Abstract
Recent studies have identified exposure to polybrominated diphenyl ethers (PBDEs) as a risk factor for deficits in cognitive functioning seen in children as well as adults. Additionally, similar alterations in learning and memory have also been observed in animal models of PBDE exposure. However, given these findings, the molecular alterations that may underlie these neurobehavioral endpoints have not been identified. As the frontal cortex is involved in modulating several cognitive functions, the purpose of our study was to investigate the possible changes to the GABAergic and glutamatergic neurotransmitter systems located in the frontal cortex following exposure to the PBDE mixture, DE-71. Primary cultured neurons isolated from the frontal cortex showed a dose-dependent reduction in neurons as well as neurite outgrowth. Furthermore, evaluation of DE-71 neurotoxicity in the frontal cortex using an in vivo model showed alterations to specific proteins involved in mediating GABA and glutamate neurotransmission, including GAD67, vGAT, vGlut, and GABA(A) 2α receptor subunit. Interestingly, these alterations appeared to be preferential for the GABA and glutamate systems located in the frontal cortex. These findings identify specific targets of PBDE neurotoxicity and provide a possible molecular mechanism for PBDE-mediated neurobehavioral deficits that arise from the frontal cortex.
Keywords: Cognition, DE-71, frontal cortex, GABA, glutamate, polybrominated diphenyl ethers
1.0 Introduction
Polybrominated diphenyl ethers (PBDEs) are a class of brominated flame retardant compounds commonly used in various consumer products including electronics, furniture, and textiles to reduce each article's flammability (Darnerud et al. 2001; de Wit 2002). In contrast to other flame-retardants, which are chemically bonded to these products, PBDEs are not chemically integrated, making them more likely to migrate out of the product and into the surrounding environment. In the past, PBDEs have been found as one of three chemical mixtures, known as pentabrominated BDE (DE-71), octabrominated BDE, and decabrominated BDE. While the manufacture of penta and octa BDEs has been discontinued, deca BDEs remain the most extensively used PBDE mixture in the United States and around the world. Similar to other halogenated compounds, like polychlorinated biphenyls (PCBs), PBDEs are highly lipophilic and resistant to degradation, allowing them to accumulate and biomagnify, making them extremely persistent in the environment (Norstrom et al. 2002). These properties could be significant contributing factors to the reported increase in PBDE levels in both the environment as well as human tissue, raising concern over the safety of PBDEs and their potential adverse health effects.
Recent epidemiological studies have identified significant associations between exposure to PBDEs and deficits in multiple psychomotor and cognitive functions (Chao et al. 2011; Eskenazi et al. 2013; Fitzgerald et al. 2012; Gascon et al. 2012; Herbstman et al. 2010; Roze et al. 2009). Similar alterations in cognitive processes, including learning and memory, as well as attention, have also been reported in animals exposed pre- or postnatally to PBDEs. Indeed, exposure to the PBDE mixture, DE-71, caused impairments in learning and attention processes (Driscoll et al. 2009; Dufault et al. 2005; Koenig et al. 2012; Yan et al. 2012). Furthermore, exposure to single PBDE congeners has also been shown to cause deficits in cognitive measures. Using a single-exposure dosing paradigm, Viberg et al., (2003; 2006) demonstrated alterations in learning and memory in mice exposed to BDE congeners 153 or 203. The molecular deficits that underlie these cognitive impairments have not been fully elucidated, with some evidence suggesting a role of the hippocampal cholinergic system (Dufault et al. 2005; Viberg et al. 2003). However, other neurotransmitter systems as well as other brain regions known to be involved in cognition have not been evaluated for PBDE-mediated deficits.
The frontal cortex has been implicated in mediating many cognitive functions often found to be altered in multiple neurobehavioral disorders, including cognitive deficits, autism spectrum disorder (ASD), which affects an individuals social cognition, communication, and behavior, and schizophrenia (DeLorey et al. 2008; Fatemi et al. 2010; Gaspar et al. 2009; Lewis and Sweet 2009; Mohler 2007; Zahr et al. 2008). The frontal cortex is extensively innervated by the GABAergic, glutamatergic and dopaminergic neurotransmitter systems (Monaghan et al. 1989; Rushworth et al. 2007; Schoch et al. 1985). While the precise molecular targets in the frontal cortex have not been identified, it appears that deficits in cognitive processes arise from disruptions in proteins critical for normal functioning of these neurotransmitter systems. Thus, our current study sought to investigate the potential neuronal targets in the frontal cortex of animals exposed to the PBDE mixture, DE-71. Using in vitro and in vivo models we initially evaluated the neurotoxic potential of DE-71 on primary cultured neurons isolated from the frontal cortex of postnatal mice followed by assessment of alterations to specific markers of the GABA, glutamate, and dopamine circuits in the frontal cortex in mice exposed to 30 mg/kg of DE-71 every day for 30 days. This information will elaborate our understanding of the neurological targets and alterations following PBDE exposure and will provide insight into the potential neuropathological endpoints responsible for subsequent neurobehavioral indices.
2.0 Methods
2.1 Chemicals and Reagents
The commercial PBDE mixture DE-71 was purchased from Wellington Labs (Guelph, Ontario, Canada). Hibernate A and Hibernate A- Calcium were purchased from BrainBits (Springfield, IL). B27, DNase1, and Neurobasal A were purchased from Life Technologies (Carlsbad, CA). Papain was obtained from Sigma (St. Louis, MO). Dispase II was purchased from Roche (Nutley, NJ). The BCA protein assay kit was obtained from Pierce (Rockford, IL). Aphidicolin was purchased from A.G. Scientific (San Diego, CA). Monoclonal anti-rat dopamine transporter and polyclonal anti-rabbit tyrosine hydroxylase and norepinephrine transporter antibodies were purchased from EMD Millipore (Billerica, MA). Monoclonal anti-mouse α-tubulin antibodies were purchased from Sigma (St. Louis, MO). Rabbit anti-GAT1, rabbit anti-vGAT, mouse anti-GABA(A) 2α receptor subunit, rabbit anti-vGlut were purchased from Synaptic Systems (Germany), mouse anti-GAD67, mouse anti-NMDAR 2B receptor subunit were purchased from BD Transduction (San Jose, CA), mouse anti-MAP2 antibodies were purchased from Abcam (San Francisco, CA). Polyclonal anti-rabbit VMAT2 antibodies were generated by Covance to the C-terminal sequence in mouse (CTQNNVQPYPVGDDEESESD). Secondary antibodies conjugated to horseradish peroxidase were obtained from Jackson Immunoresearch Laboratories (West Grove, PA). Secondary antibodies conjugated to fluorescent tags were obtained from Life Technologies. SuperSignal West Dura Extended duration substrate and stripping buffer were obtained from Pierce. 3,3’ Diaminobenzidine (DAB) was purchased from Sigma.
