Abstract
Neurotransmitters are released by synaptic vesicle exocytosis at the active zone of a presynaptic nerve terminal. In this review, I discuss the molecular composition and function of the active zone. Active zones are composed of an evolutionarily conserved protein complex containing as core constituents RIM, Munc13, RIM-BP, α-liprin, and ELKS proteins. This complex docks and primes synaptic vesicles for exocytosis, recruits Ca2+ channels to the site of exocytosis, and positions the active zone exactly opposite to post-synaptic specializations via transsynaptic cell-adhesion molecules. Moreover, this complex mediates short- and long-term plasticity in response to bursts of action potentials, thus critically contributing to the computational power of a synapse.
Introduction
Synapses are intercellular junctions between a presynaptic neuron and a postsynaptic cell, usually also a neuron. Information arrives at a presynaptic terminal in the form of an action potential and is transmitted to the postsynaptic cell via a chemical neurotransmitter. In a presynaptic terminal, neurotransmitters are packaged into synaptic vesicles. When an action potential opens presynaptic voltage-gated Ca2+ channels, the neurotransmitters are released by Ca2+-triggered synaptic vesicle exocytosis into the synaptic cleft, where they activate postsynaptic receptors. Morphologically, synapses resemble other intercellular junctions, with precisely opposed pre- and postsynaptic specializations that contain electron-dense material on their plasma membranes (Figure 1; Gray, 1963). Synaptic vesicle exocytosis is restricted to the small section of the presynaptic plasma membrane containing this electron-dense material, which led to its designation as the “active zone” (Couteaux and Pecot-Dechavassine, 1970). Thus, the active zone lies at the interface between the presynaptic terminal and the synaptic cleft, and its major function is to transform a presynaptic action potential signal into a released neurotransmitter signal (Figure 1).
Figure 1. Location of the Active Zone in a Synapse.
(A) Schematic drawing of a synapse.
(B) Electron micrograph of a conventially fixed and stained synapse in a cultured hippocampal neuron.
(C) Electron micrograph of a phosphotungstic acid stained synapse in a cultured hippocampal neuron to visualize pre- and postsynaptic specializations.
(B) and (C) are reproduced with permission from Kaeser et al. (2011).
Synapses are computational devices that not only transmit action potential-encoded information, but also transform it. Neuronal information is often encoded by bursts or trains of action potentials. Synapses process such action potential bursts or trains in a synapse-specific manner that involves use-dependent changes in neurotransmitter release during the burst or train (referred to as short-term plasticity). In addition, synapses experience use-dependent long-term changes in synaptic transmission that adjust the “gain” of a synapse, and operate either pre- and/or postsynaptically (referred to as long-term plasticity). Much of the synaptic computation of information operates in the presynaptic nerve terminal, and—as we will see below—is executed by the active zone.
Synapses reliably differ from each other in their properties, not only in terms of neurotransmitter type, but also in terms of basic synaptic parameters, such as the release probability and post-synaptic receptor composition. The mammalian brain contains hundreds of different types of neurons, which form and receive synapses that exhibit characteristic properties that depend on both the pre- and the postsynaptic neuron (Koester and Johnston, 2005). As a consequence, there are likely hundreds of different types of synapses that operate by the same fundamental mechanism, but exhibit distinct computational properties.
Presynaptic active zones perform four principal functions in neurotransmitter release. First, they dock and prime synaptic vesicles, i.e., are an intrinsic part of the synaptic vesicle release machinery; note, however, that SNARE and SM proteins which are the core fusion proteins of synaptic vesicles are not enriched in the active zone. Second, active zones recruit voltage-gated Ca2+ channels to the presynaptic membrane to allow fast synchronous excitation/release coupling. Third, active zones contribute to the precise location of pre- and postsynaptic specializations exactly opposite to each other via transsynaptic cell-adhesion molecules. Finally, active zones mediate much of the short- and long-term presynaptic plasticity observed in synapses, either directly by responding to second messengers such as Ca2+ or diacylglycerol whose production causes plasticity or indirectly by recruiting other proteins that are responsible for this plasticity. All of these functions aim to organize neuro-transmitter release, such that presynaptic vesicle exocytosis is performed with the requisite speed and plasticity needed for the information transfer and computational function of a synapse.
In the present overview, I will focus on the composition and mechanism of action of active zones. Unfortunately, length restrictions preclude a discussion of many important papers and issues in the field, and I apologize for the many omissions I am bound to commit. Despite significant progress, much about active zones remains unknown, and I will at the end of each section briefly discuss open questions and major challenges.
Evolution and Diversity of Active Zones
Synaptic vesicle exocytosis likely emerged evolutionarily from nonsynaptic forms of neurosecretion that are observed in primitive animals such as trichoplax or nomastella. Although these animals lack morphologically identifiable synapses, they contain genes homologous to synaptotagmins and complexins that mediate the Ca2+-triggering of synaptic vesicle fusion. The emergence of synapses probably depended on the evolutionary construction of the active zone that organizes the Ca2+-triggering of neurotransmitter secretion, and restricts it to a small membrane patch opposite to a cluster of postsynaptic receptors. Thus, active zones are a key component of what defines a synapse.
In central synapses of vertebrates, active zones are disc-like structures with a 0.2–0.5 μm diameter. Active zones are surrounded by a perisynaptic zone that is functionally an intrinsic part of a synapse. The perisynaptic zone is the site of synaptic vesicle endocytosis (Brodin and Shupliakov, 2006), contains transsynaptic cell-adhesion molecules such as cadherins (Uchida et al., 1996) and harbors presynaptic receptors such as endocannabinoid CB1 receptors that control neurotransmitter release (Nyíri et al., 2005).
Different from the disc-shaped active zones of central synapses, neuromuscular junctions contain elongated active zones to which synaptic vesicles are attached like pearls on a string (Harlow et al., 2001). Moreover, some sensory neurons of vertebrates form specialized ribbon synapses that are characterized by a synaptic ribbon or body that is positioned perpendicular to the plane of the plasma membrane (Matthews and Fuchs, 2010). Evolutionarily, synaptic ribbons probably arose in vertebrates with the generation of RIBEYE, their major protein component that represents a fusion of a novel N-terminal domain encoded by a single large exon with another protein called CtBP2 (Schmitz et al., 2000). Ribbon synapses contain their characteristic synaptic ribbons in addition to standard active zones, and the ribbons appear to function as accelerators in the recruitment of vesicles for exocytosis (Matthews and Fuchs, 2010). Active zones of invertebrate synapses are similar to central vertebrate synapses, except for specializations such as the t bars in Drosophila that similar to synaptic ribbons appear to function in recruiting vesicles to active zones (Kittel et al., 2006). In the present discussion, due to space constraints we will focus on the core components shared by all active zones as far known, and only refer to more specialized features in passing.
Functional Architecture of the Active Zone
Active zones are composed of a detergent insoluble protein matrix that is heterogeneous in size and composition and difficult to purify. As a result, all currently known active zone proteins were identified based on antibodies, genetic mutations, or protein-protein interactions.
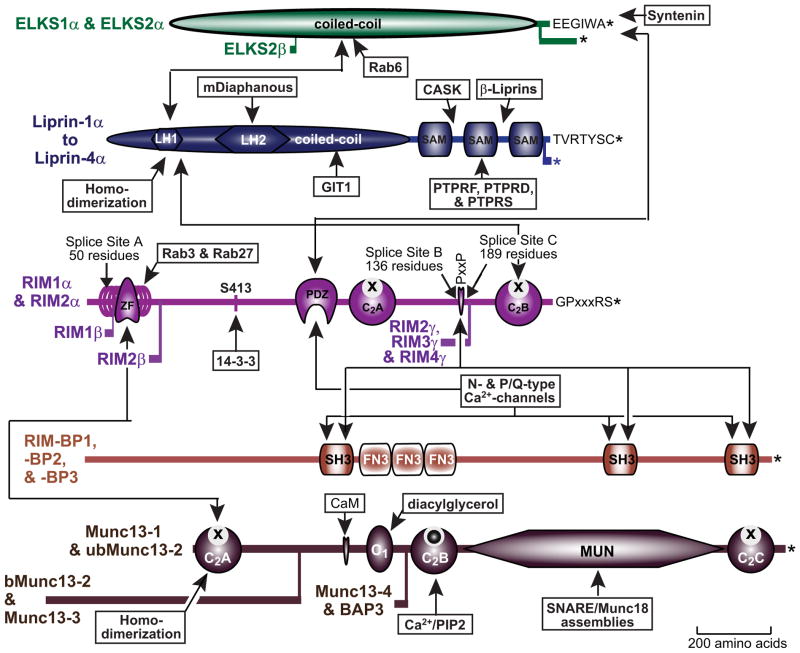
Emerging evidence suggests that five evolutionarily conserved proteins—RIM, Munc13, RIM-BP, α-liprin, and ELKS proteins—form the core of active zones (Figure 2). RIM, Munc13, and RIM-BP are multidomain proteins composed of a string of identifiable modules, whereas α-liprin and ELKS exhibit a simpler structure. These proteins are generally encoded by single genes in invertebrates and by multiple genes, often with several splice variants, in vertebrates. The five core active zone proteins form a single large protein complex that docks and primes synaptic vesicles, recruits Ca2+ channels to the docked and primed vesicles, tethers the vesicles and Ca2+ channels to synaptic cell-adhesion molecules, and mediates synaptic plasticity (Figure 3). Interestingly, the core active zone proteins are not specific for active zones, or even neurons. ELKS and α-liprins were discovered in nonneuronal cells (Serra-Pagès et al., 1995; Nakata et al.), and RIMs, Munc13s, and RIM-BPs are at least partially expressed in neuroendocrine and other secretory cells, suggesting that these proteins perform additional general functions.
Figure 2. Domain Structures of Evolutionarily Conserved Core Active Zone Proteins.
The diagram depicts the domain structures of the five core active zone proteins and their interactions with each other and with a series of other synaptic proteins. For each protein, isoforms generated by alternative promoters and major splice variants are also shown. Arrows indicate binding reactions. Proteins and second messengers binding to specific sites are shown in boxes; domain designations are indicated by standard abbreviations. For C2 domains, Ca2+-binding sites are indicated by a sphere and lack of Ca2+-binding by an “X.” Proteins and domains are drawn to scale. Identified C-terminal PDZ-domain binding sequences in ELKS, Liprins, and RIMs are shown; asterisks indicate positions of the C termini of a protein. For details, see text.
Figure 3. Molecular Model of the Active Zone Protein Complex and Its Relation to the Synaptic Vesicle Fusion Machinery, Ca2+ Channels, and Synaptic Cell-Adhesion Molecules.
The various active zone proteins and their interactions are depicted schematically (see Figure 2 and the text for details).
In addition to these five core active zone proteins, piccolo and bassoon (two large homologous proteins) are associated in vertebrates with active zones (tom Dieck et al., 1998; Wang et al., 1999; Fenster et al., 2000; Limbach et al., 2011), and proteins related to C. elegans SYD-1 are important for the assembly of active zones in invertebrates (Hallam et al., 2002; Patel et al., 2006; Owald et al., 2010). Other proteins are also likely present in active zones or close to them, but the evidence for their importance is not always strong, and only some of these proteins will be discussed below (e.g., CASK and Pick1). Besides these proteins, actin was suggested to be an active zone component, but high-resolution electron microscopy (EM) shows that actin filaments are excluded from the active zone and the vesicle cluster (Fernández-Busnadiego et al., 2010). Furthermore, as noted above, the plasma membrane SNARE proteins syntaxin and SNAP-25 and the SM protein Munc18 that are components of the synaptic vesicle fusion machinery for exocytosis (Südhof and Rothman, 2009) are not enriched in active zones but distributed all over the plasma membrane. This may appear paradoxical given that synaptic exocytosis is restricted to active zones but is consistent with the general involvement of these proteins in many types of exocytosis.
