Abstract
Background
There is a growing body of evidence that prenatal and early childhood exposure to arsenic from drinking water can have serious long-term health implications.
Objectives
Our goal was to understand the potential long-term health and disease risks associated with in utero and early life exposure to arsenic, as well as to examine parallels between findings from epidemiological studies with those from experimental animal models.
Methods
We examined the current literature and identified relevant studies through PubMed by using combinations of the search terms “arsenic”, “in utero”, “transplacental”, “prenatal” and “fetal”.
Discussion
Ecological studies have indicated associations between in utero and/or early life exposure to arsenic at high levels and increases in mortality from cancer, cardiovascular disease and respiratory disease. Additional data from epidemiologic studies suggest intermediate effects in early life that are related to risk of these and other outcomes in adulthood. Experimental animal studies largely support studies in humans, with strong evidence of transplacental carcinogenesis, atherosclerosis and respiratory disease, as well as insight into potential underlying mechanisms of arsenic’s health effects.
Conclusions
As millions worldwide are exposed to arsenic and evidence continues to support a role for in utero arsenic exposure in the development of a range of later life diseases, there is a need for more prospective studies examining arsenic’s relation to early indicators of disease and at lower exposure levels.
Keywords: arsenic, cancer, cardiovascular, in utero, prenatal, respiratory
Introduction
Environmental toxicants can profoundly impact the health of individuals and chronic exposure to toxic metals, like arsenic (As), has been implicated in the development of a variety of diseases in adults. Arsenic exposure via contaminated groundwater is a global health concern. As a known carcinogen, As can cause cancers of the lung, bladder, and skin, with mounting evidence pointing to a role in liver cancer as well (International Agency for Research on Cancer, 2004). Studies from Taiwan, Bangladesh, and Chile found that moderate-to-high levels of As exposure (200–800 µg/L) are associated with both all-cause mortality and cardiovascular-disease related mortality (Wu et al., 1989; Yuan et al., 2007b; Argos et al., 2010; Chen et al., 2011). Increases in cardiovascular disease occurrence, and modest, yet significant elevation in measures of hypertension also have been reported in As-exposed populations (States et al., 2009; Abhyankar et al., 2012).
We are now beginning to understand that pregnancy represents a particularly vulnerable window of susceptibility to toxicants for both mother and child and that many diseases may originate from environmental insults and alterations that occur during this sensitive developmental period. During pregnancy, As can pass through the placenta from mother to fetus, resulting in fetal exposure levels equivalent to those of the mother (Concha et al., 1998). Studies have found that in utero As exposure may have detrimental effects on pregnancy and birth outcomes, with higher levels of exposure associated with increased risks of spontaneous abortions and stillbirths, as well as increased infant mortality, preterm birth, low birth weight and fetal growth restriction (Hopenhayn-Rich et al., 2000; Ahmad et al., 2001; Hopenhayn et al., 2003; Milton et al., 2005; Vahter et al., 2006; von Ehrenstein et al., 2006; Huyck et al., 2007; Rahman et al., 2007; Rahman et al., 2010). Fetal growth restriction has been linked to increased risk of later metabolic disease, which in turn can lead to chronic conditions such as hypertension, diabetes and increased risks of cardiovascular disease (Valsamakis et al., 2006a).
In addition, in utero As exposure has been related to early life developmental effects, including neurodevelopmental defects in both animal studies (Martinez et al., 2008; Martinez-Finley et al., 2009; Martinez et al., 2011; Goggin et al., 2012) and epidemiological studies among children (Tsai et al., 2003; Wasserman et al., 2004; Rosado et al., 2007; von Ehrenstein et al., 2007; Wasserman et al., 2007; Hamadani et al., 2010; Hamadani et al., 2011; Parvez et al., 2011; Roy et al., 2011; Parajuli et al., 2013). While the influence of in utero or early life As exposure on neurotoxicity also may impact risk of chronic disease, the long-term consequences of these early alterations have yet to be elucidated.
Many of the early developmental effects of in utero exposure are likely influenced, at least in part, by epigenetic changes (Intarasunanont et al., 2012; Kile et al., 2012; Pilsner et al., 2012; Koestler et al., 2013) and in turn, programing of ongoing health and risk of chronic conditions. Further evidence suggests in utero or early life exposure to As increases oxidative stress signaling (Ahmed et al., 2011; Ahmed et al., 2012), and deregulation of immune and inflammatory pathways (Fry et al., 2007; Ahmed et al., 2011; Ahmed et al., 2012). These are potential mechanisms underlying observed associations between maternal As exposure and increased susceptibility to infections among their offspring (Raqib et al., 2009; Rahman et al., 2011; Farzan et al., 2013) as well as of chronic diseases. In light of accumulating epidemiological studies in conjunction with compelling animal model work, we review the literature highlighting newly published findings that address the potential role of in utero and early life exposure to As on long term health and risk of chronic disease, including cancer, respiratory disease and cardiovascular diseases.
Cardiovascular effects
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality worldwide and emerging data suggest that the determinants of CVD occur early in life. The Dutch Winter Hunger Study illuminated the link between fetal under-nutrition and growth restriction and later metabolic syndrome that leads to several chronic diseases, such as hypertension, diabetes and CVD (Valsamakis et al., 2006b). While studies in adults suggest that high levels of As adversely affect glycemic control, blood pressure, systemic inflammatory markers, vascular endothelial function and CVD occurrence, few studies have been prospectively designed to examine the effects of early life As exposure on CVD risk. Nearly 40 years ago, a set of autopsy case reports from young children in Chile suggested a possible connection between in utero and early life As exposure and cardiovascular-related disease (Rosenberg, 1973; Rosenberg, 1974). The two youngest children described in the reports, both under 3 years of age at time of death, died as a result of myocardial infarction and all five cases described had vascular lesions and thickening of the arteries; all of which are highly unusual within this age group (Table S1). These children, who all possessed hallmarks of chronic As poisoning, had resided in Region II of Chile during the period of when the public water supply was highly contaminated by As (average of 870µg/L) from 1958–1970. More recently, an ecological study conducted in Region II of Chile found that young adult men between the ages of 30–49 years born during the period of highest contamination, had about three times the rate of acute mortality from myocardial infarction as compared to the rest of Chile (Table S1) (Yuan et al., 2007a). A study of childhood mortality in As exposed children ages 5–18 years from Bangladesh found increased risks of childhood death from cancer or CVD (n= 22, HR: 2.18, 95% CI: 1.15–4.16) associated with estimated drinking water As concentrations (Rahman et al., 2013). These risks were slightly more elevated in girls and in adolescents aged 12–18 years.
