Abstract
To screen additives and their mixed ratio suitable for the mycelial growth and fruiting body formation of Oudemansiella radicata in the oak sawdust, additives such as rice bran, fermented soybean powder and wheat bran were used. Generally, the mycelial growth of O. radicata has been stable on oak sawdust mixed with rice bran of 5~20%. In case that O. radicata was cultured for about 30 days at 22 ± 1℃ under the illumination (350 lux) of 12 hours and moisture condition of 90 ± 5%, the primordia have been formed gradually from red-brown crusts covering the surface of oak sawdust media. Based on the experimental results from 9 strains of O. radicata, fruiting bodies were produced widely on oak sawdust medium mixed with rice bran of 5 to 30%. Even though fruiting bodies of O. radicata have been produced well on oak sawdust media mixed with rice bran, fruiting bodies of O. radicata were produced intensively on oak sawdust media mixed with rice bran of 10%. Therefore, this result will provide a basic information for commercial production of fruiting body of wild O. radicata. This result is the first report associated with an artificial fruiting body formation of O. radicata in Korea.
Keywords: Oudemansiella radicata, Additives, Commercial production, Fruiting bodies
Oudemansiella radicata (Relhan ex Fr.), one of edible and medicinal mushrooms belonging to Tricholomataceae, Agaricales has been known to be inhabited on the soil surface or rotted woods of the broad-leaved trees from summer to autumn (Lee, 1988). O. radicata has been collected occasionally in the remote sites of mountainous area of large parks in Korea (Kim et al., 2005). Also, fruiting body of O. radicata has been reported to possess oudenone which is one of medicinally important chemical compounds (Umezawa et al., 1973; Tsantrizos et al., 1999). The oudenone exhibited an outstanding therapeutic and inhibitory effect on the sarcoma 180 and Erhrlich carcinoma (Umezawa et al., 1973; Anke and Werle, 1990). In the submerged culture, O. radicata has been known to secrete oudemansin which exhibits high antifungal activities to plant pathogenic fungi such as Penicillium notatum, Ustilago nuda and Alternaria poli (Anke and Werle, 1990). Oudemansin was also produced from fruting body of O. mucida as well as O. radicata (Anke et al., 1979, 1983).
Although O. radicata has been considered as one of the promising edible fungi (Umezawa et al., 1973; Anke and Werle, 1990; Tsantrizos et al., 1999), there was no an experiment for producing fruiting bodies of O. radicata in Korea. As part of preliminary experiment for realizing an artificial mass production of O. radicata, Kim et al. (2005) reported the optimal culture conditions such as temperature, pH, nutrients and culture media suitable for its mycelial growth. Therefore, this study was carried out to screen an additive suitable for the mycelial growth of O. radicata on sawdust media and to test if the additive could produce fruiting body of O. radicata.
Materials and Methods
Cultures
Nine strains of O. radicata were obtained from the Culture Collection of Wild Mushroom Species (CCWM) in the Department of Biology, University of Incheon (Table 1). To facilitate various tests in oak sawdust media, O. radicata was transferred to PDA, incubated at 25℃ until it exhibited a full growth in the dark condition and kept at 5℃ for further use. Unless otherwise stateed, all the tests which the strain was used were performed at least twice.
Table 1.
Oudemansiella radicata used in this study
Screening of an additive suitable for the mycelial growth of O. radicata
The preparation of sawdust medium mixed with each of 3 additives
To screen an additive suitable for the mycelial growth and fruiting body formation of O. radicata, 3 additives such as rice bran, fermented soybean powder and wheat bran were used. Each additive was mixed with oak sawdust (Quercus variabilis) at the ratio of 5, 10, 15, 20, 25 and 30% (v/v), adjusted to the moisture content of 70%, put into the round glass column (2 × 22 cm) and steam-sterilized for 90 minutes at 121℃.
The inoculation and culture of O. radicata
Of 9 strains of O. radicata, 3 strains such as IUM 770, IUM 767 and IUM 766 were selected randomly and inoculated on the sawdust media. A 5 mm plug of inocula was removed with cork borer from 7 days old cultures of O. radicata, placed on the surface of sawdust media in the column (2 × 22 cm) and incubated for 60 days at 24 ± 1℃ under the dark condition. Based on sawdust media mixed with each of rice bran, fermented soybean powder and wheat bran, the mycelial growth of O. radicata has been measured once in every 5 days for 60 days of incubation. After 60 days of incubation, a suitable additive was screened and then used for producing fruiting body of O. radicata in the sawdust media.
