Summary
Cell polarity is essential for many cellular functions including division and cell-fate determination. Although RhoGTPase signaling and vesicle trafficking are both required for the establishment of cell polarity, the mechanisms by which they are coordinated are unclear. Here, we demonstrate that the yeast RhoGAP (GTPase activating protein), Bem3, is targeted to sites of polarized growth by the endocytic and recycling pathways. Specifically, deletion of SLA2 or RCY1 led to mislocalization of Bem3 to depolarized puncta and accumulation in intracellular compartments, respectively. Bem3 partitioned between the plasma membrane and an intracellular membrane-bound compartment. These Bem3-positive structures were polarized towards sites of bud emergence and were mostly observed during the pre-mitotic phase of apical growth. Cell biological and biochemical approaches demonstrated that this intracellular Bem3 compartment contained markers for both the endocytic and secretory pathways, which were reminiscent of the Spitzenkörper present in the hyphal tips of growing fungi. Importantly, Bem3 was not a passive cargo, but recruited the secretory Rab protein, Sec4, to the Bem3-containing compartments. Moreover, Bem3 deletion resulted in less efficient localization of Sec4 to bud tips during early stages of bud emergence. Surprisingly, these effects of Bem3 on Sec4 were independent of its GAP activity, but depended on its ability to efficiently bind endomembranes. This work unveils unsuspected and important details of the relationship between vesicle traffic and elements of the cell polarity machinery: (1) Bem3, a cell polarity and peripherally associated membrane protein, relies on vesicle trafficking to maintain its proper localization; and (2) in turn, Bem3 influences secretory vesicle trafficking.
Key words: Vesicle trafficking, Secretory pathway, Recycling pathway, Cell polarity, Bem3
Introduction
The underlying principle of cell polarity is the asymmetric distribution of proteins and lipids in specialized cellular domains for the execution of a vast spectrum of physiological processes including cell division and migration (Chant, 1999; Orlando and Guo, 2009; Pruyne and Bretscher, 2000). Cell polarity is regulated by the conserved RhoGTPase, Cdc42 (cell division cycle 42) (Etienne-Manneville, 2004; Johnson, 1999), a signaling protein first identified in the budding yeast, Saccharomyces cerevisiae, by analysis of mutants that led to a cell division arrest and loss of actin cytoskeleton polarization (Chen et al., 2000; Johnson and Pringle, 1990). Similar to other small GTPases of the Ras superfamily, the activation and inactivation of Cdc42 is regulated by the concerted action of guanine nucleotide exchange factors (GEFs) (Hart et al., 1991; Jaffe and Hall, 2005) and GTPase activating proteins (GAPs) (Scheffzek et al., 1998; Smith et al., 2002), respectively. Specifically, GEFs are primarily responsible for ‘switching on’ the GTPase by promoting GTP binding, whereas GAPs accelerate the rate of GTP hydrolysis, thereby leading to a GDP-bound state, i.e. to GTPase inactivation (Smith et al., 2002). Importantly, GAPs and GEFs are known to transduce extracellular signals into intracellular changes in Cdc42 activation (Etienne-Manneville, 2004).
Vesicle trafficking has also been proposed to participate in Cdc42 regulation (Marco et al., 2007; Orlando et al., 2011; Slaughter et al., 2009). Specifically, endocytosis contributes to the polarized distribution of Cdc42 by internalizing molecules that diffuse away from the polar cap (Marco et al., 2007), whereas the recycling and secretory pathway redirects the internalized Cdc42 back to sites of polarized growth, ensuring tight and restricted localization to regions undergoing rapid growth (Orlando et al., 2011). Although GAPs and GEFs are crucial for Cdc42 signaling control, if and how these proteins are subjected to regulation by vesicle trafficking is unclear.
Previous studies from our lab demonstrated that the epsin endocytic adaptors influence the activation levels of Cdc42 by directly interacting with all three yeast GAPs for Cdc42: Rga1, Rga2 and Bem3 (Aguilar et al., 2006). We also showed that cell division defects caused by overexpression of a hyperactive Bem3 mutant (Bem3Δ1–114) were suppressed by co-overexpression of the endocytic protein, Ent2 (Mukherjee et al., 2009). Further supporting the role of vesicle trafficking in Bem3 localization, Bem3 has been reported to localize to an unidentified intracellular compartment (Knaus et al., 2007) and actin cables have been shown to play an essential role in its transport to polar sites (Knaus et al., 2007).
Here we show that vesicle trafficking plays a major role in proper localization of Bem3. Specifically, we found that the polarized localization of Bem3 was disrupted in endocytic mutants. Moreover, Bem3 partially localized to endomembranes during early stages of bud emergence and Bem3 traffic from this compartment was dependent on Rcy1, an effector of the yeast Rab11 homolog Ypt31/32. Importantly, Bem3 was observed to co-exist in this compartment with both endocytic and secretory markers. Finally, we show that rather than being a passive cargo of the secretory pathway, Bem3 influenced the targeting of the Rab8 homolog, Sec4, to sites of polarized growth. Importantly, this Bem3 function was independent of its Cdc42 GAP activity and was conserved in the Bem3 homolog in the pathogenic fungus, Candida albicans.
This work unveils unsuspected and important details of the relationship between elements of the cell polarity machinery and vesicle trafficking pathways: (1) Bem3, a peripherally associated membrane protein, relies on vesicle traffic for its proper localization; (2) Bem3, in turn, is required for efficient targeting of secretory vesicles to sites of polarized growth. Together, our results suggest that elements of the cell polarity machinery (Bem3) and vesicle trafficking pathways (Sec4) reciprocally regulate each other, especially during the initial polarization and apical bud growth phase of the yeast cell cycle.
Results
Bem3 binds phosphoinositides through its PH domain and this interaction contributes to the membrane association and polarized distribution of Bem3
We have previously reported that cell division defects resulting from overexpression of the constitutively active Bem3 mutant (Bem3Δ1–114) in yeast (Kadota et al., 2004) are rescued by co-overexpression of the endocytic protein Ent2 (Mukherjee et al., 2009). Therefore, we hypothesized that vesicle trafficking, including endocytosis, plays an important role in the regulation of Bem3 activity. However, in order to be available as cargo for vesicle trafficking, Bem3 should interact with cellular membranes. In agreement with this, localization of Bem3 at the plasma membrane has been consistently observed by us (Fig. 1C) and others (Caviston et al., 2003; Knaus et al., 2007). Although transmembrane domains are absent in Bem3, its Phox (PX) and pleckstrin homology (PH) domains are strong candidates for mediating direct binding to membrane lipids (Itoh and Takenawa, 2002; Lemmon and Ferguson, 2000; Simonsen and Stenmark, 2001). Therefore, we tested whether the Bem3491–774 fragment (containing the PX-PH domain) could directly bind to immobilized lipids using a protein–lipid overlay assay. Our data showed that the Bem3 PX-PH domain recognized immobilized phosphatidylinositol (4,5)-bisphosphate [PtdIns(4,5)P2] and to a lesser extent, phosphatidylinositol monophosphates (Fig. 1A). Bem3491–774 also bound immobilized PtdIns(3,4)P2 strongly; however, the existence of this lipid in S. cerevisiae is dubious.
Fig. 1.
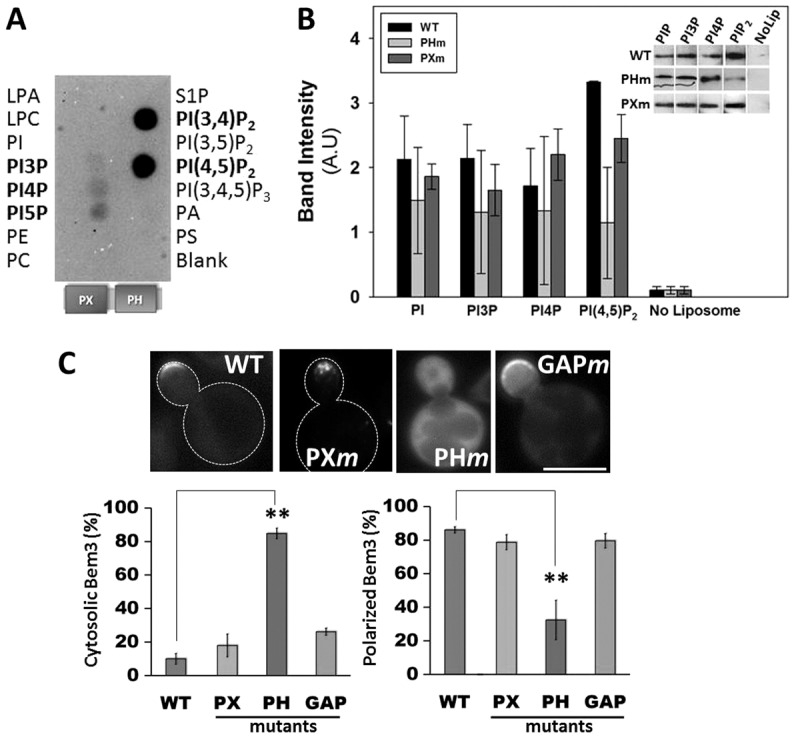
Bem3 occupies cortical membrane sites via interactions with phosphoinositols. Recombinant, purified His6-tagged PX-PH domain fragment of Bem3 was used for binding to PIP microstrips (A) and in liposome floatation assays (B). Detection by western blotting of liposome-bound Bem3 fragments corresponding to a representative experiment is shown in B. (C) Upper panel: cells expressing GFP–Bem3 (WT or mutant) were grown overnight in selective medium at 30°C and imaged at 100× using a FITC filter. Lower panel: localization patterns of GFP-tagged wild-type (WT) and mutant Bem3 were quantified by analyzing at least 200 cells. Data are the total percentage of cells with either a cytosolic (left) or polarized (right) distribution of each construct. Statistical significance was calculated using Student's t-test (**P<0.001). Scale bar: 5 µm. LPA, lysophosphatidic acid; LPC, lysophosphatidylcholine; PA, phosphatidic acid; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PI, phosphatidylinositol; PS, phosphatidylserine; S1P, sphingosine 1-phosphate.
Liposome binding assays confirmed the ability of Bem3 to bind phosphoinositides, and also, showed that introduction of specific mutations in the PH domain (R644S, R645S, K647D) impaired binding to PtdIns(4,5)P2 (Fig. 1B, grey bars). Furthermore, in the Bem3-PH mutant (PHm) there was a substantial increase in cytosolic localization and a decrease in its ability to localize to polar sites when compared with wild-type Bem3 (Fig. 1C). In contrast, mutations in the PX and GAP domains had no significant effect on the overall localization of Bem3 (Fig. 1C). In summary, our results indicate that Bem3 directly binds to PtdIns(4,5)P2 through its PH domain and that this interaction is important for its polarized localization to cortical membrane regions.
