Abstract
Balanced and precisely controlled processes between self-renewal and differentiation of hematopoietic stem cells (HSCs) into all blood lineages are critical for vertebrate definitive hematopoiesis. However, the molecular mechanisms underlying the maintenance and differentiation of HSCs have not been fully elucidated. Here, we show that zebrafish Ddx46, encoding a DEAD-box RNA helicase, is expressed in HSCs of the caudal hematopoietic tissue (CHT). The number of HSCs expressing the molecular markers cmyb or T-cell acute lymphocytic leukemia 1 (tal1) was markedly reduced in Ddx46 mutants. However, massive cell death of HSCs was not detected, and proliferation of HSCs was normal in the CHT of the mutants at 48 h postfertilization. We found that myelopoiesis occurred, but erythropoiesis and lymphopoiesis were suppressed, in Ddx46 mutants. Consistent with these results, the expression of spi1, encoding a regulator of myeloid development, was maintained, but the expression of gata1a, encoding a regulator of erythrocyte development, was downregulated in the mutants. Taken together, our results provide the first genetic evidence that zebrafish Ddx46 is required for the multilineage differentiation of HSCs during development, through the regulation of specific gene expressions.
Introduction
It has been well noted that the processes involved in vertebrate hematopoiesis during development consist of two evolutionarily conserved steps: primitive hematopoiesis followed by definitive blood formation [1–3]. In the definitive hematopoiesis stage, all blood lineages arise from self-renewing hematopoietic stem cells (HSCs), and hematopoiesis derived from HSCs persists for the lifetime of vertebrates [1–3]. In recent years, zebrafish has been used as an excellent vertebrate system for studying hematopoiesis during development [4,5]. In the definitive hematopoietic wave of zebrafish embryos, HSCs originate from the ventral wall of the dorsal aorta (VDA) in the aorta-gonad-mesonephros (AGM) region [2–4]. These HSCs then migrate to an intermediate hematopoietic site, the caudal hematopoietic tissue (CHT), and then further toward the kidney or the thymus, which are definitive hematopoietic or lymphopoietic organs, respectively, in adult zebrafish [2–4].
Several lines of genetic evidence have revealed that at least two transcription factors, cMyb and Runt-related transcription factor 1 (Runx1), have been implicated in the initiation, maintenance, and/or differentiation of HSCs during vertebrate development [5–14]. Studies on murine and human hematopoietic cell lines have shown that the cmyb proto-oncogene, encoding a transcription factor, is expressed mainly in HSCs, and its expression is downregulated in differentiated hematopoietic cells [5]. In addition, studies in a mouse model have suggested that cMyb plays critical roles in HSC maintenance and differentiation during development [6–8]. Consistent with observations that have been made in mice, zebrafish cmyb mutants have defects in definitive hematopoiesis, suggesting that cMyb function is evolutionarily conserved [9,10]. On the other hand, runx1 is expressed in the VDA of mouse and zebrafish embryos [11,12], and analyses of Runx1 knockout mice and zebrafish mutants have revealed that Runx1 is required for the emergence of HSCs at the beginning of definitive hematopoiesis [5,13,14]. In contrast to these 2 factors, analyses of mutant animals have shown that the T-cell acute lymphocytic leukemia 1 (Tal1; also known as Scl) gene, encoding a basic-helix-loop-helix transcription factor, is required for both hematopoietic and endothelial development [1,2,5]. Further, in vitro experiments using mouse embryonic stem cells revealed that Tal1 plays critical roles in both hemogenic endothelium population generation and definitive hematopoietic specification [15]. Further, the function of Tal1 is upstream of Runx1 in definitive hematopoiesis [15]. The molecular mechanisms underlying the initiation, maintenance, and differentiation of HSCs, however, remain to be elucidated.
DExD/H-box proteins belong to an evolutionarily conserved family of RNA helicases [16,17]. The DExD/H-box RNA helicases are known to function in all aspects of RNA metabolism such as pre-mRNA splicing, rRNA biogenesis, and transcription by using the energy derived from ATP hydrolysis [16,17]. By genetic screening using zebrafish, two DExD/H-box genes, Ddx18 and Dhx8, have been identified thus far as novel genes that are essential for hematopoiesis [18,19]. A recent study has reported that Ddx18 is required for primitive hematopoiesis through the regulation of p53-dependent G1 cell-cycle arrest [18]. Moreover, a sequence variation in human DDX18, which acts as a dominant-negative, was identified in samples from patients with acute myeloid leukemia [18]. A more recent report showed that a mutation in Dhx8, a zebrafish orthologue of the yeast splicing factor Prp22, led to defects in cell division, pre-mRNA splicing, and primitive hematopoiesis [19]. However, the requirement and function of the DExD/H-box RNA helicases in hematopoiesis are still largely unknown in vertebrates.
Our previous study has shown that Ddx46, a member of the DEAD-box RNA helicase family, is required for the development of digestive organs and the brain, possibly by regulating pre-mRNA splicing [20]. Here, we show zebrafish Ddx46 expression in HSCs during development. Moreover, we investigated the phenotype of a zebrafish Ddx46 mutant in definitive hematopoiesis, and we report the function of Ddx46 in HSC differentiation during development.
Materials and Methods
Ethics statement
All animal experiments were conducted according to relevant national and international guidelines “Act on Welfare and Management of Animals” (Ministry of Environment of Japan). Ethics approval from the Hiroshima University Animal Research Committee (HuARC) was not sought since this law does not mandate protection of fish.
Maintenance and staging of zebrafish
Adult zebrafish and zebrafish embryos were maintained as described by Westerfield [21]. Embryos were incubated in 1/3 Ringer's solution (39 mM NaCl, 0.97 mM KCl, 1.8 mM CaCl2, and 1.7 mM HEPES, pH 7.2) at 28.5°C and staging was performed as described by Kimmel et al. [22]. The Ddx46 allele hi2137 was isolated during an insertional mutagenesis screening (http://web.mit.edu/hopkins/group11.html) [23], and the Ddx46hi2137/+ fish was obtained from the Zebrafish International Resource Center.
Generation of Tg(tal1:EGFP) fish
Approximately 8 kb of the 5′ upstream sequence of tal1 [24,25] was polymerase chain reaction (PCR)-amplified from zebrafish genomic DNA. The amplified tal1 promoter, EGFP, and SV40 poly(A) were placed in the pT2KXIGΔin vector that has Tol2 transposable elements [26]. Microinjection of Tol2-based plasmid DNA was performed as described previously [26].
Whole-mount in situ hybridization, immunohistochemistry, detection of cell death, and genotyping
Single and double whole-mount in situ hybridizations were performed as described previously [21,27], and riboprobes were prepared according to previously published methods. To detect apoptotic cells, we performed TUNEL staining using an in situ Cell Death Detection Kit (Roche Diagnostics) according to the manufacturer's instructions. In addition to TUNEL staining, we performed acridine orange staining for apoptosis detection. Live larvae were stained with 10 μg/mL of acridine orange [acridine orange hemi (zinc chloride) salt; Sigma] in 1/3 Ringer's solution for 15 min, and then washed thrice with 1/3 Ringer's solution for 5 min each. To evaluate cell proliferation, we performed whole-mount immunohistochemistry as described previously [21]. Mouse monoclonal anti-proliferating cell nuclear antigen (PCNA) antibody (Sigma) and Alexa Fluor® 594 goat anti-mouse IgG antibody (Invitrogen, Life Technologies Corp.) were used as primary and secondary antibodies, respectively. The stained embryos/larvae were embedded in 0.5% low melting temperature agarose in 1/3 Ringer's solution and imaged on an Olympus FV1000-D confocal microscope. Following the whole-mount in situ hybridization, TUNEL staining, or immunohistochemical staining, Ddx46hi2137/hi2137 mutants were confirmed by genotyping as described previously [20].
mRNA and DNA injections
The pCS2+ vector carrying a cDNA fragment encoding Ddx46, EGFP, or Tol2-transposase [26] was used in this study. Capped mRNA was synthesized using an SP6 mMESSAGE mMACHINE (Ambion, Life Technologies Corp.). For the overexpression experiments, Ddx46 or EGFP mRNA (320 pg each) was injected at the one-cell stage.
For phenotypic rescue experiments of GATA-binding protein 1a (gata1a) [28], Tol2-mediated transgenesis was performed as described previously [29]. At the one-cell stage, 12.5 pg of pT8.1gata1Δ 3-EGFP (pT-EGFP), which contained the promoter region of gata1a and EGFP, or pT8.1gata1Δ 3-FLgata1 (pT-FLgata1), which contained the promoter region of gata1a and FLAG-tagged gata1a, was co-injected with 25 pg of Tol2-transposase mRNA [29].
