Abstract
Purpose
The combination of radiation with chemotherapy is the most effective therapy for unresectable pancreatic cancer. To improve upon this regimen, we combined the selective Chk1 inhibitor, MK8776 with gemcitabine-based chemoradiation in preclinical pancreatic cancer models.
Experimental Design
We tested the ability of MK8776 to sensitize to gemcitabine-radiation in homologous recombination repair (HRR)- proficient and deficient pancreatic cancer cells and assessed Rad51 focus formation. In vivo, we investigated the efficacy, tumor cell selectivity, and pharmacodynamic biomarkers of sensitization by MK8776.
Results
We found that MK8776 significantly sensitized HRR-proficient (AsPC-1, MiaPaCa-2, BxPC-3) but not deficient (Capan-1) pancreatic cancer cells to gemcitabine-radiation and inhibited Rad51 focus formation in HRR-proficient cells. In vivo, MiaPaCa-2 xenografts were significantly sensitized to gemcitabine-radiation by MK8776 without significant weight loss or observable toxicity in the small intestine, the dose limiting organ for chemoradiation therapy in pancreatic cancer. We also assessed pChk1 (S345), a pharmacodynamic biomarker of DNA damage in response to Chk1 inhibition in both tumor and small intestine and found that MK8776 combined with gemcitabine or gemcitabine-radiation produced a significantly greater increase in pChk1 (S345) in tumor relative to small intestine, suggesting greater DNA damage in tumor than in normal tissue. Furthermore, we demonstrated the utility of an ex vivo platform for assessment of pharmacodynamic biomarkers of Chk1 inhibition in pancreatic cancer.
Conclusions
Together, our results suggest that MK8776 selectively sensitizes HRR-proficient pancreatic cancer cells and xenografts to gemcitabine-radiation and support the clinical investigation of MK8776 in combination with gemcitabine-radiation in locally advanced pancreatic cancer.
Keywords: pancreatic cancer, Chk1, radiosensitization, homologous recombination repair, gemcitabine, cell cycle checkpoint
Introduction
Despite progress in the management of advanced pancreatic cancer, the prognosis for patients remains dismal. While the use of FOLFIRINOX in the metastatic setting and concurrent gemcitabine-based chemoradiation in the locally advanced setting has produced incremental improvements in survival, more effective therapies are desperately needed (1, 2). Local disease management is an important issue in pancreatic cancer as evidenced by the findings that 30% of patients die from local progression (3) and local control with radiation improves survival (2, 4). However, the median survival for patients with locally advanced disease remains approximately one year (although our recent results and those of others are more encouraging (5, 6)). Thus, we sought to build upon these promising clinical results by the addition of a molecularly targeted agent to chemoradiation in pancreatic cancer.
Checkpoint kinase 1 (Chk1) is an important molecular target for sensitizing cancer cells to DNA damaging agents. In response to DNA damage, Chk1 inhibits Cdc25 phosphatases resulting in cyclin-Cdk inhibition, and cell cycle arrest (notably, G2 arrest). In addition, Chk1 plays a role in activation of HRR (7, 8). Thus, impairment of the DNA damage response by Chk1 inhibition renders cells more sensitive to DNA damage. Selectivity of this sensitization for tumor cells is a critical issue, and the consensus of many independent studies supports a model in which sensitization occurs preferentially in p53 mutant tumor cells (9-11). After DNA damage, normal cells are protected by their p53-mediated G1 checkpoint in the presence of Chk1 inhibition, whereas p53 mutant tumor cells lack this G1 checkpoint and are sensitized by G2 checkpoint abrogation in response to Chk1 inhibition. Small molecule inhibitors of Chk1 have been an intense area of investigation in the last decade and as a result a number of potential clinical candidates are now under active clinical investigation: LY2603618, LY2606368, and MK8776 (formerly, SCH900776) (12, 13).
Work from our laboratory has demonstrated that Chk1 inhibition by AZD7762 sensitizes pancreatic cancer cells and tumors to gemcitabine-radiation and that inhibition of HRR is an important underlying mechanism of this sensitization (8). Furthermore, our previous work identified pChk1 (S345) as a pharmacodynamic biomarker of Chk1 inhibition through increased ATR/ATM-mediated phosphorylation of Chk1 which occurs in response to the heightened DNA damage associated with Chk1 inhibition (14, 15). Given our underlying clinical goal of combining Chk1 inhibition with gemcitabine-radiation in pancreatic cancer, in the present study we evaluated a selective Chk1 inhibitor, MK8776, which is advancing to Phase II trials, in combination with gemcitabine-radiation in preclinical models of pancreatic cancer. Although this is the first study to evaluate MK8776 in combination with radiation and chemoradiation, it has been shown to sensitize to various chemotherapies including gemcitabine and cytarabine (12, 16). Importantly, we also investigated the tumor cell selectivity of this regimen by examining the DNA damage response not only in tumors but also in normal tissues. We found that MK8776 sensitized pancreatic cancer cells to gemcitabine and gemcitabine-radiation, but only in an HRR competent setting and in association with inhibition of Rad51 focus formation. Thus, we went on to determine the effects of MK8776 in combination with gemcitabine and radiation on pancreatic tumor xenograft growth as well as on biomarkers of Chk1 inhibition in both tumor and normal tissues. Furthermore, we introduce a new platform for the ex vivo assessment of pharmacodynamic biomarkers in pancreatic cancer.
Materials and Methods
Cell culture and drug solutions
AsPC-1, MiaPaCa-2, and BxPC-3 cells were obtained from ATCC. Capan-1 Neo cells were obtained from S. Powell (17). Cells were cultured in DMEM (MiaPaCa-2), RPMI (AsPC-1, BxPC-3), or IMDM (Capan-1) supplemented with 10% fetal bovine serum, 2mM glutamine and antibiotics. MK8776 was obtained from Merck and prepared as 10mM stocks in DMSO for in vitro studies. For in vivo use, 5mg/ml MK8776 was dissolved in 5% dextrose monohydrate (pH 6.5; Sigma) and administered by IP injection. Gemcitabine (Eli Lily) was dissolved in PBS or saline for in vitro and in vivo use, respectively.
Clonogenic Survival
Exponentially growing cells were treated with drugs/radiation and then replated at cloning densities. Cells were grown for 9-14 days and then fixed and stained with methanol-acetic acid and crystal violet, respectively and scored for colonies of ≥50 cells. Drug cytotoxicity was calculated as the ratio of surviving drug-treated cells relative to untreated control cells. Radiation survival data from drug-treated cells were corrected for drug cytotoxicity. Cell survival curves were fitted using the linear-quadratic equation, and the mean inactivation dose calculated according to the method of Fertil and colleagues (18). The radiation enhancement ratio was calculated as the ratio of the mean inactivation dose under control conditions divided by the mean inactivation dose under drug-treated conditions.
