Abstract
Thioredoxins (Trxs), glutaredoxins (Grxs), and peroxiredoxins (Prxs) have been characterized as electron donors, guards of the intracellular redox state, and “antioxidants”. Today, these redox catalysts are increasingly recognized for their specific role in redox signaling. The number of publications published on the functions of these proteins continues to increase exponentially. The field is experiencing an exciting transformation, from looking at a general redox homeostasis and the pathological oxidative stress model to realizing redox changes as a part of localized, rapid, specific, and reversible redox-regulated signaling events. This review summarizes the almost 50 years of research on these proteins, focusing primarily on data from vertebrates and mammals. The role of Trx fold proteins in redox signaling is discussed by looking at reaction mechanisms, reversible oxidative post-translational modifications of proteins, and characterized interaction partners. On the basis of this analysis, the specific regulatory functions are exemplified for the cellular processes of apoptosis, proliferation, and iron metabolism. The importance of Trxs, Grxs, and Prxs for human health is addressed in the second part of this review, that is, their potential impact and functions in different cell types, tissues, and various pathological conditions. Antioxid. Redox Signal. 19, 1539–1605.
I. Introduction
Redox reactions—the transfer of electrons—are an essential requirement for cell metabolism, most notably in the form of biological energy transduction in the inner mitochondrial and plastidal membranes. As a consequence, numerous cellular compounds undergo redox modifications, and some of these redox-modified molecules function in signal transduction. Redox modifications have long been discussed to be the result of increased levels of pro-oxidants, for instance, due to irradiation or decreased levels of antioxidants (14, 714, 715). These conditions, defined as oxidative stress, were often visualized in the form of a scale and an imbalance between pro-oxidants in one pan and antioxidants in the other pan. Up to now, this dis-equilibrium has been correlated with many disorders and pathologies, including cancer, neuro- and cardiovascular diseases (86, 126, 229, 500, 561).
Often, oxidative stress was attributed to the formation of reactive oxygen species (ROS) and/or reactive nitrogen species (RNS). The biological effects of ROS were first demonstrated by Henry John Horstman Fenton in 1894 (182). He demonstrated that hydrogen peroxide (H2O2), previously isolated by Louis Jacques Thénard in 1818 as “eau oxygenée” (772), in combination with ferrous iron, was able to oxidize biomolecules. This “Fenton reaction” leads to the formation of the hydroxyl radical, which was described by Fritz Haber and Richard Willstätter in 1931 only 2 years after Fenton had passed away (249). It took until 1971 for H2O2 production to be measured in respirating mammalian mitochondria from rat liver and pigeon heart (110, 449).
The biological activity of nitric oxide (·NO), the RNS prototype, was recognized early and repeatedly, but its physiological importance remained unnoticed for many decades. In 1867, the British physician Lauder Brunton found that organic nitrates were effective in relieving pain in angina pectoris (79), a disease from which also Alfred Nobel, the inventor of the nitroglycerin-based explosive dynamite and the founder of the Nobel awards, suffered. When in the 1890s Nobel's physicians recommended nitroglycerin as a remedy for his heart disease, he declined it. In a letter to Ragnar Sohlman, his assistant and later executor of his testamentary dispositions, he noted, “Isn't it the irony of fate that I have been prescribed N/G 1 [nitroglycerin], to be taken internally! They call it Trinitrin, so as not to scare the chemist and the public.” (727). In 1979, Louis J. Ignarro and his coworkers demonstrated that·NO and·NO-releasing drugs induce the relaxation of the coronary artery through the activation of guanylate cyclase (245). However, it was not before 1986 that Robert Francis Furchgott demonstrated that the blood vessel dilating “endothelium-derived relaxing factor” which he had proposed in 1978 was, in fact, endogenously produced·NO (217). It took another decade before the reaction of NO with thiol groups was recognized as specific redox modification. As early as 1925, John Scott Haldane and coworkers presented a case in which the death of a man who was employed in a colliery was suspected to be caused by carbon monoxide poisoning. However, the victim's blood did not contain CO-modified hemoglobin but “NO-haemoglobin” (39). Eventually, in 1996, Jonathan S. Stamler demonstrated that ·NO may react not only with the heme moiety, but also specifically with thiols in the form of S-nitrosothiols on the cysteine residue at position 93 of hemoglobin's β-chain, implying new regulatory functions through the release of ·NO during arterial-venous transit (341).
The major intracellular thiol compound glutathione (GSH), γ-L-glutamyl-cysteinyl-glycine, was likely first isolated around 1888. J. de Rey-Pailhade described a nearly ubiquitous substance that he had isolated from yeast, bovine, sheep, fish, egg, and asparagus. It released hydrogen sulfide (H2S), bleached several dyes, and reacted with halogenates. Hence, de Rey-Pailhade suggested the name philothion—“sulfur-loving” (637–639). In 1921, Frederick Gowland Hopkins re-described the compound as an “autooxidizable constituent of the cell.” He originally assumed it to be a dipeptide between glutamate and cysteine and, therefore, named it “glutathione” (294); he also characterized it as an “oxidation-reduction system” (296). In 1927, George Hunter and Blythe Alfred Eagles presented evidence for the conjugation of glutamine and cysteine with additional amino acids (313). Hopkins responded that their preparation was likely impure and insisted on the dipeptide nature of GSH. Nevertheless, he ended his response letter with the words: “In any case, although I have myself no doubts as to the [dipetide] nature of GSH, the appearance of Hunter and Eagle's papers make it desirable that I should if possible give greater precision to the account of its isolation. This I hope to do in the near future” (295). It took Hopkins 2 years and 12 additional preparations of GSH, each from ∼50 kg of yeast, to confirm: “The tripeptide has been shown to constitute a large portion of the preparation […]. The description of the substance as dipeptide was therefore erroneous” (297). More than 40 years after the discovery of GSH, it was the pioneering work of Alton Meister that unraveled the enzymology and regulation of GSH metabolism, for example, (488, 489). Meister's discoveries opened up several new lines of research into the functions of GSH, for instance, its involvement in detoxification reactions (273), its role as electron donor (184, 778), and its part in redox regulation and homeostasis (495).
Over the past decade, our view of redox biochemistry evolved rapidly, realizing and establishing redox changes as physiological, rapid, specific, and reversible cell signaling events and a form that regulated the activity of key proteins (227, 354). Moreover, redox signaling was shown to be localized to distinct regions within a cell or even a compartment at a given time point, affecting distinct redox couples such as GSH/glutathione disulfide (GSSG) or NADH/NAD+ differently (239, 254). This so-called “compartmentalized redox signaling,” therefore, stands in opposition to the view of an overall cellular redox balance, which implies that all cellular redox couples are reduced or oxidized to a similar degree by the same stimuli.
Many key regulators of redox signaling and thus of the intracellular effects of ROS and RNS are members of the thioredoxin (Trx)-fold family of proteins, among them the proteins highlighted in this review: Trxs, glutaredoxins (Grxs), and peroxiredoxins (Prxs) (12, 432, 434, 554, 643). Members of these protein families are ubiquitously expressed in all organisms, tissues, cell types, and organelles. Some of these proteins can even shuttle between cellular compartments and the extracellular space.
Trxs, the first branch and the name-giving proteins of the Trx family of proteins, were discovered by Peter Reichard and coworkers in 1964 in their quest to discover the electron/hydrogen donor for ribonucleotide reductase (RNR) in Escherichia coli (418) (Fig. 1). The characteristic dithiol active site motif, Cys-Gly-Pro-Cys, which facilitates the reduction of the disulfide formed in the catalytic cycle of RNR, was determined by protein sequencing in 1968 (284). This motif is, with rare exceptions, conserved throughout all kingdoms of life. In 1976–77, Bob Buchanan and coworkers established the concept of redox regulation by identifying Trx as activator of metabolic enzymes in phototrophic organisms after light exposure (82, 291). The proteins from the second branch of the Trx family were identified as GSH-linked enzymes functioning in thiol-disulfide exchange reactions by Bengt Mannervik and coworkers around 1974 and, despite the oxidation-reduction nature of this reaction, were named “thioltransferases” (170). In parallel, Arne Holmgren faced the challenge to identify alternative electron donors for RNR, because E. coli mutants lacking Trx were still viable, despite the essential nature of RNR (285). In his studies, published between 1976 and 1979, he characterized this new group of GSH-dependent oxidoreductases as electron donors for RNR and named them Grxs (287, 288, 455). In contrast to Trxs and Grxs, Prxs reduce peroxides rather than protein disulfides. Prxs were not discovered because of their enzymatic activity. It was because of their high abundance and their distinct quaternary structure that the first Prx, isolated from human erythrocytes, became known under the name of “torin” in 1968 (265). It was not until 1993 that the previously identified thiol-specific antioxidant activity (380) could be assigned to the torin-homolog from yeast (103). The name Prx was coined by Sue Goo Rhee and coworkers in 1994 in a “note added in proof” (104).
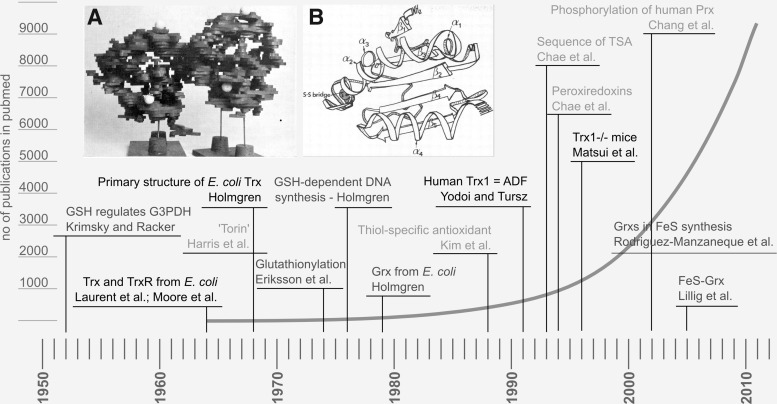
FIG. 1.
A brief history of “redoxin” research. The figure highlights some milestones of Trx, Grx, and Prx research and (in the background) the number of publications listed in pubmed with the query “Trx OR Grx OR thioltransferase OR Prx”. Black: Trx, dark gray: Grx, and light gray: Prx-related findings. Insets: (A) The first structure of Escherichia coli Trx at 4–5 Å resolution, photography of the balsa model (Söderberg et al. 1974) (723). (B) Drawing of the first high-resolution structure of E. coli Trx at 2.8 Å (Holmgren et al. 1975) (293). The work by Krimsky and Racker in 1952 (408) on GSH and glyceralaldehyde-3-phosphate dehydrogenase did not decipher the redox nature of this interaction, but first emphasized the regulatory functions of GSH. GSH, glutathione; Trx, thioredoxin; Grx, glutaredoxin; Prx, peroxiredoxin; ADF, adult T-cell leukemia-derived factor; TSA, thiol-specific antioxidant.
In this review, we summarized the past 50 years of research on Trxs, Grxs, and Prxs, focusing primarily on recent data from vertebrates and mammals (Fig. 1). We discussed redox signaling by looking at reaction mechanisms, oxidative post-translational protein modifications, and interaction partners of the proteins. In the second part of this review, we addressed the importance of Trxs, Grxs, and Prxs for human health, emphasizing the potential impact and functions of redoxins in different cell types, pathways, and pathological conditions.
A. Trx family of proteins
1. Structure and reaction mechanisms
Members of the Trx fold family share a common structural motif, which, in its most basic representation, consists of three α-helices surrounding a central core of a four-stranded β-sheet (Fig. 2A, B) (471). In higher organisms, the motif may contain additional α-helices or β-sheets (Figs. 1A, B and 2C, D). In addition, Grxs display two unique features in their Trx-fold structures: an active site environment that favors the attack of GSH moieties and a hydrophobic surface area for the interaction with protein substrates (85, 831). Trx family proteins are moreover characterized by their active site motifs, containing either one or two cysteinyl residues. These thiol groups are essential for (i) the reduction of protein disulfides, (ii) protein de-/glutathionylation and de-/trans-/nitrosylation, or (iii) the reduction of H2O2. Distinct reaction mechanisms have been described for these processes (Figs. 3 and 4). The reduction of protein disulfides depends on the active site motif Cys-X-X-Cys and is catalyzed by Trxs and Grxs via the so-called dithiol mechanism (Fig. 3). The N-terminal active site thiol has a low pKa value, allowing the initiation of a nucleophilic attack on a target disulfide and the formation of a transient covalently bound mixed disulfide intermediate (Fig. 4A, B, reaction 1). In the second step, the C-terminal active site thiol reduces the mixed disulfide, yielding the reduced substrate and an oxidized thio- or Grx (Fig. 4A, B, reaction 2). The protein disulfide in the active site of Trx is reduced by thioredoxin reductase (TrxR), receiving electrons from NADPH (Figs. 3 and 4A, reactions 3–4) (290), whereas the oxidized Grx is reduced by NADPH via glutathione reductase (GR) and GSH (Figs. 3 and 4B, reactions 3–4) (289). Reversible (de-)glutathionylation is catalyzed by the monothiol mechanism. This mechanism is unique to Grxs and depends only on the N-terminal active site cysteinyl residue (Fig. 4B, reaction 5), which forms a GSH-mixed disulfide intermediate. Thus, the substrate is reduced. The oxidized, Grx-GSH mixed disulfide is reduced by a second molecule of GSH (Fig. 4B, reaction 4) (240, 286).
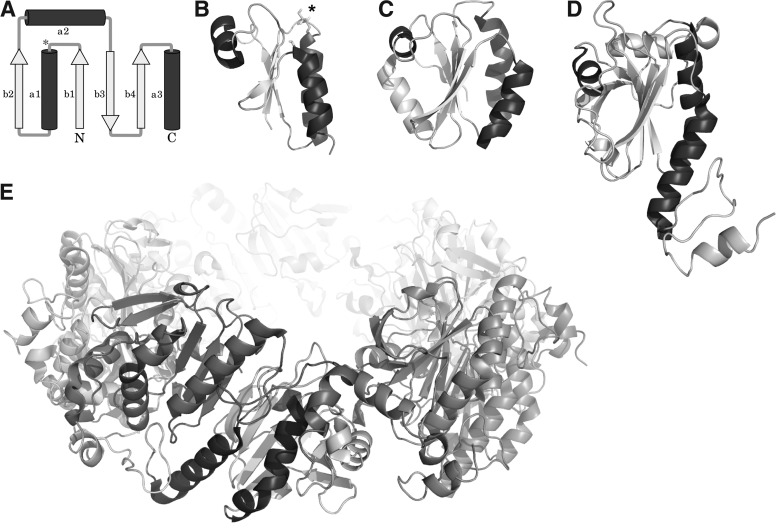
FIG. 2.
The Trx fold. (A) Schematic representation of the Trx fold, the asterisk marks the position of the proximal active site cysteinyl residue, helices are shown in dark, sheets in light gray. Bacterial Grxs, such as (B) E. coli Grx1 (PDB accession number: 1EGR), are the most basic representations of the fold. (C) Human Trx1 (PDB: 3TRX) contains an additional N-terminal sheet and helix. (D, E) The 2-Cys Prx1 is shown as monomer (D) and (E) decameric torin.
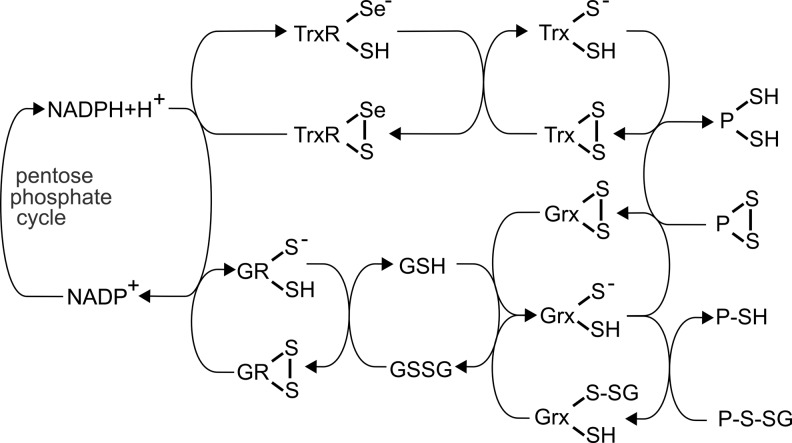
FIG. 3.
Electron flow from NADPH to substrates via the Trx and GSH/Grx systems. NADPH as the main electron source reduces the selenoprotein thioredoxin reductase (TrxR), which delivers electrons to Trx, which then reduces protein (P) disulfides. NADPH also donates electrons to glutathione reductase (GR), which reduces glutathione disulfide (GSSG), thereby generating two molecules of reduced GSH. Electrons can then be delivered to oxidized Grx, which either possesses an active site disulfide bridge due to reduction of protein disulfides or a glutathionylated N-terminal active site Cys from reducing a GSH-mixed disulfide.
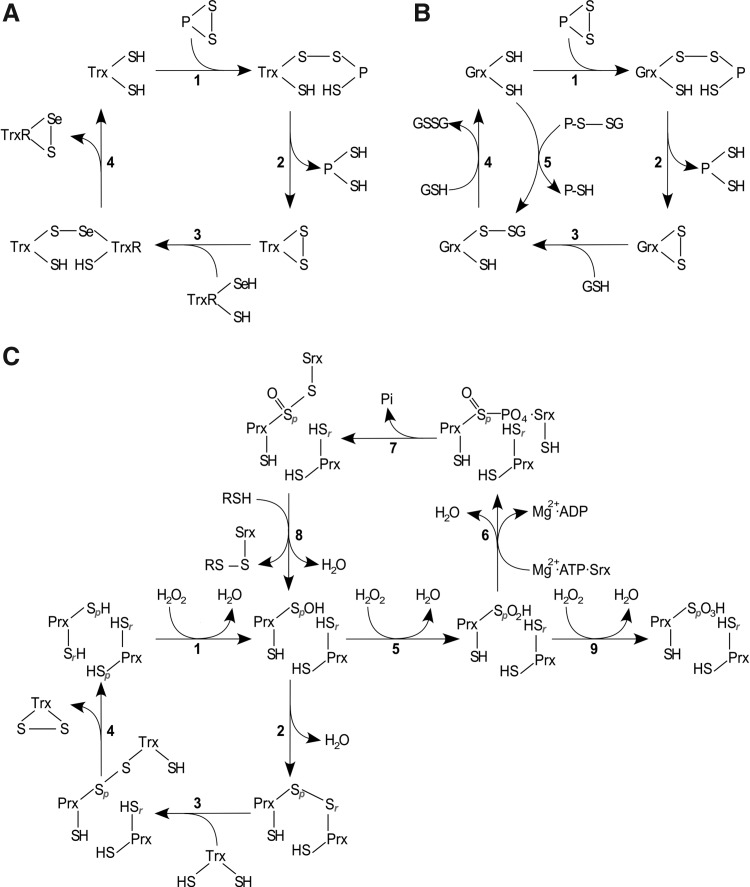
FIG. 4.
Reaction mechanisms of Trx family proteins. (A) Trxs reduce protein disulfides via the dithiol mechanism, depending on both active site cysteines. The N-terminal active site Cys forms a covalently bound mixed disulfide intermediate (A 1), which is reduced by the C-terminal active site Cys, releasing the reduced protein (A 2). Oxidized Trx is reduced by TrxR in a similar reaction sequence (A 3–4). (B) Grxs also reduce protein disulfides via the dithiol mechanism, being reduced by two GSH molecules (B 1–4). In addition, they reduce glutathionylated proteins via the monothiol mechanism (B 5–4), only depending on the N-terminal active site Cys, that attacks the GSH moiety and forms a GSH-mixed disulfide intermediate (B 5), which is reduced by another GSH molecule (B 4). (C) During the reduction of H2O2 by Prxs, the redox-active, peroxidatic Cys (labeled p) is oxidized to sulfenic acid (C 1), which either forms an inter-(2-Cys Prxs) (C 2) or an intramolecular disulfide (atypical 2-Cys Prxs) (not shown) with the resolving Cys residue (labeled r), with both being reduced by Trx as outlined in (A) (C 3–4). 1-Cys Prxs lack an additional resolving cysteine and are reduced by GSH (not shown). In the presence of H2O2, the sulfenic acid can be further oxidized (“over-oxidized”) to sulfinic acid [5] and sulfonic acid [9]. Sulfinic acid-modified Prxs can be recovered by the ATP-dependent action of sulfiredoxin (Srx) [6–8]. For a detailed discussion, see section I.A.1. H2O2, hydrogen peroxide.
Similarly, the reduction of H2O2 by Prxs is a multi-step reaction, reviewed for instance in (641). In the first step, H2O2 is partially reduced to water, leaving a sulfenic acid intermediate at the peroxidatic, N-terminal active site cysteinyl residue (Fig. 4C, reaction 1). In the second step, a resolving cysteinyl residue, outside the classical Trx family active site, forms a disulfide with the N-terminal thiol in a nucleophilic displacement reaction with water as leaving group. In the case of the 2-Cys Prxs (human Prx 1–4), the conserved releasing cysteinyl residue is located in the C-terminus of the proteins. However, these Prxs do not form intramolecular disulfides, but intermolecular disulfides between two adjacent subunits of the homo-dimeric proteins (Fig. 4C, reaction 2). In contrast, atypical 2-Cys Prxs (e.g., human Prx5) form an intramolecular disulfide, as the releasing cysteinyl residue is located in the C-terminus of the same subunit. The disulfides in both types of 2-Cys Prxs are reduced primarily by Trxs, in the dithiol reactions mechanism outlined earlier, see also Figure 4C, reactions 3–4. Members of the 1-Cys Prx family (human Prx6) lack the additional resolving cysteinyl residue and can be reduced by GSH (121). In yeast, this reduction also involves a dithiol Grx (583).
In the access of substrate, Prxs may be over-oxidized by formation of sulfinic and sulfonic acids on the peroxidatic N-terminal active site thiol (Fig. 4C, reactions 5 and 9). In most cases, the formation of sulfonic acids is an irreversible modification under physiological conditions, see section I.C.1. Prxs are, so far, the only class of proteins for which a specific reductase of the sulfinic acid has been described—sulfiredoxin (Srx); for an elaborate discussion on this topic, see Ref. (640). In brief, Srx is an ATP-dependent enzyme that activates the sulfinic acid to a sulfinic phosphoryl ester (355) (Fig. 4C, reaction 6), which subsequently reacts to a thiosulfinate with Srx (Fig. 4C, reaction 7) (356). This intermediate is reduced to a sulfenic acid on the peroxidatic cysteinyl residue of Prx, a reaction that depends on the disulfide formation between Srx and other thiols (Fig. 4C, reaction 8) (64). In addition, Srx has also been reported to specifically catalyze the de-glutathionylation of 2-Cys Prxs (576).
2. Trx, Grx, and Prx family proteins in mammals
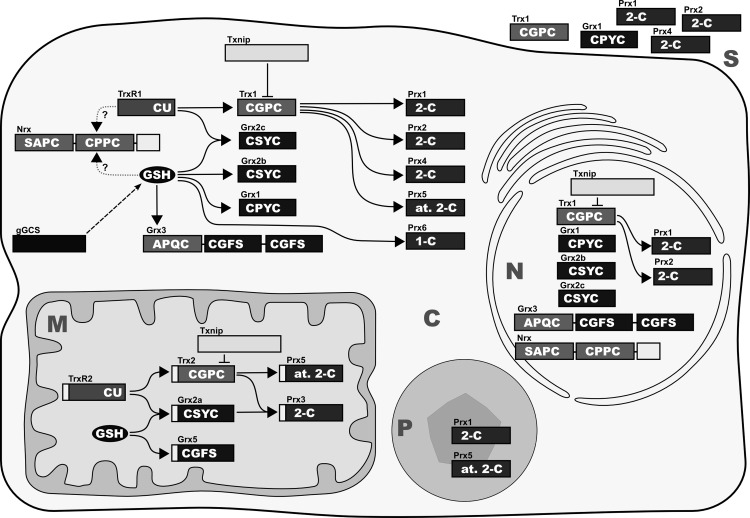
The Trx fold family of proteins comprises numerous proteins. Besides the name-giving Trxs, glutathione peroxidases (GPxs), Grxs, protein disulfide isomerases (PDIs), and Prxs share both the Trx fold and oxidoreductase activity. Moreover, various eukaryotic proteins containing one or more domains are evolutionary related to Trx, Grx, or PDI. All these proteins share similar structural motifs, but most of them have unique functions, which do not necessarily depend on the catalysis of redox reactions. Table 1 lists the more than 50 human proteins that contain Trx, Grx, or Prx domains with putative or confirmed redox activity, including their localization, structural domains, and active site motifs. Figure 5 depicts the compartmentalization of a cell into nucleus, cytosol, mitochondrium, and peroxisome as well as the localization, structural motifs, and the electron transfer between members of the Trx, Grx, and Prx systems.
Table 1.
Members of the Trx Family of Proteins with Putative or Confirmed Redox Activity Encoded in the Human Genome Are Enlisted
| |
Uniprot |
Gene |
Name |
aa |
Compartment |
Trx-domain(s) |
Active site |
|---|---|---|---|---|---|---|---|
| Thioredoxin related | |||||||
| 1 | DJC16 | DNAJC16 | DnaJ homlog subfamily C member 16 | 782 | Membrane | 119–247 | CFSC |
| 2 | NHLC2 | NHLRC2 | NHL repeat-containing protein 2 | 726 | 43–200 | CINC? | |
| 3 | NXN | NXN | Nucleoredoxin | 435 | Cytosol, nucleus | (–), 167–321 | SAPC, CPPC |
| 4 | NXNL1 | NXNL1 | Nucleoredoxin-like protein 1 | 212 | Nucleus, membrane | 1–164 | CPQC |
| 5 | NXNL2 | NXNL2 | Nucleoredoxin-like protein 2 | 156 | 9–147 | CAPS? | |
| 6 | QSOX1 | QSOX1 | Sulfhydryloxidase 1 | 747 | Golgi, membrane, secreted | 36–156 | CGHC |
| 7 | QSOX2 | QSOX2 | Sulfhydryloxidase 2 | 698 | Nucleus, membrane, secreted | 34–178 | CGHC |
| 8 | TMX1 | TMX1 | Thioredoxin-related transmembrane protein 1 | 280 | ER, membrane | 27–132 | CPAC |
| 9 | TMX2 | TMX2 | Thioredoxin-related transmembrane protein 2 | 296 | Membrane | 114–269 | SNDC? |
| 10 | TMX3 | TMX3 | Protein disulfide-isomerase TMX3 | 454 | ER, membrane | 25–128 | CGHC |
| 11 | TMX4 | TMX4 | Thioredoxin-related transmembrane protein 4 | 349 | Membrane | 30–137 | CPSC |
| 12 | THIO | TXN | Thioredoxin 1 | 105 | Cytosol, nucleus, secreted | 1–105 | CGPC |
| 13 | THIOM | TXN2 | Thioredoxin 2, mitochondrial | 166 | Mitochondria | 61–166 | CGPC |
| 14 | TXND2 | TXNDC2 | Thioredoxin domain-containing protein 2 (Sp-Trx1) | 553 | Cytosol | 429–553 | CGPC |
| 15 | TXND3 | TXNDC3 | Thioredoxin domain-containing protein 3 (Sp-Trx2) | 588 | Cytosol | 2–119 | CGPC |
| 16 | TXND5 | TXNDC5 | Thioredoxin domain-containing protein 5 | 432 | ER lumen | 36–169, 170–295, 304–429 | CGHC, CGHC, CGHC |
| 17 | Q3KNW3 | TXNDC6 | TXNDC6 protein | 174 | CGPC | ||
| 18 | B7ZME0 | TXNDC8 | TXNDC8 protein | 95 | CGPC | ||
| 19 | A9Z1W9 | TXNDC8 | Thioredoxin domain-containing 8 (Spermatozoa) (Sp-Trx3) | 108 | CGPC | ||
| 20 | TXND9 | TXNDC9 | Thioredoxin domain-containing protein 9 | 226 | 74–180 | TFRC? | |
| 21 | TXD11 | TXNDC11 | Thioredoxin domain-containing protein 11 | 985 | ER, membrane | 92–214, 649–749 | CELC, CGFC |
| 22 | TXD12 | TXNDC12 | Thioredoxin domain-containing protein 12 | 172 | ER lumen | 27–172 | CGAC |
| 23 | TXD15 | TXNDC15 | Thioredoxin domain-containing protein 15 | 360 | Membrane | 153–296 | CRFS? |
| 24 | TXD16 | TXNDC16 | Thioredoxin domain-containing protein 16 | 825 | Secreted | 392–495 | ? |
| 25 | TXD17 | TXNDC17 | Thioredoxin domain-containing protein 17 | 123 | Cytosol | 41–123 | CPDC |
| 26 | TXNL1 | TXNL1 | Thioredoxin-like protein 1 (Txl1) | 289 | Cytosol | 2–109 | CGPC |
| 27 | TXN4A | TXNL4A | Thioredoxin-like protein 4A | 142 | Nucleus | DPTC? | |
| 28 | TXN4B | TXNL4B | Thioredoxin-like protein 4B | 149 | Nucleus | DPVC? |
| Protein disulfide isomerases | |||||||
|---|---|---|---|---|---|---|---|
| 29 | ERP27 | ERP27 | Endoplasmic reticulum resident protein 27 | 273 | ER | 39–152 | ? |
| 30 | ERP44 | ERP44 | Endoplasmic reticulum resident protein 44 | 406 | ER | 30–138 | CRFS |
| 31 | PDIA1 | P4HB | Protein disulfide-isomerase | 508 | ER, membrane | 18–134, 349–475 | CGHC, CGHC |
| 32 | PDIA2 | PDIA2 | Protein disulfide-isomerase A2 | 525 | ER | 27–152, 367–496 | CGHC, CTHC |
| 33 | PDIA3 | PDIA3 | Protein disulfide-isomerase A3 | 505 | ER | 25–133, 343–485 | CGHC, CGHC |
| 34 | PDIA4 | PDIA4 | Protein disulfide-isomerase A4 | 645 | ER | 21–169, 158–301, 505–636 | CGHC, CGHC, CGHC |
| 35 | PDIA5 | PDIA5 | Protein disulfide-isomerase A5 | 519 | ER | 134–261, 270–384, 378–506 | CSMC, CGHC, CPHC |
| 36 | PDIA6 | PDIA6 | Protein disulfide-isomerase A6 | 440 | ER | 20–133, 154–287 | CGHC, CGHC |
| Glutaredoxin related | |||||||
|---|---|---|---|---|---|---|---|
| 37 | GLRX | GLRX | Glutaredoxin 1 | 106 | Cytosol, nucleus, secreted | 3–106 | CPYC |
| 38 | GLRX2 | GLRX2 | Glutaredoxin 2 | 164 | Mitochondria | 57–157 | CSYC |
| 39 | GLRX3 | GLRX3 | Glutaredoxin 3 | 335 | Cytosol, nucleus | 2–117, 144–236, 237–335 | APQC, CGFS, CGFS |
| 40 | GLRX5 | GLRX5 | Glutaredoxin 5 | 157 | Mitochondria | 42–145 | CGFS |
| 41 | GRCR1 | GRXCR1 | Glutaredoxin domain-containing Cys-rich protein 1 | 290 | 127–234 | CPSC (CSVC, CTAC) | |
| 42 | GRCR2 | GRXCR2 | Glutaredoxin domain-containing Cys-rich protein 2 | 248 | CFHC (CSLC, CPAC) | ||
| 43 | PGES2 | PTGES2 | Putative uncharacterized protein PTGES2 | 377 | Cytosol, golgi, membrane | 90–193 | CPFC |
| 44 | TRXR1 | TXNRD1_v3 | Thioredoxin reductase 1, cytoplasmic (TrxR1_v3) | 649 | Cytosol, nucleus | 56–156 | CTRC |
| 45 | TRXR3 | TXNRD3 | Thioredoxin reductase 3 (TGR) | 754 | Cytosol, nucleus, ER | 167–267 | CPHS |
| 46 | YD286 | Glutaredoxin-like protein YDR286C homolog | 115 | 1–115 | CPLC |
| Peroxiredoxins | |||||||
|---|---|---|---|---|---|---|---|
| 47 | PRDX1 | PRDX1 | Peroxiredoxin-1 | 199 | Cytosol, nucleus, Secreted | 6–165 | CPTE |
| 48 | PRDX2 | PRDX2 | Peroxiredoxin-2 | 198 | Cytosol, nucleus, secreted | 6–164 | CPTE |
| 49 | PRDX3 | PRDX3 | Peroxiredoxin-3 | 256 | Mitochondria | 63–221 | CPTE |
| 50 | PRDX4 | PRDX4 | Peroxiredoxin-4 | 271 | Cytosol, secreted, ER | 79–237 | CPTE |
| 51 | PRDX5 | PRDX5 | Peroxiredoxin-5 | 214 | Cytosol, mitochondria, peroxisomes | 56–214 | CSKT |
| 52 | PRDX6 | PRDX6 | Peroxiredoxin-6 | 224 | Cytosol, vesicles, lysosomes | 5–169 | CTTE |
FIG. 5.