Quantitative real-time PCR
Total RNA was prepared using TRIzol (Invitrogen, Life Technologies Corp.) from the tails of 50 combined samples of the 3 days postfertilization (dpf) control (con) or Ddx46hi2137/hi2137 mutant larvae that were identified morphologically or molecularly. Con larvae were sibling wild-type (WT) or Ddx46hi2137/+ larvae, and they had normal phenotypes. DNase-treated RNA (750 ng) was reverse transcribed with random 9-mer priming and reverse transcriptase XL (AMV) (TaKaRa). Quantitative PCR (qPCR) for spi1 (also known as pu.1) [30] and gata1a was performed in triplicate using the Thermal Cycler Dice® Real Time System, SYBR® Premix Ex Taq™ (TaKaRa Bio, Inc.), and total RNA prepared as described above, according to the manufacturer's instructions. The amplified signals were confirmed to be a single band by gel electrophoresis, and they were normalized to the signals of zebrafish 18S rRNA. The primers used were as follows: gata1a, 5′-GGCTAGTTCACTCCATGATC-3′ and 5′-CTCAG AGCTGGAGTAGAAAG-3′; spi1, 5′-ATGTGGAGTCCAGCC ATTTC-3′ and 5′-TTGTGAGGGTAACACACCGA-3′; 18S rRNA, 5′-CCGCTAGAGGTGAAATTCTTG-3′ and 5′-CAG CTTTGCAACCATACTCC-3′.
Reverse transcription-PCR analysis of splicing
Reverse transcription (RT)-PCR was performed using total RNA prepared as described above to monitor the splicing of spi1, gata1a, and cmyb. The primer pairs and detailed PCR conditions used to amplify each of these genes are listed in the Supplementary Tables S1 and S2 (Supplementary Data are available online at www.liebertpub.com/scd).
Results
Ddx46hi2137/hi2137 mutants have defects in definitive hematopoiesis but not primitive hematopoiesis
We have previously shown that Ddx46hi2137/hi2137 mutants have defects in the formation of digestive organs and the brain [20]. In the course of the phenotypic analyses of Ddx46hi2137/hi2137 mutants, we also found that the expression of hematopoietic markers was downregulated in the mutants. At 22 hours postfertilization (hpf), the expression of the primitive hematopoietic markers tal1, LIM domain only 2 (lmo2) [31], gata1a, hemoglobin beta embryonic-3 (hbbe3) [32], and myeloid-specific peroxidase (mpx) [33] was normal in Ddx46hi2137/hi2137 mutants (Fig. 1A–J). On the other hand, the expression of the HSC markers tal1, runx1, and cmyb was markedly reduced in Ddx46hi2137/hi2137 mutants at 3 dpf (Fig. 1K–P). These results indicate that definitive hematopoiesis, but not primitive hematopoiesis, was defective in the mutants.
FIG. 1.
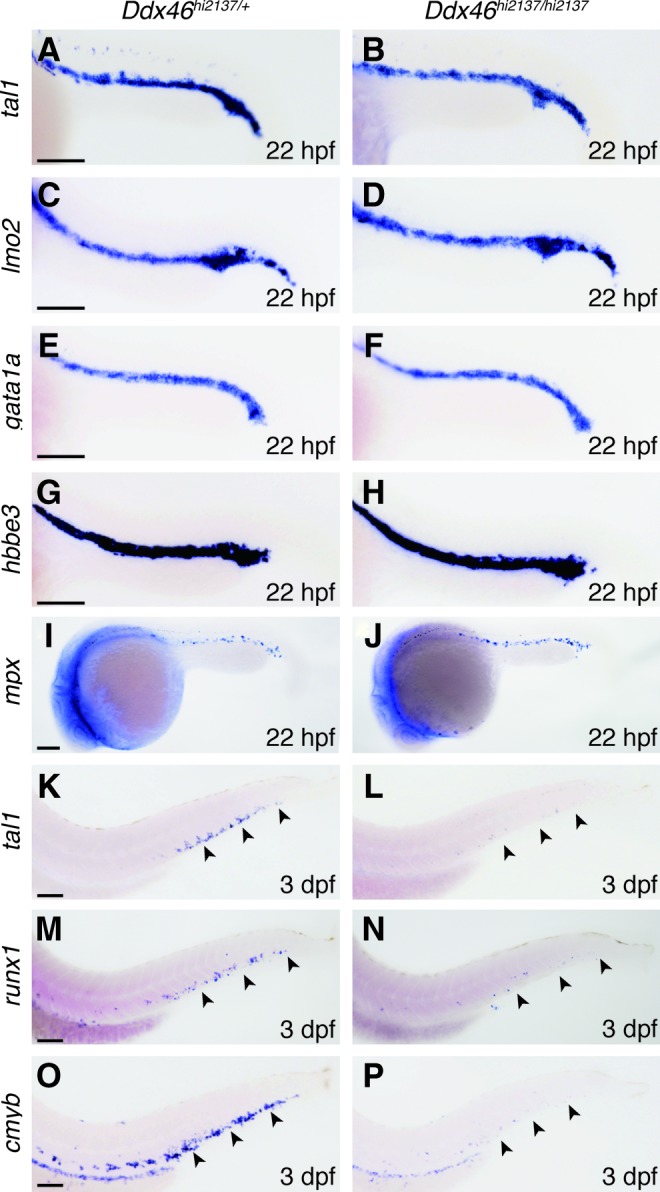
Definitive, but not primitive, hematopoiesis is defective in Ddx46hi2137/hi2137 mutants. (A–J) The expression of primitive hematopoietic markers, tal1, lmo2, gata1a, hbbe3, and mpx, and definitive hematopoietic markers, tal1, runx1, and cmyb was examined by whole-mount in situ hybridization at 22 hpf and 3 dpf, respectively. All are lateral views, anterior to the left. The expression of tal1, lmo2, gata1a, hbbe3, and mpx was indistinguishable between Ddx46hi2137/+ (tal1, n=9/9; lmo2, n=11/11; gata1a, n=9/9; hbbe3, n=12/12; mpx, n=16/16) and Ddx46hi2137/hi2137 embryos (tal1, n=9/9; lmo2, n=9/9; gata1a, n=7/7; hbbe3, n=10/10; mpx, n=7/7) at 22 hpf (A–J). In contrast, the number of cells expressing tal1, runx1, and cmyb in Ddx46hi2137/hi2137 larvae (tal1, n=6/6; runx1, n=9/9; cmyb, n=11/11) was markedly reduced compared with that in Ddx46hi2137/+ larvae (tal1, n=8/8; runx1, n=11/11; cmyb, n=13/13) at 3 dpf (arrowheads in K–P). Scale bars represent 100 μm. tal1, T-cell acute lymphocytic leukemia 1; hpf, hours postfertilization; lmo2, LIM domain only 2; hbbe3, hemoglobin beta embryonic-3; mpx, myeloid-specific peroxidase; dpf, days postfertilization. Color images available online at www.liebertpub.com/scd
To confirm that the loss of Ddx46 is responsible for the observed phenotype in definitive hematopoiesis, we compared the expression pattern of Ddx46 with that of cmyb in the CHT. Ubiquitous expression of Ddx46 in the AGM and CHT was observed at 2 dpf (Fig. 2A), and dotted expression of Ddx46 was found in the CHT at 3 dpf (Fig. 2B, C). At 4 dpf, the dotted expression pattern of Ddx46 in the CHT was similar to the expression pattern of cmyb (Fig. 2D, E). Moreover, the expression of both genes overlapped in the CHT, as analyzed by double whole-mount in situ staining (Fig. 2F), indicating that Ddx46 is expressed in the HSCs at 4 dpf. We next examined whether rescue was achieved by the overexpression of Ddx46 mRNA. The expressions of cmyb and tal1 were rescued in the Ddx46 mRNA-injected Ddx46hi2137/hi2137 mutants (cmyb, 19 of 21 Ddx46 mRNA-injected mutants were rescued; tal1, 19 of 22 Ddx46 mRNA-injected mutants were rescued; Fig. 3C, F), compared with that in the EGFP mRNA-injected Ddx46hi2137/hi2137 mutants (cmyb, 0 of 26 EGFP mRNA-injected mutants were rescued; tal1, 0 of 21 EGFP mRNA-injected mutants were rescued; Fig. 3B, E) at 3 dpf. The overlapping expression of Ddx46 and cmyb in the CHT and data from the rescue experiments clearly indicate that the defects in definitive hematopoiesis in Ddx46hi2137/hi2137 mutants are caused by the loss of Ddx46.
FIG. 2.
