Abstract
Sulfur (S) deficiency is prevailing all over the world and becoming an important issue for crop improvement through maximising its utilization efficiency by plants for sustainable agriculture. Its interaction with other regulatory molecules in plants is necessary to improve our understanding on its role under changing environment. Our knowledge on the influence of S on ethylene signaling is meagre although it is a constituent of cysteine (Cys) required for the synthesis of reduced glutathione (GSH) and S-adenosyl methionine (SAM), a precursor of ethylene biosynthesis. Thus, there may be an interaction between S assimilation, ethylene signaling and plant responses under optimal and stressful environmental conditions. The present review emphasizes that responses of plants to S involve ethylene action. This evaluation will provide an insight into the details of interactive role of S and ethylene signaling in regulating plant processes and prove profitable for developing sustainability under changing environmental conditions.
Keywords: abiotic stress, cysteine, ethylene biosynthesis, ethylene signaling, glutathione, sulfur assimilation
Introduction
Plant nutrition is a fundamental science that impacts all aspects of cropping systems, environmental sustainability, and human health and well being.1 Sulfur (S) is a critical nutrient for metabolism, plant growth and development.2,3 The importance of S as a plant nutrient has been recognized since long time but active research started in the second half of the 20th century when widespread S deficiencies were observed.4 Different crops have different demands for S, and the adequate supply of S increases crop yields appreciably.2
Sulfur plays an inevitable and imperative role in the formation of amino acids, methionine (Met; 21%) and cysteine (Cys; 27%), synthesis of protein, chlorophyll and oil in the oilseed crops.5 The process of S acquisition and assimilation play an integral role with plant metabolism, and its deficiency leads to reduced chlorophyll content, pigment system II (PS II) efficiency and ribulose 1,5-bisphosphate carboxylase (Rubisco) content.6 Excess sulfate transported into leaf cells accumulates mainly in the vacuoles and constitutes a large internal S reserve.7 Conversely, in some circumstances, S may be present in abundance, and regulatory mechanisms will favor a limitation of uptake and assimilation. The rationale is to avoid excess uptake, which is energetically wasteful and/or to avoid potential osmotic imbalances.8
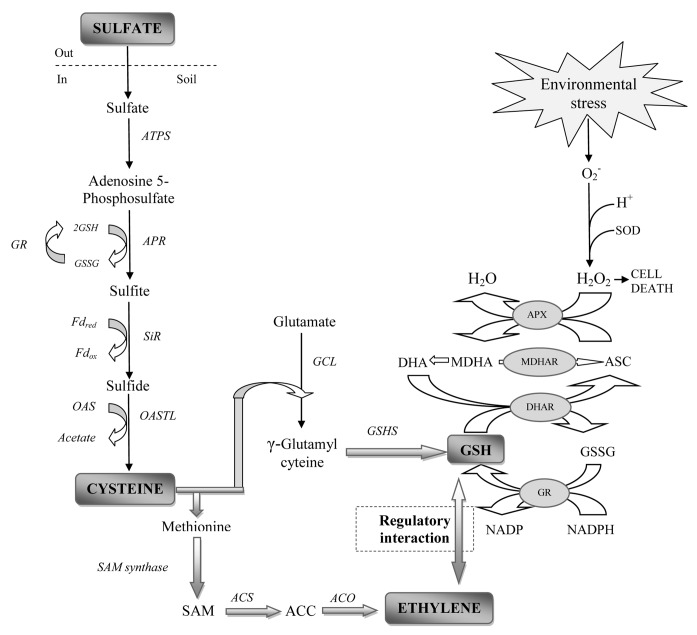
A good part of S incorporated into organic molecules in plants is located in thiol (-SH) groups in proteins (Cys-residues) or non-protein thiols, (reduced gluthione; GSH).9-12 These bonds are important for the stabilization of protein structure. The sensing of S nutrition state occurs through elaborate systems that modulate flux involving different component pathways.13 Cysteine is the donor of reduced S for the synthesis of Met and other S-containing metabolites.14 Methionine is the precursor for S-adenosyl methionine (SAM), the precursor for ethylene15 (Fig. 1), polyamines, and nicotinamine which is important for Fe nutrition in plants.16,17
Figure 1. Schematic representation of regulatory interaction between sulfur assimilation and ethylene biosynthesis linked with ascorbate-glutathione pathway. ATPS, ATP sulfurylase; APR, adenosine 5-phosphosulfate reductase; SiR, sulfite reductase; ; OAS, O-acetylserine; OASTL, O-acetylserine (thiol) lyase; GR, glutathione reductase; GSH, glutathione reduced; GSSG, glutathione oxidized; SAM, S-adenosyl methionine; ACC 1-aminocyclopropane carboxylic acid; ACS, ACC synthase; ACO, ACC oxidase; GCL, Glutamate cysteine ligase; GSHS, GSH synthetase; ASC, ascorbate; DHA, dehydroascorbate; DHAR; DHA reductase; MDHA, monodehydroascorbate; MDHAR, MDHA reductase; APX, ascorbate peroxidase ; SOD, Superoxide dismutase.
Examples of regulation of plant development by S and phytohormones are provided under optimal and abiotic stress.18-20 The present review discusses the interrelation between S assimilation, ethylene action and plant functions. This evaluation will provide an insight into the mechanisms in plants on S assimilation and ethylene signaling that can be successfully exploited in developing plant vigour under optimal and stressful environmental conditions.
Regulation of Sulfur Assimilation
Sulfate assimilation is highly regulated in a demand-driven manner.19,21,22 The pathway is induced when there is a high demand for growth and development.23 A surplus of reduced S compounds represses the pathway.14 It has been reported in barley that the uptake capacity reached a maximum after 4 d of S deprivation and even decreased after this; however, sulfate transporter mRNA abundance continued to increase.24 In a similar experiment with potato, the sulfate transporter mRNA abundance increased over an 8 d period; however, the measured increased uptake capacity showed only a transient rise.25 It was proposed that a repression mechanism operated in which some downstream reduced S compounds acted to repress uptake, probably acting on the transcription of the genes for the uptake transporters.26 When S supply becomes limiting, the levels of these compounds fall and the repression is relieved. Indirect evidence using inhibitors supported a rapid turnover of the sulfate transporter proteins and the importance of transcriptional regulation.26,27
Sulfate withdrawal from the growing medium decreases the levels of sulfate, Cys and GSH in plant tissues leading to the induction of sulfate transporter systems and key enzymes along the assimilatory pathway.21,28 The increase in steady-state levels of mRNAs for high-affinity sulfate transporters, ATP-sulfurylase (ATPS), and adenosine 5-phosphosulfate reductase (APR) upon S starvation has been detected by Northern analysis29,30 or cDNA arrays.31,32 It reveals that the de-repression is regulated at the level of transcription. This de-repression correlates with the time of exposure to S-deficiency; and the activity of APR and ATPS quickly returns to the normal levels when plants are supplied with sulfate again.21,33 O-acetylserine accumulates during S starvation and may thus serve as a signal of the S status.34 O-acetylserine acts most probably as a transcriptional regulator since its addition strongly increases mRNA levels of all the three APR isoforms and also those of sulfite reductase (SiR), chloroplastic O-acetyl serine (thiol) lyase (OAS-TL), and cytosolic serine acetyl transferase (SAT).35 O-acetylserine plays a regulatory role in the synthesis of Cys by controlling the oligomerization of the Cys synthase complex, thus coordinating between serine as the nitrogen (N) source and sulfide as the sulfate assimilation intermediate.36
An Overview of Ethylene Biosynthesis
Ethylene (C2H4) is a simple gaseous plant hormone that has profound effects upon plant growth and development. It regulates diverse aspects of plant growth and development, including germination; leaf, stem, and root growth; sex determination; fruit ripening; organ abscission; leaf and flower senescence; and cell death of the cereal endosperm.37,38 Ethylene before being recognized as a plant hormone played a role in the history of agriculture. The historical techniques to promote fruit ripening are ancient Egyptians cutting sycamore figs and the Chinese burning incense to ripen pears,39,40 all of these practices released ethylene gas, which promoted fruit ripening. It was in 1795 that ethylene was combined with chlorine gas to produce oil of the Dutch chemists. For its part in the process, ethylene was known as olefiant gas – or oil-making gas,41 and it became a compound of commercial interest. Later, with the introduction of a standard nomenclature system, olefiant gas was named ethylene. The chronology of events has been conveyed in great detail in the works of Abeles et al.38 and Chaves and de Mello-Farias.40
The biosynthesis of ethylene occurs through a relatively simple metabolic pathway that has been extensively studied and well documented in plants.42-44 Ethylene is derived from the amino acid Met. Methionine is converted to SAM by the enzyme SAM synthetase. S-adenosyl methionine is then converted by 1-aminocyclopropane carboxylic acid (ACC) synthase (ACS) to ACC and 5´-deoxy-5´-methylthioadenosine (MTA). Conversion of SAM into ACC is the rate-limiting step in the pathway. ACC oxidase (ACO) catalyzes the conversion of ACC to ethylene. Thus, these two (ACS and ACO) are the important enzymes that are involved in the formation and oxidation of the immediate precursor of ethylene i.e., ACC (Fig. 1). The final conversion of ACC to ethylene is oxygen dependent43 and yields CO2 and cyanide as by-products. ACC oxidase may play an important role in regulating ethylene biosynthesis, especially during conditions of high ethylene production.