Mammalian Trxs, Grxs, and Prxs. Isoforms, subcellular localization, and confirmed interactions between the various redox proteins discussed in this review. The active site sequences and the classes of proteins, respectively, are indicated in white. C, cytosol; M, mitochondrium; N, nucleus; P, peroxisome; S, secreted. The secretory compartments, that is, endoplasmatic reticulum, Golgi apparatus, and lysosomes, were excluded for reasons of clarity; however, these compartments contain Trx family proteins; see Table 1.
a. Trx systems
In the Trx system, electrons (in conjunction with protons) are transferred from NADPH to the flavo- and selenoprotein TrxR to the oxidoreductase Trx and are ultimately used to reduce disulfides in target proteins (Fig. 3). The 12 kDa Trx contains the active site motif Cys-Gly-Pro-Cys, which is highly conserved throughout different species from bacteria to humans (165). Due to the variety of substrates, the Trx system is required for DNA synthesis via the reduction of RNR (418), proliferation (see section II.B.2), and protection against apoptosis via for example, the reduction of the mitogen-activated protein (MAP) kinase kinase kinase apoptosis signal-regulating kinase 1 (ASK1) and initiated downstream cascades (479, 670) (see also section II.A.1), regulation of transcription by controlling the activity of nuclear factor kappa B (NF-κB) or activating protein 1 (AP-1) (1, 480), modulation of the immune response via for example, cytokine expression (685) (see also section II.B.8.b), and the H2O2 and lipid hydroperoxide levels via Prxs (55, 196, 642).
Trx1 itself is regulated both by hypoxia (54) and by oxidative conditions via binding of nuclear factor E2-related factor 2 (Nrf2) to an antioxidant responsive element in the Trx promotor (384, 761). Knockout of p53 and DJ-1 in mice resulted in either up- or down-regulation of Trx1, and also via increased or decreased levels of Nrf2, respectively (41, 320).
TrxR exists as a 55–60 kDa homo-dimer in a head-to-tail conformation, with every subunit containing a flavin adenine dinucleotide (FAD) domain, an NADPH binding domain, and an interface domain. It possesses two active site motifs; Gly-Cys-Sec-Gly at the C-terminus and Cys-Val-Asn-Val-Gly-Cys at the N-terminus, adjacent to the FAD domain (28). TrxR is known for its broad substrate specificity, which can be explained by the high accessibility and reactivity of selenocysteine. Moreover, different isoforms of TrxR have been described, giving rise to different proteins with distinct functions (657, 785). Besides its main substrate Trx, it was shown to reduce other targets, including PDI (454), Grx2 (349), and dehydroascorbate (482).
Mammalian genomes encode two Trx systems. Trx1 and TrxR1 constitute the cytosolic system (Fig. 5). Trx1 was also shown to translocate into the nucleus on various stimuli (280) or to be secreted (655) (see also section II.B.8.b). Mitochondria contain Trx2 and TrxR2 (Fig. 5). In addition, there is a third testis-specific TrxR3, also named thioredoxin glutathione reductase (TGR), which is mainly expressed in germ cells (see section II.B.10.c). Trx1 and Trx2 share 35% sequence homology and similar catalytic properties in vitro (736) with mitochondrial Trx2 possessing the active site motif of Trx1, but lacking additional structural cysteines. Another protein worth mentioning is the 43–44 kDa Trx interacting protein (Txnip) (Fig. 5), also named thioredoxin-binding protein 2 (TBP2) or Vitamin D up-regulated protein 1 (VDUP1), which does not possess a Trx fold, but belongs to the arrestin superfamily of regulatory proteins. It was found as an interaction partner for Trx in a yeast two-hybrid system (548, 689, 839). Txnip binds to the active site of Trx, inhibiting its disulfide reductase activity, and it was, thus, suggested to be an endogenous Trx inhibitor. Txnip is involved in various cellular processes, such as the regulation of the Trx1/ASK1-dependent apoptosis pathway (115). Knock-down of single components of the Trx systems, that is, Trx1, Trx2, TrxR1, or TrxR2, results in embryonic lethality (135, 270, 474, 759); however, Txnip is not essential (see also section II.B.1).
b. Grx systems
Grxs are, depending on the number of active site Cys residues, divided into dithiol (Cys-X-X-Cys) and monothiol (Cys-X-X-Ser) Grxs, the latter being moreover classified as single- and multi-domain monothiol Grxs (277, 432). Dithiol Grxs act in a system in which electrons are transferred from NADPH, via GR and GSH to Grx (Fig. 3) and subsequently to the oxidized target, conducting similar functions as the Trx system. They act in the regulation of proliferation (see section II.A.2) and differentiation via the MAP kinase ASK1 and downstream targets (82, 529), apoptosis (see section II.A.1) by inhibiting caspase activation (571) and cytochrome c release from mitochondria (167), transcription via modulating the activity of NF-κB (140), and levels of H2O2 via some Prxs (258).
Monothiol Grxs, on the other hand, have so far not been shown to be catalytically active in the Grx-specific HED assay (277). However, recent studies clearly demonstrate that they function primarily in both iron homeostasis and the biosynthesis of FeS proteins (647) (Section II.A.3.a). So far, four Grxs have been discovered in mammals: Grx1, Grx2, Grx3 (also known as protein interacting cousin of Trx—PICOT), and Grx5 (Fig. 5). The dithiol 12 kDa Grx1 is mainly localized in the cytosol, but can be translocated into the nucleus, exported from the cell, and was found in the intermembrane space of mitochondria (187, 453, 456, 565). The dithiol Grx2 is located in mitochondria, but different cancer/testis-specific isoforms, restricted to the cytosol, have been described in mouse and human (310, 447). The 14 kDa Grx2 shares 34% sequence homology with Grx1. It does not possess the active site motif Cys-Pro-Tyr-Cys, but instead Cys-Ser-Tyr-Cys. This single amino acid change is essential for the coordination of a [2Fe2S] cluster (56) (see also section II.A.3.a) and enables the protein to receive electrons from TrxR (349). TrxR is, compared with GSH, a poor electron donor for Grx2 (218); however, when GSSG levels increase, the reaction may become significant (349). The 38 kDa monothiol Grx3 is a multi-domain protein that contains two N-terminal monothiol Grx domains with the active site Cys-Gly-Phe-Ser and an additional C-terminal Trx domain with the active site motif Ala-Pro-Gln-Cys. It is localized in the cytosol and the nucleus. Grx3 was identified as a potential binding partner of protein kinase C-θ in a yeast-two hybrid screening (819) and was furthermore described as an FeS protein, with two monomers coordinating two [2Fe2S] clusters (271). The monothiol Grx5 has a molecular weight of around 17 kDa, has a mitochondrial translocation signal, shares the active site motif of Grx3, and has the ability to bind a [2Fe2S] cluster (350, 647). So far, no disulfide reductase activity was observed for the mitochondrial Grx5. Knock-down of Grxs shows severe phenotypes; however, only knockout of Grx3 in mice is embryonically lethal (105) (see also section II.B.1).
c. Peroxiredoxins
Prxs are 20–30 kDa proteins, which are expressed as different isoforms, that are located in different cellular compartments (283, 822). They are high abundance proteins that can account for up to 1% of soluble cellular proteins (102, 822). In addition to their peroxidase activity, alternative functions have been proposed, for instance, as molecular chaperones and phospholipase A2 (121, 333, 413).
Mammalian cells contain six Prxs (Fig. 5), which are divided into three groups, based on their structure and the catalytic mechanisms described earlier: 2-Cys Prxs (Prx1–4), atypical 2-Cys Prxs (Prx5), and 1-Cys Prx (Prx6) (641, 698). Most Prxs function as homo-dimers, the 2-Cys Prxs also form decamers, and the different conformations are linked to switches in function (42).
Prx1 is mainly localized in the cytosol, the nucleus, and peroxisomes, but it was also found in serum (112, 321). Prx2 is present in the cytosol and the nucleus and was shown to bind to cell membranes (109). Prx3 is exclusively located in mitochondria (98, 805). Prx4 is found in both the cytosol and the endoplasmic reticulum. It contains a leader peptide that is believed to be essential for protein secretion (558). Prx5 is localized in cytosol, mitochondria, and peroxisomes (98, 870). Prx6 is located in the cytosol, vesicles, and lysosomes (734, 735), reviewed in (195). Expression of some Prxs is regulated by hyperoxia (378, 379). Knockout mice for peroxiredoxins (Prx1–4, 6) generally showed increased ROS levels, but were viable; for details, see section II.A.1.
d. Trx-like proteins
Many multi-domain proteins contain at least one Trx fold domain. In fact, at least 723 proteins may contain at least one Trx fold domain, some with additional secondary structure elements that extend the common Trx motif (614). Various proteins share the active site motif Cys-X-X-Cys and were shown to possess oxidoreductase activity. However, there are Trx-like proteins that lack the active site and any oxidoreductase activity. Functions in disulfide bond formation, intracellular signaling, and protection from peroxides have been described (281). Until now, numerous proteins have not been analyzed thoroughly, the nomenclature is not clear, and physiological functions are rare; therefore, the impact of most of these proteins is generally not well understood.
In humans, there are various Trx-like proteins, including nucleoredoxin (Nrx), Thioredoxin-like protein (Txl) 1 and 2, the latter also known as thioredoxin domain-containing protein (TXNDC) 6, sperm-specific thioredoxin (Sp-Trx) 1–3, also known as TXNDC 2, 3, and 8 (Table 1). PDIs (180, 244, 405), GSTs (627, 673), and GPxs (199, 778) are also Trx-fold proteins, but are not a part of this review.
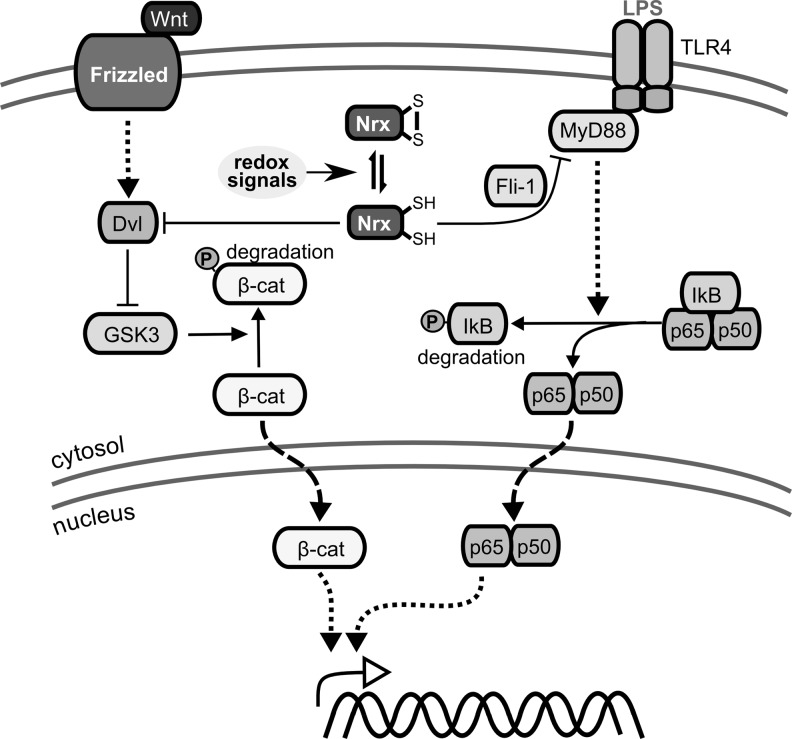
Nrx is characterized by two N-terminal Trx-like domains with the active site motif Cys-Pro-Pro-Cys and a C-terminal PDI-like domain without any redox active Cys residues (215). The 55 kDa protein is located in both the cytosol and the nucleus (Fig. 5), even though no nuclear localization sequence was identified. Nrx was shown to reduce insulin and seems to regulate distinct transcription factors, including NF-κB and AP-1 (279). It suppresses the Wnt/β-catenin pathway, essential for embryonic development, via redox-dependent associated interaction with Dishevelled (213), and regulates Toll-like receptor 4 (TLR-4) signaling (272) (see also section II.B.1). Moreover, Nrx-like protein 1 (Txl6) and 2 have been proposed (Table 1).
The ubiquitously expressed Txl1 is a two-domain protein, composed of a N-terminal Trx-domain and a C-terminal domain with unknown function (497), which was shown to receive electrons from TrxR1 (344). Due to the findings that (i) Txl1 expression is highest in tissues with high metabolic rate including stomach, testis, bone marrow (497), and the central nervous system (CNS) (344) and that (ii) Txl1 over-expression protects cells against glucose-starvation induced cytotoxicity, the protein might function in the cellular response to sugar deprivation (344). TXNDC6 (Txl2) is also ubiquitously expressed and possesses two domains, the N-terminal Trx-domain and a C-terminal domain, that are typical of the nucleoside-diphosphate (NDP) kinase family. The highest expression was detected in testis and lung. Interestingly, the protein was shown to be associated to microtubular structures, potentially regulating microtubuli physiology (664).
TXNDC2/Sp-Trx1 is exclusively located in spermatozoa. It reduces insulin in the presence of NADPH and TrxR (498). Moreover, TXNDC2/Sp-Trx1 can oxidize a specific substrate, in the presence of the electron acceptor selenite. Acting as an oxidase, TXNDC2/Sp-Trx1 might be essential for stabilizing different structures in the developing spermatid-tails via disulfide bond formation (343). TXNDC3/Sp-Trx2 is also a testis-specific protein, consisting of a N-terminal Trx-domain and three consecutive NDP kinase domains. Recombinantly expressed TXNDC3/Sp-Trx2 in E. coli does not show any oxidoreductase activity (663). TXNDC8/Sp-Trx3 comprises a unique Trx domain, which is highly homologous to Trx1. The protein is exclusively found in male germ cells, where it is located in the Golgi apparatus, even though no transit sequence was found. The protein might regulate proteins via post-translational modifications, controlling germ-cell specific functions. However, no reduction of insulin was detected in enzymatic assays (345).
B. The concept of redox signaling
The concept of cell signaling was developed from the ground-breaking analysis of signal transduction of extracellular signals to intracellular effector molecules via G-protein coupled receptors by Martin Rodbell and Alfred Goodman Gilman (232, 646). In the first step, an extracellular signal activates a receptor protein or protein complex. In the second step, this activation promotes the conversion, production or release of second-messenger molecules. These molecules might act on transducer proteins, for example, protein kinases, activate the production or release of third messenger molecules, or directly activate effector molecules. In some cases, the receptor itself might act directly as the effector molecule.
Redox regulation of cellular processes has most commonly been characterized using redox potentials, for instance, by determination of the [GSH] (or more correctly, the [GSH]2) to [GSSG] ratio. ΔE, the difference in redox potentials between products and reactants, is a measure of the change in free energy ΔG, as ΔG equals −n·F·ΔE, with n being the number of electrons and F being the Faraday constant. In a biological system held at constant pressure and temperature, ΔG determines whether a chemical reaction or reaction sequence is thermodynamically favorable and, therefore, the direction of the reaction. However, ΔG does not determine the reaction kinetics, it leaves no clues whether and at what rate the reaction actually takes place. This rate is determined by the number of molecules in the transition state, which is dependent on the activation energy. Even thermodynamically favorable reactions cannot occur if the activation energy is too high. Enzymes accelerate reaction rates by lowering this activation energy. Therefore, ΔE values by themselves are not suitable to describe or model dynamic cellular redox processes, such as transient modifications of transducer proteins in signaling pathways. The activities of the enzymes that catalyze the generation of the signals and the modifications of the effector molecules determine the transduction of the information, as long as the reactions are thermodynamically favorable. By analogy, the action of protein kinases and phosphatases in signal transduction pathways such as the MAP kinase cascades cannot be described or modeled on the basis of the ΔG values of the phosphorylation and de-phosphorylation reactions.
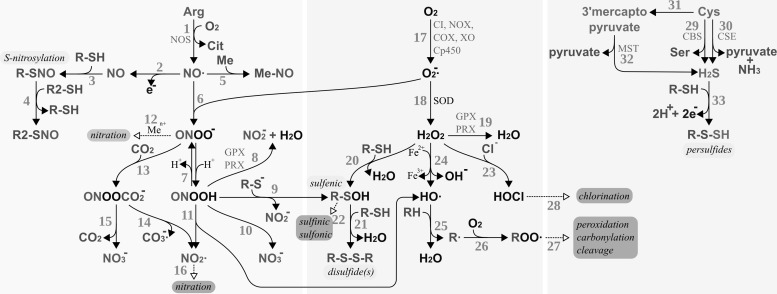
Redox signaling requires the active adjustment of the levels of redox active second messengers in response to the activation of a receptor or sensor molecule. Figure 6 summarizes potential pathways for the production, reaction, and breakdown of such redox active compounds, namely reactive oxygen, nitrogen, and sulfur species; for details, see, for instance, (311, 352, 738, 791). The key compounds, that is, the metabolites which hold the potential to induce reversible post-translational redox modifications on proteins, are H2O2, ·NO, peroxynitrite/peroxynitrous acid (ONOO−/ONOOH), and, possibly, H2S. These compounds are produced enzymatically, either as primary products of specialized enzymes, for instance, ·NO produced by nitric oxide synthase (NOS) or as by-products of enzymes, such as superoxide (O2−·) produced by complex I of the inner mitochondrial membrane and a number of other enzymes (see below). The decay of these compounds is controlled by other, independent enzymes, for instance, H2O2 and ONOOH are reduced by GPxs and Prxs. The levels of these redox-active second messengers are, thus, enzymatically regulated on both the production and the elimination side, similar to, for example, adenylate cyclases and phosphodiesterases whose combined activities determine the level of the second-messenger molecule cAMP.
FIG. 6.
Production and reactivity of reactive nitrogen, oxygen, and sulfur species. RNS (bio-)chemistry, left side: [1] Production of nitric oxide by nitric oxide synthase (NOS). [2–3] S-nitrosylation of protein thiols. [4] Trans-nitrosylation between protein thiols. [5] Reaction of nitric oxide with metals, for example, heme iron. [6] Nitric oxide reacts spontaneously with superoxide yielding peroxynitrite. [7] Reversible protonation of peroxynitrite to peroxynitrous acid. [8] Reduction of peroxynitrous acid by glutathione peroxidases (GPxs) or PRX. [9] Peroxynitrous acid reacts with protein thiolates, yielding protein sulfenic acids. [10] Spontaneous decomposition of peroxynitrous acid yielding nitrite anion. [11] Spontaneous decomposition of peroxynitrous acid to hydroxy radicals and NO2·. [12] Peroxynitrite can (metal catalyzed) lead to the nitration of, for instance, protein tyrosyl residues. [13] Peroxynitrite and carbon dioxide react spontaneously to nitrosoperoxycarbonate. [14] Spontaneous decay of nitrosoperoxycarbonate to carbonate radical anions and nitrite radicals. [15] Spontaneous decay of nitrosoperoxycarbonate to carbon dioxide and nitrate. [16] Nitration may also be initiated by NO2·. ROS (bio-)chemistry, bmiddle: [17] Production of superoxide by, for instance, mitochondrial complex I (CI), NADH oxidase (NOX), cyclooxygenases (COX), xanthine oxidase (XO), or cytochrome P450 enzymes (Cp450). [18] Superoxide is either reduced to H2O2 or oxidized to molecular oxygen (not shown) by superoxide dismutases (SOD). [19] H2O2 can be reduced to water by GPxs or PRX. [20] H2O2 may react directly with specific thiols, yielding sulfenic acids. [21] Sulfenic acids can react with other thiols, yielding disulfides. These disulfides are direct substrates of Trxs and Grxs (not depicted). [22] Sulfenic acids may be further irreversibly oxidized, for example, by H2O2, to sulfinic and sulfonic acids. [23] H2O2 may react with chloride anions, yielding hypochlorous acid. [24] The metal-catalyzed Fenton reaction yields hydroxyl anions and hydroxy radicals. [25] Hydroxy radicals remove hydrogen from volatile organic compounds, yielding water and alkyl radicals. [26–27] Alkyl radicals may react with molecular oxygen and other compounds, eventually resulting in the peroxidation, carbonylation, or cleavage of the organic molecules, for example, proteins. [28] Hypochloric acid may lead to the chlorination of organic compounds. RSS biochemistry, right side: [29–32] Hydrogen sulfide may be the product of cystathionine β-synthase [29, CBS], cystathionine γ-lyase [30, CSE], or via 3-mercaptopyruvate sulfurtransferase [31–32, MST]. [33] Hydrogen sulfide may react with thiols in the presence of an electron and hydrogen acceptor to persulfides. Modifications labeled with a light gray background are reversible and important in redox signaling, and modifications with a dark gray background are irreversible modifications; hence, “oxidative damage.” ROS, reactive oxygen species; RNS, reactive nitrogen species; RSS, reactive sulfur species.
In the next section, we will discuss how the redox second-messenger molecules mentioned earlier may transduce their information to effector proteins in the form of post-translational redox modifications and how the proteins from the Trx family might be involved in these processes.
C. Reversible post-translational redox modifications of protein thiols
Proteins can be regulated post-translationally via reversible redox modifications of susceptible amino acid side chains or cofactors. The thiol groups of cysteinyl side chains constitute the major targets, even though methionyl and selenocysteinyl residues undergo reversible redox modifications as well. Cysteinyl residues are often essential, for instance, in the form of active side residues, or for the tertiary and quaternary structure of proteins. The number of homologous proteins containing at least one cysteine expanded along with evolution, highlighting the importance of their signaling and regulatory functions in increasingly complex organisms (499).
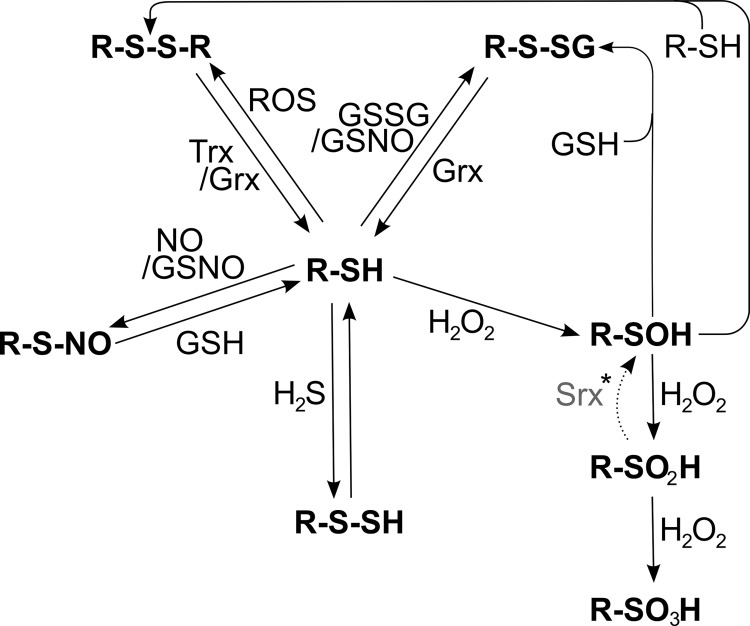
Thiol groups can be oxidized in various ways (Figs. 6 and 7). Two protein thiols can be oxidized to a disulfide, forming a strong inter- or intramolecular bridge. A single protein thiol may also form a disulfide with GSH, termed glutathionylation, or free cysteine, termed cysteinylation or thiolation. Cysteinyl thiols may also react with H2S to form persulfides, ROS or RNS to form sulfenic acids, or nitric oxide resulting in nitroso thiols, a process named S-nitrosylation. Not every surface-exposed, cysteinyl residue can undergo any or all of these oxidative modifications. It was repeatedly demonstrated that distinct thiol groups undergo specific modifications, such as glutathionylation, S-nitrosylation, or sulfenylation, in response to specific oxidants; see, for instance, (200, 202, 233, 257, 699, 765). In proximity to basic amino acids, the pKa of the SH-group is lowered from usually eight to between five and seven. At physiological pH, these thiols with lower pKa will be dissociated. The resulting thiolates are efficient nucleophiles, and their reactivity toward electrophilic targets increases by orders of magnitude. The susceptibility of cysteinyl side chains to undergo S-nitrosylation is determined by the electrostatic and hydrophobic environment of the thiol. Two motifs have been proposed that determine this specificity, the “acid-base motif,” or the “hydrophobic motif”; for details, we refer to (278). It is thus the micro-environment of the cysteinyl side chains that determines their reactivity toward different redox compounds and, therefore, the specificity of redox signaling in general.
FIG. 7.
Redox modifications at cysteinyl residues. Free thiol groups (R-SH) can be reversibly modified by ROS, leading to the formation of protein disulfides (R-S-S-R), which can be reduced by the Trx and Grx systems. Thiols can also be glutathionylated (R-S-SG) by oxidized glutathione (GSSG) or S-nitroso glutathione (GSNO). The de-glutathionylation is exclusively catalyzed by Grxs. GSNO or ·NO, in general, can lead to the nitrosylation of cysteinyl residues, which can be reversed by GSH or transferred to other thiols such as the active site of Trx1 (53) (trans-nitrosylation, not shown). Another modification, induced by peroxides, is the formation of sulfenic acid (R-SOH). In the presence of another free thiol, it can be modified to a protein disulfide. However, in the presence of excessive peroxides, it can be irreversibly over-oxidized to sulfinic (R-SO2H) and sulfonic acid (R-SO3H). *The reduction of sulfinic acids to sulfenic acids, catalyzed by Srxs, is specific for Prxs; in addition, Srxs have been reported to catalyze the de-glutathionylation of Prxs.
1. Sulfenylation
Oxidation of thiol groups to sulfenic acids may occur directly by a reaction of susceptible thiols/thiolates with H2O2, ONOO−, or ONOOH (Fig. 6). Outside peroxisomes, H2O2 may primarily be the product of superoxide dismutases (SOD), metal cofactor-dependent enzymes that are present in the cytosol (SOD1, Cu/Zn-dependent), and the mitochondrial matrix (SOD2, Mn-dependent), catalyzing the alternate reduction and oxidation of O2−· to H2O2 and O2 (212, 484, 859). O2−· is produced both actively and as byproduct by numerous enzymes in the cell, for instance, complex I, complex III, NADH oxidases, cyclooxygenases, xanthine oxidase, or cytochrome p450 enzymes (73, 88, 208, 485, 547, 821). It may also be produced through the reaction of iron sulfur clusters with oxygen (560). ONOO− and ONOOH are the product of the, only diffusion limited, chemical reaction of O2−· and ·NO (325), an RNS that is actively produced by three isoforms of NOS (358, 792).
The reactivity of most cysteinyl side chains toward H2O2 or ONOO−/ONOOH is low; however, if present in the thiolate form, some may react with H2O2 or ONOOH to form the sulfenic acid intermediates. The formation of sulfenic acids on cysteinyl side chains is, via the formation and subsequent reduction of a disulfide formed with another thiol, a reversible reaction. However, in excess of H2O2 or ONOO−/ONOOH, these intermediates may be oxidized further to sulfinic and sulfonic acids (Figs. 6 and 7) (606). With the exception of sulfinic acid formation on Prxs, these reactions are irreversible (see section I.A.2.c). Both H2O2 and ONOOH are substrates for peroxidases, that is, GPxs and Prxs (outside peroxisomes). During catalysis, these enzymes form sulfenic intermediates on their selenolate (GPx1–4 & 6) or thiolate (GPx5 & 7–8, Prx1–6) active site themselves, which are subsequently reduced to H2O along with the formation of a disulfide, that is, GSSG or protein disulfides (199).
The topic of sulfenylation in redox regulation has been comprehensively summarized by others earlier, for example, (377).
2. Protein disulfides
“Thiol redox control” via the reversible formation of intra- and intermolecular disulfide bridges was first conceptualized by Bob Buchanan and co-workers, following their studies on the regulation of photosynthesis (81). In nonphotosynthetic organisms, disulfides (outside the secretory pathway) may be formed by the reaction of a cysteinyl thiol with the sulfenic acid of a second cysteinyl residue (Figs. 6 and 7), or by direct thiol-disulfide exchange reactions. Both Trxs and Grxs catalyze the reduction of protein disulfides and have been implied in numerous regulatory processes that rely on this post-translational redox modification (432, 434, 703).
In the context of redox signaling, the most efficient way of protein disulfide formation would be via specialized transducer proteins, that is, proteins which show a very high reactivity toward, for instance, H2O2, leading to oxidation and disulfide formation on the transducer protein. This disulfide could subsequently be transferred to effector proteins. Such disulfide relay systems have been described in bacteria and lower eukaryotes; for an introduction, see (77). Although experimental evidence is missing, it is tempting to speculate about similar functions for human Prxs and Trxs with their specificities for peroxides and target proteins, respectively.
3. Glutathionylation and cysteinylation
Cysteinyl side chains may not only form disulfides with other protein thiols, but some form disulfides with low-molecular thiol compounds, such as GSH or cysteine. These post-translational redox modifications have been termed glutathionylation and cysteinylation, or, more generally, thiolation. Hundreds of proteins have been reported to undergo glutathionylation at specific cysteinyl residues, and the topic has been reviewed extensively earlier; see, for instance, (142, 205, 495, 833). Similar to protein disulfides, these disulfides may not only form via sulfenic acid intermediates and subsequent reactions with the reduced low-molecular-weight thiol (Figs. 6 and 7), but they may also result from a nucleophilic attack of a cysteinyl thiolate on the low-molecular-weight disulfide, that is, GSSG or cystine. In addition, radical pathways have been suggested to result in thiolation. Grxs have a very high affinity for the GSH moiety. They catalyze the reduction of mixed disulfides, the de-glutathionylation, with very high efficiency (240, 703), as well as, if thermodynamically favorable, the forward reaction, that is, the glutathionylation of protein thiols (658). Therefore, Grxs are central for signal transduction via glutathionylation.
4. S-nitrosylation
·NO is best known for its relaxing function in smooth muscle cells surrounding the vasculature, through the activation of guanylate cyclases by modification of their heme iron cofactor (607). In addition, it was recognized early on that ·NO leads to the reversible modification of cysteinyl residues by the formation of S-nitroso thiols (Figs. 6 and 7). By today, hundreds of proteins with susceptible cysteinyl residues have been identified; for detailed discussions on the topic, see, for instance, (203, 699). The reaction of ·NO with thiols to S-nitroso thiols is an oxidation that requires the transfer of one electron to an acceptor molecule, and thus catalysis, for instance, by protein-bound transition metals. Despite the direct modification of thiols by ·NO, redox signals may also be transduced by the transfer of S-nitroso groups between thiol groups, a process termed trans-nitrosylation (467). A source for such nitroso groups may be S-nitrosylated glutathione (GSNO). The formation of GSNO is catalyzed, for instance, by ceruloplasmin, the decay by GSNO reductases (203). Thus, GSNO may qualify as a second-messenger molecule in redox signaling (Fig. 7), although experimental evidence for this role is still incomplete. Trx and TrxR have been implied in trans-nitrosylation reactions, as well as in specific reductases of S-nitroso thiols and may thus take part in both the transduction and termination of such signaling events (697).
5. Other reversible redox modifications
a. Persulfide formation
H2S is, similar to NO, an endogenously produced gaseous signaling molecule. It is produced enzymatically by three different enzymes (Fig. 6), cystathionine β-synthase, cystathionine γ-lyase, and 3-mercaptopyruvate sulfurtransferase (MST), all of which depend on pyridoxal-5′-phosphate as cofactor (168, 241, 706, 740, 749), reviewed in (160, 382, 720). The formation of persulfides of protein thiols exposed to H2S has been reported for ATP-sensitive K+ channels, it leads to the inhibition of phosphodiesterases (801), and shows a number of additional physiological effects; see, for instance, (43, 157, 274, 650, 813). Recently, Francoleon et al. reported that protein persulfides are easily generated by a reaction of H2S with disulfides and are relatively stable (204). It remains to be established as to what extent these modifications occur in vivo, whether they modify transducer or effector molecules, and whether Trx family proteins take part in persulfide reduction or “trans-persulfidation” reactions.
b. Methionine sulfoxidation
In addition to cysteinyl residues, ROS may also react directly with methionyl residues to form protein methionine sulfoxides. This oxidation is reversible through the action of the Trx-dependent methionine sulfoxide reductases (Msr), reviewed in (739). This post-translational modification is discussed to be involved in metabolic regulation and cell signaling; for more elaborate discussions on this topic, we refer to Refs. (62, 519, 520, 739).
