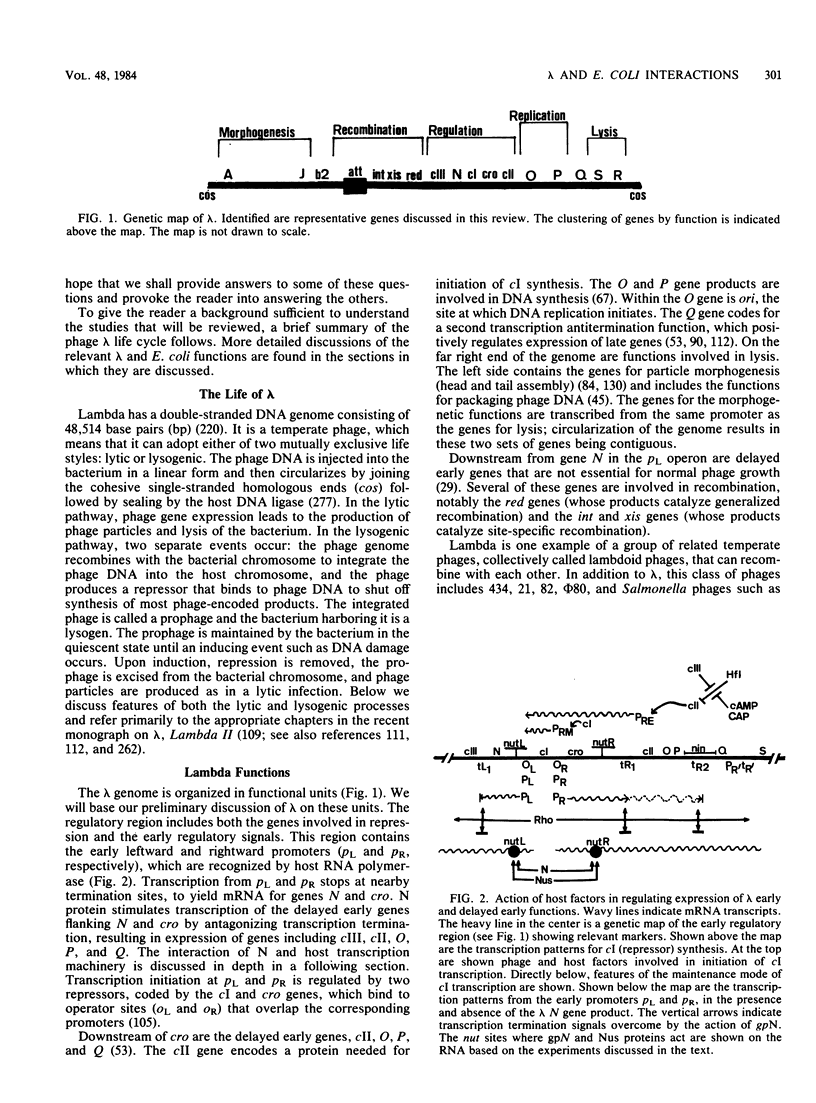
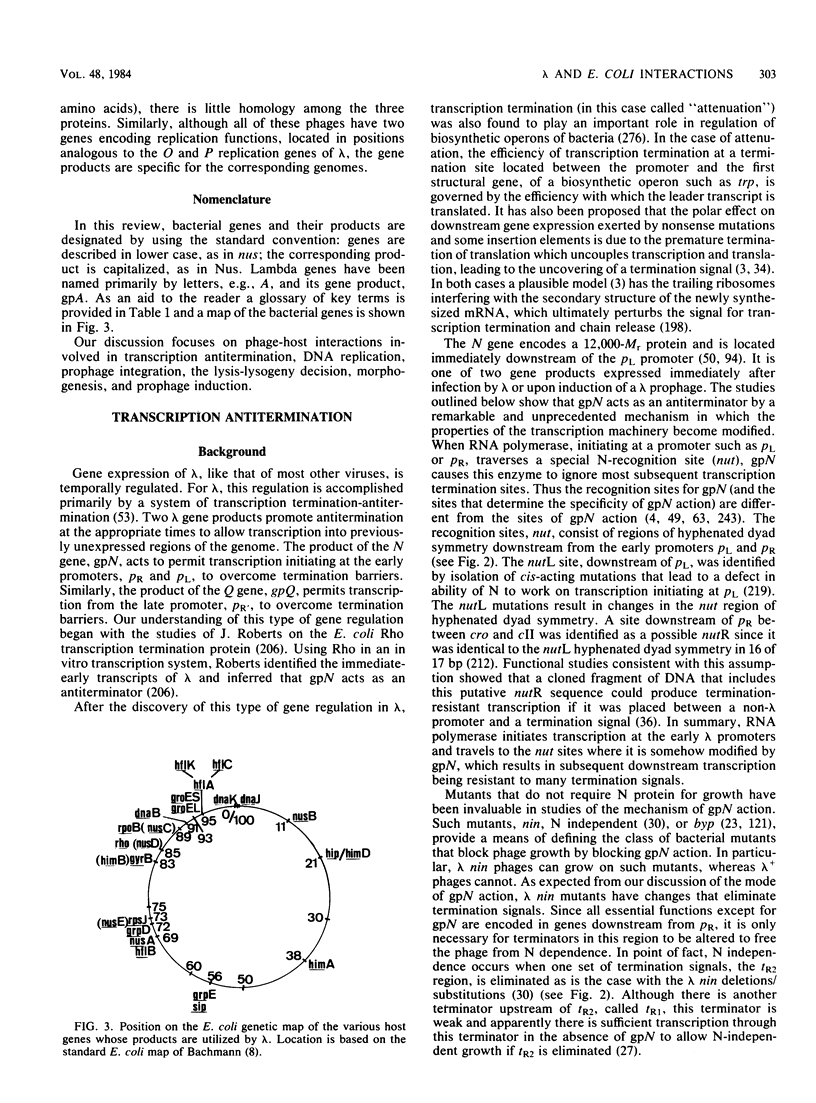
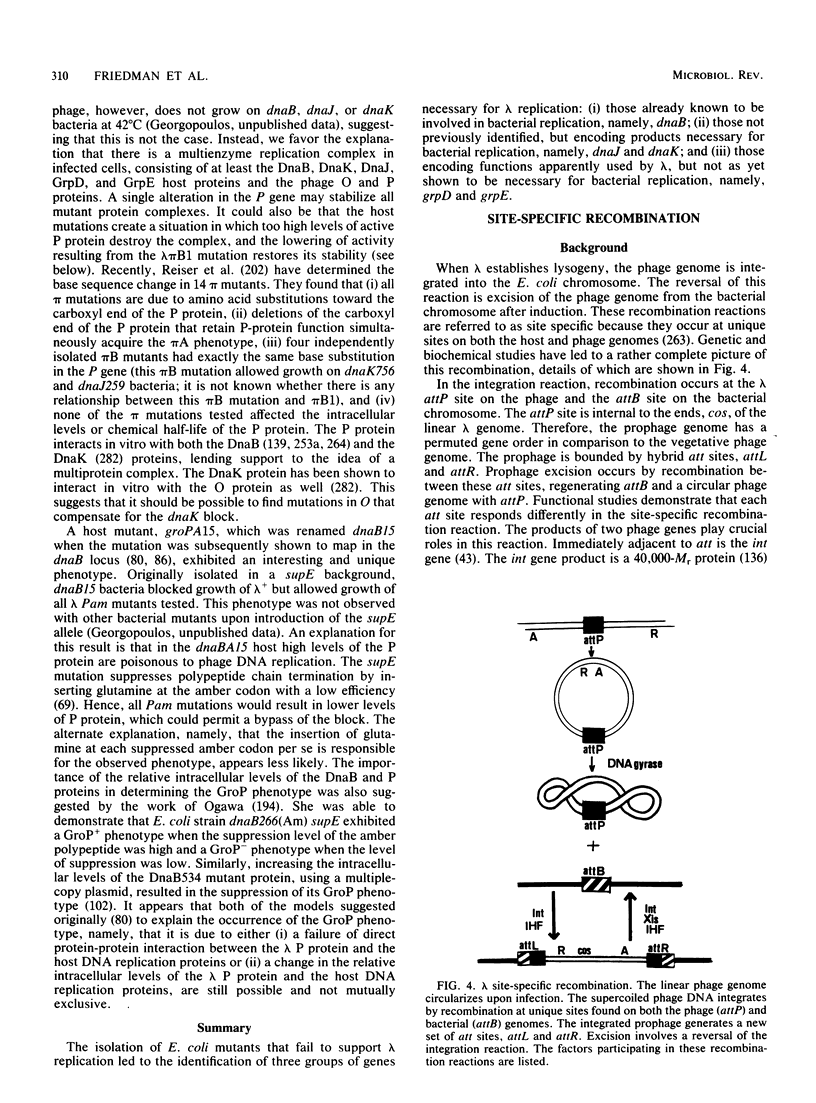
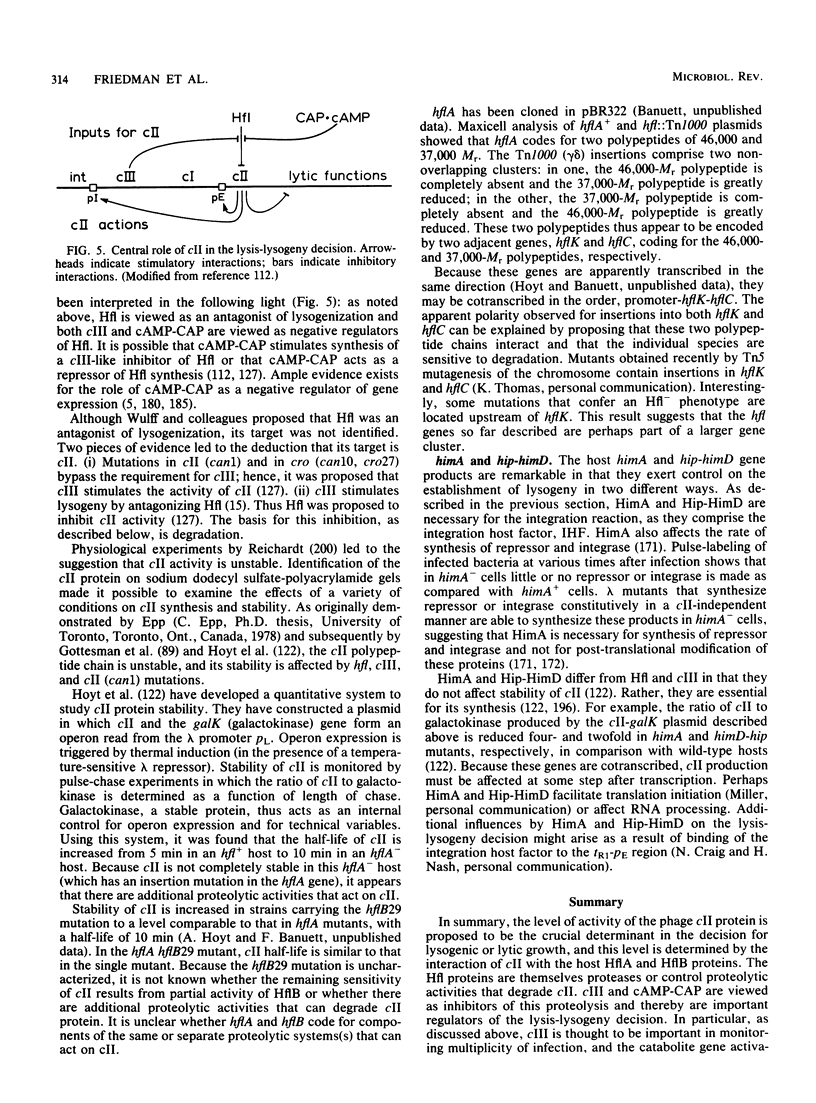
Full text
PDF






















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abremski K., Gottesman S. Purification of the bacteriophage lambda xis gene product required for lambda excisive recombination. J Biol Chem. 1982 Aug 25;257(16):9658–9662. [PubMed] [Google Scholar]
- Abremski K., Gottesman S. The form of the DNA substrate required for excisive recombination of bacteriophage lambda. J Mol Biol. 1979 Jul 5;131(3):637–649. doi: 10.1016/0022-2836(79)90012-3. [DOI] [PubMed] [Google Scholar]
- Adhya S., Gottesman M. Control of transcription termination. Annu Rev Biochem. 1978;47:967–996. doi: 10.1146/annurev.bi.47.070178.004535. [DOI] [PubMed] [Google Scholar]
- Adhya S., Gottesman M., De Crombrugghe B. Release of polarity in Escherichia coli by gene N of phage lambda: termination and antitermination of transcription. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2534–2538. doi: 10.1073/pnas.71.6.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiba H. Autoregulation of the Escherichia coli crp gene: CRP is a transcriptional repressor for its own gene. Cell. 1983 Jan;32(1):141–149. doi: 10.1016/0092-8674(83)90504-4. [DOI] [PubMed] [Google Scholar]
- Anderl A., Klein A. Replication of lambda dv DNA in vitro. Nucleic Acids Res. 1982 Mar 11;10(5):1733–1740. doi: 10.1093/nar/10.5.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin S., Ziese M., Sternberg N. A novel role for site-specific recombination in maintenance of bacterial replicons. Cell. 1981 Sep;25(3):729–736. doi: 10.1016/0092-8674(81)90180-x. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardwell J. C., Craig E. A. Major heat shock gene of Drosophila and the Escherichia coli heat-inducible dnaK gene are homologous. Proc Natl Acad Sci U S A. 1984 Feb;81(3):848–852. doi: 10.1073/pnas.81.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron L. S., Penido E., Ryman I. R., Falkow S. Behavior of coliphage lambda in hybrids between Escherichia coli and Salmonella. J Bacteriol. 1970 Apr;102(1):221–233. doi: 10.1128/jb.102.1.221-233.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann M. F., Friedman D. I. Cooperative effects of bacterial mutations affecting lambda N gene expression. II. Isolation and characterization of mutations in the rif region. Virology. 1976 Aug;73(1):128–138. doi: 10.1016/0042-6822(76)90067-2. [DOI] [PubMed] [Google Scholar]
- Bear S. E., Court D. L., Friedman D. I. An accessory role for Escherichia coli integration host factor: characterization of a lambda mutant dependent upon integration host factor for DNA packaging. J Virol. 1984 Dec;52(3):966–972. doi: 10.1128/jvi.52.3.966-972.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfort M., Wulff D. L. Genetic and biochemical investigation of the Escherichia coli mutant hfl-1 which is lysogenized at high frequency by bacteriophage lambda. J Bacteriol. 1973 Jul;115(1):299–306. doi: 10.1128/jb.115.1.299-306.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfort M., Wulff D. The roles of the lambda c3 gene and the Escherichia coli catabolite gene activation system in the establishment of lysogeny by bacteriophage lambda. Proc Natl Acad Sci U S A. 1974 Mar;71(3):779–782. doi: 10.1073/pnas.71.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Better M., Freifelder D. Studies on the replication of Escherichia coli phage lambda DNA. I. The kinetics of DNA replication and requirements for the generation of rolling circles. Virology. 1983 Apr 15;126(1):168–182. doi: 10.1016/0042-6822(83)90469-5. [DOI] [PubMed] [Google Scholar]
- Birchmeier C., Folk W., Birnstiel M. L. The terminal RNA stem-loop structure and 80 bp of spacer DNA are required for the formation of 3' termini of sea urchin H2A mRNA. Cell. 1983 Dec;35(2 Pt 1):433–440. doi: 10.1016/0092-8674(83)90176-9. [DOI] [PubMed] [Google Scholar]
- Botstein D., Maurer R. Genetic approaches to the analysis of microbial development. Annu Rev Genet. 1982;16:61–83. doi: 10.1146/annurev.ge.16.120182.000425. [DOI] [PubMed] [Google Scholar]
- Brooks K., Clark A. J. Behavior of lambda bacteriophage in a recombination deficienct strain of Escherichia coli. J Virol. 1967 Apr;1(2):283–293. doi: 10.1128/jvi.1.2.283-293.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius J., Dull T. J., Sleeter D. D., Noller H. F. Gene organization and primary structure of a ribosomal RNA operon from Escherichia coli. J Mol Biol. 1981 May 15;148(2):107–127. doi: 10.1016/0022-2836(81)90508-8. [DOI] [PubMed] [Google Scholar]
- Brunel F., Davison J. Bacterial mutants able to partly suppress the effect of N mutations in bacteriophage lambda. Mol Gen Genet. 1975;136(2):167–180. doi: 10.1007/BF00272037. [DOI] [PubMed] [Google Scholar]
- Burton Z. F., Gross C. A., Watanabe K. K., Burgess R. R. The operon that encodes the sigma subunit of RNA polymerase also encodes ribosomal protein S21 and DNA primase in E. coli K12. Cell. 1983 Feb;32(2):335–349. doi: 10.1016/0092-8674(83)90453-1. [DOI] [PubMed] [Google Scholar]
- Butler B., Echols H. Regulation of bacteriophage lambda development by gene N: properties of a mutation that bypasses N control of late protein synthesis. Virology. 1970 Feb;40(2):212–222. doi: 10.1016/0042-6822(70)90396-x. [DOI] [PubMed] [Google Scholar]
- CLARK A. J., MARGULIES A. D. ISOLATION AND CHARACTERIZATION OF RECOMBINATION-DEFICIENT MUTANTS OF ESCHERICHIA COLI K12. Proc Natl Acad Sci U S A. 1965 Feb;53:451–459. doi: 10.1073/pnas.53.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellazzi M., George J., Buttin G. [Prophage induction and cell division in E. coli. II. Linked (recA, zab) and unlinked (lex) suppressors of tif-1-mediated induction and filamentation]. Mol Gen Genet. 1972;119(2):153–174. doi: 10.1007/BF00269134. [DOI] [PubMed] [Google Scholar]
- Coppo A., Manzi A., Pulitzer J. F., Takahashi H. Abortive bacteriophage T4 head assembly in mutants of Escherichia coli. J Mol Biol. 1973 May 5;76(1):61–87. doi: 10.1016/0022-2836(73)90081-8. [DOI] [PubMed] [Google Scholar]
- Court D., Brady C., Rosenberg M., Wulff D. L., Behr M., Mahoney M., Izumi S. U. Control of transcription termination: a rho-dependent termination site in bacteriophage lambda. J Mol Biol. 1980 Apr;138(2):231–254. doi: 10.1016/0022-2836(80)90285-5. [DOI] [PubMed] [Google Scholar]
- Court D., Green L., Echols H. Positive and negative regulation by the cII and cIII gene products of bacteriophage lambda. Virology. 1975 Feb;63(2):484–491. doi: 10.1016/0042-6822(75)90321-9. [DOI] [PubMed] [Google Scholar]
- Court D., Sato K. Studies of novel transducing variants of lambda: dispensability of genes N and Q. Virology. 1969 Oct;39(2):348–352. doi: 10.1016/0042-6822(69)90060-9. [DOI] [PubMed] [Google Scholar]
- Craig N. L., Roberts J. W. E. coli recA protein-directed cleavage of phage lambda repressor requires polynucleotide. Nature. 1980 Jan 3;283(5742):26–30. doi: 10.1038/283026a0. [DOI] [PubMed] [Google Scholar]
- Daniels D. L., Blattner F. R. Nucleotide sequence of the Q gene and the Q to S intergenic region of bacteriophage lambda. Virology. 1982 Feb;117(1):81–92. doi: 10.1016/0042-6822(82)90509-8. [DOI] [PubMed] [Google Scholar]
