Abstract
Chronic use of addictive drugs produces enduring neuroadaptations in the corticostriatal glutamatergic brain circuitry. The nucleus accumbens (NAc), which integrates cortical information and regulates goal-directed behavior, undergoes long-term morphological and electrophysiological changes that may underlie the increased susceptibility for relapse in drug-experienced individuals even after long periods of withdrawal. Additionally, it has recently been shown that exposure to cues associated with drug use elicits rapid and transient morphological and electrophysiological changes in glutamatergic synapses in the NAc. This review highlights these dynamic drug-induced changes in this pathway that are specific to a drug seeking neuropathology, as well as how these changes impair normal information processing and thereby contribute to the uncontrollable motivation to relapse. Future directions for relapse prevention and pharmacotherapeutic targeting of the rapid, transient synaptic plasticity in relapse are discussed.
1.1 Introduction
Drug addiction is a leading cause of poor health and has enormous societal impact (Volkow et al., 2011). When investigating the neural mechanisms underlying various phenomena associated with drug addiction, glutamatergic input into the nucleus accumbens (NAc) emerges as a major regulator of addictive behavior. Long term changes in basal extracellular levels of glutamate (Baker et al., 2003; Peters et al., 2009b; Wydra et al., 2013) and synaptic strength at glutamatergic synapses in the NAc (Boudreau and Wolf, 2005; Conrad et al., 2008; Gipson et al., 2013; Kourrich et al., 2007; Martin et al., 2006; Moussawi et al., 2009) are induced by chronic drug use. Although these slow and persistent changes may render the individual more vulnerable to relapse, they are not the mechanism triggering the relapse event.
A relapse event is frequently triggered by environmental cues associated with drug use, which rapidly initiate an urge to use drugs. This rapid change in behavior is driven by rapid, transient increases in synaptic strength in glutamatergic synapses between prefrontal cortex (PFC) afferents and medium spiny neurons (MSNs) of the NAc. Moreover, while the rapid, transient relapse-associated changes in excitatory synapses on MSNs are shared between classes of addictive drugs including nicotine, cocaine and heroin (Gipson et al., 2012; Gipson et al., 2013; Shen et al., 2011), different classes of addictive drugs produce opposite long-term effects on excitatory transmission in the NAc. Specifically, repeated psychostimulant administration brings about an increase (Conrad et al., 2008; Gipson et al., 2012; Gipson et al., 2013; Kourrich et al., 2007), and opioids cause a decrease in synaptic strength in the NAc as measured electrophysiologically and by dendritic spine morphology (Robinson and Kolb, 1999a; Shen et al., 2011; Spiga et al., 2005; but see Wu et al., 2012 who showed synaptic potentiation in the nucleus accumbens shell (NAshell) after withdrawal from morphine). It should be noted, however, that functional relevance of structural changes in spines remains difficult to interpret, as is discussed below in section 3.1.1.
This review will focus on the glutamatergic input to the NAc and its involvement in drug relapse. We will discuss the sources of glutamatergic afferents to the NAc, as well as synaptic changes in glutamatergic input to the NAc. Special emphasis will be given to the newly discovered rapid, transient synaptic plasticity that underlies the initiation of relapse to drug seeking. Finally, we will discuss potential relevance to relapse prevention and pharmacotherapy development.
2.1 Glutamatergic Projections to the Nucleus Accumbens involved in Addiction and Relapse
Glutamate neurotransmission in the NAc has been shown to underlie drug-seeking behavior, and changes in NAc glutamatergic transmission are thought to encode the transition from occasional use of drugs to the pathological inability to control drug-seeking behavior (Kalivas and Volkow, 2011; Kasanetz et al., 2010; Peters et al., 2009b; Wolf and Ferrario, 2010). Neurons in the NAc receive convergent glutamatergic afferents from different cortical and subcortical regions (Figure 1), including innervation by projections from the prefrontal cortex (PFC) (Berendse et al., 1992; Fuller et al., 1987; Gorelova and Yang, 1997; Papp et al., 2012; Reynolds and Zahm, 2005; Stefanik et al., 2012), the basolateral amygdala (BLA) (Groenewegen et al., 1980; McDonald, 1991a, b; Papp et al., 2012; Stuber et al., 2011), the ventral hippocampus (vHipp) (Britt et al., 2012; DeFrance et al., 1985; Groenewegen et al., 1987; Papp et al., 2012; Thompson and Swanson, 2010), the midline/intralaminar thalamic nuclei (Berendse and Groenewegen, 1990; Kelley and Stinus, 1984; Smith et al., 2004; Vertes et al., 2012), and the recently described glutamatergic neurons in the ventral tegmental area (VTA) (Gorelova et al., 2012; Hnasko et al., 2012; Yamaguchi et al., 2007; Yamaguchi et al., 2011). Interestingly, the different inputs are topographically arranged, such that each projection innervates different regions in the NAc, rather than diffusely innervating the entire NAc (Voorn et al., 2004). For example, afferents from the prelimbic and infralimbic subcompartments of the medial PFC are mostly segregated and project mainly to the NAcore and NAshell, respectively (Groenewegen et al., 1999; Wright and Groenewegen, 1995; Zahm, 2000); the projections from the BLA are compartmentally organized and more densely innervate the NAshell than the NAcore (Papp et al., 2012; Wright et al., 1996); the vHipp projections are most concentrated in the medial NAshell (Britt et al., 2012); and the paraventricular nucleus of the thalamus projects mainly to the NAshell (Papp et al., 2012; Smith et al., 2004). Microstructural studies show that all of the above projections synapse on spine heads of the GABAergic MSNs (Kita and Kitai, 1990; Meredith et al., 1990; Papp et al., 2012; Sesack and Grace, 2010). It should be noted that other studies also showed that in spite of the topographical segregation between different inputs in the NAc, individual MSNs can be innervated by projections from two or more different regions (Britt et al., 2012; French and Totterdell, 2002, 2003; Sesack and Grace, 2010; Stuber et al., 2011), thus implying that the MSNs have a role in integrating glutamatergic information from multiple sources.
Figure 1. Glutamatergic afferents to the nucleus accumbens involved in addictive behavior.
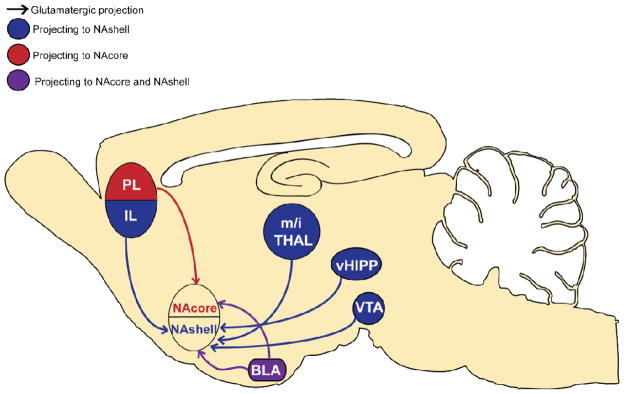
While both nucleus accumbens subregions (NAcore and NAshell) receive input from all cortical regions, there is strong topographic bias. The NAcore receives glutamatergic input mainly from the prelimbic cortex (PL) and the basolateral amygdala (BLA), whereas the NAshell receives strong glutamatergic input from a larger number of sources, including the infralimbic cortex (IL), the ventral hippocampus (vHIPP), glutamatergic neurons in the ventral tegmental area (VTA), the midline/intralaminar thalamus nuclei (m/i THAL), and the BLA.
While most research we describe below focuses on the projection from the PFC to NAc, other glutamatergic inputs are also implicated to a greater or lesser extent in the modulation of drug-seeking behavior. For example, the BLA integrates information regarding conditioned associations and affective drive (Sesack and Grace, 2010). Thus, activating the BLA and its projection to the NAc induces self-stimulation behavior (Stuber et al., 2011), while inhibiting this projection impairs drug-seeking induced by conditioned cues (Di Ciano and Everitt, 2004; Fuchs et al., 2007; McLaughlin and See, 2003; See et al., 2003). Similarly, activation of ventral hippocampal afferents to the NAshell promotes addiction-like behavior (Grace et al., 2007; Vorel et al., 2001), while inhibiting them attenuates drug-induced locomotion (Britt et al., 2012; Lodge and Grace, 2008).
A major glutamatergic input to the NAc comes from the PFC. This cortical region regulates goal-directed behaviors by integrating information from numerous brain regions and “making a decision” to execute an adaptive behavioral response (Balleine and O’Doherty, 2010; Killcross and Coutureau, 2003; Sharpe and Killcross, 2012; Smith et al., 2012). The medial portion of the PFC, which sends extensive projections to the NAc, is divided to dorsal prelimbic (PL), and ventral infralimbic (IL) regions. Although not completely segregated, the IL projects to the NAshell and has been associated with extinction of drug seeking, and the PL projects to the NAcore and is implicated in the execution of drug seeking (Capriles et al., 2003; Kalivas et al., 2005; LaLumiere et al., 2012; McFarland et al., 2004; McFarland and Kalivas, 2001; McLaughlin and See, 2003; Millan et al., 2011; Peters et al., 2009b; Peters et al., 2008; Rocha and Kalivas, 2010; Stefanik et al., 2012; Van den Oever et al., 2008). Thus, inactivation of the PL prevents reinstated drug seeking in various animal models, while inactivation of the IL increases cocaine seeking. Interestingly, it has been shown that without a period of extinction training, inactivation of the IL can inhibit drug-seeking behavior (Koya et al., 2009). The IL-NAshell pathway appears to be involved in the learning of extinction rather than simply in the suppression of drug seeking behavior (LaLumiere et al., 2010); its activation reduces lever pressing by strengthening the extinction behavior (LaLumiere et al., 2012) when such behavior was learned. Thus, it is important to emphasize differences in various animal models when integrating mechanisms underlying relapse vulnerability.
