Abstract
The axon initial segment (AIS) is highly enriched in the structural proteins ankyrin G and βIV-spectrin, the pore-forming (α) subunits of voltage-gated sodium (Nav) channels, and functional Nav channels, and is critical for the initiation of action potentials. We previously reported that FGF14, a member of the intracellular FGF (iFGF) sub-family, is expressed in cerebellar Purkinje neurons and that the targeted inactivation of Fgf14 in mice (Fgf14−/−) results in markedly reduced Purkinje neuron excitability. Here, we demonstrate that FGF14 immunoreactivity is high in the AIS of Purkinje neurons and is distributed in a decreasing, proximal to distal, gradient. This pattern is evident early in the postnatal development of Purkinje neurons and is also observed in many other types of central neurons. In ( Scn8amed) mice, which are deficient in expression of the Nav1.6 α subunit, FGF14 immunoreactivity is markedly increased and expanded in the Purkinje neuron AIS, in parallel with increased expression of the Nav1.1 (Scn1a) α subunit and expanded expression of βIV-spectrin. Although Nav1.1, FGF14, and βIV-spectrin are affected, ankyrin G immunoreactivity at the AIS of Scn8amed and wild type (WT) Purkinje neurons was not significantly different. In Fgf14−/− Purkinje neurons, βIV-spectrin and ankyrin G immunoreactivity at the AIS were also similar to WT Purkinje neurons, although both the Nav1.1 and Nav1.6 α subunits are modestly, but significantly (P<0.005), reduced within sub-domains of the AIS, changes that may contribute to the reduced excitability of Fgf14−/− Purkinje neurons.
Keywords: Purkinje neuron, axon initial segment, AIS, iFGF, FGF14, voltage-gated sodium channel, Nav1.1, Scn1a, Nav1.6, Scn8a, ankyrin G, βIV-spectrin
Introduction
The axon initial segment (AIS) is a specialized structural and functional region in neurons that is critical for the generation of action potentials. The AIS is delineated by the scaffolding protein, ankyrin G (AnkG) (Zhou et al., 1998), the cytoskeletal adaptor protein, βIV-spectrin (Yang et al., 2007), and the cell adhesion molecules, neurofascin-186 (NF-186) (Hedstrom et al., 2007) and neuronal cell adhesion molecule (NrCAM) (Davis et al., 1996). In addition, the AIS is highly enriched in voltage-gated Na+ (Nav) channels. In the absence of ankyrin G or βlV-spectrin, the localization of Nav channel pore-forming (a) subunit to the AIS is disrupted, action potential generation is impaired, and neuronal excitability is reduced (Komada and Soriano, 2002; Winkels et al., 2009; Zhou et al., 1998).
In the central nervous system, three different Nav α subunits, Nav1.1 (Scn1a), Nav1.2 (Scn2a1), and Nav1.6 (Scn8a), are enriched in the AIS (Hu et al., 2009; Kole et al., 2008; Stuart and Sakmann, 1994). Interestingly, these Nav α subunits are differentially expressed in different types of CNS neurons and display distinct distribution patterns within the AIS, differences that contribute to cell-type specific firing properties (Lorincz and Nusser, 2008). Nav1.1, for example, is restricted to a small proximal subregion of the AIS of GABAergic inhibitory neurons (Ogiwara et al., 2007) and retinal ganglion cells (Van Wart et al., 2007) and displays little overlap with Nav1.6, which localizes to the distal AIS. Purkinje neurons also express Nav1.1 or Nav1.6, and mice lacking either Nav1.1 or Nav1.6 show defects in firing, suggesting that both Nav α subunits are required for normal Purkinje neuron function (Kalume et al., 2007; Raman et al., 1997; Vega-Saenz de Miera et al., 1997). Deletion of Scn1a (Nav1.1) (Kalume et al., 2007; Yu et al., 2006) or Scn8a (Nav1.6) (Meisler et al., 2004) result in seizures, ataxia, impaired motor function, and early death, revealing that the important physiological roles of Nav channels encoded by these α subunits regulate neuronal activity in multiple regions of the central nervous system.
Intracellular FGFs (iFGFs 11–14), also called Fibroblast Growth Factor Homologous Factors (FHFs), are distinct from canonical FGFs in that the iFGFs are not secreted and do not interact with classical tyrosine kinase FGF receptors (Itoh and Ornitz, 2011). FGF14 and the other iFGFs are widely expressed throughout the nervous system (Smallwood et al., 1996; Wang et al., 2000; Xiao et al., 2007) and are concentrated at the AIS of cultured hippocampal neurons (Grubb and Burrone, 2010; Laezza et al., 2007) and in nodes of Ranvier (Wittmack et al., 2004). FGF14 interacts directly with the C-termini of Nav α subunits, including Nav1.1, Nav1.2, Nav1.6, in HEK293 cells (Laezza et al., 2007; Laezza et al., 2009; Lou et al., 2005), and the targeted deletion of Fgf14 (FGF14) results in an ataxia-like phenotype (Wang et al., 2002) and markedly attenuated excitability in cerebellar Purkinje (Shakkottai et al., 2009; Xiao et al., 2007) and granule (Goldfarb et al., 2007) neurons. Indeed, the phenotypic consequences of the loss of FGF14 are similar to those resulting from loss of function mutations in Nav1.1 and Nav1.6 (Kalume et al., 2007; Meisler et al., 2004; Yu et al., 2006). Interestingly, mutations in FGF14 have been linked to spinocerebellar ataxia (SCA27) in humans (Brusse et al., 2006; Dalski et al., 2005; Misceo et al., 2009; Van Swieten et al., 2003; Wang et al., 2002).
Here, we show that FGF14 is preferentially localized in a decreasing proximal to distal gradient along the AIS of adult Purkinje neurons, as well as in other type of central neurons. This pattern is evident early in the postnatal development of Purkinje neurons in vivo and is recapitulated in isolated Purkinje neurons maintained in vitro. Additional experiments demonstrate that the intensity of FGF14 and Nav1.1 immunostaining are increased along the AIS of Purkinje neurons in Scn8amed mice, which lack Nav1.6, whereas the intensity of βIV-spectrin and ankyrin G at the AIS of Scn8amed Purkinje neurons is similar to WT Purkinje neurons. In addition, although the AIS scaffolding proteins, βIV-spectrin and ankyrin G, are not significantly affected by the loss of FGF14, Nav1.1 and Nav1.6 immunostaining is decreased in Fgf14−/− Purkinje neurons, changes that may contribute to the reduced excitability of these cells (Shakkottai et al., 2009).
Materials and Methods
Mice
Fgf14−/− mice were generated as previously described and maintained on an inbred C57BL/6J background (Wang et al., 2002). All genotypes were confirmed by PCR analysis (Wang et al., 2002). Heterozygous male and female Scn8amed (Burgess et al., 1995) mice (C3Fe.Cg-Scn8amed/J, JAX stock number 003798) were purchased from the Jackson Lab. Scn8amed mice were bred with wild type (WT) C57BL/6J mice. Genotypes were determined by PCR (forward primer 5’-GGAGCAAGGTTCTAGGCAGCTTTAAGTGTG, reverse primer 5’-GGACTTAGAATGTACAAGGCAGGAG, WT band 280 bp, mutant band 460 bp) as described previously (Kohrman et al., 1996; Van Wart and Matthews, 2006). Animals were handled in accordance with the Guide for the Care and Use of Laboratory Animals (NIH). All protocols involving animals were approved by the Animal Studies Committee at Washington University Medical School.
Primary cerebellar Purkinje neuron cultures
Dissociated cerebellar cultures were prepared as described previously (Tabata et al., 2000) with some modifications. Briefly, the cerebellum was dissected and the meninges were removed (on ice) from individual postnatal day 0–1 (P0/1) Fgf14−/− mouse pups produced by crossing heterozygous Fgf14+/− mice. Dissected tissue from each animal was washed twice with ice-cold Ca2+ and Mg2+-free HBSS (Invitrogen) and incubated in papain (papain dissociation kit system for neural cells, Worthington Biochemical Corporation) for 23 min at 37°C in a shaking water bath. Digestion was terminated by addition of albumin-ovomucoid inhibitor solution, followed by two washes in DMEM/F-12 (1:1) medium (Invitrogen). Tissues were triturated gently using fire-polished Pasteur pipettes and the resulting cell suspensions were centrifuged at 300 g for 5 min at room temperature. Cell pellets were resuspended in media, centrifuged at 100 g for 5 min, resuspended in seeding medium (10% FBS in DMEM/F-12), and plated onto 0.01% poly-D-lysine-coated coverslips (12mm Ф, Fisher Scientific) in 24 well plates at a density of 5 × 106 cells/ml. Approximately 24 hr later, Purkinje neuron culture media (100 µM putrescine, 30 nM sodium selenite, 1.4 mM L-glutamine, 20 nM progesterone, 10 ng/ml insulin, 100 mg/ml transferrin, 0.5 ng/ml tri-iodotyronine, 10 µg/ml gentamicin in 1:1 DMEM/F-12) was added to each well. Half of the culture medium was replaced with fresh medium containing 4 µM cytosine arabinoside (AraC) (to inhibit glial proliferation) after 6 and 12 days in vitro (DIV).
Tissue preparation and immunostaining
Mice were anesthetized with sodium pentobarbital (60 mg/kg, i.p.) or ketamine-atropine-xylazine (KAX, 30 mg/ml ketamine, 0.2 mg/ml atropine, and 4 mg/ml xylazine in 1.0 ml/kg, i.p) and transcardially perfused with a vascular rinse of 0.9% NaCl followed by ice-cold 1% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). Brains were dissected, postfixed in the same solution overnight at 4°C, and cryoprotected in 30% sucrose, 0.1 M phosphate buffer until they sank. After embedding in O.C.T. compound, brains were cryosectioned (sagittal) at 14 µm or 30 µm and collected on positive charged slides. For immunohistochemistry, brain sections were washed in PBS 3X, blocked in a solution of 7.5% goat serum (Sigma) and 0.25% Triton X-100 (TX-100; Sigma) in PBS. Sections were then incubated at 4°C overnight with the primary antibodies diluted in 1% bovine serum albumin (BSA), 0.25% TX-100 in PBS. Sections were then washed 3X for 5 min at room temperature in PBS before application of secondary antibodies. Sections were then incubated in the appropriate secondary goat antibodies (labeled with Alexa 488, 594 or 647) against mouse, rabbit or guinea pig antibodies at 1:250 dilution in the same solution as used for the primary antibodies (Molecular Probes).
