Abstract
Human peripheral cannabinoid receptor CB2, a G protein-coupled receptor (GPCR) involved in regulation of immune response has become an important target for pharmaceutical drug development. Structural and functional studies on CB2 may benefit from immobilization of the purified and functional receptor onto a suitable surface at a controlled density and, preferably in a uniform orientation. The goal of this project was to develop a generic strategy for preparation of functional recombinant CB2 and immobilization at solid interfaces. Expression of CB2 as a fusion with Rho-tag (peptide composed of the last nine amino acids of rhodopsin) in E. coli was evaluated in terms of protein levels, accessibility of the tag, and activity of the receptor. The structural integrity of CB2 was tested by ligand binding to the receptor solubilized in detergent micelles, captured on tag-specific monoclonal 1D4 antibody-coated resin. Highly pure and functional CB2 was obtained by sequential chromatography on a 1D4- and Ni-NTA- resin and its affinity to the 1D4 antibody characterized by surface plasmon resonance (SPR). Either the purified receptor or fusion CB2 from the crude cell extract was captured onto a 1D4 -coated CM4 chip (Biacore) in a quantitative fashion at uniform orientation as demonstrated by the SPR signal. Furthermore, the accessibility of the extracellular surface of immobilized CB2 and the affinity of interaction with a novel monoclonal antibody NAA-1 was studied by SPR. In summary, we present an integral strategy for purification, surface immobilization, ligand- and antibody binding studies of functional cannabinoid receptor CB2.
Keywords: Cannabinoid receptor CB2, surface plasmon resonance (SPR), Rho-tag, functional immobilization
1. Introduction
Human peripheral cannabinoid receptor CB2, a 7-transmembrane domain, G protein-coupled receptor (GPCR) is a part of the endocannabinoid system and is primarily found in tissues and cells of the immune system. CB2 mediates physiological pathways implicated in regulation of the immune response primarily via inhibition of adenylate cyclase [1].
With the goal of performing structural and functional studies on CB2, procedures for expression of the receptor in E. coli, chromatographic affinity purification using His- and Strep-tagged constructs and stabilization in detergent micelles have been developed in our laboratory [2-4]. However, certain challenges inherent to this target protein still need to be overcome. For example, the necessity of using detergents for protein solubilization during purification has known negative influence on affinity of His- and Strep-tags to their respective resins, thus directly impacting protein yield and purity. Characterization of the functional properties of the recombinant CB2 by ligand binding is also non-trivial due to the strong hydrophobicity of cannabinoid ligands, the endogenous 2-AG and anandamide, but also of the plant derived- and synthetic cannabinoids like Δ9-tertahydrocannabinol (Δ9-THC) and the high-affinity agonist CP-55,940. Finally, immobilization of the receptor on a solid support for ligand binding or surface-plasmon-resonance (SPR) assays requires careful preservation of the functional structure of CB2 at conditions that are potentially harmful for stability of this very labile protein [2]. The objective of this work was to develop procedures for tight, reversible and specific surface immobilization of CB2 for purification, functional characterization, and the study of molecular interactions including binding affinity and kinetics by SPR.
In a search for a small affinity tag that would be compatible with high level expression of a functional GPCR in E. coli, efficient purification in the presence of detergents, and a specific, tight and reversible surface immobilization, we selected a peptide composed of the last nine amino acid residues of bovine rhodopsin (Rho-tag) that selectively binds to 1D4 monoclonal antibody. The 1D4 antibody was first introduced and characterized by Molday et al. [5] and is widely used for affinity purification of rhodopsin [6] and other proteins engineered to contain the epitope [7], as well as in expression of other GPCR like CB1-Rho-tag [8], the chemokine receptors CXCR4 and CCR5 [9], a series of GPCR expressed in a cell-free system [10], and for production of paramagnetic liposomes [11, 12]. For SPR, the Rho-tag was first used to capture GPCR from crude cell lysates on a hydrazide-modified L1 sensor chip (Biacore) via 1D4 antibody followed by reconstitution of a lipid environment, and on CM5 (Biacore) sensor chip without reconstitution into bilayers by Stenlund et al. [13]. Later attempts have used a CM4 chip (Biacore) for immobilization of 1D4 and consecutive capturing of GPCR with a Rho-tag to explore solubilization [14, 15] and crystallization [16] conditions for chemokine receptors, as well as binding of ligands and small-molecule inhibitor to CXCR4 and CCR5 receptors [17, 18].
Although the Rho-tag/1D4 antibody system has been used successfully for purification of several other GPCR expressed in eukaryotic cells, the expression of Rho-tagged proteins in bacterial cells has not been explored so far. Therefore, the first stage of our study was devoted to examining the suitability of the Rho-tag for expression of the fusion construct in E. coli. The functional characterization and purification of the Rho-tagged CB2 from the best performing constructs was carried out taking advantage of Rho-tag interaction with resin-immobilized 1D4 antibody. Finally, we characterized the Rho-tag/ 1D4 interaction in detergent-containing buffers and used the Rho-tag on CB2 for surface capturing on CM4-1D4 antibody-covered chips for Surface Plasmon Resonance (SPR) experiments to assess the efficiency of capturing, the feasibility of enzymatic removal of the MBP fusion partner of the immobilized receptor, and its interaction with a monoclonal NAA-1 antibody raised against CB2.
2. Materials and Methods
2.1. Chemicals and reagents
Oligonucleotides were purchased from Operon Biosciences. Restriction- and DNA-modifying enzymes were obtained from New England Biolabs. The Ni–NTA resin was from Qiagen, the CNBr-activated Sepharose from GE Healthcare. The monoclonal antibody against CB2 (NAA-1) was from Epitomics and 1D4 antibody – from the University of British Columbia, Vancouver, Canada. [3H]-CP-55,940 (specific activity 139.6 Ci/mmol) and [35S]-γ-GTP (specific activity 1250 Ci/mmol) were purchased from Perkin-Elmer. Alexa Fluor488 reactive dye was from Invitrogen. Research grade sensor chip CM4, immobilization reagents NHS, EDC, ethanolamine and HBS-N buffers for SPR experiments were from GE Healthcare.
Cholesteryl hemisuccinate Tris salt (CHS), the detergents 3[(cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS) and n-dodecyl-β-dmaltoside (DDM) were obtained from Anatrace. N-Octyl-β-d-glucopyranoside (OG) was purchased from Calbiochem. Lipids 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoserine sodium salt (POPS) were purchased from Avanti Polar Lipids Inc.
2.2. Expression vectors and strains
E. coli strain DH5α was obtained from Invitrogen and E. coli strain BL21(DE3) was purchased from Agilent Technologies. Plasmid for expression of Gα was a kind gift from Dr. J. Northup (NIDCD/NIAAA, NIH). The plasmid for expression of MBP-TEV protease (pRK1043) was a gift from Dr. D.S. Waugh (NCI-Frederick, NIH).
2.3. Expression and purification of CB2
Construction of plasmids, expression of CB2 fusion proteins1, preparation of membranes, functional characterization, solubilization of CB2 into detergent micelles, expression and purification of TEV protease, expression and purification of subunits of G proteins, preparation and regeneration of 1D4-Sepharose resin and chromatographic purification of CB2 is described in Supplemental Materials.
2.4. Ligand binding on CB2 in detergent micelles
Ligand binding on CB2 in detergent micelles was performed as follows. 2 mL of wash buffers were prepared by mixing 50 mM Tris, pH 7.5 containing 200 mM NaCl, 30 % (v/v) glycerol, 0.5% (w/v) CHAPS, 0.1% (w/v) DDM, 0.1 % (w/v) CHS (buffer A). Buffers were supplemented with a mixture of [3H]-CP-55,940 and unlabeled CP-55,940 (specific activity 50 mCi/mmol), so that the ligand concentration ranged from 0 to 50 μM, and kept on ice until use.
Fusion CB2-255(Table 1) was purified on a 1D4-Sepharose (as described in Supplemental Materials), and the resin with immobilized protein stored frozen at −80 °C until use. The resin was re-suspended in 2 bed volumes of ice-cold buffer A supplemented with 10 μM of stabilizing ligand CP-55,940, and aliquoted into 0.5 mL Ultrafree centrifugal filters (PVDF 0.45 μm pore diameter, Millipore). Typically, each sample contained 50 μL of resin with ~25 μg of immobilized CB2-255. Samples were washed 3 × 300 μL of buffer A + 10 μM CP-55,940 at 1,500 × g, 1 min each in a refrigerated Eppendorf centrifuge. The stabilizing ligand (unlabeled CP-55,940) was then removed by washing the resin with 5 × 200 μL of corresponding [3H]-CP-55,940-containing wash buffers, by centrifugation at 500 g for 1.5 min. The resin was then re-suspended in 200 μL of a wash buffer and incubated on ice for additional 2 hours. Upon incubation, samples were centrifuged at 12,000 × g for 30 sec and washed with 4 × 300 μL ice-cold buffer A (without ligand). The resin was re-suspended in 250 μL of elution buffer (buffer A supplemented with 4 mM Rho peptide and NaCl concentration raised to 1 M), incubated for 15 min on ice, and the eluate collected by centrifugation (500 × g, 2 min). The elution was repeated 3 more times, and the eluate fractions were combined. An aliquot was used to determine the content of [3H]-CP-55,940 on a scintillation counter.
Table 1. Expression levels and functional activity of CB2 in Rho-tag fusion constructs.