D. Oxidative stress in the concept of redox signaling
As outlined earlier, oxidizing second-messenger molecules, also known as “pro-oxidants,” are produced both constitutively and in response to signals as primary or side products of specific enzymes and are eliminated by reactions with target or transducer proteins. How can these regulatory circuits be brought into accordance with the oxidative damage that was demonstrated in numerous pathological conditions? If the redox circuity was disturbed, for instance by continuous activation of such signaling pathways in response to a specific pathological condition, oxidative second-messenger molecules could accumulate to such a degree that biomolecules become irreversibly modified, as outlined in Figure 6. Excessive peroxynitrite might, via different pathways, lead to the nitration of, for instance, protein tyrosyl residues. Further oxidation of sulfenic acids will lead to sulfinic and sulfonic acids. Decomposition of peroxynitrous acid or the Fenton reaction of H2O2 with metal ions may lead to the formation of the hydroxyl radical. This molecule will, only limited by diffusion, subtract hydrogen atoms from various biomolecules; subsequent reactions of the radical products will lead to peroxidation, carbonylation, or decomposition of these molecules. Catalyzed by myeloperoxidase, excess H2O2 may also react with chloride ions, yielding hypochlorous acid that may lead to the chlorination of various building blocks of the cell. These irreversible modifications do not occur randomly, instead various proteomic screenings suggest both target and side chain specificity for them as well, summarized, for instance, in (114, 242, 458, 622, 788).
Although the concept of “oxidative stress” as damage that arises from disturbed redox signaling/regulation reactions is based on an overwhelming body of knowledge and evolved for a long time, it was not explicitly written out before 2005/2006 (227, 354).
II. Mammalian Trx Family Proteins in Health and Disease
A. Specific pathways
1. Apoptosis
Trxs, Grxs, and Prxs have been implied in many aspects of programmed cell death prevention and induction, as mentioned earlier and as exemplified next. Please see also section II.B.12 for their role in degenerative disorders.
a. Cytosolic pathways
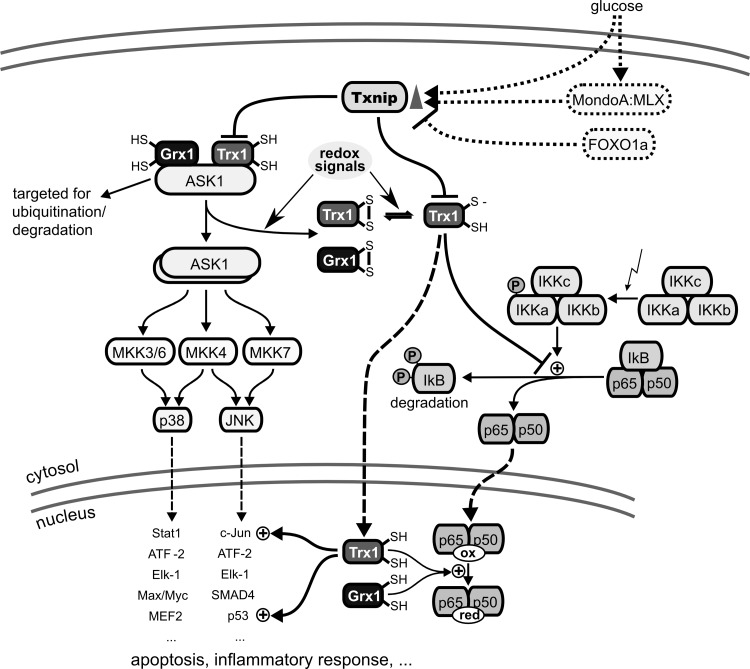
ASK1 is a MAP kinase kinase kinase that leads to the activation of JNK and p38 MAP kinase pathways, for instance, during tumor necrosis factor (TNF) α-induced apoptosis (317). ASK1 is activated by ROS through signal transduction via Trx1 and/or Grx1. Reduced Trx1 and Grx1 bind to N- and C-terminal domains of ASK1, respectively, thereby inhibiting its kinase activity (Fig. 8). Oxidation of Trx1 or Grx1 leads to the dissociation of the complex and activation of the kinase (670, 731, 732). In addition, binding of reduced Trx1 to ASK1 targets the kinase for ubiquitination and degradation (443). In agreement with a function as endogenous Trx1 inhibitor, silencing of Txnip expression attenuated high glucose-induced apoptosis and activation of ASK1 in mouse mesangial cells (710) and dexamethason-mediated apoptosis of insulin-producing cells (631). Thus, Trx1 and Grx1 act as redox signal transducers for the induction of apoptosis via the JNK and p38 MAPK pathways.
FIG. 8.
Trx, Txnip, and Grx in MAP kinase and NF-κB signaling. Txnip, whose expression is promoted by glucose via MondoA:MLX signaling and repressed by FOXO1a, was suggested to be a negative regulator of reduced Trx1. Left side: Trx and Grx as negative regulators of apoptosis signal-regulating kinase 1 (ASK1)–ASK1 is a mitogen-activated protein (MAP) kinase kinase kinase that signals downstream to the c-Jun N-terminal kinase (JNK) and the p38 MAP kinase pathways via MAP kinase kinases 3, 4, 6, and 7. Reduced Trx1 and Grx1 can bind to ASK1, leading to an inactive complex. Oxidation of Trx1 and/or Grx1 by various redox signals leads to dissociation of the complex and activation of ASK1. Moreover, the Trx1/ASK1 complex is targeted for ubiquitination and degradation. Right side: Redox regulation of NF-κB activation–the NF-κB subunit p50 contains a cysteine (Cys 62) in its DNA binding site that is susceptible to oxidation. After dissociation of the I-κB/NF-κB complex, which is not only promoted by phosphorylation of I-κB in response to a variety of signals but also inhibited by reduced Trx1, NF-κB is translocated to the nucleus. In the nucleus, reduction of Cys62 in the p50 subunit of NF-κB is necessary for binding of the transcription factor to its target site in the DNA. In the nucleus, Trx1, Grx1, and Nrx (not shown) have been reported to promote NF-κB binding to the κB site in the DNA. NF-κB, nuclear factor kappa B; Nrx, nucleoredoxin; Txnip, trx interacting protein.
Caspases, the executors of apoptosis, belong to the class of cysteine proteases, whose activity critically depends on the presence of a thiolate in their active site (72). This requirement makes them vulnerable to redox modifications such as S-nitrosylation and S-glutathionylation (307, 465). Trx1 catalyzes the trans-nitrosylation or denitrosylation of caspase-3, thereby regulating protease activity (53, 502). Silencing of Grx1 significantly inhibited TNF-α-induced endothelial cell death because of attenuated caspase-3 cleavage, for example, by caspase 8, concomitant with increased caspase-3 glutathionylation, apparently also of cysteinyl residues outside the active site (571).
Increasing evidence suggests diverse functions of the cytosolic Prxs in redox signal-induced apoptosis. Cisplatin is a chemotherapeutic that is effective in the treatment of several tumors. Prx1-deficient embryonic fibroblasts were sensitized to cisplatin-induced apoptosis, displayed an increased activation of p38 and JNK, and reduced extracellular signal-regulated kinase (ERK) activation. Thus, Prx1 modulated the cisplatin-induced MAP kinase activation that leads to apoptosis (459). Mammalian Ste20-like kinase-1 (MST1) mediates p53-dependent H2O2-induced cell death. Morinaka et al. showed that H2O2 generation by cisplatin caused Prx1 oligomer formation, dependent on the presence of p53, and subsequently MST1 activation (517). Inhibition of Prx1 by a recombinant antibody induced apoptosis in A549 lung carcinoma cells and sensitized these cells to radiation (248). Prx2 inhibited granulosa cell apoptosis during follicle atresia through the NF-κB pathway (844). Down-regulation of Prx2 expression contributed to angiotensin II-mediated podocyte apoptosis (304). Transgenic over-expression of Prx4 protected mice against high-dose streptozotocin-induced death of pancreatic β-cells (154). TNF-related apoptosis-inducing ligand (TRAIL) signaling repressed Prx4 at the transcriptional level, and over-expression of Prx4 suppressed TRAIL-induced apoptosis. Deficiency of Prx6 in lens epithelial cells evoked unfolded protein response and apoptosis (179) and over-expression attenuated cisplatin-induced apoptosis in human ovarian cancer cells (566).
b. Mitochondrial pathways
Cardiolipin is a phospholipid that is specific for energy transducing membranes such as the inner mitochondrial membrane and is important for the activity of the complexes of the electron chain (209, 282, 645). Importantly, cardiolipin anchors cytochrome c to the inner mitochondrial membrane (644); loss of this lipid causes the release of cytochrome c and to the induction of apoptosis as monitored by activation of distinct caspases (69, 330, 745). Short interfering RNA silencing of mitochondrial Grx2 in HeLa cells sensitized these cells to cell death induced by doxorubicin (50-fold) and phenylarsine oxide (40-fold), but the cells did not show signs of a general increase in oxidative damage, that is, protein carbonylation (435). HeLa cells over-expressing Grx2 were less susceptible to apoptosis induced by 2-deoxy D-glucose and doxorubicin. Grx2 prevented the loss of cardiolipin and, therefore, cytochrome c release and caspase activation (167). Corroboratively, transgenic mice over-expressing Grx2 displayed an attenuation of doxorubicin-induced cardiac inquiry, which was accompanied by an increase in protein S-glutathionylation (155).
Trx2-deficient DT 40 cells derived from chicken undergo apoptosis mediated by cytochrome c release and subsequent caspase-9 and caspase-3 activation (759). Trx2−/− mouse embryos showed massively increased apoptosis at 10.5 days and died before day 12.5 along with the maturation of mitochondria. It should be noted that even embryonic fibroblasts cultured from Trx−/− embryos were not viable (552). WEHI7.2 thymoma cells with stable over-expression of Prx3 showed a marked resistance to hypoxia-, H2O2-, tert-butyl hydroperoxide-, and imexon-induced apoptosis (551). Over-expression of Prx3 also protected pancreatic β cells from apoptosis induced by pro-inflammatory cytokines or streptozotocin (820).
2. Proliferation
Both Trxs and Grxs were initially discovered as electron donors for RNR (Fig. 1), an essential enzyme for DNA synthesis and thus proliferation.
Dysregulated proliferation is one hallmark of tumor formation. Several members of the Trx family, that is, Trxs, Grxs, and Prxs, have been suggested to fulfill crucial functions during carcinogenesis, including promotion of proliferation and thereby tumor growth (see section II.B.12). This function as growth factor has been determined not only in cancer cells, but also in normal hepatocytes and lymphocytes as well as in murine fibroblasts (540, 555, 798). Proliferation of human adipose tissue-derived mesenchymal stem cells was increased by over-expression of both Trx1 and Trx2, whereas knockout of these proteins inhibited proliferation (733). However, treatment with recombinant Trx1 and high expression of Trx1 was also described to induce growth arrest in liver cells (656). Extracellular applied Trx1—alone or in concert with interleukins (ILs)—stimulated the proliferation of human B cells immortalized by the Epstein-Barr virus via activation of protein kinase C, indicating an important role of Trx1 not only in the permanent growth of Epstein-Barr virus-infected B cells, but also for cell growth of Epstein-Barr virus negative cell lines (47, 63, 798). Trx1 might promote proliferation by increased expression and stimulation of different growth factors and proliferation-associated transcription factors such as ILs, FGF, ERK1/2, TNF-alpha, p53, NF-κB, AP-1, or Nrf2 (206, 480, 509, 609, 685, 733, 751, 787). Although it remains elusive whether all interactions with the listed factors are directly connected to the proliferative effect of Trx1, a crucial role of Trx1 during cell cycle progression appears likely (509).
Usually, Trx1 activity depends on the presence of TrxR1. Mouse hepatocytes lacking TrxR1 displayed normal supply of electrons to RNR to support DNA replication and normal proliferative growth, indicating that TrxR1 might be dispensable under certain conditions (649). In mouse liver lacking TrxR1, GSH was essential, indicating that the GSH- and TrxR1-dependent pathways constitute complementary systems of supporting RNR in this organ (611). The important role of GSH for the cellular proliferation was recognized decades earlier (403, 440); depletion of total GSH induced cell cycle arrest (171). GSH, when transported into the nucleus, seems to have a profound impact on cell cycle progression and gene expression; for details, see (153, 468).
Grx1 was suggested to be involved in controlling cell proliferation in mouse primary lens epithelial cells (445). Grx3-deficient mouse embryonic fibroblasts exhibited defects in cell cycle progression during late mitosis, one potential reason for early embryonic lethality of Grx3 knockout (116).
In human mammary epithelial cell lines, higher expression of Prx1 positively correlated with the proliferation rate (612). c-Abl and c-Myc were identified as interaction partners of Prx1, suggesting that Prx1 promotes proliferation via these important cell cycle regulating proteins (531, 810). Indeed, over-expression of Prx1 altered the transcription profile of c-Myc target genes (531). Moreover, it was proposed that phosphorylation of Prx1 by cyclin-dependent kinase 2 is an important regulatory mechanism during cell cycle progression, as the modified Prx1 was detectable during mitosis, but not during interphase (113).
Nrx inhibits activity of the Wnt/β-catenin pathway, a signaling pathway promoting proliferation. Not surprisingly, Nrx silencing accelerated proliferation (213).
3. Iron metabolism
Iron is an essential trace element that is required for a number of protein co-factors, including, for instance, heme and iron-sulfur centers. On the down side, ferrous iron in its free form is an efficient catalyst of the Fenton reaction, generating OH· radicals from H2O2. The reaction of OH· radicals with proteins, lipids, and nucleic acids generates other radical species that subsequently lead to peroxidation, carbonylation, or fragmentation of these biomolecules (Fig. 6). It is, therefore, not surprising that the dysregulation of iron metabolism was implied in the pathophysiology of various human diseases, including Alzheimer's disease (AD) (5, 460), Friedreich's Ataxia (570, 816), hemochromatosis (59, 94), and Parkinson's disease (PD) (60, 126).
a. Iron sulfur Grxs
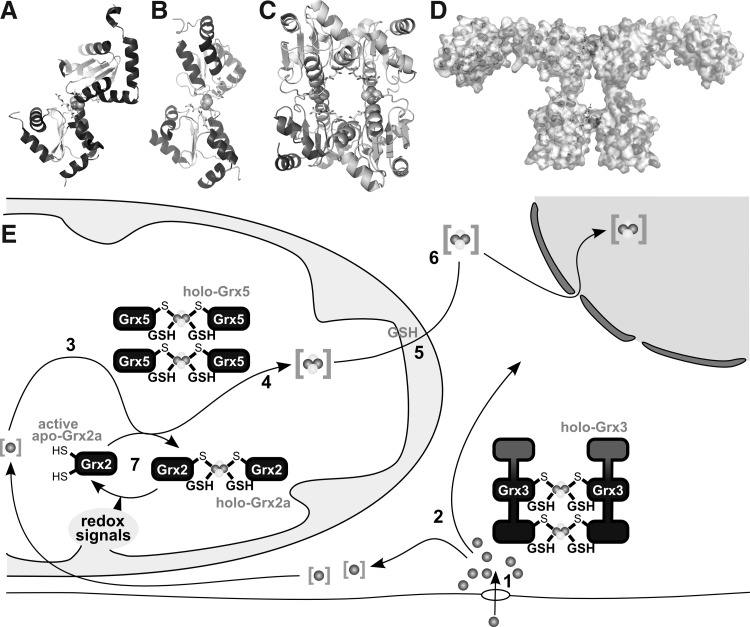
Human mitochondrial Grx2 was the first Grx that was identified to complex a [FeS] cluster (433). This, in many aspects unusual Grx (active site Cys-Ser-Tyr-Cys), contains a redox inactive [2Fe2S]2+ cluster that bridges two Grx2 molecules to form a dimeric holo Grx2 complex (Fig. 9). The [FeS]-bridged dimer lacks enzymatic activity, but degradation of the cluster and dissociation of the holo complex activated the protein. Slow degradation of the complex under aerobic conditions was efficiently prevented by GSH. GSSG promoted cluster degradation and thereby activation of Grx2 (433). The biochemical analysis of several mutants demonstrated that the iron-sulfur cluster is complexed by the two N-terminal active site thiols of two Grx2 monomers and two molecules of GSH which are bound noncovalently to the proteins and in equilibrium with GSH in solution (56). The structure of the dimeric holo Grx2 complex was solved by X-ray diffraction (Fig. 9A) (348). Astonishingly, hardly any direct molecular interactions between the two protein monomers could be identified. Besides one hydrogen bond and two small hydrophobic interactions, all molecular interactions contributing to the holo complex involve the GSH molecules. The two GSH molecules efficiently shield the iron from the solvent. Only one of the sulfur atoms of the [FeS] cluster is solvent exposed. Hence, the [2Fe2S] cluster may not be able to react with redox compounds that require direct molecular interactions with iron such as H2O2. Instead, degradation of the cluster in response to oxidative signals more likely occurs through the formation of GSSG (see above). Similar to human Grx2, many, if not all, monothiol Grxs (active site Cys-Gly-Phe-Ser) can form the dimeric holo [FeS] complex (Fig. 9B–D) (271, 490, 603). The properties that permit some Grxs to form the [FeS] bridged dimeric holo complex are likely due to the exchange of the active site Pro. This exchange allows a higher flexibility of the main chain in the active site area, providing enough room for the noncovalent binding of GSH and cluster coordination (181, 348). For human Grx2, a function as redox sensor of the [FeS] cluster was suggested, because redox-induced cluster decay activated the oxidoreductase (56, 433); the functions of the monothiol Grxs appear to lie primarily in iron metabolism (see below).
FIG. 9.
[FeS]-Grxs in cellular iron metabolism. (A) Structure of the holo-Grx2 complex consisting of two monomers Grx2 (cartoon graphics), two GSH molecules (ball and stick model), and the [2Fe2S] cluster (calotte model), derived from PDB entry 2HT9 (348). (B, C) Structures of the holo-Grx5 complex depicted as dimer (B) and tetrameric holo complexes (C), derived from PDB entry 2WUL (350). (D) Hypothetical model of the dimeric Grx3 holo complex (271). (E) Iron taken up into the cell, simplified in [1], is shuttled through the cytosol, presumably involving Grx3 [2]. Inside mitochondria, iron is used, for instance, for the biogenesis of iron-sulfur clusters [3] on a scaffold protein and transferred to target apo-proteins [4] in a reaction that requires Grx5. The export of iron-sulfur clusters in a hitherto unknown form requires GSH [5]. This compound X is used by the cytosolic iron-sulfur cluster assembly machinery for the synthesis of cytosolic and nuclear FeS proteins [6]. [7] Grx2 is usually present in the enzymatically inactive FeS-bridged dimeric holo form. On redox signals, the FeS cluster dissociates, yielding active monomeric Grx2.
Another amino acid whose presence prevents metal binding in the active site of Trx family proteins is the cis-proline (743). Exchange of this prolyl residue not only in human Grx1, but also in human Trx1 resulted in a [FeS] cluster coordinating protein. Moreover, mutation of the Thr-X-X-Cys active site in a Prx, Prxs do not contain the cis-proline, to a Cys-X-X-Cys active site resulted in a [FeS] cluster coordinating protein as well.
b. Biogenesis of iron-sulfur centers
The biogenesis of iron-sulfur centers in eukaryotic cells is an essential function of mitochondria (436). Initially, iron-sulfur centers are synthesized on the scaffold protein Isu (IscU or NifU in bacteria). In the next step, these newly assembled [FeS] units are transferred to apo-proteins with the help of a DnaK- and DnaJ-type chaperone couple (437).
Knockout of mitochondrial monothiol Grx5 in yeast led to iron accumulation in the cell and inactivation of iron-sulfur center-containing enzymes (647). These defects could be suppressed by over-expression of the Hsp70/DnaK-type chaperone Ssq1 and the potential alternative [FeS] scaffold Isa2. Moreover, depletion of Grx5 led to an accumulation of iron loaded onto the scaffold protein Isu1, implying a function of Grx5 in the transfer of [FeS] clusters from the scaffold to apo-target proteins (521). A hypochromic anemia mutant of zebrafish (Shiraz) lacking Grx5 and a human sideroblastic-like microcytic anemia patient with reduced Grx5 levels provided strong evidence that this function of yeast Grx5 was conserved in vertebrate species; in both cases, impaired [FeS] cluster assembly resulted in defects in heme biosynthesis (95, 817). The exact biochemical function of Grx5 in [FeS] center biosynthesis, however, remains to be established.
The lack of both mitochondrial Prx and mitochondrial/cytosolic dithiol Grx in yeast led to the induction the Aft1 iron regulon, despite optimal mitochondrial [FeS] biogenesis. A crosstalk between the dysfunction of mitochondrial redox homeostasis and the cytosolic iron regulation was thus suggested (486).
c. Regulation of iron metabolism
Vertebrate cells evolved a post-transcriptional regulatory mechanism for the expression of proteins involved in iron homeostasis and iron cofactor biosynthesis based on iron regulatory proteins (IRP) 1 and 2, reviewed for instance in Refs. (276, 572, 652). Loss of Grx5 in the zebrafish Shiraz mutant impaired mitochondrial [FeS] cluster assembly and promoted activation of IRP1. To some extent, knock-down of IRP1 restored hemoglobin synthesis in the Grx5 mutant, demonstrating a crosstalk between hemoglobin production and the mitochondrial [FeS] cluster assembly machinery (817) (Fig. 9E).
During exposure to nitric oxide the iron regulating function of both IRP1 and IRP2 is disrupted (161, 808). This dysregulation of ·NO-modified IRPs was restored by Trx1 in vitro and in cell cultures, indicating a crucial role of Trx as a modulator of IRP activity (559).
d. Intracellular iron distribution
As late as 2 years earlier, essentially nothing was known on how cells manage to passage iron safely to the various iron-dependent processes in the different subcellular compartments. Only recently, strong evidence was presented for an essential function of the cytosolic multi-domain monothiol glutaredoxins Grx3 and Grx4 in cellular iron trafficking in yeast (522). Combined depletion of Grx3 and Grx4 specifically impaired all iron-dependent reactions in the cytosol, mitochondria, and nucleus. These defects were caused by insufficient iron insertion into proteins and organelles, despite accumulation of cytosolic iron. Thus, in the absence of Grx3 and Grx4 iron, even though sufficient amounts were taken up into the cells, iron was not bioavailable. The ability of the monothiol Grxs to bind a [FeS] cluster themselves was an absolute requirement for this function (522).
B. Tissues, organ systems, and diseases
1. Development
Oxygen concentrations and ROS levels are known to affect cell fate and embryonic development. The expression of proteins of the Trx family was also shown to be important, because protein deficiency is correlated with severe and often fatal phenotypes (Table 2). Trx1 knockout in mice was lethal due to its importance in early differentiation and morphogenesis. These mice died already at embryonic day E3.5 (474). Deletion of mitochondrial Trx2 was also lethal. Due to increased apoptosis, homozygous mice die between E10.5 and E12.5, which coincides with maturation of mitochondria (552). In contrast, Txnip, the suggested endogenous inhibitor of the Trxs, was not essential for embryonic development (856). Grx1 knockout mice were viable (301), while in zebrafish, Grx2 has an important impact on embryonic brain development. Knock-down inhibited the outgrowth of axons and leads to neuronal apoptosis and subsequent impaired formation of a functional neuronal network (75). Grx3 knockout is lethal between E12.5 and E14 (105, 116). Most likely, Grx3 deficiency induces defects in cell cycle progression during late mitosis (116). Grx3 was identified as a direct target of serum response factor, indicating that Grx3 is important during early embryonic development of cardiac tissue (865). The mitochondrial monothiol Grx5 is also important for embryonic development and essential for [FeS] cluster and heme biosynthesis (section II.A.3) (817).
Table 2.
KnockOut Phenotypes of Trx Family (and Related) Proteins
| Gene | Protein | Embryonically lethal? | Phenotype(s) | References |
|---|---|---|---|---|
| TXN1 | Thioredoxin 1 | Yes | Died before implantation | (474) |
| TXN2 | Thioredoxin 2 | Yes, E10.5–12.5 | Massive apoptosis at the onset of respiration; open anterior neural tube | (552) |
| TRXR1 | Thioredoxin reductase 1 | Yes, E9.5–10.5 | Reduced proliferation; reduced body size; cerebellar hypoplasia | (332, 552) |
| TRXR2 | Thioredoxin reductase 2 | Yes, E13.5–15.5 | Reduced in size; anemic; reduced hematopoiesis; thinning of the ventricular myocardium, septum, and trabeculae; pleiomorphic and spongiform liver | (135, 298) |
| TXNIP | Thioredoxin interacting protein | No | Hypoglycemic; hypoinsulinemic; defects in the glucose metabolism of hepatocytes | (856) |
| NXN | Nucleoredoxin | No | Skeletal and cardiovascular defects | (216) |
| GLRX1 | Glutaredoxin 1 | No | No obvious phenotype | (301) |
| GLRX2 | Glutaredoxin 2 | No | Mouse—unpublished; loss of neurons and axonal scaffolds in zebrafish embryos | (75, 826) |
| GLRX3 | Glutaredoxin 3 | Yes, E12.5–E14.5 | Reduced body size; hemorrhage in the brain | (105) |
| GLRX5 | Glutaredoxin 5 | (Yes in D. rerio) | Anemia; iron overload | (817) |
| PRDX1 | Peroxiredoxin 1 | No | Hemolytic anemia at 9 months; more oxidative damage; more malignant tumors | (393) |
| PRDX2 | Peroxiredoxin 2 | No | Splenomegaly; abnormal erythrocyte morphology | (369) |
| PRDX3 | Peroxiredoxin 3 | No | Reduced body weight | (438) |
| PRDX4 | Peroxiredoxin 4 | No | Atrophic testes; otherwise, no obvious phenotypes | (328) |
| PRDX6 | Peroxiredoxin 6 | No | No obvious phenotype | (802) |
Transgenic mice lacking Prx1–4 and 6 were viable, but showed signs of increased ROS levels (328, 422, 438, 545, 802). In addition, Prx1 controls motor neuron differentiation in the spinal cord of chick embryos via redox-dependent regulation of glycerophosphodiester phosphodiesterase 2 (GDE2) activity, a transmembrane protein that is essential for motor neuron differentiation (846).
Nrx knockout mice show skeletal and cardiovascular defects and die around birth (216). In Xenopus embryos, it was shown earlier that Nrx interacts with dishevelled to regulate both Wnt/β-catenin and Wnt/planar cell polarity pathways during embryonic development (Fig. 10) (213, 214). Wnt signaling is one of the central pathways during embryogenesis (750). In addition, Nrx also regulates via an interaction with Flightless-1 (Fli-1) TLR-4 signaling, another important pathway during embryogenesis (272).
FIG. 10.
Nrx in Wnt/Dvl and Toll-like receptor 4 (TLR4) signaling. Nrx was shown to suppress the Wnt/β-catenin pathway, which is involved in embryonic development and cancer. Secreted Wnt proteins bind to receptors of the Frizzled family and activate a signaling cascade. This process involves the cytosolic dishevelled (Dvl) protein, which inhibits the glycogen synthase kinase-3 (GSK3)-containing destruction apparatus and thereby phosphorylation and degradation of beta-catenin (β-cat). β-cat translocates into the nucleus and activates the transcription of Wnt-regulated target genes. Reduced Nrx binds to Dvl and suppresses Wnt/β-catenin signaling, whereas via “redox signals” oxidized Nrx does not. Similarly, reduced Nrx can inhibit TLR4 signaling, which is essential for embryonic development and the innate immune response. Lipopolysaccharide (LPS) stimulates the oligomerization of TLR4, inducing the recruitment of signal transduction adaptor proteins, such as myeloid differentiation primary response protein (MyD88). MyD88 activates a cascade of IKK and MAP kinases, leading to the phosphorylation and degradation of the inhibitor protein IκB, translocation of NF-κB (comprising subunits p65 and p50) into the nucleus, and activation of target genes. Reduced Nrx binds to Flightless-1 (Fli-1), forming an inhibitory complex with Myd88, suppressing TLR4-signaling.
The electron donors of both the Trx and Grx system, TrxR and GSH, were essential for embryonic development. Mouse embryos lacking TrxR1 died between E8.5 and E10.5 because of impaired cell proliferation or gastrulation, respectively (332). TrxR2−/− mice displayed a severe anemic phenotype and partial growth retardation and died between E13.5 and E15.5 (135). However, these phenotypes were less severe compared with Trx1 or Trx2 knockout mice (see above), indicating that Trx functions during embryonic development are not entirely dependent on the respective TrxR. GSH, the electron donor of Grxs, is synthesized in two steps. Mice lacking either γ-glutamylcysteine synthetase or GSH synthase died latest at E8.5 (711, 818). Lack of GSH resulted in failed gastrulation, impaired formation of the mesoderm, and death because of increased apoptosis rather than reduced cell proliferation (711), confirming earlier studies claiming crucial functions for GSH during early embryonic development (221).
2. Central nervous system
a. Expression profile of Trxs, Grxs, Prxs, and related proteins in the CNS
Cells of the nervous system are particularly susceptible to oxidative damage due to their high oxygen consumption and metabolic activity that are accompanied by a reduced cellular regeneration capacity and the presence of redox sensitive molecules such as neurotransmitters and polyunsaturated lipids. Indicators of ROS-induced damage have been reported in the three most widespread neurodegenerative diseases, that is, PD (17, 146, 152, 684), AD (525, 620), and amyotrophic lateral sclerosis (ALS) (40, 188).
Trx family proteins are expressed in various areas of the mouse, rat, and human brain (for a more detailed comparison of the expression patterns, see Table 3). With certain exceptions, for instance the mouse striatum, the redox proteins seem to be ubiquitously expressed in the murine and human brain. Certain redox proteins show strong nuclear staining in different brain regions, as described for Trx1, Grx1, Grx3, and Nrx in the mouse. Other proteins such as Prx2, Prx3, and, most notably, Prx6 show a typical astroglial staining in different areas of the brain (26, 143, 235). Prx1 was reported to be localized in glial cells of several human brain regions, whereas in neurons, it was essentially not present (678). The same study described a reciprocal staining pattern for Prx2, which was exclusively expressed in neurons but not in glia. Mizusawa et al. identified the localization of Prx1 to be restricted to oligodendroglia and Schwann cells, whereas most neurons appeared to be negative (508). In an earlier study, Prx1 immunoreactivity was detected in oligodendrocytes in several regions of the brain (347). Prx2 is highly expressed in the hippocampus and cerebral cortex of rats and humans (26, 143) and was previously reported to be present in NeuN-positive cells in the CA3 region of the hippocampus and thalamus, as well as in neurons of the gray matter in the hippocampus, cerebral cortex, and thalamus of the mouse (347). In the mouse CNS, Trx2 was specifically detected in axonal fibers in the cerebral cortex, striatum, and white matters of the cerebellum and spinal cord, in contrast to the functional-related protein TrxR2, which was present in the cell bodies (235). This observation may imply more specific functions of Trx2 independently of its reductase, for instance, in mitochondria that are distributed along axons. Using in situ hybridization, Lippoldt et al. demonstrated transcription of Trx1 in neurons of the cerebral cortex, the piriform cortex, the medial preoptic area, the CA3/CA4 region of the hippocampal formation, the dentate gyrus, the paraventricular nucleus of the hypothalamus, the arcuate nucleus, the substantia nigra pars compacta, the locus coeruleus, the ependyma of the 4th ventricle, and the epithelial cells of the choroid plexus (441).
Table 3.