Besides formation of ACC and subsequently its conversion to ethylene, ACS is also involved in catalyzing the conversion of SAM into MTA. These enzymatic reactions appear to be the rate-limiting step in the formation of ethylene but there are situations where ACO is absent and ACS and ACO are induced, for example by wounding and the ripening stimulus.45 The conversion of ACC to ethylene catalyzed by ACO is oxygen dependent, and, under anaerobic conditions, ethylene formation is completely suppressed. In this reaction, Fe2+ and ascorbic acid (AsA) are required as a cofactor and a co-substrate, respectively. The other reaction product, MTA, must be recycled back into the Met pathway to provide an adequate supply of Met as substrate for the continual production of ethylene. The poisonous gas hydrogen cyanide formed from the decomposition of ACC to ethylene is detoxi□ed by β-cyanoalanine synthase. Different expression of ACS and ACO isozymes encoded by multigene families in response to external and internal stimuli is controlled at the transcriptional and post transcriptional level.46,47
In plants, ethylene biosynthesis is controlled by two systems: the ethylene autoinhibitory system 1, which generally operates during normal vegetative growth of plant; and system 2, regulated by a positive feedback mechanism, usually responsible for the rapid increase in ethylene production in senescing ethylene-sensitive plant organs, and in ripening climacteric fruits.48 In Arabidopsis, the N-terminal fragments of five ethylene receptors (subfamily 1, ETR1 and ERS1; subfamily 2, ETR2, ERS2, and EIN4) are involved in copper-mediated ethylene binding.
Plants under biotic or abiotic stress, such as pathogen attack, salt, wounding, drought, heat, flooding, low phosphorus,49 and low iron50 produce higher levels of ethylene than non-stressed plants. It is now becoming clear that various ethylene-regulated stress responses are essential for stress tolerance and the survival of plants. For example, the submergence tolerance in rice (Oryza sativa) is determined by the levels of ethylene and the activity of SUBMERGENCE1A (SUB1A), which is induced by ethylene.51-53 In addition, ethylene-insensitive mutants (etr1–1, ein2–1, and ein4–1) are more sensitive to high salt concentrations than the wild type, whereas a constitutive ethylene response mutant (ctr1–1) is more tolerant, supporting the important role of ethylene in salt stress signaling.54,55 Ethylene production increases when plants are deprived of potassium (K),56 and ethylene-insensitive mutants are more sensitive to low K+ in terms of leaf chlorosis and shoot growth than is the wild type. These findings show ethylene as an important component in the plant response to low K+ stress.
Plants exposed to environmental stresses speed up their rate of ethylene production. Various types of stress induce enhanced production of ethylene from plant tissues.38 However, the mechanism of ethylene biosynthesis is same under stress as under optimal environmental conditions. Stress induces the synthesis of stress ethylene because of the burst of ethylene that occurs under stress. When plants are exposed to conditions that threaten their ability to survive, the same mechanism that produces ethylene for normal development instead functions to produce what is known as stress ethylene.57 The functions of normal and stress ethylene differ considerably although both are produced by the same pathway.
Responses to Sulfur Involve Phytohormones Action
Sulfur availability affects phytohormone action and S assimilation may interact with phytohormones. Koprivova and Rennenberg23 reported that phytohormones play important role in reduced GSH synthesis. cDNA arrays revealed the induction of genes involved in auxin synthesis upon S-starvation, pointing to a possible role of phytohormones under S deficiency.23, Ohkama et al.34 used transgenic Arabidopsis plants, expressing βSR-driven green fluorescent protein (GFP) under the control of a chimeric promoter containing the sulfur-responsive element of β-conglycinin38 to test the influence of phytohormones on the sulfur-deficiency response. Whereas abscisic acid (ABA), indole-3-acetic acid (IAA), ACC (precursor of ethylene), gibberellic acid (GA), and jasmonic acid (JA) were not able to induce the expression of GFP derived from the sulfur-responsive element and, thus, mimic the sulfur-starvation response, trans-zeatin caused an increase in GFP synthesis both in sulfur-sufficient and sulfur-deficient conditions. In addition, zeatin treatment resulted in an increased accumulation of mRNA for APR and a low-affinity sulfate transporter.34
Thus, it is not surprising to see increasing evidence of coordination between nutritional and hormonal signaling.59 Schachtman and Shin60 reported that the plant hormones cytokinin, IAA, and JA were signaling components in response to S deficiency. The expression of APR1 is upregulated by S deficiency61 and also by exogenous cytokinin.34 Exogenous cytokinin downregulates the expression of the high-af□nity transporter SULTR;262, which is upregulated by S deprivation. Cytokinin acts through the cytokinin response receptor (CRE1) to regulate sulfate uptake and transporter expression. In the cre1–1 mutant, application of cytokinin only partly reduces sulfate uptake, suggesting redundancy as noted for the case of phosphate deprivation.63
Auxin is also a signaling component under sulfate limitation.32 The expression of auxin-inducible genes (IAA18, At1g51950, tryptophan synthase β chain, At5g38530, putative auxin-regulated protein, At2g33830) is upregulated by S starvation.32,64 The expression of NIT3 nitrilase, which can convert indole-3-acetonitrile to IAA, is strongly increased by S starvation.65 The increased auxin production may result in an increase in lateral root density in Arabidopsis under sulfate-limited conditions.66
Jasmonic acid is also a possible signaling component in leaves. Genes involved in JA biosynthesis are upregulated under S deficiency.67,68 These genes include 12-oxophytodienoate reductase 1 and lipoxygenase.31,32,67 Jasmonic acid may regulate the expression of genes involved in sulfate assimilation and GSH synthesis.68,69 Furthermore, methyl jasmonate is involved in regulating the activity of S assimilation enzymes such as SAT and APR.69 Although JA is a regulator of S metabolism, its levels in plants are not well characterized under deficient conditions. Recently, an ethylene insensitive like (EIL) transcription factor, SLIM1, was isolated and shown to be involved in the regulation of a high-affinity sulfate transporter in response to sulfate limitation.70
The other level of interaction of nutrient and hormone may be visualized at the level of reactive oxygen species (ROS) production. ROS have important consequences that lead to aerenchyma formation in nutrient-deprived roots,71 which may be an important adaptation that lowers the cost of maintaining roots.72 Direct evidence showing that ROS is a signaling component in S-starved plants is not yet available. However, in Bacillus subtilis several genes involved in S-assimilation and synthesis of S-containing amino acids were induced by adding paraquat.73 The amount of ROS visualized after 30 h of S deprivation in roots was greater than in sulfate-sufficient roots.60 The regulation and interaction between ROS and ascorbate-glutathione (AsA-GSH) cycle impacts the synthesis of plant hormones such as SA, GA, ABA, and ethylene,74,75 which may signal plant response to nutrients deficiency. The involvement of ROS in S signaling may be more complex than that of K deprivation because the AsA-GSH cycle, i.e., downstream of sulfate assimilation is involved in the removal of H2O2.