- Das A., Court D., Adhya S. Isolation and characterization of conditional lethal mutants of Escherichia coli defective in transcription termination factor rho. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1959–1963. doi: 10.1073/pnas.73.6.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A., Gottesman M. E., Wardwell J., Trisler P., Gottesman S. lambda mutation in the Escherichia coli rho gene that inhibits the N protein activity of phage lambda. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5530–5534. doi: 10.1073/pnas.80.18.5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiDomenico B. J., Bugaisky G. E., Lindquist S. The heat shock response is self-regulated at both the transcriptional and posttranscriptional levels. Cell. 1982 Dec;31(3 Pt 2):593–603. doi: 10.1016/0092-8674(82)90315-4. [DOI] [PubMed] [Google Scholar]
- Drahos D. J., Hendrix R. W. Effect of bacteriophage lambda infection on synthesis of groE protein and other Escherichia coli proteins. J Bacteriol. 1982 Mar;149(3):1050–1063. doi: 10.1128/jb.149.3.1050-1063.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw W. C., Hendrix R. W., King J. Structural studies of bacteriophage lambda heads and proheads by small angle X-ray diffraction. J Mol Biol. 1979 Nov 5;134(3):575–594. doi: 10.1016/0022-2836(79)90368-1. [DOI] [PubMed] [Google Scholar]
- Echols H., Green L. Establishment and maintenance of repression by bacteriophage lambda: the role of the cI, cII, and c3 proteins. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2190–2194. doi: 10.1073/pnas.68.9.2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnham P. J., Greenblatt J., Platt T. Effects of NusA protein on transcription termination in the tryptophan operon of Escherichia coli. Cell. 1982 Jul;29(3):945–951. doi: 10.1016/0092-8674(82)90457-3. [DOI] [PubMed] [Google Scholar]
- Ferrucci F., Murialdo H. Bacteriophage lambda prohead assembly: assembly of biologically active precollars in vitro. Prog Clin Biol Res. 1981;64:193–212. [PubMed] [Google Scholar]
- Floor E. Interaction of morphogenetic genes of bacteriophage T4. J Mol Biol. 1970 Feb 14;47(3):293–306. doi: 10.1016/0022-2836(70)90303-7. [DOI] [PubMed] [Google Scholar]
- Forbes D., Herskowitz I. Polarity suppression by the Q gene product of bacteriophage lambda. J Mol Biol. 1982 Oct 5;160(4):549–569. doi: 10.1016/0022-2836(82)90314-x. [DOI] [PubMed] [Google Scholar]
- Franklin N. C. Altered reading of genetic signals fused to the N operon of bacteriophage lambda: genetic evidence for modification of polymerase by the protein product of the N gene. J Mol Biol. 1974 Oct 15;89(1):33–48. doi: 10.1016/0022-2836(74)90161-2. [DOI] [PubMed] [Google Scholar]
- Franklin N. C., Bennett G. N. The N protein of bacteriophage lambda, defined by its DNA sequence, is highly basic. Gene. 1979 Dec;8(1):107–119. doi: 10.1016/0378-1119(79)90011-8. [DOI] [PubMed] [Google Scholar]
- Friden P., Voelkel K., Sternglanz R., Freundlich M. Reduced expression of the isoleucine and valine enzymes in integration host factor mutants of Escherichia coli. J Mol Biol. 1984 Feb 5;172(4):573–579. doi: 10.1016/s0022-2836(84)80024-8. [DOI] [PubMed] [Google Scholar]
- Friedman D. I., Baron L. S. Genetic characterization of a bacterial locus involved in the activity of the N function of phage lambda. Virology. 1974 Mar;58(1):141–148. doi: 10.1016/0042-6822(74)90149-4. [DOI] [PubMed] [Google Scholar]
- Friedman D. I., Baumann M., Baron L. S. Cooperative effects of bacterial mutations affecting lambda N gene expression. I. Isolation and characterization of a nusB mutant. Virology. 1976 Aug;73(1):119–127. doi: 10.1016/0042-6822(76)90066-0. [DOI] [PubMed] [Google Scholar]
- Friedman D. I., Jolly C. T., Mural R. J. Interference with the expression of the N gene function of phage lambda in a mutant of Escherichia coli. Virology. 1973 Jan;51(1):216–226. doi: 10.1016/0042-6822(73)90381-4. [DOI] [PubMed] [Google Scholar]
- Friedman D. I., Olson E. J., Carver D., Gellert M. Synergistic effect of himA and gyrB mutations: evidence that him functions control expression of ilv and xyl genes. J Bacteriol. 1984 Feb;157(2):484–489. doi: 10.1128/jb.157.2.484-489.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D. I., Olson E. R. Evidence that a nucleotide sequence, "boxA," is involved in the action of the NusA protein. Cell. 1983 Aug;34(1):143–149. doi: 10.1016/0092-8674(83)90144-7. [DOI] [PubMed] [Google Scholar]
- Friedman D. I., Plantefaber L. C., Olson E. J., Carver D., O'Dea M. H., Gellert M. Mutations in the DNA gyrB gene that are temperature sensitive for lambda site-specific recombination, Mu growth, and plasmid maintenance. J Bacteriol. 1984 Feb;157(2):490–497. doi: 10.1128/jb.157.2.490-497.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D. I., Ponce-Campos R. Differential effect of phage regulator functions on transcription from various promoters: evidence that the P22 gene 24 and the lambda gene N products distinguish three classes of promoters. J Mol Biol. 1975 Nov 5;98(3):537–549. doi: 10.1016/s0022-2836(75)80085-4. [DOI] [PubMed] [Google Scholar]
- Friedman D. I., Wilgus G. S., Mural R. J. Gene N regulator function of phage lambda immun21: evidence that a site of N action differs from a site of N recognition. J Mol Biol. 1973 Dec 25;81(4):505–516. doi: 10.1016/0022-2836(73)90519-6. [DOI] [PubMed] [Google Scholar]
- Fuerst C. R., Bingham H., Bouchard J. P. Temperature sensitivity in Escherichia coli K12: mutants unable to support normal growth of lambda phage at high temperatures. Virology. 1978 Jun 15;87(2):416–436. doi: 10.1016/0042-6822(78)90145-9. [DOI] [PubMed] [Google Scholar]
- Furth M. E., Dove W. F., Meyer B. J. Specificity determinants for bacteriophage lambda DNA replication. III. Activation of replication in lambda ric mutants by transcription outside of ori. J Mol Biol. 1982 Jan 5;154(1):65–83. doi: 10.1016/0022-2836(82)90417-x. [DOI] [PubMed] [Google Scholar]
- Furth M. E., McLeester C., Dove W. F. Specificity determinants for bacteriophage lambda DNA replication. I. A chain of interactions that controls the initiation of replication. J Mol Biol. 1978 Dec 5;126(2):195–225. doi: 10.1016/0022-2836(78)90359-5. [DOI] [PubMed] [Google Scholar]
- Furth M. E., Yates J. L. Specificity determinants for bacteriophage lambda DNA replication. II. Structure of O proteins of lambda-phi80 and lambda-82 hybrid phages and of a lambda mutant defective in the origin of replication. J Mol Biol. 1978 Dec 5;126(2):227–240. doi: 10.1016/0022-2836(78)90360-1. [DOI] [PubMed] [Google Scholar]
- Garen A., Garen S., Wilhelm R. C. Suppressor genes for nonsense mutations. I. The Su-1, Su-2 and Su-3 genes of Escherichia coli. J Mol Biol. 1965 Nov;14(1):167–178. doi: 10.1016/s0022-2836(65)80238-8. [DOI] [PubMed] [Google Scholar]
- Gautsch J. W., Wulff D. L. Fine structure mapping, complementation, and physiology of Escherichia coli hfl mutants. Genetics. 1974 Jul;77(3):435–448. doi: 10.1093/genetics/77.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M. DNA topoisomerases. Annu Rev Biochem. 1981;50:879–910. doi: 10.1146/annurev.bi.50.070181.004311. [DOI] [PubMed] [Google Scholar]
- Gellert M., Menzel R., Mizuuchi K., O'Dea M. H., Friedman D. I. Regulation of DNA supercoiling in Escherichia coli. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):763–767. doi: 10.1101/sqb.1983.047.01.087. [DOI] [PubMed] [Google Scholar]