RIM Proteins Are Central Organizers of Active Zones
RIMs (for Rab3-interacting molecules; Wang et al., 1997) contain five identifiable domains (Figure 2): an N-terminal composite domain consisting of a zinc finger surrounded by α helices, a central PDZ domain, two C-terminal C2 domains that different from classical C2 domains do not bind Ca2+, and a conserved proline-rich sequence in the linker sequence between the C2 domains (Wang and Südhof, 2003). Vertebrates express four RIM genes, of which only the RIM1 and RIM2 genes produce proteins called RIM1α and RIM2α that include all of the domains mentioned above. The RIM1 gene contains an additional internal promoter driving expression of RIM1β that lacks the N-terminal α-helix of the first domain (Kaeser et al., 2008a), and the RIM2 gene contains two internal promoters driving expression of RIM2β that lacks the entire RIM N-terminal domain, or of RIM2γ that consists of only of the second RIM2 C2B domain preceded by a short unique sequence (Wang et al., 2000; Wang and Südhof, 2003). Finally, the RIM3 and RIM4 genes encode only RIM3γ and RIM4γ isoforms, respectively, with the same domain structures as RIM2γ (Figure 2).
Genetic experiments in C. elegans and mice revealed that RIM is essential for synaptic vesicle docking and priming (Koushika et al., 2001; Schoch et al., 2002; Gracheva et al., 2008; Kaeser et al., 2011; Deng et al., 2011; Han et al., 2011), for recruiting Ca2+ channels to active zones (Kaeser et al., 2011), and for short-term plasticity of neurotransmitter release (Schoch et al., 2002; Castillo et al., 2002). RIM apparently performs these functions in all synapses, with at least some redundancy among RIM isoforms (Schoch et al., 2006; Kaeser et al., 2008a, 2011a, 2012). In vertebrates, RIM1α is additionally required for all types of long-term presynaptic plasticity analyzed (Castillo et al., 2002; Huang et al., 2005; Chevaleyre et al., 2007; Fourcaudot et al., 2008; Pelkey et al., 2008; Lachamp et al., 2009). Some of the same forms of plasticity were also shown to be dependent on Rab3A (Castillo et al., 1997; Huang et al., 2005) or Rab3B (Tsetsenis et al., 2011), suggesting that RIM1α acts in long-term plasticity via binding to Rab3. It was initially thought that PKA-dependent phosphorylation of RIM1α at serine-413 controls long-term plasticity (Lonart et al., 2003), but knockin mice with a constitutive alanine substitution of serine-413 exhibited normal presynaptic LTP, ruling out this hypothesis (Kaeser et al., 2008b).
The N-terminal zinc finger of RIMs binds to the C2A domain of Munc13-1 and ubMunc13-2, the two principal Munc13 isoforms in brain (Betz et al., 2001; Dulubova et al., 2005; Lu et al., 2006), while the α helices surrounding the zinc finger bind to Rab3 and Rab27 in a GTP-dependent manner (Wang et al., 1997, 2000; Fukuda, 2003). Interestingly, the Munc13 C2A domain forms a constitutive homodimer that is disrupted by binding of the RIM zinc finger, thereby producing a RIM/Munc13 heterodimer (Dulubova et al., 2005). The heterotrimeric complex of the N-terminal RIM domain with Munc13 and Rab3 or Rab27 (Lu et al., 2006) links synaptic vesicles to active zones in close proximity to the priming factor Munc13 and likely accounts for the function of RIM in synaptic vesicle docking (Gracheva et al., 2008; Kaeser et al., 2011).
The central PDZ-domain of RIMs binds at least two proteins: ELKS (Ohtsuka et al., 2002; Wang et al., 2002) and N- and P/Q-type but not L-type Ca2+ channels (Kaeser et al., 2011). The physiological importance of ELKS binding to RIMs is unclear since the synaptic function of ELKS remains enigmatic (see below). In contrast, the binding of the RIM PDZ-domain to Ca2+ channels is essential for recruiting Ca2+ channels to active zones (Kaeser et al., 2011; Han et al., 2011). Synapses expressing mutant RIM that lacks the PDZ-domain exhibit a selective loss of presynaptic Ca2+ channels, with a resulting shift in the Ca2+-dependence of release to a higher Ca2+-requirement and a desynchronization of release (Kaeser et al., 2011). In addition to binding directly to RIMs, Ca2+ channels are tethered to the active zone by binding to RIM-BPs which in turn bind to RIMs (Figure 2). Specifically, the SH3-domains of RIM-BPs interact with proline-rich sequences of RIMs (localized between their C2A and C2B domains) and of Ca2+ channels (in their cytoplasmic tails). A RIM fragment consisting of only its PDZ domain and proline-rich sequence is sufficient to rescue the presynaptic loss of Ca2+ channels in RIM-deficient synapses (Kaeser et al., 2011). Together, these data suggest that Ca2+ channels are recruited to active zones by a tripartite complex composed of RIMs, RIM-BPs, and the C-terminal tails of the channels (Figure 3).
The function of the RIM C2 domains remains poorly understood. The C2B domain binds to α-liprins and synaptotagmin-1 (Schoch et al., 2002), and the C2A domain may bind to SNARE proteins (Coppola et al., 2001), but it is unclear whether these interactions are physiologically relevant. The C2 domains may also bind to Ca2+ channels (Coppola et al., 2001), and the C2B domain of RIMs modulates Ca2+ channel opening (Uriu et al., 2010; Kaeser et al., 2012). A fragment containing only the C2A and C2B domains of RIM partly rescues the decrease in synaptic strength observed in RIM-deficient synapses, without reversing the loss of presynaptic Ca2+ channels, suggesting that the C2 domains of RIM perform an active function in release (Kaeser et al., 2011). However, the nature of this function and its relation to the biochemical activities of the C2 domains remain unknown.
RIM-BPs—Links of Ca2+Channels to RIMs
RIM-BPs are large multidomain proteins (Figure 2). Vertebrates express three RIM-BP genes (Wang et al., 2000; Mittelstaedt and Schoch, 2007), whereas Drosophila expresses only a single gene (Liu et al., 2011). All RIM-BPs contain one central and two C-terminal SH3 domains and three central fibronectin III domains (Wang et al., 2000; Mittelstaedt and Schoch, 2007). The sequences separating these domains lack identifiable domains and vary among RIM-BP isoforms. RIM-BPs were initially cloned as an anonymous cDNA (KIAA0318) and as a protein interacting with the mitochondrial peripheral benzodiazepine receptor (“PRAX-1”; Galiègue et al., 1999). However, the latter identification may be erroneous since subsequent studies showed that RIM-BPs are highly expressed only in brain and not peripherally and tightly bind to RIM (Wang et al., 2000) and to N-, P/Q-, and L-type Ca2+ channels (Hibino et al., 2002; Kaeser et al., 2011).
The finding that RIM-BPs biochemically form a complex with RIMs in the active zone (Wang et al., 2000), and the discovery that RIM-BPs bind to Ca2+ channels (Hibino et al., 2002) suggested that they may act to recruit Ca2+ channels to active zones. However, the initial problem with this hypothesis was that RIM-BPs bind nonsynaptic L-type Ca2+ channels as well as synaptic N- and P/Q-type Ca2+ channels and thus could not account for the specific recruitment of N- and P/Q-type Ca2+ channels to active zones (Hibino et al., 2002). This problem was resolved when the RIM PDZ-domains were found to bind to N- and P/Q-type but not L-type Ca2+ channels (Kaeser et al., 2011), indicating that Ca2+ channels are recruited to active zones by binding simultaneously to both RIM and RIM-BPs (Figure 3). This hypothesis was not only confirmed in rescue experiments with mutant RIM proteins showing that both interactions are essential for recruiting Ca2+ channels to active zones (Kaeser et al., 2011), but also in Drosophila experiments in which mutations in RIM-BP were found to disrupt Ca2+ channel localization (Liu et al., 2011). The Drosophila experiments additionally revealed that in the absence of RIM-BP, the organization of the active zone was impaired, and the ultrastructural distribution of the ELKS homolog Bruchpilot at active zones was altered, suggesting that RIM-BPs may have additional functions besides assisting RIM in the recruitment of Ca2+ channels. Indeed, the fact that the loss of presynaptic Ca2+ channels in RIM-deficient synapses can be rescued with a RIM fragment consisting only of its PDZ-domain and RIM-BP binding sequence (Kaeser et al., 2011) can only be explained by the assumption that RIM-BP engages in other interactions besides binding to RIMs and Ca2+ channels. Identifying these additional interactions of RIM-BPs will be both challenging and exciting.
MUNC13s Mediate Vesicle Priming
The C. elegans unc-13 gene was identified as a gene encoding a diacylglycerol-binding protein whose mutation caused an “uncoordinated” phenotype, but nothing was known about the localization or function of this protein (Maruyama and Brenner, 1991). Characterization of the mammalian homologs of UNC-13—named Munc13s—revealed that Munc13 proteins are active zone proteins essential for synaptic vesicle priming (Brose et al., 1995; Augustin et al., 1999).
Mammals contain five Munc13 genes (Brose et al., 1995; Song et al., 1998; Koch et al., 2000). The Munc13-1, -2, and -3 genes encode larger proteins primarily expressed in brain, while the Munc13-4 and BAP3 genes encode smaller proteins primarily expressed outside of brain. The Munc13-2 gene includes two promoters that produce a ubiquitously expressed, major isoform (called ubMunc13-2) and a brain-specific less abundant isoform (called bMunc13-2; Figure 2; Brose et al., 1995; Song et al., 1998). The N-terminal regions of Munc13-1 and ubMunc13-2 contain a Ca2+-independent C2A domain and a long sequence of unknown significance, followed by a central calmodulin-binding sequence and C1-domain. In contrast, bMunc13-2 and Munc13-3 have a different, even longer N-terminal region upstream of the C1 domain (Figure 2). The short Munc13 isoforms (Munc13-4 and BAP3), conversely, lack all domains upstream of the C2B domain, placing the C2B domain at their N terminus. In all Munc13 isoforms, the C2B domain is followed by a large domain called the MUN domain and a C-terminal Ca2+-independent C2C domain (Figure 2).
Munc13 proteins have two principal functions at the active zone: to prime the SNARE/SM protein fusion machinery for exocytosis, thus rendering synaptic vesicles fusion competent, and to mediate short-term plasticity by regulating this priming activity. Munc13s execute their priming function via the MUN domain (Basu et al., 2005; Stevens et al., 2005). The MUN domain may act to open the ‘closed’ form of the SNARE protein syntaxin-1 (Gerber et al., 2008), thereby enabling syntaxin-1 to form SNARE complexes (Richmond et al., 2001; Ma et al., 2011). This function of the MUN domain may be general for regulated exocytosis, since Munc13-4 is essential for cytotoxic granule exocytosis in NK cells (Feldmann et al., 2003). Remarkably, a recent crystal structure of a fragment of the MUN domain has revealed similarities of the MUN domain to tethering factors involved in other intracellular trafficking steps, suggesting that the MUN domain may exert a conserved function similar to those of other tethering factors (Li et al., 2011). Moreover, a distantly related protein called CAPS that also has a MUN domain is essential for dense-core vesicle priming for exocytosis and again functions by binding to SNARE/SM protein complexes (Khodthong et al., 2011). How precisely the MUN interacts with SNARE and SM proteins, however, remains unclear.
Deletion of all large Munc13 isoforms blocks synaptic vesicle exocytosis in autapses formed by hippocampal neurons but produces only a partial impairment of exocytosis in neuromuscular junction synapses (Varoqueaux et al., 2002, 2005). Thus, it may be that different types of synapses exhibit distinct requirements for Munc13. Based on knockout studies, CAPS has also been suggested to function at synapses (Jockusch et al., 2007), but its role in synaptic exocytosis may be indirect. At present, it is unclear whether Munc13 and CAPS are functionally redundant, and whether Munc13 proteins are generally required for all regulated exocytosis similar to SNARE and SM proteins.
Munc13’s are regulated at many levels. Their N-terminal C2A domain homodimerizes (Dulubova et al., 2005), which inhibits the priming function of the MUN domain (Deng et al., 2011). Binding of the RIM zinc-finger to the Munc13 C2A domain disrupts the homodimers, thereby activating Munc13. As a result, RIM-deficient synapses exhibit a severe impairment in vesicle priming that can be rescued not only by the N-terminal RIM fragment, but also by expression of mutant Munc13 that is constitutively monomeric, illustrating that the function of RIMs in priming consists of activating Munc13 (Deng et al., 2011).