Immunofluorescence
Cells cultured and treated on cover slips were fixed and processed as previously described except with a mouse monoclonal Rad51 antibody (GeneTex) (19). Samples were imaged with an Olympus IX71 FluoView confocal microscope (Olympus America) with a 60x oil objective. For quantitation of Rad51 foci, at least 100 cells from each of three independent experiments were visually scored for each condition.
Immunohistochemistry
Tissues were fixed in 10% neutral buffered formalin for 24 hours, then embedded in paraffin and sectioned at 5μm. Sections were dehydrated to buffer and pretreated with citrate buffer, pH 6.0 for 10 minutes. After peroxidase and non-specific blocking sections were stained with pChk1 (S345) antibody (Cell Signaling) at a dilution of 1:200 overnight at 4°C, followed the next day with the Envision+ Rabbit detection kit (room temperature) on a DAKO Autostainer (DAKO North America). Chromogen was applied for 5 minutes and sections were counterstained with hematoxylin. Immunohistochemistry was conducted by the University of Michigan Cancer Center Research Histology and Immunoperoxidase Lab. Specimens were evaluated microscopically with a 20× objective on an Olympus IX71 microscope (Olympus America) with a Nikon DS-Fi1 camera and NIS-Elements software (Nikon). Samples were scored blindly by counting the number positive and negative nuclei in a representative field. Between 300 and 800 cells were scored for each sample.
Immunoblotting
Pulverized tissues were lysed and immunoblotted as previously described (20). Proteins were detected with antibodies recognizing pChk1 (S345), pChk1 (S296), GAPDH (Cell Signaling), and total Chk1 (Santa Cruz).
Irradiation
Irradiations were carried out using a Philips RT250 (Kimtron Medical) at a dose rate of ~2Gy/minute in the University of Michigan Comprehensive Cancer Center Experimental Irradiation Core. Dosimetry was carried out using an ionization chamber connected to an electrometer system that is directly traceable to a National Institute of Standards and Technology calibration. For tumor irradiation, animals were anesthetized with isoflurane and positioned such that the apex of each flank tumor was at the center of a 2.4cm aperture in the secondary collimator, with the rest of the mouse shielded from radiation. For simultaneous irradiation of flank tumors and small intestines, mice were placed in a prone position and a 2cm × 8cm aperture in the secondary collimator was used.
Tumor growth studies
Animals were handled according to a protocol approved by the University of Michigan Committee for Use and Care of animals. MiaPaCa-2 cells (5×106) were suspended in a 1:1 mixture of 10% FBS/DMEM:Matrigel (BD Biosciences) and injected subcutaneously, bilaterally into the flanks of 3-5 week old, female, athymic nude mice. Treatment was initiated when the average tumor volume reached 100mm3. Tumor size was measured two times per week; tumor volume (TV) was calculated according to the equation: TV = π / 6 (ab2), where a and b are the longer and shorter dimensions of the tumor, respectively. Measurements were made until the tumor volume increased by approximately a factor of 4.
Tumor slicing
Tumors of approximately 300mm3 were collected aseptically and embedded in 3% low melting point agarose. Two hundred μm slices were prepared with a Leica VT 1200S microtome with vibrating blade (Leica Microsystems) while submerged in oxygenated, ice cold Krebs buffer, according to the previously described methodology (21). Slices were incubated in 12-well dishes containing DMEM (ATCC) supplemented with 2mM glutamine, penicillin, streptomycin, antibiotic-antimycotic (Gibco), and 20mM HEPES (pH 7.4) in a carbogen (95% O2, 5% CO2) purged chamber inside of at a 37°C, humidified, 5% CO2 incubator for up to 30 h, after which they were processed for biochemical or immunohistochemical analysis using the same methods as for intact tumors.
Statistics
For tumor growth experiments, the time required for tumor volume doubling was determined for each xenograft by identifying the earliest day on which it was at least twice as large as on the first day of treatment. The Kaplan-Meier method was used to analyze the doubling times. Log rank test (PROC LIFETEST in SAS) was used to compare the doubling times between any two treatment groups. In addition, the Bayesian hierarchical changepoint (BHC) model was used to compare tumor regression rates, regression periods, and regrowth rates between any two treatment groups. Biomarker data were analyzed using a mixed effect model (PROC MIXED in SAS), with a random effect modeling correlation between normal and tumor cells in the same animal. A Student’s t-test or one-way ANOVA with a Tukey’s post-test was used for other analyses.
Results
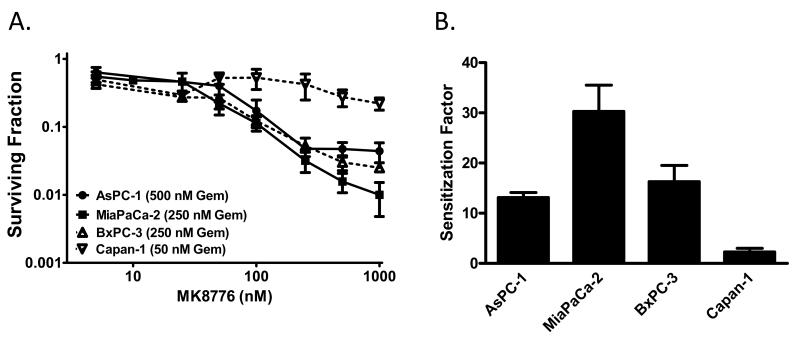
To begin to determine the ability of MK8776 to sensitize to chemoradiation in pancreatic cancer cells, we first assessed sensitization to gemcitabine by MK8776 in a panel of p53 mutant pancreatic cancer cell lines. Cells were treated with IC50 concentrations of gemcitabine followed by MK8776 at increasing concentrations. We found, in the order of magnitude of sensitization, that MiaPaCa-2, BxPC-3, and AsPC-1 cells were sensitized to gemcitabine by MK8776 (Fig. 1), while Capan-1 cells were only minimally sensitized. Sensitization occurred at concentrations of MK8776 which produced minimal single agent toxicity (Suppl. Fig. 1).
Figure 1. Chemosensitization by MK8776 in pancreatic cancer cells.
