Abstract
HDL is strongly inversely related to cardiovascular risk. Hepatic HDL uptake is controlled by ecto-F1-ATPase activity, and potentially inhibited by mitochondrial inhibitor factor 1 (IF1). We recently found that IF1 is present in serum and correlates with HDL-cholesterol (HDL-C). Here, we have evaluated the relationship between circulating IF1 and plasma lipoproteins, and we determined whether IF1 concentration is associated with the risk of coronary heart disease (CHD). Serum IF1 was measured in 648 coronary patients ages 45–74 and in 669 matched male controls, in the context of a cross-sectional study on CHD. Cardiovascular risk factors were documented for each participant, including life-style habits and biological and clinical markers. In controls, multivariate analysis demonstrated that IF1 was independently positively associated with HDL-C and apoA-I (r = 0.27 and 0.28, respectively, P < 0.001) and negatively with triglycerides (r = −0.23, P < 0.001). Mean IF1 concentration was lower in CHD patients than in controls (0.43 mg/l and 0.53 mg/l, respectively, P < 0.001). In multivariate analyses, following adjustments on cardiovascular risk factors or markers, IF1 was negatively related to CHD (P < 0.001). This relationship was maintained after adjustment for HDL-C or apoA-I. This study identifies IF1 as a new determinant of HDL-C that is inversely associated with CHD.
Keywords: HDL-cholesterol, coronary heart disease, ATP-synthase inhibitor factor 1, nucleotide receptor P2Y13, inhibitor factor 1
Despite considerable advances in the treatment of cardiovascular diseases, they remain among the leading causes of death in developed countries. Several large-scale prospective studies evidenced an independent inverse association of HDL-cholesterol (HDL-C) levels with risk of coronary heart disease (CHD) (1). Accordingly, studies in genetically modified animals, and in patients with rare disorders of HDL metabolism, support a causal relationship between low HDL and development of atherosclerotic vascular disease (1, 2). A low level of HDL-C (generally considered as <40 mg/dl in men and <50 mg/dl in women) remains predictive of future cardiovascular risk, even when the concentration of LDL-cholesterol (LDL-C) has reached low levels upon treatment with statins (3). Research to understand the protective effects of HDL against coronary artery disease (CAD) has yielded a large number of likely beneficial actions of HDL particles and their components, such as antiinflammatory, antithrombotic, antioxidative, and cytoprotective effects (1). However, the best-characterized protective action of HDL is their central contribution in a process called reverse cholesterol transport (RCT), a process whereby cholesterol is transported from peripheral cells to the liver for further excretion into bile and feces. Thus, these pleiotropic cardioprotective functions of HDL, supported by epidemiological studies, led to the idea that therapies aiming at enhancing plasma HDL levels would be antiatherogenic and protective against cardiovascular events. However, this issue was revived with recent results from large clinical trials showing that some mechanisms that enhance HDL levels are not necessarily beneficial toward atherosclerosis (4, 5). HDL is highly heterogeneous with subclasses that differ in composition and physical properties, and display different antiatherogenic properties (6). Moreover, several environmental factors, such as tobacco and alcohol consumption, physical activity, or inflammation, are known to influence plasma HDL levels and functions (1). Given the complexity of the HDL system, it has emerged that a single measurement of HDL-C level often fails to provide a reliable prediction of HDL biological activities (7). Therefore, new biological markers reflecting metabolic or vascular activities of HDL lipoproteins are needed to better evaluate patients’ cardiovascular risk or to evaluate their responsiveness to emergent HDL-related therapies (2).
In this context, we previously demonstrated the presence of a protein complex, related to mitochondrial ATP-synthase, on the surface of human hepatocytes, behaving as a high-affinity receptor for HDL apolipoprotein A-I (apoA-I). ApoA-I binds to cell surface ATP-synthase (namely, ecto-F1-ATPase), stimulating extracellular ATP hydrolysis into ADP (8). ADP further activates the nucleotide receptor P2Y13, resulting in HDL endocytosis (9). We and others (10, 11) described that P2Y13-knockout mice displayed altered HDL hepatic catabolism and impaired RCT, but HDL levels were maintained or slightly decreased, supporting the emerging concept that steady-state serum HDL levels do not necessarily reflect the ability of HDL to promote RCT (12).
Inhibitory factor 1 (IF1) is a mitochondrial protein of 81 amino acids (NCBI Reference Sequence: NM_016311.4; NP_057395.1) that specifically inhibits the ATPase activity of the mitochondrial FOF1-ATP synthase (13). Using human hepatocytes and perfused rat liver, we previously demonstrated that exogenous recombinant IF1 also inhibits F1-ATPase / P2Y13-mediated HDL uptake (8). In addition, we recently described the presence of constitutive IF1 in human serum, and we developed a specific immunoassay for this compound (14). First measurements in 100 normolipidemic subjects have shown a positive association of IF1 level with HDL-C (14). In the present work, IF1 measurements were extensively performed in the framework of a case-control study on CHD. The results argue in favor of IF1 being a new determinant of HDL levels and a potential biomarker of cardiovascular risk. IF1 measurements might be particularly relevant for CHD risk assessment in individuals with low HDL levels.
METHODS
Study sample
The Génétique et Environnement en Europe du Sud (GENES) study is a case-control study designed to assess the role of genetic, biological, and environmental determinants in the occurrence of CHD. All participants signed an informed consent form. The study protocol was approved by the local ethics committee (CCPPRB, Toulouse / Sud-Ouest, file #1-99-48, Feb 2000). A biological sample collection has been constituted (declared as DC-2008-463 #1 to the Ministry of Research and to the regional Health authority). As previously described, cases were stable CHD patients, ages 45–74, living in the Toulouse area (southwest France) and prospectively recruited from 2001 to 2004 after admission to the Cardiology Department, Toulouse University Hospital, for cardiovascular examination and referred for evaluation and management of their CHD (15, 16). Stable CHD was defined by a previous history of acute coronary syndrome, a previous history of coronary artery revascularization, a documented myocardial ischemia, a stable angina, or the presence at coronary angiography of a coronary stenosis of 50% or more. Patients who had presented an acute coronary episode during the past eight days were not included in the study, because they were considered unstable. During the same period, male controls, ages 45–74, were selected from the general population using electoral rolls. Stratification into decadal age groups was used to approximately match the age distribution of the people with and without CHD.