In addition to the regulation of the Munc13 MUN domain by RIMs, the MUN domain is controlled by the central signaling domains of Munc13 that comprise a calmodulin-binding sequence and the C1 and C2B domains (Figure 2) and that perform essential functions in regulating release (Rhee et al., 2002; Junge et al., 2004; Shin et al., 2010; see discussion below). It is unknown, however, whether the N-terminal sequences of bMunc13-2 and Munc13-3 have a regulatory role since they do not bind to RIMs, and no function has been observed yet for the conserved C2C domain of Munc13s. Addressing these questions may have general implications not only for synaptic exocytosis, but also for other forms of exocytosis, for example cytotoxic granule exocytosis in NK cells which requires Munc13-4 (Feldmann et al., 2003).
α-Liprins
α- and β-liprins are related proteins composed of an N-terminal half with a predicted coiled-coil domain, and three C-terminal SAM domains (Serra-Pagès et al., 1995). Two highly conserved sequence motifs in the N-terminal coiled-coil region are referred to as “liprin homology domains” LH1 and LH2 (Taru and Jin, 2011). The N-terminal half of α-liprins binds to itself to form homodimers (Taru and Jin, 2011), to the RIM C2B domain (Schoch et al., 2002), to ELKS (Ko et al., 2003a; Dai et al., 2006), to mDiaphanous, a rho effector protein (Sakamoto et al., 2012), and to GIT1 (Ko et al., 2003b). The C-terminal SAM-domains, in turn, bind to β-liprins to form heterodimers (Serra-Pagès et al., 1995), to CASK (Olsen et al., 2005), and to LAR-type receptor phosphotyrosine phosphatases (PTPRF, PTPRD, and PTPRS; Serra-Pagès et al., 1995). Of these interactions, α-liprin binding to β-liprins, to receptor phosphotyrosine phosphatases, to ELKS, and to itself have been functionally validated (Kaufmann et al., 2002; Ackley et al., 2005; Dai et al., 2006; Taru and Jin, 2011; Astigarraga et al., 2010).
α-Liprins were first linked to presynaptic active zones when a loss-of-function mutation in C. elegans α-liprin was found to apparently increase the size of the active zone and to disrupt synaptic vesicle accumulation (Zhen and Jin, 1999; Dai et al., 2006), a finding that was confirmed in Drosophila (Kaufmann et al., 2002). No studies on α-liprin function in vertebrate presynaptic terminals exist, but a rich body of work in C. elegans primarily from the Jin laboratory uncovered a fascinating interaction network that links trans-synaptic cell adhesion via a LAR-type receptor phosphotyrosine phosphatase to presynaptic active zone assembly. Specifically, a deletion of a synaptic isoform of the LAR-type receptor phosphotyrosine phosphatase PTP-3 was found to cause mislocalization of α-liprin, whereas a deletion of α-liprin caused mislocalization of the synaptic isoform of PTP-3 (Ackley et al., 2005). Moreover, a gain-of-function point mutation in the LH1 domain of α-liprin suppressed the phenotype caused by a loss of SYD-1 (a rho GAP that is essential for synapse assembly in invertebrates, but whose vertebrate homolog has not yet been identified; Dai et al., 2006; Owald et al., 2010). Strikingly, the α-liprin gain-of-function mutation increased α-liprin binding to ELKS. In addition, the ability of the α-liprin gain-of-function mutation to rescue the syd-1 mutation required ELKS (Dai et al., 2006), although ELKS mutations otherwise did not appear to cause any phenotype in C. elegans (Deken et al., 2005). Furthermore, the homodimerization of α-liprin appears to be essential for its ability to suppress the loss-of-function effect of syd-1 mutations (Taru and Jin, 2011), suggesting overall that the syd-1 loss-of-function is rescued by an α-liprin homodimer that exhibits increased binding to ELKS.
Together, these data established that active zone formation with recruitment of synaptic vesicles and of LAR-type receptor phosphotyrosine phosphatases requires α-liprin, possibly by simultaneous binding of α-liprin to the receptor phosphotyrosine phosphatase, RIM, ELKS, Syd-1, and itself. The data thus suggest a model whereby α-liprin acts to link synaptic cell adhesion to the RIM/Munc13/RIM-BP core complex that recruits vesicles and Ca2+ channels to active zones. However, the current understanding of α-liprins is incomplete. Many pressing questions remain, from simple questions about the possible role of β-liprins (see Wang and Wang, 2009; Astigarraga et al., 2010), to complex issues such as how α-liprins exactly organize a nerve terminal. Why does the active zone become apparently bigger in α-liprin mutants? What is the role of the LAR tyrosine phosphatase activity in synapse assembly and function, if any? How do α-liprin mutations affect neurotransmitter release, which is—after all—what the nerve terminal does? And finally, is the α-liprin function uncovered in C. elegans paradigmatic of its function elsewhere? Moreover, a more fundamental biophysical description of the protein complexes involving α-liprins is needed, as illustrated by the puzzling observation that the gain-of-function α-liprin mutation in C. elegans that increases ELKS binding (Dai et al., 2006) is in a region of the protein that in studies of mammalian proteins was not involved in ELKS binding (Ko et al., 2003a).
ELKS
Of the five core active zone proteins, ELKS is the most enigmatic. ELKS was discovered when a translocation in papillary thyroid carcinoma was found to place the ELKS gene upstream of the RET tyrosine kinase, thereby activating it (Nakata et al., 1999). It was then rediscovered as a Rab6-binding protein and named Rab6IP2 (Monier et al., 2002), and subsequently identified as an active zone protein and renamed CAST (Ohtsuka et al., 2002) or ERC (Wang et al., 2002). The field agreed on the original ELKS name for the protein, although the CAST and ERC names are still used occasionally.
ELKS consist largely of predicted coiled-coil sequences with no apparent domain structure. The mammalian genome contains two ELKS genes encoding structurally similar proteins, whereas C. elegans expresses a single ELKS gene highly homologous to mammalian ELKS. Mammalian ELKS genes contain alternative N-terminal promoters and alternatively spliced C-terminal sequences, with a shorter C-terminal sequence that is primarily expressed in brain and a longer C-terminal sequence that is primarily expressed in peripheral tissues (Wang et al., 2002; Kaeser et al., 2009). In contrast to other organisms, Drosophila expresses an ELKS fusion protein called “bruchpilot” (German for “crash pilot”) that consists of an N-terminal ELKS-related domain and a C-terminal plectin-related domain (Wagh et al., 2006).
As documented by its repeated rediscovery, ELKS likely functions in several cellular processes, and engages in multiple protein-protein interactions (Figure 2). It binds to Rab6 in a GTP-dependent manner, implicating it in membrane traffic involving the trans-Golgi complex (Monier et al., 2002). Its active zone localization was discovered by virtue of its binding to the RIM PDZ domains (Wang et al., 2002). The C terminus of ELKS probably also binds to other PDZ domain proteins, as described for syntenin-1 (Ko et al., 2006), and ELKS furthermore directly binds to α-liprins (Ko et al., 2003a; see discussion above).
Although initial overexpression and peptide injection experiments suggested a major function for ELKS2 in neurotransmitter release (Takao-Rikitsu et al., 2004), deletion of ELKS in C. elegans and of ELKS2 in mice did not impair neurotransmitter release (Deken et al., 2005; Kaeser et al., 2009). Interestingly, however, ELKS was required in C. elegans for the ability of the α-liprin gain-of-function mutation to suppress the syd-1 mutation (Dai et al., 2006; see discussion above). This result shows that at least for synapse formation and function under basal conditions, the synaptic function of ELKS is dispensible. Moreover, although acute or constitutive deletion of ELKS2 in mice did not produce a decrease in neurotransmitter release, they caused an increase in the readily releasable pool of synaptic vesicles (Kaeser et al., 2009). In contrast, constitutive deletion of ELKS1 caused embryonic lethality in mice, suggesting that the protein is essential for survival in a nonneuronal function (P.S. Kaeser and T.C.S., unpublished data).
At first glance, ELKS appears to have a more important function in Drosophila where deletion of bruchpilot produces a loss of the t bars characteristic of Drosophila synapses (Wagh et al., 2006). However, the bruchpilot deletion decreased neurotransmitter release only by ~30% (Kittel et al., 2006). Strikingly, a deletion of the 17 C-terminal residues of bruchpilot (which are part of the plectin-homology region) impaired attachment of synaptic vesicles to t bars in Drosophila synapses and altered synaptic transmission, suggesting that a major contribution to bruchpilot function is derived from the plectin-homology region (Hallermann et al., 2010). Overall, these studies suggest that bruchpilot performs a double function in Drosophila synapses, with the N-terminal ELKS component acting like a standard ELKS protein, and the C-terminal plectin-homology region acting in vesicle recruitment analogous to piccolo and bassoon in mammalian synapses (see below).
Piccolo and Bassoon Provide a Presynaptic Skeleton
Primarily due to pioneering work by the Gundelfinger laboratory, piccolo and bassoon are among the best studied presynaptic proteins. Piccolo and bassoon are large proteins specific to vertebrates whose major function appears to be to guide synaptic vesicles from the backfield of the synapse to the active zone (Mukherjee et al., 2010; Hallermann et al., 2010). Most piccolo and bassoon sequences are homologous and are predicted to form N-terminal zinc finger domains followed by extended coiled-coil structures without clear domain boundaries (tom Dieck et al., 1998; Wang et al., 1999). Moreover, piccolo contains a C-terminal PDZ domain and two C2 domains. Different from other C2 domains, the first C2 domain of piccolo undergoes a major conformational change upon Ca2+ binding, while the second C2 domain does not bind Ca2+ but is alternatively spliced (Wang et al., 1999; Gerber et al., 2001; Garcia et al., 2004).
Partial knockout of bassoon causes partial lethality and impairs neurotransmitter release (Altrock et al., 2003), whereas deletion of piccolo has no significant effect on survival or on neurotransmitter release in cultured neurons or in acute slices (Mukherjee et al., 2010). In synapses with a partial loss of both piccolo and bassoon, synaptic vesicle clusters are disrupted, indicating a possible role for these proteins in vesicle clustering (Mukherjee et al., 2010). Given the size of piccolo and bassoon, the interesting C-terminal domains of piccolo, and the reactive changes observed in bassoon knockout mice (Heyden et al., 2011), it seems likely that the more peripheral active zone function of piccolo and bassoon will have an important role in overall brain performance. This role may be particularly important in specialized synapses such as hippocampal mossy fiber synapses or retinal ribbon synapses. Among the problems in characterizing this role, however, has been the difficulty in generating conditional knockouts and the large size of the proteins which makes biochemical studies nearly impossible.
CASK/VELI/MINT1 Complexes
CASK is composed of an N-terminal CaM kinase-like domain that constitutes a catalytically active, unusual protein kinase (Mukherjee et al., 2008), and a C-terminal set of domains characteristic of MAGUKs (for membrane-associated guanylate kinases; Hata et al., 1996). CASK was discovered because its PDZ-domain—a component of the MAGUK domains—tightly binds to the C terminus of neurexins, thereby constituting the first example of a “type II” PDZ-domain interaction (Hata et al., 1996). In addition, the CASK PDZ-domain binds to SynCAMs (Biederer et al., 2002), CASPRs (Spiegel et al., 2002), and syndecans (Hsueh et al., 1998; Cohen et al., 1998). Both CASK and its C. elegans homolog Lin-2 form a tripartite complex with two other PDZ-domain proteins called Velis (also named MALS, originally discovered in C. elegans as Lin-7) and Mints (also called Lin-10; Butz et al., 1998; Kaech et al., 1998). CASK additionally binds in vertebrates but not in invertebrates to α-liprins (Olsen et al., 2005; Wei et al., 2011) and to CASKIN (Tabuchi et al., 2002). CASKIN in Drosophila interacts with LAR-type receptor phosphotyrosine phosphatases (that in turn also bind to α-liprins [Serra-Pagès et al., 1995]) and thus provides another possible link of CASK to presynaptic terminals. Together, these interactions create the potential for a large protein assembly that links the core active zone components to a secondary complex composed of CASK and its various interactors, including a variety of putative synaptic cell-adhesion molecules.