Bem3 is also present in an intracellular compartment in un-budded and small-budded cells
The association of Bem3 with PtdIns(4,5)P2, a lipid enriched at the plasma membrane, along with evidence that endocytic proteins can downregulate the signaling activity of constitutively active Bem3 (Mukherjee et al., 2009) led us to investigate a putative role of endocytosis in Bem3 localization. We first analyzed the localization of GFP–Bem3 (Caviston et al., 2003) in the endocytosis-deficient yeast strain, sla2Δ (Baggett et al., 2003; Engqvist-Goldstein et al., 1999; Wesp et al., 1997) (see supplementary material Table S2 for genotype and phenotype description). Whereas wild-type cells displayed a tight localization of Bem3 at sites of polarized growth, there was a significant increase in the number of cells with depolarized Bem3 puncta in the sla2Δ background (Fig. 2A; P<0.001), suggesting a role for the endocytic pathway in efficiently targeting Bem3 to its normal polar sites. In addition, we observed that depolarized Bem3 puncta partially colocalized with the endocytic marker Ede1 and associated with abnormal cortical actin structures typical of the sla2Δ strain (supplementary material Fig. S1A). These observations suggest that under endocytosis-deficient conditions Bem3 accumulates in cortical puncta. Indeed, Bem3 depolarized puncta could be also observed using total internal reflection fluorescence microscopy, also suggesting a cortical localization (data not shown). Similar observations were made using another endocytic mutant strain, ent1Δ/ent2Δ/yap1801Δ/yap1802Δ. These cells, which lack most plasma-membrane-located endocytic adaptors, also showed a more depolarized Bem3 distribution (supplementary material Fig. S1B).
Fig. 2.
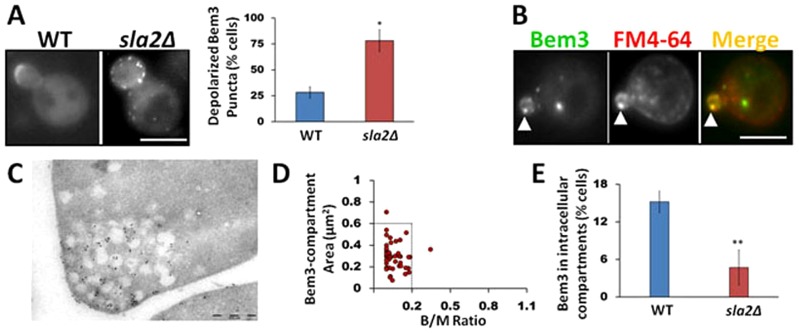
Bem3 occupies an intracellular compartment within the endocytic pathway in unbudded and small budded cells. (A) WT (SEY6210) and sla2Δ cells expressing GFP-Bem3 were imaged at 100× magnification using a FITC filter. Bem3 localization defects were quantified by analyzing at least 200 cells (*P<0.01, t-test). (B) WT (W303) cells expressing GFP-Bem3 were labeled with FM4-64 and observed using FITC and Rhodamine filters at 100×. Arrowheads point to Bem3 and FM4-64 colocalization. (C) Immunolocalization using 10 nm gold particles shows Bem3 localization to endomembranes near sites of bud emergence. (D) The area of the Bem3-containing compartment and bud/mother size dependence was quantified as described in Materials and Methods in W303 WT cells expressing GFP-Bem3. (E) WT and sla2Δ cells were imaged as in A and at least 200 cells were analyzed to determine the percentage of cells bearing Bem3 compartments. Scale bars: 5 µm (A,B); 0.5 µm (C).
Next, we labeled the plasma membrane of cells expressing GFP–Bem3 with the lipophilic dye FM4-64 and chased the cells for 10 minutes at 30°C to mark endocytic compartments (Lewis et al., 2000; Zahn et al., 2001), followed by colocalization analysis between Bem3 and FM4-64. Our results revealed that GFP–Bem3-containing compartments colocalized extensively with FM4-64 (Fig. 2B; Table 1). Furthermore, immunoelectron micrographs clearly showed the presence of Bem3 on intracellular, membrane-bound compartments polarized at the tips of growing buds (Fig. 2C).
Table 1. Colocalization of the Bem3-containing compartment with intracellular markers.

Images were obtained using GFP (Bem3) and Rhodamine (marker) filters. If the intensity value in the red channel was above the bleedthrough/crosstalk thresholds, a colocalization value of 1 was assigned to that particular structure; otherwise a score of 0 was assigned. At least 50 cells were analyzed per marker. Percentage colocalization was obtained as (sum of colocalization values/total number of compartments analyzed)×100.
Interestingly, the presence of Bem3 in intracellular compartments is temporally regulated during the cell cycle. Therefore, we only observed the Bem3-containing compartment in cells where the bud area was 20% or less than the area of the mother cell (B/M = 0.2, Fig. 2D). Because bud size is an intrinsic indicator of the cell cycle in yeast (Pruyne and Bretscher, 2000) our results suggest that Bem3 localizes to intracellular compartments only during early stages of the cell cycle. Bem3 is also recruited to the bud neck, and the proportion of cells displaying Bem3 at this location increases as the cell cycle progresses.
Interestingly, sla2Δ cells showed a drastic decrease in the percentage of cells containing the distinct Bem3-containing intracellular compartment, even when the analysis was restricted to cells with bud areas less than 20% of the mother cell area (Fig. 2E), further supporting the link between Bem3 localization and the endocytic pathway. A similar result was observed using the ent1Δ/ent2Δ/yap1801Δ/yap1802Δ strain (supplementary material Fig. S1).
To test whether other members of the polarity machinery were also present on distinct intracellular compartments during bud emergence, we analyzed the localization of Rga1, Cdc24 and Gic1 fused to GFP in un-budded and small-budded cells. We did not observe any intracellular compartment labeled with the GFP-polarity proteins listed above at any stage of the cell cycle (supplementary material Fig. S1C). We concluded that Bem3 was the only analyzed cell polarity protein abundant enough at endomembranes to be detectable by regular epifluorescence microscopy.
Importantly, we observed the presence of Bem3 in intracellular compartments independent of the expression levels of GFP–Bem3. Specifically, GFP-Bem3 expressed at endogenous levels from its genomic locus, or from different plasmid constructs (supplementary material Fig. S2), consistently showed Bem3 in dynamic intracellular membrane-bound structures (see below and supplementary material Movie 1). However, the size of the Bem3-containing compartment directly correlated with the expression levels of Bem3 (Fig. 3; supplementary material Fig. S2). When Bem3 was expressed from a strong GAL1 promoter and induced for increasing amounts of time, the size of the Bem3-containing compartment progressively increased (Fig. 3). We also noted that at higher Bem3 concentrations, the B/M dependence was more relaxed and the Bem3 compartment was present at B/M>0.2.
Fig. 3.

The size of the intracellular Bem3 compartment is proportional to the expression levels of Bem3. Cells expressing either HA-Bem3 or GFP-Bem3 from a GAL1 promoter were grown overnight at 30°C in medium containing 2% glucose, transferred to 2% galactose-containing mediim and aliquots obtained for western blotting (anti-HA antibody) or microscopy (100×, FITC filter) at the indicated times. Scale bar: 5 µm. The size of the Bem3-containing compartment was measured using ImageJ as described before. Horizontal lines on the graph indicate median values of the size of the Bem3-containing compartment for the respective induction times.
Because expression of Bem3 from its endogenous promoter in a high-copy plasmid (Yep13, 2μ, LEU2) did not affect cell growth or morphology (supplementary material Fig. S2, middle panel), but improved signal-to-noise ratios as compared to GFP–Bem3 expressed from its genomic locus (RLY 3090; supplementary material Fig. S2, top panel), we used the Yep13-GFP-Bem3 construct in most of the experiments described in this work.
Time-lapse videomicroscopy showed that the Bem3-containing compartment is highly dynamic, with bidirectional motility (supplementary material Movie 1). Near the bud tip, these Bem3-positive structures often appeared to interact closely with the Bem3 pool localized at the polar cap (supplementary material Movie 1). We found this behavior to be typical for most of the Bem3-positive compartments in a variety of strain backgrounds (W303, SEY6210 and BY4741, data not shown).
The Bem3 compartment contains both endocytic and secretory markers
In order to investigate the nature of the Bem3-containing compartment, we first measured its colocalization with various intracellular compartment markers, including early and late endosomes, trans-Golgi network (TGN) and the secretory pathway. We found that FM4-64 (endocytic pathway) always colocalized with the Bem3-containing compartment (Fig. 2B; Table 1). Moreover, although the early endosomal marker, Snc1, also colocalized substantially (Fig. 4A; Table 1) with Bem3 in the intracellular compartment, late endosome [Vps21, Markgraf et al., 2009); Vps4 (Vajjhala et al., 2007)] or TGN (Sec7) markers exhibited lower levels of colocalization (Table 1).
Fig. 4.
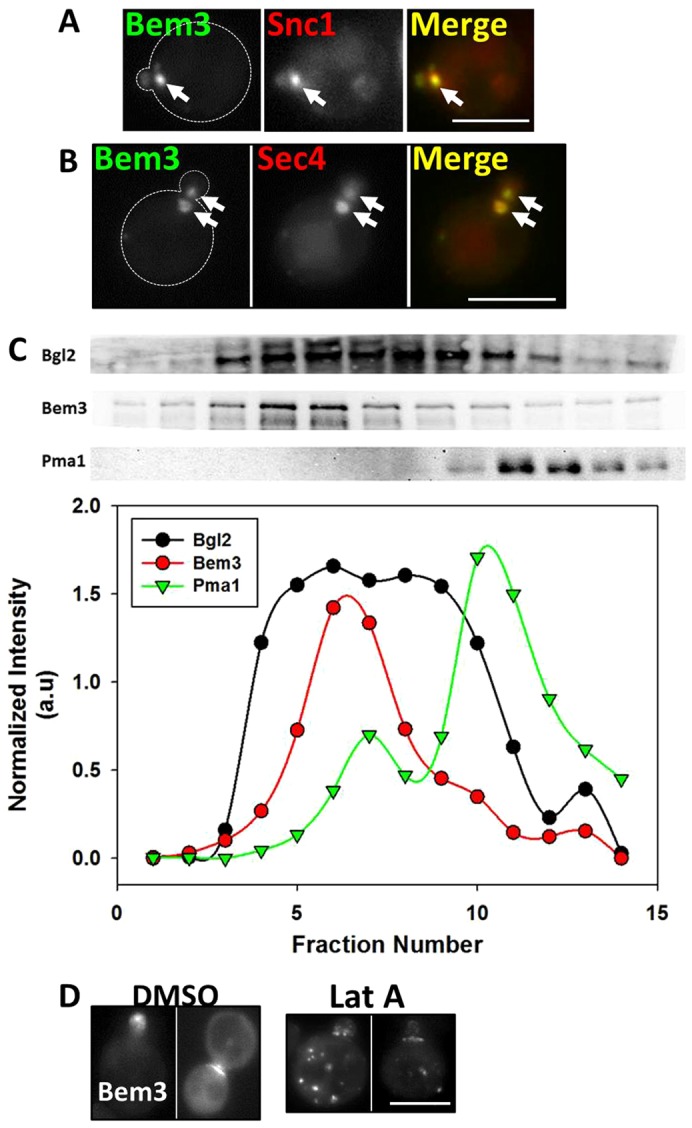
The Bem3-containing compartment colocalizes substantially with markers of the early endosome and secretory vesicles and traffics to sites of polarized growth on actin cables. (A) W303 cells transformed withYep13-GFP-Bem3 and pRS316-HA-mRFP-cSnc1were grown overnight in selective medium at 30°C and imaged at 100× using FITC and Rhodamine filters. Arrows point to the Bem3-containing compartment. (B) W303 cells expressing pGAL1.416-Bem3-GFP and pAD54-RFP-Sec4 were grown overnight in 2% glucose-containing medium at 30°C and transferred to 2% galactose medium for 2 hours before imaging at 100× using FITC and Rhodamine filters. Arrows point to the Bem3 compartment. Scale bar: 5 µm. (C) sec10-2 cells expressing HA-Bem3 from a GAL1 promoter were grown in glucose-containing medium overnight, then transferred to 2% galactose-containing medium and shifted to 37°C for 2 hours before cell lysis. The P3 pellet (see Materials and Methods) was subjected to Percoll density gradient fractionation. Fractionation profiles of Bem3, Bgl2 and PmaI were determined by western blotting (upper panels) with appropriate antibodies (see supplementary material Table S3), followed by band densitometry (lower panels). (D) W303cells expressing Yep13-GFP-Bem3 were grown overnight in selective medium, followed by addition of 200 µM Latrunculin A in DMSO, or vehicle alone, for 60 minutes and imaged at 100× using a FITC filter. Scale bar: 5 µm.