The following primary antibodies were used: mouse monoclonal anti-panNav (1:1000, Sigma); mouse monoclonal anti-Nav1.1 (1:1000, NeuroMab); rabbit polyclonal anti-Nav1.1 (1:250, Sigma); mouse monoclonal anti-Nav1.6 (1:1000, NeuroMab); rabbit polyclonal anti-Nav1.6 (1:500, Alomone) and mouse monoclonal anti-FGF14 (1:1000, NeuroMab); mouse monoclonal anti-ankyrin G (1:1000, gift from P. Mohler or NeuroMab); rabbit polyclonal anti-ankyrin G (1:500, gift from P. Mohler or Santa Cruz); chick polyclonal anti-βIV-spectrin (1:1000, gift from M. Komada); mouse monoclonal anti-calbindin D (Calb1) (1:20,000, Swant); rabbit polyclonal anti-calbinding1 (D-28K; 1:20,000; Swant); guinea pig polyclonal anti-calbindin 1 (D-28K; 1:2000; Synaptic Systems).
Western blotting
Mice were sacrificed by CO2 asphyxiation, decapitated, and cerebella were removed and placed in ice-cold brain homogenization buffer (20 mM Tris, pH 7.4, 150 mM NaCl, 1% Triton X-100) containing protease inhibitors (1 mM PMSF and Calbiochem, set #3). Cerebella were homogenized on ice in a Dounce homogenizer. Homogenates were rotated for 15 min at 4°C and then centrifuged at 3000 rpm for 10 min at 4°C to pellet nuclei. Protein concentrations were measured using a BCA assay (Pierce). Proteins were electrophoresed on 4–15% polyacrylamide gels (BioRad). Resolved proteins were transferred to PVDF membranes (Millipore) for 1.5 h at 4°C and blocked in tris-buffered saline (TBS) with 3% nonfat dry milk and 0.1% Tween-20. Membranes were incubated in primary antibodies diluted in TBS with 0.5% nonfat dry milk and 0.1% Tween-20.
The following primary antibodies were used: mouse monoclonal anti-panNav (1:1000, Sigma); mouse monoclonal anti-Nav1.1 (1:1000, NeuroMab); rabbit polyclonal anti-Nav1.6 (1:300, gift from J. Trimmer); and rabbit polyclonal anti-FGF14 (1:1000). Rabbit polyclonal serum against FGF14 was made by inoculating rabbits with an FGF14 peptide (Lou et al., 2005). Washed membranes were incubated with goat-anti-mouse-HRP or goat-anti-rabbit-HRP (1:5000, Santa-Cruz) and visualized with the Super Signal Fempto chemiluminescence substrate (Pierce).
Imaging
All imaging was performed on a Zeiss Apotome microscope fitted with an AxioCam digital camera (Carl Zeiss). AxioVision software was used for collection of fluorescence images. Comparison of WT and Fgf14−/− or WT and Scn8amed tissue was performed on slices prepared in parallel and images were acquired using identical exposure times. All images were collected by Z-scan with 0.5 µm steps. Fluorescence intensity (FI) was measured using NIH ImageJ software. The contrast and brightness of displayed images were adjusted using Canvas X software. Image stacks for double immunostained slides were converted into the same single average intensity Z-axis projection and exported as TIFF files. Fluorescence intensities for FGF14, Nav1.1 and Nav1.6 double stained with ankyrin G or βIV-spectrin were measured using the NIH ImageJ line-scan module starting within the soma and extending through the length of the AIS, as defined by the pattern of ankyrin G or βIV-spectrin immunostaining. Quantification of the lengths and fluorescence intensities of the AIS staining was performed using a previously described method (Grubb and Burrone, 2010). Briefly, the start and end positions of the AIS were defined as 33% of the maximum fluorescence intensity of ankyrin G or βIV-spectrin. The background (for ankyrin G, βIV-spectrin or FGF14) was subtracted based on a 1 µm segment within the soma along the vector of the AIS. For Nav1.1 and Nav1.6 immunostaining, the background was subtracted based on a 1 µm segment distal to the AIS. AIS fluorescence density was defined as the sum of AIS fluorescence intensity divided by AIS length.
Statistical analysis
Two-tailed Student’s t tests were performed where appropriate. All the results are presented as mean ± SEM as indicated.
Results
FGF14 is localized in a proximal to distal gradient along AIS in CNS neurons
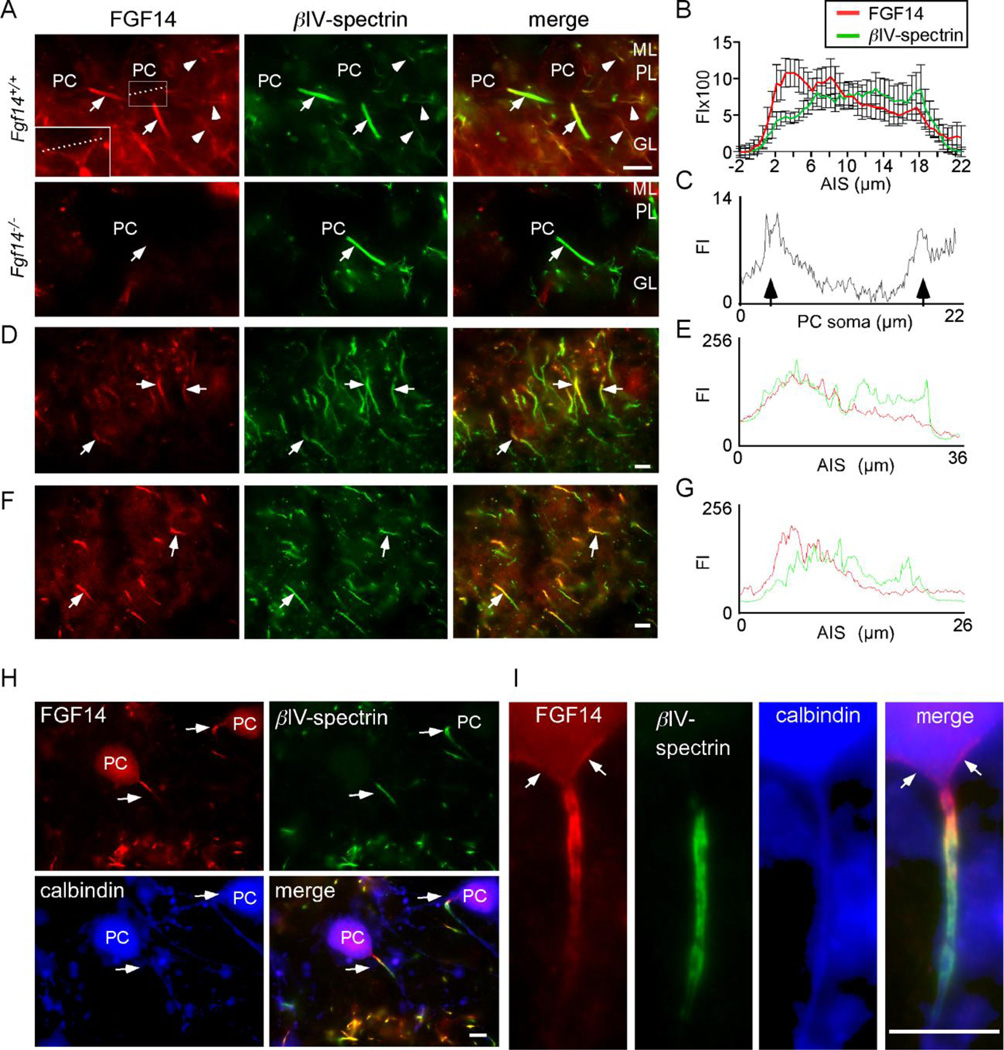
To examine the distribution and localization of FGF14 in situ in the adult CNS, we used an anti-FGF14 monoclonal antibody on cryostat sections prepared from adult WT (FgF+/+) and Fgf14−/− (Wang et al., 2002) mice (Figure 1). In situ immunoreactivity for FGF14 was detected in the AIS of neurons in several regions of the Fgf14+/+ CNS, including Purkinje neurons, hippocampal CA1 pyramidal neurons, and neurons in layers II to VI of the motor cortex (Figure 1A, D, F). In the cerebellum, FGF14 immunostaining was also observed at the AIS of granule and basket neurons (Figure 1A). No AIS FGF14 immunoreactivity, however, was detected in sections from Fgf14−/− mice (Figure 1A, lower panels), validating the specificity of the anti-FGF14 antibody. Quantitative analysis of the intensity of the FGF14 immunolabeling along the length of the AIS revealed higher expression proximally and lower expression distally (Figure 1A, B). In contrast, the intensity of the anti-βIV-spectrin staining appeared to be uniform along the AIS. Graded expression of FGF14 was also observed in the AIS of hippocampal CA1 pyramidal neurons (Figure 1D,E), as well as in neurons in layers II to VI of the motor cortex (Figure 1F, G). Although less intense, FGF14 immunostaining was also evident on Purkinje neuron somata in WT, but not in Fgf14−/−, mice (Figure 1A inset).
Figure 1. Proximal to distal gradient in FGF14 immunoreactivity in the AIS of multiple CNS neurons.