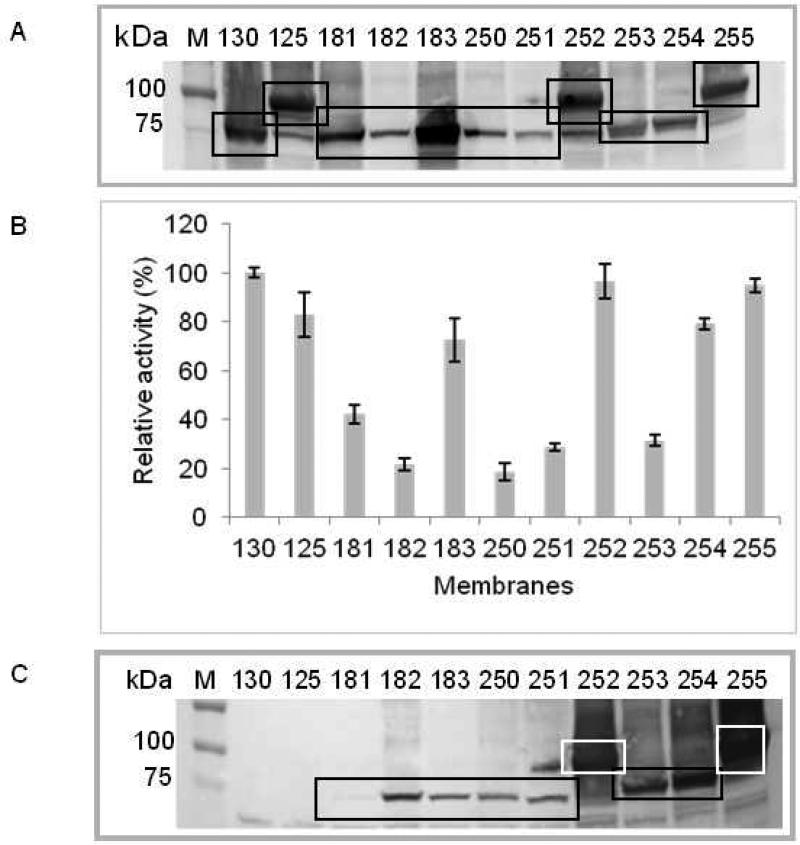
To quantify the relative levels of the constructs expressing Rho-tagged CB2 with respect to the control (CB2-130), the analysis of Western blot probed with anti- CB2 antibody (Fig. 1A) was performed by densitometry using the Kodak 1D program for data analysis. The intensity corresponding to CB2-130 was arbitrary chosen as 100%. The activity values for each construct presented in Fig. 1 B were calculated as a percentage of the activity displayed by the control CB2-130. For calculation of specific activity (%), the activity values (CPM) were divided by the amount of CB2 present in the corresponding band, normalized to 1 μg of total protein in membranes, and calculated as a percentage of the specific activity of CB2-130. The expression level was estimated to be 3 ng of CB2-130 per μg of total protein [2]. For qualitative assessment of Rho-tag accessibility in the fusion CB2, intensity of bands from Western blots visualized with 1D4 (Fig. 1 C) were normalized by dividing them by intensities of bands visualized with anti-CB2 antibody (Fig. 1 A). Constructs with ratios of 2-3 were classified as having highly accessible tag (+++); ratios of 1-2, medium accessible tag (++), ratios of 0.5-1, low accessible tag (+) and ratio <0.5 as not accessible (−).
| Construct | Fusion protein components | CB2 % | Activity % (G-protein activation) | SA4 %G-protein activation) | Accessibility of lD4 | Bmax (pmo1 CP 55940/mg total protein) | KD (nM) |
|---|---|---|---|---|---|---|---|
| 130 (control) | MBP-TEV-Strep-CB2-His10 | 100 | 100 | 100 | NA | 2.4 ± 0.2 | 2.2 ± 0.4 |
| 125 (control) | MBP-TEV-Strep-CB2-TEV-TrxA-His10 | 123 | 80.4 | 65 | NA | ND | ND |
| 181 | MBP-TEV-Step-Rho-CB2-His10 | 71 | 33.4 | 47 | − | 0.4 00.4 | 2.2 ± 0.5 |
| 182 | MBP-TEV-CB2-Rho | 30 | 9.6 | 32 | ++ | ambiguous | ambiguous |
| 183 | MBP-TEV-Strep-CB2-Rho-His10 | 177 | 68.4 | 39 | − | 1.9 ± 0.3 | 3.0 ± 0.8 |
| 250 | MBP-TEV-CB2-His10-Rho | 43 | 6.2 | 15 | + | ND | ND |
| 251 | MBP-TEV-His10-CB2-Rho | 18 | 17.9 | 98 | ++ | ND | ND |
| 252 | MBP-TEV-His10-CB2-TEV-TrxA-Rho | 120 | 96.1 | 80 | +++ | 2.8 ± 0.7 | 2.0 ± 1.2 |
| 253 | MBP-TEV-CB2-spacer 1-Rho1 | 53 | 21.0 | 40 | +++ | ND | ND |
| 254 | MBP-TEV-CB2-spacer 2-Rho2 | 55 | 76.1 | 138 | +++ | ND | ND |
| 255 | MBP-TEV-His10-CB2-spacer 3-TrxA-Rho3 | 98 | 94.0 | 96 | +++ | 7.8 ± 4.2 | 6.2 ± 4.6 |
Spacer 1: A2HMG3SAS
Spacer 2: A2HMA3N5AS
Spacer 3: A3N5G3SENLYG2SG3SEF
Specific activity
Since the concentration of the radiolabeled CP-55,940 in this assay is in the micromolar range, it is not feasible to determine levels of non-specific binding by adding much higher concentrations of non-labeled ligand. Therefore, to correct for non-specific binding of CP-55,940, we measured the amount of the radiolabeled ligand that remained bound to the receptor immobilized to the resin upon exposure of the sample to 65 °C for 1 hour. We have shown earlier that such treatment completely inhibits specific ligand binding to CB2 [2]. The levels of nonspecific binding to the resin with immobilized receptor did not exceed 25% of total binding (results not shown).
2.5. Thermoinactivation of CB2 in micelles
The thermoinactivation of CB2 in detergent micelles was measured by quantifying the loss of binding of [3H]-CP-55,940 to resin-immobilized fusion CB2-255. The experiment was performed as follows: 50 μL of 1D4-Sepharose containing 25 μg of purified fusion CB2-255 were dispensed in Ultrafree centrifugal filters (Millipore) on ice and washed with 3 × 250 μL of a wash buffer (either buffer A or buffer A without CHS) supplemented with 20 μM of CP-55,940 by centrifugation in a refrigerated centrifuge at 1,500 × g for 1 min. The resin was re-suspended in 200 μL of the wash buffer and placed into a water bath kept at 4 °C. A set of samples was then exposed to a linear temperature gradient of 1 °C/ min from 4 to 84 °C and one sample withdrawn at temperature intervals of 10 °C and placed on ice. Samples then were centrifuged at 1,500 × g for 1 min, and washed 3 times with 250 μL of buffer A supplemented with 20 μM CP-55,940 and 3.6 nM of [3H]-CP-55,940 by centrifugation at 500 × g for 2 min. The resin was re-suspended in 200 μL of the same buffer and incubated on ice for 2 hours. Samples were centrifuged at 12,000 × g for 1 min, and washed 4 times with ice-cold buffer A (without ligand) at 12,000 × g, 1 min. The CB2 protein was eluted by addition of 4 mM Rho peptide to buffer A at an elevated NaCl concentration of 1M, as described above, the eluted fractions combined, and the radioactivity counted.
2.6. Reconstitution of the purified receptor into liposomes for antibody production
Reconstitution of the purified CB2 protein into liposomes was performed using Pierce detergent removal resin (Thermo Scientific). Purified protein (13 μg) in buffer A was mixed with 132 μg of lipids POPC/POPS (4/1, mol/mol) dissolved at a concentration of 3.3 mg/mL in 1% (w/v) CHAPS [2]. The solution (80 μL) was loaded onto a 0.5 mL Pierce detergent removing spin column and proteoliposomes were eluted following manufacturer's instructions.
2.7. Preparation of antibody against CB2
Monoclonal antibody NAA-1 against CB2 was raised by Epitomics, Inc. (Burlingame, CA) in rabbit using purified, liposome-reconstituted CB2-130 as antigen [3] . Briefly, two rabbits were immunized, and the antisera titer was evaluated using ELISA in MaxiSorb plates (Millipore) containing antigen (functional proteoliposome-reconstituted CB2). One rabbit was then selected, splenectomy performed, and lymphocytes isolated. Lymphocyte fusion was then constructed, and an ELISA screen in 96-well plates against the screening antigen performed. The IgG heavy chain and light chain cDNAs of the best performing clones were cloned into a mammalian expression vector and transiently expressed in mammalian HEK293-derived cell lines, and the expressed recombinant antibody was tested in standard ELISA.
2.8. Surface Plasmon Resonance experiments
CM4 sensor chips were used for SPR experiments performed in a Biacore 3000 biosensor (GE Healthcare) at a temperature of 25 °C or 10 °C as indicated in the text.
2.8.1. Immobilization of 1D4 antibody
The 1D4 antibody was immobilized on the CM4 chip surface at 25 °C by amine coupling using 10 mM HEPES pH 7.4 and 150 mM NaCl (HBS-N) as a running buffer at a flow rate of 5 μL/min following the standard protocol recommended by the manufacturer with minor modifications. Briefly, upon activation of surfaces with 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS) for 7 min, 1D4 antibody diluted in 10 mM sodium acetate, pH 5.5 was applied onto the surface. The surface density of the immobilized antibody was controlled by varying the concentration of antibody from 2 to 10 μg/mL and the injection time so that densities ranging from 300 to 7000 Response Unites (RU) [19] were achieved. Excess activated groups were blocked with two consecutive injections (35 and 5 μL) of 1 M ethanolamine-HCl, pH 8.5. Then the surface was stabilized by several consecutive 50 μL injections of 2M NaCl and running buffer HBS-N at a flow rate 50 μL/min. For each sensor chip, a reference surface was generated by treatment with amine coupling reagents with or without antibody, depending on the experiment.
2.8.2. Surface-capture of Rho-tagged CB2
Capturing of CB2 on 1D4 antibody coated surfaces was performed at 25°C, at a flow rate of 2 μL/min using buffer CSPR (50 mM Tris, pH 7.5, 100 mM NaCl, 10% glycerol, 0.5% CHAPS, 0.1% DDM, 0.1% CHS and 10 μM CP-55,940) as running buffer. Quantitative capturing of CB2 (2500-3500 RU) was performed by passing 40 μL of either undiluted crude protein extract or purified CB2 solution (0.5 μM) in running buffer over a surface coated with 6000-7000 RU of 1D4 antibody, whereas lower density capture of CB2 (140-420 RU) was achieved by injection of purified CB2 solution (0.2 μM) in running buffer over a surface with 1D4 antibody immobilized at lower density (1000 RU).
2.8.3. On-chip proteolytic cleavage of CB2-fusion with TEV protease
Following the capture of the CB2-fusion from crude extract, a TEV protease solution (1 mg/mL) was injected over 1D4 antibody-coated (reference) and CB2-coated flow cells at a flow rate of 2 μL/min for 20 min at 25 °C. Then the running buffer was injected for another 10 min at the same flow rate.
