Abstract
Epstein-Barr virus (EBV) latency III infection converts B lymphocytes into lymphoblastoid cell lines (LCLs) by expressing EBV nuclear and membrane proteins, EBNAs, and latent membrane proteins (LMPs), which regulate transcription through Notch and tumor necrosis factor receptor pathways. The role of NF-κB in LMP1 and overall EBV latency III transcriptional effects was investigated by treating LCLs with BAY11-7082 (BAY11). BAY11 rapidly and irreversibly inhibited NF-κB, decreased mitochondrial membrane potential, induced apoptosis, and altered LCL gene expression. BAY11 effects were similar to those of an NF-κB inhibitor, ΔN-IκBα, in effecting decreased JNK1 expression and in microarray analyses. More than 80% of array elements that decreased with ΔN-IκBα expression decreased with BAY11 treatment. Newly identified NF-κB-induced, LMP1-induced, and EBV-induced genes included pleckstrin, Jun-B, c-FLIP, CIP4, and IκBɛ. Of 776 significantly changed array elements, 134 were fourfold upregulated in EBV latency III, and 74 were fourfold upregulated with LMP1 expression alone, whereas only 28 were more than fourfold downregulated by EBV latency III. EBV latency III-regulated gene products mediate cell migration (EBI2, CCR7, RGS1, RANTES, MIP1α, MIP1β, CXCR5, and RGS13), antigen presentation (major histocompatibility complex proteins and JAW1), mitogen-activated protein kinase pathway (DUSP5 and p62Dok), and interferon (IFN) signaling (IFN-γRα, IRF-4, and STAT1). Comparison of EBV latency III LCL gene expression to immunoglobulin M (IgM)-stimulated B cells, germinal-center B cells, and germinal-center-derived lymphomas clustered LCLs with IgM-stimulated B cells separately from germinal-center cells or germinal-center lymphoma cells. Expression of IRF-2, AIM1, ASK1, SNF2L2, and components of IFN signaling pathways further distinguished EBV latency III-infected B cells from IgM-stimulated or germinal-center B cells.
Epstein-Barr virus (EBV) initially establishes latency III infection in B lymphocytes. Latency III infection is characterized by expression of EBV nuclear proteins (EBNA1, -2, -3A, -3B, -3C, and -LP), of integral latent membrane proteins (LMP1, -2A, and -2B), of the BamA rightward transcripts (BARTs), and of small RNAs (EBERs) and by infected cell proliferation. A robust T-lymphocyte immune response eliminates most latency III-infected cells. Subsequently, EBV persists in resting memory B lymphocytes that express EBNA1, LMP2a, EBERs, and BARTs (17, 68, 85). EBNA1 is protected from proteosome degradation and is not presented by major histocompatibility complex (MHC) class I on the infected cell surface, enabling infected cells to evade CD8+ cytotoxic T lymphocytes. High-level T-lymphocyte immunity to latency III-infected B lymphocytes persists for life. In the absence of an effective immune response, infected B lymphocytes can proliferate without restraint and cause malignant lymphoproliferative diseases. EBV-associated lymphoproliferative diseases occur with primary infection after organ transplantation or in previously infected people with profound immune suppression for transplantation or as a consequence of AIDS (reviewed in reference 76).
EBV infection of B lymphocytes in vitro also results in latency III and sustained cell proliferation as lymphoblastoid cell lines (LCLs). EBV reverse genetic analyses in the context of primary B-lymphocyte outgrowth into LCLs indicate that EBNA2, EBNALP, EBNA3A, EBNA3C, and LMP1 are the critical EBV genes for LCL growth and survival. Latency III induces B-lymphocyte proliferation and survival by constitutively activating cellular signaling pathways. EBNA2, -LP, -3A, -3B, and -3C associate with the cellular protein RBP-Jκ/CBF1 and regulate the transcription of promoters that are downstream of Notch receptor signaling, whereas LMP1 associates with tumor necrosis factor receptor-associated factors (TRAFs), tumor necrosis factor receptor-associated death domain protein (TRADD), and receptor-interacting protein (RIP), and activates the NF-κB and stress activated kinase pathways (reviewed in reference 50).
The objective of the studies reported here is to further assess the importance of NF-κB and LMP1 in LCL survival and in the overall effects of latency III-regulated cell gene expression. Mutations of the LMP1 C-terminal TRAF or TRADD/RIP engagement sites render LMP1 ineffective in LCL outgrowth and diminish NF-κB activation (38-40, 46-48). NF-κB inhibition in two LCL cell lines that have been in culture for many years resulted in IB4 LCL apoptosis and sensitization of an LCL to daunorubicin-induced apoptosis (14, 25). Transcripts from 1,405 of 4,146 arrayed cDNAs have been evaluated for differences in abundance in IB4 LCLs versus latency III EBV-infected and uninfected BL41 cells (16).
MATERIALS AND METHODS
Cell lines and antibodies.
IB4 is an in vitro EBV-infected cord blood derived LCLs (35). BL41, BL2, BL30, and Ramos are EBV-negative Burkitt's lymphoma (BL) cell lines. BL41 was infected, in vitro, with the B95-8 type I EBV strain to establish BL41/EBV (5). SUDHL4 and -6 are diffuse large cell lymphomas of a germinal-center-like phenotype, and OCI-LY3 and -10 are diffuse large cell lymphomas of an activated B-cell phenotype (3). LCLs were established by using the B95-8 EBV strain and were used within 4 months of initial outgrowth. IB4 cells with tetracycline (TET)-regulated ΔN-IκBα expression and BL41 cells expressing tTA were grown in complete medium with 1 μg of TET/ml for tTA inactivation and in complete medium without TET for tTA activation (14, 20). Flag-tagged LMP1 cDNA was cloned into pJEF4 (26), transfected into BL41 tTA clone 2B4, and selected with neomycin (0.8 mg/ml). Two clones with regulated LMP1 were isolated (F3 and F10). TET almost abolishes LMP1 expression in these lines and TET withdrawal induces LMP1 expression >25-fold to levels three or four times that of IB4 cells. All lines were maintained in RPMI 1640 media supplemented with 10% fetal calf serum and l-glutamine. TET-regulated cell lines were grown in complete medium with 0.4 mg of hygromycin/ml and 0.5 mg of neomycin/ml. Antibodies to JNK1, phospho-JNK1, p38, and phospho-p38 were purchased from New England Biolabs.
BAY11-7082 treatment of cells.
BAY11-7082 [E-3-(4-methylphenylsulfonyl)-2-propenenitrile; BAY11] was purchased from Calbiochem and reconstituted in dimethyl sulfoxide (DMSO). LCLs in log-phase growth were adjusted to 2.5 × 105 cells per ml. BAY11 or DMSO was added at the indicated concentrations. Cells were washed and lysed in sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer for Western blot analysis. For fluorescence-activated cell sorting (FACS) and confocal analyses for DNA content, cells were fixed in 70% ethanol in phosphate-buffered saline and stained with propidium iodide. Changes in mitochondrial membrane potential (MMP) were assessed by loading cells with 3,3′-dihexyloxacarbocyanine iodide (DiOC6; Molecular Probes) for 30 min, followed by two washes and propidium iodide staining without fixation. Only propidium iodide-negative cells were included in the FACS analyses. CCCP (carbonyl cyanide m-chlorophenylhydrazone; Sigma-Aldrich) was used as a positive control for loss of MMP.
Transcriptional profiling.
Polyadenylated RNAs were selected with FastTrack 2.0 (Invitrogen) or Oligotex (Qiagen) after RNA extraction by using RNAzol (Teltest). In experiment 1, RNAs were collected at the start and at 24 h with or without ΔN-IκBα expression. RNAs were arrayed in comparison to lymphopool RNA, an RNA standard collected from multiple lymphomas (1). The 24-h RNAs with or without ΔN-IκBα expression were also compared directly. In the second experiment, RNAs obtained from cells after 8, 16, or 24 h of induced ΔN-IκBα expression were directly compared to RNAs from control cells manipulated in parallel without ΔN-IκBα expression. RNAs from IB4 cells or LCL4 that were treated with 6 or 3 μM BAY11, respectively, for 8 h were compared to control cultures treated with DMSO. RNAs from BL41 cells that conditionally express LMP1 in response to growth in TET-minus complete medium for 10 or 24 h were directly compared to RNAs from BL41 cells with continuously repressed LMP1 expression. In other experiments, RNAs from IB4, BL2, BL30, RAMOS, BL41, BL41-B95, LCL2, LCL14, SUDHL4 and -6, and OCI-LY3 and -10 cells, resting and immunoglobulin-activated B cells, and purified germinal-center cells were arrayed in comparison to lymphopool RNA. B lymphocytes (>98% pure by flow cytometry) were isolated from peripheral blood mononuclear cells by negative magnetic selection by using StemCell Technologies human B-cell enrichment mixture and cultured for 0, 1, 3, 6, or 24 h at 5 × 106 cells/ml in complete RPMI medium containing 50 μg of anti-immunoglobulin M (IgM; Jackson Laboratories)/ml. Germinal-cells were purified as described previously (58). Probe cDNAs were prepared and hybridized to Lymphochip arrays, and the data were collected and analyzed (1-3).
Analysis.