Distribution and Expression Pattern of Trx Family Proteins in the Mouse, Rat, and Human Brain
| |
Tissue or cell type |
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
|
|
|
|
|
|
|
|
|
|
|
|
Cerebellum |
|
|
||||||||
| |
Hippocampus |
Substantia nigra |
Striatum |
Cerebral cortex |
Purkinje cells |
Granular layer |
Mol. layer |
Plexus coroideus |
|||||||||||||||
| ProteinSpecies | M | R | H | M | R | H | M | R | H | M | R | H | M | R | H | M | R | H | M | R | H | M | R |
| Trx1 | +* | + | +a | +* | ++ | n.a. | − | + | n.a. | + | ++ | + | ++* | ++ | − | ++ | + | − | ++ | ++ | − | ++* | n.a. |
| TrxR1 | + | + | ++a | − | + | n.a. | − | + | n.a. | + | + | ++ | + | + | ++ | + | + | + | + | + | ++ | + | n.a. |
| TrxR1-v3 | n.a. | n.a. | ++a | n.a. | n.a | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | ++* | n.a. | n.a. | ++ | n.a. | n.a. | +* | n.a. | n.a. | ++ | n.a. | n.a. |
| Trx2 | + | + | +a | + | ++ | n.a. | +x | ++ | n.a. | +x | + | +* | + | + | + | + | + | − | + | + | + | + | n.a. |
| TrxR2 | + | + | + | + | + | n.a. | − | + | n.a. | + | + | − | ++ | ++d | + | + | + | + | ++ | ++ | + | + | n.a. |
| Txnip | n.a | + | n.a | n.a. | + | n.a. | n.a. | + | n.a. | n.a. | + | n.a | n.a. | + | n.a | n.a. | + | n.a. | n.a. | + | n.a. | n.a. | n.a. |
| Nrx | +* | n.a. | n.a | +*x | n.a | n.a. | − | n.a. | n.a. | ++* | n.a. | n.a. | +* | n.a. | n.a. | ++ | n.a. | n.a. | ++ | n.a. | n.a. | ++ | n.a. |
| Grx1 | ++* | ++a | n.a | ++* | + | n.a. | − | + | n.a. | ++* | ++ | n.a | +* | +* | n.a | + | + | n.a. | + | + | n.a. | + | n.a. |
| Grx2 | ++ | + | n.a | + | ++ | n.a. | − | ++ | n.a. | ++ | + | n.a | + | +* | n.a | + | + | n.a. | + | + | n.a. | + | n.a. |
| Grx3 | +* | + | n.a | ++*x | + | n.a. | +x | ++ | n.a. | +* | + | n.a | +* | + | n.a | + | + | n.a. | + | + | n.a. | ++* | n.a. |
| Grx5 | + | + | n.a | + | ++ | n.a. | − | ++ | n.a. | + | + | n.a | + | + | n.a | + | + | n.a. | + | + | n.a. | + | n.a. |
| γGCS | + | + | +a | + | + | n.a. | − | + | n.a. | + | + | + | − | + | + | − | + | + | − | + | + | − | n.a. |
| GR | n.a. | n.a. | +a | n.a. | n.a | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | + | n.a. | n.a. | + | n.a. | n.a. | +* | n.a. | n.a. | ++* | n.a. | n.a. |
| Prx1 | + | + | +a | + | + | n.a. | − | + | n.a. | + | ++ | + | ++* | + | − | + | + | +* | + | ++ | +* | + | n.a. |
| Prx2 | − | ++a | ++a | + | ++ | n.a. | − | + | n.a. | + | ++ | ++n | − | ++ | ++* | + | + | +* | + | ++ | ++* | + | n.a. |
| Prx3 | +a | + | ++a | + | + | n.a. | +a | + | n.a. | +a | ++ | ++ | + | + | + | + | + | + | + | ++ | + | ++* | n.a. |
| Prx4 | − | ++ | + | + | ++ | n.a. | − | + | n.a. | + | ++ | + | − | + | + | − | + | + | − | ++ | + | − | n.a. |
| Prx5 | + | ++ | + | + | ++ | n.a. | − | + | n.a. | + | + | ++ | + | + | + | + | + | + | ++ | + | + | ++* | n.a. |
| Prx6 | +a | ++a | ++a | −x | ++ | n.a. | +a | ++a | n.a. | +a | ++a | ++* | − | + | +* | + | + | + | + | + | ++* | + | n.a. |
Immunohistochemistry data adapted from Godoy et al. 2011 (mouse) (235), Aon-Bertolino et al. 2011 (rat) (26), and Dammeyer and Arnér 2011 (human) (143).
M, mouse; R, rat; H, human; −, absent, not detected;+, present, weak expressed; ++, strong expressed; n.a., not analyzed; a, glial staining; *, strong nuclear staining; x, axonal staining; n, neurofilaments; d, dendrites; Grx, glutaredoxin; GR, glutathione reductase; Nrx, nucleoredoxin; Prx, peroxiredoxin.
Padilla et al. reported the immunolocalization of Trx1 and Grx1 in the hypophysis (563). Trx1 and Grx1 were prominently detected in the folliculo-stellatae cells of the adenohypophysis, while only a minor proportion of the glandular cells were stained. In the pituicytes and the clusters of synaptic terminals of the neurohypophysis, Trx1 was intensely stained. Grx1 immunoreactivity, in contrast, was detected in the neurosecretory terminals and Herring bodies.
In the dopaminergic neurons of the substantia nigra from mouse and rat, strong immunoreactivities were reported for Trx1, Trx2, Grx1, and Grx2 (26, 235). Using confocal microscopy, co-localization of these proteins with the specific marker tyrosine hydroxylase (TH) was demonstrated (Godoy and Lillig, unpublished data). The most notable observation was the high correlation between Grx2 and the cytosolic-localized TH, pointing out the presence of a cytosolic isoform of Grx2 in these neurons, likely Grx2c. By in situ hybridization and immunofluorescence, Grx2 was identified in both neurons and glia cells of mouse brain and co-localized with TH in the substantia nigra as well (371).
In the Purkinje cells as well as in different layers of the cerebellum, Trx1, TrxR2, and Prx1 were strongly detected (26, 143, 235).
Some of the Trx-related redox proteins were detected in areas of the central nervous system where active secretion takes place. In the plexus coroideus of the mouse, Grx3, Prx3, Prx5, Nrx, and Trx1 were notably expressed. Trx1 and Nrx are also strongly expressed in the ependymal cells of the cerebral ventricles (235). Active secretion of Trx1 was reported for a variety of cells (655), and increased Trx1 plasma levels have been detected in many diseases (342, 363, 542, 543). The putative presence of Trx1 in the cerebrospinal fluid might contribute to the defense of the central nervous system.
Trx1, Prx1, and Prx5 were strongly expressed in the motor neurons of the mouse and rat spinal cord (26, 235). In the mouse spinal cord, some of the proteins (i.e., Grx1, Prx3, and Nrx) were abundantly present in both the gray and the white matter; whereas other, such as Trx2, showed a clear regional distribution, being most notably expressed in the white matter (235). Prx2 was detected in the cytoplasm, proximal dendrites, and nuclei of anterior horn neurons of human, rats, and mice (374).
b. Trxs, Grxs, Prxs, and pathologies of the CNS
In this section, we summarized the role of Trx family members in cellular and animal models, as well as in patients suffering, from AD, PD, ALS, ischemia/stroke (see also section II.B.11 for details on hypoxic insults), and neuroinflammation.
Amyloid β treatment, a common model for AD, led to oxidation of Trx1 in the neuroblastoma cell line SH-SY5Y (16). Over-expression of Trx1 protected SH-SY5Y cells as well as rat primary hippocampal neurons against amyloid β-induced cell death (16, 450). Trx1 expression was suppressed in a rat pheochromocytoma cell line (PC12) after treatment with 1-methyl-4-phenylpyridinium (MPP+), an active metabolite of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), which causes Parkinsonism (36). Over-expression of Trx1 attenuated MPP+-induced neurotoxicity of PC12 cells (36). Trx1 is also induced after cerebral ischemia induced by middle cerebral artery occlusion (399), and mice over-expressing Trx1 showed attenuation of apoptosis and thereby neuroprotection after both permanent and transient focal ischemia (752, 868). Infarct volume and neurological deficits after transient focal ischemia were also ameliorated by an intravenous injection of recombinant human Trx1 in mice (269). In patients, during amnestic mild cognitive impairment, a transition stage between normal aging and AD, as well as in several regions of Alzheimer's brains, Trx1 protein levels were markedly decreased (16, 159, 450). In contrast, Cumming et al. reported no significant differences in Trx levels between control and AD patients (138). Trx1 levels were elevated in diseases associated with neuroinflammation, for example, in cerebrospinal fluid and blood of multiple sclerosis patients (592), and in spinal cords of ALS patients (462).
Although mitochondrial integrity is crucial for the progression of most of the neurological diseases, almost nothing is known about the role of the mitochondrial Trx2, except for the finding that Trx2 levels increased in hippocampus of gerbils after ischemia reperfusion (316). The electron donor of Trx2, TrxR2, is not essential for the development of the central nervous system. Mice lacking TrxR2 specifically in the nervous system developed normally, whereas nervous system-specific deletion of TrxR1 displayed massive malformation of the hippocampus and cerebellum, resulting in ataxia and tremor (724). Treatment of rat primary hippocampal neurons with TrxR1 attenuated amyloid β-mediated toxicity (450). In Alzheimer's patients, TrxR1 activity was generally enhanced compared with controls (450), whereas TrxR1 levels in the cerebrospinal fluid and blood of multiple sclerosis patients were decreased (592). Moreover, single-nucleotide polymorphisms of the TRNRD1 gene were significantly associated with familial ALS (503).
Amyloid β treatment of SH-SY5Y cells led to the oxidized of both Trx1 and Grx1. Over-expression of Grx1 protected SH-SY5Y cells against amyloid β-induced cell death (16). Several proteins have been described to be involved in PD development and progression. The expression of one of these proteins, DJ-1 (137), correlates with the expression of Grx1 (665). In a mouse model for PD based on MPTP toxicity, loss of dopaminergic neurons was associated with inactivation of mitochondrial complex I, a hallmark of the disease. Recovery of complex I activity correlated with an increase of Grx activity after MPTP treatment (376). Although knockout of both Grx1 and Grx2 inhibited this recovery (371, 376), over-expression of Grx2 diminished MPTP-nduced neuronal apoptosis via decreased complex I activity (371, 419). Aggregation of mutant SOD1 has been proposed as one reason for the degeneration of motoneurons during ALS. Over-expression of both Grx1 and Grx2 in immortalized motoneurons increased solubility of mutant SOD1, but only Grx2 protected against subsequent apoptosis (191). After induction of focal ischemia in rat brains, Grx1 levels decreased parallel to the rate of neuronal damage (752). In Alzheimer's brain tissue, Grx1 was up-regulated in healthy neurons of the hippocampus and the frontal cortex, but down-regulated in degenerating neurons (16).
De-/glutathionylation, specifically catalyzed by Grxs (see also section I.C.3), is associated with several aspects of neurodegeneration, such as apoptosis, mitochondrial function, and plaque formation, summarized for instance in (661). Several studies demonstrated the important role of GSH in pathologies of the central nervous system. Amyloid β treatment of SH-SY5Y cells decreased the total cellular GSH amount (16). In the substantia nigra of Parkinson patients, not only total GSH levels were decreased, but also GSH was virtually absent. This loss of GSH is one of the first signs of the disease (597, 598). Knock-down of GSH synthesis in PC12 cells, rat dopaminergic N27 cells, as well as in mice by catecholaminergic neuron-specific down-regulation of γ-glutamyl cysteine ligase, the rate-limiting enzyme in the de novo GSH synthesis, resulted in inhibition of complex I activity (127, 305, 340, 419). These data highlight the importance of the GSH/Grx system in the maintenance of mitochondrial function in the early onset of PD.
In amyloid β-resistant clones of the PC12 cell line, Prxs1, 2, and 6 expression was significantly increased. PC12 cells and primary neurons over-expressing Prx1 exhibited attenuated amyloid β and MPP+/MPTP toxicity (138, 615). Treatment with 6-hydroxydopamine (6-OHDA) led to an oxidation of Prx1. Increased levels of Prx1 as well as Prx2 protected dopaminergic cells both in vitro and in vivo against 6-OHDA-induced apoptosis, whereas silencing of Prx1 sensitized the cells (315, 423). In addition, elevated Prx2 levels protected against amyloid β toxicity in a transgenic mouse model for AD (847). In whole brain samples, the expression levels of Prx1 and Prx2 were elevated in Alzheimer's patients (138, 381). Two other studies, however, could not confirm a higher Prx1 expression in Alzheimer's brains (406, 746). Prx2 levels were also increased in the hippocampus and the frontal cortex of AD patients (746), in substantia nigra of PD patients (44), and in motor neurons during ALS (374). Prx3 expression was decreased in the brains of Alzheimer's patients and in the motor neurons of ALS patients (381, 391). In addition, Prx4 was down-regulated in motor neurons during ALS (391). Prx6 was up-regulated in astrocytes of AD patients and familial ALS (608, 741). In PD patients, peroxidase activity of Prx2 was inhibited by S-nitrosylation (174) and phosphorylation (615). The redox states of Prx2 and Prx6 were more oxidized in the brains and serum of Alzheimer's patients (138, 853). In circulating endothelial progenitor cells of ischemia stroke patients, Prx1 was tenfold higher expressed than in healthy controls (76). Prx2, in rat brains down-regulated after cerebral ischemia (399), protected against stroke-related insults, such as ischemia and glutamate treatment in vitro and in vivo (70, 624). Prx3 was increased in the hippocampus of gerbils after cerebral ischemia reperfusion and protected against ischemic damage (316). Inflammation is known to be a characteristic for several neurological diseases such as multiple sclerosis, ALS, and PD. Thus, the protection of Prx2 against neuroinflammation via suppression of pro-inflammatory signaling pathways in microglia (556) may be become helpful to combat these diseases. Based on the importance of the Trx family proteins in other inflammatory processes (see section II.B.8), it is likely that more of these proteins may protect against neuroinflammation.
3. Sensory organs
a. Expression profile of Trx-related proteins in sensory organs
Sensory organs are directly exposed to various sources of ROS. In this section, we will discuss expression patterns and pathological implications of Trx family proteins in different sensory organs, with emphasis on the eye, which has been the most intensively studied so far. The ear, tongue, and olfactory part of the nose have up to now only been sparsely investigated with regard to the Trx, Grx, and Prx systems.
Both Trx1 and Grx1 were detected after E13.5 in the mouse lens and retina (396). Trx1 and TrxR1 expression was also demonstrated in neurons and photoreceptor cells in the developing rat retina (259). In adult rats, Trx1 was detected in the outer and inner plexiform layers with especially strong expression in the ganglionar cell layer but only faint staining in the photoreceptors. In contrast, TrxR1 was primarily observed in the photoreceptors (26). In the same study, no or only weak immunoreactivities were detected for Grx2 and Grx3 in photoreceptor cells. In the mouse eye, with the exception of γGCS, Trx family proteins were abundantly expressed in the corneal epithelium and stroma, the lens, ciliary body, retina, and the underlying pigmented epithelium (235) (Fig. 11A). In the retina, the layer of rods and cones showed clear differences in the localization of certain Trx-related proteins. The inner segment of the photoreceptors is rich in mitochondria and was intensely stained for Grx1, Grx5, Prx5, Trx2, and TrxR2. TrxR2 also displayed the strongest immunoreactivity in the outer photoreceptor segment (235). An eye-specific Trx-like protein, the rod-derived cone viability factor (RDCVF or Nrx-like protein 1, Nxnl1, see Table 1), was detected in photoreceptor outer segments and the interphotoreceptor matrix (428). The outermost layer of the retina, the retinal pigmented epithelium, functions in photoreceptor nourishment and contributes to the formation of the blood-retinal barrier (162). Several Trx-related proteins have been detected in this epithelium, with the strongest staining detected for Nrx (Fig. 11A). However, the high content of melanin in these cells makes qualitative analyses of immunoreactivities generally difficult.
FIG. 11.
Expression pattern of selected members of the Trx family in various organs of the mouse. (A) Sensory organs. Upper panel: In the mouse eye, Trx1 is highly expressed in the corneal epithelium; Grx5 is intensely stained the lens fibers. Prx6 immunoreactivities were detected in several layers of the retina (from the bottom up: layer of rods and cones, outer nuclear layer, outer plexiform layer, inner nuclear layer, inner plexiform layer, and ganglionar cells). Lower panel: Despite the high melanin content in the retinal pigmented epithelium, Nrx staining in this layer is evident (arrows). Grx1 and Prx6 were abundantly detected in the olfactory epithelium of the nose (arrows). Prx6 showed intense nuclear staining patterns in the outermost layer of the olfactory epithelium and also appeared distributed in the olfactory nerve bundles (arrows). (B) Lymph nodes and spleen. Upper panel: In contrast to the other members of the Trx family, which appear to be uniformly expressed in the lymph nodes (Trx1 was weakly expressed, and Grx5 particular strongly expressed), Grx3 yielded a strong immunoreactivity that was concentrated in the germinal centers. Lower panel: Trx1, Grx5, and Grx3 immunoreactivity suggests nuclear localization in the megakaryocytes of the mouse spleen (arrows). (C) Pancreas and duodenum. Trx1 and Grx5 show intense nuclear staining in the islets cells (arrows). Prx2 was abundantly detected in intercellular spaces of both endocrine and exocrine components of the mouse pancreas (arrows). In contrast to other tissues, where γGCS was weakly detected, the islets of Langerhans show intense immunoreactivities. Note that this enzyme is absent from the exocrine part of the pancreas. In the duodenal epithelium, Trx family proteins show a high variability in the compartmentalization. Trx1 and Prx5 appear homogenously distributed within the cells, Whereas Grx5 and Nrx immunoreactivities seem to be concentrated in specific areas of the cells, that is, the apical pole for Grx5 and the lateral sides for Nrx. As in the pancreas, Trx1 also shows a consistent nuclear staining pattern in the duodenal epithelium (arrows). All pictures are derived from the freely accessible redox atlas of the mouse (www.lillig.de/redoxatlas). INS, islets of Langerhans.
Several Trx family proteins were detected in the mouse lens and cornea; Grx5 immunoreactivity was the strongest of all analyzed proteins in the lens fibers (Fig. 11A) (235).
Most Trxs, Grxs, and Prxs have been observed in the stratified squamous epithelium of the mouse tongue and the underlying skeletal muscles (235). Grx1 was also detected in the calf tongue by immunohistochemistry (653).
The olfactory epithelium of the developing mouse was intensely stained for Trx1 and Grx1 at E13.5 (396). With the exception of Prx2, proteins of the Trx family were abundantly distributed in both the olfactory and the respiratory epithelium of the mouse nose. An apparently nuclear staining was described for Trx1, Prx1, and Prx6 but only in the outermost cell layer of this epithelium (Fig. 11A). Protein expression of Prx3 and Trx1 appeared very strong in ganglion cells, whereas Prx4 and Prx6 were prominently stained in olfactory nerve bundles (Fig. 11A) (235). Immunoreactivities for both Trx1 and TrxR1 were seen in epithelial cells, glands, and vascular endothelium of human nasal mucosa and nasal polyps (421).
In the cochlea of guinea pigs, TrxR1 was expressed in the inner and outer hair cells of the organ of Corti as well as in the lateral wall and the neurons of spiral ganglion (275).
b. Pathologies of the eye
The next section focuses on the role of Trxs, Grxs, and Prxs in pathological features that are common for several diseases of the eye, for example, glaucoma, age-related macular degeneration (AMD), retinopathy, and cataract. Glaucoma is one of the leading causes of blindness in the world that is connected to selective death of retinal ganglion cells. The disease is characterized by an elevation in intraocular pressure (IOP), which leads to increased levels of glutamate and pro-inflammatory cytokines and subsequently to deleterious formation of ROS (771). Diabetic retinopathy and AMD are associated with hypertension-induced oxidative stress and inflammation, causing loss of vision (360, 448). Formation of cataracts, opaque areas of the lens, is mainly based on oxidative stress that is induced by intrinsic factors such as hypertension, or by extrinsic factors such as UV light exposure (782).
In the retina of albino rats, as well as in mice exposed to cell-damaging high light, Trx1 and TrxR1 were increased (762, 764). In line, Trx1 expression and activity were increased in the lens of a mouse model for human cataract after the induction of photochemical oxidative stress (630). Over-expression of Trx1 in mice, as well as an intravitreous injection of recombinant human Trx1, suppressed the reduction of photoreceptors and the apoptosis induced by high light exposure in mice retina (401, 763). Trx1 was down-regulated after IOP in a rat glaucoma model. Consistently, over-expression of Trx1 attenuated cell death after IOP (526). Administration of N-methyl-D-aspartate (NMDA) to mammalian eyes stimulated glutamate receptors and induced retinal damage, mimicking retinal ischemia and glaucoma (717). An intravitreous injection of Trx1 effectively attenuated NMDA-induced retinal cell damage (323). An NMDA injection into the rat retina increased the levels of Txnip, the proposed Trx1 antagonist, which may contribute to inflammation and subsequent apoptosis (19). Moreover, decreased levels of Txnip attenuated early signs of diabetic retinopathy (595), and Txnip expression levels increased after IOP (528). Over-expression of Trx2 in the retina and the optic nerve attenuated degeneration after intra-ocular pressure (526, 527).
The absence of Grx1 worsened cataract morphology in mouse lens after exposure to ultraviolet radiation (409, 493). Knockout of Grx1 in rat Müller cells exposed to hyperglycemia increased the pro-inflammatory response. Corroboratively, over-expression showed the opposite effect. Grx1 may, thus, be important for the protection against diabetic retinopathy by regulating both autocrine and paracrine pro-inflammatory responses (704, 705). In general, knockout of Grx1 (445) and Grx2 (826, 827) in human lens epithel cells increased oxidative stress-induced apoptosis; over-expression of Grx2 protected the cells (185, 827).
GSH was implied in several pathologies of the eye. GSH levels were decreased in the retina of mice exposed to damaging light (764), in rats with glaucoma (514), and both decreased and oxidized in the cataractous lens (107). Plasma and whole blood of patients with AMD displayed reduced levels of the total GSH pool and an increase of the GSH/GSSG redox potential (136, 674); GSH treatment of cultured retinal endothelial cells protected against oxidative damage (91).
In patients with diabetic retinopathy (see also section II.B.9.a), Prx1 was increased in the vitreous (219). Nipradilol and timolol, two drugs used in glaucoma therapy, induced the expression of Prx2, thereby protecting cells of the tabecular meshwork, the tissue surrounding the base of the cornea, against H2O2-induced apoptosis (506). Prx3 was induced in human and mouse lens cells after treatment with H2O2 (420). Over-expression of Prx5 in Xenopus embryos reduced alcohol-induced eye malformation, indicating that Prx5 might be involved in protection against alcohol-induced fetal ocular injury (590). Over-expression of Prx6 attenuated hypoxia-induced retinal ganglion cell death (784), and treatment of retinal ganglion cells with Prx6 decreased glutamate and TNF-α induced cell death (177). In primary human cells of the tabecular meshwork, Prx6 levels were enhanced in glaucoma patients with increased signs of inflammation (178). Prx6 levels were 10-fold decreased in cataractous lenses of rats and mice (410). In addition, in patients, a negative correlation between severity of cataracts and Prx6 expression was reported (266, 567). Prx6-deficient lens epithelial cells were sensitized to apoptotic cell death that was induced by UV-B exposure, a major cause of the development of cataracts (411). Over-expression of Prx6 delayed development of cataracts in rat and mouse lenses (410).
c. Pathologies related to tongue, olfactory system, and ear
Trx1 was significantly increased in tongue squamous cell carcinoma tissue (773, 871). TrxR1, as well as Prx1 and Prx6 levels were also elevated in this type of malignancy (306, 842, 871). For more details on Trx-fold proteins in cancer, see section II.B.12.a.
No significant change of expression of Trx1 and TrxR1 was detected in nasal polyps compared with normal human nasal mucosa (421), whereas Grx1 was reported to be over-expressed in nasal polyps, that is, in the surface epithelial cells and the submucosal glandular cells (823). Levels of Trx1 were positively correlated with the respiratory disturbance in patients with obstructive sleep apnea. After nasal continuous positive airway pressure therapy, Trx1 levels significantly decreased (754).
Several anticancer drugs induce ototoxicity. Cisplatin is strongly ototoxic to cochlea hair cells in a guinea pig model, likely by targeting TrxR1 (275). In these cochlea hair cells, ototoxicity is induced not only by cisplatin, but also by gentamicin. The toxicity was increased after depletion of Prx3 (128). Ménière's disease is characterized by fluctuating hearing loss and tinnitus. In patients with this disease, decreased levels of Trx1 and the GSH/GSSG ratio were reported (93). In families with sensorineural autosomal-recessive nonsyndromic hearing impairment, a splice-site mutation was found, which leads to an impaired expression of the GRXCR1 gene (see Table 1) and that resulted in a protein with partial or complete loss of its Grx domain (686). Interestingly, a mutation in the same gene region was determined as a reason for hearing loss in the pirouette mouse mutant (557).
4. Cardiovascular system
a. Expression pattern of Trxs, Grxs, and Prxs in cardiovascular tissue
In 1978, Trx1 was detected in hearts and erythrocytes from a calf by a radioimmunoassay (292). Seven years later, both Trx1 and TrxR1 were detected by immunohistochemistry in the cytosol of epithelial cells in adult rats (654). More recently, investigations revealed that as early as embryonic day E8.5, Trx1 and Grx1 are detectable in the heart and great vessels of the mouse embryo, whereas most tissues were still negative for these proteins. The myocardium and the wall of great vessels showed strong immunoreactivities for Trx1 and Grx1 and remained positive until adulthood (396). Recently, several members of the Trx family have been detected in the adult mouse and human heart (143, 235). In the mouse cardiomyocytes, Trx1 and Grx3 showed a typical nuclear staining pattern, whereas Prx2 and TrxR2 were localized in the intercellular space of cardiomyocytes, most probably in the connective tissue (235). In the human heart, this expression pattern of TrxR2 was not confirmed. The mitochondrial reductase, as well as Prx1, was the only redox protein out of the 14 proteins analyzed as being absent from heart muscle cells (143).
b. Trxs, Grxs, and Prxs in pathologies of the cardiovascular system
The anti-apoptotic functions of Trx1 (see section II.A.1) are related to its role in protecting cardiac tissue against ischemia/reperfusion injury (see section II.B.11). This function is inhibited by nitration of Trx1 (769) as detected in diabetic (849) and aged hearts (864). In contrast, S-nitrosylation of Trx1 increases protective effects (767). Since Trx1 knockout is embryonically lethal for mice (see section II.B.1), the group of Sadoshima decreased the activity of Trx1 in mice via expression of a dominant negative form of Trx1 in the cardiovascular system (838). These mice displayed cardiac hypertrophy, a risk factor for sudden death caused by heart failure, already without induction of this disease. In contrast, mice with cardiac-specific over-expression of wildtype Trx1 were protected against hypertrophy. Recently, the same group described that Trx1 modulates cardiac hypertrophy via up-regulation of miR98 transcription, which down-regulates cyclin D2, an essential mediator of angiotensin II-induced cardiac hypertophy (845). Trx1 activity potentially depends on the presence of the endogenous inhibitor Txnip. Not surprisingly, modulation of Txnip has opposite effects on the development of cardiac hypertrophy as described for Trx1 (856, 857), although Txnip-dependent regulation of cardiac hypertrophy is not only related to Trx1 inhibition (856). Besides attenuated cardiac hypertrophy, Txnip knockout mice display reduced infarct size after reversible coronary ligation (855, 856). Interestingly, a recent genetic study revealed that individuals carrying polymorphisms up-regulating Txnip expression show increased susceptibility to hypertension (190). After infarction, Trx1 is important for neovascularization, as seen not only in over-expressing mice (6), but also in infarcted myocardium in diabetic rat hearts in which Trx1 was increased via intramyocardial administration of an adenoviral vector encoding for Trx1 (675). This Trx1 gene delivery also protected rats against cardiac failures associated with hypertension (400). Over-expression of human Trx1 in mouse hearts conferred protection against doxorubicin-induced cardiotoxicity (707). It is important to mention that doxorubicin (Adriamycin) is one of the most effective drugs in the treatment of several types of cancers; however, its therapeutic use is limited due to its high cardiotoxicity (873). Moreover, Trx1 prevented damage of cardiac tissue via inhibition of pro-inflammatory cytokine expression, adhesion of neutrophils (see also section II.B.8), and subsequent inflammatory injury of hearts occurring in response to ischemia/reperfusion (166). In addition, severity of myocarditis, an inflammation of the heart most likely due to virus infection (395), is correlated to Trx1. In mouse models and patients, Trx1 was up-regulated in the acute stage (505, 708) and enhanced in plasma in more severe forms of myocarditis, respectively (129, 546). Temocapril treatment of rats in the beginning of experimental autoimmune myocarditis ameliorated the severity via up-regulation of Trx1 in the acute phase (858). As described earlier, increased levels of Trx1 protect against several pathological conditions in the cardiovascular system. Even though Trx1 was down-regulated after ischemia/reperfusion in isolated rat hearts, it is up-regulated in adapted hearts after ischemic preconditioning (IPC) (786) (see also section II.B.11), potentially explaining the protective effects of this powerful technique for cardioprotection (166, 530). In line with this aspect, it is not surprising that treatment with human recombinant Trx1 attenuated several pathological processes affecting the cardiovascular system. It attenuated cardiac hypertrophy in aged mice (9), reduced infarct size after reperfusion in mice and rats (768, 829), lowered inflammatory cell infiltration in rats (829), diminished severity of myocarditis (442), and protected against reperfusion-induced arrhythmias in isolated rat hearts (27). Recent studies related the protective effect against arrhythmias with Trx1-dependent regulation of expression of ventricular K+ channels (429, 444, 760). Trx1 levels in blood and serum of patients with dilated cardiomyopathy, acute coronary syndrome, or chronic heart failure were higher compared with controls, which correlated to the severity of the diseases (335, 392).
Surprisingly, knockout of TrxR1 had no effect on heart formation. TrxR1 seemed to be essential for development of most tissues, except for the heart, as heart-specific deletion of TrxR1 resulted in normally developed and viable mice (332). However, cardiac-specific knockout of mitochondrial TrxR2 resulted in embryonic lethality due to dilated cardiomyopathy and congestive heart failure (135). Moreover, knockout of TrxR2 attenuated myocardial protection after ischemia/reperfusion (298). Recently, it was described that mutations in the TXNRD2 gene may correlate with dilated cardiomyopathy in patients. The resulting proteins were not able to restore TrxR2 function in mouse fibroblasts lacking TrxR2 (713).
Although the role of Trx1 in cardiovascular pathology has been extensively investigated, only little is known about mitochondrial Trx2. Mice over-expressing Trx2 were protected against angiotensin II-induced cardiac hypertrophy and hypertension (814), generated less arteriolosclerotic lesions (863), and displayed enhanced arteriogenesis as well as angiogenesis (141).
In Grx1 knockout mice, attenuated cardiac hypertrophy was detected after induction via angiotensin II infusion (35). Grx1 knockout inhibited functional recovery and increased infarct size in coronary occlusion/reperfusion models of heart infarction (464); whereas in an earlier study, no role of Grx1 in this animal model was identified (301). In agreement, Grx1 over-expressing mice exhibited a reduced infarct size, as seen for increased Grx1 expression after IPC or gene therapy (426, 464). In addition, over-expression of Grx2 in myocardial mitochondria reduced infarct size (535). Similar to Trx1, over-expression of Grx2 protected mice against doxorubicin-induced cardiotoxicity (155). Grx3 over-expression in cardiomyocytes inhibited cardiac hypertrophy induced by treatment with enthothelin-1 and phenylephrine (337). In hearts of adult rats as well as in neonatal rat cardiomyocytes, Grx3 was up-regulated after treatment with enthothelin-1 and phenylephrine (337). This effect was mediated by interference of Grx3 with calcineurin-nuclear factor of activated T cells (NFAT) signaling (338). Using Grx3−/+ gene-targeted mice as well as Grx3 over-expressing mice as models, this specific function was confirmed in vivo (105, 338). A patient with reduced levels of Grx5 exhibited sideroblastic-like hypochromic anemia (95) (see also section II.A.3).
Both Prx1 and Prx2 knock-out mice developed hemolytic anemia (422, 545), and exacerbate formation of atherosclerotic plaques (393, 575). Prx2 deficiency suppressed angiogenesis during tumor development, supporting the important role of Prxs in cancer progression (see also section II.B.12) (368). In mice over-expressing Prx3, cardiac failure after myocardial infarction was inhibited (476); a similar phenotype was observed in Prx6 knockout mice. In an ischemia-reperfusion model, mice lacking Prx6 were more susceptible to ischemia-reperfusion injury such as increased infarct size (534). In contrast, neither mice with elevated Prx6 levels nor those with reduced Prx6 levels yielded hints for an involvement of this protein in the development of atherosclerosis (601, 803).
Mice with the targeted disruption of the Nrx gene displayed several cardiovascular defects, for example, a ventricular septal defect and persistent truncus arteriosus (216).