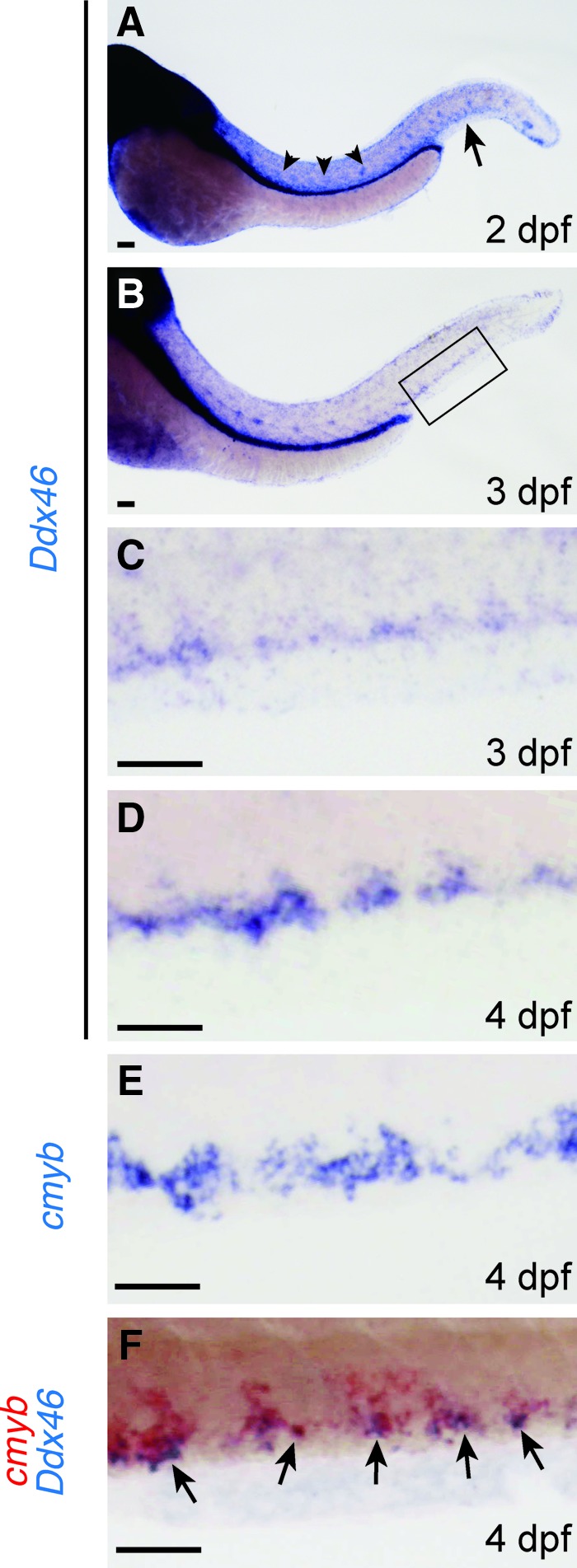
Ddx46 expression in HSCs. The expression of Ddx46 and cmyb in wild-type larvae was examined by whole-mount in situ hybridization at 2, 3, and 4 dpf. All are lateral views, anterior to the left. (A-C) Ddx46 is ubiquitously expressed in the AGM (arrowheads in A) and CHT (arrow in A) at 2 dpf (n=6/6) and is specifically expressed in the CHT (boxed area in B) at 3 dpf (n=6/6). The boxed area in (B) is shown enlarged in (C). (D, E) The transcripts of both genes (Ddx46, n=11/11; cmyb, n=9/9) were detected in the CHT at 4 dpf. (F) Double whole-mount in situ staining showed that the expression domains of Ddx46 (blue) and cmyb (red) overlapped in the CHT (arrows) (n=7/7) at 4 dpf. Scale bars represent 50 μm. HSCs, hematopoietic stem cells; AGM, aorta-gonad-mesonephros; CHT, caudal hematopoietic tissue. Color images available online at www.liebertpub.com/scd
FIG. 3.
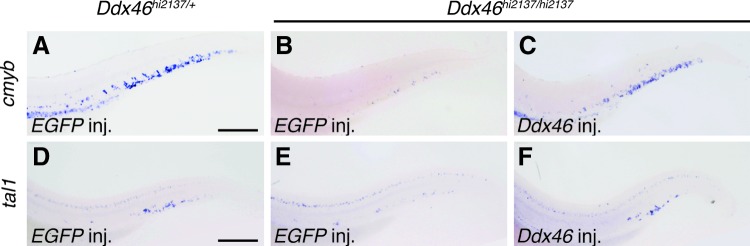
Expression of cmyb and tal1 in Ddx46hi2137/hi2137 mutants is rescued by overexpression of Ddx46 mRNA. (A–F) The expression of cmyb and tal1 was examined by whole-mount in situ hybridization at 3 dpf. All are lateral views, anterior to the left. The number of cmyb- or tal1-expressing HSCs in the EGFP mRNA-injected Ddx46hi2137/hi2137 larvae (cmyb, 0 of 26 EGFP mRNA-injected mutants were rescued; tal1, 0 of 21 EGFP mRNA-injected mutants were rescued) was markedly lower than that in the EGFP mRNA-injected Ddx46hi2137/+ larvae (cmyb, n=17/17; tal1, n=16/16) at 3 dpf (A, B, D, E). The overexpression of Ddx46 mRNA was able to rescue the number of cmyb- or tal1-expressing HSCs in Ddx46hi2137/hi2137 larvae (cmyb, 19 of 21 Ddx46 mRNA-injected mutants were rescued; tal1, 19 of 22 Ddx46 mRNA-injected mutants were rescued) at 3 dpf (B, C, E, F). Scale bars represent 100 μm. Color images available online at www.liebertpub.com/scd
Expression of molecular markers for HSCs decreases in Ddx46hi2137/hi2137 mutants without cell death or cell growth defects
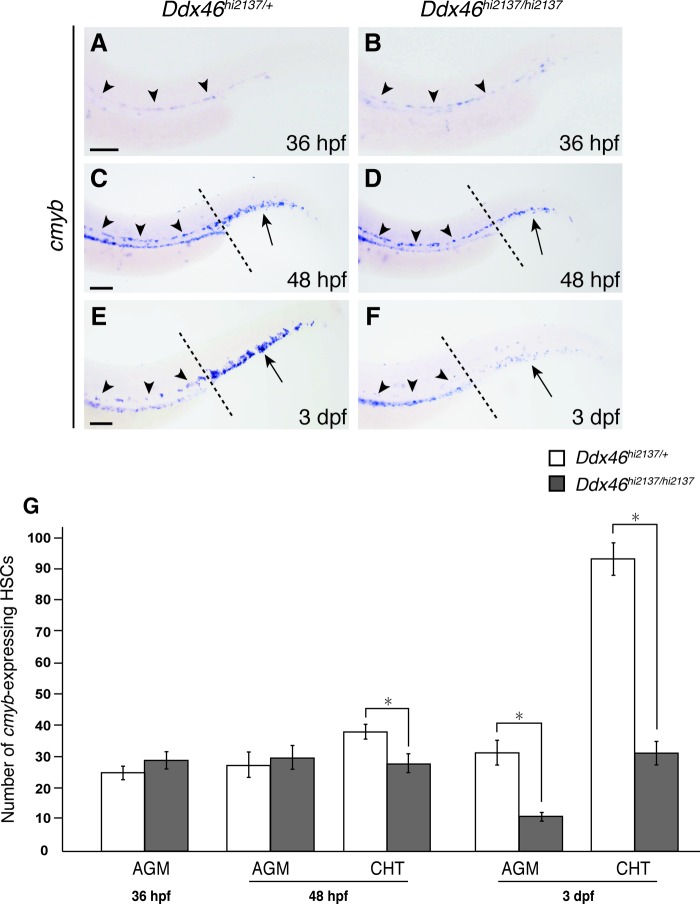
Previous studies on zebrafish and mouse development showed that HSCs originate from the VDA in the AGM, and they then migrate to the CHT [2–4]. To determine when definitive hematopoiesis was affected in Ddx46hi2137/hi2137 larvae, we next counted the number of cmyb-expressing HSCs at 36 hpf, 48 hpf, and 3 dpf. The number of cmyb-expressing HSCs was indistinguishable between Ddx46hi2137/+ (Fig. 4A, G) and Ddx46hi2137/hi2137 larvae (Fig. 4B, G) at 36 hpf. In contast, the number of cmyb-expressing HSCs in the CHT, but not in the AGM, at 48 hpf and in both the AGM and CHT at 3 dpf was lower in Ddx46hi2137/hi2137 larvae (Fig. 4D, F, G) than in Ddx46hi2137/+ larvae (Fig. 4C, E, G). To exclude the possibility that the formation of the VDA is affected in Ddx46hi2137/hi2137 mutants, we examined the expressions of runx1 and kinase insert domain receptor like (kdrl; also known as flk1) [31], an endothelial marker, in the VDA at 48 hpf. The expression of these genes was normal in the mutants at this stage (Supplementary Fig. S1). These results suggest that the emergence of HSCs from the VDA is normal, but the expression of cmyb, a molecular marker for HSCs is not maintained in Ddx46hi2137/hi2137 mutants.