RNA was considered detected if the signal strength was greater than twice the mean background in both the Cy3 and the Cy5 channels or if there were >1,000 relative fluorescent units in either channel. Q values were determined by analysis using significance analysis of microarray data (SAM) (86). Mean centering and cluster analyses used CLUSTER (24). The data sets referenced to lymphopool RNA were mean centered in groups. Group 1 included IB4 (twice), LCL2, LCL14, BL2, BL30, and RAMOS. Group 2 included duplicate RNAs from BL41and BL41/B958. Group 3 included RNAs from IB4 ΔN-IκBα cells at 0 and 24 h of continuous ΔN-IκBα repression versus 24 h of ΔN-IκBα-induced expression. Group 4 included all of the data from group 1 plus SUDHL4 and -6, OCI-LY3 and -10, purified germinal-center cells (twice), and peripheral blood B cells stimulated with anti-immunoglobulin at 0, 1, 3, 6, and 24 h. When RNAs were directly compared to each other, the resultant data were used without modification. The data are presented in log base 2.
To identify EBV up- and downregulated RNAs, the average of mean centered data for latency III EBV-infected cells was referenced to the average of the mean centered data for BL cells. LMP1 up- and downregulated RNAs were initially identified from a single array (see Fig. 3A, lane 13) in which LMP1 expression was induced for 24 h. EBV identifier genes have an average mean centered value of >2 in latency III EBV-infected cells and <1 in activated B cells, germinal-center lymphocytes, and germinal-center derived lymphomas. Hybridization values were averaged when one gene had multiple cDNA array elements.
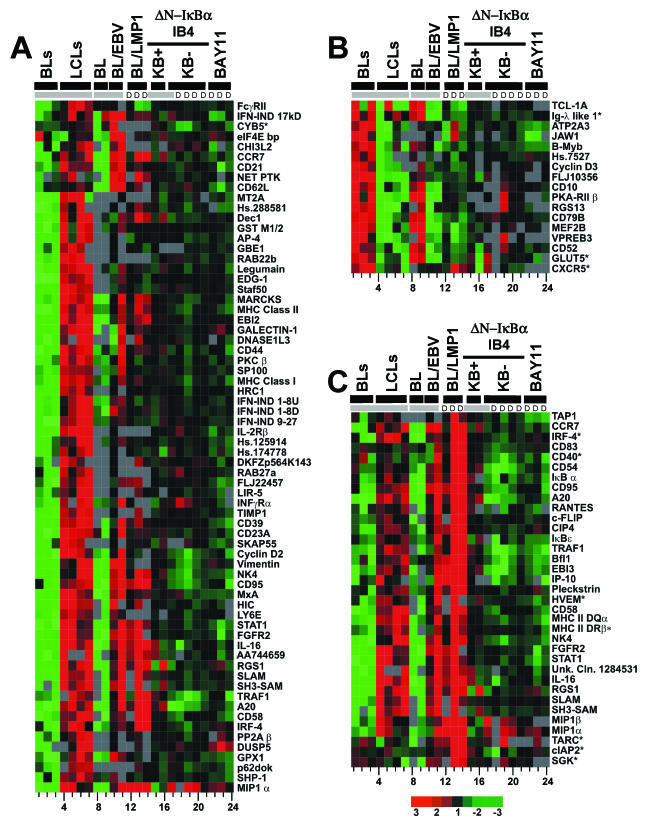
FIG. 3.
Transcriptional profiling by using Lymphochip. (A) Clustering of 776 significantly changed array elements. EBV-induced array elements are indicated by a red bar; EBV-repressed array elements are indicated by a green bar; LMP1-induced array elements are indicated by the yellow and black bar; LMP1-repressed array elements are indicated by a purple and black bar; NF-κB-induced array elements are indicated by a black bar; EBV-induced, LMP1-induced, and NF-κB induced array elements are indicated by a blue bar; EBV-induced, LMP1-induced, and NF-κB-repressed array elements are indicated by a pink bar; and ferritin light-chain array elements are indicated by the aqua bar. (B) 47 EBV-induced, LMP-1 induced and NF-κB induced array elements. For both panels A and B, the lanes were as follows. Lanes 1 to 11 show the results with mRNAs compared to a reference lymphopool mRNA. Lanes 1, 2, and 3 show the findings for Ramos, BL30, and BL2 cells, respectively. Lanes 4 and 5 show the results for the recently established LCL2 and LCL14 lines. Lanes 6 and 7 show the results for IB4, lanes 8 and 9 show the results for BL41, and lanes 10 and 11 show the results for BL41EBV. Lanes 12 and 13 show the findings for mRNAs at 24 h after LMP1 induction. Lane 14 shows the findings for mRNAs at 10 h after induction. Lanes 12 to 14 show a direct comparison to the nonexpressing condition at the same time points. In lanes 15 to 17, mRNAs are compared to a reference lymphopool mRNA. Lane 15 and 16 show results for mRNAs from conditionally expressing ΔN-IκBα IB4 cells with high levels of NF-κB activity, i.e., with ΔN-IκBα repressed for 24 h or from the initiation of the experiment. Lane 17 shows the results for mRNAs from the same cells with low NF-κB activity due to the expression of ΔN-IκBα for 24 h. In lanes 18 to 24, mRNAs from the low-NF-κB condition are directly compared to mRNAs from the high-NF-κB condition. In lanes 18 and 19, the results are for 24 h of ΔN-IκBα expression in IB4 cells; in lanes 20 and 21, the results are for 16 and 8 h of expression, respectively. mRNA abundance was directly compared between BAY11- and DMSO-treated LCLs for 8 h. Lane 22 shows the findings for mRNAs from LCL4 that was treated with 3 μM BAY11. Lanes 23 and 24 show the findings from two independent experiments in which IB4 was treated with 6 μM BAY11. Groups that were arrayed in comparison to lymphopool RNA and then mean centered together are indicated at the top in gray. The letter “D” indicates RNAs are compared directly. cDNAs with an asterisk have Q values between 2 and 5%; those without an asterisk have Q values of <2%.
RESULTS
BAY11-7082 (BAY11) rapidly and irreversibly inhibits NF-κB in LCLs.
To further assess the importance of NF-κB for LCL survival and gene expression, BAY11, an NF-κB inhibitor that prevents tumor necrosis factor-induced IκBα phosphorylation in human umbilical vein endothelial cells and blocks constitutive NF-κB activity in pleural effusion lymphoma cells (49, 73), was used to inhibit NF-κB activity in IB4 and newly derived LCLs. NF-κB inhibition was evident within 5 min of LCL treatment with 5 to 10 μM BAY11 (Fig. 1A, lane 2, data shown for LCL5). Treatment for 1 h was equally effective in inactivating NF-κB for the ensuing 23 h as treatment for 24 h (Fig. 1A, lanes 5 and 6). Thus, BAY11 rapidly and irreversibly inhibits NF-κB in LCLs.
FIG. 1.
(A) BAY11 inhibition of NF-κB in LCLs. Electrophoretic mobility shift assay with cell lysates after treatment for the indicated times with DMSO (lanes 1 and 4) or 10 μM BAY11 (lanes 2, 3, 5, and 6). In lane 6, cells were treated with BAY11 for only 1 h. The asterisk indicates a nonspecific complex. (B) BAY11 slowly downregulates JNK expression in LCLs. LCLs were treated with DMSO or 6 μM BAY11 for 4, 8, or 24 h. Total cell lysates were analyzed by using JNK (top) or phospho-JNK (bottom) antibodies. (C) JNK1 expression is downregulated by ΔN-IκBα. IB4 cells that conditionally express ΔN-IκBα under TET control were induced for 1, 2, or 3 days. Total cell extracts were examined for JNK expression. (D) BAY11 augments p38 activity. Cells were treated as in panel B. Western blot analyses were performed with antibodies to p38 (top) or phospho-p38 (bottom).
BAY11 stimulates p38 and slowly alters JNK1 levels but does not affect JNK1 activity.
Since BAY11 activates JNK1 and p38 in human umbilical vein endothelial cells (73), we assessed whether BAY11 affects JNK1 or p38 kinases in LCLs. JNK1, phospho-JNK1, p38, and phospho-p38 antibodies were used to assess BAY11 effects in two recently transformed LCLs (LCL1 or LCL3) and in IB4 cells (data not shown). Cells in log-phase growth were treated with 0 or 6 μM BAY11 for 4, 8, and 24 h. BAY11 did not affect JNK1 levels or phospho-JNK1 in LCL1, LCL3, and IB4 cells at 4 or 8 h but JNK1 expression was slightly diminished at 24 h. JNK1 expression was similarly decreased 2 days after ΔN-IκBα induction in IB4 LCLs (Fig. 1B and C).
The expression of p38 was not consistently altered over 24 h of treatment of any LCLs (Fig. 1D, lanes 2 and 3, and data not shown). However, phospho-p38 was higher in cells at 4 and 8 h in BAY11-treated cells (Fig. 1D, lanes 2, 3, 5, and 6) than in untreated cells (Fig. 1D, lanes 1 and 4). By 24 h, phospho-p38 was barely detectable in untreated or BAY11-treated cells (Fig. 1D, lanes 7 to 9), a finding consistent with the notion that BAY11 enhances a serum effect on p38 activity (18, 61, 64). Thus, enhanced p38 activity may contribute to BAY11 effects on LCLs.
BAY11 treatment decreased MMP and induced apoptosis.
Decreased MMP as assayed by DiOC6 fluorescence is a prominent manifestation of ΔN-IκBα apoptotic effects at 2 or 3 days after initiation of ΔN-IκBα induction in IB4 LCLs (14). BAY11 treatment at 10 μM had similar effects on the DiOC6 fluorescence of IB4 or newly transformed LCLs at 16 to 24 h (Fig. 2A, data shown for LCL5). Loss of MMP preceded a loss of plasma membrane integrity, as assessed by the lack of propidium iodide staining, and preceded decreased cell volume, as assessed by forward light scatter (Fig. 2A and data not shown). By 24 h, 15% of BAY11-treated cells were sub-2n in DNA content compared to 4% of those treated with DMSO (Fig. 2B). Confocal analysis of 24-h BAY11-treated, fixed, and stained LCLs revealed condensed and fragmented nuclei indicative of apoptosis (Fig. 2C).