- Gellert M., Mizuuchi K., O'Dea M. H., Itoh T., Tomizawa J. I. Nalidixic acid resistance: a second genetic character involved in DNA gyrase activity. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4772–4776. doi: 10.1073/pnas.74.11.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M., Mizuuchi K., O'Dea M. H., Nash H. A. DNA gyrase: an enzyme that introduces superhelical turns into DNA. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3872–3876. doi: 10.1073/pnas.73.11.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos C. P. A new bacterial gene (groPC) which affects lambda DNA replication. Mol Gen Genet. 1977 Feb 28;151(1):35–39. doi: 10.1007/BF00446910. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C. P. Bacterial mutants in which the gene N function of bacteriophage lambda is blocked have an altered RNA polymerase. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2977–2981. doi: 10.1073/pnas.68.12.2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos C. P., Eisen H. Bacterial mutants which block phage assembly. J Supramol Struct. 1974;2(2-4):349–359. doi: 10.1002/jss.400020224. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C. P., Hendrix R. W., Casjens S. R., Kaiser A. D. Host participation in bacteriophage lambda head assembly. J Mol Biol. 1973 May 5;76(1):45–60. doi: 10.1016/0022-2836(73)90080-6. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C. P., Hohn B. Identification of a host protein necessary for bacteriophage morphogenesis (the groE gene product). Proc Natl Acad Sci U S A. 1978 Jan;75(1):131–135. doi: 10.1073/pnas.75.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos C. P., Lam B., Lundquist-Heil A., Rudolph C. F., Yochem J., Feiss M. Identification of the C. coli dnaK (groPC756) gene product. Mol Gen Genet. 1979 May 4;172(2):143–149. doi: 10.1007/BF00268275. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C. P., Swindle J., Keppel F., Ballivet M., Bisig R., Eisen H. Studies on the E. coli groNB (nusB) gene which affects bacteriophage lambda N gene function. Mol Gen Genet. 1980;179(1):55–61. doi: 10.1007/BF00268446. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C., Tilly K., Drahos D., Hendrix R. The B66.0 protein of Escherichia coli is the product of the dnaK+ gene. J Bacteriol. 1982 Mar;149(3):1175–1177. doi: 10.1128/jb.149.3.1175-1177.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghysen A., Pironio M. Relationship between the N function of bacteriophage lambda and host RNA polymerase. J Mol Biol. 1972 Mar 28;65(2):259–272. doi: 10.1016/0022-2836(72)90281-1. [DOI] [PubMed] [Google Scholar]
- Gold M., Becker A. The bacteriophage lambda terminase. Partial purification and preliminary characterization of properties. J Biol Chem. 1983 Dec 10;258(23):14619–14625. [PubMed] [Google Scholar]
- Gottesman S., Gottesman M., Shaw J. E., Pearson M. L. Protein degradation in E. coli: the lon mutation and bacteriophage lambda N and cII protein stability. Cell. 1981 Apr;24(1):225–233. doi: 10.1016/0092-8674(81)90518-3. [DOI] [PubMed] [Google Scholar]
- Grayhack E. J., Roberts J. W. The phage lambda Q gene product: activity of a transcription antiterminator in vitro. Cell. 1982 Sep;30(2):637–648. doi: 10.1016/0092-8674(82)90260-4. [DOI] [PubMed] [Google Scholar]
- Greenblatt J., Li J., Adhya S., Friedman D. I., Baron L. S., Redfield B., Kung H. F., Weissbach H. L factor that is required for beta-galactosidase synthesis is the nusA gene product involved in transcription termination. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1991–1994. doi: 10.1073/pnas.77.4.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt J., Li J. Interaction of the sigma factor and the nusA gene protein of E. coli with RNA polymerase in the initiation-termination cycle of transcription. Cell. 1981 May;24(2):421–428. doi: 10.1016/0092-8674(81)90332-9. [DOI] [PubMed] [Google Scholar]
- Greenblatt J., Li J. Properties of the N gene transcription antitermination protein of bacteriophage lambda. J Biol Chem. 1982 Jan 10;257(1):362–365. [PubMed] [Google Scholar]
- Greenblatt J., Li J. The nusA gene protein of Escherichia coli. Its identification and a demonstration that it interacts with the gene N transcription anti-termination protein of bacteriophage lambda. J Mol Biol. 1981 Mar 25;147(1):11–23. doi: 10.1016/0022-2836(81)90076-0. [DOI] [PubMed] [Google Scholar]
- Greenblatt J., McLimont M., Hanly S. Termination of transcription by nusA gene protein of Escherichia coli. Nature. 1981 Jul 16;292(5820):215–220. doi: 10.1038/292215a0. [DOI] [PubMed] [Google Scholar]
- Grodzicker T., Arditti R. R., Eisen H. Establishment of repression by lambdoid phage in catabolite activator protein and adenylate cyclase mutants of Escherichia coli. Proc Natl Acad Sci U S A. 1972 Feb;69(2):366–370. doi: 10.1073/pnas.69.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarneros G., Echols H. New mutants of bacteriophage lambda with a specific defect in excision from the host chromosome. J Mol Biol. 1970 Feb 14;47(3):565–574. doi: 10.1016/0022-2836(70)90323-2. [DOI] [PubMed] [Google Scholar]
- Guarneros G., Galindo J. M. The regulation of integrative recombination by the b2 region and the cII gene of bacteriophage lambda. Virology. 1979 May;95(1):119–126. doi: 10.1016/0042-6822(79)90406-9. [DOI] [PubMed] [Google Scholar]
- Guarneros G., Montañez C., Hernandez T., Court D. Posttranscriptional control of bacteriophage lambda gene expression from a site distal to the gene. Proc Natl Acad Sci U S A. 1982 Jan;79(2):238–242. doi: 10.1073/pnas.79.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest J. R., Nice H. M. Chromosomal location of the mop (groE) gene necessary for bacteriophage morphogenesis in escherichia coli. J Gen Microbiol. 1978 Dec;109(2):329–333. doi: 10.1099/00221287-109-2-329. [DOI] [PubMed] [Google Scholar]
- Günther E., Bagdasarian M., Schuster H. Cloning of the dnaB gene of Escherichia coli: the dnaB gene of groPB534 and groPB612 and the replication of phage lambda. Mol Gen Genet. 1984;193(2):225–230. doi: 10.1007/BF00330672. [DOI] [PubMed] [Google Scholar]
- Günther E., Lanka E., Mikolajczyk M., Schuster H. The dnaB protein of Escherichia coli groPB mutants. J Biol Chem. 1981 Oct 25;256(20):10712–10716. [PubMed] [Google Scholar]
- Günther E., Mikolajczyk M., Schuster H. Stabilization by ATP and ADP of Escherichia coli dnaB protein activity. J Biol Chem. 1981 Dec 10;256(23):11970–11973. [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. The effect of a lambda repressor mutation on the activation of transcription initiation from the lambda PRM promoter. Cell. 1983 Feb;32(2):327–333. doi: 10.1016/0092-8674(83)90452-x. [DOI] [PubMed] [Google Scholar]
- Hendrix R. W., Casjens S. R. Protein fusion: a novel reaction in bacteriophage lambda head assembly. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1451–1455. doi: 10.1073/pnas.71.4.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix R. W. Purification and properties of groE, a host protein involved in bacteriophage assembly. J Mol Biol. 1979 Apr 15;129(3):375–392. doi: 10.1016/0022-2836(79)90502-3. [DOI] [PubMed] [Google Scholar]