Like α-liprins, CASK is present in both pre- and postsynaptic specializations (Hsueh et al., 1998) but is also widely expressed outside of brain (Hata et al., 1996). Mutations of CASK produce a developmental phenotype in invertebrates, mice, and humans (Hoskins et al., 1996; Atasoy et al., 2007; Moog et al., 2011) and cause major changes in neuronal function, including a general impairment of synaptic transmission (Zordan et al., 2005; Atasoy et al., 2007; Sun et al., 2009; Chen and Featherstone, 2011).
Although much data thus link CASK to synapses, its precise role remains unclear. The CASK mutants were relatively uninformative given their complex phenotypes. It is possible that CASK is involved in different functions performed by distinct types of intercellular junctions. To address these questions, conditional deletion of CASK in either only pre- or postsynaptic neurons will be essential, as will be a better definition of the physiologically relevant protein interactions of CASK.
Membrane Proteins of Active Zones
P/Q- (Cav2.1) and N-type Ca2+ channels (Cav2.2) are localized to active zones, and the related R-type Ca2+ channel (Cav2.3) may also be present (Gasparini et al., 2001; Li et al., 2007). Besides Ca2+ channels, active zones contain at least two other classes of membrane proteins: presynaptic neurotransmitter receptors and transsynaptic cell-adhesion molecule.
Elegant immuno-EM studies demonstrated that group III metabotropic glutamate receptors (mGluR4, mGluR7, and mGluR8) are concentrated in active zones (Shigemoto et al., 1996; Corti et al., 2002; Kogo et al., 2004; Ferraguti et al., 2005). In the hippocampus, metabotropic group III receptors expressed by pyramidal neurons are selectively targeted to synapses formed by these neurons onto interneurons and excluded from synapses formed by the same pyramidal neurons onto other pyramidal neurons (Shigemoto et al., 1996). Similarly, metabotropic group III receptors expressed by GABAergic neurons are only enriched in the active zones of synapses formed by these neurons onto other interneurons, but not in synapses onto pyramidal neurons (Corti et al., 2002; Kogo et al., 2004; Ferraguti et al., 2005). Moreover, agonists of group III metabotropic receptors selectively suppress GABAergic synaptic transmission at synapses formed onto inhibitory inter-neurons, but not at synapses formed onto pyramidal neurons (Kogo et al., 2004). Together, these results describe a novel role for glutamate as an autoinhibitory neurotransmitter at a subset of excitatory synapses, and as a heterosynaptic suppressor of release at some inhibitory synapses, a role that is likely to greatly influence synaptic transmission at these synapses during stimulus trains. Protein interaction studies revealed that metabotropic group III glutamate receptors bind to the intracellular PDZ-domain protein PICK1, suggesting that PICK1 may recruit these receptors to active zones (Dev et al., 2000; Boudin et al., 2000).
Apart from group III metabotropic receptors, presynaptic GABAB-receptors appear to be at least partly localized to active zones (Luján et al., 2004). In contrast, CB1 receptors for endocannabinoids are excluded from active zones, but enriched in the perisynaptic region (Nyíri et al., 2005).
At present, no presynaptic cell-adhesion molecule has been definitively localized to the active zone. Cadherins appear to surround the active zone (Uchida et al., 1996), but two other pre-synapic cell-adhesion molecules may be in the active zone: the LAR-type receptor phosphotyrosine phosphatases PTPRF, PTPRD, and PTPRS, and neurexins. For LAR-type PTPRs, their molecular tethering to α-liprins which in turn are part of the active zone (see Figure 3) strongly suggests a localization either in the active zone or on the fringe of the active zone. For neurexins, the localization of the neurexin ligands neuroligin-1 and neuroli-gin-2 to the postsynaptic density (Song et al., 1999; Lorincz and Nusser, 2010) suggests that neurexins might also localize to the active zone opposite to the postsynaptic density.
Ultrastructure of the Active Zone
EM studies of chemically fixed and stained central synapses showed that the active zone contains a hexagonal grid of dense projections with intercalated vesicles (Figure 4A; Akert et al., 1972; Pfenninger et al., 1972; Limbach et al., 2011). Immuno-EM experiments suggested that RIM and Munc13, arguably the two most important active zone proteins, are localized between the dense projections adjacent to the plasma membrane, whereas the cytomatrix proteins piccolo and bassoon are more distant and appear to be attached to the tips of the dense projections (Limbach et al., 2011). These localizations agree well with ultra high-resolution light microscopy studies that place RIM closer to the plasma membrane than piccolo and bassoon (Dani et al., 2010). Interestingly, ultra-high resolution light microscopy has also been used in Drosophila to reconstruct at least part of an active zone with the t-bar that is characteristic for Drosophila active zones (Figure 4B; Liu et al., 2011). EM tomography in C. elegans synapses also revealed dense projections to which synaptic vesicles are attached (Stigloher et al., 2011). Gratifyingly, mutations in RIM or α-liprin disrupted the attachment of synaptic vesicles in C. elegans active zones, consistent with the functional assignments of these proteins described above. Together, these results support the notion that the core complex of active zone proteins is involved in linking synaptic vesicles, Ca2+ channels, and the fusion machinery to each other at the plasma membrane (Figure 3).
Figure 4. Ultrastructure of the Mammalian and Drosophila Active Zone.
(A) The left and central drawing depict a schematic view of the structure of the active zone as it emerges from studies of chemically fixed synapses, shown in a cross section (left) and top-down view (right; data from Akert et al. [1972] and Limbach et al. [2011]). The right drawing depicts a similarly schematic view of the active zone as it emerges from cryo-EM tomography of unfixed materials (based on Fernández-Busnadiego et al. [2010]). The nature and sizes of key features are explained below the drawings.
(B) Structure of the Drosophila neuromuscular active zone and t-bar as revealed by high-resolution LM (adapted with permission from Liu et al. [2011]).
In cryo-EM studies of unfixed and unstained synapse preparations, however, no dense projections are detectable. The only structures visible are the plasma membrane, synaptic vesicles, and sparse filaments that either connect vesicles to each others (“connectors,” average length ~10 nm) or tether vesicles to the presynaptic plasma membrane (“tethers”—5–20 nm; Landis et al., 1988; Fernández-Busnadiego et al., 2010). No other structures are visible, even though the cytosol clearly must contain abundant protein complexes as described above.
Is the view of the active zone obtained with fixed or with un-fixed materials correct? It has been argued that EM with unfixed preparations is superior to EM on chemically fixed preparations because chemical fixatives, by their very nature, crosslink proteins, and thus may create structures that are not normally present (Siksou et al., 2009; Fernández-Busnadiego et al., 2010). However, high-pressure freezing of samples is not devoid of potential problems since it generally involves a long preincubation in hyperosmotic medium, is not instantaneous, and subjects a sample to very high pressures. Clearly the fact that in cryo-EM images the protein complexes that are known to mediate the functions of the active zone are invisible does not mean these complexes are not there. Nevertheless, the dense projections observed in chemically fixed preparations would have been seen in cryo-EM images given their size, suggesting that these projections represent the result of chemical fixation. A plausible hypothesis thus is that chemical cross-linking of the active zone core protein complexes generates these dense projections. In order to account for the regularity and reproducibility of the dense projections, however, one has to assume that they reflect the normal spatial architecture of these protein complexes, with the proteins involved in vesicle fusion and recruiting Ca2+ channels (RIMs and ELKS) localized at the base of the projections, and proteins involved in guiding vesicles to the active zone (bassoon and piccolo) at the tip of the projections. In that sense, chemically fixed preparations reveal an underlying organization of the active zone that is missed in the cryo-EM studies, and the two EM approaches—EM on chemically fixed and on unfixed preparations—provide complementary insights in the organization of active zones.
How then can we interpret the results obtained with EM studies of mutant synapses? For example, in Munc13-1 KO mice synaptic vesicle docking appears to be normal as analyzed by EM of chemically fixed synapses (Augustin et al., 1999) but impaired as analyzed by cryo-EM of unfixed samples (Siksou et al., 2009). A possible interpretation of this finding is that docking analyzed with fixed samples is prone to artifacts, but the situation is not as straightforward as it seems. If in chemically fixed samples even nondocked vesicles always appear docked, no mutation should cause a loss of docking as analyzed by this method. However, in chemically fixed RIM mutant synapses, vesicles are at least partially undocked (Kaeser et al., 2011). The fundamental problem here is that docking as defined by EM is not a functional definition, and both EM approaches may provide a technique-dependent limited view, with neither allowing the claim of absolute conclusions.
Short- and Long-Term Synaptic Plasticity
Short-term synaptic plasticity can increase or decrease the strength of a synaptic signal several-fold (Fioravante and Regehr, 2011). This change dramatically alters the size of a postsynaptic response elicited by a train of presynaptic action potentials. Many different mechanisms of short-term synaptic plasticity were identified, nearly all of which involve the active zone, although in different ways.
At the most basic level, short-term plasticity of release is due to the interplay between the buildup of residual Ca2+ and the loss of releasable vesicles from one action potential to the next. Ca2+ entering the active zone via Ca2+ channels is buffered away quickly, leading to Ca2+-transients of less than 1 ms (Meinrenken et al., 2003). However, during repeated action potentials, especially at frequencies of >10 Hz, residual Ca2+ accumulates because the decay of Ca2+-transients decelerates as Ca2+-buffers become saturated, resulting in an increase of the release probability and thus facilitation. At the same time, vesicles undergoing exocytosis need to be replenished. Although the precise rate of vesicle replenishment differs among synapses and is also regulated by Ca2+ (see discussion below), replenishment of release-ready vesicles can be rate-limiting during action-potential trains, leading to synaptic depression. Thus, short-term plasticity due to the interplay of residual Ca2+ and vesicle depletion depends on synapse-specific factors such as available Ca2+-buffers, the size of the readily releasable pool of vesicles, and the basal release probability.
A second major mechanism of short-term plasticity at the active zone is mediated by the Ca2+- and G protein-dependent modulation of presynaptic Ca2+ channels (Catterall and Few, 2008). Ca2+ can inhibit or activate Ca2+ channels, mediated by several EF-hand Ca2+-binding proteins. Specifically, calmodulin appears to both facilitate and inhibit voltage-dependent activation of Cav2.1 P/Q-type Ca2+ channels via binding to discrete sites in the cytoplasmic Ca2+ channel tail sequences (DeMaria et al., 2001; Lee et al., 2003). In addition, another EF-hand Ca2+-binding protein called “calcium-binding protein 1” (CaBP1) increases inactivation of P/Q-type Ca2+ channels (Lee et al., 2002), whereas a third EF-hand Ca2+-binding protein called visinin-like protein 2 (VILIP-2) slows the rate of Ca2+ channel inactivation and enhances facilitation (Lautermilch et al., 2005). Moreover, Ca2+ channels are powerfully inhibited by G protein mediated mechanisms activated by presynaptic receptors, and such inhibition can also contribute to short-term synaptic plasticity. For example, GABAB-autoreceptors mediate short-term synaptic depression of inhibitory synapses during stimulus trains in insular cortex, illustrating this mode of short-term synaptic plasticity (Kobayashi et al., 2012). However, most G protein mediated presynaptic inhibition of release by suppression of Ca2+ channel activation probably does not operate via autoreceptors, but via receptors for neuromodulators such as neuropeptides, endocannabinoids, acetylcholine, and catecholamines. The most prominent example of this process is depolarization-induced suppression of inhibition, a form of short-term plasticity where postsynaptically released endocannabinoids suppress presynaptic release of GABA by inhibiting presynaptic Ca2+ channels (Wilson and Nicoll, 2001). This widespread mechanism also operates outside of short-term plasticity to modulate entire neuronal ensembles, as seen for example in the suppression of excitatory synaptic transmission at Schaffer collateral synapses in the CA1 region of the hippocampus by presynaptic muscarinic receptors (Vogt and Regehr, 2001).
