Abstract
A highly enantioselective carboalkoxylation of alkynes catalyzed by cationic (DTBM-MeO-Biphep) gold(I) complexes is reported. Various optically active beta-alkoxy indanone derivatives were obtained in good yields and high enantioselectivities. Furthermore, this methodology was extended to enantioselective syntheses 3-methoxycyclopentenones. The reaction is proposed to proceed through an enantioselective cyclization of intermediates containing vinylgold(I) and prochiral oxocarbenium moieties.
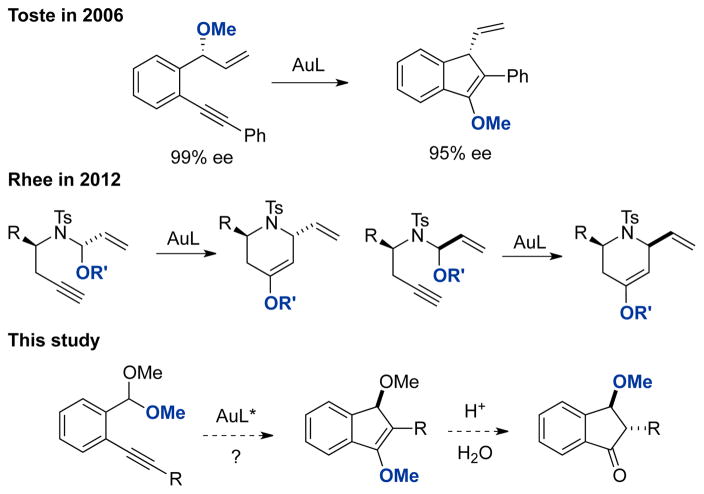
Gold(I)-catalyzed carboalkoxylation of alkynes affords a direct and atom-economical synthetic approach to diversified cyclic enol ethers bearing stereogenic centers at the β position.1,2 We2a and Rhee2d reported that carboalkoxylation occurs with efficient chirality transfer from enantioenriched benzylic ethers (Scheme 1a) and N,O-acetals (Scheme 1b), respectively. Despite the intensive development of homogeneous gold(I) catalyzed enantioselective reactions, enantioselective carboalkoxylation of alkynes posed an unsolved challenge.3 This could be attributed in part to the low reactivity of the ether C-O bond, which necessitates the use of highly electrophilic catalytic systems and precludes many others developed for enantioselective gold catalysis. In addition, our group’s previous work pertaining to chirality transfer suggested that initial desymmetrization of sterically modest ether linkages might be required.2a,4
Scheme 1.
Ether nucleophiles in gold(I)-catalyzed carboalkoxylation
Acetals are widely used protecting groups for aldehydes due to their chemical inertness under many reaction conditions. Nevertheless, the use of transition metal catalysts affords the opportunity to use them as reactive functionalities.5 In 2004, Yamamoto reported the palladium and platinum catalyzed carboalkoxylation of alkyne using acetals.1b,1c Inspired by their work, we reasoned that acetals might be better nucleophiles than benzylic ethers for gold-catalyzed carboalkoxylation, due to the stronger resonance stabilization of oxocarbenium ions. We also posited that an additional benefit of the increased electronic stabilization could be a reduction of chirality transfer efficiency, which would provide additional opportunities for enantioinduction. Hydrolysis of the initially formed enol ether product would provide enantioselective access to 3-alkoxyindanones and cyclopentenones (Scheme 1c). The prevalence of these structural motifs in natural products and bioactive molecules makes them valuable targets for enantioselective synthesis.6 However, few methods have been reported for their preparation, and no catalytic enantioselective approaches are available.7 Herein, we report the highly enantioselective gold-catalyzed carboalkoxylation of alkynylacetals as a concise and convenient means of accessing diverse enantioenriched 3-alkoxyindanone and cyclopentenones. Furthermore, we present evidence for trapping of the vinylgold intermediate as the enantiodetermining step, in contrast to our previous work with benzylic ethers.