Data collection
Male participants with and without CHD were examined in the morning after an overnight fast. Patients with stable CHD (cases) were examined in the clinics of the Cardiology Department, Toulouse University Hospital and underwent a coronary angiography. Control subjects were examined in the same health center. The medical examination started with standardized face-to-face interviews performed by a trained physician using standardized methods. During this interview, participants received oral and written information on the aim of the study and signed a consent form. Then a blood sample was taken from each participant. Each participant then completed standardized questionnaires, lasting for about 40 min, covering age, socioeconomic variables, educational level (number of years spent at school), smoking status, alcohol consumption, physical activity, information on cardiovascular risk factors, and past medical (personal and parental) history. The medical examination was continued with anthropometric and clinical measurements. Smoking status was classified as current smokers, past smokers who had quit smoking for at least three years before the interview, and nonsmokers. For current smokers, cigarette consumption was also recorded as the average number of cigarettes per day. Alcohol consumption was assessed using typical weekly patterns. Physical activity (PA) was categorized into four levels: no PA, light PA for 20 min once a week, moderate PA for 20 min twice a week, and intense PA for 20 min at least three times a week or more. In this study, PA was considered and studied as a dichotomous variable: PA > (“high”) or ≤ (“low”) 20 min, once a week. Presence of dyslipidemia, diabetes or hypertension was assessed from the subjects’ current treatments. In those with CHD, their medication at discharge was also considered. Anthropometrical measurements included waist circumference and body mass index (BMI). Blood pressure and heart rate were measured with an automatic sphygmomanometer. Right arm blood pressure was measured twice, and the average value was recorded. Measurements were performed after a 5 min rest. Blood samples were analyzed for serum total cholesterol, HDL-C, triglycerides (TG), glucose, γ-glutamyltransferase (γ-GT), and sensitive C-reactive protein (CRP) with enzymatic reagents on an automated analyzer (Hitachi 912, Roche Diagnostics, Meylan, France). ApoA-I, apoB, and lipoprotein (a) [Lp(a)] were assayed with an immunoturbidimetric method on an automated analyzer (Roche Diagnostics, France). LDL-C was calculated using the Friedewald formula, with VLDL-cholesterol (VLDL-C) (g/l) = TG (g/l)/5, as long as TG concentration was below 4 g/l (17). Lipoproteins containing apoB and either apoCIII (Lp B:CIII) or apoE (Lp B:E) were assayed by a specific immunoelectrodiffusion assay (Sebia, France).
Immunoassay of IF1
A competitive immunoassay was developed to quantify IF1 in human sera as previously described (14). Repeatability within the same day and reproducibility (10 measurements over a six-month period) gave variation coefficients of 6–7%. An average recovery of 96.8% was measured in dilution experiments. Resistance to freezing and thawing was assessed on different occasions with a 95–103% agreement between measurements. With regard to possible correlations of IF1 with HDL markers, competition experiments with purified apolipoproteins A-I and A-II indicated no cross reactivity in this immunoassay.
Statistical analyses
In the control group, associations of IF1, HDL-C, and apoA-I with clinical characteristics and biological markers were tested using Spearman's rank correlations. Independent associations of IF1 with HDL-C and apoA-I were analyzed using a multivariate linear regression model. Two separate regression analyses of HDL-C on IF1 and apoA-I on IF1 were performed. Conversely, regression analyses of IF1 on HDL-C or apoA-I and other lipoprotein variables were also made. A stepwise selection method was used to adjust for confounding variables. The F statistics for a variable to be added in the model had to be significant at P < 0.10. Variables were further maintained in the model when the F statistic was significant at P < 0.05. However, although associations with LDL-C were not significant, LDL-C, as an established lipid risk factor, was forcibly included in final models. For each quantitative variable, regression analyses were performed with polynomial models to look for possible nonlinear relationships with dependent variables. Lack of multicolinearity for IF1 and HDL-C or apoA-I was assessed by examining the variance inflation factors in the final models. Interactions between IF1 and other explanatory variables were tested. The specific contribution to the model for each explanatory variable was estimated.
In the tables, data are presented as percentages for qualitative variables and as means with standard deviations for quantitative ones. The chi-square test was used to compare the distribution of qualitative variables between cases and controls. The mean values of quantitative variables were compared by Student t-test. Shapiro-Wilks was used to test the normality of distribution of residuals and Levene test to test the homogeneity of variances. When the basic assumptions of Student t-test were not satisfied, the data were logarithmically transformed or subjected to a Wilcoxon-Mann-Whitney test.
Two separate multivariate logistic regressions were carried out to analyze the statistical association of IF1, HDL-C, and apoA-I quartiles with CHD. Adjustments were made systematically for classical cardiovascular risk factors: (treatment for) dyslipidemia, hypertension, diabetes, smoking, and also for physical activity, alcohol consumption, C reactive protein, Lp(a), and triglycerides, which were found associated with CHD (supplemental Table IV). For the IF1 logistic regression model, a further adjustment was made for HDL-C or apoA-I. Distributions of IF1, HDL-C, and apoA-I were dichotomized (the median cut-off value was 0.47 mg/l for IF1, 0.43 g/l for HDL-C, and 1.37 g/l for apoA-I) to test the statistical interaction between IF1 and HDL-C or apoA-I. The Hosmer-Lemeshow chi-square statistic was used to assess model fit.
The performance of the final model was evaluated by analysis of the area under the receiver operating characteristic (ROC) curve (AUC). The internal validity of the prediction model was evaluated by bootstrapping (18) using 100 random samples. ROC curves were constructed, and models with and without IF1 or HDL-C, separately or in combination, were compared (19). Analyses were two-tailed, and P < 0.05 was considered to be significant. Computation was carried out with SAS software, version 9.2 (SAS Institute, Cary, IL) and with STATA statistical software (release 11.2, Stata Corporation, College Station, TX).