In addition to short-term synaptic plasticity due to the interplay of residual Ca2+ and vesicle depletion and to the modulation of presynaptic Ca2+ channels, a third class of mechanisms mediates short-term plasticity via direct changes in the release machinery. Mutations in several proteins associated with the release machinery alter short-term plasticity in a manner independent of the first two sets of mechanisms, for example mutations in synapsins (Rosahl et al., 1995), Munc13 (Augustin et al., 1999), and RIMs (Schoch et al., 2002). The mechanisms by which these mutations cause such changes are largely unclear, except for one protein: Munc13. As we discussed earlier, Munc13 is an active zone protein that is essential for synaptic vesicle priming, probably because it catalyzes SNARE-complex formation via its MUN domain, and that is directly regulated by RIM proteins. However, Munc13 contains three additional signaling domains that appear to regulate its MUN domain and thereby to mediate short-term plasticity, namely its central C2B domain, C1 domain, and a short Ca2+/calmodulin-binding sequence (Figure 5A).
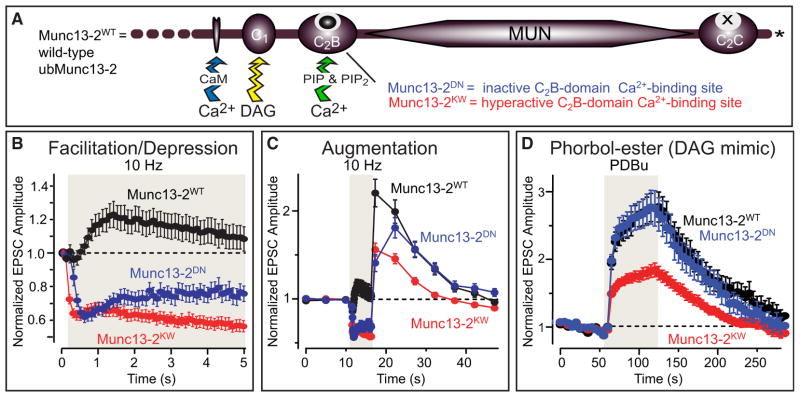
Figure 5. Ca2+ Binding to the Munc13 C2B Domain Mediates Short-Term Synaptic Plasticity.
(A) Schematic drawing of the signaling domain architecture of Munc13-1 and ubMunc13-2 (see Figure 2).
(B–D) Role of Ca2+-binding to the Munc13 C2B domain as analyzed in three different forms of short-term synaptic plasticity, use-dependent depression (B), augmentation (C), and diacylglycerol-induced synaptic facilitation (D). Graphs show normalized EPSC amplitudes in autapses from neurons lacking Munc13-1 and Munc13-2, and expressing wild-type ubMunc13-2 (Munc13-2WT) or two different ubMunc13-2 mutants, namely a mutant in which the C2B domain is unable to bind Ca2+ (Munc13-2DN), or in which the C2B domain Ca2+-binding site is activated to allow Ca2+-binding even in the absence of phosphatidylinositol-phosphates (Munc13-2KW). Note that the activating mutation greatly increases the basal release probability in synapses, thus resulting in use-dependent depression because readily releasable vesicles become rapidly depleted. The inactivating mutation, conversely, has no effect on basal release probability but causes use-dependent depression and impairs augmentation because Ca2+-binding to the Munc13 C2B domain normally accelerates vesicle recruitment during stimulus trains. Note also that the activating mutation decreases the relative efficacy of phorbol esters in enhancing release because it already enhances the release probability, thereby partly occluding the phorbol ester effect. Modified with permission from Shin et al. (2010).
The C2B domain of Munc13—the only Munc13 signaling domain shared by all isoforms (Figure 2)—binds Ca2+ with a relatively high affinity, but only in the presence of phosphatidyl-inositolphosphates (Shin et al., 2010). Blocking of Ca2+ binding to the C2B domain by a mutation in Munc13 dramatically depresses neurotransmitter release during action potential trains, suggesting that this domain serves to convert increasing Ca2+-concentrations during stimulus trains into an increased priming rate for synaptic vesicles (Figures 5B and 5C; Shin et al., 2010). Rendering the C2B domain Ca2+-binding sites independent of phosphatidylinositolphosphates by a mutation in Munc13, conversely, produces depression during stimulus trains because it increases the basal release probability (Figures 5B and 5C). The central C1 domain of Munc13’s binds to diacyl-glycerol as an endogenous ligand and to phorbol esters as a pharmacological activator (Betz et al., 1998), and its activation also dramatically activates neurotransmitter release (Figure 5D; Rhee et al., 2002). Again, this activation appears to normally occur during stimulus trains since mutation of the domain produces a change in short-term synaptic transmission (Rhee et al., 2002). The effects of the C2B and the C1 domain likely occlude each other, since the activating mutation in the C2B domain decreases the effectiveness of the C1 domain activation by phorbol esters in increasing release (Figure 5C). The third signaling motif of Munc13’s, their calmodulin-binding sequence, is also implicated in short-term synaptic plasticity, suggesting that this contributes to the effects of the other two signaling domains (Junge et al., 2004). The close proximity of three signaling sequences in Munc13 is fascinating, as it indicates that the three motifs may act together to integrate intracellular Ca2+-signals, possibly by sensing different time frames, or that they may form a computational node that is sensitive to different types of intracellular messengers.
The active zone not only plays a dominant role in short-term plasticity, but also in long-term plasticity. Strikingly, up to now all forms of presynaptic long-term plasticity investigated—both long-term potentiation (LTP) and long-term depression (LTD)—are blocked by the constitutive knockout of RIM1α (which only partly impairs basal release since all other RIM isoforms are still present), suggesting that the amount of RIM1α available at a synapse is crucial (Castillo et al., 2002; Chevaleyre et al., 2007; Fourcaudot et al., 2008; Lachamp et al., 2009). Moreover, deletions of the corresponding Rab3 isoform in a synapse appears to also block long-term synaptic plasticity, consistent with the notion that long-term plasticity requires the RIM/Rab3 interaction (Castillo et al., 1997; Tsetsenis et al., 2011). However, the mechanisms by which RIM1α acts in presynaptic long-term plasticity remain unknown.
Finally, short-term plasticity is mediated by presynaptic receptors. Many terminals contain presynaptic neurotransmitter receptors, whose role in short-term plasticity is obvious for autoreceptors that recognize the very transmitter being released from a terminal. However, presynaptic endocannabinoid CB1 receptors also have a major role in short- and long-term plasticity, and presynaptic neuropeptide receptors may additionally mediate short-term plasticity if they are for a neuropeptide that is secreted by the terminal upon prolonged stimulation. Presynaptic receptors usually act by inhibiting presynaptic Ca2+ channels and thus represent a major mechanism by which release can be modulated via a uniform pathway that overlaps with other short-term plasticity pathways.
Acknowledgments
I would like to thank Drs. Y. Jin, S. Sigrist, and P. Kaeser for advice and comments on this manuscript and S. Sigrist for Figure 4B. Work on synaptic transmission in my laboratory is supported by the NIMH (grants MH086403 and MH052804), NINDS (grants NS053862 and NS077906), and Simons Foundation (grant 177850).
References
- Ackley BD, Harrington RJ, Hudson ML, Williams L, Kenyon CJ, Chisholm AD, Jin Y. The two isoforms of the Caenorhabditis elegans leukocyte-common antigen related receptor tyrosine phosphatase PTP-3 function independently in axon guidance and synapse formation. J Neurosci. 2005;25:7517–7528. doi: 10.1523/JNEUROSCI.2010-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akert K, Pfenninger K, Sandri C, Moor H. Freeze-etching and cytochemistry of vesicles and membrane complexes in synapses of the C.N.S. In: Pappas GD, Purpura DP, editors. Structure and Function of Synapses. New York: Raven Press; 1972. pp. 67–86. [Google Scholar]
- Altrock WD, tom Dieck S, Sokolov M, Meyer AC, Sigler A, Brakebusch C, Fässler R, Richter K, Boeckers TM, Potschka H, et al. Functional inactivation of a fraction of excitatory synapses in mice deficient for the active zone protein bassoon. Neuron. 2003;37:787–800. doi: 10.1016/s0896-6273(03)00088-6. [DOI] [PubMed] [Google Scholar]
- Astigarraga S, Hofmeyer K, Farajian R, Treisman JE. Three Drosophila liprins interact to control synapse formation. J Neurosci. 2010;30:15358–15368. doi: 10.1523/JNEUROSCI.1862-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atasoy D, Schoch S, Ho A, Nadasy KA, Liu X, Zhang W, Mukherjee K, Nosyreva ED, Fernandez-Chacon R, Missler M, et al. Deletion of CASK in mice is lethal and impairs synaptic function. Proc Natl Acad Sci USA. 2007;104:2525–2530. doi: 10.1073/pnas.0611003104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin I, Rosenmund C, Südhof TC, Brose N. Munc13-1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature. 1999;400:457–461. doi: 10.1038/22768. [DOI] [PubMed] [Google Scholar]
- Basu J, Shen N, Dulubova I, Lu J, Guan R, Guryev O, Grishin NV, Rosenmund C, Rizo J. A minimal domain responsible for Munc13 activity. Nat Struct Mol Biol. 2005;12:1017–1018. doi: 10.1038/nsmb1001. [DOI] [PubMed] [Google Scholar]
- Betz A, Ashery U, Rickmann M, Augustin I, Neher E, Südhof TC, Rettig J, Brose N. Munc13-1 is a presynaptic phorbol ester receptor that enhances neurotransmitter release. Neuron. 1998;21:123–136. doi: 10.1016/s0896-6273(00)80520-6. [DOI] [PubMed] [Google Scholar]
- Betz A, Thakur P, Junge HJ, Ashery U, Rhee JS, Scheuss V, Rosenmund C, Rettig J, Brose N. Functional interaction of the active zone proteins Munc13-1 and RIM1 in synaptic vesicle priming. Neuron. 2001;30:183–196. doi: 10.1016/s0896-6273(01)00272-0. [DOI] [PubMed] [Google Scholar]
- Biederer T, Sara Y, Mozhayeva M, Atasoy D, Liu X, Kavalali ET, Südhof TC. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science. 2002;297:1525–1531. doi: 10.1126/science.1072356. [DOI] [PubMed] [Google Scholar]
- Boudin H, Doan A, Xia J, Shigemoto R, Huganir RL, Worley P, Craig AM. Presynaptic clustering of mGluR7a requires the PICK1 PDZ domain binding site. Neuron. 2000;28:485–497. doi: 10.1016/s0896-6273(00)00127-6. [DOI] [PubMed] [Google Scholar]
- Brodin L, Shupliakov O. Giant reticulospinal synapse in lamprey: molecular links between active and periactive zones. Cell Tissue Res. 2006;326:301–310. doi: 10.1007/s00441-006-0216-2. [DOI] [PubMed] [Google Scholar]
- Brose N, Hofmann K, Hata Y, Südhof TC. Mammalian homologues of Caenorhabditis elegans unc-13 gene define novel family of C2 domain proteins. J Biol Chem. 1995;270:25273–25280. doi: 10.1074/jbc.270.42.25273. [DOI] [PubMed] [Google Scholar]
- Butz S, Okamoto M, Südhof TC. A tripartite protein complex with the potential to couple synaptic vesicle exocytosis to cell adhesion in brain. Cell. 1998;94:773–782. doi: 10.1016/s0092-8674(00)81736-5. [DOI] [PubMed] [Google Scholar]
- Castillo PE, Janz R, Südhof TC, Tzounopoulos T, Malenka RC, Nicoll RA. Rab3A is essential for mossy fibre long-term potentiation in the hippocampus. Nature. 1997;388:590–593. doi: 10.1038/41574. [DOI] [PubMed] [Google Scholar]
- Castillo PE, Schoch S, Schmitz F, Südhof TC, Malenka RC. RIM1α is required for presynaptic long-term potentiation. Nature. 2002;415:327–330. doi: 10.1038/415327a. [DOI] [PubMed] [Google Scholar]