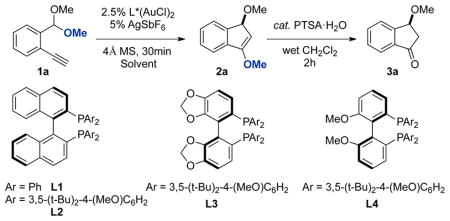
We began our investigation by examining the carboalkoxylation of alkyne 1a with cationic gold(I) catalysts bearing different chiral bisphosphine ligands (Table 1).8 Although these catalyst systems all gave good yields, only DTBM-MeO-Biphep(AuCl)2/AgSbF6 induced any significant enantioselectivity. (entry 4). To our delight, simply changing the solvent from CH2Cl2 to CCl4, the ee improved dramatically to 94% without any loss in yield (entry 5). Other nonpolar solvents were screened. Among them, toluene gave the best result and the desired indanone 3 was obtained in 88% yield and 94% ee (entry 7). Finally, decreasing the loading of AgSbF6 to 2.5 mol % further increased both yield and enantioselectivity to 92% isolated yield and 95% ee. (entry 8).
Table 1.
Optimization of the Reaction Conditions
| ||||
|---|---|---|---|---|
| entrya | L | Solvent | yield (%)b | ee (%)c |
| 1 | L1 | CH2Cl2 | 75 | 8 |
| 2 | L2 | CH2Cl2 | 77 | 2 |
| 3 | L3 | CH2Cl2 | 71 | 5 |
| 4 | L4 | CH2Cl2 | 74 | 24 |
| 5 | L4 | CCl4 | 74 | 94 |
| 6 | L4 | Benzene | 80 | 89 |
| 7 | L4 | Toluene | 88 | 94 |
| 8d | L4 | Toluene | 92 | 95 |
Reaction conditions: 1) 2.5 mol % gold catalyst, 5 mol % AgSbF2, 0.05 mmol 1a, 10 mg 4Å molecular sieve, 1 mL solvent. 2) 2.5 mol % PTSA • H2O, 1 mL CH2Cl2, 0.1 mL H2O.
Isolated yields.
Determined by chiral HPLC. Absolute stereochemistry assigned by analogy to 3k, see the Supporting Information.
2.5 mol % AgSbF6 was used.
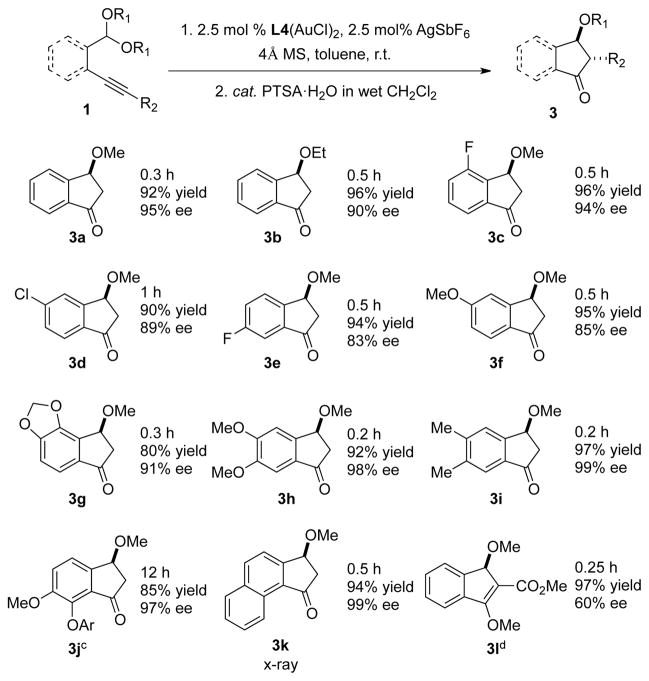
With these optimized conditions in hand, we next investigated the scope of the gold(I)-catalyzed enantioselective carboalkoxylation reaction of alkynes (Table 2). Changing from a dimethyl acetal to a diethyl acetal produced the corresponding indanone in slightly lower enantioselectivity (3a vs. 3b). The impact of substituents on the aromatic ring was also investigated. Substrates with electron-withdrawing group (Cl, F), electron-donating group (MeO) and two substituents on the aryl ring all were well-tolerated (3c–3i). While the gold-catalyzed reaction to form 7-substituted indanone 3j required prolonged reaction time, excellent enantioselectivity was still obtained. On the other hand, the reaction of a naphthalene-derived substrate proceeded smoothly under the standard reaction conditions (3k).9 We next explored reactivities of internal alkynes. Phenyl (R2 = Ph) and alkyl (R2 = iPr) substituted of the alkynes were unreactive. Electronic-withdrawing ester substrate (R2 = CO2Me) afforded nearly quantitative yield of enol-ether product (3l) but the enantioselectivity was moderate (60% ee).10
Table 2.