2.8.4. Binding of anti CB2 monoclonal antibody NAA-1 to immobilized CB2 on chip surface
Binding of monoclonal antibody NAA-1 to captured fusion CB2 (before and after removal of MBP with TEV protease), was performed in a running buffer Tris-buffered saline + 0.05% (v/v) of Tween-20 (TBS-T) or CSPR, depending on the experiment at a flow rate of 2 μL/min using a 1D4 antibody-coated surface as a reference. Anti-CB2 antibody NAA-1 (1 μM) was injected for 10 min over both surfaces, followed by running buffer for another 10 min or longer.
2.8.5. Determination of the KD value for 1D4-CB2 interaction
Equilibrium titration experiments were performed at 10 °C at a flow rate of 5 μL/min, using surfaces coated with 1D4 antibody at densities of 300, 600 and 1000 RU and a reference generated by mock coupling without antibody. Running buffer, CSPR, was kept on ice during the entire experiment, and samples were kept on ice until injection. CB2-255 solutions (0, 1.9, 5.8, 17.2, 51.8 and 155.3 nM) were successively injected over both the active and reference surfaces; the contact time was kept long enough to ensure that the sensorgram approached a steady reading. The first three injections lasted for 1 hour or longer and additional injections for 40 minutes or longer. Then the running buffer was passed through the cells for 1 hour and the surface was regenerated as described below. The same experiment was repeated using CSPR buffer for all injections and the results used for double referencing to correct the signal for effects not related to CB2 binding.
2.8.6. Determination of the KD value for CB2 - anti CB2 antibody interaction
Equilibrium titration experiments were conducted at 25 °C at a flow rate of 30 μL/min. Purified CB2 was captured at densities of 140 and 420 RU on surfaces coated with 1D4 antibody (1000 RU), while the third antibody-coated surface served as a reference. Anti-CB2 Ab NAA-1 was diluted from a stock solution into buffer CSPR, dialyzed against the same buffer for 2 hours at 4 °C and further diluted to working concentrations. Antibody solutions at concentrations 0, 0.62, 1.83, 5.5, 16.6, 50.0, 150.0 and 450.0 nM were successively injected over CB2-coated and reference surfaces. The buffer injection lasted for 5 minutes, the first four antibody injections lasted for 10 minutes, and the remaining injections for 5 minutes. Then the running buffer was passed through the cells for 1 hour and surfaces were regenerated as described below. The same experiment was repeated using CSPR buffer for all injections, for double referencing to correct the signal for effects not related to CB2 capture.
2.8.7. Regeneration of the sensor chip surface
Removal of captured Rho-tagged CB2 was performed by displacement of the protein by Rho peptide. Protocols were somewhat different depending on temperature, 25°C or 10 °C. At 25 °C, a solution of 4 mM Rho peptide in 1M NaCl was injected at a flow rate of 1 μL/min for 10 min, followed by two additional 5 min injections. Bound peptide was then removed at a flow rate of 50 μL/min injecting 5 μL of 1% OG in 10 mM NaOH twice followed by two injections of 50 μL of running buffer. At 10 °C, three Rho-peptide/ NaCl injections (30, 10 and 10 min, respectively) at 2 μL/min and three OG/NaOH injections were performed.
2.8.8. SPR data analysis
Sensorgrams were processed using version 4.0 BIAevaluation software (Biacore, GE HealthCare) to subtract the signal response from the designated reference surface and to subtract remaining background signals measured in blank buffer injections. The values of the thermodynamic dissociation constant, KD, and the kinetic rate constant of enzymatic cleavage were calculated using GraphPad Prism software.
3. Results
3.1.Expression of Rho-tagged CB2
Selection for a high level expression of functionally active CB2 in E. coli cells was performed by testing nine constructs with alternative locations of the Rho-tag with respect to CB2 and to other fusion partners (Table 1, Fig. S1). All of these constructs encode human CB2 as a fusion with N-terminal maltose binding protein (MBP) followed by a tobacco etch virus (TEV) protease recognition site, as well as one or more of the following epitopes: His-tag, Strep-tag, Thioredoxin A and Rho-tag1. The expression levels and accessibility of the Rho-tag in membrane preparations were evaluated by Western blot while the functional state of CB2 was assessed by radioligand binding and G protein activation assays. As the positive controls for CB2 expression levels and activity, the previously characterized constructs CB2-130 and CB2-125 were used [3]. Expression levels were determined by Western blots analyzed by densitometry, and specific activity was estimated as described in Materials and Methods. Table 1 provides a summary of results, and Fig. 1 (A-C) and Fig. S2 depict results of the Western blots and functional assays.
Figure 1. Expression and activity of fusion CB2 proteins in E. coli membranes.
A. Expression levels of fusion CB2 proteins in E. coli membranes. Membrane preparations containing 67 μg of total protein were resolved on SDS-PAGE, electroblotted onto nitrocellulose membranes, and probed with NAA-1, rabbit monoclonal anti-CB2 antibody. Squares indicate bands corresponding to CB2.
B. Functional activity of fusion CB2. Membrane preparations containing 2 μg of total protein were analyzed in a G protein activation assay. Relative activity values were calculated as follows: from average radioactivity of duplicate measurements, the background measured in the absence of membranes was subtracted and expressed as percentage of the activity of a standard, CB2-130 in E. coli membranes.
C. Accessibility of the Rho-tag in fusion CB2 constructs for interaction with 1D4 antibody. Membrane preparations containing 67 μg of total protein were dissolved on SDS-PAGE, electroblotted onto nitrocellulose membranes, and probed with 1D4 antibody. Squares indicate bands corresponding to Rho-tagged CB2.
In constructs CB2-181, 182 and 183, the position of the sequence coding the Rho-tag was varied, such that the expressed proteins carry the tag either immediately upstream or downstream of CB2. In the case of CB2-183, a C-terminal decahistidine tag was also introduced. While the Rho-tag was highly accessible in the fusion CB2-182, this protein was expressed at a very low level (Fig. 1A and B). On the other hand, the construct CB2-183 was highly expressed but the Rho-tag was inaccessible. Since both the high expression level and the accessibility of the Rho-tag in a fusion protein are important for development of a successful purification strategy for recombinant CB2, we prepared several more fusion proteins (constructs 250-255) to select for a construct that meets both conditions. Here, the Rho-tag was placed at the C-terminal end of the fusion to ensure its better accessibility for interaction with the 1D4 antibody. In addition, the position of the His-tag was varied and different spacers between the CB2 and the Rho-tag were introduced.
Our results suggest that the presence of a spacer between CB2 and the Rho-tag is essential for high expression levels of the recombinant protein (constructs 250, 252-255) while a position of the Rho-tag at the C-terminus of the fusion (constructs182, 251-255) is critical for accessibility of this tag for interaction with 1D4 antibody (see Fig. S1 for location and the sequence of spacers). Not only the length but also the nature of the spacer affects the accessibility of the Rho-tag. The His10 tag, when placed directly upstream of the Rho-tag (CB2-250), resulted in a sharp decrease of binding to 1D4 antibody while other spacers resulted in increased binding. As shown in Table 1, out of nine fusion constructs tested, two (CB2-252 and CB2-255) were highly expressed and functional as demonstrated by specific binding of the high affinity ligand CP-55,940 (Fig. S2) and robust activation of G protein in response to agonist binding (Fig. 1B). Importantly, in these fusion proteins, the Rho-tag efficiently binds to 1D4 monoclonal antibody enabling affinity purification of the receptor and its subsequent surface immobilization. Therefore, these two constructs were selected for large scale expression, purification, and further characterization of CB2.
3.2.Purification of CB2
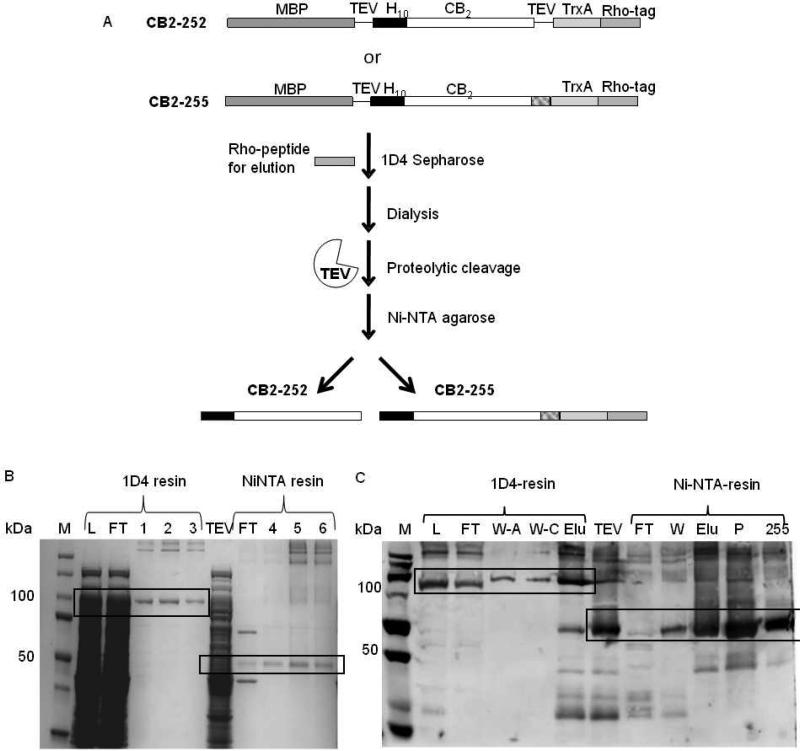
For purification of the Rho-tagged CB2, a resin was prepared by incubating 1D4 antibody with CNBr-activated Sepharose, and the binding capacity of the resin determined as described in Supplemental Materials. Purification protocols for the constructs CB2-252 and CB2-255 expressed in E. coli cells were developed by taking advantage of the C-terminal Rho-tag. Cells were cultured either in a 5L bioreactor (for CB2-252) or in shaker flasks (total volume of 5L for CB2-255) that resulted in production of a wet cell biomass of 236 g and 40 g, respectively. A two steps purification protocol was developed (Fig. 2 A), described in detail in Supplemental Materials. Briefly, after solubilizing proteins in a mixture of detergents DDM and CHAPS supplemented with stabilizers CHS and the high affinity ligand CP-55,940, the recombinant CB2 was captured onto 1D4-Sepharose resin by overnight exposure at 4 °C. The resin was collected and washed, and CB2 eluted by gravity in three cycles of 10-min incubation with Rho peptide. The 10 minute incubations are essential to efficiently dissociate the receptor from the 1D4 resin. This purification step results in a highly pure fusion protein as shown in Fig. 2 B and Fig S3 B. After removal of expression partners by treatment with TEV protease, the CB2 was further purified by Ni-NTA chromatography taking advantage of the N-terminal decahistidine tag (Fig. 2 C and S3 A). Additional bands in the Coomassie blue-stained gel correspond mainly to TEV protease and fusion-CB2 cleavage products. Purity of the resulting CB2 preparation was ≥ 90% as confirmed by Coomassie blue staining (Fig. 2 B).
