Abstract
Polarized growth in yeast requires cooperation between the polarized actin cytoskeleton and delivery of post-Golgi secretory vesicles. We have previously reported that loss of the major tropomyosin isoform, Tpm1p, results in cells sensitive to perturbations in cell polarity. To identify components that bridge these processes, we sought mutations with both a conditional defect in secretion and a partial defect in polarity. Thus, we set up a genetic screen for mutations that conferred a conditional growth defect, showed synthetic lethality with tpm1Δ, and simultaneously became denser at the restrictive temperature, a hallmark of secretion-defective cells. Of the 10 complementation groups recovered, the group with the largest number of independent isolates was functionally null alleles of RAS2. Consistent with this, ras2Δ and tpm1Δ are synthetically lethal at 35°C. We show that ras2Δ confers temperature-sensitive growth and temperature-dependent depolarization of the actin cytoskeleton. Furthermore, we show that at elevated temperatures ras2Δ cells are partially defective in endocytosis and show a delocalization of two key polarity markers, Myo2p and Cdc42p. However, the conditional enhanced density phenotype of ras2Δ cells is not a defect in secretion. All the phenotypes of ras2Δ cells can be fully suppressed by expression of yeast RAS1 or RAS2 genes, human Ha-ras, or the double disruption of the stress response genes msn2Δmsn4Δ. Although the best characterized pathway of Ras function in yeast involves activation of the cAMP-dependent protein kinase A pathway, activation of the protein kinase A pathway does not fully suppress the actin polarity defects, suggesting that there is an additional pathway from Ras2p to Msn2/4p. Thus, Ras2p regulates cytoskeletal polarity in yeast under conditions of mild temperature stress through the stress response pathway.
INTRODUCTION
Growth during the cell cycle in budding yeast is polarized to ensure correct assembly of a new bud and correct septum formation before cell division. These processes require coordination between the synthesis of cell growth material by the secretory pathway and its delivery by the polarized cytoskeleton. The actin cytoskeleton, consisting of cortical patches and cables, is polarized toward regions of cell growth and is responsible for delivery of the post-Golgi vesicles (Adams and Pringle, 1984; Novick and Botstein, 1985; Ayscough et al., 1997; reviewed by Finger and Novick, 1998; Pruyne and Bretscher, 2000b). The organization of the actin cytoskeleton is under the control of the cyclin-dependent kinase Cdc28p, which directs, in an unknown manner, a cascade of proteins centered on Cdc42p that ultimately sets up and maintains polarity (reviewed by Lew and Reed, 1995; Pruyne and Bretscher, 2000a). For example, when G1 cyclins, Cln1/2p, activate Cdc28p early in the cell cycle, Cdc42p along with several polarisome components, including Bni1p, Spa2, and Bud6p/Aip3p, localizes to the site of bud emergence to direct the assembly of a polarized actin cytoskeleton (Snyder, 1989; Snyder et al., 1991; Ziman et al., 1993; Amberg et al., 1997; Evangelista et al., 1997; Sheu et al., 1998; Jin and Amberg, 2000). Recently, distinct functions have been assigned to cortical patches and cables: many of the components of actin cortical patches are necessary for endocytosis (reviewed by Geli and Riezman, 1998; Wendland et al., 1998; Pruyne and Bretscher, 2000b); whereas actin cables are necessary for Myo2p-dependent polarized delivery of secretory vesicles, vacuolar elements, and factors necessary for initial spindle orientation (Johnston et al., 1991; Govindan et al., 1995; Hill et al., 1996; Catlett and Weisman, 1998; Schott et al., 1999; Yin et al., 2000), as well as for delivery of Ash1p mRNA by Myo4p (Bobola et al., 1996; Takizawa et al., 1997).
So far four components of actin cables have been identified: actin (Adams and Pringle, 1984), fimbrin (Drubin et al., 1988), Abp140p (Asakura et al., 1998), and tropomyosin (Liu and Bretscher, 1989), of which only tropomyosin is restricted to the cables. Tropomyosins are encoded by two genes, TPM1 and TPM2, which overlap in providing an essential polarizing function (Drees et al., 1995). Disruption of TPM1, which encodes the major tropomyosin isoform, is not lethal but results in yeast cells with a greatly reduced number of cables and with a slightly reduced cortical patch polarity (Liu and Bretscher, 1989, 1992). Elimination of all tropomyosin function leads to the complete loss of cables, depolarization of secretion, and depolarization of cortical patches (Pruyne et al., 1998).
In earlier studies aimed at identifying components that participate with tropomyosins in the structure or the regulation of the actin cytoskeleton, we set up a genetic screen for mutations that were lethal in the absence, but not in the presence, of Tpm1p. We analyzed seven mutations that fell into six complementation groups, all of which disrupted the normal polarity of the actin cytoskeleton (Wang and Bretscher, 1995, 1997). Thus, the tpm1Δ synthetic lethality screen appears to cast a wide net for mutations affecting polarity of the actin cytoskeleton. We therefore reasoned that an additional constraint to this screen might recover mutations affecting more defined aspects of polarization.
Because secretion and polarization of the actin cytoskeleton are closely coordinated events, there might be molecules that integrate these two processes. To search for such components, we sought to identify mutations that showed both synthetic lethality with tpm1Δ and were conditionally defective in secretion. In their classic screen for mutations that defined essential genes necessary for secretion, Novick et al. (1980) made use of the fact that cells conditionally defective for secretion become denser at their restrictive temperature. We therefore set out to look for mutations that resulted in synthetic lethality with tpm1Δ, conferred a conditional growth phenotype, and caused the cells to become denser at their restrictive temperature.
In addition to the expected isolation of mutations in SEC genes to be described elsewhere, this strategy resulted in the unexpected isolation of mutations in the RAS2 gene. Yeast has two Ras genes, RAS1 and RAS2, which are functional homologues of the mammalian proto-oncogene Ha-ras (DeFeo-Jones et al., 1985; Kataoka et al., 1985). In mammalian cells, Ras signals to multiple pathways that regulate nuclear gene expression as well as the actin cytoskeleton (Vojtek and Der, 1998; Shields et al., 2000). Ras regulation of the actin cytoskeleton is of particular interest in the study of cancers because rearrangements of the actin cytoskeleton are thought to be necessary for metastasis (Barbacid, 1987).
In contrast to the multiple Ras pathways present in mammalian cells, there exists only one well characterized pathway in yeast. This is a regulatory pathway from Ras1/2p through the cyclase-associated protein Srv2p to adenyl cyclase, Cyr1p (Shima et al., 2000). Production of cAMP activates the three protein kinase (PK) A catalytic subunits (Tpk1p, Tpk2p and Tpk3p) by binding to the PKA regulatory unit (Bcy1p) (Broach, 1991). In turn, PKA signals to the nucleus to carry out many functions of which an essential one is G1 progression (Thevelein and de Winde, 1999). The Ras2/PKA pathway has also been found to be a negative regulator of the stress response pathway (Marchler et al., 1993; Gorner et al., 1998). Many pathways, including the PKA pathway, converge on the related transcription factors Msn2p and Msn4p, which bind to the stress response element STRE to induce stress response genes (Martinez-Pastor et al., 1996; Schmitt and McEntee, 1996; Gorner et al., 1998).
Here we show that yeast Ras proteins are important for maintaining the polarity of the yeast actin cytoskeleton under conditions of mild heat stress. Moreover, loss of polarity is accompanied by depolarization of some key polarity markers. Because this effect of ras2Δ is abrogated in cells lacking Msn2/4p, Ras2p must normally signal through the stress response pathway to regulate cytoskeletal organization and polarization in yeast.
MATERIALS AND METHODS
Strains, Plasmids, and Media
Yeast strains used in this study are listed in Table 1. Plasmids used in this study and their method of construction are described in Table 2. Transformation of yeast cells was performed with the use of Frozen-EZ Yeast Transformation Kit (ZYMO Research, Orange, CA). Standard genetic techniques were used for strain construction and linkage analysis (Guthrie and Fink, 1991). Yeast were grown with the use of standard rich yeast extract-peptone-dextrose (YPD) and complete minimal (CM) dropout media (Ausubel et al., 2000; Difco Laboratories, Detroit, MI)
Table 1.
Yeast strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| NY10 | MATα ura3-52 | P. Novick, Yale University |
| NY13 | MATa ura3-52 | P. Novick |
| CUY570 | MATa ura3-52 leu2-3,112 his3-Δ200 trp1-Δ1 | T. Huffaker, Cornell University |
| RCY250 | MATα ura3-52 leu2-3,112 sec5-24 | R. Collins, Cornell University |
| ABY153 | MATα met4 | B. Tye, Cornell University |
| ABY1075 | MATa ura3-52 leu2Δ∷kanMX | This study |
| ABY1078 | MATα ura3-52 tpm1Δ∷kanMX +pTPM1-URA3 | This study |
| ABY1082B | MATα ura3-52 leu2Δ∷kanMX | This study |
| ABY1084B | MATα ura3-52 leu2Δ∷kanMX tpm1Δ∷kanMX+ pTPM1-URA3 | This study |
| ABY1087B | MATa ura3-52 leu2Δ∷kanMX tpm1Δ∷kanMX+ pTPM1-URA3 | This study |
| ABY1089 | MATα ura3-52 leu2Δ∷kanMX RAS1∷LEU2 | This study |
| ABY1204 | MATa ura3-52 leu2Δ∷kanMX ras2Δ∷URA3 | This study |
| ABY1205 | MATα ura3-52 leu2-3,112 his3-Δ200 trp1-Δ1 | This study |
| ABY1206 | MATa ura3-52 leu2-3,112 his3-Δ200 trp1-Δ1 | This study |
| ABY1207 | MATα ura3-52 leu2-3,112 his3-Δ200 trp1-Δ1 ras1Δ∷LEU2 | This study |
| ABY1209 | MATa ura3-52 leu2-3,112 his3-Δ200 trp1-Δ1 ras2Δ∷LEU2 | This study |
| ABY1210 | MATα ura3-52 leu2-3,112 his3-Δ200 trp1-Δ1 ras2Δ∷LEU2 | This study |
| ABY1226 | MATα ura3-52 leu2-3,112 his3-Δ200 trp1-Δ1 tpm1Δ∷kanMX | This study |
| ABY1228 | MATa ura3-52 leu2-3,112 his3-Δ200 trp1-Δ1 ras2Δ∷LEU2 tpm1Δ∷kanMX | This study |
| ABY1240 | MATα ura3-52 leu2-3,112 his3-Δ200 trp1-Δ1 ras1Δ∷LEU2 ras2Δ∷LEU2+ pTPK1 | This study |
| ABY1354 | MATa ura3-52 leu2Δ∷kanMX tpm1Δ∷kanMX tsl7-1+ pTPM1-URA3 | This study |
| SP1 | MATα his3 leu2ura3 trp1 ade8 Can | Stanhill et al., 1999 |
| SP1ras2Δ | Isogenic to SP1 but ras2∷LEU2 | Stanhill et al., 1999 |
| SP1ras2Δmsn2Δmsn4Δ | Isogenic to SP1 but ras2∷LEU2 msn2∷HIS3 msn4∷URA3 | Stanhill et al., 1999 |
Table 2.