Opposite roles for the PL and the IL, such as controlling drug seeking, have been reported for fear expression and extinction (Peters et al., 2009a) as well as cue-induced cocaine seeking (McLaughlin and See, 2003). In fear conditioning experiments, the PL promoted fear behavior and its inhibition reduced expression of fear to contextual stimuli (Corcoran and Quirk, 2007; Peters et al., 2009a; Sotres-Bayon and Quirk, 2010). The IL, on the other hand, is involved in fear extinction, and its inhibition decreases fear expression (Corcoran and Quirk, 2007; Peters et al., 2009a; Sotres-Bayon and Quirk, 2010). The anatomically segregated pathways of the PL-to-NAcore and IL-to-NAshell projections, together with the results showing opposite involvement of PL and IL in the control of drug-seeking behavior, has led to a more simplified hypothesis of two parallel pathways in the control of drug-seeking behavior – the PL-to-NAcore pathway which promotes drug-seeking behavior and the IL-to-NAshell pathway which is responsible for the extinction of drug-seeking behavior (Peters et al., 2009b). It should be noted, however, that studies examining the role of IL inactivation on reinstated drug seeking show inconsistent results, depending on the regimen used (Willcocks and McNally, 2013). Thus, IL inactivation has been shown to increase, decrease, or have no effect on reinstated drug seeking when using different behavioral paradigms, such as contextual renewal (Bossert et al., 2012; Willcocks and McNally, 2013), reinstatement after extinction (Peters et al., 2008), and cue-induced reinstatement after extinction from cocaine or methamphetamine self-administration (McLaughlin and See, 2003; Rocha and Kalivas, 2010).
The glutamatergic input from the PL to the NAcore is of particular interest when studying the mechanisms underlying the reinstatement of drug-seeking. The PL has been shown to be important for action-outcome contingencies and adjusting goal-directed behavior in response to changes in the environment or task conditions (Dalley et al., 2004; Sharpe and Killcross, 2012; Smith et al., 2012), such as those used in animal models of cue-induced reinstatement of drug seeking. Thus, exposure to a drug-associated cue after extinction training, a manipulation known to induce PL-dependent reinstatement of drug-seeking behavior, is likely to involve changes in the PL-NAcore projections. In the next sections we review the alterations in glutamatergic neurotransmission in the NAcore that occur during various phases of the addiction cycle, with a focus on our recent findings showing rapid cue-induced changes induced by the presentation of a drug-associated cue to reinstate drug-seeking.
3.1 Enduring Drug-Induced Synaptic Plasticity: Alterations in Nucleus Accumbens Morphology and Synaptic Currents
Addictive drugs exert their reinforcing properties by targeting the mesocorticolimbic dopamine pathway, which includes the cell bodies in the VTA and axon terminals in the NAc, BLA and PFC. Although different drugs of abuse have specific targets (e.g., nicotine is an agonist for nicotinic acetylcholine receptors, cocaine is an inhibitor of the dopamine transporter, and heroin is an agonist at mu opioid receptors), they share a common ability to increase dopamine concentrations in efferents from the VTA to NAc, as well as within the VTA itself (Corrigall et al., 1994; Di Chiara and Imperato, 1988; Nestler, 2005). Addiction has been associated with altered neuroplasticity in this mesocorticolimbic brain circuitry, which is important in guiding goal-directed behavior (Everitt and Robbins, 2005; Peters et al., 2009b). Within a simplified PFC-NAc-VTA circuit, the NAc serves as a “gateway” through which information regarding the direction of behavioral output is processed from limbic cortex to motor subcircuits (Groenewegen et al., 1996; Yin and Knowlton, 2006). It is thought that the transition to compulsive drug seeking arises from an impaired ability of this subcircuit to effectively process information about negative environmental contingencies, leading to an inability to inhibit prepotent drug-associated responses; thereby the addict is rendered prone to relapse.
Due to its critical importance in governing behavior, it is important to examine the cellular changes induced by drugs of abuse within this circuit. Opposite changes in excitatory synaptic transmission have been found at early versus late withdrawal as well as with re-exposure to cocaine (Grueter et al., 2012). One surrogate measure commonly used to examine excitatory synaptic transmission is the ratio of alpha-amino-3-hydroxy-5-methyl-4- isoxazole propionic acid (AMPA) receptor- to N-methyl-d-aspartate (NMDA) receptor-mediated excitatory postsynaptic currents (EPSCs). At 24 hr after repeated cocaine injections, depression of synaptic transmission, quantified as a decrease in AMPA:NMDA ratio, was found in the NAc (Kourrich et al., 2007; Thomas et al., 2001). A current interpretation of this decrease is an increase in NMDA receptor-mediated currents typical in silent synapses, the number of which is increased in the NAcore after repeated cocaine treatment (Huang et al., 2009; Wolf and Ferrario, 2010). In contrast to the first 24 hr after discontinuing cocaine administration, excitatory synapses in NAc MSNs are potentiated following a protracted withdrawal phase (Boudreau and Wolf, 2005; Kourrich et al., 2007; Ortinski et al., 2012); consistent with an increase in dendritic spine head diameter (dh) and spine density of NAc MSNs after three weeks withdrawal from daily cocaine injection (Shen et al., 2009) or an increase in density after one month withdrawal from cocaine self-administration (Robinson et al., 2001). Dendritic spines are highly plastic (Nimchinsky et al., 2002), and changes in their morphology are a structural correlate of alterations in synaptic strength (De Roo et al., 2008; Dietz et al., 2012; Dumitriu et al., 2012; Kasai et al., 2010). Indeed, parallel drug-induced increases in both spine dh as well as AMPA:NMDA ratio have been observed in NAc following two weeks of withdrawal from cocaine self-administration (Gipson et al., 2013). It is hypothesized that the increased dh results from increased actin cycling and trafficking of AMPA receptors to the cell surface (Dietz et al., 2012; Kopec et al., 2006; Shen et al., 2009), leading to an increase in synaptic strength. Based on these data with chronic cocaine, it is tempting to posit that increased synaptic strength in NAc MSNs is an important mediator of relapse. However, extended withdrawal from heroin or morphine elicits an opposite, enduring reduction in dh and AMPA:NMDA ratio (Robinson and Kolb, 1999b; Shen et al., 2011). Thus, the enduring increase or decrease in synaptic strength observed after withdrawal from addictive drugs is not a consistent neuroadaptation between addictive drugs and may therefore not mediate the shared characteristic of relapse vulnerability.
3.1.1 Functional Relevance of Changes in Spine Morphology
A question remains whether the changes in the spine’s morphology per se are of functional importance or even a good marker for changes in synaptic strength. It is widely accepted that behavior is driven by neuronal activity determined by the sum of electrical signals generated by ionotropic receptors embedded in the postsynaptic membrane of the spine. In the frame of this notion the increase in spine head size might be a side effect of recruitment of AMPA receptors to the spine, a result of addition of membrane to the spine when AMPA-containing vesicles fuse with the spine membrane (Kopec and Malinow, 2006; Park et al., 2004; Park et al., 2006). Indeed, theoretical studies have shown that changes in spine morphology, while important for chemical compartmentalization within the spine, have a negligible effect on the size of the electrical signal in the soma (Gulledge et al., 2012; Jaslove, 1992; Kawato and Tsukahara, 1984; Koch and Zador, 1993; Segev et al., 1995; Wilson, 1984). However, recent studies reveal a more complicated scenario, reflecting a bidirectional interaction between structure (i.e spine morphology) and function (i.e. glutamate currents) in the spine (Hotulainen and Hoogenraad, 2010; Nimchinsky et al., 2002). In particular, a complicated interaction between glutamatergic ionotropic receptors (AMPA and NMDA) and actin, a main structural element in the spine (Fifkova and Delay, 1982; Matus et al., 1982), has been observed in a number of studies. Activation of AMPA receptors during LTP increases dh (Fischer et al., 2000; Zhao et al., 2012a, b) and this increase is attributed to the stabilization of spines through actin-dependent mechanisms (Fischer et al., 2000) or to a change in the balance between the filament (F-actin) and monomer (G-actin) forms of actin (Zhao et al., 2012a). This would imply that changes in AMPA receptor activation drive the changes in spine morphology. However, the quantity of AMPA receptors embedded in the spine is itself affected by actin activation. Thus, pharmacological manipulation of F-actin activity was sufficient to change the amplitude and frequency of spontaneous AMPA currents in mature cultured hippocampal neurons (Ivanov et al., 2009). In addition, pharmacological inhibition of F-actin affected the movement of receptors into and out of the synapse (Charrier et al., 2006; Cingolani and Goda, 2008) and its activation drastically increased the levels of CaMKII in the postsynaptic compartment (Okamoto et al., 2004). Since CaMKII is essential for recruitment of AMPA receptors into the postsynaptic membrane, activation of F-actin per se could increase AMPA receptor density in the postsynaptic membrane. This would imply the morphological changes might themselves drive changes in synaptic AMPA receptor expression that underlie synaptic potentiation. Along these lines, during chemical LTP (LTP induced by chemical depolarization) it was shown that morphological enlargement of the spine occurs before the increase in synaptic AMPA expression (Kopec et al., 2006). It should be noted that in addition to the large amount of data linking changes in spine morphology and synaptic function, an emerging view notes that the signaling pathways that trigger structural and functional plasticity are, in some cases, dissociable (Cingolani and Goda, 2008). However, it has not yet been shown that spine morphological changes unaccompanied by changes in synaptic strength have an effect on behavior.
