidized nicotinamide adenine dinucleotide (phosphate) transhydrogenase and glutamate dehydrogenase activities in enteric bacteria during nitrogen limitation. J Bacteriol. 1981 Jun;146(3):997–1002. doi: 10.1128/jb.146.3.997-1002.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin E. C. Glycerol dissimilation and its regulation in bacteria. Annu Rev Microbiol. 1976;30:535–578. doi: 10.1146/annurev.mi.30.100176.002535. [DOI] [PubMed] [Google Scholar]
- Lohmeier E., Hagen D. S., Dickie P., Weiner J. H. Cloning and expression of fumarate reductase gene of Escherichia coli. Can J Biochem. 1981 Mar;59(3):158–164. doi: 10.1139/o81-023. [DOI] [PubMed] [Google Scholar]
- Lovitt R. W., Wimpenny J. W. Physiological behaviour of Escherichia coli grown in opposing gradients of oxidant and reductant in the gradostat. J Gen Microbiol. 1981 Dec;127(2):269–276. doi: 10.1099/00221287-127-2-269. [DOI] [PubMed] [Google Scholar]
- Lutkenhaus J. F., Moore B. A., Masters M., Donachie W. D. Individual proteins are synthesized continuously throughout the Escherichia coli cell cycle. J Bacteriol. 1979 May;138(2):352–360. doi: 10.1128/jb.138.2.352-360.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARUYAMA Y. Biochemical aspects of the cell growth of Escherichia coli as studied by the method of synchronous culture. J Bacteriol. 1956 Dec;72(6):821–826. doi: 10.1128/jb.72.6.821-826.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOSS F. The influence of oxygen tension on respiration and cytochrome a2 formation of Escherichia coli. Aust J Exp Biol Med Sci. 1952 Dec;30(6):531–540. doi: 10.1038/icb.1952.51. [DOI] [PubMed] [Google Scholar]
- MacGregor C. H. Biosynthesis of membrane-bound nitrate reductase in Escherichia coli: evidence for a soluble precursor. J Bacteriol. 1976 Apr;126(1):122–131. doi: 10.1128/jb.126.1.122-131.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor C. H., Bishop C. W. Do cytochromes function as oxygen sensors in the regulation of nitrate reductase biosynthesis? J Bacteriol. 1977 Jul;131(1):372–373. doi: 10.1128/jb.131.1.372-373.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor C. H., Christopher A. R. Asymmetric distribution of nitrate reductase subunits in the cytoplasmic membrane of Escherichia coli: evidence derived from surface labeling studies with transglutaminase. Arch Biochem Biophys. 1978 Jan 15;185(1):204–213. doi: 10.1016/0003-9861(78)90160-1. [DOI] [PubMed] [Google Scholar]
- MacGregor C. H., McElhaney G. E. New mechanism for post-translational processing during assembly of a cytoplasmic membrane protein? J Bacteriol. 1981 Nov;148(2):551–558. doi: 10.1128/jb.148.2.551-558.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor C. H., Schnaitman C. A. Alterations in the cytoplasmic membrane proteins of various chlorate-resistant mutants of Escherichia coli. J Bacteriol. 1971 Oct;108(1):564–570. doi: 10.1128/jb.108.1.564-570.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor C. H., Schnaitman C. A., Normansell D. E. Purification and properties of nitrate reductase from Escherichia coli K12. J Biol Chem. 1974 Aug 25;249(16):5321–5327. [PubMed] [Google Scholar]
- MacGregor C. H. Solubilization of Escherichia coli nitrate reductase by a membrane-bound protease. J Bacteriol. 1975 Mar;121(3):1102–1110. doi: 10.1128/jb.121.3.1102-1110.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macy J., Kulla H., Gottschalk G. H2-dependent anaerobic growth of Escherichia coli on L-malate: succinate formation. J Bacteriol. 1976 Feb;125(2):423–428. doi: 10.1128/jb.125.2.423-428.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason T. G., Richardson G. Escherichia coli and the human gut: some ecological considerations. J Appl Bacteriol. 1981 Aug;51(1):1–16. doi: 10.1111/j.1365-2672.1981.tb00903.x. [DOI] [PubMed] [Google Scholar]
- Mather M., Blake R., Koland J., Schrock H., Russell P., O'Brien T., Hager L. P., Gennis R. B., O'Leary M. Escherichia coli pyruvate oxidase: interaction of a peripheral membrane protein with lipids. Biophys J. 1982 Jan;37(1):87–88. doi: 10.1016/S0006-3495(82)84613-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M., Schopfer L. M., Massey V., Gennis R. B. Studies of the flavin adenine dinucleotide binding region in Escherichia coli pyruvate oxidase. J Biol Chem. 1982 Nov 10;257(21):12887–12892. [PubMed] [Google Scholar]
- Mathews F. S., Bethge P. H., Czerwinski E. W. The structure of cytochrome b562 from Escherichia coli at 2.5 A resolution. J Biol Chem. 1979 Mar 10;254(5):1699–1706. [PubMed] [Google Scholar]
- Matsushita K., Patel L., Gennis R. B., Kaback H. R. Reconstitution of active transport in proteoliposomes containing cytochrome o oxidase and lac carrier protein purified from Escherichia coli. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4889–4893. doi: 10.1073/pnas.80.16.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavis R. D., Vagelos P. R. The effect of phospholipid fatty acid composition in membranous enzymes in Escherichia coli. J Biol Chem. 1972 Feb 10;247(3):652–659. [PubMed] [Google Scholar]
- McRee D. E., Richardson D. C. Preliminary X-ray diffraction studies on the hemoprotein subunit of Escherichia coli sulfite reductase. J Mol Biol. 1982 Jan 5;154(1):179–180. doi: 10.1016/0022-2836(82)90426-0. [DOI] [PubMed] [Google Scholar]
- Meyer D. J. Interaction of cytochrome oxidases aa3 and d with nitrite. Nat New Biol. 1973 Oct 31;245(148):276–277. doi: 10.1038/newbio245276a0. [DOI] [PubMed] [Google Scholar]