RESULTS
Serum IF1 levels in the general population
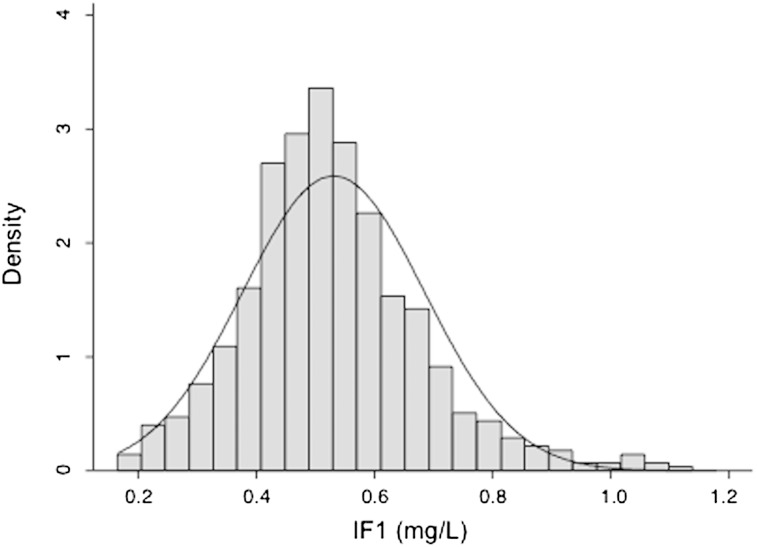
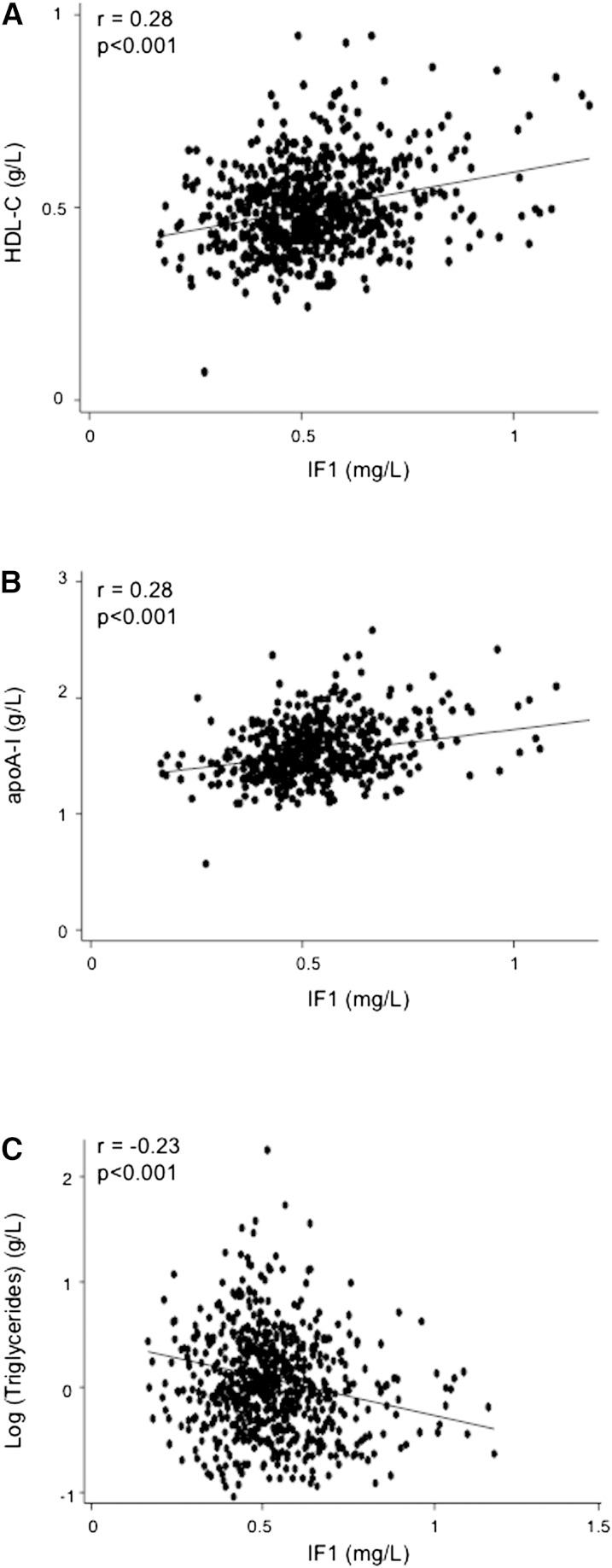
Plotting of IF1 levels in the general male population (669 controls ages 45–74) demonstrated a normal distribution with a median value at 0.53 ± 0.15 mg/l and a 95% confidence interval (CI) of 0.24–0.82 mg/l (Fig. 1 and Table 1). Serum IF1 was positively correlated with HDL-C and apoA-I, and negatively with triglycerides and triglyceride-containing lipoproteins (Fig. 2 and Table 2). Both HDL-C and apoA-I regularly increased as a function of quintiles of IF1 in the control population (supplemental Table I). Although the GENES study dealt only with male subjects, it is most likely that the relationship between serum IF1 and lipids also exists among women. In a preliminary approach, we measured IF1 concentrations among 70 normolipemic women who participated in a population study in the same geographical area. Mean IF1 level was 0.52 mg/l ± 0.21. A positive correlation with HDL-C and a negative one with TG concentration were also observed (A. Genoux, unpublished observations).
Fig. 1.
Density histogram with normal density plot of IF1. IF1 was measured in 669 controls ages 45–74.
TABLE 1.
Clinical and biological characteristics of the study population
| Characteristics | Cases (n = 648) | Controls (n = 669) | P |
| Age (year) | 60.4 (±7.9) | 59.2 (±8.3) | 0.007 |
| School (years of education) | 9.6 (±2.9) | 13.1 (±4.1) | 0.001 |
| Alcohol (g/day) | 28.1 (±30.6) | 24.0 (±22.4) | 0.37 |
| Waist (cm) | 99.0 (±10.9) | 95.3 (±9.6) | 0.001 |
| BMI (kg/m2) | 27.4 (±4.1) | 26.8 (±3.5) | 0.002 |
| SBP (mmHg) | 139.7 (±20.6) | 136.9 (±16.0) | 0.002 |
| Heart rate (beat/min) | 63.8 (±12.0) | 63.4 (±9.3) | 0.41 |
| Glucose (mmol/l) a | 5.94 (±2.1) | 5.47 (±1.1) | 0.001 |
| Triglycerides (g/l) a | 1.70 (±1.01) | 1.23 (±0.76) | 0.001 |
| Total cholesterol (g/l) | 2.01 (±0.44) | 2.25 (±0.37) | 0.001 |
| LDL-C (g/l) | 1.29 (±0.38) | 1.51 (±0.34) | 0.001 |
| HDL-C (g/l) | 0.39 (±0.11) | 0.50 (±0.11) | 0.001 |
| γ-GT (IU/l) | 63.5 (±74.8) | 45.9 (±52.8) | 0.001 |
| Lp(a) ≥0.30 versus <0.30 g/l (%) | 52.6 | 34.1 | 0.001 |
| CRP ≥ 5 mg/l versus < (%) | 53.6 | 16.6 | 0.001 |
| Metabolic syndrome (NCEP-ATPIII %) | 50.4 | 17.8 | 0.001 |
| Treatment for diabetes (%) | 25.0 | 6.4 | 0.001 |
| Treatment for dyslipidemia (%) | 65.6 | 23.6 | 0.001 |
| Treatment for hypertension (%) | 43.8 | 20.6 | 0.001 |
| Cigarettes (cig/day) | 3.2 (±8.1) | 1.9 (±6.4) | 0.001 |
| Smoking habits (%) | 0.001 | ||
| Current smoking | 20.4 | 12.7 | |
| Past smoking | 61.9 | 53.2 | |
| Never smoking | 17.7 | 34.1 | |
| Physical activity (high level) (%) b | 11.5 | 34.2 | 0.001 |
| IF1 (mg/l) | 0.43 (±0.13) | 0.53 (±0.15) | 0.001 |
Data are expressed as mean (± SD) or percentage.
Analyses performed on log-transformed data.
“High” physical activity during 20 min at least twice a week versus “low” physical activity once a week or less.
Fig. 2.
Relationships between IF1 and HDL-C (A), IF1 and apoA-I (B), and IF1 and triglycerides (C) in control samples. All individual data of measurement in 669 normolipemic male subjects are represented.
TABLE 2.