3.2 Alterations in Receptor Composition Following Drug Exposure: Return to Developmental Conditions?
Drug-induced alterations in AMPA and NMDA- receptor composition has been observed at various time-points after drug exposure, such as increased calcium permeable synaptic GluA2-lacking AMPA and extrasynaptic GluN2B-containing NMDA receptors (Bellone and Luscher, 2012; Conrad et al., 2008; Shen et al., 2011). These receptors are highly expressed during early postnatal synaptic development (Kumar et al., 2002; Monyer et al., 1994), and it is possible that drugs of abuse reopen this critical period of augmented synaptic plasticity by increasing GluN2B and GluA1 expression. This possibility is also consistent with an apparent increase in actin cycling in the NAc after discontinuing daily injections of either cocaine or morphine (Russo et al., 2010; Toda et al., 2006). Given that heroin reduces while cocaine increases synaptic strength, it is reasonable to speculate that the shared susceptibility to undergo synaptic plasticity is a more accurate correlate of addiction rather than the resting state of synaptic strength after discontinuing chronic drug treatment. How these alterations contribute to compulsive drug-seeking is not understood, but it is hypothesized that the increase in calcium permeable AMPA receptors may enhance learning to discriminate among different environmental stimuli to select the reward with the highest value in the decision making process (Bellone and Luscher, 2012). Accordingly, the enduring alterations in synaptic plasticity may provide a neural substrate for maladaptive processing of highly salient drug-paired stimuli in individuals with substance abuse disorders.
Following prolonged withdrawal from extended access to cocaine self-administration (but not experimenter-delivered cocaine), formation of Ca2+-permeable (CP) GluA2-lacking AMPA receptors in the NAc has been observed (McCutcheon et al., 2011b). Additionally, these CP-AMPA receptors have been implicated in the incubation of cocaine craving, in which greater withdrawal periods from cocaine lead to proportionally increased drug-seeking behavior (Conrad et al., 2008). Interestingly, the switch in AMPA receptor subunits occurs between day 25–30 of cocaine withdrawal and is maintained through day 70 (Wolf and Tseng, 2012). As well, accumulation of these receptors appears to take place under very specific conditions, only occurring after withdrawal from extended access to cocaine self-administration (Purgianto et al., 2013). Increased GluN2B NMDA receptors in the VTA has been observed after an acute cocaine injection (Schilstrom et al., 2006), as well as in the NAc after early (Huang et al., 2009) and extended withdrawal from experimenter-delivered cocaine (Schumann and Yaka, 2009) and self-administered heroin (Shen et al., 2011) and nicotine (Gipson et al., submitted). The increase in GluN2B-containing NMDA receptors has been linked to an increase in the generation of silent synapses after exposure to cocaine (Brown et al., 2011). Silent synapses are glutamatergic connections found during development as well as after exposure to cocaine, and defined by the presence NMDA receptor-mediated responses in the absence of stable AMPA receptor responses (Lee and Dong, 2011). Indeed, these silent excitatory synapses were found in the NAshell at 24–48 hours after cocaine exposure, and it is thought that they allow NAc MSNs to more readily recruit AMPA receptors and thus increase synaptic transmission (Huang et al., 2009). Although this may be an underlying mechanism for the rapid, transient increases in synaptic plasticity we see in the NAcore during cue-induced cocaine seeking (Gipson et al., 2013), it is unclear if these silent synapses endure into periods of extended withdrawal and if they occur in the NAcore, as the NAshell was examined at an early withdrawal time-point (Huang et al., 2009). Additionally, the paradigms used in these two studies differ between experimenter- and self-administered cocaine, extinction training versus abstinence, and two weeks of withdrawal versus two days. Finally, it is important to note that Gipson et al. (2013) used a short access (2-hr) self-administration condition, and it is unknown if the rapid cue-induced changes in plasticity occur after withdrawal from conditions of extended cocaine access (e.g., 6-hr sessions).
Persistently impaired NAc synaptic plasticity is hypothesized to underlie the transition from goal-directed to habitual behavior in addiction. In the NAc drug-induced loss of both long-term potentiation (LTP) and long-term depression (LTD) occurs after withdrawal from chronic cocaine or heroin self-administration (Knackstedt et al., 2010b; Martin et al., 2006; Moussawi et al., 2009; Shen and Kalivas, 2012). A discrepancy exists in the literature, however, where one study found loss of LTD NAcore of cocaine-extinguished but not abstinent animals (Knackstedt et al., 2010b), and another study found that animals in 21 days abstinence from cocaine showed the loss of LTD (Martin et al., 2006). In the latter study, however, the loss of LTD is also not observed in non-contingent yoked cocaine or in sucrose trained animals (Martin et al., 2006), indicating that this deficit is specific to the pathological state induced by a history of voluntary cocaine self-administration (Madsen et al., 2012). In contrast, LTD can be induced both 24 hr after 50–72 days of cocaine self-administration (Kasanetz et al., 2010) and after abstinence from extended access to cocaine self-administration (McCutcheon et al., 2011b). Taken together, these data suggest that loss of LTD may be paradigm-specific. Also as discussed in section 4.1, it is possible that while the NAc is in an enduring potentiated or depotentiated state after extended withdrawal from cocaine or heroin, respectively (Gipson et al., 2013; Kourrich et al., 2007; Shen et al., 2011; Shen et al., 2009), these cells do not have great flexibility to undergo experimenter-induced synaptic plasticity, but perhaps have capacity to undergo rapid synaptic plasticity induced by a motivated behavior.
3.3 Relationship Between Synaptic Plasticity and Glutamate Homeostasis
Some types of long term plasticity depend on the activity of extrasynaptic glutamatergic receptors, such as extrasynaptic NMDA receptors or metabotropic glutamate receptors (Asrar and Jia, 2013; Liu et al., 2012a; Oh et al., 2012; Papouin et al., 2012), and it has been hypothesized that changes in the extracellular basal levels of glutamate may contribute to the loss of synaptic plasticity in MSNs after cocaine or heroin self-administration (Peters et al., 2009b). Indeed, basal extracellular glutamate levels in the NAc are decreased after extinction from cocaine self-administration, a result of downregulated catalytic subunit (xCT) of the glial cystine-glutamate exchanger (system xc-). The relative paucity of glutamate prevents activation of extrasynaptic mGluRs necessary for the expression of long-term plasticity (Moussawi et al., 2009). In this way, it is possible that cocaine-induced loss of metaplasticity at cortico-accumbens synapses after withdrawal from cocaine underlies the failure of the PFC to regulate drug-seeking behavior. However, neither the reduction in xCT nor a reduction in basal extracellular glutamate is measured in the NAc after withdrawal from heroin self-administration (Shen et al., unpublished), indicating that this effect of cocaine is not central to relapse in addiction to all drugs of abuse.
In contrast with xCT, self-administration of all addictive drugs studied thus far induces an enduring reduction of the glial glutamate transporter GLT-1 in the NAc, including cocaine, heroin and nicotine (Gipson et al., 2012; Knackstedt et al., 2010a; Shen et al., unpublished data). The enduring reduction in glutamate uptake from the vicinity of the synapse would be predicted to increase the overflow of glutamate from the synapse during periods of high synaptic activity, as might occur during a relapse event. Indeed, the reinstatement of heroin or cocaine seeking is associated with a transient increase in extracellular glutamate that is prevented by inhibiting the glutamatergic projection from PFC to NAc (LaLumiere and Kalivas, 2008; McFarland et al., 2003). Given that this transient rise in extracellular glutamate could stimulate extrasynaptic GluN2B-containing NMDA receptors and dysregulate postsynaptic plasticity (Papouin et al., 2012; Petralia, 2012), we recently used the reinstatement model of relapse to demonstrate that relapse is associated with the rapid and transient induction of LTP-like changes in synaptic strength (Gipson et al., 2013).
4.1 Rapid, Transient Plasticity during Relapse to Drug-Seeking
Although alterations in synaptic plasticity have been examined during withdrawal from drugs of abuse, it has remained unclear until recently if synaptic changes are initiated by and contribute to relapse. Cues associated with cocaine use can precipitate relapse, and using a rat model of cue-induced cocaine reinstatement, two measures of synaptic plasticity in the NAcore were recently quantified – spine head diameter (dh) and AMPA- to-NMDA ratio (A/N) ((Gipson et al., 2013); see Figure 2). The presentation of cocaine-conditioned cues elicited rapid (within 15 min of initiation of contingent cue reinstatement) and transient increases in both dh and A/N. The magnitude of synaptic potentiation was positively correlated with the intensity of 5 or 15 min of reinstated cocaine seeking, and inhibiting PL glutamatergic inputs to the NAcore prevented both synaptic potentiation and reinstatement. These results show that rapid cue-evoked synaptic potentiation in the NAcore may underpin relapse to cocaine use. Further supporting the possibility that the induction of synaptic potentiation may mediate the initiation of reinstated behavior, active lever presses were significantly correlated with dh and A/N only during the first 5 min bin after initiating the reinstatement session, and not during the second and third 5 min bins (Figure 3). Similar results, although with a somewhat slower timescale, were found in another recent study with d-amphetamine, where re-exposure to the drug-paired context elicited rapid (within 30 min) NAc dh enlargement in FosB (+) cells (Vezina et al., 2012). Also, heroin-induced reinstatement of heroin seeking was associated with a short-term (45 min) increase in dh and LTP measured in vivo by electrically stimulating the PFC and recording field potentials in the NAc (Shen et al., 2011). Thus, in contrast with opposite changes in resting synaptic strength in NAc MSNs by cocaine and nicotine versus heroin, a rapid, reversible synaptic potentiation occurs during drug or cue induced reinstatement for both drugs (Figure 4); indicating that reinstatement-associated synaptic potentiation might be a better correlate of relapse vulnerability than enduring drug-induced synaptic plasticity in the NAc. This possibility is supported by the fact that operant reinstatement after withdrawal from sucrose self-administration did not result in rapid synaptic plasticity in NAc MSNs (Gipson et al., 2013).
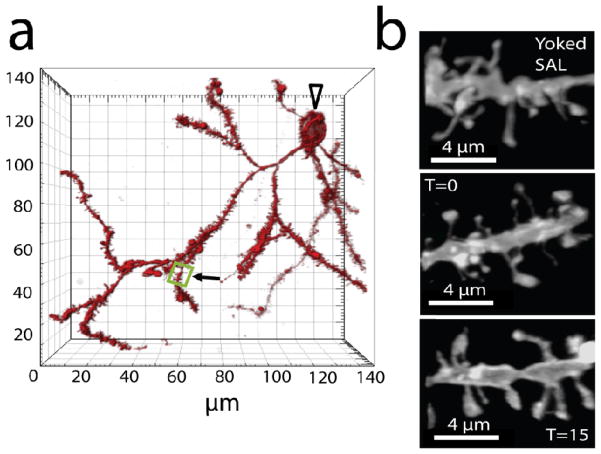
Figure 2. Spine enlargement after 15 min of cue-induced cocaine reinstatement in NAcore medium spiny neurons.