Ethylene Cross-Talk Associated with Sulfur
The interaction between ethylene and S has been shown to control the regulation of plants processes and abiotic stress tolerance. The first organic compound synthesized in the sulfate assimilatory pathway is Cys. Cysteine is the final product of S-assimilation. It is the precursor and S donor for the majority of other organic S compounds present in plants14,76 such as Met, SAM, S-methyl methionine, [Fe/S] clusters, hormones, vitamins and enzyme cofactors.76 The main pathway for ethylene biosynthesis comes from Met. Methionine is a fundamental metabolite in plant cells because it controls the level of several key metabolites, such as ethylene, polyamines and biotin through its first metabolite, SAM. It is first converted to SAM, then ACC, and finally ethylene in three consecutive reactions catalyzed by the enzymes of SAM synthetase, ACS and ACO, respectively77 (Fig. 1).
Further, Bürstenbinder et al.15 using an mtk mutant, that has a disruption of the Yang cycle, reported that the Yang cycle contributes to SAM homeostasis, especially when de novo SAM synthesis is limited, such as at S starvation.78 They also showed that this cycle was required to sustain a high level of ethylene synthesis. However, additional evidence suggests that in addition to recycling the Met moieties via the Yang cycle, the de novo synthesis of Met is required when high rates of ethylene production are induced.79
Moreover, S availability and ethylene have been shown to regulate GSH synthesis and stress tolerance to ozone80 and Cd81,82 stress. Glutathione acts as a storage and transport form for Cys; otherwise the excess Cys present in the cell becomes toxic.83-86 Glutathione acts as a signal, controlling the inter-organ regulation of S nutrition and is mainly confined to the leaves.87,88 During S deficiency GSH content rapidly decreased in tobacco cell cultures but was rebuilt upon the re-supply of sulfate and/or Cys.89 Glutathione is reduced in plants subjected to S deficiency.32 The reduced form of glutathione, GSH, is an abundant compound in plant tissue that exists interchangeably with the oxidized form, GSSG. GSH is abundant (3–10 mM) in cytoplasm, nuclei and mitochondria and is the major soluble antioxidant in these cell compartments. Glutathione has been associated with several growth and development related events in plants, including cell differentiation, cell death and senescence, pathogen resistance and enzymatic regulation86 and its content is affected by S nutrition.90,91 Glutathione is the major reservoir of non-protein S. It is the major redox buffer in most aerobic cells, and plays an important role in physiological functions, including redox regulation, conjugation of metabolites, detoxification of xenobiotics and homeostasis and cellular signaling that triggers adaptive responses.86 It also plays an indirect role in protecting membranes by maintaining α-tocopherol and zeaxanthin in the reduced state. It can also function directly as a free radical scavenger by reacting with superoxide, singlet oxygen and hydroxyl radicals. Glutathione protects proteins against denaturation caused by the oxidation of protein thiol groups under stress. The central role of GSH in the antioxidative defense system is due to its ability to regenerate another water soluble antioxidant, AsA, in ascorbate-glutathione (AsA-GSH) cycle.86 The increased demand for GSH can be met by the activation of pathways involved in S assimilation and Cys biosynthesis. Its concentration is controlled by a complex homeostatic mechanism where the availability of S seems to be required.92 Manipulation of GSH biosynthesis increases resistance to oxidative stress.19,20
Glutathione reductase (GR) is a flavo-protein oxidoreductase, found in both prokaryotes and eukaryotes86 and maintains the balance between GSH and AsA pools, which in turn maintain cellular redox state. The enzyme protein, although synthesized in the cytoplasm, can be targeted to both chloroplast and mitochondria.93 In higher plants, GR is involved in defense against oxidative stress, whereas, GSH plays an important role within the cell system, which includes participation in the AsA-GSH cycle, maintenance of the -SH group and a substrate for glutathione-S-transferases.94 Glutathione reductase and GSH play a crucial role in determining the tolerance of a plant during environmental stresses.95 In almost all the biological functions, GSH is oxidized to oxidised glutathione (GSSG) which should be converted back to GSH in plant cell to perform normal physiological functions. Supplementary S fertilization to high S loving crops such as brassicas and leguminous crops has been shown to enhance plant-stress-defense operations and act indirectly by improving general plant performance under abiotic and biotic stresses as well through improving GSH and AsA.12,96
There are reports that demonstrate a functional link between ethylene and S. Ethylene plays important roles in selenite resistance in Arabidopsis.97 A comprehensive gene expression analysis showed that transcripts regulating ethylene synthesis (ACS6) and signaling (ERF) were upregulated by selenate treatment, and plants overexpressing ERF1 exhibited an increase in selenium (Se) resistance.98 These results indicate that Se resistance achieved through ethylene signaling is not mediated by S starvation resulting from the Se treatment but is a Se-specific response. The resistance mechanism may involve ethylene-enhanced S uptake and assimilation, as observed in Arabidopsis thaliana accessions, Columbia (Col)-0. The higher levels of organic S compounds observed in Col-0 may enable it to more efficiently prevent Se analogs from replacing S in proteins and other S compounds,97 but no direct study is available regarding ethylene and S. However, the role of ethylene in various nutrients deficiency is available.49,99-107 Reports on the interaction of S and ethylene are still in its infancy. Koprivova et al.108reported that the application of 0.2 mM ACC, which stimulates ethylene production increased accumulation of APR activity. Recently, it has been shown that ethylene action in mustard is dependent on S availability.81,82 Disruption of JA and ethylene signaling pathway prevented GSH accumulation under salt stress. There is an indication that APR activity is increased in salt stress if ethylene signaling is disturbed, but GSH will not accumulate suggesting that components of GSH biosynthesis are under the control of ethylene108 (Fig. 1). Selenate induces S starvation and activates genes controlling S assimilation.109 A Se-induced S starvation response is further suggested by the 30-fold upregulation of a thioglucosidase (At3g60140), thought to break down glucosinolates; these compounds are S-containing secondary metabolites and their catabolism may recycle a limited pool of S.31,110 Microarray analysis also suggests that Se treatment induces the synthesis of ethylene.98 Thus Se reduces S assimilation and induces ethylene synthesis.
In plants exposed to various types of abiotic and biotic stresses, increased ethylene levels correspond to increased damage, implying that stress ethylene is deleterious to plants. However, it was also reported that transcriptional activation of ERF in ethylene signaling process enhances stress tolerance in tobacco seedlings by decreasing ROS accumulation in response to salt, drought and freezing.111 It may be that these discrepancies are due to differences in the amount of endogenous ethylene production, and in the period of stress treatment, in addition to the plant tissue studied.112 Studies have shown that MPK3/6 can be activated by many stress signals in minutes, which are also able to trigger ethylene production in hours.113-115 Yoshida et al.80 studied the defensive roles of ethylene and SA against ozone. Macroarray analysis suggested that the ethylene and SA defects influenced GSH metabolism and they protected against ozone-induced leaf injury by increasing de novo biosynthesis of glutathione. Elevated oxidative stresses caused by environmental stimuli, including ozone, UV-B, and wounding have been demonstrated to enhance ethylene production via ACS and ACO.116 In ozone treatment, ethylene also enhanced ROS generation, which in turn leads to cell death.116 In sweet potato, a wound-inducible ipomoelin (IPO) gene expression can be induced by ethylene,117 but was completely repressed by diphenylene iodonium, an inhibitor of NADPH oxidase.118 These studies suggest that elevated oxidative stress may play important role in ethylene biosynthesis, ethylene signaling, and ethylene-mediated effects. Several studies suggest that shoot elongation in submerged plants may be controlled by phytohormones, and in particular by ethylene production, as in flood-tolerant Rumex species.119,120 Zhang and Huang121 reported that treatment of ethylene biosynthesis inhibitor or ethylene receptor antagonist, i.e., blockage of ethylene biosynthesis or the ethylene signaling pathway decreases freezing tolerance of overexpressing TERF2/LeERF2 tobaccos. In addition, gene profiling studies also showed that ethylene might be involved in cold response.122 Zhang et al.123 reported that ERF proteins regulate a variety of stress responses in plant. JERF1, a tomato ERF protein, can be induced by ABA. Overexpression of JERF1 enhanced the tolerance of transgenic tobacco to high salt concentration, osmotic stress, and low temperature by regulating the expression of stress-responsive genes by binding to DRE/CRT and GCC-box cis-elements. The internodes elongation response, which serves as an important adaptation response in deepwater rice, is also controlled by ethylene.124 Ethylene responsive factors (ERFs) play an important role in plant responses to stresses. SlERF1 played a positive role in the salt tolerance of tomato plants.125 Considering the cross-talk between S and ethylene signaling, the performance of plants could be achieved under optimal and changing environment.