- Meyer D. J., Jones C. W. Oxidative phosphorylation in bacteria which contain different cytochrome oxidases. Eur J Biochem. 1973 Jul 2;36(1):144–151. doi: 10.1111/j.1432-1033.1973.tb02894.x. [DOI] [PubMed] [Google Scholar]
- Miki K., Lin E. C. Anaerobic energy-yielding reaction associated with transhydrogenation from glycerol 3-phosphate to fumarate by an Escherichia coli system. J Bacteriol. 1975 Dec;124(3):1282–1287. doi: 10.1128/jb.124.3.1282-1287.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki K., Lin E. C. Electron transport chain from glycerol 3-phosphate to nitrate in Escherichia coli. J Bacteriol. 1975 Dec;124(3):1288–1294. doi: 10.1128/jb.124.3.1288-1294.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki K., Lin E. C. Enzyme complex which couples glycerol-3-phosphate dehydrogenation to fumarate reduction in Escherichia coli. J Bacteriol. 1973 May;114(2):767–771. doi: 10.1128/jb.114.2.767-771.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki K., Silhavy T. J., Andrews K. J. Resolution of glpA and glpT loci into separate operons in Escherichia coli K-12 strains. J Bacteriol. 1979 Apr;138(1):268–269. doi: 10.1128/jb.138.1.268-269.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki K., Wilson T. H. Proton translocation associated with anaerobic transhydrogenation from glycerol 3-phosphate to fumarate in Escherichia coli. Biochem Biophys Res Commun. 1978 Aug 29;83(4):1570–1575. doi: 10.1016/0006-291x(78)91400-6. [DOI] [PubMed] [Google Scholar]
- Miller J. B., Amy N. K. Molybdenum cofactor in chlorate-resistant and nitrate reductase-deficient insertion mutants of Escherichia coli. J Bacteriol. 1983 Aug;155(2):793–801. doi: 10.1128/jb.155.2.793-801.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. J., Gennis R. B. The purification and characterization of the cytochrome d terminal oxidase complex of the Escherichia coli aerobic respiratory chain. J Biol Chem. 1983 Aug 10;258(15):9159–9165. [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Respiratory-chain protonmotive stoicheiometry. Biochem Soc Trans. 1979 Oct;7(5):887–894. doi: 10.1042/bst0070887. [DOI] [PubMed] [Google Scholar]
- Myer Y. P., Bullock P. A. Cytochrome b562 from Escherichia coli: conformational, configurational, and spin-state characterization. Biochemistry. 1978 Sep 5;17(18):3723–3729. doi: 10.1021/bi00611a008. [DOI] [PubMed] [Google Scholar]
- MØLLER V. Diagnostic use of the Braun KCN test within the Enterobacteriaceae. Acta Pathol Microbiol Scand. 1954;34(2):115–126. doi: 10.1111/j.1699-0463.1954.tb00809.x. [DOI] [PubMed] [Google Scholar]
- NICHOLAS D. J., WILSON P. W., HEINEN W., PALMER G., BEINERT H. Use of electron paramagnetic resonance spectroscopy in investigations of functional metal components in micro-organisms. Nature. 1962 Nov 3;196:433–436. doi: 10.1038/196433a0. [DOI] [PubMed] [Google Scholar]
- Narindrasorasak S., Goldie A. H., Sanwal B. D. Characteristics and regulation of a phospholipid-activated malate oxidase from Escherichia coli. J Biol Chem. 1979 Mar 10;254(5):1540–1545. [PubMed] [Google Scholar]
- Newton N. A., Cox G. B., Gibson F. The function of menaquinone (vitamin K 2 ) in Escherichia coli K-12. Biochim Biophys Acta. 1971 Jul 20;244(1):155–166. doi: 10.1016/0304-4165(71)90132-2. [DOI] [PubMed] [Google Scholar]
- O'Brien T. A., Blake R., 2nd, Gennis R. B. Regulation by lipids of cofactor binding to a peripheral membrane enzyme: binding of thiamin pyrophosphate to pyruvate oxidase. Biochemistry. 1977 Jul 12;16(14):3105–3109. doi: 10.1021/bi00633a010. [DOI] [PubMed] [Google Scholar]
- O'Brien T. A., Schrock H. L., Russell P., Blake R., 2nd, Gennis R. B. Preparation of Escherichia coli pyruvate oxidase utilizing a thiamine pyrophosphate affinity column. Biochim Biophys Acta. 1976 Nov 8;452(1):13–29. doi: 10.1016/0005-2744(76)90054-1. [DOI] [PubMed] [Google Scholar]
- O'Brien T. A., Shelton E., Mather M., Gennis R. B. Conformational studies of Escherichia coli pyruvate oxidase. Biochim Biophys Acta. 1982 Aug 10;705(3):321–329. doi: 10.1016/0167-4838(82)90254-0. [DOI] [PubMed] [Google Scholar]
- OTA A., YAMANAKA T., OKUNUKI K. OXIDATIVE PHOSPHORYLATION COUPLED WITH NITRATE RESPIRATION. II. PHOSPHORYLATION COUPLED WITH ANAEROBIC NITRATE REDUCTION IN A CELL-FREE EXTRACT OF ESCHERICHIA COLI. J Biochem. 1964 Feb;55:131–135. [PubMed] [Google Scholar]
- Ohnishi T., Lim J., Winter D. B., King T. E. Thermodynamic and EPR characteristics of a HiPIP-type iron-sulfur center in the succinate dehydrogenase of the respiratory chain. J Biol Chem. 1976 Apr 10;251(7):2105–2109. [PubMed] [Google Scholar]
- Ohnishi T., Salerno J. C. Thermodynamic and EPR characteristics of two ferredoxin-type iron-sulfur centers in the succinate-ubiquinone reductase segment of the respiratory chain. J Biol Chem. 1976 Apr 10;251(7):2094–2104. [PubMed] [Google Scholar]
- Oki M. Correlation between metabolism of phosphatidylglycerol and membrane synthesis in Escherichia coli. J Mol Biol. 1972 Jul 21;68(2):249–264. doi: 10.1016/0022-2836(72)90212-4. [DOI] [PubMed] [Google Scholar]
- Olden K., Hempfling W. P. The 503-nm pigment of Escherichia coli B: characterization and nutritional conditions affecting its accumulation. J Bacteriol. 1973 Feb;113(2):914–921. doi: 10.1128/jb.113.2.914-921.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsiewski P. J., Kaczorowski G. J., Walsh C. T., Kaback H. R. Reconstitution of Escherichia coli membrane vesicles with D-amino acid dehydrogenase. Biochemistry. 1981 Oct 13;20(21):6272–6279. doi: 10.1021/bi00524a056. [DOI] [PubMed] [Google Scholar]