Fruiting body formation of O. radicata in sawdust media
The preparation of spawn
A total of 9 strains of O. radicata were used to test fruiting body formation (Table 1). To prepare each spawn of 9 inoculum sources, oak sawdust was mixed throughly with 20% rice bran (v/v), adjusted to the moisture content of 70%, put into an Erlenmayer flask (250 ml), steam-sterilized for 90 minutes at 121℃. To inoculate each of 9 strains, a 5 mm plug of inocula was removed with cork borer from 7 days old cultures of O. radicata, placed on the surface of sawdust media into an Erlenmayer flask (250 ml) and cultured for 10 days at 24 ± 1℃.
The preparation of suitable sawdust media
After an additive such as rice bran suitable for the mycelial growth of O. radicata was screened, sawdust medium was prepared to produce its fruiting body. The oak sawdust was mixed throughly with 5%, 10%, 15%, 20%, 25% and 30% rice bran (v/v), adjusted to the moisture content of 70%, put into plastic bottles (800 ml), marking a hole with glass bar (diameter 1.5 × depth 8 cm) in the center of sawdust medium and steam-sterilized for 90 minutes at 121℃.
The inoculation and incubation of O. radicata
About 20 grams of each inoculum was removed from fully grown sawdust spawn, inoculated on the surface of sawdust medium in the polyethylene plastic bottle (800 ml), and cultured for 30 days at 24 ± 1℃ under the dark condition.
Conditions for primordium formation
When the mycelia of O. radicata were colonized completely from the top of cultivation bottle to bottom, the mycelia of O. radicata on the top of the medium was scratched slightly with a spatula, transferred to an another room to induce an occurrence of primordia and cultured for 2 days at 15 ± 1℃ under the illumination (350 lux) of 12 hours and 90 ± 5% of humidity in a day.
Conditions for fruiting body formation
After 2 days of culture at 15 ± 1℃, the cultures of O. radicata were transferred to incubation room, cultured at 22 ± 1℃ under the illumination (350 lux) of 12 hours and relative humidity of 90 ± 5% in a day and checked to identify fruiting body formation once in a day.
Results and Discussions
Screening of an additive suitable for the mycelial growth of O. radicata
After 60 days of incubation in the columns at 24 ± 1℃, the mycelial growth of O. radicata was analyzed. Of 3 additives used for measuring the myceilal growth of 3 strains of O. radicata, rice bran stimulated its mycelial growth. The oak sawdust which was mixed with rice bran of 5~20% showed a favorable mycelial growth (Table 2). Generally, the mycelial growth and density of O. radicata seemed to be more favorable on oak sawdust mixed with rice bran than fermented soybean powder or wheat bran. Particularly, all 3 strains of O. radicata showed their outstanding mycelial growth on oak sawdust mixed with 10% rice bran. Rew et al. (2004) reported that the mycelial growth of Phellinus baumii was outstanding in oak sawdust mixed with 20% of rice bran (v/v). Therefore, it seems to be reasonable that rice bran contains some ingredients to promote the favorable mycelial growth of O. radicata and the other mushroom.
Table 2.
Effect of 3 additives for the mycelial growth of Oudemansiella radicata in oak sawdust media
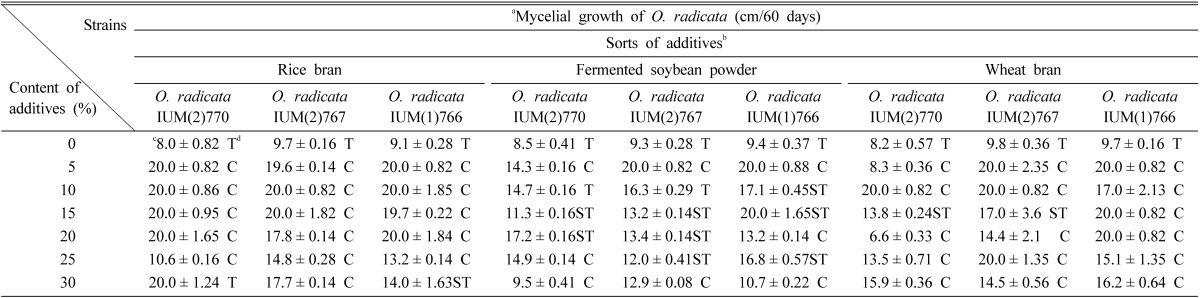
aThree of 9 strains were selected randomly, inoculated on the sawdust media and cultured to check their mycelial growth.
bEach of 3 additives was mixed with oak sawdust (such as sawdust of Quercus variabilis) at the ratio of 5%, 10%, 15%, 20%, 25% and 30%, respectively and then put in the column (2 × 22 cm).
cValues are an average of 4 replications. and, within columns, are significantly different at p = 0.01.
dC, Compact; ST, Somewhat thin; T, thin.
