Dear Editor,
The traditional approach for the generation of gene-targeted animals is homologous recombination (HR) conducted in embryonic stem (ES) cells followed by chimera technology, such as in mice and rats, or HR in somatic cells combined with nuclear transfer in those animals in which germline-competent ES cells are not available, such as pigs, sheep, goats, and cattle. However, no germline-competent rabbit ES cells are yet available. Furthermore, the efficiency of rabbit cloning is so low that no gene-targeted rabbits have been produced thus far by somatic cell nuclear transfer approaches. Over the past several years, zinc-finger nucleases (ZFNs) technology has been employed to produce gene knockout (KO) animals, such as rats1, pigs2, and rabbits3. Recently, transcription activator-like effector nucleases (TALENs), a new genome-modifying technology, has been employed for in vivo genetic engineering in vertebrates. Unlike ZFNs, TALENs have fewer off-target effects and lower toxicity. Given the success of the TALEN technology in reported species 4,5,6, we attempted to explore the feasibility of producing KO rabbits using this technology. In the current study, we chose recombination activation genes (RAGs), including RAG1 and RAG2, as the first genes of interest. RAG gene product have a crucial function as enzymes that activate or catalyze the V(D)J recombination reaction in primary lymphoid tissues. RAG gene-targeted mice without mature B and T cells are powerful tools for studies of allografts, xenografts, tumors, vaccine development, and infectious diseases7,8. Owing to their suitable size and similar physiology to humans, RAG-deficient rabbits would be valuable models in biomedicine research.
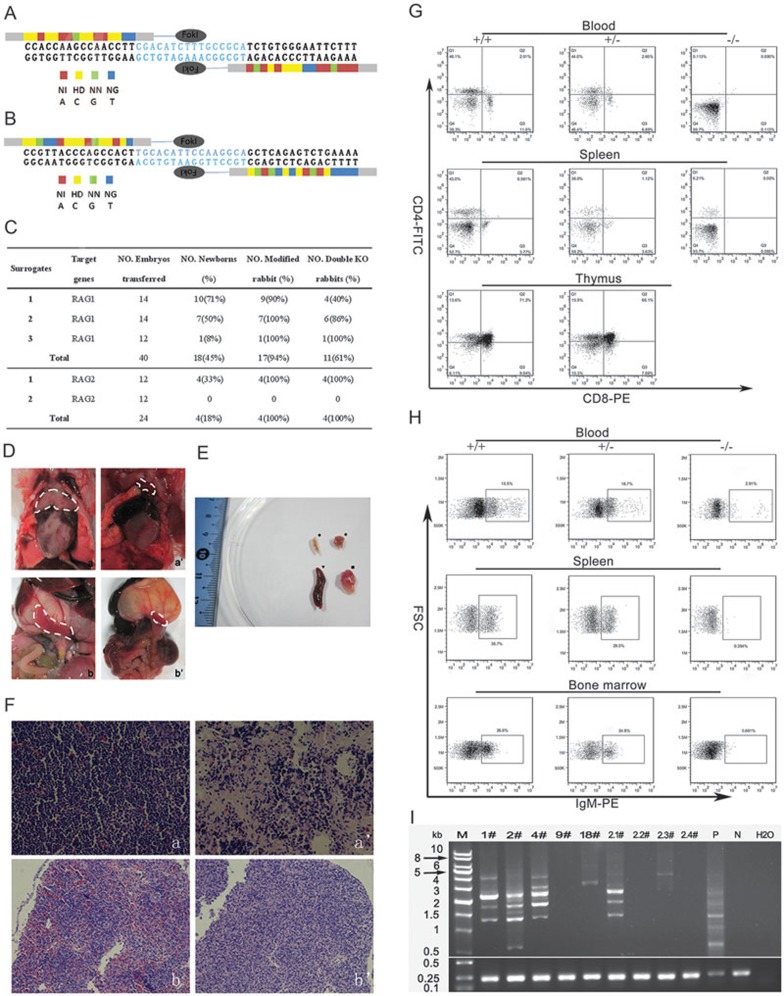
According to the Golden Gate assembly method with the following simple codes: NI for adenine, NG for thymine, HD for cytosine, and NN for guanine, we designed and assembled one pair of TALENs each for rabbit RAG1 exon and RAG2 exon (Figure 1A and 1B). After in vitro transcription, 50 ng/μl TALEN-coding mRNA was injected into the cytoplasm of rabbit pronuclear-stage embryos. The early in vitro development of the embryos appeared to be not substantially affected by the injection of TALENs, as 73% (27/37) of the embryos injected with RAG1 TALENs mRNA and 36% (9/25) of those injected with RAG2 TALENs mRNA were able to develop to the blastocyst stage (Supplementary information, Table S1). To evaluate the targeting efficiencies of the TALENs, the PCR products corresponding to the target sites were amplified from individual embryos in the morula or blastocyst stage and sequenced. Up to 92% (23/25) of the rabbit embryos injected with RAG1 TALENs mRNA were modified at the target site. Among the embryos injected with RAG2 TALENs mRNA, 75% (12/16) were modified at the target site and 19% (3/16) were biallelically modified (Supplementary information, Table S1, Figure S1A and S1B).