2.2 Primary culture of cortical neurons
Primary cortical cultures were generated as previous described (Bradner et al. 2012), with modifications. Briefly, neuron cultures from postnatal mice (postnatal day 1-3) were isolated from the frontal cortex, which included the medial aspect of the frontal cortex but did not include tissue from the striatum or limbic system. Mouse brains were dissected in ice cold Hibernate A supplemented with B27. Following isolation of the relevant region and the removal of meninges, tissue pieces were chemically treated with a dissociation solution containing Papain (1 mg/ml), Dispase II (1.2U/ml), and DNase 1 (1ul/ml) dissolved in Hibernate A- Calcium for 20 min at 37°C and gently agitated every 5 min. Tissue was then rinsed in plating media containing Neurobasal-A, 10% heat inactivated fetal bovine serum, pen-strep, and mechanically dissociated using gentle trituration. Cells were plated on poly-d-lysine pre-coated 96 well plates at 40,000 cells per well. Plating media was removed and immediately switched to Neurobasal-A based culture media containing B27, 1% L-glutamine, 1% penicillin-streptomycin, and aphidicolin (1 μg/ml) after 2 hrs, in vitro. Primary cultures were treated 2 hrs after plating with four concentrations of DE-71 dissolved in DMSO and further diluted in cell culture media. For all control and DE-71 treatment experiments the final concentration of DMSO was <0.05% and no toxicity was observed at this percentage. After 24 hrs, cells were fixed in 4% PFA for 20 mins and incubated overnight in mouse anti-MAP2 at 4°C. The following day, cultures were incubated with fluorescent secondary antibodies, goat anti-mouse 572 for 1hr at room temperature. After staining with DAPI, cells were rinsed and stored in PBS. Images of treated cultures were obtained using the 10x objective on an Array Scan VTI HCS (Cellomics; Pittsburgh, PA). Twenty-five contiguous fields were taken per well, DAPI+ nuclei were counted, MAP2+ cell bodies were identified, and neurite length was recorded for all fields containing at least one MAP2+ cell body. Objects were identified and measured using the neuronal profiling bioapplication from Thermo Scientific. Statistical significance between the control and treatment groups for neuron count and neurite length was determined using GraphPad analysis software.
2.3 Animals and Treatment
Four month old male mice C57Bl/6J were orally gavaged with 30 mg/kg of DE-71 dissolved in corn oil vehicle daily for 30 days, as previously described (Bradner et al. 2012). This dosing paradigm was intended to represent the primary route of human exposure to DE-71 and is based upon previously published no observable adverse effect levels (NOAEL) and lowest observable adverse effect levels (LOAEL) for tetra-BDEs (Darnerud et al. 2001; Hallgren and Darnerud 2002; Hallgren et al. 2001). Furthermore, DE-71 was chosen based upon its composition of tetra- and penta PBDE congeners, which represents a mixture of the most prevalent congeners found in the environment, as well as in human tissue (Darnerud 2003; de Wit 2002). One day following the last dose, mice were sacrificed with frontal cortex (without inclusion of striatal or limbic tissue), collected for subsequent experimental assays. Standard rodent chow and tap water were available ad libitum. All procedures were conducted in accordance with the Guide for Care and Use of Laboratory Animals (National Institutes of Health) and have been approved by the Institutional Animal Care and Use Committee at Emory University.
2.4 Western Blot Analysis
Western blots were used to quantify the amount of several targets, including GABAergic GAD67, GABA transporter 1 (GAT1), vesicular GABA transporter (vGAT), GABA(A) 2α receptor subunit, vesicular glutamate transporter (vGlut), NMDA 2B receptor subunit, dopamine transporter (DAT), tyrosine hydroxylase (TH), vesicular monoamine transporter 2 (VMAT2), and norepinephrine transporter (NET), in samples of frontal cortex, striatum, hippocampus, and cerebellum from treated and control mice. Analysis was performed as previously described (Bradner et al. 2012). Briefly, samples were homogenized, subjected to polyacrylamide gel electrophoresis and electrophoretically transferred to polyvinylidene difluoride membranes. Nonspecific sites were blocked in 7.5% nonfat dry milk in Tris-buffered saline and then membranes incubated overnight in primary antibody. Antibody binding was detected using a horseradish peroxidase secondary antibody (1:10,000) and enhanced chemiluminescence. The luminescence signal was captured on an Alpha Innotech Fluorochem imaging system and stored as a digital image. Membranes were stripped for 15 min at room temperature with Pierce Stripping Buffer and sequentially reprobed with additional antibodies. α-Tubulin blots were used to ensure equal protein loading across samples.
2.5 Immunohistochemistry
Tissue staining was performed as described previously (Caudle et al. 2006). Briefly, whole brains were immersion fixed in 4% paraformaldehyde and serially sectioned at 40 μm. Sections were incubated with a polyclonal anti-GABA(A) 2α receptor subunit antibody overnight and then incubated in a biotinylated goat anti-rabbit secondary antibody for 1 hr at room temperature. Visualization was performed using DAB for 3 mins at room temperature. After DAB, tissue was mounted on slides, dehydrated, and coverslipped.
2.6 Statistical Analysis
All analysis was performed on raw data for each treatment group by one-way ANOVA or Students t-test. Post hoc analysis was performed using Tukey's post hoc test. Significance is reported at the p < 0.05 level.