FIG. 4.
Number of cmyb-expressing cells decreases in Ddx46hi2137/hi2137 mutants. (A–G) The expression of cmyb was examined by whole-mount in situ hybridization at 36 hpf, 48 hpf, or 3 dpf. All are lateral views, anterior to the left. The number of cmyb-expressing HSCs in the AGM (arrowheads) and CHT (arrows) was counted at 36 hpf, 48 hpf, and 3 dpf (G). At 36 hpf, the number of cmyb-expressing HSCs in the AGM (arrowheads) was indistinguishable between Ddx46hi2137/+ and Ddx46hi2137/hi2137 larvae (A, B, G). In contrast, the number of cmyb-expressing HSCs in the CHT (arrows), but not in the AGM (arrowheads), at 48 hpf (C, D, G) and in both the AGM (arrowheads) and CHT (arrows) at 3 dpf (E, F, G) of Ddx46hi2137/hi2137 larvae was significantly reduced compared with that of Ddx46hi2137/+ larvae. Ddx46hi2137/+ larvae: n=13/13 (36 hpf), n=18/18 (48 hpf), n=13/13 (3 dpf); Ddx46hi2137/hi2137 larvae: n=9/9 (36 hpf), n=14/14 (48 hpf), n=11/11 (3 dpf). Black dotted lines in (C–F) indicate the boundary between the AGM and CHT. Error bars represent the standard error. *P<0.01 by the Student's t-test. Scale bars represent 100 μm. Color images available online at www.liebertpub.com/scd
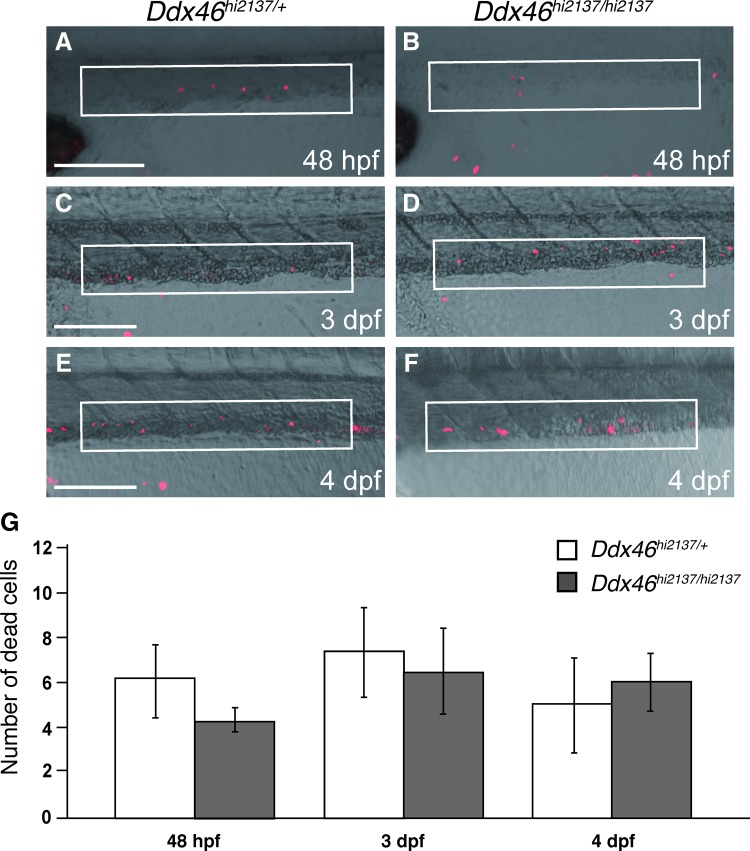
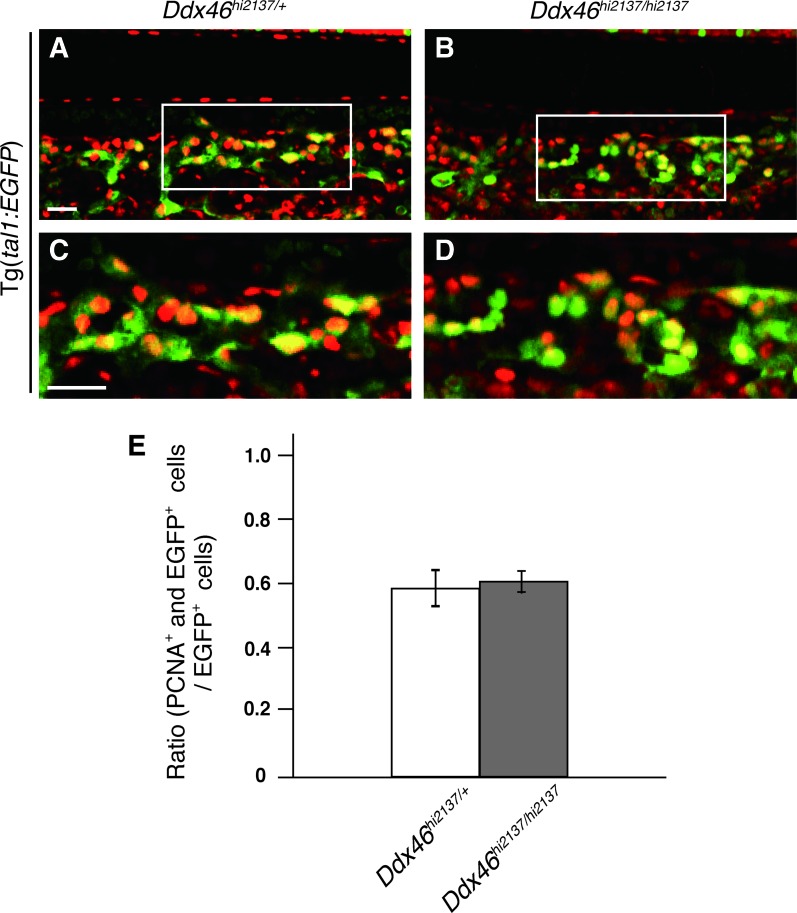
Possible explanations for the reduction of tal1-, runx1-, or cmyb-expressing HSCs in Ddx46hi2137/hi2137 larvae are the upregulation of cell death or the downregulation of cell proliferation. We first examined cell death of HSCs in the AGM and CHT from 36 hpf to 4 dpf by TUNEL analysis and acridine orange staining because massive apoptosis was observed in digestive organs and the brain of Ddx46hi2137/hi2137 larvae at 3 dpf, and these larvae cannot survive beyond 5 dpf [20]. From 24 hpf to 4 dpf, increased cell death was not detected in the AGM or CHT of the Ddx46hi2137/hi2137 larvae (Fig. 5 and Supplementary Fig. S2, and data not shown) compared with that in the Ddx46hi2137/+ larvae. We next examined cell proliferation using the Ddx46hi2137; Tg(tal1:EGFP) transgenic line. Confocal images of the anti-PCNA immunostaining and EGFP fluorescence indicated that the proliferation of tal1-expressing HSCs was indistinguishable between Ddx46hi2137/+ and Ddx46hi2137/hi2137 larvae at 48 hpf (Fig. 6). It was very difficult to estimate cell proliferation of HSCs after 2.5 dpf because the EGFP fluorescence and the number of EGFP+ cells were profoundly reduced in Ddx46hi2137/hi2137 larvae at these stages (Supplementary Fig. S3). These results indicate that the reduction of tal1-expressing HSCs in Ddx46hi2137/hi2137 larvae is not caused by the upregulation of cell death in the AGM and CHT or by the downregulation of cell proliferation of HSCs at 48 hpf.
FIG. 5.
Cell death is not upregulated in the CHT of Ddx46hi2137/hi2137 mutants. (A–F) Confocal microscopic images of dead cells (red) detected by the TUNEL method at 48 hpf, 3 dpf, or 4 dpf. All are lateral views, anterior to the left. The white, boxed regions show an area of the CHT. (G) The number of labeled cells in the white, boxed regions (A–F) was counted. The number of dead cells in the CHT was indistinguishable between Ddx46hi2137/+ and Ddx46hi2137/hi2137 larvae at 48 hpf, 3 dpf, and 4 dpf. Ddx46hi2137/+ larvae: n=7/7 (48 hpf), n=5/5 (3 dpf), n=5/5 (4 dpf); Ddx46hi2137/hi2137 larvae: n=5/5 (48 hpf), n=6/6 (3 dpf), n=8/8 (4 dpf). Error bars represent the standard error. Scale bars represent 75 μm. Color images available online at www.liebertpub.com/scd
FIG. 6.
