Abstract
Endometrial cancer is preceded by endometrial hyperplasia, unopposed estrogen exposure and genetic alterations, but the precise causes of endometrial cancer remain uncertain. Mig-6, mainly known as a negative regulator of the EGF receptor, is an important mediator of progesterone signaling in the uterus, where it mediates tumor suppression by modulating endometrial stromal-epithelial communications. In this study, we investigated the function of Mig-6 in the uterine epithelium using a tissue-specific gene knockout strategy, in which floxed Mig-6 (Mig-6f/f) mice were crossed to Wnt7a-Cre mice (Wnt7acre+ Mig-6f/f). Wnt7acre+ Mig-6f/f mice developed endometrial hyperplasia and estrogen-dependent endometrial cancer, exhibiting increased proliferation in epithelial cells as well as apoptosis in sub-epithelial stromal cells. We documented increased expression of NOTCH1 and BIRC3 in epithelial cells of Wnt7acre+ Mig-6f/f mice and decreased expression of the progesterone receptor (PR) in stromal cells. Progesterone therapy controls endometrial growth and prevents endometrial cancer, but the effectiveness of progesterone as a treatment for women with endometrial cancer is less clear. We noted that the hyperplasic phenotype of Wnt7acre+ Mig-6f/f mice was prevented by progesterone treatment, whereas this treatment had no effect in PRcre/+ Mig-6f/f mice where Mig-6 was deleted in both the epithelial and stromal compartments of the uterus. In contrast, activation of progesterone signaling in the stroma regulated proliferation and apoptosis in the epithelium via suppression of ERα signaling there. In summary, our results establish that epithelial Mig-6 functions as a critical tumor suppressor that mediates the ability of progesterone to prevent the development of endometrial cancer.
Keywords: Mig-6, Endometrial cancer, Tumor suppressor, Progesterone, Uterus
Introduction
Endometrial carcinoma, commonly referred to as uterine cancer, is the most common malignancy of the female genital tract. In the United States, approximately 49,560 cases are diagnosed and about 8,190 women die from the disease each year (1). The most common type of endometrial carcinoma, approximately 85% of cases, is endometrioid carcinoma (2). Endometrial hyperplasia which is a proliferative process in epithelium is associated with endometrioid carcinoma. This process is commonly associated with unopposed estrogen stimulation (3).
The ability of ovarian steroids to regulate uterine cell proliferation depends upon the ability of hormonal stimulation to regulate growth factor communication networks between the uterine stroma and epithelium. E2 stimulates proliferation of uterine epithelial cells while P4 is inhibitory to estrogen-mediated proliferation of the epithelium. P4 achieves this inhibition of proliferation by coordinating stromal-epithelial cross-talk (4-6). Elucidating the molecular mechanisms by which the steroid hormones control uterine physiology is paramount to understanding female infertility and tumorigenesis of endometrial cancer.
Progestin therapy has been used in the conservative endocrine treatment of endometrial complex atypical hyperplasia; the direct precursor lesion to endometrial cancer in women in order to preserve their fertility; as well as in palliative treatment to advanced-stage patients who are poor surgical candidates (7-9). Expression of PR has been positively correlated with good prognosis and response to progestin treatment (10). However, more than 30% of patients do not respond to progestin due to de novo or acquired progestin resistance (8, 11-14). The mechanism of progestin resistance is still unknown.
Mitogen Inducible Gene 6, Mig-6, (Errfi1, RALT, or gene 33) is an immediate early response gene that can be induced by various mitogens and commonly occurring chronic stress stimuli. It contains a CRIB domain, a src homology 3 (SH3) binding domain, a 14-3-3 binding domain and an epidermal growth factor receptor (EGFR) binding domain as an adaptor protein, but no domain with enzymatic activity (15). Ablation of Mig-6 in mice has led to the development of animals with epithelial hyperplasia, adenoma, and adenocarcinomas in organs, such as the uterus, lung, gallbladder, and bile duct (16-18). We identified Mig-6 as a target of progesterone receptor (PR) action using a genome-wide gene expression profiling (19, 20), and showed that targeted ablation of Mig-6 gene in the mouse uterus led to the development of endometrial hyperplasia and estrogen-induced endometrial carcinoma (18, 20). Using this mouse model, we further demonstrated that Mig-6 suppresses estrogen signaling in the presence of progesterone and that Mig-6 regulates PTEN/PI3K/AKT signaling (21). In searching for relevance of the findings from the animal study to human endometrial cancer, we found a significantly lower MIG-6 expression in human endometrioid carcinoma cells compared to normal uterine cells, suggesting a similar Mig-6 regulated system present in the human uterus (20). Tumor suppressor function of Mig-6 coordinates endometrial stromal-epithelial communication. The expression of Mig-6 in these cellular compartments is under tight temporal and endocrine control. However, the function of Mig-6 in endometrial cancer has remained elusive.
In this study, we used endometrial epithelial cell specific Mig-6 knockout (Wnt7acre+ Mig-6f/f) mice to assess the role of epithelial Mig-6 in steroid hormone responsiveness and tumorigenesis (18, 22). We observed that Wnt7acre+ Mig-6f/f mice display normal P4 attenuation of E2-mediated uterine hypertrophy. However, Wnt7acre+ Mig-6f/f mice develop endometrial hyperplasia as well as estrogen-induced endometrial cancer. The development of endometrial hyperplasia in Wnt7acre+ Mig-6f/f mice is prevented by P4 treatment. These data suggest that P4-induced stromal Mig-6 prevents hyperplasia seen in Wnt7acre+ Mig-6f/f mice by regulating estrogen signaling.
Materials and Methods
Animals and Tissue Collection
Mice were cared for and used in the designated animal care facility according to the Michigan State University institutional guidelines. All animal procedures were approved by the Institutional Animal Care and Use Committee of Michigan State University. We generated uterine epithelial-specific Mig-6 knockout mice using the Wnt7a-cre mouse model (23, 24). Control mice and Wnt7acre+ Mig-6f/f mice were ovariectomized at six weeks of age. Two weeks later, ovariectomized control and Wnt7acre+ Mig-6f/f mice were injected with one of the following: vehicle (sesame oil), P4 (1 mg/mouse), E2 (0.1 μg/mouse), P4 plus E2. Five mice from each group were injected with one of the above treatments every 24 hrs and uteri were collected at 72 hrs. For the determination of the development of endometrial hyperplasia and estrogen/progesterone effects, either vehicle (beeswax), E2 (20μg/pellet) or P4 (40mg/pellet) pellets were placed subcutaneously into ovariectomized control and Wnt7acre+ Mig-6f/f mice for every one month and aged for three months before euthanization. Uterine tissues were flash frozen at the time of dissection and stored at −80 °C for RNA isolation or fixed with 4% (vol/vol) paraformaldehyde for histological analysis.
Quantitative real-time PCR
RNA was extracted from the uterine tissues using the RNeasy total RNA isolation kit (Qiagen, Valencia, CA). Expression levels of mRNA transcripts for Mig-6, estrogen receptor α (ERα) target genes: Muc-1, Clca3, Ltf, Birc1a and Birc1b, PR target genes: Mig-6, Fst and Il13ra2, and 18S rRNA (for normalization) were measured by quantitative RT-PCR analysis using the Applied Biosystems StepOnePlus™ qRT-PCR systems according to the manufacturer’s instructions (PE Applied Biosystems, Foster City, CA). Prevalidated TaqMan probes and primers were purchased from Applied Biosystems. cDNA was produced from 1 μg of total RNA using random hexamers and MMLV Reverse Transcriptase (Invitrogen Corp., Carlsbad, CA). RT-PCR was performed using RT-PCR Universal Master Mix reagent (Applied Biosystems, Foster City, CA) according to the manufacturer’s instructions. All real-time PCR was done by using five independent RNA sets. The relative expression of each transcript was normalized to 18S rRNA using ABI rRNA control reagents. Statistical analyses were performed using Student’s t test and one-way ANOVA. Differences between multiple groups were determined by Tukey’s post hoc multiple comparisons test. Statistical analyses were performed using the Instat package from GraphPad (San Diego, CA).