3.0 Results
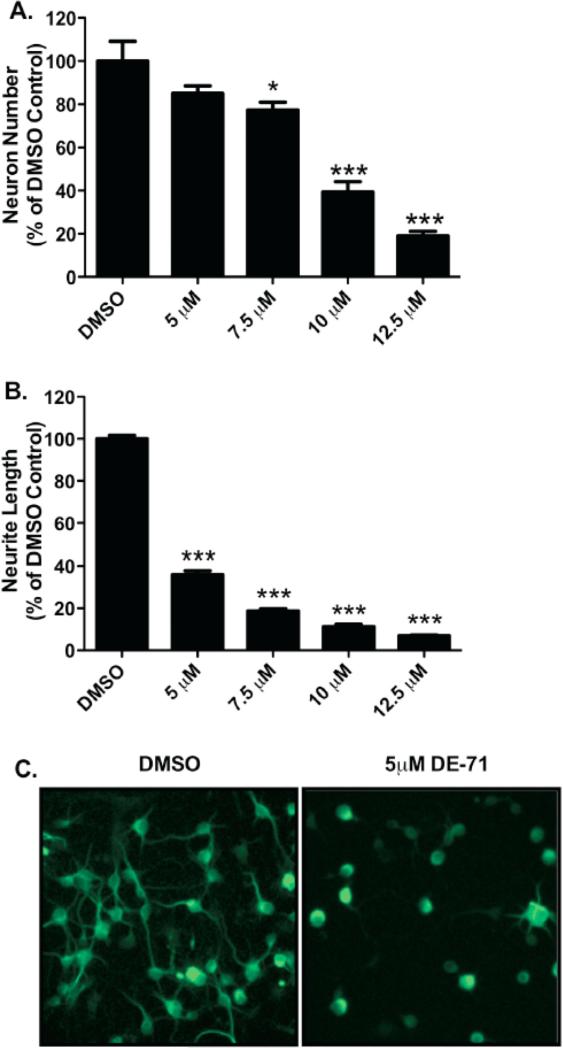
In order to get a general indication of the neurotoxic effects of DE-71 we generated primary cultures isolated from the frontal cortex of newborn mice, verified the presence of GABAergic and glutamatergic neuronal populations (Figure 1), and then exposed these cultures to increasing concentrations of DE-71 (0, 5 μM, 7.5 μM, 10 μM, 12.5 μM) for 24 hrs. Following treatment the number of neurons was analyzed as well as the total length of the neuronal processes (neurites) per neuron. As seen in Figure 2A, a dose dependent reduction in neuron density is observed in these cultures, with a significant reduction occurring with 7.5 μM DE-71. These findings demonstrate the neurotoxicity of DE-71 to frontal cortical neurons. Evaluation of damage to neurite length demonstrated a similar dose-dependent response. However, the response to DE-71 was much more sensitive for this measure (Figure 2B and C), suggesting that DE-71 may cause alterations to specific cellular functions and morphology in the cortical neuronal processes prior to impacting the neuronal cell body. Previous work from our lab has demonstrated similar neurotoxic effects of DE-71 on dopaminergic neurons, in culture (Bradner et al. 2012).
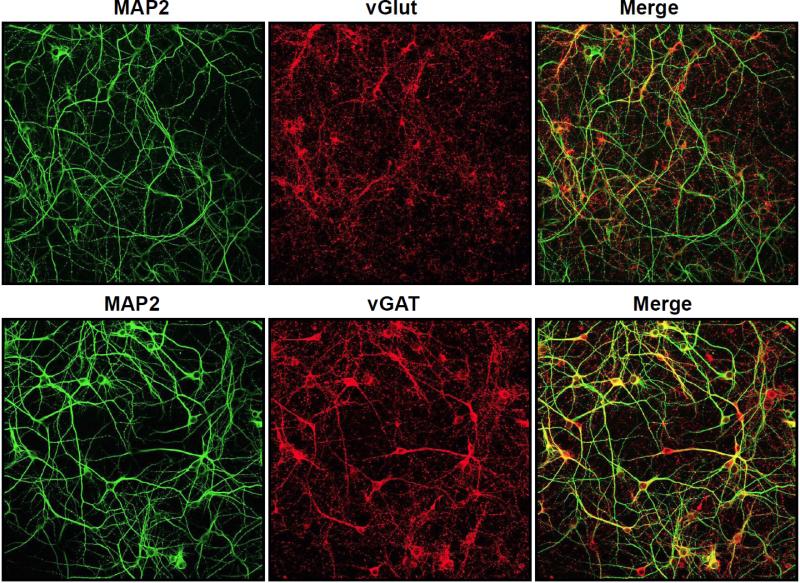
Figure 1.
GABAergic and glutamatergic neuronal populations are highly enriched in primary cultured neurons isolated from the frontal cortex of postnatal day 1 (PND1) mice. Neurons were grown in culture for 7 days and then incubated with MAP2 to visualize all neurons and vGlut or vGAT to localize all glutamatergic or GABAergic neurons, respectively in culture.
Figure 2.
Exposure of cortical primary cultures to DE-71 causes a loss of cortical neurons as well as reductions in neurite outgrowth. (A) Treatment of cortical cultures caused a significant reduction in the number of MAP2+ neurons, compared with DMSO control (52.3 ± 11.7) at 7.5 (40.5 ± 4.6), 10 (20.6 ± 6.0), and 12.5 μM (9.9 ± 2.8) DE-71. (B) Assessment of neurite length in these neurons demonstrated a greater loss of neurite outgrowth with a substantial reduction, compared with DMSO control (45.3 μm ± 1.8) at 5 μM (16.3 μm ± 2.0), 7.5 μM (8.5 μm ± 1.0), 10 μM (5.1 μm ± 1.4), and 12.5 μM (3.2 μm ± 0.4) DE-71. Columns represent the percent change from DMSO control for each genotype. Data represent the mean ± SEM of 4 experimental replicates per treatment group performed across 3 separate experiments. *Values for treatments significantly different from their respective genotype DMSO control (p < 0.05). ***Values significantly different from their respective genotype DMSO control (p < 0.001). (C) Representative cortical cultures stained for MAP2 and treated with DMSO or 5 μM DE-71.