|
Reaction conditions: 1) 2.5 mol % (R)-DTBM-MeO-Biphep(AuCl)2, 2.5 mol % AgSbF6, 0.2 mmol 1, 50 mg 4Å molecular sieve, 2 mL toluene. 2) 2.5 mol % PTSA • H2O, 4 mL CH2Cl2, 0.4 mL H2O.
Isolated yields. ee was determined by chiral HPLC. Absolute stereochemistry assigned by analogy to 3k, see the Supporting Information.
Ar = 4-bromobenzenesulfonyl, 5.0 mol % gold catalyst and 10 mol % AgSbF6 was used.
Enol ether hydrolysis was not performed.
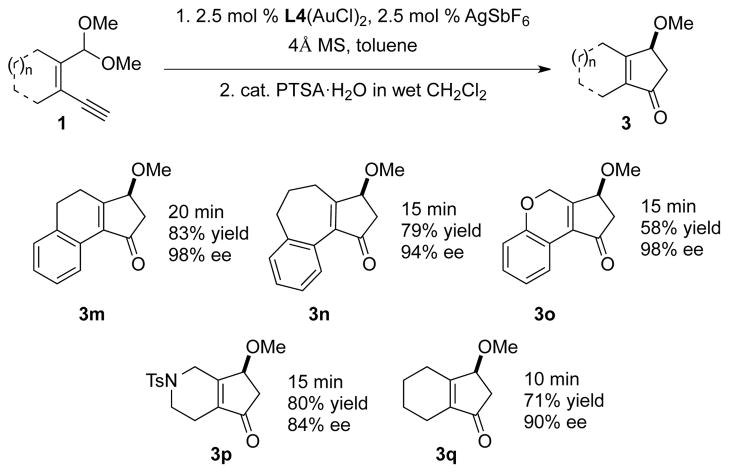
We next explored the possibility of generating 4-methoxylcyclopentenones from the gold-catalyzed simple vinyl acetylenes (Table 3). Gratifyingly, the reaction displayed high enantioselectivities for different ring size substrates. Although the yields for these substrates were slightly lower compared to phenyl acetylenes, the rapid assemble of various bicyclic cyclopentenones from easily prepared substrates11 greatly expands the synthetic versatility of the gold-catalyzed reaction.
Table 3.
|
Reaction conditions see table 2.
Isolated yields. ee was determined by chiral HPLC.
We envisioned two possible pathways to explain the origin of the enantioinduction. In the first possibility, coordination of chiral cationic gold(I) to the alkyne moiety results in a desymmetrizing alkoxylation of the triple bond.12 In analogy to the previously studied chirality transfer of benzyl ethers,2a the resulting intermediate A bearing a chiral acetal-derived cation would undergo rearrangement through chirality-preserving intermediate B-chiral to provide C and subsequently 2a. In this case the first step would be the enantiodetermining step (EDS). On the other hand, it is possible that oxocarbenium intermediate B is sufficiently long lived to relax to an achiral conformation, B′-achiral, that would undergo enantioselective cyclization to afford C. In this second possibility, the nucleophilic addition of a vinylgold species to the oxocarbenium intermediate would be enantiodetermining.5,13–15
In order to distinguish between these two plausible mechanisms, the gold-catalyzed reaction of mixed acetal 4a (d.r. = 1.3:1) was examined (Scheme 3). We anticipated that the sterically-hindered oxygen atom on the methyl lactate moiety would be unable to participate in nucleophilic attack of the alkyne. If the first mechanism were operative, a kinetic resolution would be expected to occur, to return diastereomerically enriched 4a and the product 5a or 5b. As showed in scheme 3, the gold-catalyzed reaction of mixed acetal 4a selectively gave the cyclized products 5a/5b and 3a and 4a were not detected in the crude reaction mixtures; therefore, if the chirality transfer pathway were operative, the products (5a/5b) would be expected to form in approximately the dr of the starting material. However, epimers 5a and 5b were formed with similar diastereoselectivity using (S) and (R) catalysts, respectively. The observation that the catalyst controls the diastereoselectivity in this process, irrespective of the the dr of the starting acetal, is more consistent with the second mode of enantioinduction presented.16
Scheme 3.