- Olsiewski P. J., Kaczorowski G. J., Walsh C. Purification and properties of D-amino acid dehydrogenase, an inducible membrane-bound iron-sulfur flavoenzyme from Escherichia coli B. J Biol Chem. 1980 May 25;255(10):4487–4494. [PubMed] [Google Scholar]
- Orth V., Chippaux M., Pascal M. C. A mutant defective in electron transfer to nitrate in Escherichia coli K12. J Gen Microbiol. 1980 Mar;117(1):257–262. doi: 10.1099/00221287-117-1-257. [DOI] [PubMed] [Google Scholar]
- Owen P., Kaback H. R. Antigenic architecture of membrane vesicles from Escherichia coli. Biochemistry. 1979 Apr 17;18(8):1422–1426. doi: 10.1021/bi00575a005. [DOI] [PubMed] [Google Scholar]
- Owen P., Kaback H. R. Immunochemical analysis of membrane vesicles from Escherichia coli. Biochemistry. 1979 Apr 17;18(8):1413–1422. doi: 10.1021/bi00575a004. [DOI] [PubMed] [Google Scholar]
- Owen P., Kaback H. R. Molecular structure of membrane vesicles from Escherichia coli. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3148–3152. doi: 10.1073/pnas.75.7.3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen P., Kaczorowski G. J., Kaback H. R. Resolution and identification of iron-containing antigens in membrane vesicles from Escherichia coli. Biochemistry. 1980 Feb 5;19(3):596–600. doi: 10.1021/bi00544a032. [DOI] [PubMed] [Google Scholar]
- PATEMAN J. A., COVE D. J., REVER B. M., ROBERTS D. B. A COMMON CO-FACTOR FOR NITRATE REDUCTASE AND XANTHINE DEHYDROGENASE WHICH ALSO REGULATES THE SYNTHESIS OF NITRATE REDUCTASE. Nature. 1964 Jan 4;201:58–60. doi: 10.1038/201058a0. [DOI] [PubMed] [Google Scholar]
- PECK H. D., Jr, GEST H. Formic dehydrogenase and the hydrogenlyase enzyme complex in coli-aerogenes bacteria. J Bacteriol. 1957 Jun;73(6):706–721. doi: 10.1128/jb.73.6.706-721.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PINSENT J. The need for selenite and molybdate in the formation of formic dehydrogenase by members of the coli-aerogenes group of bacteria. Biochem J. 1954 May;57(1):10–16. doi: 10.1042/bj0570010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padan E., Zilberstein D., Rottenberg H. The proton electrochemical gradient in Escherichia coli cells. Eur J Biochem. 1976 Apr 1;63(2):533–541. doi: 10.1111/j.1432-1033.1976.tb10257.x. [DOI] [PubMed] [Google Scholar]
- Pascal M. C., Casse F., Chippaux M., Lepelletier M. Genetic analysis of mutants of Escherichia coli K12 and Salmonella typhimurium LT2 deficient in hydrogenase activity. Mol Gen Genet. 1975 Nov 24;141(2):173–179. doi: 10.1007/BF00267682. [DOI] [PubMed] [Google Scholar]
- Polglase W. J., Pun W. T., Withaar J. Lipoquinones of Escherichia coli. Biochim Biophys Acta. 1966 May 5;118(2):425–426. doi: 10.1016/s0926-6593(66)80053-x. [DOI] [PubMed] [Google Scholar]
- Poole R. K. Bacterial cytochrome oxidases. A structurally and functionally diverse group of electron-transfer proteins. Biochim Biophys Acta. 1983 Sep 15;726(3):205–243. doi: 10.1016/0304-4173(83)90006-x. [DOI] [PubMed] [Google Scholar]
- Poole R. K., Blum H., Scott R. I., Collinge A., Ohnishi T. The orientation of cytochromes in membrane multilayers prepared from aerobically grown Escherichia coli K12. J Gen Microbiol. 1980 Jul;119(1):145–154. doi: 10.1099/00221287-119-1-145. [DOI] [PubMed] [Google Scholar]
- Poole R. K., Chance B. The reaction of cytochrome o in Escherichia coli K12 with oxygen. Evidence for a spectrally and kinetically distinct cytochrome o in cells from oxygen-limited cultures. J Gen Microbiol. 1981 Oct;126(2):277–287. doi: 10.1099/00221287-126-2-277. [DOI] [PubMed] [Google Scholar]
- Poole R. K., Haddock B. A. Effects of sulphate-limited growth in continuous culture on the electron-transport chain and energy conservation in Escherichia coli K12. Biochem J. 1975 Dec;152(3):537–546. doi: 10.1042/bj1520537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole R. K., Haddock B. A. Energy-linked reduction of nicotinamide--adenine dinucleotide in membranes derived from normal and various respiratory-deficient mutant strains of Escherichia coli K12. Biochem J. 1974 Oct;144(1):77–85. doi: 10.1042/bj1440077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole R. K., Kumar C., Salmon I., Chance B. The 650 and chromophore in Escherichia coli is an 'oxy-' or oxygenated compound, not the oxidized form of cytochrome oxidase d: an hypothesis. J Gen Microbiol. 1983 May;129(5):1335–1344. doi: 10.1099/00221287-129-5-1335. [DOI] [PubMed] [Google Scholar]
- Poole R. K., Salmon I., Chance B. The reaction with oxygen of cytochrome oxidase (cytochrome d) in Escherichia coli K12: optical studies of intermediate species and cytochrome b oxidation at sub-zero temperatures. J Gen Microbiol. 1983 May;129(5):1345–1355. doi: 10.1099/00221287-129-5-1345. [DOI] [PubMed] [Google Scholar]
- Poole R. K., Scott R. I., Blum H. Respiratory biogenesis during the cell cycle of aerobically grown Escherichia coli K12. The accumulation of iron-sulphur clusters and their orientation in the membrane. J Gen Microbiol. 1981 May;124(1):181–185. doi: 10.1099/00221287-124-1-181. [DOI] [PubMed] [Google Scholar]