Cryostat sections of the cerebellum were cut from the brains of adult WT (Fgf14+/+) mice and mice lacking FGF14 (Fgf14−/−) and stained with antibodies against FGF14 and βIV-spectrin as described in Materials and Methods. A. FGF14 immunostaining (red) is prominent at the AIS of Fgf14+/+ Purkinje neurons (arrows) and is higher proximal to the soma and lower distally; immunostaining is also seen in cell bodies of Purkinje neurons (dashed line) and in the AIS of basket/granule neurons (arrowheads). In contrast, no anti-FGF staining is evident in Fgf14−/− cerebellar sections. An antibody against βIV-spectrin (green) was used to define the AIS (arrows). B. Relative anti-FGF14 (red) and anti-βIV-spectrin (green) fluorescence intensities (FI, mean ± SEM), determined from linescan analyses of individual Fgf14+/+ neurons (n = 11) along the AIS. C. Linescan of a single Purkinje neuron along the dashed line shown in (A) demonstrating FGF14 immunofluorescence in the Purkinje neuron soma membrane (arrows). D. Immunostaining of CA1 hippocampal pyramidal neurons reveals a proximal to distal gradient of FGF14 (red) along the AIS (arrows). E. Linescan analysis of anti-FGF14 (red) and anti-βIV-spectrin (green) labeling of the AIS in a representative CA1 hippocampal pyramidal neuron. F. Immunostaining of neurons in layer III of the motor cortex reveal a proximal to distal gradient of FGF14 (red) along the AIS (arrows). G. Linescan analysis of anti-FGF14 (red) and anti-βIV-spectrin (green) labeling of the AIS in a representative neuron in layer III of the motor cortex. H. Isolated Purkinje neurons maintained in culture for 14 days were stained for FGF14 (red) and βIV-spectrin (green), as well as for calbindin (blue; to identify Purkinje neurons). I. High magnification view of an isolated Purkinje neuron showing FGF14 (red) and βIV-spectrin (green) immunoreactivity and a proximal to distal gradient similar to that seen in Purkinje neurons in situ (A). FGF14 immunostaining can also be seen on the soma (arrows). ML, molecular layer, PL, Purkinje neuron layer; GL, granule neuron layer; PC, Purkinje neuron cell body; scale bars are 10 µm.
Anti-FGF14 staining was also examined in WT cerebellar Purkinje neurons isolated at postnatal day 0–1 and allowed to mature in vitro for approximately two weeks. In culture, Purkinje neurons were identified by their large somata and by the presence of the Ca2+ binding protein, calbindin1 (Figure 1H, I) (Jenkins and Bennett, 2001). Labeling of these cells with the anti-FGF14 antibody also revealed a decreasing proximal to distal gradient of FGF14 immunostaining along the AIS, whereas βIV-spectrin immunofluorescence intensity was uniform along the AIS, but was not observed at the axon hillock (Figure 1H, I). Interestingly, FGF14 immunoreactivity was evident in the axon hillock, proximal to detectable anti-βIV-spectrin labeling (Figure 1I, arrows). Taken together, these data reveal that, in vivo and in vitro, endogenous FGF14 is localized in a decreasing proximal to distal gradient along the AIS in Purkinje and other CNS neurons.
FGF14 is not required for assembly of the AIS during Purkinje neuron development
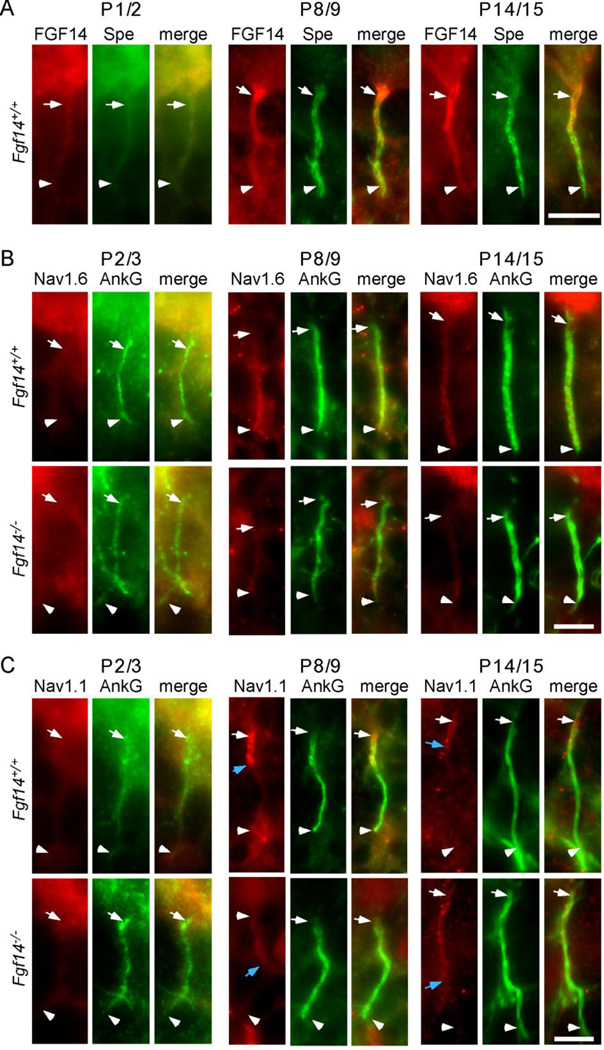
To explore the possible relationship(s) between FGF14 expression/localization and assembly of the AIS, we examined labeling with anti-FGF14, anti-ankyrin G, and anti-βIV-spectrin antibodies at different times during Fgf14+/+ and Fgf14−/− Purkinje neuron maturation in situ. Similar to the adult (Figure 1), immature Purkinje neurons were readily identified by immunostaining for calbindin 1 (data not shown) as early as postnatal day 1–2. As illustrated in Figure 2, weak staining with the anti-FGF14 (Figure 2A), anti-βIV-spectrin (Figure 2A), and anti-ankyrin G (Figure 2B,C) antibodies was detected in the AIS of Fgf14+/+ Purkinje neurons as early as postnatal day 1/2. At this stage, FGF14 immunofluorescence appears to be uniformly distributed along the length of the AIS (Figure 2A). In contrast, at postnatal days 8/9 and 14/15, FGF14 immunoreactivity was decidedly less uniform, similar to that of adult Purkinje neurons (Figure 1), where anti-FGF14 staining was higher in the proximal and lower in the distal AIS. Similar to postnatal day1/2, however, anti-ankyrin G (Figure 2B, C) and anti-βIV-spectrin immunostaining (data not shown) appeared to be uniformly distributed along the length of the AIS at postnatal days 8/9 and 14/15. In addition, the pattern of anti-ankyrin G immunostaining at the AIS of Fgf14+/+ and Fgf14−/− Purkinje neurons at postnatal days 2/3, 8/9 and 14/15 (Figure 2B,C) was similar.
Figure 2. Localization of AIS-associated proteins during Purkinje neuron maturation in vivo.
A. Representative images showing immunostaining for FGF14 (red) and βIV-spectrin (green) in Fgf14+/+ and Fgf14−/− Purkinje neuron AIS at postnatal days 1/2, 8/9, and 14/15. B. Representative images of Nav1.6 (red) and ankyrin G (green) immunostaining in Fgf14+/+ and Fgf14−/− Purkinje neuron AIS at postnatal days 2/3, 8/9 and 14/15. C. Representative images of immunostaining for Nav1.1 (red) and ankyrin G (green) in Fgf14+/+ and Fgf14−/− Purkinje neuron AIS at postnatal days 2/3, 8/9 and 14/15. The white arrows in each panel indicate the positions of the proximal AIS and the white arrowheads, the distal AIS. In C, the blue arrows mark the distal extent of detectable Nav1.1 immunostaining at the AIS. Scale bars are 10 µm.
In early postnatal (day 2/3) cerebellum, faint labeling with the anti-Nav1.6- and anti-Nav1.1-specific antibodies was also detectable in Purkinje neuron AIS (Figure 2B,C), although no clear staining patterns were resolved. The distinct patterns of anti-Nav1.1 and anti-Nav1.6 immunofluorescence that are evident in the AIS of many mature neuronal cell types (Duflocq et al., 2008; Lorincz and Nusser, 2008; Ogiwara et al., 2007; Van Wart et al., 2007), including mature Purkinje neurons (see also Figure 6), become apparent in early postnatal (days 8/9 and14/15) Fgf14+/+ Purkinje neurons: Nav1.6 immunoreactivity was evident in a proximal (low) to distal (high) gradient in the AIS (Figure 2B), whereas Nav1.1 immunoreactivity appeared restricted to the proximal third of the AIS (Figure 2C), in Fgf14+/+ Purkinje neurons. The adult patterns of Nav1.1 and Nav1.6 α subunit localization in the AIS, therefore, begin to emerge early during the developmental maturation of Purkinje neurons in vivo. Interestingly, the patterns of anti-Nav1.1 and anti-Nav1.6 immunoreactivity at the AIS of Fgf14+/+ and Fgf14−/− Purkinje neurons at postnatal days 2/3, 8/9 and 14/15 (Figure 2B,C) were qualitatively similar, although it does appear that Nav1.1 immunofluorescence was expanded along the AIS of Fgf14−/−, compared with Fgf14+/+, Purkinje neurons (see blue arrows in Figure 2C), suggesting a relationship between FGF14 and Nav1.1 expression and/or localization.
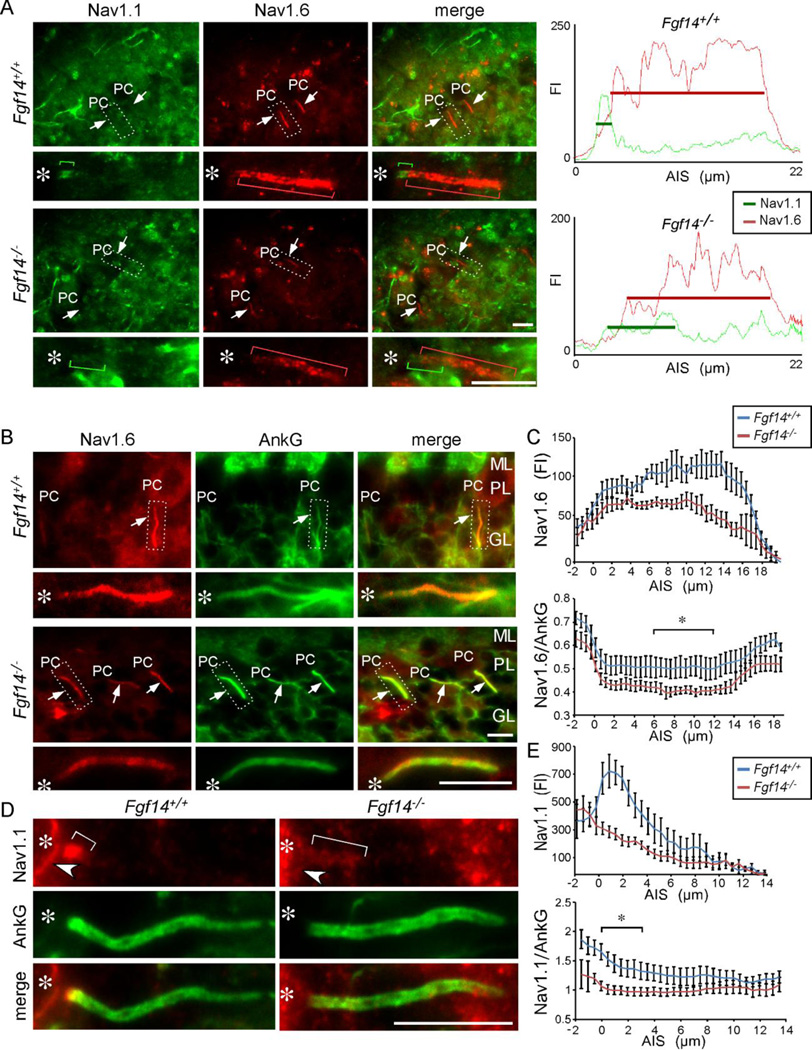
Figure 6. Altered distributions of Nav1.1 and Nav1.6 in Fgf14−/− Purkinje neuron AIS.