FIG. 2.
BAY11 treatment of LCLs causes loss of MMP and apoptosis. (A) LCLs were treated with DMSO or 10 μM BAY11 for 16 h. DiOC6 staining (indicative of MMP) of propidium iodide-negative cells was compared to forward light scatter (indicative of cell size). (B) LCLs were treated with DMSO or 10 μM BAY11 for 24 h, fixed, and stained with propidium iodide for DNA content and analyzed by FACS. (C) A portion of cells from panel B were examined by confocal microscopy.
Transcriptional profiling.
Transcriptional profiling by the Lymphochip cDNA microarray (2) was used to investigate the contribution of both NF-κB and LMP1 to EBV effects on cell gene expression. Three sets of comparisons were made. First, the effects of latency III EBV infection were investigated by comparing RNAs in EBV-negative BL (BL30, BL2, and Ramos) cells with RNAs in EBV-transformed latency III-infected LCLs [IB4 (twice), LCL2, and LCL14]. Another component of this experiment was to compare RNAs in EBV-negative BL (BL41) cells with RNAs in the stable EBV latency III-infected counterpart, BL41/EBV. Second, the effects of conditional LMP1 expression on cell RNAs in BL41 cells were compared to RNAs in BL41. Third, the role of NF-κB in RNA abundance in EBV-transformed latency III-infected LCLs was investigated by comparing RNAs from IB4 LCLs conditionally expressing the dominant IκBα, ΔN-IκBα, with RNAs of the same cells grown under ΔN-IκBα repressed conditions for 0, 8, 16, and 24 h. As a corollary to these latter experiments, RNAs in IB4 and recently established LCL4 cells were compared to RNAs from these LCLs after NF-κB inhibition with BAY11 for 8 h. Changes in gene expression can be compared across the arrays; however, the relative abundance of any array element is only quantitative within the arrays that were mean centered together as indicated by the gray bars above the lanes in Fig. 3 and 4.
FIG. 4.
Genes that change ≥4-fold with EBV infection or LMP1 expression. (A) EBV-induced genes; (B) EBV-repressed genes; (C) LMP1-induced genes. No genes were repressed by LMP1 fourfold. Arrays are arranged as described for Fig. 3. Groups that were arrayed in comparison to lymphopool RNA and then mean centered together are indicated in gray. The letter “D” indicates RNAs were compared directly. cDNAs with an asterisk have Q values of between 2 and 5%; those without an asterisk have Q values of <2%.
Labeled cDNA hybridized to 8,843 of 17,856 cDNA array elements by >2-fold over the background level in at least one experiment and to 3,628 cDNA array elements at 2-fold over the background in at least 70% of the arrays (complete data are available [http://kiefflab.bwh.harvard.edu/]). Using multiclass SAM (86), 776 (Fig. 3A) of the 3,628 elements showed changes accepting a Q value of ≤5% (Q values are the lowest false detection rate at which these genes are significantly different from no change). Cluster analysis (24) based on Pearson correlation coefficients identified EBV latency III-induced genes in LCLs versus BL30, BL2, and Ramos BL cells, EBV latency III-induced genes in BL41, LMP1-induced genes in BL41 cells, and NF-κB induced genes in LCLs (Fig. 3A, blue bar, and Fig. 3B).
Cluster analysis of all of the array elements revealed that RNAs in IB4 LCLs were most similar to recently derived LCLs despite extensive passage of IB4 LCLs over the past 20 years (Fig. 3A, lanes 4 and 5, are RNAs from early-passage LCLs versus lanes 6 and 7, which are RNAs from extensively passaged IB4). This similarity is consistent with IB4 cells still being dependent on EBNA2 interaction with RBP-Jκ for c-myc expression and cell proliferation and on NF-κB activation for cell survival (14, 20). Cell RNAs in the EBV latency III-infected LCLs (Fig. 3A lanes 4 to 7) were also quite similar to cell RNAs in EBV latency III-infected BL41/EBV cells (Fig. 3A, lanes 10 to 11) or LMP1-expressing BL41 cells (Fig. 3A lanes 12 to 14) and differed extensively from RNAs in BL cells (Fig. 3A lanes 1 to 3 and 8 to 9). Thus, latency III EBV infection or merely LMP1 expression substantially alters lymphoblast cell gene expression.
To assess the role of NF-κB activation in EBV-transformed LCL latency III cell RNA abundances, we examined the transcriptional effects of ΔN-IκBα expression on IB4 cells and the effect of BAY11 on both IB4 and recently transformed LCLs. Despite the effect on p38 activation, the transcriptional effects of BAY11 were remarkably similar to those caused by ΔN-IκBα expression. NF-κB inhibition in IB4 LCLs by expression of ΔN-IκBα for 8, 16, or 24 h (Fig. 3A, lanes 18 to 21) was compared to NF-κB inhibition in both IB4 cells and LCL4 by treatment with BAY11 for 8 h (Fig. 3A, lanes 23 and 24 [IB4] and lane 22 [LCL4]). Note that in all lanes labeled D in Fig. 3A, RNAs were directly compared on the same Lymphochip. Overall, two-thirds of the array elements were affected similarly by BAY11 and ΔN-IκB. A total of 424 (55%) array elements decreased and 81 (11%) increased with both treatments. BAY11 treatment at 8 h was most similar to ΔN-IκBα expression at 24 h, reflecting the difference of the kinetics of NF-κB inhibition (Fig. 3A, compare lane 19, which shows ΔN-IκBα induction at 24 h, with lane 24, which shows BAY11 treatment at 8 h). BAY11 treatment (Fig. 3A, lanes 22 to 24) differed from ΔN-IκBα expression (Fig. 3A, lanes 18 to 21) by causing increased ferritin light-chain expression (Fig. 3A, aqua bar). This discrepancy may be due to BAY11-mediated activation of p38.
The greatest decrease in expression levels after NF-κB inhibition occurred for 47 cDNA array elements (Fig. 3B). These array elements were also EBV latency III and LMP1 induced. On average, these cDNAs were 1.6-fold less abundant in LCLs in which NF-κB was inhibited, 4-fold more abundant with LMP1 expression, and 1.9-fold more abundant in EBV latency III-infected cells than in BL cells. These 47 array elements were significantly changed in all of the arrays, with an average Q value (false detection rate) of 1%. The 47 array elements are encoded by 21 unique genes. The abundance of these RNAs began to decrease after only 8 h of ΔN-IκBα induction. Newly identified EBV-induced, LMP1-induced, and NF-κB-induced genes in LCLs include the pleckstrin, Jun-B, c-FLIP, NF-κB1 and -κB2, IκBɛ, and CIP4 genes. Other genes in this cluster have been identified as EBV or LMP1 induced (RANTES, CD54, TRAF1, BFL-1, CD40, EBI3, CD83, CD95, and A20 genes) (22, 23, 54, 84, 92), as NF-κB induced in LCLs (A20, TRAF1, EBI3, CD54, CD95, BFL-1, and c-IAPs genes) (14, 22), or as NF-κB induced in other cells (c-FLIP, Jun-B, c-IAP, CD83, BCL-XL, NF-κB1 and -κB2, and IκBα genes) (4, 8, 12, 19, 36, 37, 57, 59, 63, 65, 66, 89, 96). Many of these genes regulate NF-κB (e.g., IκBα, IκBɛ, and NF-κB1 and -κB2 genes), protect cells from apoptosis (e.g., c-FLIP, A20, c-IAPs, BFL-1, BCL-XL, and TRAF1 genes), or regulate other cellular responses (e.g., RANTES, CD54, CD83, pleckstrin, and CIP4 genes).
NF-κB has a more significant role in EBV regulation of cell gene expression than is evident from consideration of the 47 most highly NF-κB upregulated RNAs. Of all NF-κB-upregulated array elements, 277 were EBV upregulated, and 84 were EBV downregulated. As anticipated, most NF-κB- and EBV-induced array elements were also LMP1 induced (Fig. 3A). NF-κB-induced and EBV-repressed array elements were usually LMP1 repressed (Fig. 3A). Only MIP1α and MIP1β, both EBV- and LMP1-induced genes, showed increased expression with NF-κB inhibition (Fig. 3A, small pink bar, and Fig. 4C).
Of the 776 significantly changed array elements, 480 were EBV latency III induced since they were expressed at higher levels in EBV latency III converted BL cells than in EBV-negative BL cells (Fig. 3A, red bar) and 296 were EBV latency III repressed since they were expressed at lower levels in EBV latency III-converted BL cells than in EBV-negative cells (Fig. 3A, green bar). Overall, the effects were modest. Only 383 array elements were EBV induced more than twofold; of these, 134 were induced more than fourfold. Similarly, only 219 array elements were EBV repressed more than twofold; of these, only 28 were repressed more than fourfold.
The 134 array elements induced by EBV more than fourfold are encoded by 68 genes (Fig. 4A). All 68 genes changes were significant, with Q values of <2%, except for CYB5, which had a Q value of <5% (Fig. 4A). Of these 68 gene changes, 34 were induced by LMP1 and 18 were NF-κB induced. Most other EBV-induced genes are probably upregulated by EBNA2. Many of these 68 genes had been identified as EBV induced by comparison of EBV-positive and -negative BL cells by FACS, previous microarray studies, or by subtractive hybridization; these genes include MIP1α, DNASE1L3, CCR7, EBI2, MARCKS, CD23, CD39, MHC class I and II molecules, A20, TRAF1, CD58, vimentin, CD83, CD95, SHP-1, SLAM, STAT1, CD21, NK4, MXA, and the interferon (IFN)-induced genes 1-8D, 1-8U, and 9-27 (7, 9, 10, 16, 23, 54, 70, 84, 91). Some of the new EBV-induced genes encode components of the IFN pathway (IRF-4 and IFN-γRα), B-lymphocyte receptor-mediated NF-κB activation (PKCβ) (82), tyrosine kinase signaling (SKAP55) (62, 95), mitogen-activated protein kinase signaling (DUSP5 and p62Dok) (44, 52, 94), vesicular transport (RAB27A and RAB22B) (45, 78, 94), or chemotaxis (RGS1) (69, 75).