- Hendrix R. W., Tsui L. Role of the host in virus assembly: cloning of the Escherichia coli groE gene and identification of its protein product. Proc Natl Acad Sci U S A. 1978 Jan;75(1):136–139. doi: 10.1073/pnas.75.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herendeen S. L., VanBogelen R. A., Neidhardt F. C. Levels of major proteins of Escherichia coli during growth at different temperatures. J Bacteriol. 1979 Jul;139(1):185–194. doi: 10.1128/jb.139.1.185-194.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskowitz I. Control of gene expression in bacteriophage lambda. Annu Rev Genet. 1973;7:289–324. doi: 10.1146/annurev.ge.07.120173.001445. [DOI] [PubMed] [Google Scholar]
- Herskowitz I., Hagen D. The lysis-lysogeny decision of phage lambda: explicit programming and responsiveness. Annu Rev Genet. 1980;14:399–445. doi: 10.1146/annurev.ge.14.120180.002151. [DOI] [PubMed] [Google Scholar]
- Hertman I., Luria S. E. Transduction studies on the role of a rec+ gene in the ultraviolet induction of prophage lambda. J Mol Biol. 1967 Jan 28;23(2):117–133. doi: 10.1016/s0022-2836(67)80021-4. [DOI] [PubMed] [Google Scholar]
- Ho Y. S., Wulff D. L., Rosenberg M. Bacteriophage lambda protein cII binds promoters on the opposite face of the DNA helix from RNA polymerase. Nature. 1983 Aug 25;304(5928):703–708. doi: 10.1038/304703a0. [DOI] [PubMed] [Google Scholar]
- Hochschild A., Irwin N., Ptashne M. Repressor structure and the mechanism of positive control. Cell. 1983 Feb;32(2):319–325. doi: 10.1016/0092-8674(83)90451-8. [DOI] [PubMed] [Google Scholar]
- Hohn T., Flick H., Hohn B. Petit lambda, a family of particles from coliphage lambda infected cells. J Mol Biol. 1975 Oct 15;98(1):107–120. doi: 10.1016/s0022-2836(75)80104-5. [DOI] [PubMed] [Google Scholar]
- Hohn T., Hohn B., Engel A., Wurtz M., Smith P. R. Isolation and characterization of the host protein groE involved in bacteriophage lambda assembly. J Mol Biol. 1979 Apr 15;129(3):359–373. doi: 10.1016/0022-2836(79)90501-1. [DOI] [PubMed] [Google Scholar]
- Holowachuk E. W., Friesen J. D. Isolation of a recombinant lambda phage carrying nusA and surrounding region of the Escherichia coli K-12 chromosome. Mol Gen Genet. 1982;187(2):248–253. doi: 10.1007/BF00331126. [DOI] [PubMed] [Google Scholar]
- Hopkins N. Bypassing a positive regulator: isolation of a lambda mutant that does not require N product to grow. Virology. 1970 Feb;40(2):223–229. doi: 10.1016/0042-6822(70)90397-1. [DOI] [PubMed] [Google Scholar]
- Hoyt M. A., Knight D. M., Das A., Miller H. I., Echols H. Control of phage lambda development by stability and synthesis of cII protein: role of the viral cIII and host hflA, himA and himD genes. Cell. 1982 Dec;31(3 Pt 2):565–573. doi: 10.1016/0092-8674(82)90312-9. [DOI] [PubMed] [Google Scholar]
- Inoko H., Imai M. Isolation and genetic characterization of the nitA mutants of Escherichia coli affecting the termination factor rho. Mol Gen Genet. 1976 Jan 16;143(2):211–221. doi: 10.1007/BF00266924. [DOI] [PubMed] [Google Scholar]
- Ishii S., Hatada E., Maekawa T., Imamoto F. Molecular cloning and nucleotide sequencing of the nusB gene of E. coli. Nucleic Acids Res. 1984 Jun 25;12(12):4987–4995. doi: 10.1093/nar/12.12.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii S., Ihara M., Maekawa T., Nakamura Y., Uchida H., Imamoto F. The nucleotide sequence of the cloned nusA gene and its flanking region of Escherichia coli. Nucleic Acids Res. 1984 Apr 11;12(7):3333–3342. doi: 10.1093/nar/12.7.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB F., CAMPBELL A. Sur le système de répression assurant l'immunité chez les bactéries lysogenes. C R Hebd Seances Acad Sci. 1959 Jun 1;248(22):3219–3221. [PubMed] [Google Scholar]
- Jarvik J., Botstein D. Conditional-lethal mutations that suppress genetic defects in morphogenesis by altering structural proteins. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2738–2742. doi: 10.1073/pnas.72.7.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. O., Herskowitz I. Mutants of bacteriophage lambda which do not requre the cIII gene for efficient lysogenization. Virology. 1978 Jul 15;88(2):199–212. doi: 10.1016/0042-6822(78)90277-5. [DOI] [PubMed] [Google Scholar]
- Jordan E., Green L., Echols H. Establishment of repression by bacteriophage lambda: lack of a direct regulatory effect of cyclic AMP. Virology. 1973 Oct;55(2):521–523. doi: 10.1016/0042-6822(73)90194-3. [DOI] [PubMed] [Google Scholar]
- KAISER A. D. Mutations in a temperate bacteriophage affecting its ability to lysogenize Escherichia coli. Virology. 1957 Feb;3(1):42–61. doi: 10.1016/0042-6822(57)90022-3. [DOI] [PubMed] [Google Scholar]
- Katzir N., Oppenheim A., Belfort M., Oppenheim A. B. Activation of the lambda int gene by the cii and ciii gene products. Virology. 1976 Oct 15;74(2):324–331. doi: 10.1016/0042-6822(76)90339-1. [DOI] [PubMed] [Google Scholar]
- Kelley P. M., Schlesinger M. J. Antibodies to two major chicken heat shock proteins cross-react with similar proteins in widely divergent species. Mol Cell Biol. 1982 Mar;2(3):267–274. doi: 10.1128/mcb.2.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C. J., Walker G. C. DNA-damaging agents stimulate gene expression at specific loci in Escherichia coli. Proc Natl Acad Sci U S A. 1980 May;77(5):2819–2823. doi: 10.1073/pnas.77.5.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppel F., Georgopoulos C. P., Eisen H. Host interference with expression of the lambda N gene product. Biochimie. 1974;56(11-12):1505–1509. doi: 10.1016/s0300-9084(75)80273-2. [DOI] [PubMed] [Google Scholar]
- Kikuchi Y., Nash H. A. The bacteriophage lambda int gene product. A filter assay for genetic recombination, purification of int, and specific binding to DNA. J Biol Chem. 1978 Oct 25;253(20):7149–7157. [PubMed] [Google Scholar]
- Kikuchi Y., Nash H. Integrative recombination of bacteriophage lambda: requirement for supertwisted DNA in vivo and characterization of int. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):1099–1109. doi: 10.1101/sqb.1979.043.01.122. [DOI] [PubMed] [Google Scholar]
- Kingston R. E., Chamberlin M. J. Pausing and attenuation of in vitro transcription in the rrnB operon of E. coli. Cell. 1981 Dec;27(3 Pt 2):523–531. doi: 10.1016/0092-8674(81)90394-9. [DOI] [PubMed] [Google Scholar]
- Kirby E. P., Jacob F., Goldthwait D. A. Prophage induction and filament formation in a mutant strain of Escherichia coli. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1903–1910. doi: 10.1073/pnas.58.5.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A., Lanka E., Schuster H. Isolation of a complex between the P protein of phage lambda and the dnaB protein of Escherichia coli. Eur J Biochem. 1980 Mar;105(1):1–6. doi: 10.1111/j.1432-1033.1980.tb04467.x. [DOI] [PubMed] [Google Scholar]
- Klinkert J., Klein A. Roles of bacteriophage lambda gene products O and P during early and late phases of infection cycle. J Virol. 1978 Mar;25(3):730–737. doi: 10.1128/jvi.25.3.730-737.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll B. J. An analysis of repressor overproduction by the lambda cIIIs mutant. J Mol Biol. 1979 Aug 15;132(3):551–555. doi: 10.1016/0022-2836(79)90276-6. [DOI] [PubMed] [Google Scholar]
- Knoll B. J. Interactions of the lambda cIIIs1 and E. coli hfl-1 mutations. Virology. 1980 Aug;105(1):270–272. doi: 10.1016/0042-6822(80)90178-6. [DOI] [PubMed] [Google Scholar]