A. Immunostaining showing Nav1.1 (green) and Nav1.6 (red) in the AIS of adult Fgf14+/+ and Fgf14−/− Purkinje neurons. In Fgf14−/− Purkinje neurons, the intensity of anti-Nav1.1 immunostaining is decreased and expanded distally (green bracket), compared with the pattern evident in Fgf14+/+ Purkinje neurons. Similarly, the intensity of anti-Nav1.6 immunostaining is decreased (red bracket) in Fgf14−/−, compared with Fgf14+/+, Purkinje neurons. Right panels: linescans of the AIS of individual Fgf14+/+ and Fgf14−/− Purkinje neurons. B. Immunostaining showing Nav1.6 (red) and ankyrin G (green) distribution in adult Fgf14+/+ and Fgf14−/− Purkinje neuron AIS. C. Linescan analysis showing reduced Nav1.6 immunostaining in the AIS of Fgf14−/−, compared to Fgf14+/+, Purkinje neurons (n = 6𠄷, values presented are means ± SEM). Lower panel: ratio of Nav1.6 to ankyrin G immunofluorescence intensity throughout the AIS. Bracket indicates the region between 6.7 and 10.5 µm that are significantly (p < 0.05) different between Fgf14+/+ and Fgf14−/− Purkinje neurons. D. Immunostaining showing Nav1.1 (red) and ankyrin G (green) localization in adult Fgf14+/+ and Fgf14−/− Purkinje neuron AIS. E. Linescan analysis showing a proximal peak of Nav1.1 immunostaining in Fgf14+/+ compared to reduced immunostaining in Fgf14−/− Purkinje neuron AIS (n=6–10, values presented are means ± SEM). Lower panel: ratio of Nav1.1 to ankyrin G throughout the AIS. Bracket indicates the region between 0 and 3.9 µm that are significantly (p < 0.05) different between Fgf14+/+ and Fgf14−/− Purkinje neurons. Regions in dashed box are enlarged below. Asterisk marks the location of the Purkinje neuron soma. FI, Fluorescence intensity; ML, molecular layer, PC, Purkinje neuron soma; PL, Purkinje neuron layer; GL, granule neuron layer. Scale bars equal 10 µm.
FGF14 and Nav1.1 are increased in neurons lacking Nav1.6
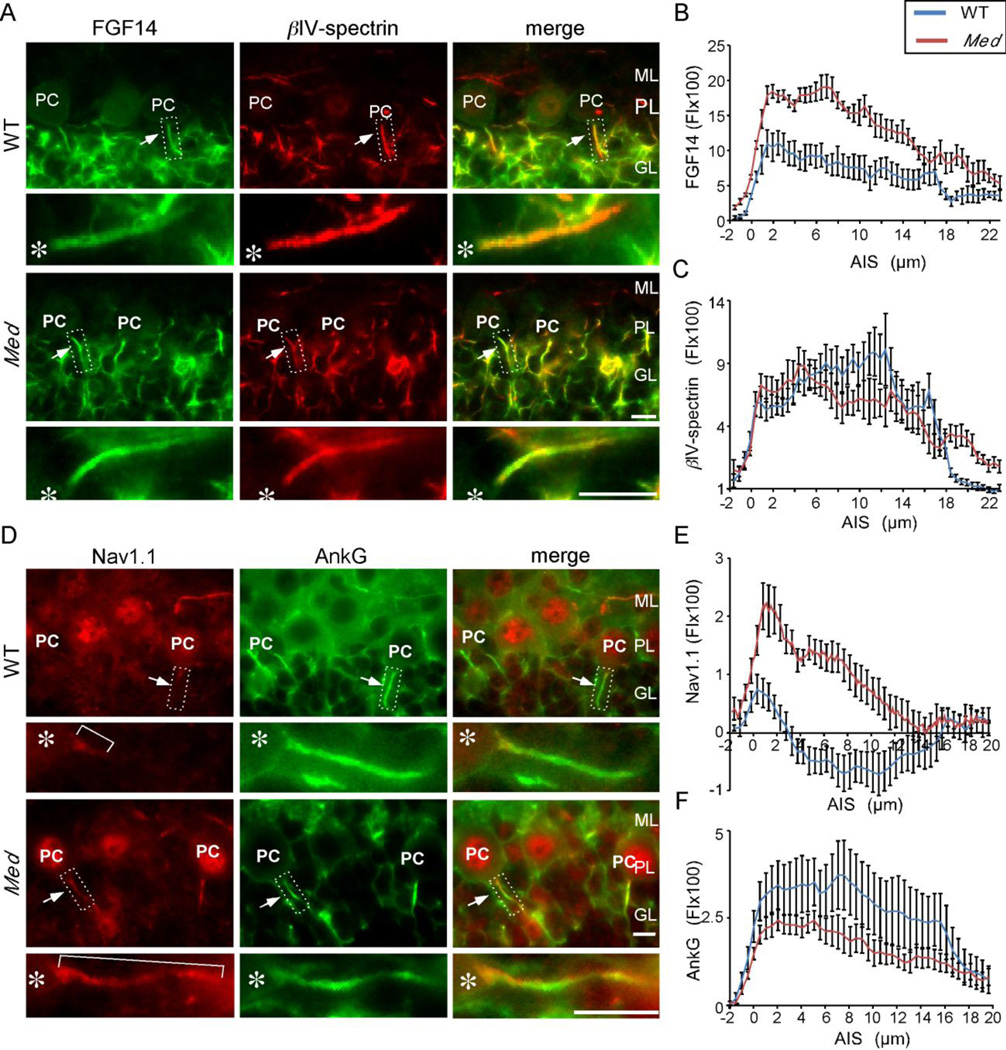
It has previously been reported that Purkinje neuron spontaneous firing is impaired in (Scn8amed) mice lacking Nav1.6 (Khaliq et al., 2003) and, in addition, that Nav1.1 immunoreactivity is expanded along the length of the AIS in Scn8amed Purkinje neurons (Van Wart and Matthews, 2006). To determine if the distribution of FGF14 is also affected by the loss of Nav1.6 and the alterations in Nav1.1, we compared the relative levels and the patterns of anti-FGF14 and anti-Nav1.1 labeling at the AIS of Purkinje neurons in Scn8amed and WT mice. These experiments revealed that the pattern of anti-FGF14 immunostaining was similar in the AIS of WT and Scn8amed Purkinje neurons, with higher levels in the proximal and lower levels in the distal AIS (Figure 3A). Quantitative linescan analysis, however, revealed that the intensity of anti-FGF14 immunostaining was significantly (p < 0.01) higher throughout the length of the AIS of Scn8amed, compared with WT, Purkinje neurons and extended more distally (Figure 3B, Table 1). Analysis of βIV-spectrin immunofluorescence revealed no significant differences in staining intensity in WT and Scn8amed Purkinje neurons, although anti-βIV-spectrin labeling was increased distally along the axons of Scn8amed, compared with WT, Purkinje neurons (Figure 3C, Table 1), suggesting expansion of the AIS (see Discussion).
Figure 3. Changes in the AIS inScn8amed Purkinje neurons.
A. Immunostaining showing FGF14 (green) and βIV-spectrin (red) localization in WT and Scn8amed (Med) Purkinje neuron AIS. B, C. Line scan analysis showing increased FGF14 immunostaining (B) and similar βIV-spectrin immunostaining (C) in Scn8amed, compared to WT, Purkinje neuron AIS. Note that the length of the AIS, as demarcated by βIV-spectrin immunostaining, is increased in Scn8amed Purkinje neurons. D. Immunostaining showing Nav1.1 (red) and ankyrin G (green) localization in WT and Scn8amed (Med) Purkinje neuron AIS. E, F. Linescan analysis showing increased Nav1.1 immunostaining (E) and similar ankyrin G immunostaining (F) in Scn8amed, compared to WT, Purkinje neuron AIS. Asterisks mark the locations of the Purkinje neuron soma. FI, Fluorescence intensity; ML, molecular layer, PC, Purkinje neuron soma; PL, Purkinje neuron layer; GL, granule neuron layer. Scale bars are 10 µm. For linescan analyses, n=7–11 values presented are means ± SEM.
Table 1.
AIS parameters of wild type (WT) and Scn8amed Purkinje neurons
| Length (µm) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Ankyrin G | ± SEM | βIV-spectrin | ± SEM | Nav1.1 | ± SEM | FGF14 | ± SEM | |
| WT | 18.6 | 0.9 | 17.8 | 0.7 | 3.3 | 0.4 | 17.3 | 1.0 |
| Scn8amed | 20.9 | 0.9 | 21.7 | 0.4 | 8.9 | 0.3 | 22.4 | 0.9 |
| Scn8amed/WT | 1.12 | 1.22 | 2.70 | 1.30 | ||||
| P value | ns | <0.001 | <0.01 | <0.01 | ||||
| number (WT, Scn8amed) | (7,7) | (8, 7) | (8,11) | (7, 8) | ||||
| Fluorescence intensity/µm | ||||||||
|---|---|---|---|---|---|---|---|---|
| Ankyrin Ga | ± SEM | βIV-spectrina | ± SEM | Nav1.1a | ± SEM | FGF14a | ± SEM | |
| WT | 3066 | 671 | 6863 | 959 | 641 | 123 | 7956 | 1231 |
| Scn8amed | 1372 | 115 | 5945 | 737 | 1690 | 225 | 14812 | 722 |
| Scn8amed/WT | 0.45 | 0.87 | 2.63 | 1.86 | ||||
| P value | <0.05 | ns | <0.01 | <0.001 | ||||
| number (WT, Scn8amed) | (7,7) | (8, 7) | (8,11) | (7, 8) | ||||
Background corrected fluorescence intensity divided by the length of the wild type AIS.
