Abstract
The brain is naturally considered as a network of interacting elements which, when functioning properly, produces an enormous range of dynamic, adaptable behavior. However, when elements of this network fail, pathological changes ensue, including epilepsy, one of the most common brain disorders. This review examines some aspects of cortical network organization that distinguish epileptic cortex from normal brain as well as the dynamics of network activity before and during seizures, focusing primarily on focal seizures. The review is organized around four phases of the seizure: the interictal period, onset, propagation, and termination. For each phase, the authors discuss the most common rhythmic characteristics of macroscopic brain voltage activity and outline the observed functional network features. Although the characteristics of functional networks that support the epileptic seizure remain an area of active research, the prevailing trends point to a complex set of network dynamics between, before, and during seizures.
Keywords: seizure, coupling, functional network, structural network, rhythms
Epilepsy, the condition of recurrent unprovoked seizures resulting from a wide variety of causes, is the world’s most prominent serious brain disorder, affecting some 50 million people worldwide. For an estimated 30% of these patients, seizures remain poorly controlled despite maximal medical management (Duncan and others 2006). Moreover, control of epilepsy through medication and surgery often results in significant, sometimes debilitating, side effects. Advancing the therapeutic management of epilepsy requires a detailed understanding of the neurophysiological underpinnings that give rise to seizures and how seizures are initiated and subsequently spread across interconnected brain regions. The majority of work in this field has focused on the molecular, anatomical, and cellular physiological changes involved in the development of epilepsy (epileptogenesis) and in the initiation of seizures (ictogenesis). Until recently, little attention had been paid to interactions between activities in different brain regions. This type of analysis—focused on functional connectivity and resulting network maps—has expanded enormously in the last 5 years and has offered important new perspectives and insights into the nature of epilepsy. Research into the network organization of seizures has spanned a variety of different model systems and data sources, explored the rhythmic nature of epileptic activity, and both used and motivated the development of innovative analytic techniques. In this review, we briefly discuss some of the relevant background information on these different aspects of epilepsy, provide a short course on network analysis, and then delve more deeply into recent findings and implications of network investigations in patients with epilepsy.
Overview of Epilepsy and Seizures, Rhythms, and Networks
Different Kinds of Seizures and Implications for Spatial Characterization of Epilepsy
Seizures and epilepsy have traditionally been divided into two different types: primary generalized seizures and focal seizures. Primary generalized seizures involve all of the brain or large portions of the brain at the outset. A substantial body of work has indicated that generalized seizures likely involve a perturbation in existing thalamocortical network interrelationships (for an excellent review, see Chang and Lowenstein 2003). Focal seizures, in contrast, originate from a circumscribed region of the brain and may or may not spread. As implied by the name, the focus of the seizure may determine exactly what symptoms occur. In addition, localization of the focus can be enormously important in trying to understand the cause of the disease, the prognosis, and—especially when surgical options are considered—targets for therapy. Although the division between these types of seizures or epileptic syndromes seems clear, there is mounting evidence that these phenomena exist on a spectrum with overlapping or at least related mechanisms.
Sources of Data
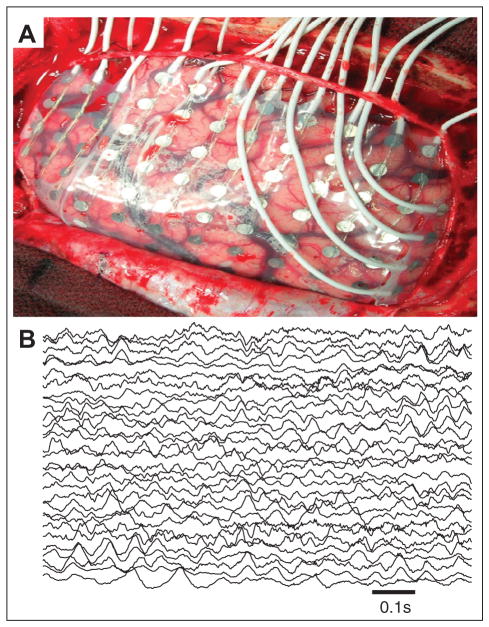
Animal models, both in vitro and in vivo, provide powerful techniques to address the biophysical mechanisms that support the seizure (McCormick and Contreras 2001) and have been the mainstay of much of the research in epilepsy. However, both methods possess important qualifications. First, in vitro preparations necessarily remove a brain section from its surrounding network. Second, animal models of epilepsy are just that—models. A powerful alternative to animal models of epilepsy is to study spontaneously occurring seizures in vivo from human patients. Although significant work has been done based on electroencephalogram (EEG) recordings made from the scalp surface of patients with epilepsy, even more detail has been derived from intracranial recordings. In some cases of pharmaco-resistant epilepsy (when medications fail to prevent seizures), invasive electrocorticogram (ECoG) recordings are performed. These recordings of voltage activity—directly from the brain’s surface or deep brain regions—provide both high temporal and spatial resolution as well as adequate brain coverage (Fig. 1).
Figure 1.
Example ECoG recording. (A) An 8 × 8 electrode grid (1 cm spacing between electrodes) is placed directly on the cortical surface and recordings are made usually for several days or even weeks. The voltage data (B) recorded continuously for multiple days typically exhibit complicated dynamic activity.
Neuronal Rhythms of Seizures
Understanding the vast quantities of voltage data recorded in the ECoG typically requires analysis beyond visual inspection. One technique to characterize these activities is the quantitative assessment of neuronal rhythms that appear during the seizure. Epilepsy is perhaps best characterized as a disease of brain rhythms—a paroxysmal cerebral dysrhythmia (Gibbs and others 2002)—and the voltage activity recorded from an ECoG electrode often reveals stereotyped dynamics (e.g., spike-wave complexes during the “absence seizures” of a primary generalized epilepsy). During a typical secondarily generalized seizure (i.e., a seizure that spreads from a focus to include a large portion of the brain network), there is often a characteristic sequence of neuronal rhythms evolving from low amplitude, fast activity to large amplitude, slow activity. The appearance and characterization of these changing neuronal rhythms provide critical information about the different stages of the seizure (Pinto and others 2005; Schiff and others 2005), as we described in detail below.
Characterization of Coupling and Networks
Characterizing the synchronization—or coupling—between brain areas during seizures is critical for developing a deeper understanding of epilepsy. The synchronization between a few electrode pairs is readily visualized and interpreted. However, for the high- density, multi-electrode recordings of interest here (Fig. 1), complex patterns of network connectivity may emerge whose quantitative understanding requires graph theory and network analysis techniques. In the next section, we provide a brief introduction to the growing field of networks in neuroscience. There already exist many excellent and thorough reviews of this subject (Bullmore and Sporns 2009; Sporns 2010; Stam and Reijneveld 2007); our purpose in the next section is simply to familiarize the reader with some terms from network science that will be helpful in understanding the results for seizure networks presented below.