Immunohistochemistry
Uterine sections from paraffin-embedded tissue were cut at 5 μm and mounted on silane-coated slides, deparaffinized, and rehydrated in a graded alcohol series. Sections were preincubated with 10% normal goat serum in PBS (pH 7.5) and then incubated with primary antibody diluted in 10% normal goat serum in PBS (pH 7.5) overnight at 4°C at the following dilutions: 1:200 for anti-PR antibody (sc-7208, Santa Cruz Biotech., Santa Cruz, CA), 1:500 for anti-ERα (M-7047, DAKO Corp., Capinteria, CA), 1:50 for anti-MIG-6 (PE-16, Sigma, St. Louis, MO), 1:1000 for anti-phospho-histone H3 (06-570, Millipore., Billerica, MA), 1:500 for anti-cleaved caspase 3 (#9661, Cell Signaling., Danvers, MA), 1:200 for anti-NOTCH1 (sc-6014-R, Santa Cruz Biotech., Santa Cruz, CA), 1:200 for anti-BIRC3 (ab32059, Abcam Inc., Cambridge, MA), 1:1000 for anti-STAT3 (#4904, Cell Signaling., Danvers, MA), 1:100 for anti-phospho-STAT3 (#9131, Cell Signaling., Danvers, MA). On the following day, sections were washed in PBS and incubated with a secondary antibody (5 μl/ml; Vector Laboratories, Burlingame, CA) for one hour at room temperature. Immunoreactivity was detected using the Vectastain Elite DAB kit (Vector Laboratories, Burlingame, CA).
Isolation of the uterine epithelium
Isolated uteri were placed into Hanks balanced salt solution (HBSS; Ca2+-free, Mg2+free), and cut into 1-mm segments. The cut uteri were placed into 1% trypsin/HBSS solution for 1.5 hrs at room temperature and then washed with cold HBSS. The uteri were placed into 20% FBS/HBSS solution for five minutes, and washed with cold HBSS. The uteri were incubated with DNase I solution for 1 min to break down DNA. The uterine luminal epithelium was gently removed from the uterine stroma under a dissecting microscope.
Results
Generation of Epithelial Mig-6 Ablation in the Murine Uterus
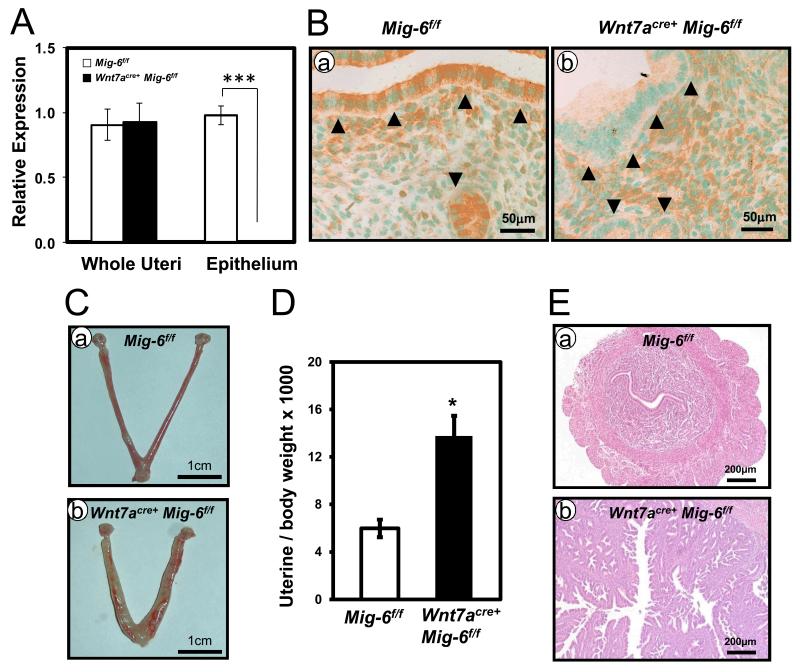
Previously, we generated conditional ablation of Mig-6 in all compartments of the uterus (PRcre/+ Mig-6f/f) which lead to the development of endometrial hyperplasia and estrogen-induced endometrial cancer (18, 20). Mig-6 is a critical mediator of stromal-epithelial communication in steroid hormone regulation and tumor suppressor function. In order to investigate the role of epithelial Mig-6 in the uterus, we bred Mig-6f/f mice to Wnt7a-Cre mice (22-24). Ablation of epithelial Mig-6 in Wnt7acre+ Mig-6f/f mice was confirmed by qRT-PCR and immunohistochemical analysis. After isolating epithelium from control (PRcre/+ and Mig-6f/f) mice and Wnt7acre+ Mig-6f/f mice, Mig-6 mRNA expression was detected in control epithelium whereas it was not detected in Wnt7acre+ Mig-6f/f epithelium (Figure 1A). In control mice, MIG-6 was expressed in the luminal epithelium, glandular epithelium and stroma. However, MIG-6 was only detected in stroma but not the epithelial cells of Wnt7acre+ Mig-6f/f mice (Figure 1B). These results suggested that we successfully generated epithelial Mig-6 ablation in the uterus of mice.
Figure 1.
Generation of epithelial Mig-6 ablation in the murine uterus and the development of endometrial hyperplasia. (A) Quantitative RT-PCR analysis of Mig-6 transcripts levels in whole uteri and isolated epithelium from control mice and Wnt7acre+ Mig-6f/f mice. The results represent the mean ± SE of five independent RNA sets. Mig-6 transcripts are significantly reduced in the epithelium of Wnt7acre+ Mig-6f/f mice compared to controls, ***, p<0.001. (B) Immunohistochemical analysis of MIG-6 in control mice (a) and Wnt7acre+ Mig-6f/f mice (b). Arrowheads indicate luminal and 5 glandular epithelium. (C) Gross morphology of control mice (a) and Wnt7acre+ Mig-6f/f mice (b) at five months of age. (D) Wnt7acre+ Mig-6f/f mice show an increase uterine weight compared with control mice at five months of age. (E) Hematoxylin and eosin staining of control mice (a) and Wnt7acre+ Mig-6f/f mice (b) at five months of age. The results represent the mean ± SE (n = 5 per genotype). *, p < 0.05.
Steroid Hormone Regulation and Tumor Suppressor Function of Epithelial Mig-6
We demonstrated that PRcre/+ Mig-6f/f mice result in the inability of P4 to inhibit E2-induced uterine weight gain and expression of E2-responsive target genes (20). To examine the effect of ovarian steroid hormone regulation on epithelial Mig-6 expression, ovariectomized control and Wnt7acre+ Mig-6f/f mice were injected daily with either vehicle (sesame oil), P4, E2 and E2+P4 for 3 days (n = 5 per genotype per treatment). Wnt7acre+ Mig-6f/f mice displayed a normal P4 attenuation of E2-mediated uterine hypertrophy (Supplemental Figure 1). These results suggest that stromal Mig-6 has an important role in acute steroid hormone responsiveness.