Exponentially growing pancreatic cancer cells were treated with an IC50 concentration of gemcitabine (AsPC-1, 500nM; MiaPaCa-2 and BxPC-3, 250nM; Capan-1, 50nM) for 2 hours (t=0-2h) followed by increasing concentrations of MK8776 (t=24-48h). At the end of drug exposure, cells were processed for clonogenic survival. The surviving fraction was normalized to the MK8776 alone plating efficiency for each respective concentration. B, The Sensitization Factor was calculated as (surviving fraction, Gem only)/(surviving fraction, Gem + MK8776). The MK8776 concentration used to calculate the Sensitization Factor was 500nM except in AsPC-1 which was 250nM. Data are the mean ± standard error of n = 3 - 4 independent experiments.
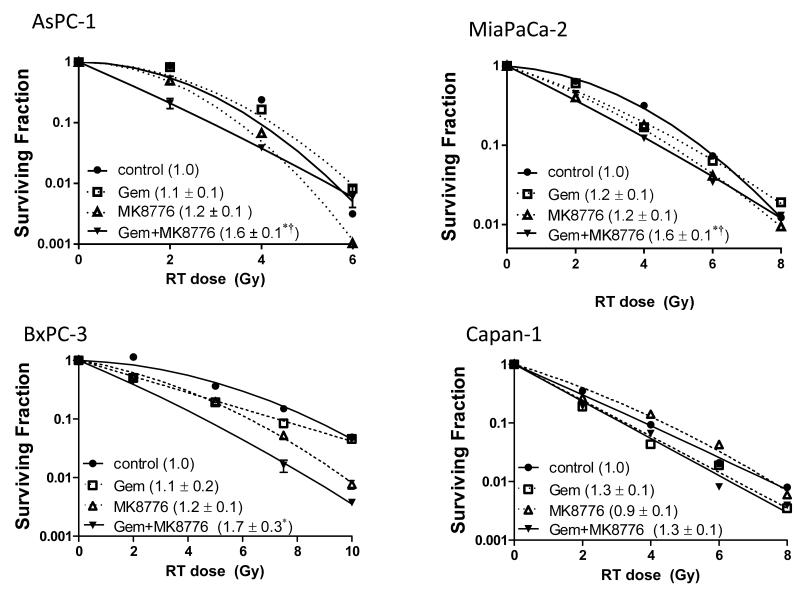
We next assessed whether MK8776 would sensitize pancreatic cancer cells to gemcitabine-based chemoradiation. Cells were treated with a previously optimized schedule of gemcitabine followed by MK8776 and radiation (8, 14). We utilized non-toxic concentrations of gemcitabine that produced little radiosensitization alone and minimally toxic concentrations of MK8776, which were sufficient to abrogate the G2 checkpoint (Suppl. Fig. 2) and inhibit Chk1 autophosphorylation (16). Treatment of AsPC-1, MiaPaCa-2, and BxPC-3 cells with MK8776 did not produce significant radiosensitization although there was a trend toward marginal radiosensitization under these minimally toxic MK8776 conditions (Fig. 2, Suppl. Table 1). Importantly, MK8776 did significantly sensitize AsPC-1, MiaPaCa-2, and BxPC-3 cells to gemcitabine-radiation but not Capan-1 cells. Consistent with this observation, MK8776 treatment caused persistent DNA damage, reflected by γH2AX expression in response to gemcitabine-radiation in MiaPaCa-2 but not Capan-1 cells (Suppl. Fig. 3). Given that Capan-1 cells harbor a BRCA2 mutation rendering them HRR incompetent (17) while no BRCA2 mutation is present in the other pancreatic cancer cell lines, these findings suggest that HRR may play a role in sensitization by MK8776.
Figure 2. Sensitization to chemoradiation by MK8776 in pancreatic cancer cells.
Pancreatic cancer cell lines were treated with minimally cytotoxic concentrations of gemcitabine (AsPC-1, 100nM, MiaPaCa-2 and BxPC-3, 50nM; Capan-1, 25nM) for 2 hours (t=0-2h) followed by MK8776 (500nM except in AsPC-1 250nM; t=23-48h), and radiation (0-8Gy; t=24h). At the end of treatment cells were processed for clonogenic survival. Data are from a single representative experiment (plots) or are the mean radiation enhancement ratio ± standard error for n = 3 - 4 independent experiments (in parenthesis). Statistical significance (P<0.05) versus control* or Gem† is indicated. Cytotoxicity is summarized in Suppl. Table 1.
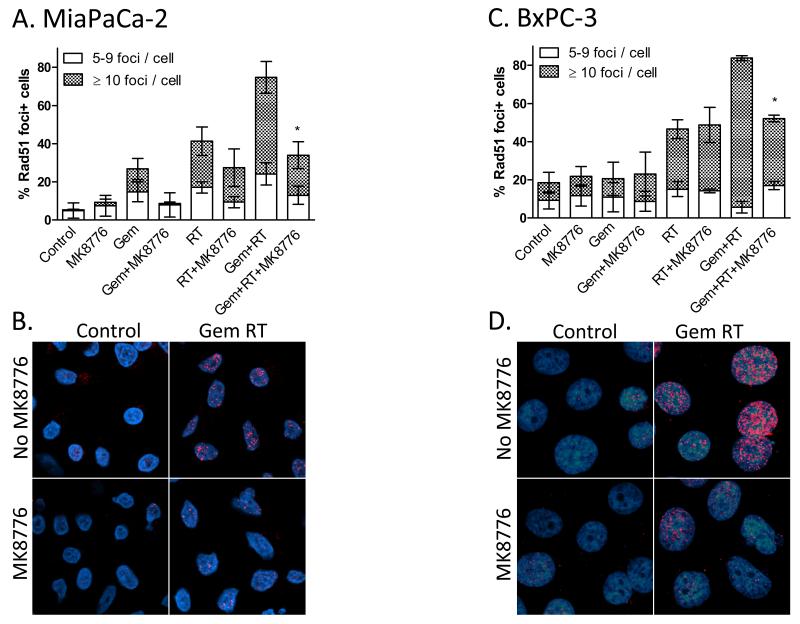
In order to specifically assess HRR we measured Rad51 focus formation in response to gemcitabine, MK8776, and radiation. Rad51, a DNA recombinase that promotes template strand invasion, is a key intermediary in HRR and forms foci at sites of HRR-mediated DNA double strand break repair (22). In MiaPaCa-2 cells, Rad51 focus formation was induced in response to gemcitabine and/or radiation treatment, and as anticipated, MK8776 inhibited Rad51 focus formation in response to gemcitabine/radiation although the magnitude of this effect appeared greatest in the presence of gemcitabine (Fig. 3A-B). In BxPC-3 cells, the inhibitory effect of MK8776 on Rad51 foci was only evident in response to gemcitabine plus radiation (Fig. 3C-D). This result is consistent with the chemoradiosensitization by MK8776 observed in these cells (Fig. 2). Together these results demonstrate that the ability of MK8776 to inhibit Rad51 focus formation after gemcitabine-radiation is associated with sensitization.