Fruiting body formation of O. radicata
Conditions for primordium formation
After a slight scratch of fully grown mycelia on oak sawdust media filled in the polyethylene plastic bottles, the polyethylene plastic bottles were kept for 2 days at 15 ± 1℃ under the illumination (350 lux) of 12 hours and relative humidity of 90 ± 5% in a day. After O. radicata was kept for 2 days at 15 ± 1℃, O. radicata was transferred to another incubation room and cultured for 30 days at 22 ± 1℃ under the illumination (350 lux) of 12 hours and relative humidity of 90 ± 5% in a day. O. radicata has been started to form primordia from its red-brown mycelial crusts covering the surface of oak sawdust medium 30 days after the culture (Fig. 1). The primordia have been observed firstly from O. radicata IUM 1259 and IUM 1260, respectively.
Fig. 1.
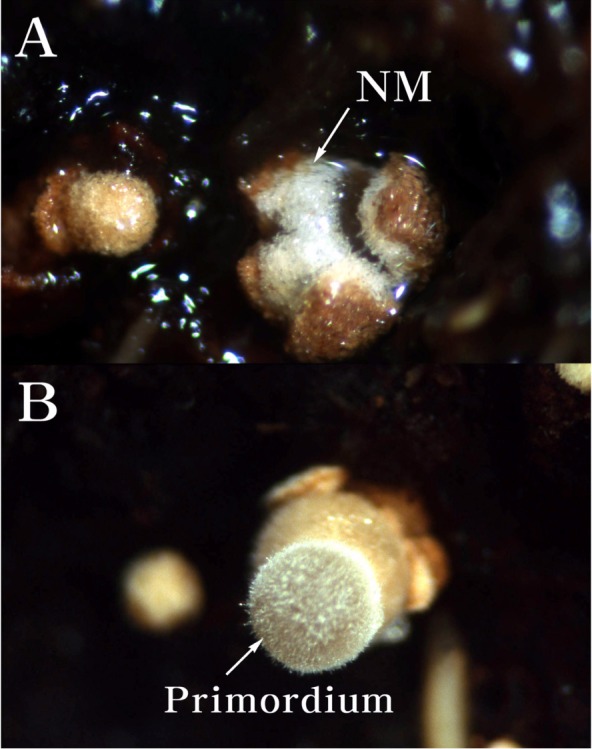
The primordium of Oudemansiella radicata formed on sawdust media of Quercus variabilis mixed with 10% rice bran. A, The protrusion (18×) of new mycelium (NM) originated from a mycelial mass of O. radicata covering the surface of oak sawdust media. B, The primitive primordium (18×) of O. radicata on the surface of oak sawdust media.
Conditions for fruiting body formation
After primordia of O. radicata were formed, fruiting bodies were produced on oak sawdust medium mixed with rice bran of 5 to 30% (Table 3). Of 9 strains, O. radicata IUM 1260 formed its mature fruiting body 6 days after an occurrence of primordia (Fig. 2).
Table 3.
The fruiting body formation of Oudemansiella radicata on oak sawdust mediaa
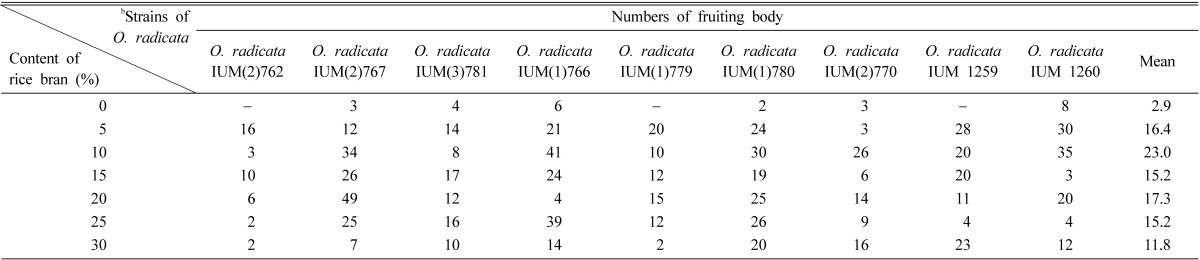
aThe sawdust of oriental oak (Quercus variabilis) was mixed with 5%, 10%, 15%, 20%, 25% and 30% rice bran (v/v), respectively.
bEach of nine strains was treated by 5 replications.
