Abstract
Single-stranded adenovirus-associated virus type 2 deoxyribonucleic acid (AAV-2 DNA) has been isolated from the virion after enzymatic pretreatment of the particles by heating at 53 C for 1 hr in 0.015 m NaCl plus 0.0015 m sodium citrate in the presence of 1% sodium dodecyl sulfate. Double-stranded AAV-2 DNA present as a marker is not denatured by this treatment. AAV-2 single-stranded DNA is composed of two complementary species which can be separated in neutral CsCl when 5-bromodeoxyuridine has been substituted for thymidine in the DNA. The present report is the first documented instance of the separation of complementary strands of an animal virus DNA.
Full text
PDF
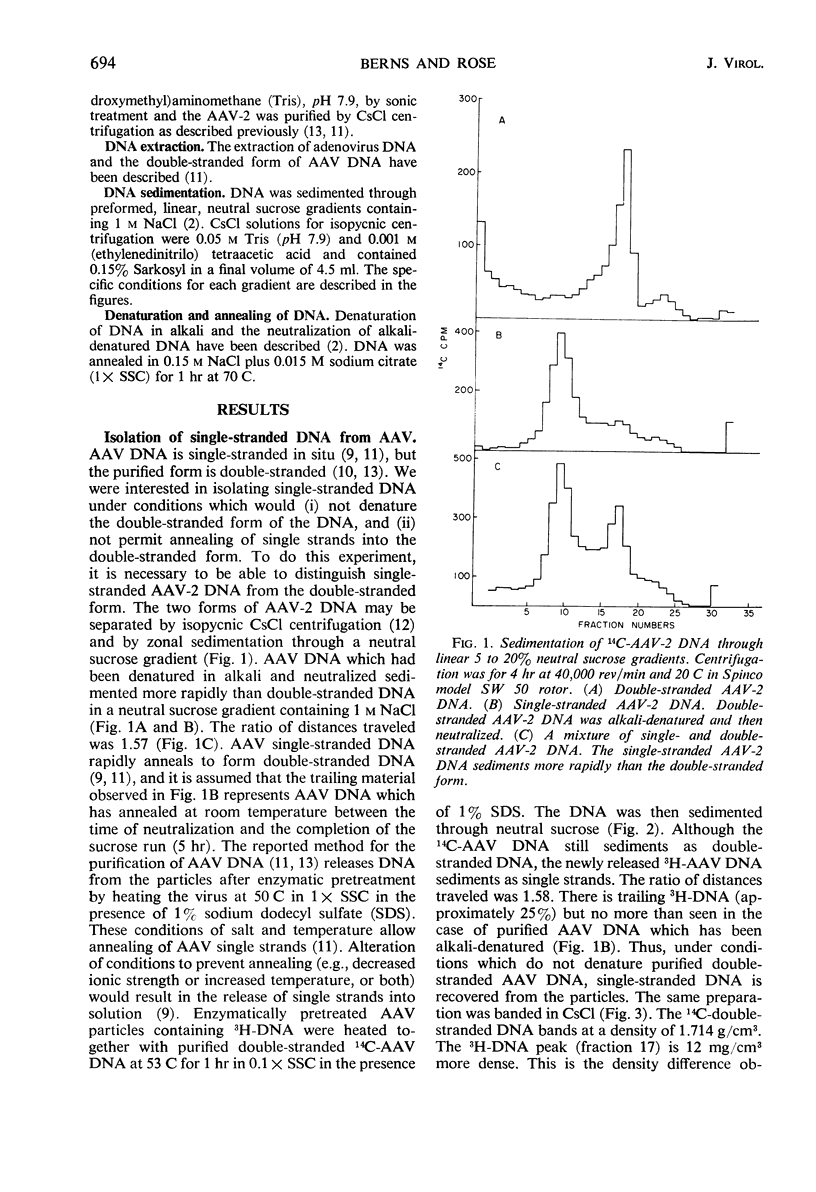
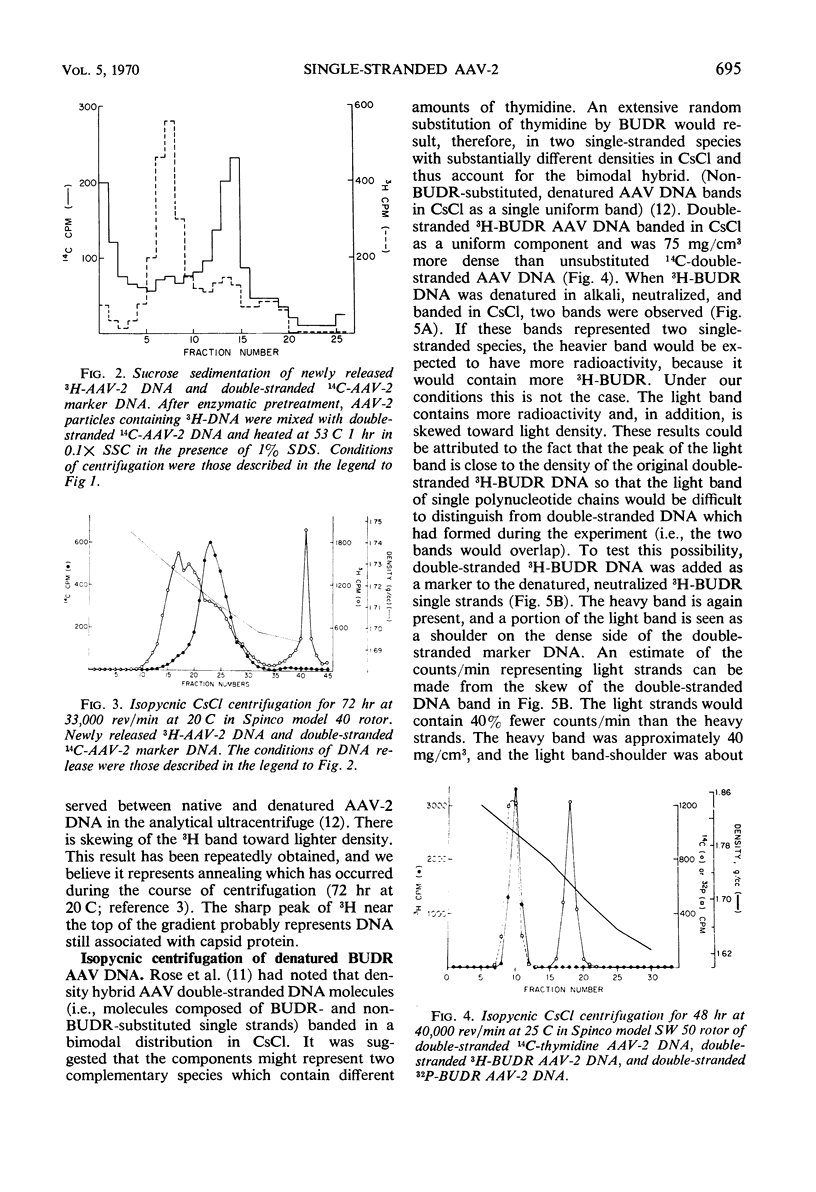
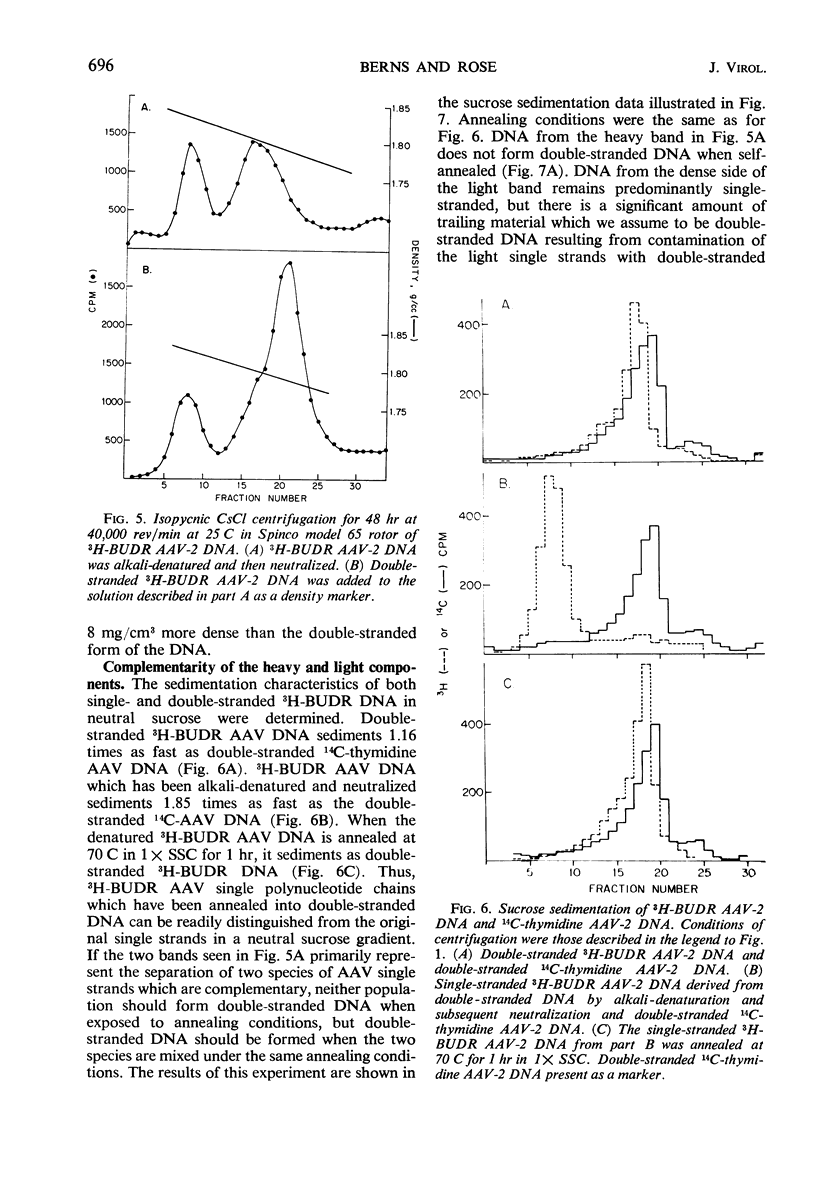
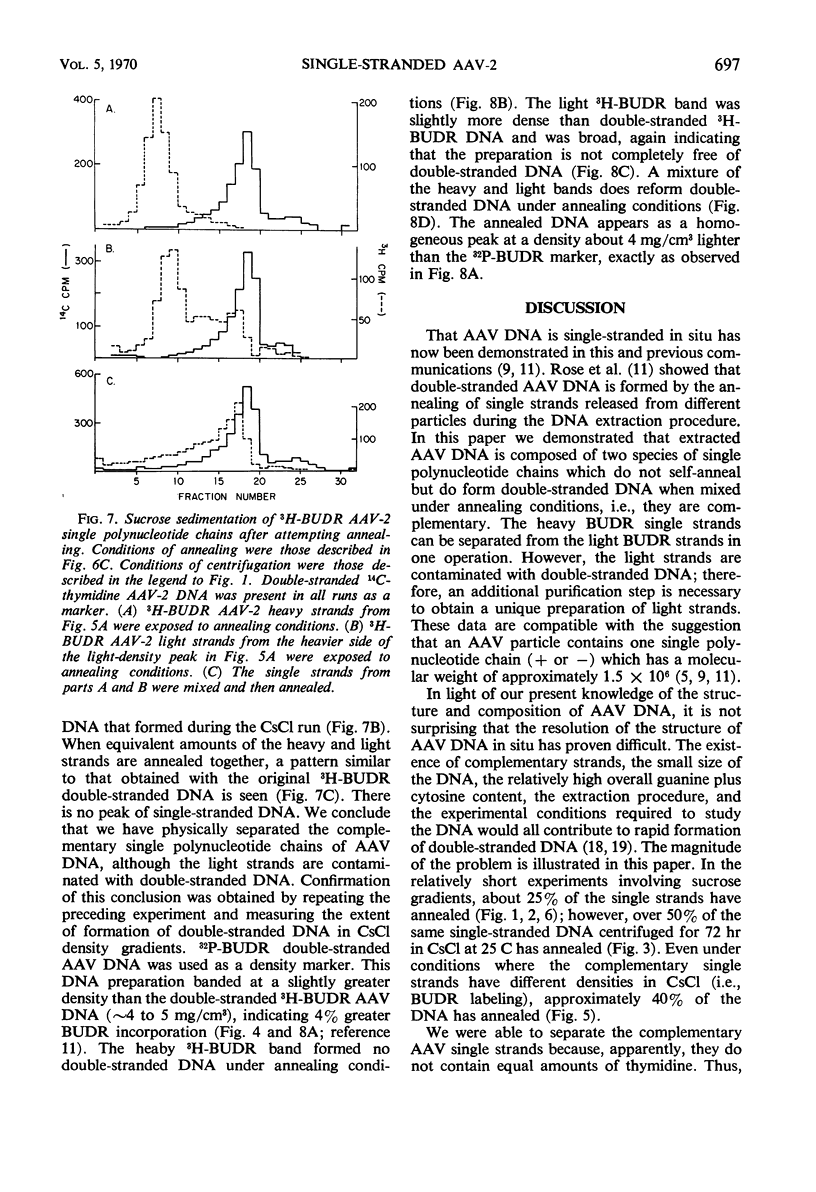

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATCHISON R. W., CASTO B. C., HAMMON W. M. ADENOVIRUS-ASSOCIATED DEFECTIVE VIRUS PARTICLES. Science. 1965 Aug 13;149(3685):754–756. doi: 10.1126/science.149.3685.754. [DOI] [PubMed] [Google Scholar]