- Poole R. K., Scott R. I., Chance B. Low-temperature spectral and kinetic properties of cytochromes in Escherichia coli K-12 grown at lowered oxygen tension. Biochim Biophys Acta. 1980 Jul 8;591(2):471–482. doi: 10.1016/0005-2728(80)90177-2. [DOI] [PubMed] [Google Scholar]
- Poole R. K., Scott R. I., Chance B. The light-reversible binding of carbon monoxide to cytochrome a1 in Escherichia coli K12. J Gen Microbiol. 1981 Aug;125(2):431–438. doi: 10.1099/00221287-125-2-431. [DOI] [PubMed] [Google Scholar]
- Poole R. K., Sivaram A., Salmon I., Chance B. Photolysis at very low temperatures of co-liganded cytochrome oxidase (cytochrome d) in oxygen-limited Escherichia coli. FEBS Lett. 1982 May 17;141(2):237–241. doi: 10.1016/0014-5793(82)80056-2. [DOI] [PubMed] [Google Scholar]
- Poole R. K. The influence of growth substrate and capacity for oxidative phosphorylation on respiratory oscillations in synchronous cultures of Escherichia coli K12. J Gen Microbiol. 1977 Apr;99(2):369–377. doi: 10.1099/00221287-99-2-369. [DOI] [PubMed] [Google Scholar]
- Poole R. K., Waring A. J., Chance B. Evidence for a functional oxygen-bound intermediate in the reaction of Escherichia coli cytochrome o with oxygen. FEBS Lett. 1979 May 1;101(1):56–58. doi: 10.1016/0014-5793(79)81293-4. [DOI] [PubMed] [Google Scholar]
- Poole R. K., Waring A. J., Chance B. The reaction of cytochrome omicron in Escherichia coli with oxygen. Low-temperature kinetic and spectral studies. Biochem J. 1979 Nov 15;184(2):379–389. doi: 10.1042/bj1840379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope N. R., Cole J. A. Generation of a membrane potential by one of two independent pathways for nitrite reduction by Escherichia coli. J Gen Microbiol. 1982 Jan;128(1):219–222. doi: 10.1099/00221287-128-1-219. [DOI] [PubMed] [Google Scholar]
- Porra R. J., Barnes R., Jones O. T. The over-production of porphyrins by semi-anaerobic yeast. Enzyme. 1973;16(1):1–8. doi: 10.1159/000459357. [DOI] [PubMed] [Google Scholar]
- Porter N., Drozd J. W., Linton J. D. The effects of cyanide on the growth and respiration of Enterobacter aerogenes in continuous culture. J Gen Microbiol. 1983 Jan;129(1):7–16. doi: 10.1099/00221287-129-1-7. [DOI] [PubMed] [Google Scholar]
- Poulis M. I., Shaw D. C., Campbell H. D., Young I. G. In vitro synthesis of the respiratory NADH dehydrogenase of Escherichia coli. Role of UUG as initiation codon. Biochemistry. 1981 Jul 7;20(14):4178–4185. doi: 10.1021/bi00517a035. [DOI] [PubMed] [Google Scholar]
- Poulson R., Whitlow K. J., Polglase W. J. Catabolite repression of protoporhyrin IX biosynthesis in Escherichia coli K-12. FEBS Lett. 1976 Mar 1;62(3):351–353. doi: 10.1016/0014-5793(76)80092-0. [DOI] [PubMed] [Google Scholar]
- Pratt E. A., Fung L. W., Flowers J. A., Ho C. Membrane-bound D-lactate dehydrogenase from Escherichia coli: purification and properties. Biochemistry. 1979 Jan 23;18(2):312–316. doi: 10.1021/bi00569a013. [DOI] [PubMed] [Google Scholar]
- Pratt E. A., Jones J. A., Cottam P. F., Dowd S. R., Ho C. A biochemical study of the reconstitution of D-lactate dehydrogenase-deficient membrane vesicles using fluorine-labeled components. Biochim Biophys Acta. 1983 Apr 6;729(2):167–175. doi: 10.1016/0005-2736(83)90482-0. [DOI] [PubMed] [Google Scholar]
- Pudek M. R., Bragg P. D. Inhibition by cyanide of the respiratory chain oxidases of Escherichia coli. Arch Biochem Biophys. 1974 Oct;164(2):682–693. doi: 10.1016/0003-9861(74)90081-2. [DOI] [PubMed] [Google Scholar]
- Pudek M. R., Bragg P. D. Reaction of cyanide with cytochrome d in respiratory particles from exponential phase Escherichia coli. FEBS Lett. 1975 Feb 1;50(2):111–113. doi: 10.1016/0014-5793(75)80468-6. [DOI] [PubMed] [Google Scholar]
- Pudek M. R., Bragg P. D. Redox potentials of the cytochromes in the respiratory chain of aerobically grown Escherichia coli. Arch Biochem Biophys. 1976 Jun;174(2):546–552. doi: 10.1016/0003-9861(76)90382-9. [DOI] [PubMed] [Google Scholar]
- Pudek M. R., Bragg P. D. Trapping of an intermediate in the oxidation-reduction cycle of cytochrome d in Escherichia coli. FEBS Lett. 1976 Mar 1;62(3):330–333. doi: 10.1016/0014-5793(76)80087-7. [DOI] [PubMed] [Google Scholar]
- Puig J., Azoulay E. Etude génétique et biochimique des mutants résistant au Clo minus 3 (gènes chl A, chl B, chl C) C R Acad Sci Hebd Seances Acad Sci D. 1967 Apr 10;264(15):1916–1918. [PubMed] [Google Scholar]
- Rainnie D. J., Bragg P. D. The effect of iron deficiency on respiration and energy-coupling in Escherichia coli. J Gen Microbiol. 1973 Aug;77(2):339–349. doi: 10.1099/00221287-77-2-339. [DOI] [PubMed] [Google Scholar]
- Raj T., Russell P., Flygare W. H., Gennis R. B. Quasi-elastic light scattering studies on pyruvate oxidase. Biochim Biophys Acta. 1977 Mar 15;481(1):42–49. doi: 10.1016/0005-2744(77)90135-8. [DOI] [PubMed] [Google Scholar]
- Ramos S., Kaback H. R. The relationship between the electrochemical proton gradient and active transport in Escherichia coli membrane vesicles. Biochemistry. 1977 Mar 8;16(5):854–859. doi: 10.1021/bi00624a007. [DOI] [PubMed] [Google Scholar]
- Raunio R. P., Jenkins W. T. D-alanine oxidase form Escherichia coli: localization and induction by L-alanine. J Bacteriol. 1973 Aug;115(2):560–566. doi: 10.1128/jb.115.2.560-566.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raunio R. P., Straus L. D., Jenkins W. T. D-alanine oxidase from Escherichia coli: participation in the oxidation of L-alanine. J Bacteriol. 1973 Aug;115(2):567–573. doi: 10.1128/jb.115.2.567-573.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recny M. A., Hager L. P. Reconstitution of native Escherichia coli pyruvate oxidase from apoenzyme monomers and FAD. J Biol Chem. 1982 Nov 10;257(21):12878–12886. [PubMed] [Google Scholar]