Conclusion
Sulfur is an important macronutrient crucial for plant growth and vigour, and crop yield, and resistance to stressful environments. Plants assimilate sulfate and reduce to cysteine and methionine, which constitute about 80–90% of total sulfur in most plants. Cysteine is an important link for the formation of non-protein thiol, GSH, a major plant constituent of storage S, and ethylene through the formation of SAM. Under optimal environmental conditions, S and ethylene regulate physiological processes interdependently, while conditions of stressful environments lead to enhanced S assimilation capacity and GSH synthesis. Under these conditions, ethylene signaling also regulates GSH synthesis for better adaptation of plants. Thus, an interaction exists between S and ethylene to control plant development under optimal and stressful environments. Although our scientific understanding of the molecular mechanisms of ethylene biosynthesis, perception and signaling has been improved considerably, but there are still major challenges concerning interaction or cross-talk between ethylene and S in crop plants under various environmental conditions.
Ethylene is a pivotal signaling molecule, and while its connection to all plant processes is yet not completely understood, a picture is emerging of a simple molecule whose concentration can be manipulated to create many desirable traits in plants. Study of the ethylene biosynthesis pathway in plants made it possible to modify and insert genes that alter the level of ethylene produced in response to various stimuli. Enzymes that degrade SAM or ACC, the precursors of ethylene, have been shown to effectively reduce ethylene levels without drastically altering the physiology of the plant. Expression of sense or antisense versions of enzymes from the ethylene biosynthesis pathway should also allow for genetic control of ethylene levels. It is also crucial to address how ethylene signaling and changes in gene expression are integrated into specific agronomic traits. A detailed understanding of molecular and cellular details in crop plants on ethylene signaling and S assimilation under different environmental conditions will provide innovative tools for sustainable plant development.
Acknowledgments
Financial assistance for the research by the Council of Scientific and Industrial Research, University Grants Commission and Department of Science and Technology, New Delhi is gratefully acknowledged.
Glossary
Abbreviations:
- S
sulfur
- Cys
cysteine
- GSH
reduced glutathione
- SAM
S-adenosyl methionine
- Met
methionine
- Rubisco
ribulose 1,5-bisphosphate carboxylase
- ATPS
ATP-sulfurylase
- APR
adenosine 5-phosphosulfate reductase
- SiR
sulfite reductase
- OAS-TL
O-acetyl serine (thiol) lyas
- ACS
1-aminocyclopropane carboxylic acid synthase
- ACO
ACC oxidase
- ABA
abscisic acid
- IAA
indole-3-acetic acid
- GA
gibberellic acid
- JA
jasmonic acid
- SAT
serine acetyl transferase
- GR
glutathione reductase
- GSSG
oxidized glutathione
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/22478
References
- 1.Six J. Plant nutrition for sustainable development and global health. Soil Sci. 2011;339:1–2. [Google Scholar]
- 2.Marschner H. Mineral nutrition of higher plants. New York: Academic Press, 1995:229-99. [Google Scholar]
- 3.Dubousset L, Etienne P, Avice JC. Is the remobilization of S and N reserves for seed filling of winter oilseed rape modulated by sulphate restrictions occurring at different growth stages? J Exp Bot. 2010;61:4313–24. doi: 10.1093/jxb/erq233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duke SH, Reisenauer HM. Role and requirements of sulphur in plant nutrition. In: Tabatabai MA, ed. Sulphur in agriculture, agronomy monograph. 27. Madison WI USA:American society of agronomy, crop science society of America, soil science society of America, 1986:123-68. [Google Scholar]
- 5.Aulakh MS, Pasricha NS, Sahota NS. Yield, nutrient concentration and quality of mustard crop as influenced by nitrogen and sulphur fertilization. J Agric Sci. 1980;84:545–9. doi: 10.1017/S0021859600028549. [DOI] [Google Scholar]
- 6.Lunde C, Zygadlo A, Simonsen HT, Nielsen PL, Blennow A, Haldrup A. Sulfur starvation in rice: the effect on photosynthesis, carbohydrate metabolism, and oxidative stress protective pathways. Physiol Plant. 2008;134:508–21. doi: 10.1111/j.1399-3054.2008.01159.x. [DOI] [PubMed] [Google Scholar]
- 7.Bell CI, Cram WJ, Clarkson DT. Compartmental analysis of 35SO42- exchange kinetics in roots and leaves of a tropical legume Macroptilium atropurpureum cv. Siratro. J Exp Bot. 1994;45:879–86. doi: 10.1093/jxb/45.7.879. [DOI] [Google Scholar]
- 8.Hawkesford MJ, De Kok LJ. Managing sulphur metabolism in plants. Plant Cell Environ. 2006;29:382–95. doi: 10.1111/j.1365-3040.2005.01470.x. [DOI] [PubMed] [Google Scholar]
- 9.Noji M, Saito K. Sulfur amino acids: biosynthesis of cysteine and methionine. In: Abrol YP, Ahmad A, eds. Sulphur in plants. The Netherlands:Kluwer Academic Publishers, 2003:135-44. [Google Scholar]
- 10.Tausz M, Gullner G, Komives T, Grill D. The role of thiols in plant adaptation to environmental stress. In: Abrol YP, Ahmad A, eds. Sulphur in plants. The Netherlands:Kluwer Academic Publishers, 2003:221-44. [Google Scholar]
- 11.De Kok LJ, Castro A, Durenkamp M, Kralewska A, Posthumus FS, Elisabeth C, et al. Pathways of plant sulphur uptake and metabolism-an overview. Landbauforschung Volkenrode. 2005;283:5–13. [Google Scholar]
- 12.Anjum NA, Umar S, Ahmad A, Iqbal M, Khan NA. Sulphur protects mustard (Brassica campestris L.) from cadmium toxicity by improving leaf ascorbate and glutathione. Plant Growth Regul. 2008;54:271–9. doi: 10.1007/s10725-007-9251-6. [DOI] [Google Scholar]
- 13.Yi H, Galant A, Ravilious GE, Preuss ML, Jez JM. Sensing sulfur conditions: simple to complex protein regulatory mechanisms in plant thiol metabolism. Mol Plant. 2010;3:269–79. doi: 10.1093/mp/ssp112. [DOI] [PubMed] [Google Scholar]
- 14.Kopriva S. Regulation of sulphate assimilation in Arabidopsis and beyond. Ann Bot (Lond) 2006;97:479–95. doi: 10.1093/aob/mcl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bürstenbinder K, Rzewuski G, Wirtz M, Hell R, Sauter M. The role of methionine recycling for ethylene synthesis in Arabidopsis. Plant J. 2007;49:238–49. doi: 10.1111/j.1365-313X.2006.02942.x. [DOI] [PubMed] [Google Scholar]
- 16.Ling HQ, Koch G, Bäumlein H, Ganal MW. Map-based cloning of chloronerva, a gene involved in iron uptake of higher plants encoding nicotianamine synthase. Proc Natl Acad Sci USA. 1999;96:7098–103. doi: 10.1073/pnas.96.12.7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zuchi S, Cesco S, Varanini Z, Pinton R, Astolfi S. Sulphur deprivation limits Fe-deficiency responses in tomato plants. Planta. 2009;230:85–94. doi: 10.1007/s00425-009-0919-1. [DOI] [PubMed] [Google Scholar]