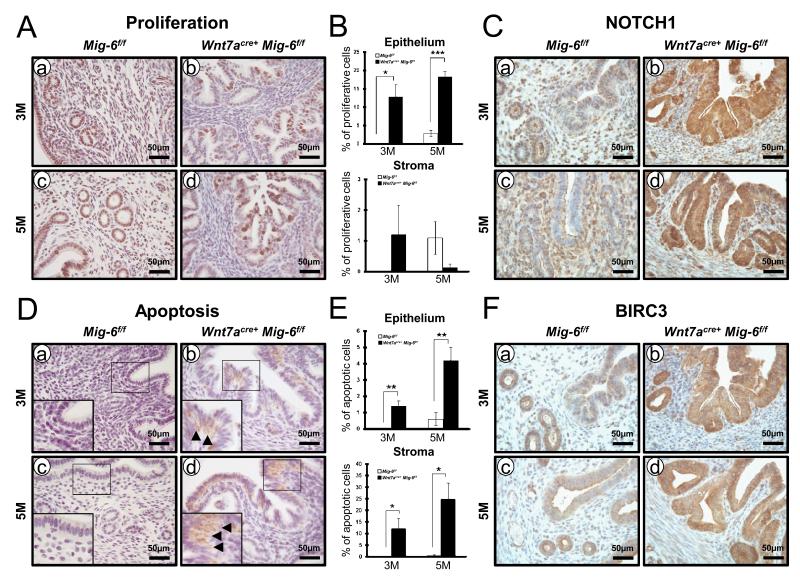
PRcre/+ Mig-6f/f mice developed endometrial hyperplasia and cancer in a hormone-dependent manner (20). We examined the development of endometrial hyperplasia and steroid hormone-dependent endometrial cancer in the Wnt7acre+Mig-6f/f mice. To investigate the impact of epithelial Mig-6 ablation on endometrial hyperplasia development, control and Wnt7acre+ Mig-6f/f mice were sacrificed at 5 months of age. Uterine weight, gross and histological morphology were examined (n = 5 per genotype). Wnt7acre+ Mig-6f/f mice showed an increased gross morphology when compared to control mice (Figure 1C). Uterine weight was significantly increased in Wnt7acre+ Mig-6f/f mice when compared to control mice (Figure 1D). Histological analysis revealed that Wnt7acre+ Mig-6f/f mice developed endometrial hyperplasia (Figure 1E). In addition, Wnt7acre+ Mig-6f/f mice developed estrogen-dependent endometrial cancer (Supplemental Figure 2). To address whether endometrial hyperplasia in Wnt7acre+ Mig-6f/f mice is caused by an alteration in endometrial epithelial cell proliferation, we performed immunohistochemical analysis for phospho-histone H3, a mitotic marker, in endometrium from mice at 3 months and 5 months of age. The levels of phospho-histone H3 were significantly increased in epithelial cells of Wnt7acre+Mig-6f/f mice compared to controls at both 3 months and 5 months of age (Figure 2, A and B). However, proliferation in stroma cells of Wnt7acre+Mig-6f/f mice was not changed. Notch pathway activation leads to increased proliferation and tumor progression in endometrial cancers (25). Because ablation of Notch1 in PR-positive cells demonstrated decreased cellular proliferation (26), we examined NOTCH1 expression in highly proliferative epithelial cells of Wnt7acre+Mig-6f/f mice. Wnt7acre+Mig-6f/f uteri showed a robust expression of NOTCH1 in the luminal and glandular epithelium, whereas the Mig-6f/f mice display NOTCH1 expression only in the stroma (Figure 2C).
Figure 2.
Regulation of proliferation and apoptosis by epithelial Mig-6 (A) Immunostaining of phospho-histone H3 was significantly increased in the endometrial epithelial cells of Wnt7acre+ Mig-6f/f mice (b and d) compared to control mice (a and c) at three months of age (a and b) and five months of age (c and d). (B) Quantification of phospho-histone H3-positive in endometrial stroma and epithelial cells. (C) Immunohistochemical analysis of NOTCH1 in the uteri of control mice (a and c) and Wnt7acre+ Mig-6f/f mice (b and d) at three months of age (a and b) and five months of age (c and d). (D) Immunohistochemistry of cleaved caspase-3 was increased in epithelial cells and sub-epithelial storma cells of Wnt7acre+ Mig-6f/f mice (b and d) compared to control mice (a and c) at three months of age (a and b) and five months of age (c and d). (E) Quantification of cleaved caspase-3-positive in endometrial stroma and epithelial cells. (F) Immunohistochemical analysis of BIRC3 in the uteri of control mice (a and c) and Wnt7acre+ Mig-6f/f mice (b and d) at three months of age (a and b) and five months of age (c and d). Arrowheads indicate positive-cleaved caspase-3 cells. The results represent the mean ± SE. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
The number of cleaved caspase 3 positive cells was significantly increased in epithelial cells of Wnt7acre+Mig-6f/f mice compared to controls. Interestingly, apoptosis in sub-epithelial stroma cells of Wnt7acre+Mig-6f/f mice was significantly increased compared to control mice at 3 months and 5 months of age (Figure 2, C and D). BIRC3 contributes to the survival of endometrial cancer cells against apoptosis mediated by inhibition of AKT (27). Therefore, it was determined whether Wnt7acre+Mig-6f/f mice altered regulation of BIRC3 during endometrial hyperplasia development. The expression of BIRC3 was increased in the luminal and glandular epithelium of Wnt7acre+Mig-6f/f mice compared to Mig-6f/f mice, whereas the expression of BIRC3 was not observed in sub-epithelial stroma cells of Wnt7acre+Mig-6f/f mice (Figure 3F).
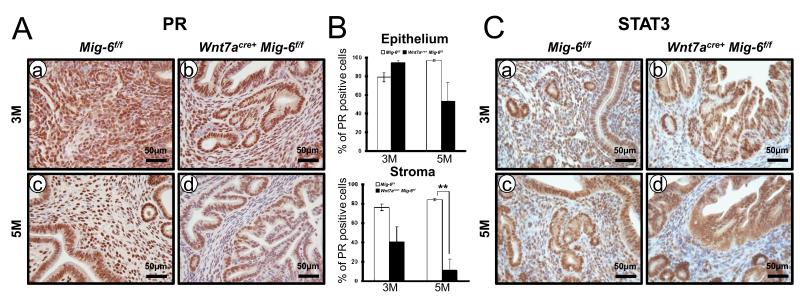
Figure 3.
The reduction of stromal PR in the Wnt7acreMig-6f/f mice compared to control mice. (A) Immunohistochemistry for PR in the uteri of control mice (a and c) and Wnt7acre+ Mig-6f/f mice (b and d) at three months of age (a and b) and five months of age (c and d). (B) Quantification of PR-positive cells in epithelial and stromal cells of control mice and Wnt7acre+ Mig-6f/f mice. The results represent the mean ± SE. **, p < 0.01. (C) Immunohistochemical analysis of STAT3 at three months of age (a and b) and five months of age (c and d).