Primer on Network Analysis and Synthetic Networks
Structural versus Functional Networks
The human brain is naturally conceived as a network consisting of two fundamental components: nodes (e.g., individual neurons or brain regions) and edges (e.g., synaptic connections or white matter tracts) that connect node pairs. In neuroscience, “brain networks” (i.e., graphs representing the connectivity of brain components) are typically divided into two categories: structural networks and functional networks. In structural networks, the edges represent physical connections between nodes (Fig. 2). At the microscopic spatial scale, these include synaptic or gap junctional connections between individual neurons. The only complete brain structural network mapped at this scale is for the 302 neurons of the nematode worm Caenorhabditis elegans (White and others 1986). For the human brain, consisting of more than 1010 neurons and 1012 synapses, defining the complete structural network remains intractable. At a coarser spatial scale, noninvasive in vivo neuroimaging techniques (e.g., magnetic resonance imaging or MRI) can be used to infer the brain’s white matter tracks and construct millimeter scale, macroscopic structural networks in humans (Gong and others 2009; Hagmann and others 2008; Iturria-Medina and others 2007).
Figure 2.
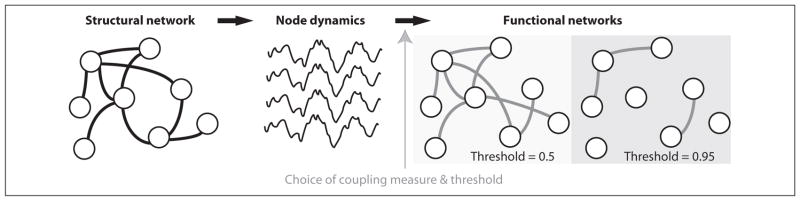
Structural networks (left) represent physical connections between nodes (e.g., axons or white matter tracks). Here the nodes represent macroscopic brain regions that generate population voltage activity (middle). From the coupling between the node dynamics, functional networks are inferred (right) whose structure depends upon the choice of coupling measure and threshold.
In contrast to the anatomically defined structural network, functional networks represent the coupling between dynamic activity recorded from separate brain areas (Fig. 2) (Friston 1994). Different types of multivariate neuroimaging data, from single neuron recordings to the functional magnetic resonance imaging (fMRI) blood oxygen level dependent (BOLD) signal, are used to construct functional networks at different spatial and temporal scales (Sporns 2010). Critical to the establishment of a functional network is the choice of coupling measure, of which there are many options (Pereda and others 2005). An open question, and area of active research, is determining which coupling measures are most appropriate. Different measures exist that focus on linear interactions, nonlinear interactions, wavelet coherence, causality, and many other methods (Pereda and others 2005). Each measure provides a different view of the coupling and requires different processing methods and assumptions (e.g., filtering the data in a specific frequency band to extract phase information, or choice of embedding dimension). Recent studies suggest that linear and nonlinear coupling measures perform equally well when applied to macroscopic voltage data, although subtle changes in the physiological state of the brain may require more sophisticated approaches (Ansari-Asl and others 2006; Mormann and others 2005; Osterhage and others 2007).
After selecting a coupling measure for building a functional network, additional choices must be made. One of the most important is determining the level of coupling that constitutes an edge. In Figure 2 we connected electrode pairs with edges whose coupling measure exceeded a threshold value (e.g., 0.5), and different choices of threshold may result in different networks. For example, a higher threshold choice (e.g., 0.95) results in fewer edges (Fig. 2). No technique yet exists to choose the most appropriate coupling threshold. One approach is to examine the networks produced for a variety of threshold values and seek consistent results (e.g., Kramer and others 2008; Ponten and others 2007).
Another approach to defining coupling threshold is first to apply a statistical hypothesis test to the coupling result computed for each electrode pair. Doing so, a P value may be assigned to each edge and these P values thresholded, rather than the original coupling measure value. An advantage of this approach is that multiple comparisons (a P value exists for each electrode pair) may be addressed using sophisticated statistical techniques and a measure of network uncertainty deduced, namely the number of spurious edges in the network (Kramer and others 2009). One difficulty of this approach is the development of an appropriate method to assess the statistical significance of a coupling value. For classical linear measures (such as the coherence or cross-correlation), analytic techniques exist to determine the statistical significance of the measure, although these typically require specific assumptions about the data (e.g., the asymptotic case of extremely large sample sizes). For modern nonlinear measures, no such analytic methods to assess statistical significance exist. Instead, one might use a bootstrapping procedure; however, bootstrapping techniques are computationally expensive and may not be tractable for large networks.
Linking Structural and Functional Brain Networks
Brain structural and functional networks are intimately related. In general, structural networks constrain functional networks—coupled activity between two areas typically requires (direct or indirect) structural connections. But brain dynamics (used to infer the functional networks) modify brain structure, through processes such as spike-timing-dependent plasticity (Rubinov and others 2009). Computational studies have examined the relationship between brain structural and functional networks (Ponten and others 2010; Zhou and others 2006) and suggested that the structure–function relationship depends on the time scale of activity (Honey and others 2007). At the slow time scale of fMRI and BOLD observations (i.e., on the order of seconds), a general relationship exists between brain structural and functional connectivity (Hagmann and others 2008). But the voltage dynamics of the seizure evolve on a much faster time scale, and recent observations suggest that so do the functional networks (Kramer and others 2010). How functional networks inferred at the faster time scale of ECoG voltage recordings relate to structural networks remains incompletely understood.
Definition of Network Measures and Simple Network Models
Many network models and measures to characterize networks exist. We outline below the network models and measures used to describe the cortical networks of seizure. In doing so we attempt to provide some intuition for these characterizations, and the interested reader is referred to the literature for many excellent and thorough discussions (e.g., Kolaczyk 2009; Newman 2003; Rubinov and Sporns 2010; Stam and Reijneveld 2007).
Network Measures: Density, Path Length, Clustering Coefficient
Brain functional networks are typically complicated structures that require characterization tools beyond visual inspection. Many such tools exist, of which we describe three of the most important. Perhaps the most fundamental network measure is the density—the actual number of edges in the network divided by the number of possible edges. A network with density 0 contains no edges, whereas a density of 1 indicates that all possible edges exist. The path length is the minimum number of edges traversed to travel from one node to another in the network, and the average path length is calculated as the path length between all possible node pairs. Short average path lengths typically suggest fast communication in a network. Finally, the average clustering coefficient measures the number of completed triangles in a network. In social networks, clustering is typically high (i.e., near 1); for example, the friends (nearest neighbors) of an individual (the chosen node) also tend to be friends. These three measures of network structure provide simple numeric summaries to characterize complex networks, consisting of many nodes and edges. There are many additional possible measures that characterize the topology of the network in more detailed and specific fashion (Kolaczyk 2009; Rubinov and Sporns 2010).
Network Models: Regular, Random, and Small-World
Many different types of model networks exist (Newman 2003). We focus here on three simple models important to understanding the cortical networks in epilepsy (Fig. 3). In a regular network, each node connects to its k nearest neighbors. Regular networks have a mesh-like connectivity, resulting in many completed triangles (i.e., high average clustering coefficient) and a large average path length—traveling between nodes requires proceeding along the circumference of the circle in Figure 3A. In a traditional random network, node pairs are connected randomly with some probability P. The result is a network with low average clustering coefficient (the probability that a node’s “friends” are also friends in a random network is P) and low average path length (because travel is no longer restricted to the circumference in Fig. 3C). Finally, a small-world network possesses high clustering coefficients (like regular networks) and short average path lengths (like random networks) (Bassett and Bullmore 2006; Watts and Strogatz 1998). To construct a small-world network, a small number of edges in a regular network are “rewired.” The modified edges typically serve as shortcuts through the network, allowing quick traversal from one side of the network to the other. Because only a few edges are modified, the clustering coefficient remains large (Fig. 3B). We will see that all three network models can be used to represent different stages of functional network progression during seizures and may characterize ictogenic cortex in general.