Correlation between IF1, HDL-C, apoA-I, and other cardiovascular risk factors in controls
| Characteristics | IF1 (mg/l) | HDL-C (g/l) | ApoA-I (g/l) |
| Age (years) | −0.02 (−0.10; 0.05) | 0.03 (−0.04; 0.11) | 0.05 (−0.02; 0.13) |
| Alcohol (g/day) | 0.09 (0.02; 0.16)* | 0.20 (0.12; 0.27)*** | 0.28 (0.21; 0.31)*** |
| Smoking (cig/day) | −0.03 (−0.11; 0.05) | −0.10 (−0.17; −0.02)* | −0.10 (−0.17; −0.02)* |
| Physical activity score | 0.01 (−0.07; 0.08) | 0.20 (0.13; 0.27)*** | 0.09 (0.02; 0.17)* |
| BMI (kg/m2) | −0.17 (−0.24; −0.09)*** | −0.33 (−0.40; −0.26)*** | −0.20 (−0.27; −0.12)*** |
| Waist (cm) | −0.15 (−0.22; −0.08)*** | −0.34 (−0.41; −0.27)*** | −0.17 (−0.24; −0.10)*** |
| SBP (mmHg) | −0.02 (−0.09; 0.06) | −0.08 (−0.15; −0.01)* | 0.01 (−0.08; 0.08) |
| Heart rate (beat/min) | −0.04 (−0.12; 0.03) | −0.07 (−0.14; 0.01) | −0.05 (−0.12; 0.03) |
| Total cholesterol (g/l) | −0.07 (−0.14; 0.01) | 0.16 (0.09; 0.24)*** | 0.23 (0.16; 0.30)*** |
| LDL-C (g/l) | −0.07 (−0.15; 0.01) | 0.01 (−0.07; 0.08) | 0.03 (−0.05; 0.10) |
| HDL-C (g/l) | 0.24 (0.16−0.31)*** | — | 0.82 (0.80; 0.84)*** |
| ApoA-I (g/l) | 0.27 (0.20−0.34)*** | 0.82 (0.80; 0.84)*** | — |
| ApoB (g/l) | −0.17 (−0.24; −0.09)*** | −0.15 (−0.22; −0.08)*** | −0.07 (−0.14; 0.01) |
| Triglyceride (g/l) | −0.25 (−0.32; −0.17)*** | −0.48 (−0.54; −0.42)*** | −0.19 (−0.26; −0.12)*** |
| LpB:CIII (mg/l) | −0.14 (−0.32; −0.17)*** | −0.34 (−0.41; −0.27)*** | −0.06 (−0.14; 0.02) |
| LpB:E (mg/l) | −0.26 (−0.33; −0.19)*** | −0.25 (−0.32; −0.18)*** | −0.21 (−0.28; −0.13)*** |
| Lp(a) (g/l) | 0.06 (−0.02; 0.13) | 0.09 (0.01; 0.16)* | 0.02 (−0.06; 0.09) |
| Glucose (mmol/l) | −0.02 (−0.08; 0.07) | −0.04 (−0.11; 0.04) | 0.03 (−0.06; 0.09) |
| γ-GT (IU/l) | −0.06 (−0.13; 0.02) | −0.03 (−0.11; 0.04) | 0.06 (−0.01; 0.14) |
| CRP (mg/l) | −0.01 (−0.08; 0.07) | −0.19 (−0.26; −0.12)*** | −0.13 (−0.20; −0.06)*** |
Spearman rank correlation (95% CI). *P < 0.05, **P < 0.01,***P < 0.001.
Correlations between IF1 levels and individual metabolic parameters or cardiovascular risk markers were investigated and compared with equivalent correlations for HDL-C and apoA-I (Table 2). All three parameters (IF1, HDL-C, and apoA-I) were negatively correlated with BMI and waist circumference. Environmental factors, such as cigarette smoking, alcohol consumption, physical activity, and an inflammatory condition, as documented by elevated CRP, displayed expected correlations with HDL-C or apoA-I, but correlations with IF1 were absent, or much weaker in the case of alcohol (Table 2). This suggests that IF1 levels, for most part, are little influenced by environmental variables that are known to affect HDL-C or apoA-I levels. This hypothesis was supported by multivariate analyses conducted among control subjects. First, we developed multiple regression models that included IF1 as an explanatory variable of HDL-C or apoA-I (Table 3). In model 1, which explained 34.2% of HDL-C variability, triglyceride concentration was the major determinant of HDL-C levels (56.3%), being inversely related, followed by serum IF1, positively associated (10.1%). In model 2, which explained 21.2% of apoA-I variability, alcohol consumption and serum IF1 were the strongest positive contributors of apoA-I variability (27.0% and 30.5%, respectively). As expected, BMI, tobacco smoking, or an inflammatory condition was a negative determinant, whereas physical activity was positively associated. Serum IF1 was then studied as the dependent variable in multiple regression analyses. Two models were developed that included either HDL-C or apoA-I as explanatory variables (supplemental Table II). The two models explained 10.4% and 12.3%, respectively, of IF1 variability. HDL-C or apoA-I were the strongest contributors of the models, followed by triglyceride and apoB levels as negative determinants. Alcohol consumption also contributed positively and significantly to IF1 variability, although to a lesser extent (supplemental Table II).
TABLE 3.
Multiple linear regression analysis of HDL-C and apoA-I on IF1 in the control group
| Regression of HDL-C on IF1 (Model 1), R2 = 34.2% |
Regression of apoA-I on IF1 (Model 2), R2 = 21.2% |
|||||||
| β | SE | P | % b | β | SE | P | % b | |
| IF1 (mg/l) | 0.129 | 0.027 | 0.001 | 10.1 | 0.347 | 0.055 | 0.001 | 30.5 |
| Triglycerides (g/l) a | −0.108 | 0.009 | 0.001 | 56.3 | −0.055 | 0.019 | 0.01 | 6.1 |
| γ-GT (by 10 IU/l) | 0.003 | 0.001 | 0.001 | 6.3 | 0.006 | 0.002 | 0.001 | 8.1 |
| CRP ≥ 5 mg/l versus < 5 mg/l | −0.036 | 0.01 | 0.001 | 4.8 | −0.084 | 0.023 | 0.001 | 10.7 |
| BMI ≥ 30 kg/m2 versus < 30 kg/m2 | −0.036 | 0.01 | 0.001 | 4.5 | −0.066 | 0.023 | 0.004 | 6.1 |
| Alcohol (10 g / day) | 0.008 | 0.002 | 0.001 | 8.4 | 0.023 | 0.004 | 0.001 | 27.0 |
| Physical activity (high versus low) c | 0.032 | 0.009 | 0.001 | 6.0 | 0.041 | 0.018 | 0.01 | 4.0 |
| Current smoker (yes versus no) | −0.028 | 0.012 | 0.03 | 2.3 | −0.074 | 0.025 | 0.008 | 6.6 |
| LDL-C (g/l) | 0.021 | 0.012 | 0.09 | 1.3 | 0.027 | 0.025 | 0.28 | 0.9 |
Log-transformed data.
Percentage of variance explained.
“High” physical activity for 20 min at least twice a week versus “low” physical activity once a week or less.