Plasmids used in this study
| Name | Characteristics and Source |
|---|---|
| pTPM1-URA3 | TPM1 in pRS316 (CEN, URA3) (Bretscher Lab Stock) |
| pTPM1-LEU2 | = pTW119, TPM1 in pRS315 (CEN, LEU2) (Wang and Bretscher, 1997) |
| pRAS1 | = pJH71, RAS1 in pRS315 (CEN, LEU2). Isolated as a suppressor of ras2 mutant from a genomic library in pRS315 (Wang and Bretscher, 1997). |
| pRAS2 | = pJH92, RAS2 in pRS416 (CEN, URA3). Made by subcloning a 1.9-kb HindIII-XbaI RAS2 containing genomic fragment from a genomic library in pRS315 (Wang and Bretscher, 1997) into pRS416. |
| PADH1-Ha-ras | = AAH5 H-ras, Ha-ras under the control of the ADH1 promoter in AAH5 (2μ, LEU2) (Marshall et al., 1987) |
| PRAS2-Ha-ras | = pJH139, Ha-ras under the control of the RAS2 promoter in pRS414 (CEN, TRP1). PCR was used to generate a RAS2 promoter/Ha-ras fusion. The fusion fragment was amplified by using the primer pair 5′-AACGTTTTCGAATTGAAAGGAGATATACAGAAAAAAAAATGACAGAATACAAGCTTGT-3′ spanning the RAS2 promoter/Ha-ras junction and 5′-AGACTCGAGACTGCCCAGATGTCTTGCTG-3′ downstream of Ha-ras. The NspV and XhoI restriction sites are underlined and the Ha-ras open reading frame is in bold. This PCR product was cloned into the pCR-BluntII-Topo vector (Invitrogen, Carlsbad, CA) and then checked for errors by sequencing. The DNA was then cut with NspV and XhoI and inserted into a plasmid containing a genomic fragment of RAS2 in pRS414 (CEN, TRP1) which replaced the ORF of RAS2 with Ha-ras but left the promoter intact. |
| pCDC42 | = pPB102, CDC42 (2μ, URA3) (Bender and Pringle, 1989) |
| pCDC42Val12 | = pRS315(42/Val12)BH-2, CDC42VAL12 in pRS315 (CEN, LEU2) (Ziman et al., 1991) |
| pRHO1 | = pWT-URAE, RHO1 (2μ, URA3) (Madaule et al., 1987) |
| pRHO2 | = pC-186, RHO2 (2μ, URA3) (Madaule et al., 1987) |
| pRHO3 | = pPB1070, RHO3 (2μ, URA3) (Bender et al., 1996) |
| pRHO4 | = pOPR4, RHO4 (2μ, TRP1) (Matsui and Toh, 1992) |
| pTEM1-HA3 | = pSJ56, C-terminally 3× HA tagged TEM1 in pRS426 (2μ, URA3) (Jaspersen et al., 1998) |
| pHA3-DBF2 | = pSJ57, N-terminally 3× HA tagged DBF2 in pRS426 (2μ, URA3) (Jaspersen et al., 1998) |
| pCDC15-HA3 | = pSJ103, C-terminally 3× HA tagged CDC15 in pRS426 (2μ, URA3) (Jaspersen et al., 1998) |
| pHA-CDC5 | = p29, N-terminally HA tagged CDC5 in pRS426 (2μ, URA3) (Jaspersen et al., 1998) |
| pSPO12 | = pSJ19, SPO12 in pRS426 (2μ, URA3) (Jaspersen et al., 1998) |
| pRSR1 | = pJH140, RSR1 in pRS424 (2μ, TRP1). Made by subcloning a PstI-SpeI PCR-generated region of genomic RSR1 into pRS424 (2μ, TRP1). The PCR construct was checked for errors by sequencing. |
| pSRV2 | = pJH129, SRV2 in pRS424 (2μ, TRP1). Made by subcloning a XhoI-SstI PCR-generated region of genomic SRV2 into pRS424 (2μ, TRP1). PCR construct was checked for errors by sequencing. |
| pVPS34 | = pPHY52, VPS34 in pRS324 (2μ, TRP1) (Schu et al., 1993) |
| pTPK1 | = pJH126, TPK1 in pRS423 (2μ, HIS3). Made by subcloning a HindIII-SphI TPK1 containing fragment from B1373 (Toda et al., 1987) in PUC19. Then from this plasmid, a HindIII-SstI TPK1 containing fragment was subcloned into pRS416. Then from this plasmid, a XhoI-SstI TPK1 containing fragment was subcloned into pRS423 (2μ, HIS3). |
| pTPK2 | = pXP3, TPK2 in Yeplac195 (2μ, URA3) (Pan and Heitman, 1999) |
| pTPK3 | = pXP4, TPK3 in Yeplac195 (2μ, URA3) (Pan and Heitman, 1999) |
| pPFY1 | = p593, PFY1 in YEp24 (2μ, URA3) (Evangelista et al., 1997) |
Strain Construction
ABY1075 was constructed by replacing LEU2 with kanMX in NY13. LEU2 was replaced by transforming a polymerase chain reaction (PCR)-generated DNA fragment containing the kanMX module (Wach et al., 1994) flanked by 50 bases homologous to a region immediately upstream of the LEU2 start codon and directly downstream of the LEU2 stop codon. ABY1078 was constructed by replacing TPM1 with kanMX in NY10 and then transforming in pTPM1-URA3. ABY1082B, ABY1084B, and ABY1087B were obtained by mating ABY1075 with ABY1078. Because kanMX was used in the disruption of both LEU2 and TPM1, leu2− and LEU2+ spores were differentiated on the basis of their ability to grow on CM medium lacking leucine, and tpm1− and TPM1+ spores were differentiated by immunoblot analysis after having grown on 5-fluoroorotic acid (5-FOA) to select for loss of pTPM1-URA3. To tag the RAS1 locus with the LEU2 marker in ABY1089, a PCR-generated fragment containing the LEU2 marker replaced a region of the chromosomal DNA from position −617 to −864, relative to the start of the RAS1 open reading frame. Proper insertion of the LEU2 marker was verified by PCR. ABY1204 was constructed by replacing RAS2 with URA3 in ABY1075. To disrupt RAS2, a segment of the RAS2 open reading frame (from +42 to +543) was replaced with a PCR-generated DNA fragment containing URA3 from the pRS series of vectors (Sikorski and Hieter, 1989) flanked by 625 bases homologous to a region upstream and 676 bases homologous to a region downstream of the replaced segment. Proper disruption of RAS2 was verified by PCR. ABY1205 and ABY1206 were obtained by mating ABY1082B with CUY570 followed by sporulation of the generated diploid. ABY1207 was generated by first replacing RAS1 with LEU2 in ABY1082B and then mating the resultant strain to CUY570 followed by sporulation of the generated diploid. To disrupt RAS1, the first 480 bases and much of the upstream promoter sequence was replaced with a PCR-generated DNA fragment containing LEU2 flanked by 575 bases homologous to a region upstream and 428 bases homologous to a region downstream of the replaced segment. Proper disruption of RAS1 was verified by PCR. ABY1209 and ABY1210 were constructed by replacing RAS2 with LEU2 in ABY1082B (method of replacement is the same as the method used to construct ABY1204) and then mating the resultant strain to CUY570 followed by sporulation of the generated diploid. ABY1226 and ABY1228 were obtained in a two-step process: first, ABY1206 was mated to ABY1078 to obtain a strain that was MATa his3 trp1 leu2 ura3 tpm1Δ::kanMX + pTPM1-URA3; second, the resulting strain was mated to ABY1210 and then sporulated, and the dissected spores were grown on 5-FOA. To construct ABY1240, pRAS2 was transformed into ABY1209; this strain was then mated to ABY1207 and the generated diploid was sporulated. Because a double knockout of Ras is lethal, a ras1Δras2Δ strain was selected on the basis of 5-FOA sensitivity; the selected strain was then transformed with pTPK1 and grown on 5-FOA to select against cells retaining the pRAS2 plasmid.
Isolation of tpm1Δ Sythetic Lethal Mutants
Six stationary-phase cultures of ABY1084B and three stationary phase cultures of ABY1087B were harvested, washed twice with water, and resuspended in 5 ml of 0.5 M sodium acetate (pH 4.8) and 20 mg of sodium nitrite to induce mutations. After 10 min at 30°C, 5 ml of 2.7% Na2HPO4 containing 1% yeast extract was added, and cells were harvested and washed with water. This treatment resulted in approximately a 50% loss in viability. The mutagenized cells were resuspended in 50 ml of YPD medium to give an OD600 of ∼0.015 and then allowed to recover at 25°C for 16 h to an OD600 of 0.1–0.25. The cultures were then incubated at 37°C for 3 h, placed in an ice bath for 15 min, spun down, and resuspended in 0.8 ml of water. To enrich for dense cells, a sample was mixed with 9.2 ml of Percoll (colloidal silica; Sigma Chemical, St Louis, MO) in a 10-ml Sorvall Polypropylene Oak Ridge bottle (Sorvall, Newton, CT) and centrifuged in an HB-4 swinging bucket rotor (20,000 × g, 20 min, 4°C). One-milliliter fractions were taken from the top of the gradient and the two densest fractions, containing ∼2% of the cells, were diluted into 50 ml of YPD medium. The cells were then subjected to another round of growth at 25°C, a temperature shift to 37°C, and density enrichment. After the second round of density enrichment, the two densest fractions were diluted into 10 ml of YPD medium containing 25% glycerol and frozen (−80°C).