- Catterall WA, Few AP. Calcium channel regulation and presynaptic plasticity. Neuron. 2008;59:882–901. doi: 10.1016/j.neuron.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Chen K, Featherstone DE. Pre and postsynaptic roles for Drosophila CASK. Mol Cell Neurosci. 2011;48:171–182. doi: 10.1016/j.mcn.2011.07.009. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Heifets BD, Kaeser PS, Südhof TC, Castillo PE. Endocannabinoid-mediated long-term plasticity requires cAMP/PKA signaling and RIM1α. Neuron. 2007;54:801–812. doi: 10.1016/j.neuron.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AR, Woods DF, Marfatia SM, Walther Z, Chishti AH, Anderson JM. Human CASK/LIN-2 binds syndecan-2 and protein 4.1 and localizes to the basolateral membrane of epithelial cells. J Cell Biol. 1998;142:129–138. doi: 10.1083/jcb.142.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppola T, Magnin-Luthi S, Perret-Menoud V, Gattesco S, Schiavo G, Regazzi R. Direct interaction of the Rab3 effector RIM with Ca2+ channels, SNAP-25, and synaptotagmin. J Biol Chem. 2001;276:32756–32762. doi: 10.1074/jbc.M100929200. [DOI] [PubMed] [Google Scholar]
- Corti C, Aldegheri L, Somogyi P, Ferraguti F. Distribution and synaptic localisation of the metabotropic glutamate receptor 4 (mGluR4) in the rodent CNS. Neuroscience. 2002;110:403–420. doi: 10.1016/s0306-4522(01)00591-7. [DOI] [PubMed] [Google Scholar]
- Couteaux R, Pecot-Dechavassine M. L’Ouverture des vesicules synaptiques au niveau des ‘zones actives.’. Septieme Congres International de Microscopie Electronique; Grenoble, France. 1970. pp. 709–710. [Google Scholar]
- Dai Y, Taru H, Deken SL, Grill B, Ackley B, Nonet ML, Jin Y. SYD-2 Liprin-α organizes presynaptic active zone formation through ELKS. Nat Neurosci. 2006;9:1479–1487. doi: 10.1038/nn1808. [DOI] [PubMed] [Google Scholar]
- Dani A, Huang B, Bergan J, Dulac C, Zhuang X. Superresolution imaging of chemical synapses in the brain. Neuron. 2010;68:843–856. doi: 10.1016/j.neuron.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deken SL, Vincent R, Hadwiger G, Liu Q, Wang ZW, Nonet ML. Redundant localization mechanisms of RIM and ELKS in Caenorhabditis elegans. J Neurosci. 2005;25:5975–5983. doi: 10.1523/JNEUROSCI.0804-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMaria CD, Soong TW, Alseikhan BA, Alvania RS, Yue DT. Calmodulin bifurcates the local Ca2+ signal that modulates P/Q-type Ca2+ channels. Nature. 2001;411:484–489. doi: 10.1038/35078091. [DOI] [PubMed] [Google Scholar]
- Deng L, Kaeser PS, Xu W, Südhof TC. RIM proteins activate vesicle priming by reversing autoinhibitory homodimerization of Munc13. Neuron. 2011;69:317–331. doi: 10.1016/j.neuron.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dev KK, Nakajima Y, Kitano J, Braithwaite SP, Henley JM, Nakanishi S. PICK1 interacts with and regulates PKC phosphorylation of mGLUR7. J Neurosci. 2000;20:7252–7257. doi: 10.1523/JNEUROSCI.20-19-07252.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulubova I, Lou X, Lu J, Huryeva I, Alam A, Schneggenburger R, Südhof TC, Rizo J. A Munc13/RIM/Rab3 tripartite complex: from priming to plasticity? EMBO J. 2005;24:2839–2850. doi: 10.1038/sj.emboj.7600753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann J, Callebaut I, Raposo G, Certain S, Bacq D, Dumont C, Lambert N, Ouachée-Chardin M, Chedeville G, Tamary H, et al. Munc13-4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3) Cell. 2003;115:461–473. doi: 10.1016/s0092-8674(03)00855-9. [DOI] [PubMed] [Google Scholar]
- Fenster SD, Chung WJ, Zhai R, Cases-Langhoff C, Voss B, Garner AM, Kaempf U, Kindler S, Gundelfinger ED, Garner CC. Piccolo, a presynaptic zinc finger protein structurally related to bassoon. Neuron. 2000;25:203–214. doi: 10.1016/s0896-6273(00)80883-1. [DOI] [PubMed] [Google Scholar]
- Fernández-Busnadiego R, Zuber B, Maurer UE, Cyrklaff M, Baumeister W, Lucic V. Quantitative analysis of the native presynaptic cytomatrix by cryoelectron tomography. J Cell Biol. 2010;188:145–156. doi: 10.1083/jcb.200908082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraguti F, Klausberger T, Cobden P, Baude A, Roberts JD, Szucs P, Kinoshita A, Shigemoto R, Somogyi P, Dalezios Y. Metabotropic glutamate receptor 8-expressing nerve terminals target subsets of GABAergic neurons in the hippocampus. J Neurosci. 2005;25:10520–10536. doi: 10.1523/JNEUROSCI.2547-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fioravante D, Regehr WG. Short-term forms of presynaptic plasticity. Curr Opin Neurobiol. 2011;21:269–274. doi: 10.1016/j.conb.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourcaudot E, Gambino F, Humeau Y, Casassus G, Shaban H, Poulain B, Lüthi A. cAMP/PKA signaling and RIM1α mediate presynaptic LTP in the lateral amygdala. Proc Natl Acad Sci USA. 2008;105:15130–15135. doi: 10.1073/pnas.0806938105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M. Distinct Rab binding specificity of Rim1, Rim2, rabphilin, and Noc2. Identification of a critical determinant of Rab3A/Rab27A recognition by Rim2. J Biol Chem. 2003;278:15373–15380. doi: 10.1074/jbc.M212341200. [DOI] [PubMed] [Google Scholar]
- Galiègue S, Jbilo O, Combes T, Bribes E, Carayon P, Le Fur G, Casellas P. Cloning and characterization of PRAX-1. A new protein that specifically interacts with the peripheral benzodiazepine receptor. J Biol Chem. 1999;274:2938–2952. doi: 10.1074/jbc.274.5.2938. [DOI] [PubMed] [Google Scholar]
- Garcia J, Gerber SH, Sugita S, Südhof TC, Rizo J. A conformational switch in the Piccolo C2A domain regulated by alternative splicing. Nat Struct Mol Biol. 2004;11:45–53. doi: 10.1038/nsmb707. [DOI] [PubMed] [Google Scholar]
- Gasparini S, Kasyanov AM, Pietrobon D, Voronin LL, Cherubini E. Presynaptic R-type calcium channels contribute to fast excitatory synaptic transmission in the rat hippocampus. J Neurosci. 2001;21:8715–8721. doi: 10.1523/JNEUROSCI.21-22-08715.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber SH, Garcia J, Rizo J, Südhof TC. An unusual C(2)-domain in the active-zone protein piccolo: implications for Ca(2+) regulation of neurotransmitter release. EMBO J. 2001;20:1605–1619. doi: 10.1093/emboj/20.7.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber SH, Rah JC, Min SW, Liu X, de Wit H, Dulubova I, Meyer AC, Rizo J, Arancillo M, Hammer RE, et al. Conformational switch of syntaxin-1 controls synaptic vesicle fusion. Science. 2008;321:1507–1510. doi: 10.1126/science.1163174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracheva EO, Hadwiger G, Nonet ML, Richmond JE. Direct interactions between C. elegans RAB-3 and Rim provide a mechanism to target vesicles to the presynaptic density. Neurosci Lett. 2008;444:137–142. doi: 10.1016/j.neulet.2008.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray EG. Electron microscopy of presynaptic organelles of the spinal cord. J Anat. 1963;97:101–106. [PMC free article] [PubMed] [Google Scholar]
- Hallam SJ, Goncharov A, McEwen J, Baran R, Jin Y. SYD-1, a presynaptic protein with PDZ, C2 and rhoGAP-like domains, specifies axon identity in C. elegans. Nat Neurosci. 2002;5:1137–1146. doi: 10.1038/nn959. [DOI] [PubMed] [Google Scholar]
- Hallermann S, Fejtova A, Schmidt H, Weyhersmüller A, Silver RA, Gundelfinger ED, Eilers J. Bassoon speeds vesicle reloading at a central excitatory synapse. Neuron. 2010;68:710–723. doi: 10.1016/j.neuron.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Kaeser PS, Südhof TC, Schneggenburger R. RIM determines Ca2+ channel density and vesicle docking at the presynaptic active zone. Neuron. 2011;69:304–316. doi: 10.1016/j.neuron.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow ML, Ress D, Stoschek A, Marshall RM, McMahan UJ. The architecture of active zone material at the frog’s neuromuscular junction. Nature. 2001;409:479–484. doi: 10.1038/35054000. [DOI] [PubMed] [Google Scholar]
- Hata Y, Butz S, Südhof TC. CASK: a novel dlg/PSD95 homolog with an N-terminal calmodulin-dependent protein kinase domain identified by interaction with neurexins. J Neurosci. 1996;16:2488–2494. doi: 10.1523/JNEUROSCI.16-08-02488.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyden A, Ionescu MC, Romorini S, Kracht B, Ghiglieri V, Calabresi P, Seidenbecher C, Angenstein F, Gundelfinger ED. Hippocampal enlargement in Bassoon-mutant mice is associated with enhanced neurogenesis, reduced apoptosis, and abnormal BDNF levels. Cell Tissue Res. 2011;346:11–26. doi: 10.1007/s00441-011-1233-3. [DOI] [PubMed] [Google Scholar]
- Hibino H, Pironkova R, Onwumere O, Vologodskaia M, Hudspeth AJ, Lesage F. RIM binding proteins (RBPs) couple Rab3-interacting molecules (RIMs) to voltage-gated Ca(2+) channels. Neuron. 2002;34:411–423. doi: 10.1016/s0896-6273(02)00667-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskins R, Hajnal AF, Harp SA, Kim SK. The C. elegans vulval induction gene lin-2 encodes a member of the MAGUK family of cell junction proteins. Development. 1996;122:97–111. doi: 10.1242/dev.122.1.97. [DOI] [PubMed] [Google Scholar]
- Hsueh YP, Yang FC, Kharazia V, Naisbitt S, Cohen AR, Weinberg RJ, Sheng M. Direct interaction of CASK/LIN-2 and syndecan heparan sulfate proteoglycan and their overlapping distribution in neuronal synapses. J Cell Biol. 1998;142:139–151. doi: 10.1083/jcb.142.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Zakharenko SS, Schoch S, Kaeser PS, Janz R, Südhof TC, Siegelbaum SA, Kandel ER. Genetic evidence for a protein-kinase-A-mediated presynaptic component in NMDA-receptor-dependent forms of long-term synaptic potentiation. Proc Natl Acad Sci USA. 2005;102:9365–9370. doi: 10.1073/pnas.0503777102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jockusch WJ, Speidel D, Sigler A, Sørensen JB, Varoqueaux F, Rhee JS, Brose N. CAPS-1 and CAPS-2 are essential synaptic vesicle priming proteins. Cell. 2007;131:796–808. doi: 10.1016/j.cell.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Junge HJ, Rhee JS, Jahn O, Varoqueaux F, Spiess J, Waxham MN, Rosenmund C, Brose N. Calmodulin and Munc13 form a Ca2+ sensor/effector complex that controls short-term synaptic plasticity. Cell. 2004;118:389–401. doi: 10.1016/j.cell.2004.06.029. [DOI] [PubMed] [Google Scholar]
- Kaech SM, Whitfield CW, Kim SK. The LIN-2/LIN-7/LIN-10 complex mediates basolateral membrane localization of the C. elegans EGF receptor LET-23 in vulval epithelial cells. Cell. 1998;94:761–771. doi: 10.1016/s0092-8674(00)81735-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeser PS, Kwon HB, Chiu CQ, Deng L, Castillo PE, Südhof TC. RIM1α and RIM1β are synthesized from distinct promoters of the RIM1 gene to mediate differential but overlapping synaptic functions. J Neurosci. 2008a;28:13435–13447. doi: 10.1523/JNEUROSCI.3235-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeser PS, Kwon HB, Blundell J, Chevaleyre V, Morishita W, Malenka RC, Powell CM, Castillo PE, Südhof TC. RIM1α phosphorylation at serine-413 by protein kinase A is not required for presynaptic long-term plasticity or learning. Proc Natl Acad Sci USA. 2008b;105:14680–14685. doi: 10.1073/pnas.0806679105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeser PS, Deng L, Chávez AE, Liu X, Castillo PE, Südhof TC. ELKS2α/CAST deletion selectively increases neurotransmitter release at inhibitory synapses. Neuron. 2009;64:227–239. doi: 10.1016/j.neuron.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeser PS, Deng L, Wang Y, Dulubova I, Liu X, Rizo J, Südhof TC. RIM proteins tether Ca2+ channels to presynaptic active zones via a direct PDZ-domain interaction. Cell. 2011;144:282–295. doi: 10.1016/j.cell.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeser PS, Deng L, Fan M, Südhof TC. RIM genes differentially contribute to organizing presynaptic release sites. Proc Natl Acad Sci USA. 2012 doi: 10.1073/pnas.1209318109. Published online July 2, 2012. http://dx.doi.org/10.1073/pnas. 1209318109. [DOI] [PMC free article] [PubMed]