Cell proliferation is not downregulated in the CHT of Ddx46hi2137/hi2137 mutants at 48 hpf. (A–D) Confocal microscopic images of EGFP fluorescence (green) and anti-PCNA (red) whole-mount immunostaining of the CHT in Ddx46hi2137/+;Tg(tal1:EGFP) and Ddx46hi2137/hi2137;Tg(tal1:EGFP) larvae at 48 hpf. All are lateral views, anterior to the left. Merged single-slice images of cells expressing EGFP (HSCs) and PCNA (proliferating cells). The boxed areas in (A) and (B) are shown enlarged in (C) and (D), respectively. (E) Quantification of the experiments in panels (C) and (D) was performed by plotting the ratio of EGFP+ and PCNA+ cells (yellow) to the total number of EGFP+ cells (green and yellow). No significant difference between Ddx46hi2137/+;Tg(tal1:EGFP) and Ddx46hi2137/hi2137;Tg(tal1:EGFP) larvae was observed. Cells were counted from four single slices from four embryos for each condition. Error bars represent the standard error. The scale bar represents 20 μm. PCNA, proliferating cell nuclear antigen. Color images available online at www.liebertpub.com/scd
Myelopoiesis occurs, but erythropoiesis and lymphopoiesis are suppressed in the CHT of Ddx46hi2137/hi2137 mutants
An alternative possibility for the reduction of HSCs in Ddx46hi2137/hi2137 larvae is that the multipotency of HSCs may be lost, and premature differentiation of HSCs to blood lineages may occur in Ddx46hi2137/hi2137 mutants. To test this hypothesis, we examined the expression of various molecular markers for definitive hematopoiesis by whole-mount in situ hybridization. The expression of an erythroid marker hemoglobin alpha embryonic-1 (hbae1) [32] was markedly reduced in Ddx46hi2137/hi2137 mutants at 3 dpf (Fig. 7A, B). We further found that the expression of lymphoid makers, IKAROS family zinc finger 1 (ikzf1) [34] and recombination activating gene 1 (rag1) [35], was lost in the thymus of Ddx46hi2137/hi2137 mutants at 4 dpf (Fig. 7C–F). However, the expression of forkhead box N1 (foxn1) [36], a thymus epithelial marker, was indistinguishable between Ddx46hi2137/+ and Ddx46hi2137/hi2137 larvae (Fig. 7G, H), indicating that thymus formation is normal in these mutants. Next, we tested the expression of myeloid markers such as lymphocyte cytosolic plastin 1 (lcp1) [37] and mpx. In contrast to the erythroid and lymphoid markers, myeloid markers were not reduced in Ddx46hi2137/hi2137 mutants at 3 dpf (Fig. 7I–L). To exclude the possibility that the reduction of erythroid and lymphoid markers was not caused by the deficiency of Ddx46, we examined the results of the rescue experiments. We found that hbae1 and ikzf1 expression was partially rescued by Ddx46 mRNA overexpression (Supplementary Fig. S4 and data not shown). Together, these results suggest that HSCs have defects in multilineage differentiation in Ddx46hi2137/hi2137 mutants: they were able to differentiate to the myeloid fate, but differentiation to the erythroid or lymphoid fate was suppressed in the mutants.
FIG. 7.
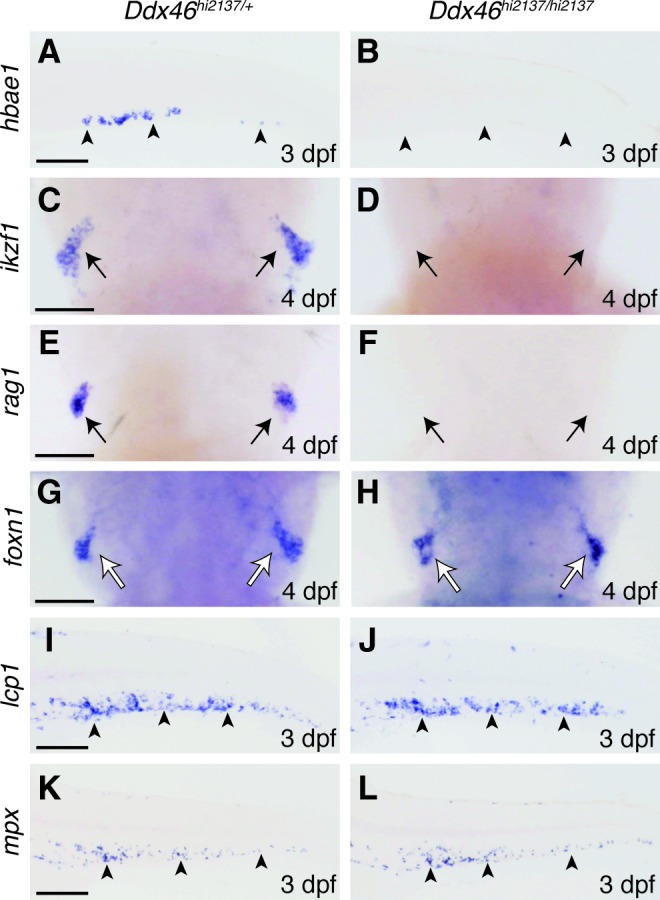
Myelopoiesis occurs, but erythropoiesis and lymphopoiesis are suppressed in Ddx46hi2137/hi2137 mutants. (A–L) The expression of molecular markers for erythrocytes, lymphocytes, myelocytes, and a thymus epithelium was examined by whole-mount in situ hybridization at 3 and 4 dpf. Lateral views, anterior to the left (A, B, I–L). Dorsal views, anterior to the top (C–H). The expression of a definitive erythroid marker hbae1 was markedly reduced in Ddx46hi2137/hi2137 larvae at 3 dpf (arrowheads in A, B) (Ddx46hi2137/hi2137 larvae, n=7/7; Ddx46hi2137/+ larvae, n=9/9). The expression of lymphoid markers, ikzf1 (Ddx46hi2137/hi2137 larvae, n=9/9; Ddx46hi2137/+ larvae, n=9/9) and rag1 (Ddx46hi2137/hi2137 larvae, n=9/9; Ddx46hi2137/+ larvae, n=7/7), was lost in Ddx46hi2137/hi2137 larvae (black arrows in C–F), whereas the expression of a thymus epithelial marker foxn1 was indistinguishable between Ddx46hi2137/+ (n=7/7) and Ddx46hi2137/hi2137 larvae (n=9/9) at 4 dpf (white arrows in G, H). In contrast, the expression of myeloid markers, lcp1 (Ddx46hi2137/hi2137 larvae, n=8/8; Ddx46hi2137/+ larvae, n=8/8) and mpx (Ddx46hi2137/hi2137 larvae, n=12/13; Ddx46hi2137/+ larvae, n=8/8), was maintained in Ddx46hi2137/hi2137 larvae at 3 dpf (arrowheads in I–L). Scale bars represent 100 μm. hbae1, hemoglobin alpha embryonic-1; ikzf1, IKAROS family zinc finger 1; rag1, recombination activating gene 1; foxn1, forkhead box N1; lcp1, lymphocyte cytosolic plastin 1. Color images available online at www.liebertpub.com/scd
Reduction of gata1a expression leads to erythropoiesis defects in Ddx46hi2137/hi2137 mutants
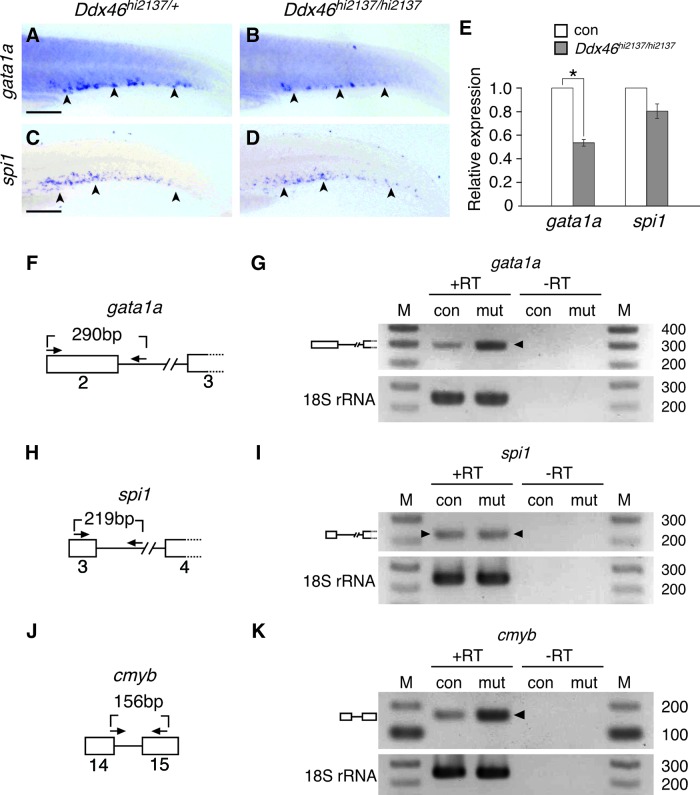
Because recent articles have reported that the cross-antagonistic interactions between Gata1a and Spi1 transcription factors are critical for deciding the differentiation to the erythroid or myeloid fate [2,4,5,38], we examined the expression of these genes in Ddx46hi2137/hi2137 mutants at 3 dpf (Fig. 8A–E). Analyses of in situ hybridization and qPCR revealed that although gata1a expression was significantly reduced, spi1 expression was maintained in the mutants (Fig. 8A–E). To test whether the deficiency of erythropoiesis was caused by the reduction of gata1a in Ddx46hi2137/hi2137 mutants, we carried out rescue experiments. Because phenotypic rescue of vlad tepes (vlt), a zebrafish gata1a mutant, was not achieved by overexpression of gata1a mRNA [39], we tried to use an efficient transient rescue method using the Tol2 transposable element [29]. We found that the expression of an erythroid marker, hbae1, was partially rescued by this Tol2-mediated transgenesis method at 3 dpf (Supplementary Fig. S5). These results suggest that the suppression of erythropoiesis in Ddx46hi2137/hi2137 mutants was due to the reduction of gata1a expression.