Cells were thawed, diluted 1:20, and plated onto YPD to give ∼100 colonies per plate, with a total of 360 plates used for the nine independent cultures. The plates were incubated at room temperature (20–25°C) for 2 d, and colonies were then replicated onto a CM 5-FOA plate and a CM uracil dropout plate. After 2 d the replica plates were compared, and those colonies that were able to grow on CM lacking uracil and not on CM 5-FOA were saved for further analysis.
Microscopy and Imaging
Fluorescence microscopy of actin was performed as described by Pringle et al. (1989). Affinity-purified actin antibodies (Wang and Bretscher, 1995) were used at a 1:25 dilution. Myo2p staining was performed with the use of affinity-purified Myo2p antibody (Schott et al., 1999) at a 1:50 dilution. Cdc42p staining was performed as described by Kozminski et al. (2000) with the use of an affinity-purified α-Cdc42p antibody, generously provided by David Drubin. Tpm1p staining was performed with the use of affinity-purified Tpm1p antibody (Pruyne et al., 1998) at a 1:50 dilution. In all cases, a methanol/acetone postfixation step and a goat anti-rabbit IgG fluorescein isothiocyanate (ICN BioChemicals, Aurora, OH) secondary antibody at a 1:150 dilution were used. Staining with lucifer yellow-carbohydrazide (LY; Molecular Probes, Eugene, OR) staining was performed as described by Riezman (1985). N-(3-Triethylammoniumpropyl)-4-(p-diethylaminophenylhexatrienyl) pyridium dibromide (FM4–64; Molecular Probes) staining was performed as described by Vida and Emr (1995).
For brightfield microscopy, cells were first fixed in formaldehyde for 2 h and visualized with the use of differential interference contrast (DIC).
DIC and fluorescence images were acquired with a RTC/CCD digital camera (Princeton Instruments, Trenton, NJ) with the use of a Zeiss Axiovert 100 TV microscope (Carl Zeiss, Oberkochen, Germany) and the Metamorph Imaging System (Universal Imaging, West Chester, PA). All images were processed through Abode Photoshop 5.0 (Adobe Systems, Mountain View, CA).
Determination of Internal Levels of Invertase and Bgl2p
Cells were grown in YPD to midlog phase and preshifted to 37°C for 30 min. The yeast were then washed once with water, pelleted, and resuspended in low-glucose (0.1%) YPD and kept at 37°C for 3 h. Afterward, cells were washed twice in chilled 10 mM NaN3 solution, once in a basic solution (0.1 M Tris-SO4, pH 9.4) to loosen the cell wall, once in spheroplast buffer (0.1 M Tris-PO4, pH 7.5, with 1.2 M sorbitol and 14 mM β-mercaptoethanol), and then spheroplasted by the addition of 0.05 mg/ml 100T zymolyase for 30 min at 37°C. Samples were centrifuged to separate internal from external fractions. The supernatant (external fraction) was separated and the pellet (internal fraction) was resuspended in an equal volume of spheroplast buffer. Triton X-100 was added to one-half of both the external and internal fractions to a 0.5% final concentration and used for invertase assays. The other half was boiled in 6× SDS sample buffer, subjected to SDS-PAGE, and immunoblotted for Bgl2p with an antibody generously provided by Dr. Franz Klebl. Invertase was assayed at 30°C as described by Novick and Schekman (1979).
Quantitation of LY uptake
Quantitation of LY uptake was done through analysis of digitally acquired images with the use of the Metamorph Imaging System. A fixed area smaller than the size of a single yeast vacuole (a circle with a radius of 0.3 μm) was chosen as the standard area of measurement. Within each cell the amount of fluorescence contained in the standard area was measured when the standard area was placed within the vacuole and when it was placed in the cytoplasm. The difference between these two measurements was considered the amount of LY taken up by the cell. This process was repeated for n = 30 RAS2+ cells and n = 30 ras2Δ cells.
RESULTS
Identification of Conditional Mutants That Show Synthetic Lethality with tpm1Δ and Become Denser at 37°C
To isolate mutants that are synthetically lethal with tpm1Δ, show temperature-sensitive growth, and get denser at the restrictive temperature, we generated tpm1Δ strains that carried the pTPM1-URA3 plasmid in both mating types (ABY1084B and ABY1087B). Nine independent cultures were mutagenized with nitrous acid to ∼50% viability. After recovering from mutagenesis, exponentially growing cultures were shifted to 37°C for 3 h and fractionated on a Percoll density gradient. The denser cells, representing ∼2% of the population, were recovered and then subjected to a second temperature-dependent density enrichment step. After the second enrichment, cells were spread onto YPD plates. After growth at 25°C, ∼34,000 colonies were replica plated onto control and 5-FOA-containing plates. A total of 1064 colonies failed to grow on the 5-FOA plates, indicating that they were probably unable to grow without the pTPM1-URA3 plasmid. Of these colonies, 112 were temperature sensitive for growth at 37°C. From these, 72 were able to grow on 5-FOA if an alternate plasmid containing TPM1 (pTPM1-LEU2) was introduced, demonstrating a dependence on TPM1. On retesting, all 72 potential Tsl (TPM1 synthetic lethal) mutants showed temperature sensitivity and became denser after growth at 37°C.
Complementation tests were used to assess dominance of the mutations and to place recessive mutants into complementation groups. After this, selected Tsl mutants were backcrossed to the appropriate parental strains and sporulated. Tetrads were analyzed for segregation of the three phenotypes: synthetic lethality with tpm1Δ, conditional growth at 37°C, and increased density at 37°C. Thirty-three of the mutants were discarded either because the phenotypes did not cosegregate or were too leaky. The remaining 39 mutants contained recessive mutations that fell into 10 complementation groups, designated TSL7 through TSL16. The two largest groups, TSL7 and TSL8, showed a clear cosegregation of the temperature sensitivity and enhanced density phenotypes, although through successive backcrosses the tpm1Δ synthetic lethal phenotype became leaky. Because TSL7 contained the largest number of independent isolates, we explored it further. In this report we characterize the TSL7 gene; the other TSL complementation groups will be described elsewhere.
TSL7 Is RAS2
The TSL7 gene was cloned from a CEN-based genomic library (Wang and Bretscher, 1995) by complementation of tsl7-1 temperature sensitivity. Seventeen plasmids were recovered, eight of which contained genomic DNA covering the RAS1 locus and nine of which contained genomic DNA covering the RAS2 locus. Further subcloning demonstrated that RAS2 complemented the tsl7 mutation but not the adjacent open reading frames. RAS1 on a CEN plasmid was also able to complement the temperature sensitivity of tsl7. To distinguish whether TSL7 was RAS1 or RAS2, the linkage of tsl7-1 (ABY1354) to RAS1::LEU2 (ABY1089) and the linkage of tsl7-1 to met4 (ABY153), which is closely linked to RAS2, was determined. Analysis of 14 tetrads revealed no linkage between tsl7-1 and RAS1::LEU2, whereas analysis of 17 tetrads revealed a genetic distance of 5.5 cM between tsl7-1 and met4, close to the reported distance of 4.4 cM between RAS2 and MET4. Next, we sequenced the RAS2 locus in four of the five independent isolates from the TSL7 complementation group. All four isolates contained mutations (Gln29→Stop, Gly67→Arg, Gln136→stop, Gln156→stop) in RAS2 that are predicted to destroy Ras2p function.
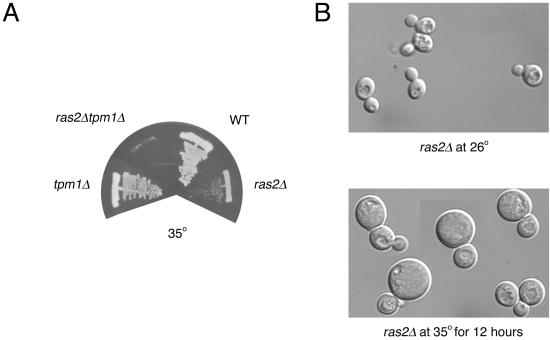
Consistent with the linkage analysis, we generated a ras2Δ strain that conferred both a temperature-sensitive phenotype and a temperature-dependent increase in density; whereas our ras1Δ conferred neither a temperature-sensitive phenotype nor a temperature-dependent increase in density. ras2Δ cells were crossed to tpm1Δ cells and, surprisingly, the double mutant combination was viable at room temperature. However, whereas the tpm1Δ and ras2Δ strains grew at 35°C, the tpm1Δ ras2Δ double mutant did not (Figure 1A). From these data, we conclude that TSL7 is RAS2 and that ras2Δ shows synthetic lethality with tpm1Δ at 35°C.
Figure 1.
Growth properties of mutants. (A) Wild-type (WT; ABY1205), ras2Δ (ABY1209), tpm1Δ (ABY1226), and ras2Δtpm1Δ (ABY 1228) were first grown on selective media and then streaked onto YPD plates at 35°C. Growth after 2 d is shown. (B) DIC images of ras2Δ (ABY1209) grown at 26°C and after a 12-h 35°C temperature shift.
When ras2Δ cells are shifted to 35°C for 12 h, cells enlarge and become heterogeneous in size (Figure 1B). This phenotype is in contrast to phenotypes in which there are conditional defects in secretion, where cells stop growing completely, but remarkably similar tpm1Δ and other mutants defective in the cytoskeleton, such as act1, myo2, and cdc42, which continue to grow isotropically when shifted to their restrictive temperatures (Novick and Botstein, 1985; Adams et al., 1990; Johnston et al., 1991).
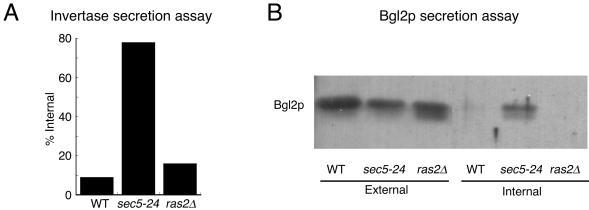
The ras2Δ Mutant Is Not Defective in the Secretion of Periplasmic Enzymes Invertase and Bgl2p
To determine whether the density phenotype of ras2Δ cells at 37°C was due to a defect in secretion, we examined the ability of ras2Δ strains to secrete the periplasmic enzymes invertase and Bgl2p. Wild-type, ras2Δ, and sec5–24 strains were shifted to low glucose to induce invertase expression at 37°C, the restrictive temperature for ras2Δ strains. The amount of internal and external invertase was determined in three separate experiments, and the percentage of internal invertase is shown in Figure 2A. As expected, the classical secretion mutant sec5-24 accumulates internal invertase and the wild-type strain secretes invertase normally. Similar to the wild-type strain, the ras2Δ mutant secretes invertase normally.