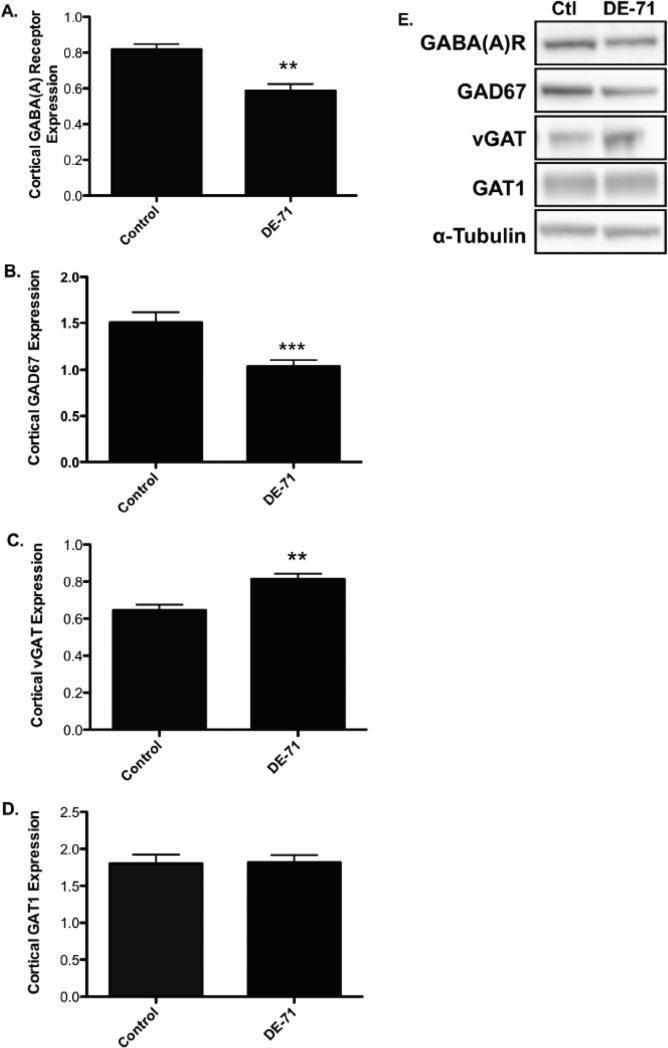
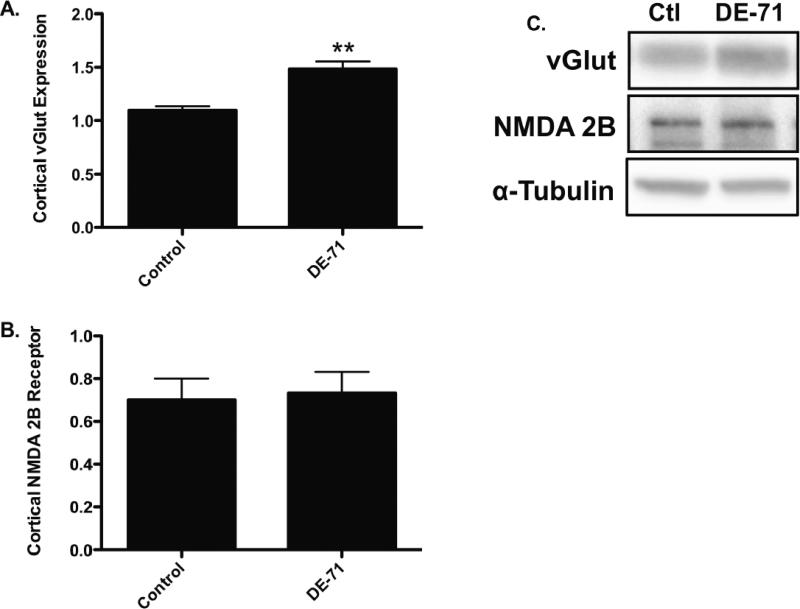
In order to further investigate these findings we exposed mice to DE-71 for 30 days and assessed the expression of multiple cortical proteins associated with the GABA, glutamate, and dopamine neurotransmitter systems in the frontal cortex. Although this concentration of DE-71 was approximately 4-fold higher than that used to treat our cortical cultures administration of this dosing paradigm did not result in overt signs of toxicity, indicated by no significant change in body weight, appearance, or gross behavior (data not shown). However, mice exposed to DE-71 showed a significant reduction in expression of the postsynaptic GABA(A) 2α receptor subunit as well as the GABA synthesizing enzyme, GAD67 (Figure 3A, B, and E; Figure 5 for GABA(A) 2α receptor subunit). In contrast, a significant increase in the expression of the vesicular GABA transporter, vGAT was observed (Figure 3C and E). No change was seen in GAT1, the plasmalemmal GABA transporter (Figure 3D and E). Evaluation of specific markers of the glutamate system in the frontal cortex showed a significant increase in the expression of the vesicular glutamate transporter, vGlut (Figure 4A and C). However, the postsynaptic NMDA 2B receptor subunit was not altered by DE-71 (Figure 4B and C). These data suggest that exposure to DE-71 causes significant alterations to specific proteins involved in GABAergic and glutamatergic neurotransmission that could affect the normal functioning of these neurotransmitter systems and their neural connections in the frontal cortex.
Figure 3.
Exposure of mice to 30 mg/kg DE-71 for 30 days show an alteration in the expression of pre- and postsynaptic GABAergic proteins in the frontal cortex. (A) Treatment with DE-71 significantly reduced cortical GABA(A) 2α receptor subunit expression compared with untreated mice. (B) Similarly, DE-71 treatment resulted in a significant reduction in cortical GAD67 expression compared with untreated animals. (C) In contrast, DE-71 caused an increase in cortical vGAT expression, relative to untreated mice. (D) Expression of GAT1 in the cortex was unchanged following DE-71 treatments. (E) Representative immunoblots for each protein examined. Data represent mean ± SEM (4-6 animals per treatment group). **Values for animals that are significantly different from controls (p < 0.01). ***Values for animals significantly different from controls (p < 0.001).
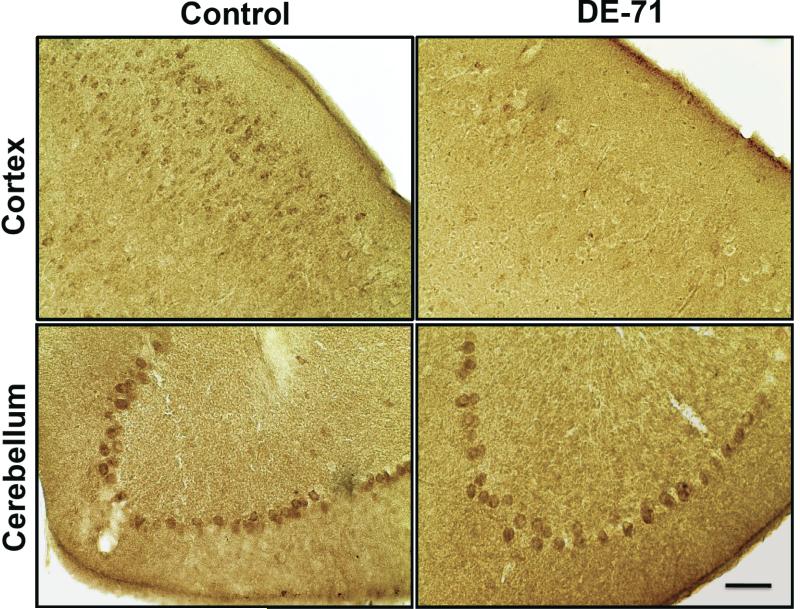
Figure 5.