Few prospective studies have been done to address this question with individual measures of exposure. Blood pressure was assessed in a subset of children in the MINIMat cohort in Bangladesh (Hawkesworth et al., 2012). Higher in utero exposure to As (measured by As concentrations at 8 and 30 weeks gestation during pregnancy) was associated with increased blood pressure at 4.5 years of age, such that a 1mg/L increase in maternal urinary As was associated with a 3.7mmHg increase in systolic and a 2.9mmHg increase in diastolic blood pressure. The magnitude of the association was greater for As exposure measured in the children at 18 months of age (8.3mmHg per 1mg/L increase in urinary As). Although the changes may appear modest, sustained elevations in blood pressure from an early age may be damaging long-term, particularly in genetically susceptible populations.
Recent work has evaluated other sub-clinical indicators of CVD, including carotid intima-media thickness (cIMT). In a cross-sectional study of children ages 3 to 14 in Zimapan, Mexico, total urinary As was associated with increases in cIMT, as well as increases in plasma levels of asymmetric dimethylarginine (ADMA), an endogenous inhibitor of nitric oxide production that is predictive of CVD (Osorio-Yanez et al., 2013). Although cross-sectional, 83% of the mothers of the participants reported living in the same highly contaminated area during their pregnancies, suggesting a possible contribution of in utero As exposure to the observed effects.
A valuable model for studying the mechanisms underlying the cardiovascular effects of As exposure has been the apolipoprotein E deficient (ApoE−/−) mouse, which is highly susceptible to atherosclerosis compared to wild-type mice, due to the lack of this particular lipoprotein carrier molecule. These mice spontaneously develop atherosclerotic disease, but As exposure has been shown to accelerate the process and increase the formation of atherosclerotic plaques in adult mice (Simeonova et al., 2003). More recently, this model has been utilized to test for cardiovascular effects of in utero As exposure (Table S2) (Srivastava et al., 2007). Pregnant ApoE−/− mice were exposed to 850µg/L sodium arsenite in their drinking water until birth, and their male offspring were examined for evidence of early life atherogenesis. In utero exposure doubled the number of atherosclerotic plaques in pups at both 10 and 16 weeks of age and also appeared to affect endothelial cell function and vascular tone. Follow-up experiments found that early postnatal As exposure of ApoE−/− mice had even more profound effects (Srivastava et al., 2009). Mice exposed to a 7-week treatment of 490µg/L As in drinking water beginning at postnatal week 3, had up to 5-fold increases in atherosclerotic lesion formation in the aorta. Further, the investigators observed both dose and time-dependent effects, with prolonged exposure leading to continued increases in lesion formation up to 36 weeks of age. Expression profiling of these vascular lesions showed increases in markers of inflammation (MCP-1, IL-6) and of oxidative stress (HNE- and MDA-protein adducts). Together, these experiments strongly support a role for transplacental and early life As exposure in atherogenic plaque promotion.
States and colleagues hypothesized that As’s in utero effect on hepatic development could in turn effect later life cardiovascular disease risk and tested this by examining liver tissue from pups born to pregnant ApoE−/− mice exposed to As in their drinking water from gestational day 8 to until birth (Table S2) (States et al., 2012). When hepatic mRNA and microRNA abundance was measured in the pups at postnatal days 1 and 70, in utero As exposure appeared to have significantly altered liver development, as demonstrated by differential expression of 51 genes over two postnatal time points, in comparison to controls. These deregulated genes indicated that pathways for gluconeogenesis and glycolysis were suppressed, while processes related to inflammation, stress, and lipid synthesis were upregulated, potentially contributing to early onset atherosclerosis. In addition to altering developmental programming in the liver, in utero As exposure may also lead to long-term dysregulation of stress and inflammatory responses that can contribute to atherosclerosis, as mechanistic evidence suggests that prenatal As exposure may cause a transient state of stress in early life, in part due to delayed induction of Hsp70 (Ngalame et al., 2012). This aberrant gene regulation in early life could potentially alter disease susceptibility in adulthood, leading to increases in liver carcinogenesis and cardiovascularrelated outcomes.
Carcinogenesis and cancer-related mortality
Much of what we have learned about the carcinogenic effects of in utero and early As exposure in humans has come from Region II in Chile. One of the first studies focused on lung cancer mortality in young adults, who were 30–49 years of age at the time of death (Smith et al., 2006). Among of residents of Region II, those who had experienced the peak As exposure period in early childhood had strikingly increased mortality rates from lung cancer compared to the rest of Chile (SMR: 7.0). Likewise, lung cancer mortality rates were similarly high among individuals who were born during the period of high-exposure (1958–1970) (SMR: 6.1).
In another study of Region II in Chile, the childhood mortality rate from liver cancer from 1950–2000 among those born just prior to the highest contamination period was ten-fold higher than less exposed counterparts in Region V of Chile (Table S1) (Liaw et al., 2008). Rates of other childhood cancers remained relatively steady and mortality rates for those born during the high contamination period were not elevated. It is possible that As exposure differentially targets liver development and/or function in early childhood, and may not only account for this observation, but could relate to As’s impact on cardiovascular health and inflammation. Although also based on a small number of outcomes, a more recent study examined mortality among adults (30–49 years) in Region II born between 1958 and 1970 (with likely in utero or early life peak exposures) and found striking increases in bladder cancer mortality (SMR = 18.1), large increases in laryngeal cancer (SMR = 2.5) and elevated liver cancer (SMR= 8.5), compared to the rest of Chile (Table S1) (Smith et al., 2012).
Increased cancer rates also have been observed in Japan, where in 1955 As contamination of a popular milk powder brand poisoned thousands -- an estimated 2,000 young children and infants in Okayama Prefecture alone. An ecological study of this area indicated increases in the incidence rates of all cancers and liver cancers among those exposed before one year of age, as well as pancreatic and hematopoietic cancers among those exposed before five years of age (Yorifuji et al., 2011). Increased total cancer mortality rates and mortality specifically from skin cancers, pancreatic cancer, leukemia and liver cancer also were observed for those exposed to the As contaminated milk powder before the age of five years (Yorifuji et al., 2010). Albeit at relatively high levels of exposures, these ecologic epidemiologic studies provide compelling evidence that early life is a key exposure window for As’s carcinogenic effects via ingestion.
In addition to epidemiologic data, a series of laboratory studies examining in utero As exposure in mice conducted by the Waalkes group were among the first to establish data on detrimental health effects of prenatal As exposure (Table S2) (Waalkes et al., 2003; Waalkes et al., 2004b; Waalkes et al., 2004a; Waalkes et al., 2004c). These studies, performed in several strains of wild-type mice, mirror the carcinogenic effects observed with As exposure in ecological studies and provided direct evidence that gestational exposure to As alone elevates the risk of cancer in exposed offspring. Offspring of mice given up to 850µg/L sodium arsenite in drinking water during gestation developed hepatocellular carcinomas and tumors of the adrenal glands, liver, lungs, ovaries and uterus. Males tended to develop hepatocellular and adrenal carcinomas, while lung carcinoma was significantly increased in females across studies (Waalkes et al., 2003; Waalkes et al., 2004b; Waalkes et al., 2004c). Interestingly, previous work had shown that these doses of As were generally well tolerated by adult mice and were only carcinogenic to adult mice in the presence of a secondary insult, further confirming that fetal development is specifically sensitive to the effects of toxicants (Waalkes et al., 2000; Waalkes et al., 2007).