Figure 3. MK8776 inhibits Rad51 focus formation in response to chemoradiation.
MiaPaCa-2 (A, B) or BxPC-3 cells (C, D) were treated with Gem (t=0-2h), MK8776 (t=23-30h), and radiation (7.5Gy; t=24h). Six hours post-radiation, cells were fixed and stained for Rad51 (red) and with DAPI (blue). Rad51 foci were scored and are presented as the percentage of cells containing 5-9 foci or ≥ 10 foci. Data are the mean ± standard error of n=3 independent experiments (A, C) where statistical significance versus GemRT for 10+ foci is indicated *P<0.05. Representative fields are shown (B, D).
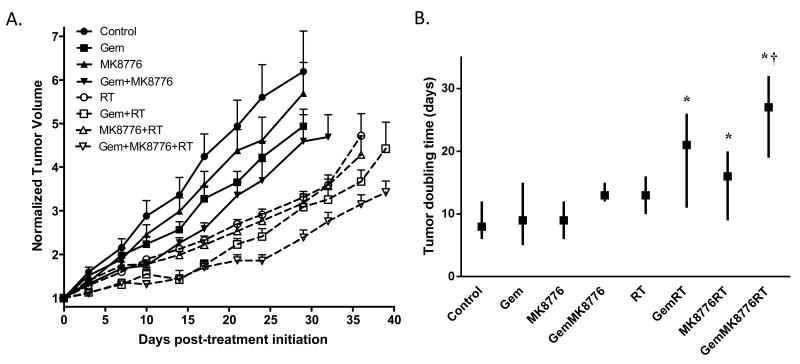
We then wished to determine the efficacy of MK8776 on pancreatic tumor growth in response to gemcitabine and radiation. Mice bearing MiaPaCa-2 xenografts were treated with MK8776 in combination with gemcitabine and radiation for two weeks and tumor growth was monitored during and after therapy. We found that treatment with modest doses of gemcitabine or MK8776 alone produced minimal effect on tumor growth (Fig. 4). While the combination of gemcitabine and MK8776 appeared to inhibit tumor growth more than either single agent, this effect did not reach significance. As expected, radiation treatment prolonged the time required for tumor volume doubling. Likewise, the combination of modest doses of gemcitabine or MK8776 with radiation prolonged the time required for tumor volume doubling relative to untreated tumors (p=0.0001 or p=0.0195, respectively) but this effect was not significant relative to radiation treatment. Importantly, the combination of these same modest doses of MK8776 with gemcitabine-radiation significantly delayed the tumor volume doubling time relative to gemcitabine-radiation treatment (p=0.0087) without a significant effect on weight loss (Suppl. Fig. 4). In addition, using the BHC statistical model (23), we noted that during treatment with the combination of gemcitabine, MK8776, and radiation, that tumors did not grow for an average of 10 days (90% confidence interval 5 to 14 days) whereas tumor growth occurred throughout treatment with gemcitabine-radiation. These results support the conclusion that MK8776 sensitizes pancreatic tumors to chemoradiation.
Figure 4. MK8776 sensitizes pancreatic tumor xenografts to chemoradiation.
Athymic nude mice bearing bilateral, subcutaneous MiaPaCa-2 xenografts were treated with Gem (120mg/kg, Mon, t=0h), MK8776 (50mg/kg, Mon-Fri, t=2h), and radiation (1Gy/fraction, Mon-Fri, t=3h) for 2 cycles. Data are the mean normalized tumor volume ± standard error of n = 12 - 18 tumors per condition (A) or are the median tumor volume doubling time (symbols) with upper and lower limits (lines) for n = 12 - 18 tumors per condition (B). Statistical significance determined by the log-rank test versus control* or Gem RT† is indicated.
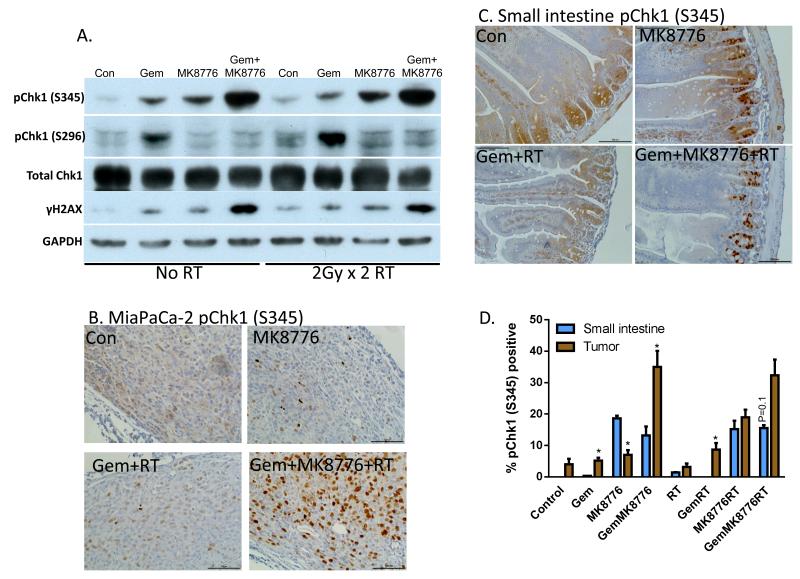
In order to assess the Chk1-mediated DNA damage response in tumors, mice bearing MiaPaCa-2 xenografts were acutely treated with MK8776, gemcitabine, and radiation. The levels of both ATR/ATM-mediated phosphorylation (S345) as well as autophosphorylation (S296) of Chk1 in tumors were then measured. We found that pChk1 (S345) levels increased in response to either gemcitabine or MK8776, while the combination of the two produced an even greater level of pChk1 (S345) (Fig. 5A). In tumors treated with radiation there was a slight increase in pChk1 (S345) which was maximal in response to the combination of MK8776+gemcitabine+radiation. This increase in pChk1 (S345) in response to Chk1 inhibition has been previously established and is thought to occur as a result of increased DNA damage in response to Chk1 inhibition as well as a feedback loop with protein phosphatase 2A (14, 24). Indeed, we found that induction of pChk1 (S345) was in general agreement with γH2AX expression in response to MK8776, gemcitabine, and radiation. Consistent with Chk1 inhibition by MK8776, Chk1 autophosphorylation (S296) was inhibited under all conditions in which MK8776 was present. The effects of treatment on pChk1 (S345) were largely reproduced by immunohistochemistry, where strong nuclear pChk1 (S345) staining was observed in response to MK8776+gemcitabine+radiation (Fig. 5B, Suppl. Fig. 5).