Expression of PR has been reported as prognostic factors for endometrial carcinoma (28-30). We evaluated the expression of PR by immunohistochemistry in mice at 3 months and 5 months of age. Immunostaining of PR showed a significant decrease of stromal PR expression in the endometrium of Wnt7acre+Mig-6f/f mice when compared to control mice at 5 months of age (Figure 3, A and B). These results indicate that Wnt7acre+Mig-6f/f mice exhibited endometrial cancer development and progression as observed in human. Activation of the signal transducer and activator of transcription-3 (STAT3) interacts with PR for decidualization in uterus. The expression of stromal PR was decreased during decidualization and pre-implantation period in PRcre/+ Stat3f/f mice and PR target genes were significantly down-regulated after progesterone treatment (31). Therefore, we examined the expression of STAT3 by immunohistochemistry in endometrial hyperplasia from Wnt7acre+Mig-6f/f mice at 3 months and 5 months of age. The immunostaining results showed a decreased STAT3 protein in endometrial stroma of Wnt7acre+Mig-6f/f mice when compared to control mice at 3 and 5 months of age (Figure 3C).
Prevention of the Development of Endometrial Hyperplasia by Progesterone Treatment
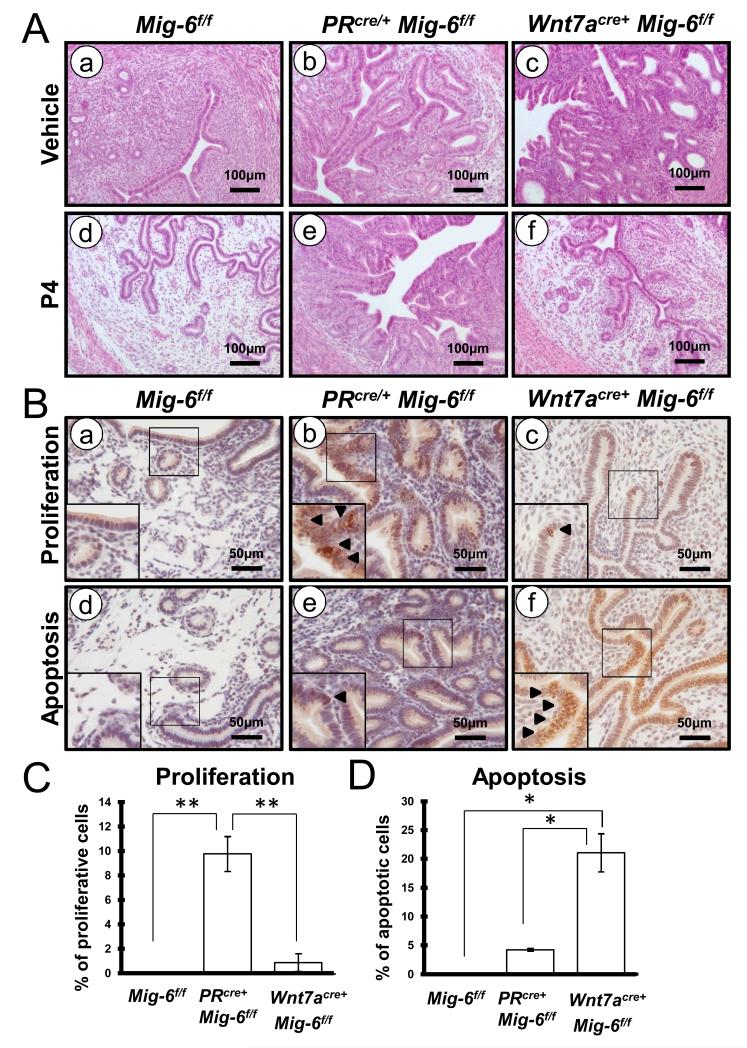
P4 has been used as a therapeutic agent for the treatment of early stage endometrial cancer in patients (11). However, the effectiveness of P4 for women with endometrial cancer is less clear. To assess the effect of P4 on epithelial ablation of Mig-6, we placed P4 or vehicle pellets subcutaneously into the control, PRcre/+ Mig-6f/f and Wnt7acre+ Mig-6f/f mice at 6 weeks of age and treated for 3 months (n = 8 per genotype per treatment). There was no reduction in the development of endometrial hyperplasia with P4 treatment in the PRcre/+ Mig-6f/f mice. However, the development of endometrial hyperplasia in the Wnt7acre+ Mig-6f/f mice was prevented by P4 treatment (Figure 4A).
Figure 4.
The endometrial hyperplasia in the Wnt7acre+ Mig-6f/f mice, but not the PRcre/+ Mig-6f/f mice, was prevented with P4 treatment. (A) Hematoxylin and eosin staining of control mice (a and d), PRcre/+ Mig-6f/f mice (b and e) and Wnt7acre+ Mig-6f/f mice (c and f) before (a,b and c) and after (d,e and f) P4 treatment. (B) Immunostaining for phospho-histone H3 (a,b and c) and cleaved caspase-3 (d,e and f) in the uteri of control mice (a and d), PRcre/+ Mig-6f/f mice (b and e) and Wnt7acre+ Mig-6f/f mice (c and f) after P4 treatment. Arrowheads indicate positive-phospho-histone H3 (b and c) and positive-cleaved caspase-3 cells (e and f). (C) Quantification of phospho-histone H3 and cleaved caspase-3-positive cells in epithelial cells. The results represent the mean ± SE. *, p < 0.05; **, p < 0.01.
To determine whether the prevention of endometrial hyperplasia in Wnt7acre+ Mig-6f/f mice was caused by an alteration in cell proliferation and apoptosis, we examined the immunohistochemistry for phospho-histone H3 and cleaved caspase 3 following P4 treatment. Immunostaining of phospho-histone H3 showed a significant decreased expression in endometrial epithelium of Wnt7acre+ Mig-6f/f mice compared with PRcre/+ Mig-6f/f mice after P4 treatment (Figure 4, B and C). This indicates that P4 is decreased in epithelial proliferation in Wnt7acre+ Mig-6f/f mice but not PRcre/+ Mig-6f/f mice. Immunostaining of cleaved caspase 3 showed that apoptosis were significantly increased in the endometrial epithelium of Wnt7acre+ Mig-6f/f mice compared to PRcre/+ Mig-6f/f mice (Figure 4, B and D). In addition, apoptosis of stromal cells was not observed in Wnt7acre+ Mig-6f/f mice after P4 treatment. These results suggest that activation of P4 signaling including Mig-6 in stroma induces epithelial cell apoptosis.