Figure 2. Purification of CB2.
A. Purification strategy. Crude cell extracts are treated with 1D4 antibody affinity resin, and the CB2 fusion protein eluted with the Rho peptide. Fusion partners are removed by treatment with TEV protease, and the released CB2 is further purified by Ni-NTA affinity chromatography. Final products are either N-terminally His-tagged CB2 (CB2-252) or dual His/Rho-tagged CB2 (CB2-255).
B. Purification of CB2-255 followed by SDS-PAGE electrophoresis and coomassie blue staining. M, marker; L, load before 1D4 antibody chromatography; FT, flowthrough from indicated chromatographic step; 1, 2, 3, 4, 5, 6 elution fractions from indicated chromatographic step; TEV, proteins after TEV protease digest. Relevant bands corresponding to CB2 are highlighted by squares.
C. Purification of CB2-255 followed by Western blot analysis. M, molecular weight marker; L, load at the start of 1D4-resin chromatography; FT, W-A, W-C, W and Elu, flowthrough, wash with buffer A, wash with buffer C, wash and combined elution fractions from indicated chromatographic steps, respectively; TEV, reaction products of TEV protease digest; P, pellet after concentration of purified CB2-255; 255, purified CB2-255. Relevant bands corresponding to CB2 are highlighted by squares.
The structural integrity of the purified CB2 was confirmed in a G protein activation assay on the receptor reconstituted into liposomes (see Supplemental materials) [2]. For fluorometric quantification of the reconstituted protein, trace amounts of AlexaFluor 488-labeled CB2 were included in the reconstitution mixture. The G protein activation assay was performed in the presence of 2 μM CP-55,940, using 2-5 nM concentrations of CB2 in proteoliposomes and saturating concentrations of G protein subunits in the presence of the non-hydrolizable analog of GTP, 35S-γ-GTP [2, 20]. The specific activity of the reconstituted CB2 was calculated using rates of accumulation of a complex of Gαi1 with radiolabeled 35S-γ-GTP, and was comparable to that of CB2-130 standard in E. coli membrane preparations (results not shown).
The estimated yield for CB2-252 was 4.1 mg from a 5 L culture (17 μg/g of cell pellet), corresponding to a recovery of ~87% (Appendix A). The estimated yield for CB2-255 was 1.5 mg from 5 L of culture, corresponding to a 64% recovery (Appendix A). In the latter case, though, some precipitate formed when the purified protein was concentrated to ~1.5 mg/mL in detergent solution which decreased the yield to 600 μg (15 μg/ g of cell pellet).
In summary, we demonstrated that the Rho-tag separated from the C-terminus of CB2 by an appropriate spacer is compatible with the expression of the receptor in E. coli cells and subsequent purification by affinity chromatography. Furthermore, the Rho-tagged CB2 can be expressed in functionally active form and at reasonably high yield in E. coli cells cultivated in minimal medium in a bioreactor, potentially enabling selective incorporation of stable isotope-labeled amino acids. Due to the high yield and purity of CB2-252 and the absence of a large C-terminal tag, this construct is particularly suited for structural studies by NMR. CB2-255 can be immobilized on a solid support through a specific interaction of the Rho-tag with 1D4 monoclonal antibody, facilitating studies of ligand exchange on the surface-immobilized receptor in detergent micelles.
3.3.Interaction between Rho-tagged CB2 and immobilized 1D4 antibody studied by SPR
Tight, specific and reversible binding of the Rho-tagged CB2 to resin-immobilized 1D4 antibody is essential for development of an efficient purification protocol and for studies of ligand binding on resin-captured CB2 receptor. To assess the affinity between the Rho-tagged CB2 and 1D4 antibody in the presence of detergents, CHS, glycerol and salts, we determined the value of the corresponding thermodynamic dissociation constant at these conditions. The 1D4 monoclonal antibody was first immobilized on the surface of a CM4 sensor chip at 25 °C at three different surface densities, 300, 600, and 1000 RU. The reference surface was generated by mock amino coupling without antibody. Interactions with CB2 were investigated at 10 °C to mimic the conditions for purification of Rho-tagged CB2 on 1D4 coupled to Sepharose. For this purpose, the biosensor was equilibrated at 10 °C overnight. A fresh aliquot of the purified CB2-255 was defrosted, dialyzed at 4°C against running buffer CSPR for 2 hours, filtered, and diluted to appropriate concentrations. Samples and the running buffer were kept refrigerated before use as described in Materials and Methods.
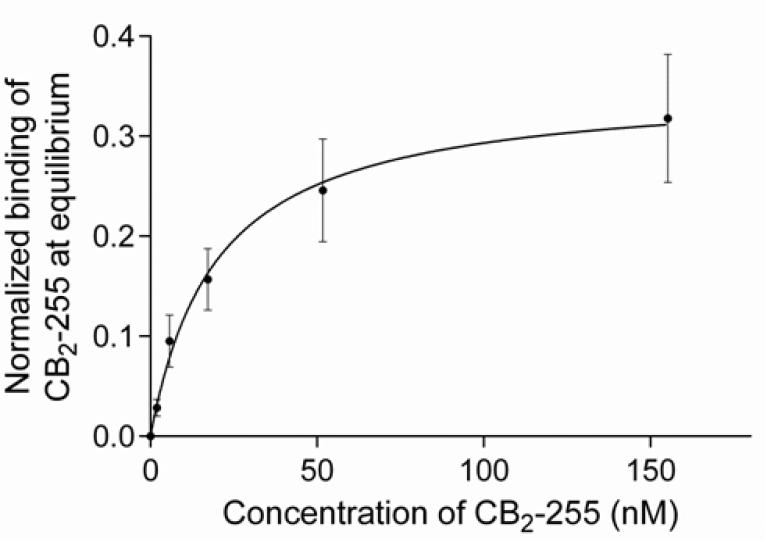
For determination of the thermodynamic dissociation constant, KD, at 10 °C, we performed the titration experiment similar to the stepwise titration procedure described earlier [21]. Sample injections with increasing concentrations of CB2 were performed sequentially over both active and reference surfaces and sensorgrams recorded until readings approached a steady-state, without washing or regeneration of surfaces between injections. After the sample with the highest concentration was applied and the binding response had stabilized, the running buffer was injected for 1 hour. The estimated value of KD (10 °C) was 19.9 ± 4.9 nM according to steady state affinity analysis of normalized data (Fig. 3). Dissociation of the complex appeared to be slow; the recorded time was insufficient for determination of dissociation rates.
Figure 3. Thermodynamic characterization of the interaction between purified CB2 and the 1D4 antibody by SPR.
The normalized binding response of purified CB2-255 to 1D4 antibody-coated surfaces as a function of CB2 concentration is shown. 1D4 antibody was immobilized at three different surface densities while a reference flow cell was kept empty (see Materials and Methods). The steady-state responses from a stepwise titration for each CB2 concentration were determined at 1 hour (0, 1.9, 5.7 nM) and 40 minutes (17, 51, 155 nM) after injection at 5 μL/min. For normalization, the CB2-binding response was divided by the corresponding surface density RU-value of immobilized 1D4 antibody.
Steady state affinity analysis was performed using GraphPad Prism software. The value for KD (10 °C) of 19.9 ± 4.9 nM was obtained by non-linear regression analysis considering a 1:1 binding model.
The estimated concentrations of the Rho-tagged CB2 in crude extracts from cells obtained by fermentation in rich 2xYT medium in shaker flasks or in minimal salt medium (bioreactor) are ~65 nM and 40 nM, respectively (Appendix A) [2, 22], slightly above the value of KD. Therefore, the measured binding capacity of 1D4 resin for the CB2-fusion protein as ~1.8 mg/mL (section 3.2) is about ~75% of the maximal expected binding capacity of the resin due to low concentration of CB2 in the crude cell extracts. Nevertheless, the Rho-tag/ 1D4 purification is a suitable alternative to the His-tag or Strep-tag for isolation of the recombinant receptors. The use of an excess of resin is advised to compensate for the low concentration of receptor in crude cell extract. Because of the high specificity of the binding of Rho-tag to 1D4, the Rho-tagged CB2 preparations tend to have somewhat higher purity compared to CB2 purification solely by His- and/or Strep-tag.
Taken together, these results show that the affinity of the interaction of the Rho-tagged CB2 with 1D4 antibody is quite high at 10 °C even in the presence of detergents used to solubilize and stabilize CB2. This enables efficient purification of the tagged CB2 using 1D4 resin as well as studies of interaction of solid interface-immobilized receptor with various binding partners.
3.4. Ligand binding to Rho-tagged CB2 in detergent micelles
The structural integrity of the recombinant receptor solubilized in detergent micelles was assessed by monitoring its ligand binding properties at 4 °C. Because of the small size of hydrophobic cannabinoid ligands and their non-specific partitioning into protein-detergent micelles SPR technique is not well suited to study ligand interaction with CB2. A radioligand binding assay has superior sensitivity but requires separation of the ligand-receptor complex from nonspecifically bound ligand which is difficult to achieve because of the high affinity of the hydrophobic cannabinoids for detergent micelles and hydrophobic interfaces. Therefore, we immobilized the receptor-ligand complex on 1D4 resin, and removed loosely bound ligands by rapid washing with ligand-free buffer. This reduced the non-specific binding of radioligand [3H]-CP-55,940 to no more than ~25% of total binding which greatly improved the quality of ligand binding studies. For the ligand binding assay, the resin was loaded with the Rho-tagged fusion CB2, exposed to variable concentrations of [3H]-CP-55,940 for several hours, and washed rapidly as described in Materials and Methods. The receptor-ligand complex was then eluted with the Rho peptide.