Immunohistochemical assessment of GABA(A) 2α receptor subunit expression in mice exposed to 30 mg/kg DE-71 for 30 days. Exposure to DE-71 caused a reduction in the expression of GABA(A) 2α receptor subunit in the frontal cortex, compared with untreated controls. In contrast, no change in GABA(A) 2α receptor subunit expression was observed in the Purkinje cells of the cerebellum of DE-71 exposed mice. Scale Bar: 100 μm.
Figure 4.
Exposure of mice to 30 mg/kg DE-71 for 30 days shows an alteration in the expression of a presynaptic glutamatergic protein in the frontal cortex. (A) Exposure to DE-71 caused a significant increase in expression of the cortical vGlut compared with untreated animals. (B) In contrast, DE-71 treatment did not have an effect on the expression of the NMDA 2B receptor in the frontal cortex. (C) Representative immunoblots for each protein examined. Data represent mean ± SEM (4-6 animals per treatment group). **Values for animals that are significantly different from controls (p < 0.01).
Given these changes we next asked whether the alterations observed in the GABA and glutamate systems were specific to the frontal cortex. To address these concerns we evaluated expression of the GABA(A) 2α receptor subunit and vGlut in the striatum, hippocampus, and cerebellum. Interestingly, there was no difference in expression in either of these above markers in these brain regions, suggesting that the DE-71-mediated alterations to the GABA and glutamate systems are preferential for the frontal cortex (Table 1; Figure 5 for the GABA(A) 2α receptor subunit). Additionally, we were interested in examining the specificity of the changes demonstrated in the frontal cortex for the GABA and glutamate systems. As our previous work revealed a significant reduction in dopaminergic proteins, such as the dopamine transporter (DAT) and vesicular monoamine transporter 2 (VMAT2), in the striatum of mice exposed to DE-71, we first evaluated potential alterations in key proteins that mediate dopamine neurotransmission in the frontal cortex (Bradner et al. 2012). In contrast to our findings in the striatum we did not detect alterations in expression of DAT, VMAT2, or tyrosine hydroxylase (TH) in the frontal cortex (Table 1). Similar results were seen when the expression of the norepinephrine transporter was examined in the frontal cortex (Table 1). These data suggest that the alterations observed in the frontal cortex are specific to the GABA and glutamate neurotransmitter systems.
Table 1.
Effects of DE-71 on relevant neurotransmitter proteins in specific brain regions.
| Frontal Cortex | Striatum | Hippocampus | Cerebellum | |
|---|---|---|---|---|
| GABA(A) 2α Receptor Subunit | Decreased | No Change | No Change | No Change |
| vGlut | Increased | No Change | No Change | No Change |
| DAT | No Change | Decreaseda | Not Determined | Not Determined |
| TH | No Change | No Changea | Not Determined | Not Determined |
| VMAT2 | No Change | Decreaseda | Not Determined | Not Determined |
| NET | No Change | Not Determined | Not Determined | Not Determined |
Abbreviations: Vesicular Glutamate Transporter (vGlut), Dopamine Transporter (DAT), Tyrosine Hydroxylase (TH), Vesicular Monoamine Transporter 2 (VMAT2), Norepinephrine Transporter (NET).
Results previously published (Bradner et al., 2012).
4.0 Discussion
PBDEs are highly persistent in our environment and exposure to these compounds has been associated with significant deficits in psychomotor and cognitive development in children (Chao et al. 2011; Eskenazi et al. 2013; Fitzgerald et al. 2012; Gascon et al. 2012; Herbstman et al. 2010; Roze et al. 2009). Although the precise mechanisms that may underlie these deficits have not been identified, alterations to the GABA, glutamatergic and dopaminergic neurotransmitter systems, which are highly expressed in the frontal cortex and have been implicated in many neurobehavioral processes, including cognition, ASD, and schizophrenia, may be involved (DeLorey et al. 2008; Fatemi et al. 2010; Gaspar et al. 2009; Lewis and Sweet 2009; Mohler 2007; Zahr et al. 2008). Therefore, this study utilized in vitro and in vivo models to investigate alterations to the GABAergic, glutamatergic and dopaminergic neurotransmitter systems located in the frontal cortex following exposure to the PBDE mixture, DE-71.
As the frontal cortex is highly enriched with GABAergic, glutamatergic, and dopaminergic neuronal cell bodies and fibers (Monaghan et al. 1989; Rushworth et al. 2007; Schoch et al. 1985) we first sought to confirm that our primary culture model would appropriately represent the robust expression of the GABA and glutamate neuronal populations in this region. Our findings suggest that the frontal cortex is highly enriched with GABAergic and glutamatergic neurons and these populations are sensitive to exposure to low micromolar concentrations of DE-71, with neurite outgrowth appearing to be more vulnerable to these effects than the neuronal cell bodies. These findings are important as neurite extensions are involved in forming neuron-neuron synaptic connections, which is imperative for the proper formation of neural circuitry and neuronal communication and function. Indeed, deficits in neuronal connectivity, particularly in the frontal cortex, have been implicated in several neurological disorders, including ASD (Courchesne and Pierce 2005). Other groups have also evaluated the neurotoxic effects of DE-71 and specific PBDE congeners in primary cultured cortical neurons. Similar to our results, Giordano et al., (2008) demonstrated neuronal loss in the same range of DE-71 concentrations as we used in our study. While our study appears to be the first to utilize this model system to evaluate alterations to neurite outgrowth, other investigators have provided valuable support to our findings. Indeed, proteomic assessments of the effects of the PBDE congener, BDE-99 found significant alterations to a suite of proteins involved in cytoskeletal function, which would be involved in neurite outgrowth (Alm et al. 2008; Alm et al. 2006).