(a) Example of NAcore medium spiny neuron. Clear arrow - location of soma; boxed region - segment from yoked saline animal shown in (b). (b) Sample dendrites from NAcore spiny cells in yoked saline or cocaine-trained rats at 0 min (no reinstatement; T=0) and 15 min after initiating cue-induced reinstatement (T=15).
Figure 3. Synaptic potentiation is correlated with behavior in the first 5 min after initiating relapse.
Linear regressions of either (a) mean spine head diameter or (b) AMPA/NMDA ratio and active lever presses in either the first (0–5 min), second (6–10 min), or third (11–15 min) 5 min bin of the 15 min cue relapse session, indicating that only the initiation (first 5 min bin) of relapse is significantly correlated with synaptic potentiation.
Figure 4. Timeline of postsynaptic changes in NAcore MSNs during withdrawal from and relapse of cocaine seeking.
(a) A drug naïve state. (b) In cocaine-withdrawn animals (T=0), dh is enlarged compared to drug-naïve animals, with increased AMPA and GluN2B-containing NMDA receptors, resulting in an increased A/N. (c) Fifteen minutes into a relapse event (T=15) more AMPA receptors are inserted into the postsynaptic membrane; the result is a further increase in dh and A/N. (d) The increased dh and A/N rapidly reverses, and 120 minutes after the initiation of the relapse event (T=120), both measures return to pre-relapse levels.
The biochemical mechanisms underlying the rapid plasticity associated with drug seeking are unknown, although recent evidence indicates this may be mediated by CaMKII. Indeed, both L-type Ca2+ channels (linked to NAc GluA1 receptor plasticity in cocaine sensitization (Schierberl et al., 2011)) and CaMKII (important in transporting AMPA receptors to synapses (Boehm and Malinow, 2005)), were shown to mediate interaction between glutamatergic and dopaminergic signaling in the NAshell during initiation of cocaine-induced cocaine seeking (Anderson et al., 2008). Interestingly, increased CaMKII phosphorylation was found in the NAshell but not core during reinstatement of morphine seeking (Liu et al., 2012b), indicating that this mechanism may underlie relapse vulnerability across different drug classes.
An intriguing finding in the cue-induced drug-seeking results presented above is the strikingly similar changes in synaptic strength and spine morphology. This similarity suggests that spine morphology is intimately linked with synaptic strength. Indeed, although still somewhat controversial (see Cingolani and Goda, 2008), it is generally accepted that spine head enlargement reflects strengthening of the synapse (Alvarez and Sabatini, 2007; Holtmaat and Svoboda, 2009; Hoogenraad and Akhmanova, 2010). Larger spine volumes are positively correlated with the area covered by the postsynaptic density (PSD) (Harris et al., 1992; Schikorski and Stevens, 1999), which in turn is proportional to synaptic AMPA receptors expression (Cingolani and Goda, 2008; Kharazia and Weinberg, 1999; Nusser et al., 1998; Takumi et al., 1999) and sensitivity to glutamate (Matsuzaki et al., 2001) (although see Beique et al., 2006; Elias and Nicoll, 2007 who show that spines of the same size can show very different AMPA receptor function). Also, electrically driving LTP or LTD is known to cause an increase (Allison et al., 1998; Goldin et al., 2001) or a decrease (Nagerl et al., 2004; Okamoto et al., 2004; Zhou et al., 2004) in dh, respectively.
It is noteworthy that the rapid synaptic potentiation observed after reinstatement of drug seeking is seemingly contradictory to the lack of ability to induce either LTP or LTD following withdrawal discussed above. However, an essential difference between these two sets of findings is that while the loss of LTP or LTD is revealed using artificial electrical stimulation of NAc glutamatergic afferents, the presentation of a drug-associated cue induces specific, physiological activation of the relevant NAc afferents. This indicates that while MSNs have become relatively refractory to the induction of synaptic plasticity by stimuli unrelated to the experience of drug use, stimuli associated with drug use (i.e. conditioned cues or the drug itself) efficiently induce LTP-like synaptic plasticity. We propose that the loss of LTP or LTD in response to a stimulus unrelated to drug use combined with the selective induction of synaptic plasticity related to drug associated stimuli may contribute to two key characteristics of relapse vulnerabilities: 1) the difficulty addicts have in developing behavior that competes with relapse to drug use (i.e. reduced plasticity by nondrug-associated stimuli), and 2) the overwhelming desire to obtain drug (i.e. the robust LTP induced by drug-associated stimuli).
5.1 Clinical Implications and Future Directions
Drug-paired environmental cues abnormally affect the morphology and physiology of synapses in the accumbens, and this may mediate the uncontrollable effect cues have in initiating relapse to drug use. As mentioned above, the rapid plasticity associated with initiating relapse to drug seeking may be linked to alterations in glutamate homeostasis, and supports the following sequence of events: 1) Following chronic cocaine or heroin, reduced basal glutamate levels result in lower binding to presynaptic mGluR2/3 and increased levels of activator of G protein signaling 3 (AGS3) (Bowers et al., 2004; Yao et al., 2005), thereby decreasing inhibitory tone on presynaptic glutamate release (Xi et al., 2002); 2) withdrawal from addictive drug use is associated with downregulated glial glutamate uptake (GLT-1) which allows glutamate to spill over from the synapse; 3) drug-associated stimulus increases activity at glutamatergic synapses resulting in excessive glutamate spillover from the synapse and stimulating extrasynaptic receptors such as GluN2B-containing NMDA receptors and mGluR5; 4) activation of GluN2B and mGluR5 (Cosgrove et al., 2011) signals rapid increases in dh and A/N which drive and perhaps perpetuate drug seeking behavior, likely via increasing calcium influx or release from internal stores, respectively (Figure 5). Based on this sequence of events, targeting key proteins involved in synaptic plasticity may be crucial in the development of drug relapse prevention pharmacotherapies.
Figure 5. Alterations in glutamate homeostasis and synaptic plasticity in addiction and relapse.
Impaired glutamate homeostasis after withdrawal from chronic use of an addictive drug. Expression of both xCT and GLT-1 is decreased, which results in decreased basal levels of extrasynaptic glutamate. This results in decreased glutamatergic tone on presynaptic inhibitory mGluR2/3 receptors and upregulation of activator of G protein signaling 3 (AGS3), which in turn disinhibits glutamatergic transmission. Upon activation of the presynaptic terminal (for example, by presentation of a drug-associated cue) an abnormally high amount of glutamate is released, which, together with the decreased GLT-1 expression, causes glutamate spillover; the net result is enhanced activation of synaptic and extrasynaptic ionotropic glutamate (i.e., AMPA, NMDA, and kainite receptors) and mGluR5 receptors. This in turn signals the rapid increase in dendritic spine head diameter (dh) and AMPA:NMDA ratio (A/N).
Because impaired glutamate homeostasis has been associated with reinstated drug-seeking, repairing glutamate levels via various drugs that target dysregulated proteins of the tripartite synapse (presynapse, postsynapse and glia; see Figure 5) has been of pharmacotherapeutic interest. Of these pharmacotherapies, one is the commonly prescribed antibiotic ceftriaxone, and another is the antioxidant N-acetylcysteine (NAC), both of which restore levels of GLT1 and extracellular glutamate in the NAc of rats trained to self-administer cocaine, nicotine or heroin (Knackstedt et al., 2010a; Moussawi et al., 2011; Shen et al., submitted). NAC also restores the ability to electrically induce LTP and LTD in cocaine-withdrawn animals (Moussawi et al., 2009). In humans, NAC decreases cigarette use (Knackstedt et al., 2009), marijuana use (Gray et al., 2010), and cocaine craving and symptoms of withdrawal, as well as restores glutamate levels in cocaine-dependent individuals (Amen et al., 2011; Schmaal et al., 2012). Although NAC holds promise as a pharmacotherapy, in some cases data in various disorders including autism, schizophrenia, depression, cocaine, marijuana, smoking, bipolar disorder, gambling, among others remain preliminary and require replication (Berk et al., 2013). Indeed, as NAC continues to be tested in various disorders, it is likely to show relatively higher efficacy in some disorders.
In addition to targeting glial proteins involved in glutamate homeostasis, both presynaptic and postsynaptic metabotropic and ionotropic glutamate receptors have been targeted in animal models of relapse. Given that the overflow of glutamate would produce excessive stimulation of extrasynaptic GluN2B-containing NMDA receptors and metabotropic glutamate receptors, antagonists to these glutamate receptors would be postulated to reduce reinstated drug seeking. Pharmacological inhibition of mGluR5 and stimulation of mGluR2/3 receptors reduces cue-and cocaine-induced reinstatement of cocaine-seeking behavior, as well as nicotine self-administration (Kenny et al., 2003; Peters and Kalivas, 2006; Wang et al., 2013). Stimulation of mGluR1 under conditions of increased CP-AMPA receptor accumulation may also represent a novel pharmacotherapeutic approach (Loweth et al., 2013; McCutcheon et al., 2011a), as activation of these receptors inhibits CP-AMPA receptor mediated synaptic transmission. As well, targeting GluN2B-containing NMDA receptors inhibits heroin seeking (Shen et al., 2011). Interestingly, the effects of NAC on metaplasticity (Moussawi et al., 2009) and glutamate transmission in the PFC-NAc synapse (Kupchik et al., 2012) are themselves mediated by mGluR2/3 and mGluR5.