Figure 5. Phosphorylated Chk1 (S345) is a biomarker of MK8776 activity.
A-D, Mice bearing MiaPaCa-2 xenografts (approximately 250mm3) were treated with Gem (120mg/kg, t=0h), MK8776 (50mg/kg, t=2 and 23h), and radiation (2Gy/fraction, t=3 and 24h). One hour post-radiation (t=25h), tumors were lysed for immunoblotting (A) or fixed for immunohistochemistry (B). Small intestines from the same animals were harvested in parallel and prepared for immunohistochemistry (C). D, Quantitation of pChk1 (S345) immunostaining was conducted by scoring the percentage of tumor cells or intestinal crypt cells with nuclear pChk1 (S345) staining. Data are the mean ± standard error of 4 tumors per condition or 2 segments of small intestine from 2 independent animals. Bars represent between 1600 and 3000 scored cells. Statistically significant differences between small intestines and tumor are indicated (*P<0.05). Images/blots are representative of 4 tumors (A, B) or 2 small intestines (C). Complete image sets are included in Suppl. Fig. 5.
Clinically, the combination of gemcitabine with radiation for pancreatic cancer is limited by toxicity to the small intestine. We hypothesized that DNA damage measured by phosphorylation of Chk1 (S345) would be increased by MK8776 in combination with gemcitabine-radiation in tumor cells, but not in normal cells, as normal cells should be protected by an intact p53-mediated G1 checkpoint, while p53 mutant tumor cells should not. Small intestines were harvested from treated, tumor bearing mice and assessed by immunohistochemistry for pChk1 (S345) protein levels. MK8776 treatment alone increased pChk1 (S345) levels in the intestinal crypt cells but not in the intestinal villar cells, a result likely related to the role Chk1 plays during DNA synthesis in rapidly dividing cells (12, 25) (Fig. 5C-D). However, this early increase in pChk1 (S345) in the intestinal crypt cells in response to MK8776 was not associated with any observable long term toxicity (Suppl. Fig. 4). More importantly, the addition of MK8776 to gemcitabine or gemcitabine-radiation produced a significantly greater effect on pChk1 (S345) in tumor relative to small intestine. In order to specifically test whether MK8776 sensitized small intestine to gemcitabine-radiation, we conducted a jejunal crypt assay, as we have done previously (26). We found, with the exception of positive control-treated mice, that none of the treatments, including MK8776 combined with gemcitabine-radiation produced any observable loss of crypt viability (Suppl. Fig. 6). This result is consistent with a minimal effect of MK8776 in combination with gemcitabine-radiation on weight loss (Suppl. Fig. 4). Collectively, these results demonstrate that Chk1 inhibition produces more DNA damage and thus greater sensitization in response to gemcitabine/radiation in tumors than in normal tissues.
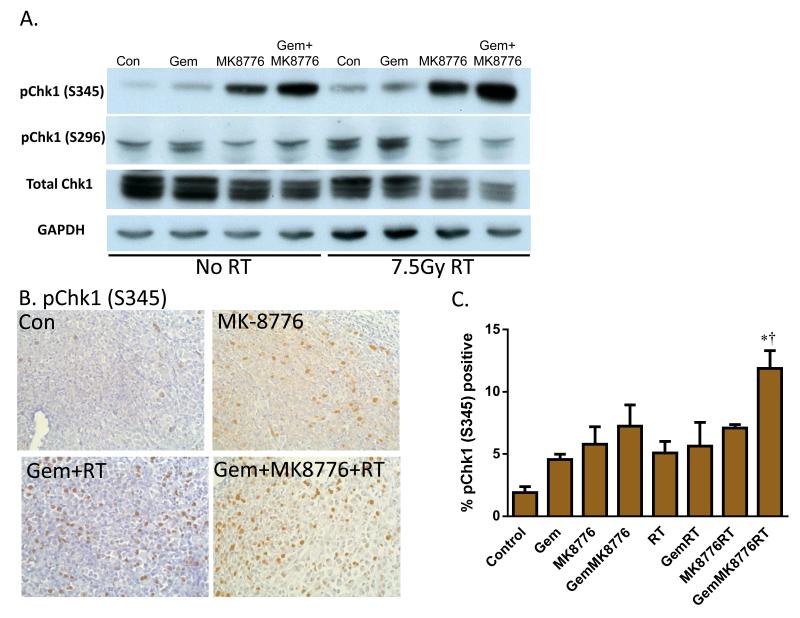
Given the need for pharmacodynamic biomarkers of response in pancreatic cancer and the complexity associated with pre- and post-treatment biopsies in patients, we initiated an ex vivo biomarker system with the long term goal of being able to assess pharmacodynamic biomarkers in patient tumors from a single biopsy. To this end, 200 μm slices of MiaPaCa-2 xenografts were treated ex vivo with MK8776, gemcitabine, and radiation. Similar to the results obtained following in vivo treatment (Fig. 5A-B), pChk1 (S345) was induced in response to MK8776 and, to a greater extent, in combination with gemcitabine/radiation (Fig. 6). Consistent with phosphorylation of Chk1 at S345 triggering ubiquitin-mediated degradation of Chk1 (19, 27), we observed a decrease in total Chk1 protein in response to treatment with gemcitabine and MK8776 (with or without radiation). Induction of pChk1 (S296) was inhibited by MK8776. Immunohistochemistry revealed that the induction of pChk1 (S345) mirrored that seen in the in vivo-treated xenografts and also demonstrated that the structural integrity of the tumors was maintained using this tissue slicing approach (Fig. 6B-C; Suppl. Fig. 7). Taken together, these results demonstrate that this ex vivo biomarker approach can recapitulate an in vivo pharmacodynamic response and warrant further studies to determine whether this platform has potential utility in the clinic.
Figure 6. Ex vivo-treated tumor slices as a biomarker platform in pancreatic tumors.
MiaPaCa-2 xenografts were collected, embedded in low melting point agarose, and sectioned into 200um slices. Slices were treated with Gem (50nM, t=0-2h), MK8776 (500nM, t=23-30h), and radiation (7.5Gy, t=24h). At time 30 hours, samples were lysed for immunoblotting (A) or fixed for immunohistochemistry (B), respectively. Data are representative of 3 independent experiments. C, Quantification of pChk1 (S345) immunostaining was conducted by scoring the percentage of cells with nuclear pChk1 (S345) staining. Data are the mean ± standard error of 3 tumors per condition with an average of 1000 cells scored per sample. Statistical significance (P<0.05) versus control* or GemRT† is indicated. A complete image set is included in Suppl. Fig. 7.