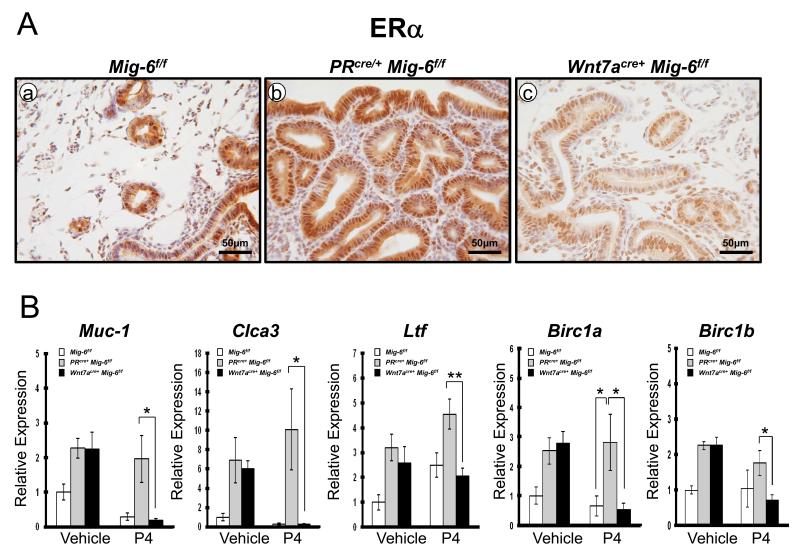
To determine if the suppression of hyperplastic phenotype observed was due to altered ovarian steroid hormone signaling, we examined the expression of ERα and PR using immunohistochemisty. The expression of ERα was significantly decreased in Wnt7acre+ Mig-6f/f mouse endometrium as compared to control and PRcre/+ Mig-6f/f mice after P4 treatment (Figure 5A). Transcript levels of ERα target genes, Muc-1, Clca3 and Ltf were also significantly decreased in the Wnt7acre+ Mig-6f/f mice as compared to the PRcre/+ Mig-6f/f after P4 treatment (Figure 5B). It is known that E2 can tip this balance toward cell survival in uterine epithelial cells by inducing the expression of baculoviral inhibitors of apoptosis repeat-containing 1 (Birc1), a family of anti-apoptotic proteins (32). To determine if P4 treatment suppresses uterine epithelial apoptosis by suppressing Birc1 expression, the expression of Birc1a and Birc1b was determined in control, PRcre/+ Mig-6f/f and Wnt7acre+ Mig-6f/f mice treated with P4 for 3 months by real-time RT-PCR. Interestingly, the expression of Birc1a and Birc1b was significantly decreased in the Wnt7acre+ Mig-6f/f mice as compared to the PRcre/+ Mig-6f/f after P4 treatment (Figure 5B). These results suggest that P4 treatment induce uterine epithelial apoptosis via down-regulation of Birc1 expression.
Figure 5.
A decrease in expression of ERα protein and ERα–regulated genes in Wnt7acre+ Mig-6f/f mice compared to PRcre/+ Mig-6f/f mice after P4 treatment. (A) Immunohistochemical analysis of ERα in the uteri of control mice (a), PRcre/+ Mig-6f/f mice (b) and Wnt7acre+ Mig-6f/f mice (c) after P4 treatment. (B) Quantitative RT-PCR analysis of ERα target genes (Muc-1, Clca3, Ltf, Birc1a and Birc1b) was performed on uteri of control mice, PRcre/+ Mig-6f/f mice and Wnt7acre+ Mig-6f/f mice before and after vehicle or P4 treatment. The results represent the relative expression of transcripts (normalized to 18S rRNA) mean ± SE for RNA isolated from five mice per group. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
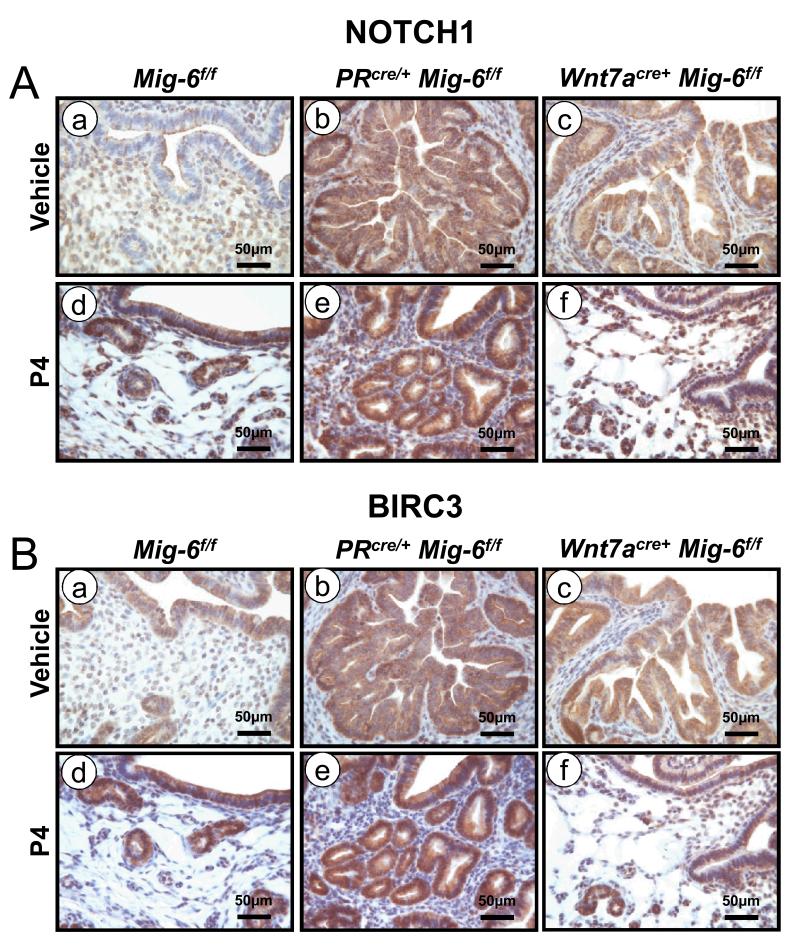
The expression of PR was not significantly different between control and Wnt7acre+ Mig-6f/f mice after P4 treatment. However, transcript levels of PR target genes Mig-6, Fst and Il13ra2 were increased in the Wnt7acre+ Mig-6f/f mice as compared to the PRcre/+ Mig-6f/f after P4 treatment (Supplemental Figure 3). Interestingly, the levels of BIRC3 and NOTCH1 were decreased in endometrial epithelium of Wnt7acre+ Mig-6f/f mice compared with PRcre/+ Mig-6f/f mice after P4 treatment (Figure 6). Therefore, BIRC3 and NOTCH1 may play an important role in hyperplasia development in Wnt7acre+ Mig-6f/f mice. These results suggest that activated stromal P4 signaling including Mig-6 prevent the development of endometrial epithelial hyperplasia seen in the Wnt7acre+ Mig-6f/f mice by regulating estrogen signaling.
Figure 6.
Regulation of NOTCH1 and BIRC3 by P4 treatment. Immunohistochemical analysis of (A) BIRC3 and (B) NOTCH1 in the uteri of control mice (a and d), PRcre/+ Mig-6f/f mice (b and e) and Wnt7acre+ Mig-6f/f mice (c and f) before (a,b and c) and after (d,e and f) P4 treatment.
Discussion
P4 and E2, acting through their cognate nuclear receptors, play critical roles in uterine functions associated with the establishment and maintenance of pregnancy (33, 34). E2 is required for proliferation and differentiation of the uterine epithelium whereas the coordinated action of E2 and P4 promotes stromal cell differentiation (35). Elucidating P4-regulated pathways in the uterus is thus critical for understanding the impairments that underlie disruption of steroid hormone control of uterine cell proliferation and differentiation. The progesterone-induced gene Mig-6 suppresses E2 signaling as a tumor suppressor by regulating proliferation and apoptosis in endometrial cancer (20, 21). The expression of Mig-6 in these cellular compartments is under tight temporal and endocrine control (15). However, the in vivo role of Mig-6 in uterine epithelium has remained elusive.