Figure 1.
(A) Design of TALENs targeting the exon of rabbit RAG1. (B) Design of TALENs targeting the exon of rabbit RAG2. (C) Generation of RAG KO rabbits via TALENs cleavage. (D) Thymus and spleen from wild-type and RAG1-KO rabbits. Thymus (a) and spleen (b) of wild-type rabbit; Thymus (a') and spleen (b') of RAG1 KO rabbit 12#. (E) Thymus (★) and spleen (♦) from RAG1 KO rabbit 12# were obviously smaller than thymus (▪) and spleen (▾) from wild-type. (F) Histological analysis of thymus (400×) and spleen (200×). Normal thymus (a) and spleen (b) were collected from a wild-type rabbit, while thymus with decreased thymocytes (a') and hypoplastic spleen (b') were derived from RAG1 KO rabbit 12#. (G) FACS analysis of mature T cells from the peripheral blood, spleen and thymus. Cells were stained with antibodies of anti-CD4 and anti-CD8. The percentages of CD4/CD8 double-positive and single-positive cells were much lower in the peripheral blood, spleen and thymus from double RAG-KO rabbits (−/−), compared with that from heterozygous (+/−) and wild-type rabbits (+/+). (H) FACS analysis of mature B cells in peripheral blood, spleen and bone marrow. Single-cell suspensions isolated from peripheral blood, spleen and bone marrow were stained with anti-IgM. Anti-mouse IgG-PE was used as a labeled secondary antibody binding to the primary antibody. Only a small amount of cells expressed IgM in peripheral blood of double-KO rabbits (−/−), compared with heterozygous (+/−) and wild-type rabbits (+/+). No IgM-positive cells were observed in spleen and bone marrow of double-KO rabbits. (I) PCR analysis of recombination at the TCRβ locus. Genomic DNAs derived from ear (N) and blood (P) of wild-type rabbit were used as negative and positive controls for TCRβ recombination, respectively. 1#, 2#, 4#, 9#, and 18# represent the number of RAG1 KO rabbits; 2.1#, 2.2#, 2.3#, and 2.4# represent the number of RAG2 KO rabbits.
A total of 40 embryos injected with RAG1 TALENs mRNA were transferred into 3 surrogate rabbits. All foster mothers developed to term and gave birth to 18 bunnies. Four of the founders grew up healthily, while others died before sexual maturation, due to unknown reasons. Up to 24 embryos injected with RAG2 TALENs mRNA were transferred into 2 surrogate mothers. One surrogate rabbit developed to term and delivered 4 bunnies (Figure 1C). DNA sequencing analysis revealed that 17 of the RAG1 newborns (Figure 1C and Supplementary information, Figure S2) and all 4 RAG2 newborns (Figure 1C and Supplementary information, Figure S3) carried the expected mutation at the target locus (Supplementary information, Table 2 and Supplementary information, Figure S4). Among the RAG-KO rabbits, a variety of mutations were found at the target loci, including deletions from 1 bp to 108 bp and insertions from 3 bp to 5 bp (Supplementary information, Figures S2 and S3). Most of the rabbits were mosaic animals with at least two different genetic modifications at the RAG locus. Interestingly, we also found that 11 RAG1-KO and all RAG2-KO rabbits were biallelically modified, and two RAG1-KO rabbits and one RAG2-KO rabbit carried the same mutation at both RAG loci. The mutations in the majority of the newborn rabbits were frame-shift mutants.