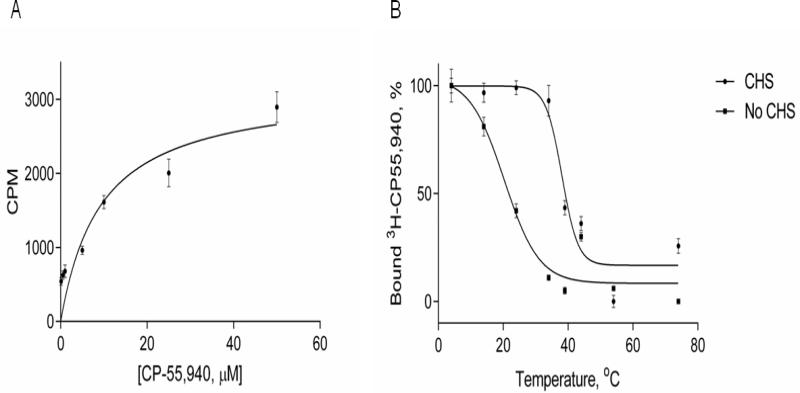
A typical experiment yielded a number of ligand binding sites (Bmax) in the range of ~80-85% of expectations assuming a 1:1 complex of ligand and CB2 (Fig. 4 A). Taking into account that the washing procedure may have removed some of the ligand that was specifically bound to the receptor, the amount of ligand binding-competent receptor could be even higher.
Figure 4. Ligand binding on CB2-255 fusion protein in micelles.
A. Ligand binding on CB2 in detergent micelles. Saturation ligand binding was performed to determine the number of ligand binding sites (Bmax) and affinity (KD) of the interaction between CB2-255 fusion protein and CP-55,940 ligand. Aliquots of CB2-255 fusion protein from crude cell lysate, solubilized in buffer A supplemented with 10 μM CP-55,940 and immobilized onto 1D4-Sepharose resin (50 μL/sample) were washed with solutions of CP-55,940 (0-60 μM) in buffer A, supplemented with [3H]-CP-55,940 and analyzed for ligand binding as described in Material and Methods. Each point represents an average of duplicate measurements. Results of a representative experiment (out of three) are presented.
B. Thermoinactivation of CB2 protein in micelles. The CB2-255 fusion protein from crude lysate in buffer A supplemented with 10 μM CP-55,940 was immobilized on 1D4-Sepharose resin (50 μL/sample) and subjected to a temperature increase from 4 °C to 84 °C at a rate of 1 °C/min. Functional activity of CB2 was analyzed by measuring ligand binding upon addition of [3H] CP-55,940 as described in Materials and Methods. Measurements were performed in duplicate; a representative experiment is shown. Squares- buffer without CHS, circles- buffer with CHS. Results of a representative experiment (three total) are presented.
While the KD for the CP-55,940 ligand/receptor interaction is expected to be in the low nanomolar range as determined for recombinant CB2 in bacterial membranes, the exact value could not be determined in the current experiment because of the high, micromolar concentrations of ligand required for this assay.
3.5. Thermoinactivation of CB2 in detergent micelles
The thermoinactivation of Rho-tagged CB2 was studied in micelles with or without stabilizer CHS. Aliquots of resin-immobilized fusion CB2-255 were subjected to a temperature increase as described in Materials and Methods, incubated on ice for two hours in the presence of [3H]-CP-55,940 and Bmax determined as described above (Fig. 4 B). The apparent Tm50 for CB2 denaturation in CHAPS/DDM detergent micelles supplemented with 0.1% CHS was 44.8 °C. The apparent Tm50 for CB2 in detergent micelles without the stabilizer CHS was significantly lower (~20 °C) supporting the notion that CHS stabilizes this receptor in the micellar phase, in good agreement with our earlier studies using a G protein activation assay [2].
In summary, immobilization of Rho-tagged CB2 onto 1D4-resin enables ligand binding studies in detergent micelles using a few micrograms of purified receptor per sample.
3.6. Preparation of CM4 chips for SPR studies
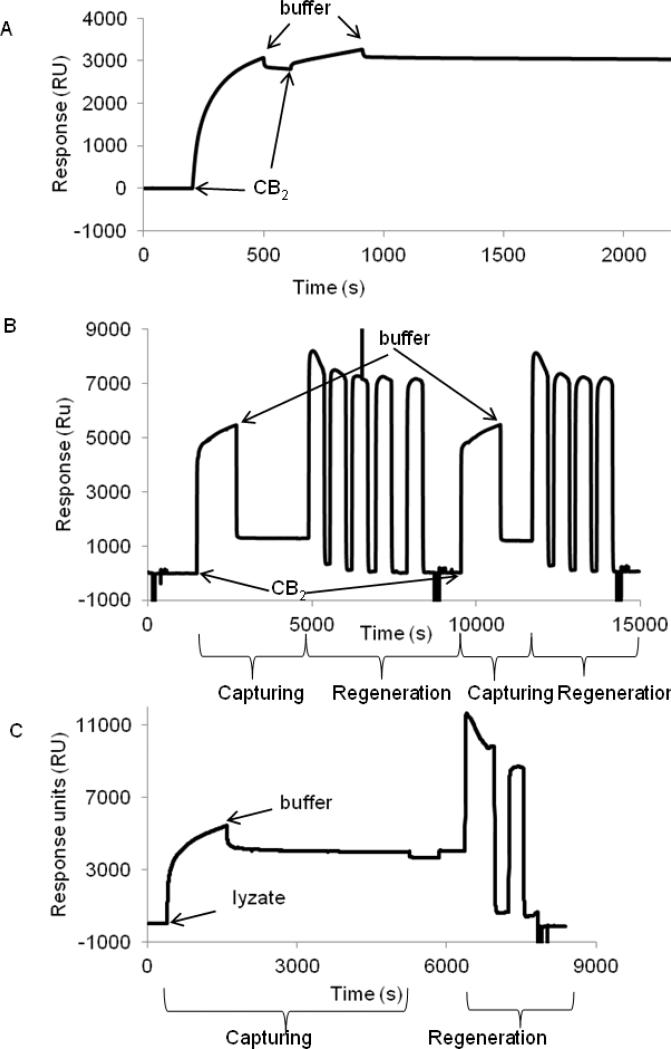
For binding studies, CB2 was immobilized on a CM4 chip surface coated with 1D4 antibody at high density (6000-7000 RU) as described above. Purified CB2-255 in detergent micelles supplemented with CHS in running buffer CSPR was captured at 25 °C. The reference cell contained a surface without 1D4 antibody. The capturing was specific and quantitative (about 3000 RU), and the SPR signal remained steady for at least 1 hour (Fig. 5 A). Denaturation of CB2 in the presence of CHS in relatively short experiments was not expected as demonstrated above.
Figure 5. Capture of purified CB2 and fusion CB2-255 from crude lysate on a CM4 sensor chip surface.
A. Surface capture of CB2. Purified CB2-255 (0.5 μM) was injected at 2 μL/min for 10 min over empty (reference) - and 1D4 antibody-coated (~6000 RU) surfaces, followed by flow of running buffer (CSPR). The procedure was repeated for a second time. The experiment was carried out at 25 °C. The response from the reference surface was subtracted from the value of the experimental surface.
B. Regeneration of CB2-coated surface. Purified CB2-255 was captured on a 1D4 antibody-coated surface. For removal of CB2, a solution of Rho peptide (4 mM) in buffer CSPR supplemented with 1 M NaCl was injected four or five times slowly (1 μL/min) with contact times of 5-10 minutes each, followed by 1% OG in 10 mM NaOH two times at 50 μL/min for 5 sec each and buffer CSPR at the same flow rate for 1 min each. Two cycles of capturing and regeneration are shown.
C. Capture of fusion CB2 from cell lysate and regeneration of surface. Fusion protein was captured on a 1D4 antibody-coated surface by injecting the cell lysate at a rate of 2 μL/min for 20 min followed by injection of a running buffer.
For removal of captured protein, a solution of Rho peptide (4 mM) in buffer CSPR supplemented with 1 M NaCl was injected two times (1 μL/min) with contact times of 10, and 5 minutes each, followed by OG/NaOH and running buffer injections as described in B. The corresponding (not-referenced) sensorgram is shown.
The CB2 was quantitatively removed from the surface by gently displacing it with 4 mM Rho-peptide in buffer CSPR supplemented with 1M NaCl, thus mimicking the elution conditions for CB2-255 from the 1D4 antibody resin during purification. The peptide/ NaCl solution was passed at a low rate of 1 μL/min, to allow sufficient time for the peptide to compete with the Rho-tag - 1D4 antibody interaction. The peptide was then removed from the chip surface by treatment with 1% OG in 10 mM NaOH. As much as 90-99% of the surface was regenerated by this procedure (Fig. 5B). Please note that data of a single flow cell without referencing are shown. The abrupt increase and decrease of the SPR response at the beginning and end of sample injections reflect changes in the refractive index of solutions due to differences in glycerol content between the running buffer and the protein sample. The good performance of chips was maintained for up to three cycles of regeneration.
Attempts to remove the captured receptor from the surface using 1% OG in 10 mM NaOH as reported earlier for other GPCR [15, 16], or other conditions including 4M MgCl2; 10 mM glycine pH 2; 100 mM NaOH or 100 mM HCl in running buffer, in isopropanol, in combination with OG or other detergents failed (data not shown). The reason most likely was irreversible precipitation of CB2 on the surface of the chip.
3.7.Capture of Rho-tagged fusion CB2 from crude cell lysate on 1D4-coated CM4 chips
Capturing Rho-tagged fusion CB2 directly from crude cell lysates eliminates the need for purification of the receptor. The membrane fraction of the cell lysate was solubilized using running buffer CSPR at a concentration of ~8 mg/mL total protein corresponding to ~24 μg/mL of CB2 fusion). Without further dilution, the protein solution was injected slowly (2 μL/min) over a 1D4 antibody-coated surface. Figure 5C shows the non- referenced sensorgram. The observed signal increase corresponds to the combined specific and non-specific binding of materials to the chip surface. Here the contribution of the SPR response from changes of the refractive index of solutions is lower compared to experiments with purified protein, because differences in the glycerol content of solutions were smaller. During the wash that followed injection, a rapid decrease in signal was initially observed due to a removal of non-specifically-bound material. Towards the end of the wash cycle, the signal approached values in the range of 3500-4000 RU which is close to the values obtained for binding of purified CB2-255. The regeneration protocol to remove captured fusion CB2-255 was effective as well (Fig 5C).
These results demonstrate that fusion CB2 from crude lysates is quantitatively and specifically captured on and removed from a 1D4 antibody-coated CM4 sensor surface at high efficiency, which greatly reduces efforts when conducting SPR experiments.