EBV repressed 28 array elements more than fourfold. These 28 elements were encoded by 17 genes (Fig. 4B). Most genes changes were highly significant, with Q values of <2%; only Ig-λ-like 1, GLUT5, and CXCR5 had Q values of between 2 and 5%. PKA-RIIβ, CD10, and Ig-λ-like 1 had been identified as EBV repressed (16, 92). New EBV-repressed genes included transcription factors B-Myb and MEF2B, cyclin D3, chemokine receptor CXCR5, the GαiGTPase-activating protein RGS13, the B-lymphocyte receptor signaling component CD79B, the Campath1 antigen CD52, and the TAP-independent MHC class I peptide delivery protein JAW1 (Fig. 4B). Genes such as CDC25C, HMG-I, PCNA, RFC, E2F5, ZAP70, SOD1, and 14-3-3ɛ, which have been reported to be EBV repressed more than twofold, were confirmed (data not shown) (7, 16).
LMP1 significantly contributes to EBV effects and induced at least 210 of the 480 EBV-induced array elements (Fig. 3A, yellow striped bars); 161 were induced ≥2-fold. LMP1 also repressed 172 of 296 EBV-repressed array elements (Fig. 3A, purple areas). Only 24 elements were repressed more than twofold, and none were repressed more than fourfold. LMP1 effects were concordant with EBV effects except for FcRγII and Glutaredoxin (Fig. 4A and data not shown), which were EBV induced and LMP1 repressed and CXCR5 (Fig. 4B), which was EBV repressed and LMP1 induced.
LMP1 induced 74 array elements ≥4-fold; these 74 array elements are encoded by 35 genes (Fig. 4C). Newly identified LMP1-induced genes include IRF-4, HVEM, NK4, FGFR2, RGS1, pleckstrin, MIP1β, SLAM, and SH3-SAM. Many of the 35 are known targets of the NF-κB pathway and are NF-κB induced in LCLs (Fig. 4C).
LCLs resemble antigen-activated B lymphoblasts in the expression of adhesion and activation markers. The extent to which LCL gene expression resembles that of antigen-activated B cells was assessed by comparing LCL RNA abundances with those of anti-IgM-stimulated peripheral blood B cells, BL cells, purified germinal-center cells, and four diffuse large cell lymphoma cell lines, two of which have a GC-like phenotype (SUDHLs) and two of which have an activated phenotype (OCI-LYs). Multiclass SAM analysis indicated that 3,399 cDNA array elements were significantly different between cell types, with Q values of <5%. These 3,399 were further characterized by Pearson correlation coefficients by using cluster analysis of both the genes and the arrays. LCLs clustered with IgM-stimulated B cells and were separate from germinal-center cells or B-cell lymphomas with a germinal-center origin (Fig. 5, array tree).
FIG.5.
LCLs are more similar to IgM-stimulated B cells than germinal-center B cells or diffuse large-cell lymphomas (DLCLs). mRNAs abundance for 3,399 cDNAs was compared among LCLs (lanes 1 to 4), IgM-stimulated B cells (lanes 5 to 9), germinal-center B cells (lanes 10 to 11), DLCLs (lanes 12 and 16 to 18), and BL cells (lanes 13 to 15). Cluster analysis determined the order of the arrays shown by the array tree. Two nodes exemplifying the differences between the two main branches of the array tree are shown. (A) Genes highly expressed in LCLs and IgM-stimulated B cells. Genes labeled in pink were identified as EBV induced, genes labeled in orange were identified as part of an activated signature, genes labeled in green were identified as LMP1 induced, genes labeled in purple were identified as EBV and LMP1 induced, genes labeled in black were identified as EBV induced and part of the activated signature, and genes labeled with asterisks were identified as EBV induced, LMP1 induced, and part of the activation signature. (B) Genes expressed at low levels in LCLs and IgM-stimulated B cells. Genes labeled in purple were identified as EBV repressed, genes labeled in blue were identified as part of the germinal-center signature, and genes labeled in black were identified as both EBV repressed and part of the germinal-center signature.
Two nodes, comprising 633 array elements encoded by 440 genes, clearly show the distinction between activated lymphocytes and LCLs versus GC cells (Fig. 5). The first node encompasses genes that are expressed at relatively high level in LCLs and IgM-simulated B cells. Within this group are genes that were previously characterized as differentially expressed in activated versus germinal-center cells (Fig. 5A, orange text), including TCPTP, APR, BCL-2, CYP2a1, PBEF, and Id2 (3). Many EBV-induced genes (Fig. 5A, pink text), LMP1-induced genes (Fig. 5A, green text), and EBV- and LMP1-induced genes (Fig. 5A, purple text) are in this node. Some, such as EBI2, SP100, and PP2Aβ, are activation signature genes and EBV induced (Fig. 5A, black text), and others, such as IRF-4 and interleukin-6, are activation signature genes and both EBV and LMP1 induced (Fig. 5A, asterisks). The second node encompasses genes that are expressed at lower levels in LCLs and IgM-stimulated B cells than in germinal-center B cells or germinal-center-derived lymphomas. TTG-2, PKCδ, A-myb, BCL-7, OGG1, and BCL-7A are germinal-center signature genes that are expressed at low levels in LCLs (Fig. 5B, blue text) (3). Some genes in this cluster, such as MEF2b, CD79B, and GLUT5, were identified as EBV repressed in these studies and are not part of the germinal-center signature (Fig. 5B, purple text), whereas JAW1, CD10, and RGS13 are EBV-repressed and germinal-center signature genes (Fig. 5B, black text). Overall, EBV latency III gene expression is similar to that of IgM-stimulated B cells.
Distinguishers of EBV latency III infection.
The EBV signature overlapped so extensively with the activation cell signature that we used the term “distinguishers” to designate a set of 32 genes that are expressed at high levels in latency III EBV-infected cells and are expressed at low levels in both germinal-center and anti-IgM-stimulated B cells (Fig. 6). We did not find any genes that were uniquely repressed by EBV infection. These EBV distinguishers included 15 genes that were induced more than fourfold by EBV latency III infection relative to BL cells (BL cells have a GC phenotype). These included STAT1, interleukin-2Rβ, NK4, CD39, Galectin-1, MHC class II DP α, DNASE1L3, TIMP-1, IFN-IND, protein 9-27, FcγRII, STAF50, GBE1, FGFR2, FLJ22457, and Legumain. Interestingly, only NK4, RANTES, STAT1, and FGFR2 were LMP1 induced, and only NK4 and RANTES showed any NF-κB induction in these studies.
FIG. 6.
EBV latency III distinguishers. Genes expressed at high levels in LCLs and not in IgM-stimulated B cells or germinal-center cells. Genes identified as EBV induced by ≥2-fold in these studies are indicated. The genes were identified as IFN responsive in a separate study (21). Genes with GTGGGAA within 5 kb 5′ of the transcriptional start site are indicated. cDNAs with an asterisk have a Q value of between 2 and 5%; those without an asterisk have Q values of <2%.
DISCUSSION
In the experiments described here, we treated six recently transformed LCLs with BAY11 to inhibit NF-κB and observed transcriptional and apoptotic effects similar to those previously observed after overexpression of the dominant-positive mutant IkBα in IB4, a long-term LCL (14). All six LCLs were dependent on NF-κB for survival. Further, survival genes were identified that are temporally and critically dependent on NF-κB activity in these LCLs. Thus, NF-κB activation and genes regulated by NF-κB activation are attractive targets for treating EBV-associated lymphoproliferative diseases.
BAY11 caused the rapid and irreversible inhibition of NF-κB, loss of MMP and apoptosis. BAY11 is a propenenitrile that may act by modifying cysteine residues. Since IKKβ-C179 is critical for arsenite inhibition of NF-κB activation, BAY11 effects could be mediated by IKKβ-C179 modification (43).
BAY11-induced apoptosis was similar to ΔN-IκBα-mediated apoptosis. Although BAY11 decreased MMP and induced apoptosis with a condensed time course relative to ΔN-IκBα, the more rapid effect of BAY11 is consistent with its almost immediate effect on NF-κB inhibition versus the 24-h delay in NF-κB inhibition that is necessary for the induction of ΔN-IκBα expression (14). BAY11 was also similar to ΔN-IκBα in inhibiting expression of the antiapoptotic genes CD40, NF-κB2, NF-κB1, A20, BFL-1, BCL-XL, TRAF1, c-FLIP, and c-IAPs (14) (Fig. 4B).
BAY11 did affect stress-activated kinases, transiently increased p38 phosphorylation, and slowly decreased JNK1 expression, but these effects are unlikely to substantively differentiate BAY11-mediated apoptosis from that mediated by IκBα. Bay11 and IκBα had similar transcriptional effects overall (Fig. 3), including effects on JNK1 expression (Fig. 1 and data not shown). Since JNK activity is thought to promote apoptosis (60, 83), decreased JNK1 expression would be anticipated to have a survival effect. With regard to p38, BAY11 inhibits NF-κB and induces apoptosis in pleural effusion lymphoma cells without affecting p38 activity (49). Moreover, in preliminary experiments with BAY11 and a specific p38 inhibitor, SB203580, p38 inhibition did not affect BAY11-mediated apoptosis in LCLs.