Serum IF1 levels and coronary heart disease
All characteristics of CHD and control individuals were considered (Table 1). Increased prevalence of classical risk factors was recorded in CHD individuals: hypertension, diabetes, dyslipidemia, and smoking habits. BMI and waist circumference were higher in CHD-affected than in control individuals, while educational level and physical activity were lower. Averages of alcohol consumption were not significantly different between cases and controls. However, alcohol patterns were different, with a higher percentage of moderate alcohol consumption (<40 g/day) in controls and, conversely, a higher percentage of both abstinent and excessive drinkers (≥40 g/day) in cases (data not shown). Among metabolic markers, total or LDL-C were lower in CHD individuals, probably reflecting effects of lipid-lowering drugs in patients. However, CHD individuals displayed hyperglycemia, higher levels of triglyceride-containing lipoproteins and Lp(a), increased CRP, and lower HDL markers. Altogether, 50% of CAD individuals had features of metabolic syndrome, according to the NCEP-ATPIII definition versus 18% of control subjects. Average IF1 was 0.43 ± 0.13 in CHD individuals versus 0.53 ± 0.15 mg/l in controls (P < 0.001, Table 1). We also determined IF1 levels in cases and controls taking or not taking treatments for dyslipidemia, diabetes, or hypertension, and we observed no impact of those treatments on serum IF1 (supplemental Table III).
A prior logistic regression analysis of established risk factors associated with occurrence of CHD in our study identified smoking, diabetes, dyslipidemia or hypertension, elevated CRP, TG, Lp(a), and low physical activity (supplemental Table IV). A trend to positive association was observed for alcohol. Thus, all these factors were taken into account for further statistical adjustments. Logistic regression analyses were conducted regarding the CHD status and studying HDL-C, apoA-I, and IF1 as explanatory variables, following multiple adjustments on cardiovascular risk factors and markers having shown significant differences between the two subpopulations. All three parameters were divided into quartiles, and odds ratios (OR) with 95% CI were determined (Table 4). A negative association with CHD was recorded with increasing levels of the three investigated parameters, yet effects of IF1 were somewhat less pronounced and with a more gradual decrease than those observed with HDL-C or apoA-I. Effects of IF1 were studied after a further adjustment on HDL-C. Quartiles 3 and 4 of IF1 distribution still remained associated with a significant reduction of CHD risk (−50% and −70%, respectively). When adjustment was done on apoA-I levels instead of HDL-C, only quartile 4 of IF1 appeared associated with a significant reduction (−60%) (Table 4). To ensure that our results on IF1 were not influenced by the proximity of a coronary event, analyses were resumed after excluding those patients having presented an acute event in the last three months, but OR were identical to those in the whole population (supplemental Table V). Quartile ranking was performed to provide a convenient illustration of those relationships, but significant associations were also found when HDL-C, apoA-I, and IF1 were considered as continuous variables (data not shown). The linearity of the relationships was verified. Thus, multivariate analyses argues in favor of IF1 being independently negatively associated with CHD. A statistical interaction existed on CHD risk, between IF1 and HDL-C or apoA-I levels (P = 0.04 and P = 0.009, respectively, Table 5). To illustrate this interaction, IF1, HDL-C, and apoA-I concentrations were considered from both sides of their medians, and the ORs were calculated. At low levels of HDL-C (<0.43 g/l), an elevated IF1 (≥0.47 mg/l) conferred a 69% reduction of the OR for CHD (P < 0.001). A similar decrease was observed in the reverse situation; i.e., at low levels of IF1 but with elevated HDL-C. However, risk reduction was significantly greater (−86%, P < 0.001) when both markers were above medians. Combination of elevated IF1 (≥0.47 mg/l) and HDL-C (≥0.43 g/l) was associated with an OR reduction of greater magnitude than either one of the two markers alone. At low levels of apoA-I (<1.37 g/l), an elevated IF1 (≥0.47 mg/l) conferred a 68% risk reduction (P < 0.001). Risk reduction was significantly greater (P < 0.001) when both markers were above medians (−90%). However, an elevated apoA-I by itself achieved a similar reduction of CHD occurrence. Pairwise comparisons (Table 5) clearly show that the beneficial effect of elevated IF1 (above median) in terms of reduced association to CHD is more pronounced at low levels of HDL-C or apoA-I (below median). Again, data were considered across medians for presentation clarity, but similar relationships were observed for continuous variables (not shown).
TABLE 4.
Risk of coronary heart disease using HDL-C, apoA-I, and IF1 as explanatory variables
| Q2 | Q3 | Q4 | |||
| Models with | Q1 | OR (95% CI) | OR (95% CI) | OR (95% CI) | P for Trend |
| HDL-C | 1 | 0.29 (0.19; 0.47) | 0.15 (0.09; 0.24) | 0.10 (0.06; 0.16) | 0.001 |
| ApoA-I | 1 | 0.19 (0.12; 0.32) | 0.08 (0.05; 0.14) | 0.04 (0.02; 0.07) | 0.001 |
| IF1 | 1 | 0.67 (0.45; 1.02) | 0.38 (0.25; 0.58) | 0.20 (0.13; 0.32) | 0.001 |
| IF1 a | 1 | 0.80 (0.52; 1.24) | 0.53 (0.34; 0.82) | 0.31 (0.19; 0.48) | 0.001 |
| IF1 b | 1 | 0.84 (0.53; 1.32) | 0.69 (0.43; 1.09) | 0.41 (0.25; 0.65) | 0.001 |
All models adjusted on treatments for dyslipidemia, hypertension and diabetes; on smoking, physical activity, and alcohol consumption; and on CRP, Lp(a), and triglyceride. Quartiles for HDL-C: 0.36, 0.43, 0.51 g/l. Quartiles for apoA-I: 1.19, 1.37, 1.55 g/l. Quartiles for IF1: 0.382, 0.466, 0.560 mg/l.
Plus HDL-C.
Plus apoA-I.
TABLE 5.
Concomitant risks of CHD for HDL-C, apoA-I, and IF1
| IF1 (mg/l) |
|||
| < 0.47 | ≥ 0.47 | ||
| OR (95% CI) | OR (95% CI) | ||
| HDL-C (g/l) | < 0.43 | 1 | 0.31 (0.20; 0.48) a |
| ≥ 0.43 | 0.24 (0.15; 0.37) b | 0.14 (0.09; 0.21) c | |
| ApoA-I (g/l) | < 1.37 | 1 | 0.32 (0.20; 0.51) d |
| ≥ 1.37 | 0.14 (0.09; 0.21) e | 0.10 (0.07; 0.15) f | |
Adjusted for dyslipidemia, hypertension, diabetes, smoking, physical activity, alcohol consumption, CRP, Lp(a), and triglyceride. IF1 × HDL-C interaction, P = 0.04. IF1 × apoA-I interaction, P = 0.009. Median cutoff values for IF1, HDL-C and apoA-I analyses were considered from both sides of median values.
Post-hoc pairwise comparisons with Benjamini-Hochberg correction for P value: a versus b: P = 0.30; a versus c: P < 0.001; b versus c: P < 0.02; d versus e: P < 0.001; d versus f: P < 0.001; e versus f: P = 0.20.