5. Skin
The skin is exposed to several chemical and physical injuries. Oxidative equivalents in the skin are produced, among others, by gaseous airborne environmental pollutants, UV radiation, cosmetic products, drugs, and certain food constituents/contaminants (32, 61). Trx-related proteins are already present in the skin of the fetal organism, even though they are not detectable in the surface ectoderm from mouse embryos at E8.5 and only faintly in the epidermis at E10.5. Trx1 and Grx1 immunoreactivities become very prominent in the epidermis and hair follicles of mouse fetuses at E16.5 (396). Later, in the adult mouse, 16 members of the Trx family of proteins have been identified in the different layers of the epidermis (235). In the outermost layer of the epidermis, the stratum corneum, Trx1 is the most abundantly detected Trx family protein. The three layers underneath the stratum corneum (i.e., stratum granulosum, stratum spinosum, and stratum basale) were uniformly and strongly stained for Grx2, Grx5, Nrx, Prx3, Prx4, Prx6, and Trx1. In the hair follicles and sebaceous glands, Grx2 and Grx5, Prx5 and Prx6, and Trx1 displayed strong immunoreactivities (235). In the epidermal cells of the human skin, Trx1 and TrxR1_v3 were strongly expressed; whereas mitochondrial Trx2 displayed low or no staining (143). In bovine, Grx1 was prominently detected in the epithelium, and the expression pattern suggested functions during differentiation (653).
6. Skeletal muscle
ROS and RNS are continuously generated in skeletal muscle cells and increased during contraction and fatigue (189, 267, 397). In older rats, skeletal muscles are the main sources of ROS and RNS generation (404). With the exception of Trx1 and TrxR2, all Trxs and Prxs are present in the human skeletal myocytes; the strongest expression was detected for Prx2 (143). In mouse skeletal muscle, Grx2, Nrx, Prx1, and Prx5 displayed the strongest staining. Prx5 imunoreactivity was localized in the periphery of the myocytes, close to the plasma membrane; whereas Prx2 and TrxR2 were more diffusely detected in the intercellular space, which was reported as a characteristic attribute for these proteins in several tissues (235). Supported by the findings in other tissues (for instance, mammary gland), localization of Prx2 and TrxR2 was detected in the connective tissue. In the case of TrxR2 and in contrast to other analyzed members of the Trx system, a significantly reduced expression in aging skeletal and cardiac muscle was demonstrated, which could be renormalized by caloric restriction (648).
Trx family proteins have been implied in muscular and joint diseases. Protein levels of Trx1 and Prx3 were decreased in the late phase of disuse muscle atrophy in rats. In the same study, the mRNA level of Txnip was significantly increased before the muscle loss and the concomitant decrease in Trx1 levels (478).
Human chondrocytes constitutively express Prx5, an expression that was increased in osteoarthritis (800). The cytosolic Trx system seemed to be more implicated in rheumatoid arthritis than in osteoarthritis. Trx1 was significantly increased in the synovial fluid of rheumatoid arthritis patients, and also along with TrxR1 in synovial tissues of the same patients (481). In rheumatoid arthritis patients, Trx1 was detected on the surface of the synovial lining layer and in mononuclear cells of the synovial sublining layer. Trx1 levels in synovial fluids from rheumatoid arthritis were significantly higher compared with those from osteoarthritis patients and were correlated with inflammatory indicators in the serum and synovial fluid (342).
7. Respiratory system
a. Expression of Trx family proteins in the respiratory system
The airway system is the main oxygen delivering interface between the host and the environment and is, consequently, especially susceptible to oxygen-mediated injury. The constant and combined exposure of airborne gases and particles and endogenously produced ROS and RNS requires sophisticated lines of defense. GSH plays a vital role here. The first and probably most important protective barrier is the highly heterogeneous layer of respiratory-tract lining fluid covering the respiratory epithelium. An about 100-fold higher concentration of GSH compared with serum levels underlines its general importance, combined with GSH-dependent oxidoreductases, that is, Prx6 and/or GPx3 (253). In fetal mice, Trx1 and Grx1 were detected in the epithelial cells of the airway (future bronchial and alveolar epithelia) and the lung parenchyme at E13.5, increasing at later developmental stages (396). Postnatal exposure to oxygen induces elevated expression of Trx and TrxR (148). In combination with the ROS-evoked chronic rise of intracellular buffer capacity, it supports the role of Trx and related molecules as key activators for oxidative stress-inactivated proteins and during development of the human lung (186). In the human lung, Trx1 is detected from week 35 onward and is located in the bronchial epithelium, alveolar macrophages, chondroid cells, and cells of the bronchial glandular epithelium (361). However, in the mature, adult lung, Trx1 and TrxR1 immunoreactivities were reported to be weak or moderate only in pneumocytes, macrophages, and bronchial epithelial cells (774). In a recent study, some redox proteins were detected in alveolar cells (i.e., TrxR1, GR, Prx2, and Prx6) as well as in macrophages of human lungs (i.e., Trx2, TrxR1, TrxR1_v3, TrxR2, Prx2, and Prx3) (143). Grxs have not been intensively investigated in the human lung. In a study analyzing Grx1 and Grx2 expression, only the former showed strong immunoreactivities in the alveolar macrophages and weakly positive signals in the bronchial epithelium (589).
In the adult mouse and rat, only the mitochondrial Trx system along with the cytosolic peroxiredoxins, Prx2 and Prx6, were detected in pneumocytes; whereas Grxs and Trx2 were either not or only weakly expressed (235, 359, 654). In the upper respiratory tract of the mouse, only Prx6 was highly expressed in the pseudostratified epithelium of the trachea (235).
b. Trxs, Grxs, and Prxs in pathologies of the lung—interplay between ROS and inflammation
Many airway-related disorders, including acute lung injury, asthma bronchiale, chronic obstructive pulmonary disease (COPD), and pulmonary fibrosis, display an alteration of the cellular redox profile (71, 147). Asthma has an approximate prevalence of 5% of the worldwide population and is one of the most common chronic inflammatory airway diseases (809). In several studies, a direct correlation between oxidative damage as a fundamental consequence of characteristic-reversible airway obstruction and airway hyper-responsiveness and the severity of asthma was found (532, 662, 672).
GSH was attributed a key role in the maintenance of the integrity of the epithelial barrier. Not surprisingly, inflammatory airway diseases such as asthma or COPD show altered ratios of GSH to GSSG (198, 617) and its biosynthesizing enzyme γGCS (261, 618). An increase of γGCS, especially in the epithelium, might be a counter-regulation of ongoing inflammatory responses in pulmonary diseases (626), and an external augmentation of GSH levels in the lung appears to be a logical therapeutic approach to combat COPD or asthma (74).
Asthma is a mainly a type-2 helper T-cell (Th2)-driven disease that is characterized by eosinophilia and the activation of a broad array of inflammatory cells such as macrophages, neutrophils, and mast cells. GSH depletion in mouse T-cells and macrophages leads to an impaired interferon-gamma production and favors a Th2 response, underlining its vital importance in the polarization of the immune system (599). High concentrations of circulating Trx1 were found in infectious and inflammatory diseases, inhibiting monocyte and neutrophil responses to chemokines by acting downstream of the chemokine receptors eotaxin and CCR3 (538, 564). In patients, Trx serum concentrations correlated with increasing serum eosinophil cationic protein concentrations and were conversely proportionate to the expiratory peak flow rate during asthmatic attacks (835). Mouse models demonstrated that both exogenous application of Trx1 and transgenic over-expression suppress asthmatic key features such as airway hyperresponsiveness and inflammation. The combined role as regulator/transducer of ROS and regulator of the macrophage inhibitory factor (MIF) (see also section II.B.8.b) might be the key to maintaining the integrity of the epithelial cell and, subsequently, of the healthy lung (318, 779), reviewed in (327). In addition, an intraperitoneal injection of recombinant human Trx 1 suppressed bleomycin-induced or inflammatory cytokine-induced acute interstitial pneumonia in mice, suppressed lipopolysaccharide (LPS)- or bleomycin-induced acute lung injury (539), and was also shown to efficiently prevent elastase-induced emphysema (390).
In the case of COPD, which is highly induced by smoking, an up-regulation of Trx1 and TrxR1 in epithelial cells was detected (251, 604). Grx1 was up-regulated during monocytic differentiation and highly expressed in macrophages (589, 755). The expression in sarcoidosis and allergic alveolitis patient samples is decreased, implying further functions in both inflammatory and fibrotic lung diseases. In an ovalbumin-induced murine model of allergic airway disease, an increased amount of Grx1 and total Grx-activity was detected (636). Prxs exert a broad spectrum of peroxidase activity in the lung and show a cell-type specific expression (389). Prx1 was induced in mouse peritoneal macrophages exposed to oxidative stress and in endothelial cells, fibroblasts, and leukocytes (324, 326). It functions not only as a mere ROS scavenger in the Th2-driven asthmatic phenotype, but also by suppressing the IL4 cytokine. High expression of Prx1 and Prx3 in granulomas and alveolar macrophages of sarcoidosis parenchyma suggest a significant role in the control of the oxidant burden and the progression of lung injury (389). KO-mice of Prx2 displayed an increased asthmatic phenotype along with a decreased expression of the LPS-detecting TLR-4 (512). In addition, Prx3, Prx5, and Prx6 were abundantly expressed in the bronchial epithelium, protecting epithelial cells from oxidative stress-induced cell death (34, 389). COPD patient lungs appear to express all Prxs at equal levels, and only Prx6 was found to be induced in sputum supernatant (424).
8. Infection, inflammation, and immune response
a. Expression pattern of Trx-related proteins in lymphoid tissues
The cytosolic Trx system has been detected in lymphoid cells of human lymph nodes (143). In mouse lymph nodes, Trx1 was not so strongly present, compared with the spleen, where it appeared in both functional areas: the white and the red pulp (235). In contrast, certain Trx-related proteins showed more intense immunoreactivities in defined areas. Grx2 and TrxR2 signals were more pronounced in the red pulp than in the white pulp; whereas Grx1, Grx3, Grx5, and TrxR1 immunoreactivites were more significant in the white pulp. Moreover, a difference in the subcellular localization in both zones was reported. Using confocal microscopy, Grx2a was demonstrated in the mitochondria of red pulp cells; whereas in white pulp cells, mainly cytosolic Grx2c was detected (310). In mouse lymph nodes, the expression of 16 Trx family proteins has been described (235). Grx5 and Prx2 were strongly expressed in the cortex and medulla. Grx3 was the only protein of the family that showed a specific staining pattern in the germinal centers of the lymph nodes (Fig. 11B).
b. Immune system
An adequate host immune response to viral, bacterial, and parasitic infections and airborne macromolecules is vital to regulate effector mechanisms. A proper activation of cells of the innate immune system via their so-called pattern recognition receptors has been demonstrated to play a crucial role in early shaping of the immune system. A fine balancing of Th1, Th2, and regulatory T-cell responses triggered by altered or missing innate immune cell activation depends on the reduction–oxidation equilibrium of tissues, and disturbances are implied in a broad array of diseases (231). The phagocytic cells of the innate immune system, including macrophages, monocytes, dendritic cells (DCs), and neutrophils, destroy pathogens via the NADPH oxidase-dependent formation of ROS. NADPH oxidases are membrane-spanning enzyme complexes that transfer electrons from NADPH across biological membranes to molecular oxygen, forming superoxide and its downstream metabolite H2O2 (414), as well as other radical species in the presence of myeloperoxidase, nitric oxide, or iron. Potential mechanisms killing pathogens involve changes in the phagosomal pH and ion concentrations as well as the inactivation of virulence factors, for instance, redox-sensitive elements, as described for bacterial pheromones (651), reviewed in (51). ROS can activate an increased immune response via redox-sensitive signaling pathways, including, for example, NF-κB-activation and expression of pro-inflammatory cytokines such as IL-1β and TNF-α (494, 687). This is especially important for the activation of the adaptive immunity, established by antibody-producing B cells and T cells, which depends on the binding of the T-cell receptor to a peptide bound to the major histocompatibility complex on antigen-presenting cells and additional co-stimulating and pro-inflammatory signals (139, 336). In addition, it was shown that ROS levels increase in T cells in the presence of mitogenic stimuli and during activation, for example, on antigen presentation. On the contrary, in the presence of ROS-scavenging enzymes, T cells lose their ability to respond to cytokine- or receptor-mediated signaling (473, 783). Similarly, DCs also produce ROS during antigen presentation, which is essential for cytokine production (473). Moreover, they generate cysteine from imported cystine after LPS- or TNF-α-stimulation and secrete Trx1 on interaction with T cells. Interestingly, DC activation can be inhibited by glutamate, prohibiting cystine uptake, or antagonistic antibodies against Trx. The authors hypothesized that DCs may activate T cells, which lack cystine transporters, by creating a reducing milieu in the immunological synapse, providing them with free thiols (25). This is especially important, because intracellular GSH, which is generated from cysteine, is essential in the response to mitogenic and antigenic stimuli (491). This is also valid in the case of polymorphonuclear leukocytes, reviewed in (780). GSH can inhibit the binding of NF-κB to DNA by specific glutathionylation of the p50 (605) and the p65 subunit (613).
Cytosolic proteins of the Trx family have been shown to be secreted in various cell and animal models as well as in various clinical conditions. This section summarizes these findings and discusses potential functions in the modulation of the immune response. The extracellular environment is more oxidizing than the intracellular room; the GSH plasma levels, for instance, range between 2 and 20 μM compared with cellular levels in the millimolar range (353). Therefore, the redox state of the individual proteins is believed to be rather oxidized than reduced; which is also underlined by the lack of sufficient electron donors such as NADPH or TrxR. However, a few studies show that the latter can also be secreted by some cell types (23, 722).
Besides the missing information on the specific redox state of the individual proteins and thereby their enzymatic activity, not many specific targets and mechanisms have been revealed and analyzed. It is possible that extracellular functions are not based on the reduction of disulfides, but rather the transfer of disulfides to target-soluble proteins or surface-exposed cellular structures.
Trx1 was shown to be secreted by various cell lines, including primary and cancer cells, following a secretory pathway independent from ER and the Golgi apparatus (655). The oxidoreductase was detected in blood plasma in various clinical conditions, such as cancer (541), rheumatoid arthritis (342), type-2 diabetes (363), and HIV infection (543). Indeed, Trx, secreted from human T-lymphotropic virus-1 transformed T cells, was originally identified as T-cell leukemia-derived factor, inducing the expression of the IL-2 receptor (751). B and T cells secrete detectable levels of Trx1 when activated by specific compounds. Ericson and coworkers demonstrated that activated B cells, isolated from B-type chronic lymphocytic leukemia patients and healthy donors, strongly increased the expression of Trx1, with two thirds being secreted (169). Potential functions include the reduction of ROS, which can freely pass or leak through the membranes of phagosomes, via endogenous GPx3 (66) or Prxs. Extracellular Trx1 was also shown to act as a cytokine or chemokine, that is, signaling molecules, which can either alter the cellular expression of distinct genes and transcription factors or attract and activate immune cells, respectively. Even though no specific receptor has been identified so far, exogenous administered Trx1 was shown to induce the expression of antioxidant genes, including Grx1, Grx2, SOD2, and Prx4 in human lens epithelial cells (848), of various cytokines, such as TNF-α, IL-1, IL-2, and IL-8 in monocytes and dose dependently of IL-6 in fibrosarcoma and endothelial cells (685). Furthermore, Trx regulates NF-κB activation. Trx1 translocates from the cytosol into the nucleus on TNF-α treatment and was shown to suppress NF-κB activation induced by TNF-α, phorbol myristate acetate (PMA), or IL-1 (279, 757).
Controversially, Trx1 was also shown to reduce a general inflammatory response (58). Wu and coworkers showed that the injection of recombinant Trx1 in a rat model for myocardial ischemia (see also section II.B.11), significantly reduced the number of infiltrating immune cells after 24 h of reperfusion (829). The anti-inflammatory function could be explained by regulating or rather inhibiting the macrophage migration inhibitor factor, a pro-inflammatory protein released from immune cells. MIF is characterized by the active site motif Cys-Ala-Leu-Cys and exhibits disulfide reductase activity in the insulin and the HED assay (394). Various studies have analyzed Trx1 and MIF, demonstrating potential regulatory mechanisms. Son and coworkers, for instance, showed a direct association between the two proteins (730), inhibiting the release of MIF or directly regulating protein expression, thereby inhibiting, for example, a general inflammatory response against cigarette smoking (537, 681).
The third described function as a chemoattractant in the nanomolar range also depends on the redox-active cysteine residues and was revealed as a receptor- and G protein-independent process in monocytes, polymorphonuclear leukocytes, and T cells. Trx1 might act as a redox sensor on a yet unknown substrate protein, promoting the transmigration of immune cells by its disulfide isomerase activity or generally amplifying the cellular response at a site of inflammation (58). Higher chemokine levels potentially have the oppositional effect, leading to impaired leukocyte migration and an enhanced infection rate, as described for IL-8 (718), human monocyte chemoattractant protein 1 (660), or murine CXC chemokine KC (815). Bertini and coworkers discuss that elevated, exogenous Trx levels might inhibit leukocyte migration and enhance the general infection rate, which could explain the early mortality rate of HIV patients with elevated, plasma Trx levels (>30 ng/ml), compared with patients with similar symptoms, but regular Trx levels (58, 543).
The truncated Trx80 comprises the N-terminal first 80–84 amino acids of the cytosolic Trx1 and was originally described as an eosinophil cytotoxicity-enhancing factor, which was detected in the plasma of humans infected with chronic schistosomiasis mansoni infection (150). Trx80 was detected in human B and T lymphocytes, monocytes, granulocytes, and melanomas (667), and it was shown to be secreted by monocytes and transformed leukocytes (666, 716), but is preferably localized or rather incorporated into the plasma membrane, which is externally oriented. Only minor levels of Trx1 are located to the membrane (667). The truncated protein differs from the full-length protein, because it is not a substrate for TrxR, lacks parts of the active site, and, thus, does not exhibit oxidoreductase activity (587). Nonetheless, both proteins share similar functions, such as proliferative effects on peripheral blood mononuclear cells (PBMCs) and monocytes (586, 587), induction and secretion of pro-inflammatory mediators into the plasma, role as a chemoattractant for monocytes and polymorphonuclear cells (65), and the activation of MAPK signaling pathways (585). It furthermore induces the expression of numerous “cluster of differentiation” (CD) surface antigens (584) and is involved in the production of the anti-inflammatory cytokine IL-10 (585).
The Trx-like protein Nrx functions in the innate immune response via the stabilization of the interaction of flightless homolog 1 and myeloid differentiation primary response protein MyD88, suppressing LPS-induced NF-κB activity (272). In addition, potential regulatory functions of gene expression were determined. Nrx over-expression in HEK293 cells led to an increased NF-κB activation, following stimulation by TNF-α or PMA (279).
Prx1, Prx2, and Prx4 were extracellularly detected. Prx1 was secreted from lung cancer adenocarcinoma cells and was also detected in the serum of patients suffering from nonsmall cell lung cancer, as well as through specific antibodies against the peroxidase (112). Prx2 was detected in plasma, potentially due to endogenous hemolysis or secretion by T cells, of patients suffering from multiple sclerosis with severe acute respiratory syndrome (118). Prx1 and Prx2 are also known under the name “natural killer cell enhancing factors A and B,” because they increase the cytotoxicity of natural killer cells, which belong to the innate immune system (682, 683, 701). Prx4 possesses a leader peptide and is processed and secreted from the cell via the ER and Golgi apparatus within minutes. The secreted form is potentially enzymatically active and might act in scavenging extracellular ROS or regulating biological processes via binding heparan sulfate attached to cell surfaces or the extracellular matrix (558). It regulates H2O2-induced signaling pathways, for instance, the H2O2-mediated activation of NF-κB via modulation of the inhibitory protein IκB, modulating specific gene expression and the immune response (346). Macrophages with depleted levels of Prx6 displayed increased levels of H2O2 and an elevated apoptosis rate (804).
In addition, the cytosolic Grx1 was demonstrated in the extracellular compartment. Lundberg et al. detected Grx1 in human plasma and demonstrated that the protein was secreted by unstimulated PBMCs, suggesting general extracellular functions (453). Peltoniemi et al. showed that alveolar macrophages expressed Grx1 and that Grx1 levels were decreased in homogenates of the lung and increased in the sputum of patients with COPD, with the levels correlating to the stage of the disease and lung function (588). Grx1−/− mice were characterized by lower levels of pro-inflammatory markers, after LPS stimulation, potentially due to a disruption of redox signaling via de-glutathionylation of specific proteins and signaling pathways (8). On the contrary, over-expression of Grx1 in HEK293 cells led to an increased TNF-α-and PMA-induced NF-κB activation (279). Furthermore, intracellular Grx1 was shown to affect the NF-κB-dependent expression of intercellular adhesion molecule 1 (ICAM-1), an adhesion molecule in endothelial- and immune cells, that facilitates cell-cell interactions and leukocyte transmigration into tissues. Furthermore, over-expression of Grx1 increases the secretion of IL6. Administering IL6 itself to the medium of cells also induces Grx1 and ICAM-1 expression, revealing pro-inflammatory functions in both autocrine and paracrine signaling (704). Another proposed function includes the decomposition of peroxides via the reduction of plasma GPx3 (66). Unlike Trx1, Grx1 does not seem to act as a chemokine (58).
Grx3 was identified as an interaction partner of protein kinase C-θ (819), which regulates TCR-mediated signaling and the activation of transcription factors, including NF-κB and AP-1 in antigen-stimulated T cells, reviewed in (747). Over-expression of Grx3 in T cells led to decreased phosphorylation and activation of c-Jun N-terminal kinase (JNK) and NF-κB (819). In another study, Grx3 over-expressing RBL-2H3 cells were characterized by increased degranulation, elevated activation of the transcription factor NFAT and the expression of IL-4 and TNF-α, a decreased phosphorylation state of JNK, and no changes in the phosphorylation state of ERK and NF-κB activation, implying new functions in FcɛRI-mediated mast cell activation (373).
Down-regulation of Grx3 in HeLa cells affected the expression of numerous genes involved in the organization of the cytosceleton, cytokine secretion, and processes, including apoptosis, differentiation, and migration; that is, for instance, ICAM-1, IL-8, IL receptor 4, or the dual-specific phosphatases DUSP4 and DUSP6, which regulate MAP kinase signaling (unpublished data, P. Haunhorst and C.H. Lillig).
c. Infectious diseases
Infectious diseases arise from the presence of pathogenic organisms such as bacteria, fungi, parasites and viruses, or pathogenic agents called prions. Consequently, human defense mechanisms such as the formation of ROS, pore-forming immune toxins (PFTs), and pathogen-binding immunoglobulines evolved. To overcome continuous ROS exposure by the infected host and to maintain their own intracellular redox conditions, many bacterial and parasite-specific Trx systems have developed, reviewed, for instance, in (616, 860). The pivotal role of GPx1 in viral and bacterial infections was summarized in (49). It is worth mentioning that parasitic protozoa of the order Kinetoplastida, such as trypanosomes and leishmania, lack the eukaryotic (GSH)/GR and (Trx)/TrxR system and have instead developed a tryparedoxin/trypanothione [bis(glutathionyl) spermidine; T(SH)2]/trypanothione reductase system (407, 550). However, trypanosomatids contain not only the parasite-specific tryparedoxin (451, 629), but also prominent levels of GSH and various monothiol and dithiol Grxs (101, 193, 407, 469).
Trx secreted by the host performs a variety of physiological and pathophysiological functions (see also section II.B.8.b). In addition, its role as an antibiotic agent, on the one hand, and species-specific expression as a counter-measurement to adapt to changing environmental conditions, on the other hand, are quite diverse, affecting the outcome of an infection. Numerous bacteria that multiply extracellularly, including Staphylococcus species, secrete PFTs to alter host cell membranes. Extracellular TrxR and Trx can modulate the activity of the pore-forming cysteine-containing NK-lysin, an effector peptide of T-lymphocytes (23, 722). Furthermore, secreted Trx exerts differential regulatory functions on circulating immunoglobulins by reducing intermolecular disulfides between heavy and light chains, thereby affecting the adaptive immune response (461). The potent antimicrobial peptide human β-defensin 1 is activated by Trx (688), which shows that secreted Trx from both host and intruding agents is being used to counteract respective defense mechanisms. Intracellular bacterial Trxs function as hydrogen donors, affect DNA synthesis in cell division, and regulate the transcriptome, phage assembly, and propagation (860). For instance, Mycobacterium tuberculosis resides in mononuclear phagocytes and, as most bacteria, has developed an individual set of Trxs to counteract intracellular oxidative killing (15). Prxs can detoxify cells from ROS and RNS. In M. tuberculosis, the alkyl hydroperoxide reductase AhpC and a thioredoxin peroxidase appear to play leading roles in the detoxification process (331). In the case of the diphteria toxin, secreted by Corynebacterium diphteriae, TrxR1 is essential as a part of the so-called “cytosolic translocation factor complex,” which enables receptor-mediated endocytosis of the A domain of the toxin (625). Due to the central role of TrxR as an effector enzyme for bacteria, parasites, and cancerous cells, a vast number of inhibitors was developed to trigger lethal effects (28, 48, 616).
As for viral infections, the Trx and Grx systems play a major part in both life cycle and virus-host interaction. Species-specific Grxs function in viral DNA biosynthesis when levels of the corresponding host cell proteins are depleted (619). The cytoplasmic vaccinia virus encodes its own Grx, which functions as a redox shuttle between membrane-associated enzymes that play an essential role in virion morphogenesis, assembly, and growth circle (11, 811, 812). An unusual monothiol Grx has been found in the Chlorella virus PBCV-1 genome; the fact that it is expressed throughout the entire virus life cycle implies its importance in viral replication (197). Moreover, Trx is relevant for DNA replication as a subunit of the T7 bacteriophage DNA polymerase and filamentous phage assembly (308, 659). The infection of bacteria by viruses or so-called “bacteriophages” was reviewed in (659).
Enzyme expression involved in GSH homeostasis affects host susceptibility and progression of many diseases such as cancer, neurodegenerative diseases, cystic fibrosis, and human immunodeficient virus (HIV) (780). GSH levels of HIV-infected individuals and in AIDS patients are depleted in plasma, epithelial lining fluid, PBMCs, and monocytes (83). Decreased GSH levels in viral-affected CD4+ T lymphocytes and NF-κB-dependent HIV gene activation underlines the importance of a specific GSH/GSSG ratio in HIV-positive cells (737). Oral application of N-acetyl cysteine (NAC) provided beneficial effects for HIV-infected patients and might be a sufficient tool to counteract virus-related apoptosis in lymphocytes (230). HIV has a major impact on Trx expression and distribution. Decreased expression of the Trx system correlated with a decreased rate of activated macrophages and DCs and a general higher apoptosis rate of CD4+ cells (22, 238), thereby preventing an effective immune mounting against virus-infected cells. An initial down-regulation of the pro-apoptotic Bcl-2 and Trx allows a replication boost; subsequent up-regulation might reflect a way of inducing a persistent infection (13). Innate immune mechanisms are further compromised by elevated Trx serum levels that impair CD4+ cell survival by blocking pathogen-induced chemotaxis (542). Concordantly, TrxR was found to negatively regulate HIV-1 encoded transcriptional activator Tat in human macrophages (364). Grx1 was identified at the HI-virus surface, and a regulation and/or maintenance of protease activity in HIV-1 infected cells was suggested (149). Lundberg and co-workers have demonstrated that the interaction between the viral glycoprotein gp120 and the host cell receptor CD4 is modulated by both Grx1 and Trx1. Blocking antibodies could reduce the disulfide-dependent HIV-1 entry and could, therefore, constitute novel pharmacological therapeutic targets (33, 633). Prx1 and Prx2 can be up-regulated in activated CD8+ T cells and are found in the plasma of HIV-infected patients. However, the secretion seems to be independent of the state of T cell activation (223). T cells over-expressing either Prx1 or Prx2 were resistant to HIV-1 infection, and HIV-1 replication was inhibited by the presence of the recombinant proteins in HIV-1 cultures (223, 501). This could be explained by the peroxidase function, because H2O2 promotes the NF-κB-dependent expression and replication of HIV-1 in Jurkat cells, which has been shown for cytoplasmic Prx4 and its effects on HIV infection (346).
9. Metabolic and digestive system
In the mouse liver, both Trx1 and Grx1 are already detectable by immunohistochemistry at E11.5. In contrast to Grx1, which is expressed continuously in the liver during the adult life, Trx1 expression was reported to decrease (396). Sixteen members of the Trx family were detected in the adult mouse liver. Trx1 and Grx5 were abundantly distributed in the cytosol of hepatocytes (235). In this study, clear differences in immunoreactivities between the areas surrounding the portal and central veins were reported, with the latter stronger stained for the majority of the analyzed proteins (e.g., Trx2, Grx1, Grx3, and Prx3). Other Trx family proteins, such as Grx2, Prx4, and TrxR2, appeared to be uniformly distributed in both areas. In the Kupffer cells, the specialized macrophages of the liver, Grx1, Grx3, and Prx5, were significantly stained. Prx6 was also detected in hepatocytes, Kupffer cells, and endothelial cells in the mouse liver (802). Prx6 along with Prx2, Prx3, and Trx2 were also detected in human hepatocytes (143).
During embryonic development, Trx1 and Grx1 were not detectable in acini and islet cells of the pancreas before E16.5. Both proteins are distributed through the nucleus of acinous and islet cells and also in the cytoplasm of islet cells (396). γGCS, which was weakly detected in most tissues of the adult mice, including the exocrine component of the pancreas, showed the strongest staining in the endocrine cells of the Langerhans' islets (Fig. 11C). Trx1 was detected in the cytoplasm but not in nuclei of endocrine and exocrine cells. In the same study, TrxR1 staining was more pronounced in the endocrine cells of the islets. Both Trx1 and TrxR1 expression was affected in starving mice (260). In our expression analysis of the mouse pancreas, Trx1 along with Grx3 and Grx5 showed clear nuclear staining patterns. Prx2 and TrxR2 were detected in the intercellular spaces in both the endocrine and the exocrine components of the pancreas (see Fig. 11C) (235). In the human exocrine part of the pancreas, Trx2, TrxR1, GR, and Prx2, Prx4, and Prx6 showed the most evident staining. TrxR1 and its transcript variant v3 displayed reversed expression patterns. TrxR1_v3 showed a stronger staining in the central part of the acinus cells, whereas TrxR1 staining was weak (143).
The intestinal epithelium represents a barrier between the body and the luminal environment and is exposed to oxidants generated both in the intestinal mucosa and in the lumen (e.g., ingesta and bacterial metabolites/toxins) (52, 133). Although faintly positive in the mouse intestinal epithelium at E11.5 (Trx1) and E13.5 (Grx1), immunoreactivities of Trx1 and Grx1 were very strong in the villous epithelium of the fetal intestine at E16.5 (396). Trx family proteins have been intensively analyzed in the gastrointestinal tract of adult mice (235). Since Trx family proteins are abundantly present in the different layers along the gastrointestinal tract (for details please see Table 4 and Fig. 11C), we will focus on the intestinal epithelium, where interesting expression patterns were observed (Fig. 11C). This epithelium harbors different populations of specialized cells, and the redox protein levels seem to be especially strong in some of them. Probably the most evident staining pattern has been observed in the enteroendocrine cells. These hormone-secreting cells were found to be strongly stained for Trx2, TrxR2, Grx1, Grx2, Prx2, and Nrx in the mouse stomach epithelium (235). In the duodenal enterocytes, strong immunoreactivities were detected for Grx2, Nrx, Prx5, Trx2, and TrxR1. Especially interesting is the localization pattern of certain Trx-related proteins in the intestinal epithelium (see Fig. 11C). Grx5 and Prx4 accumulated in the apical membrane, whereas Nrx was the only analyzed protein that was located at the lateral side of the enterocytes (Fig. 11C). The implication of such a “polarization” in the intestinal epithelium has to be investigated, as well as potential secretory pathways and extracellular functions of the proteins should be explored.
Table 4.