These experiments strongly support the hypothesis that LCLs depend on NF-κB for survival. Using transcriptional profiling, we delineated a group of NF-κB-dependent genes that are EBV- and LMP1-induced. LMP1 has oncogene effects and provides survival signals in BL cells (33, 79, 90); the cluster of EBV-induced, LMP1-induced, and NF-κB-dependent genes are excellent candidates for regulators of LCL growth and survival. This cluster (Fig. 4B) includes the antiapoptotic A20, c-FLIP, c-IAP2, BCL-X, and BFL-1 genes. Decreased c-IAP2 likely contributes to caspase-3 activation and decreased c-FLIP to caspase-8 activation. c-IAP2 mRNA levels decreased at 16 h after ΔN-IκBα expression (Fig. 4B, lane 21), whereas c-FLIP mRNA levels decrease at a later time; this likely explains the more immediate effect of ΔN-IκBα expression on caspase-3 activation than on caspase-8 activation (14).
Although NF-κB inhibition by ΔN-IκBα expression results in caspase activation, cells treated with zVAD.fmk still die, indicating that another critical cell death pathway is activated (14). The loss of MMP by both ΔN-IκBα and BAY11 points to a mitochondrial target of NF-κB-mediated protection in LCLs. Consistent with earlier findings (81), MCL1, BCL-2, BCL-X, and BFL-1 were the antiapoptotic BCL-2 family members expressed in the LCL studies here. Both BFL-1 and BCL-X RNAs decreased twofold after 24 h of ΔN-IκBα expression, but BCL-X protein levels did not decrease over 3 days of ΔN-IκBα expression (14).
BAX may be a key effector of apoptosis after NF-κB inhibition. BAX is easily detected by the microarray, whereas BAK is only detected in IB4 cells and not in the BL cells or other LCLs tested (data not shown). NF-κB inhibition resulted in BAX activation as assessed by immunodetection of the amino terminus (data not shown). Furthermore, forced dimerization of BAX causes apoptosis in a manner similar to ΔN-IκBα expression (14, 28). BAX activation after NF-κB inhibition may be mediated by decreased in BFL-1 expression and/or by tBID via the caspase pathway. Decreased BFL-1 expression may liberate BAX directly or by liberating a BH3-only family member, which can activate BAX. BH3-only family members that were detected in LCLs by these arrays included BAD, BID, BIK, and BNIP3 (data not shown). BNIP3 is of potential interest since overexpression causes apoptosis in a caspase-independent manner by effects on the transition pore (87).
In addition to genes that directly inhibit apoptosis, the cluster of EBV-induced, LMP1-induced, and NF-κB-dependent genes included genes that regulate the NF-κB pathway, as well as adhesion, the cytoskeleton, and cytokines. With NF-κB inhibition, LCLs deaggregate due to decreased ICAM1 and LFA-1 expression (22, 91). Decreased integrin signaling may contribute to LCL apoptosis since LCL growth is dependent on cell-cell contact (27). The effects of downregulation of pleckstrin and CIP4 on the LCL cytoskeleton have not been characterized.
NF-κB has an important role in the upregulation of many more genes than are grouped in the EBV- and LMP-induced and NF-κB-dependent mRNA cluster (Fig. 4B). Although these mRNAs showed the greatest decrease in expression after NF-κB inhibition, this reflects both NF-κB dependence and a shorter mRNA half-life. Most if not all RNAs in this cluster have half-lives of <2 h, with the JUN-B RNA half-life being as short as 11 min (55, 74). Given their dynamic regulation, the proteins of this cluster are likely to be particularly important in regulating cell survival, but other equally NF-κB-dependent, prosurvival mRNAs may simply have longer half-lives; some of these may encode less-stable proteins.
EBV-induced changes in gene expression are largely mediated by LMP1 and EBNA2. Here we assess directly the ability of LMP1 and EBV to change cell gene expression in a BL background. LMP1 expression in BL41 cells induced changes in gene expression of a greater magnitude than were evident with stable latency III EBV infection in BL cells. In part this may be due to three- to fivefold overexpression of LMP1 in the BL41 cells at 24 h after induction. Nevertheless, the effects were concordant with EBV infection, and overall, most of the gene changes observed in the entire array could be attributed to LMP1 expression in the absence of all of the other latency proteins. However, if we examine the genes that are induced more than fourfold by EBV latency III infection, many were unaffected by LMP1 expression and are likely EBNA2 induced. Or, like CD23 (92), other EBV-induced genes may require both EBNA2 and LMP1 for maximal expression.
The similarity between antigen-activated B lymphocytes and EBV latency III infection is further evidence that EBV has evolved to mimic normal B-cell activation and proliferation. EBNA2 drives cell proliferation through c-Myc activation (20, 42), whereas LMP1 and NF-κB engender an antiapoptotic state that allows cell survival (14) (Fig. 2). EBV latency III-infected cells may alter migration through the induction of the chemokine receptors, EBI2 and EBI1/CCR7, and repression of CXCR5 (9) (Fig. 4). These changes may redirect the homing of LCLs away from the B-cell zone of germinal centers (71). Latency III infection, by mimicking antigen activation, may enable the survival of the infected cell and thereby permit differentiation toward latency I and a memory cell phenotype, independent of antigen stimulation or the germinal-center reaction (6).
EBV latency III infection increases MHC-based antigen presentation to ensure the limited proliferation and timely elimination of excess latency III-infected cells by cytotoxic T cells. Latency III is associated with a switch from proteosome-independent to proteosome-dependent antigen processing and presentation, as well as high-level MHC class I and II expression. EBV infection induced MHC class I and II mRNAs fourfold and β2-microglobulin and LMP2 proteosome subunit mRNAs two- to threefold (data not shown), and repressed JAW1 mRNA fourfold; JAW1 delivers peptides to the class I pathway independent of TAP proteins (80). Thus, EBV infection, LMP1 expression, and NF-κB activation provide survival signals engender immune clearance of latency III cells and likely enable the transition into latency I-infected B cells.
The extent that EBV is similar to antigen-activated B cells reflects LMP1 mimicking of CD40 signaling and, to a limited extent, LMP2a mimicking of BCR signaling (13, 15, 31, 51, 67). However, of the EBV latency III distinguishers, only NK4, RANTES, STAT1, and FGFR2 were LMP1 induced. EBV latency III most obviously differs from antigen-activated cells because the Notch and PU.1 pathways are constitutively activated or regulated by EBNA2, -LP, -3A, and -3C (29, 30, 34, 41, 53, 56, 77, 88, 98, 99). Therefore, genes expressed at high levels in LCLs and not in anti-immunoglobulin G-stimulated peripheral B cells or GC cells are likely to be regulated by the EBNAs. EBNA2 associates with RBP-Jκ, and 15 of the 32 genes in the EBV latency III distinguisher group have RBP-Jκ binding sites within 5 kb upstream of their transcriptional start sites (Fig. 6).
LCLs also differ from antigen activated B cells by the expression of alpha IFN (IFN-α), IFN-β, and IFN-γ (11, 16, 93; data not shown). This may be a reminder of the importance of the presence of the EBV genome, which encodes RNAs from both DNA strands in latency III EBV infection (32, 72). EBV latency III distinguishers that may be downstream of IFN induction include IRF-2, PML, MxB, RANTES, STAT1, MHC class II proteins, IFN-IND 9-27, and STAF50 (21). We also note that EBV induced IFN-γRα, STAT1, tyk2, and IRF-4, all of which may contribute to the increase IFN effects (7, 16).
Acknowledgments
We thank E. Cesarman for sharing unpublished data regarding the use of BAY11 as an NF-κB inhibitor in the LCLs and members of the Kieff Lab for their input, particularly Eric Johannsen, Micah Luftig, Jimmy Duong, and Bo Zhao.
E.D.C.-M., K.C., and E.K. were supported by PHS grants CA87661, CA47006, and CA85180. E.D.C.-M. is a Special Fellow of the Leukemia and Lymphoma Society (LLS-3499).