5.2 Conclusions
The successes to date of targeting drug-induced changes in proteins and physiology in the tripartite synapse shown in Figure 5 are encouraging. However, with the exception of NAC, all studies have been conducted in the reinstatement animal model of relapse. While this model possesses face validity, and the NAC studies lend some criterion validity to the model (Epstein et al., 2006), more fully translating these findings into clinical trials is necessary. Within the animal model additional future studies are needed to determine if these treatments induced temporary antagonism of the vulnerability to reinstate, or if chronic treatment can provide enduring protection. While encouraging data in this regard has been obtained with daily NAC treatments during extinction training after cocaine or heroin self-administration (Moussawi et al., 2011; Reichel et al., 2011; Zhou et al., 2009), substantial additional work into the mechanisms of not only relapse associated changes in synaptic transmission but how to target these changes to provide enduring protection from relapse remains to be conducted.
Highlights.
Glutamatergic input to the NA and its involvement in drug relapse is discussed.
Cue-induced relapse to cocaine requires rapid, transient plasticity in accumbens.
Future directions for relapse prevention are discussed.
Acknowledgments
We thank the Kalivas lab members for helpful comments on an earlier version of this manuscript. This work was supported by DA033690 (CDG), and DA03906, DA012513 and DA015369 (PWK) grants from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allison DW, Gelfand VI, Spector I, Craig AM. Role of actin in anchoring postsynaptic receptors in cultured hippocampal neurons: differential attachment of NMDA versus AMPA receptors. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1998;18:2423–2436. doi: 10.1523/JNEUROSCI.18-07-02423.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez VA, Sabatini BL. Anatomical and physiological plasticity of dendritic spines. Annual review of neuroscience. 2007;30:79–97. doi: 10.1146/annurev.neuro.30.051606.094222. [DOI] [PubMed] [Google Scholar]
- Amen SL, Piacentine LB, Ahmad ME, Li SJ, Mantsch JR, Risinger RC, Baker DA. Repeated N-acetyl cysteine reduces cocaine seeking in rodents and craving in cocaine-dependent humans. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2011;36:871–878. doi: 10.1038/npp.2010.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SM, Famous KR, Sadri-Vakili G, Kumaresan V, Schmidt HD, Bass CE, Terwilliger EF, Cha JH, Pierce RC. CaMKII: a biochemical bridge linking accumbens dopamine and glutamate systems in cocaine seeking. Nature neuroscience. 2008;11:344–353. doi: 10.1038/nn2054. [DOI] [PubMed] [Google Scholar]
- Asrar S, Jia Z. Molecular mechanisms coordinating functional and morphological plasticity at the synapse: Role of GluA2/N-cadherin interaction-mediated actin signaling in mGluR-dependent LTD. Cellular signalling. 2013;25:397–402. doi: 10.1016/j.cellsig.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nature neuroscience. 2003;6:743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- Balleine BW, O’Doherty JP. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2010;35:48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beique JC, Lin DT, Kang MG, Aizawa H, Takamiya K, Huganir RL. Synapse-specific regulation of AMPA receptor function by PSD-95. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:19535–19540. doi: 10.1073/pnas.0608492103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellone C, Luscher C. Drug-evoked plasticity: do addictive drugs reopen a critical period of postnatal synaptic development? Front Mol Neurosci. 2012;5:75. doi: 10.3389/fnmol.2012.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendse HW, Galis-de Graaf Y, Groenewegen HJ. Topographical organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. The Journal of comparative neurology. 1992;316:314–347. doi: 10.1002/cne.903160305. [DOI] [PubMed] [Google Scholar]
- Berendse HW, Groenewegen HJ. Organization of the thalamostriatal projections in the rat, with special emphasis on the ventral striatum. The Journal of comparative neurology. 1990;299:187–228. doi: 10.1002/cne.902990206. [DOI] [PubMed] [Google Scholar]
- Berk M, Malhi GS, Gray LJ, Dean OM. The promise of N-acetylcysteine in neuropsychiatry. Trends in pharmacological sciences. 2013;34:167–177. doi: 10.1016/j.tips.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Boehm J, Malinow R. AMPA receptor phosphorylation during synaptic plasticity. Biochemical Society transactions. 2005;33:1354–1356. doi: 10.1042/BST0331354. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Stern AL, Theberge FR, Marchant NJ, Wang HL, Morales M, Shaham Y. Role of projections from ventral medial prefrontal cortex to nucleus accumbens shell in context-induced reinstatement of heroin seeking. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:4982–4991. doi: 10.1523/JNEUROSCI.0005-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2005;25:9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers MS, McFarland K, Lake RW, Peterson YK, Lapish CC, Gregory ML, Lanier SM, Kalivas PW. Activator of G protein signaling 3: a gatekeeper of cocaine sensitization and drug seeking. Neuron. 2004;42:269–281. doi: 10.1016/s0896-6273(04)00159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt JP, Benaliouad F, McDevitt RA, Stuber GD, Wise RA, Bonci A. Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron. 2012;76:790–803. doi: 10.1016/j.neuron.2012.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TE, Lee BR, Mu P, Ferguson D, Dietz D, Ohnishi YN, Lin Y, Suska A, Ishikawa M, Huang YH, Shen H, Kalivas PW, Sorg BA, Zukin RS, Nestler EJ, Dong Y, Schluter OM. A silent synapse-based mechanism for cocaine-induced locomotor sensitization. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:8163–8174. doi: 10.1523/JNEUROSCI.0016-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capriles N, Rodaros D, Sorge RE, Stewart J. A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2003;168:66–74. doi: 10.1007/s00213-002-1283-z. [DOI] [PubMed] [Google Scholar]
- Charrier C, Ehrensperger MV, Dahan M, Levi S, Triller A. Cytoskeleton regulation of glycine receptor number at synapses and diffusion in the plasma membrane. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26:8502–8511. doi: 10.1523/JNEUROSCI.1758-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cingolani LA, Goda Y. Actin in action: the interplay between the actin cytoskeleton and synaptic efficacy. Nature reviews Neuroscience. 2008;9:344–356. doi: 10.1038/nrn2373. [DOI] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Quirk GJ. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:840–844. doi: 10.1523/JNEUROSCI.5327-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain research. 1994;653:278–284. doi: 10.1016/0006-8993(94)90401-4. [DOI] [PubMed] [Google Scholar]
- Cosgrove KE, Galvan EJ, Barrionuevo G, Meriney SD. mGluRs modulate strength and timing of excitatory transmission in hippocampal area CA3. Molecular neurobiology. 2011;44:93–101. doi: 10.1007/s12035-011-8187-z. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neuroscience and biobehavioral reviews. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- De Roo M, Klauser P, Muller D. LTP promotes a selective long-term stabilization and clustering of dendritic spines. PLoS biology. 2008;6:e219. doi: 10.1371/journal.pbio.0060219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFrance JF, Marchand JF, Sikes RW, Chronister RB, Hubbard JI. Characterization of fimbria input to nucleus accumbens. Journal of neurophysiology. 1985;54:1553–1567. doi: 10.1152/jn.1985.54.6.1553. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Direct interactions between the basolateral amygdala and nucleus accumbens core underlie cocaine-seeking behavior by rats. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24:7167–7173. doi: 10.1523/JNEUROSCI.1581-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz DM, Sun H, Lobo MK, Cahill ME, Chadwick B, Gao V, Koo JW, Mazei-Robison MS, Dias C, Maze I, Damez-Werno D, Dietz KC, Scobie KN, Ferguson D, Christoffel D, Ohnishi Y, Hodes GE, Zheng Y, Neve RL, Hahn KM, Russo SJ, Nestler EJ. Rac1 is essential in cocaine-induced structural plasticity of nucleus accumbens neurons. Nature neuroscience. 2012;15:891–896. doi: 10.1038/nn.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitriu D, Laplant Q, Grossman YS, Dias C, Janssen WG, Russo SJ, Morrison JH, Nestler EJ. Subregional, dendritic compartment, and spine subtype specificity in cocaine regulation of dendritic spines in the nucleus accumbens. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:6957–6966. doi: 10.1523/JNEUROSCI.5718-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias GM, Nicoll RA. Synaptic trafficking of glutamate receptors by MAGUK scaffolding proteins. Trends Cell Biol. 2007;17:343–352. doi: 10.1016/j.tcb.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology. 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nature neuroscience. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fifkova E, Delay RJ. Cytoplasmic actin in neuronal processes as a possible mediator of synaptic plasticity. J Cell Biol. 1982;95:345–350. doi: 10.1083/jcb.95.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer M, Kaech S, Wagner U, Brinkhaus H, Matus A. Glutamate receptors regulate actinbased plasticity in dendritic spines. Nature neuroscience. 2000;3:887–894. doi: 10.1038/78791. [DOI] [PubMed] [Google Scholar]
- French SJ, Totterdell S. Hippocampal and prefrontal cortical inputs monosynaptically converge with individual projection neurons of the nucleus accumbens. The Journal of comparative neurology. 2002;446:151–165. doi: 10.1002/cne.10191. [DOI] [PubMed] [Google Scholar]
- French SJ, Totterdell S. Individual nucleus accumbens-projection neurons receive both basolateral amygdala and ventral subicular afferents in rats. Neuroscience. 2003;119:19–31. doi: 10.1016/s0306-4522(03)00150-7. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Eaddy JL, Su ZI, Bell GH. Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. The European journal of neuroscience. 2007;26:487–498. doi: 10.1111/j.1460-9568.2007.05674.x. [DOI] [PubMed] [Google Scholar]
- Fuller TA, Russchen FT, Price JL. Sources of presumptive glutamergic/aspartergic afferents to the rat ventral striatopallidal region. The Journal of comparative neurology. 1987;258:317–338. doi: 10.1002/cne.902580302. [DOI] [PubMed] [Google Scholar]
- Gipson CD, Kupchik YM, Reissner KJ, Kalivas PW. Alterations in synaptic potentiation and glutamatergic signaling in nicotine abuse. American College of Neuropsychopharmacology 2012 [Google Scholar]