Figure 3.
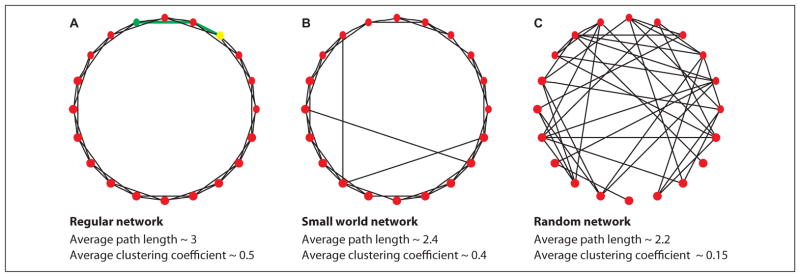
Examples of three networks structures: regular (A), small-world (B), and random (C). In the regular network, a path between two nodes (green and yellow) is shown in green. For each network, the approximate average path length and clustering coefficient are shown.
Networks That Characterize the Epileptic Brain between Seizures
Classically seizures are thought to represent a hyper-synchronous state (Penfield and Jasper 1954) and epilepsy is rudimentarily considered to be a disease of hypersynchronization—a problem of regions and neurons connected or communicating too readily. With this baseline idea in mind, several groups have sought evidence of pathological network relationships in the resting (i.e., not seizing) brain of patients with epilepsy. Even the healthy brain at rest is expected to show some synchronized activity, typically dominated by low-frequency rhythms. The expectation, however, is that in patients with epilepsy the degree of connectivity will be higher, particularly but not necessarily exclusively in the seizure-onset zone. A variety of different studies suggest that this concept has some validity. In patients with mesial temporal lobe epilepsy, for example, fMRI (Zhang and others 2009) and EEG (Bettus and others 2008; Liao and others 2010) studies have indicated that there is increased connectivity in the temporal lobes. In these same studies there was decreased connectivity in frontal and parietal lobes—regions outside of the location of seizure initiation. Furthermore, abnormal connectivity and network topology have also been reported, specifically in the δ and θ bands, in patients with focal epilepsy (Horstmann and others 2010; Wilke and others 2010). This later study also reported a more regular network topology, again in the θ band, in patients with epilepsy compared with healthy controls as measured with EEG (Horstmann and others 2010).
This emerging area of translational neuroscience has already begun to deepen our understanding of how epilepsy arises and suggests some new pathways for diagnosis and treatment. For example, it may soon be possible to take a short segment of non-invasive physiological data (EEG or magnetoencephalogram) or imaging data (fMRI or even structural MRI) and make direct inferences about the underlying disease. For example, the development of network analysis techniques may allow researchers to (1) predict that a patient with a single seizure will go on to develop epilepsy or (2) help localize the area of seizure onset in a patient with intractable focal epilepsy.
Recent research points to the feasibility of both approaches. For example, if global synchronization differs between healthy subjects and epilepsy patients in the interictal state (the periods between seizures), these differences may be present early in the disease and enable an early diagnosis. Indeed, EEG-based measures of functional connectivity from children with absence seizures (Rosso and others 2009) and mixed types of idiopathic epilepsy (Righi and others 2008) differed from healthy controls, suggesting a diagnostic utility of such measures. Similarly, synchronization likelihood analysis of EEG from patients with new-onset seizures could predict later development of epilepsy, albeit with sensitivity and specificity of 62% and 76%, respectively (Douw and others 2010).
These functional network approaches provide new techniques not only for diagnosis but also for localization of seizure-onset areas in focal epilepsy. It is reasonable to expect that ictogenic regions will show some form of increased correlation with other brain areas—a form of hypersynchrony (for review, see Lehnertz and others 2009). Indeed, a variety of different studies using widely different measures of synchrony in the interictal EEG have shown some ability to delineate epileptogenic cortex (Arnhold and others 1999; Ben-Jacob and others 2007; Mormann and others 2000; Ortega and others 2008; Schevon and others 2007; Towle and others 1999). Nonetheless, much work is needed to more completely understand which measures of connectivity work best to delineate ictogenic regions and how to incorporate these topological results into clinical determinations.
In summary, a rapidly expanding body of literature has identified differences in functional connectivity between healthy subjects and patients with epilepsy during seizure free intervals. These differences might be seen in patients with generalized epilepsy and those with focal forms of epilepsy, and the differences may be robust enough to be used as a marker of epilepsy and ictal onset areas. This growing body of information is also beginning to illuminate why seizures actually arise. What happens in the brain during the seizure itself—namely, its initiation, spread, and termination—is the focus of the next section of this review.
Networks That Characterize the Seizure Itself
Synchronization during the Seizure
From a simplistic perspective, focal seizures can be understood as local events that begin in a circumscribed region. This local activity may then spread, from the central focus outward, through the recruitment of other brain areas in a cascade of activity that includes both pathological and normal brain tissue. Presumably, this spread manifests as increased synchronization throughout the brain network. Mathematical measures now allow a formal characterization of this claim, and recent observations challenge the assertion that seizures are uniformly hypersynchronous events (Bartolomei and others 2004; Netoff and others 2004; Kramer and others 2010; Truccolo and others 2011). In the next sections, we describe the different seizure stages and how neural rhythms and synchronization evolve during the seizure. To organize our discussion, we focus on three stages of the focal seizure: onset, propagation, and termination. Within each stage, we consider three issues: (1) the types of rhythmic activity observed, (2) the coupling between voltage data recorded from separate spatial locations, and (3) the types of network structures observed within each stage. As described in detail below, these insights have altered the traditional perspective of the seizure as a purely hypersynchronous state.
Seizure Onset: HFO, Decoupling, and the Axon Plexus
Rhythms
There exist many informal definitions for seizure onset. Perhaps the most obvious manifestation of seizure onset is the emergence of clinical symptoms (e.g., convulsions in tonic–clonic seizures). But more subtle changes in brain voltage activity typically precede this clinical onset. These include rhythmic phenomena, such as the low-amplitude, high-frequency “beta buzz” (near 20 Hz) (Curtis and Gnatkovsky 2009). These rapid discharges are associated with the epileptogenic zone, and optimal resection may target these localized sites of high-frequency activity (Alarcon and others 1995). Recently, even faster rhythms have been associated with seizure onset. These include high-frequency oscillations (HFOs) with frequency ranges that typically exceed 100 Hz (Bragin and others 1999). Localization of seizure onset, through identification of pathological HFO activity, may also help target the epileptogenic zone critical for seizure resection (Fisher and others 1992; Jirsch and others 2006).
A proposed biological mechanism supporting HFO, of particular relevance to this review, involves a structural network between individual neurons: the axon plexus. The axon plexus is a collection of pyramidal cells with axons connected by gap junctions. To support HFO, relatively infrequent spontaneous action potentials propagate across gap junctions between pyramidal cell axons. In this network model, the period of the HFO is determined by the global topological structure of the axon plexus network (Traub and Whittington 2010). We note that the biological mechanisms supporting HFO remain under investigation; another possible mechanism—inhibitory interneuron discharges on pyramidal cells (Penttonen and others 1998)—does not depend on the network structure of the axon plexus.