Surprisingly, we found complete colocalization between the Bem3 compartment and the small Rab GTPase and secretory vesicle marker, Sec4 (Table 1; Fig. 4B). Bem3 association with secretory vesicles was further investigated by using 20–55% Percoll density gradients (Orlando et al., 2011) to resolve the P100 fraction (secretory vesicles, endosomal membranes, Golgi components and portions of plasma membrane) of lysates from the temperature-sensitive strain sec10-2 (accumulates secretory vesicles at restrictive temperatures). Bem3 co-fractionated with the known secretory vesicle marker Bgl2 (Harsay and Bretscher, 1995; He et al., 2007) (Fig. 4C), further confirming the presence of Bem3 on secretory vesicles. Because sec10-2 cells show a defect in secretory vesicle fusion with the plasma membrane at the restrictive temperature (Roth et al., 1998) only a minor fraction of Bem3 co-fractionated with the plasma membrane marker, Pma1 (Ferreira et al., 2001; Luo and Chang, 2000) (Fig. 4C).
Additionally, it is known that secretory vesicles traffic on filamentous actin cables in budding yeast to reach sites of polarized growth (Mulholland et al., 1997). When we treated cells with Latrunculin A to disrupt actin cables, Bem3 redistributed into dispersed intracellular compartments (Fig. 4D) confirming results first reported by Knaus et al. (Knaus et al., 2007). Nevertheless, we cannot rule out the possibility that Bem3 is directly or indirectly binding actin filaments.
In summary, our data demonstrates that the Bem3-containing compartment is a very dynamic conglomerate of vesicles containing both endocytic (e.g. FM4-64-stained) and secretory (Sec4-positive) cargo (Figs 1–4). Moreover, its localization to sites of polarized growth depends on the integrity of the actin cytoskeleton (Fig. 4D). The Bem3-containing compartment is only observed during a restricted period of time during bud emergence in the vicinity of sites of polarized growth (Fig. 2D). Interestingly, all these are defining characteristics of the cell-polarity-linked organelle present in several filamentous fungi, known as the Spitzenkörper (Crampin et al., 2005; Steinberg, 2007; Virag and Harris, 2006). Importantly, a recent report has indicated that such a membrane-bound structure can also be found in S. cerevisiae (Chapa-y-Lazo et al., 2011).
Bem3 localizes to a Spitzenkörper-like compartment
Based on the observations described above, we hypothesized that Bem3 occupies a ‘Spitzenkörper-like’ compartment during the initial phases of bud emergence. Since the Spitzenkörper is known to function as a recycling center and is involved in polarized bud growth during hyphal development in filamentous fungi (Steinberg, 2007; Taheri-Talesh et al., 2008; Virag and Harris, 2006), we tested (1) whether Bem3 could recycle from its intracellular compartment, and (2) if the presence of Bem3 on this endomembrane compartment correlates with polarized growth.
Bem3 recycling
Our results demonstrated that Bem3 colocalized substantially with Snc1, a v-SNARE protein known to recycle from endosomes in a Ypt31/32- and Rcy1-dependent manner (Furuta et al., 2007; Galan et al., 2001; Lewis et al., 2000). Therefore, we tested what consequences RCY1 deletion (rcy1Δ) would have on the characteristics of the Bem3-containing compartment. Although the mean fluorescence intensity per unit area of GFP–Bem3 within the compartments and the Bem3 protein content remained unchanged, in contrast to the situation in wild-type cells, Bem3 accumulated in many, enlarged intracellular compartments in rcy1Δcells (Fig. 5A). Moreover, the presence of Bem3 on intracellular endomembranes could be detected in large budded cells as well (Fig. 5B), indicating a relaxation of the wild-type temporal regulation of the presence of Bem3 in intracellular compartments (Fig. 2D). Because Rcy1 is an effector of the Rab-GTPase Ypt31/32 (Chen et al., 2005), overexpression of the constitutively inactive Ypt31N126I mutant (Yoo et al., 1999) led to similar intracellular accumulations of Bem3 as expected (supplementary material Fig. S3). Altogether, these results demonstrate that Bem3 undergoes Rcy1-mediated recycling for exit from its intracellular compartment. In contrast, we found that the total size and bud/mother size dependence for detection of Bem3 in endomembranes were not affected in cells deficient for the retromer complex (Schellmann and Pimpl, 2009) (vps29Δ, Fig. 5A–C), majorly involved in recycling cargo from late endosomes back to the Golgi.
Fig. 5.
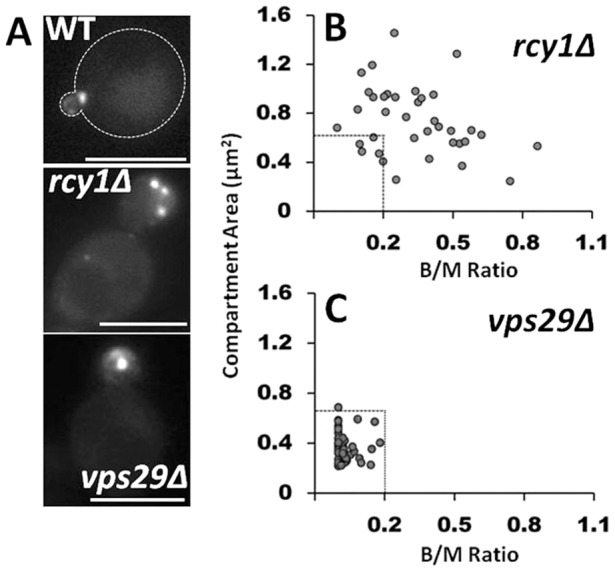
Bem3 traffics from its compartment in an Rcy1-dependent manner. (A) GFP-Bem3-expressing WT, rcy1Δ and vps29Δ cells were grown overnight in selective medium at 30°C and imaged at 100× using a FITC filter. The total area of the Bem3 compartment present within a cell was measured using ImageJ (see Material and Methods) and plotted as a function of bud/mother area ratio in rcy1Δ (B) and vps29Δ (C) cells. Dashed lines in B and C indicate the boundaries of compartment size and bud/mother area ratios in WT cells. Scale bars: 5 µm.
The size of the Bem3-containing compartment correlates with the length of the apical growth phase
The Spitzenkörper is only observed during hyphal development in filamentous fungi, when the bud experiences extended periods of apical growth. Similarly, we observed that Bem3 localizes to an intracellular compartment only in unbudded and small budded cells (Fig. 2D), a stage when the bud is reported to undergo the most rapid expansion (Goranov et al., 2009; He et al., 2007). Moreover, the Spitzenkörper contains markers for both the endocytic and secretory pathways and is essentially a recycling center. Therefore, if the Bem3-containing compartment is functionally related to the Spitzenkörper, the presence of Bem3 in the intracellular compartment should be enhanced during extended periods of apical growth. In order to test this prediction, we experimentally extended the period of apical bud growth by two different methods: pseudohyphal induction (Gancedo, 2001), and growth of a temperature-sensitive septin mutant (Kim et al., 1991) at restrictive temperatures. We then analyzed the total area of the Bem3-containing compartment under these conditions and compared them with their respective controls.
Pseudohyphal growth is a form of filamentous bud growth characterized by unipolar budding patterns and extensive periods of apical growth (Gancedo, 2001). We observed that the total size of the Bem3-containing compartment was significantly larger (P<0.05, Wilcoxon test) in cells grown under pseudohyphal induction conditions [1% butanol (Lorenz et al., 2000)] than in cells grown in the absence of butanol (median areaPseudophypha = 0.928 µm2 versus median areaControl = 0.524 µm2; Fig. 6A).
Fig. 6.
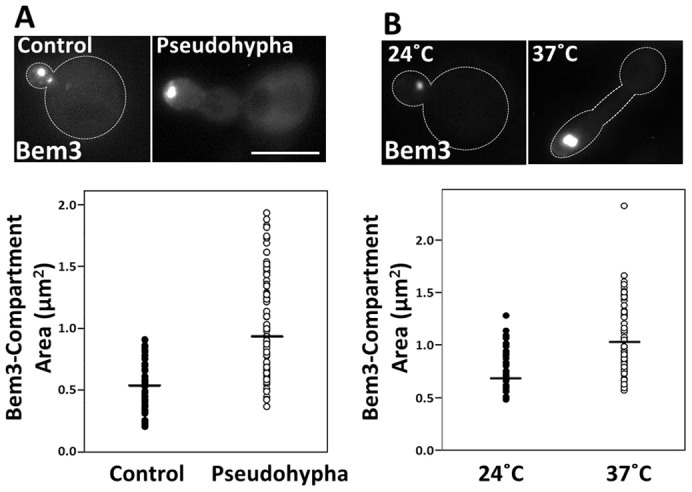
The area of the Bem3-containing compartment is enlarged in cells experiencing extended periods of apical growth. (A) Diploid cells expressing GFP-Bem3 were grown in the absence (Control) or presence (Pseudohypha) of 1% butanol for 6 hours and imaged at 100× using a FITC filter. (B) cdc3ts mutant cells transformed with Yep13-GFP-Bem3 were grown at 24°C or shifted to 37°C for 6 hours before imaging at 100× using a FITC filter. The areas of the Bem3-containing compartments of control and pseudohypha-induced cells were quantified using ImageJ. Statistical significance was tested using the Wilcoxon test (**P<0.001). Scale bar: 5 µm.