FIG. 8.
Expression and pre-mRNA splicing of gata1a, but not spi1, are defective in Ddx46hi2137/hi2137 mutants. (A–D) The expression of gata1a and spi1 was examined by whole-mount in situ hybridization at 3 dpf. All are lateral views, anterior to the left. The expression of gata1a in the CHT of Ddx46hi2137/hi2137 larvae (n=10/10) was markedly reduced compared with that of Ddx46hi2137/+ larvae (n=10/10) (arrowheads in A, B). In contrast, spi1 expression in the CHT of Ddx46hi2137/hi2137 larvae (n=10/10) was maintained compared with that of Ddx46hi2137/+ larvae (n=9/9) (arrowheads in C, D). Scale bars represent 100 μm. (E) Relative expression of gata1a and spi1 genes in control (con) larvae compared with that in Ddx46hi2137/hi2137 larvae at 3 dpf, by qPCR. Although no significant difference of spi1 expression was found between con and Ddx46hi2137/hi2137 larvae, gata1a expression in Ddx46hi2137/hi2137 larvae was significantly lower than that in con larvae. *P<0.01 by the Student's t-test. Error bars represent the standard error. (F–K) Schematic drawings of the gata1a, spi1, and cmyb pre-mRNA regions analyzed for splicing (boxes, exons; lines, introns; arrows, primers) (F, H, J). The splicing status of gata1a, spi1, or cmyb pre-mRNA was monitored by RT-PCR with the primers indicated in schemes (F), (H), or (J), respectively. The reverse primer for gata1a or spi1 mRNA was designed within the intron (F, H). The forward primer for cmyb crosses the exon14/intron14 boundary (J). Unspliced gata1a or cmyb mRNA was retained at a higher level in Ddx46hi2137/hi2137 mutant (mut) larvae than in con larvae (arrowhead in G=290 bp; arrowhead in K=156 bp). In contrast, the level of unspliced spi1 mRNA was indistinguishable between the mut larvae and con larvae (arrowheads in I=219 bp). Unspliced PCR products were verified by sequencing.+RT refers to the validation reaction itself, and −RT represents the respective control reaction without reverse transcriptase. 18S rRNA is a loading control. Control larvae were sibling WT or Ddx46hi2137/+ larvae, and they had normal phenotypes. qPCR, quantitative polymerase chain reaction; RT, reverse transcription. Color images available online at www.liebertpub.com/scd
Ddx46hi2137/hi2137 mutants have defects in pre-mRNA splicing in the hematopoietic cells
We have previously reported that pre-mRNA splicing of specific genes is defective in Ddx46hi2137/hi2137 mutants [20]; hence, we examined the pre-mRNA splicing of gata1a and spi1 by RT-PCR in this study. The RT-PCR analyses showed that although the unspliced mRNAs of gata1a were retained, the pre-mRNA splicing of spi1 was normal in Ddx46hi2137/hi2137 mutants at 3 dpf (Fig. 8F–I). It is possible that the defects in pre-mRNA splicing lead to the reduction of gata1a expression and suppression of erythropoiesis in Ddx46hi2137/hi2137 mutants. In addition to gata1a and spi1, we tested the pre-mRNA splicing of cmyb to examine the effect in HSCs. Similarly to gata1a, the unspliced mRNAs of cmyb were retained in Ddx46hi2137/hi2137 mutants at 3 dpf (Fig. 8J, K), suggesting that the defects in pre-mRNA splicing may affect the multilineage differentiation of HSCs.
Discussion
Status of HSCs in Ddx46hi2137/hi2137 mutants
Although the expression of molecular markers for HSCs, such as tal1, runx1, or cmyb, was markedly reduced and proliferation of the HSCs was normal at 48 hpf, massive cell death of HSCs was not detected in Ddx46hi2137/hi2137 mutants. There are several possibilities to explain the status of HSCs in Ddx46hi2137/hi2137 mutants. One possible explanation is that, although the differentiation of HSCs is restricted to myeloid fate at the beginning of definitive hematopoiesis (around 30 hpf), the ability of differentiation to myeloid fate is lost at later stages, probably due to the reduction of tal1, runx1, and cmyb expressions in Ddx46hi2137/hi2137 mutants at 3 dpf. Since previous studies have reported that Cmyb could regulate the expression of tal1 and runx1 in zebrafish and mouse HSCs, respectively [9,40], it is possible that splicing defects in cmyb result in the reduction of tal1 and runx1 expressions in HSCs of Ddx46hi2137/hi2137 mutants. Another possibility is that HSCs continue to produce myeloid cells without the expressions of tal1, runx1, and cmyb during definitive hematopoiesis in Ddx46hi2137/hi2137 mutants. Alternative possibility is that HSCs are lost, and they prematurely differentiate into myeloid cells. In both scenarios, the number of lcp1- or mpx-expressing myeloid cells should be increased in the mutants throughout definitive hematopoiesis. Since we have not analyzed the expression of molecular markers for myeloid cells at the beginning of definitive hematopoiesis, it is interesting to examine spi1 expression in Ddx46hi2137/hi2137 mutants at around 30 hpf. However, we showed that there was no striking difference in the number of myeloid cells between Ddx46hi2137/+ and Ddx46hi2137/hi2137 larvae at 3 dpf (Fig. 7). Therefore, it is possible that the proliferation of the HSCs is reduced after 2.5 dpf. Unfortunately, due to the downregulation of EGFP fluorescence at 3 dpf, it was very difficult to evaluate the proliferation of HSCs using the Tg(tal1:EGFP) line (Supplementary Fig. S3). In contrast to tal1, the number of cmyb-expressing cells is higher than that of tal1-expressing cells during definitive hematopoiesis; this finding could be related to the fact that mouse cmyb is expressed in HSCs and progenitor cells [5]. In addition, cmyb-expressing cells are still present in Ddx46hi2137/hi2137 mutants at 3 dpf (Fig. 4G). Therefore, it may be important to analyze the cell proliferation after 2.5 dpf by using the cmyb:EGFP transgenic line. Currently, the presence of HSCs in Ddx46hi2137/hi2137 mutants remains unknown because molecular markers of HSCs for maintenance and differentiation, except tal1, runx1, and cmyb, have not yet been reported in zebrafish. Further studies will therefore be necessary to identify the key target genes affected by the loss of Ddx46 function for the maintenance and differentiation of HSCs.