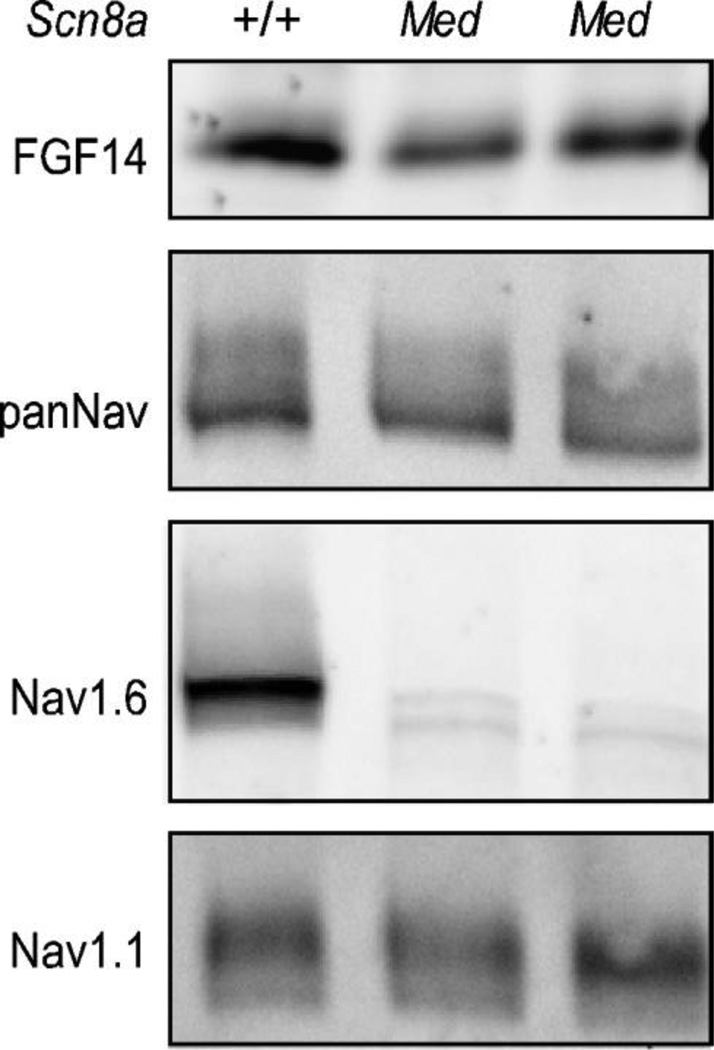
Consistent with previously published observations (Van Wart and Matthews, 2006), ant-Nav1.1 immunofluorescence intensity was increased in the proximal AIS and expanded distally along the length of Scn8amed Purkinje neuron axons, whereas ankyrin G immunofluorescence intensity was decreased (Figure 3D–F, Table 1). Comparison of Nav1.1 and FGF14 distributions in Scn8amed Purkinje neurons revealed similar increases in both (Table 1). Although anti-FGF14 and anti-Nav1.1 immunostaining were increased in Scn8amed animals in parallel with the loss of Nav1.6, Western blot analysis (Figure 4) revealed that the total protein expression levels of FGF14 and Nav1.1 are qualitatively not different in WT and Scn8amed cerebellar lysates.
Figure 4. FGF14 and Nav α subunit protein levels in cerebellum lacking Nav1.6.
Whole cerebellar lysates from WT (+/+) and Scn8amed mice were fractionated and Western blots were probed with anti-FGF14, anti-panNav, anti-Nav1.1, and anti-Nav1.6 antibodies. As expected, Nav1.6 protein expression is deficient in Med mice cerebellum; However, FGF14, total Nav, and Nav1.1 protein levels are not affected.
Remodeling of Nav channel α subunits at the AIS in Fgf14−/− Purkinje neurons
The observation that FGF14 immunofluorescence is expanded along the AIS of Scn8amed Purkinje neurons in parallel with the increase in anti-Nav1.1 immunoreactivity (Figure 3) suggests that FGF14 and Nav1.1 may be coordinately regulated. The observation that Nav1.1 labeling appears to be expanded along the AIS of developing Fgf14x−/x−, compared with Fgf14+/+, Purkinje neurons (see blue arrows in Figure 2C) also suggests a relationship between the localization of the FGF14 and Nav1.1 proteins. To explore this hypothesis further, we examined the distributions of the AIS scaffold proteins, ankyrin G and βIV-spectrin, and of the Nav1.1 and Nav1.6 α subunits at the AIS of adult Fgf14+/+ and Fgf14−/− Purkinje neurons.
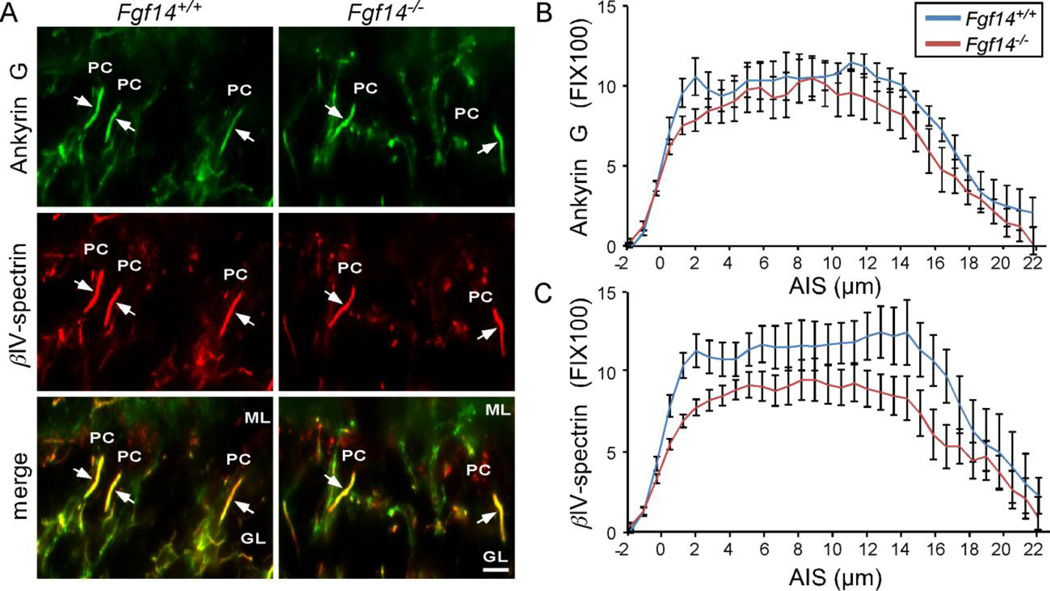
As illustrated in Figure 5, anti-ankyrin G and anti-βIV-spectrin immunoreactivity were colocalized at the AIS of adult Fgf14+/+ and Fgf14−/− Purkinje neurons (Figure 5A). Quantitative linescan analysis revealed no differences in the overall length of the AIS or in the intensity of anti-ankyrin G immunofluorescence in the AIS of Fgf14−/−, compared to Fgf14+/+, Purkinje neurons (Figure 5A,B, Table 2). Parallel analyses revealed that βIV-spectrin immunofluorescence intensity was modestly lower (by ~30%) in Fgf14−/−, compared with Fgf14+/+, Purkinje neuron AIS (Figure 5A,C Table 2). The distribution patterns and the intensities of anti-ankyrin G and anti-βIV-spectrin labeling were also similar in Fgf14−/− and Fgf14+/+ cortical (layers II to VI) neurons and in hippocampal CA1 pyramidal neurons (data not shown). FGF14, therefore, appears not to be required for the maintenance of the AIS in mature (Purkinje or other central) neurons.
Figure 5. Structural components of the AIS are localized normally in Purkinje neurons in adult Fgf14−/− mice.
A. Immunostaining showing βIV-spectrin (red) and ankyrin G (green) localization in Fgf14+/+ and Fgf14−/− Purkinje neuron AIS. B. Linescan analysis of ankyrin G in the AIS showing similar intensities. C. Linescan analysis revealed reduced βIV-spectrin labeling in the AIS of Fgf14−/− , compared with Fgf14+/+, Purkinje neurons. FI, Fluorescence intensity; ML, molecular layer, PC, Purkinje neuron soma; GL, granule neuron layer. Scale bars are 10 µm. For linescan analysis, n=7–11, error bars equal ± SEM.
Table 2.
AIS parameters inFgf14+/+;(WT) andFgf14−/− Purkinje neurons.
| Length (µm) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Ankyrin G | ± SEM | βIV-spectrin | ± SEM | Nav1.1 | ± SEM | Nav1.6 | ± SEM | |
| Fgf14+/+ | 19.4 | 0.9 | 19.1 | 1.0 | 2.5 | 0.5 | 16.8 | 0.3 |
| Fgf14−/− | 19.0 | 0.8 | 18.8 | 1.0 | 6.4 | 1.4 | 15.6 | 0.6 |
| Fgf14−/−/Fgf14+/+ | 0.98 | 0.98 | 2.56 | 0.93 | ||||
| P value | ns | ns | <0.05 | ns | ||||
| number (WT, Fgf14−/−) | (8, 7) | (8, 7) | (6,10) | (6,7) | ||||
| Fluorescence intensity/µm | ||||||||
|---|---|---|---|---|---|---|---|---|
| Ankyrin Gsupa | ± SEM | βIV-spectrina | ± SEM | Nav1.1b | ± SEM | Nav1.6b | ± SEM | |
| Fgf14+/+ | 6358 | 529 | 7202 | 715 | 6682 | 919 | 1019 | 123 |
| Fgf14−/− | 5237 | 630 | 4851 | 423 | 2565 | 375 | 655 | 39 |
| Fgf14−/−Fgf14+/+ | 0.82 | 0.67 | 0.38 | 0.64 | ||||
| P value | ns | <0.05 | <0.001 | <0.05 | ||||
| number (WT, Fgf14−/−) | (8, 7) | (8, 7) | (6,10) | (6, 7) | ||||
Background corrected fluorescence intensity divided by the length of the Fgf14−/− AIS.