A similar observation was made using a temperature-sensitive septin mutant (cdc3ts; Fig. 6B). When these cells were grown at a restrictive temperature (37°C), they experienced a cell cycle delay at the G2 phase and therefore, experienced extended periods of apical growth (Kim et al., 1991; Sakchaisri et al., 2004); (Longtine et al., 2000). Although the size of the Bem3-containing compartment remained unaffected when wild-type cells were grown at the restrictive temperature (supplementary material Fig. S4A), the size of the Bem3-containing compartment in cdc3tscells grown at 37°C was significantly larger (Fig. 6B) compared to those grown at room temperature (median area37°C = 1.023 µm2 versus median area24°C = 0.665 µm2; P<0.05, Wilcoxon test). We established that these conditions proceed with no changes in Bem3 protein levels (data not shown), but with enhanced recruitment to endomembranes. Collectively, these observations suggest that the size of the Bem3-containing compartment correlates with the extent of apical bud growth. A logical prediction is that Bem3 overexpression should enhance bud growth. However, it is known that Bem3 overexpression is toxic (Knaus et al., 2007). We hypothesized that this toxicity is due to GAP-mediated inhibition of Cdc42 signaling affecting cell polarity establishment and bud formation. In fact, Bem3K1003A mutant (unable to bind Cdc42; i.e. lacking GAP activity) overexpression was not toxic and importantly, it induced bud-elongation and greatly enlarged Bem3 compartments (supplementary material Fig. S4B).
Bem3 enhances Sec4 recruitment to endomembranes and targeting to sites of polarized growth
Given the extensive colocalization between Sec4 and the Bem3-containing compartment (100%; Fig. 4B; Table 1), we sought to gain insights into the relationship between these two proteins by first analyzing Sec4 localization in bem3Δ cells. In the absence of Bem3, Sec4 localization to newly emerging buds was significantly reduced (P<0.0001, Wilcoxon test; Fig. 7A). Specifically, Bem3 deletion resulted in a decrease in the fraction of total Sec4 present at the tip of small buds (up to B/M = 0.2) compared with wild-type (WT) cells (medians: 57% and 76% for bem3Δ and WT, respectively, Fig. 7A). At this stage it is interesting to note that small-budded and unbudded cells (B/M<0.2) are the populations in which Bem3 localizes to the Spitzenkörper-like compartment (Fig. 2D). Interestingly, GFP–Sec4 was observed to also populate an intracellular structure of similar characteristics to the Bem3 compartment (Fig. 7A).
Fig. 7.
Bem3 and secretory machinery proteins reciprocally regulate each other. (A) WT (BY4741) and bem3Δ cells expressing GFP-Sec4 (endogenous promoter) were grown overnight in selective medium at 30°C and imaged at 100× using a FITC filter. Fluorescence intensity of GFP–Sec4 was quantified using ImageJ and the polarized GFP–Sec4 fluorescence versus the bud/mother area ratio was plotted. *P<0.0001. (B) W303 WT cells expressing RFP-Sec4 (GAL1 promoter) and GFP-Bem3 were grown overnight in 2% glucose-containing medium, transferred to 2% galactose-containing medium for the indicated times and imaged at 100× using FITC and Rhodamine filters. Both Bem3 and Sec4 cluster in internal compartments of increasing size as Bem3 dosage increases. (C,D) W303 WT cells expressing GFP-Sec4 and Bem3PHm (C) or Bem3K1003A (D) were grown overnight in 2% glucose-containing medium, transferred to medium containing 2% galactose for 4 hours and then imaged at 100× using a FITC filter. (E) W303 WT cells expressing the indicated GFP-Sec4 mutants and Bem3 were grown overnight in 2% glucose-containing medium, transferred to medium containing 2% galactose for 4 hours and then imaged at 100× using a FITC filter. 24% of cells expressing GFP-Sec4Q79L showed the clustered phenotype upon Bem3 overexpression compared with 4% for GFP-Sec4S29N. (F,G) Cells expressing GFP-Bem3 and overexpressing Sec15 (F) or Sro7 (G) from a GAL1 promoter (pYES2.1) were grown in 2% galactose-containing medium for 15 hours before imaging at 100× using a FITC filter. Areas of the Bem3 compartments were measured as described above and plotted as a function of the bud/mother area ratio. Dashed lines indicate the boundaries of the corresponding bud/mother area ratios in WT cells grown under control conditions. (H) Working model of the relationship between the cell polarity protein Bem3 and vesicle trafficking. STEP I: Bem3 (red bars) recognizes sites of polarized growth through interactions with Cdc42 and PtdIns(4,5)P2, is retrieved from the plasma membrane by internalization and trafficked to a compartment reminiscent of the Spitzenkörper. STEP II: Bem3 actively recruits Sec4 (black asterisks) to this compartment. STEP III: Bem3 is recycled back to the bud tip from this Spitzenkörper-like compartment, in secretory vesicles in an Rcy1/Sec4-dependent manner. Scale bars: 5 µm.
Importantly, Bem3 overexpression resulted in Sec4 accumulation in Bem3-containing compartments in a dose-dependent manner (Fig. 7B). Specifically, we varied the protein concentration of Bem3 by varying the GAL1 promoter induction time. We observed that progressively higher doses of Bem3 led to the previously described size increment of the Bem3-positive compartment (Fig. 3; supplementary material Fig. S2; Fig. 7B, top panel). In addition, Sec4 increasingly accumulated in these Bem3-positive compartments as Bem3 expression increased with time, leading to a switch from a mainly cytosolic (probably as a result of overexpression) to completely endomembrane localized (Fig. 7B). Importantly, the PH domain mutant of Bem3 (Bem3R644S,R645S,K647D), previously described as being unable to efficiently bind endomembranes (Fig. 1B,C) and therefore, largely absent from intracellular compartments, is unable to cluster Sec4 when overexpressed (Fig. 7C). To test whether upon Bem3 overexpression its PH impaired PtdIns(4,5)P2/PtdIns(3)P availability, we determined whether a Bem3 PX-PH fragment would led or not to Sec4 clustering. Supplementary material Fig. S4C, shows that as opposed to Bem3 full length, the PX-PH fragment was unable to induce Sec4 clustering. Therefore, this result indicates that phospholipid sequestering is not the mechanism by which Bem3 triggers Sec4 intracellular clustering.
Interestingly, Bem3K1003A also led to Sec4 clustering (Fig. 7D) when overexpressed. To our knowledge, this is the first report of a GAP-activity-independent function of Bem3. In vitro binding assays with purified proteins and yeast two-hybrid experiments yielded negative results for a direct interaction between Bem3 and Sec4 (data not shown), suggesting an indirect functional relationship between these proteins. In addition, the Bem3 homolog in Candida albicans (Ca-Bem3) also clustered Sec4 in intracellular compartments (supplementary material Fig. S5A) when overexpressed in Saccharomyces cerevisiae, suggesting that this novel Bem3 function of recruiting Sec4 to endomembrane compartments is conserved in filamentous, pathogenic fungi as well.
Furthermore, comparable to the sharp increase in the presence of depolarized Bem3 puncta in sla2Δ cells described above (Fig. 2A), Sec4 localization is also affected in a similar way in sla2Δ cells. Specifically, Sec4 puncta were significantly (P<0.01) more depolarized in sla2Δ cells (40.86±2.32%) compared with wild-type cells (24.36±3.05%). Therefore, deficient endocytosis causes both Sec4 and Bem3 to mislocalize to depolarized puncta, strongly suggesting that the localization of both Sec4 and Bem3 is heavily dependent on efficient vesicle trafficking, including the endocytic pathway. In turn, Bem3 had a depolarized distribution in the sec4-8 temperature-sensitive strain (supplementary material Fig. S5B), suggesting a reciprocal requirement between Bem3 and Sec4 for polarized localization.
The Bem3-overexpression-mediated accumulation of Sec4 in endomembranes is dependent on the nucleotide-bound status of Sec4
Like other small GTPases of the Ras superfamily, Sec4 reversibly switches between the active (GTP-bound) and inactive (GDP-bound) conformations (Barr and Lambright, 2010; Prekeris, 2003). In order to test whether Sec4 accumulation in intracellular compartments upon Bem3 overexpression was dependent on its nucleotide-bound status, we analyzed the effect of Bem3 overexpression on constitutively active (Sec4Q79L) and inactive (Sec4S29N) mutants of Sec4 fused to GFP (Calero et al., 2003; Walworth et al., 1992). We observed that the penetrance of the clustering phenotype and the size of the Sec4 clusters were substantially reduced in cells expressing the constitutively inactive or GDP-bound conformation of Sec4 (Sec4S29N) when compared with the wild-type or active form (Fig. 7E). Therefore, our results suggest that Bem3 overexpression primarily leads to the clustering of GTP-bound Sec4. Colocalization analysis of GFP–Bem3 with Sec4Q79L–RFP showed that GTP–Sec4 colocalized extensively (data not shown) with the Bem3-containing compartment.
Overexpression of the exocyst subunits, Sec15 and Sro7, affects the cell-cycle dependence of the Bem3-containing compartment
The existence of at least two independent mechanisms for Sec4 tethering to the exocyst complex has been suggested, one dependent on the Sec15 exocyst subunit (Guo et al., 1999; Novick et al., 2006) and the other on the late secretory marker, Sro7 (Grosshans et al., 2006; Rossi and Brennwald, 2011; Zanolari et al., 2011; Zhang et al., 2005). Interestingly, overexpression of these proteins also leads to clustering of Sec4-containing secretory vesicles (Rossi and Brennwald, 2011; Salminen and Novick, 1989). Since Bem3 and Sec4 colocalized extensively, we tested whether the intracellular localization of Bem3 could be also influenced by Sec15 or Sro7. We observed that both Sec15 and Sro7 overexpression led to a relaxation of the strict bud/mother size dependence of cells in which a Bem3-containing compartment could be found (Fig. 7F,G). These results further support a functional link between Bem3 and the secretory pathway.
Discussion
This work describes a novel crosstalk mechanism between two processes essential for cell polarity establishment: Cdc42 signaling and vesicle trafficking. Specifically, we show for the first time that a negative regulator of Cdc42 signaling, Bem3, requires and regulates vesicle trafficking (see Fig. 7H).