We next sought to elaborate our in vitro findings in an in vivo model system and investigate whether exposure to DE-71 would elicit alterations to proteins in the frontal cortex that are responsible for mediating GABAergic, glutamatergic, and dopaminergic neurotransmission. Based on previously published work, exposure to DE-71 resulted in a significant deposition of PBDE congeners in the brain, with PBDE-28, -47, -66, -85, -99, -100, -153, and -154 being present in the highest concentrations (Bradner et al. 2012). In addition, exposure to DE-71 caused significant alterations to several GABAergic and glutamatergic proteins in the frontal cortex. We found that exposure to DE-71 resulted in a significant reduction in the expression of the GABA(A) 2α receptor subunit as well as GAD67. In contrast, expression of vGAT was increased while GAT1 was unchanged. Although changes in expression of GABAergic proteins has not been previously identified following exposure to PBDEs, Hendriks et al., (2010) found that the hydroxylated metabolite of BDE-47 (6-OH-BDE-47) was a partial agonist for the GABA(A) receptor in Xenopous oocytes. This finding is interesting as other GABA(A) receptor agonists, such as ethanol have also been shown to reduce the expression of the receptor in the cerebral cortex (Davies 2003). Thus, it can be speculated that the reductions that we observed in the GABA(A) 2α receptor subunit may be due to its interaction with specific PBDE metabolites. To our knowledge, our study is the first to identify significant alterations to other GABAergic proteins, such as GAD67 and vGAT. While the implications of changes to these proteins is not known, a reduction in GAD67, which is the major enzyme involved in synthesizing GABA from glutamate in the nerve terminal would suggest a potential for loss of GABAergic neurons or less severe, a decrease in GABA being produced in these neurons. Related to this, an increase in vGAT, as seen in our study suggests further deficits in GABAergic neurotransmission by acting as a compensatory mechanism by the GABA neurons to attempt to sequester and package any available GABA into synaptic vesicles in order to maintain neuronal homeostasis. Taken in concert, prolonged exposure to PBDE congeners or their metabolites could affect GABAergic signaling in the frontal cortex. As GABA is the major inhibitory neurotransmitter in the brain, a reduction in its signaling, both from the presynaptic and postsynaptic neuronal aspects could have deleterious results in maintaining a normal balance between excitation-inhibition neuronal activity, which could result in over excitation, similar to that seen in epilepsy as well as ASD (Rubenstein and Merzenich 2003). Interestingly, alterations in GABA signaling have been observed in the frontal cortex of patients with ASD, as well as schizophrenia (Fatemi et al. 2010; Lewis et al. 2005).
In addition to changes in the GABAergic system we also identified an increase in vGlut expression while the expression of the postsynaptic NMDA 2B receptor subunit was not changed following exposure to DE-71. As with several of the GABAergic markers, our study appears to be the first to identify alterations in the glutamate vesicular transporter following exposure to PBDEs. Similar to the elevations in vGAT expression, an increase in vGlut in the frontal cortex could signify an effort by the glutamatergic neurons to compensate for a disruption in an unidentified mechanism involved in glutamate neurotransmission. Although we did not observe a change in the expression of the NMDA 2B receptor subunit in or study, other in vivo studies have examined alterations to glutamatergic receptors and routinely found reductions in expression of specific NMDA receptor subunits, including NR1, NR2A and NR2B in the hippocampus and cortex following exposure to BDE-47 (Dingemans et al. 2007; Suvorov and Takser 2011; Yan et al. 2012). Additionally, studies by Llansola et al., (2007; 2009) found prenatal exposure to BDE-99 altered the glutamate-nitric oxide-cGMP cascade via NMDA receptor activation, resulting in enhanced learning and memory capabilities of exposed offspring. Although the reasons for these differences can not be clearly defined, it can be speculated that exposure to BDE-47 or BDE-99 during critical periods of neurodevelopment, as seen in the Dingemans et al., (2007), Llansola (2007; 2009), and Suvorov and Takser (2011) studies, as well as the explicit use of BDE-47 and BDE-99 in each study rather than a mixture of BDE congeners could be contributing factors.
Previous work from our lab identified significant reductions in expression of DAT and VMAT2 in the striatum following exposure to DE-71 (Bradner et al. 2012). However, in contrast, we did not observe alterations in expression of these proteins, or TH in the frontal cortex. Additionally, we did not observe a change in expression of the norepinephrine transporter in this region. Although other studies have not evaluated these proteins in the brain and specifically the frontal cortex, a study by Gee and Moser, (2011) observed an increase in dopamine in the cortex of mice exposed to a single dose of 1, 10, or 30 mg/kg of BDE-47 on postnatal day 10. Finally, to assess the specificity of our changes in the GABAergic and glutamatergic neurotransmitter systems observed in the frontal cortex we also evaluated the expression of the GABA(A) 2α receptor subunit and vGlut in the striatum, hippocampus, and cerebellum. Again, the changes observed in the GABA and glutamate systems appear to be specific for the frontal cortex as no change was seen in either of these protein markers in any of the other regions tested. The reason for the specificity related to the GABAergic and glutamatergic neurotransmitter systems in the frontal cortex is unclear. However, we have observed a similar regional and neurotransmitter system specificity following exposure of mice to the polychlorinated biphenyl mixtures, Aroclor 1254 and 1260 (Caudle et al. 2006). These mice exhibited a significant reduction of the DAT and VMAT2 in the striatum, which were specific for the striatum, as these proteins did not change in other dopaminergic regions, including the frontal cortex, hypothalamus, and midbrain. Additionally, these changes were selective for the dopamine system as no effect was seen in GABAergic, glutamatergic, noradrenergic, and serotonergic neurotransmitter systems in the striatum.
In conclusion, using in vitro and in vivo models systems we have demonstrated significant alterations to the GABAergic and glutamatergic neurotransmitter systems located in the frontal cortex. These alterations appear to be preferential, not only for these neurotransmitters, but also for the frontal cortex. These findings are of interest as numerous studies have identified exposure to PBDEs as a risk factor for deficits in various cognitive and psychomotor endpoints in children and adults. Moreover, as changes in the GABA and glutamate systems has been identified in other neurobehavioral disorders, such as ASD and schizophrenia, these data could be a first step in elucidating possible environmental risk factors for these deficits.
Acknowledgments
FUNDING SOURCES
This work was supported by National Institutes of Health grants [R00ES017477 and P01ES016731 to W.M.C.]