Distribution and Expression Pattern of Trx Family Proteins in the Gastrointestinal Tract from the Mouse
| |
Esophagus |
Stomach |
Duodenum |
Jejunum |
Ileum |
Colon |
||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein | E | LP | SM | MM | ME | E | LP | SM | MM | ME | E | LP | SM | MM | ME | E | LP | SM | MM | ME | E | LP | SM | MM | ME | E | LP | SM | MM | ME |
| Trx1 | + | + | + | + | + | +n | + | + | + | + | +n | + | + | + | + | +P | + | ++ | + | + | +Pn | + | + | + | + | + | + | + | + | + |
| TrxR1 | + | + | − | + | + | + | + | + | + | + | ++ | + | + | + | + | ++ | − | − | + | + | + | + | − | + | + | + | − | − | + | + |
| Trx2 | + | + | + | + | + | ++e | + | + | + | + | ++ | + | + | + | + | ++ | + | +M | + | + | + | + | + | + | + | ++ | + | + | ++ | + |
| TrxR2 | ++ | + | + | + | + | ++e | + | + | + | + | + | + | + | + | + | +P | + | +M | + | + | + | + | + | + | + | + | + | + | + | + |
| Grx1 | + | + | + | ++ | ++ | ++e | + | + | + | + | +e | + | + | + | + | + | + | + | + | + | +e | + | + | + | + | + | + | + | ++ | + |
| Grx2 | + | + | + | + | ++ | ++e | + | + | + | + | ++ | + | ++B | ++MA | ++ | ++P | + | + | + | + | ++ | + | − | + | + | + | + | + | + | + |
| Grx3 | ++ | + | + | + | ++ | + | + | + | + | + | +al | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Grx5 | +s | − | − | − | + | + | + | + | + | + | +ea | + | ++B | ++ | ++ | + | + | + | + | + | ++n | + | − | + | + | ++ | + | + | + | + |
| γGCS | + | + | + | ++ | + | + | + | + | ++ | ++ | +e | + | + | ++ | ++ | + | + | +M | + | + | + | + | + | +A | + | + | + | + | ++ | + |
| Prx1 | + | − | − | + | ++ | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Prx2 | − | ++ | ++ | + | + | ++e | + | + | + | + | +e | ++ | + | + | + | + | + | −M | + | + | + | ++ | + | + | + | + | + | + | + | + |
| Prx3 | ++ | + | + | ++ | + | ++nm | + | + | + | + | + | + | ++B | + | + | +e | + | + | + | + | ++ | + | + | + | + | ++ | + | + | + | + |
| Prx4 | ++ | + | + | + | + | ++m | + | + | + | + | +a | + | + | + | + | ++ | + | + | + | + | + | + | + | + | + | ++ | + | + | + | + |
| Prx5 | ++ | + | + | + | ++ | ++p | + | + | + | + | ++ | + | + | + | + | + | + | +M | +A | + | ++ | ++ | + | + | + | ++ | + | + | + | + |
| Prx6 | ++ | + | + | + | + | +n | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ++ | + | − | + | + |
| Nrx | + | + | + | + | + | ++e | + | + | + | + | ++l | + | + | +A | + | ++e | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
Within each gastrointestinal segment, different layers (E, epithelial layer; LP, lamina propria; SM, submucosa; MM, muscularis mucosae, ME, muscularis externa) were analyzed separately for each redoxin. Immunohistochemistry data adapted from Godoy et al. 2011 (235).
−, absent, not detected; +, present, weak expressed; ++, strong expressed; n.a., not analyzed; s, squamous cell layer of esophageal epithelium; m, surface mucous cells; p, parietal cells; e, enteroendocrine cells; n, nuclear staining; a, apical localization; l, lateral localization; B, Brunner's glands; P, Paneth cells; M, Meissner's plexus; A, Auerbach's plexus.
Serum Trx1 levels are significantly higher in patients with inflammatory bowel disease compared with controls, and over-expression of Trx1 ameliorated dextran sulfate sodium-induced colitis (758). Grx3 was reported to be strongly expressed in colon and lung cancer cells. mRNA and protein levels of Grx3 in colon cancer were the highest among several analyzed Grxs, Trxs, and Prxs (108).
a. Diabetes mellitus
The chronic disease diabetes mellitus is an increasing health issue with 220 million cases worldwide as stated by the World Health Organization in 2010. Type-I or juvenile diabetes is an autoimmune disease (207). The immune system attacks pancreatic β-cells, attenuating the synthesis and release of the hormone insulin into the portal vein. Type-II diabetes arises from constant glucose uptake and emerging insulin resistance, a condition in which insulin cannot be efficiently used anymore and β-cells progressively die from metabolic stress (721). The pivotal role of ROS/redox signaling was highlighted by the development of type-II diabetes in mice over-expressing GPx1; for an overview and details, see (425).
Consequently, both conditions prohibit the regulation and cellular uptake of glucose and lead to hyperglycemia. Long-term, chronic hyperglycemia or “glucose toxicity” can affect various tissues, manifesting, for instance, in increased susceptibility to infections, micro-vascular complications as in retinopathy and neuropathy, atherosclerosis, and cardiovascular and neurological damage (375, 850). Generation of ROS potentially via the reduction of sugars (366), the hexosamine pathway (367), and/or the mitochondrial respiratory chain (671) seems to be correlated to chronic hyperglycemia and the establishment of diabetes (78, 375) and diabetic complications (388). Moreover, mitochondrial dysfunction plays an essential role. Increases in mitochondrial ROS levels seem to lead to morphologically and functionally altered mitochondria, attenuating ATP production, glucose-dependent insulin secretion and potentially lead to apoptosis and β-cell mass reduction, reviewed in (483, 851).
Insulin secretion was shown to be inhibited by ROS; a process that could be restored by treatment with N-acetylcysteine (475) and GSH (247). In addition, NADPH was shown to stimulate exocytosis of insulin in pancreatic β cells. Cytosolic Grx1 increased this effect, whereas Trx1 counteracted the secretion (329). Knock-down of Grx1 inhibited glucose-mediated insulin secretion in distinct β-cell models, whereas knock-down of Trx1 did not do so. Furthermore, the authors showed that the excitatory effects of NADPH depended on the expression of Grx1 (632).
NADPH levels were decreased by 32%, and the general NADPH/NADP was significantly lower in streptozotocin-induced diabetic rats even though the pentose phosphate shunt did not seem to be affected. Streptozotocin is a β-cell toxin that is experimentally used to induce a phenotype resembling type-1 diabetes. Within this study, the total GSH level and the GSH/GSSG ratio were also significantly decreased (246). However, there are studies showing the opposite or no significant effect on GSH (691, 719). The Langerhans' islets of the pancreas showed the highest expression of γGCS, compared with all other analyzed mouse tissues (235). In diabetes patients, the GSH synthesis itself did not seem to be impaired; however, a lack of the GSH precursors cysteine and glycine was detected. Dietary supplementation of these amino acids or N-acetylcysteine restored GSH synthesis in platelets of diabetic patients and decreased ROS levels and, accordingly, oxidative modifications (228, 692). The expression of GR is especially high in islets and inhibition of the enzyme sensitizes β-cells to streptozotocin-induced diabetes (533). Furthermore, it was shown to be increased in diabetic patients (691, 719).
Grx1 expression and activity were decreased in platelets of diabetic patients (691, 719). Over-expression of Grx1 via gene therapy, that is, lentivirus-mediated over-expression in mice, attenuated diabetes-related cardiac pathologies in diabetic hearts exposed to ischemia-reperfusion (I/R) (426). Grx1 is known to regulate NF-κB via deglutathionylation, a hallmark in the generation of insulin resistance (712) and in various diabetes-related diseases, implying regulatory functions for Grx1 in various diabetic complications (96, 704). For instance, Grx1 was shown to be induced by glucose in rat retinal Müller cells, inducing NF-κB activation, ICAM-1 expression, and potentially diabetic retinopathy (705). Various other proteins have been shown to be regulated via de-/glutathionylation and were implicated in diabetes, for instance, aldose reductase, a substrate for Grx1, which catalyzes the conversion of glucose to sorbitol, or potassium and calcium channels, reviewed in (495).
Over-expression of Trx1 in models for type-I and type-II diabetes minimized cellular damage and improved the survival of β-cells (300, 837). Moreover, it reduced and prevented associated conditions such as diabetic embryopathy (365) and diabetic osteopenia (255). An intravenous administration of recombinant Trx1 into nonobese diabetic (NOD) mice protected islets and prevented the development of type-1 diabetes by modulating or inhibiting aberrant reactions of the immune system (125). In addition, administering recombinant Trx1 to diabetic mice exposed to myocardial I/R reduced apoptosis, infarct size, and had a positive impact on the overall cardiac function (849). This was confirmed in a different study by myocardial over-expression of Trx1 using a specific adenoviral vector in diabetic rats. Over-expression of Trx1 induced heme oxygenase (HO)-1, VEGF, and p38 MAPK-β expression; decreased the levels of phosphorylated p38 MAPK-α and JNK; and reduced the overall apoptosis rate of cardiomyocytes and endothelial cells (675). So far, not much is known about the role of the mitochondrial Trx-system. Development of diabetes induced in rats by streptozotocin led to a reduction in Trx2 mRNA in the aorta after 2 weeks, which was normalized via insulin treatment. In addition, human umbilical vein endothelial cells (HUVEC) cultured at high glucose concentrations showed around 20% reduced Trx2 mRNA levels. Knock-down of Trx2-sensitized HUVEC to glucose-induced cellular alterations, including decreased levels of free thiols, increased lipid peroxidation and increased cytochrome c expression, probably due to elevated glucose transporter 1-mediated glucose uptake and metabolism (430). Mice with the impaired ability to incorporate selenocysteine in proteins, for instance TrxR, did not seem to exacerbate streptozotocin-induced nephropathy (67).
Generally, decreased intracellular levels or inactivation of Trx1 has been correlated to increased diabetes susceptibility. Increased secretion of Trx1 or rather elevated plasma levels were detected in patients with increased glucose tolerance and manifested diabetes (507), with the latter correlating to increased nonesterified fatty acid levels (363). Nitrative inactivation of the oxidoreductase sensitives streptozotocin-induced diabetic mouse hearts to myocardial I/R injury (849). In addition, a genetic analysis revealed distinct Trx1 polymorphisms correlating with the development of diabetes (319). Especially the endogenous inhibitor of Trx1, Txnip, has gained a lot of attention in the diabetes field. Txnip has been shown to be dramatically induced by glucose, a process that is p38 dependent and mediated via inhibition of the PI3-kinase/Akt pathway during hyperglycemia (690), suppressed by insulin (700), and strongly up-regulated in diabetic animal models and patients, inhibiting Trx activity (573, 690). It is not induced by the presence of fatty acids (117). Txnip was actually the highest changing transcript in models of diabetes (623). Glucose-induced Txnip expression was associated with increased transcription of IL-1β and its precursor, as well as an elevated secretion rate (398) and induction of the intrinsic mitochondrial apoptosis pathway (120). Deletion of the protein enhanced insulin sensitivity and glucose-mediated insulin secretion, promoting adipositas but protecting against diabetes (119, 132, 621, 854). Txnip expression was moreover induced and involved in diabetic complications such as diabetic retinopathy (595, 596), diabetic nephropathy (7, 623, 766), and by glucocorticoid hormones used as treatment of numerous inflammatory and immune diseases, which in the long run can lead to glucose intolerance and diabetes (631).
Streptozotocin, H2O2, and cytokines induce the transcription of Prx1 and Prx2 mRNA in the rat insulinoma cell line INS-1 (45). Furthermore, Prx1 protein levels were shown to be elevated in erythrocytes of type-2 diabetes patients (510). Wolf and colleagues stated that down-regulation of Prx3 in rat insulinoma cells led to insufficient insulin secretion, while over-expression protected the cells against various agents, including H2O2, NO, proinflammatory cytokines, and the β-cell toxin streptozotocin (820). Transgenic mice over-expressing Prx3, on the other hand, were characterized by lower mitochondrial H2O2 concentrations and were protected against hyperglycemia and glucose intolerance (122). Prx4 over-expressing mice were generally less susceptible to streptozotocin-induced diabetes, showed less hyperglycemia and glucose tolerance, down-regulation of various inflammatory-related proteins, less infiltration of CD3+ lymphocytes, and a lower apoptosis rate (154).
It is known that not only several transcriptional and signaling pathways which are important for islet cell development, including PI3K, Wnt/β-catenin, PDX-1, TGF/Smad, and notch [for an overview on the topic see, for instance, (357)] can be redox modified, but also protocols for β-cell culture and differentiation depend on redox control; for instance, by adding reductants to the cell medium. The formation of definitive endoderm is controlled by TGF-β. By defining conditions of β-cell differentiation, it might be possible to improve the promising therapeutic strategy of β-cell transplantation. However, ROS are generated during islet isolation and transplantation, as well as general inflammation reactions, which may prevent long-term survival and regeneration of β-cells and the restored insulin secretion. Chou and Sytwu transfected healthy mouse islets with a lentivirus vector coding for Trx1, before transplantation into NOD mice. Trx1 over-expressing islets were resistant against inflammatory processes and significantly prolonged islet survival after transplantation, without showing any differences in the glucose-dependent insulin secretion in vitro (131).
Regular exercise is a preventive and, in a way, therapeutic strategy against diabetes. General exercise training was shown to affect GSH levels and enzyme activities in a tissue-dependent way in various animal- and disease models, including catalase, GPx, GR, and SOD (151, 416, 729). Trx1 levels were also shown to be increased in healthy animals on exercise, an effect that was attenuated in diabetic animals, potentially due to the increase in Txnip mRNA (417). Furthermore, exercise was shown to significantly increase Prx2 expression in obese type-2 diabetes patients (510).
10. Urinary tract and reproductive systems
a. Kidney
The kidney exhibits a high complexity due to different functional areas and segments of the nephron that execute special functions. Described expression patterns of Trx family proteins reflect this complexity; several of the oxidoreductases show segment-specific distributions. In the glomeruli of fetal and adult mice, both Trx1 and Grx1 immunoreactivities could not be detected (396). In calf, Grx1 imunoreactivity was also absent from the glomerulus, but strong staining of juxtaglomerular cells was reported, suggesting functions in the renin-angiotensin system (653). Kasuno et al. analyzed the effects of Trx1 in a renal ischemia and reperfusion model (372). In sham-operated mice, Trx1 showed strong immunoreactivity in the cortex, but weak staining in the medulla. The same expression pattern was observed while analyzing Trx1 mRNA and protein in rat and human kidneys, with a strong expression in the cortex, particularly in the proximal tubules, compared with the distal segments of the nephron (7). Txnip mRNA and protein levels were, in contrast to Trx1, most abundantly expressed in the glomeruli and in the distal nephron of rats and human kidneys. Recently, an exhaustive expression analysis of the Trx family in the mouse kidney confirmed this expression profile (see also section II.B.11). Although Trx1 staining was abundantly distributed over the whole mouse kidney, it was particularly strong in the cortex, specifically in the proximal tubule cells (235). Such a regional strong distribution was also observed for Grx2 and Prx3 in the cortex and the medulla. In contrast, Prx2 was more abundant in the medulla, and γGCS immunoreactivity was especially strong in the outer medullary area. Prx2 and TrxR2 immunoreactivites were described as the strongest in glomeruli and renal connective tissue. Prx2 was also detected in podocytes of rat kidneys (304). All proteins were present in the proximal tubule cells, where Grx3 and Prx1 display strong nuclear localization. In the human kidney, several Trxs and Prxs have been detected in glomeruli and tubule cells. Prx5 was found in endothelial cells, forming the lining of the Bowman's capsule (143). Prx6 was abundantly detected in renal cells (802).
The clinical involvement of Trx family proteins in the kidney has been analyzed in renal ischemia and reperfusion (236, 372); see section II.B.11, in diabetic nephropathy (7, 256) and in angiotensin II-induced podocyte injury (304). The Trx system seems to play a role in the progression of diabetic nephropathy, because the over-expression of Trx1 in mice led to the suppression of pathophysiological changes after streptozotocin-induced diabetes (see also section II.B.9.a) (256). The increased mRNA levels of Txnip after the induction of diabetes in rats supports these findings (7). The podocytes are essential components of the glomerular filtration barrier. In rats, angiotensin II treatment and podocyte-specific over-expression of angiotensin II type-1 receptors led to a decrease in Prx2 expression, implying a role of the peroxidase or rather increased H2O2 levels in angiotensin II-induced podocyte injury (304).
b. Urinary bladder
Members of the Trx family are also significantly expressed in the urinary bladder. The epithelial cells of uriniferous tubules express Trx1 and Grx1 from day E11.5 onward up to adult age (396). In the urothelium, most Trx family proteins showed stronger immunoreactivities in the basal cells as compared with the superficial cells, where only Grx2 and Grx5 showed strong immunoreactivity (235).
c. Male reproductive system
Tissues of the reproductive system are generally diverse due to distinct functions, hormonal status, high differentiation rates, and the stage of cellular development of primary reproductive cells. Interestingly, reproductive organs, especially testis, are equipped with distinct sets and even specific isoforms of antioxidants and Trx family proteins (see Table 5). However, not much is known about the specific functions of these proteins in the testis, even though disulfide formation and isomerization are important during sperm development and maturation (744). Selenium was shown to be essential for this process, which was confirmed by the findings that the selenoproteins GPx4 and TrxR3/TGR function in the disulfide-dependent sperm formation (549, 744, 794), thereby being essential for fertility. In addition, TXNDC2/Sp-Trx1 might also play a role in disulfide formation (343); whereas TXNDC8/Sp-Trx3 seems to regulate proteins via post-translational modifications, controlling germ-cell-specific functions (345).
Table 5.
Testis-Specific Proteins and Isoforms from the Trx Family
| Protein isoform | Cell types | Specific properties | Reference(s) |
|---|---|---|---|
| mouse Grx2c and Grx2d | Spermatogonia, spermatids | Unlike Grx2c, Grx2d is inactive and does not form iron-sulfur cluster bridged dimers | (310) |
| human Grx2b and Grx2c | Spermatids, Sertoli cells, cancer cells | Unlike Grx2c, Grx2b does not form iron-sulfur cluster bridged dimers | (447) |
| TXNRD1_v3 | Leydig cells, cancer cells | TXNRD1_v3 guides actin polymerization in relation to cell membrane restructuring | (144) |
| TGR and TGR-1 | Elongating spermatids | CUG serves as an inefficient start codon in mouse and rat | (224, 744) |
| TXNDC2/Sp-Trx1 | Spermatids | Nucleation center in fibrous sheath (suspected) | (498) |
| TXNDC3/Sp-Trx2 | Spermatids | Structural component of fibrous sheath (suspected) | (663) |
| TXNDC8/Sp-Trx3 | Spermatids | Likely required in later steps of spermiogenesis or mature spermatozoa | (345) |
| Txl-2 | Mainly associated with cilia and flagella | Novel regulator of microtubule physiology (suspected) | (664) |
| snGPx | Spermatocytes and early spermatids | Restricted to late stages of spermatogenesis | (515) |
| Prx4 31kda | Membrane bound in the elongating spermatid and the residual body | Acrosome formation during vesicular reorganization in spermiogenesis | (558, 679) |
The seminiferous tubules of the testis show a high of rate cell division, and Trx family proteins are present in these tubules in mice, as well as in the interstitial cells. The Trx fold proteins display a high variability in the testicular tissue, as several testis-specific proteins have been detected in distinct cell types. In Leydig cells, which function in the testosterone production, several proteins were strongly expressed, that is, Grx2, Prx2, Prx3, Prx5, Trx1, Trx2, and TrxR2 (235). Trx1 was highly expressed in these cells, showing a strong correlation with nuclei; a similar strong Trx1 immunoreactivity was also reported for calf Lydig cells (653). In contrast, virtually no signals were detected for the analyzed proteins in Sertoli cells, a specialized cell type that harbors the spermatogenesis. In contrast, strong staining for Grx1 was reported in bovine Sertoli cells (653). In mice, Grx1 displayed a strong staining mainly in the nuclei of progenitors cells, that is, spermatogonia. The developing spermatocytes abundantly express Grxs, Trxs, and Prxs. In early spermatids, Grx2 staining is characterized by a cup-like pattern capping the nucleus (235, 309). Further analyses will have to define the localization of Grx2 in this cellular stadium, for instance, in the acrosome, the anterior end of the head of a spermatide, which is essential for ovum penetration during fertilization. Grx3 also showed a notably strong nuclear staining in the early spermatids. In elongated spermatids, Prx5 and Trx2 were highly expressed in the mitochondrial rich tail region; whereas Grx2 and TrxR1 signals were situated in the acrosomal region (235).
In the human prostate, only TrxR1, GR, Prx3, and Prx6 were reported to display strong staining in both glandular cells and stroma (143). Malignant transformation of cells from the reproductive system displayed characteristic alterations of the redox systems (795). The invasiveness of human prostate carcinoma cell lines was shown to correlate to levels of ROS/RNS and the GSH/GSSG ratio. The invasive PC3 cells were characterized by a comparably lower amount of ROS/RNS, lower lipid peroxidation, and an increased GSH/GSSG ratio during cell growth (523). An up-regulation of the Trx system may play important roles in prostate cancer progression and responses to personalized cancer therapies. The majority of androgen-independent or hormone refractory prostate cancers expressing androgen receptors and Prx1–4 were up-regulated, regulating the receptor's activity (46, 578, 709).
d. Female reproductive system
As mentioned earlier, ROS are essential for reproductive processes. It was shown that ROS play an important role during ovulation and that suppression of an inflammatory response, as well as SOD or catalase, inhibit ovulation (210). Several members of the Trx family of proteins have been identified in the stroma and follicles cells of the mouse ovary as well as in the oviduct (235), with the highest expression detected for Trx1 and TrxR2. TrxR2, as well as Prx2, which was absent in these cell types, was expressed in the extracellular matrix of the ovarian stroma and the corpus luteum. In calf, Trx1, but not Grx1, was demonstrated immunohistochemically in follicular cells in the ovary. Grx1 immunoreactivity was particularly strong in both bovine and rat oocytes (237, 653). Grx1 was also suggested to play a role in corpus luteum regulation, because it was detected at different periods of the luteal phase in the human corpus luteum (220).
Trx family proteins have been shown to correlate with poor prognosis in ovarian cancers. A low cytoplasmic expression of Trx correlated significantly with better progression-free survival (824). In ovarian carcinomas, cytoplasmic Prx4 expression was associated with a better prognosis, whereas cytoplasmic Prx5 and 6 were associated with a higher stage (370).
In the mouse uterus, the expression pattern of 16 Trx family proteins was described in the endometrium (235). Several proteins showed stronger expression signals in specific areas, such as the uterine gland epithelium (Grx3, Prx3, Prx4, Prx6, and Trx1) compared with the surface epithelium. In term pregnant women, both Trx1 and Grx1 expression was increased in the cervix compared with nonpregnant individuals; the proteins are believed to be involved in cervical ripening (457, 668).
11. Ischemia and hypoxia
Conditions of insufficient oxygen supply to the whole organism or to single organs or tissues, for instance by insufficient blood supply, were implicated in various pathologies due to the general inhibition of proliferation and induction of apoptosis. However, oxygen concentrations are especially important in the regulation of embryonic development, often determing cell fate. Hypoxia regulates the survival and promotes proliferation and differentiation of some cell types, including neural crest stem cells (518) and CNS precursors (742). Human embryonic stem cells cultured under low oxygen concentrations proliferate, but do not differentiate, a process that in this case depends on hyperoxia (172). The same is valid for placental development (3). The formation of the vascular system is also oxygen dependent, because the proliferation of endothelial cells (602) and hematopoietic progenitors (145) depends on low oxygen concentrations. Of course, oxygen levels also regulate the development of the fetal and postnatal lung. Hypoxia modulates the expression of angiogenic factors and potentially affects lung microvascular development and lung morphogenesis (696). Transitional changes that occur in the pulmonary blood vessels at birth and with the postnatal adaptation to the extra-uterine environment are accompanied by an abrupt increase of NOS activity (30) and the Trx/TrxR system (148), as well as the differential regulation of Prxs (211, 378).
Cells have developed response mechanisms to cope with low oxygen concentrations. Hypoxia-inducible factors (HIF) 1 and 2 constitute transcription factors that regulate the expression of more than 180 genes under hypoxic conditions. HIF comprises two subunits, HIF-1α and the nuclear translocator HIF-1β. On normoxia, the α-subunit is degraded by the proteasome after hydroxylation of oxygen-sensing prolyl hydroxylases (PHD); while HIF-1β is constitutively expressed. On hypoxia, PHDs are inhibited, preventing HIF-1α ubiquitination and degradation, and the protein accumulates. The α-subunit translocates into the nucleus, dimerizes with HIF-1β, forming HIF-1, which can then regulate gene expression by binding to hypoxia-responsive elements, regulating angiogenesis, erythropoiess, vasomotor control, energy metabolism, and cell survival (4, 492, 694, 695). The HIF-1 target anti-TNFa-induced-apoptosis was shown to protect cells against hypoxia-induced apoptosis via Trx2 and the generation of ROS (130). Moreover, hypoxia-induced mitochondrial ROS are essential for stabilizing HIF-1α and HIF-1β, ensuring HIF activation (466, 677). Among others, they also regulate inflammatory responses via IL-6 production (581) and apoptosis via p38 phosphorylation (412). Increasing mitochondrial ROS, as well as exposing cells to H2O2, induced an activation of HIF-1 under normoxic conditions (111). Controversial reports state that nitric oxide is involved in the regulation of HIF-1, either by inhibiting HIF-1 activation (725) or by stabilizing and increasing the levels of the active HIF-1α subunit and the DNA binding capacity of HIF-1 under oxygen deprivation (38, 383) and normoxia (569), with the latter being potentially important on inflammation (676). NO potentially inactivates PHDs via nitrosylation, as seen in HEK293 cells treated with S-nitroso glutathione (492), or can nitrosylate HIF-1α at a Cys residue within the degradation domain, preventing its destruction (431). Another regulatory mechanism of HIF-1 is the generally increased translation due to phosphorylation of essential proteins such as by phosphatidylinositol 3 kinase/Akt-dependent or MAPK signaling pathways (695). Stress- and MAP kinase pathways represent cellular mechanisms to cope with altered O2 levels. Over-expression of Trx1 led to elevated HIF-1α levels in cells cultured under normoxic and hypoxic conditions, whereas inhibition of TrxR1 activity blocked the activation of HIF-1α (513). Zhou and coworkers confirmed these data, showing that Trx1 increased the levels of HIF-1α by activating Akt-dependent translation. Trx1 might also be involved in depleting HIF-1α levels on reoxygenation (339). Over-expression of mitochondrial Trx2, on the other hand, either prevented or diminished hypoxia-induced HIF-1α accumulation (869). In addition, there are mechanisms regulating hypoxia-induced gene expression, independently from HIF. For instance, Txnip mRNA and protein levels rapidly decreased on hypoxia, potentially due to a cascade of changes preventing the activation of the MondoA:Mlx transcription factor and the binding to the carbohydrate response elements in the Txnip promoter. The authors hypothesized that down-regulation of Txnip is essential for cancer cells, adjusting their metabolism to the hypoxic conditions (106).
Tumors develop due to elevated cell- or tissue growth, exceeding the general blood supply; with tumor hypoxia correlating to poor prognosis, increased tumor growth, and resistance to drug and radiation therapy (264). In addition, over-expression of HIF-1α was accompanied by an elevated mortality rate in patients suffering from distinct cancer types (695). Many Trx family proteins show distinct expression patterns in cancer (see also section II.B.12). Prx1, for instance, was over-expressed in several cancer cell lines (840, 841) and up-regulated in A549 cells, after 4 h of hypoxia and 2–24 h of reoxygenation (385). Knock-down of Prx1 protein levels in lung carcinoma cell lines generally impaired cell growth, and transplantation of xenografts into nude mice resulted in delayed formation of tumors and metastasis and higher sensitivity toward irradiation therapy (123); see also section II.B.12. These proliferative and anti-apoptotic functions of the peroxidase Prx1 were confirmed in human lung cancer 1170i cells and explained by the inhibition of the JNK-signaling pathway via an interaction with the GST-JNK complex (385, 386). Both HIF-1α and Prx1 were appointed potential targets in cancer therapy. In addition, Prx3 stably over-expressing thymoma cells were also more resistant to hypoxia-induced production of H2O2 and apoptosis (551). On the contrary, retinal ganglion cells cultured in a hypoxic atmosphere showed reduced Prx6 levels, increased levels of ROS, NF-κB-activation, and induction of apoptosis. This potential dysregulation of redox signaling events can be prevented, by over-expressing the peroxidase, resulting in reduced hypoxia-induced cell death and neuroprotection. The authors speculate that Prx6 could be used in the clinic, intervening with the progression of hypoxia-related disorders such as glaucoma (784). Moreover, treatment with recombinant Trx1 protected retinal ischemia-reperfusion injury in rats (673)
Wound areas are susceptible to hypoxia as a result of tissue damage. Due to the loss of microcirculation and the presence of transmigrating inflammatory cells, the wound gets hypoxic, with an oxygen gradient being present between the last perfused capillary and the wound space. Angiogenesis, the formation of new blood vessels, is also induced by hypoxia and promoted in wound healing (602). Trx1 has been analyzed in burn injuries, demonstrating, among others, a potential function in wound healing (2), which is also seen for N-acetylcysteine (20).
Other pathological conditions, including cerebral stroke (please see also section II.B.2) and heart infarction (compare with section II.B.4), are triggered by hypoxic insults, induced by the blockage or general reduction of the blood flow, resulting not only in the lack of oxygen but also ATP and other nutrients. The return of blood, although necessary, leads to dramatic consequences in the injured tissue. This process, called ischemia-reperfusion injury, induces molecular and cellular changes, affecting cell morphology, cell polarity, osmoregulation, protein synthesis, and, for example, in the brain, release of neurotransmitters (156, 351). The reoxygenation phase can be divided into (i) an early, acute phase, induced by O2, O2−·, and rapid changes in the redox properties of the affected tissue and (ii) the late, subacute phase, induced by increased cytokine and chemokine levels and the infiltration of immune cells (173). Not surprisingly, members of the Trx family have been described to protect against ischemic injuries. Increased levels of Trx1, Grx1, Grx2, and Prx2 by either over-expression or treatment attenuated ischemic damage of neurons (70, 752, 868) and cardiac cells (426, 464, 535, 768, 829), respectively (see also sections II.B.2 and II.B.4).
Besides the pathological conditions, I/R injury is also induced surgically and during cell and organ transplantation. Lung transplantation in patients with progressive pulmonary diseases is limited by early graft dysfunction and rejection, implicated with I/R injury and elevated secreted Trx1 levels in the bronchoalveolar fluid (580). Even though Trx1 is a potential marker protein for graft rejection, a different study has demonstrated that pretreatment of rat donor lungs with Trx1 decreased tissue rejection, due to inhibition of I/R injury, NF-κB activation, and the inflammatory response (see also section II.B.8) (312). Hepatic I/R injury is induced by liver transplantation. The expression of Prx1 and Prx2 was shown to be induced by I/R in transplanted organs (702). Moreover, over-expression of Prx5, which among other organelles is located in mitochondria, decreased hepatocellular injury in a rat model for liver transplantation (828). Hepatic I/R injury was shown to affect mitochondrial function; indeed, the expression of 234 proteins was altered in a mouse model for I/R, detected by a proteomic analysis of liver mitochondria. Interestingly, Prx6 was shown to translocate from the cytosol into mitochondria after I/R. The peroxidase seems to function in the degradation of mitochondrial H2O2, because Prx6 knockout mice demonstrated increased levels of the second messenger, mitochondrial dysfunction, and hepatocellular injury (164). The important role of Prx6 in I/R injury was confirmed in a different study using Prx6 knockout mice in a model for myocardial I/R injury. Compared with wildtype organs, knockout hearts showed reduced recovery of the left ventricular function, increased myocardial infarct size, and elevated levels of apoptotic cardiomyocytes (534).
We have recently analyzed the expression of 16 Trx-related proteins in a mouse model for renal ischemia-reperfusion injury, revealing nephron segment-specific responses of Trx family proteins to the ischemic insult (236). I/R kidneys showed significantly increased levels of Trx1, Trx2, and Grx5 and decreased levels of Grx1, which might have resulted from specific secretion of the protein into the urine. Moreover, an analysis of contralateral kidneys revealed increased Grx5 and Prx6 levels and decreased protein levels of TrxR2, Prx4, and Prx5. We believe that these differences contribute to the distinct susceptibilities of different parts of the nephron toward the I/R insult; the glomeruli and the inner medulla cells, for instance, have been described to be very resistant, while medullary thick limb cells are extremely susceptible (234, 314). Moreover, Trx family proteins probably function in a systemic inflammatory response, due to their versatile extra- and intracellular functions (see also section II.B.8.b).
Trx1 and Trx2 are expressed in mTAL cells, and Kasuno et al. analyzed Trx1 over-expressing mice demonstrating attenuated reperfusion-induced mTAL injury (372). Induced by I/R, protein levels of Grx2, Prx3, and Prx6 were significantly increased in proximal tubule cells (236), a cell type that is characterized by the ability to regenerate after an I/R insult (511, 553). Over-expression of these proteinss in HeLa and HEK293 cells attenuated oxidative damage to the DNA and led to a higher cell survival and proliferation rate, compared with controls, implying functions in the regeneration process of cells after I/R (236). In addition, Yang and coworkers demonstrated that Prx5 is essential for the regulation of kidney homeostasis under hypoxic conditions, for example, in terms of mitochondrial function and fatty acid metabolism (843).