- 18.Khan NA, Mir MR, Nazar R, Singh S. The application of ethephon (an ethylene releaser) increases growth, photosynthesis and nitrogen accumulation in mustard (Brassica juncea L.) under high nitrogen levels. Plant Biol (Stuttg) 2008;10:534–8. doi: 10.1111/j.1438-8677.2008.00054.x. [DOI] [PubMed] [Google Scholar]
- 19.Nazar R, Iqbal N, Masood A, Syeed S, Khan NA. Understanding the significance of sulfur in improving salinity tolerance in plants. Environ Exp Bot. 2011;70:80–7. doi: 10.1016/j.envexpbot.2010.09.011. a. [DOI] [Google Scholar]
- 20.Nazar R, Iqbal N, Syeed S, Khan NA. Salicylic acid alleviates decreases in photosynthesis under salt stress by enhancing nitrogen and sulfur assimilation and antioxidant metabolism differentially in two mungbean cultivars. J Plant Physiol. 2011;168:807–15. doi: 10.1016/j.jplph.2010.11.001. b. [DOI] [PubMed] [Google Scholar]
- 21.Lappartient AG, Touraine B. Demand-driven control of root ATP-sulfurylase activity and SO42- uptake in intact canola. Plant Physiol. 1996;111:147–57. doi: 10.1104/pp.111.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davidian JC, Kopriva S. Regulation of sulfate uptake and assimilation--the same or not the same? Mol Plant. 2010;3:314–25. doi: 10.1093/mp/ssq001. [DOI] [PubMed] [Google Scholar]
- 23.Kopriva S, Rennenberg H. Control of sulphate assimilation and glutathione synthesis: interaction with N and C metabolism. J Exp Bot. 2004;55:1831–42. doi: 10.1093/jxb/erh203. [DOI] [PubMed] [Google Scholar]
- 24.Smith FW, Hawkesford MJ, Ealing PM, Clarkson DT, Vanden Berg PJ, Belcher AR, et al. Regulation of expression of a cDNA from barley roots encoding a high affinity sulphate transporter. Plant J. 1997;12:875–84. doi: 10.1046/j.1365-313X.1997.12040875.x. [DOI] [PubMed] [Google Scholar]
- 25.Hopkins L, Parmar S, Błaszczyk A, Hesse H, Hoefgen R, Hawkesford MJ. O-acetylserine and the regulation of expression of genes encoding components for sulfate uptake and assimilation in potato. Plant Physiol. 2005;138:433–40. doi: 10.1104/pp.104.057521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rennenberg H, Kemper O, Thoene B. Recovery of sulfate transport into heterotrophic tobacco cells from inhibition by reduced glutathione. Physiol Plant. 1989;76:271–6. [Google Scholar]
- 27.Clarkson DT, Hawkesford MJ, Davidian J-C, Grignon C. Contrasting responses of sulfate and phosphate-transport in barley (Hordeum vulgare L.) roots to protein-modifying reagents and inhibition of protein synthesis. Planta. 1992;187:306–14. doi: 10.1007/BF00195653. [DOI] [PubMed] [Google Scholar]
- 28.Lappartient AG, Vidmar JJ, Leustek T, Glass ADM, Touraine B. Interorgan signalling in plant: regulation of ATP sulfurylase and sulfate transporter genes expression in roots mediated by phloem-translocated compounds. Planta. 1999;18:89–95. doi: 10.1046/j.1365-313X.1999.00416.x. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi H, Yamazaki M, Sasakura N, Watanabe A, Leustek T, Engler JA, et al. Regulation of sulfur assimilation in higher plants: a sulfate transporter induced in sulfate-starved roots plays a central role in Arabidopsis thaliana. Proc Natl Acad Sci USA. 1997;94:11102–7. doi: 10.1073/pnas.94.20.11102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamaguchi Y, Nakamura T, Harada E, Koizumi N, Sano H. Differential accumulation of transcripts encoding sulfur assimilation enzymes upon sulfur and/or nitrogen deprivation in Arabidopsis thaliana. Biosci Biotechnol Biochem. 1999;63:762–6. doi: 10.1271/bbb.63.762. [DOI] [PubMed] [Google Scholar]
- 31.Maruyama-Nakashita A, Inoue E, Watanabe-Takahashi A, Yamaya T, Takahashi H. Transcriptome profiling of sulfur-responsive genes in Arabidopsis reveals global effects of sulfur nutrition on multiple metabolic pathways. Plant Physiol. 2003;132:597–605. doi: 10.1104/pp.102.019802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nikiforova VJ, Freitag J, Kempa S, Adamik M, Hesse H, Hoefgen R. Transcriptome analysis of sulfur depletion in Arabidopsis thaliana: interlacing of biosynthetic pathways provides response specificity. Plant J. 2003;33:633–50. doi: 10.1046/j.1365-313X.2003.01657.x. [DOI] [PubMed] [Google Scholar]
- 33.Reuveny Z, Filner P. Regulation of adenosine triphosphate sulfurylase in cultured tobacco cells. Effects of sulfur and nitrogen sources on the formation and decay of the enzyme. J Biol Chem. 1977;252:1858–64. [PubMed] [Google Scholar]
- 34.Ohkama N, Takei K, Sakakibara H, Hayashi H, Yoneyama T, Fujiwara T. Regulation of sulfur-responsive gene expression by exogenously applied cytokinins in Arabidopsis thaliana. Plant Cell Physiol. 2002;43:1493–501. doi: 10.1093/pcp/pcf183. [DOI] [PubMed] [Google Scholar]
- 35.Koprivova A, Suter M, den Camp RO, Brunold C, Kopriva S. Regulation of sulfate assimilation by nitrogen in Arabidopsis. Plant Physiol. 2000;122:737–46. doi: 10.1104/pp.122.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kopriva S, Mugford SG, Matthewman CA, Koprivova A. Plant sulfate assimilation genes: redundancy versus specialization. Plant Cell Rep. 2009;28:1769–80. doi: 10.1007/s00299-009-0793-0. [DOI] [PubMed] [Google Scholar]
- 37.Mattoo AK, Suttle CS. The plant hormone ethylene. FL Boca Raton:CRC Press, 1991. [Google Scholar]
- 38.Abeles FB, Morgan PW, Saltveit ME Jr. Ethylene in plant biology, 2nd edn. New York:Academic, 1992. [Google Scholar]
- 39.Wright TJ. Amos and the “sycomore fig”. Vetus Testamentum. 1976;26:362–8. doi: 10.1163/156853376X00529. [DOI] [Google Scholar]
- 40.Chaves ALS, de Mello-Farias PC. Ethylene and fruit ripening: from illumination gas to the control of gene expression, more than a century of discoveries. Genet Mol Biol. 2006;29:508–15. doi: 10.1590/S1415-47572006000300020. [DOI] [Google Scholar]
- 41.Snelders HAM. Het gezelschap der Hollandsche scheikundigen: Amsterdamse chemici uit het einde van de achttiende eeus. Amsterdam (In Dutch):Rodopi Publishers, 1980. [Google Scholar]
- 42.Yang SF, Hoffman NE. Ethylene biosynthesis and its regulation in higher plants. Annu Rev Plant Physiol. 1984;35:155–89. doi: 10.1146/annurev.pp.35.060184.001103. [DOI] [Google Scholar]
- 43.Kende H. Ethylene biosynthesis. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:283–307. doi: 10.1146/annurev.pp.44.060193.001435. [DOI] [Google Scholar]
- 44.Zarembinski TI, Theologis A. Ethylene biosynthesis and action: a case of conservation. Plant Mol Biol. 1994;26:1579–97. doi: 10.1007/BF00016491. [DOI] [PubMed] [Google Scholar]