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alm H, Kultima K, Scholz B, Nilsson A, Andren PE, Fex-Svenningsen A, Dencker L, Stigson M. Exposure to brominated flame retardant PBDE-99 affects cytoskeletal protein expression in the neonatal mouse cerebral cortex. Neurotoxicology. 2008;29:628–637. doi: 10.1016/j.neuro.2008.04.021. [DOI] [PubMed] [Google Scholar]
- Alm H, Scholz B, Fischer C, Kultima K, Viberg H, Eriksson P, Dencker L, Stigson M. Proteomic evaluation of neonatal exposure to 2,2 ,4,4 ,5-pentabromodiphenyl ether. Environ Health Perspect. 2006;114:254–259. doi: 10.1289/ehp.8419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradner JM, Suragh TA, Wilson WW, Lazo CR, Stout KA, Kim HM, Wang MZ, Walker DI, Pennell KD, Richardson JR, Miller GW, Caudle WM. Exposure to the Polybrominated Diphenyl Ether Mixture DE-71 Damages the Nigrostriatal Dopamine System: Role of Dopamine Handling in Neurotoxicity. Exp Neurol. 2012 doi: 10.1016/j.expneurol.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudle WM, Richardson JR, Delea KC, Guillot TS, Wang M, Pennell KD, Miller GW. Polychlorinated biphenyl-induced reduction of dopamine transporter expression as a precursor to Parkinson's disease-associated dopamine toxicity. Toxicol Sci. 2006;92:490–499. doi: 10.1093/toxsci/kfl018. [DOI] [PubMed] [Google Scholar]
- Chao HR, Tsou TC, Huang HL, Chang-Chien GP. Levels of breast milk PBDEs from southern Taiwan and their potential impact on neurodevelopment. Pediatr Res. 2011;70:596–600. doi: 10.1203/PDR.0b013e3182320b9b. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Pierce K. Why the frontal cortex in autism might be talking only to itself: local over-connectivity but long-distance disconnection. Curr Opin Neurobiol. 2005;15:225–230. doi: 10.1016/j.conb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Darnerud PO. Toxic effects of brominated flame retardants in man and in wildlife. Environ Int. 2003;29:841–853. doi: 10.1016/S0160-4120(03)00107-7. [DOI] [PubMed] [Google Scholar]
- Darnerud PO, Eriksen GS, Johannesson T, Larsen PB, Viluksela M. Polybrominated diphenyl ethers: occurrence, dietary exposure, and toxicology. Environ Health Perspect. 2001;109(Suppl 1):49–68. doi: 10.1289/ehp.01109s149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies M. The role of GABAA receptors in mediating the effects of alcohol in the central nervous system. J Psychiatry Neurosci. 2003;28:263–274. [PMC free article] [PubMed] [Google Scholar]
- de Wit CA. An overview of brominated flame retardants in the environment. Chemosphere. 2002;46:583–624. doi: 10.1016/s0045-6535(01)00225-9. [DOI] [PubMed] [Google Scholar]
- DeLorey TM, Sahbaie P, Hashemi E, Homanics GE, Clark JD. Gabrb3 gene deficient mice exhibit impaired social and exploratory behaviors, deficits in non-selective attention and hypoplasia of cerebellar vermal lobules: a potential model of autism spectrum disorder. Behav Brain Res. 2008;187:207–220. doi: 10.1016/j.bbr.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemans MM, Ramakers GM, Gardoni F, van Kleef RG, Bergman A, Di Luca M, van den Berg M, Westerink RH, Vijverberg HP. Neonatal exposure to brominated flame retardant BDE-47 reduces long-term potentiation and postsynaptic protein levels in mouse hippocampus. Environ Health Perspect. 2007;115:865–870. doi: 10.1289/ehp.9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll LL, Gibson AM, Hieb A. Chronic postnatal DE-71 exposure: effects on learning, attention and thyroxine levels. Neurotoxicol Teratol. 2009;31:76–84. doi: 10.1016/j.ntt.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Dufault C, Poles G, Driscoll LL. Brief postnatal PBDE exposure alters learning and the cholinergic modulation of attention in rats. Toxicol Sci. 2005;88:172–180. doi: 10.1093/toxsci/kfi285. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Chevrier J, Rauch SA, Kogut K, Harley KG, Johnson C, Trujillo C, Sjodin A, Bradman A. In utero and childhood polybrominated diphenyl ether (PBDE) exposures and neurodevelopment in the CHAMACOS study. Environ Health Perspect. 2013;121:257–262. doi: 10.1289/ehp.1205597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Reutiman TJ, Folsom TD, Rooney RJ, Patel DH, Thuras PD. mRNA and protein levels for GABAAalpha4, alpha5, beta1 and GABABR1 receptors are altered in brains from subjects with autism. J Autism Dev Disord. 2010;40:743–750. doi: 10.1007/s10803-009-0924-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald EF, Shrestha S, Gomez MI, McCaffrey RJ, Zimmerman EA, Kannan K, Hwang SA. Polybrominated diphenyl ethers (PBDEs), polychlorinated biphenyls (PCBs) and neuropsychological status among older adults in New York. Neurotoxicology. 2012;33:8–15. doi: 10.1016/j.neuro.2011.10.011. [DOI] [PubMed] [Google Scholar]
- Gascon M, Fort M, Martinez D, Carsin AE, Forns J, Grimalt JO, Santa Marina L, Lertxundi N, Sunyer J, Vrijheid M. Polybrominated diphenyl ethers (PBDEs) in breast milk and neuropsychological development in infants. Environ Health Perspect. 2012;120:1760–1765. doi: 10.1289/ehp.1205266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar PA, Bustamante ML, Silva H, Aboitiz F. Molecular mechanisms underlying glutamatergic dysfunction in schizophrenia: therapeutic implications. J Neurochem. 2009;111:891–900. doi: 10.1111/j.1471-4159.2009.06325.x. [DOI] [PubMed] [Google Scholar]
- Gee JR, Moser VC, McDanie KL, Herr DW. Neurochemical changes following a single dose of polybrominated diphenyl ether 47 in mice. Drug Chem Toxicol. 2011;34:213–219. doi: 10.3109/01480545.2010.536768. [DOI] [PubMed] [Google Scholar]