Figure 2.
ras2Δ mutants accumulate neither invertase nor Bgl2p. (A) ras2Δ (ABY1205) mutants shifted to 37°C for 3 h accumulate invertase to the same degree as wild-type (WT) strains (ABY1209) and to a lesser degree than sec5-24 mutants (RCY250). Percentage of internal invertase is calculated as internal/(internal plus external). (B) Immunoblot analysis of Bgl2p shows that ras2Δ (ABY1205) mutants shifted to 37°C for 3 h does not accumulate Bgl2p internally, similar to wild-type strains (ABY1205) but in contrast to sec5-24 mutants (RCY250).
There are two distinct post-Golgi vesicle populations in yeast, with invertase being specific to the lighter population and Bgl2p, a cell wall endoglucanase, being specific to the denser population (Harsay and Bretscher, 1995). We reasoned that ras2Δ mutants might accumulate the denser population of vesicles rather than the lighter population and therefore examined whether Bgl2p accumulated in ras2Δ mutants shifted to 37°C for 3 h. Because Bgl2p is constitutively expressed, immunoblots revealed that it was present in all external fractions and, as expected, wild-type strains did not accumulate significant Bgl2p internally, whereas sec5-24 did (Figure 2B). As with wild-type cells, the ras2Δ mutant did not accumulate Bgl2p internally, showing that ras2Δ mutants do not have a defect in Bgl2p secretion.
The ras2Δ Mutant Has a Temperature-dependent Actin Cytoskeletal Polarization Defect
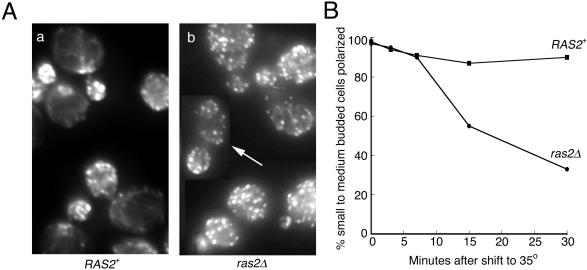
Because the synthetic lethality between tpm1Δ and ras2Δ suggested that Ras2p might be involved in the organization of the actin cytoskeleton, we examined actin localization in ras2Δ cells. At room temperature, the actin organization of ras2Δ cells was similar to wild-type. However, upon a 30-min shift to 35°C, the actin cytoskeleton of the ras2Δ mutant became disorganized, whereas in wild-type cells the actin organization remained normal (Figure 3A). To quantitate this phenotype, we counted the number of small to medium budded cells that were polarized at various times after shifting to 35°C (Figure 3B). A cell was counted as polarized if >50% of the actin patches detected by fluorescence microscopy were within the bud. Before the temperature shift (0 time point), >95% of both RAS2+ and ras2Δ cells were polarized. After a 30-min shift, only ∼30% of ras2Δ cells remained polarized, whereas ∼90% of RAS2+ remained polarized; of that 30% in ras2Δ cells, many cells were qualitatively depolarized but were not counted as depolarized because the majority of actin patches remained in the bud (arrow in Figure 3A shows an example of a cell counted as polarized). This result argues that Ras2p is essential for maintaining actin cytoskeletal organization under mild heat stress conditions but is not essential for actin organization at room temperature. It also suggests that the reason ras2Δ and tpm1Δ show synthetic lethality only at 35°C is because the ras2Δ depolarization phenotype is manifested only at higher temperatures.
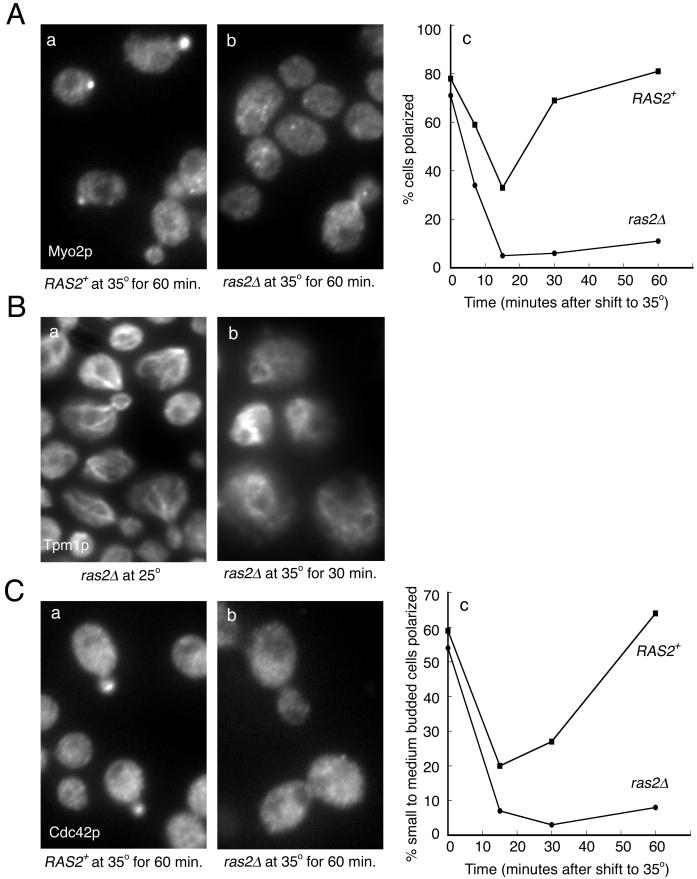
Figure 3.
The actin cytoskeleton becomes depolarized in ras2Δ cells at 35°C. (A) RAS2+ (ABY1205) and ras2Δ (ABY1209) cells growing in YPD were shifted to 35°C for 30 min, fixed, and stained for actin. ras2Δ mutants became depolarized after a 30-min shift, whereas wild-type cells remained polarized. The arrow indicates a cell that was scored as polarized, although qualitatively the cell is not completely polarized. (B) RAS2+ (▪, ABY1205) and ras2Δ (●, ABY 1209) cells were shifted to 35°C, fixed at 0, 3, 7, 15, and 30 min, and stained for actin. One hundred small and medium budded cells were scored for actin polarization. A cell was scored polarized if >50% of total actin patch fluorescence was concentrated in the bud.
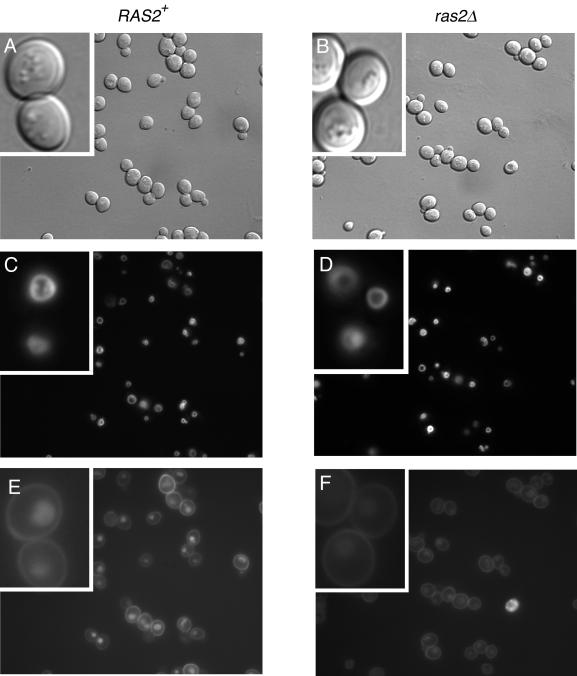
The ras2Δ Mutant Has a Partial Defect in the Endocytosis of LY
Mutations in cortical actin patch components frequently cause a loss of actin polarity as well as a defect in endocytosis (reviewed by Geli and Riezman, 1998; Wendland et al., 1998; Pruyne and Bretscher, 2000b). Because ras2Δ cells display a loss of actin patch polarity and Ras2p binds directly to the patch component Srv2p, we examined whether ras2Δ cells would display a temperature-dependent endocytic defect. Although no defect could be detected in the endocytosis of the lipophilic dye FM4–64 (our unpublished results), ras2Δ mutant cells displayed a partial defect in the uptake of LY at 37°C (Figure 4, E and F). Quantitation of fluorescence intensity indicated a 50% reduction of LY staining in ras2Δ compared with wild-type cells (an average of 16,620 arbitrary units of fluorescence in RAS2+ vs. 9098 U in ras2Δ mutants.)
Figure 4.
ras2Δ cells are partially defective for endocytosis of LY. Corresponding DIC (A and B), FM4-64 (C and D), and LY (E and F) images of wild-type (ABY1205) and ras2Δ (ABY1209) cells. Cells were grown and stained with the vacuolar marker FM4-64 at 26°C and then shifted to 37°C for 1.5 h before incubating with LY for 1 h at 37°C.
Because it was difficult to see the vacuole clearly in DIC images of mutant cells after the temperature shift (Figure 4, A and B), we were concerned that the reduced level of LY staining might reflect a disruption of the vacuole rather than a defect in endocytosis. To examine this, the vacuole was labeled at room temperature with FM4-64 and then the cells were shifted to 37°C to examine endocytosis of LY. The results showed no significant alteration in vacuole morphology (Figure 4, C and D) and therefore indicated that ras2Δ confers a mild defect on endocytosis of LY.
Two Markers of Polarity, Myo2p and Cdc42p, Are Depolarized in ras2Δ Mutants in a Temperature-dependent Manner
Polarized secretion in yeast has been shown to occur by the delivery of post-Golgi vesicles, carried by the myosin V encoded by MYO2, along polarized actin cables (Govindan et al., 1995; Pruyne et al., 1998). In wild-type cells, Myo2p shows a highly polarized distribution (Lillie and Brown, 1994). To examine whether loss of Ras2p affected this distribution, ras2Δ cells were shifted to 35°C for various times and Myo2p localized by immunofluorescence microscopy. In wild-type cells, Myo2p underwent a transient depolarization within 15 min and then repolarized back to its former distribution (Figure 5A; Lillie and Brown, 1994). In contrast, ras2Δ cells, although initially having a highly polarized Myo2p distribution, depolarized to a greater extent than wild-type cells and remained depolarized for up to 60 min. But consistent with the ability of ras2Δ cells to grow in temperatures up to 35°C, after 3 h at 35°C, 60% of ras2Δ cells regained a polarized distribution of Myo2p (Ho and Bretscher, unpublished results). This is in contrast to a ras2Δtpm1Δ mutant that was unable to live at 35°C and in which Myo2p distribution remained depolarized after 3 h at 35°C (Ho and Bretscher, unpublished results). Because Myo2p translocates down actin cables (Lillie and Brown, 1994; Pruyne et al., 1998), we examined cable organization. Immunolocalization of the cable-specific component Tpm1p in ras2Δ cells revealed that they are well organized at 25°C but completely disorganized after shifting to 35°C for 30 min (Figure 5B).