Coupling and networks
Analysis of voltage activity recorded at seizure onset has typically revealed decreases in coupling between brain regions, although not always. Decoupling has been observed in the 80- to 200-Hz frequency band among different gyri (although correlated activity appears within a gyrus) (Grenier and others 2001) and during β frequency discharges from patients with mesial temporal lobe epilepsy (Bartolomei and others 2004). In addition, depth electrode recordings of initial fast ictal discharges (60–90 Hz) in patients with partial epilepsy exhibit spatial decorrelations, compared with intervals immediately preceding and following (Wendling and others 2003). Decorrelations also appear immediately at seizure onset in high-frequency bands (80–200 Hz) in depth and strip electrode recordings from patients with complex-partial and secondarily generalized seizures (Schindler and others 2010). However, other analyses that focused on lower frequency bands have revealed increased correlation at seizure onset. A measure of non-linear coupling, the synchronization likelihood, increases in the α (8–13 Hz), β (13–30 Hz), and δ (1–4 Hz) frequency bands during the rapid discharges characteristic of seizure onset. The associated functional networks tend to exhibit increased clustering coefficients and path lengths during the rapid discharges compared with interictal intervals (Ponten and others 2007). In addition, cross-correlation analysis of wideband ECoG data from surface and depth electrodes suggests increased correlation (and therefore increased network density) immediately at seizure onset (Kramer and others 2010; Schindler, Leung, and others 2007). The different results may occur for many reasons, including differences in coupling measures, patient populations, recording electrodes, definitions of seizure onset, and selection of “nodes” included in the analysis. The observation of decorrelation in high-frequency bands at seizure onset may be interpreted as an initial functional disconnection between distant brain regions, whereas the observation of increased correlation in low-frequency bands at onset may reflect recruitment of brain regions preceding seizure spread. Thus the properties of brain functional connectivity at seizure onset remain an open area of active research.
Propagation: Ictal Chirps and Decoupling
Rhythms
Following a focal onset, the pathological seizure activity spreads throughout the cortical network. At this point, the macroscopic brain voltage dynamics (i.e., as recorded in the ECoG) typically transition from low-amplitude, fast rhythmic activity in spatially focal regions to large-amplitude, slower rhythmic activity across spatially widespread areas. Different types of widespread rhythmic patterns appear, including spike-wave complexes (Gibbs and others 2002) and voltage rhythms that decrease in frequency with time—the “brain chirp” (Schiff and others 2000)—in generalized seizures (Fig. 4).
Figure 4.
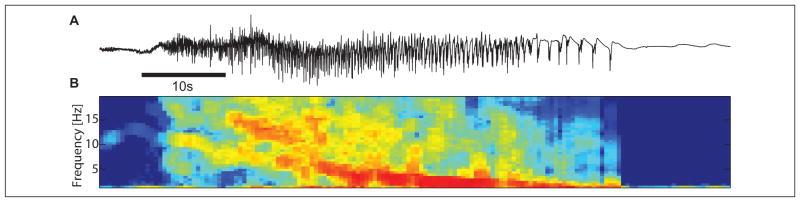
Example of ictal dynamics. (A) Voltage trace from a single ECoG electrode during seizure. Visual inspection begins to suggest the different dynamic regimes. (B) A time–frequency spectrum of the voltage signal in (A). Warm (cool) colors indicate high (low) amplitude oscillations. As time evolves, the dominant rhythms slow.
In generalized seizures, this rhythmic activity is widespread, appearing throughout the brain. The large-amplitude oscillations characteristic of seizure are commonly thought of as “hypersynchronous” events (Schindler, Leung, and others 2007). This may be true at the mesoscopic spatial scale in which the summed postsynaptic activity of many thousands of synapses generates population voltage activity observable in a single EEG electrode (Penfield and Jasper 1954). However, whether hypersynchrony persists at the microscopic scale of individual neurons or larger macroscopic spatial scales is questionable. Individual neuronal activity during focal seizures in humans seems not to show the highly ordered synchrony that would be expected (Truccolo and others 2011). Furthermore, recent analysis has shown that at the macroscopic spatial scale of ECoG recordings, brain activity decouples, as we now describe.
Coupling and networks
At the macroscopic spatial scale (e.g., in ECoG recordings) the transition from seizure onset to propagation may result in a period of decreased (linear) correlation between brain regions (Kramer and others 2010; Schindler and others 2008; Schindler, Leung, and others 2007). This decoupling appears independent of the anatomical location of seizure onset, duration of seizure, and number of recording channels (Schindler, Leung, and others 2007). However other measures of coupling, including phase or amplitude correlations (Schiff and others 2005) and the synchronization likelihood (Ponten and others 2007), remain elevated during the seizure. The elevated synchronization likelihood has also been observed in different seizure types and frequency bands, including the 8- to 12-Hz band from patients with nocturnal frontal lobe seizures (Ferri and others 2004) and broadband in neonatal patients (Altenburg and others 2003). Again, this apparent inconsistency in coupling changes during the middle part of seizure may result from many factors, including differences in the coupling measures (e.g., linear versus nonlinear) and differences in seizure types.
As coupling of voltage activity changes between brain regions, so too does the functional network structure. Changes in the simplest measure of network structure—the density—follow patterns consistent with some of the coupling results; decreased linear correlation during seizure propagation results in sparser functional networks (i.e., network with fewer edges) (Schindler and others 2008), and the dominant connected components fracture into smaller structures (Kramer and others 2010). Measures of more subtle network properties include the average path length (PL) and clustering coefficient (CC). Functional networks (based on correlation measures) suggest that the CC and PL increase during the first half of the seizure and then gradually decrease (Kramer and others 2010; Schindler and others 2008). A nonlinear measure of coupling (synchronization likelihood) also results in functional networks whose CC and PL increase during the seizure (Ponten and others 2007). These results all suggest a shift toward a more regular (less random) network topology during seizure propagation. In addition, the PL and CC permit assessment of the small-world characteristics of the networks. The small-world property has been proposed to become more significant during seizure (Ponten and others 2007; Wu and others 2006) but remains controversial (Antiqueira and others 2010; Bialonski and others 2010; Gerhard and others 2011).
The network mechanisms that support seizure propagation remain unknown. One proposal, consistent with the decrease in coupling during propagation, is that an initial interval of extremely intense neuronal firing at seizure onset saturates “hub” neurons—which maintain many connections to other neurons. When these hub neurons shut off, the result is a functional disconnection between local substructures and decreased coupling (Schindler and others 2008). In computational models of seizure (Morgan and Soltesz 2008) and in observations of human seizures (Kramer and others 2008), hubs serve important roles and may perhaps be useful as targets for surgical treatment of epilepsy (Wilke and others 2011). In addition, simulation studies suggest that the pathological organization of seizing activity is supported by small-world topologies (Netoff and others 2004; Percha and others 2005). Although network topology itself influences the neuronal activity, the interaction of network structure and intrinsic neuronal properties is also crucial (Bogaard and others 2009; Dyhrfjeld-Johnsen and others 2007) but remains poorly understood.