- Gipson CD, Kupchik YM, Shen H, Reissner KJ, Thomas CA, Kalivas PW. Relapse induced by cues predicting cocaine depends on rapid, transient synaptic potentiation. Neuron. 2013 doi: 10.1016/j.neuron.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin M, Segal M, Avignone E. Functional plasticity triggers formation and pruning of dendritic spines in cultured hippocampal networks. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2001;21:186–193. doi: 10.1523/JNEUROSCI.21-01-00186.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelova N, Mulholland PJ, Chandler LJ, Seamans JK. The glutamatergic component of the mesocortical pathway emanating from different subregions of the ventral midbrain. Cerebral cortex. 2012;22:327–336. doi: 10.1093/cercor/bhr107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelova N, Yang CR. The course of neural projection from the prefrontal cortex to the nucleus accumbens in the rat. Neuroscience. 1997;76:689–706. doi: 10.1016/s0306-4522(96)00380-6. [DOI] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends in neurosciences. 2007;30:220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Gray KM, Watson NL, Carpenter MJ, Larowe SD. N-acetylcysteine (NAC) in young marijuana users: an open-label pilot study. The American journal on addictions / American Academy of Psychiatrists in Alcoholism and Addictions. 2010;19:187–189. doi: 10.1111/j.1521-0391.2009.00027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Becker NE, Lohman AH. Subcortical afferents of the nucleus accumbens septi in the cat, studied with retrograde axonal transport of horseradish peroxidase and bisbenzimid. Neuroscience. 1980;5:1903–1916. doi: 10.1016/0306-4522(80)90038-x. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Vermeulen-Van der Zee E, te Kortschot A, Witter MP. Organization of the projections from the subiculum to the ventral striatum in the rat. A study using anterograde transport of Phaseolus vulgaris leucoagglutinin. Neuroscience. 1987;23:103–120. doi: 10.1016/0306-4522(87)90275-2. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Beijer AV. The nucleus accumbens: gateway for limbic structures to reach the motor system? Progress in brain research. 1996;107:485–511. doi: 10.1016/s0079-6123(08)61883-x. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Beijer AV, Voorn P. Convergence and segregation of ventral striatal inputs and outputs. Annals of the New York Academy of Sciences. 1999;877:49–63. doi: 10.1111/j.1749-6632.1999.tb09260.x. [DOI] [PubMed] [Google Scholar]
- Grueter BA, Rothwell PE, Malenka RC. Integrating synaptic plasticity and striatal circuit function in addiction. Current opinion in neurobiology. 2012;22:545–551. doi: 10.1016/j.conb.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulledge AT, Carnevale NT, Stuart GJ. Electrical advantages of dendritic spines. PloS one. 2012;7:e36007. doi: 10.1371/journal.pone.0036007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM, Jensen FE, Tsao B. Three-dimensional structure of dendritic spines and synapses in rat hippocampus (CA1) at postnatal day 15 and adult ages: implications for the maturation of synaptic physiology and long-term potentiation. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1992;12:2685–2705. doi: 10.1523/JNEUROSCI.12-07-02685.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnasko TS, Hjelmstad GO, Fields HL, Edwards RH. Ventral tegmental area glutamate neurons: electrophysiological properties and projections. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:15076–15085. doi: 10.1523/JNEUROSCI.3128-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nature reviews Neuroscience. 2009;10:647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- Hoogenraad CC, Akhmanova A. Dendritic spine plasticity: new regulatory roles of dynamic microtubules. Neuroscientist. 2010;16:650–661. doi: 10.1177/1073858410386357. [DOI] [PubMed] [Google Scholar]
- Hotulainen P, Hoogenraad CC. Actin in dendritic spines: connecting dynamics to function. J Cell Biol. 2010;189:619–629. doi: 10.1083/jcb.201003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YH, Lin Y, Mu P, Lee BR, Brown TE, Wayman G, Marie H, Liu W, Yan Z, Sorg BA, Schluter OM, Zukin RS, Dong Y. In vivo cocaine experience generates silent synapses. Neuron. 2009;63:40–47. doi: 10.1016/j.neuron.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov A, Esclapez M, Pellegrino C, Shirao T, Ferhat L. Drebrin A regulates dendritic spine plasticity and synaptic function in mature cultured hippocampal neurons. J Cell Sci. 2009;122:524–534. doi: 10.1242/jcs.033464. [DOI] [PubMed] [Google Scholar]
- Jaslove SW. The integrative properties of spiny distal dendrites. Neuroscience. 1992;47:495–519. doi: 10.1016/0306-4522(92)90161-t. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. New medications for drug addiction hiding in glutamatergic neuroplasticity. Molecular psychiatry. 2011 doi: 10.1038/mp.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H, Fukuda M, Watanabe S, Hayashi-Takagi A, Noguchi J. Structural dynamics of dendritic spines in memory and cognition. Trends in neurosciences. 2010;33:121–129. doi: 10.1016/j.tins.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Kasanetz F, Deroche-Gamonet V, Berson N, Balado E, Lafourcade M, Manzoni O, Piazza PV. Transition to addiction is associated with a persistent impairment in synaptic plasticity. Science. 2010;328:1709–1712. doi: 10.1126/science.1187801. [DOI] [PubMed] [Google Scholar]
- Kawato M, Tsukahara N. Electrical properties of dendritic spines with bulbous end terminals. Biophys J. 1984;46:155–166. doi: 10.1016/S0006-3495(84)84008-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Stinus L. The distribution of the projection from the parataenial nucleus of the thalamus to the nucleus accumbens in the rat: an autoradiographic study. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 1984;54:499–512. doi: 10.1007/BF00235475. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Paterson NE, Boutrel B, Semenova S, Harrison AA, Gasparini F, Koob GF, Skoubis PD, Markou A. Metabotropic glutamate 5 receptor antagonist MPEP decreased nicotine and cocaine self-administration but not nicotine and cocaine-induced facilitation of brain reward function in rats. Annals of the New York Academy of Sciences. 2003;1003:415–418. doi: 10.1196/annals.1300.040. [DOI] [PubMed] [Google Scholar]
- Kharazia VN, Weinberg RJ. Immunogold localization of AMPA and NMDA receptors in somatic sensory cortex of albino rat. The Journal of comparative neurology. 1999;412:292–302. doi: 10.1002/(sici)1096-9861(19990920)412:2<292::aid-cne8>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Killcross S, Coutureau E. Coordination of actions and habits in the medial prefrontal cortex of rats. Cerebral cortex. 2003;13:400–408. doi: 10.1093/cercor/13.4.400. [DOI] [PubMed] [Google Scholar]
- Kita H, Kitai ST. Amygdaloid projections to the frontal cortex and the striatum in the rat. The Journal of comparative neurology. 1990;298:40–49. doi: 10.1002/cne.902980104. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, LaRowe S, Mardikian P, Malcolm R, Upadhyaya H, Hedden S, Markou A, Kalivas PW. The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biological psychiatry. 2009;65:841–845. doi: 10.1016/j.biopsych.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Melendez RI, Kalivas PW. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biological psychiatry. 2010a;67:81–84. doi: 10.1016/j.biopsych.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Moussawi K, Lalumiere R, Schwendt M, Klugmann M, Kalivas PW. Extinction training after cocaine self-administration induces glutamatergic plasticity to inhibit cocaine seeking. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010b;30:7984–7992. doi: 10.1523/JNEUROSCI.1244-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C, Zador A. The function of dendritic spines: devices subserving biochemical rather than electrical compartmentalization. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1993;13:413–422. doi: 10.1523/JNEUROSCI.13-02-00413.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopec C, Malinow R. Neuroscience. Matters of size. Science. 2006;314:1554–1555. doi: 10.1126/science.1137595. [DOI] [PubMed] [Google Scholar]
- Kopec CD, Li B, Wei W, Boehm J, Malinow R. Glutamate receptor exocytosis and spine enlargement during chemically induced long-term potentiation. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26:2000–2009. doi: 10.1523/JNEUROSCI.3918-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourrich S, Rothwell PE, Klug JR, Thomas MJ. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:7921–7928. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koya E, Uejima JL, Wihbey KA, Bossert JM, Hope BT, Shaham Y. Role of ventral medial prefrontal cortex in incubation of cocaine craving. Neuropharmacology. 2009;56(Suppl 1):177–185. doi: 10.1016/j.neuropharm.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar SS, Bacci A, Kharazia V, Huguenard JR. A developmental switch of AMPA receptor subunits in neocortical pyramidal neurons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22:3005–3015. doi: 10.1523/JNEUROSCI.22-08-03005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupchik YM, Moussawi K, Tang XC, Wang X, Kalivas BC, Kolokithas R, Ogburn KB, Kalivas PW. The effect of N-acetylcysteine in the nucleus accumbens on neurotransmission and relapse to cocaine. Biological psychiatry. 2012;71:978–986. doi: 10.1016/j.biopsych.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Kalivas PW. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:3170–3177. doi: 10.1523/JNEUROSCI.5129-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Niehoff KE, Kalivas PW. The infralimbic cortex regulates the consolidation of extinction after cocaine self-administration. Learning & memory. 2010;17:168–175. doi: 10.1101/lm.1576810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Smith KC, Kalivas PW. Neural circuit competition in cocaine-seeking: roles of the infralimbic cortex and nucleus accumbens shell. The European journal of neuroscience. 2012;35:614–622. doi: 10.1111/j.1460-9568.2012.07991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BR, Dong Y. Cocaine-induced metaplasticity in the nucleus accumbens: silent synapse and beyond. Neuropharmacology. 2011;61:1060–1069. doi: 10.1016/j.neuropharm.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu DD, Yang Q, Li ST. Activation of extrasynaptic NMDA receptors induces LTD in rat hippocampal CA1 neurons. Brain research bulletin. 2012a doi: 10.1016/j.brainresbull.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Liu Z, Zhang JJ, Liu XD, Yu LC. Inhibition of CaMKII activity in the nucleus accumbens shell blocks the reinstatement of morphine-seeking behavior in rats. Neuroscience letters. 2012b;518:167–171. doi: 10.1016/j.neulet.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. Amphetamine activation of hippocampal drive of mesolimbic dopamine neurons: a mechanism of behavioral sensitization. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:7876–7882. doi: 10.1523/JNEUROSCI.1582-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loweth JA, Tseng KY, Wolf ME. Using metabotropic glutamate receptors to modulate cocaine’s synaptic and behavioral effects: mGluR1 finds a niche. Current opinion in neurobiology. 2013 doi: 10.1016/j.conb.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen HB, Brown RM, Lawrence AJ. Neuroplasticity in addiction: cellular and transcriptional perspectives. Front Mol Neurosci. 2012;5:99. doi: 10.3389/fnmol.2012.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M, Chen BT, Hopf FW, Bowers MS, Bonci A. Cocaine self-administration selectively abolishes LTD in the core of the nucleus accumbens. Nature neuroscience. 2006;9:868–869. doi: 10.1038/nn1713. [DOI] [PubMed] [Google Scholar]