Figure 5.
The polarity markers Myo2p and Cdc42p show sustained depolarization in ras2Δ cells. The percentage of RAS2+ (▪, ABY1205) and ras2Δ (●, ABY1209) cells showing a polarized distribution of Myo2p and Cdc42p was determined after shifting cells growing in YPD to 35°C for the indicated times and then staining with specific antibodies. (A) For Myo2p staining, cells at all stages of the cell cycle were scored. (C) For Cdc42p staining, cells at the small and medium budded stages were scored. Examples of staining after 60-min shifts (a and b). (B) Tpm1p localization in ras2Δ cells (ABY1209), before (a) and after (b) a 30-min shift to 35°C.
Cdc42p is one of the key molecules directing the polarity of the actin cytoskeleton in yeast. During the cell cycle, Cdc42p is localized to sites of active growth (Ziman et al., 1993). To see whether the distribution of this molecule was also affected, Cdc42p was localized by immunofluorescence microscopy in wild-type and ras2Δ cells after a temperature shift to 35°C. In wild-type cells, the percentage of cells with a polarized distribution of Cdc42p dropped to 20% in 15 min but then recovered (Figure 5C). In ras2Δ cells, Cdc42p was localized at 25°C, but the percentage of cells polarized dropped rapidly after shifting to 35°C and it remained low for up to 60 min (Figure 5C). Thus, two proteins that play key roles in polarized growth, Myo2p and Cdc42p, depend on ras2Δ for their polarized distribution at 35°C.
Human Ha-ras Is Able to Complement the ras2Δ Actin Depolarization Phenotype
Classic studies have shown that at 25°C human Ha-ras can provide the essential function in yeast normally provided by Ras1p and Ras2p (DeFeo-Jones et al., 1985; Kataoka et al., 1985). We therefore wished to examine whether human Ha-ras could also suppress the growth defect of ras2Δ cells at 37°C and/or provide the function necessary for polarization of the actin cytoskeleton at 35°C. The human Ha-ras on a 2 μ plasmid expressed from the strong ADH1 promoter (PADH1-Ha-ras) or on a CEN plasmid and behind the yeast RAS2 promoter (PRAS2-Ha-ras), as well as yeast RAS1 and RAS2 behind their endogenous promoters on CEN plasmids (pRAS1 and pRAS2), were all able to complement the temperature sensitivity at 37°C caused by ras2Δ (Figure 6A; Table 3). To test for suppression of the actin polarization defect, cells were shifted to 35°C for 30 min, and the percentage of small to medium budded cells with a polarized actin distribution was determined. Whereas ras2Δ mutant cells are 20% polarized under these conditions, Ha-ras behind either promoter or yeast RAS1 or RAS2 on CEN plasmids were able to rescue the actin depolarization phenotype (Figure 6B). These results indicate that not only can an enhanced level of Ras1p substitute for Ras2p function involving regulation of the polarity of the actin cytoskeleton but also that human Ras can provide this function as well. The reason ras2Δ, but not ras1Δ, cells display a mutant phenotype may be because Ras2p is more abundant than Ras1p in wild-type cells (cited as unpublished data in Mosch et al., 1999).
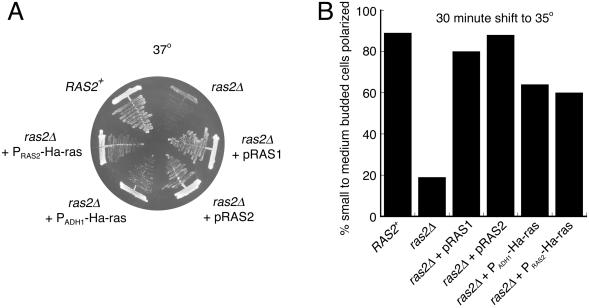
Figure 6.
Suppression of both temperature sensitivity and actin depolarization ras2Δ phenotypes by yeast and human Ras proteins. (A) Growth of wild-type (ABY1205), ras2Δ (ABY1209 or ABY1204) alone or carrying pRAS1, pRAS2, PADH1-Ha-ras, or PRAS2-Ha-ras on CM selective media at 37°C after 3 d. (B) Cells were also grown in CM selective media, shifted to 35°C for 30 min, fixed, stained for actin, and scored for actin polarization.
Table 3.
Ability of various genes expressed from plasmids to suppress the conditional growth and actin depolarization phenotypes of a ras2Δ mutant
| Plasmid | Growth at 37°C | Actin polarity at 35°C | Plasmid | Growth at 37°C | Actin polarity at 35°C |
|---|---|---|---|---|---|
| Vector | − | − | pTPK2 | − | − |
| pRAS1 | + | + | pTPK3 | − | − |
| pRAS2 | + | + | pTEM1-HA3 | − | − |
| PRAS2-Ha-ras | + | + | pHA3-DBF2 | − | − |
| PADH1-Ha-ras | + | + | pCDC15-HA3 | − | − |
| pRHO1 | − | − | pHA-CDC5 | − | − |
| pRHO2 | − | − | pSPO12 | − | − |
| pRHO3 | − | − | pRSR1 | − | − |
| pRHO4 | − | − | pSRV2 | − | − |
| pCDC42 | − | − | pPFY1 | − | − |
| pCDC42V12 | − | − | pVPS34 | − | − |
| pTPK1 | − | − |
ras2Δ (ABY1209) cells were transformed with the various plasmids. After growth on selective media, strains were streaked to YPD media for growth at 37°C and after 3 d were scored to test for suppression of the conditional growth defect. Alternatively, cells were grown in liquid YPD at 26°C before being shifted to 35°C for 30 min and then fixed, stained for actin, and scored for actin polarization. + indicates growth or polarized actin; − indicates no growth or depolarized actin.
The Ras2p-Mediated Polarization of the Actin Cytoskeleton Is Not Mediated Solely by the PKA Pathway
The best characterized downstream pathway of Ras1/2p in yeast involves the activation of adenylate cyclase, which produces cAMP to activate the cAMP-dependent protein kinases (Tpk1/2/3p; Broach, 1991). Overexpression of Tpk1p rescues the double ras1Δ ras2Δ mutant at 25°C (Toda et al., 1987). However, ras1Δ ras2Δ rescued by Tpk1p is still temperature sensitive, indicating a function of Ras that is not provided by Tpk1p (Wigler et al., 1988). In agreement with these results, overexpression of Tpk1p in our ras2Δ strain does not rescue the temperature sensitivity at 37°C (Figure 7A; Table 3) but does rescue the temperature-dependent increase in density (Ho and Bretscher, unpublished results).
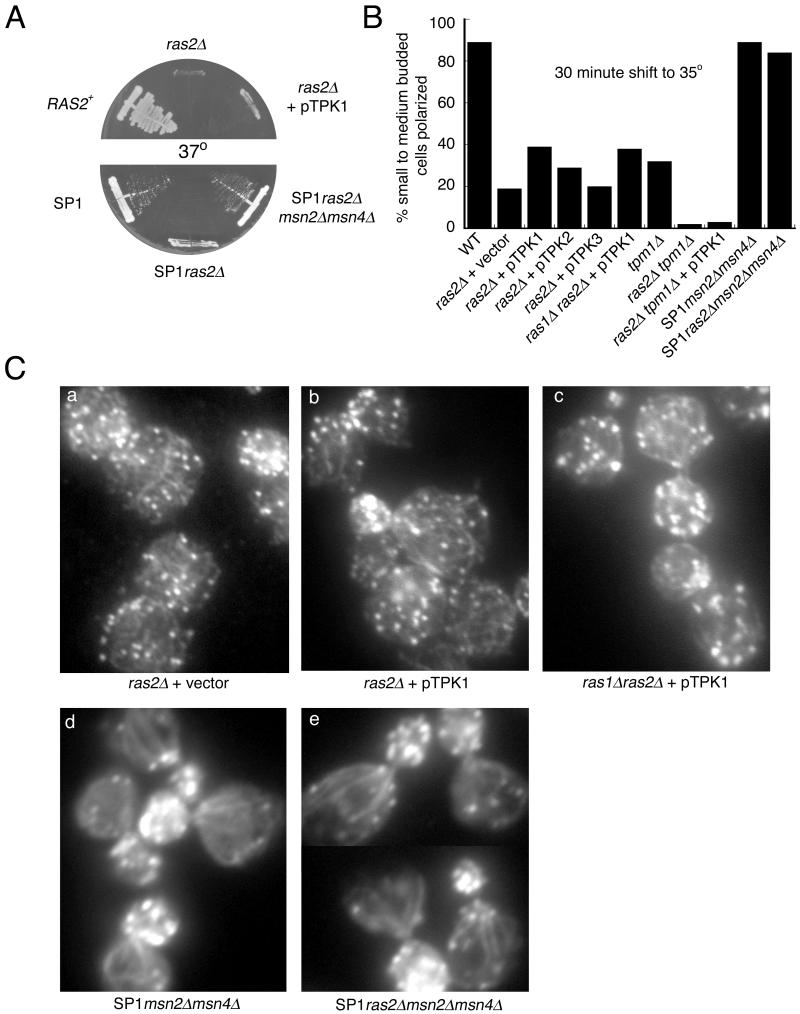
Figure 7.
An msn2msn4 double disruption suppresses the temperature sensitivity and actin depolarization phenotypes of ras2Δ cells, whereas activation of the PKA pathway does not. (A) Growth of RAS2+ (ABY1205), ras2Δ (ABY1209) alone or carrying pTPK1, SP1, SP1ras2Δ, or SP1ras2Δmsn2Δmsn4Δ on YPD medium after 2 d at 37°C. (B and C) Actin phenotype of wild-type (ABY1205); ras2Δ (ABY1209) carrying an empty vector, pTPK1, pTPK2, or pTPK3; ras1Δras2Δ carrying pTPK1 (ABY1240); tpm1Δ (ABY1226); ras2Δtpm1Δ (ABY1228) alone or carrying pTPK1; SP1msn2Δmsn4Δ; and SP1ras2Δmsn2Δmsn4Δ. (B) Cells were grown in CM selective media or YPD media, shifted to 35°C for 30 min, fixed, stained for actin, and scored for actin polarization. (C) Images of actin localization. ras2Δ cells have similar actin phenotypes whether they carried pTPK1 or an empty vector, and ras2Δmsn2Δmsn4Δ cells have well polarized actin similar to msn2Δmsn4Δ cells. (a) ras2Δ (ABY1209) with empty vector, (b) ras2Δ (ABY1209) with pTPK1, (c) ras1Δras2Δ with pTPK1 (ABY1240), (d) SP1msn2Δmsn4Δ, and (e) SP1ras2Δmsn2Δmsn4Δ.