A further study attempted to mimic the type of exposure an individual person might have over a lifetime by exposing mice to lower doses (up to 240µg/L) of As in drinking water, beginning 2 weeks prior to breeding, during pregnancy, and continuing through the offspring’s adult life (Tokar et al., 2011). Interestingly, these whole-life exposed mice developed tumors at sites very similar to those of mice that were only exposed in utero, such as hepatocellular, lung, and adrenal carcinomas, but sex-specific differences in the types of carcinomas were no longer apparent, as previously observed with those only exposed in utero (Table S2). Tumors tended to occur more frequently and to be more invasive, indicating that As exposure during gestation may play a role in dictating which tissues are targeted, while the dose and length of exposure may drive the aggressiveness of the disease.
To define early molecular changes in response to in utero As exposure, Shen et al. gave As in drinking water (850µg/L) to pregnant C3H mice on gestational days 8–18 and then examined lung tissue from the pups for gene expression alterations (Shen et al., 2007). In the fetal lung, they found increased expression of estrogen receptor-α, as well as changes in other estrogen-related genes, insulin growth factor, α-fetoprotein, epidermal growth factor receptor, L-myc, and metallothionein-1, all of which have been implicated in lung oncogenesis or progression. In other experiments, mice exposed to As prenatally appear to be sensitized in adulthood to the toxic and carcinogenic effects of other compounds, including estrogen-like molecules DES and tamoxifen (Table S2). Offspring of mice that received 850µg/L As in drinking water during pregnancy and were postnatally dosed with DES or tamoxifen, had increased incidence of urogenital tumors in females and hepatocellular or urinary bladder tumors in males (Waalkes et al., 2006a; Waalkes et al., 2006b). Similarly, a pair of studies assessed the tumorigenic effects of topical application of tumor-promoting molecule 12-O-tetradecanoyl phorbol-13-acetate (TPA) in offspring born to mothers prenatally exposed to As (Waalkes et al., 2008; Tokar et al., 2010). Although TPA alone can promote tumorigenesis, in these studies, fetal As exposure before TPA treatment increased the occurrence and aggressiveness of tumors at both skin and non-skin sites. Examination of skin tumors in As-exposed mice revealed increases in CD34-positive cells, a stem cell marker, and expression of Rac1, a stimulator of self-renewal, suggesting that in utero As exposure may target stem cells, leading to deregulated self-renewal capacity in later life and perhaps accounting in part for the increased sensitivity to secondary insults.
Respiratory disease and pulmonary function
Studies also have examined the non-cancerous effects of early life As exposure on the respiratory system (Table S1). In the ecological work from Chile, in addition to increased lung cancer mortality, individuals born just before the high-exposure period (1950–1957) and exposed in early childhood had elevated mortality rates from bronchiectasis (SMR: 12.4), which was even higher among those born during the peak exposure years (1958–1970) (SMR: 46.2), although based on only nine events (Smith et al., 2006). This increase may relate to the further observation in this region that early-life As exposure was associated with multiple measures of reduced lung function in adulthood (Dauphine et al., 2011). Compared to less exposed individuals, those with high early-life As exposure (i.e., living as a child or born during the peak exposure period), had on average an 11.5% lower forced expiratory volume in one second (FEV1), a 12.2% lower forced vital capacity (FVC) and increased self-reported breathlessness.
Several studies in experimental systems have supported the idea that prenatal As exposure may lead to lung disease by showing that As can induce alterations in lung structure and function (Table S2). Mice exposed to lower doses (<100µg/L) of As in utero and in early postnatal life were found to have significantly altered airway reactivity to methacholine challenge at 28 days of age (Lantz et al., 2009). Moreover, in utero and early life exposure appeared to be unique in their effect, as adult mice exposed to similar levels of As in drinking water were unaffected. Smooth muscle mass was decreased around airways, particularly in small (<100µm) airways, and concomitant decreases in extracellular matrix proteins suggest that early As exposure can alter both the structure and function of the lungs. Even at low doses, in utero exposure to As via drinking water resulted in impaired lung function in early life (Ramsey et al., 2013b). Offspring of C57BL/6 mice that were given drinking water containing 0, 10 (current US EPA and WHO maximum contaminant level) or 100µg/L As were assessed for lung volume, lung mechanics, pressure-volume curves and the volume dependence of lung mechanics at 2, 4, 6 and 8 weeks of age. These low doses resulted in deficits in lung function at 2 weeks of age, with higher tissue elastance and tissue damping in exposed mice compared to controls, which translates to increased stiffness of the parenchymal lung tissue. Male pups appeared to be more susceptible to the toxic effects. However, in these studies, alterations to lung mechanics following in utero As exposure appeared to be recovered by adulthood.
Further, when embryonic lungs of Sprague-Dawley rats exposed to 500µg/L As in utero were analyzed for altered gene and protein expression, researchers identified 59 genes and 34 proteins that were specifically altered in As-exposed rats (Petrick et al., 2009). Many of these proteins, which play roles in lung structure and have been implicated in aberrant growth, were related to cell motility and integrin signaling through the beta-catenin pathway, resulting in alterations in c-myc, while others encoded key extracellular matrix proteins. The potential for altered infection clearance or immune response were suggested by a microarray analysis of lung tissue from three strains of mice (BALB/c, C57BL/6, C3H/HeARC) exposed to 100µg/L As via drinking water in utero that showed that As exposure increased the expression of mucus-production genes (Clca3, Muc5b, Scgb3a1), innate immune regulators (Reg3γ, Tff2, Dynlrb2, Lplunc1) and lung morphogenesis gene, Sox2 (Ramsey et al., 2013a). Thus, the overall effect of in utero As exposure on lung mechanics, mucociliary clearance, innate immunity, and essential extracellular matrix proteins, could impact not only long-term respiratory health but could potentially enhance vulnerability to infections, and in turn enhancing sequelae such as bronchiectasis.
Summary and Conclusions
We now understand that the critical developmental period, beginning in utero and continuing into early postnatal life, is uniquely sensitive to environmental insults. Emerging data from both epidemiologic studies and experimental systems suggest that As is one such environmental toxicant for which exposure during this period may impact lifelong health. In particular, there is a fairly consistent picture of in utero or early-life exposure to As at high levels in relation to elevated risk of respiratory disease, impaired lung function, cancer and CVD. However, data on effects at lower levels of exposure are not available. Experimental data, including from animal studies, appear to closely parallel effects observed in humans. They provide important mechanistic insights indicating several pathways by which in utero or early-life exposure may influence these outcomes, i.e., via alterations in innate immunity, inflammatory response, and extracellular matrix integrity, as well as through developmental deregulation of pathways implicated in carcinogenesis.