Termination: Slow Rhythms and Recoupling
Rhythms
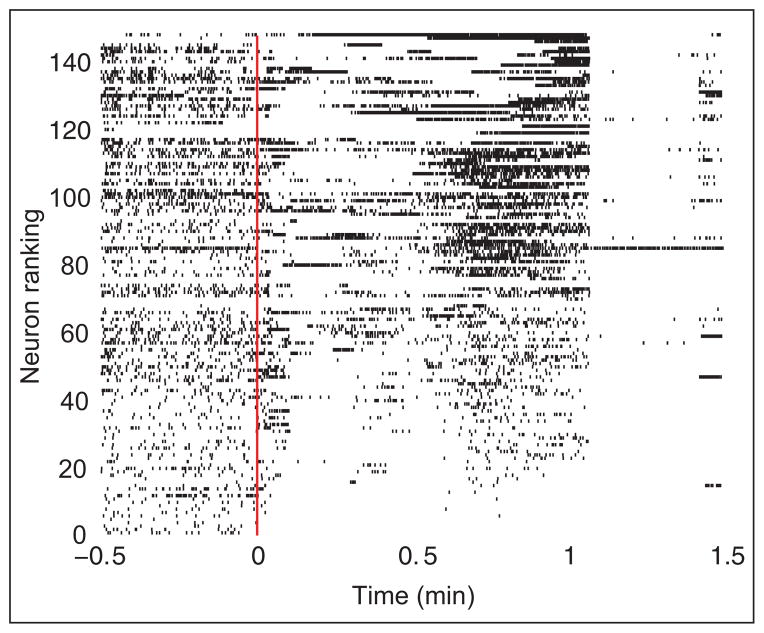
Like the other stages of the seizure, the voltage dynamics of seizure termination exhibit characteristic behaviors. The first, as mentioned in the previous section, is the slowing of the voltage rhythms (i.e., the brain chirp; Schiff and others 2000) in the approach to seizure termination. The second is the nearly simultaneous cessation of voltage activity across the brain at termination, as observed in both macroscale ECoG recording and the microscale activity of individual neurons (Fig. 5). Analyses of functional network topologies in the approach to seizure termination are providing additional information.
Figure 5.
Neuronal spike raster recorded in vivo from a human subject during seizure. The neurons (n = 149) are arranged according to increasing mean spike rate. The seizure begins at t = 0 min (solid red line) and ends near t = 1 min. Visual inspection suggests a nearly simultaneous cessation of spiking activity for most neurons at seizure termination. Adapted from Truccolo and others (2011).
Coupling and networks
One hypothesis is that in the approach to seizure termination, the coupling of brain activity increases. This has been observed using (linear) measures of cross-correlation in the broadband (Kramer and others 2010; Schindler and others 2008; Schindler, Elger, and others 2007) and high-frequency (80–200 Hz) band (Schindler and others 2010), appeared independent of anatomical location of seizure-onset zone and duration of seizure, and occurred for both partial complex and secondarily generalized seizures (Schindler, Leung, and others 2007). In addition, the non-linear correlation coefficient, applied to voltage activity recorded at temporal lobe and thalamus, increased in the approach to seizure termination, and the maximal values of synchrony were observed during the last part of the seizure (Guye and others 2006). These results are consistent with animal models showing that synchrony progressively increased toward the late seizure stage (Topolnik and others 2003).
This coupling increase affects the functional network structure by increasing the network density (i.e., the number of edges) (Kramer and others 2010; Schindler and others 2008) and supporting the emergence of a giant connected component—the coalescence of the functional network (Kramer and others 2010). More specific measures of the network structure suggest that in the approach to seizure termination the PL and CC decrease, and therefore the networks move in the direction of becoming more random (Kramer and others 2010; Schindler and others 2008).
Understanding the mechanisms that end the seizure remains an important goal, and these analyses of coupling and functional network structure at seizure termination are beginning to provide additional clues. An important question to address is whether the increase in synchrony before termination is an epiphenomenon of another process or an active mechanism for seizure termination. One proposal for seizure termination is that the self-organization of neuronal activity (as reflected by the increased coupling) drives neuronal populations into a hypo-excitable, refractory state (Schindler, Elger, and others 2007). The biophysical mechanisms that may support this transition include the activation of potassium currents across large neuronal networks that overcome hyperpolarization- activated depolarizing currents (Schindler, Elger, and others 2007). The role of deep brain structures, such as the thalamus, in seizures remains an area of active research with some observations from animal models suggesting an important role for the thalamus (Bertram and others 2001), and others not (Timofeev and Steriade 2004). Whether the thalamus, or another deep brain region, serves to mediate coordination between cortical regions and thereby terminate activity remains unknown (Lado and Moshé 2008).
Conclusions and Open Questions
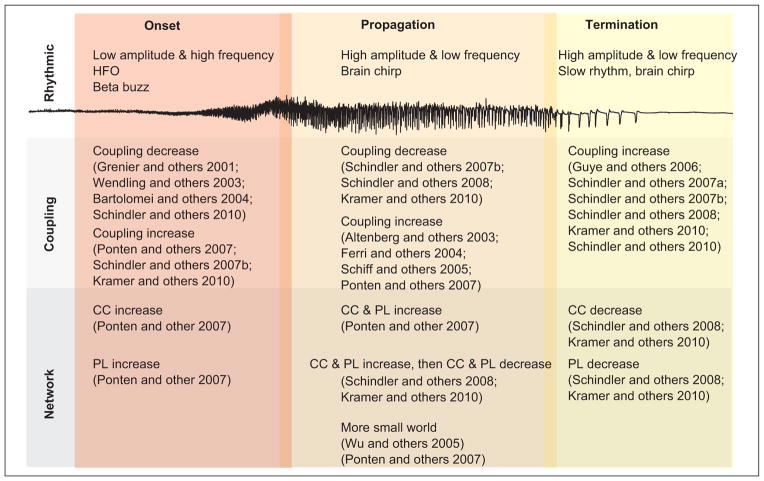
In this review we considered the rhythmic activity and functional networks observed during seizures and in the interictal state. We focused on three stages of seizure—onset, propagation, and termination—and summarized the types of rhythms and functional networks observed in each stage. In general, the rhythmic stages of seizure are fairly well characterized, although new recording modalities have allowed the observation of new phenomena (e.g., high-frequency voltage recordings and HFO). The inference and characterization of functional networks during seizures remain an active area of research; nonetheless, we attempted to summarize some of most common observations (Fig. 6). These include changes in coupling during the seizure and in particular increased coupling at seizure termination. In terms of network structure, observations suggest that networks acquire larger path lengths and clustering coefficients near the beginning of the seizure and that networks become more small-world during seizure propagation and more random at seizure termination. For each stage, we also noted contradicting observations. These discrepancies may exist for many reasons, including differences in the types of coupling measures used (e.g., linear versus nonlinear) and the types of patients analyzed. We hope that future research will help reduce these discrepancies and will further understanding of the functional networks of seizure.
Figure 6.
Summary of functional network organization during different seizure stages. Three different stages of seizure—onset, propagation, and termination—are shown with a typical voltage trace during seizure. The characteristics of the rhythms (top row), coupling (middle row), and networks (bottom row) are indicated as observed in each state and with references to the literature. HFO = high-frequency oscillation, CC = clustering coefficient, PL = path length.