To understand the role of epithelial Mig-6 in the uterus, we generated ablation of uterine epithelial Mig-6 using Wnt7a-cre mice (Figure 1). Ablation of epithelial Mig-6 in the murine uterus did not show any alterations in ovarian morphology, ovulation, or fertilization. In addition, there were normal levels of P4 and E2 in the serum of Wnt7acre+ Mig-6f/f mice (data not shown). One of the endocrine risk factors for developing endometrial cancer and endometriosis is exposure to E2, conversely a lower incidence of these diseases in women is associated with decreased endogenous E2 production (36). Even though Wnt7acre+ Mig-6f/f mice have normal acute steroid hormone responsiveness (Supplemental Figure 1), Wnt7acre+ Mig-6f/f mice developed endometrial hyperplasia (Figure 1). Endometrial hyperplasia is defined as an increased proliferation of the endometrial glands relative to the stroma, resulting in an increased gland-to-stroma ratio when compared with normal proliferative endometrium (37). Endometrial hyperplasia deserves special attention because of its relationship with endometrial carcinoma. Clinicopathologic and epidemiologic studies have supported the malignant potential of endometrial hyperplasia and the concept of a continuum of proliferative glandular lesions culminating, in some cases, in carcinoma. However, detail molecular signaling during development of endometrial hyperplasia has remained elusive. Our mouse models are invaluable for further study of endometrial tumorigenesis.
Proliferation in epithelial cells and apoptosis in sub-epithelial stroma cells were significantly increased in Wnt7acre+ Mig-6f/f mice compared to control mice in epithelial cells at 5 months of age (Figure 2). These results indicate that these increases lead to development of endometrial hyperplasia. Notch signaling plays an important role in the regulation of cellular proliferation, differentiation and apoptosis (38). Deregulation of Notch signaling was found in a variety of cancers (38). Moreover, Notch signaling is prominently regulated by estrogen (39, 40). Here, it is demonstrated that epithelial Mig-6 inhibits epithelial proliferation through its regulation of NOTCH1 protein (Figure 2C). The inhibitor of apoptosis proteins (IAPs) are a negative key regulators of apoptosis (41). Alterations in IAPs are found in many types of human cancer and are connected with chemoresistance, disease progression and poor prognosis (42, 43). IAPs have important roles in estrogen-mediated apoptosis suppression in the uterine epithelium (32). Since the cellular inhibitor of apoptosis genes including Birc1 and Birc3 can tip toward cell survival in uterine epithelial cells (27, 32). The expression of Birc1 and Birc3 was significantly increased in Wnt7acre+ Mig-6f/f mice (Figure 2 and 5). These results suggest that an increase of epithelial proliferation in Wnt7acre+ Mig-6f/f mice leads to the development of endometrial hyperplasia through BIRC3 and NOTCH1. The mechanism by which this is achieved is suggested to be through EGFR/ERK and PI3K/AKT signaling pathway (27). Therefore, future studies are needed to determine whether it is through this pathway or others that epithelial Mig-6 regulates proliferation and apoptosis.
Since P4 attenuates E2 regulation of proliferation and gene expression by regulating the expression of a yet to be identified paracrine signal from the stromal cells to the epithelial cells, the regulation of the expression of PR in the endometrial stromal cells by epithelial Mig-6 is critical for the ability of P4 to attenuate the E2 regulated proliferation, apoptosis and expression of ERα target genes. Wnt7acre+ Mig-6f/f mice exhibited reduced PR expression in stromal cells (Figure 3) as observed in human endometrial cancer (30, 44). It has been reported that PR is essential for uterine biology as a key regulator of uterine epithelial-stromal crosstalk (45, 46). P4 was unable to stimulate the expression of its epithelial target genes and inhibit neonatal endometrial glandular development in conditional ablation of epithelial PR in the uterus of Wnt7acre+ PRf/− mice (24). PR directly interacts with STAT3 through protein-protein interactions (31, 47). STAT3 signaling pathways are activated by cytokines and growth factors. The expression of stromal PR was decreased during decidualization and pre-implantation period in PRcre/+Statf/f mice and PR target genes were significantly down-regulated after progesterone induction (31). The expression of PR and STAT3 was decreased during endometrial hyperplasia development in the stroma of Wnt7acre+ Mig-6f/f mice (Figure 3). There are differential roles in uterine stromal and epithelial compartments, and stromal-epithelial communication is critical in pregnancy, steroid hormone regulation and in tumor suppressor function. Therefore, these results suggest that dysregulation of STAT3 and PR crosstalk is important for endometrial hyperplasia development.
In contrast, a negative risk factor for these endometrial diseases is exposure to P4 (48). It is well known that endometrial cancer is an estrogen-dependent disease and that progestin therapy has been used successfully to slow the growth of endometrial tumors in women that are poor surgical candidates as well as reverse endometrial complex atypical hyperplasia in women who wish to retain their fertility (5-8). The mechanism by which progestins slow endometrial cancer cell growth is due to its inhibitory effects on E2 action (49). After P4 treatment, Wnt7acre+ Mig-6f/f mice did not develop endometrial hyperplastic lesions (Figure 4). Proliferation is significantly decreased in Wnt7acre+ Mig-6f/f mice compared to PRcre/+ Mig-6f/f in epithelial cells and apoptosis is highly increased in Wnt7acre+ Mig-6f/f mice compared to PRcre/+ Mig-6f/f in epithelial cells after P4 treatment. It is known that baculoviral inhibitors of apoptosis repeat-containing 1 (Birc1), a family of anti-apoptotic proteins as functional targets of estrogen through its receptor can suppress uterine epithelial cells (32). To determine if activated stromal Mig-6 by P4 promotes uterine epithelial apoptosis by suppressing Birc1 expression, we determined transcription levels of Birc1a and Birc1b. These genes were significantly decreased in Wnt7acre+ Mig-6f/f mice compared to PRcre/+ Mig-6f/f mice after P4 treatment (Figure 5). ERα protein level and ERα target genes (Muc-1, Clca3 and Ltf) levels were decreased in Wnt7acre+ Mig-6f/f mice compared to PRcre/+ Mig-6f/f after P4 treatment (Figure 5). PR protein level was not changed between PRcre/+ Mig-6f/f and Wnt7acre+ Mig-6f/f mice. However, PR target genes, Mig-6, Fst and Il13ra2 expression were highly increased in Wnt7acre+ Mig-6f/f mice compared to PRcre/+ Mig-6f/f mice after P4 treatment (Supplement Figure 3). BIRC3 is induced by progestins through PRB and contributes to the survival of endometrial cancer cells against apoptosis mediated by inhibition of AKT (27). The levels of BIRC3 was decreased in Wnt7acre+ Mig-6f/f mice while the high levels of BIRC3 was not changed in PRcre+ Mig-6f/f mice after P4 treatment (Figure 6). It suggested that the induction of BIRC3 by P4 plays a role in the resistance to P4 therapy observed in some women with endometrial carcinoma (27). Our results support the function of BIRC3 in the resistance of P4 therapy. It is sufficient to play as a tumor suppressor and/or mediator of PR-P4 signaling, even though expression of Mig-6 in Wnt7acre+ Mig-6f/f mice is lower than control mice after P4 treatment. Our results suggest that activated stromal Mig-6 can regulate proliferation and apoptosis via regulating ERα activity in epithelium, can contribute to the prevention of endometrial hyperplasia and that epithelial Mig-6 is a critical tumor suppressor involved in P4 mediated protection against the development endometrial cancer. These results suggest that epithelial Mig-6 is critical for a tumor suppressor function in endometrial cancer.
In conclusion, our results show the role of epithelial Mig-6 in steroid hormone regulation and endometrial cancer. Ablation of epithelial Mig-6 in the murine uterus developed endometrial hyperplasia and P4 treatment prevented this endometrial hyperplastic phenotype with which occurs via Mig-6 regulation of ERα activity (Table 1). The Wnt7acre+ Mig-6f/f model is useful for studying new targets during cancer progression and can be exploited therapeutically in order to identify new therapies for the prevention and treatment of endometrial cancer. Determining the role of Mig-6 in stromal-epithelial cross talk will be critical in understanding the role of steroid hormone signaling in endometrial function and dysfunction associated with infertility and endometriosis and in developing therapy for both of these common uterine diseases.