REFERENCES
- 1.Alizadeh, A., M. Eisen, D. Botstein, P. O. Brown, and L. M. Staudt. 1998. Probing lymphocyte biology by genomic-scale gene expression analysis. J. Clin. Immunol. 18:373-379. [DOI] [PubMed] [Google Scholar]
- 2.Alizadeh, A., M. Eisen, R. E. Davis, C. Ma, H. Sabet, T. Tran, J. I. Powell, L. Yang, G. E. Marti, D. T. Moore, J. R. Hudson, Jr., W. C. Chan, T. Greiner, D. Weisenburger, J. O. Armitage, I. Lossos, R. Levy, D. Botstein, P. O. Brown, and L. M. Staudt. 1999. The Lymphochip: a specialized cDNA microarray for the genomic-scale analysis of gene expression in normal and malignant lymphocytes. Cold Spring Harbor Symp. Quant. Biol. 64:71-78. [DOI] [PubMed] [Google Scholar]
- 3.Alizadeh, A. A., M. B. Eisen, R. E. Davis, C. Ma, I. S. Lossos, A. Rosenwald, J. C. Boldrick, H. Sabet, T. Tran, X. Yu, J. I. Powell, L. Yang, G. E. Marti, T. Moore, J. Hudson, Jr., L. Lu, D. B. Lewis, R. Tibshirani, G. Sherlock, W. C. Chan, T. C. Greiner, D. D. Weisenburger, J. O. Armitage, R. Warnke, L. M. Staudt, et al. 2000. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 403:503-511. [DOI] [PubMed] [Google Scholar]
- 4.Ardeshna, K. M., A. R. Pizzey, S. Devereux, and A. Khwaja. 2000. The PI3 kinase, p38 SAP kinase, and NF-κB signal transduction pathways are involved in the survival and maturation of lipopolysaccharide-stimulated human monocyte-derived dendritic cells. Blood 96:1039-1046. [PubMed] [Google Scholar]
- 5.Avila-Carino, J., S. Torsteinsdottir, B. Ehlin-Henriksson, G. Lenoir, G. Klein, E. Klein, and M. G. Masucci. 1987. Paired Epstein-Barr virus (EBV)-negative and EBV-converted Burkitt lymphoma lines: stimulatory capacity in allogeneic mixed lymphocyte cultures. Int. J. Cancer 40:691-697. [DOI] [PubMed] [Google Scholar]
- 6.Babcock, G. J., L. L. Decker, M. Volk, and D. A. Thorley-Lawson. 1998. EBV persistence in memory B cells in vivo. Immunity 9:395-404. [DOI] [PubMed] [Google Scholar]
- 7.Baran-Marszak, F., R. Fagard, B. Girard, S. Camilleri-Broet, F. Zeng, G. M. Lenoir, M. Raphael, and J. Feuillard. 2002. Gene array identification of Epstein-Barr virus-regulated cellular genes in EBV-converted Burkitt lymphoma cell lines. Lab. Investig. 82:1463-1479. [DOI] [PubMed] [Google Scholar]
- 8.Berchtold, S., P. Muhl-Zurbes, E. Maczek, A. Golka, G. Schuler, and A. Steinkasserer. 2002. Cloning and characterization of the promoter region of the human CD83 gene. Immunobiology 205:231-246. [DOI] [PubMed] [Google Scholar]
- 9.Birkenbach, M., K. Josefsen, R. Yalamanchili, G. Lenoir, and E. Kieff. 1993. Epstein-Barr virus-induced genes: first lymphocyte-specific G protein-coupled peptide receptors. J. Virol. 67:2209-2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birkenbach, M., D. Liebowitz, F. Wang, J. Sample, and E. Kieff. 1989. Epstein-Barr virus latent infection membrane protein increases vimentin expression in human B-cell lines. J. Virol. 63:4079-4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brewster, F. E., and J. L. Sullivan. 1983. Epstein-Barr virus-infected B lymphoblastoid cell lines: dynamics of interferon and 2′5′-oligoadenylate synthetase activity. Antivir. Res. 3:195-209. [DOI] [PubMed] [Google Scholar]
- 12.Brown, R. T., I. Z. Ades, and R. P. Nordan. 1995. An acute phase response factor/NF-κB site downstream of the junB gene that mediates responsiveness to interleukin-6 in a murine plasmacytoma. J. Biol. Chem. 270:31129-31135. [DOI] [PubMed] [Google Scholar]
- 13.Busch, L. K., and G. A. Bishop. 1999. The EBV transforming protein, latent membrane protein 1, mimics and cooperates with CD40 signaling in B lymphocytes. J. Immunol. 162:2555-2561. [PubMed] [Google Scholar]
- 14.Cahir-McFarland, E. D., D. M. Davidson, S. L. Schauer, J. Duong, and E. Kieff. 2000. NF-kappa B inhibition causes spontaneous apoptosis in Epstein-Barr virus-transformed lymphoblastoid cells. Proc. Natl. Acad. Sci. USA 97:6055-6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caldwell, R. G., J. B. Wilson, S. J. Anderson, and R. Longnecker. 1998. Epstein-Barr virus LMP2A drives B-cell development and survival in the absence of normal B-cell receptor signals. Immunity 9:405-411. [DOI] [PubMed] [Google Scholar]
- 16.Carter, K. L., E. Cahir-McFarland, and E. Kieff. 2002. Epstein-Barr virus-induced changes in B-lymphocyte gene expression. J. Virol. 76:10427-10436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen, H., P. Smith, R. F. Ambinder, and S. D. Hayward. 1999. Expression of Epstein-Barr virus BamHI-A rightward transcripts in latently infected B cells from peripheral blood. Blood 93:3026-3032. [PubMed] [Google Scholar]
- 18.Cheng, H. L., and E. L. Feldman. 1998. Bidirectional regulation of p38 kinase and c-Jun N-terminal protein kinase by insulin-like growth factor-I. J. Biol. Chem. 273:14560-14565. [DOI] [PubMed] [Google Scholar]
- 19.Cogswell, P. C., R. I. Scheinman, and A. S. Baldwin, Jr. 1993. Promoter of the human NF-κB p50/p105 gene: regulation by NF-κB subunits and by c-REL. J. Immunol. 150:2794-2804. [PubMed] [Google Scholar]
- 20.Cooper, A., E. Johannsen, S. Maruo, E. Cahir-McFarland, D. Illanes, D. Davidson, and E. Kieff. 2003. EBNA3A association with RBP-Jκ down-regulates c-myc and Epstein-Barr virus-transformed lymphoblast growth. J. Virol. 77:999-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Veer, M. J., M. Holko, M. Frevel, E. Walker, S. Der, J. M. Paranjape, R. H. Silverman, and B. R. Williams. 2001. Functional classification of interferon-stimulated genes identified using microarrays. J. Leukoc. Biol. 69:912-920. [PubMed] [Google Scholar]
- 22.Devergne, O., E. Cahir-McFarland, G. Mosialos, K. M. Izumi, C. F. Ware, and E. Kieff. 1998. Role of the TRAF binding site and NF-κB activation in Epstein-Barr virus latent membrane protein 1-induced cell gene expression. J. Virol. 72:7900-7908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dudziak, D., A. Kieser, U. Dirmeier, F. Nimmerjahn, S. Berchtold, A. Steinkasserer, G. Marschall, W. Hammerschmidt, G. Laux, and G. W. Bornkamm. 2003. Latent membrane protein 1 of Epstein-Barr virus induces CD83 by the NF-κB signaling pathway. J. Virol. 77:8290-8298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eisen, M. B., P. T. Spellman, P. O. Brown, and D. Botstein. 1998. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. USA 95:14863-14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feuillard, J., M. Schuhmacher, S. Kohanna, M. Asso-Bonnet, F. Ledeur, R. Joubert-Caron, P. Bissieres, A. Polack, G. W. Bornkamm, and M. Raphael. 2000. Inducible loss of NF-κB activity is associated with apoptosis and Bcl-2 down-regulation in Epstein-Barr virus-transformed B lymphocytes. Blood 95:2068-2075. [PubMed] [Google Scholar]
- 26.Floettmann, J. E., K. Ward, A. B. Rickinson, and M. Rowe. 1996. Cytostatic effect of Epstein-Barr virus latent membrane protein-1 analyzed using tetracycline-regulated expression in B-cell lines. Virology 223:29-40. [DOI] [PubMed] [Google Scholar]
- 27.Frisch, S. M., and E. Ruoslahti. 1997. Integrins and anoikis. Curr. Opin. Cell Biol. 9:701-706. [DOI] [PubMed] [Google Scholar]
- 28.Gross, A., J. Jockel, M. C. Wei, and S. J. Korsmeyer. 1998. Enforced dimerization of BAX results in its translocation, mitochondrial dysfunction, and apoptosis. EMBO J. 17:3878-3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grossman, S. R., E. Johannsen, X. Tong, R. Yalamanchili, and E. Kieff. 1994. The Epstein-Barr virus nuclear antigen 2 transactivator is directed to response elements by the Jκ recombination ignal binding protein. Proc. Natl. Acad. Sci. USA 91:7568-7572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harada, S., and E. Kieff. 1997. Epstein-Barr virus nuclear protein LP stimulates EBNA-2 acidic domain-mediated transcriptional activation. J. Virol. 71:6611-6618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hatzivassiliou, E., W. E. Miller, N. Raab-Traub, E. Kieff, and G. Mosialos. 1998. A fusion of the EBV latent membrane protein-1 (LMP1) transmembrane domains to the CD40 cytoplasmic domain is similar to LMP1 in constitutive activation of epidermal growth factor receptor expression, nuclear factor-κB, and stress-activated protein kinase. J. Immunol. 160:1116-1121. [PubMed] [Google Scholar]
- 32.Hayward, S. D., and E. D. Kieff. 1976. Epstein-Barr virus-specific RNA. I. Analysis of viral RNA in cellular extracts and in the polyribosomal fraction of permissive and nonpermissive lymphoblastoid cell lines. J. Virol. 18:518-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henderson, S., M. Rowe, C. Gregory, D. Croom-Carter, F. Wang, R. Longnecker, E. Kieff, and A. Rickinson. 1991. Induction of bcl-2 expression by Epstein-Barr virus latent membrane protein 1 protects infected B cells from programmed cell death. Cell 65:1107-1115. [DOI] [PubMed] [Google Scholar]
- 34.Henkel, T., P. D. Ling, S. D. Hayward, and M. G. Peterson. 1994. Mediation of Epstein-Barr virus EBNA2 transactivation by recombination signal-binding protein Jκ. Science 265:92-95. [DOI] [PubMed] [Google Scholar]
- 35.Hurley, E. A., L. D. Klaman, S. Agger, J. B. Lawrence, and D. A. Thorley-Lawson. 1991. The prototypical Epstein-Barr virus-transformed lymphoblastoid cell line IB4 is an unusual variant containing integrated but no episomal viral DNA. J. Virol. 65:3958-3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ito, C. Y., N. Adey, V. L. Bautch, and A. S. Baldwin, Jr. 1995. Structure and evolution of the human IKBA gene. Genomics 29:490-495. [DOI] [PubMed] [Google Scholar]