As we begin to elucidate the relationship between fetal and early postnatal As exposure and disease, several methodological issues need to be considered in future epidemiologic studies. First, the lack of prospective studies with data at the individual level represents a critical gap in our knowledge. While ecological studies have contributed greatly to our current understanding of the long-term effects of in utero exposure in relation to increased disease mortality, individual exposure assessments are needed to distinguish effects at lower levels of exposures such as those common to the US, and ones that examine disease incidence rather than mortality. Second, examination of how prenatal exposure to As affects intermediary clinical endpoints, such as blood pressure and pulmonary function, in childhood and early adulthood may help to establish a causal role for in utero As exposure in the development of later life disease and provide avenues for early intervention. Recent methodological advancements have made it feasible to examine impacts of exposure on multiple biomarkers in epidemiological studies, including epigenetic alteration, microbiome profiling, immunologic phenotyping and assessment of early molecular changes such as gene expression in cord blood and placenta, leading researchers to a fuller mechanistic understanding of human disease development in response to early As exposure.
Third, as mentioned, our current understanding is limited by the fact that most studies have examined higher levels of exposure, in both humans and animal models, necessitating work to elucidate the lower end of the dose curve. It is conceivable that certain systems and tissues may be uniquely affected by lower doses of As. Indeed, the cardiovascular system may be particularly sensitive to As, as indicated by reports of adverse effects in vascular smooth muscle and endothelial cells at doses much lower than those required to induce cancer in animal models (Soucy et al., 2005; Straub et al., 2007). In some cases, lower exposures may cause more toxicity, as suggested in a study of adult ApoE−/− mice that found a greater number of As-related atherosclerotic plaques in animals given doses of 200µg/L, as compared to those that were exposed to higher doses of As (i.e. 1000µg/L) (Lemaire et al., 2011). Additionally, it is possible that certain doses of As may sensitize individuals to secondary insults, as indicated by animal work. This phenomenon has yet to be explored epidemiologically. Fourth, recent evidence that consumption of rice and rice products may also contribute to exposure levels has raised concerns of low-level As exposure from diet (Gilbert-Diamond et al., 2011; Davis et al., 2012; Jackson et al., 2012). Rice products, such as organic brown rice syrup, are often used in prepared foods and can contain high levels of inorganic As (Jackson et al., 2012), and consumption of rice and rice products has been shown to increase total urinary arsenic levels in both pregnant women and children (Gilbert-Diamond et al., 2011; Davis et al., 2012). As regulatory limits on As in rice have yet to be established in many parts of the world, including in the US, dietary intake of As may contribute significantly to an individual’s overall exposure level, especially in populations largely not exposed to As from groundwater. Studies that take advantage of biomarkers of As exposure, such as levels in urine or nail clippings, as a measure of exposure from all sources can more precisely examine the effects at lower levels and in different populations, including the US population. Lastly, studies that are able to assess the impact of timing of exposure during the life course are needed. If animal models are a good indicator of effects in humans, exposure timing and length of exposure have the potential to lead to very different effects, such as sex-specific outcomes.
Millions of individuals worldwide continue to be chronically exposed to As via contaminated water, and millions more may be unknowingly exposed to As via their diet, as consumption of rice and rice products may be a common source of As exposure (Gilbert-Diamond et al., 2011; Jackson et al., 2012). Thus, the need for further work to examine in utero and early life exposure to As is evident and essential to defining the potential long-term health consequences of this widespread toxicant.
Supplementary Material
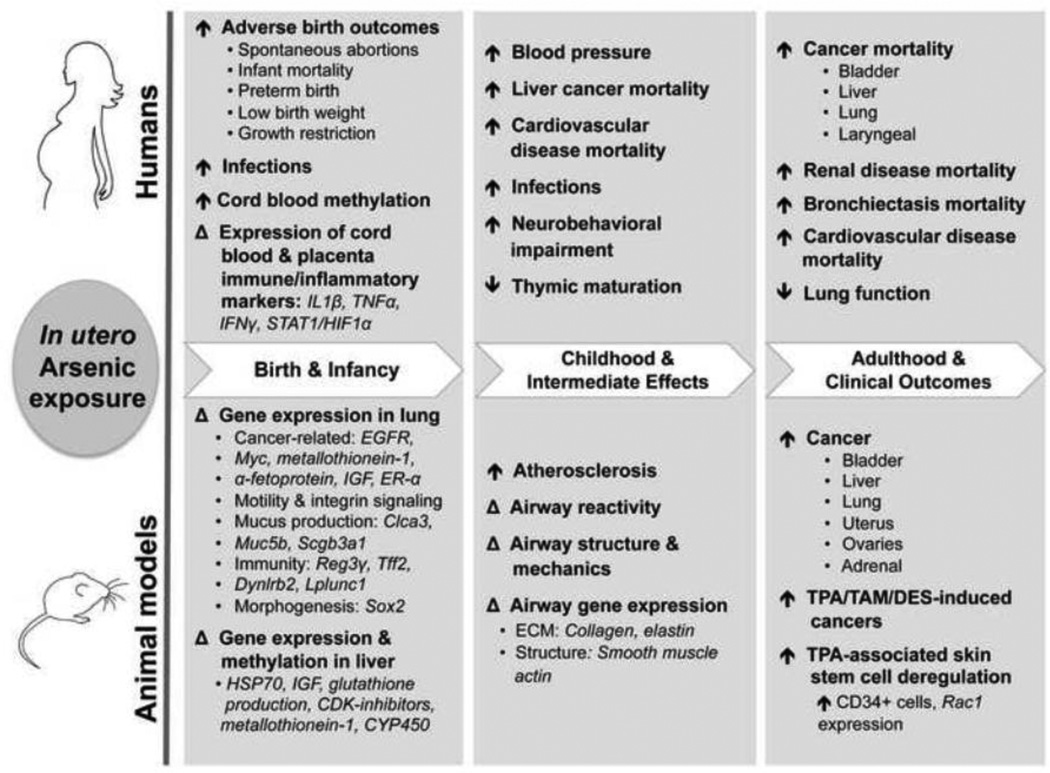
Figure 1.
In utero arsenic exposure and lifelong health effects. Human epidemiological studies (top) and experimental animal studies (bottom) demonstrate that in utero exposure to arsenic can impact health and disease development at different stages of life, from birth (leftmost gray panel) to childhood (center panel) and into adulthood (rightmost panel). In utero arsenic exposure may lead to different clinical presentation depending on the stage of life investigated, but examination of clinically relevant, intermediate endpoints in childhood may help to elucidate arsenic-related pathogenesis and later-life disease susceptibility.
Highlights.