Background corrected fluorescence intensity divided by the length of the Fgf14+/+ (WT) AIS.
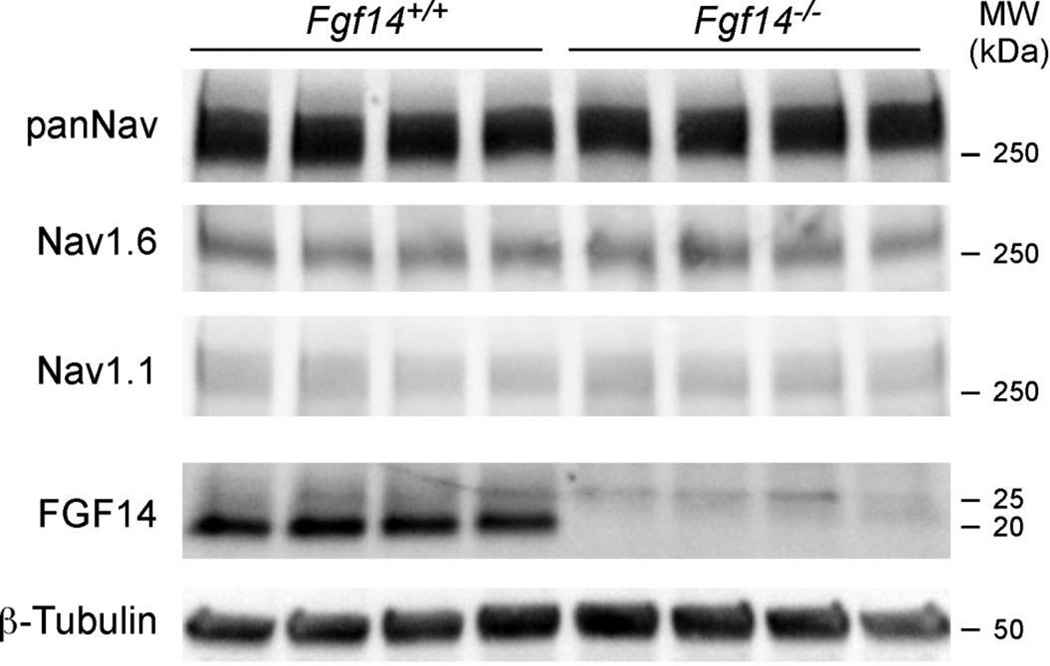
Experiments were also completed to examine the localization and relative distribution levels of Nav1.1 and Nav1.6 at the AIS of mature Fgf14+/+ and Fgf14−/− Purkinje neurons (Figure 6). Linescan analysis revealed increased anti-Nav1.6 immunofluorescence intensity over most of the length of the AIS in Fgf14+/+ Purkinje neurons (Figure 6A,C), consistent with a proximal to distal gradient in Nav1.6. In contrast, anti-Nav1.1 immunoreactivity at the AIS was highest proximal to the cell body (Figure 6A). Anti-Nav1.6 and anti-Nav1.1 immunofluorescence intensities were decreased at the AIS of Fgf14−/−, compared with Fgf14+/+, Purkinje neurons (Figure 6A). Western blot analyses, however, revealed no differences in the (total) expression levels of the Nav1.1 and Nav1.6 proteins in Fgf14+/+ and Fgf14−/− cerebellar lysates (Figure 7), suggesting that the apparent differences in anti-Nav1.6 and anti-Nav1.1 immunoreactivity at the AIS of Fgf14+/+ and Fgf14−/− Purkinje neurons (Figure 6A) reflect changes in the distribution/localization of Nav α subunits in parallel with the loss of FGF14.
Figure 7. Nav1.1 and 1.6 protein levels in whole cerebellar lysates are not affected by loss of FGF14.
Cerebellar lysates from four Fgf14+/+ and four Fgf14−/− adult mice were probed with the anti-FGF14, anti-panNav, anti-Nav1.1, anti-Nav1.6 and anti-β-tubulin antibodies.
To explore this hypothesis, additional immunofluorescence experiments were completed to quantify anti-Nav1.1 and anti-Nav1.6 immunoreactivities in the AIS of individual Fgf14+/+ and Fgf14−/− Purkinje neurons. In these experiments, cerebellar sections were double labeled with antibodies to ankyrin G and Nav1.1 or to ankyrin G and Nav1.6 (Figure 6B–D) and anti-Na1.1, anti-Nav1.6, and anti-ankyrin G staining intensities, were measured along the AIS. In Fgf14−/− Purkinje neurons, mean ± SEM anti-Nav1.6 immunofluorescence intensity was lower throughout the length of the AIS than in Fgf14+/+ Purkinje neurons (Figure 6B,C, Table 2). Linescan analysis of anti-Nav1.1 immunofluorescence in Fgf14−/− Purkinje neurons also revealed reduced mean ± SEM Nav1.1 labeling throughout the AIS, which was most prominent in the proximal 6 µm of the AIS (Figure 6D,E, Table 2). For further quantification, anti-Nav1.1 and anti-Nav1.6 labeling intensities along the AIS in individual Purkinje neurons were measured and normalized to the anti-ankyrin G immunofluorescence staining intensity at the same location in the same cell. As illustrated in Figure 6C, the mean ± SEM anti-Nav1.6/anti-ankyrin G relative fluorescence intensity along the middle 6–12 µm of the AIS was significantly (p < 0.05) lower in Fgf14−/−, compared with Fgf14+/+, Purkinje neurons. Plotting the mean ± SEM anti-Nav1.1/anti-ankyrin G relative fluorescence intensity ratios as a function of distance along the AIS revealed a significant (p < 0.05) decrease in the Nav1.1/ankyrin G fluorescence intensity ratio in Fgf14−/−, compared with Fgf14+/+, Purkinje neurons particularly along the first ~4 µm of the AIS (Figure 6E, Table 2). Taken together, these analyses suggest that FGF14, which is localized in a proximal (high) to distal (low) gradient along the AIS, is required to attain/maintain high proximal Nav1.1 expression and maximal Nav1.6 expression over the length of the AIS.
Discussion
Here, we demonstrate that FGF14 is prominently expressed in a unique, proximal to distal, gradient along the AIS of mature cerebellar Purkinje neurons and that this localization pattern develops early during the postnatal development of these cells. A similar pattern of AIS labeling was seen in mature hippocampal and cortical neurons. Additional data are presented here demonstrating that FGF14 and Nav1.1 immunostaining were increased and expanded distally along the AIS of Purkinje neurons in mice lacking Nav1.6 (ScnSamed), suggesting a regulatory interaction(s) between these proteins. Conversely, anti-Nav1.1 and anti-Nav1.6 immunolabeling were both decreased and anti-Nav1.1 staining was expanded along the AIS of Fgf14−/− Purkinje neurons, suggesting a role for FGF14 in regulating the densities and distributions of Nav1.1-encoded and Nav1.6-encoded Nav channels in the AIS of mature Purkinje neurons. Through regulation of Nav α subunit expression and patterning, FGF14 and other iFGFs may modulate the firing properties of Purkinje (and other types of central) neurons.
Recent studies have revealed diverse AIS expression patterns of Nav α subunits in different neuronal types (Lorincz and Nusser, 2008), suggesting that cell type-specific mechanisms are operative that organize and maintain AIS Nav α subunit composition, resulting in the generation of specific physiological properties (Clark et al., 2009). The major Nav α subunit, Nav1.6, for example, is expressed alone or with Nav1.1/Nav1.2 in different neurons and in different sub-domains of the AIS (Duflocq et al., 2008; Hu et al., 2009; Lorincz and Nusser, 2008). Complementary expression patterns of Nav1.1 and Nav1.6 have been identified in the AIS of cortical and cerebellar interneurons, as well as cerebellar Purkinje neurons, spinal cord neurons, and retinal ganglion cells (Boiko et al., 2003; Duflocq et al., 2008; Ogiwara et al., 2007; Van Wart and Matthews, 2006). In each of these neuronal cell types, Nav1.1 is preferentially localized in the very proximal AIS and Nav1.6 is distributed more uniformly along the length of the AIS, distal to Nav1.1.
The cellular and molecular mechanisms that regulate the complex patterns of Nav α subunit localization in the AIS are likely to be complex. For Nav α subunits distributed at high levels proximally and low levels distally, for example, the amount of available Nav α subunit protein could be limiting and, once directed towards the axonal compartment, available protein could be sequestered at the proximal AIS or, alternatively, degraded distally. In contrast, for Nav α subunits distributed at low levels proximally and at higher levels distally, there may be mechanisms that limit the affinities of these subunits for AIS scaffolding proteins in the proximal, compared to the distal, AIS, perhaps by competition with higher affinity proximally localized Nav α subunits for scaffold proteins. In this model, the molecules that function to effect the differential organization of the AIS are themselves also likely to be asymmetrically localized along the length of the axon. One such molecule is neurofascin (NF186), which is an extracellular matrix protein that is localized at high levels proximally and low levels distally (Ango et al., 2004). It is not known, however, whether the distribution of neurofascin affects the localization of Nav α subunits in the AIS.
In contrast with the differential AIS localization of the Nav1.1 and Nav1.6 α subunits, the ankyrin G and βIV-spectrin scaffolding proteins appear to be uniform along the AIS of Purkinje neurons. Although ankyrin G and βIV-spectrin are required for Nav α subunit localization in the AIS, it is unlikely that either directly regulates the observed localization patterns of the Nav1.1 and/or Nav1.6 α subunits. The intracellular location (Itoh and Ornitz, 2011; Smallwood et al., 1996; Wang et al., 2000; Xiao et al., 2007), ability to interact directly with the C termini of Nav α subunits (Wang et al., 2011), and the graded AIS distribution (present study), suggest possible roles for FGF14 in modulating the localization of Nav α subunits in Purkinje neuron AIS. Clearly, however, FGF14 cannot be the sole determinant of the pattern(s) of Nav α subunit localization because the AIS distribution of FGF14 is perturbed in response to changes Nav α subunit expression, as observed in Scn8amed Purkinje neurons, which lack Nav1.6 and have increased FGF14 (and Nav1.1).