- Knoll B. J. Isolation and characterization of mutations in the cIII gene of bacteriophage lambda which increase the efficiency of lysogenization of Escherichia coli K-12. Virology. 1979 Jan 30;92(2):518–531. doi: 10.1016/0042-6822(79)90154-5. [DOI] [PubMed] [Google Scholar]
- Kochan J., Carrascosa J. L., Murialdo H. Bacteriophage lambda preconnectors. Purification and structure. J Mol Biol. 1984 Apr 15;174(3):433–447. doi: 10.1016/0022-2836(84)90330-9. [DOI] [PubMed] [Google Scholar]
- Kochan J., Murialdo H. Stimulation of groE synthesis in Escherichia coli by bacteriophage lambda infection. J Bacteriol. 1982 Mar;149(3):1166–1170. doi: 10.1128/jb.149.3.1166-1170.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourilsky P., Gros D. Lysogenization by bacteriophage lambda IV inhibition of phage DNA synthesis by the products of genes cII and cIII. Biochimie. 1976;58(11-12):1321–1327. doi: 10.1016/s0300-9084(77)80015-1. [DOI] [PubMed] [Google Scholar]
- Kourilsky P., Knapp A. Lysogenization by bacteriophage lambda. III. Multiplicity dependent phenomena occuring upon infection by lambda. Biochimie. 1974;56(11-12):1517–1523. [PubMed] [Google Scholar]
- Kourilsky P. Lysogenization by bacteriophage lambda. I. Multiple infection and the lysogenic response. Mol Gen Genet. 1973 Apr 12;122(2):183–195. doi: 10.1007/BF00435190. [DOI] [PubMed] [Google Scholar]
- Kourilsky P. Lysogenization by bacteriophage lambda. II. Identification of genes involved in the multiplicity dependent processes. Biochimie. 1974;56(11-12):1511–1516. [PubMed] [Google Scholar]
- Krueger J. H., Walker G. C. groEL and dnaK genes of Escherichia coli are induced by UV irradiation and nalidixic acid in an htpR+-dependent fashion. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1499–1503. doi: 10.1073/pnas.81.5.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung H., Spears C., Weissbach H. Purification and properties of a soluble factor required for the deoxyribonucleic acid-directed in vitro synthesis of beta-galactosidase. J Biol Chem. 1975 Feb 25;250(4):1556–1562. [PubMed] [Google Scholar]
- Künzler P., Hohn T. Stages of bacteriophage lambda head morphogenesis: physical analysis of particles in solution. J Mol Biol. 1978 Jun 25;122(2):191–211. doi: 10.1016/0022-2836(78)90035-9. [DOI] [PubMed] [Google Scholar]
- LWOFF A. Lysogeny. Bacteriol Rev. 1953 Dec;17(4):269–337. doi: 10.1128/br.17.4.269-337.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LWOFF A., SIMINOVITCH L., KJELDGAARD N. Induction de la production de bacteriophages chez une bactérie lysogène. Ann Inst Pasteur (Paris) 1950 Dec;79(6):815–859. [PubMed] [Google Scholar]
- Lau L. F., Roberts J. W., Wu R. RNA polymerase pausing and transcript release at the lambda tR1 terminator in vitro. J Biol Chem. 1983 Aug 10;258(15):9391–9397. [PubMed] [Google Scholar]
- Lau L. F., Roberts J. W., Wu R. Transcription terminates at lambda tR1 in three clusters. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6171–6175. doi: 10.1073/pnas.79.20.6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecocq J. P., Dambly C., Lathe R., Babinet C., Bailone A., Devoret R., Gathoye A. M., Garcia H., De Wilde M., Cabezon T. Nomenclature and location of bacterial mutations modifying the frequency of lysogenization of E. coli by lambdoid phages. Mol Gen Genet. 1976 Apr 23;145(1):63–64. doi: 10.1007/BF00331558. [DOI] [PubMed] [Google Scholar]
- Lecocq J., Dambly C. A bacterial RNA polymerase mutant that renders lambda growth independent of the N and cro functions at 42 degrees C. Mol Gen Genet. 1976 Apr 23;145(1):53–64. doi: 10.1007/BF00331557. [DOI] [PubMed] [Google Scholar]
- Lieb M. Studies of heat-inducible lambda bacteriophage. I. Order of genetic sites and properties of mutant prophages. J Mol Biol. 1966 Mar;16(1):149–163. doi: 10.1016/s0022-2836(66)80269-3. [DOI] [PubMed] [Google Scholar]
- Lipińska B., Podhajska A., Taylor K. Synthesis and decay of lambda DNA replication proteins in minicells. Biochem Biophys Res Commun. 1980 Jan 15;92(1):120–126. doi: 10.1016/0006-291x(80)91528-4. [DOI] [PubMed] [Google Scholar]
- Little J. W. Autodigestion of lexA and phage lambda repressors. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1375–1379. doi: 10.1073/pnas.81.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W., Mount D. W. The SOS regulatory system of Escherichia coli. Cell. 1982 May;29(1):11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- Loomis W. F., Wheeler S., Schmidt J. A. Phosphorylation of the major heat shock protein of Dictyostelium discoideum. Mol Cell Biol. 1982 May;2(5):484–489. doi: 10.1128/mcb.2.5.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupski J. R., Smiley B. L., Godson G. N. Regulation of the rpsU-dnaG-rpoD macromolecular synthesis operon and the initiation of DNA replication in Escherichia coli K-12. Mol Gen Genet. 1983;189(1):48–57. doi: 10.1007/BF00326054. [DOI] [PubMed] [Google Scholar]
- MAKMAN R. S., SUTHERLAND E. W. ADENOSINE 3',5'-PHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1965 Mar;240:1309–1314. [PubMed] [Google Scholar]
- McMacken R., Mantei N., Butler B., Joyner A., Echols H. Effect of mutations in the c2 and c3 genes of bacteriophage lambda on macromolecular synthesis in infected cells. J Mol Biol. 1970 May 14;49(3):639–655. doi: 10.1016/0022-2836(70)90288-3. [DOI] [PubMed] [Google Scholar]
- Miller H. I., Friedman D. I. An E. coli gene product required for lambda site-specific recombination. Cell. 1980 Jul;20(3):711–719. doi: 10.1016/0092-8674(80)90317-7. [DOI] [PubMed] [Google Scholar]
- Miller H. I., Kikuchi A., Nash H. A., Weisberg R. A., Friedman D. I. Site-specific recombination of bacteriophage lambda: the role of host gene products. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):1121–1126. doi: 10.1101/sqb.1979.043.01.125. [DOI] [PubMed] [Google Scholar]
- Miller H. I., Kirk M., Echols H. SOS induction and autoregulation of the himA gene for site-specific recombination in Escherichia coli. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6754–6758. doi: 10.1073/pnas.78.11.6754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller H. I., Mozola M. A., Friedman D. I. int-h: An int mutation of phage lambda that enhances site-specific recombination. Cell. 1980 Jul;20(3):721–729. doi: 10.1016/0092-8674(80)90318-9. [DOI] [PubMed] [Google Scholar]
- Miller H. I. Multilevel regulation of bacteriophage lambda lysogeny by the E. coli himA gene. Cell. 1981 Jul;25(1):269–276. doi: 10.1016/0092-8674(81)90252-x. [DOI] [PubMed] [Google Scholar]
- Miller H. I., Nash H. A. Direct role of the himA gene product in phage lambda integration. Nature. 1981 Apr 9;290(5806):523–526. doi: 10.1038/290523a0. [DOI] [PubMed] [Google Scholar]
- Mizuuchi K., Gellert M., Nash H. A. Involement of supertwisted DNA in integrative recombination of bacteriophage lambda. J Mol Biol. 1978 May 25;121(3):375–392. doi: 10.1016/0022-2836(78)90370-4. [DOI] [PubMed] [Google Scholar]
- Mizuuchi K., Mizuuchi M. Integrative recombination of bacteriophage lambda: in vitro study of the intermolecular reaction. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):1111–1114. doi: 10.1101/sqb.1979.043.01.123. [DOI] [PubMed] [Google Scholar]
- Movva R. N., Green P., Nakamura K., Inouye M. Interaction of cAMP receptor protein with the ompA gene, a gene for a major outer membrane protein of Escherichia coli. FEBS Lett. 1981 Jun 15;128(2):186–190. doi: 10.1016/0014-5793(81)80077-4. [DOI] [PubMed] [Google Scholar]