- Kaufmann N, DeProto J, Ranjan R, Wan H, Van Vactor D. Drosophila liprin-α and the receptor phosphatase Dlar control synapse morphogenesis. Neuron. 2002;34:27–38. doi: 10.1016/s0896-6273(02)00643-8. [DOI] [PubMed] [Google Scholar]
- Khodthong C, Kabachinski G, James DJ, Martin TF. Munc13 homology domain-1 in CAPS/UNC31 mediates SNARE binding required for priming vesicle exocytosis. Cell Metab. 2011;14:254–263. doi: 10.1016/j.cmet.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittel RJ, Wichmann C, Rasse TM, Fouquet W, Schmidt M, Schmid A, Wagh DA, Pawlu C, Kellner RR, Willig KI, et al. Bruchpilot promotes active zone assembly, Ca2+ channel clustering, and vesicle release. Science. 2006;312:1051–1054. doi: 10.1126/science.1126308. [DOI] [PubMed] [Google Scholar]
- Ko J, Na M, Kim S, Lee JR, Kim E. Interaction of the ERC family of RIM-binding proteins with the liprin-α family of multidomain proteins. J Biol Chem. 2003a;278:42377–42385. doi: 10.1074/jbc.M307561200. [DOI] [PubMed] [Google Scholar]
- Ko J, Kim S, Valtschanoff JG, Shin H, Lee JR, Sheng M, Premont RT, Weinberg RJ, Kim E. Interaction between liprin-alpha and GIT1 is required for AMPA receptor targeting. J Neurosci. 2003b;23:1667–1677. doi: 10.1523/JNEUROSCI.23-05-01667.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko J, Yoon C, Piccoli G, Chung HS, Kim K, Lee JR, Lee HW, Kim H, Sala C, Kim E. Organization of the presynaptic active zone by ERC2/CAST1-dependent clustering of the tandem PDZ protein syntenin-1. J Neurosci. 2006;26:963–970. doi: 10.1523/JNEUROSCI.4475-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Takei H, Yamamoto K, Hatanaka H, Koshikawa N. Kinetics of GABAB autoreceptor-mediated suppression of GABA release in rat insular cortex. J Neurophysiol. 2012;107:1431–1442. doi: 10.1152/jn.00813.2011. [DOI] [PubMed] [Google Scholar]
- Koch H, Hofmann K, Brose N. Definition of Munc13-homology-domains and characterization of a novel ubiquitously expressed Munc13 isoform. Biochem J. 2000;349:247–253. doi: 10.1042/0264-6021:3490247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koester HJ, Johnston D. Target cell-dependent normalization of transmitter release at neocortical synapses. Science. 2005;308:863–866. doi: 10.1126/science.1100815. [DOI] [PubMed] [Google Scholar]
- Kogo N, Dalezios Y, Capogna M, Ferraguti F, Shigemoto R, Somogyi P. Depression of GABAergic input to identified hippocampal neurons by group III metabotropic glutamate receptors in the rat. Eur J Neurosci. 2004;19:2727–2740. doi: 10.1111/j.0953-816X.2004.03394.x. [DOI] [PubMed] [Google Scholar]
- Koushika SP, Richmond JE, Hadwiger G, Weimer RM, Jorgensen EM, Nonet ML. A post-docking role for active zone protein Rim. Nat Neurosci. 2001;4:997–1005. doi: 10.1038/nn732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachamp PM, Liu Y, Liu SJ. Glutamatergic modulation of cerebellar interneuron activity is mediated by an enhancement of GABA release and requires protein kinase A/RIM1α signaling. J Neurosci. 2009;29:381–392. doi: 10.1523/JNEUROSCI.2354-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis DM, Hall AK, Weinstein LA, Reese TS. The organization of cytoplasm at the presynaptic active zone of a central nervous system synapse. Neuron. 1988;1:201–209. doi: 10.1016/0896-6273(88)90140-7. [DOI] [PubMed] [Google Scholar]
- Lautermilch NJ, Few AP, Scheuer T, Catterall WA. Modulation of CaV2.1 channels by the neuronal calcium-binding protein visinin-like protein-2. J Neurosci. 2005;25:7062–7070. doi: 10.1523/JNEUROSCI.0447-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Westenbroek RE, Haeseleer F, Palczewski K, Scheuer T, Catterall WA. Differential modulation of Ca(v)2.1 channels by calmodulin and Ca2+-binding protein 1. Nat Neurosci. 2002;5:210–217. doi: 10.1038/nn805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Zhou H, Scheuer T, Catterall WA. Molecular determinants of Ca(2+)/calmodulin-dependent regulation of Ca(v)2.1 channels. Proc Natl Acad Sci USA. 2003;100:16059–16064. doi: 10.1073/pnas.2237000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Bischofberger J, Jonas P. Differential gating and recruitment of P/Q-, N-, and R-type Ca2+ channels in hippocampal mossy fiber boutons. J Neurosci. 2007;27:13420–13429. doi: 10.1523/JNEUROSCI.1709-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Ma C, Guan R, Xu Y, Tomchick DR, Rizo J. The crystal structure of a Munc13 C-terminal module exhibits a remarkable similarity to vesicle tethering factors. Structure. 2011;19:1443–1455. doi: 10.1016/j.str.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limbach C, Laue MM, Wang X, Hu B, Thiede N, Hultqvist G, Kilimann MW. Molecular in situ topology of Aczonin/Piccolo and associated proteins at the mammalian neurotransmitter release site. Proc Natl Acad Sci USA. 2011;108:E392–E401. doi: 10.1073/pnas.1101707108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu KS, Siebert M, Mertel S, Knoche E, Wegener S, Wichmann C, Matkovic T, Muhammad K, Depner H, Mettke C, et al. RIM-binding protein, a central part of the active zone, is essential for neurotransmitter release. Science. 2011;334:1565–1569. doi: 10.1126/science.1212991. [DOI] [PubMed] [Google Scholar]
- Lonart G, Schoch S, Kaeser PS, Larkin CJ, Südhof TC, Linden DJ. Phosphorylation of RIM1α by PKA triggers presynaptic long-term potentiation at cerebellar parallel fiber synapses. Cell. 2003;115:49–60. doi: 10.1016/s0092-8674(03)00727-x. [DOI] [PubMed] [Google Scholar]
- Lorincz A, Nusser Z. Molecular identity of dendritic voltage-gated sodium channels. Science. 2010;328:906–909. doi: 10.1126/science.1187958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Machius M, Dulubova I, Dai H, Südhof TC, Tomchick DR, Rizo J. Structural basis for a Munc13-1 homodimer to Munc13-1/RIM heterodimer switch. PLoS Biol. 2006;4:e192. doi: 10.1371/journal.pbio.0040192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luján R, Shigemoto R, Kulik A, Juiz JM. Localization of the GABAB receptor 1a/b subunit relative to glutamatergic synapses in the dorsal cochlear nucleus of the rat. J Comp Neurol. 2004;475:36–46. doi: 10.1002/cne.20160. [DOI] [PubMed] [Google Scholar]
- Ma C, Li W, Xu Y, Rizo J. Munc13 mediates the transition from the closed syntaxin-Munc18 complex to the SNARE complex. Nat Struct Mol Biol. 2011;18:542–549. doi: 10.1038/nsmb.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama IN, Brenner S. A phorbol ester/diacylglycerol-binding protein encoded by the unc-13 gene of Caenorhabditis elegans. Proc Natl Acad Sci USA. 1991;88:5729–5733. doi: 10.1073/pnas.88.13.5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews G, Fuchs P. The diverse roles of ribbon synapses in sensory neurotransmission. Nat Rev Neurosci. 2010;11:812–822. doi: 10.1038/nrn2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinrenken CJ, Borst JG, Sakmann B. Local routes revisited: the space and time dependence of the Ca2+ signal for phasic transmitter release at the rat calyx of Held. J Physiol. 2003;547:665–689. doi: 10.1113/jphysiol.2002.032714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelstaedt T, Schoch S. Structure and evolution of RIM-BP genes: identification of a novel family member. Gene. 2007;403:70–79. doi: 10.1016/j.gene.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Monier S, Jollivet F, Janoueix-Lerosey I, Johannes L, Goud B. Characterization of novel Rab6-interacting proteins involved in endosome-to-TGN transport. Traffic. 2002;3:289–297. doi: 10.1034/j.1600-0854.2002.030406.x. [DOI] [PubMed] [Google Scholar]
- Moog U, Kutsche K, Kortüm F, Chilian B, Bierhals T, Apeshiotis N, Balg S, Chassaing N, Coubes C, Das S, et al. Phenotypic spectrum associated with CASK loss-of-function mutations. J Med Genet. 2011;48:741–751. doi: 10.1136/jmedgenet-2011-100218. [DOI] [PubMed] [Google Scholar]
- Mukherjee K, Sharma M, Urlaub H, Bourenkov GP, Jahn R, Südhof TC, Wahl MC. CASK Functions as a Mg2+-independent neurexin kinase. Cell. 2008;133:328–339. doi: 10.1016/j.cell.2008.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee K, Yang X, Gerber SH, Kwon HB, Ho A, Castillo PE, Liu X, Südhof TC. Piccolo and bassoon maintain synaptic vesicle clustering without directly participating in vesicle exocytosis. Proc Natl Acad Sci USA. 2010;107:6504–6509. doi: 10.1073/pnas.1002307107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata T, Kitamura Y, Shimizu K, Tanaka S, Fujimori M, Yokoyama S, Ito K, Emi M. Fusion of a novel gene, ELKS, to RET due to translocation t(10;12)(q11;p13) in a papillary thyroid carcinoma. Genes Chromosomes Cancer. 1999;25:97–103. doi: 10.1002/(sici)1098-2264(199906)25:2<97::aid-gcc4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Nyíri G, Cserép C, Szabadits E, Mackie K, Freund TF. CB1 cannabinoid receptors are enriched in the perisynaptic annulus and on preterminal segments of hippocampal GABAergic axons. Neuroscience. 2005;136:811–822. doi: 10.1016/j.neuroscience.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Ohtsuka T, Takao-Rikitsu E, Inoue E, Inoue M, Takeuchi M, Matsubara K, Deguchi-Tawarada M, Satoh K, Morimoto K, Nakanishi H, Takai Y. Cast: a novel protein of the cytomatrix at the active zone of synapses that forms a ternary complex with RIM1 and munc13-1. J Cell Biol. 2002;158:577–590. doi: 10.1083/jcb.200202083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen O, Moore KA, Fukata M, Kazuta T, Trinidad JC, Kauer FW, Streuli M, Misawa H, Burlingame AL, Nicoll RA, Bredt DS. Neurotransmitter release regulated by a MALS-liprin-α presynaptic complex. J Cell Biol. 2005;170:1127–1134. doi: 10.1083/jcb.200503011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owald D, Fouquet W, Schmidt M, Wichmann C, Mertel S, Depner H, Christiansen F, Zube C, Quentin C, Körner J, et al. A Syd-1 homologue regulates pre- and postsynaptic maturation in Drosophila. J Cell Biol. 2010;188:565–579. doi: 10.1083/jcb.200908055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel MR, Lehrman EK, Poon VY, Crump JG, Zhen M, Bargmann CI, Shen K. Hierarchical assembly of presynaptic components in defined C. elegans synapses. Nat Neurosci. 2006;9:1488–1498. doi: 10.1038/nn1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkey KA, Topolnik L, Yuan XQ, Lacaille JC, McBain CJ. State-dependent cAMP sensitivity of presynaptic function underlies metaplasticity in a hippocampal feedforward inhibitory circuit. Neuron. 2008;60:980–987. doi: 10.1016/j.neuron.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfenninger K, Akert K, Moor H, Sandri C. The fine structure of freeze-fractured presynaptic membranes. J Neurocytol. 1972;1:129–149. doi: 10.1007/BF01099180. [DOI] [PubMed] [Google Scholar]