To further explore the associations with CHD, ROC curves were constructed using classical cardiovascular risk factors and markers with or without IF1, HDL-C, or IF1+HDL-C (supplemental Fig. I). Classical cardiovascular risk factors and markers having shown differences between the two subpopulations included (treatment for) dyslipidemia, hypertension, and diabetes; smoking, physical activity, and alcohol consumption; and CRP, Lp(a), and TG. The AUC for this “basal panel” was 0.866 as regards CHD. AUC was increased after addition to the model of either IF1 (AUC = 0.880, P < 0.001 for the difference) or HDL-C (AUC = 0.892, P < 0.001). Furthermore, in the latter case (basal panel + HDL-C), inclusion of IF1 concentration still significantly increased AUC, compared with HDL-C alone (0.897 with HDL-C+IF1 versus 0.892 with HDL-C, P < 0.01, supplemental Fig. I).
DISCUSSION
A few years ago, we found that membrane ecto-F1-ATPase, an enzyme complex related to mitochondrial ATP-synthase, was a high-affinity receptor for apoA-I, leading secondarily to liver uptake of HDL particles (8). More recently, we detected the presence in human serum of IF1, the natural inhibitor of mitochondrial F1-ATPase, and we developed a specific immunoassay for serum IF1 (14). In the present study, for the first time, large-scale measurements of serum IF1 were performed in the context of a case-control study on CHD. In control subjects taken from the general population, we again found correlations between serum IF1 and HDL-C, apoA-I, and TG, extending previous observations on a limited sample of 100 normolipemic patients (14). Moreover, multiple adjustments enabled us to demonstrate that IF1 is an independent and important contributor of HDL-C or apoA-I variability in the general population and, reciprocally, that HDL-related markers are the major determinants of IF1 levels. Noteworthy, IF1 could not be detected in isolated human HDL (data not shown) using both immunoassay and anti-IF1 immunoprecipitation techniques as previously described (14). Accordingly, IF1 has never been reported to be associated with HDL in proteomics studies and other biochemical approaches, such as immunoprecipitation and in vitro binding studies, suggesting IF1 is not likely associated with HDL (20, 21). Whether IF1 is circulating as an intact and active protein will require further investigation. However, our previous data showed that IF1 could be immunoprecipitated from serum into two immunoreactive products of ∼20 kDa each (14). Although this size could correspond to a dimer of IF1, which is the main active form of IF1 within the mitochondria (22), we cannot exclude that the immunoprecipitated products reflect some posttranslational modifications or alternative splicing of IF1 or that circulating IF1 is associated with other serum compounds.
The observed relationships between circulating IF1 and HDL levels lend support to the view that, in humans, this new F1-ATPase pathway is physiologically relevant in HDL metabolism. At present, only hypotheses can be proposed to explain this reciprocal relationship. IF1 is a mitochondrial protein, able to block the ATP hydrolytic activity of mitochondrial ATP-synthase (23). Components of this enzymatic complex, and particularly of the F1-ATPase catalytic domain, have been found present at the surface of various cells, including hepatocytes (24). We have shown that apoA-I binds to cell surface F1-ATPase and stimulates its ATP hydrolyzing activity, triggering P2Y13 ADP-receptor activation and a signaling cascade, which further leads to the uptake by hepatocytes of both protein and lipid moieties of the HDL particle, a process called holo-HDL endocytosis (8). This endocytosis pathway for HDL is distinct from the so-called selective HDL cholesterol uptake mediated by the scavenger receptor B1 (SR-BI), a mechanism by which cholesterol is preferentially taken up, while the protein components of the HDL particle are not (25). Interestingly, addition of exogenous IF1 was able to reduce HDL uptake by cultured human hepatocytes and perfused rodent livers, most likely by inhibiting apoA-I-induced F1-ATPase activity (8). Thus, serum IF1 activity might slow down HDL hepatic catabolism, increasing the residence time of HDL in serum. IF1 concentrations were also negatively correlated to levels of TG-rich, apoB-containing lipoproteins. The inverse relationship between metabolism of HDL and TG-rich lipoproteins is well known, being mediated by lipid transfer proteins and lipoprotein lipase. Thus, as IF1 concentration is closely related to HDL-C, its inverse relation with TG markers might reflect the same metabolic interactions. However, in multivariate analysis, TGs were independent determinants of IF1. Thus we cannot exclude a direct impact of IF1, either on the metabolism of TG-rich lipoproteins or an impact on adipocytes, which control TG storage and mobilization (26). Interestingly, functional ecto-F1-ATPase has been described at the surface of adipocytes (27).
IF1 concentration was found 20% lower in CHD cases than in control subjects. Noteworthy, the lower IF1 level in the cases was not related to treatment for dyslipidemia, diabetes, or hypertension. Furthermore, multivariate analyses demonstrated an independent inverse association of serum IF1 levels with CHD. Indeed, even though serum IF1 is highly related to HDL-C, IF1 remained negatively associated to CHD following adjustments on multiple risk factors or markers, including HDL-C. Determination of ROC curves regarding CHD risk yielded similar conclusions, as introduction of IF1 significantly increased AUC, even after HDL-C was included in the model. All those observations suggest that beyond its close association with HDL, IF1 concentration would be negatively related to CHD through other mechanisms. In our laboratory, as mentioned above, we have experimental evidence of the impact of exogenous IF1 on HDL catabolism. However, we still have no evidence regarding the origin of circulating IF1. As a hypothesis, since IF1 is first synthesized with a mitochondrial import signal, it is likely serum IF1 might route from the mitochondrial matrix toward the plasma membrane to be eventually secreted outside the cell. This hypothesis is supported by our detection of IF1 in serum-free media from human endothelial cells (HUVEC) and hepatocytes (HepG2) (L. O. Martinez, unpublished observations), which are two cell lines previously described to express endogenous IF1 at their cell surface (28–30). The secretory pathway of IF1 has not been characterized yet. However, it has been reported that the calcium-modulated protein calmodulin could sequester IF1 on the plasma membrane of hepatocytes, suggesting that the IF1 sorting pathway could depend on calcium signaling (29). Also, under acute cholestasis induced by short-term bile duct ligation in rat, the level of IF1 was found increased at the liver plasma membrane, indicating that the IF1-trafficking pathway might be regulated by hepatobiliary cholesterol metabolism (30). Moreover, mobilization of mitochondrial IF1 in cardiomyocytes in response to experimental ischemia has been previously reported (31, 32); hence, serum IF1 concentration might partly reflect a subtle balance between its utilization and release, depending on the cell energetic status. Finally, environmental factors, which are known to modulate HDL levels, had little or no influence on IF1 concentrations, suggesting that individual factors (genetic and metabolic) might control serum IF1 levels.