Observations that suggest that Bem3 requires vesicle trafficking are: (1) although Bem3 is not a transmembrane or membrane-anchored protein, it depends on vesicle trafficking to properly localize to sites of polarized growth; (2) efficient endocytosis is required for the proper/efficient localization of both Bem3 and Sec4; (3) Bem3 partitions between a Spitzenkörper-like compartment and the plasma membrane.
Observations that suggest Bem3 affects secretory traffic are: (1) Bem3 affects endomembrane recruitment and polarized delivery of the secretory Rab, Sec4; (2) Bem3 controls the size of the Bem3-containing Spitzenkörper-like compartment; (3) these novel functions of Bem3 are the first to be described as independent of its GAP activity.
Our results demonstrate that Bem3 requires vesicle trafficking for proper localization to sites of polarized growth and that it partitions between the plasma membrane and a Spitzenkörper-like endomembrane compartment. Specifically, we observed that in the sla2Δ endocytic mutant strain, Bem3 accumulated in depolarized puncta (Fig. 2A). As opposed to a transmembrane endocytic cargo, Bem3 can also directly associate and dissociate from the plasma membrane. This dynamic process would mostly be dependent on Bem3 binding to PtdIns(4,5)P2, which is present at sites of polarized growth and cytokinesis, as well as on the cell cortex as dispersed, discrete puncta. We speculate that upon endocytosis deficiency (and in contrast to integral membrane proteins that homogeneously accumulate on the membrane surface), Bem3 would remain present in dispersed PtdIns(4,5)P2-containing puncta from where endocytosis fails to remove it. Furthermore, upon Sla2 deletion, there was a significant decrease in the fraction of cells that showed endomembrane-localized Bem3, suggesting that the endocytic pathway is required for Bem3 trafficking and/or to generate the endosomal platforms from where Bem3 exerts some of its novel functions.
When its exit from intracellular compartments was hampered by the deletion of RCY1 (Wiederkehr et al., 2000), Bem3 accumulated in endomembranes whose collective area was significantly larger than in wild-type strains. Since Rcy1 is an effector of Ypt31/32 (Chen et al., 2005), the Rab11 yeast homolog involved in recycling cargo to the plasma membrane (Chen and Wandinger-Ness, 2001; Lapierre and Goldenring, 2005), the dependence of Bem3 on Rcy1 suggests that Bem3 follows a recycling route. In this regard, the secretory machinery is a well-documented player in the Rcy1-mediated cargo recycling back to the plasma membrane (Chen and Wandinger-Ness, 2001; Lapierre and Goldenring, 2005). Indeed, we demonstrated that Bem3 not only colocalized with the secretory vesicle marker, Sec4, but it was present in biochemically characterized secretory vesicles. Therefore, this study shows that Bem3 localizes to an intracellular compartment at the intersection of the endocytic and secretory pathways, much like the Spitzenkörper in filamentous fungi (Steinberg, 2007; Taheri-Talesh et al., 2008; Virag and Harris, 2006). Similar to the Bem3-containining compartment, the Spitzenkörper is dependent on the actin cytoskeleton for function and can be labeled with FM4-64 (Köhli et al., 2008) and Sec4 (Jones and Sudbery, 2010). Furthermore, the Spitzenkörper is present during periods of robust polarized growth (Sudbery, 2011), i.e. it forms the ‘recycling center’ from where materials can efficiently be trafficked and reused to support extended periods of hyphal growth (Steinberg, 2007). A recent report suggests that a Spitzenkörper-like structure, which appears as a spot marked by FM4-64 is also present near the tips of mating projections in budding yeast (Chapa-y-Lazo et al., 2011). Therefore, we propose that Bem3 occupies a Spitzenkörper-like compartment during the initial stages of bud emergence.
A dependence on vesicle trafficking has also been proposed for the localization of Cdc42 itself (Zajac et al., 2005; Osmani et al., 2010; Slaughter et al., 2009). In addition, cellular control of Cdc42 signaling activation is classically known to be exerted by regulation of the corresponding GAPs and GEFs and, to the best of our knowledge, no link between these proteins and vesicle trafficking has been demonstrated before in yeast. Importantly, Bem3 is a GAP that unlike Cdc42, is not membrane anchored, but predicted to be peripherally associated. This report, along with others (Bai et al., 2010) indicate that peripherally associated membrane proteins can be subjected to vesicle trafficking. We believe that such traffic events are possible by the association of Bem3 with membranes, mediated largely by its PH domain. Indeed, although this protein does not contain a transmembrane domain or a membrane anchoring mechanism, it has been classically observed at the cell cortex and enriched at the polar cap and bud-neck regions (Caviston et al., 2003) (Fig. 1C). Accordingly, following subcellular fractionation and based on colocalization experiments, we have been able to detect more than 50% of Bem3 associated with membrane fractions in general, a part of which consisted of secretory vesicles (Fig. 4C). Furthermore, in vitro overlay and liposome-binding experiments showed that purified Bem3 fragments are capable of binding the plasma membrane-enriched lipid, PtdIns(4,5)P2 (Fig. 1A). Importantly, this lipid is especially abundant at endocytic and polarized growth sites (Doherty and McMahon, 2009; Souza and Pichler, 2007; Takahashi and Pryciak, 2007) and it is known to play a role in protein recruitment to the plasma membrane (Sun et al., 2007; Takenawa and Itoh, 2001).
Our study also indicates that Bem3, instead of behaving like a passive secretory cargo, directly affects the physical and molecular characteristics of the Spitzenkörper-like endomembrane compartment. Specifically, we observed a strong correlation between Bem3 dosage and the total size occupied by the Bem3-containing compartment (Fig. 3). Indeed, electron microscopy showed the presence of Bem3 on clustered membrane-bound structures near the tips of emerging buds (Fig. 2C). We hypothesize that these structures, not described before, may arise from Bem3-induced enlargement of the Spitzenkörper-like compartment.
Bem3 also exerted effects on polarized delivery of secretory vesicles. Specifically, although overexpression of Bem3 led to accumulation of Sec4 in the internal compartment (Fig. 7B–D), deletion of Bem3 led to a defect in Sec4 polarization to the bud tip (Fig. 7A) during the earliest stages of the cell cycle. These results could explain how mis-directed delivery of secretory vesicles upon Bem3 deletion leads to the generation of cells with abnormal shapes (Park and Bi, 2007). Interestingly, the Bem3 activity described above is the first report of a GAP-independent property of Bem3. In turn, our data suggest that Sec4 is involved in the polarized delivery of Bem3 (supplementary material Fig. S5B).
Overexpression of two functionally distinct proteins of the secretory pathway – the exocyst component Sec15 and the SNARE regulator Sro7, were also shown to cluster Sec4 in internal compartments (Rossi and Brennwald, 2011). Interestingly, we found that overexpression of these proteins also affected the cell-cycle stage at which the Bem3-compartment could be found (Fig. 7F,G).
The presence of the Bem3-containing compartment at the tips of growing buds (Fig. 2C) and its colocalization with early endosome, recycling and secretory machineries (Fig. 4) suggested that early endosomes could be playing a role in polarized secretion for apical bud growth. Moreover, Bem3 was only visible on the compartment in unbudded and small budded cells (Fig. 2D), a stage when the bud primarily experiences apical growth (Pruyne and Bretscher, 2000). Therefore, we tested the consequences of extending the period of apical growth on the presence and total size of this Bem3-positive compartment. Regardless of the method used (pseudohyphal induction or temperature-sensitive septin mutants at their restrictive temperature), increased periods of apical growth correlated with a significantly enlarged Bem3-containing intracellular compartments (Fig. 6), suggesting that the compartment plays a critical role in apical bud growth, predominant during early stages of the cell cycle when the bud emerges.
The crucial role played by endocytosis and recycling in polarized growth has been explicitly demonstrated in several cases. In U. maydis, the early endosome plays a crucial role in coordinating the processes of exo- and endocytosis to ensure polar and directed bud growth (Wedlich-Söldner et al., 2000). The Yup1-stained BSD (brightly stained dot) described by the authors (Wedlich-Söldner et al., 2000) shares striking similarities with the structure and function of the Bem3-containing compartment we have described in this study. In addition, the exocyst complex has been reported to be present on recycling endosomes in mammalian cells (Zhang et al., 2004), analogous to the colocalization of Sec4 with the Bem3-containing compartment shown in this work.
The Spitzenkörper is an essential recycling compartment for the efficient development of long, filamentous hyphae in infectious yeast species such as Candida, and a prerequisite for switching to the yeast pathogenic form. Therefore, considering the parallels between the compartment marked by Bem3 in budding yeast and the Spitzenkörper in filamentous fungi, the findings reported here promise to be particularly useful for better understanding of the basis for pathogenicity of infectious yeast species. In this regard, we have found that overexpression of Bem3 from Candida albicans is also competent in clustering Sec4 in S. cerevisiae (supplementary material Fig. S5A). Therefore, in addition to unraveling the intricate interplay between signaling (Bem3) and vesicle trafficking (Sec4 and Rcy1) machineries, our results suggest that the Bem3-containing compartment may represent a conserved hub for efficient polarized traffic of the cell polarity machinery during rapid apical bud growth, especially during bud emergence in S. cerevisiae and hyphal growth in C. albicans.
Materials and Methods
Reagents and DNA constructs
Materials were purchased from Fisher Scientific (Fairlawn, NJ) or Sigma (St. Louis, MO) unless stated otherwise. Plasmids, strains and antibodies used in this study are listed in supplementary material Tables S1–S3. DNA constructs were prepared using standard techniques. Site directed mutagenesis was performed using a QuikChange® Lightning Site Directed Mutagenesis kit (Agilent Technologies, Inc., Santa Clara, CA).
Yeast culture conditions and transformation procedures
Yeast strains were grown overnight at 30°C (or room temperature for temperature sensitive strains) with shaking at 250 r.p.m. in standard yeast extract–peptone–dextrose (YPD) or synthetic selective medium supplemented with dextrose or galactose and lacking appropriate amino acids for plasmid maintenance. Yeast was transformed by the lithium acetate method.
PIP strip assay
A recombinant His6-tagged PX-PH domain fragment of Bem3 (Bem3491–774) was purified from bacterial lysates by affinity purification as described before (Aguilar et al., 2003) and 15 µg was used for binding to PIP microstrips (Frontier Scientific Inc., Logan, UT) using the company recommended protocol. Bem3 was detected using anti-His6 antibody (see supplementary material Table S3).