So far, no effective therapeutic approaches to prevent or treat the damage caused by the hypoxia/ischemia insult are available. However, since the middle of the twentieth century hypothermia has been used as a clinical approach to reduce I/R injury, for instance, of the brain (487, 496) or the cardiovascular system (322, 402). The mechanisms underlying the protective effects are not fully understood. However, it was shown that hypothermia reduces ROS levels (99), NF-κB activation, and inflammation (806). In a proteomic approach, 1089 proteins were shown to be differently expressed in human coronary artery endothelial cells when cultured at 25°C, compared with at 37°C. These proteins were assigned to different categories, including oxidoreductase activity, cell redox homeostasis, and response to stress. Grx1, Prx2, Prx4, Prx6, and mitochondrial SOD showed changes. However, only Trx1, TrxR1 isoform 5, TrxR3, Prx1, GST π, and GST ω-1 were significantly increased; whereas protein-glutamine γ-glutamyltransferase 4, PDI 1, and PDI 4 were decreased. The reduction of the temperature to 25°C did not seem to affect the ratio of GSH/GSSG or the levels of reduced protein thiols and glutathionylation. These cold-adapted cells, as well as cells grown constantly at 37°C, were exposed to 0°C, which was followed by a rewarming period. Cold-adapted cells showed increased levels of glutathionylated proteins, higher levels of reduced protein thiols, due to higher activities of Trx, TrxR, and GSTs (872). Hypothermal preconditioning can be compared with general IPC, where resistance to I/R damage is induced by exposing a tissue to several ischemic episodes (516). IPC induced ROS, which are believed to alter various cellular targets including transcription factors, explaining the potential cyto- and tissue-protective role of this approach; for a detailed review, see Ref. (669). In a cardiovascular rat model, IPC increased Trx1 levels and the translocation from the cytosol to the nucleus, where it binds to Ref-1 and induces NF-κB and Akt1. Suppression of Trx1 by shRNA in rat hearts diminished cardioprotective effects of the preconditioning (463). IPC also induced production of ·NO, increased mitochondrial function, and altered the expression levels of various proteins, including mitochondrial SOD; see Ref. (524) and references within. Another approach is the so called “postconditioning,” which is defined as brief intermittent cycles of ischemia alternating with reperfusion applied after the ischemic event. This approach is important for the treatment of patients, when the reperfusion period is induced and has been proved to have protective effects on the affected tissues, for instance, cardioprotection. Both approaches were discussed and compared in Ref. (797).
12. Cancer
a. Carcinogenesis
Malignant transformation is the consequence of numerous dynamic changes in the genome, regulation of transcription, signaling pathways, proliferation, cellular architecture, and cell-cell interactions. Since essentially all of these cellular functions are at some level controlled by redox signaling, it comes as no surprise that Trx protein family members play a pivotal role in the process of carcinogenesis. Many aspects of the potential role of Trx family members both as oncogenes and as tumor suppressor genes have been extensively analyzed and reviewed earlier; for the Trx system, see, for instance, (29, 84, 362, 541, 693); for the Grx system (432); and for Prxs (87, 544, 728, 830). The various roles of the proteins in tumor development and progression are as complex as the multiple redox-regulated signaling pathways that contribute to malignant transformation. It is, thus, not possible to plainly classify Trx family proteins as oncogenes or tumor suppressor genes.
Trx1 was up-regulated in various cancers investigated, that is, from liver (540), lung (222), and colon (54). Trx may promote the growth of tumors, and its levels are negatively correlated with apoptosis in cancer cells (243). On the other hand, mice transgenically over-expressing human Trx1 do not show an increase in malignant diseases; instead, their life expectancy is increased (see also section II.B.13) (504, 594, 752, 852). The DNA-binding activity of the tumor suppressor p53 is controlled by the redox state of some critical cysteinyl side chains in its DNA-binding domain (252, 577). The redox state of these residues appears to be regulated by both Ref-1 and Trx. Trx can stimulate the DNA binding activity of p53 and potentiate Ref-1-stimulated p53 activity both in vitro and in vivo (789). In yeast, TrxR mutants failed to induce p53-dependent gene activation (100, 582).
Cytosolic Grx1 and mitochondrial Grx2a expression showed a significant correlation with the degree of differentiation in adenocarcinomas and an inverse correlation with proliferation (183). By mechanisms of alternative transcription initiation and alternative splicing, two nonmitochondrial Grx2 isoforms, Grx2b and Grx2c, are generated, whose expression in humans could so far only be demonstrated in testis and various tumor cells (447). Contradictory to a potential role as oncogene, Grx2c reduces the proliferation rate in stably transfected cells (183).
In many aspects, Prxs appear to qualify as tumor “preventers”; for an elaborate discussion on this topic, see (544); however, many cancer cells showed increased levels of Prxs, and their expression sometimes correlated with progression. Epigenetic down-regulation has been demonstrated for Prx1 in 1p/19q-deleted oligodendroglial tumors (158), Prx2 in acute myeloid leukemia (10), and Prx4 in acute promyelocytic leukemia (568). Prx1 interacted with a region of the c-Myc transcriptional regulatory domain that is essential for transformation. Therefore, c-Myc-mediated transformation was inhibited, implying a tumor suppressor role for Prx1 (163, 531). Moreover, Prx1 inhibits tumorigenesis via regulating phosphatase and tensin homolog (PTEN)/AKT activity; binding of Prx1 protected PTEN from oxidation-induced inactivation (97). Corroboratively to these findings, Prx1 knockout mice showed a higher rate of age-dependent malignancies (163). On the contrary, increased levels of Prxs have, for instance, been described for Prx1 in ovarian cancer proximal fluids (299), and Prx3 in lung cancer (387). Srx and Prx4 promoted the progression of human lung cancer (807).
13. Aging
In the 1950s, Denham Harman hypothesized that the aging process is linked to species-specific metabolic activities, which depend on heritage and various stress factors. He proposed the so-called “free radical theory of aging,” depicting the production and over-time accumulation of ROS and the irreversible macromolecular damage, as main reasons for the aging process (262). The special contribution of mitochondria was demonstrated later in 1972 (263). This theory was supported by a body of evidence, including that (i) cells grown in hypoxia have a prolonged lifespan (562), while cells grown under hyperoxia have a reduced lifespan (861); (ii) cells treated with extracellular H2O2 undergo rapid, senescence-like growth arrest (124); and (iii) caloric restriction was shown to enhance the lifespan in a wide range of organisms from yeast to mammals (194, 472). Moreover, various studies in distinct cell and animal models have demonstrated an age-related increase in the intracellular ROS levels (134, 726), mitochondrial dysfunction (427, 635, 680), accumulation of oxidative-damaged mitochondrial (192, 834) and nuclear DNA (50), oxidized lipids, and protein-mixed disulfides (68, 628), with the latter also being the result of disturbed degradation and repair mechanisms of oxidatively modified proteins (176, 790). Intracellular as well as plasma GSH/GSSG ratios are known to decrease with age (628) and in age-related diseases such as atherosclerosis (31), macular degeneration (see also section II.B.3.b), and type-2 diabetes (674) (see also section II.B.9.a). The expression and/or activity of various mammalian Trx family and related proteins is also known to be reduced during aging, including, for instance, Trx1 (21, 832, 864), Trx2 (439), TrxR1 (832), TrxR2 (37), GR (68), Grx1 (69, 832), and Prx3 (439).
Mutations in various genes that interfere with the aging process have been characterized. These encode for proteins functioning either in the regulation of energy use or in redox regulation and signaling, including, for instance, catalase (770) and Prx (194, 775). Various model organisms, including Sacharomyces cerevisiae, Cenorhabtitis elegans, Drosophily melanogaster, or Mus musculus, depleted from SOD (446), Trx (748), TrxR Prx, and PDIs (250), were characterized by a reduced lifespan, reviewed in (334). Vice versa, protein over-expression can lead to a prolonged lifespan, which is especially significant in the case of Trx1, where protein over-expression led to a 35% increase in median and a 22% increase in maximum lifespan (504, 594). However, the absence of individual genes or proteins not always leads to a life-shortening phenotype; for instance, in SOD2−/− (634), SOD2−/− and GPx1−/− (866), or Trx2+/− mice, reviewed in detail in (334), which might be explained by compensatory effects of their functional homologs. Similarly, the over-expression of these antioxidants does not necessarily lead to an elongated lifespan (593). Despite all this supporting evidence, the contribution of ROS to organismic changes is highly controversial (574), because, first of all, ROS formation is very complex and verification procedures are difficult; and second, numerous studies did not confirm Harman's theory, which originally implied that the targets of ROS were random, indiscriminate, and cumulative (194)—which, as we know more than half a century later, is not true.
A different hypothesis suggested that mitochondrial mutations are created during replication errors during embryogenesis and then undergo clonal expansion in adult life, leading to mitochondrial and age-related diseases (574). The so-called “mutator mouse” expresses a proofreading-deficient mitochondrial DNA polymerase, leading to the accumulation of point mutations in the mitochondrial DNA, impaired respiratory chain functions, and premature aging (574). This phenotype was shown not to be associated with significant increased levels of ROS or a cellular anti-stress response (781), confirming the findings of the comparable Tfam knockout mice (799). However, even though these models disagree with the “free radical theory of aging,” they do not exclude redox regulation. Many aging-related proteins are specifically redox regulated, including the lifespan regulator p66Shc (225, 226), MAP kinases (90, 94, 302, 303), the Trx-dependent Msr (89, 520, 600), as well as processes including protein folding and degradation (57, 470, 536). Furthermore, recent findings correlate specific oxidative changes to the aging process, demonstrating that these changes are distinctly redox couple-, subcellular compartment-, and tissue-specific. Using genetically encoded redox probes in Drosophila melanogaster, the authors showed that age-dependent pro-oxidants were not equally distributed throughout the organism, but rather restricted to specific regions. Moreover, they showed that the increased lifespan of the chico1/+ mutant strain, carrying a mutation in the insulin/IGF signaling pathway, is correlated with increased oxidant levels (18), confirming previous data supporting a potential beneficial role for free radicals in longevity, reviewed in (593) and (415).
In the future, we might be able to assess oxidative changes during aging, elucidate critical mechanisms, and understand the impact of these modifications on the aging process as well as on age-related pathological conditions.
C. Therapeutic approaches
As described earlier, proteins of the Trx family show specific alterations in various pathological conditions, including changes in protein expression, enzymatic activity, tissue distribution, and intra- and extracellular localization. In the future, we might be able to use these findings in the clinic and apply Trxs, Grxs, and Prxs as specific biomarkers in early diagnosis, disease progression, or in order to determine the state of a disease, allowing for a more precise prognosis and choice of treatment.
The secretion of specific Trxs, Grxs, and Prxs has been described in various clinical disorders (see section II.B.8.b), and the analysis of, for example, sputum, blood, or urine samples for specific proteins has been done through a well-established and noninvasive approach to confirm potential pathological changes in patients. Elevated levels of Trx1 in HIV-infected patients have been correlated with a reduced immune response, an increased progression of the infection, and an early mortality rate (543). Grx1 was only detected in the urine of mice after I/R. This specific secretion of the oxidoreductase into urine has to be confirmed in patients; but might be applied as a marker for renal I/R injury in the future (236). Moreover, specific auto antibodies against Trx-related proteins could be used as diagnostic markers (112).
The specific expression and distribution of Trxs, Grxs, and Prxs is especially obvious in various cancer pathologies, with elevated protein levels of Trx1 or even the presence of cancer-specific Grx2 isoforms (see section II.B.12). These potential tumor markers could help determine the state of the disease and establish a patient- and disease-specific treatment. Even though Prxs are also highly expressed in various cancers, they cannot be implicated as medical biomarkers, due to the general high cellular abundance of these peroxidases. However, clinical information on the expression of Prxs might help determine the best medical care and improve the outcome of radiation therapy (825, 862).
Targeting the proteins that increase the proliferation or differentiation of cells and which are implicated in the development and progression of cancer cells (see section II.B.12) has been an ongoing challenge. So far, no inhibitors for Grxs have been found, but various drugs were shown to affect proteins of the Trx family. Here, a prominent role is attributed to TrxR—the key enzyme of the Trx system, which also bears unique functions of its own (see also section I.A.2.a). Various drugs, foremost electrophilic compounds that target the selenocysteinyl residue in TrxR, have been developed, inhibiting the oxidoreductase activity. For an indepth discussion on this topic, we refer to the following articles: (29, 92, 591, 610, 693, 777, 793). Due to the finding that the mammalian reductase differs dramatically from the bacterial or parasitic enzyme, numerous inhibitors have been developed to counteract infectious diseases; for instance, affecting the malaria parasite Plasmodium falciparum (24, 48, 616). In addition, specific substrates for TrxR have been subjected to clinical trials. For instance, ebselen, reviewed in (175, 452), a “chemical mimic of GPx,” which was shown to reduce H2O2 levels and oxidize Trx (867), was analyzed as a potential drug, for example, for cerebral ischemia (579) and stroke (836). Another target of TrxR is motexafin gadolineum. The drug has been suggested as a radiosensitizer in cancer therapy due to its tumor-specific uptake and its induction of ROS (201, 268). In the future, a strategy might be developed to specifically target Prxs in cancer cells, which contribute to resistance against radiotherapy (862).
It has been shown that the induction of protein expression, the addition of recombinant proteins, or over-expression via gene therapy is beneficial in various pathological conditions in distinct cell- and animal models, as well as in medical patient-based studies. Especially the treatment with recombinant Trx1 was described to decrease several pathological conditions affecting, for instance, the cardiovascular system, reviewed in (477) (see also section II.B.4.b), retinal cell damage (323) (see also section II.B.3.b), diabetes (125, 131, 849) (see also section II.B.9.a), and pathologies of the lung (see also section II.B.7.b). It was also shown to have a positive impact in the therapeutic approach of transplantation (see also section II.B.11). Pretreatment or preconditioning of rat donor lungs with the cytosolic oxidoreductase abolished tissue rejection (312) and transfection of mouse donor pancreatic β-cells with a Trx1 lentivirus vector extended cell survival after transplantation (131). Interestingly, Trx also bears functions in reducing allergenicity to, for instance, milk by reduction of its major whey protein β-lactoglobulin (756, 796). In addition, major protein wheat fractions (gliadins, glutenins) are enzymatically reduced (80). Trx may, thus, be useful for the production of hypoallergenic and more digestible food. Notably, breast milk concentrations of Trx during the early postpartum stage are highly increased, supporting this role (776). Elevated Grx1 protein levels in mice also diminished cardiac pathologies in diabetic hearts suffering from I/R-induced injury (426). Moreover, oral applications of GSH and precursor amino acids, including NAC, provide some degree of protection for HIV patients (230) (see also section II.B.8.b) and diabetes patients (228, 692) (see also section II.B.9.a). The activity of Prx2 was enhanced by obovatol, a compound extracted from the medical plant Magnolia obovata, revealing Prx2 as a new target protecting microglia against neuroinflammation (556). Two drugs, nipradilol and timolol, used in glaucoma therapy, increase the protein levels of Prx2, thereby protecting cells of the tabecular meshwork, the tissue surrounding the base of the cornea, against H2O2-induced apoptosis (506). Another potential target in the clinic is Prx6. Over-expression of the peroxidase could be used to prevent the progression of hypoxia-dependent disorders, such as glaucoma (784).
III. Concluding Remarks
The importance of redox signaling is increasingly recognized, despite the highly transient and volatile nature of the redox modifications that makes them very difficult to access for broad indepth investigations. The various redox modifications are highly target- and site specific, and the Trx, Grx, and Prx systems are pivotal players in redox signal transduction both as transducers and as regulators of second-messenger levels. One of the most striking findings of the last years was that these events might have to be defined individually for each tissue, each cell type within, and the condition of interest. Among others, our joint approach mapping Trxs, Grxs, and Prxs in physiological tissues of human, mouse, and rat “Human and murine redox atlases” highlighted the complexity of redox regulatory networks and implied more specific functions and interactions between the proteins themselves and with other proteins than those previously assumed. What will the future bring? Even though numerous open questions are awaiting bold hypotheses and brilliant new strategies, we should also continuously reassess previous findings and opinions. New issues involve the dynamics of subcellular localization—how can Trx fold proteins be secreted through the plasma membrane or taken up into cells? How do the proteins shuttle into and out of the nucleus? More targets and interactions will have to be defined. The molecular mechanisms of redox signal transduction are worthy of closer attention. Will we see more redox circuits with specific oxidases and reductases? Do redoxins transduce oxidative signals from, for instance, H2O2 to target proteins? […] Undoubtedly, the redoxin and redox signaling research field will continue to grow, and biological understanding will contribute to the development of clinical applications.
Abbreviations Used
- 6-OHDA
6-hydroxydopamine
- aa
amino acid
- AD
Alzheimer's disease
- ADF
adult T-cell leukemia-derived factor
- ALS
amyotrophic lateral sclerosis
- AMD
age-related macular degeneration
- AP-1
activating protein 1
- ASK1
apoptosis signal-regulating kinase 1
- CBS
cystathionine β-synthase
- CD
cluster of differentiation
- CNS
central nervous system
- COPD
chronic obstructive pulmonary disease
- COX
cyclooxygenases
- Cp450
cytochrome P450 enzymes
- CSE
cystathionine y-base
- DCs
dendritic cells
- ER
endoplasmic reticulum
- FAD
flavin adenine dinucleotide
- Fli-1
Flightless-1
- GPx
glutathione peroxidase
- GR
glutathione reductase
- Grx
glutaredoxin
- GSH
glutathione
- GSNO
S-nitrosylated glutathione
- GSSG
glutathione disulfide
- H2O2
hydrogen peroxide
- HIF
hypoxia-inducible factors
- HIV
human immunodeficient virus
- HUVEC
human umbilical vein endothelial cells
- ICAM-1
intercellular adhesion molecule 1
- IL
interleukin
- INS
islets of Langerhans
- IOP
intraocular pressure
- IPC
ischemic preconditioning
- IRP
iron regulatory proteins
- JNK
C-Jun N-terminal kinase
- LPS
lipopolysaccharide
- MAP
mitogen-activated protein
- MIF
macrophage inhibitory factor
- MPP+
1-methyl-4-phenylpyridinium
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- Msr
methionine sulfoxide reductases
- MST
3-mercaptopyruvate sulfurtransferase
- NDP
nucleoside-diphosphate
- NFAT
nuclear factor of activated T cells
- NF-κB
nuclear factor kappa B
- NMDA
N-methyl-D-aspartate
- ·NO
nitric oxide
- NOD
nonobese diabetic
- NOS
nitric oxide synthase
- NOX
NADH oxidase
- Nrf2
nuclear factor E2-related factor 2
- Nrx
nucleoredoxin
- P
protein
- PBMCs
peripheral blood mononuclear cells
- PC12
pheochromocytoma cell line
- PD
Parkinson's disease
- PDI
protein disulfide isomerase
- PFTs
pore forming immune toxins
- PHD
prolyl hydroxylases
- Prxs
peroxiredoxins
- RNR
ribonucleotide reductase
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- RSS
reactive sulfur species
- SOD
superoxide dismutases
- SP-Trx
sperm-specific thioredoxin
- Srx
sulfiredoxin
- TBP2
thioredoxin binding protein 2
- TGR
thioredoxin glutathione reductase
- TH
tyrosine hydroxylase
- TLR
Toll-like receptor
- TNF
tumor necrosis factor
- TRAIL
TNF-related apoptosis-inducing ligand
- Trx
thioredoxin
- TrxR
thioredoxin reductase
- TSA
thiol-specific antioxidant
- Txl1
thioredoxin-like protein 1
- TXNDC
thioredoxin domain-containing protein
- Txnip
trx interacting protein
- VDUP1
Vitamin D up-regulated protein 1
- XO
xanthine oxidase
Acknowledgments
The authors' work was funded by the Deutsche Forschungsgemeinschaft (SFB593-N01), Karolinska Institutet, the P.E. Kempkes Foundation, and the von Behring-Röntgen Foundation.
References
- 1.Abate C. Patel L. Rauscher FJ., 3rd Curran T. Redox regulation of fos and jun DNA-binding activity in vitro. Science. 1990;249:1157–1161. doi: 10.1126/science.2118682. [DOI] [PubMed] [Google Scholar]
- 2.Abdiu A. Nakamura H. Sahaf B. Yodoi J. Holmgren A. Rosén A. Thioredoxin blood level increases after severe burn injury. Antioxid Redox Signal. 2000;2:707–716. doi: 10.1089/ars.2000.2.4-707. [DOI] [PubMed] [Google Scholar]
- 3.Adelman DM. Gertsenstein M. Nagy A. Simon MC. Maltepe E. Placental cell fates are regulated in vivo by HIF-mediated hypoxia responses. Genes Dev. 2000;14:3191–3203. doi: 10.1101/gad.853700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adelman DM. Maltepe E. Simon MC. Multilineage embryonic hematopoiesis requires hypoxic ARNT activity. Genes Dev. 1999;13:2478–2483. doi: 10.1101/gad.13.19.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adlard PA. Bush AI. Metals and Alzheimer's disease. J Alzheimers Dis. 2006;10:145–163. doi: 10.3233/jad-2006-102-303. [DOI] [PubMed] [Google Scholar]
- 6.Adluri RS. Thirunavukkarasu M. Zhan L. Akita Y. Samuel SM. Otani H. Ho Y-S. Maulik G. Maulik N. Thioredoxin 1 enhances neovascularization and reduces ventricular remodeling during chronic myocardial infarction: a study using thioredoxin 1 transgenic mice. J Mol Cell Cardiol. 2011;50:239–247. doi: 10.1016/j.yjmcc.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Advani A. Gilbert RE. Thai K. Gow RM. Langham RG. Cox AJ. Connelly KA. Zhang Y. Herzenberg AM. Christensen PK. Pollock CA. Qi W. Tan SM. Parving H-H. Kelly DJ. Expression, localization, and function of the thioredoxin system in diabetic nephropathy. J Am Soc Nephrol. 2009;20:730–741. doi: 10.1681/ASN.2008020142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aesif SW. Anathy V. Kuipers I. Guala AS. Reiss JN. Ho Y-S. Janssen-Heininger YMW. Ablation of glutaredoxin-1 attenuates lipopolysaccharide-induced lung inflammation and alveolar macrophage activation. Am J Respir Cell Mol Biol. 2011;44:491–499. doi: 10.1165/rcmb.2009-0136OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ago T. Sadoshima J. Thioredoxin and ventricular remodeling. J Mol Cell Cardiol. 2006;41:762–773. doi: 10.1016/j.yjmcc.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agrawal-Singh S. Isken F. Agelopoulos K. Klein H-U. Thoennissen NH. Koehler G. Hascher A. Bäumer N. Berdel WE. Thiede C. Ehninger G. Becker A. Schlenke P. Wang Y. McClelland M. Krug U. Koschmieder S. Büchner T. Yu D-Y. Singh SV. Hansen K. Serve H. Dugas M. Müller-Tidow C. Genome-wide analysis of histone H3 acetylation patterns in AML identifies PRDX2 as an epigenetically silenced tumor suppressor gene. Blood. 2012;119:2346–2357. doi: 10.1182/blood-2011-06-358705. [DOI] [PubMed] [Google Scholar]
- 11.Ahn BY. Moss B. Glutaredoxin homolog encoded by vaccinia virus is a virion-associated enzyme with thioltransferase and dehydroascorbate reductase activities. Proc Natl Acad Sci U S A. 1992;89:7060–7064. doi: 10.1073/pnas.89.15.7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahsan MK. Lekli I. Ray D. Yodoi J. Das DK. Redox regulation of cell survival by the thioredoxin superfamily: an implication of redox gene therapy in the heart. Antioxid Redox Signal. 2009;11:2741–2758. doi: 10.1089/ars.2009.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aillet F. Masutani H. Elbim C. Raoul H. Chêne L. Nugeyre MT. Paya C. Barré-Sinoussi F. Gougerot-Pocidalo MA. Israël N. Human immunodeficiency virus induces a dual regulation of Bcl-2, resulting in persistent infection of CD4(+) T- or monocytic cell lines. J Virol. 1998;72:9698–9705. doi: 10.1128/jvi.72.12.9698-9705.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akerboom TP. Sies H. Assay of glutathione, glutathione disulfide, and glutathione mixed disulfides in biological samples. Methods Enzymol. 1981;77:373–382. doi: 10.1016/s0076-6879(81)77050-2. [DOI] [PubMed] [Google Scholar]
- 15.Akif M. Khare G. Tyagi AK. Mande SC. Sardesai AA. Functional studies of multiple thioredoxins from Mycobacterium tuberculosis. J Bacteriol. 2008;190:7087–7095. doi: 10.1128/JB.00159-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akterin S. Cowburn RF. Miranda-Vizuete A. Jiménez A. Bogdanovic N. Winblad B. Cedazo-Minguez A. Involvement of glutaredoxin-1 and thioredoxin-1 in beta-amyloid toxicity and Alzheimer's disease. Cell Death Differ. 2006;13:1454–1465. doi: 10.1038/sj.cdd.4401818. [DOI] [PubMed] [Google Scholar]
- 17.Alam ZI. Daniel SE. Lees AJ. Marsden DC. Jenner P. Halliwell B. A generalised increase in protein carbonyls in the brain in Parkinson's but not incidental Lewy body disease. J Neurochem. 1997;69:1326–1329. doi: 10.1046/j.1471-4159.1997.69031326.x. [DOI] [PubMed] [Google Scholar]
- 18.Albrecht SC. Barata AG. Grosshans J. Teleman AA. Dick TP. In vivo mapping of hydrogen peroxide and oxidized glutathione reveals chemical and regional specificity of redox homeostasis. Cell Metab. 2011;14:819–829. doi: 10.1016/j.cmet.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 19.Al-Gayyar MMH. Abdelsaid MA. Matragoon S. Pillai BA. El-Remessy AB. Thioredoxin interacting protein is a novel mediator of retinal inflammation and neurotoxicity. Br J Pharmacol. 2011;164:170–180. doi: 10.1111/j.1476-5381.2011.01336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Jawad FH. Sahib AS. Al-Kaisy AA. Role of antioxidants in the treatment of burn lesions. Ann Burns Fire Disasters. 2008;21:186–191. [PMC free article] [PubMed] [Google Scholar]
- 21.Altschmied J. Haendeler J. Thioredoxin-1 and endothelial cell aging: role in cardiovascular diseases. Antioxid Redox Signal. 2009;11:1733–1740. doi: 10.1089/ars.2008.2379. [DOI] [PubMed] [Google Scholar]
- 22.Ameisen JC. Programmed cell death (apoptosis) and cell survival regulation: relevance to AIDS and cancer. AIDS. 1994;8:1197–1213. doi: 10.1097/00002030-199409000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Andersson M. Holmgren A. Spyrou G. NK-lysin, a disulfide-containing effector peptide of T-lymphocytes, is reduced and inactivated by human thioredoxin reductase. Implication for a protective mechanism against NK-lysin cytotoxicity. J Biol Chem. 1996;271:10116–10120. doi: 10.1074/jbc.271.17.10116. [DOI] [PubMed] [Google Scholar]
- 24.Andricopulo AD. Akoachere MB. Krogh R. Nickel C. McLeish MJ. Kenyon GL. Arscott LD. Williams CH., Jr. Davioud-Charvet E. Becker K. Specific inhibitors of Plasmodium falciparum thioredoxin reductase as potential antimalarial agents. Bioorg Med Chem Lett. 2006;16:2283–2292. doi: 10.1016/j.bmcl.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 25.Angelini G. Gardella S. Ardy M. Ciriolo MR. Filomeni G. Di Trapani G. Clarke F. Sitia R. Rubartelli A. Antigen-presenting dendritic cells provide the reducing extracellular microenvironment required for T lymphocyte activation. Proc Natl Acad Sci U S A. 2002;99:1491–1496. doi: 10.1073/pnas.022630299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aon-Bertolino ML. Romero JI. Galeano P. Holubiec M. Badorrey MS. Saraceno GE. Hanschmann E-M. Lillig CH. Capani F. Thioredoxin and glutaredoxin system proteins-immunolocalization in the rat central nervous system. Biochim Biophys Acta. 2011;1810:93–110. doi: 10.1016/j.bbagen.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 27.Aota M. Matsuda K. Isowa N. Wada H. Yodoi J. Ban T. Protection against reperfusion-induced arrhythmias by human thioredoxin. J Cardiovasc Pharmacol. 1996;27:727–732. doi: 10.1097/00005344-199605000-00016. [DOI] [PubMed] [Google Scholar]
- 28.Arnér ESJ. Focus on mammalian thioredoxin reductases—Important selenoproteins with versatile functions. Biochim Biophys Acta. 2009;1790:495–526. doi: 10.1016/j.bbagen.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 29.Arnér ESJ. Holmgren A. The thioredoxin system in cancer. Semin Cancer Biol. 2006;16:420–426. doi: 10.1016/j.semcancer.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 30.Arrigoni FI. Hislop AA. Pollock JS. Haworth SG. Mitchell JA. Birth upregulates nitric oxide synthase activity in the porcine lung. Life Sci. 2002;70:1609–1620. doi: 10.1016/s0024-3205(02)01471-6. [DOI] [PubMed] [Google Scholar]