These observations suggest a refinement of the traditional idea that seizures are hypersynchronous events. Although the current analysis of interictal data points to at least some degree of increased coupling between brain regions, particularly within the seizure-onset zone in patients with focal epilepsy, this hypothesis needs substantiation across larger patient groups and situations and will likely lead to a more nuanced conclusion regarding what regions show hypersynchronization at a network level. At the microscopic spatial scale of individual neurons, some correlated neuronal activity must occur to produce the large-amplitude, macroscopic fields observable in the ECoG during the seizure (although seizure-like activity induced in rat hippocampal slices also shows desynchronization of neuronal firing; Netoff and Schiff 2002). However, hypersynchrony at the microscopic spatial scale does not imply correlated activity between macroscopic brain regions. In fact, variability in propagation between brain regions may act to decorrelate macroscopic brain activity during the seizure (Schindler, Leung, and others 2007). The results reviewed here and other investigations are beginning to reveal more details of the entire scope of interictal and ictal activity.
Epilepsy is a disease with many causes and manifestations and one for which many research questions remain. For example, are the network characteristics between and during seizures of different causes the same; in other words, is there a “final common pathway” linking the different causes at a mechanistic level? Perhaps the most important remaining issue, and perhaps the most difficult to approach, is how to link these network-level descriptions with the vast but still incomplete understanding of seizures and epilepsy at the cellular and subcellular levels. Linking these different spatial scales and intellectual frameworks would provide a comprehensive description of the disease and undoubtedly lead to novel therapeutic interventions.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: M.A.K. holds a Career Award at the Scientific Interface from the Burroughs Wellcome Fund. S.S.C. was supported by funds from the National Institute of Neurological Disorders and Stroke Grant R01 NS062092.
Footnotes
Reprints and permission: http://www.sagepub.com/journalsPermissions.nav
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Alarcon G, Binnie CD, Elwes RD, Polkey CE. Power spectrum and intracranial EEG patterns at seizure onset in partial epilepsy. Electroencephalogr Clin Neurophysiol. 1995;94(5):326–37. doi: 10.1016/0013-4694(94)00286-t. [DOI] [PubMed] [Google Scholar]
- Altenburg J, Vermeulen RJ, Strijers RLM, Fetter WPF, Stam CJ. Seizure detection in the neonatal EEG with synchronization likelihood. Clin Neurophysiol. 2003;114(1):50–5. doi: 10.1016/s1388-2457(02)00322-x. [DOI] [PubMed] [Google Scholar]
- Ansari-Asl K, Senhadji L, Bellanger J-J, Wendling F. Quantitative evaluation of linear and nonlinear methods characterizing interdependencies between brain signals. Phys Rev E. 2006;74(3 pt 1):031916. doi: 10.1103/PhysRevE.74.031916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antiqueira L, Rodrigues FA, van Wijk BCM, da F Costa L, Daffertshofer A. Estimating complex cortical networks via surface recordings—a critical note. Neuroimage. 2010;53(2):439–49. doi: 10.1016/j.neuroimage.2010.06.018. [DOI] [PubMed] [Google Scholar]
- Arnhold J, Grassberger P, Lehnertz K, Elger C. A robust method for detecting interdependences: application to intracranially recorded EEG. Physica D. 1999;134(4):419–30. [Google Scholar]
- Bartolomei F, Wendling F, Régis J, Gavaret M, Guye M, et al. Pre-ictal synchronicity in limbic networks of mesial temporal lobe epilepsy. Epilepsy Res. 2004;61(1–3):89–104. doi: 10.1016/j.eplepsyres.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Bassett DS, Bullmore E. Small-world brain networks. Neuroscientist. 2006;12(6):512–23. doi: 10.1177/1073858406293182. [DOI] [PubMed] [Google Scholar]
- Ben-Jacob E, Doron I, Gazit T, Rephaeli E, Sagher O, Towle VL. Mapping and assessment of epileptogenic foci using frequency-entropy templates. Phys Rev E. 2007;76(5 pt 1):051903. doi: 10.1103/PhysRevE.76.051903. [DOI] [PubMed] [Google Scholar]
- Bettus G, Wendling F, Guye M, Valton L, Régis J, Chauvel P, et al. Enhanced EEG functional connectivity in mesial temporal lobe epilepsy. Epilepsy Res. 2008;81(1):58–68. doi: 10.1016/j.eplepsyres.2008.04.020. [DOI] [PubMed] [Google Scholar]
- Bertram EH, Mangan PS, Zhang D, Scott CA, Williamson JM. The midline thalamus: alterations and a potential role in limbic epilepsy. Epilepsia. 2001;42(8):967–78. doi: 10.1046/j.1528-1157.2001.042008967.x. [DOI] [PubMed] [Google Scholar]
- Bialonski S, Horstmann M-T, Lehnertz K. From brain to earth and climate systems: small-world interaction networks or not? Chaos (Woodbury, NY) 2010;20(1):013134. doi: 10.1063/1.3360561. [DOI] [PubMed] [Google Scholar]
- Bogaard A, Parent J, Zochowski M, Booth V. Interaction of cellular and network mechanisms in spatiotemporal pattern formation in neuronal networks. J Neurosci. 2009;29(6):1677–87. doi: 10.1523/JNEUROSCI.5218-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin A, Engel J, Wilson CL, Fried I, Buzsáki G. High-frequency oscillations in human brain. Hippocampus. 1999;9(2):137–42. doi: 10.1002/(SICI)1098-1063(1999)9:2<137::AID-HIPO5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10(3):186–98. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- de Curtis M, Gnatkovsky V. Reevaluating the mechanisms of focal ictogenesis: the role of low-voltage fast activity. Epilepsia. 2009;50(12):2514–25. doi: 10.1111/j.1528-1167.2009.02249.x. [DOI] [PubMed] [Google Scholar]
- Chang BS, Lowenstein DH. Epilepsy. N Engl J Med. 2003;349(13):1257–66. doi: 10.1056/NEJMra022308. [DOI] [PubMed] [Google Scholar]
- Douw L, de Groot M, van Dellen E, Heimans JJ, Ronner HE, et al. “Functional connectivity” is a sensitive predictor of epilepsy diagnosis after the first seizure. PLoS ONE. 2010;5(5):e10839. doi: 10.1371/journal.pone.0010839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan JS, Sander JW, Sisodiya SM, Walker MC. Adult epilepsy. Lancet. 2006;367(9516):1087–100. doi: 10.1016/S0140-6736(06)68477-8. [DOI] [PubMed] [Google Scholar]
- Dyhrfjeld-Johnsen J, Santhakumar V, Morgan RJ, Huerta R, Tsimring L, et al. Topological determinants of epileptogenesis in large-scale structural and functional models of the dentate gyrus derived from experimental data. J Neurophysiol. 2007;97(2):1566–87. doi: 10.1152/jn.00950.2006. [DOI] [PubMed] [Google Scholar]