Table 1.
The comparison between whole uterine and epithelial specific Mig-6 ablation
|
PR cre/+ Mig-6 f/f (Total Uterine KO) |
Wnt7a cre+ Mig-6 f/f (Epithelial KO) |
|
|---|---|---|
| Steroid hormone response | No P4 inhibition | Normal P4 inhibition |
| Hyperplasia | Yes | Yes |
| Hormone Dependence of Hyperplasia |
Yes | Yes |
| Cancer development by chronic E2 treatment |
Yes | Yes |
| P4 treatment | No effect | Suppression of hyperplasia |
Supplementary Material
Acknowlegements
We thank Francesco J. DeMayo and Sophia Y. Tsai (Baylor College of Medicine, Houston, TX) for helpful discussions; Sharra A. Poncil and Thuy L. Tran for manuscript preparation. This work was supported by NIH R01HD057873, American Cancer Society Research Scholar Grant RSG-12-084-01-TBG and World Class University program (R31-10056) through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (to J.W.J.), NIH HD30284 and SPORE in Uterine Cancer CA098258 (to R.R.B.), and the National Cancer Institute CA09299 Training Program in the Molecular Genetics of Cancer (to G.D.O.).
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Deligdisch L, Holinka CF. Endometrial carcinoma: two diseases? Cancer Detect Prev. 1987;10:237–46. [PubMed] [Google Scholar]
- 3.Kurman RJ, Kaminski PF, Norris HJ. The behavior of endometrial hyperplasia. A long-term study of “untreated” hyperplasia in 170 patients. Cancer. 1985;56:403–12. doi: 10.1002/1097-0142(19850715)56:2<403::aid-cncr2820560233>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 4.Huet-Hudson YM, Andrews GK, Dey SK. Cell type-specific localization of c-myc protein in the mouse uterus: modulation by steroid hormones and analysis of the periimplantation period. Endocrinology. 1989;125:1683–90. doi: 10.1210/endo-125-3-1683. [DOI] [PubMed] [Google Scholar]
- 5.Paria BC, Hudson YM, Dey SK. Blastocyst’s state of activity determines the “window” of implantation in the receptive mouse uterus. Proc Natl Acad Sci U S A. 1993;90:10159–62. doi: 10.1073/pnas.90.21.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarke CL, Sutherland RL. Progestin regulation of cellular proliferation. Endocr Rev. 1990;11:266–301. doi: 10.1210/edrv-11-2-266. [DOI] [PubMed] [Google Scholar]
- 7.Benshushan A. Endometrial adenocarcinoma in young patients: evaluation and fertility-preserving treatment. Eur J Obstet Gynecol Reprod Biol. 2004;117:132–7. doi: 10.1016/j.ejogrb.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 8.Hahn HS, Yoon SG, Hong JS, Hong SR, Park SJ, Lim JY, et al. Conservative treatment with progestin and pregnancy outcomes in endometrial cancer. Int J Gynecol Cancer. 2009;19:1068–73. doi: 10.1111/IGC.0b013e3181aae1fb. [DOI] [PubMed] [Google Scholar]
- 9.Yamazawa K, Hirai M, Fujito A, Nishi H, Terauchi F, Ishikura H, et al. Fertility-preserving treatment with progestin, and pathological criteria to predict responses, in young women with endometrial cancer. Hum Reprod. 2007;22:1953–8. doi: 10.1093/humrep/dem088. [DOI] [PubMed] [Google Scholar]
- 10.Ehrlich CE, Young PC, Stehman FB, Sutton GP, Alford WM. Steroid receptors and clinical outcome in patients with adenocarcinoma of the endometrium. Am J Obstet Gynecol. 1988;158:796–807. doi: 10.1016/0002-9378(88)90075-0. [DOI] [PubMed] [Google Scholar]
- 11.Ramirez PT, Frumovitz M, Bodurka DC, Sun CC, Levenback C. Hormonal therapy for the management of grade 1 endometrial adenocarcinoma: a literature review. Gynecol Oncol. 2004;95:133–8. doi: 10.1016/j.ygyno.2004.06.045. [DOI] [PubMed] [Google Scholar]
- 12.Hoekstra AV, Kim JJ, Keh P, Schink JC. Absence of progesterone receptors in a failed case of fertility-sparing treatment in early endometrial cancer: a case report. J Reprod Med. 2008;53:869–73. [PMC free article] [PubMed] [Google Scholar]
- 13.Kim JJ, Chapman-Davis E. Role of progesterone in endometrial cancer. Semin Reprod Med. 2010;28:81–90. doi: 10.1055/s-0029-1242998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaku T, Yoshikawa H, Tsuda H, Sakamoto A, Fukunaga M, Kuwabara Y, et al. Conservative therapy for adenocarcinoma and atypical endometrial hyperplasia of the endometrium in young women: central pathologic review and treatment outcome. Cancer Lett. 2001;167:39–48. doi: 10.1016/s0304-3835(01)00462-1. [DOI] [PubMed] [Google Scholar]
- 15.Kim TH, Lee DK, Franco HL, Lydon JP, Jeong JW. ERBB receptor feedback inhibitor 1 regulation of estrogen receptor activity is critical for uterine implantation in mice. Biol Reprod. 2010;82:706–13. doi: 10.1095/biolreprod.109.081307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anastasi S, Sala G, Huiping C, Caprini E, Russo G, Iacovelli S, et al. Loss of RALT/MIG-6 expression in ERBB2-amplified breast carcinomas enhances ErbB-2 oncogenic potency and favors resistance to Herceptin. Oncogene. 2005;24:4540–8. doi: 10.1038/sj.onc.1208658. [DOI] [PubMed] [Google Scholar]
- 17.Ferby I, Reschke M, Kudlacek O, Knyazev P, Pante G, Amann K, et al. Mig6 is a negative regulator of EGF receptor-mediated skin morphogenesis and tumor formation. Nat Med. 2006;12:568–73. doi: 10.1038/nm1401. [DOI] [PubMed] [Google Scholar]
- 18.Jin N, Gilbert JL, Broaddus RR, Demayo FJ, Jeong JW. Generation of a Mig-6 conditional null allele. Genesis. 2007;45:716–21. doi: 10.1002/dvg.20348. [DOI] [PubMed] [Google Scholar]
- 19.Jeong JW, Lee KY, Kwak I, White LD, Hilsenbeck SG, Lydon JP, et al. Identification of murine uterine genes regulated in a ligand-dependent manner by the progesterone receptor. Endocrinology. 2005;146:3490–505. doi: 10.1210/en.2005-0016. [DOI] [PubMed] [Google Scholar]