- BURGI E., HERSHEY A. D. Sedimentation rate as a measure of molecular weight of DNA. Biophys J. 1963 Jul;3:309–321. doi: 10.1016/s0006-3495(63)86823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns K. I., Silverman C. Natural occurrence of cross-linked vaccinia virus deoxyribonucleic acid. J Virol. 1970 Mar;5(3):299–304. doi: 10.1128/jvi.5.3.299-304.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford L. V., Follett E. A., Burdon M. G., McGeoch D. J. The DNA of a minute virus of mice. J Gen Virol. 1969 Jan;4(1):37–46. doi: 10.1099/0022-1317-4-1-37. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Hoggan M. D., Blacklow N. R., Rowe W. P. Studies of small DNA viruses found in various adenovirus preparations: physical, biological, and immunological characteristics. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1467–1474. doi: 10.1073/pnas.55.6.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoggan M. D., Shatkin A. J., Blacklow N. R., Koczot F., Rose J. A. Helper-dependent infectious deoxyribonucleic acid from adenovirus-associated virus. J Virol. 1968 Aug;2(8):850–851. doi: 10.1128/jvi.2.8.850-851.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor H. D., Torikai K., Melnick J. L., Mandel M. Plus and minus single-stranded DNA separately encapsidated in adeno-associated satellite virions. Science. 1969 Dec 5;166(3910):1280–1282. doi: 10.1126/science.166.3910.1280. [DOI] [PubMed] [Google Scholar]
- Parks W. P., Green M., Piña M., Melnick J. L. Physicochemical characterization of adeno-associated satellite virus type 4 and its nucleic acid. J Virol. 1967 Oct;1(5):980–987. doi: 10.1128/jvi.1.5.980-987.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. A., Berns K. I., Hoggan M. D., Koczot F. J. Evidence for a single-stranded adenovirus-associated virus genome: formation of a DNA density hybrid on release of viral DNA. Proc Natl Acad Sci U S A. 1969 Nov;64(3):863–869. doi: 10.1073/pnas.64.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. A., Hoggan M. D., Koczot F., Shatkin A. J. Genetic relatedness studies with adenovirus-associated viruses. J Virol. 1968 Oct;2(10):999–1005. doi: 10.1128/jvi.2.10.999-1005.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. A., Hoggan M. D., Shatkin A. J. Nucleic acid from an adeno-associated virus: chemical and physical studies. Proc Natl Acad Sci U S A. 1966 Jul;56(1):86–92. doi: 10.1073/pnas.56.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINSHEIMER R. L., STARMAN B., NAGLER C., GUTHRIE S. The process of infection with bacteriophage phi-XI74. I. Evidence for a "replicative form". J Mol Biol. 1962 Mar;4:142–160. doi: 10.1016/s0022-2836(62)80047-3. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Schildkraut C. L., Maio J. J. Fractions of HeLa DNA differing in their content of guanine+cytosine. J Mol Biol. 1969 Dec 14;46(2):305–312. doi: 10.1016/0022-2836(69)90423-9. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Effects of the conformation of single-stranded DNA on renaturation and aggregation. J Mol Biol. 1969 Apr;41(2):199–209. doi: 10.1016/0022-2836(69)90385-4. [DOI] [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]