Role of pre-mRNA splicing factors in hematopoiesis
Because pre-mRNA splicing of gata1a and cmyb, but not spi1, is defective in Ddx46hi2137/hi2137 mutants (Fig. 8), it is possible that aberrant pre-mRNAs lead to reduced gata1a and cmyb expressions. Our results suggest that pre-mRNA splicing is associated with hematopoiesis in zebrafish. Recently, numerous studies using whole-exome sequencing revealed that recurrent mutations in spliceosome subunits have been implicated in hematopoietic malignancies [41–44]. The 4 genes encoding spliceosome components, U2 small nuclear RNA auxiliary factor 1 (U2AF1; also known as U2AF35), splicing factor 3B subunit 1 (SF3B1), U2AF1-related protein (ZRSR2; also known as Urp), and serine/arginine-rich splicing factor 2 (SRSF2), are frequently mutated in chronic lymphocytic leukemia (CLL) and/or myelodysplastic syndrome (MDS) [41–44]. It is well known that these 4 components are involved in the initial steps of pre-mRNA splicing for the establishment of spliceosome complexes E and A: U2AF1 and SRSF2 bind to the 3′ splice acceptor site of the pre-mRNA: ZRSR2 interacts with U2AF1 and a serine/arginine-rich SR protein: and SF3B1, which is a component of the U2 small nuclear ribonucleoprotein (U2snRNP), binds to the branch point sequence of the pre-mRNA [45,46]. These results suggest that the initial steps of pre-mRNA splicing are closely related to hematopoietic malignancies in mammals.
Yeast DExD/H-box proteins, Sub2, Prp5, Prp28, Brr2, Prp2, Prp16, Prp22, and Prp43, act in specific steps of the splicing cycles to catalyze RNA–RNA rearrangements and RNP remodeling [16,17]. Among these, it has been determined that Saccharomyces cerevisiae Prp5 (a yeast orthologue of vertebrate Ddx46) and human DDX46 are able to interact with U2snRNP [16,17,47]. These reports, combined with the splicing factor studies in hematopoietic malignancies, suggest that the 4 splicing components (U2AF1, SF3B1, ZRSR2, and SRSF2) and Ddx46 play critical roles in the initial steps of pre-mRNA splicing, and these factors may function in the maintenance and/or differentiation of HSCs. Although recurrent mutations in DDX46 have not yet been reported in patients with CLL and/or MDS by whole-exome sequencing, it is possible that mutations in DDX46 cause hematopoietic malignancies.
Control of hematopoiesis by DExD/H-box RNA helicases
A recent report has revealed that Dhx8, a zebrafish orthologue of the yeast splicing factor Prp22, is involved in pre-mRNA splicing and is required for primitive hematopoiesis [19]. In contrast to Prp5/Ddx46, the function of yeast Prp22 is critical for spliceosome disassembly when splicing reactions have been completed [16,17]. Although both Ddx46 and Dhx8 are maternal genes and are ubiquitously expressed during early somitogenesis, Dhx8 mutants, but not Ddx46hi2137/hi2137 mutants, showed defects in primitive hematopoiesis. One possible explanation for this phenotypic difference between Ddx46hi2137/hi2137 and Dhx8 mutants is that the function of Ddx46 is not necessary for primitive hematopoiesis and is specific for the control of HSC differentiation in zebrafish larvae. Alternatively, it is possible that because maternal transcripts of Ddx46 or maternally derived Ddx46 proteins are more stable than those of Dhx8, defects in primitive hematopoiesis are rescued in Ddx46hi2137/hi2137 mutants. Further studies will be needed to elucidate the detailed mechanisms that lead to hematopoiesis deficiencies and related diseases that are caused by DExD/H-box RNA helicases and/or splicing factors.
Supplementary Material
Acknowledgements
We thank the members of Kikuchi and Atsushi Suzuki laboratories in Hiroshima University for helpful discussion and critical comments. We also thank Drs. Atsuo Kawahara and Makoto Kobayashi for providing DNA templates. This work was supported by a grant from the Sasakawa Foundation to R.H., The Sasakawa Foundation and the Kao Foundation for Arts and Sciences to S.H., and grant-in-aid for Scientific Research from the JSPS (KAKENHI 15370094 and 19570204) to Y.K.
Author Disclosure Statement
There are no conflicts of interest in this article.
References
- 1.Cumano A. Godin I. Ontogeny of the hematopoietic system. Annu Rev Immunol. 2007;25:745–785. doi: 10.1146/annurev.immunol.25.022106.141538. [DOI] [PubMed] [Google Scholar]
- 2.Orkin SH. Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132:631–644. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Medvinsky A. Rybtsov S. Taoudi S. Embryonic origin of the adult hematopoietic system: advances and questions. Development. 2011;138:1017–1031. doi: 10.1242/dev.040998. [DOI] [PubMed] [Google Scholar]
- 4.Carradice D. Lieschke GJ. Zebrafish in hematology: sushi or science? Blood. 2008;111:3331–3342. doi: 10.1182/blood-2007-10-052761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paik EJ. Zon LI. Hematopoietic development in the zebrafish. Int J Dev Biol. 2010;54:1127–1137. doi: 10.1387/ijdb.093042ep. [DOI] [PubMed] [Google Scholar]
- 6.Mucenski ML. McLain K. Kier AB. Swerdlow SH. Schreiner CM. Miller TA. Pietryga DW. Scott WJ. Potter SS. A functional c-myb gene is required for normal murine fetal hepatic hematopoiesis. Cell. 1991;65:677–689. doi: 10.1016/0092-8674(91)90099-k. [DOI] [PubMed] [Google Scholar]
- 7.Sumner R. Crawford A. Mucenski M. Frampton J. Initiation of adult myelopoiesis can occur in the absence of c-Myb whereas subsequent development is strictly dependent on the transcription factor. Oncogene. 2000;19:3335–3342. doi: 10.1038/sj.onc.1203660. [DOI] [PubMed] [Google Scholar]
- 8.Lieu YK. Reddy EP. Conditional c-myb knockout in adult hematopoietic stem cells leads to loss of self-renewal due to impaired proliferation and accelerated differentiation. Proc Natl Acad Sci U S A. 2009;106:21689–21694. doi: 10.1073/pnas.0907623106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soza-Ried C. Hess I. Netuschil N. Schorpp M. Boehm T. Essential role of c-myb in definitive hematopoiesis is evolutionarily conserved. Proc Natl Acad Sci U S A. 2010;107:17304–17308. doi: 10.1073/pnas.1004640107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y. Jin H. Li L. Qin FX. Wen Z. cMyb regulates hematopoietic stem/progenitor cell mobilization during zebrafish hematopoiesis. Blood. 2011;118:4093–4101. doi: 10.1182/blood-2011-03-342501. [DOI] [PubMed] [Google Scholar]
- 11.North TE. de Bruijn MF. Stacy T. Talebian L. Lind E. Robin C. Binder M. Dzierzak E. Speck NA. Runx1 expression marks long-term repopulating hematopoietic stem cells in the midgestation mouse embryo. Immunity. 2002;16:661–672. doi: 10.1016/s1074-7613(02)00296-0. [DOI] [PubMed] [Google Scholar]
- 12.Burns CE. DeBlasio T. Zhou Y. Zhang J. Zon L. Nimer SD. Isolation and characterization of runxa and runxb, zebrafish members of the runt family of transcriptional regulators. Exp Hematol. 2002;30:1381–1389. doi: 10.1016/s0301-472x(02)00955-4. [DOI] [PubMed] [Google Scholar]
- 13.Chen MJ. Yokomizo T. Zeigler BM. Dzierzak E. Speck NA. Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature. 2009;457:887–891. doi: 10.1038/nature07619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sood R. English MA. Belele CL. Jin H. Bishop K. Haskins R. McKinney MC. Chahal J. Weinstein BM. Wen Z. Liu PP. Development of multilineage adult hematopoiesis in the zebrafish with a runx1 truncation mutation. Blood. 2010;115:2806–2809. doi: 10.1182/blood-2009-08-236729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lancrin C. Sroczynska P. Stephenson C. Allen T. Kouskoff V. Lacaud G. The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature. 2009;457:892–895. doi: 10.1038/nature07679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rocak S. Linder P. DEAD-box proteins: the driving forces behind RNA metabolism. Nat Rev Mol Cell Biol. 2004;5:232–241. doi: 10.1038/nrm1335. [DOI] [PubMed] [Google Scholar]