We next examined the polarization of the actin cytoskeleton after shifting cells to 35°C for 30 min. ras2Δ overexpressing Tpk1p and ras1Δras2Δ overexpressing Tpk1p have normally polarized actin cytoskeletons at room temperature, and both show very modest suppression of the temperature-dependent actin depolarization phenotype (Figure 7, B and C). Overexpression of Tpk2p showed similar levels of suppression and overexpression of Tpk3p showed almost no suppression of the actin phenotype (Figure 7B). The suppression by TPK1 of the ras2Δtpm1Δ actin polarization defect was slight (Figure 7B).
The ras2Δ Actin Depolarization Phenotype Is Not Suppressed by Several Candidate Downstream Effectors but Is Suppressed by Loss of the Stress Response Pathway
Because the PKA pathway did not seem to be the primary conduit for the regulation of cytoskeletal polarity contributed by Ras2p, we examined other potential pathways. Previous studies have indicated a potential physical link between Ras and the cytoskeleton through Srv2p, the yeast homologue of cyclase-associated protein (Shima et al., 2000). A component of cortical actin patches (Lila and Drubin, 1997; Yu et al., 1999), Srv2p, has been identified as a suppressor of an activated Ras allele (Fedor-Chaiken et al., 1990). In yeast, Srv2p has two independent functions, one linking activated Ras proteins to stimulation of adenylate cyclase and the other having a role in actin filament organization (Gerst et al., 1991). In addition, profilin (PFY1) overexpression can suppress the loss of Srv2p's actin filament organization function (Vojtek et al., 1991). Furthermore, overexpression of any one of a group of mitotic regulatory genes, TEM1, DBF2, CDC15, CDC5, and SPO12, or of the Ras-like gene, RSR1, have been reported to suppress the temperature sensitivity of the triple ras1Δras2Δcyr1Δ mutant maintained alive at 25°C by overexpression of Tpk1p (Morishita et al., 1995). In our background, overexpression of these genes, of SRV2, or of PFY1, was unable to suppress the temperature sensitivity associated with ras2Δ or the actin depolarization phenotype (Table 3).
In mammals, there are multiple effectors of Ras, including PI-3 kinase and members of the Rho family of G-proteins that regulate the actin cytoskeleton (Scita et al., 2000; Shields et al., 2000). We therefore tested the ability of overexpression of these candidate genes to suppress the temperature sensitivity of ras2Δ or to rescue the temperature-dependent depolarization phenotype. Overexpression of genes encoding proteins of the Rho family, CDC42, RHO1, RHO2, RHO3, and RHO4, of an activated allele of CDC42, CDC42Val12, or of a PI-3 kinase, encoded by VPS34, was unable to suppress the ras2Δ temperature sensitivity or the actin depolarization phenotypes (Table 3).
Although PKA is one of the regulators of the stress response pathway (Gorner et al., 1998), we explored whether the temperature dependence of the actin depolarization phenotype in our ras2Δ strains might nevertheless be mediated through the transcriptional activators Msn2/4p of the stress response pathway. Recently, Stanhill et al. (1999) showed that introduction of ras2Δ into the SP1 genetic background eliminated its ability to undergo invasive growth, and this could be restored by deletion of the MSN2 and MSN4 genes. We therefore obtained these strains and confirmed that ras2Δ in the SP1 background confers both temperature-sensitive growth and temperature-dependent depolarization of the actin cytoskeleton. Remarkably, disruption of msn2Δmsn4Δ rescued both the temperature sensitivity conferred by ras2Δ (Figure 7A) and completely suppressed the actin depolarization phenotype (Figure 7, B and C). This argues that an important function of Ras2p is to suppresses the stress response through the Msn2/4p pathway.
DISCUSSION
There is enormous interest in Ras-signaling pathways in vertebrate cells because mutations in human Ras genes are found in a very large percentage of cancers (Barbacid, 1987). Throughout the years, vertebrate Ras has been implicated in multiple signaling pathways that regulate the actin cytoskeleton as well as nuclear gene expression, thus ultimately contributing to the transformed phenotype (Vojtek and Der, 1998).
In this paper, we show that in yeast Ras also contributes to the maintenance of cytoskeletal polarity in response to mild temperature stresses, and because human Ras can supply this polarity function in yeast perhaps mammalian Ras contributes to the maintenance of mammalian cytoskeletal organization via a similar mechanism. As far as we are aware, this is the first demonstration of the involvement of Ras in regulating the actin cytoskeleton in vegetatively growing yeast.
We identified RAS2 as a regulator of actin polarity in a screen for genes important to both cytoskeletal polarity and secretion. Mutations in such genes were expected to confer synthetic lethality with deletion of the gene encoding the actin-binding protein tropomyosin (TPM1; indicative of a polarity defect) and to confer increased cell density (indicative of a secretion defect). Although the increased density in the ras2 mutants is not due a secretory defect, the ras2 mutants show profound defects in cytoskeletal polarity under mild temperature stresses.
The localization of Myo2p is a sensitive indicator of cytoskeletal polarity. Myo2p, a molecular motor that normally localizes to regions of cell growth during bud formation through rapid translocation along polarized actin cables (Lillie and Brown, 1994; Pruyne et al., 1998), shows a transient and reversible depolarization (within 30 min) in wild-type cells upon shifting to 35°C. In contrast, in ras2Δ cells shifted to 35°C the normal polarized distribution of Myo2p is lost rapidly, depolarized to a greater extent, and takes up to 3 h to recover. This loss of polarization correlates with the loss of organization of the actin cables, which are necessary for Myo2p polarization.
Another indicator of cell polarity is the small GTPase Cdc42p. Cdc42p is essential for the establishment of actin polarity for bud emergence and regulates polarity through the cell cycle (reviewed by Johnson, 1999; Pruyne and Bretscher, 2000a). Again, wild-type cells show a partial transient delocalization of Cdc42p from growth sites, and the ras2Δ mutants show a complete delocalization, indicating Ras2p plays a role in restoring the polarity of Cdc42p during mild temperature shifts.
Studies in yeast have suggested that during normal vegetative growth Ras signals primarily through stimulation of adenylate cyclase with subsequent activation of the PKA pathway—a pathway not used by Ras in mammalian cells. Under normal vegetative growth conditions, loss of both RAS1 and RAS2 is lethal. However, the ras1Δ ras2Δ lethality at room temperature is rescued simply by overexpression of the cAMP-dependent protein kinase Tpk1p, and indeed Tpk1p even suppresses loss of both RAS genes and adenylate cyclase. However, overexpression of Tpk1p is unable to rescue the temperature-sensitive growth phenotype at 37°C of ras2Δ both in this and in other studies (Wigler et al., 1988; Morishita et al., 1995). Because overexpression of Tpk1p, Tpk2p, or Tpk3p does not restore cytoskeletal polarity to ras2Δ cells at 35°C, it appears that the PKA pathway does not play a major role in the maintenance of cytoskeletal polarity by Ras2p in vegetatively growing yeast.
We have shown that inactivation of the stress response pathway by disruption of the MSN2 and MSN4 genes is able to rescue both the temperature sensitivity and actin phenotypes of a ras2Δ strain. Msn2p and Msn4p are Zn2+-finger transcription factors involved in the activation of stress response genes that contain the stress response element or STRE (Martinez-Pastor et al., 1996; Schmitt and McEntee, 1996). The Msn2/4p pathway is negatively regulated by the PKA pathway (Marchler et al., 1993; Gorner et al., 1998): therefore, it was surprising that activation of the PKA pathway by overexpression of Tpk1p, Tpk2p, or Tpk3p was not able to rescue the ras2Δ temperature-sensitive and actin depolarization phenotypes. This suggests that the fully activated PKA pathway is unable to negatively regulate Msn2/4p sufficiently to suppress the phenotype associated with loss of Ras2p. It suggests that another parallel pathway exists to Msn2/4p that allows Ras2p to inhibit the stress response in a cAMP-independent manner.
What is this PKA-independent pathway? One interesting possibility is via the Cdc42p pathway shown to be necessary for yeast pseudohyphal growth. Previous studies have indicated a link among Ras, Cdc42p, and polarity during the pseudohyphal differentiation in yeast (Mosch et al., 1996; Cook et al., 1997). In response to particular nutritional signals, yeast assume a pseudohyphal form, showing exaggerated polarized growth and alterations in gene expression, cell cycle progression, and budding pattern. Pseudohyphal development requires a functional Ras2p, which can act through either of two pathways: 1) via adenylate cyclase and the PKA pathway and 2) via Cdc42p. Although the Cdc42p pathway indicates a link between Ras2p and polarity during pseudohyphal development, it might also be a route whereby Ras2p polarizes the cytoskeleton during mild temperature stress. Roberts et al. (1997) have shown that Ras2p signals to a Bmh1/2p-Ste20p complex during pseudohyphal development. Bmh2p has also been shown to bind and negatively regulate Msn2/4p nuclear localization (Beck and Hall, 1999). An attractive hypothesis is that Ras2p signals through both the PKA pathway and through a PKA-independent pathway such as via Cdc42p and Bmh1/2p. It will be of interest to determine whether such a pathway exists.
ACKNOWLEDGMENTS
The authors thank A. Bender, C. Boone, J. Broach, S. Emr, M. Evangelista, J. Hegemann, J. Heitman, S. Jaspersen, D. Johnson, and K. Tatchell for providing plasmids used in this study. We are also grateful to D. Drubin for providing antibodies to Cdc42p and to F. Klebl for antibodies to Bgl2p. We thank D. Engelberg and A. Stanhill for their generous gift of strains. We also thank D. Pruyne and D. Schott for critical advice and comments. A.B. is very grateful to the National Institutes of Health for a Fogarty Senior International Fellowship and to Hugh Pelham (Medical Research Council Laboratory of Molecular Biology, Cambridge, England) in whose lab preliminary studies for this work were initiated. Finally, we are indebted to one of the referees who suggested that the effect of ras2Δ that we uncovered might involve the Mns2/4p stress response pathway. This work was supported by National Institutes of Health grant GM39066.