- Ferri R, Stam CJ, Lanuzza B, Cosentino FII, Elia M, et al. Different EEG frequency band synchronization during nocturnal frontal lobe seizures. Clin Neurophysiol. 2004;115(5):1202–11. doi: 10.1016/j.clinph.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Fisher RS, Webber WR, Lesser RP, Arroyo S, Uematsu S. High-frequency EEG activity at the start of seizures. J Clin Neurophysiol. 1992;9(3):441–8. doi: 10.1097/00004691-199207010-00012. [DOI] [PubMed] [Google Scholar]
- Friston K. Functional and effective connectivity in neuroimaging: a synthesis. Hum Brain Mapp. 1994;2(1–2):56–78. [Google Scholar]
- Gerhard F, Pipa G, Lima B, Neuenschwander S, Gerstner W. Extraction of network topology from multi-electrode recordings: is there a small-world effect? Front Comput Neurosci. 2011;5:4. doi: 10.3389/fncom.2011.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs F, Gibbs E, Lennox W. Epilepsy: a paroxysmal cerebral dysrhythmia. Epilepsy Behav. 2002;3(4):395–401. doi: 10.1016/s1525-5050(02)00050-1. [DOI] [PubMed] [Google Scholar]
- Gong G, He Y, Concha L, Lebel C, Gross DW, Evans AC, et al. Mapping anatomical connectivity patterns of human cerebral cortex using in vivo diffusion tensor imaging tractography. Cereb Cortex. 2009;19(3):524–36. doi: 10.1093/cercor/bhn102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenier F, Timofeev I, Steriade M. Focal synchronization of ripples (80–200 Hz) in neocortex and their neuronal correlates. J Neurophysiol. 2001;86(4):1884–98. doi: 10.1152/jn.2001.86.4.1884. [DOI] [PubMed] [Google Scholar]
- Guye M, Régis J, Tamura M, Wendling F, McGonigal A, Chauvel P, et al. The role of corticothalamic coupling in human temporal lobe epilepsy. Brain. 2006;129(pt 7):1917–28. doi: 10.1093/brain/awl151. [DOI] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey C, Wedeen V, et al. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6(7):e159 EP. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey CJ, Kötter R, Breakspear M, Sporns O. Network structure of cerebral cortex shapes functional connectivity on multiple time scales. Proc Natl Acad Sci U S A. 2007;104(24):10240–5. doi: 10.1073/pnas.0701519104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horstmann M-T, Bialonski S, Noennig N, Mai H, Prusseit J, Wellmer J, et al. State dependent properties of epileptic brain networks: comparative graph-theoretical analyses of simultaneously recorded EEG and MEG. Clin Neurophysiol. 2010;121(2):172–85. doi: 10.1016/j.clinph.2009.10.013. [DOI] [PubMed] [Google Scholar]
- Iturria-Medina Y, Canales-Rodríguez EJ, Melie-García L, Valdés-Hernández PA, Martínez-Montes E, Alemán-Gómez Y, et al. Characterizing brain anatomical connections using diffusion weighted MRI and graph theory. Neuroimage. 2007;36(3):645–60. doi: 10.1016/j.neuroimage.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Jirsch JD, Urrestarazu E, LeVan P, Olivier A, Dubeau F, Gotman J. High-frequency oscillations during human focal seizures. Brain. 2006;129(pt 6):1593–608. doi: 10.1093/brain/awl085. [DOI] [PubMed] [Google Scholar]
- Kolaczyk ED. Statistical Analysis of Network Data: Methods and Models. New York: Springer; 2009. [Google Scholar]
- Kramer MA, Eden UT, Cash SS, Kolaczyk ED. Network inference with confidence from multivariate time series. Phy Rev E. 2009;79(6 pt 1):061916. doi: 10.1103/PhysRevE.79.061916. [DOI] [PubMed] [Google Scholar]
- Kramer MA, Eden UT, Kolaczyk ED, Zepeda R, Eskandar EN, Cash SS. Coalescence and fragmentation of cortical networks during focal seizures. J Neurosci. 2010;30(30):10076–85. doi: 10.1523/JNEUROSCI.6309-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer MA, Kolaczyk ED, Kirsch HE. Emergent network topology at seizure onset in humans. Epilepsy Res. 2008;79(2–3):173–86. doi: 10.1016/j.eplepsyres.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Lado FA, Moshé SL. How do seizures stop? Epilepsia. 2008;49(10):1651–64. doi: 10.1111/j.1528-1167.2008.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnertz K, Bialonski S, Horstmann M-T, Krug D, Rothkegel A, Staniek M, et al. Synchronization phenomena in human epileptic brain networks. J Neurosci Meth. 2009;183(1):42–8. doi: 10.1016/j.jneumeth.2009.05.015. [DOI] [PubMed] [Google Scholar]
- Liao W, Zhang Z, Pan Z, Mantini D, Ding J, Duan X, et al. Altered functional connectivity and small-world in mesial temporal lobe epilepsy. PLoS ONE. 2010;5(1):e8525. doi: 10.1371/journal.pone.0008525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Contreras D. On the cellular and network bases of epileptic seizures. Ann Rev Physiol. 2001;63:815–46. doi: 10.1146/annurev.physiol.63.1.815. [DOI] [PubMed] [Google Scholar]
- Morgan RJ, Soltesz I. Nonrandom connectivity of the epileptic dentate gyrus predicts a major role for neuronal hubs in seizures. Proc Natl Acad Sci U S A. 2008;105(16):6179–84. doi: 10.1073/pnas.0801372105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormann F, Kreuz T, Rieke C, Andrzejak RG, Kraskov A, David P, et al. On the predictability of epileptic seizures. Clin Neurophysiol. 2005;116(3):569–87. doi: 10.1016/j.clinph.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Mormann F, Lehnertz K, David P, Elger C. Mean phase coherence as a measure for phase synchronization and its application to the EEG of epilepsy patients. Physica D. 2000;144(3–4):358–69. [Google Scholar]
- Netoff TI, Clewley R, Arno S, Keck T, White JA. Epilepsy in small-world networks. J Neurosci. 2004;24(37):8075–83. doi: 10.1523/JNEUROSCI.1509-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netoff TI, Schiff SJ. Decreased neuronal synchronization during experimental seizures. J Neurosci. 2002;22(16):7297–307. doi: 10.1523/JNEUROSCI.22-16-07297.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman M. The structure and function of complex networks. SIAM Rev. 2003;45(2):167. [Google Scholar]
- Ortega GJ, de la Prida LM, Sola RG, Pastor J. Synchronization clusters of interictal activity in the lateral temporal cortex of epileptic patients: intraoperative electrocorticographic analysis. Epilepsia. 2008;49(2):269–80. doi: 10.1111/j.1528-1167.2007.01266.x. [DOI] [PubMed] [Google Scholar]
- Osterhage H, Mormann F, Staniek M. Measuring synchronization in the epileptic brain: a comparison of different approaches. Int J Bifurcat Chaos. 2007;17(10):3539–3555. [Google Scholar]
- Penfield W, Jasper H. Epilepsy and the Functional Anatomy of the Human Brain. Little Brown; 1954. [Google Scholar]
- Penttonen M, Kamondi A, Acsády L, Buzsáki G. Gamma frequency oscillation in the hippocampus of the rat: intracellular analysis in vivo. Eur J Neurosci. 1998;10(2):718–28. doi: 10.1046/j.1460-9568.1998.00096.x. [DOI] [PubMed] [Google Scholar]
- Percha B, Dzakpasu R, Zochowski M, Parent J. Transition from local to global phase synchrony in small world neural network and its possible implications for epilepsy. Phys Rev E. 2005;72(3 pt 1):031909. doi: 10.1103/PhysRevE.72.031909. [DOI] [PubMed] [Google Scholar]