- Reddy T. L., Hendler R. W. Reconstitution of escherichia coli succinoxidase from soluble components. J Biol Chem. 1978 Nov 10;253(21):7972–7979. [PubMed] [Google Scholar]
- Reeves J. P., Hong J. S., Kaback H. R. Reconstitution of D-lactate-dependent transport in membrane vesicles from a D-lactate dehydrogenase mutant of Escherichia coli. Proc Natl Acad Sci U S A. 1973 Jul;70(7):1917–1921. doi: 10.1073/pnas.70.7.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves J. P. Transient pH changes during D-lactate oxidation by membrane vesicles. Biochem Biophys Res Commun. 1971 Nov;45(4):931–936. doi: 10.1016/0006-291x(71)90427-x. [DOI] [PubMed] [Google Scholar]
- Reid G. A., Haddock B. A., Ingledew W. J. Assembly of functional b-type cytochromes in membranes from a 5-aminolaevulinic acid-requiring mutant of Escherichia coli. FEBS Lett. 1981 Aug 31;131(2):346–350. doi: 10.1016/0014-5793(81)80400-0. [DOI] [PubMed] [Google Scholar]
- Reid G. A., Ingledew W. J. Characterization and phenotypic control of the cytochrome content of Escherichia coli. Biochem J. 1979 Aug 15;182(2):465–472. doi: 10.1042/bj1820465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid G. A., Ingledew W. J. The purification of a respiratory oxidase complex from Escherichia coli. FEBS Lett. 1980 Jan 1;109(1):1–4. doi: 10.1016/0014-5793(80)81297-x. [DOI] [PubMed] [Google Scholar]
- Rice C. W., Hempfling W. P. Oxygen-limited continuous culture and respiratory energy conservation in Escherichia coli. J Bacteriol. 1978 Apr;134(1):115–124. doi: 10.1128/jb.134.1.115-124.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich P. R. The organization of the quinone pool. Biochem Soc Trans. 1982 Dec;10(6):482–484. doi: 10.1042/bst0100482. [DOI] [PubMed] [Google Scholar]
- Riviere C., Giordano G., Pommier J., Azoulay E. Membrane reconstitution in chl-r mutants of Escherichia coli K 12. VIII. Purification and properties of the FA factor, the product of the chl B gene. Biochim Biophys Acta. 1975 May 6;389(2):219–235. doi: 10.1016/0005-2736(75)90317-x. [DOI] [PubMed] [Google Scholar]
- Robinson J. J., Weiner J. H. Molecular properties of fumarate reductase isolated from the cytoplasmic membrane of Escherichia coli. Can J Biochem. 1982 Aug;60(8):811–816. doi: 10.1139/o82-101. [DOI] [PubMed] [Google Scholar]
- Robinson J. J., Weiner J. H. The effect of amphipaths on the flavin-linked aerobic glycerol-3-phosphate dehydrogenase from Escherichia coli. Can J Biochem. 1980 Oct;58(10):1172–1178. doi: 10.1139/o80-157. [DOI] [PubMed] [Google Scholar]
- Robinson J. J., Weiner J. H. The effects of anions on fumarate reductase isolated from the cytoplasmic membrane of Escherichia coli. Biochem J. 1981 Dec 1;199(3):473–477. doi: 10.1042/bj1990473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg H., Cox G. B., Butlin J. D., Gutowski S. J. Metabolite transport in mutants of Escherichia coli K12 defective in electron transport and coupled phosphorylation. Biochem J. 1975 Feb;146(2):417–423. doi: 10.1042/bj1460417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruch F. E., Jr, Lin E. C., Kowit J. D., Tang C. T., Goldberg A. L. In vivo inactivation of glycerol dehydrogenase in Klebsiella aerogenes: properties of active and inactivated proteins. J Bacteriol. 1980 Mar;141(3):1077–1085. doi: 10.1128/jb.141.3.1077-1085.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Herrera J., Showe M. K., DeMoss J. A. Nitrate reductase complex of Escherichia coli K-12: isolation and characterization of mutants unable to reduce nitrate. J Bacteriol. 1969 Mar;97(3):1291–1297. doi: 10.1128/jb.97.3.1291-1297.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell P., Schrock H. L., Gennis R. B. Lipid activation and protease activation of pyruvate oxidase. Evidence suggesting a common site of interaction on the protein. J Biol Chem. 1977 Nov 10;252(21):7883–7887. [PubMed] [Google Scholar]
- Ruíz-Herrera J., Alvarez A., Figueroa I. Solubilization and properties of formate dehydrogenases from the membrane of Escherichia coli. Biochim Biophys Acta. 1972 Dec 7;289(2):254–261. doi: 10.1016/0005-2744(72)90075-7. [DOI] [PubMed] [Google Scholar]
- Ruíz-Herrera J., García L. G. Regulation of succinate dehydrogenase in Escherichia coli. J Gen Microbiol. 1972 Aug;72(1):29–35. doi: 10.1099/00221287-72-1-29. [DOI] [PubMed] [Google Scholar]
- SMITH P. H., HUNGATE R. E. Isolation and characterization of Methanobacterium ruminantium n. sp. J Bacteriol. 1958 Jun;75(6):713–718. doi: 10.1128/jb.75.6.713-718.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos E., Kung H., Young I. G., Kaback H. R. In vitro synthesis of the membrane-bound D-lactate dehydrogenase of Escherichia coli. Biochemistry. 1982 Apr 27;21(9):2085–2091. doi: 10.1021/bi00538a016. [DOI] [PubMed] [Google Scholar]
- Savage D. C. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977;31:107–133. doi: 10.1146/annurev.mi.31.100177.000543. [DOI] [PubMed] [Google Scholar]
- Scarpulla R. C., Soffer R. L. Membrane-bound proline dehydrogenase from Escherichia coli. Solubilization, purification, and characterization. J Biol Chem. 1978 Sep 10;253(17):5997–6001. [PubMed] [Google Scholar]