- 20.Jeong JW, Lee HS, Lee KY, White LD, Broaddus RR, Zhang YW, et al. Mig-6 modulates uterine steroid hormone responsiveness and exhibits altered expression in endometrial disease. Proc Natl Acad Sci U S A. 2009;106:8677–82. doi: 10.1073/pnas.0903632106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim TH, Franco HL, Jung SY, Qin J, Broaddus RR, Lydon JP, et al. The synergistic effect of Mig-6 and Pten ablation on endometrial cancer development and progression. Oncogene. 2010;29:3770–80. doi: 10.1038/onc.2010.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang CC, Orvis GD, Wang Y, Behringer RR. Stromal-to-epithelial transition during postpartum endometrial regeneration. PLoS One. 2012;7:e44285. doi: 10.1371/journal.pone.0044285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winuthayanon W, Hewitt SC, Orvis GD, Behringer RR, Korach KS. Uterine epithelial estrogen receptor alpha is dispensable for proliferation but essential for complete biological and biochemical responses. Proc Natl Acad Sci U S A. 2010;107:19272–7. doi: 10.1073/pnas.1013226107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franco HL, Rubel CA, Large MJ, Wetendorf M, Fernandez-Valdivia R, Jeong JW, et al. Epithelial progesterone receptor exhibits pleiotropic roles in uterine development and function. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2012;26:1218–27. doi: 10.1096/fj.11-193334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitsuhashi Y, Horiuchi A, Miyamoto T, Kashima H, Suzuki A, Shiozawa T. Prognostic significance of Notch signalling molecules and their involvement in the invasiveness of endometrial carcinoma cells. Histopathology. 2012;60:826–37. doi: 10.1111/j.1365-2559.2011.04158.x. [DOI] [PubMed] [Google Scholar]
- 26.Afshar Y, Jeong JW, Roqueiro D, DeMayo F, Lydon J, Radtke F, et al. Notch1 mediates uterine stromal differentiation and is critical for complete decidualization in the mouse. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2012;26:282–94. doi: 10.1096/fj.11-184663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neubauer NL, Ward EC, Patel P, Lu Z, Lee I, Blok LJ, et al. Progesterone receptor-B induction of BIRC3 protects endometrial cancer cells from AP1-59-mediated apoptosis. Horm Cancer. 2011;2:170–81. doi: 10.1007/s12672-011-0065-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kleine W, Maier T, Geyer H, Pfleiderer A. Estrogen and progesterone receptors in endometrial cancer and their prognostic relevance. Gynecol Oncol. 1990;38:59–65. doi: 10.1016/0090-8258(90)90012-a. [DOI] [PubMed] [Google Scholar]
- 29.Nyholm HC, Nielsen AL, Lyndrup J, Dreisler A, Thorpe SM. Estrogen and progesterone receptors in endometrial carcinoma: comparison of immunohistochemical and biochemical analysis. Int J Gynecol Pathol. 1993;12:246–52. doi: 10.1097/00004347-199307000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Fukuda K, Mori M, Uchiyama M, Iwai K, Iwasaka T, Sugimori H. Prognostic significance of progesterone receptor immunohistochemistry in endometrial carcinoma. Gynecol Oncol. 1998;69:220–5. doi: 10.1006/gyno.1998.5023. [DOI] [PubMed] [Google Scholar]
- 31.Lee JH, Kim TH, Oh SJ, Yoo JY, Akira S, Ku BJ, et al. Signal transducer and activator of transcription-3 (Stat3) plays a critical role in implantation via progesterone receptor in uterus. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2013 doi: 10.1096/fj.12-225664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yin Y, Huang WW, Lin C, Chen H, MacKenzie A, Ma L. Estrogen suppresses uterine epithelial apoptosis by inducing birc1 expression. Mol Endocrinol. 2008;22:113–25. doi: 10.1210/me.2007-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Conneely OM, Mulac-Jericevic B, Lydon JP, De Mayo FJ. Reproductive functions of the progesterone receptor isoforms: lessons from knock-out mice. Mol Cell Endocrinol. 2001;179:97–103. doi: 10.1016/s0303-7207(01)00465-8. [DOI] [PubMed] [Google Scholar]
- 34.Conneely OM, Mulac-Jericevic B, DeMayo F, Lydon JP, O’Malley BW. Reproductive functions of progesterone receptors. Recent Prog Horm Res. 2002;57:339–55. doi: 10.1210/rp.57.1.339. [DOI] [PubMed] [Google Scholar]
- 35.Wang H, Dey SK. Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet. 2006;7:185–99. doi: 10.1038/nrg1808. [DOI] [PubMed] [Google Scholar]
- 36.Stovall DW, Halme J. Endometriosis and associated pathology. Curr Opin Obstet Gynecol. 1991;3:853–8. [PubMed] [Google Scholar]
- 37.Montgomery BE, Daum GS, Dunton CJ. Endometrial hyperplasia: a review. Obstet Gynecol Surv. 2004;59:368–78. doi: 10.1097/00006254-200405000-00025. [DOI] [PubMed] [Google Scholar]
- 38.Dotto GP. Notch tumor suppressor function. Oncogene. 2008;27:5115–23. doi: 10.1038/onc.2008.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ranganathan P, Weaver KL, Capobianco AJ. Notch signalling in solid tumours: a little bit of everything but not all the time. Nat Rev Cancer. 2011;11:338–51. doi: 10.1038/nrc3035. [DOI] [PubMed] [Google Scholar]
- 40.Rizzo P, Osipo C, Foreman K, Golde T, Osborne B, Miele L. Rational targeting of Notch signaling in cancer. Oncogene. 2008;27:5124–31. doi: 10.1038/onc.2008.226. [DOI] [PubMed] [Google Scholar]
- 41.Fulda S, Vucic D. Targeting IAP proteins for therapeutic intervention in cancer. Nature reviews Drug discovery. 2012;11:109–24. doi: 10.1038/nrd3627. [DOI] [PubMed] [Google Scholar]
- 42.Wang S, Bai L, Lu J, Liu L, Yang CY, Sun H. Targeting inhibitors of apoptosis proteins (IAPs) for new breast cancer therapeutics. J Mammary Gland Biol Neoplasia. 2012;17:217–28. doi: 10.1007/s10911-012-9265-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salvesen GS, Duckett CS. IAP proteins: blocking the road to death’s door. Nat Rev Mol Cell Biol. 2002;3:401–10. doi: 10.1038/nrm830. [DOI] [PubMed] [Google Scholar]
- 44.Mote PA, Johnston JF, Manninen T, Tuohimaa P, Clarke CL. Detection of progesterone receptor forms A and B by immunohistochemical analysis. J Clin Pathol. 2001;54:624–30. doi: 10.1136/jcp.54.8.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tan J, Paria BC, Dey SK, Das SK. Differential uterine expression of estrogen and progesterone receptors correlates with uterine preparation for implantation and decidualization in the mouse. Endocrinology. 1999;140:5310–21. doi: 10.1210/endo.140.11.7148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cunha GR, Cooke PS, Kurita T. Role of stromal-epithelial interactions in hormonal responses. Arch Histol Cytol. 2004;67:417–34. doi: 10.1679/aohc.67.417. [DOI] [PubMed] [Google Scholar]
- 47.Liu T, Ogle TF. Signal transducer and activator of transcription 3 is expressed in the decidualized mesometrium of pregnancy and associates with the progesterone receptor through protein-protein interactions. Biol Reprod. 2002;67:114–8. doi: 10.1095/biolreprod67.1.114. [DOI] [PubMed] [Google Scholar]
- 48.Grosskinsky CM, Halme J. Endometriosis: the host response. Baillieres Clin Obstet Gynaecol. 1993;7:701–13. doi: 10.1016/s0950-3552(05)80459-6. [DOI] [PubMed] [Google Scholar]
- 49.Kaunitz AM. Injectable depot medroxyprogesterone acetate contraception: an update for U.S. clinicians. Int J Fertil Womens Med. 1998;43:73–83. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.