Discussion
In this study we have found that inhibition of Chk1 by MK8776 sensitizes pancreatic cancers to gemcitabine-radiation through mechanisms involving inhibition of HRR. Importantly, our animal studies suggest that sensitization of tumor cells to gemcitabine-radiation by Chk1 inhibition occurs selectively in tumor cells relative to small intestines. In addition, we demonstrate that pChk1 (S345) is a useful pharmacodynamic biomarker of MK8776 sensitization in tumor cells and introduce an ex vivo biomarker platform for the assessment of pancreatic tumor specimens. These results support clinical investigation of MK8776 with gemcitabine-radiation in locally advanced pancreatic cancer patients.
A number of Chk1 inhibitors have been developed preclinically as sensitizers with the intent of combining them with standard chemotherapy/radiation in the clinic. In our previous work we investigated the Chk1/Chk2 inhibitor AZD7762 in combination with chemoradiation in pancreatic cancer. Unlike AZD7762, which has equal affinity for Chk1 and Chk2 (28), MK8776 is approximately 500 times more selective for Chk1 than Chk2 (12). We noted differences between AZD7762 and MK8776 in terms of their pure radiosensitizing properties. Both in vitro and in vivo, AZD7762 was a very effective radiosensitizer, while single agent MK8776 produced less radiosensitization (8, 10). Although our previous studies suggested Chk1 was the key target of AZD7762 for modulating radiosensitization (8), it is still intriguing to consider the possibility that the ability of AZD7762 to radiosensitize is in part attributable to its activity against Chk2 or some other target. Others have found that inhibition of Chk2 radiosensitizes cancer cells (29). In terms of chemosensitization, MK8776 and AZD7762 are similar (14). Most importantly, both AZD7762 and MK8776 produced significant sensitization to gemcitabine-radiation, which is crucial in the context of developing a new treatment for locally advanced pancreatic cancer.
Tumor cell selectivity is a critical issue in the development of any new cancer treatment. Specifically, with regard to Chk1 inhibitors there are at least two factors to consider: tumor cell selectivity of sensitization and tumor cell selectivity of Chk1 inhibitor monotherapy. Sensitization by Chk1 inhibition is thought to occur in a p53 mutation-dependent manner whereby normal cells are protected by their p53-mediated G1 checkpoint, while p53 mutant tumor cells are not. There is substantial literature to support this model (10, 11), although it is likely that other genetic aberrations contribute to this selectivity (30). With respect to the effects of Chk1 inhibitors as monotherapy, the emerging evidence that Chk1 inhibition causes DNA damage in replicating cells (31-33) has led to the hypothesis that the deleterious effects of Chk1 inhibition should preferentially occur in tumor cells based on their higher fraction of cycling cells and elevated levels of endogenous DNA damage due to genetic aberrations relative to normal cells (25). Since small intestine is the dose limiting toxicity for chemoradiation therapy of pancreatic cancer, we investigated the effects of Chk1 inhibition on sensitization of the small intestine to chemoradiation. To our knowledge this is the first study to examine the sensitizing effects of a small molecule Chk1 inhibitor on a normal tissue. Consistent with the role of Chk1 in replicating cells, we found pS345 Chk1 was induced in both tumor and small intestines following treatment with MK8776 alone. Of note, the pChk1 (S345) induction in the small intestines was restricted only to the replicating crypt cells. Although we have noted that pS345 Chk1 is a surrogate marker for DNA damage in tumors (14), the meaning of this marker in crypt cells is less clear, since there was no evidence of intestinal toxicity (weight loss or loss of crypt viability). More importantly, in the small intestines, the addition of gemcitabine/radiation to MK8776 did not produce any additional pChk1 (S345) or intestinal crypt toxicity suggesting that no sensitization occurred in the small intestines. In tumors however, the addition of gemcitabine/radiation to MK8776 produced a significant increase in pChk1 (S345) relative to MK8776 alone and was associated with the greatest tumor growth delay. Taken together these results suggest that Chk1 inhibition by MK8776 selectively sensitizes tumor but not small intestine to gemcitabine/radiation.
An interesting finding of this study is that MK8776 did not sensitize to chemoradiation in HRR-deficient pancreatic cancer cells. Our previous work suggested that HRR inhibition was a mechanism of radiosensitization by Chk1 inhibitors, but left unclear the relative contributions of HRR inhibition versus G2 checkpoint abrogation to sensitization. The finding that Capan-1 cells, which are rendered HRR-incompetent by a BRCA2 mutation, are not sensitized to gemcitabine/radiation by MK8776 (despite G2 checkpoint abrogation by MK8776; data not shown) suggests that HRR inhibition is a major mechanism of sensitization. Consistent with previous reports, the effects of MK8776 on Rad51 focus formation (Fig. 3) support the hypothesis that HRR is a major target of Chk1 inhibition. Given that BRCA1/2 mutations are quite rare in pancreatic cancer (34), these results are favorable in the context of the broader applicability of Chk1 inhibitors as sensitizers in pancreatic cancers.
The development of a molecularly targeted agent in pancreatic cancer should involve the confirmation that both the target and the subsequent downstream signaling are inhibited. In the absence of a primary resection, patient pancreatic tumor specimens are generally limited to a one-time fine needle aspirate or a small core needle biopsy. While this may be adequate for assessing static biomarkers (such p53 or K-Ras mutation status), it is insufficient for the assessment of pharmacodynamic responses which require pre- and post- treatment comparison. Furthermore, while p53 mutation status is a useful biomarker to some extent, it does not capture the degree of sensitization, as even in p53 mutant cells Chk1 inhibition produces a wide range of sensitization (Fig. 1). Thus, the development of pharmacodynamic biomarkers of Chk1 inhibition is important and has the potential to be predictive of the degree of tumor sensitization. To begin to develop a system which would permit pharmacodynamic assessment of a biomarker from a one-time biopsy in pancreatic cancer, we have integrated methodology which utilizes thinly sliced fresh tissue (21) into an ex vivo treatment platform. It will be important in future studies to determine if ex vivo biomarker analyses are feasible on patient pancreatic tumor biopsies and whether they are predictive of therapeutic efficacy (35).