- Schrock H. L., Gennis R. B. Specific ligand enhancement of the affinity of E. coli pyruvate oxidase for dipalmitoyl phosphatidylcholine. Biochim Biophys Acta. 1980 Jul 10;614(1):215–220. doi: 10.1016/0005-2744(80)90182-5. [DOI] [PubMed] [Google Scholar]
- Schryvers A., Lohmeier E., Weiner J. H. Chemical and functional properties of the native and reconstituted forms of the membrane-bound, aerobic glycerol-3-phosphate dehydrogenase of Escherichia coli. J Biol Chem. 1978 Feb 10;253(3):783–788. [PubMed] [Google Scholar]
- Schryvers A., Weiner J. H. The anaerobic sn-glycerol-3-phosphate dehydrogenase of Escherichia coli. Purification and characterization. J Biol Chem. 1981 Oct 10;256(19):9959–9965. [PubMed] [Google Scholar]
- Scott R. I., Poole R. K. A re-examination of the cytochromes of Escherichia coli using fourth-order finite difference analysis: their characterization under different growth conditions and accumulation during the cell cycle. J Gen Microbiol. 1982 Aug;128(8):1685–1696. doi: 10.1099/00221287-128-8-1685. [DOI] [PubMed] [Google Scholar]
- Scott R. I., Poole R. K., Chance B. Respiratory biogenesis during the cell cycle of aerobically grown Escherichia coli K 12. The accumulation and ligand binding of cytochrome o. J Gen Microbiol. 1981 Feb;122(2):255–261. doi: 10.1099/00221287-122-2-255. [DOI] [PubMed] [Google Scholar]
- Shaw-Goldstein L. A., Gennis R. B., Walsh C. Identification, localization, and function of the thiamin pyrophosphate and flavin adenine dinucleotide dependent pyruvate oxidase in isolated membrane vesicles of Escherichia coli B. Biochemistry. 1978 Dec 26;17(26):5605–5613. doi: 10.1021/bi00619a004. [DOI] [PubMed] [Google Scholar]
- Shaw D. J., Guest J. R. Amplification and product identification of the fnr gene of Escherichia coli. J Gen Microbiol. 1982 Oct;128(10):2221–2228. doi: 10.1099/00221287-128-10-2221. [DOI] [PubMed] [Google Scholar]
- Shaw D. J., Guest J. R. Molecular cloning of the fnr gene of Escherichia coli K12. Mol Gen Genet. 1981;181(1):95–100. doi: 10.1007/BF00339011. [DOI] [PubMed] [Google Scholar]
- Shipp W. S. Cytochromes of Escherichia coli. Arch Biochem Biophys. 1972 Jun;150(2):459–472. doi: 10.1016/0003-9861(72)90063-x. [DOI] [PubMed] [Google Scholar]
- Shipp W. S., Piotrowski M., Friedman A. E. Apparent cytochrome gene dose effects in F-lac and F-gal heterogenotes of Escherichia coli. Arch Biochem Biophys. 1972 Jun;150(2):473–481. doi: 10.1016/0003-9861(72)90064-1. [DOI] [PubMed] [Google Scholar]
- Short S. A., Kaback H. R., Hawkins T., Kohn L. D. Immunochemical properties of the membrane-bound D-lactate dehydrogenase from Escherichia coli. J Biol Chem. 1975 Jun 10;250(11):4285–4290. [PubMed] [Google Scholar]
- Short S. A., Kaback H. R., Kohn L. D. D-lactate dehydrogenase binding in Escherichia coli dld- membrane vesicles reconstituted for active transport. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1461–1465. doi: 10.1073/pnas.71.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short S. A., Kaback H. R., Kohn L. D. Localization of D-lactate dehydrogenase in native and reconstituted Escherichia coli membrane vesicles. J Biol Chem. 1975 Jun 10;250(11):4291–4296. [PubMed] [Google Scholar]
- Siegel L. M., Davis P. S. Reduced nicotinamide adenine dinucleotide phosphate-sulfite reductase of enterobacteria. IV. The Escherichia coli hemoflavoprotein: subunit structure and dissociation into hemoprotein and flavoprotein components. J Biol Chem. 1974 Mar 10;249(5):1587–1598. [PubMed] [Google Scholar]
- Simoni R. D., Shallenberger M. K. Coupling of energy to active transport of amino acids in Escherichia coli. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2663–2667. doi: 10.1073/pnas.69.9.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A. P., Bragg P. D. Anaerobic transport of amino acids coupled to the glycerol-3-phosphate-fumarate oxidoreductase system in a cytochrome-deficient mutant of Escherichia coli. Biochim Biophys Acta. 1976 Mar 12;423(3):450–461. doi: 10.1016/0005-2728(76)90200-0. [DOI] [PubMed] [Google Scholar]
- Singh A. P., Bragg P. D. Energization of phenylalanine transport and energy-dependent transhydrogenase by ATP in cytochrome-deficient Escherichia coli K12. Biochem Biophys Res Commun. 1974 Apr 23;57(4):1200–1206. doi: 10.1016/0006-291x(74)90824-9. [DOI] [PubMed] [Google Scholar]
- Singh A. P., Bragg P. D. Reduced nicotinamide adenine dinucleotide dependent reduction of fumarate coupled to membrane energization in a cytochrome deficient mutant of Escherichia coli K12. Biochim Biophys Acta. 1975 Aug 11;396(2):229–241. doi: 10.1016/0005-2728(75)90037-7. [DOI] [PubMed] [Google Scholar]
- Singh A. P., Bragg P. D. Transport of alpha-methyl glucoside in a cytochrome-deficient mutant of Escherichia coli K-12. FEBS Lett. 1976 Apr 15;64(1):169–172. doi: 10.1016/0014-5793(76)80275-x. [DOI] [PubMed] [Google Scholar]