- 45.Alexander L, Grierson D. Ethylene biosynthesis and action in tomato: a model for climacteric fruit ripening. J Exp Bot. 2002;53:2039–55. doi: 10.1093/jxb/erf072. [DOI] [PubMed] [Google Scholar]
- 46.Lin Z, Zhong S, Grierson D. Recent advances in ethylene research. J Exp Bot. 2009;60:3311–36. doi: 10.1093/jxb/erp204. [DOI] [PubMed] [Google Scholar]
- 47.Tsuchisaka A, Yu G, Jin H, Alonso JM, Ecker JR, Zhang X, et al. A combinatorial interplay among the 1-aminocyclopropane-1-carboxylate isoforms regulates ethylene biosynthesis in Arabidopsis thaliana. Genetics. 2009;183:979–1003. doi: 10.1534/genetics.109.107102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim JH, Kim WT, Kang BG. IAA and N(6)-benzyladenine inhibit ethylene-regulated expression of ACC oxidase and ACC synthase genes in mungbean hypocotyls. Plant Cell Physiol. 2001;42:1056–61. doi: 10.1093/pcp/pce133. [DOI] [PubMed] [Google Scholar]
- 49.Borch K, Bouma TJ, Lynch JP, Brown KM. Ethylene: a regulator of root architectural responses to soil phosphorus availability. Plant Cell Environ. 1999;22:425–31. doi: 10.1046/j.1365-3040.1999.00405.x. [DOI] [Google Scholar]
- 50.Romera FJ, Alcántara E, de la Guardia MD. Ethylene production by Fe-deficient roots and its involvement in the regulation of Fe-deficiency stress responses by Strategy I plants. Ann Bot (Lond) 1999;83:51–5. doi: 10.1006/anbo.1998.0793. [DOI] [Google Scholar]
- 51.Xu K, Xu X, Fukao T, Canlas P, Maghirang-Rodriguez R, Heuer S, et al. Sub1A is an ethylene-response-factor-like gene that confers submergence tolerance to rice. Nature. 2006;442:705–8. doi: 10.1038/nature04920. [DOI] [PubMed] [Google Scholar]
- 52.Perata P, Voesenek LA. Submergence tolerance in rice requires Sub1A, an ethylene-response-factor-like gene. Trends Plant Sci. 2007;12:43–6. doi: 10.1016/j.tplants.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 53.Fukao T, Bailey-Serres J. Submergence tolerance conferred by Sub1A is mediated by SLR1 and SLRL1 restriction of gibberellin responses in rice. Proc Natl Acad Sci USA. 2008;105:16814–9. doi: 10.1073/pnas.0807821105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Achard P, Cheng H, De Grauwe L, Decat J, Schoutteten H, Moritz T, et al. Integration of plant responses to environmentally activated phytohormonal signals. Science. 2006;311:91–4. doi: 10.1126/science.1118642. [DOI] [PubMed] [Google Scholar]
- 55.Cao WH, Liu J, He XJ, Mu RL, Zhou HL, Chen SY, et al. Modulation of ethylene responses affects plant salt-stress responses. Plant Physiol. 2007;143:707–19. doi: 10.1104/pp.106.094292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shin R, Schachtman DP. Hydrogen peroxide mediates plant root cell response to nutrient deprivation. Proc Natl Acad Sci USA. 2004;101:8827–32. doi: 10.1073/pnas.0401707101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stearns JC, Glick BR. Transgenic plants with altered ethylene biosynthesis or perception. Biotechnol Adv. 2003;21:193–210. doi: 10.1016/S0734-9750(03)00024-7. [DOI] [PubMed] [Google Scholar]
- 58.Awazuhara M, Kim H, Goto DB, Matsui A, Hayashi H, Chino M, et al. A 235-bp region from a nutritionally regulated soybean seed-specific gene promoter can confer its sulfur and nitrogen response to a constitutive promoter in aerial tissues of Arabidopsis thaliana. Plant Sci. 2002;163:75–82. doi: 10.1016/S0168-9452(02)00064-X. [DOI] [Google Scholar]
- 59.Krouk G, Ruffel S, Gutiérrez RA, Gojon A, Crawford NM, Coruzzi GM, et al. A framework integrating plant growth with hormones and nutrients. Trends Plant Sci. 2011;16:178–82. doi: 10.1016/j.tplants.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 60.Schachtman DP, Shin R. Nutrient sensing and signaling: NPKS. Annu Rev Plant Biol. 2007;58:47–69. doi: 10.1146/annurev.arplant.58.032806.103750. [DOI] [PubMed] [Google Scholar]
- 61.Setya A, Murillo M, Leustek T. Sulfate reduction in higher plants: molecular evidence for a novel 5′-adenylylsulfate reductase. Proc Natl Acad Sci USA. 1996;93:13383–8. doi: 10.1073/pnas.93.23.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maruyama-Nakashita A, Nakamura Y, Watanabe-Takahashi A, Yamaya T, Takahashi H. Induction of SULTR1;1 sulfate transporter in Arabidopsis roots involves protein phosphorylation/dephosphorylation circuit for transcriptional regulation. Plant Cell Physiol. 2004;45:340–5. doi: 10.1093/pcp/pch029. a. [DOI] [PubMed] [Google Scholar]
- 63.Maruyama-Nakashita A, Nakamura Y, Yamaya T, Takahashi H. Regulation of high-affinity sulphate transporters in plants: towards systematic analysis of sulphur signalling and regulation. J Exp Bot. 2004;55:1843–9. doi: 10.1093/jxb/erh175. b. [DOI] [PubMed] [Google Scholar]
- 64.Hirai MY, Yano M, Goodenowe DB, Kanaya S, Kimura T, Awazuhara M, et al. Integration of transcriptomics and metabolomics for understanding of global responses to nutritional stresses in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2004;101:10205–10. doi: 10.1073/pnas.0403218101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kutz A, Müller A, Hennig P, Kaiser WM, Piotrowski M, Weiler EW. A role for nitrilase 3 in the regulation of root morphology in sulphur-starving Arabidopsis thaliana. Plant J. 2002;30:95–106. doi: 10.1046/j.1365-313X.2002.01271.x. [DOI] [PubMed] [Google Scholar]
- 66.López-Bucio J, Cruz-Ramírez A, Herrera-Estrella L. The role of nutrient availability in regulating root architecture. Curr Opin Plant Biol. 2003;6:280–7. doi: 10.1016/S1369-5266(03)00035-9. [DOI] [PubMed] [Google Scholar]
- 67.Hirai MY, Fujiwara T, Awazuhara M, Kimura T, Noji M, Saito K. Global expression profiling of sulfur-starved Arabidopsis by DNA macroarray reveals the role of O-acetyl-l-serine as a general regulator of gene expression in response to sulfur nutrition. Plant J. 2003;33:651–63. doi: 10.1046/j.1365-313X.2003.01658.x. [DOI] [PubMed] [Google Scholar]
- 68.Xiang C, Oliver DJ. Glutathione metabolic genes coordinately respond to heavy metals and jasmonic acid in Arabidopsis. Plant Cell. 1998;10:1539–50. doi: 10.1105/tpc.10.9.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jost R, Altschmied L, Bloem E, Bogs J, Gershenzon J, Hähnel U, et al. Expression profiling of metabolic genes in response to methyl jasmonate reveals regulation of genes of primary and secondary sulfur-related pathways in Arabidopsis thaliana. Photosynth Res. 2005;86:491–508. doi: 10.1007/s11120-005-7386-8. [DOI] [PubMed] [Google Scholar]
- 70.Maruyama-Nakashita A, Nakamura Y, Tohge T, Saito K, Takahashi H. Arabidopsis SLIM1 is a central transcriptional regulator of plant sulfur response and metabolism. Plant Cell. 2006;18:3235–51. doi: 10.1105/tpc.106.046458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Konings H, Verschuren G. Formation of aerenchyma in roots of Zea mays in aerated solutions, and its relation to nutrient supply. Physiol Plant. 1980;49:265–79. doi: 10.1111/j.1399-3054.1980.tb02661.x. [DOI] [Google Scholar]