- Murialdo H., Becker A. A genetic analysis of bacteriophage lambda prohead assembly in vitro. J Mol Biol. 1978 Oct 15;125(1):57–74. doi: 10.1016/0022-2836(78)90254-1. [DOI] [PubMed] [Google Scholar]
- Murialdo H., Becker A. Assembly of biologically active proheads of bacteriophage lambda in vitro. Proc Natl Acad Sci U S A. 1977 Mar;74(3):906–910. doi: 10.1073/pnas.74.3.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murialdo H., Becker A. Head morphogenesis of complex double-stranded deoxyribonucleic acid bacteriophages. Microbiol Rev. 1978 Sep;42(3):529–576. doi: 10.1128/mr.42.3.529-576.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murialdo H. Early intermediates in bacteriophage lambda prohead assembly. Virology. 1979 Jul 30;96(2):341–367. doi: 10.1016/0042-6822(79)90094-1. [DOI] [PubMed] [Google Scholar]
- Musso R. E., Di Lauro R., Adhya S., de Crombrugghe B. Dual control for transcription of the galactose operon by cyclic AMP and its receptor protein at two interspersed promoters. Cell. 1977 Nov;12(3):847–854. doi: 10.1016/0092-8674(77)90283-5. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Uchida H. Isolation of conditionally lethal amber mutations affecting synthesis of the nusA protein of Escherichia coli. Mol Gen Genet. 1983;190(2):196–203. doi: 10.1007/BF00330640. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Yura T. Induction of sigma factor synthesis in Escherichia coli by the N gene product of bacteriophage lambda. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4405–4409. doi: 10.1073/pnas.73.12.4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash H. A. Integration and excision of bacteriophage lambda: the mechanism of conservation site specific recombination. Annu Rev Genet. 1981;15:143–167. doi: 10.1146/annurev.ge.15.120181.001043. [DOI] [PubMed] [Google Scholar]
- Nash H. A., Robertson C. A. Purification and properties of the Escherichia coli protein factor required for lambda integrative recombination. J Biol Chem. 1981 Sep 10;256(17):9246–9253. [PubMed] [Google Scholar]
- Neidhardt F. C., VanBogelen R. A., Lau E. T. Molecular cloning and expression of a gene that controls the high-temperature regulon of Escherichia coli. J Bacteriol. 1983 Feb;153(2):597–603. doi: 10.1128/jb.153.2.597-603.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., VanBogelen R. A. Positive regulatory gene for temperature-controlled proteins in Escherichia coli. Biochem Biophys Res Commun. 1981 May 29;100(2):894–900. doi: 10.1016/s0006-291x(81)80257-4. [DOI] [PubMed] [Google Scholar]
- Nevins J. R. Induction of the synthesis of a 70,000 dalton mammalian heat shock protein by the adenovirus E1A gene product. Cell. 1982 Jul;29(3):913–919. doi: 10.1016/0092-8674(82)90453-6. [DOI] [PubMed] [Google Scholar]
- Ogawa T. Analysis of dnaB function of Escherichia coli K12 and the dnaB-like function of P1 prophage. J Mol Biol. 1975 May 25;94(3):327–340. doi: 10.1016/0022-2836(75)90206-5. [DOI] [PubMed] [Google Scholar]
- Olson E. R., Flamm E. L., Friedman D. I. Analysis of nutR: a region of phage lambda required for antitermination of transcription. Cell. 1982 Nov;31(1):61–70. doi: 10.1016/0092-8674(82)90405-6. [DOI] [PubMed] [Google Scholar]
- Oppenheim A. B., Gottesman S., Gottesman M. Regulation of bacteriophage lambda int gene expression. J Mol Biol. 1982 Jul 5;158(3):327–346. doi: 10.1016/0022-2836(82)90201-7. [DOI] [PubMed] [Google Scholar]
- Parkinson J. S., Huskey R. J. Deletion mutants of bacteriophage lambda. I. Isolation and initial characterization. J Mol Biol. 1971 Mar 14;56(2):369–384. doi: 10.1016/0022-2836(71)90471-2. [DOI] [PubMed] [Google Scholar]
- Plumbridge J. A., Howe J. G., Springer M., Touati-Schwartz D., Hershey J. W., Grunberg-Manago M. Cloning and mapping of a gene for translational initiation factor IF2 in Escherichia coli. Proc Natl Acad Sci U S A. 1982 Aug;79(16):5033–5037. doi: 10.1073/pnas.79.16.5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt L. F. Control of bacteriophage lambda repressor synthesis after phage infection: the role of the N, cII, cIII and cro products. J Mol Biol. 1975 Apr 5;93(2):267–288. doi: 10.1016/0022-2836(75)90132-1. [DOI] [PubMed] [Google Scholar]
- Reichardt L., Kaiser A. D. Control of lambda repressor synthesis. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2185–2189. doi: 10.1073/pnas.68.9.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser W., Leibrecht I., Klein A. Structure and function of mutants in the P gene of bacteriophage lambda leading to the pi phenotype. Mol Gen Genet. 1983;192(3):430–435. doi: 10.1007/BF00392186. [DOI] [PubMed] [Google Scholar]
- Revel H. R., Stitt B. L., Lielausis I., Wood W. B. Role of the host cell in bacteriophage T4 development. I. Characterization of host mutants that block T4 head assembly. J Virol. 1980 Jan;33(1):366–376. doi: 10.1128/jvi.33.1.366-376.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson J. P., Fink P., Blanchard K., Macy M. Bacteria with defective rho factors suppress the effects of N mutations in bacteriophage lambda. Mol Gen Genet. 1977 May 20;153(1):81–85. doi: 10.1007/BF01035999. [DOI] [PubMed] [Google Scholar]
- Richardson J. P., Grimley C., Lowery C. Transcription termination factor rho activity is altered in Escherichia coli with suA gene mutations. Proc Natl Acad Sci U S A. 1975 May;72(5):1725–1728. doi: 10.1073/pnas.72.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W., Craig N. L. Escherichia coli recA gene product inactivates phage lambda repressor. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4714–4718. doi: 10.1073/pnas.75.10.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W., Mount D. W. Inactivation and proteolytic cleavage of phage lambda repressor in vitro in an ATP-dependent reaction. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2283–2287. doi: 10.1073/pnas.74.6.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W. Proteolytic cleavage of bacteriophage lambda repressor in induction. Proc Natl Acad Sci U S A. 1975 Jan;72(1):147–151. doi: 10.1073/pnas.72.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. W. Termination factor for RNA synthesis. Nature. 1969 Dec 20;224(5225):1168–1174. doi: 10.1038/2241168a0. [DOI] [PubMed] [Google Scholar]
- Roberts J. W. Transcription termination and late control in phage lambda. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3300–3304. doi: 10.1073/pnas.72.9.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D., Shimatake H., Brady C., Wulff D. L. The relationship between function and DNA sequence in an intercistronic regulatory region in phage lambda. Nature. 1978 Mar 30;272(5652):414–423. doi: 10.1038/272414a0. [DOI] [PubMed] [Google Scholar]
- Ross W., Landy A. Bacteriophage lambda int protein recognizes two classes of sequence in the phage att site: characterization of arm-type sites. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7724–7728. doi: 10.1073/pnas.79.24.7724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross W., Landy A. Patterns of lambda Int recognition in the regions of strand exchange. Cell. 1983 May;33(1):261–272. doi: 10.1016/0092-8674(83)90355-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan M. J. Coumermycin A1: A preferential inhibitor of replicative DNA synthesis in Escherichia coli. I. In vivo characterization. Biochemistry. 1976 Aug 24;15(17):3769–3777. doi: 10.1021/bi00662a020. [DOI] [PubMed] [Google Scholar]