- Smith M. W., Neidhardt F. C. Proteins induced by aerobiosis in Escherichia coli. J Bacteriol. 1983 Apr;154(1):344–350. doi: 10.1128/jb.154.1.344-350.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. W., Neidhardt F. C. Proteins induced by anaerobiosis in Escherichia coli. J Bacteriol. 1983 Apr;154(1):336–343. doi: 10.1128/jb.154.1.336-343.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth C. J., Siegel J., Salton M. R., Owen P. Immunochemical analysis of inner and outer membranes of Escherichia coli by crossed immunoelectrophoresis. J Bacteriol. 1978 Jan;133(1):306–319. doi: 10.1128/jb.133.1.306-319.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer M. E., Guest J. R. Isolation and properties of fumarate reductase mutants of Escherichia coli. J Bacteriol. 1973 May;114(2):563–570. doi: 10.1128/jb.114.2.563-570.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer M. E., Guest J. R. Molecular cloning of four tricarboxylic acid cyclic genes of Escherichia coli. J Bacteriol. 1982 Aug;151(2):542–552. doi: 10.1128/jb.151.2.542-552.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer M. E., Guest J. R. Proteins of the inner membrane of Escherichia coli: identification of succinate dehydrogenase by polyacrylamide gel electrophoresis with sdh amber mutants. J Bacteriol. 1974 Mar;117(3):947–953. doi: 10.1128/jb.117.3.947-953.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart V., MacGregor C. H. Nitrate reductase in Escherichia coli K-12: involvement of chlC, chlE, and chlG loci. J Bacteriol. 1982 Aug;151(2):788–799. doi: 10.1128/jb.151.2.788-799.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart V. Requirement of Fnr and NarL functions for nitrate reductase expression in Escherichia coli K-12. J Bacteriol. 1982 Sep;151(3):1320–1325. doi: 10.1128/jb.151.3.1320-1325.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroobant P., Kaback H. R. Reconstitution of ubiquinone-linked functions in membrane vesicles from a double quinone mutant of Escherichia coli. Biochemistry. 1979 Jan 9;18(1):226–231. doi: 10.1021/bi00568a035. [DOI] [PubMed] [Google Scholar]
- Synthesis and sideedness of membrane-bound respiratory nitrate reductase (EC1.7.99.4) in Escherichia coli lacking cytochromes. Biochem J. 1975 May;148(2):329–333. [PMC free article] [PubMed] [Google Scholar]
- Sánchez Crispín J. A., Dubourdieu M., Chippaux M. Localization and characterization of cytochromes from membrane vesicles of Escherichia coli K-12 grown in anaerobiosis with nitrate. Biochim Biophys Acta. 1979 Aug 14;547(2):198–210. doi: 10.1016/0005-2728(79)90003-3. [DOI] [PubMed] [Google Scholar]
- Săsărman A., Surdeanu M., Horodniceanu T. Locus determining the synthesis of delta-aminolevulinic acid in Escherichia coli K-12. J Bacteriol. 1968 Nov;96(5):1882–1884. doi: 10.1128/jb.96.5.1882-1884.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TARR H. L. Microbiological deterioration of fish post mortem, its detection and control. Bacteriol Rev. 1954 Mar;18(1):1–15. doi: 10.1128/br.18.1.1-15.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR W. H., TAYLOR M. L. ENZYMES OF THE PYRIMIDINE PATHWAY IN ESCHERICHIA COLI. II. INTRACELLULAR LOCALIZATION AND PROPERTIES OF DIHYDROOROTIC DEHYDROGENASE. J Bacteriol. 1964 Jul;88:105–110. doi: 10.1128/jb.88.1.105-110.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi M., Tsuchiya T., Ishimoto M. Proton translocation coupled to trimethylamine N-oxide reduction in anaerobically grown Escherichia coli. J Bacteriol. 1981 Dec;148(3):762–768. doi: 10.1128/jb.148.3.762-768.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y., Anraku Y., Futai M. Escherichia coli membrane D-lactate dehydrogenase. Isolation of the enzyme in aggregated from and its activation by Triton X-100 and phospholipids. J Biochem. 1976 Oct;80(4):821–830. doi: 10.1093/oxfordjournals.jbchem.a131343. [DOI] [PubMed] [Google Scholar]
- Tarmy E. M., Kaplan N. O. Chemical characterization of D-lactate dehydrogenase from Escherichia coli B. J Biol Chem. 1968 May 25;243(10):2579–2586. [PubMed] [Google Scholar]
- Tarmy E. M., Kaplan N. O. Kinetics of Escherichia coli B D-lactate dehydrogenase and evidence for pyruvate-controlled change in conformation. J Biol Chem. 1968 May 25;243(10):2587–2596. [PubMed] [Google Scholar]
- Thomson J. W., Shapiro B. M. The respiratory chain NADH dehydrogenase of Escherichia coli. Isolation of an NADH:quinone oxidoreductase from membranes and comparison with the membrane-bound NADH:dichlorophenolindophenol oxidoreductase. J Biol Chem. 1981 Mar 25;256(6):3077–3084. [PubMed] [Google Scholar]
- Tomsett A. B., Garrett R. H. The isolation and characterization of mutants defective in nitrate assimilation in Neurospora crassa. Genetics. 1980 Jul;95(3):649–660. doi: 10.1093/genetics/95.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya T., Misawa A., Miyake Y., Yamasaki K., Niiya S. Solubilization and reconstitution of membrane energy-transducing systems of Escherichia coli. FEBS Lett. 1982 Jun 7;142(2):231–234. doi: 10.1016/0014-5793(82)80141-5. [DOI] [PubMed] [Google Scholar]
- Van Wielink J. E., Oltmann L. F., Leeuwerik F. J., De Hollander J. A., Stouthamer A. H. A method for in situ characterization of b- and c-type cytochromes in Escherichia coli and in complex III from beef heart mitochondria by combined spectrum deconvolution and potentiometric analysis. Biochim Biophys Acta. 1982 Aug 20;681(2):177–190. doi: 10.1016/0005-2728(82)90021-4. [DOI] [PubMed] [Google Scholar]
- Venables W. A., Guest J. R. Transduction of nitrate reductase loci of Escherichia coli by phages P-1 and lambda. Mol Gen Genet. 1968;103(2):127–140. doi: 10.1007/BF00427140. [DOI] [PubMed] [Google Scholar]