We review in utero and early-life As exposure impacts on lifelong disease risks
Evidence indicates that early-life As increases risks of lung disease, cancer and CVD
Animal work largely parallels human studies and may lead to new research directions
Prospective studies and individual exposure assessments with biomarkers are needed
Assessing intermediary endpoints may aid early intervention and establish causality
Acknowledgements
The authors would like to thank Crystal Flaherty for her assistance with figure artwork. This work was supported by grant numbers R01-ES017541, RD-83459901 (EPA), P20-ES018175 (NIEHS) and CA134286.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no competing interests to declare.
References
- Abhyankar LN, Jones MR, Guallar E, Navas-Acien A. Arsenic exposure and hypertension: a systematic review. Environmental health perspectives. 2012;120:494–500. doi: 10.1289/ehp.1103988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad SA, Sayed MH, Barua S, Khan MH, Faruquee MH, Jalil A, Hadi SA, Talukder HK. Arsenic in drinking water and pregnancy outcomes. Environmental health perspectives. 2001;109:629–631. doi: 10.1289/ehp.01109629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S, Ahsan KB, Kippler M, Mily A, Wagatsuma Y, Hoque AM, Ngom PT, Arifeen SE, Raqib R, Vahter M. In utero arsenic exposure is associated with impaired thymic function in newborns possibly via oxidative stress and apoptosis. Toxicological sciences : an official journal of the Society of Toxicology. 2012 doi: 10.1093/toxsci/kfs202. [DOI] [PubMed] [Google Scholar]
- Ahmed S, Mahabbat-e Khoda S, Rekha RS, Gardner RM, Ameer SS, Moore S, Ekstrom EC, Vahter M, Raqib R. Arsenic-associated oxidative stress, inflammation, and immune disruption in human placenta and cord blood. Environmental health perspectives. 2011;119:258–264. doi: 10.1289/ehp.1002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argos M, Kalra T, Rathouz PJ, Chen Y, Pierce B, Parvez F, Islam T, Ahmed A, Rakibuz-Zaman M, Hasan R, Sarwar G, Slavkovich V, van Geen A, Graziano J, Ahsan H. Arsenic exposure from drinking water, and all-cause and chronic-disease mortalities in Bangladesh (HEALS): a prospective cohort study. Lancet. 2010;376:252–258. doi: 10.1016/S0140-6736(10)60481-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Graziano JH, Parvez F, Liu M, Slavkovich V, Kalra T, Argos M, Islam T, Ahmed A, Rakibuz-Zaman M, Hasan R, Sarwar G, Levy D, van Geen A, Ahsan H. Arsenic exposure from drinking water and mortality from cardiovascular disease in Bangladesh: prospective cohort study. Bmj. 2011;342:d2431. doi: 10.1136/bmj.d2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concha G, Vogler G, Lezcano D, Nermell B, Vahter M. Exposure to inorganic arsenic metabolites during early human development. Toxicological sciences : an official journal of the Society of Toxicology. 1998;44:185–190. doi: 10.1006/toxs.1998.2486. [DOI] [PubMed] [Google Scholar]
- Dauphine DC, Ferreccio C, Guntur S, Yuan Y, Hammond SK, Balmes J, Smith AH, Steinmaus C. Lung function in adults following in utero and childhood exposure to arsenic in drinking water: preliminary findings. International archives of occupational and environmental health. 2011;84:591–600. doi: 10.1007/s00420-010-0591-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MA, Mackenzie TA, Cottingham KL, Gilbert-Diamond D, Punshon T, Karagas MR. Rice consumption and urinary arsenic concentrations in U.S. children. Environmental health perspectives. 2012;120:1418–1424. doi: 10.1289/ehp.1205014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzan SF, Korrick S, Li Z, Enelow R, Gandolfi AJ, Madan J, Nadeau K, Karagas MR. In utero arsenic exposure and infant infection in a United States cohort: A prospective study. Environmental research. 2013 doi: 10.1016/j.envres.2013.05.001. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry RC, Navasumrit P, Valiathan C, Svensson JP, Hogan BJ, Luo M, Bhattacharya S, Kandjanapa K, Soontararuks S, Nookabkaew S, Mahidol C, Ruchirawat M, Samson LD. Activation of inflammation/NF-kappaB signaling in infants born to arsenic-exposed mothers. PLoS genetics. 2007;3:e207. doi: 10.1371/journal.pgen.0030207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert-Diamond D, Cottingham KL, Gruber JF, Punshon T, Sayarath V, Gandolfi AJ, Baker ER, Jackson BP, Folt CL, Karagas MR. Rice consumption contributes to arsenic exposure in US women. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:20656–20660. doi: 10.1073/pnas.1109127108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goggin SL, Labrecque MT, Allan AM. Perinatal exposure to 50 ppb sodium arsenate induces hypothalamic-pituitary-adrenal axis dysregulation in male C57BL/6 mice. Neurotoxicology. 2012;33:1338–1345. doi: 10.1016/j.neuro.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamadani JD, Grantham-McGregor SM, Tofail F, Nermell B, Fangstrom B, Huda SN, Yesmin S, Rahman M, Vera-Hernandez M, Arifeen SE, Vahter M. Pre- and postnatal arsenic exposure and child development at 18 months of age: a cohort study in rural Bangladesh. International journal of epidemiology. 2010;39:1206–1216. doi: 10.1093/ije/dyp369. [DOI] [PubMed] [Google Scholar]
- Hamadani JD, Tofail F, Nermell B, Gardner R, Shiraji S, Bottai M, Arifeen SE, Huda SN, Vahter M. Critical windows of exposure for arsenic-associated impairment of cognitive function in pre-school girls and boys: a population-based cohort study. International journal of epidemiology. 2011;40:1593–1604. doi: 10.1093/ije/dyr176. [DOI] [PubMed] [Google Scholar]
- Hawkesworth S, Wagatsuma Y, Kippler M, Fulford AJ, Arifeen SE, Persson LA, Moore SE, Vahter M. Early exposure to toxic metals has a limited effect on blood pressure or kidney function in later childhood, rural Bangladesh. Int J Epidemiol. 2012 doi: 10.1093/ije/dys215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopenhayn C, Ferreccio C, Browning SR, Huang B, Peralta C, Gibb H, Hertz-Picciotto I. Arsenic exposure from drinking water and birth weight. Epidemiology. 2003;14:593–602. doi: 10.1097/01.ede.0000072104.65240.69. [DOI] [PubMed] [Google Scholar]