Abbreviations used:
- CM
complete minimal
- DIC
differential interference contrast
- 5-FOA
5-fluoroorotic acid
- LY
lucifer yellow-carbohydrazide
- PCR
polymerase chain reaction
- YPD
yeast extract-peptone-dextrose medium
REFERENCES
- Adams AE, Johnson DI, Longnecker RM, Sloat BF, Pringle JR. CDC42 and CDC43, two additional genes involved in budding and the establishment of cell polarity in the yeast Saccharomyces cerevisiae. J Cell Biol. 1990;111:131–142. doi: 10.1083/jcb.111.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams AE, Pringle JR. Relationship of actin and tubulin distribution to bud growth in wild-type and morphogenetic-mutant Saccharomyces cerevisiae. J Cell Biol. 1984;98:934–945. doi: 10.1083/jcb.98.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amberg DC, Zahner JE, Mulholland JW, Pringle JR, Botstein D. Aip3p/Bud6p, a yeast actin-interacting protein that is involved in morphogenesis and the selection of bipolar budding sites. Mol Biol Cell. 1997;8:729–753. doi: 10.1091/mbc.8.4.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakura T, Sasaki T, Nagano F, Satoh A, Obaishi H, Nishioka H, Imamura H, Hotta K, Tanaka K, Nakanishi H, Takai Y. Isolation and characterization of a novel actin filament-binding protein from Saccharomyces cerevisiae. Oncogene. 1998;16:121–130. doi: 10.1038/sj.onc.1201487. [DOI] [PubMed] [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current Protocols in Molecular Biology. Vol. 2. New York: John Wiley & Sons, Unit 13.2; 2000. pp. 1–12. [Google Scholar]
- Ayscough KR, Stryker J, Pokala N, Sanders M, Crews P, Drubin DG. High rates of actin filament turnover in budding yeast and roles for actin in establishment and maintenance of cell polarity revealed using the actin inhibitor latrunculin-A. J Cell Biol. 1997;137:399–416. doi: 10.1083/jcb.137.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Beck T, Hall MN. The TOR signaling pathway controls nuclear localization of nutrient-regulated transcription factors. Nature. 1999;402:689–692. doi: 10.1038/45287. [DOI] [PubMed] [Google Scholar]
- Bender A, Pringle JR. Multicopy suppression of the cdc24 budding defect in yeast by CDC42 and three newly identified genes including the ras-related gene. RSR1. Proc Natl Acad Sci USA. 1989;86:9976–9980. doi: 10.1073/pnas.86.24.9976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender L, Lo HS, Lee H, Kokojan V, Peterson V, Bender A. Associations among PH and SH3 domain-containing proteins and Rho-type GTPases in yeast. J Cell Biol. 1996;133:879–894. doi: 10.1083/jcb.133.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobola N, Jansen RP, Shin TH, Nasmyth K. Asymmetric accumulation of Ash1p in postanaphase nuclei depends on a myosin and restricts yeast mating-type switching to mother cells. Cell. 1996;84:699–709. doi: 10.1016/s0092-8674(00)81048-x. [DOI] [PubMed] [Google Scholar]
- Broach JR. RAS genes in Saccharomyces cerevisiae: signal transduction in search of a pathway. Trends Genet. 1991;7:28–33. doi: 10.1016/0168-9525(91)90018-l. [DOI] [PubMed] [Google Scholar]
- Catlett NL, Weisman LS. The terminal tail region of a yeast myosin-V mediates its attachment to vacuole membranes and sites of polarized growth. Proc Natl Acad Sci USA. 1998;95:14799–14804. doi: 10.1073/pnas.95.25.14799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JG, Bardwell L, Thorner J. Inhibitory and activating functions for MAPK Kss1 in the S. cerevisiae filamentous-growth signaling pathway. Nature. 1997;390:85–88. doi: 10.1038/36355. [DOI] [PubMed] [Google Scholar]
- DeFeo-Jones D, Tatchell K, Robinson LC, Sigal IS, Vass WC, Lowy DR, Scolnick EM. Mammalian and yeast ras gene products: biological function in their heterologous systems. Science. 1985;228:179–184. doi: 10.1126/science.3883495. [DOI] [PubMed] [Google Scholar]
- Drees B, Brown C, Barrell BG, Bretscher A. Tropomyosin is essential in yeast, yet the TPM1 and TPM2 products perform distinct functions. J Cell Biol. 1995;128:383–392. doi: 10.1083/jcb.128.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drubin DG, Miller KG, Botstein D. Yeast actin-binding proteins: evidence for a role in morphogenesis. J Cell Biol. 1988;107:2551–2561. doi: 10.1083/jcb.107.6.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangelista M, Blundell K, Longtine MS, Chow CJ, Adames N, Pringle JR, Peter M, Boone C. Bni1p, a yeast formin linking cdc42p and the actin cytoskeleton during polarized morphogenesis. Science. 1997;276:118–122. doi: 10.1126/science.276.5309.118. [DOI] [PubMed] [Google Scholar]
- Fedor-Chaiken M, Deschenes RJ, Broach JR. SRV2, a gene required for RAS activation of adenylate cyclase in yeast. Cell. 1990;61:329–340. doi: 10.1016/0092-8674(90)90813-t. [DOI] [PubMed] [Google Scholar]
- Finger FP, Novick P. Spatial regulation of exocytosis: lessons from yeast. J Cell Biol. 1998;142:609–612. doi: 10.1083/jcb.142.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geli MI, Riezman H. Endocytic internalization in yeast and animal cells: similar and different. J Cell Sci. 1998;111:1031–1037. doi: 10.1242/jcs.111.8.1031. [DOI] [PubMed] [Google Scholar]
- Gerst JE, Ferguson K, Vojtek A, Wigler M, Field J. CAP is a bifunctional component of the Saccharomyces cerevisiae adenylyl cyclase complex. Mol Cell Biol. 1991;11:1248–1257. doi: 10.1128/mcb.11.3.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorner W, Durchschlag E, Martinez-Pastor MT, Estruch F, Ammerer G, Hamilton B, Ruis H, Schuller C. Nuclear localization of the C2H2 zinc finger protein Msn2p is regulated by stress and protein kinase A activity. Genes Dev. 1998;12:586–597. doi: 10.1101/gad.12.4.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindan B, Bowser R, Novick P. The role of Myo2, a yeast class V myosin, in vesicular transport. J Cell Biol. 1995;128:1055–1068. doi: 10.1083/jcb.128.6.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie C, Fink GR. Guide to yeast genetics and molecular biology. Methods Enzymol. 1991;194:3–38. [PubMed] [Google Scholar]
- Harsay E, Bretscher A. Parallel secretory pathways to the cell surface in yeast. J Cell Biol. 1995;131:297–310. doi: 10.1083/jcb.131.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill KL, Catlett NL, Weisman LS. Actin and myosin function in directed vacuole movement during cell division in Saccharomyces cerevisiae. J Cell Biol. 1996;135:1535–1549. doi: 10.1083/jcb.135.6.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaspersen SL, Charles JF, Tinker-Kulberg RL, Morgan DO. A late mitotic regulatory network controlling cyclin destruction in Saccharomyces cerevisiae. Mol Biol Cell. 1998;9:2803–2817. doi: 10.1091/mbc.9.10.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Amberg DC. The secretory pathway mediates localization of the cell polarity regulator Aip3p/Bud6p. Mol Biol Cell. 2000;11:647–661. doi: 10.1091/mbc.11.2.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DI. Cdc42: an essential Rho-type GTPase controlling eukaryotic cell polarity. Microbiol Mol Biol Rev. 1999;63:54–105. doi: 10.1128/mmbr.63.1.54-105.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston GC, Prendergast JA, Singer RA. The Saccharomyces cerevisiae MYO2 gene encodes an essential myosin for vectorial transport of vesicles. J Cell Biol. 1991;113:539–551. doi: 10.1083/jcb.113.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka T, Powers S, Cameron S, Fasano O, Goldfarb M, Broach J, Wigler M. Functional homology of mammalian and yeast RAS genes. Cell. 1985;40:19–26. doi: 10.1016/0092-8674(85)90304-6. [DOI] [PubMed] [Google Scholar]
- Kozminski KG, Chen AJ, Rodal AA, Drubin DG. Functions and functional domains of the GTPase Cdc42p. Mol Biol Cell. 2000;11:339–354. doi: 10.1091/mbc.11.1.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew DJ, Reed SI. A cell cycle checkpoint monitors cell morphogenesis in budding yeast. J Cell Biol. 1995;129:739–749. doi: 10.1083/jcb.129.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lila T, Drubin DG. Evidence for physical and functional interactions among two Saccharomyces cerevisiae SH3 domain proteins, an adenylyl cyclase- associated protein and the actin cytoskeleton. Mol Biol Cell. 1997;8:367–385. doi: 10.1091/mbc.8.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillie SH, Brown SS. Immunofluorescence localization of the unconventional myosin, Myo2p, and the putative kinesin-related protein, Smy1p, to the same regions of polarized growth in Saccharomyces cerevisiae. J Cell Biol. 1994;125:825–842. doi: 10.1083/jcb.125.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Bretscher A. Characterization of TPM1 disrupted yeast cells indicates an involvement of tropomyosin in directed vesicular transport. J Cell Biol. 1992;118:285–299. doi: 10.1083/jcb.118.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HP, Bretscher A. Disruption of the single tropomyosin gene in yeast results in the disappearance of actin cables from the cytoskeleton. Cell. 1989;57:233–242. doi: 10.1016/0092-8674(89)90961-6. [DOI] [PubMed] [Google Scholar]
- Madaule P, Axel R, Myers AM. Characterization of two members of the rho gene family from the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1987;84:779–783. doi: 10.1073/pnas.84.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchler G, Schuller C, Adam G, Ruis H. A Saccharomyces cerevisiae UAS element controlled by protein kinase A activates transcription in response to a variety of stress conditions. EMBO J. 1993;12:1997–2003. doi: 10.1002/j.1460-2075.1993.tb05849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall MS, Gibbs JB, Scolnick EM, Sigal IS. Regulatory function of the Saccharomyces cerevisiae RAS C-terminus. Mol Cell Biol. 1987;7:2309–2315. doi: 10.1128/mcb.7.7.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Pastor MT, Marchler G, Schuller C, Marchler-Bauer A, Ruis H, Estruch F. The Saccharomyces cerevisiae zinc finger proteins Msn2p and Msn4p are required for transcriptional induction through the stress response element (STRE) EMBO J. 1996;15:2227–2235. [PMC free article] [PubMed] [Google Scholar]