- Rhee JS, Betz A, Pyott S, Reim K, Varoqueaux F, Augustin I, Hesse D, Südhof TC, Takahashi M, Rosenmund C, Brose N. Phorbol ester- and diacylglycerol-induced augmentation of neurotransmitter release from hippocampal neurons is mediated by Munc13s and not by PKCs. Cell. 2002;108:121–133. doi: 10.1016/s0092-8674(01)00635-3. [DOI] [PubMed] [Google Scholar]
- Richmond JE, Weimer RM, Jorgensen EM. An open form of syntaxin bypasses the requirement for UNC-13 in vesicle priming. Nature. 2001;412:338–341. doi: 10.1038/35085583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosahl TW, Spillane D, Missler M, Herz J, Selig DK, Wolff JR, Hammer RE, Malenka RC, Südhof TC. Essential functions of synapsins I and II in synaptic vesicle regulation. Nature. 1995;375:488–493. doi: 10.1038/375488a0. [DOI] [PubMed] [Google Scholar]
- Sakamoto S, Ishizaki T, Okawa K, Watanabe S, Arakawa T, Watanabe N, Narumiya S. Liprin-α controls stress fiber formation by binding to mDia and regulating its membrane localization. J Cell Sci. 2012;125:108–120. doi: 10.1242/jcs.087411. [DOI] [PubMed] [Google Scholar]
- Schmitz F, Königstorfer A, Södhof TC. RIBEYE, a component of synaptic ribbons: a protein’s journey through evolution provides insight into synaptic ribbon function. Neuron. 2000;28:857–872. doi: 10.1016/s0896-6273(00)00159-8. [DOI] [PubMed] [Google Scholar]
- Schoch S, Castillo PE, Jo T, Mukherjee K, Geppert M, Wang Y, Schmitz F, Malenka RC, Södhof TC. RIM1α forms a protein scaffold for regulating neurotransmitter release at the active zone. Nature. 2002;415:321–326. doi: 10.1038/415321a. [DOI] [PubMed] [Google Scholar]
- Schoch S, Mittelstaedt T, Kaeser PS, Padgett D, Feldmann N, Chevaleyre V, Castillo PE, Hammer RE, Han W, Schmitz F, et al. Redundant functions of RIM1α and RIM2α in Ca(2+)-triggered neuro-transmitter release. EMBO J. 2006;25:5852–5863. doi: 10.1038/sj.emboj.7601425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra-Pagès C, Kedersha NL, Fazikas L, Medley Q, Debant A, Streuli M. The LAR transmembrane protein tyrosine phosphatase and a coiled-coil LAR-interacting protein co-localize at focal adhesions. EMBO J. 1995;14:2827–2838. doi: 10.1002/j.1460-2075.1995.tb07282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemoto R, Kulik A, Roberts JD, Ohishi H, Nusser Z, Kaneko T, Somogyi P. Target-cell-specific concentration of a metabotropic glutamate receptor in the presynaptic active zone. Nature. 1996;381:523–525. doi: 10.1038/381523a0. [DOI] [PubMed] [Google Scholar]
- Shin OH, Lu J, Rhee JS, Tomchick DR, Pang ZP, Wojcik SM, Camacho-Perez M, Brose N, Machius M, Rizo J, et al. Munc13 C2B domain is an activity-dependent Ca2+ regulator of synaptic exocytosis. Nat Struct Mol Biol. 2010;17:280–288. doi: 10.1038/nsmb.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siksou L, Varoqueaux F, Pascual O, Triller A, Brose N, Marty S. A common molecular basis for membrane docking and functional priming of synaptic vesicles. Eur J Neurosci. 2009;30:49–56. doi: 10.1111/j.1460-9568.2009.06811.x. [DOI] [PubMed] [Google Scholar]
- Song Y, Ailenberg M, Silverman M. Cloning of a novel gene in the human kidney homologous to rat munc13s: its potential role in diabetic nephropathy. Kidney Int. 1998;53:1689–1695. doi: 10.1046/j.1523-1755.1998.00942.x. [DOI] [PubMed] [Google Scholar]
- Song JY, Ichtchenko K, Südhof TC, Brose N. Neuroligin 1 is a postsynaptic cell-adhesion molecule of excitatory synapses. Proc Natl Acad Sci USA. 1999;96:1100–1105. doi: 10.1073/pnas.96.3.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel I, Salomon D, Erne B, Schaeren-Wiemers N, Peles E. Caspr3 and caspr4, two novel members of the caspr family are expressed in the nervous system and interact with PDZ domains. Mol Cell Neurosci. 2002;20:283–297. doi: 10.1006/mcne.2002.1110. [DOI] [PubMed] [Google Scholar]
- Stevens DR, Wu ZX, Matti U, Junge HJ, Schirra C, Becherer U, Wojcik SM, Brose N, Rettig J. Identification of the minimal protein domain required for priming activity of Munc13-1. Curr Biol. 2005;15:2243–2248. doi: 10.1016/j.cub.2005.10.055. [DOI] [PubMed] [Google Scholar]
- Stigloher C, Zhan H, Zhen M, Richmond J, Bessereau JL. The presynaptic dense projection of the Caenorhabditis elegans cholinergic neuromuscular junction localizes synaptic vesicles at the active zone through SYD-2/liprin and UNC-10/RIM-dependent interactions. J Neurosci. 2011;31:4388–4396. doi: 10.1523/JNEUROSCI.6164-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Liu L, Zeng X, Xu M, Liu L, Fang M, Xie W. Genetic interaction between Neurexin and CAKI/CMG is important for synaptic function in Drosophila neuromuscular junction. Neurosci Res. 2009;64:362–371. doi: 10.1016/j.neures.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Tabuchi K, Biederer T, Butz S, Südhof TC. CASK participates in alternative tripartite complexes in which Mint 1 competes for binding with caskin 1, a novel CASK-binding protein. J Neurosci. 2002;22:4264–4273. doi: 10.1523/JNEUROSCI.22-11-04264.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takao-Rikitsu E, Mochida S, Inoue E, Deguchi-Tawarada M, Inoue M, Ohtsuka T, Takai Y. Physical and functional interaction of the active zone proteins, CAST, RIM1, and Bassoon, in neurotransmitter release. J Cell Biol. 2004;164:301–311. doi: 10.1083/jcb.200307101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taru H, Jin Y. The Liprin homology domain is essential for the homomeric interaction of SYD-2/Liprin-α protein in presynaptic assembly. J Neurosci. 2011;31:16261–16268. doi: 10.1523/JNEUROSCI.0002-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- tom Dieck S, Sanmartí-Vila L, Langnaese K, Richter K, Kindler S, Soyke A, Wex H, Smalla KH, Kämpf U, Fränzer JT, et al. Bassoon, a novel zinc-finger CAG/glutamine-repeat protein selectively localized at the active zone of presynaptic nerve terminals. J Cell Biol. 1998;142:499–509. doi: 10.1083/jcb.142.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsetsenis T, Younts TJ, Chiu CQ, Kaeser PS, Castillo PE, Südhof TC. Rab3B protein is required for long-term depression of hippocampal inhibitory synapses and for normal reversal learning. Proc Natl Acad Sci USA. 2011;108:14300–14305. doi: 10.1073/pnas.1112237108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N, Honjo Y, Johnson KR, Wheelock MJ, Takeichi M. The catenin/cadherin adhesion system is localized in synaptic junctions bordering transmitter release zones. J Cell Biol. 1996;135:767–779. doi: 10.1083/jcb.135.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uriu Y, Kiyonaka S, Miki T, Yagi M, Akiyama S, Mori E, Nakao A, Beedle AM, Campbell KP, Wakamori M, Mori Y. Rab3-interacting molecule gamma isoforms lacking the Rab3-binding domain induce long lasting currents but block neurotransmitter vesicle anchoring in voltage-dependent P/Q-type Ca2+ channels. J Biol Chem. 2010;285:21750–21767. doi: 10.1074/jbc.M110.101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varoqueaux F, Sigler A, Rhee JS, Brose N, Enk C, Reim K, Rosenmund C. Total arrest of spontaneous and evoked synaptic transmission but normal synaptogenesis in the absence of Munc13-mediated vesicle priming. Proc Natl Acad Sci USA. 2002;99:9037–9042. doi: 10.1073/pnas.122623799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varoqueaux F, Sons MS, Plomp JJ, Brose N. Aberrant morphology and residual transmitter release at the Munc13-deficient mouse neuromuscular synapse. Mol Cell Biol. 2005;25:5973–5984. doi: 10.1128/MCB.25.14.5973-5984.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt KE, Regehr WG. Cholinergic modulation of excitatory synaptic transmission in the CA3 area of the hippocampus. J Neurosci. 2001;21:75–83. doi: 10.1523/JNEUROSCI.21-01-00075.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagh DA, Rasse TM, Asan E, Hofbauer A, Schwenkert I, Dürrbeck H, Buchner S, Dabauvalle MC, Schmidt M, Qin G, et al. Bruchpilot, a protein with homology to ELKS/CAST, is required for structural integrity and function of synaptic active zones in Drosophila. Neuron. 2006;49:833–844. doi: 10.1016/j.neuron.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Wang Y, Südhof TC. Genomic definition of RIM proteins: evolutionary amplification of a family of synaptic regulatory proteins(small star, filled) Genomics. 2003;81:126–137. doi: 10.1016/s0888-7543(02)00024-1. [DOI] [PubMed] [Google Scholar]
- Wang DY, Wang Y. HLB-1 functions as a new regulator for the organization and function of neuromuscular junctions in nematode Caenorhabditis elegans. Neurosci Bull. 2009;25:75–86. doi: 10.1007/s12264-009-0119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Okamoto M, Schmitz F, Hofmann K, Südhof TC. Rim is a putative Rab3 effector in regulating synaptic-vesicle fusion. Nature. 1997;388:593–598. doi: 10.1038/41580. [DOI] [PubMed] [Google Scholar]
- Wang X, Kibschull M, Laue MM, Lichte B, Petrasch-Parwez E, Kilimann MW. Aczonin, a 550-kD putative scaffolding protein of presynaptic active zones, shares homology regions with Rim and Bassoon and binds profilin. J Cell Biol. 1999;147:151–162. doi: 10.1083/jcb.147.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Sugita S, Südhof TC. The RIM/NIM family of neuronal C2 domain proteins. Interactions with Rab3 and a new class of Src homology 3 domain proteins. J Biol Chem. 2000;275:20033–20044. doi: 10.1074/jbc.M909008199. [DOI] [PubMed] [Google Scholar]
- Wang Y, Liu X, Biederer T, Südhof TC. A family of RIM-binding proteins regulated by alternative splicing: Implications for the genesis of synaptic active zones. Proc Natl Acad Sci USA. 2002;99:14464–14469. doi: 10.1073/pnas.182532999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z, Zheng S, Spangler SA, Yu C, Hoogenraad CC, Zhang M. Liprin-mediated large signaling complex organization revealed by the liprin-α/CASK and liprin-α/liprin-β complex structures. Mol Cell. 2011;43:586–598. doi: 10.1016/j.molcel.2011.07.021. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- Zhen M, Jin Y. The liprin protein SYD-2 regulates the differentiation of presynaptic termini in C. elegans. Nature. 1999;401:371–375. doi: 10.1038/43886. [DOI] [PubMed] [Google Scholar]
- Zordan MA, Massironi M, Ducato MG, Te Kronnie G, Costa R, Reggiani C, Chagneau C, Martin JR, Megighian A. Drosophila CAKI/CMG protein, a homolog of human CASK, is essential for regulation of neurotransmitter vesicle release. J Neurophysiol. 2005;94:1074–1083. doi: 10.1152/jn.00954.2004. [DOI] [PubMed] [Google Scholar]