- Giordano G, Kavanagh TJ, Costa LG. Neurotoxicity of a polybrominated diphenyl ether mixture (DE-71) in mouse neurons and astrocytes is modulated by intracellular glutathione levels. Toxicol Appl Pharmacol. 2008;232:161–168. doi: 10.1016/j.taap.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgren S, Darnerud PO. Polybrominated diphenyl ethers (PBDEs), polychlorinated biphenyls (PCBs) and chlorinated paraffins (CPs) in rats-testing interactions and mechanisms for thyroid hormone effects. Toxicology. 2002;177:227–243. doi: 10.1016/s0300-483x(02)00222-6. [DOI] [PubMed] [Google Scholar]
- Hallgren S, Sinjari T, Hakansson H, Darnerud PO. Effects of polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) on thyroid hormone and vitamin A levels in rats and mice. Archives of toxicology. 2001;75:200–208. doi: 10.1007/s002040000208. [DOI] [PubMed] [Google Scholar]
- Hendriks HS, Antunes Fernandes EC, Bergman A, van den Berg M, Westerink RH. PCB-47, PBDE-47, and 6-OH-PBDE-47 differentially modulate human GABAA and alpha4beta2 nicotinic acetylcholine receptors. Toxicol Sci. 2010;118:635–642. doi: 10.1093/toxsci/kfq284. [DOI] [PubMed] [Google Scholar]
- Herbstman JB, Sjodin A, Kurzon M, Lederman SA, Jones RS, Rauh V, Needham LL, Tang D, Niedzwiecki M, Wang RY, Perera F. Prenatal exposure to PBDEs and neurodevelopment. Environ Health Perspect. 2010;118:712–719. doi: 10.1289/ehp.0901340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig CM, Lango J, Pessah IN, Berman RF. Maternal transfer of BDE-47 to offspring and neurobehavioral development in C57BL/6J mice. Neurotoxicol Teratol. 2012;34:571–580. doi: 10.1016/j.ntt.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Sweet RA. Schizophrenia from a neural circuitry perspective: advancing toward rational pharmacological therapies. J Clin Invest. 2009;119:706–716. doi: 10.1172/JCI37335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llansola M, Erceg S, Monfort P, Montoliu C, Felipo V. Prenatal exposure to polybrominated diphenylether 99 enhances the function of the glutamate-nitric oxide-cGMP pathway in brain in vivo and in cultured neurons. Eur J Neurosci. 2007;25:373–379. doi: 10.1111/j.1460-9568.2006.05289.x. [DOI] [PubMed] [Google Scholar]
- Llansola M, Hernandez-Viadel M, Erceg S, Montoliu C, Felipo V. Increasing the function of the glutamate-nitric oxide-cyclic guanosine monophosphate pathway increases the ability to learn a Y-maze task. J Neurosci Res. 2009;87:2351–2355. doi: 10.1002/jnr.22064. [DOI] [PubMed] [Google Scholar]
- Mohler H. Molecular regulation of cognitive functions and developmental plasticity: impact of GABAA receptors. J Neurochem. 2007;102:1–12. doi: 10.1111/j.1471-4159.2007.04454.x. [DOI] [PubMed] [Google Scholar]
- Monaghan DT, Bridges RJ, Cotman CW. The excitatory amino acid receptors: their classes, pharmacology, and distinct properties in the function of the central nervous system. Annu Rev Pharmacol Toxicol. 1989;29:365–402. doi: 10.1146/annurev.pa.29.040189.002053. [DOI] [PubMed] [Google Scholar]
- Norstrom RJ, Simon M, Moisey J, Wakeford B, Weseloh DV. Geographical distribution (2000) and temporal trends (1981-2000) of brominated diphenyl ethers in Great Lakes hewing gull eggs. Environ Sci Technol. 2002;36:4783–4789. doi: 10.1021/es025831e. [DOI] [PubMed] [Google Scholar]
- Roze E, Meijer L, Bakker A, Van Braeckel KN, Sauer PJ, Bos AF. Prenatal exposure to organohalogens, including brominated flame retardants, influences motor, cognitive, and behavioral performance at school age. Environ Health Perspect. 2009;117:1953–1958. doi: 10.1289/ehp.0901015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MF, Buckley MJ, Behrens TE, Walton ME, Bannerman DM. Functional organization of the medial frontal cortex. Curr Opin Neurobiol. 2007;17:220–227. doi: 10.1016/j.conb.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Schoch P, Richards JG, Haring P, Takacs B, Stahli C, Staehelin T, Haefely W, Mohler H. Co-localization of GABA receptors and benzodiazepine receptors in the brain shown by monoclonal antibodies. Nature. 1985;314:168–171. doi: 10.1038/314168a0. [DOI] [PubMed] [Google Scholar]
- Suvorov A, Takser L. Delayed response in the rat frontal lobe transcriptome to perinatal exposure to the flame retardant BDE-47. J Appl Toxicol. 2011;31:477–483. doi: 10.1002/jat.1667. [DOI] [PubMed] [Google Scholar]
- Viberg H, Fredriksson A, Eriksson P. Neonatal exposure to polybrominated diphenyl ether (PBDE 153) disrupts spontaneous behaviour, impairs learning and memory, and decreases hippocampal cholinergic receptors in adult mice. Toxicol Appl Pharmacol. 2003;192:95–106. doi: 10.1016/s0041-008x(03)00217-5. [DOI] [PubMed] [Google Scholar]
- Viberg H, Johansson N, Fredriksson A, Eriksson J, Marsh G, Eriksson P. Neonatal exposure to higher brominated diphenyl ethers, hepta-, octa-, or nonabromodiphenyl ether, impairs spontaneous behavior and learning and memory functions of adult mice. Toxicol Sci. 2006;92:211–218. doi: 10.1093/toxsci/kfj196. [DOI] [PubMed] [Google Scholar]
- Viberg H, Mundy W, Eriksson P. Neonatal exposure to decabrominated diphenyl ether (PBDE 209) results in changes in BDNF, CaMKII and GAP-43, biochemical substrates of neuronal survival, growth, and synaptogenesis. Neurotoxicology. 2008;29:152–159. doi: 10.1016/j.neuro.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Yan T, Xiang L, Xuejun J, Chengzhi C, Youbin Q, Xuelan Y, Yang L, Changyan P, Hui C. Spatial learning and memory deficit of low level polybrominated diphenyl ethers-47 in male adult rat is modulated by intracellular glutamate receptors. J Toxicol Sci. 2012;37:223–233. doi: 10.2131/jts.37.223. [DOI] [PubMed] [Google Scholar]
- Zahr NM, Mayer D, Pfefferbaum A, Sullivan EV. Low striatal glutamate levels underlie cognitive decline in the elderly: evidence from in vivo molecular spectroscopy. Cereb Cortex. 2008;18:2241–2250. doi: 10.1093/cercor/bhm250. [DOI] [PMC free article] [PubMed] [Google Scholar]