- 72.Fan MS, Zhu JM, Richards C, Brown KM, Lynch JP. Physiological roles for aerenchyma in phosphorus-stressed roots. Funct Plant Biol. 2003;30:493–506. doi: 10.1071/FP03046. [DOI] [PubMed] [Google Scholar]
- 73.Mostertz J, Scharf C, Hecker M, Homuth G. Transcriptome and proteome analysis of Bacillus subtilis gene expression in response to superoxide and peroxide stress. Microbiology. 2004;150:497–512. doi: 10.1099/mic.0.26665-0. [DOI] [PubMed] [Google Scholar]
- 74.Conklin PL, Barth C. Ascorbic acid, a familiar small molecule intertwined in the response of plants to ozone, pathogens, and the onset of senescence. Plant Cell Environ. 2004;27:959–71. doi: 10.1111/j.1365-3040.2004.01203.x. [DOI] [Google Scholar]
- 75.Pastori GM, Kiddle G, Antoniw J, Bernard S, Veljovic-Jovanovic S, Verrier PJ, et al. Leaf vitamin C contents modulate plant defense transcripts and regulate genes that control development through hormone signaling. Plant Cell. 2003;15:939–51. doi: 10.1105/tpc.010538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Khan MIR, Asgher M, Iqbal N, Khan NA. Potentiality of sulfur-containing compounds in salt stress tolerance. In: Ahmad P, Azooz MM, Prasad MNV, eds. Ecophysiology and responses of plants under salt stress. 2012; DOI: 10.1007/978-1-4614-4747-4_17. [Google Scholar]
- 77.Bleecker AB, Kende H. Ethylene: a gaseous signal molecule in plants. Annu Rev Cell Dev Biol. 2000;16:1–18. doi: 10.1146/annurev.cellbio.16.1.1. [DOI] [PubMed] [Google Scholar]
- 78.Nikiforova VJ, Bielecka M, Gakière B, Krueger S, Rinder J, Kempa S, et al. Effect of sulfur availability on the integrity of amino acid biosynthesis in plants. Amino Acids. 2006;30:173–83. doi: 10.1007/s00726-005-0251-4. [DOI] [PubMed] [Google Scholar]
- 79.Katz YS, Galili G, Amir R. Regulatory role of cystathionine-gamma-synthase and de novo synthesis of methionine in ethylene production during tomato fruit ripening. Plant Mol Biol. 2006;61:255–68. doi: 10.1007/s11103-006-0009-8. [DOI] [PubMed] [Google Scholar]
- 80.Yoshida S, Tamaoki M, Ioki M, Ogawa D, Sato Y, Aono M, et al. Ethylene and salicylic acid control glutathione biosynthesis in ozone-exposed Arabidopsis thaliana. Physiol Plant. 2009;136:284–98. doi: 10.1111/j.1399-3054.2009.01220.x. [DOI] [PubMed] [Google Scholar]
- 81.Masood A, Iqbal N, Khan NA. Role of ethylene in alleviation of cadmium-induced photosynthetic capacity inhibition by sulphur in mustard. Plant Cell Environ. 2012;35:524–33. doi: 10.1111/j.1365-3040.2011.02432.x. a. [DOI] [PubMed] [Google Scholar]
- 82.Masood A, Iqbal N, Khan MIR, Khan NA. The coordinated role of ethylene and glucose in sulfur-mediated protection of photosynthetic inhibition by cadmium. Plant Signal Behav. 2012;7 doi: 10.4161/psb.22079. b. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cobbett CS, Goldsbrough P. Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annu Rev Plant Biol. 2002;53:159–82. doi: 10.1146/annurev.arplant.53.100301.135154. [DOI] [PubMed] [Google Scholar]
- 84.Droux M. Sulfur assimilation and the role of sulfur in plant metabolism: a survey. Photosynth Res. 2004;79:331–48. doi: 10.1023/B:PRES.0000017196.95499.11. [DOI] [PubMed] [Google Scholar]
- 85.Foyer CH, Theodoulou FL, Delrot S. The functions of inter- and intracellular glutathione transport systems in plants. Trends Plant Sci. 2001;6:486–92. doi: 10.1016/S1360-1385(01)02086-6. [DOI] [PubMed] [Google Scholar]
- 86.Noctor G, Mhamdi A, Chaouch S, Han Y, Neukermans J, Marquez-Garcia B, et al. Glutathione in plants: an integrated overview. Plant Cell Environ. 2012;35:454–84. doi: 10.1111/j.1365-3040.2011.02400.x. [DOI] [PubMed] [Google Scholar]
- 87.Hartmann T, Hönicke P, Wirtz M, Hell R, Rennenberg H, Kopriva S. Regulation of sulphate assimilation by glutathione in poplars (Populus tremula x P. alba) of wild type and overexpressing γ-glutamylcysteine synthetase in the cytosol. J Exp Bot. 2004;55:837–45. doi: 10.1093/jxb/erh094. [DOI] [PubMed] [Google Scholar]
- 88.Nocito FF, Lancilli C, Crema B, Fourcroy P, Davidian JC, Sacchi GA. Heavy metal stress and sulfate uptake in maize roots. Plant Physiol. 2006;141:1138–48. doi: 10.1104/pp.105.076240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Smith IK. Regulation of sulfate assimilation in tobacco cells. Effect of nitrogen and sulfur nutrition on sulfate permease and O-acetylserine sulfhydrylase. Plant Physiol. 1980;66:877–83. doi: 10.1104/pp.66.5.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Blake-Kalff MMA, Hawkesford MJ, Zhao FJ, McGrath SP. Diagnosing sulfur deficiency in field-grown oilseed rape (Brassica napus L.) and wheat (Triticum aestivum L.) Plant Soil. 2000;225:95–107. doi: 10.1023/A:1026503812267. [DOI] [Google Scholar]
- 91.Khan NA, Nazar R, Anjum NA. Growth, photosynthesis and antioxidant metabolism in mustard (Brassica juncea L.) cultivars differing in ATP-sulfurylase activity under salinity stress. Sci Hortic (Amsterdam) 2009;122:455–60. doi: 10.1016/j.scienta.2009.05.020. [DOI] [Google Scholar]
- 92.May MJ, Vernoux T, Leaver C, van Montagu M, Inzé D. Glutathione homeostasis in plants: implications for environmental sensing and plant development. J Exp Bot. 1998;49:649–67. [Google Scholar]
- 93.Mullineaux PM, Creissen GP. Glutathione reductase: regulation and role in oxidative stress. In: Scandalios JG, ed. Oxidative stress and the molecular biology of antioxidants. NY:Cold Spring Harbor Laboratory Press, 1997:667-713. [Google Scholar]
- 94.Reddy AR, Raghavendra AS. Photooxidative stress. In: Madhava Rao KV, Raghavendra AS, Reddy KJ, eds. Physiology and molecular biology of stress tolerance in plants. The Netherlands:Springer, 2006:157-86. [Google Scholar]
- 95.Rao ASVC, Reddy A. Glutathione reductase: a putative redox regulatory system in plant cells. In: Khan NA, Singh S, Umar S eds. Sulfur assimilation and abiotic stress in plants. Berlin Heidelberg:Springer-Verlag, 2008:111-47. [Google Scholar]
- 96.Rausch T, Wachter A. Sulfur metabolism: a versatile platform for launching defence operations. Trends Plant Sci. 2005;10:503–9. doi: 10.1016/j.tplants.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 97.Tamaoki M, Freeman JL, Pilon-Smits EAH. Cooperative ethylene and jasmonic acid signaling regulates selenite resistance in Arabidopsis. Plant Physiol. 2008;146:1219–30. doi: 10.1104/pp.107.110742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Van Hoewyk D, Takahashi H, Inoue E, Hess A, Tamaoki M, Pilon-Smits EA. Transcriptome analyses give insights into selenium-stress responses and selenium tolerance mechanisms in Arabidopsis. Physiol Plant. 2008;132:236–53. doi: 10.1111/j.1399-3054.2007.01002.x. [DOI] [PubMed] [Google Scholar]