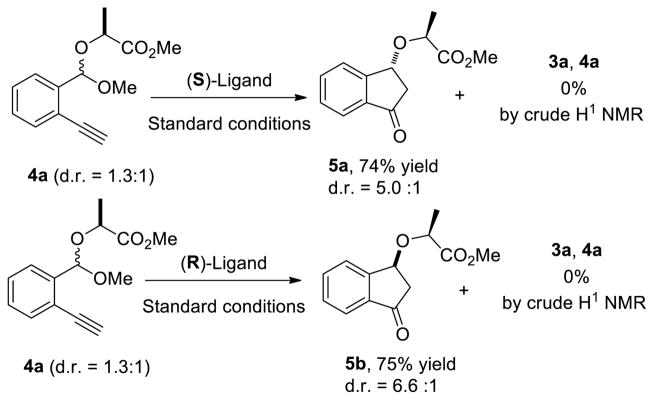
Mixed Acetal Study.
In conclusion, we have developed the first gold-catalyzed enantioselective carboalkoxylation of alkynes, including phenyl acetylenes and vinyl acetylenes, which allowed a variety of highly enantioenriched 3-methoxyindanones and cyclopentenones to be prepared.17 Mechanistic studies suggest that a vinylgold species and a prochiral oxocarbenium are involved the enantioselectivity determining cyclization. Despite the prevalence of vinylgold intermediates in gold-catalyzed reactions of allenes and alkynes,18 this reaction constitutes a rare example of enantioselective carbon-carbon bond formation from this organometallic species.
Supplementary Material
Scheme 2.
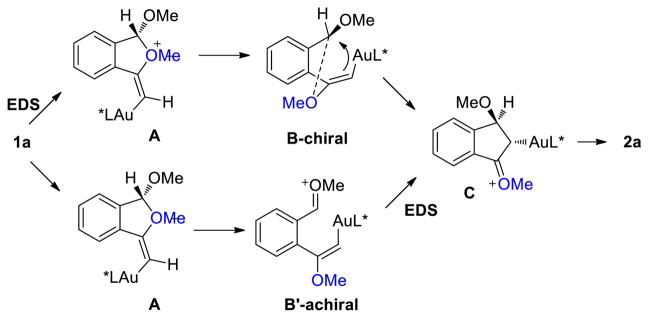
Two Possible Pathways
Acknowledgments
We gratefully acknowledge NIHGMS (RO1 GM073932) for financial support. W. Z. is grateful to Shanghai Institute of Organic Chemistry (SIOC) and Zhejiang Medicine for a joint postdoctoral fellowship. We thank Yi-Ming Wang for helpful discussions and proofreading this manuscript.
Footnotes
The authors declare no competing financial interest.
Experimental procedures and compound characterization data. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.For platinum and palladium-catalyzed carboalkoxylation of alkynes: Nakamura I, Bajracharya GB, Mizushima Y, Yamamoto Y. Angew Chem Int Ed. 2002;41:4328. doi: 10.1002/1521-3773(20021115)41:22<4328::AID-ANIE4328>3.0.CO;2-G.Nakamura I, Bajracharya GB, Wu H, Oishi K, Mizushima Y, Gridnev ID, Yamamoto Y. J Am Chem Soc. 2004;126:15423. doi: 10.1021/ja044603c.Nakamura I, Mizushima Y, Yamamoto Y. J Am Chem Soc. 2005;127:15022. doi: 10.1021/ja055202f.Fürstner A, Davies PW. J Am Chem Soc. 2005;127:15024. doi: 10.1021/ja055659p.Ting CM, Wang CD, Chaudhuri R, Liu RS. Org Lett. 2011;13:1702. doi: 10.1021/ol2002144.