Our preclinical finding that MK8776 improves the efficacy of gemcitabine-radiation in pancreatic cancer xenografts provides further support for the clinical development of Chk1 inhibitors in combination with chemoradiation. In order to best utilize Chk1 inhibitors such as MK8776 with chemoradiation, however, a few outstanding issues need to be addressed. Although p53 is one of several candidate biomarkers for tumor sensitization following Chk1 inhibition, the relationship of p53 mutation status, or that of other candidate biomarkers such as K-Ras, BRCA1/2 or DPC4, with clinical outcome remains to be established. Similarly, while pChk1 (S345) appears to be a reliable biomarker for Chk1 inhibition and DNA damage, it is still unclear whether changes in pChk1 (S345), or other dynamic biomarkers such as γH2AX or Rad51, correlate with clinical response. Furthermore, with the clinical development of agents that target different molecules in the Chk1-response network, such as Wee1, it is plausible that different subsets of tumors may respond more to Chk1 inhibitors compared to another targeted agent, and vice versa. Thus it will be important to compare these agents to Chk1 inhibitors in terms of their efficacy and tumor cell selectivity, as well as to identify biomarkers which predict the superiority of one agent over the others. Finally, it is of interest to begin to understand the mechanisms of resistance which may be incurred in response to Chk1 inhibition in tumor cells.
Supplementary Material
Statement of Translational Relevance.
Chemoradiation (versus chemotherapy alone) improves survival in locally advanced pancreatic cancer, and thus represents the best standard platform on which to improve outcome. Chk1 inhibitors in combination with chemotherapy in pancreatic cancer are now entering Phase II clinical trials, and it is likely that Chk1 inhibitors in combination with chemoradiation will produce an even greater therapeutic benefit in the locally advanced disease setting. One outstanding issue in the development of Chk1 inhibitors is whether sensitization can be achieved selectively in tumors. In this study we demonstrate the efficacy and tumor cell selectivity of sensitization to chemoradiation by Chk1 inhibition and assess pharmacodynamic biomarkers of this sensitization. The findings of this study should aid in the design of planned clinical trials using Chk1 inhibitors with chemoradiation in pancreatic cancer.
Acknowledgements
Grant support: This work was funded by NIH Grants R01CA163895 (MM), R01CA138723 (JM, TL), P50CA130810 (JM, TL), and Cancer Center Core Grant P30 CA046592 (TL), and an Alfred B. Taubman Scholarship (TL).
Abbreviations
- Chk1
checkpoint kinase 1
- ER
radiation enhancement ratio
- Gem
gemcitabine
- HRR
homologous recombination repair
- IMRT
intensity modulated radiation therapy
- pS345 Chk1
phosphorylated S345 Chk1
Footnotes
Disclosure of potential conflicts of interest: None.
References
- 1.Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–25. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 2.Loehrer PJ, Sr., Feng Y, Cardenes H, Wagner L, Brell JM, Cella D, et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an eastern cooperative oncology group trial. J Clin Oncol. 2011;29:4105–12. doi: 10.1200/JCO.2011.34.8904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iacobuzio-Donahue CA, Fu B, Yachida S, Luo M, Abe H, Henderson CM, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol. 2009;27:1806–13. doi: 10.1200/JCO.2008.17.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ben-Josef E, Lawrence TS. Radiotherapy: the importance of local control in pancreatic cancer. Nat Rev Clin Oncol. 2012;9:9–10. doi: 10.1038/nrclinonc.2011.182. [DOI] [PubMed] [Google Scholar]
- 5.Ben-Josef E, Schipper M, Francis IR, Hadley S, Ten-Haken R, Lawrence T, et al. A Phase I/II Trial of Intensity Modulated Radiation (IMRT) Dose Escalation With Concurrent Fixed-dose Rate Gemcitabine (FDR-G) in Patients With Unresectable Pancreatic Cancer. Int J Radiat Oncol Biol Phys. 2012 doi: 10.1016/j.ijrobp.2012.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crane CH, Varadhachary GR, Yordy JS, Staerkel GA, Javle MM, Safran H, et al. Phase II trial of cetuximab, gemcitabine, and oxaliplatin followed by chemoradiation with cetuximab for locally advanced (T4) pancreatic adenocarcinoma: correlation of Smad4(Dpc4) immunostaining with pattern of disease progression. J Clin Oncol. 2011;29:3037–43. doi: 10.1200/JCO.2010.33.8038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sorensen CS, Hansen LT, Dziegielewski J, Syljuasen RG, Lundin C, Bartek J, et al. The cell-cycle checkpoint kinase Chk1 is required for mammalian homologous recombination repair. Nat Cell Biol. 2005;7:195–201. doi: 10.1038/ncb1212. [DOI] [PubMed] [Google Scholar]
- 8.Morgan MA, Parsels LA, Zhao L, Parsels JD, Davis MA, Hassan MC, et al. Mechanism of radiosensitization by the Chk1/2 inhibitor AZD7762 involves abrogation of the G2 checkpoint and inhibition of homologous recombinational DNA repair. Cancer Res. 2010;70:4972–81. doi: 10.1158/0008-5472.CAN-09-3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma CX, Janetka JW, Piwnica-Worms H. Death by releasing the breaks: CHK1 inhibitors as cancer therapeutics. Trends Mol Med. 2010 doi: 10.1016/j.molmed.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitchell JB, Choudhuri R, Fabre K, Sowers AL, Citrin D, Zabludoff SD, et al. In vitro and in vivo radiation sensitization of human tumor cells by a novel checkpoint kinase inhibitor, AZD7762. Clin Cancer Res. 2010;16:2076–84. doi: 10.1158/1078-0432.CCR-09-3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vance S, Liu E, Zhao L, Parsels JD, Parsels LA, Brown JL, et al. Selective radiosensitization of p53 mutant pancreatic cancer cells by combined inhibition of Chk1 and PARP1. Cell Cycle. 2011;10:4321–9. doi: 10.4161/cc.10.24.18661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guzi TJ, Paruch K, Dwyer MP, Labroli M, Shanahan F, Davis N, et al. Targeting the Replication Checkpoint Using SCH 900776, a Potent and Functionally Selective CHK1 Inhibitor Identified via High Content Screening. Mol Cancer Ther. 2011;10:591–602. doi: 10.1158/1535-7163.MCT-10-0928. [DOI] [PubMed] [Google Scholar]