Liposome flotation assay
Lipids in chloroform or powder were purchased from Avanti Polar Lipids (Alabaster, AL). A dried film of the indicated lipids was prepared by evaporation under nitrogen gas. Lipids (5% of the indicated phosphatidylinositol or phosphatidylinositol phosphate and 95% PC) were resuspended in 50 mM Hepes (pH 7.2), 120 mM potassium acetate buffer by vortexing and brief sonication. The liposome suspension was extruded 20 times through polycarbonate filters of 0.2 mm pore size (LiposoFast; Avestin, Ottawa, ON, Canada). Binding of Bem3 constructs to liposomes were analyzed using liposome floatation assays. Purified His6-tagged proteins (0.25 mM) and liposomes (1 mM) were incubated in HKM buffer [50 mM Hepes, pH 7.2, 120 mM potassium acetate, 1 mM MgCl2, 1 mM dithiothreitol (DTT)] for 10 minutes at room temperature. Then, 75% (w/v) sucrose solution was added and homogenized with the liposome and protein mixture to make a 30% final sucrose solution. HKM buffer containing 25% (w/v) sucrose was gently layered on top of the 30% sucrose suspension, followed by addition of buffer containing no sucrose. The samples were centrifuged at 55,000 r.p.m. for 1 hour in a Beckman swinging rotor (TLS-55). The two interfacial layers were manually collected and analyzed by SDS-PAGE and western blotting using monoclonal antibodies (Clontech) raised against the His6 epitope.
Drug treatments
Disruption of actin structures with Latrunculin A
Latrunculin A (Invitrogen, Carlsbad, CA) was added to a final concentration of 200 µM to cells growing in selective medium and incubated for 60 minutes at 30°C prior to imaging.
Pseudohyphal growth induction using butanol
Diploid yeast cells when grown in selective medium overnight, harvested and resuspended in YPD containing 1% butanol for 6 hours at 30°C with shaking at 250 r.p.m. to induce pseudohyphae.
Compartment labeling with FM4-64
Cells were grown overnight in selective medium, pelleted and resuspended in YPD (1 OD/ml). Cells were allowed to chill on ice for 10 minutes before addition of FM4-64 (1∶50 dilution from a stock of 1 mg/ml in DMSO) and incubated on ice for another 20 minutes. After removing excess dye by washing with cold YPD twice, cells were resuspended in pre-warmed medium and incubated at 30°C for 10 minutes. Cells were then returned to ice, washed once with cold YPD containing 10 mM sodium azide and 10 mM sodium fluoride and imaged at 100× using the FITC and Rhodamine filters.
Microscopy
Images were acquired using a Zeiss Axiovert 200M microscope equipped with Zeiss Axiocam MRm monochrome digital camera and Carl Zeiss Axiovision image acquisition software (version 4.4) in wide-field mode. For imaging of live cells expressing fluorescently tagged proteins, 2–5 OD600 nm of cells were pelleted and resuspended in 100 µl of selective medium (pH 7.5). 10 µl of cells were spotted on a precleaned glass slide, uniformly spread using 22×22 mm coverslips and imaged at 100× with the appropriate optics.
Quantification of Bem3 compartment size and cell cycle dependence
Cells expressing GFP–Bem3 were imaged at 100× using a FITC filter. The size of the Bem3 compartment under all conditions tested was measured by drawing a freehand ROI demarcating the compartment using ImageJ software (Sheffield, 2007).
To determine the cell cycle dependence of the Bem3 compartment, the sizes of the bud and mother cells were also measured along with the compartment size using freehand ROIs in ImageJ. The data were represented as a scatter plot of compartment size as a function of the bud to mother area ratios.
Colocalization quantification of GFP–Bem3 with early and late endosome markers
We adapted the methods used in Poulter et al. to quantify colocalization of the Bem3 compartment with various cellular markers (Poulter et al., 2010). Briefly, images were obtained using both FITC (Bem3) and Rhodamine (endosome marker) filters. Bleedthrough of the Bem3 compartment signal into the red channel was accounted for by imaging single transformants in both channels, followed by measurement of the average pixel intensity of an ROI demarcating the Bem3 compartment in both channels. This procedure was repeated for 75 Bem3 compartments of varying pixel intensities. A ‘bleedthrough’ plot was constructed of the average pixel intensity in the green channel versus their corresponding values in the red channel. The data were processed using regression analysis software (Mac Curve Fit) to obtain the errors associated with the y-intercept and slope of the regression line. We determined that a threshold of y = (a+3SD)x+(b+3SD) [y = ax+b, where a = 0.0861±0.01084 and b = 2.088±2.011] discounted bleedthrough with 95% confidence. Finally, cells co-transformed with both GFP-Bem3 and the appropriate endosome marker were imaged in both channels and the average pixel intensity of the ROI demarcating the Bem3 compartment was measured for both channels. If the intensity value in the red channel was above the threshold, a colocalization value of 1 was assigned to that particular compartment. Otherwise the compartment received a score of 0. At least 50 cells were analyzed per marker. Percentage colocalization was obtained as (sum of colocalization values/total number of compartments)×100.
Quantification of GFP–Bem3 localization
Images of cells expressing wild-type and mutant GFP-Bem3 constructs were acquired as described above. Cells were scored according to whether Bem3 was present at sites of polarized growth, in depolarized puncta or in the cytosol. The cytosolic content of wild-type Bem3, as measured by the mean of the maximum fluorescence intensity of a line scan across the cytosol of 50 cells was 139.11±31.9 arbitrary fluorescence units. Subsequently, cells were considered to have a significant cytosolic Bem3 fraction if the maximum fluorescence intensity of a line scan through the cytosol was greater than the mean +1 s.d. (171.9 arbitrary units) of wild-type cells. At least 200 cells were counted per construct. Data are presented as the means ± s.d. from three experiments. Statistical significance was determined using Student's t-test.
Biochemical analysis of secretory vesicles by differential centrifugation and Percoll density gradients
In order to test for the presence of Bem3 on secretory vesicles, sec10-2 cells expressing HA-tagged Bem3 from a GAL1 inducible promoter were grown overnight in selectable medium containing 2% glucose. Next morning, cells were transferred to medium containing 2% galactose and shifted to 37°C for 2 hours to induce Bem3-HA and to begin the accumulation of secretory vesicles. Then, cells were harvested by centrifugation at 2200 r.p.m. for 5 minutes and treated with 0.1 M Tris-HCl (pH 9.4) and 10 mM DTT for 10 minutes at room temperature without shaking. Cells were spheroplasted by incubation at 30°C in the presence of 1 U/OD Zymolase (Zymo Research, Orange, CA) in S-buffer (20 mM Tris-HCl pH 7.4, 1 M sorbitol, 2% glucose, 0.5 mM MgCl2) at a density of 5 OD/ml for 45 minutes with gentle shaking. After a wash with S-buffer, cells were resuspended in lysis buffer (10 mM triethanolamine, 1 mM EDTA, 0.8 M sorbitol) containing protease inhibitors and lysed with 20 strokes in a Dounce homogenizer. Unbroken cells were removed by centrifugation at 500 g for 3 minutes, followed by lysate clarification by centrifugation at 13,000 g for 20 minutes. The supernatant was then subjected to ultracentrifugation at 100,000 g for 60 minutes to collect the P3 pellet. Finally, this pellet was resuspended in 375 µl of lysis buffer and fractionated in a 10 ml 20–55% Percoll density gradient. 500 µl fractions were collected from the top, and samples were resolved using SDS-PAGE in 4–20% polyacrylamide precast gradient minigels (Thermo Fisher Scientific Inc., Waltham, MA) at 120 V constant voltage and transferred onto nitrocellulose at 80 V for 75 minutes. Bem3–HA, Bgl2 and Pma1 were detected by western blotting with appropriate antibodies (supplementary material Table S3) and fractionation profiles of the individual proteins were generated by quantification of band intensities of each fraction.
Immunoelectron microscopy
Cells were processed according to the method of Collins et al. (Collins et al., 2000) to preserve the cell wall integrity. Briefly, cells were fixed with 3% glutaraldehyde and embedded in 2% ultralow-temperature agarose. The cells were post-fixed in 3% KMnO4 and embedded in Spurr's resin. Sections were cut, Yep13-HA-Bem3 labeled with anti-HA and secondary antibody tagged with 10 nm gold particles and observed on a Phillips EM410 microscope.
Supplementary Material
Acknowledgments
We thank Beverly Wendland (The Johns Hopkins University), Howard Riezman (University of Geneva), Chris Burd (Yale University), Dan Szymanski, Jessica Henty, Henry Chang, Donald Ready (Purdue University) for stimulating discussions, critical reading of the manuscript and reagents. We thank Claudia B. Hanna (Purdue University) and Onaidy Teresa Torres (University of Miami) for excellent technical assistance.
Footnotes
Author contributions
D.M. and R.C.A. conceived and designed the experiments; D.M., A.S., D.R.B, G.D.F, D.S., J.M.C. and F.J.B.V performed the experiments; J.M.C., C.J.S., S.G., S.K.L. and R.C.A., analyzed the data; T.H., D.M. and A.S. contributed reagents, materials and analysis tools; D.M. and R.C.A. wrote the paper.
Funding
This work was supported by the National Science Foundation [grant number 1021377 to R.C.A.]; D.R.B and S.K.L. are supported by the National Institutes of Health [grants numbers F32-GM084677 and R01-GM055796, respectively]. Deposited in PMC for release after 12 months. GM084677 to D.R.B., R01-GM055796 to S.K.L.].