- Hopenhayn-Rich C, Browning SR, Hertz-Picciotto I, Ferreccio C, Peralta C, Gibb H. Chronic arsenic exposure and risk of infant mortality in two areas of Chile. Environmental health perspectives. 2000;108:667–673. doi: 10.1289/ehp.00108667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huyck KL, Kile ML, Mahiuddin G, Quamruzzaman Q, Rahman M, Breton CV, Dobson CB, Frelich J, Hoffman E, Yousuf J, Afroz S, Islam S, Christiani DC. Maternal arsenic exposure associated with low birth weight in Bangladesh. Journal of occupational and environmental medicine / American College of Occupational and Environmental Medicine. 2007;49:1097–1104. doi: 10.1097/JOM.0b013e3181566ba0. [DOI] [PubMed] [Google Scholar]
- Intarasunanont P, Navasumrit P, Waraprasit S, Chaisatra K, Suk WA, Mahidol C, Ruchirawat M. Effects of arsenic exposure on DNA methylation in cord blood samples from newborn babies and in a human lymphoblast cell line. Environmental health : a global access science source. 2012;11:31. doi: 10.1186/1476-069X-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Agency for Research on Cancer I. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Some Drinking-water Disinfectants and Contaminants, including Arsenic. Lyon, France: 2004. [PMC free article] [PubMed] [Google Scholar]
- Jackson BP, Taylor VF, Karagas MR, Punshon T, Cottingham KL. Arsenic, organic foods, and brown rice syrup. Environmental health perspectives. 2012;120:623–626. doi: 10.1289/ehp.1104619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kile ML, Baccarelli A, Hoffman E, Tarantini L, Quamruzzaman Q, Rahman M, Mahiuddin G, Mostofa G, Hsueh YM, Wright RO, Christiani DC. Prenatal arsenic exposure and DNA methylation in maternal and umbilical cord blood leukocytes. Environmental health perspectives. 2012;120:1061–1066. doi: 10.1289/ehp.1104173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koestler DC, Avissar-Whiting M, Houseman EA, Karagas MR, Marsit CJ. Differential DNA Methylation in Umbilical Cord Blood of Infants Exposed to Low Levels of Arsenic in-utero. Environmental health perspectives. 2013 doi: 10.1289/ehp.1205925. Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantz RC, Chau B, Sarihan P, Witten ML, Pivniouk VI, Chen GJ. In utero and postnatal exposure to arsenic alters pulmonary structure and function. Toxicology and applied pharmacology. 2009;235:105–113. doi: 10.1016/j.taap.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire M, Lemarie CA, Molina MF, Schiffrin EL, Lehoux S, Mann KK. Exposure to moderate arsenic concentrations increases atherosclerosis in ApoE−/− mouse model. Toxicological sciences : an official journal of the Society of Toxicology. 2011;122:211–221. doi: 10.1093/toxsci/kfr097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liaw J, Marshall G, Yuan Y, Ferreccio C, Steinmaus C, Smith AH. Increased Childhood Liver Cancer Mortality and Arsenic in Drinking Water in Northern Chile. Cancer Epidemiology Biomarkers & Prevention. 2008;17:1982–1987. doi: 10.1158/1055-9965.EPI-07-2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez EJ, Kolb BL, Bell A, Savage DD, Allan AM. Moderate perinatal arsenic exposure alters neuroendocrine markers associated with depression and increases depressive-like behaviors in adult mouse offspring. Neurotoxicology. 2008;29:647–655. doi: 10.1016/j.neuro.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez L, Jimenez V, Garcia-Sepulveda C, Ceballos F, Delgado JM, Nino-Moreno P, Doniz L, Saavedra-Alanis V, Castillo CG, Santoyo ME, Gonzalez-Amaro R, Jimenez-Capdeville ME. Impact of early developmental arsenic exposure on promotor CpG-island methylation of genes involved in neuronal plasticity. Neurochemistry international. 2011;58:574–581. doi: 10.1016/j.neuint.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Martinez-Finley EJ, Ali AM, Allan AM. Learning deficits in C57BL/6J mice following perinatal arsenic exposure: consequence of lower corticosterone receptor levels? Pharmacology, biochemistry, and behavior. 2009;94:271–277. doi: 10.1016/j.pbb.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milton AH, Smith W, Rahman B, Hasan Z, Kulsum U, Dear K, Rakibuddin M, Ali A. Chronic arsenic exposure and adverse pregnancy outcomes in bangladesh. Epidemiology. 2005;16:82–86. doi: 10.1097/01.ede.0000147105.94041.e6. [DOI] [PubMed] [Google Scholar]
- Ngalame NN, Micciche AF, Feil ME, States JC. Delayed Temporal Increase of Hepatic Hsp70 in ApoE Knockout Mice After Prenatal Arsenic Exposure. Toxicological sciences : an official journal of the Society of Toxicology. 2012;131:225–233. doi: 10.1093/toxsci/kfs264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio-Yanez C, Ayllon-Vergara JC, Aguilar-Madrid G, Arreola-Mendoza L, Hernandez-Castellanos E, Barrera-Hernandez A, De Vizcaya-Ruiz A, Del Razo LM. Carotid Intima-Media Thickness and Plasma Asymmetric Dimethylarginine in Mexican Children Exposed to Inorganic Arsenic. Environ Health Perspect. 2013 doi: 10.1289/ehp.1205994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parajuli RP, Fujiwara T, Umezaki M, Watanabe C. Association of cord blood levels of lead, arsenic, and zinc with neurodevelopmental indicators in newborns: a birth cohort study in Chitwan Valley, Nepal. Environmental research. 2013;121:45–51. doi: 10.1016/j.envres.2012.10.010. [DOI] [PubMed] [Google Scholar]
- Parvez F, Wasserman GA, Factor-Litvak P, Liu X, Slavkovich V, Siddique AB, Sultana R, Sultana R, Islam T, Levy D, Mey JL, van Geen A, Khan K, Kline J, Ahsan H, Graziano JH. Arsenic exposure and motor function among children in Bangladesh. Environmental health perspectives. 2011;119:1665–1670. doi: 10.1289/ehp.1103548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrick JS, Blachere FM, Selmin O, Lantz RC. Inorganic arsenic as a developmental toxicant: in utero exposure and alterations in the developing rat lungs. Molecular nutrition & food research. 2009;53:583–591. doi: 10.1002/mnfr.200800019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilsner JR, Hall MN, Liu X, Ilievski V, Slavkovich V, Levy D, Factor-Litvak P, Yunus M, Rahman M, Graziano JH, Gamble MV. Influence of prenatal arsenic exposure and newborn sex on global methylation of cord blood DNA. PloS one. 2012;7:e37147. doi: 10.1371/journal.pone.0037147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Persson LA, Nermell B, El Arifeen S, Ekstrom EC, Smith AH, Vahter M. Arsenic exposure and risk of spontaneous abortion, stillbirth, and infant mortality. Epidemiology. 2010;21:797–804. doi: 10.1097/EDE.0b013e3181f56a0d. [DOI] [PubMed] [Google Scholar]