3.8.On-chip TEV protease cleavage of fusion CB2
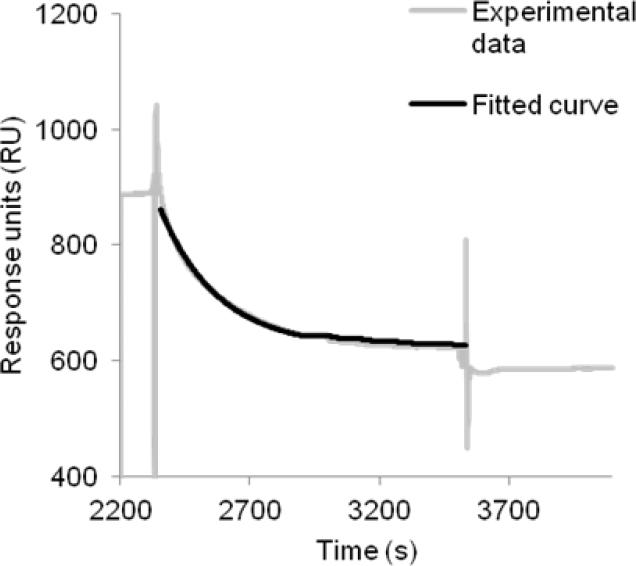
The presence of the large N-terminal fusion partner MBP may limit access of binding partners to CB2. Therefore, we tested whether the cleavage of the fusion CB2 with TEV protease could be performed at the surface of a sensor chip (Fig. 6). For cleavage, we slowly passed a solution of TEV protease in running buffer over the surfaces containing either fusion CB2 captured onto 1D4 or the reference (1D4 only) followed by the running buffer as described in Materials and Methods. The response decreased exponentially as a function of time (Fig. 6). The decline in response after 20 minutes is close to 100% of the expected decrease for complete removal of the MBP portion of the fusion protein. Analysis of the TEV enzymatic cleavage kinetics assuming a first order reaction yielded a rate constant of 4.5 × 10−3 s−1 (25 °C) corresponding to half-time of removal of MBP of 2.5 minutes (Fig. 6).
Figure 6. Enzymatic cleavage of fusion CB2-255 monitored by SPR.
The experiment was performed at 25 °C using buffer CSPR as a running buffer. After capture of fusion CB2 from crude extract to the high density 1D4 antibody-coated surface and removal of non-specifically retained material by injection of the running buffer, TEV protease (1 mg/mL in buffer CSPR) was injected at a flow rate of 2 μL/min for 20 min followed by injection of buffer for another 10 min. The specific response (after subtraction of a reference) as a function of time (dotted line) is shown. Data were fitted using an equation for exponential decay-one phase dissociation (black curve) using GraphPad Prism software. The rate constant for the enzymatic reaction was determined to be k (25 °C) = 4.5 × 10−3 s−1. The fit to the curve (solid line) is presented in the same graph.
3.9.Binding of anti-CB2 monoclonal antibody to captured CB2
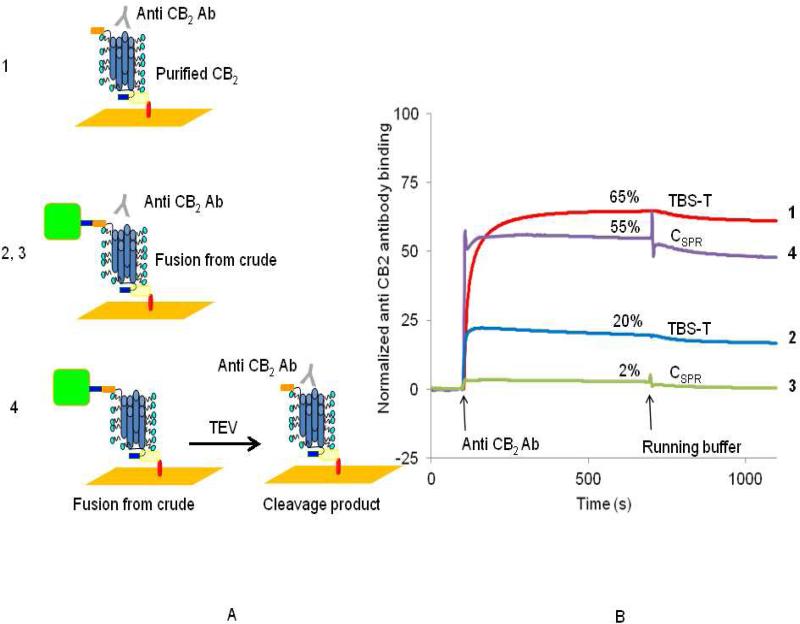
We studied interaction of a novel monoclonal antibody, NAA-1 with surface-immobilized CB2 (Fig. 7). The antibody was raised against purified, proteoliposome-reconstituted CB2. Due to the randomness of receptor orientation in the proteoliposomes [2, 23], the antibody may recognize water-exposed epitope(s) located on either extracellular (N-terminal) or cytoplasmic (C-terminal) surface of CB2. Experiments were performed at 25 °C using two different running buffers, TBS-T or CSPR. As a reference, a 1D4-antibody coated flow cell surface without CB2 was used A quantitative and specific response was observed when the NAA-1 antibody was injected in TBS-T as a running buffer over the surface containing captured purified CB2-255 from which the MBP fusion partner was already removed during purification (Fig. 7A, B (curve 1)). The SPR response from antibody binding is equivalent to interaction with about 65 % of the CB2 molecules at the surface (Table 2, binding estimate in appendix B). However, when CB2-255 was captured from a bacterial cell lysate without prior cleavage of the fusion, only ~20% of the CB2-255 molecules interacted with the antibody under otherwise identical conditions (Fig. 7 A, B (curve 2)). The apparent lower antibody binding is likely the result of restricted accessibility to the binding epitope. The binding of the NAA-1 antibody was even lower (~2%) in buffer CSPR (Fig. 7A, B (curve 3)) but it was completely restored after removal of MBP from fusion-CB2 by treatment of the sensor chip surface with TEV protease (Fig. 7A, B (curve 4)). After cleavage, binding of NAA-1 antibody in buffer CSPR reached 55% of the expected maximal change of response suggesting that MBP blocks access to the epitope on CB2 especially in the presence of DDM/CHAPS detergent micelles.
Figure 7. Accessibility of surface-immobilized CB2 for interaction with specific antibody.
Anti-CB2 monoclonal antibody NAA-1 (1 μM in TBS-T or buffer CSPR as indicated) was injected at a flow rate of 2 μL/min for 10 min over reference (1D4-coated surface) and either surfaces covered with purified CB2 (1), fusion CB2 captured from cell lysate (2,3) or fusion CB2 captured from cell lysate and cleaved by TEV protease (4). The scheme of the experimental design is shown in A. Referenced and normalized sensorgrams corresponding to specific binding of antibody to CB2 are shown in B.
Table 2. Binding of monoclonal anti- CB2 antibodyNAA-1 to surface-immobilized CB2.
The calculation of the fraction of CB2 that binds NAA-1 is explained in appendix B.
| Running Buffer | Source of protein | Protein immobilized | Treatment after immobilization | Running Buffer | Antibody binding (%) |
|---|---|---|---|---|---|
| TBS-T | Homogeneous preparation | CB2 | none | TBS-T | 64% |
| TBS-T | Cell Iyzate | Fusion | none | TBS-T | 20% |
| C-SPR | Cell Iyzate | Fusion | none | C-SPR | 2% |
| C-SPR | Cell Iyzate | Fusion | TEV protease cleavage | C-SPR | 60% |
In summary, the accessibility of CB2 for interaction with monoclonal NAA-1antibody significantly increased upon removal of the MBP-fusion partner by treatment with TEV protease. This strongly suggests that the binding epitope is located on the N-terminal side of CB2.
3.10. Affinity of the CB2/anti-CB2 antibody interaction studied by SPR
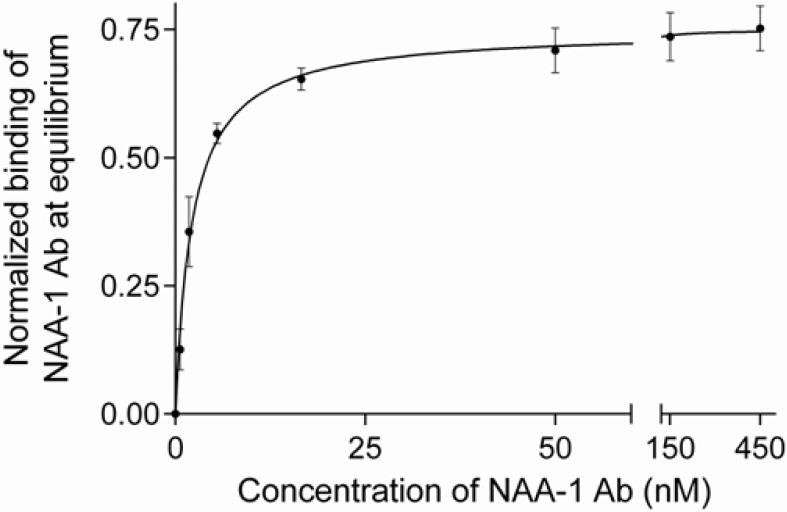
To further characterize the binding of CB2 to the NAA-1 monoclonal antibody, we determined the value of the thermodynamic dissociation constant (KD) of the interaction by equilibrium titration at 25 °C. Relatively low surface densities of CB2 of 140 and 420 RU and a high flow rate (30 μL/min) were chosen to minimize mass transfer limitations and to increase the likelihood to reach a steady signal at each concentration of the analyte. Increasing concentrations of NAA-1 antibody in buffer CSPR were injected stepwise over CB2- and reference surfaces, and the final dissociation step was performed in running buffer. For data analysis, the same injection protocol was repeated using running buffer instead of antibody solutions, and the resulting sensorgrams were subtracted. An example is shown in Fig. S4.
The estimated value of KD (25 °C) was 2.2 ± 0.2 nM according to the steady state affinity analysis of normalized data (Fig. 8).Taken together the results indicate that the anti-CB2 antibody NAA-1 interacts with an extracellular domain of CB2 receptor with high affinity.
Figure 8. Thermodynamic characterization of the interaction between purified CB2 and an anti CB2 monoclonal antibody by SPR.
The normalized binding response of NAA-1 antibody to CB2–coated surfaces as a function of antibody concentration is shown. For normalization, the response obtained from equilibrium titration experiments (Material and methods, and figure S4) at the end of each antibody injection (0-450 nM) was divided by the corresponding surface density RU-value of captured CB2. Steady state affinity analysis was performed using GraphPad Prism software. The value for KD (25 °C) of 2.2 ± 0.2 nM was obtained by non-linear regression analysis considering a 1:1 binding model.