- Pereda E, Quiroga R, Bhattacharya J. Nonlinear multivariate analysis of neurophysiological signals. Prog Neurobiol. 2005;77:1–37. doi: 10.1016/j.pneurobio.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Pinto DJ, Patrick SL, Huang WC, Connors BW. Initiation, propagation, and termination of epileptiform activity in rodent neocortex in vitro involve distinct mechanisms. J Neurosci. 2005;25(36):8131–40. doi: 10.1523/JNEUROSCI.2278-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponten SC, Bartolomei F, Stam CJ. Small-world networks and epilepsy: graph theoretical analysis of intracerebrally recorded mesial temporal lobe seizures. Clin Neurophysiol. 2007;118(4):918–27. doi: 10.1016/j.clinph.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Ponten SC, Daffertshofer A, Hillebrand A, Stam CJ. The relationship between structural and functional connectivity: graph theoretical analysis of an EEG neural mass model. Neuroimage. 2010;52(3):985–94. doi: 10.1016/j.neuroimage.2009.10.049. [DOI] [PubMed] [Google Scholar]
- Righi M, Barcaro U, Starita A, Karakonstantaki E, Micheloyannis S. Detection of signs of brain dysfunction in epileptic children by recognition of transient changes in the correlation of seizure-free EEG. Brain Topogr. 2008;21(1):43–51. doi: 10.1007/s10548-008-0057-2. [DOI] [PubMed] [Google Scholar]
- Rosso OA, Mendes A, Rostas JA, Hunter M, Moscato P. Distinguishing childhood absence epilepsy patients from controls by the analysis of their background brain electrical activity. J Neurosci Meth. 2009;177(2):461–8. doi: 10.1016/j.jneumeth.2008.10.017. [DOI] [PubMed] [Google Scholar]
- Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52(3):1059–69. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Rubinov M, Sporns O, van Leeuwen C, Breakspear M. Symbiotic relationship between brain structure and dynamics. BMC Neurosci. 2009;10:55. doi: 10.1186/1471-2202-10-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schevon CA, Cappell J, Emerson R, Isler J, Grieve P, Goodman R, et al. Cortical abnormalities in epilepsy revealed by local EEG synchrony. Neuroimage. 2007;35(1):140–8. doi: 10.1016/j.neuroimage.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff SJ, Colella D, Jacyna GM, Hughes E, Creekmore JW, Marshall A, et al. Brain chirps: spectrographic signatures of epileptic seizures. Clin Neurophysiol. 2000;111(6):953–8. doi: 10.1016/s1388-2457(00)00259-5. [DOI] [PubMed] [Google Scholar]
- Schiff SJ, Sauer T, Kumar R, Weinstein SL. Neuronal spatiotemporal pattern discrimination: the dynamical evolution of seizures. Neuroimage. 2005;28(4):1043–55. doi: 10.1016/j.neuroimage.2005.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler KA, Bialonski S, Horstmann M-T, Elger CE, Lehnertz K. Evolving functional network properties and synchronizability during human epileptic seizures. Chaos (Woodbury, NY) 2008;18(3):033119. doi: 10.1063/1.2966112. [DOI] [PubMed] [Google Scholar]
- Schindler K, Amor F, Gast H, Müller M, Stibal A, Mariani L, et al. Peri-ictal correlation dynamics of high-frequency (80–200 Hz) intracranial EEG. Epilepsy Res. 2010;89(1):72–81. doi: 10.1016/j.eplepsyres.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Schindler K, Elger CE, Lehnertz K. Increasing synchronization may promote seizure termination: evidence from status epilepticus. Clin Neurophysiol. 2007;118(9):1955–68. doi: 10.1016/j.clinph.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Schindler K, Leung H, Elger CE, Lehnertz K. Assessing seizure dynamics by analysing the correlation structure of multichannel intracranial EEG. Brain. 2007;130(pt 1):65–77. doi: 10.1093/brain/awl304. [DOI] [PubMed] [Google Scholar]
- Sporns O. Networks of the Brain. Cambridge, MA: MIT Press; 2010. [Google Scholar]
- Stam CJ, Reijneveld JC. Graph theoretical analysis of complex networks in the brain. Nonlinear Biomed Phys. 2007;1(1):3. doi: 10.1186/1753-4631-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeev I, Steriade M. Neocortical seizures: initiation, development and cessation. Neuroscience. 2004;123(2):299–336. doi: 10.1016/j.neuroscience.2003.08.051. [DOI] [PubMed] [Google Scholar]
- Topolnik L, Steriade M, Timofeev I. Partial cortical deafferentation promotes development of paroxysmal activity. Cereb Cortex. 2003;13(8):883–93. doi: 10.1093/cercor/13.8.883. [DOI] [PubMed] [Google Scholar]
- Towle VL, Carder RK, Khorasani L, Lindberg D. Electrocorticographic coherence patterns. J Clin Neurophysiol. 1999;16(6):528–47. doi: 10.1097/00004691-199911000-00005. [DOI] [PubMed] [Google Scholar]
- Traub RD, Whittington MA. Cortical Oscillations in Health and Disease. New York: Oxford University Press; 2010. [Google Scholar]
- Truccolo W, Donoghue J, Hochberg L, Eskandar E, Madsen J, Anderson W, et al. Single-neuron dynamics in human focal epilepsy. Nat Neurosci. 2011;14(5):635–41. doi: 10.1038/nn.2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts DJ, Strogatz SH. Collective dynamics of “small-world” networks. Nature. 1998;393(6684):440–2. doi: 10.1038/30918. [DOI] [PubMed] [Google Scholar]
- Wendling F, Bartolomei F, Bellanger JJ, Bourien J, Chauvel P. Epileptic fast intracerebral EEG activity: evidence for spatial decorrelation at seizure onset. Brain. 2003;126(pt 6):1449–59. doi: 10.1093/brain/awg144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J, Southgate E, Thomson J, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1986;314(1165):1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- Wilke C, van Drongelen W, Kohrman M, He B. Neocortical seizure foci localization by means of a directed transfer function method. Epilepsia. 2010;51(4):564–72. doi: 10.1111/j.1528-1167.2009.02329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke C, Worrell G, He B. Graph analysis of epileptogenic networks in human partial epilepsy. Epilepsia. 2011;52(1):84–93. doi: 10.1111/j.1528-1167.2010.02785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Li X, Guan X. Networking property during epileptic seizure with multi-channel EEG recordings. In: Wang J, et al., editors. Lecture Notes in Computer Science. Vol. 3976. 2006. pp. 573–578. [Google Scholar]
- Zhang Z, Lu G, Zhong Y, Tan Q, Liao W, Chen Z, et al. Impaired perceptual networks in temporal lobe epilepsy revealed by resting fMRI. J Neurol. 2009;256(10):1705–13. doi: 10.1007/s00415-009-5187-2. [DOI] [PubMed] [Google Scholar]
- Zhou C, Zemanová L, Zamora G, Hilgetag CC, Kurths J. Hierarchical organization unveiled by functional connectivity in complex brain networks. Phys Rev Lett. 2006;97(23):238103. doi: 10.1103/PhysRevLett.97.238103. [DOI] [PubMed] [Google Scholar]