- 37.Ito, C. Y., A. G. Kazantsev, and A. S. Baldwin, Jr. 1994. Three NF-κB sites in the IκB-alpha promoter are required for induction of gene expression by TNF alpha. Nucleic Acids Res. 22:3787-3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Izumi, K. M., E. Cahir-McFarland, A. T. Ting, E. A. Riley, B. Seed, and E. D. Kieff. 1999. The Epstein-Barr virus oncoprotein latent membrane protein 1 engages the tumor necrosis factor receptor-associated proteins TRADD and receptor-interacting protein (RIP) but does not induce apoptosis or require RIP for NF-κB activation. Mol. Cell. Biol. 19:5759-5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Izumi, K. M., K. M. Kaye, and E. D. Kieff. 1997. The Epstein-Barr virus LMP1 amino acid sequence that engages tumor necrosis factor receptor associated factors is critical for primary B lymphocyte growth transformation. Proc. Natl. Acad. Sci. USA 94:1447-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Izumi, K. M., and E. D. Kieff. 1997. The Epstein-Barr virus oncogene product latent membrane protein 1 engages the tumor necrosis factor receptor-associated death domain protein to mediate B lymphocyte growth transformation and activate NF-κB. Proc. Natl. Acad. Sci. USA 94:12592-12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johannsen, E., C. L. Miller, S. R. Grossman, and E. Kieff. 1996. EBNA-2 and EBNA-3C extensively and mutually exclusively associate with RBPJκ in Epstein-Barr virus-transformed B lymphocytes. J. Virol. 70:4179-4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaiser, C., G. Laux, D. Eick, N. Jochner, G. W. Bornkamm, and B. Kempkes. 1999. The proto-oncogene c-myc is a direct target gene of Epstein-Barr virus nuclear antigen 2. J. Virol. 73:4481-4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kapahi, P., T. Takahashi, G. Natoli, S. R. Adams, Y. Chen, R. Y. Tsien, and M. Karin. 2000. Inhibition of NF-κB activation by arsenite through reaction with a critical cysteine in the activation loop of IκB kinase. J. Biol. Chem. 275:36062-36066. [DOI] [PubMed] [Google Scholar]
- 44.Kashige, N., N. Carpino, and R. Kobayashi. 2000. Tyrosine phosphorylation of p62dok by p210bcr-abl inhibits RasGAP activity. Proc. Natl. Acad. Sci. USA 97:2093-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kauppi, M., A. Simonsen, B. Bremnes, A. Vieira, J. Callaghan, H. Stenmark, and V. M. Olkkonen. 2002. The small GTPase Rab22 interacts with EEA1 and controls endosomal membrane trafficking. J. Cell Sci. 115:899-911. [DOI] [PubMed] [Google Scholar]
- 46.Kaye, K. M., K. M. Izumi, and E. Kieff. 1993. Epstein-Barr virus latent membrane protein 1 is essential for B-lymphocyte growth transformation. Proc. Natl. Acad. Sci. USA 90:9150-9154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaye, K. M., K. M. Izumi, H. Li, E. Johannsen, D. Davidson, R. Longnecker, and E. Kieff. 1999. An Epstein-Barr virus that expresses only the first 231 LMP1 amino acids efficiently initiates primary B-lymphocyte growth transformation. J. Virol. 73:10525-10530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaye, K. M., K. M. Izumi, G. Mosialos, and E. Kieff. 1995. The Epstein-Barr virus LMP1 cytoplasmic carboxy terminus is essential for B-lymphocyte transformation: fibroblast cocultivation complements a critical function within the terminal 155 residues. J. Virol. 69:675-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Keller, S. A., E. J. Schattner, and E. Cesarman. 2000. Inhibition of NF-κB induces apoptosis of KSHV-infected primary effusion lymphoma cells. Blood 96:2537-2542. [PubMed] [Google Scholar]
- 50.Kieff, E., and A. B. Rickinson. 2001. Epstein-Barr virus and its replication, p. 2511-2574. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott/The Williams & Wilkins Co., Philadelphia, Pa.
- 51.Kilger, E., A. Kieser, M. Baumann, and W. Hammerschmidt. 1998. Epstein-Barr virus-mediated B-cell proliferation is dependent upon latent membrane protein 1, which simulates an activated CD40 receptor. EMBO J. 17:1700-1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kovanen, P. E., A. Rosenwald, J. Fu, E. M. Hurt, L. T. Lam, J. M. Giltnane, G. Wright, L. M. Staudt, and W. J. Leonard. 2003. Analysis of gamma c-family cytokine target genes. Identification of dual-specificity phosphatase 5 (DUSP5) as a regulator of mitogen-activated protein kinase activity in interleukin-2 signaling. J. Biol. Chem. 278:5205-5213. [DOI] [PubMed] [Google Scholar]
- 53.Krauer, K. G., N. Kienzle, D. B. Young, and T. B. Sculley. 1996. Epstein-Barr nuclear antigen-3 and -4 interact with RBP-2N, a major isoform of RBP-Jκ in B lymphocytes. Virology 226:346-353. [DOI] [PubMed] [Google Scholar]
- 54.Laherty, C. D., H. M. Hu, A. W. Opipari, F. Wang, and V. M. Dixit. 1992. The Epstein-Barr virus LMP1 gene product induces A20 zinc finger protein expression by activating nuclear factor κB. J. Biol. Chem. 267:24157-24160. [PubMed] [Google Scholar]
- 55.Lam, L. T., O. K. Pickeral, A. C. Peng, A. Rosenwald, E. M. Hurt, J. M. Giltnane, L. M. Averett, H. Zhao, R. E. Davis, M. Sathyamoorthy, L. M. Wahl, E. D. Harris, J. A. Mikovits, A. P. Monks, M. G. Hollingshead, E. A. Sausville, and L. M. Staudt. 2001. Genomic-scale measurement of mRNA turnover and the mechanisms of action of the anti-cancer drug flavopiridol. Genome Biol. 2:(10):research0041.1-0041.11. [Online.] http://genomebiology.com. [DOI] [PMC free article] [PubMed]
- 56.Laux, G., B. Adam, L. J. Strobl, and F. Moreau-Gachelin. 1994. The Spi-1/PU. 1 and Spi-B ets family transcription factors and the recombination signal binding protein RBP-Jκ interact with an Epstein-Barr virus nuclear antigen 2 responsive cis-element. EMBO J. 13:5624-5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee, H. H., H. Dadgostar, Q. Cheng, J. Shu, and G. Cheng. 1999. NF-κB-mediated up-regulation of Bcl-x and BFL-1/A1 is required for CD40 survival signaling in B lymphocytes. Proc. Natl. Acad. Sci. USA 96:9136-9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu, Y. J., and J. Banchereau. 1996. Human peripheral B cell subsets, p. 93.1-93.9. In D. Weir, C. Blackwell, L. Herzenberg, and L. Herzenberg (ed.), Handbook of experimental immunology. Blackwell Scientific, Oxford, England.
- 59.Lombardi, L., P. Ciana, C. Cappellini, D. Trecca, L. Guerrini, A. Migliazza, A. T. Maiolo, and A. Neri. 1995. Structural and functional characterization of the promoter regions of the NFKB2 gene. Nucleic Acids Res. 23:2328-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maeda, S., L. Chang, Z. W. Li, J. L. Luo, H. Leffert, and M. Karin. 2003. IΚKβ is required for prevention of apoptosis mediated by cell-bound but not by circulating TNF-α. Immunity 19:725-737. [DOI] [PubMed] [Google Scholar]
- 61.Maher, P. 1999. p38 mitogen-activated protein kinase activation is required for fibroblast growth factor-2-stimulated cell proliferation but not differentiation. J. Biol. Chem. 274:17491-17498. [DOI] [PubMed] [Google Scholar]
- 62.Marie-Cardine, A., E. Bruyns, C. Eckerskorn, H. Kirchgessner, S. C. Meuer, and B. Schraven. 1997. Molecular cloning of SKAP55, a novel protein that associates with the protein tyrosine kinase p59fyn in human T-lymphocytes. J. Biol. Chem. 272:16077-16080. [DOI] [PubMed] [Google Scholar]
- 63.Mathas, S., M. Hinz, I. Anagnostopoulos, D. Krappmann, A. Lietz, F. Jundt, K. Bommert, F. Mechta-Grigoriou, H. Stein, B. Dorken, and C. Scheidereit. 2002. Aberrantly expressed c-Jun and JunB are a hallmark of Hodgkin lymphoma cells, stimulate proliferation and synergize with NF-κB. EMBO J. 21:4104-4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Matsumoto, T., K. Yokote, K. Tamura, M. Takemoto, H. Ueno, Y. Saito, and S. Mori. 1999. Platelet-derived growth factor activates p38 mitogen-activated protein kinase through a Ras-dependent pathway that is important for actin reorganization and cell migration. J. Biol. Chem. 274:13954-13960. [DOI] [PubMed] [Google Scholar]
- 65.McKinsey, T. A., Z. Chu, T. F. Tedder, and D. W. Ballard. 2000. Transcription factor NF-κB regulates inducible CD83 gene expression in activated T lymphocytes. Mol. Immunol. 37:783-788. [DOI] [PubMed] [Google Scholar]
- 66.Micheau, O., S. Lens, O. Gaide, K. Alevizopoulos, and J. Tschopp. 2001. NF-κB signals induce the expression of c-FLIP. Mol. Cell. Biol. 21:5299-5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miller, C. L., A. L. Burkhardt, J. H. Lee, B. Stealey, R. Longnecker, J. B. Bolen, and E. Kieff. 1995. Integral membrane protein 2 of Epstein-Barr virus regulates reactivation from latency through dominant-negative effects on protein-tyrosine kinases. Immunity 2:155-166. [DOI] [PubMed] [Google Scholar]
- 68.Miyashita, E. M., B. Yang, G. J. Babcock, and D. A. Thorley-Lawson. 1997. Identification of the site of Epstein-Barr virus persistence in vivo as a resting B-cell. J. Virol. 71:4882-4891. (Erratum, 72:9419, 1998.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moratz, C., V. H. Kang, K. M. Druey, C. S. Shi, A. Scheschonka, P. M. Murphy, T. Kozasa, and J. H. Kehrl. 2000. Regulator of G protein signaling 1 (RGS1) markedly impairs Gi alpha signaling responses of B lymphocytes. J. Immunol. 164:1829-1838. [DOI] [PubMed] [Google Scholar]
- 70.Mosialos, G., M. Birkenbach, R. Yalamanchili, T. VanArsdale, C. Ware, and E. Kieff. 1995. The Epstein-Barr virus transforming protein LMP1 engages signaling proteins for the tumor necrosis factor receptor family. Cell 80:389-399. [DOI] [PubMed] [Google Scholar]
- 71.Muller, G., U. E. Hopken, and M. Lipp. 2003. The impact of CCR7 and CXCR5 on lymphoid organ development and systemic immunity. Immunol. Rev. 195:117-135. [DOI] [PubMed] [Google Scholar]
- 72.Orellana, T., and E. Kieff. 1977. Epstein-Barr virus-specific RNA. II. Analysis of polyadenylated viral RNA in restringent, abortive, and productive infections. J. Virol. 22:321-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pierce, J. W., R. Schoenleber, G. Jesmok, J. Best, S. A. Moore, T. Collins, and M. E. Gerritsen. 1997. Novel inhibitors of cytokine-induced IκBα phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J. Biol. Chem. 272:21096-21103. [DOI] [PubMed] [Google Scholar]
- 74.Raghavan, A., R. L. Ogilvie, C. Reilly, M. L. Abelson, S. Raghavan, J. Vasdewani, M. Krathwohl, and P. R. Bohjanen. 2002. Genome-wide analysis of mRNA decay in resting and activated primary human T lymphocytes. Nucleic Acids Res. 30:5529-5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reif, K., and J. G. Cyster. 2000. RGS molecule expression in murine B lymphocytes and ability to down-regulate chemotaxis to lymphoid chemokines. J. Immunol. 164:4720-4729. [DOI] [PubMed] [Google Scholar]
- 76.Rickinson, A., and E. Kieff. 2001. Epstein-Barr virus, p. 2575-2628. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott/The Williams & Wilkins Co., Philadelphia, Pa.