In mice lacking FGF14, the overall level of Nav1.6 was decreased along the length of the Purkinje neuron AIS. In contrast, the intense proximal localization of Nav1.1 is lost Fgf14−/− Purkinje neurons, and weak anti-Nav1.1 immunostaining was evident distally suggesting expansion of the Nav1.1, although the overall amount of Nav1.1 protein within the AIS may not be changed. The distribution patterns of the scaffolding proteins, ankyrin G and βIV-spectrin, in contrast, appear not to be affected by the absence of FGF14, although there was a modest decrease in the intensity of anti-βIV-spectrin immunoreactivity. These observations suggest that FGF14 is not required to initiate or maintain the structural components of the AIS. We hypothesize that FGF14 may function to regulate the affinity of Nav α subunits to scaffolding proteins in the AIS or to other intermediary proteins that could couple Nav α subunits to the protein components of the AIS scaffold. These scaffolding proteins include the intracellular proteins ankyrin G and βIV-spectrin, as well as the extracellular cell adhesion molecules neurofascin-186 and neuron glia-related cell adhesion molecule, NrCAM (Jenkins and Bennett, 2001; Zhou et al., 1998).
There are four members of the iFGF subfamily and these proteins display distinct abilities to interact with the carboxyl terminal tails of different Nav α subunits. For example, FGF14 interacts differentially with the carboxyl terminal tails of Nav1.1, 1.2, 1.5 and 1.6 (Laezza et al., 2009; Lou et al., 2005). Similarly, FGF13 does, whereas FGF12 does not, interact with the carboxyl terminal tail of Nav1.1, whereas both FGF12 and FGF13 interact with the carboxyl terminal tails of other Nav α subunits (Wang et al., 2011). Differential interactions between iFGFs and Nav α subunits may contribute to the ability of the iFGFs to regulate the distributions of Nav α subunits in the AIS. Although the affinity of FGF14 for either the Nav1.1 or the Nav1.6 α subunit has not been determined directly, FGF14 and the carboxyl terminus of Nav1.1 are efficiently co-immunoprecipitated, suggesting a relatively high affinity interaction (Lou et al., 2005). Interestingly, there were increased levels of FGF14 and Nav1.1 in the AIS of Purkinje neurons in Scn8amed mice, consistent with a model (Figure 8) in which the distributions of FGF14 and Nav1.1 are coordinately regulated. In the absence of FGF14, all AIS Nav α subunit levels were reduced, suggesting that FGF14 may be limiting. Consistent with this suggestion, overexpression of FGF14 in hippocampal neurons resulted in increased levels of Nav α subunits in the AIS (Laezza et al., 2007; Lou et al., 2005). These observations suggest a model in which limiting amounts of FGF14, different FGF14-Nav α subunit binding affinities, competition among the Nav α subunits for FGF14 binding and, ultimately, the ability of FGF14 to regulate interactions between/among individual Nav α subunits and AIS scaffolding proteins, combine to determine the variable distributions of Nav α subunits along the AIS.
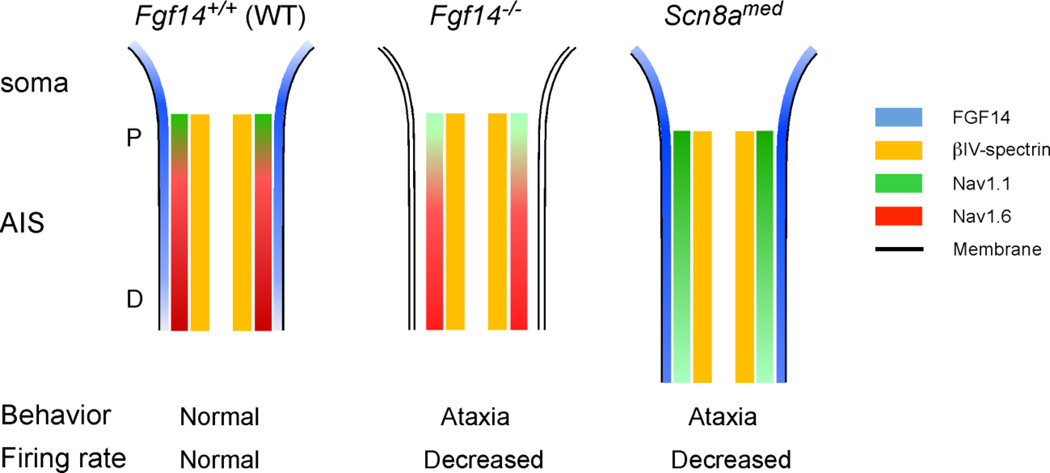
Figure 8. Protein distribution patterns in the Purkinje neuron AIS in mice lacking FGF14 or Nav1.6.
FGF14 (blue) is distributed in a decreasing proximal (P) to distal (D) gradient along the AIS of Fgf14+/+ cerebellar Purkinje neurons and weakly on the soma membrane. Nav1.1 (green) is distributed in a similar pattern, with high levels proximally and lower levels distally, whereas Nav1.6 (red) is distributed in an inverse pattern with lower levels proximally and higher levels distally. In the absence of FGF14 (Fgf14−/−), the intensity of both the anti-Nav1.1 and the anti-Nav1.6 immunolabeling is decreased. In addition, the distributions of these subunits in the AIS is also shifted such that the Nav1.1 domain is expanded and the Nav1.6 domain is constricted. In Scn8amed mice, which lack Nav1.6, immunostaining for FGF14 and Nav1.1 at the AIS are increased in parallel with an expanded domain for βIV-spectrin, suggesting lengthening of the AIS in Scn8amed Purkinje neurons.
The contributions of other members of the iFGF family to AIS organization are not known. Functional redundancy between FGF12 and FGF14 (FHF1 and FHF4) in cerebellar granule cells in vivo, however, has been demonstrated (Goldfarb et al., 2007). It has also been demonstrated that FGF14 can form homodimers (Laezza et al., 2007), as well as heterodimers with other iFGFs (MKB., unpublished data) in vitro, suggesting that complex combinatorial interactions among iFGFs may further regulate the functioning, as well, perhaps, as the patterning of Nav α subunit localization in the AIS.
Acknowledgements
We thank L. Li for technical assistance and Y. Carrasquillo for helpful discussions. This work was supported by National Institutes of Health grant R01-NS065761 (DMO and JMN). The mouse monoclonal anti-FGF14, anti-Nav1.1 and anti-Nav1.6 antibodies were developed by and obtained from the UC Davis/NIH NeuroMab Facility, supported by NIH grant U24NS050606 and maintained by the University of California, Davis, CA 95616.
Abbreviations
- AIS
axon initial segment
- FGF14
fibroblast growth factor 14
- iFGF
intracellular fibroblast growth factor
- Nav
voltage gated sodium channel
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ango F, di Cristo G, Higashiyama H, Bennett V, Wu P, Huang ZJ. Ankyrin-Based Subcellular Gradient of Neurofascin, an Immunoglobulin Family Protein, Directs GABAergic Innervation at Purkinje Axon Initial Segment. Cell. 2004;119:257–272. doi: 10.1016/j.cell.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Boiko T, Van Wart A, Caldwell JH, Levinson SR, Trimmer JS, Matthews G. Functional specialization of the axon initial segment by isoform-specific sodium channel targeting. J. Neurosci. 2003;23:2306–2313. doi: 10.1523/JNEUROSCI.23-06-02306.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusse E, de Koning I, Maat-Kievit A, Oostra BA, Heutink P, van Swieten JC. Spinocerebellar ataxia associated with a mutation in the fibroblast growth factor 14 gene (SCA27): A new phenotype. Mov. Disord. 2006;21:396–401. doi: 10.1002/mds.20708. [DOI] [PubMed] [Google Scholar]
- Burgess DL, Kohrman DC, Galt J, Plummer NW, Jones JM, Spear B, Meisler MH. Mutation of a new sodium channel gene, Scn8a, in the mouse mutant ‘motor endplate disease’. Nat. Genet. 1995;10:461–465. doi: 10.1038/ng0895-461. [DOI] [PubMed] [Google Scholar]
- Clark BD, Goldberg EM, Rudy B. Electrogenic tuning of the axon initial segment. Neuroscientist. 2009;15:651–668. doi: 10.1177/1073858409341973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalski A, Atici J, Kreuz FR, Hellenbroich Y, Schwinger E, Zuhlke C. Mutation analysis in the fibroblast growth factor 14 gene: frameshift mutation and polymorphisms in patients with inherited ataxias. Eur. J. Hum. Genet. 2005;13:118–120. doi: 10.1038/sj.ejhg.5201286. [DOI] [PubMed] [Google Scholar]
- Davis JQ, Lambert S, Bennett V. Molecular composition of the node of Ranvier: identification of ankyrin-binding cell adhesion molecules neurofascin (mucin+/third FNIII domain-) and NrCAM at nodal axon segments. J. Cell Biol. 1996;135:1355–1367. doi: 10.1083/jcb.135.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duflocq A, Le Bras B, Bullier E, Couraud F, Davenne M. Nav1.1 is predominantly expressed in nodes of Ranvier and axon initial segments. Mol. Cell. Neurosci. 2008;39:180–192. doi: 10.1016/j.mcn.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Goldfarb M, Schoorlemmer J, Williams A, Diwakar S, Wang Q, Huang X, Giza J, Tchetchik D, Kelley K, Vega A, Matthews G, Rossi P, Ornitz DM, D’Angelo E. Fibroblast Growth Factor Homologous Factors Control Neuronal Excitability through Modulation of Voltage-Gated Sodium Channels. Neuron. 2007;55:449–463. doi: 10.1016/j.neuron.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb MS, Burrone J. Activity-dependent relocation of the axon initial segment fine-tunes neuronal excitability. Nature. 2010;465:1070–1074. doi: 10.1038/nature09160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedstrom KL, Xu X, Ogawa Y, Frischknecht R, Seidenbecher CI, Shrager P, Rasband MN. Neurofascin assembles a specialized extracellular matrix at the axon initial segment. J. Cell Biol. 2007;178:875–886. doi: 10.1083/jcb.200705119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Tian C, Li T, Yang M, Hou H, Shu Y. Distinct contributions of Na(v)1.6 and Na(v)1.2 in action potential initiation and backpropagation. Nat. Neurosci. 2009;12:996–1002. doi: 10.1038/nn.2359. [DOI] [PubMed] [Google Scholar]