- 2.For gold-catalyzed carboalkoxylation of alkynes: Dube P, Toste FD. J Am Chem Soc. 2006;128:12062. doi: 10.1021/ja064209+.Bae HJ, Baskar B, An SE, Cheong JY, Thangadurai DT, Hwang IC, Rhee YH. Angew Chem Int Ed. 2008;47:2263. doi: 10.1002/anie.200705117.Kim C, Bae HJ, Lee JH, Jeong W, Kim H, Sampath V, Rhee YH. J Am Chem Soc. 2009;131:14660. doi: 10.1021/ja906744r.Kim H, Rhee YH. J Am Chem Soc. 2012;134:4011. doi: 10.1021/ja2116298.Schultz DM, Babij NR, Wolfe JP. Adv, Synth, Catal. 2012;354:3451.Zhang M, Wang Y, Yang Y, Hu X. Adv Synth Catal. 2012;354:981. doi: 10.1002/adsc.201100831.
- 3.For selected recent reviews of gold catalysis, see: Corma A, Leyva-Pérez A, Sabater MJ. Chem Rev. 2011;111:1657. doi: 10.1021/cr100414u.Bandini M. Chem Soc Rev. 2011;40:1358. doi: 10.1039/c0cs00041h.Hashmi ASK, Bührle M. Aldrichimica Acta. 2010;43:27.Nevado C. Chimia. 2010;64:247. doi: 10.2533/chimia.2010.247.Shapiro ND, Toste FD. Synlett. 2010;5:675. doi: 10.1055/s-0029-1219369.Fürstner A. Chem Soc Rev. 2009;38:3208. doi: 10.1039/b816696j.For reviews of enantioselective gold catalysis, see: Sengupta S, Shi X. ChemCatChem. 2010;2:609.Pradal A, Toullec PY, Michelet V. Synthesis. 2011;10:1501.Watson IDG, Toste FD. Chem Sci. 2012;3:2899.
- 4.Faza ON, López CS, de Lera AR. J Org Chem. 2011;76:3791. doi: 10.1021/jo200090j.For review on chirality transfer of gold catalysis, see: Patil NT. Chem Asian J. 2012;7:2186. doi: 10.1002/asia.201200194.
- 5.For transition-metal-catalyzed enantioselective transformations of acetals: Umebayashi N, Hamashima Y, Hashizume D, Sodeoka M. Angew Chem Int Ed. 2008;47:4196. doi: 10.1002/anie.200705344.Moquist PN, Kodama T, Schaus SE. Angew Chem Int Ed. 2010;49:7096. doi: 10.1002/anie.201003469.Maity P, Srinivas HD, Watson MP. J Am Chem Soc. 2011;133:17142. doi: 10.1021/ja207585p.
- 6.(a) Benkrief R, Skaltsounis A-L, Tillequin F, Koch M, Pusset J Planta Med. 1991;57:79. doi: 10.1055/s-2006-960025. [DOI] [PubMed] [Google Scholar]; (b) Uddin SJ, Jason TLH, Beattie KD, Grice D, Tiralongo E. J Nat Prod. 2011;74:2010. doi: 10.1021/np2004598. [DOI] [PubMed] [Google Scholar]; (c) Kim SH, Kwon SH, Park SH, Lee JK, Bang HS, Nam SJ, Kwon HC, Shin J, Oh DC. Org Lett. 2013;15:1834. doi: 10.1021/ol4004417. [DOI] [PubMed] [Google Scholar]
- 7.For catalytic asymmetric synthesis of substituted indanes: Arp FO, Fu GC. J Am Chem Soc. 2005;127:10482. doi: 10.1021/ja053751f.Troutman MV, Appella DH, Buchwald SL. J Am Chem Soc. 1999;121:4916.Fillion E, Wilsily A. J Am Chem Soc. 2006;128:2774. doi: 10.1021/ja056692e.
- 8.For additional conditions examined see Supporting Information.
- 9.The structure and absolute configuration of 3k were determined by single-crystal X-ray diffraction (see the Supporting Information). The stereochemistry of the remaining products was assigned by analogy.
- 10.In some cases, the obtained enol ether product partly hydrolyzed to ketone during the first step.
- 11.Vinyl acetylene substrates were prepared from commercially available ketones in four steps, see supporting information for details.