To assay the elimination of RAG function, phenotypes of the founder were characterized. Agenesis of lymphoid organs, including undersize or deficiency of thymus and spleen, was found in the double RAG1-KO rabbit through autopsy of the dead founder (Figure 1D and 1E). Compared with wild-type (WT) rabbits, a remarkable decrease in the lymphocyte population in the RAG1-KO rabbit was observed by histological analysis with hematoxylin and eosin (H&E) staining (Figure 1F).
To verify whether there is deficiency in mature T and B lymphocytes in RAG-KO rabbits, cells from peripheral blood, spleen, thymus and bone marrow were stained and subjected to flow cytometry analysis. No CD4/CD8 double-positive T cells or mature CD4/CD8 single-positive T cells were detected in the peripheral blood from RAG-deficient rabbits (−/−). Only 6.2% of splenocytes from RAG-deficient rabbits (−/−) expressed CD4, while 36.0% of splenocytes from heterozygous (+/−) and 43.0% of splenocytes from wild-type rabbits (+/+) were CD4-positive (Figure 1G). The thymuses isolated from RAG-KO rabbits (−/−) were too small to contain enough thymocytes for FACS analysis. We characterized the B-cell development in RAG-KO rabbits by detecting the expression of Immunoglobulin M (IgM). In RAG-deficient rabbits, IgM+ cells could not be detected from splenocytes or bone marrow cells. Compared with wild-type (15.5%) and heterozygous (18.7%) animals, only 2.91% of white blood cells from RAG-KO peripheral blood expressed IgM (Figure 1H). In summary, these results indicate that the T- and B-cell development were both blocked at early stages in RAG-deficient rabbits.
To further examine whether or not the rearrangement of the somatic cell DNA had been blocked in double-KO rabbits, we amplified the T-cell receptor β chain (TCRβ) locus by PCR (Supplementary information, Table S2). No segments of rearrangement were observed at the TCRβ locus in blood DNA from double-KO rabbits (9#, 2.2#, and 2.4#). Blood DNA from heterozygous rabbits (1#, 2#, and 4#) and homozygous rabbits (2.1#, 2.3#) without frame-shift mutations exhibited several PCR-amplified rearrangement segments (Figure 1I). Arrested lymphocyte development at an immature stage and the absence of V(D)J recombination indicate that the function of RAG is lost in the double-KO rabbits.
To test the specificity of RAG TALENs cleavage, we used e-PCR (www.ncbi.nlm.nih.gov/sutils/e-pcr) to predict potential off-target sequences in the mutant rabbits using the criterion for determining off-target sites previously reported9. No off-target mutations were found at predicted sites of genomic DNA from TALEN-injected rabbits by DNA sequencing (Supplementary information, Tables S3-S5).
In summary, for the first time, we have successfully obtained RAG-KO rabbits by embryo microinjection of TALENs mRNA. The efficiency of KO in founder offspring is extremely high, reaching 94% for RAG1 TALENs and 100% for RAG2 TALENs. These efficiencies are much higher than those observed from the production of random integration transgenic rabbits by pronuclear injection (< 10%)10. Moreover, the biallelic modification efficiency is very high (61% for RAG1; 100% for RAG2). The issue of mosaicism in founder animals generated by embryo injection of TALEN mRNA, which may have been caused by different TALEN activities during embryogenesis, could be overcome by breeding. Rabbits with a special mutation can be obtained among the F1 offspring by genotype screening. Considering their easy availability and high efficiency, we believe that the application of TALENs would tremendously facilitate specific genetic modifications of rabbits for biomedicine and agriculture purposes. Furthermore, the established RAG-deficient rabbits without mature T and B cells could be used as valuable animal models for drug discovery and development, translational research, and stem cell research.