- SMITH H. O., LEVINE M. TWO SEQUENTIAL REPRESSIONS OF DNA SYNTHESIS IN THE ESTABLISHMENT OF LYSOGENY BY PHAGE P22 AND ITS MUTANTS. Proc Natl Acad Sci U S A. 1964 Aug;52:356–363. doi: 10.1073/pnas.52.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., Nakamura Y., Uchida H. A transducing lambda phage carrying grpE, a bacterial gene necessary for lambda DNA replication, and two ribosomal protein genes, rpsP (S16) and rplS (L19). Mol Gen Genet. 1978 Oct 24;165(3):247–256. doi: 10.1007/BF00332523. [DOI] [PubMed] [Google Scholar]
- Saito H., Uchida H. Initiation of the DNA replication of bacteriophage lambda in Escherichia coli K12. J Mol Biol. 1977 Jun 15;113(1):1–25. doi: 10.1016/0022-2836(77)90038-9. [DOI] [PubMed] [Google Scholar]
- Saito H., Uchida H. Organization and expression of the dnaJ and dnaK genes of Escherichia coli K12. Mol Gen Genet. 1978 Aug 4;164(1):1–8. doi: 10.1007/BF00267592. [DOI] [PubMed] [Google Scholar]
- Salstrom J. S., Szybalski W. Coliphage lambdanutL-: a unique class of mutants defective in the site of gene N product utilization for antitermination of leftward transcription. J Mol Biol. 1978 Sep 5;124(1):195–221. doi: 10.1016/0022-2836(78)90156-0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Hong G. F., Hill D. F., Petersen G. B. Nucleotide sequence of bacteriophage lambda DNA. J Mol Biol. 1982 Dec 25;162(4):729–773. doi: 10.1016/0022-2836(82)90546-0. [DOI] [PubMed] [Google Scholar]
- Sauer R. T. DNA sequence of the bacteriophage gama cI gene. Nature. 1978 Nov 16;276(5685):301–302. doi: 10.1038/276301a0. [DOI] [PubMed] [Google Scholar]
- Schmeissner U., Court D., Shimatake H., Rosenberg M. Promoter for the establishment of repressor synthesis in bacteriophage lambda. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3191–3195. doi: 10.1073/pnas.77.6.3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. C., Chamberlin M. J. Amplification and isolation of Escherichia coli nusA protein and studies of its effects on in vitro RNA chain elongation. Biochemistry. 1984 Jan 17;23(2):197–203. doi: 10.1021/bi00297a004. [DOI] [PubMed] [Google Scholar]
- Schnös M., Inman R. B. Position of branch points in replicating lambda DNA. J Mol Biol. 1970 Jul 14;51(1):61–73. doi: 10.1016/0022-2836(70)90270-6. [DOI] [PubMed] [Google Scholar]
- Schwarz E., Scherer G., Hobom G., Kössel H. Nucleotide sequence of cro, cII and part of the O gene in phage lambda DNA. Nature. 1978 Mar 30;272(5652):410–414. doi: 10.1038/272410a0. [DOI] [PubMed] [Google Scholar]
- Shih M. C., Gussin G. N. Differential effects of mutations on discrete steps in transcription initiation at the lambda PRE promoter. Cell. 1983 Oct;34(3):941–949. doi: 10.1016/0092-8674(83)90551-2. [DOI] [PubMed] [Google Scholar]
- Shih M. C., Gussin G. N. Role of cII protein in stimulating transcription initiation at the lambda PRE promoter. Enhanced formation and stabilization of open complexes. J Mol Biol. 1984 Feb 5;172(4):489–506. doi: 10.1016/s0022-2836(84)80019-4. [DOI] [PubMed] [Google Scholar]
- Simatake H., Rosenberg M. Purified lambda regulatory protein cII positively activates promoters for lysogenic development. Nature. 1981 Jul 9;292(5819):128–132. doi: 10.1038/292128a0. [DOI] [PubMed] [Google Scholar]
- Simon L. D., Gottesman M., Tomczak K., Gottesman S. Hyperdegradation of proteins in Escherichia coli rho mutants. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1623–1627. doi: 10.1073/pnas.76.4.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons R. W., Kleckner N. Translational control of IS10 transposition. Cell. 1983 Sep;34(2):683–691. doi: 10.1016/0092-8674(83)90401-4. [DOI] [PubMed] [Google Scholar]
- Sternberg N. A class of rifR RNA polymerase mutations that interferes with the expression of coliphage lambda late gene. Virology. 1976 Aug;73(1):139–154. doi: 10.1016/0042-6822(76)90068-4. [DOI] [PubMed] [Google Scholar]
- Sternberg N. Properties of a mutant of Escherichia coli defective in bacteriophage lambda head formation (groE). I. Initial characterization. J Mol Biol. 1973 May 5;76(1):1–23. doi: 10.1016/0022-2836(73)90078-8. [DOI] [PubMed] [Google Scholar]
- Sternberg N. Properties of a mutant of Escherichia coli defective in bacteriophage lambda head formation (groE). II. The propagation of phage lambda. J Mol Biol. 1973 May 5;76(1):25–44. doi: 10.1016/0022-2836(73)90079-x. [DOI] [PubMed] [Google Scholar]
- Strauch M., Friedman D. I. Identification of the nusB gene product of Escherichia coli. Mol Gen Genet. 1981;182(3):498–501. doi: 10.1007/BF00293941. [DOI] [PubMed] [Google Scholar]
- Sugino A., Peebles C. L., Kreuzer K. N., Cozzarelli N. R. Mechanism of action of nalidixic acid: purification of Escherichia coli nalA gene product and its relationship to DNA gyrase and a novel nicking-closing enzyme. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4767–4771. doi: 10.1073/pnas.74.11.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner-Smith M., Becker A., Gold M. DNA packaging in the lambdoid phages: the role of lambda genes Nu1 and A. Virology. 1981 Jun;111(2):642–646. doi: 10.1016/0042-6822(81)90363-9. [DOI] [PubMed] [Google Scholar]
- Sunshine M., Feiss M., Stuart J., Yochem J. A new host gene (groPC) necessary for lambda DNA replication. Mol Gen Genet. 1977 Feb 28;151(1):27–34. doi: 10.1007/BF00446909. [DOI] [PubMed] [Google Scholar]
- Swindle J., Ajioka J., Dawson D., Myers R., Carroll D., Georgopoulos C. The nucleotide sequence of the Escherichia coli K12 nusB (groNB) gene. Nucleic Acids Res. 1984 Jun 25;12(12):4977–4985. doi: 10.1093/nar/12.12.4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindle J., Ajioka J., Georgopoulos C. Identification of the E. coli groNB(nusB) gene product. Mol Gen Genet. 1981;182(3):409–413. doi: 10.1007/BF00293928. [DOI] [PubMed] [Google Scholar]
- Szybalski W. Initiation and regulation of transcription in coliphage lambda. Basic Life Sci. 1974;3:201–212. doi: 10.1007/978-1-4613-4529-9_16. [DOI] [PubMed] [Google Scholar]
- Tabor H., Hafner E. W., Tabor C. W. Construction of an Escherichia coli strain unable to synthesize putrescine, spermidine, or cadaverine: characterization of two genes controlling lysine decarboxylase. J Bacteriol. 1980 Dec;144(3):952–956. doi: 10.1128/jb.144.3.952-956.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H., Coppo A., Manzi A., Martire G., Pulitzer J. F. Design of a system of conditional lethal mutations (tab/k/com) affecting protein-protein interactions in bacteriophage T4-infected Escherichia coli. J Mol Biol. 1975 Aug 25;96(4):563–578. doi: 10.1016/0022-2836(75)90139-4. [DOI] [PubMed] [Google Scholar]
- Takano T., Kakefuda T. Involvement of a bacterial factor in morphogenesis of bacteriophage capsid. Nat New Biol. 1972 Sep 13;239(89):34–37. doi: 10.1038/newbio239034a0. [DOI] [PubMed] [Google Scholar]
- Taylor D. E., Levine J. G. Characterization of a plasmid mutation affecting maintenance, transfer and elimination by novobiocin. Mol Gen Genet. 1979 Jul 13;174(2):127–133. doi: 10.1007/BF00268350. [DOI] [PubMed] [Google Scholar]
- Tilly K., Georgopoulos C. Evidence that the two Escherichia coli groE morphogenetic gene products interact in vivo. J Bacteriol. 1982 Mar;149(3):1082–1088. doi: 10.1128/jb.149.3.1082-1088.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly K., McKittrick N., Georgopoulos C., Murialdo H. Studies on Escherichia coli mutants which block bacteriophage morphogenesis. Prog Clin Biol Res. 1981;64:35–45. [PubMed] [Google Scholar]