- Matsui Y, Toh EA. Yeast RHO3 and RHO4 ras superfamily genes are necessary for bud growth, and their defect is suppressed by a high dose of bud formation genes CDC42 and. BEM1. Mol Cell Biol. 1992;12:5690–5699. doi: 10.1128/mcb.12.12.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita T, Mitsuzawa H, Nakafuku M, Nakamura S, Hattori S, Anraku Y. Requirement of Saccharomyces cerevisiae Ras for completion of mitosis. Science. 1995;270:1213–1215. doi: 10.1126/science.270.5239.1213. [DOI] [PubMed] [Google Scholar]
- Mosch HU, Kubler E, Krappmann S, Fink GR, Braus GH. Crosstalk between the Ras2p-controlled mitogen-activated protein kinase and cAMP pathways during invasive growth of Saccharomyces cerevisiae. Mol Biol Cell. 1999;10:1325–1335. doi: 10.1091/mbc.10.5.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosch HU, Roberts RL, Fink GR. Ras2 signals via the Cdc42/Ste20/mitogen-activated protein kinase module to induce filamentous growth in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1996;93:5352–5356. doi: 10.1073/pnas.93.11.5352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P, Botstein D. Phenotypic analysis of temperature-sensitive yeast actin mutants. Cell. 1985;40:405–416. doi: 10.1016/0092-8674(85)90154-0. [DOI] [PubMed] [Google Scholar]
- Novick P, Field C, Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980;21:205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- Novick P, Schekman R. Secretion and cell-surface growth are blocked in a temperature-sensitive mutant of Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1979;76:1858–1862. doi: 10.1073/pnas.76.4.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X, Heitman J. Cyclic AMP-dependent protein kinase regulates pseudohyphal differentiation in Saccharomyces cerevisiae. Mol Cell Biol. 1999;19:4874–4887. doi: 10.1128/mcb.19.7.4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle JR, Preston RA, Adams AE, Stearns T, Drubin DG, Haarer BK, Jones EW. Fluorescence microscopy methods for yeast. Methods Cell Biol. 1989;31:357–435. doi: 10.1016/s0091-679x(08)61620-9. [DOI] [PubMed] [Google Scholar]
- Pruyne D, Bretscher A. Polarization of cell growth in yeast. I. Establishment and maintenance of polarity states. J Cell Sci. 2000a;113:571–585. doi: 10.1242/jcs.113.3.365. [DOI] [PubMed] [Google Scholar]
- Pruyne D, Bretscher A. Polarization of cell growth in yeast. II. The role of the cortical actin cytoskeleton. J Cell Sci. 2000b;113:365–375. doi: 10.1242/jcs.113.3.365. [DOI] [PubMed] [Google Scholar]
- Pruyne DW, Schott DH, Bretscher A. Tropomyosin-containing actin cables direct the Myo2p-dependent polarized delivery of secretory vesicles in budding yeast. J Cell Biol. 1998;143:1931–1945. doi: 10.1083/jcb.143.7.1931. [DOI] [PubMed] [Google Scholar]
- Riezman H. Endocytosis in yeast: several of the yeast secretory mutants are defective in endocytosis. Cell. 1985;40:1001–1009. doi: 10.1016/0092-8674(85)90360-5. [DOI] [PubMed] [Google Scholar]
- Roberts RL, Mosch HU, Fink GR. 14–3-3 proteins are essential for RAS/MAPK cascade signaling during pseudohyphal development in S. cerevisiae. Cell. 1997;89:1055–1065. doi: 10.1016/s0092-8674(00)80293-7. [DOI] [PubMed] [Google Scholar]
- Schmitt AP, McEntee K. Msn2p, a zinc finger DNA-binding protein, is the transcriptional activator of the multistress response in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1996;93:5777–5782. doi: 10.1073/pnas.93.12.5777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott D, Ho J, Pruyne D, Bretscher A. The COOH-terminal domain of Myo2p, a yeast myosin V, has a direct role in secretory vesicle targeting. J Cell Biol. 1999;147:791–808. doi: 10.1083/jcb.147.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schu PV, Takegawa K, Fry MJ, Stack JH, Waterfield MD, Emr SD. Phosphatidylinositol 3-kinase encoded by yeast VPS34 gene essential for protein sorting. Science. 1993;260:88–91. doi: 10.1126/science.8385367. [DOI] [PubMed] [Google Scholar]
- Scita G, Tenca P, Frittoli E, Tocchetti A, Innocenti M, Giardina G, Di Fiore PP. Signaling from Ras to Rac and beyond: not just a matter of GEFs. EMBO J. 2000;19:2393–2398. doi: 10.1093/emboj/19.11.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu YJ, Santos B, Fortin N, Costigan C, Snyder M. Spa2p interacts with cell polarity proteins and signaling components involved in yeast cell morphogenesis. Mol Cell Biol. 1998;18:4053–4069. doi: 10.1128/mcb.18.7.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields JM, Pruitt K, McFall A, Shaub A, Der CJ. Understanding Ras: 'it ain't over 'til it's over'. Trends Cell Biol. 2000;10:147–154. doi: 10.1016/s0962-8924(00)01740-2. [DOI] [PubMed] [Google Scholar]
- Shima F, Okada T, Kido M, Sen H, Tanaka Y, Tamada M, Hu CD, Yamawaki-Kataoka Y, Kariya K, Kataoka T. Association of yeast adenylyl cyclase with cyclase-associated protein CAP forms a second Ras-binding site which mediates its Ras-dependent activation. Mol Cell Biol. 2000;20:26–33. doi: 10.1128/mcb.20.1.26-33.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder M. The SPA2 protein of yeast localizes to sites of cell growth. J Cell Biol. 1989;108:1419–1429. doi: 10.1083/jcb.108.4.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder M, Gehrung S, Page BD. Studies concerning the temporal and genetic control of cell polarity in Saccharomyces cerevisiae. J Cell Biol. 1991;114:515–532. doi: 10.1083/jcb.114.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanhill A, Schick N, Engelberg D. The yeast ras/cyclic AMP pathway induces invasive growth by suppressing the cellular stress response. Mol Cell Biol. 1999;19:7529–7538. doi: 10.1128/mcb.19.11.7529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa PA, Sil A, Swedlow JR, Herskowitz I, Vale RD. Actin-dependent localization of an RNA encoding a cell-fate determinant in yeast. Nature. 1997;389:90–93. doi: 10.1038/38015. [DOI] [PubMed] [Google Scholar]
- Thevelein JM, de Winde JH. Novel sensing mechanisms and targets for the cAMP-protein kinase A pathway in the yeast Saccharomyces cerevisiae. Mol Microbiol. 1999;33:904–918. doi: 10.1046/j.1365-2958.1999.01538.x. [DOI] [PubMed] [Google Scholar]
- Toda T, Cameron S, Sass P, Zoller M, Wigler M. Three different genes in S. cerevisiae encode the catalytic subunits of the cAMP-dependent protein kinase. Cell. 1987;50:277–287. doi: 10.1016/0092-8674(87)90223-6. [DOI] [PubMed] [Google Scholar]
- Vida TA, Emr SD. A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J Cell Biol. 1995;128:779–792. doi: 10.1083/jcb.128.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vojtek A, Haarer B, Field J, Gerst J, Pollard TD, Brown S, Wigler M. Evidence for a functional link between profilin and CAP in the yeast S. cerevisiae. Cell. 1991;66:497–505. doi: 10.1016/0092-8674(81)90013-1. [DOI] [PubMed] [Google Scholar]
- Vojtek AB, Der CJ. Increasing complexity of the Ras signaling pathway. J Biol Chem. 1998;273:19925–19928. doi: 10.1074/jbc.273.32.19925. [DOI] [PubMed] [Google Scholar]
- Wach A, Brachat A, Pohlmann R, Philippsen P. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast. 1994;10:1793–1808. doi: 10.1002/yea.320101310. [DOI] [PubMed] [Google Scholar]
- Wang T, Bretscher A. The rho-GAP encoded by BEM2 regulates cytoskeletal structure in budding yeast. Mol Biol Cell. 1995;6:1011–1024. doi: 10.1091/mbc.6.8.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Bretscher A. Mutations synthetically lethal with tpm1delta lie in genes involved in morphogenesis. Genetics. 1997;147:1595–1607. doi: 10.1093/genetics/147.4.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland B, Emr SD, Riezman H. Protein traffic in the yeast endocytic and vacuolar protein sorting pathways. Curr Opin Cell Biol. 1998;10:513–522. doi: 10.1016/s0955-0674(98)80067-7. [DOI] [PubMed] [Google Scholar]
- Wigler M, Field J, Powers S, Broek D, Toda T, Cameron S, Nikawa J, Michaeli T, Colicelli J, Ferguson K. Studies of RAS function in the yeast Saccharomyces cerevisiae. Cold Spring Harbor Symp Quant Biol. 1988;53:649–655. doi: 10.1101/sqb.1988.053.01.074. [DOI] [PubMed] [Google Scholar]
- Yin H, Pruyne D, Huffaker TC, Bretscher A. Myosin V orientates the mitotic spindle in yeast. Nature. 2000;406:1013–1015. doi: 10.1038/35023024. [DOI] [PubMed] [Google Scholar]
- Yu J, Wang C, Palmieri SJ, Haarer BK, Field J. A cytoskeletal localizing domain in the cyclase-associated protein, CAP/Srv2p, regulates access to a distant SH3-binding site. J Biol Chem. 1999;274:19985–19991. doi: 10.1074/jbc.274.28.19985. [DOI] [PubMed] [Google Scholar]
- Ziman M, O'Brien JM, Ouellette LA, Church WR, Johnson DI. Mutational analysis of CDC42Sc, a Saccharomyces cerevisiae gene that encodes a putative GTP-binding protein involved in the control of cell polarity. Mol Cell Biol. 1991;11:3537–3544. doi: 10.1128/mcb.11.7.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziman M, Preuss D, Mulholland J, O'Brien JM, Botstein D, Johnson DI. Subcellular localization of Cdc42p, a Saccharomyces cerevisiae GTP- binding protein involved in the control of cell polarity. Mol Biol Cell. 1993;4:1307–1316. doi: 10.1091/mbc.4.12.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]