Abstract
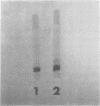
Heat treatment of highly purified staphylococcal enterotoxin B causes a more rapid loss of immunological activity at 70 to 80 C than at 90 to 100 C. Toxicological results based on intravenous injection of dogs paralleled the results obtained by immunological means (single gel diffusion). The loss of immunological activity did not follow first-order kinetics. Results are given on the effects on heat inactivation of changing pH, ionic strength, and initial concentration of enterotoxin. Disc-gel electrophoresis of purified enterotoxin B showed a major and minor band. The minor band was a size isomer of the major band.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chu F. S. Hydrogen ion equilibrai of staphylococcal enterotoxin B. J Biol Chem. 1968 Aug 25;243(16):4342–4349. [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- Joseph R. L., Baird-Parker A. C. Fractions of staphylococcal enterotoxin B. Nature. 1965 Aug 7;207(997):663–664. doi: 10.1038/207663a0. [DOI] [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- Read R. B., Jr, Bradshaw J. G. Thermal inactivation of staphylococcal enterotoxin B in veronal buffer. Appl Microbiol. 1966 Jan;14(1):130–132. doi: 10.1128/am.14.1.130-132.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH L. D., GARDNER M. V. The anomalous heat inactivation of Clostridium perfringens lecithinase. Arch Biochem. 1950 Jan;25(1):54–60. [PubMed] [Google Scholar]
- Satterlee L. D., Kraft A. A. Effect of meat and isolated meat proteins on the thermal inactivation of staphylococcal enterotoxin B. Appl Microbiol. 1969 Jun;17(6):906–909. doi: 10.1128/am.17.6.906-909.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schantz E. J., Roessler W. G., Wagman J., Spero L., Dunnery D. A., Bergdoll M. S. Purification of staphylococcal enterotoxin B. Biochemistry. 1965 Jun;4(6):1011–1016. doi: 10.1021/bi00882a005. [DOI] [PubMed] [Google Scholar]
- WILLIAMS D. E., REISFELD R. A. DISC ELECTROPHORESIS IN POLYACRYLAMIDE GELS: EXTENSION TO NEW CONDITIONS OF PH AND BUFFER. Ann N Y Acad Sci. 1964 Dec 28;121:373–381. doi: 10.1111/j.1749-6632.1964.tb14210.x. [DOI] [PubMed] [Google Scholar]
- Weirether F. J., Lewis E. E., Rosenwald A. J., Lincoln R. E. Rapid quantitative serological assay of staphylococcal enterotoxin B. Appl Microbiol. 1966 Mar;14(2):284–291. doi: 10.1128/am.14.2.284-291.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]