- Matsuzaki M, Ellis-Davies GC, Nemoto T, Miyashita Y, Iino M, Kasai H. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nature neuroscience. 2001;4:1086–1092. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus A, Ackermann M, Pehling G, Byers HR, Fujiwara K. High actin concentrations in brain dendritic spines and postsynaptic densities. Proceedings of the National Academy of Sciences of the United States of America. 1982;79:7590–7594. doi: 10.1073/pnas.79.23.7590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JE, Loweth JA, Ford KA, Marinelli M, Wolf ME, Tseng KY. Group I mGluR activation reverses cocaine-induced accumulation of calcium-permeable AMPA receptors in nucleus accumbens synapses via a protein kinase C-dependent mechanism. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011a;31:14536–14541. doi: 10.1523/JNEUROSCI.3625-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JE, Wang X, Tseng KY, Wolf ME, Marinelli M. Calcium-permeable AMPA receptors are present in nucleus accumbens synapses after prolonged withdrawal from cocaine selfadministration but not experimenter-administered cocaine. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011b;31:5737–5743. doi: 10.1523/JNEUROSCI.0350-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ. Organization of amygdaloid projections to the prefrontal cortex and associated striatum in the rat. Neuroscience. 1991a;44:1–14. doi: 10.1016/0306-4522(91)90247-l. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Topographical organization of amygdaloid projections to the caudatoputamen, nucleus accumbens, and related striatal-like areas of the rat brain. Neuroscience. 1991b;44:15–33. doi: 10.1016/0306-4522(91)90248-m. [DOI] [PubMed] [Google Scholar]
- McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24:1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drugseeking behavior. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology. 2003;168:57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- Meredith GE, Wouterlood FG, Pattiselanno A. Hippocampal fibers make synaptic contacts with glutamate decarboxylase-immunoreactive neurons in the rat nucleus accumbens. Brain research. 1990;513:329–334. doi: 10.1016/0006-8993(90)90476-r. [DOI] [PubMed] [Google Scholar]
- Millan EZ, Marchant NJ, McNally GP. Extinction of drug seeking. Behavioural brain research. 2011;217:454–462. doi: 10.1016/j.bbr.2010.10.037. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Moussawi K, Pacchioni A, Moran M, Olive MF, Gass JT, Lavin A, Kalivas PW. NAcetylcysteine reverses cocaine-induced metaplasticity. Nature neuroscience. 2009;12:182–189. doi: 10.1038/nn.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Zhou W, Shen H, Reichel CM, See RE, Carr DB, Kalivas PW. Reversing cocaine-induced synaptic potentiation provides enduring protection from relapse. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:385–390. doi: 10.1073/pnas.1011265108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagerl UV, Eberhorn N, Cambridge SB, Bonhoeffer T. Bidirectional activity-dependent morphological plasticity in hippocampal neurons. Neuron. 2004;44:759–767. doi: 10.1016/j.neuron.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Is there a common molecular pathway for addiction? Nature neuroscience. 2005;8:1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- Nimchinsky EA, Sabatini BL, Svoboda K. Structure and function of dendritic spines. Annu Rev Physiol. 2002;64:313–353. doi: 10.1146/annurev.physiol.64.081501.160008. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Lujan R, Laube G, Roberts JD, Molnar E, Somogyi P. Cell type and pathway dependence of synaptic AMPA receptor number and variability in the hippocampus. Neuron. 1998;21:545–559. doi: 10.1016/s0896-6273(00)80565-6. [DOI] [PubMed] [Google Scholar]
- Oh WC, Hill TC, Zito K. Synapse-specific and size-dependent mechanisms of spine structural plasticity accompanying synaptic weakening. Proceedings of the National Academy of Sciences of the United States of America. 2012 doi: 10.1073/pnas.1214705110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K, Nagai T, Miyawaki A, Hayashi Y. Rapid and persistent modulation of actin dynamics regulates postsynaptic reorganization underlying bidirectional plasticity. Nature neuroscience. 2004;7:1104–1112. doi: 10.1038/nn1311. [DOI] [PubMed] [Google Scholar]
- Ortinski PI, Vassoler FM, Carlson GC, Pierce RC. Temporally dependent changes in cocaine-induced synaptic plasticity in the nucleus accumbens shell are reversed by D1-like dopamine receptor stimulation. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2012;37:1671–1682. doi: 10.1038/npp.2012.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papouin T, Ladepeche L, Ruel J, Sacchi S, Labasque M, Hanini M, Groc L, Pollegioni L, Mothet JP, Oliet SH. Synaptic and extrasynaptic NMDA receptors are gated by different endogenous coagonists. Cell. 2012;150:633–646. doi: 10.1016/j.cell.2012.06.029. [DOI] [PubMed] [Google Scholar]
- Papp E, Borhegyi Z, Tomioka R, Rockland KS, Mody I, Freund TF. Glutamatergic input from specific sources influences the nucleus accumbens-ventral pallidum information flow. Brain Struct Funct. 2012;217:37–48. doi: 10.1007/s00429-011-0331-z. [DOI] [PubMed] [Google Scholar]
- Park M, Penick EC, Edwards JG, Kauer JA, Ehlers MD. Recycling endosomes supply AMPA receptors for LTP. Science. 2004;305:1972–1975. doi: 10.1126/science.1102026. [DOI] [PubMed] [Google Scholar]
- Park M, Salgado JM, Ostroff L, Helton TD, Robinson CG, Harris KM, Ehlers MD. Plasticity-induced growth of dendritic spines by exocytic trafficking from recycling endosomes. Neuron. 2006;52:817–830. doi: 10.1016/j.neuron.2006.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Kalivas PW. The group II metabotropic glutamate receptor agonist, LY379268, inhibits both cocaine- and food-seeking behavior in rats. Psychopharmacology. 2006;186:143–149. doi: 10.1007/s00213-006-0372-9. [DOI] [PubMed] [Google Scholar]
- Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem. 2009a;16:279–288. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learning & memory. 2009b;16:279–288. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, LaLumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:6046–6053. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS. Distribution of extrasynaptic NMDA receptors on neurons. TheScientificWorldJournal. 2012;2012:267120. doi: 10.1100/2012/267120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purgianto A, Scheyer A, Loweth JA, Ford KA, Tseng KY, Wolf ME. Different adaptations in AMPA receptor transmission in the nucleus accumbens after short versus long access cocaine self-administration regimens. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Moussawi K, Do PH, Kalivas PW, See RE. Chronic N-acetylcysteine during abstinence or extinction following cocaine self-administration produces enduring reductions in drugseeking. The Journal of pharmacology and experimental therapeutics. 2011 doi: 10.1124/jpet.111.179317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SM, Zahm DS. Specificity in the projections of prefrontal and insular cortex to ventral striatopallidum and the extended amygdala. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2005;25:11757–11767. doi: 10.1523/JNEUROSCI.3432-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Gorny G, Mitton E, Kolb B. Cocaine self-administration alters the morphology of dendrites and dendritic spines in the nucleus accumbens and neocortex. Synapse. 2001;39:257–266. doi: 10.1002/1098-2396(20010301)39:3<257::AID-SYN1007>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. The European journal of neuroscience. 1999a;11:1598–1604. doi: 10.1046/j.1460-9568.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Morphine alters the structure of neurons in the nucleus accumbens and neocortex of rats. Synapse. 1999b;33:160–162. doi: 10.1002/(SICI)1098-2396(199908)33:2<160::AID-SYN6>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Rocha A, Kalivas PW. Role of the prefrontal cortex and nucleus accumbens in reinstating methamphetamine seeking. The European journal of neuroscience. 2010;31:903–909. doi: 10.1111/j.1460-9568.2010.07134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Dietz DM, Dumitriu D, Morrison JH, Malenka RC, Nestler EJ. The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends in Neurosciences. 2010;33:267–276. doi: 10.1016/j.tins.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schierberl K, Hao J, Tropea TF, Ra S, Giordano TP, Xu Q, Garraway SM, Hofmann F, Moosmang S, Striessnig J, Inturrisi CE, Rajadhyaksha AM. Cav1.2 L-type Ca(2)(+) channels mediate cocaine-induced GluA1 trafficking in the nucleus accumbens, a long-term adaptation dependent on ventral tegmental area Ca(v)1.3 channels. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:13562–13575. doi: 10.1523/JNEUROSCI.2315-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schikorski T, Stevens CF. Quantitative fine-structural analysis of olfactory cortical synapses. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:4107–4112. doi: 10.1073/pnas.96.7.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilstrom B, Yaka R, Argilli E, Suvarna N, Schumann J, Chen BT, Carman M, Singh V, Mailliard WS, Ron D, Bonci A. Cocaine enhances NMDA receptor-mediated currents in ventral tegmental area cells via dopamine D5 receptor-dependent redistribution of NMDA receptors. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26:8549–8558. doi: 10.1523/JNEUROSCI.5179-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaal L, Veltman DJ, Nederveen A, van den Brink W, Goudriaan AE. N-acetylcysteine normalizes glutamate levels in cocaine-dependent patients: a randomized crossover magnetic resonance spectroscopy study. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2012;37:2143–2152. doi: 10.1038/npp.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann J, Yaka R. Prolonged withdrawal from repeated noncontingent cocaine exposure increases NMDA receptor expression and ERK activity in the nucleus accumbens. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:6955–6963. doi: 10.1523/JNEUROSCI.1329-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE, Fuchs RA, Ledford CC, McLaughlin J. Drug addiction, relapse, and the amygdala. Annals of the New York Academy of Sciences. 2003;985:294–307. doi: 10.1111/j.1749-6632.2003.tb07089.x. [DOI] [PubMed] [Google Scholar]