- Rahman A, Vahter M, Ekstrom EC, Persson LA. Arsenic exposure in pregnancy increases the risk of lower respiratory tract infection and diarrhea during infancy in Bangladesh. Environmental health perspectives. 2011;119:719–724. doi: 10.1289/ehp.1002265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Vahter M, Ekstrom EC, Rahman M, Golam Mustafa AH, Wahed MA, Yunus M, Persson LA. Association of arsenic exposure during pregnancy with fetal loss and infant death: a cohort study in Bangladesh. American journal of epidemiology. 2007;165:1389–1396. doi: 10.1093/aje/kwm025. [DOI] [PubMed] [Google Scholar]
- Rahman M, Sohel N, Yunus M, Chowdhury ME, Hore SK, Zaman K, Bhuiya A, Streatfield PK. Increased Childhood Mortality and Arsenic in Drinking Water in Matlab, Bangladesh: A Population-Based Cohort Study. PloS one. 2013;8:e55014. doi: 10.1371/journal.pone.0055014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey KA, Bosco A, McKenna KL, Carter KW, Elliot JG, Berry LJ, Sly PD, Larcombe AN, Zosky GR. In utero exposure to arsenic alters lung development and genes related to immune and mucociliary function in mice. Environmental health perspectives. 2013a;121:244–250. doi: 10.1289/ehp.1205590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey KA, Larcombe AN, Sly PD, Zosky GR. In utero exposure to low dose arsenic via drinking water impairs early life lung mechanics in mice. BMC pharmacology & toxicology. 2013b;14:13. doi: 10.1186/2050-6511-14-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raqib R, Ahmed S, Sultana R, Wagatsuma Y, Mondal D, Hoque AM, Nermell B, Yunus M, Roy S, Persson LA, Arifeen SE, Moore S, Vahter M. Effects of in utero arsenic exposure on child immunity and morbidity in rural Bangladesh. Toxicology letters. 2009;185:197–202. doi: 10.1016/j.toxlet.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Rosado JL, Ronquillo D, Kordas K, Rojas O, Alatorre J, Lopez P, Garcia-Vargas G, Del Carmen Caamano M, Cebrian ME, Stoltzfus RJ. Arsenic exposure and cognitive performance in Mexican schoolchildren. Environmental health perspectives. 2007;115:1371–1375. doi: 10.1289/ehp.9961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg HG. Systemic arterial disease with myocardial infarction. Report on two infants. Circulation. 1973;47:270–275. doi: 10.1161/01.cir.47.2.270. [DOI] [PubMed] [Google Scholar]
- Rosenberg HG. Systemic arterial disease and chronic arsenicism in infants. Archives of pathology. 1974;97:360–365. [PubMed] [Google Scholar]
- Roy A, Kordas K, Lopez P, Rosado JL, Cebrian ME, Vargas GG, Ronquillo D, Stoltzfus RJ. Association between arsenic exposure and behavior among first-graders from Torreon, Mexico. Environmental research. 2011;111:670–676. doi: 10.1016/j.envres.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Shen J, Liu J, Xie Y, Diwan BA, Waalkes MP. Fetal onset of aberrant gene expression relevant to pulmonary carcinogenesis in lung adenocarcinoma development induced by in utero arsenic exposure. Toxicological sciences : an official journal of the Society of Toxicology. 2007;95:313–320. doi: 10.1093/toxsci/kfl151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeonova PP, Hulderman T, Harki D, Luster MI. Arsenic exposure accelerates atherogenesis in apolipoprotein E(−/−) mice. Environmental health perspectives. 2003;111:1744–1748. doi: 10.1289/ehp.6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AH, Marshall G, Liaw J, Yuan Y, Ferreccio C, Steinmaus C. Mortality in young adults following in utero and childhood exposure to arsenic in drinking water. Environmental health perspectives. 2012;120:1527–1531. doi: 10.1289/ehp.1104867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AH, Marshall G, Yuan Y, Ferreccio C, Liaw J, von Ehrenstein O, Steinmaus C, Bates MN, Selvin S. Increased mortality from lung cancer and bronchiectasis in young adults after exposure to arsenic in utero and in early childhood. Environmental health perspectives. 2006;114:1293–1296. doi: 10.1289/ehp.8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soucy NV, Mayka D, Klei LR, Nemec AA, Bauer JA, Barchowsky A. Neovascularization and angiogenic gene expression following chronic arsenic exposure in mice. Cardiovasc Toxicol. 2005;5:29–41. doi: 10.1385/ct:5:1:029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S, D'Souza SE, Sen U, States JC. In utero arsenic exposure induces early onset of atherosclerosis in ApoE−/− mice. Reprod Toxicol. 2007;23:449–456. doi: 10.1016/j.reprotox.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S, Vladykovskaya EN, Haberzettl P, Sithu SD, D'Souza SE, States JC. Arsenic exacerbates atherosclerotic lesion formation and inflammation in ApoE−/− mice. Toxicol Appl Pharmacol. 2009;241:90–100. doi: 10.1016/j.taap.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- States JC, Singh AV, Knudsen TB, Rouchka EC, Ngalame NO, Arteel GE, Piao Y, Ko MS. Prenatal arsenic exposure alters gene expression in the adult liver to a proinflammatory state contributing to accelerated atherosclerosis. PloS one. 2012;7:e38713. doi: 10.1371/journal.pone.0038713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- States JC, Srivastava S, Chen Y, Barchowsky A. Arsenic and cardiovascular disease. Toxicological sciences : an official journal of the Society of Toxicology. 2009;107:312–323. doi: 10.1093/toxsci/kfn236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub AC, Stolz DB, Vin H, Ross MA, Soucy NV, Klei LR, Barchowsky A. Low level arsenic promotes progressive inflammatory angiogenesis and liver blood vessel remodeling in mice. Toxicol Appl Pharmacol. 2007;222:327–336. doi: 10.1016/j.taap.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokar EJ, Diwan BA, Waalkes MP. Arsenic exposure in utero and nonepidermal proliferative response in adulthood in Tg.AC mice. International journal of toxicology. 2010;29:291–296. doi: 10.1177/1091581810362804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokar EJ, Diwan BA, Ward JM, Delker DA, Waalkes MP. Carcinogenic effects of "whole-life" exposure to inorganic arsenic in CD1 mice. Toxicological sciences : an official journal of the Society of Toxicology. 2011;119:73–83. doi: 10.1093/toxsci/kfq315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai SY, Chou HY, The HW, Chen CM, Chen CJ. The effects of chronic arsenic exposure from drinking water on the neurobehavioral development in adolescence. Neurotoxicology. 2003;24:747–753. doi: 10.1016/S0161-813X(03)00029-9. [DOI] [PubMed] [Google Scholar]