- Tilly K., McKittrick N., Zylicz M., Georgopoulos C. The dnaK protein modulates the heat-shock response of Escherichia coli. Cell. 1983 Sep;34(2):641–646. doi: 10.1016/0092-8674(83)90396-3. [DOI] [PubMed] [Google Scholar]
- Tilly K., Murialdo H., Georgopoulos C. Identification of a second Escherichia coli groE gene whose product is necessary for bacteriophage morphogenesis. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1629–1633. doi: 10.1073/pnas.78.3.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly K., VanBogelen R. A., Georgopoulos C., Neidhardt F. C. Identification of the heat-inducible protein C15.4 as the groES gene product in Escherichia coli. J Bacteriol. 1983 Jun;154(3):1505–1507. doi: 10.1128/jb.154.3.1505-1507.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurimoto T., Hase T., Matsubara H., Matsubara K. Bacteriophage lambda initiators: preparation from a strain that overproduces the O and P proteins. Mol Gen Genet. 1982;187(1):79–86. doi: 10.1007/BF00384387. [DOI] [PubMed] [Google Scholar]
- Tsurimoto T., Matsubara K. Purified bacteriophage lambda O protein binds to four repeating sequences at the lambda replication origin. Nucleic Acids Res. 1981 Apr 24;9(8):1789–1799. doi: 10.1093/nar/9.8.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurimoto T., Matsubara K. Replication of lambda dv plasmid in vitro promoted by purified lambda O and P proteins. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7639–7643. doi: 10.1073/pnas.79.24.7639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanBogelen R. A., Vaughn V., Neidhardt F. C. Gene for heat-inducible lysyl-tRNA synthetase (lysU) maps near cadA in Escherichia coli. J Bacteriol. 1983 Feb;153(2):1066–1068. doi: 10.1128/jb.153.2.1066-1068.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada M., Itikawa H. Participation of Escherichia coli K-12 groE gene products in the synthesis of cellular DNA and RNA. J Bacteriol. 1984 Feb;157(2):694–696. doi: 10.1128/jb.157.2.694-696.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward D. F., DeLong A., Gottesman M. E. Escherichia coli nusB mutations that suppress nusA1 exhibit lambda N specificity. J Mol Biol. 1983 Jul 25;168(1):73–85. doi: 10.1016/s0022-2836(83)80323-4. [DOI] [PubMed] [Google Scholar]
- Ward D. F., Gottesman M. E. The nus mutations affect transcription termination in Escherichia coli. Nature. 1981 Jul 16;292(5820):212–215. doi: 10.1038/292212a0. [DOI] [PubMed] [Google Scholar]
- Wickner S. H. DNA replication proteins of Escherichia coli and phage lambda. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):303–310. doi: 10.1101/sqb.1979.043.01.037. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold M. S., Mallory J. B., Roberts J. D., LeBowitz J. H., McMacken R. Initiation of bacteriophage lambda DNA replication in vitro with purified lambda replication proteins. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6176–6180. doi: 10.1073/pnas.79.20.6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson J. S., Hooper D. C., Swartz M. N., McHugh G. L. Antagonism of the B subunit of DNA gyrase eliminates plasmids pBR322 and pMG110 from Escherichia coli. J Bacteriol. 1982 Oct;152(1):338–344. doi: 10.1128/jb.152.1.338-344.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A. M., Platt T. Transcription termination: nucleotide sequence at 3' end of tryptophan operon in Escherichia coli. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5442–5446. doi: 10.1073/pnas.75.11.5442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulff D. L., Beher M., Izumi S., Beck J., Mahoney M., Shimatake H., Brady C., Court D., Rosenberg M. Structure and function of the cy control region of bacteriophage lambda. J Mol Biol. 1980 Apr;138(2):209–230. doi: 10.1016/0022-2836(80)90284-3. [DOI] [PubMed] [Google Scholar]
- Wulff D. L., Mahoney M., Shatzman A., Rosenberg M. Mutational analysis of a regulatory region in bacteriophage lambda that has overlapping signals for the initiation of transcription and translation. Proc Natl Acad Sci U S A. 1984 Jan;81(2):555–559. doi: 10.1073/pnas.81.2.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamori T., Ito K., Nakamura Y., Yura T. Transient regulation of protein synthesis in Escherichia coli upon shift-up of growth temperature. J Bacteriol. 1978 Jun;134(3):1133–1140. doi: 10.1128/jb.134.3.1133-1140.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamori T., Yura T. Genetic control of heat-shock protein synthesis and its bearing on growth and thermal resistance in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1982 Feb;79(3):860–864. doi: 10.1073/pnas.79.3.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky C. Attenuation in the control of expression of bacterial operons. Nature. 1981 Feb 26;289(5800):751–758. doi: 10.1038/289751a0. [DOI] [PubMed] [Google Scholar]
- Yochem J., Uchida H., Sunshine M., Saito H., Georgopoulos C. P., Feiss M. Genetic analysis of two genes, dnaJ and dnaK, necessary for Escherichia coli and bacteriophage lambda DNA replication. Mol Gen Genet. 1978 Aug 4;164(1):9–14. doi: 10.1007/BF00267593. [DOI] [PubMed] [Google Scholar]
- Yoshida R. K., Miller J. L., Miller H. I., Friedman D. I., Howe M. M. Isolation and mapping of Mu nu mutants which grow in him mutants of E. coli. Virology. 1982 Jul 15;120(1):269–272. doi: 10.1016/0042-6822(82)90027-7. [DOI] [PubMed] [Google Scholar]
- Zaret K. S., Sherman F. DNA sequence required for efficient transcription termination in yeast. Cell. 1982 Mar;28(3):563–573. doi: 10.1016/0092-8674(82)90211-2. [DOI] [PubMed] [Google Scholar]
- Zweig M., Cummings D. J. Cleavage of head and tail proteins during bacteriophage T5 assembly: selective host involvement in the cleavage of a tail protein. J Mol Biol. 1973 Nov 5;80(3):505–518. doi: 10.1016/0022-2836(73)90418-x. [DOI] [PubMed] [Google Scholar]
- Zylicz M., Georgopoulos C. Purification and properties of the Escherichia coli dnaK replication protein. J Biol Chem. 1984 Jul 25;259(14):8820–8825. [PubMed] [Google Scholar]
- Zylicz M., Gorska I., Taylor K., Georgopoulos C. Bacteriophage lambda replication proteins: formation of a mixed oligomer and binding to the origin of lambda DNA. Mol Gen Genet. 1984;196(3):401–406. doi: 10.1007/BF00436186. [DOI] [PubMed] [Google Scholar]
- Zylicz M., LeBowitz J. H., McMacken R., Georgopoulos C. The dnaK protein of Escherichia coli possesses an ATPase and autophosphorylating activity and is essential in an in vitro DNA replication system. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6431–6435. doi: 10.1073/pnas.80.21.6431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylicz M., Taylor K. Interactions between phage lambda replication proteins, lambda DNA and minicell membrane. Eur J Biochem. 1981 Jan;113(2):303–309. doi: 10.1111/j.1432-1033.1981.tb05067.x. [DOI] [PubMed] [Google Scholar]
- de Crombrugghe B., Mudryj M., DiLauro R., Gottesman M. Specificity of the bacteriophage lambda N gene product (pN): nut sequences are necessary and sufficient for antitermination by pN. Cell. 1979 Dec;18(4):1145–1151. doi: 10.1016/0092-8674(79)90227-7. [DOI] [PubMed] [Google Scholar]