- Segev I, Friedman A, White EL, Gutnick MJ. Electrical consequences of spine dimensions in a model of a cortical spiny stellate cell completely reconstructed from serial thin sections. J Comput Neurosci. 1995;2:117–130. doi: 10.1007/BF00961883. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Grace AA. Cortico-Basal Ganglia reward network: microcircuitry. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2010;35:27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe MJ, Killcross S. The Prelimbic Cortex Contributes to the Down-Regulation of Attention Toward Redundant Cues. Cerebral cortex. 2012 doi: 10.1093/cercor/bhs393. [DOI] [PubMed] [Google Scholar]
- Shen H, Kalivas PW. Reduced LTP and LTD in prefrontal cortex synapses in the nucleus accumbens after heroin self-administration. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2012:1–3. doi: 10.1017/S1461145712001071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Moussawi K, Zhou W, Toda S, Kalivas PW. Heroin relapse requires long-term potentiation-like plasticity mediated by NMDA2b-containing receptors. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:19407–19412. doi: 10.1073/pnas.1112052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Scofield MD, Boger HA, Hensley-Simon ME, Kalivas PW. Synaptic Glutamate Spillover Mediates Heroin Relapse. doi: 10.1523/JNEUROSCI.4564-13.2014. unpublished. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HW, Toda S, Moussawi K, Bouknight A, Zahm DS, Kalivas PW. Altered dendritic spine plasticity in cocaine-withdrawn rats. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:2876–2884. doi: 10.1523/JNEUROSCI.5638-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Virkud A, Deisseroth K, Graybiel AM. Reversible online control of habitual behavior by optogenetic perturbation of medial prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:18932–18937. doi: 10.1073/pnas.1216264109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, Raju DV, Pare JF, Sidibe M. The thalamostriatal system: a highly specific network of the basal ganglia circuitry. Trends in neurosciences. 2004;27:520–527. doi: 10.1016/j.tins.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Quirk GJ. Prefrontal control of fear: more than just extinction. Curr Opin Neurobiol. 2010;20:231–235. doi: 10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiga S, Puddu MC, Pisano M, Diana M. Morphine withdrawal-induced morphological changes in the nucleus accumbens. Eur J Neurosci. 2005;22:2332–2340. doi: 10.1111/j.1460-9568.2005.04416.x. [DOI] [PubMed] [Google Scholar]
- Stefanik MT, Moussawi K, Kupchik YM, Smith KC, Miller RL, Huff ML, Deisseroth K, Kalivas PW, Lalumiere RT. Optogenetic inhibition of cocaine seeking in rats. Addiction biology. 2012 doi: 10.1111/j.1369-1600.2012.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, Tye KM, Kempadoo KA, Zhang F, Deisseroth K, Bonci A. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475:377–380. doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takumi Y, Ramirez-Leon V, Laake P, Rinvik E, Ottersen OP. Different modes of expression of AMPA and NMDA receptors in hippocampal synapses. Nature neuroscience. 1999;2:618–624. doi: 10.1038/10172. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Beurrier C, Bonci A, Malenka RC. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nature neuroscience. 2001;4:1217–1223. doi: 10.1038/nn757. [DOI] [PubMed] [Google Scholar]
- Thompson RH, Swanson LW. Hypothesis-driven structural connectivity analysis supports network over hierarchical model of brain architecture. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:15235–15239. doi: 10.1073/pnas.1009112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda S, Shen HW, Peters J, Cagle S, Kalivas PW. Cocaine increases actin cycling: effects in the reinstatement model of drug seeking. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26:1579–1587. doi: 10.1523/JNEUROSCI.4132-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Oever MC, Goriounova NA, Li KW, Van der Schors RC, Binnekade R, Schoffelmeer AN, Mansvelder HD, Smit AB, Spijker S, De Vries TJ. Prefrontal cortex AMPA receptor plasticity is crucial for cue-induced relapse to heroin-seeking. Nature neuroscience. 2008;11:1053–1058. doi: 10.1038/nn.2165. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, Rodriguez JJ. Projections of the central medial nucleus of the thalamus in the rat: node in cortical, striatal and limbic forebrain circuitry. Neuroscience. 2012;219:120–136. doi: 10.1016/j.neuroscience.2012.04.067. [DOI] [PubMed] [Google Scholar]
- Vezina P, Singer B, Bubula N, Bindokas V. Drug-unpaired environments regulate dendritic spine dynamics in the nucleus accumbens. American College of Neuropsychopharmacology. 2012 doi: 10.1038/npp.2016.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Baler RD, Goldstein RZ. Addiction: pulling at the neural threads of social behaviors. Neuron. 2011;69:599–602. doi: 10.1016/j.neuron.2011.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM. Putting a spin on the dorsal-ventral divide of the striatum. Trends in neurosciences. 2004;27:468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Vorel SR, Liu X, Hayes RJ, Spector JA, Gardner EL. Relapse to cocaine-seeking after hippocampal theta burst stimulation. Science. 2001;292:1175–1178. doi: 10.1126/science.1058043. [DOI] [PubMed] [Google Scholar]
- Wang X, Moussawi K, Knackstedt L, Shen H, Kalivas PW. Role of mGluR5 neurotransmission in reinstated cocaine-seeking. Addiction biology. 2013;18:40–49. doi: 10.1111/j.1369-1600.2011.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcocks AL, McNally GP. The role of medial prefrontal cortex in extinction and reinstatement of alcohol-seeking in rats. The European journal of neuroscience. 2013;37:259–268. doi: 10.1111/ejn.12031. [DOI] [PubMed] [Google Scholar]
- Wilson CJ. Passive cable properties of dendritic spines and spiny neurons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1984;4:281–297. doi: 10.1523/JNEUROSCI.04-01-00281.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME, Ferrario CR. AMPA receptor plasticity in the nucleus accumbens after repeated exposure to cocaine. Neuroscience and biobehavioral reviews. 2010;35:185–211. doi: 10.1016/j.neubiorev.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME, Tseng KY. Calcium-permeable AMPA receptors in the VTA and nucleus accumbens after cocaine exposure: when, how, and why? Front Mol Neurosci. 2012;5:72. doi: 10.3389/fnmol.2012.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CI, Beijer AV, Groenewegen HJ. Basal amygdaloid complex afferents to the rat nucleus accumbens are compartmentally organized. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1996;16:1877–1893. doi: 10.1523/JNEUROSCI.16-05-01877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CI, Groenewegen HJ. Patterns of convergence and segregation in the medial nucleus accumbens of the rat: relationships of prefrontal cortical, midline thalamic, and basal amygdaloid afferents. The Journal of comparative neurology. 1995;361:383–403. doi: 10.1002/cne.903610304. [DOI] [PubMed] [Google Scholar]
- Wu X, Shi M, Wei C, Yang M, Liu Y, Liu Z, Zhang X, Ren W. Potentiation of synaptic strength and intrinsic excitability in the nucleus accumbens after 10 days of morphine withdrawal. Journal of neuroscience research. 2012;90:1270–1283. doi: 10.1002/jnr.23025. [DOI] [PubMed] [Google Scholar]
- Wydra K, Golembiowska K, Zaniewska M, Kaminska K, Ferraro L, Fuxe K, Filip M. Accumbal and pallidal dopamine, glutamate and GABA overflow during cocaine self-administration and its extinction in rats. Addiction biology. 2013 doi: 10.1111/adb.12031. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Ramamoorthy S, Baker DA, Shen H, Samuvel DJ, Kalivas PW. Modulation of group II metabotropic glutamate receptor signaling by chronic cocaine. The Journal of pharmacology and experimental therapeutics. 2002;303:608–615. doi: 10.1124/jpet.102.039735. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Sheen W, Morales M. Glutamatergic neurons are present in the rat ventral tegmental area. The European journal of neuroscience. 2007;25:106–118. doi: 10.1111/j.1460-9568.2006.05263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Wang HL, Li X, Ng TH, Morales M. Mesocorticolimbic glutamatergic pathway. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:8476–8490. doi: 10.1523/JNEUROSCI.1598-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, McFarland K, Fan P, Jiang Z, Inoue Y, Diamond I. Activator of G protein signaling 3 regulates opiate activation of protein kinase A signaling and relapse of heroin-seeking behavior. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:8746–8751. doi: 10.1073/pnas.0503419102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nature reviews Neuroscience. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- Zahm DS. An integrative neuroanatomical perspective on some subcortical substrates of adaptive responding with emphasis on the nucleus accumbens. Neuroscience and biobehavioral reviews. 2000;24:85–105. doi: 10.1016/s0149-7634(99)00065-2. [DOI] [PubMed] [Google Scholar]
- Zhao S, Studer D, Chai X, Graber W, Brose N, Nestel S, Young C, Rodriguez EP, Saetzler K, Frotscher M. Structural plasticity of hippocampal mossy fiber synapses as revealed by highpressure freezing. The Journal of comparative neurology. 2012a;520:2340–2351. doi: 10.1002/cne.23040. [DOI] [PubMed] [Google Scholar]
- Zhao S, Studer D, Chai X, Graber W, Brose N, Nestel S, Young C, Rodriguez EP, Saetzler K, Frotscher M. Structural plasticity of spines at giant mossy fiber synapses. Front Neural Circuits. 2012b;6:103. doi: 10.3389/fncir.2012.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Homma KJ, Poo MM. Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron. 2004;44:749–757. doi: 10.1016/j.neuron.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Zhou W, Zhang F, Liu H, Tang S, Lai M, Zhu H, Kalivas PW. Effects of training and withdrawal periods on heroin seeking induced by conditioned cue in an animal of model of relapse. Psychopharmacology. 2009;203:677–684. doi: 10.1007/s00213-008-1414-2. [DOI] [PubMed] [Google Scholar]