4. Discussion
Small affinity tags offer advantages for expression, purification, and surface immobilization of recombinant G protein-coupled receptors facilitating biochemical and biophysical studies. Here we demonstrate that the C-terminal Rho-tag nanopeptide is a suitable tag for isolation and characterization of recombinant, functional cannabinoid receptor CB2 expressed in E. coli.
Although the Rho-tag has been previously used for isolation of several other recombinant GPCR expressed in mammalian cells [6, 7, 24], yeast [25, 26] and in a cell-free systems [27], the usefulness of this tag for recombinant expression of membrane receptors in E. coli had not been reported yet. We observed that the C-terminal location of the Rho-tag in the fusion construct is obligatory to ensure its accessibility for interaction with specific 1D4 antibody while an appropriate spacer (4 amino acid residues or longer) introduced between the C-terminus of the receptor and the Rho- tag is necessary for high expression levels. Not surprisingly, the nature of the spacer affects the level of expression as well as structural integrity of the receptor. In comparison to G3S and A3N5, the H10 tag reduces, and the C-terminal extension with TrxA, increases expression levels and functional activity of the fusion protein. While the mechanism by which the spacer may affect the folding and stability of the recombinant receptor was not investigated, it is clear that in addition to the location of the tag, the nature and length of the spacer are important considerations when designing fusion Rho-tagged constructs for expression of recombinant membrane proteins in E. coli.
The affinity of the Rho epitope (Rho-tag) to1D4 antibody in PBS buffer was assessed in the past with a solid-phase radioimmune assay by measuring the Rho-peptide concentration required for half-maximum inhibition of binding of the unpurified monoclonal antibody to crude preparations of rhodopsin (I50 = 1.3 μM) [28]. Here, we determined a thermodynamic dissociation constant of the Rho-tagged CB2 to the immobilized 1D4 antibody of about 20 nM at 10 °C. The strong interaction allows efficient capture of the tagged GPCR on a biosensor chip surface or on Sepharose resin for studies on epitope accessibility as well as ligand binding in micellar solution. The high selectivity of the Rho-tag – 1D4 interaction guarantees a high purity of protein even at relatively low expression levels of functional GPCR.
We developed a radioligand binding assay utilizing 1D4-Sepharose-immobilized CB2 and demonstrated its performance for studying interaction of the receptor with hydrophobic ligands in detergent micelles. Compared to the previously reported NMR-based determination of ligand binding [4], the radioligand-binding offers distinct advantages: it is relatively fast and requires low-microgram quantities of purified receptor only, compared to 100 μg or more for NMR. Furthermore, the resin-immobilized CB2 can be utilized in a wide variety of experiments requiring ligand exchange on the receptor and high recovery of ligand binding-competent CB2.
The purified recombinant CB2 has been characterized by three different assays reporting on functional properties and correct fold of the receptor: (i) a G protein activation assay performed on liposome-reconstituted CB2; (ii) a ligand binding, and (iii) a thermoinactivation assay performed in detergent solution. Remarkably, the Tm50 of thermoinactivation determined via ligand-binding competence of the receptor in this work closely matches Tm50 values we previously determined for detergent-solubilized CB2 subjected to thermoinactivation in the micellar phase and reconstituted for analyzed by G protein-activation activity [2]. It strongly suggests that the loss of agonist binding was the primary cause for loss of function.
Furthermore, the specific, oriented capture of functional Rho-tagged CB2 at high concentrations on the surface of a commercial CM4 chip coated with 1D4 antibody was demonstrated. Both, crude cell extracts containing fusion CB2 as well as purified CB2 serve as efficient source of recombinant receptor. We describe experimental conditions that allow preservation of structural integrity of the captured receptor and avoid its unfolding and non-specific aggregation on a surface. We developed an efficient surface regeneration protocol that permits repeated use of the CM4 chip for CB2 binding studies. It differs from previously published protocols developed for recombinant chemokine receptors by releasing the captured protein first with a Rho-peptide before applying the detergent OG in the presence of NaOH [14]. Those conditions proved to be too harsh for CB2 probably causing an irreversible precipitation/aggregation of the protein at the surface that prevented its efficient removal.
The application of SPR to monitor the enzymatic reaction of a protease to immobilized peptide substrates was described in the past [29]. Here we demonstrate the efficient TEV proteolytic cleavage of surface-immobilized fusion CB2 where SPR may be used to facilitate optimization of proteolysis conditions. The estimated rate constant of the proteolytic reaction of 4.5 × 10−3 s−1 (25 °C) suggests efficient access of active protease to the cleavage site. The results confirm our earlier report of efficient cleavage of the MBP-CB2 fusion in solution [3], though in that case the reaction was performed at 4°C and, therefore, required a longer time (typically several hours) for completion.
The efficient binding of a specific anti-CB2 monoclonal antibody (NAA-I) that was raised against CB2 reconstituted into liposomes has been demonstrated. The low value of the thermodynamic dissociation constant indicates high affinity of receptor-antibody interaction even in the presence of detergents. The antibody interacts most likely with the epitope on the N-terminal (extra-cellular) side of the CB2 receptor since the presence of the MBP in the fusion greatly inhibited interactions while removal of MBP with TEV protease facilitated it. A novel specific monoclonal antibody against CB2 will be of potential interest for applications in cell biology and pharmacology.
While the functional activity of CB2 was established by ligand binding and G protein activation assays prior to chip-surface immobilization, we did not monitor the functional state of the receptor in the course of SPR experiments for the following reasons. The small size and hydrophobicity of cannabinoid ligands do not allow sufficiently accurate measurement of their specific binding to CB2 captured at the chip surface. Likewise, monitoring of binding of G protein to micelle-solubilized CB2 at a sensor chip surface could also be ruled out at our experimental conditions since detergents are known to disrupt CB2-G protein interaction [2]. Monitoring the binding of a conformation-specific antibody in the presence of detergents would be feasible provided that such an antibody is available. SPR studies using a conformation-specific antibody to confirm structural integrity of immobilized receptors were reported previously for chemokine receptors [30] [31] [13]. The experimental procedures described in the present study allow screening of antibody libraries for a desired binding affinity and selectivity for epitopes on CB2.
The following observations suggest that the fold and function of the CB2 on a surface were preserved, and that precipitation/aggregation of the receptor on the biosensor surface is unlikely: (i) we were able to entirely displace the captured CB2 from the immobilized 1D4 antibody by competing it off with Rho peptide at an appropriate concentration; (ii) the TEV protease cleavage of fusion CB2 performed on the sensor surface was complete, suggesting that the cleavage site was fully accessible, and last but not least (iii) CB2 captured and released from 1D4 Sepharose under identical experimental conditions retained full functionality as demonstrated by ligand binding and G protein activation.
In summary, the C-terminal Rho-tag is a valuable addition to the set of tools for purification and surface immobilization of bacterially expressed, recombinant G protein-coupled membrane receptors. It allows specific, reversible, high-affinity interaction between the recombinant CB2 and resin-immobilized 1D4 antibody for efficient purification of functional receptor from crude cell extracts that contain the receptor at rather low concentrations. We also demonstrated efficient immobilization of Rho-tagged CB2 on a 1D4-coated SPR sensor surface. The ability to follow binding and to characterize the interaction of the monoclonal NAA-1 antibody with CB2 by SPR suggests feasibility of studying interactions between CB2 and other binding partners in detergent micelles. Further development of this technology includes reconstitution of CB2 into lipid bilayers at a surface that will allow SPR studies of interaction of this receptor with cognate G protein.
Supplementary Material
Highlights.
Expressed and purified functional recombinant cannabinoid receptor CB2
Immobilized CB2 in oriented fashion onto antibody-coated Biacore CM4 chip
Characterized functional recombinant CB2 by ligand binding in detergent micelles
Characterized binding of Rho-tagged CB2 to surface-immobilized 1D4 antibody by SPR
Characterized binding of monoclonal antibody to surface-immobilized CB2
Acknowledgements
This work was supported by the Intramural program of the NIAAA and NIBIB, NIH. The authors thank Mrs. Lioudmila Zoubak for assistance with expression and purification of CB2, Dr. Grzegorz Pizczek (NHLBI) for making the Biacore 3000 SPR instrument available, and Dr. John Northup for assistance with expression of G protein subunits.
Abbreviations
- CB2
peripheral cannabinoid receptor
- GPCR
G protein-coupled receptor
- MBP
maltose binding protein
- NTA
nitrilotriacetic acid
- SPR
surface plasmon resonance
- TEV
tobacco etch virus
- CHS
cholesteryl hemisuccinate Tris salt
- CHAPS
3[(cholamidopropyl)dimethylammonio]-1-propanesulfonate
- DDM
n-dodecyl-β-d-maltoside
- OG
N-octyl-β-d-glucopyranosid
- POPC
1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine
- POPS
1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoserine
- DTPA
diethylentriamine pentaacetate
Appendix A
Estimation of concentration of Rho-tagged CB2 in cell crude extracts
The total amount of CB2-255 (60 kDa) in crude extracts from cells obtained by cultivation in rich 2xYT medium in shaker flasks was estimated as follows. One liter of culture media processed to obtain E. coli membranes typically yields 115 mg of total protein. The amount of CB2-130 (44 kDa)/mg total membrane protein was estimated to be 3 μg [2]. From Table 1, the expression levels of CB2-130 and CB2-255 are almost identical; therefore it is assumed that this number can be used for estimation of amount of CB2-255 in membranes. After correction for molecular weight, 115 mg of total protein contain 470 μg of CB2-255; 5L of cell culture contain 2.35 mg of CB2. After disruption of cells and solubilization of CB2, the volume is reduced to 600 mL and the concentration of CB2 reaches 3.9 mg/ L or 65 nM. Since one molecule of CB2 was obtained from one molecule of the fusion CB2, the molar concentration of the fusion CB2 in the starting material is about the same.
If CB2 is produced with cells grown in minimal salt medium in a bioreactor, the total amount of CB2 increases by a factor of 2 but the final volume after extraction is higher by a factor of 3.3 [22] yielding the concentration of CB2 in crude extracts of ~ 40 nM.
Appendix B
Estimation of molecular weight of CB2 in detergents
The effective molecular weight of the fusion CB2 and purified CB2 were estimated assuming that each monomer receptor molecule is solubilized in one detergent micelle. The contribution of a micelle composed of CHAPS, DDM and CHS to the molecular weight of the protein was estimated to be ~30 kDa [32]. Therefore, the fusion CB2 –containing micelle has an estimated molecular weight of 130 kDa, and the purified CB2 – containing micelle- 90 kDa. We assume that the effective molecular weight of the antibody (150,000 kDa) is not affected by the presence of detergents in the buffer.