- 99.Tari I, Szen L. Effect of nitrite and nitrate nutrition on ethylene production by wheat seedlings. Acta Phytopathol Entomol Hung. 1995;30:99–104. [Google Scholar]
- 100.Lynch J, Brown KM. Ethylene and plant responses to nutritional stress. Physiol Plant. 1997;100:613–9. doi: 10.1111/j.1399-3054.1997.tb03067.x. [DOI] [Google Scholar]
- 101.Khan NA, Mir MR, Nazar R, Singh S. The application of ethephon (an ethylene releaser) increases growth, photosynthesis and nitrogen accumulation in mustard (Brassica juncea L.) under high nitrogen levels. Plant Biol (Stuttg) 2008;10:534–8. doi: 10.1111/j.1438-8677.2008.00054.x. [DOI] [PubMed] [Google Scholar]
- 102.Kim HJ, Lynch JP, Brown KM. Ethylene insensitivity impedes a subset of responses to phosphorus deficiency in tomato and petunia. Plant Cell Environ. 2008;31:1744–55. doi: 10.1111/j.1365-3040.2008.01886.x. [DOI] [PubMed] [Google Scholar]
- 103.Garnica M, Houdusse F, Claude Yvin J, Garcia-Mina JM. Nitrate supply induces changes in polyamine content and ethylene production in wheat plants grown with ammonium. J Plant Physiol. 2009;166:363–74. doi: 10.1016/j.jplph.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 104.Jung JY, Shin R, Schachtman DP. Ethylene mediates response and tolerance to potassium deprivation in Arabidopsis. Plant Cell. 2009;21:607–21. doi: 10.1105/tpc.108.063099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Benlloch-González M, Romera J, Cristescu S, Harren F, Fournier JM, Benlloch MKK. + starvation inhibits water-stress-induced stomatal closure via ethylene synthesis in sunflower plants. J Exp Bot. 2010;61:1139–45. doi: 10.1093/jxb/erp379. [DOI] [PubMed] [Google Scholar]
- 106.Iqbal N, Nazar R, Syeed S, Masood A, Khan NA. Exogenously-sourced ethylene increases stomatal conductance, photosynthesis, and growth under optimal and deficient nitrogen fertilization in mustard. J Exp Bot. 2011;62:4955–63. doi: 10.1093/jxb/err204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Iqbal N, Nazar R, Khan MIR, Khan NA. Variation in photosynthesis and growth of mustard cultivars: role of ethylene sensitivity. Sci Hortic (Amsterdam) 2012;135:1–6. doi: 10.1016/j.scienta.2011.12.005. [DOI] [Google Scholar]
- 108.Koprivova A, North KA, Kopriva S. Complex signaling network in regulation of adenosine 5′-phosphosulfate reductase by salt stress in Arabidopsis roots. Plant Physiol. 2008;146:1408–20. doi: 10.1104/pp.107.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Terry N, Zayed AM, De Souza MP, Tarun AS. Selenium in higher plants. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:401–32. doi: 10.1146/annurev.arplant.51.1.401. [DOI] [PubMed] [Google Scholar]
- 110.Dan H, Yang G, Zheng ZL. A negative regulatory role for auxin in sulphate deficiency response in Arabidopsis thaliana. Plant Mol Biol. 2007;63:221–35. doi: 10.1007/s11103-006-9084-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wu L, Zhang Z, Zhang H, Wang XC, Huang R. Transcriptional modulation of ethylene response factor protein JERF3 in the oxidative stress response enhances tolerance of tobacco seedlings to salt, drought, and freezing. Plant Physiol. 2008;148:1953–63. doi: 10.1104/pp.108.126813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wi SJ, Jang SJ, Park KY. Inhibition of biphasic ethylene production enhances tolerance to abiotic stress by reducing the accumulation of reactive oxygen species in Nicotiana tabacum. Mol Cells. 2010;30:37–49. doi: 10.1007/s10059-010-0086-z. [DOI] [PubMed] [Google Scholar]
- 113.Tena G, Asai T, Chiu WL, Sheen J. Plant mitogen-activated protein kinase signaling cascades. Curr Opin Plant Biol. 2001;4:392–400. doi: 10.1016/S1369-5266(00)00191-6. [DOI] [PubMed] [Google Scholar]
- 114.Zhang S, Klessig DF. MAPK cascades in plant defense signaling. Trends Plant Sci. 2001;6:520–7. doi: 10.1016/S1360-1385(01)02103-3. [DOI] [PubMed] [Google Scholar]
- 115.Kim CY, Liu Y, Thorne ET, Yang H, Fukushige H, Gassmann W, et al. Activation of a stress-responsive mitogen-activated protein kinase cascade induces the biosynthesis of ethylene in plants. Plant Cell. 2003;15:2707–18. doi: 10.1105/tpc.011411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang KLC, Li H, Ecker JR. Ethylene biosynthesis and signaling networks. Plant Cell. 2002;14(Suppl):S131–51. doi: 10.1105/tpc.001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen YC, Lin HH, Jeng ST. Calcium influxes and mitogen-activated protein kinase kinase activation mediate ethylene inducing ipomoelin gene expression in sweet potato. Plant Cell Environ. 2008;31:62–72. doi: 10.1111/j.1365-3040.2007.01742.x. [DOI] [PubMed] [Google Scholar]
- 118.Jih PJ, Chen YC, Jeng ST. Involvement of hydrogen peroxide and nitric oxide in expression of the ipomoelin gene from sweet potato. Plant Physiol. 2003;132:381–9. doi: 10.1104/pp.102.015701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Van der Sman AJM, Voesenek LACJ, Blom CWPM, Harren FJM, Reuss J. The role of ethylene in shoot elongation with respect to survival and seed output of flooded Rumex maritimus L. plants. Funct Ecol. 1991;5:304–13. doi: 10.2307/2389269. [DOI] [Google Scholar]
- 120.Banga M, Blom CWPM, Voesenek LACJ. Sensitivity to ethylene: the key factor in submergence-induced shoot elongation of Rumex. Plant Cell Environ. 1996;19:1423–30. doi: 10.1111/j.1365-3040.1996.tb00021.x. [DOI] [Google Scholar]
- 121.Zhang Z, Huang R. Enhanced tolerance to freezing in tobacco and tomato overexpressing transcription factor TERF2/LeERF2 is modulated by ethylene biosynthesis. Plant Mol Biol. 2010;73:241–9. doi: 10.1007/s11103-010-9609-4. [DOI] [PubMed] [Google Scholar]
- 122.Fowler S, Thomashow MF. Arabidopsis transcriptome profiling indicates that multiple regulatory pathways are activated during cold acclimation in addition to the CBF cold response pathway. Plant Cell. 2002;14:1675–90. doi: 10.1105/tpc.003483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang W, Hu W, Wen CK. Ethylene preparation and its application to physiological experiments. Plant Signal Behav. 2010;5:453–7. doi: 10.4161/psb.5.4.10875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hattori Y, Nagai K, Furukawa S, Song XJ, Kawano R, Sakakibara H, et al. The ethylene response factors SNORKEL1 and SNORKEL2 allow rice to adapt to deep water. Nature. 2009;460:1026–30. doi: 10.1038/nature08258. [DOI] [PubMed] [Google Scholar]
- 125.Lu CW, Shao Y, Li L, Chen AJ, Xu WQ, Wu KJ, et al. Overexpression of SlERF1 tomato gene encoding an ERF-type transcription activator enhances salt tolerance. Russ J Plant Physiol. 2011;58:118–25. doi: 10.1134/S1021443711010092. [DOI] [Google Scholar]