- 12.For an example of a gold-catalyzed enantioselective desymmetrization of nucleophiles by alkyne activation see: Huang L, Yang HB, Zhang DH, Zhang Z, Tang XY, Xu Q, Shi M. Angew Chem Int Ed. 2013;52:6767. doi: 10.1002/anie.201302632.For additional examples of gold-catalyzed reactions involving alkyne activation, see: Mourad AK, Leutzow J, Czekelius C. Angew Chem Int Ed. 2012;51:11149. doi: 10.1002/anie.201205416.Brazeau JF, Zhang S, Colomer I, Corkey BK, Toste FD. J Am Chem Soc. 2012;134:2742. doi: 10.1021/ja210388g.Cera G, Chiarucci M, Mazzanti A, Mancinelli M, Bandini M. Org Lett. 2012;14:1350. doi: 10.1021/ol300297t.Sethofer SG, Mayer T, Toste FD. J Am Chem Soc. 2010;132:8276. doi: 10.1021/ja103544p.
- 13.For an example of enantioselective trapping of a enamide-gold species generated from an alleneamide see: Suárez-Pantiga S, Hernández-Diaz C, Rubio E, González JM. Angew Chem Int Ed. 2012;51:11552. doi: 10.1002/anie.201206461.For enantioselective trapping of an allylgold intermediate see: Wang YM, Kuzniewski CN, Rauniyar V, Hoong C, Toste FD. J Am Chem Soc. 2011;133:12972. doi: 10.1021/ja205068j.
- 14.For examples of organocatalytic enantioselective additions to oxocarbenium ions, see: Reisman SE, Doyle AG, Jacobsen EN. J Am Chem Soc. 2008;130:7198. doi: 10.1021/ja801514m.Coric I, List B. Nature. 2012;483:315. doi: 10.1038/nature10932.Sun Z, Winschel GA, Borovika A, Nagorny P. J Am Chem Soc. 2012;134:8074. doi: 10.1021/ja302704m.Rueping M, Volla CMR, Atodiresei I. Org Lett. 2012;14:4642. doi: 10.1021/ol302084q.
- 15.Alternatively, and particularly for the reactions in Table 3, the cyclization event can be viewed as a enantioselective electrocyclization of a gold-substituted pentadienyl cation. For related gold-catalyzed electrocyclizations see: Shi X, Gorin DJ, Toste FD. J Am Chem Soc. 2005;127:5803. doi: 10.1021/ja051689g.Zhang L, Wang S. J Am Chem Soc. 2006;128:1442. doi: 10.1021/ja057327q.Lee HH, Toste FD. Angew Chem Int Ed. 2007;46:912. doi: 10.1002/anie.200604006.Lin CC, Teng TM, Tsai CC, Liao HY, Liu RS. J Am Chem Soc. 2008;130:16417. doi: 10.1021/ja806415t.Bhunia S, Liu RS. J Am Chem Soc. 2008;130:16488. doi: 10.1021/ja807384a.Lemiere G, Gandon V, Cariou K, Hours A, Fukuyama T, Dhimane AL, Fensterbank L, Malacria M. J Am Chem Soc. 2009;131:2993. doi: 10.1021/ja808872u.
-
16.A pathway involving rapid thermodynamic acetal equilibration followed by enantiodetermining cyclization was excluded by the following experiments:
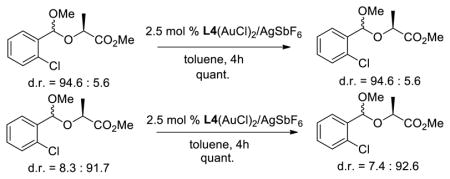
- 17.During the preparation of this manuscript, a gold-catalyzed cycloaddition of 2-ethynylbenzyl ethers with organic oxides and α-diazoesters was reported: Pawar PK, Wang C-D, Bhunia S, Jadhav AM, Liu R-S. Angew Chem, Int Ed. 2013;52:7559. doi: 10.1002/anie.201303016.
- 18.Enantiodetermining trapping of a vinylgold species has been proposed as the enantiodetermining step in the addition of nucleophiles to allenes: Brown TJ, Weber D, Gagné MR, Widenhoefer RA. J Am Chem Soc. 2012;134:9134. doi: 10.1021/ja303844h.Miles DH, Veguillas M, Toste FD. Chem Sci. 2013;4:3427.An enantiodeterming rearrangement of a pentadienyl cation is proposed in: Sethofer SSG, Staben ST, Hung OY, Toste FD. Org Lett. 2008;10:4315. doi: 10.1021/ol801760w.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.