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.117663/-/DC1
References
- Aguilar R. C., Watson H. A., Wendland B. (2003). The yeast Epsin Ent1 is recruited to membranes through multiple independent interactions. J. Biol. Chem. 278, 10737–10743 10.1074/jbc.M211622200 [DOI] [PubMed] [Google Scholar]
- Aguilar R. C., Longhi S. A., Shaw J. D., Yeh L. Y., Kim S., Schön A., Freire E., Hsu A., McCormick W. K., Watson H. A. et al. (2006). Epsin N-terminal homology domains perform an essential function regulating Cdc42 through binding Cdc42 GTPase-activating proteins. Proc. Natl. Acad. Sci. USA 103, 4116–4121 10.1073/pnas.0510513103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronov S., Gerst J. E. (2004). Involvement of the late secretory pathway in actin regulation and mRNA transport in yeast. J. Biol. Chem. 279, 36962–36971 [DOI] [PubMed] [Google Scholar]
- Baggett J. J., D'Aquino K. E., Wendland B. (2003). The Sla2p talin domain plays a role in endocytosis in Saccharomyces cerevisiae. Genetics 165, 1661–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai J. H., Hu Z. T., Dittman J. S., Pym E. C. G., Kaplan J. M. (2010). Endophilin functions as a membrane-bending molecule and is delivered to endocytic zones by exocytosis. Cell 143, 430–441 10.1016/j.cell.2010.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr F., Lambright D. G. (2010). Rab GEFs and GAPs. Curr. Opin. Cell Biol. 22, 461–470 10.1016/j.ceb.2010.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calero M., Chen C. Z., Zhu W. Y., Winand N., Havas K. A., Gilbert P. M., Burd C. G., Collins R. N. (2003). Dual prenylation is required for Rab protein localization and function. Mol. Biol. Cell 14, 1852–1867 10.1091/mbc.E02-11-0707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviston J. P., Longtine M., Pringle J. R., Bi E. (2003). The role of Cdc42p GTPase-activating proteins in assembly of the septin ring in yeast. Mol. Biol. Cell 14, 4051–4066 10.1091/mbc.E03-04-0247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chant J. (1999). Cell polarity in yeast. Annu. Rev. Cell Dev. Biol. 15, 365–391 10.1146/annurev.cellbio.15.1.365 [DOI] [PubMed] [Google Scholar]
- Chapa-y-Lazo B., Lee S., Regan H., Sudbery P., Chapa-y-Lazo, Lee, Regan, Sudbery (2011). The mating projections of Saccharomyces cerevisiae and Candida albicans show key characteristics of hyphal growth. Fungal Biol. 115, 547–556 10.1016/j.funbio.2011.02.001 [DOI] [PubMed] [Google Scholar]
- Chen W., Wandinger-Ness A. (2001). Expression and functional analyses of Rab8 and Rab11a in exocytic transport from trans-Golgi network. Methods Enzymol. 329, 165–175 [DOI] [PubMed] [Google Scholar]
- Chen F., Ma L., Parrini M. C., Mao X., Lopez M., Wu C., Marks P. W., Davidson L., Kwiatkowski D. J., Kirchhausen T. et al. (2000). Cdc42 is required for PIP(2)-induced actin polymerization and early development but not for cell viability. Curr. Biol. 10, 758–765 10.1016/S0960-9822(00)00571-6 [DOI] [PubMed] [Google Scholar]
- Chen S. H., Chen S., Tokarev A. A., Liu F. L., Jedd G., Segev N. (2005). Ypt31/32 GTPases and their novel F-box effector protein Rcy1 regulate protein recycling. Mol. Biol. Cell 16, 178–192 10.1091/mbc.E04-03-0258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins C. S., Kalish J. E., Morrel J. C., McCaffery J. M., Gould S. J. (2010). The peroxisome biogenesis factors pex4p, pex22p, pex1p, and pex6p act in the terminal steps of peroxisomal matrix protein import Mol. Cell Biol. 20, 7516–7526 10.1128/MCB.20.20.7516-7526.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crampin H., Finley K., Gerami-Nejad M., Court H., Gale C., Berman J., Sudbery P. (2005). Candida albicans hyphae have a Spitzenkörper that is distinct from the polarisome found in yeast and pseudohyphae. J. Cell Sci. 118, 2935–2947 10.1242/jcs.02414 [DOI] [PubMed] [Google Scholar]
- Davies B. A., Azmi I. F., Payne J., Shestakova A., Horazdovsky B. F., Babst M., Katzmann D. J. (2010). Coordination of Substrate Binding and ATP Hydrolysis in Vps4-Mediated ESCRT-III Disassembly. Mol. Biol. Cell 21, 3396–3408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty G. J., McMahon H. T. (2009). Mechanisms of endocytosis. Annu. Rev. Biochem. 78, 857–902 10.1146/annurev.biochem.78.081307.110540 [DOI] [PubMed] [Google Scholar]
- Engqvist-Goldstein A. E. Y., Kessels M. M., Chopra V. S., Hayden M. R., Drubin D. G. (1999). An actin-binding protein of the Sla2/Huntingtin interacting protein 1 family is a novel component of clathrin-coated pits and vesicles. J. Cell Biol. 147, 1503–1518 10.1083/jcb.147.7.1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne-Manneville S. (2004). Cdc42 – the centre of polarity. J. Cell Sci. 117, 1291–1300 10.1242/jcs.01115 [DOI] [PubMed] [Google Scholar]
- Ferreira T., Mason A. B., Slayman C. W. (2001). The yeast Pma1 proton pump: a model for understanding the biogenesis of plasma membrane proteins. J. Biol. Chem. 276, 29613–29616 10.1074/jbc.R100022200 [DOI] [PubMed] [Google Scholar]
- Furuta N., Fujimura-Kamada K., Saito K., Yamamoto T., Tanaka K. (2007). Endocytic recycling in yeast is regulated by putative phospholipid translocases and the Ypt31p/32p-Rcy1p pathway. Mol. Biol. Cell 18, 295–312 10.1091/mbc.E06-05-0461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan J. M., Wiederkehr A., Seol J. H., Haguenauer-Tsapis R., Deshaies R. J., Riezman H., Peter M. (2001). Skp1p and the F-box protein Rcy1p form a non-SCF complex involved in recycling of the SNARE Snc1p in yeast. Mol. Cell. Biol. 21, 3105–3117 10.1128/MCB.21.9.3105-3117.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gancedo J. M. (2001). Control of pseudohyphae formation in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 25, 107–123 10.1111/j.1574-6976.2001.tb00573.x [DOI] [PubMed] [Google Scholar]
- Goranov A. I., Cook M., Ricicova M., Ben-Ari G., Gonzalez C., Hansen C., Tyers M., Amon A. (2009). The rate of cell growth is governed by cell cycle stage. Genes Dev. 23, 1408–1422 10.1101/gad.1777309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosshans B. L., Andreeva A., Gangar A., Niessen S., Yates J. R., 3rd, Brennwald P., Novick P. (2006). The yeast lgl family member Sro7p is an effector of the secretory Rab GTPase Sec4p. J. Cell Biol. 172, 55–66 10.1083/jcb.200510016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Roth D., Walch-Solimena C., Novick P. (1999). The exocyst is an effector for Sec4p, targeting secretory vesicles to sites of exocytosis. EMBO J. 18, 1071–1080 10.1093/emboj/18.4.1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsay E., Bretscher A. (1995). Parallel secretory pathways to the cell surface in yeast. J. Cell Biol. 131, 297–310 10.1083/jcb.131.2.297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart M. J., Eva A., Evans T., Aaronson S. A., Cerione R. A. (1991). Catalysis of guanine nucleotide exchange on the CDC42Hs protein by the dbl oncogene product. Nature 354, 311–314 10.1038/354311a0 [DOI] [PubMed] [Google Scholar]
- He B., Xi F. G., Zhang J., TerBush D., Zhang X. Y., Guo W. (2007). Exo70p mediates the secretion of specific exocytic vesicles at early stages of the cell cycle for polarized cell growth. J. Cell Biol. 176, 771–777 10.1083/jcb.200606134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh W-K., Falvo J. V., Gerke L. C., Carroll A. S., Howson R. W., Weissman J. S., O'Shea E. K. (2003). Global analysis of protein localization in budding yeast. Nature 425, 686–691 [DOI] [PubMed] [Google Scholar]
- Itoh T., Takenawa T. (2002). Phosphoinositide-binding domains: Functional units for temporal and spatial regulation of intracellular signalling. Cell. Signal. 14, 733–743 10.1016/S0898-6568(02)00028-1 [DOI] [PubMed] [Google Scholar]
- Jaffe A. B., Hall A. (2005). Rho GTPases: biochemistry and biology. Annu. Rev. Cell Dev. Biol. 21, 247–269 10.1146/annurev.cellbio.21.020604.150721 [DOI] [PubMed] [Google Scholar]
- Johnson D. I. (1999). Cdc42: An essential Rho-type GTPase controlling eukaryotic cell polarity. Microbiol. Mol. Biol. Rev. 63, 54–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. I., Pringle J. R. (1990). Molecular characterization of CDC42, a Saccharomyces cerevisiae gene involved in the development of cell polarity. J. Cell Biol. 111, 143–152 10.1083/jcb.111.1.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L. A., Sudbery P. E. (2010). Spitzenkorper, exocyst, and polarisome components in Candida albicans hyphae show different patterns of localization and have distinct dynamic properties. Eukaryot. Cell 9, 1455–1465 10.1128/EC.00109-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadota J., Yamamoto T., Yoshiuchi S., Bi E. F., Tanaka K. (2004). Septin ring assembly requires concerted action of polarisome components, a PAK kinase Cla4p, and the actin cytoskeleton in Saccharomyces cerevisiae. Mol. Biol. Cell 15, 5329–5345 10.1091/mbc.E04-03-0254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. B., Haarer B. K., Pringle J. R. (1991). Cellular morphogenesis in the Saccharomyces cerevisiae cell cycle: localization of the CDC3 gene product and the timing of events at the budding site. J. Cell Biol. 112, 535–544 10.1083/jcb.112.4.535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus M., Pelli-Gulli M. P., van Drogen F., Springer S., Jaquenoud M., Peter M. (2007). Phosphorylation of Bem2p and Bem3p may contribute to local activation of Cdc42p at bud emergence. EMBO J. 26, 4501–4513 10.1038/sj.emboj.7601873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhli M., Galati V., Boudier K., Roberson R. W., Philippsen P. (2008). Growth-speed-correlated localization of exocyst and polarisome components in growth zones of Ashbya gossypii hyphal tips. J. Cell Sci. 121, 3878–3889 10.1242/jcs.033852 [DOI] [PubMed] [Google Scholar]
- Lapierre L. A., Goldenring J. R. (2005). Interactions of myosin vb with rab11 family members and cargoes traversing the plasma membrane recycling system. Methods Enzymol. 403, 715–723 [DOI] [PubMed] [Google Scholar]