- Itoh N, Ornitz DM. Fibroblast growth factors: from molecular evolution to roles in development, metabolism and disease. J Biochem. 2011;149:121–130. doi: 10.1093/jb/mvq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins SM, Bennett V. Ankyrin-G coordinates assembly of the spectrin-based membrane skeleton, voltage-gated sodium channels, and L1 CAMs at Purkinje neuron initial segments. J. Cell Biol. 2001;155:739–746. doi: 10.1083/jcb.200109026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalume F, Yu FH, Westenbroek RE, Scheuer T, Catterall WA. Reduced sodium current in Purkinje neurons from Nav1.1 mutant mice: implications for ataxia in severe myoclonic epilepsy in infancy. J. Neurosci. 2007;27:11065–11074. doi: 10.1523/JNEUROSCI.2162-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaliq ZM, Gouwens NW, Raman IM. The contribution of resurgent sodium current to high-frequency firing in Purkinje neurons: an experimental and modeling study. J. Neurosci. 2003;23:4899–4912. doi: 10.1523/JNEUROSCI.23-12-04899.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohrman DC, Harris JB, Meisler MH. Mutation detection in the med and medJ alleles of the sodium channel Scn8a. Unusual splicing due to a minor class AT-AC intron. J. Biol. Chem. 1996;271:17576–17581. doi: 10.1074/jbc.271.29.17576. [DOI] [PubMed] [Google Scholar]
- Kole MH, Ilschner SU, Kampa BM, Williams SR, Ruben PC, Stuart GJ. Action potential generation requires a high sodium channel density in the axon initial segment. Nat. Neurosci. 2008;11:178–186. doi: 10.1038/nn2040. [DOI] [PubMed] [Google Scholar]
- Komada M, Soriano P. [Beta]IV-spectrin regulates sodium channel clustering through ankyrin-G at axon initial segments and nodes of Ranvier. J. Cell Biol. 2002;156:337–348. doi: 10.1083/jcb.200110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laezza F, Gerber BR, Lou J, Kozel MA, Hartman H, Craig AM, Ornitz DM, Nerbonne JM. The Fgf14(F145S) mutation disrupts the interaction of FGF14 with voltage-gated Na+ channels and impairs neuronal excitability. J. Neurosci. 2007;27:12033–12044. doi: 10.1523/JNEUROSCI.2282-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laezza F, Lampert A, Kozel MA, Gerber BR, Rush AM, Nerbonne JM, Waxman SG, Dib-Hajj SD, Ornitz DM. FGF14 N-Terminal Splice Variants Differentially Modulate Nav1.2 and Nav1.6-Encoded Sodium Channels. Mol. Cell. Neurosci. 2009;42:90–101. doi: 10.1016/j.mcn.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorincz A, Nusser Z. Cell-type-dependent molecular composition of the axon initial segment. J. Neurosci. 2008;28:14329–14340. doi: 10.1523/JNEUROSCI.4833-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou JY, Laezza F, Gerber BR, Xiao M, Yamada KA, Hartmann H, Craig AM, Nerbonne JM, Ornitz DM. Fibroblast Growth Factor 14 is an Intracellular Modulator of Voltage-Gated Sodium Channels. J. Physiol. (Lond) 2005;569:179–193. doi: 10.1113/jphysiol.2005.097220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisler MH, Plummer NW, Burgess DL, Buchner DA, Sprunger LK. Allelic mutations of the sodium channel SCN8A reveal multiple cellular and physiological functions. Genetica. 2004;122:37–45. doi: 10.1007/s10709-004-1441-9. [DOI] [PubMed] [Google Scholar]
- Misceo D, Fannemel M, Baroy T, Roberto R, Tvedt B, Jaeger T, Bryn V, Stromme P, Frengen E. SCA27 caused by a chromosome translocation: further delineation of the phenotype. Neurogenetics. 2009 doi: 10.1007/s10048-009-0197-x. [DOI] [PubMed] [Google Scholar]
- Ogiwara I, Miyamoto H, Morita N, Atapour N, Mazaki E, Inoue I, Takeuchi T, Itohara S, Yanagawa Y, Obata K, Furuichi T, Hensch TK, Yamakawa K. Na(v)1.1 localizes to axons of parvalbumin-positive inhibitory interneurons: a circuit basis for epileptic seizures in mice carrying an Scn1a gene mutation. J. Neurosci. 2007;27:5903–5914. doi: 10.1523/JNEUROSCI.5270-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman IM, Sprunger LK, Meisler MH, Bean BP. Altered subthreshold sodium currents and disrupted firing patterns in Purkinje neurons of Scn8a mutant mice. Neuron. 1997;19:881–891. doi: 10.1016/s0896-6273(00)80969-1. [DOI] [PubMed] [Google Scholar]
- Shakkottai VG, Xiao M, Xu L, Wong M, Nerbonne JM, Ornitz DM, Yamada KA. FGF14 regulates the intrinsic excitability of cerebellar Purkinje neurons. Neurobiol. Dis. 2009;33:81–88. doi: 10.1016/j.nbd.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood PM, Munoz-Sanjuan I, Tong P, Macke JP, Hendry SH, Gilbert DJ, Copeland NG, Jenkins NA, Nathans J. Fibroblast growth factor (FGF) homologous factors: new members of the FGF family implicated in nervous system development. Proc. Natl. Acad. Sci. USA. 1996;93:9850–9857. doi: 10.1073/pnas.93.18.9850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart GJ, Sakmann B. Active propagation of somatic action potentials into neocortical pyramidal cell dendrites. Nature. 1994;367:69–72. doi: 10.1038/367069a0. [DOI] [PubMed] [Google Scholar]
- Tabata T, Sawada S, Araki K, Bono Y, Furuya S, Kano M. A reliable method for culture of dissociated mouse cerebellar cells enriched for Purkinje neurons. J. Neurosci. Methods. 2000;104:45–53. doi: 10.1016/s0165-0270(00)00323-x. [DOI] [PubMed] [Google Scholar]
- Van Swieten JC, Brusse E, De Graaf BM, Krieger E, Van De Graaf R, De Koning I, Maat-Kievit A, Leegwater P, Dooijes D, Oostra BA, Heutink P. A mutation in the fibroblast growth factor 14 gene is associated with autosomal dominant cerebellar ataxia. Am. J. Hum. Genet. 2003;72:191–199. doi: 10.1086/345488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wart A, Matthews G. Impaired firing and cell-specific compensation in neurons lacking nav1.6 sodium channels. J. Neurosci. 2006;26:7172–7180. doi: 10.1523/JNEUROSCI.1101-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wart A, Trimmer JS, Matthews G. Polarized distribution of ion channels within microdomains of the axon initial segment. J. Comp. Neurol. 2007;500:339–352. doi: 10.1002/cne.21173. [DOI] [PubMed] [Google Scholar]
- Vega-Saenz de Miera EC, Rudy B, Sugimori M, Llinas R. Molecular characterization of the sodium channel subunits expressed in mammalian cerebellar Purkinje cells. Proc Natl Acad Sci U S A. 1997;94:7059–7064. doi: 10.1073/pnas.94.13.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Hoch EG, Pitt GS. Identification of novel interaction sites that determine specificity between fibroblast growth factor homologous factors and voltage gated sodium channels. J. Biol. Chem. 2011;286:24253–24263. doi: 10.1074/jbc.M111.245803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Bardgett ME, Wong M, Wozniak DF, Lou J, McNeil BD, Chen C, Nardi A, Reid DC, Yamada K, Ornitz DM. Ataxia and paroxysmal dyskinesia in mice lacking axonally transported FGF14. Neuron. 2002;35:25–38. doi: 10.1016/s0896-6273(02)00744-4. [DOI] [PubMed] [Google Scholar]
- Wang Q, McEwen DG, Ornitz DM. Subcellular and developmental expression of alternatively spliced forms of fibroblast growth factor 14. Mech. Dev. 2000;90:283–287. doi: 10.1016/s0925-4773(99)00241-5. [DOI] [PubMed] [Google Scholar]
- Winkels R, Jedlicka P, Weise FK, Schultz C, Deller T, Schwarzacher SW. Reduced excitability in the dentate gyrus network of betaIV-spectrin mutant mice in vivo. Hippocampus. 2009;19:677–686. doi: 10.1002/hipo.20549. [DOI] [PubMed] [Google Scholar]
- Wittmack EK, Rush AM, Craner MJ, Goldfarb M, Waxman SG, Dib-Hajj SD. Fibroblast growth factor homologous factor 2B: association with Nav1.6 and selective colocalization at nodes of ranvier of dorsal root axons. J. Neurosci. 2004;24:6765–6775. doi: 10.1523/JNEUROSCI.1628-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao M, Xu L, Laezza F, Yamada K, Feng S, Ornitz DM. Impaired hippocampal synaptic transmission and plasticity in mice lacking fibroblast growth factor 14. Mol. Cell. Neurosci. 2007;34:366–377. doi: 10.1016/j.mcn.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Yang Y, Ogawa Y, Hedstrom KL, Rasband MN. betaIV spectrin is recruited to axon initial segments and nodes of Ranvier by ankyrinG. J. Cell Biol. 2007;176:509–519. doi: 10.1083/jcb.200610128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FH, Mantegazza M, Westenbroek RE, Robbins CA, Kalume F, Burton KA, Spain WJ, McKnight GS, Scheuer T, Catterall WA. Reduced sodium current in GABAergic interneurons in a mouse model of severe myoclonic epilepsy in infancy. Nat. Neurosci. 2006;9:1142–1149. doi: 10.1038/nn1754. [DOI] [PubMed] [Google Scholar]
- Zhou D, Lambert S, Malen PL, Carpenter S, Boland LM, Bennett V. AnkyrinG is required for clustering of voltage-gated Na channels at axon initial segments and for normal action potential firing. J. Cell Biol. 1998;143:1295–1304. doi: 10.1083/jcb.143.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]