Detailed Materials and Methods are described in the Supplementary information, Data S1.
Acknowledgments
This work was supported by grants from the Ministry of Science and Technology National Basic Research Program of China (973 program) (2011CB944203), “Strategic Priority Research Program” of the Chinese Academy of Sciences (XDA01020107), the National Natural Science Foundation of China (31071293), National Science and Technology Major Project (No 2009ZX10004-405), Science and Technology Planning Project of Guangdong Province, China (2011A060901019) and Innovative Research Team in University (IRT1248).
Footnotes
(Supplementary information is linked to the online version of the paper on the Cell Research website.
Supplementary Information
In vitro development of embryos injected with mRNA.
Sequencing analysis for TALENs-induced mutations in the RAG1 alleles in newborn rabbits.
Sequencing analysis for TALENs-induced mutations in the RAG2 alleles in newborn rabbits.
T7 endonuclease I (T7E1) assay was performed using peripheral blood genomic DNA from survival founder rabbit derived from mRNA injection
Primer pairs used for sequence analysis
TALEN modification of RAG1 (A) and RAG2 (B) gene in in-vitro cultured rabbit blastocysts with T7 endonuclease I assay.
Potential off-target sites of RAG1 TALENs predicted by e-PCR in the rabbit genome
Potential off-target sites of RAG2 TALENs predicted by e-PCR in the rabbit genome
Primers used for identification of potential off-target sites
All operations and experiments involving rabbits were approved by the Animal Research Ethics Committee of the Guangzhou Institute of Biomedicine and Health, Chinese Academy of Sciences.
References
- Geurts AM, Cost GJ, Freyvert Y, et al. Science. 2009. pp. 433–433. [DOI] [PMC free article] [PubMed]
- Yang D, Yang H, Li W, et al. Cell Res. 2011. pp. 979–982. [DOI] [PMC free article] [PubMed]
- Flisikowska T, Thorey IS, Offner S, et al. PLoS One. 2011. p. e21045. [DOI] [PMC free article] [PubMed]
- Lei Y, Guo X, Liu Y, et al. Proc Natl Acad Sci USA. 2012. pp. 17484–17489. [DOI] [PMC free article] [PubMed]
- Tesson L, Usal C, Menoret S, et al. Nat Biotechnol. 2011. pp. 695–696. [DOI] [PubMed]
- Carlson DF, Tan W, Lillico SG, et al. Proc Natl Acad Sci USA. 2012. pp. 17382–17387. [DOI] [PMC free article] [PubMed]
- Mombaerts P, Iacomini J, Johnson RS, et al. Cell. 1992. pp. 869–877. [DOI] [PubMed]
- Shinkai Y, Rathbun G, Lam KP, et al. Cell. 1992. pp. 855–867. [DOI] [PubMed]
- Miller JC, Tan S, Qiao G, et al. Nat Biotechnol. 2011. pp. 143–148. [DOI] [PubMed]
- Chrenek P, Vasicek D, Makarevich A, et al. Transgenic Res. 2005. pp. 417–428. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In vitro development of embryos injected with mRNA.
Sequencing analysis for TALENs-induced mutations in the RAG1 alleles in newborn rabbits.
Sequencing analysis for TALENs-induced mutations in the RAG2 alleles in newborn rabbits.
T7 endonuclease I (T7E1) assay was performed using peripheral blood genomic DNA from survival founder rabbit derived from mRNA injection
Primer pairs used for sequence analysis
TALEN modification of RAG1 (A) and RAG2 (B) gene in in-vitro cultured rabbit blastocysts with T7 endonuclease I assay.
Potential off-target sites of RAG1 TALENs predicted by e-PCR in the rabbit genome
Potential off-target sites of RAG2 TALENs predicted by e-PCR in the rabbit genome
Primers used for identification of potential off-target sites
All operations and experiments involving rabbits were approved by the Animal Research Ethics Committee of the Guangzhou Institute of Biomedicine and Health, Chinese Academy of Sciences.