Statistically significant interactions were evidenced between IF1 and HDL-C or apoA-I concentrations regarding their association to CHD. Most particularly, in subjects with low HDL-C or apoA-I levels, a high IF1 was associated with a 70% reduction in the OR for CHD. The relative decrease was much less pronounced in subjects with elevated HDL. In mechanistic terms, this suggests that curbing HDL liver catabolism might be beneficial in patients who have low levels of circulating HDL. This raises the question of how depressing HDL catabolism might be beneficial against atherosclerosis. Although a major role of HDL is its central involvement in RCT, HDL particles by themselves exert pleiotropic effects on vascular cells, impairing LDL-oxidation and enhancing endothelial function, probably by downregulating endothelial expression of monocyte adhesion molecules, while promoting synthesis and bioavailability of nitric oxide (33, 34). It can thus be hypothesized that slowing down HDL catabolism would prolong residence time of HDL particles, enabling them to exert pleiotropic beneficial effects. This might not have a major impact on the whole process of RCT since, in humans, the final steps (liver uptake) occur not only through selective HDL-C uptake via SR-BI (25) or possibly holo-HDL endocytosis via ecto-F1-ATPase pathway (8) but also through LDL uptake following lipid transfers between lipoproteins (35). Interestingly, it has been shown that the catabolism of HDL particles is accelerated in diabetes, which leads to a significant reduction in HDL plasma residence time. This increased HDL turnover, potentially detrimental regarding HDL vascular effects, was corrected by rosuvastatin (36). Thus, IF1 and other molecules that slow down the hepatic catabolism of HDL, like SR-BI inhibitors (37, 38) or niacin (39), might exert beneficial effects through increasing the residence time of HDL particles in serum, prolonging their multiple effects on the vascular wall.
Of course the study design, a case-control comparison, limits our conclusions in several ways and no causal relationship can be established. Therefore, further measurements are foreseen within the framework of a large prospective cohort to establish more firmly the predictive value of IF1 determinations.
In conclusion, serum IF1 measurements may prove valuable in the assessment of CHD risk along with established risk factors, including HDL-C. Its diagnostic potential might be particularly important in people who have low levels of HDL-C. Further studies will be needed to explore its regulation in different pathophysiological contexts.
Supplementary Material
Acknowledgments
The authors thank all the participants, both healthy individuals and CHD patients, for their participation in the GENES Study. The authors thank Dr. Dany Deckers for her help in recruiting healthy individuals and carrying out examinations.
Footnotes
Abbreviations:
- AUC
- area under the ROC curve
- BMI
- body mass index
- CAD
- coronary artery disease
- CHD
- coronary heart disease
- CI
- confidence interval
- CRP
- C-reactive protein
- γ-GT
- γ-glutamyltransferase
- HDL-C
- HDL-cholesterol
- IF1
- inhibitor factor 1
- LDL-C
- LDL-cholesterol
- OR
- odds ratio
- RCT
- reverse cholesterol transport
- ROC
- receiver operating characteristic
- SBP
- systolic blood pressure
- SR-BI
- scavenger receptor B1
- TG
- triglyceride
This study was supported by the INSERM Avenir Grant, the National Research Agency (ANR Emergence and GENO #102 01), l'Agence de Valorisation de la Recherche en Midi-Pyrénées (AVAMIP), la Région Midi-Pyrénées, Le Centre Hospitalier Universitaire de Toulouse (AOL-99-13-L and local grant 2012, Délégation à la Recherche Clinique), La Fédération Française de Cardiologie, La Fondation de France, L'Office National Interprofessionnel des Vins, and Bayer-Pharma France. L.L. is a recipient of the Marie Curie IEF fellowship.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of one figure and five tables.
REFERENCES
- 1.Navab M., Reddy S. T., Van Lenten B. J., Fogelman A. M. 2011. HDL and cardiovascular disease: atherogenic and atheroprotective mechanisms. Nat. Rev. Cardiol. 8: 222–232. [DOI] [PubMed] [Google Scholar]
- 2.Francis G. A. 2010. The complexity of HDL. Biochim. Biophys. Acta. 1801: 1286–1293. [DOI] [PubMed] [Google Scholar]
- 3.Barter P., Gotto A. M., LaRosa J. C., Maroni J., Szarek M., Grundy S. M., Kastelein J. J. P., Bittner V., Fruchart J-C. 2007. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N. Engl. J. Med. 357: 1301–1310. [DOI] [PubMed] [Google Scholar]
- 4.Boden W. E., Probstfield J. L., Anderson T., Chaitman B. R., Desvignes-Nickens P., Koprowicz K., McBride R., Teo K., Weintraub W. 2011. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N. Engl. J. Med. 365: 2255–2267. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz G. G., Olsson A. G., Abt M., Ballantyne C. M., Barter P. J., Brumm J., Chaitman B. R., Holme I. M., Kallend D., Leiter L. a., et al. 2012. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N. Engl. J. Med. 367: 2089–2099. [DOI] [PubMed] [Google Scholar]
- 6.Camont L., Chapman M. J., Kontush A. 2011. Biological activities of HDL subpopulations and their relevance to cardiovascular disease. Trends Mol. Med. 17: 594–603. [DOI] [PubMed] [Google Scholar]
- 7.Calabresi L., Gomaraschi M., Franceschini G. 2010. High-density lipoprotein quantity or quality for cardiovascular prevention? Curr. Pharm. Des. 16: 1494–1503. [DOI] [PubMed] [Google Scholar]
- 8.Martinez L. O., Jacquet S., Esteve J. P., Rolland C., Cabezon E., Champagne E., Pineau T., Georgeaud V., Walker J. E., Terce F., et al. 2003. Ectopic beta-chain of ATP synthase is an apolipoprotein A-I receptor in hepatic HDL endocytosis. Nature. 421: 75–79. [DOI] [PubMed] [Google Scholar]
- 9.Jacquet S., Malaval C., Martinez L. O., Sak K., Rolland C., Perez C., Nauze M., Champagne E., Terce F., Gachet C., et al. 2005. The nucleotide receptor P2Y13 is a key regulator of hepatic high-density lipoprotein (HDL) endocytosis. Cell. Mol. Life Sci. 62: 2508–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fabre A. C., Malaval C., Ben Addi A., Verdier C., Pons V., Serhan N., Lichtenstein L., Combes G., Huby T., Briand F., et al. 2010. P2Y13 receptor is critical for reverse cholesterol transport. Hepatology. 52: 1477–1483. [DOI] [PubMed] [Google Scholar]
- 11.Blom D., Yamin T. T., Champy M. F., Selloum M., Bedu E., Carballo-Jane E., Gerckens L., Luell S., Meurer R., Chin J., et al. 2010. Altered lipoprotein metabolism in P2Y(13) knockout mice. Biochim. Biophys. Acta. 1801: 1349–1360. [DOI] [PubMed] [Google Scholar]