- 17.Bleichert F. Baserga S. The long unwinding road of RNA helicases. Mol Cell. 2007;27:339–352. doi: 10.1016/j.molcel.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 18.Payne EM. Bolli N. Rhodes J. Abdel-Wahab OI. Levine R. Hedvat CV. Stone R. Khanna-Gupta A. Sun H, et al. Ddx18 is essential for cell-cycle progression in zebrafish hematopoietic cells and is mutated in human AML. Blood. 2011;118:903–915. doi: 10.1182/blood-2010-11-318022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.English MA. Lei L. Blake T. Wincovitch SM. Sood R. Azuma M. Hickstein D. Paul Liu P. Incomplete splicing, cell division defects and hematopoietic blockage in dhx8 mutant zebrafish. Dev Dyn. 2012;241:879–889. doi: 10.1002/dvdy.23774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hozumi S. Hirabayashi R. Yoshizawa A. Ogata M. Ishitani T. Tsutsumi M. Kuroiwa A. Itoh M. Kikuchi Y. DEAD-box protein Ddx46 is required for the development of the digestive organs and brain in zebrafish. PLoS One. 2012;7:e33675. doi: 10.1371/journal.pone.0033675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Westerfield M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio) 4th. University of Oregon Press; Eugene, OR: 2000. [Google Scholar]
- 22.Kimmel CB. Ballard WW. Kimmel SR. Ullmann B. Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 23.Amsterdam A. Nissen R. Sun Z. Swindell E. Farrington S. Hopkins N. Identification of 315 genes essential for early zebrafish development. Proc Natl Acad Sci U S A. 2004;101:12792–12797. doi: 10.1073/pnas.0403929101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gering M. Rodaway AR. Göttgens B. Patient RK. Green AR. The SCL gene specifies haemangioblast development from early mesoderm. EMBO J. 1998;17:4029–4045. doi: 10.1093/emboj/17.14.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang XY. Rodaway AR. SCL-GFP transgenic zebrafish: in vivo imaging of blood and endothelial development and identification of the initial site of definitive hematopoiesis. Dev Biol. 2007;307:179–194. doi: 10.1016/j.ydbio.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Urasaki A. Morvan G. Kawakami K. Functional dissection of the Tol2 transposable element identified the minimal cis-sequence and a highly repetitive sequence in the subterminal region essential for transposition. Genetics. 2006;174:639–649. doi: 10.1534/genetics.106.060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mizoguchi T. Verkade H. Heath JK. Kuroiwa A. Kikuchi Y. Sdf1/Cxcr4 signaling controls the dorsal migration of endodermal cells during zebrafish gastrulation. Development. 2008;135:2521–2529. doi: 10.1242/dev.020107. [DOI] [PubMed] [Google Scholar]
- 28.Detrich HW. Kieran MW. Chan FY. Barone LM. Yee K. Rundstadler JA. Pratt S. Ransom D. Zon LI. Intraembryonic hematopoietic cell migration during vertebrate development. Proc Natl Acad Sci U S A. 1995;92:10713–10717. doi: 10.1073/pnas.92.23.10713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takeuchi M. Kaneko H. Nishikawa K. Kawakami K. Yamamoto M. Kobayashi M. Efficient transient rescue of hematopoietic mutant phenotypes in zebrafish using Tol2-mediated transgenesis. Dev Growth Differ. 2010;52:245–250. doi: 10.1111/j.1440-169X.2009.01168.x. [DOI] [PubMed] [Google Scholar]
- 30.Lieschke GJ. Oates AC. Paw BH. Thompson MA. Hall NE. Ward AC. Ho RK. Zon LI. Layton JE. Zebrafish SPI-1 (PU.1) marks a site of myeloid development independent of primitive erythropoiesis: implications for axial patterning. Dev Biol. 2002;246:274–295. doi: 10.1006/dbio.2002.0657. [DOI] [PubMed] [Google Scholar]
- 31.Thompson MA. Ransom DG. Pratt SJ. MacLennan H. Kieran MW. Detrich HW. Vail B. Huber TL. Paw B, et al. The cloche and spadetail genes differentially affect hematopoiesis and vasculogenesis. Dev Biol. 1998;197:248–269. doi: 10.1006/dbio.1998.8887. [DOI] [PubMed] [Google Scholar]
- 32.Brownlie A. Hersey C. Oates AC. Paw BH. Falick AM. Witkowska HE. Flint J. Higgs D. Jessen J, et al. Characterization of embryonic globin genes of the zebrafish. Dev Biol. 2003;255:48–61. doi: 10.1016/s0012-1606(02)00041-6. [DOI] [PubMed] [Google Scholar]
- 33.Bennett CM. Kanki JP. Rhodes J. Liu TX. Paw BH. Kieran MW. Langenau DM. Delahaye-Brown A. Zon LI. Fleming MD. Look AT. Myelopoiesis in the zebrafish, Danio rerio. Blood. 2001;98:643–651. doi: 10.1182/blood.v98.3.643. [DOI] [PubMed] [Google Scholar]
- 34.Willett CE. Kawasaki H. Amemiya CT. Lin S. Steiner LA. Ikaros expression as a marker for lymphoid progenitors during zebrafish development. Dev Dyn. 2001;222:694–698. doi: 10.1002/dvdy.1223. [DOI] [PubMed] [Google Scholar]
- 35.Willett CE. Zapata AG. Hopkins N. Steiner LA. Expression of zebrafish rag genes during early development identifies the thymus. Dev Biol. 1997;182:331–341. doi: 10.1006/dbio.1996.8446. [DOI] [PubMed] [Google Scholar]
- 36.Schorpp M. Leicht M. Nold E. Hammerschmidt M. Haas-Assenbaum A. Wiest W. Boehm T. A zebrafish orthologue (whnb) of the mouse nude gene is expressed in the epithelial compartment of the embryonic thymic rudiment. Mech Dev. 2002;118:179–185. doi: 10.1016/s0925-4773(02)00241-1. [DOI] [PubMed] [Google Scholar]
- 37.Herbomel P. Thisse B. Thisse C. Ontogeny and behaviour of early macrophages in the zebrafish embryo. Development. 1999;126:3735–3745. doi: 10.1242/dev.126.17.3735. [DOI] [PubMed] [Google Scholar]
- 38.Monteiro R. Pouget C. Patient R. The gata1/pu.1 lineage fate paradigm varies between blood populations and is modulated by tif1γ. EMBO J. 2011;30:1093–1103. doi: 10.1038/emboj.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lyons SE. Lawson ND. Lei L. Bennett PE. Weinstein BM. Liu PP. A nonsense mutation in zebrafish gata1 causes the bloodless phenotype in vlad tepes. Proc Natl Acad Sci U S A. 2002;99:5454–5459. doi: 10.1073/pnas.082695299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dai G. Sakamoto H. Shimoda Y. Fujimoto T. Nishikawa S. Ogawa M. Over-expression of c-Myb increases the frequency of hemogenic precursors in the endothelial cell population. Genes Cells. 2006;11:859–870. doi: 10.1111/j.1365-2443.2006.00985.x. [DOI] [PubMed] [Google Scholar]
- 41.Yoshida K. Sanada M. Shiraishi Y. Nowak D. Nagata Y. Yamamoto R. Sato Y. Sato-Otsubo A. Kon A, et al. Frequent pathway mutations of splicing machinery in myelodysplasia. Nature. 2011;478:64–69. doi: 10.1038/nature10496. [DOI] [PubMed] [Google Scholar]
- 42.Hahn CN. Scott HS. Spliceosome mutations in hematopoietic malignancies. Nat Genet. 2012;44:9–10. doi: 10.1038/ng.1045. [DOI] [PubMed] [Google Scholar]
- 43.Quesada V. Conde L. Villamor N. Ordóñez GR. Jares P. Bassaganyas L. Ramsay AJ. Beà S. Pinyol M, et al. Exome sequencing identifies recurrent mutations of the splicing factor SF3B1 gene in chronic lymphocytic leukemia. Nat Genet. 2012;44:47–52. doi: 10.1038/ng.1032. [DOI] [PubMed] [Google Scholar]
- 44.Graubert TA. Shen D. Ding L. Okeyo-Owuor T. Lunn CL. Shao J. Krysiak K. Harris CC. Koboldt DC, et al. Recurrent mutations in the U2AF1 splicing factor in myelodysplastic syndromes. Nat Genet. 2012;44:53–57. doi: 10.1038/ng.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tronchère H. Wang J. Fu XD. A protein related to splicing factor U2AF35 that interacts with U2AF65 and SR proteins in splicing of pre-mRNA. Nature. 1997;388:397–400. doi: 10.1038/41137. [DOI] [PubMed] [Google Scholar]
- 46.Wahl MC. Will CL. Lührmann R. The spliceosome: design principles of a dynamic RNP machine. Cell. 2009;136:701–718. doi: 10.1016/j.cell.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 47.Xu YZ. Newnham CM. Kameoka S. Huang T. Konarska MM. Query CC. Prp5 bridges U1 and U2 snRNPs and enables stable U2 snRNP association with intron RNA. EMBO J. 2004;23:376–385. doi: 10.1038/sj.emboj.7600050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.