- 77.Robertson, E. S., J. Lin, and E. Kieff. 1996. The amino-terminal domains of Epstein-Barr virus nuclear proteins 3A, 3B, and 3C interact with RBPJ(kappa). J. Virol. 70:3068-3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rodriguez-Gabin, A. G., M. Cammer, G. Almazan, M. Charron, and J. N. Larocca. 2001. Role of rRAB22b, an oligodendrocyte protein, in regulation of transport of vesicles from trans Golgi to endocytic compartments. J. Neurosci. Res. 66:1149-1160. [DOI] [PubMed] [Google Scholar]
- 79.Rowe, M., M. Peng-Pilon, D. S. Huen, R. Hardy, D. Croom-Carter, E. Lundgren, and A. B. Rickinson. 1994. Upregulation of bcl-2 by the Epstein-Barr virus latent membrane protein LMP1: a B-cell-specific response that is delayed relative to NF-κB activation and to induction of cell surface markers. J. Virol. 68:5602-5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Snyder, H. L., I. Bacik, J. R. Bennink, G. Kearns, T. W. Behrens, T. Bachi, M. Orlowski, and J. W. Yewdell. 1997. Two novel routes of transporter associated with antigen processing (TAP)-independent major histocompatibility complex class I antigen processing. J. Exp. Med. 186:1087-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Spender, L. C., E. J. Cannell, M. Hollyoake, B. Wensing, J. M. Gawn, M. Brimmell, G. Packham, and P. J. Farrell. 1999. Control of cell cycle entry and apoptosis in B lymphocytes infected by Epstein-Barr virus. J. Virol. 73:4678-4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Su, T. T., B. Guo, Y. Kawakami, K. Sommer, K. Chae, L. A. Humphries, R. M. Kato, S. Kang, L. Patrone, R. Wall, M. Teitell, M. Leitges, T. Kawakami, and D. J. Rawlings. 2002. PKC-beta controls I kappa B kinase lipid raft recruitment and activation in response to BCR signaling. Nat. Immunol. 3:780-786. [DOI] [PubMed] [Google Scholar]
- 83.Tang, G., Y. Minemoto, B. Dibling, N. H. Purcell, Z. Li, M. Karin, and A. Lin. 2001. Inhibition of JNK activation through NF-κB target genes. Nature 414:313-317. [DOI] [PubMed] [Google Scholar]
- 84.Teruya-Feldstein, J., E. S. Jaffe, P. R. Burd, D. W. Kingma, J. E. Setsuda, and G. Tosato. 1999. Differential chemokine expression in tissues involved by Hodgkin's disease: direct correlation of eotaxin expression and tissue eosinophilia. Blood 93:2463-2470. [PubMed] [Google Scholar]
- 85.Tierney, R. J., N. Steven, L. S. Young, and A. B. Rickinson. 1994. Epstein-Barr virus latency in blood mononuclear cells: analysis of viral gene transcription during primary infection and in the carrier state. J. Virol. 68:7374-7385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tusher, V. G., R. Tibshirani, and G. Chu. 2001. Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. USA 98:5116-5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vande Velde, C., J. Cizeau, D. Dubik, J. Alimonti, T. Brown, S. Israels, R. Hakem, and A. H. Greenberg. 2000. BNIP3 and genetic control of necrosis-like cell death through the mitochondrial permeability transition pore. Mol. Cell. Biol. 20:5454-5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Waltzer, L., F. Logeat, C. Brou, A. Israel, A. Sergeant, and E. Manet. 1994. The human Jκ recombination signal sequence binding protein (RBP-Jκ) targets the Epstein-Barr virus EBNA2 protein to its DNA responsive elements. EMBO J. 13:5633-5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang, C. Y., M. W. Mayo, R. G. Korneluk, D. V. Goeddel, and A. S. Baldwin, Jr. 1998. NF-κB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 281:1680-1683. [DOI] [PubMed] [Google Scholar]
- 90.Wang, D., D. Liebowitz, and E. Kieff. 1985. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell 43:831-840. [DOI] [PubMed] [Google Scholar]
- 91.Wang, D., D. Liebowitz, F. Wang, C. Gregory, A. Rickinson, R. Larson, T. Springer, and E. Kieff. 1988. Epstein-Barr virus latent infection membrane protein alters the human B-lymphocyte phenotype: deletion of the amino terminus abolishes activity. J. Virol. 62:4173-4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang, F., C. Gregory, C. Sample, M. Rowe, D. Liebowitz, R. Murray, A. Rickinson, and E. Kieff. 1990. Epstein-Barr virus latent membrane protein (LMP1) and nuclear proteins 2 and 3C are effectors of phenotypic changes in B lymphocytes: EBNA-2 and LMP1 cooperatively induce CD23. J. Virol. 64:2309-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wickramasinghe, S. N., R. Hasan, and J. Smythe. 1997. Reduced interferon-alpha production by Epstein-Barr virus transformed B-lymphoblastoid cell lines and lectin-stimulated lymphocytes in congenital dyserythropoietic anaemia type I. Br. J. Hematol. 98:295-298. [DOI] [PubMed] [Google Scholar]
- 94.Wilson, S. M., R. Yip, D. A. Swing, T. N. O'Sullivan, Y. Zhang, E. K. Novak, R. T. Swank, L. B. Russell, N. G. Copeland, and N. A. Jenkins. 2000. A mutation in Rab27a causes the vesicle transport defects observed in ashen mice. Proc. Natl. Acad. Sci. USA 97:7933-7938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu, L., Z. Yu, and S. H. Shen. 2002. SKAP55 recruits to lipid rafts and positively mediates the MAPK pathway upon T-cell receptor activation. J. Biol. Chem. 277:40420-40427. [DOI] [PubMed] [Google Scholar]
- 96.Wu, M., H. Lee, R. E. Bellas, S. L. Schauer, M. Arsura, D. Katz, M. J. FitzGerald, T. L. Rothstein, D. H. Sherr, and G. E. Sonenshein. 1996. Inhibition of NF-κB/Rel induces apoptosis of murine B cells. EMBO J. 15:4682-4690. [PMC free article] [PubMed] [Google Scholar]
- 97.Yamanashi, Y., and D. Baltimore. 1997. Identification of the Abl- and rasGAP-associated 62 kDa protein as a docking protein, Dok. Cell 88:205-211. [DOI] [PubMed] [Google Scholar]
- 98.Zhao, B., D. R. Marshall, and C. E. Sample. 1996. A conserved domain of the Epstein-Barr virus nuclear antigens 3A and 3C binds to a discrete domain of Jκ. J. Virol. 70:4228-4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zimber-Strobl, U., L. J. Strobl, C. Meitinger, R. Hinrichs, T. Sakai, T. Furukawa, T. Honjo, and G. W. Bornkamm. 1994. Epstein-Barr virus nuclear antigen 2 exerts its transactivating function through interaction with recombination signal binding protein RBP-Jκ, the homologue of Drosophila Suppressor of Hairless. EMBO J. 13:4973-4982. [DOI] [PMC free article] [PubMed] [Google Scholar]