- 12.Rothblat G. H., Phillips M. C. 2010. High-density lipoprotein heterogeneity and function in reverse cholesterol transport. Curr. Opin. Lipidol. 21: 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pullman M. E., Monroy G. C. 1963. A naturally occurring inhibitor of mitochondrial adenosine triphosphatase. J. Biol. Chem. 238: 3762–3769. [PubMed] [Google Scholar]
- 14.Genoux A., Pons V., Radojkovic C., Roux-Dalvai F., Combes G., Rolland C., Malet N., Monsarrat B., Lopez F., Ruidavets J-B. B., et al. 2011. Mitochondrial inhibitory factor 1 (IF1) is present in human serum and is positively correlated with HDL-cholesterol. PLoS ONE. 6: e23949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bouisset F., Bongard V., Ruidavets J-B., Hascoët S., Taraszkiewicz D., Roncalli J., Carrié D., Galinier M., Elbaz M., Ferrières J. 2012. Prognostic usefulness of clinical and subclinical peripheral arterial disease in men with stable coronary heart disease. Am. J. Cardiol. 110: 197–202. [DOI] [PubMed] [Google Scholar]
- 16.Hascoet S., Elbaz M., Bongard V., Bouisset F., Verdier C., Vindis C., Genoux A., Taraszkiewicz D., Perret B., Galinier M., et al. 2013. Adiponectin and long-term mortality in coronary artery disease participants and controls. Arterioscler. Thromb. Vasc. Biol. 33: e19–e29. [DOI] [PubMed] [Google Scholar]
- 17.Friedewald W. T., Levy R. I., Fredrickson D. S. 1972. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 18: 499–502. [PubMed] [Google Scholar]
- 18.Harrell F. E., Lee K. L., Mark D. B. 1996. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat. Med. 15: 361–387. [DOI] [PubMed] [Google Scholar]
- 19.DeLong E. R., DeLong D. M., Clarke-Pearson D. L. 1988. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 44: 837–845. [PubMed] [Google Scholar]
- 20.Vaisar T. 2012. Proteomics investigations of HDL: challenges and promise. Curr. Vasc. Pharmacol. 10: 410–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah A. S., Tan L., Lu Long J., Davidson W. S. The proteomic diversity of high density lipoproteins: our emerging understanding of its importance in lipid transport and beyond. J. Lipid Res. Epub ahead of print. February 24, 2013; doi:10.1194/jlr.R035725. [DOI] [PMC free article] [PubMed]
- 22.Cabezon E., Arechaga I., Jonathan P., Butler G., Walker J. E. 2000. Dimerization of bovine F1-ATPase by binding the inhibitor protein, IF1. J. Biol. Chem. 275: 28353–28355. [DOI] [PubMed] [Google Scholar]
- 23.Cabezon E., Montgomery M. G., Leslie A. G., Walker J. E. 2003. The structure of bovine F1-ATPase in complex with its regulatory protein IF1. Nat. Struct. Biol. 10: 744–750. [DOI] [PubMed] [Google Scholar]
- 24.Vantourout P., Radojkovic C., Lichtenstein L., Pons V., Champagne E., Martinez L. O. 2010. Ecto-F1-ATPase: a moonlighting protein complex and an unexpected apoA-I receptor. World J. Gastroenterol. 16: 5925–5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trigatti B., Rigotti A., Krieger M. 2000. The role of the high-density lipoprotein receptor SR-BI in cholesterol metabolism. Curr. Opin. Lipidol. 11: 123–131. [DOI] [PubMed] [Google Scholar]
- 26.Large V., Peroni O., Letexier D., Ray H., Beylot M. 2004. Metabolism of lipids in human white adipocyte. Diabetes Metab. 30: 294–309. [DOI] [PubMed] [Google Scholar]
- 27.Arakaki N., Kita T., Shibata H., Higuti T. 2007. Cell-surface H+-ATP synthase as a potential molecular target for anti-obesity drugs. FEBS Lett. 581: 3405–3409. [DOI] [PubMed] [Google Scholar]
- 28.Cortes-Hernandez P., Dominguez-Ramirez L., Estrada-Bernal A., Montes-Sanchez D. G., Zentella-Dehesa A., de Gomez-Puyou M. T., Gomez-Puyou A., Garcia J. J. 2005. The inhibitor protein of the F1F0-ATP synthase is associated to the external surface of endothelial cells. Biochem. Biophys. Res. Commun. 330: 844–849. [DOI] [PubMed] [Google Scholar]
- 29.Contessi S., Comelli M., Cmet S., Lippe G., Mavelli I. 2007. IF(1) distribution in HepG2 cells in relation to ecto-F(0)F (1)ATPsynthase and calmodulin. J. Bioenerg. Biomembr. 39: 291–300. [DOI] [PubMed] [Google Scholar]
- 30.Giorgio V., Bisetto E., Franca R., Harris D. A., Passamonti S., Lippe G. 2010. The ectopic F(O)F(1) ATP synthase of rat liver is modulated in acute cholestasis by the inhibitor protein IF1. J. Bioenerg. Biomembr. 42: 117–123. [DOI] [PubMed] [Google Scholar]
- 31.Hassinen I. E., Vuorinen K. H., Ylitalo K., Ala-Rämi A. 1998. Role of cellular energetics in ischemia-reperfusion and ischemic preconditioning of myocardium. Mol. Cell. Biochem. 184: 393–400. [PubMed] [Google Scholar]
- 32.Penna C., Pagliaro P., Rastaldo R., Di Pancrazio F., Lippe G., Gattullo D., Mancardi D., Samaja M., Losano G., Mavelli I. 2004. F0F1 ATP synthase activity is differently modulated by coronary reactive hyperemia before and after ischemic preconditioning in the goat. Am. J. Physiol. Heart Circ. Physiol. 287: H2192–H2200. [DOI] [PubMed] [Google Scholar]
- 33.von Eckardstein A., Hersberger M., Rohrer L. 2005. Current understanding of the metabolism and biological actions of HDL. Curr. Opin. Clin. Nutr. Metab. Care. 8: 147–152. [DOI] [PubMed] [Google Scholar]
- 34.Mineo C., Deguchi H., Griffin J. H., Shaul P. W. 2006. Endothelial and antithrombotic actions of HDL. Circ. Res. 98: 1352–1364. [DOI] [PubMed] [Google Scholar]
- 35.Rader D. J. 2007. Illuminating HDL - is it still a viable therapeutic target? N. Engl. J. Med. 357: 2180–2183. [DOI] [PubMed] [Google Scholar]
- 36.Vergès B., Florentin E., Baillot-Rudoni S., Petit J-M., Brindisi M. C., Pais de Barros J-P., Lagrost L., Gambert P., Duvillard L. 2009. Rosuvastatin 20 mg restores normal HDL-apoA-I kinetics in type 2 diabetes. J. Lipid Res. 50: 1209–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nieland T. J., Penman M., Dori L., Krieger M., Kirchhausen T. 2002. Discovery of chemical inhibitors of the selective transfer of lipids mediated by the HDL receptor SR-BI. Proc. Natl. Acad. Sci. USA. 99: 15422–15427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Masson D., Koseki M., Ishibashi M., Larson C. J., Miller S. G., King B. D., Tall A. R. 2009. Increased HDL cholesterol and apoA-I in humans and mice treated with a novel SR-BI inhibitor. Arterioscler. Thromb. Vasc. Biol. 29: 2054–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang L. H., Kamanna V. S., Zhang M. C., Kashyap M. L. 2008. Niacin inhibits surface expression of ATP synthase beta chain in HepG2 cells: implications for raising HDL. J. Lipid Res. 49: 1195–1201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.