- 13.Carrassa L, Damia G. Unleashing Chk1 in cancer therapy. Cell Cycle. 2011;10:2121–8. doi: 10.4161/cc.10.13.16398. [DOI] [PubMed] [Google Scholar]
- 14.Parsels LA, Qian Y, Tanska DM, Gross M, Zhao L, Hassan MC, et al. Assessment of chk1 phosphorylation as a pharmacodynamic biomarker of chk1 inhibition. Clin Cancer Res. 2011;17:3706–15. doi: 10.1158/1078-0432.CCR-10-3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McNeely S, Conti C, Sheikh T, Patel H, Zabludoff S, Pommier Y, et al. Chk1 inhibition after replicative stress activates a double strand break response mediated by ATM and DNA-dependent protein kinase. Cell Cycle. 2010;9:995–1004. doi: 10.4161/cc.9.5.10935. [DOI] [PubMed] [Google Scholar]
- 16.Montano R, Chung I, Garner KM, Parry D, Eastman A. Preclinical development of the novel Chk1 inhibitor SCH900776 in combination with DNA-damaging agents and antimetabolites. Mol Cancer Ther. 2012;11:427–38. doi: 10.1158/1535-7163.MCT-11-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xia F, Taghian DG, DeFrank JS, Zeng ZC, Willers H, Iliakis G, et al. Deficiency of human BRCA2 leads to impaired homologous recombination but maintains normal nonhomologous end joining. Proc Natl Acad Sci U S A. 2001;98:8644–9. doi: 10.1073/pnas.151253498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fertil B, Dertinger H, Courdi A, Malaise EP. Mean inactivation dose: a useful concept for intercomparison of human cell survival curves. Radiat Res. 1984;99:73–84. [PubMed] [Google Scholar]
- 19.Parsels LA, Morgan MA, Tanska DM, Parsels JD, Palmer BD, Booth RJ, et al. Gemcitabine sensitization by checkpoint kinase 1 inhibition correlates with inhibition of a Rad51 DNA damage response in pancreatic cancer cells. Mol Cancer Ther. 2009;8:45–54. doi: 10.1158/1535-7163.MCT-08-0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morgan MA, Parsels LA, Kollar LE, Normolle DP, Maybaum J, Lawrence TS. The combination of epidermal growth factor receptor inhibitors with gemcitabine and radiation in pancreatic cancer. Clin Cancer Res. 2008;14:5142–9. doi: 10.1158/1078-0432.CCR-07-4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Geer MA, Kuhlmann KF, Bakker CT, ten Kate FJ, Oude Elferink RP, Bosma PJ. Ex-vivo evaluation of gene therapy vectors in human pancreatic (cancer) tissue slices. World J Gastroenterol. 2009;15:1359–66. doi: 10.3748/wjg.15.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krejci L, Altmannova V, Spirek M, Zhao X. Homologous recombination and its regulation. Nucleic Acids Res. 2012;40:5795–818. doi: 10.1093/nar/gks270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao L, Morgan MA, Parsels LA, Maybaum J, Lawrence TS, Normolle D. Bayesian hierarchical changepoint methods in modeling the tumor growth profiles in xenograft experiments. Clin Cancer Res. 2011;17:1057–64. doi: 10.1158/1078-0432.CCR-10-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leung-Pineda V, Ryan CE, Piwnica-Worms H. Phosphorylation of Chk1 by ATR is antagonized by a Chk1-regulated protein phosphatase 2A circuit. Mol Cell Biol. 2006;26:7529–38. doi: 10.1128/MCB.00447-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sorensen CS, Syljuasen RG. Safeguarding genome integrity: the checkpoint kinases ATR, CHK1 and WEE1 restrain CDK activity during normal DNA replication. Nucleic Acids Res. 2012;40:477–86. doi: 10.1093/nar/gkr697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morgan MA, El Shaikh M, Abu-Isa E, Davis MA, Lawrence TS. Radiosensitization by gemcitabine fixed-dose-rate infusion versus bolus injection in a pancreatic cancer model. Translational Oncology. 2008;1:44–9. doi: 10.1593/tlo.07118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang YW, Otterness DM, Chiang GG, Xie W, Liu YC, Mercurio F, et al. Genotoxic stress targets human Chk1 for degradation by the ubiquitin-proteasome pathway. Mol Cell. 2005;19:607–18. doi: 10.1016/j.molcel.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 28.Zabludoff SD, Deng C, Grondine MR, Sheehy AM, Ashwell S, Caleb BL, et al. AZD7762, a novel checkpoint kinase inhibitor, drives checkpoint abrogation and potentiates DNA-targeted therapies. Mol Cancer Ther. 2008;7:2955–66. doi: 10.1158/1535-7163.MCT-08-0492. [DOI] [PubMed] [Google Scholar]
- 29.Jobson AG, Lountos GT, Lorenzi PL, Llamas J, Connelly J, Cerna D, et al. Cellular inhibition of checkpoint kinase 2 (Chk2) and potentiation of camptothecins and radiation by the novel Chk2 inhibitor PV1019 [7-nitro-1H-indole-2-carboxylic acid {4-[1-(guanidinohydrazone)-ethyl]-phenyl}-amide] J Pharmacol Exp Ther. 2009;331:816–26. doi: 10.1124/jpet.109.154997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gilad O, Nabet BY, Ragland RL, Schoppy DW, Smith KD, Durham AC, et al. Combining ATR suppression with oncogenic Ras synergistically increases genomic instability, causing synthetic lethality or tumorigenesis in a dosage-dependent manner. Cancer Res. 2010;70:9693–702. doi: 10.1158/0008-5472.CAN-10-2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Syljuasen RG, Sorensen CS, Hansen LT, Fugger K, Lundin C, Johansson F, et al. Inhibition of human Chk1 causes increased initiation of DNA replication, phosphorylation of ATR targets, and DNA breakage. Mol Cell Biol. 2005;25:3553–62. doi: 10.1128/MCB.25.9.3553-3562.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson R, Montano R, Eastman A. The mre11 nuclease is critical for the sensitivity of cells to chk1 inhibition. PLoS One. 2012;7:e44021. doi: 10.1371/journal.pone.0044021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forment JV, Blasius M, Guerini I, Jackson SP. Structure-specific DNA endonuclease Mus81/Eme1 generates DNA damage caused by Chk1 inactivation. PLoS One. 2011;6:e23517. doi: 10.1371/journal.pone.0023517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rozenblum E, Schutte M, Goggins M, Hahn SA, Panzer S, Zahurak M, et al. Tumor-suppressive pathways in pancreatic carcinoma. Cancer Res. 1997;57:1731–4. [PubMed] [Google Scholar]
- 35.Clark DP. Ex vivo biomarkers: functional tools to guide targeted drug development and therapy. Expert Rev Mol Diagn. 2009;9:787–94. doi: 10.1586/erm.09.62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.