- Vincent S. P., Bray R. C. Electron-paramagnetic-resonance studies on nitrate reductase from Escherichia coli K12. Biochem J. 1978 Jun 1;171(3):639–647. doi: 10.1042/bj1710639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent S. P. Oxidation--reduction potentials of molybdenum and iron--sulphur centres in nitrate reductase from Escherichia coli. Biochem J. 1979 Feb 1;177(2):757–759. doi: 10.1042/bj1770757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voellmy R. W., Goldberg A. L. ATP-stimulated endoprotease is associated with the cell membrane of E. coli. Nature. 1981 Apr 2;290(5805):419–421. doi: 10.1038/290419a0. [DOI] [PubMed] [Google Scholar]
- WIMPENNY J. W., RANLETT M., GRAY C. T. Repression and derepression of cytochrome c biosynthesis in Escherichia coli. Biochim Biophys Acta. 1963 May 7;73:170–172. doi: 10.1016/0006-3002(63)90436-0. [DOI] [PubMed] [Google Scholar]
- Wallace B. J., Young I. G. Aerobic respiration in mutants of Escherichia coli accumulating quinone analogues of ubiquinone. Biochim Biophys Acta. 1977 Jul 7;461(1):75–83. doi: 10.1016/0005-2728(77)90070-6. [DOI] [PubMed] [Google Scholar]
- Wallace B. J., Young I. G. Role of quinones in electron transport to oxygen and nitrate in Escherichia coli. Studies with a ubiA- menA- double quinone mutant. Biochim Biophys Acta. 1977 Jul 7;461(1):84–100. doi: 10.1016/0005-2728(77)90071-8. [DOI] [PubMed] [Google Scholar]
- Wang E., Walsh C. Suicide substrates for the alanine racemase of Escherichia coli B. Biochemistry. 1978 Apr 4;17(7):1313–1321. doi: 10.1021/bi00600a028. [DOI] [PubMed] [Google Scholar]
- Weiner J. H., Dickie P. Fumarate reductase of Escherichia coli. Elucidation of the covalent-flavin component. J Biol Chem. 1979 Sep 10;254(17):8590–8593. [PubMed] [Google Scholar]
- Weiner J. H. The localization of glycerol-3-phosphate dehydrogenase in Escherichia coli. J Membr Biol. 1974;15(1):1–14. doi: 10.1007/BF01870078. [DOI] [PubMed] [Google Scholar]
- Wild J., Klopotowski T. D-Amino acid dehydrogenase of Escherichia coli K12: positive selection of mutants defective in enzyme activity and localization of the structural gene. Mol Gen Genet. 1981;181(3):373–378. doi: 10.1007/BF00425614. [DOI] [PubMed] [Google Scholar]
- Wimpenny J. W., Cole J. A. The regulation of metabolism in facultative bacteria. 3. The effect of nitrate. Biochim Biophys Acta. 1967 Oct 9;148(1):233–242. doi: 10.1016/0304-4165(67)90298-x. [DOI] [PubMed] [Google Scholar]
- Xavier A. V., Czerwinski E. W., Bethge P. H., Mathews F. S. Identification of the haem ligands of cytochrome b562 by X-ray and NMR methods. Nature. 1978 Sep 21;275(5677):245–247. doi: 10.1038/275245a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto I., Ishimoto M. Anaerobic growth of Escherichia coli on formate by reduction of nitrate, fumarate, and trimethylamine N-oxide. Z Allg Mikrobiol. 1977;17(3):235–242. doi: 10.1002/jobm.3630170309. [DOI] [PubMed] [Google Scholar]
- Yamamoto I., Ishimoto M. Hydrogen-dependent growth of Escherichia coli in anaerobic respiration and the presence of hydrogenases with different functions. J Biochem. 1978 Sep;84(3):673–679. doi: 10.1093/oxfordjournals.jbchem.a132172. [DOI] [PubMed] [Google Scholar]
- Yamato I., Futai M., Anraku Y., Nonomura Y. Cytoplasmic membrane vesicles of Escherichia coli. II. Orientation of the vesicles studied by localization of enzymes. J Biochem. 1978 Jan;83(1):117–128. doi: 10.1093/oxfordjournals.jbchem.a131882. [DOI] [PubMed] [Google Scholar]
- Yoch D. C., Carithers R. P. Bacterial iron-sulfur proteins. Microbiol Rev. 1979 Sep;43(3):384–421. doi: 10.1128/mr.43.3.384-421.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young I. G. Biosynthesis of bacterial menaquinones. Menaquinone mutants of Escherichia coli. Biochemistry. 1975 Jan 28;14(2):399–406. doi: 10.1021/bi00673a029. [DOI] [PubMed] [Google Scholar]
- Young I. G., Jaworowski A., Poulis M. I. Amplification of the respiratory NADH dehydrogenase of Escherichia coli by gene cloning. Gene. 1978 Sep;4(1):25–36. doi: 10.1016/0378-1119(78)90012-4. [DOI] [PubMed] [Google Scholar]
- Young I. G., Jaworowski A., Poulis M. Cloning of the gene for the respiratory D-lactate dehydrogenase of Escherichia coli. Biochemistry. 1982 Apr 27;21(9):2092–2095. doi: 10.1021/bi00538a017. [DOI] [PubMed] [Google Scholar]
- Young I. G., Rogers B. L., Campbell H. D., Jaworowski A., Shaw D. C. Nucleotide sequence coding for the respiratory NADH dehydrogenase of Escherichia coli. UUG initiation codon. Eur J Biochem. 1981 May;116(1):165–170. doi: 10.1111/j.1432-1033.1981.tb05314.x. [DOI] [PubMed] [Google Scholar]
- Young I. G., Wallace B. J. Mutations affecting the reduced nicotinamide adenine dinucleotide dehydrogenase complex of Escherichia coli. Biochim Biophys Acta. 1976 Dec 6;449(3):376–385. doi: 10.1016/0005-2728(76)90149-3. [DOI] [PubMed] [Google Scholar]
- Zahl K. J., Rose C., Hanson R. L. Isolation and partial characterization of a mutant of Escherichia coli lacking pyridine nucleotide transhydrogenase. Arch Biochem Biophys. 1978 Oct;190(2):598–602. doi: 10.1016/0003-9861(78)90315-6. [DOI] [PubMed] [Google Scholar]
- de Silva A. O., Fraenkel D. G. The 6-phosphogluconate dehydrogenase reaction in Escherichia coli. J Biol Chem. 1979 Oct 25;254(20):10237–10242. [PubMed] [Google Scholar]
- van der Plas J., Hellingwerf K. J., Seijen H. G., Guest J. R., Weiner J. H., Konings W. N. Identification and localization of enzymes of the fumarate reductase and nitrate respiration systems of escherichia coli by crossed immunoelectrophoresis. J Bacteriol. 1983 Feb;153(2):1027–1037. doi: 10.1128/jb.153.2.1027-1037.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]