Normalization of SPR signal in figure 7
The expected total number of binding sites (RUexpected) was estimated by dividing the response (RUo) for the captured purified CB2 or fusion CB2 by the corresponding molecular weight of CB2 or fusion CB2 in detergents, respectively. The expected binding response (RUexpected) of the antibody (100%) was estimated by multiplying the number of binding sites by the MW of the antibody (assuming 1:1 antigen-antibody interaction). Data corresponding to the experimental binding response of the antibody (RUexperimental) were transformed as follows: Normalized response = (RUexperimental – RUo)/RUexpected × 100.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
For convenience we define fusion as CB2 fused at its N-terminus with the E. coli maltose binding protein (MBP), while in the purified CB2 this expression partner is no longer present. Other tags may or may not be present in the purified CB2, depending on a construct (see Table 1).
Supplementary materials related to this article can be found online.
References
- 1.Cabral GA, Griffin-Thomas L. Emerging role of the cannabinoid receptor CB2 in immune regulation: therapeutic prospects for neuroinflammation, Expert Rev. Molecular Med. 2009;11 doi: 10.1017/S1462399409000957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vukoti K, Kimura T, Macke L, Gawrisch K, Yeliseev A. Stabilization of Functional Recombinant Cannabinoid Receptor CB2 in Detergent Micelles and Lipid Bilayers. Plos One. 2012;7 doi: 10.1371/journal.pone.0046290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yeliseev A, Zoubak L, Gawrisch K. Use of dual affinity tags for expression and purification of functional peripheral cannabinoid receptor. Protein Expr Purif. 2007;53:153–163. doi: 10.1016/j.pep.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yeliseev AA, Wong KK, Soubias O, Gawrisch K. Expression of human peripheral cannabinoid receptor for structural studies. Protein Sci. 2005;14:2638–2653. doi: 10.1110/ps.051550305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molday RS, MacKenzie D. Monoclonal antibodies to rhodopsin: characterization, cross-reactivity, and application as structural probes. Biochemistry. 1983;22:653–660. doi: 10.1021/bi00272a020. [DOI] [PubMed] [Google Scholar]
- 6.Oprian DD, Molday RS, Kaufman RJ, Khorana HG. Expression of a synthetic bovine rhodopsin gene in monkey kidney cells. Proc Natl Acad Sci U S A. 1987;84:8874–8878. doi: 10.1073/pnas.84.24.8874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimada M, Chen X, Cvrk T, Hilfiker H, Parfenova M, Segre GV. Purification and characterization of a receptor for human parathyroid hormone and parathyroid hormone-related peptide. J Biol Chem. 2002;277:31774–31780. doi: 10.1074/jbc.M204166200. [DOI] [PubMed] [Google Scholar]
- 8.Farrens DL, Dunham TD, Fay JF, Dews IC, Caldwell J, Nauert B. Design, expression, and characterization of a synthetic human cannabinoid receptor and cannabinoid receptor/ G-protein fusion protein. J Pept Res. 2002;60:336–347. doi: 10.1034/j.1399-3011.2002.21066.x. [DOI] [PubMed] [Google Scholar]
- 9.Mirzabekov T, Bannert N, Farzan M, Hofmann W, Kolchinsky P, Wu LJ, Wyatt R, Sodroski J. Enhanced expression, native purification, and characterization of CCR5, a principal HIV-1 coreceptor. Journal of Biological Chemistry. 1999;274:28745–28750. doi: 10.1074/jbc.274.40.28745. [DOI] [PubMed] [Google Scholar]
- 10.Corin K, Baaske P, Ravel DB, Song JY, Brown E, Wang XQ, Geissler S, Wienken CJ, Jerabek-Willemsen M, Duhr S, Braun D, Zhang SG. A robust and rapid method of producing soluble, stable, and functional G-protein coupled receptors. PLoS One. 2011;6 doi: 10.1371/journal.pone.0023036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Babcock GJ, Mirzabekov T, Wojtowicz W, Sodroski J. Ligand binding characteristics of CXCR4 incorporated into paramagnetic proteoliposomes. J Biol Chem. 2001;276:38433–38440. doi: 10.1074/jbc.M106229200. [DOI] [PubMed] [Google Scholar]
- 12.Mirzabekov T, Kontos H, Farzan M, Marasco W, Sodroski J. Paramagnetic proteoliposomes containing a pure, native, and oriented seven-transmembrane segment protein. CCR5, Nat Biotechnol. 2000;18:649–654. doi: 10.1038/76501. [DOI] [PubMed] [Google Scholar]
- 13.Stenlund P, Babcock GJ, Sodroski J, Myszka DG. Capture and reconstitution of G protein-coupled receptors on a biosensor surface. Anal Biochem. 2003;316:243–250. doi: 10.1016/s0003-2697(03)00046-0. [DOI] [PubMed] [Google Scholar]
- 14.Navratilova I, Sodroski J, Myszka DG. Solubilization, stabilization, and purification of chemokine receptors using biosensor technology. Anal Biochem. 2005;339:271–281. doi: 10.1016/j.ab.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 15.Rich RL, Miles AR, Gale BK, Myszka DG. Detergent screening of a G-protein-coupled receptor using serial and array biosensor technologies. Anal Biochem. 2009;386:98–104. doi: 10.1016/j.ab.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Navratilova I, Pancera M, Wyatt RT, Myszka DG. A biosensor-based approach toward purification and crystallization of G protein-coupled receptors. Anal Biochem. 2006;353:278–283. doi: 10.1016/j.ab.2006.03.049. [DOI] [PubMed] [Google Scholar]
- 17.Navratilova I, Dioszegi M, Myszka DG. Analyzing ligand and small molecule binding activity of solubilized GPCRs using biosensor technology. Anal Biochem. 2006;355:132–139. doi: 10.1016/j.ab.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 18.Navratilova I, Besnard J, Hopkins AL. Screening for GPCR ligands using surface plasmon resonance. ACS Med Chem Lett. 2011;2:549–554. doi: 10.1021/ml2000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stenberg E, Persson B, Roos H, Urbaniczky C. Quantitative-determination of surface concentration of protein with surface plasmon resonance using radiolabeled proteins. J. Colloid and Interface Sci. 1991;143:513–526. [Google Scholar]
- 20.Locatelli-Hoops S, Sheen FC, Zoubak L, Gawrisch K, Yeliseev AA. Application of HaloTag technology to expression and purification of cannabinoid receptor CB2. Protein Expression and Purification. 2013;89:62–72. doi: 10.1016/j.pep.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hall DR, Winzor DJ. Use of a resonant mirror biosensor to characterize the interaction of carboxypeptidase A with an elicited monoclonal antibody. Analytical Biochemistry. 1997;244:152–160. doi: 10.1006/abio.1996.9867. [DOI] [PubMed] [Google Scholar]
- 22.Berger C, Ho JT, Kimura T, Hess S, Gawrisch K, Yeliseev A. Preparation of stable isotope-labeled peripheral cannabinoid receptor CB2 by bacterial fermentation. Protein Expr Purif. 2010;70:236–247. doi: 10.1016/j.pep.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimura T, Yeliseev AA, Vukoti K, Rhodes SD, Cheng K, Rice KC, Gawrisch K. Recombinant cannabinoid type 2 receptor in liposome model activates G protein in response to anionic lipid constituents. J. Biol. Chem. 2012;287:4076–4087. doi: 10.1074/jbc.M111.268425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Min KC, Jin Y, Hendrickson WA. Large-scale production of a disulfide-stabilized constitutively active mutant opsin. Protein Expression and Purification. 2011;75:236–241. doi: 10.1016/j.pep.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 25.Lee B-K, Jung K-S, Son C, Kini H, VerBerknioes NC, Arshava B, Naider F, Becker JM. Affinity purification and characterization of a G-protein coupled receptor, Saccharomyces cerevisiae Ste2p. Protein Expression and Purification. 2007;56:62–71. doi: 10.1016/j.pep.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barak LS, Warabi K, Feng X, Caron MG, Kwatra MM. Real-time visualization of the cellular redistribution of G protein-coupled receptor kinase 2 and beta-arrestin 2 during homologous desensitization of the substance P receptor. J Biol Chem. 1999;274:7565–7569. doi: 10.1074/jbc.274.11.7565. [DOI] [PubMed] [Google Scholar]
- 27.Corin K, Baaske P, Ravel DB, Song J, Brown E, Wang X, Geissler S, Wienken CJ, Jerabek-Willemsen M, Duhr S, Braun D, Zhang S. A robust and rapid method of producing soluble, stable, and functional G-protein coupled receptors. Plos One. 2011;6:e23036. doi: 10.1371/journal.pone.0023036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hodges RS, Heaton RJ, Parker JMR, Molday L, Molday RS. Antigen-antibody interaction - synthetic peptides define linear antigenic determinants recognized by monoclonal antibodies directed to the cytoplasmic carboxyl terminus of rhodopsin. J. Biol. Chem. 1988;263:11768–11775. [PubMed] [Google Scholar]
- 29.Wegner GJ, Wark AW, Lee HJ, Codner E, Saeki T, Fang SP, Corn RM. Real-time surface plasmon resonance imaging measurements for the multiplexed determination of protein adsorption/desorption kinetics and surface enzymatic reactions on peptide microarrays. Analytical Chemistry. 2004;76:5677–5684. doi: 10.1021/ac0494275. [DOI] [PubMed] [Google Scholar]
- 30.Hoffman TL, Canziani G, Jia L, Rucker J, Doms RW. A biosensor assay for studying ligand-membrane receptor interactions: Binding of antibodies and HIV-1 Env to chemokine receptors. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:11215–11220. doi: 10.1073/pnas.190274097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoshiura C, Kofuku Y, Ueda T, Mase Y, Yokogawa M, Osawa M, Terashima Y, Matsushima K, Shimada I. NMR Analyses of the Interaction between CCR5 and Its Ligand Using Functional Reconstitution of CCR5 in Lipid Bilayers. Journal of the American Chemical Society. 2010;132:6768–6777. doi: 10.1021/ja100830f. [DOI] [PubMed] [Google Scholar]
- 32.O'Malley MA, Helgeson ME, Wagner NJ, Robinson AS. Toward rational design of protein detergent complexes: determinants of mixed micelles that are critical for the in vitro stabilization of a G-protein coupled receptor. Biophys J. 101:1938–1948. doi: 10.1016/j.bpj.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.