- Vahter ME, Li L, Nermell B, Rahman A, El Arifeen S, Rahman M, Persson LA, Ekstrom EC. Arsenic exposure in pregnancy: a population-based study in Matlab, Bangladesh. Journal of health, population, and nutrition. 2006;24:236–245. [PubMed] [Google Scholar]
- Valsamakis G, Kanaka-Gantenbein C, Malamitsi-Puchner A, Mastorakos G. Causes of intrauterine growth restriction and the postnatal development of the metabolic syndrome. Annals of the New York Academy of Sciences. 2006a;1092:138–147. doi: 10.1196/annals.1365.012. [DOI] [PubMed] [Google Scholar]
- Valsamakis G, Kanaka-Gantenbein C, Malamitsi-Puchner A, Mastorakos G. Causes of intrauterine growth restriction and the postnatal development of the metabolic syndrome. Ann N Y Acad Sci. 2006b;1092:138–147. doi: 10.1196/annals.1365.012. [DOI] [PubMed] [Google Scholar]
- von Ehrenstein OS, Guha Mazumder DN, Hira-Smith M, Ghosh N, Yuan Y, Windham G, Ghosh A, Haque R, Lahiri S, Kalman D, Das S, Smith AH. Pregnancy outcomes, infant mortality, and arsenic in drinking water in West Bengal, India. American journal of epidemiology. 2006;163:662–669. doi: 10.1093/aje/kwj089. [DOI] [PubMed] [Google Scholar]
- von Ehrenstein OS, Poddar S, Yuan Y, Mazumder DG, Eskenazi B, Basu A, Hira-Smith M, Ghosh N, Lahiri S, Haque R, Ghosh A, Kalman D, Das S, Smith AH. Children's intellectual function in relation to arsenic exposure. Epidemiology. 2007;18:44–51. doi: 10.1097/01.ede.0000248900.65613.a9. [DOI] [PubMed] [Google Scholar]
- Waalkes MP, Keefer LK, Diwan BA. Induction of proliferative lesions of the uterus, testes, and liver in swiss mice given repeated injections of sodium arsenate: possible estrogenic mode of action. Toxicology and applied pharmacology. 2000;166:24–35. doi: 10.1006/taap.2000.8963. [DOI] [PubMed] [Google Scholar]
- Waalkes MP, Liu J, Diwan BA. Transplacental arsenic carcinogenesis in mice. Toxicology and applied pharmacology. 2007;222:271–280. doi: 10.1016/j.taap.2006.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waalkes MP, Liu J, Germolec DR, Trempus CS, Cannon RE, Tokar EJ, Tennant RW, Ward JM, Diwan BA. Arsenic exposure in utero exacerbates skin cancer response in adulthood with contemporaneous distortion of tumor stem cell dynamics. Cancer research. 2008;68:8278–8285. doi: 10.1158/0008-5472.CAN-08-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waalkes MP, Liu J, Ward JM, Diwan BA. Animal models for arsenic carcinogenesis: inorganic arsenic is a transplacental carcinogen in mice. Toxicology and applied pharmacology. 2004a;198:377–384. doi: 10.1016/j.taap.2003.10.028. [DOI] [PubMed] [Google Scholar]
- Waalkes MP, Liu J, Ward JM, Diwan BA. Mechanisms underlying arsenic carcinogenesis: hypersensitivity of mice exposed to inorganic arsenic during gestation. Toxicology. 2004b;198:31–38. doi: 10.1016/j.tox.2004.01.017. [DOI] [PubMed] [Google Scholar]
- Waalkes MP, Liu J, Ward JM, Diwan BA. Enhanced urinary bladder and liver carcinogenesis in male CD1 mice exposed to transplacental inorganic arsenic and postnatal diethylstilbestrol or tamoxifen. Toxicology and applied pharmacology. 2006a;215:295–305. doi: 10.1016/j.taap.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Waalkes MP, Liu J, Ward JM, Powell DA, Diwan BA. Urogenital carcinogenesis in female CD1 mice induced by in utero arsenic exposure is exacerbated by postnatal diethylstilbestrol treatment. Cancer research. 2006b;66:1337–1345. doi: 10.1158/0008-5472.CAN-05-3530. [DOI] [PubMed] [Google Scholar]
- Waalkes MP, Ward JM, Diwan BA. Induction of tumors of the liver, lung, ovary and adrenal in adult mice after brief maternal gestational exposure to inorganic arsenic: promotional effects of postnatal phorbol ester exposure on hepatic and pulmonary, but not dermal cancers. Carcinogenesis. 2004c;25:133–141. doi: 10.1093/carcin/bgg181. [DOI] [PubMed] [Google Scholar]
- Waalkes MP, Ward JM, Liu J, Diwan BA. Transplacental carcinogenicity of inorganic arsenic in the drinking water: induction of hepatic, ovarian, pulmonary, and adrenal tumors in mice. Toxicology and applied pharmacology. 2003;186:7–17. doi: 10.1016/s0041-008x(02)00022-4. [DOI] [PubMed] [Google Scholar]
- Wasserman GA, Liu X, Parvez F, Ahsan H, Factor-Litvak P, Kline J, van Geen A, Slavkovich V, Loiacono NJ, Levy D, Cheng Z, Graziano JH. Water arsenic exposure and intellectual function in 6-year-old children in Araihazar, Bangladesh. Environmental health perspectives. 2007;115:285–289. doi: 10.1289/ehp.9501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman GA, Liu X, Parvez F, Ahsan H, Factor-Litvak P, van Geen A, Slavkovich V, LoIacono NJ, Cheng Z, Hussain I, Momotaj H, Graziano JH. Water arsenic exposure and children's intellectual function in Araihazar, Bangladesh. Environmental health perspectives. 2004;112:1329–1333. doi: 10.1289/ehp.6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MM, Kuo TL, Hwang YH, Chen CJ. Dose-response relation between arsenic concentration in well water and mortality from cancers and vascular diseases. Am J Epidemiol. 1989;130:1123–1132. doi: 10.1093/oxfordjournals.aje.a115439. [DOI] [PubMed] [Google Scholar]
- Yorifuji T, Tsuda T, Doi H, Grandjean P. Cancer excess after arsenic exposure from contaminated milk powder. Environmental health and preventive medicine. 2011;16:164–170. doi: 10.1007/s12199-010-0182-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorifuji T, Tsuda T, Grandjean P. Unusual cancer excess after neonatal arsenic exposure from contaminated milk powder. Journal of the National Cancer Institute. 2010;102:360–361. doi: 10.1093/jnci/djp536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Marshall G, Ferreccio C, Steinmaus C, Selvin S, Liaw J, Bates MN, Smith AH. Acute myocardial infarction mortality in comparison with lung and bladder cancer mortality in arsenic-exposed region II of Chile from 1950 to 2000. American journal of epidemiology. 2007a;166:1381–1391. doi: 10.1093/aje/kwm238. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Marshall G, Ferreccio C, Steinmaus C, Selvin S, Liaw J, Bates MN, Smith AH. Acute Myocardial Infarction Mortality in Comparison with Lung and Bladder Cancer Mortality in Arsenic-exposed Region II of Chile from 1950 to 2000. Am J Epidemiol. 2007b;166:1381–1391. doi: 10.1093/aje/kwm238. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.