Fig. 2.

The fruiting bodies of Oudemansiella radicata on sawdust media of Q. variabilis mixed with 10% rice bran.
Although fruiting bodies of 9 strains have been produced widely on oak sawdust media mixed with rice bran of 5 to 30%, the contamination of oak sawdust media seemed to be increased in proportion to the high mixture of rice bran. Generally, fruiting bodies of O. radicata were produced intensively on oak sawdust media mixed with rice bran of 10% (Table 3). According to Semerdzieva et al. (1988), the fruiting body of O. radicata has been known to be produced 10 weeks after the culture in case that both stationary and submerged cultures of O. radicata were cultured fully in the dark at 24℃, transferred to a lower temperature and periodically illuminated. In the further experiments, the more progressive method to produce fruiting body of O. radicata can be developed in the process of comparing and innovating our method with that of Semerdzieva et al. (1988). After all, it is a meaningful fact that this result is the first report associated with fruiting body formation of O. radicata in Korea.
Acknowledgement
This work was supported by research grant (No. 2040393) from Agricultural Promotion Research Center (APRC) in the Ministry of Agriculture and Forestry.
References
- 1.Anke T, Hecht HJ, Schramm G, Steglich W. Antibiotics from basidiomycetes IX. Oudemansin, an antifungal antibiotic from Oudemansiella mucida (Schrader ex Fr.) Hoehnel (Agaricales) J Antibiot (Tokyo) 1979;32:1112–1117. doi: 10.7164/antibiotics.32.1112. [DOI] [PubMed] [Google Scholar]
- 2.Anke T, Besl H, Mocek U, Steglich W. Antibiotics from basidiomycetes XVIII. Strobilurin C and oudemansin B, two new antifungal metabolites from Xerula species (Agaricales) J Antibiot (Tokyo) 1983;36:661–666. doi: 10.7164/antibiotics.36.661. [DOI] [PubMed] [Google Scholar]
- 3.Anke T, Werle A, Bros M, Steglich W. Antibiotics from basidiomycetes XXXIII. Oudemansin X, A new antifungal F-β-methoxyacrylate from Oudemansiella radicata (Relhan ex Fr.) sing. J Antibiot (Tokyo) 1990;48(8):1010–1011. doi: 10.7164/antibiotics.43.1010. [DOI] [PubMed] [Google Scholar]
- 4.Kim SB, Kim SH, Lee KL, Shim JO, Lee MW, Shim MJ, Lee UY, Lee TS. The optimal culture conditions for the mycelial growth of Oudemansiella radicata. Mycobiology. 2005;33:230–234. doi: 10.4489/MYCO.2005.33.4.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee JY. Coloured Korean mushrooms. Seoul, Korea: Academy Press; 1988. [Google Scholar]
- 6.Rew YH, Cho WS, Lee JM, Kim JK. Cultural characteristics of Phellinus baumii grown in bottle. Korean J Mycol. 2004;32:101–104. [Google Scholar]
- 7.Sbmerdžleva M, Buchalo AS, Hübsch P, Zakordonec OA, Wasser SP, Musilek V. Comparative study of cultures of four species of the genus Oudemansiella. Folia Microbiol. 1988;33:115–120. [Google Scholar]
- 8.Tsantrizos YS, Yang X, McClory A. Studies on the biosynthesis of the fungal metabolite oudenone. 2. synthesis and enzymatic cyclization of an diketone, open-chain procurser into oudenone in cultures of Oudemansiella radicata. J Org Chem. 1999;64:6609–6614. doi: 10.1021/jo9901135. [DOI] [PubMed] [Google Scholar]
- 9.Umezawa H, Tanabe O, Takeuchi T. A new hypotensive agent, oudenone, its salts and processes for production and preparation thereof. Res Found [JP] - Patent No., GB1333935. Microbial Chem. 1973 (Complete Specification)