- Lemmon M. A., Ferguson K. M. (2000). Signal-dependent membrane targeting by pleckstrin homology (PH) domains. Biochem. J. 350, 1–18 10.1042/0264-6021:3500001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis M. J., Nichols B. J., Prescianotto-Baschong C., Riezman H., Pelham H. R. B. (2000). Specific retrieval of the exocytic SNARE Snc1p from early yeast endosomes. Mol. Biol. Cell 11, 23–38 10.1091/mbc.11.1.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine M. S., Theesfeld C. L., McMillan J. N., Weaver E., Pringle J. R., Lew D. J. (2000). Septin-dependent assembly of a cell cycle-regulatory module in Saccharomyces cerevisiae. Mol. Cell. Biol. 20, 4049–4061 10.1128/MCB.20.11.4049-4061.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz M. C., Cutler N. S., Heitman J. (2000). Characterization of alcohol-induced filamentous growth in Saccharomyces cerevisiae. Mol. Biol. Cell 11, 183–199 10.1091/mbc.11.1.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W., Chang A. (2000). An endosome-to-plasma membrane pathway involved in trafficking of a mutant plasma membrane ATPase in yeast. Mol. Biol. Cell 11, 579–592 10.1091/mbc.11.2.579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco E., Wedlich-Soldner R., Li R., Altschuler S. J., Wu L. F. (2007). Endocytosis optimizes the dynamic localization of membrane proteins that regulate cortical polarity. Cell 129, 411–422 10.1016/j.cell.2007.02.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markgraf D. F., Ahnert F., Arlt H., Mari M., Peplowska K., Epp N., Griffith J., Reggiori F., Ungermann C. (2009). The CORVET subunit Vps8 cooperates with the Rab5 homolog Vps21 to induce clustering of late endosomal compartments. Mol. Biol. Cell 20, 5276–5289 10.1091/mbc.E09-06-0521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee D., Coon B. G., Edwards D. F., 3rd, Hanna C. B., Longhi S. A., McCaffery J. M., Wendland B., Retegui L. A., Bi E., Aguilar R. C. (2009). The yeast endocytic protein Epsin 2 functions in a cell-division signaling pathway. J. Cell Sci. 122, 2453–2463 10.1242/jcs.041137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland J., Wesp A., Riezman H., Botstein D. (1997). Yeast actin cytoskeleton mutants accumulate a new class of Golgi-derived secretary vesicle. Mol. Biol. Cell 8, 1481–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P., Medkova M., Dong G., Hutagalung A., Reinisch K., Grosshans B. (2006). Interactions between Rabs, tethers, SNAREs and their regulators in exocytosis. Biochem. Soc. Trans. 34, 683–686 10.1042/BST0340683 [DOI] [PubMed] [Google Scholar]
- Orlando K., Guo W. (2009). Membrane organization and dynamics in cell polarity. Cold Spring Harb. Perspect. Biol. 1, a001321 10.1101/cshperspect.a001321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlando K., Sun X. L., Zhang J. A., Lu T., Yokomizo L., Wang P. Y., Guo W. (2011). Exo-endocytic trafficking and the septin-based diffusion barrier are required for the maintenance of Cdc42p polarization during budding yeast asymmetric growth. Mol. Biol. Cell 22, 624–633 10.1091/mbc.E10-06-0484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmani N., Peglion F., Chavrier P., Etienne-Manneville S. (2010). Cdc42 localization and cell polarity depend on membrane traffic. J. Cell Biol. 191, 1261–1269 10.1083/jcb.201003091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H. O., Bi E. F. (2007). Central roles of small GTPases in the development of cell polarity in yeast and beyond. Microbiol. Mol. Biol. Rev. 71, 48–96 10.1128/MMBR.00028-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulter N. S., Staiger C. J., Rappoport J. Z., Franklin-Tong V. E. (2010). Actin-binding proteins implicated in the formation of the punctate actin foci stimulated by the self-incompatibility response in Papaver. Plant Physiol. 152, 1274–1283 10.1104/pp.109.152066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prekeris R. (2003). Rabs, Rips, FIPs, and endocytic membrane traffic. ScientificWorldJournal 3, 870–880 10.1100/tsw.2003.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruyne D., Bretscher A. (2000). Polarization of cell growth in yeast. I. Establishment and maintenance of polarity states. J. Cell Sci. 113, 365–375 [DOI] [PubMed] [Google Scholar]
- Robinson M., Poon P. P., Schindler C., Murray L. E., Kama R., Gabriely G., Singer R. A., Spang A., Johnston G. C., Gerst J. E. (2006). The Gcs1 Arf-GAP mediates Snc1,2 v-SNARE retrieval to the Golgi in yeast. Mol. Biol. Cell 17, 1845–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi G., Brennwald P. (2011). Yeast homologues of lethal giant larvae and type V myosin cooperate in the regulation of Rab-dependent vesicle clustering and polarized exocytosis. Mol. Biol. Cell 22, 842–857 10.1091/mbc.E10-07-0570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth D., Guo W., Novick P. (1998). Dominant negative alleles of SEC10 reveal distinct domains involved in secretion and morphogenesis in yeast. Mol. Biol. Cell 9, 1725–1739 10.1091/mbc.9.7.1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakchaisri K., Asano S., Yu L. R., Shulewitz M. J., Park C. J., Park J. E., Cho Y. W., Veenstra T. D., Thorner J., Lee K. S. (2004). Coupling morphogenesis to mitotic entry. Proc. Natl. Acad. Sci. USA 101, 4124–4129 10.1073/pnas.0400641101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A., Novick P. J. (1989). The Sec15 protein responds to the function of the GTP binding protein, Sec4, to control vesicular traffic in yeast. J. Cell Biol. 109, 1023–1036 10.1083/jcb.109.3.1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffzek K., Ahmadian M. R., Wittinghofer A. (1998). GTPase-activating proteins: helping hands to complement an active site. Trends Biochem. Sci. 23, 257–262 10.1016/S0968-0004(98)01224-9 [DOI] [PubMed] [Google Scholar]
- Schellmann S., Pimpl P. (2009). Coats of endosomal protein sorting: retromer and ESCRT. Curr. Opin. Plant Biol. 12, 670–676 10.1016/j.pbi.2009.09.005 [DOI] [PubMed] [Google Scholar]
- Sheffield J. B. (2007). ImageJ, a useful tool for biological image processing and analysis. Microsc. Microanal. 13, 200–201 10.1017/S1431927607076611 [DOI] [Google Scholar]
- Simonsen A., Stenmark H. (2001). PX domains: attracted by phosphoinositides. Nat. Cell Biol. 3, E179–E182 10.1038/35087112 [DOI] [PubMed] [Google Scholar]
- Slaughter B. D., Das A., Schwartz J. W., Rubinstein B., Li R. (2009). Dual modes of cdc42 recycling fine-tune polarized morphogenesis. Dev. Cell 17, 823–835 10.1016/j.devcel.2009.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. R., Givan S. A., Cullen P., Sprague G. F., Jr (2002). GTPase-activating proteins for Cdc42. Eukaryot. Cell 1, 469–480 10.1128/EC.1.3.469-480.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza C. M., Pichler H. (2007). Lipid requirements for endocytosis in yeast. Biochim. Biophys. Acta 1771, 442–454 10.1016/j.bbalip.2006.08.006 [DOI] [PubMed] [Google Scholar]
- Stefan C. J., Padilla S. M., Audhya A., Emr S. D. (2005). The phosphoinositide phosphatase Sjl2 is recruited to cortical actin patches in the control of vesicle formation and fission during endocytosis. Mol. Cel. Biol. 25, 2910–2923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg G. (2007). Hyphal growth: a tale of motors, lipids, and the Spitzenkörper. Eukaryot. Cell 6, 351–360 10.1128/EC.00381-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudbery P. E. (2011). Growth of Candida albicans hyphae. Nat. Rev. Microbiol. 9, 737–748 10.1038/nrmicro2636 [DOI] [PubMed] [Google Scholar]
- Sun Y. D., Carroll S., Kaksonen M., Toshima J. Y., Drubin D. G. (2007). PtdIns(4,5)P2 turnover is required for multiple stages during clathrin- and actin-dependent endocytic internalization. J. Cell Biol. 177, 355–367 10.1083/jcb.200611011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taheri-Talesh N., Horio T., Araujo-Bazán L., Dou X. W., Espeso E. A., Peñalva M. A., Osmani S. A., Oakley B. R. (2008). The tip growth apparatus of Aspergillus nidulans. Mol. Biol. Cell 19, 1439–1449 10.1091/mbc.E07-05-0464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S., Pryciak P. M. (2007). Identification of novel membrane-binding domains in multiple yeast Cdc42 effectors. Mol. Biol. Cell 18, 4945–4956 10.1091/mbc.E07-07-0676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenawa T., Itoh T. (2001). Phosphoinositides, key molecules for regulation of actin cytoskeletal organization and membrane traffic from the plasma membrane. Biochim. Biophys. Acta 1533, 190–206 10.1016/S1388-1981(01)00165-2 [DOI] [PubMed] [Google Scholar]
- Vajjhala P. R., Catchpoole E., Nguyen C. H., Kistler C., Munn A. L. (2007). Vps4 regulates a subset of protein interactions at the multivesicular endosome. FEBS J. 274, 1894–1907 10.1111/j.1742-4658.2007.05736.x [DOI] [PubMed] [Google Scholar]
- Virag A., Harris S. D. (2006). The Spitzenkörper: a molecular perspective. Mycol. Res. 110, 4–13 10.1016/j.mycres.2005.09.005 [DOI] [PubMed] [Google Scholar]
- Walworth N. C., Brennwald P., Kabcenell A. K., Garrett M., Novick P. (1992). Hydrolysis of GTP by Sec4 protein plays an important role in vesicular transport and is stimulated by a GTPase-activating protein in Saccharomyces cerevisiae. Mol. Cell. Biol. 12, 2017–2028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedlich-Söldner R., Bölker M., Kahmann R., Steinberg G. (2000). A putative endosomal t-SNARE links exo- and endocytosis in the phytopathogenic fungus Ustilago maydis. EMBO J. 19, 1974–1986 10.1093/emboj/19.9.1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesp A., Hicke L., Palecek J., Lombardi R., Aust T., Munn A. L., Riezman H. (1997). End4p/Sla2p interacts with actin-associated proteins for endocytosis in Saccharomyces cerevisiae. Mol. Biol. Cell 8, 2291–2306 10.1091/mbc.8.11.2291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederkehr A., Avaro S., Prescianotto-Baschong C., Haguenauer-Tsapis R., Riezman H. (2000). The F-box protein Rcy1p is involved in endocytic membrane traffic and recycling out of an early endosome in Saccharomyces cerevisiae. J. Cell Biol. 149, 397–410 10.1083/jcb.149.2.397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo J. S., Grabowski R., Xing L., Trepte H. H., Schmitt H. D., Gallwitz D. (1999). Functional implications of genetic interactions between genes encoding small GTPases involved in vesicular transport in yeast. Mol. Gen. Genet. 261, 80–91 10.1007/s004380050944 [DOI] [PubMed] [Google Scholar]
- Zahn R., Stevenson B. J., Schröder-Köhne S., Zanolari B., Riezman H., Munn A. L. (2001). End13p/Vps4p is required for efficient transport from early to late endosomes in Saccharomyces cerevisiae. J. Cell Sci. 114, 1935–1947 [DOI] [PubMed] [Google Scholar]
- Zajac A., Sun X. L., Zhang J., Guo W. (2005). Cyclical regulation of the exocyst and cell polarity determinants for polarized cell growth. Mol. Biol. Cell 16, 1500–1512 10.1091/mbc.E04-10-0896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanolari B., Rockenbauch U., Trautwein M., Clay L., Barral Y., Spang A. (2011). Transport to the plasma membrane is regulated differently early and late in the cell cycle in Saccharomyces cerevisiae. J. Cell Sci. 124, 1055–1066 10.1242/jcs.072371 [DOI] [PubMed] [Google Scholar]
- Zhang X. M., Ellis S., Sriratana A., Mitchell C. A., Rowe T. (2004). Sec15 is an effector for the Rab11 GTPase in mammalian cells. J. Biol. Chem. 279, 43027–43034 10.1074/jbc.M402264200 [DOI] [PubMed] [Google Scholar]
- Zhang X. Y., Wang P. Y., Gangar A., Zhang J., Brennwald P., TerBush D., Guo W. (2005). Lethal giant larvae proteins interact with the exocyst complex and are involved in polarized exocytosis. J. Cell Biol. 170, 273–283 10.1083/jcb.200502055 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