- 31.Ashfaq S. Abramson JL. Jones DP. Rhodes SD. Weintraub WS. Hooper WC. Vaccarino V. Harrison DG. Quyyumi AA. The relationship between plasma levels of oxidized and reduced thiols and early atherosclerosis in healthy adults. J Am Coll Cardiol. 2006;47:1005–1011. doi: 10.1016/j.jacc.2005.09.063. [DOI] [PubMed] [Google Scholar]
- 32.Athar M. Oxidative stress and experimental carcinogenesis. Indian J Exp Biol. 2002;40:656–667. [PubMed] [Google Scholar]
- 33.Auwerx J. Isacsson O. Söderlund J. Balzarini J. Johansson M. Lundberg M. Human glutaredoxin-1 catalyzes the reduction of HIV-1 gp120 and CD4 disulfides and its inhibition reduces HIV-1 replication. Int J Biochem Cell Biol. 2009;41:1269–1275. doi: 10.1016/j.biocel.2008.10.031. [DOI] [PubMed] [Google Scholar]
- 34.Avila PC. Kropotov AV. Krutilina R. Krasnodembskay A. Tomilin NV. Serikov VB. Peroxiredoxin V contributes to antioxidant defense of lung epithelial cells. Lung. 2008;186:103–114. doi: 10.1007/s00408-007-9066-2. [DOI] [PubMed] [Google Scholar]
- 35.Bachschmid MM. Xu S. Maitland-Toolan KA. Ho Y-S. Cohen RA. Matsui R. Attenuated cardiovascular hypertrophy and oxidant generation in response to angiotensin II infusion in glutaredoxin-1 knockout mice. Free Radic Biol Med. 2010;49:1221–1229. doi: 10.1016/j.freeradbiomed.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bai J. Nakamura H. Hattori I. Tanito M. Yodoi J. Thioredoxin suppresses 1-methyl-4-phenylpyridinium-induced neurotoxicity in rat PC12 cells. Neurosci Lett. 2002;321:81–84. doi: 10.1016/s0304-3940(02)00058-7. [DOI] [PubMed] [Google Scholar]
- 37.Bai X-Y. Ma Y. Ding R. Fu B. Shi S. Chen X-M. miR-335 and miR-34a Promote renal senescence by suppressing mitochondrial antioxidative enzymes. J Am Soc Nephrol. 2011;22:1252–1261. doi: 10.1681/ASN.2010040367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ball KA. Nelson AW. Foster DG. Poyton RO. Nitric oxide produced by cytochrome c oxidase helps stabilize HIF-1α in hypoxic mammalian cells. Biochem Biophys Res Commun. 2012;420:727–732. doi: 10.1016/j.bbrc.2012.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Banham HAL. Haldane JS. Savage T. The presence post mortem of nitricoxide-haemoglobin. Br Med J. 1925;2:187–189. [Google Scholar]
- 40.Barber SC. Mead RJ. Shaw PJ. Oxidative stress in ALS: a mechanism of neurodegeneration and a therapeutic target. Biochim Biophys Acta. 2006;1762:1051–1067. doi: 10.1016/j.bbadis.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 41.Barone E. Cenini G. Sultana R. Di Domenico F. Fiorini A. Perluigi M. Noel T. Wang C. Mancuso C. St Clair DK. Butterfield DA. Lack of p53 decreases basal oxidative stress levels in the brain through upregulation of thioredoxin-1, biliverdin reductase-A, manganese superoxide dismutase, and nuclear factor kappa-B. Antioxid Redox Signal. 2012;16:1407–1420. doi: 10.1089/ars.2011.4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barranco-Medina S. Lázaro J-J. Dietz K-J. The oligomeric conformation of peroxiredoxins links redox state to function. FEBS Lett. 2009;583:1809–1816. doi: 10.1016/j.febslet.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 43.Baskar R. Bian J. Hydrogen sulfide gas has cell growth regulatory role. Eur J Pharmacol. 2011;656:5–9. doi: 10.1016/j.ejphar.2011.01.052. [DOI] [PubMed] [Google Scholar]
- 44.Basso M. Giraudo S. Corpillo D. Bergamasco B. Lopiano L. Fasano M. Proteome analysis of human substantia nigra in Parkinson's disease. Proteomics. 2004;4:3943–3952. doi: 10.1002/pmic.200400848. [DOI] [PubMed] [Google Scholar]
- 45.Bast A. Wolf G. Oberbäumer I. Walther R. Oxidative and nitrosative stress induces peroxiredoxins in pancreatic beta cells. Diabetologia. 2002;45:867–876. doi: 10.1007/s00125-002-0846-1. [DOI] [PubMed] [Google Scholar]
- 46.Basu A. Banerjee H. Rojas H. Martinez SR. Roy S. Jia Z. Lilly MB. De León M. Casiano CA. Differential expression of peroxiredoxins in prostate cancer: consistent upregulation of PRDX3 and PRDX4. Prostate. 2011;71:755–765. doi: 10.1002/pros.21292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bazzichi A. Incaprera M. Garzelli C. Effect of Escherichia-coli thioredoxin, a homolog to the adult T-cell leukemia-derived factor, on epstein-barr virus-transformed B-lymphocytes. Int J Oncol. 1994;5:41–46. doi: 10.3892/ijo.5.1.41. [DOI] [PubMed] [Google Scholar]
- 48.Becker K. Gromer S. Schirmer RH. Müller S. Thioredoxin reductase as a pathophysiological factor and drug target. Eur J Biochem. 2000;267:6118–6125. doi: 10.1046/j.1432-1327.2000.01703.x. [DOI] [PubMed] [Google Scholar]
- 49.Beck M. Selenium and viral infections. In: Hatfield DL, editor; Berry MJ, editor; Gladyshev VN, editor. Selenium—Its Molecular Biology and Role in Human Health. Heidelberg, Germany: Springer; 2006. pp. 287–298. [Google Scholar]
- 50.Beckman KB. Ames BN. The free radical theory of aging matures. Physiol Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- 51.Bedard K. Krause K-H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 52.Benard O. Balasubramanian KA. Purification and properties of thioltransferase from monkey small intestinal mucosa: its role in protein-S-thiolation. Int J Biochem Cell Biol. 1996;28:1051–1059. doi: 10.1016/1357-2725(96)00031-3. [DOI] [PubMed] [Google Scholar]
- 53.Benhar M. Forrester MT. Hess DT. Stamler JS. Regulated protein denitrosylation by cytosolic and mitochondrial thioredoxins. Science. 2008;320:1050–1054. doi: 10.1126/science.1158265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berggren M. Gallegos A. Gasdaska JR. Gasdaska PY. Warneke J. Powis G. Thioredoxin and thioredoxin reductase gene expression in human tumors and cell lines, and the effects of serum stimulation and hypoxia. Anticancer Res. 1996;16:3459–3466. [PubMed] [Google Scholar]
- 55.Berggren MI. Husbeck B. Samulitis B. Baker AF. Gallegos A. Powis G. Thioredoxin peroxidase-1 (peroxiredoxin-1) is increased in thioredoxin-1 transfected cells and results in enhanced protection against apoptosis caused by hydrogen peroxide but not by other agents including dexamethasone, etoposide, and doxorubicin. Arch Biochem Biophys. 2001;392:103–109. doi: 10.1006/abbi.2001.2435. [DOI] [PubMed] [Google Scholar]
- 56.Berndt C. Hudemann C. Hanschmann E-M. Axelsson R. Holmgren A. Lillig CH. How does iron-sulfur cluster coordination regulate the activity of human glutaredoxin 2? Antioxid Redox Signal. 2007;9:151–157. doi: 10.1089/ars.2007.9.151. [DOI] [PubMed] [Google Scholar]
- 57.Berndt C. Lillig CH. Holmgren A. Thioredoxins and glutaredoxins as facilitators of protein folding. Biochim Biophys Acta. 2008;1783:641–650. doi: 10.1016/j.bbamcr.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 58.Bertini R. Howard OM. Dong HF. Oppenheim JJ. Bizzarri C. Sergi R. Caselli G. Pagliei S. Romines B. Wilshire JA. Mengozzi M. Nakamura H. Yodoi J. Pekkari K. Gurunath R. Holmgren A. Herzenberg LA. Herzenberg LA. Ghezzi P. Thioredoxin, a redox enzyme released in infection and inflammation, is a unique chemoattractant for neutrophils, monocytes, and T cells. J Exp Med. 1999;189:1783–1789. doi: 10.1084/jem.189.11.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beutler E. Iron storage disease: facts, fiction and progress. Blood Cells Mol Dis. 2007;39:140–147. doi: 10.1016/j.bcmd.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bharath S. Hsu M. Kaur D. Rajagopalan S. Andersen JK. Glutathione, iron and Parkinson's disease. Biochem Pharmacol. 2002;64:1037–1048. doi: 10.1016/s0006-2952(02)01174-7. [DOI] [PubMed] [Google Scholar]
- 61.Bickers DR. Athar M. Oxidative stress in the pathogenesis of skin disease. J Invest Dermatol. 2006;126:2565–2575. doi: 10.1038/sj.jid.5700340. [DOI] [PubMed] [Google Scholar]
- 62.Bigelow DJ. Squier TC. Thioredoxin-dependent redox regulation of cellular signaling and stress response through reversible oxidation of methionines. Mol Biosyst. 2011;7:2101–2109. doi: 10.1039/c1mb05081h. [DOI] [PubMed] [Google Scholar]
- 63.Biguet C. Wakasugi N. Mishal Z. Holmgren A. Chouaib S. Tursz T. Wakasugi H. Thioredoxin increases the proliferation of human B-cell lines through a protein kinase C-dependent mechanism. J Biol Chem. 1994;269:28865–28870. [PubMed] [Google Scholar]
- 64.Biteau B. Labarre J. Toledano MB. ATP-dependent reduction of cysteine-sulphinic acid by S. cerevisiae sulphiredoxin. Nature. 2003;425:980–984. doi: 10.1038/nature02075. [DOI] [PubMed] [Google Scholar]
- 65.Bizzarri C. Holmgren A. Pekkari K. Chang G. Colotta F. Ghezzi P. Bertini R. Requirements for the different cysteines in the chemotactic and desensitizing activity of human thioredoxin. Antioxid Redox Signal. 2005;7:1189–1194. doi: 10.1089/ars.2005.7.1189. [DOI] [PubMed] [Google Scholar]
- 66.Björnstedt M. Xue J. Huang W. Akesson B. Holmgren A. The thioredoxin and glutaredoxin systems are efficient electron donors to human plasma glutathione peroxidase. J Biol Chem. 1994;269:29382–29384. [PubMed] [Google Scholar]
- 67.Blauwkamp MN. Yu J. Schin MA. Burke KA. Berry MJ. Carlson BA. Brosius FC., 3rd Koenig RJ. Podocyte specific knock out of selenoproteins does not enhance nephropathy in streptozotocin diabetic C57BL/6 mice. BMC Nephrol. 2008;9:7. doi: 10.1186/1471-2369-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bokov A. Chaudhuri A. Richardson A. The role of oxidative damage and stress in aging. Mech Ageing Dev. 2004;125:811–826. doi: 10.1016/j.mad.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 69.Borisenko G. Kagan VE. Bayir HA. Belikova NA. Kapralov O. Tyurina YY. Tyurin VA. Jiang J. Stoyanovsky DA. Wipf P. Kochanek PM. Greenberger JS. Pitt B. Shvedova AA. Cytochrome c/cardiolipin relations in mitochondria: a kiss of death. Free Radic Biol Med. 2009;46:1439–1453. doi: 10.1016/j.freeradbiomed.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boulos S. Meloni BP. Arthur PG. Bojarski C. Knuckey NW. Peroxiredoxin 2 overexpression protects cortical neuronal cultures from ischemic and oxidative injury but not glutamate excitotoxicity, whereas Cu/Zn superoxide dismutase 1 overexpression protects only against oxidative injury. J Neurosci Res. 2007;85:3089–3097. doi: 10.1002/jnr.21429. [DOI] [PubMed] [Google Scholar]
- 71.Bowler RP. Crapo JD. Oxidative stress in allergic respiratory diseases. J Allergy Clin Immunol. 2002;110:349–356. doi: 10.1067/mai.2002.126780. [DOI] [PubMed] [Google Scholar]
- 72.Brady KD. Giegel DA. Grinnell C. Lunney E. Talanian RV. Wong W. Walker N. A catalytic mechanism for caspase-1 and for bimodal inhibition of caspase-1 by activated aspartic ketones. Bioorg Med Chem. 1999;7:621–631. doi: 10.1016/s0968-0896(99)00009-7. [DOI] [PubMed] [Google Scholar]
- 73.Brand MD. The sites and topology of mitochondrial superoxide production. Exp Gerontol. 2010;45:466–472. doi: 10.1016/j.exger.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Braskett M. Riedl MA. Novel antioxidant approaches to the treatment of upper airway inflammation. Curr Opin Allergy Clin Immunol. 2010;10:34–41. doi: 10.1097/ACI.0b013e328334f613. [DOI] [PubMed] [Google Scholar]
- 75.Bräutigam L. Schütte LD. Godoy JR. Prozorovski T. Gellert M. Hauptmann G. Holmgren A. Lillig CH. Berndt C. Vertebrate-specific glutaredoxin is essential for brain development. Proc Natl Acad Sci U S A. 2011;108:20532–20537. doi: 10.1073/pnas.1110085108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brea D. Rodríguez-González R. Sobrino T. Rodríguez-Yañez M. Blanco M. Castillo J. Proteomic analysis shows differential protein expression in endothelial progenitor cells between healthy subjects and ischemic stroke patients. Neurol Res. 2011;33:1057–1063. doi: 10.1179/1743132811Y.0000000038. [DOI] [PubMed] [Google Scholar]
- 77.Brigelius-Flohé R. Flohé L. Basic principles and emerging concepts in the redox control of transcription factors. Antioxid Redox Signal. 2011;15:2335–2381. doi: 10.1089/ars.2010.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 79.Brunton TL. Experimental Investigation of the Action of Medicines. London: J. and A. Churchill; 1875. [Google Scholar]
- 80.Buchanan BB. Adamidi C. Lozano RM. Yee BC. Momma M. Kobrehel K. Ermel R. Frick OL. Thioredoxin-linked mitigation of allergic responses to wheat. Proc Natl Acad Sci U S A. 1997;94:5372–5377. doi: 10.1073/pnas.94.10.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Buchanan BB. Balmer Y. Redox regulation: a broadening horizon. Annu Rev Plant Biol. 2005;56:187–220. doi: 10.1146/annurev.arplant.56.032604.144246. [DOI] [PubMed] [Google Scholar]
- 82.Buchanan BB. Wolosiuk RA. Photosynthetic regulatory protein found in animal and bacterial cells. Nature. 1976;264:669–670. doi: 10.1038/264669a0. [DOI] [PubMed] [Google Scholar]
- 83.Buhl R. Jaffe HA. Holroyd KJ. Wells FB. Mastrangeli A. Saltini C. Cantin AM. Crystal RG. Systemic glutathione deficiency in symptom-free HIV-seropositive individuals. Lancet. 1989;2:1294–1298. doi: 10.1016/s0140-6736(89)91909-0. [DOI] [PubMed] [Google Scholar]
- 84.Burke-Gaffney A. Callister MEJ. Nakamura H. Thioredoxin: friend or foe in human disease? Trends Pharmacol Sci. 2005;26:398–404. doi: 10.1016/j.tips.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 85.Bushweller JH. Aslund F. Wüthrich K. Holmgren A. Structural and functional characterization of the mutant Escherichia coli glutaredoxin (C14—-S) and its mixed disulfide with glutathione. Biochemistry. 1992;31:9288–9293. doi: 10.1021/bi00153a023. [DOI] [PubMed] [Google Scholar]
- 86.Butterfield DA. Perluigi M. Sultana R. Oxidative stress in Alzheimer's disease brain: new insights from redox proteomics. Eur J Pharmacol. 2006;545:39–50. doi: 10.1016/j.ejphar.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 87.Butterfield LH. Merino A. Golub SH. Shau H. From cytoprotection to tumor suppression: the multifactorial role of peroxiredoxins. Antioxid Redox Signal. 1999;1:385–402. doi: 10.1089/ars.1999.1.4-385. [DOI] [PubMed] [Google Scholar]
- 88.Bylund J. Brown KL. Movitz C. Dahlgren C. Karlsson A. Intracellular generation of superoxide by the phagocyte NADPH oxidase: how, where, and what for? Free Radic Biol Med. 2010;49:1834–1845. doi: 10.1016/j.freeradbiomed.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 89.Cabreiro F. Picot CR. Friguet B. Petropoulos I. Methionine sulfoxide reductases: relevance to aging and protection against oxidative stress. Ann N Y Acad Sci. 2006;1067:37–44. doi: 10.1196/annals.1354.006. [DOI] [PubMed] [Google Scholar]
- 90.Cagnol S. Chambard J-C. ERK and cell death: mechanisms of ERK-induced cell death—apoptosis, autophagy and senescence. FEBS J. 2010;277:2–21. doi: 10.1111/j.1742-4658.2009.07366.x. [DOI] [PubMed] [Google Scholar]
- 91.Cai J. Nelson KC. Wu M. Sternberg P., Jr. Jones DP. Oxidative damage and protection of the RPE. Prog Retin Eye Res. 2000;19:205–221. doi: 10.1016/s1350-9462(99)00009-9. [DOI] [PubMed] [Google Scholar]
- 92.Cai W. Zhang L. Song Y. Wang B. Zhang B. Cui X. Hu G. Liu Y. Wu J. Fang J. Small molecule inhibitors of mammalian thioredoxin reductase. Free Radic Biol Med. 2012;52:257–265. doi: 10.1016/j.freeradbiomed.2011.10.447. [DOI] [PubMed] [Google Scholar]
- 93.Calabrese V. Cornelius C. Maiolino L. Luca M. Chiaramonte R. Toscano MA. Serra A. Oxidative stress, redox homeostasis and cellular stress response in Ménière's disease: role of vitagenes. Neurochem Res. 2010;35:2208–2217. doi: 10.1007/s11064-010-0304-2. [DOI] [PubMed] [Google Scholar]
- 94.Camaschella C. Understanding iron homeostasis through genetic analysis of hemochromatosis and related disorders. Blood. 2005;106:3710–3717. doi: 10.1182/blood-2005-05-1857. [DOI] [PubMed] [Google Scholar]
- 95.Camaschella C. Campanella A. De Falco L. Boschetto L. Merlini R. Silvestri L. Levi S. Iolascon A. The human counterpart of zebrafish shiraz shows sideroblastic-like microcytic anemia and iron overload. Blood. 2007;110:1353–1358. doi: 10.1182/blood-2007-02-072520. [DOI] [PubMed] [Google Scholar]
- 96.Cameron NE. Cotter MA. Pro-inflammatory mechanisms in diabetic neuropathy: focus on the nuclear factor kappa B pathway. Curr Drug Targets. 2008;9:60–67. doi: 10.2174/138945008783431718. [DOI] [PubMed] [Google Scholar]
- 97.Cao J. Schulte J. Knight A. Leslie NR. Zagozdzon A. Bronson R. Manevich Y. Beeson C. Neumann CA. Prdx1 inhibits tumorigenesis via regulating PTEN/AKT activity. EMBO J. 2009;28:1505–1517. doi: 10.1038/emboj.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cao Z. Lindsay JG. Isaacs NW. Mitochondrial peroxiredoxins. Subcell Biochem. 2007;44:295–315. doi: 10.1007/978-1-4020-6051-9_14. [DOI] [PubMed] [Google Scholar]
- 99.Capani F. Loidl CF. Piehl LL. Facorro G. De Paoli T. Hager A. Long term production of reactive oxygen species during perinatal asphyxia in the rat central nervous system: effects of hypothermia. Int J Neurosci. 2003;113:641–654. doi: 10.1080/00207450390200099. [DOI] [PubMed] [Google Scholar]
- 100.Casso D. Beach D. A mutation in a thioredoxin reductase homolog suppresses p53-induced growth inhibition in the fission yeast Schizosaccharomyces pombe. Mol Gen Genet. 1996;252:518–529. doi: 10.1007/BF02172398. [DOI] [PubMed] [Google Scholar]
- 101.Ceylan S. Seidel V. Ziebart N. Berndt C. Dirdjaja N. Krauth-Siegel RL. The dithiol glutaredoxins of african trypanosomes have distinct roles and are closely linked to the unique trypanothione metabolism. J Biol Chem. 2010;285:35224–35237. doi: 10.1074/jbc.M110.165860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chae HZ. Kim HJ. Kang SW. Rhee SG. Characterization of three isoforms of mammalian peroxiredoxin that reduce peroxides in the presence of thioredoxin. Diabetes Res Clin Pract. 1999;45:101–112. doi: 10.1016/s0168-8227(99)00037-6. [DOI] [PubMed] [Google Scholar]
- 103.Chae HZ. Kim IH. Kim K. Rhee SG. Cloning, sequencing, and mutation of thiol-specific antioxidant gene of Saccharomyces cerevisiae. J Biol Chem. 1993;268:16815–16821. [PubMed] [Google Scholar]
- 104.Chae HZ. Robison K. Poole LB. Church G. Storz G. Rhee SG. Cloning and sequencing of thiol-specific antioxidant from mammalian brain: alkyl hydroperoxide reductase and thiol-specific antioxidant define a large family of antioxidant enzymes. Proc Natl Acad Sci U S A. 1994;91:7017–7021. doi: 10.1073/pnas.91.15.7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cha H. Kim JM. Oh JG. Jeong MH. Park CS. Park J. Jeong HJ. Park BK. Lee Y-H. Jeong D. Yang DK. Bernecker OY. Kim DH. Hajjar RJ. Park WJ. PICOT is a critical regulator of cardiac hypertrophy and cardiomyocyte contractility. J Mol Cell Cardiol. 2008;45:796–803. doi: 10.1016/j.yjmcc.2008.09.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chai TF. Leck YC. He H. Yu F-X. Luo Y. Hagen T. Hypoxia-inducible factor independent down-regulation of thioredoxin-interacting protein in hypoxia. FEBS Lett. 2011;585:492–498. doi: 10.1016/j.febslet.2010.12.033. [DOI] [PubMed] [Google Scholar]
- 107.Chakrapani B. Yedavally S. Leverenz V. Giblin FJ. Reddy VN. Simultaneous measurement of reduced and oxidized glutathione in human aqueous humor and cataracts by electrochemical detection. Ophthalmic Res. 1995;27(Suppl 1):69–77. doi: 10.1159/000267843. [DOI] [PubMed] [Google Scholar]
- 108.Cha M-K. Kim I-H. Preferential overexpression of glutaredoxin3 in human colon and lung carcinoma. Cancer Epidemiol. 2009;33:281–287. doi: 10.1016/j.canep.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 109.Cha MK. Yun CH. Kim IH. Interaction of human thiol-specific antioxidant protein 1 with erythrocyte plasma membrane. Biochemistry. 2000;39:6944–6950. doi: 10.1021/bi000034j. [DOI] [PubMed] [Google Scholar]
- 110.Chance B. Oshino N. Kinetics and mechanisms of catalase in peroxisomes of the mitochondrial fraction. Biochem J. 1971;122:225–233. doi: 10.1042/bj1220225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chandel NS. McClintock DS. Feliciano CE. Wood TM. Melendez JA. Rodriguez AM. Schumacker PT. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem. 2000;275:25130–25138. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- 112.Chang JW. Lee SH. Jeong JY. Chae HZ. Kim YC. Park Z-Y. Yoo YJ. Peroxiredoxin-I is an autoimmunogenic tumor antigen in non-small cell lung cancer. FEBS Lett. 2005;579:2873–2877. doi: 10.1016/j.febslet.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 113.Chang T-S. Jeong W. Choi SY. Yu S. Kang SW. Rhee SG. Regulation of peroxiredoxin I activity by Cdc2-mediated phosphorylation. J Biol Chem. 2002;277:25370–25376. doi: 10.1074/jbc.M110432200. [DOI] [PubMed] [Google Scholar]
- 114.Chang Y-C. Huang C-N. Lin C-H. Chang H-C. Wu C-C. Mapping protein cysteine sulfonic acid modifications with specific enrichment and mass spectrometry: an integrated approach to explore the cysteine oxidation. Proteomics. 2010;10:2961–2971. doi: 10.1002/pmic.200900850. [DOI] [PubMed] [Google Scholar]
- 115.Chen C-L. Lin C-F. Chang W-T. Huang W-C. Teng C-F. Lin Y-S. Ceramide induces p38 MAPK and JNK activation through a mechanism involving a thioredoxin-interacting protein-mediated pathway. Blood. 2008;111:4365–4374. doi: 10.1182/blood-2007-08-106336. [DOI] [PubMed] [Google Scholar]
- 116.Cheng N-H. Zhang W. Chen W-Q. Jin J. Cui X. Butte NF. Chan L. Hirschi KD. A mammalian monothiol glutaredoxin, Grx3, is critical for cell cycle progression during embryogenesis. FEBS J. 2011;278:2525–2539. doi: 10.1111/j.1742-4658.2011.08178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen J. Fontes G. Saxena G. Poitout V. Shalev A. Lack of TXNIP protects against mitochondria-mediated apoptosis but not against fatty acid-induced ER stress-mediated beta-cell death. Diabetes. 2010;59:440–447. doi: 10.2337/db09-0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen J-H. Chang Y-W. Yao C-W. Chiueh T-S. Huang S-C. Chien K-Y. Chen A. Chang F-Y. Wong C-H. Chen Y-J. Plasma proteome of severe acute respiratory syndrome analyzed by two-dimensional gel electrophoresis and mass spectrometry. Proc Natl Acad Sci U S A. 2004;101:17039–17044. doi: 10.1073/pnas.0407992101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen J. Hui ST. Couto FM. Mungrue IN. Davis DB. Attie AD. Lusis AJ. Davis RA. Shalev A. Thioredoxin-interacting protein deficiency induces Akt/Bcl-xL signaling and pancreatic beta-cell mass and protects against diabetes. FASEB J. 2008;22:3581–3594. doi: 10.1096/fj.08-111690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chen J. Saxena G. Mungrue IN. Lusis AJ. Shalev A. Thioredoxin-interacting protein: a critical link between glucose toxicity and beta-cell apoptosis. Diabetes. 2008;57:938–944. doi: 10.2337/db07-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen JW. Dodia C. Feinstein SI. Jain MK. Fisher AB. 1-Cys peroxiredoxin, a bifunctional enzyme with glutathione peroxidase and phospholipase A2 activities. J Biol Chem. 2000;275:28421–28427. doi: 10.1074/jbc.M005073200. [DOI] [PubMed] [Google Scholar]
- 122.Chen L. Na R. Gu M. Salmon AB. Liu Y. Liang H. Qi W. Van Remmen H. Richardson A. Ran Q. Reduction of mitochondrial H2O2 by overexpressing peroxiredoxin 3 improves glucose tolerance in mice. Aging Cell. 2008;7:866–878. doi: 10.1111/j.1474-9726.2008.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen M-F. Keng PC. Shau H. Wu C-T. Hu Y-C. Liao S-K. Chen W-C. Inhibition of lung tumor growth and augmentation of radiosensitivity by decreasing peroxiredoxin I expression. Int J Radiat Oncol Biol Phys. 2006;64:581–591. doi: 10.1016/j.ijrobp.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 124.Chen Q. Ames BN. Senescence-like growth arrest induced by hydrogen peroxide in human diploid fibroblast F65 cells. Proc Natl Acad Sci U S A. 1994;91:4130–4134. doi: 10.1073/pnas.91.10.4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chernatynskaya AV. Looney B. Hu H. Zhu X. Xia C-Q. Administration of recombinant human thioredoxin-1 significantly delays and prevents autoimmune diabetes in nonobese diabetic mice through modulation of autoimmunity. Diabetes Metab Res Rev. 2011;27:809–812. doi: 10.1002/dmrr.1232. [DOI] [PubMed] [Google Scholar]
- 126.Chinta SJ. Andersen JK. Redox imbalance in Parkinson's disease. Biochim Biophys Acta. 2008;1780:1362–1367. doi: 10.1016/j.bbagen.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chinta SJ. Kumar MJ. Hsu M. Rajagopalan S. Kaur D. Rane A. Nicholls DG. Choi J. Andersen JK. Inducible alterations of glutathione levels in adult dopaminergic midbrain neurons result in nigrostriatal degeneration. J Neurosci. 2007;27:13997–14006. doi: 10.1523/JNEUROSCI.3885-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Choi HS. Park KJ. Hwang SC. Park HY. Kim YS. Park K. The role of peroxiredoxin III in the ototoxic drug-induced mitochondrial apoptosis of cochlear hair cells. Acta Otolaryngol. 2008;128:944–951. doi: 10.1080/00016480701805463. [DOI] [PubMed] [Google Scholar]
- 129.Choi J-O. Yun S-H. Sung K. Lee YT. Park J-I. Ju E-S. Lee S-C. Park SW. Kim D-K. Oh JK. Jeon E-S. Thioredoxin, adiponectin and clinical course of acute fulminant myocarditis. Heart. 2011;97:1067–1073. doi: 10.1136/hrt.2010.219568. [DOI] [PubMed] [Google Scholar]
- 130.Choksi S. Lin Y. Pobezinskaya Y. Chen L. Park C. Morgan M. Li T. Jitkaew S. Cao X. Kim Y-S. Kim H-S. Levitt P. Shih G. Birre M. Deng C-X. Liu Z-G. A HIF-1 target, ATIA, protects cells from apoptosis by modulating the mitochondrial thioredoxin, TRX2. Mol Cell. 2011;42:597–609. doi: 10.1016/j.molcel.2011.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chou F-C. Sytwu H-K. Overexpression of thioredoxin in islets transduced by a lentiviral vector prolongs graft survival in autoimmune diabetic NOD mice. J Biomed Sci. 2009;16:71. doi: 10.1186/1423-0127-16-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chutkow WA. Birkenfeld AL. Brown JD. Lee H-Y. Frederick DW. Yoshioka J. Patwari P. Kursawe R. Cushman SW. Plutzky J. Shulman GI. Samuel VT. Lee RT. Deletion of the alpha-arrestin protein Txnip in mice promotes adiposity and adipogenesis while preserving insulin sensitivity. Diabetes. 2010;59:1424–1434. doi: 10.2337/db09-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Circu ML. Aw TY. Redox biology of the intestine. Free Radic Res. 2011;45:1245–1266. doi: 10.3109/10715762.2011.611509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cochemé HM. Quin C. McQuaker SJ. Cabreiro F. Logan A. Prime TA. Abakumova I. Patel JV. Fearnley IM. James AM. Porteous CM. Smith RAJ. Saeed S. Carré JE. Singer M. Gems D. Hartley RC. Partridge L. Murphy MP. Measurement of H2O2 within living Drosophila during aging using a ratiometric mass spectrometry probe targeted to the mitochondrial matrix. Cell Metab. 2011;13:340–350. doi: 10.1016/j.cmet.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Conrad M. Jakupoglu C. Moreno SG. Lippl S. Banjac A. Schneider M. Beck H. Hatzopoulos AK. Just U. Sinowatz F. Schmahl W. Chien KR. Wurst W. Bornkamm GW. Brielmeier M. Essential role for mitochondrial thioredoxin reductase in hematopoiesis, heart development, and heart function. Mol Cell Biol. 2004;24:9414–9423. doi: 10.1128/MCB.24.21.9414-9423.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Coral K. Raman R. Rathi S. Rajesh M. Sulochana KN. Angayarkanni N. Paul PG. Ramakrishnan S. Plasma homocysteine and total thiol content in patients with exudative age-related macular degeneration. Eye (Lond) 2006;20:203–207. doi: 10.1038/sj.eye.6701853. [DOI] [PubMed] [Google Scholar]
- 137.Da Costa CA. DJ-1: a newcomer in Parkinson's disease pathology. Curr Mol Med. 2007;7:650–657. doi: 10.2174/156652407782564426. [DOI] [PubMed] [Google Scholar]
- 138.Cumming RC. Dargusch R. Fischer WH. Schubert D. Increase in expression levels and resistance to sulfhydryl oxidation of peroxiredoxin isoforms in amyloid beta-resistant nerve cells. J Biol Chem. 2007;282:30523–30534. doi: 10.1074/jbc.M700869200. [DOI] [PubMed] [Google Scholar]
- 139.Curtsinger JM. Schmidt CS. Mondino A. Lins DC. Kedl RM. Jenkins MK. Mescher MF. Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J Immunol. 1999;162:3256–3262. [PubMed] [Google Scholar]
- 140.Daily D. Vlamis-Gardikas A. Offen D. Mittelman L. Melamed E. Holmgren A. Barzilai A. Glutaredoxin protects cerebellar granule neurons from dopamine-induced apoptosis by activating NF-kappa B via Ref-1. J Biol Chem. 2001;276:1335–1344. doi: 10.1074/jbc.M008121200. [DOI] [PubMed] [Google Scholar]
- 141.Dai S. He Y. Zhang H. Yu L. Wan T. Xu Z. Jones D. Chen H. Min W. Endothelial-specific expression of mitochondrial thioredoxin promotes ischemia-mediated arteriogenesis and angiogenesis. Arterioscler Thromb Vasc Biol. 2009;29:495–502. doi: 10.1161/ATVBAHA.108.180349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dalle-Donne I. Rossi R. Colombo G. Giustarini D. Milzani A. Protein S-glutathionylation: a regulatory device from bacteria to humans. Trends Biochem Sci. 2009;34:85–96. doi: 10.1016/j.tibs.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 143.Dammeyer P. Arnér ESJ. Human Protein Atlas of redox systems—what can be learnt? Biochim Biophys Acta. 2011;1810:111–138. doi: 10.1016/j.bbagen.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 144.Dammeyer P. Damdimopoulos AE. Nordman T. Jiménez A. Miranda-Vizuete A. Arnér ESJ. Induction of cell membrane protrusions by the N-terminal glutaredoxin domain of a rare splice variant of human thioredoxin reductase 1. J Biol Chem. 2008;283:2814–2821. doi: 10.1074/jbc.M708939200. [DOI] [PubMed] [Google Scholar]
- 145.Danet GH. Pan Y. Luongo JL. Bonnet DA. Simon MC. Expansion of human SCID-repopulating cells under hypoxic conditions. J Clin Invest. 2003;112:126–135. doi: 10.1172/JCI17669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Danielson SR. Andersen JK. Oxidative and nitrative protein modifications in Parkinson's disease. Free Radic Biol Med. 2008;44:1787–1794. doi: 10.1016/j.freeradbiomed.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Daniil ZD. Papageorgiou E. Koutsokera A. Kostikas K. Kiropoulos T. Papaioannou AI. Gourgoulianis KI. Serum levels of oxidative stress as a marker of disease severity in idiopathic pulmonary fibrosis. Pulm Pharmacol Ther. 2008;21:26–31. doi: 10.1016/j.pupt.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 148.Das KC. Guo XL. White CW. Induction of thioredoxin and thioredoxin reductase gene expression in lungs of newborn primates by oxygen. Am J Physiol. 1999;276:L530–L539. doi: 10.1152/ajplung.1999.276.3.L530. [DOI] [PubMed] [Google Scholar]
- 149.Davis DA. Newcomb FM. Starke DW. Ott DE. Mieyal JJ. Yarchoan R. Thioltransferase (glutaredoxin) is detected within HIV-1 and can regulate the activity of glutathionylated HIV-1 protease in vitro. J Biol Chem. 1997;272:25935–25940. doi: 10.1074/jbc.272.41.25935. [DOI] [PubMed] [Google Scholar]
- 150.Dessein AJ. Lenzi HL. Bina JC. Carvalho EM. Weiser WY. Andrade ZA. David JR. Modulation of eosinophil cytotoxicity by blood mononuclear cells from healthy subjects and patients with chronic schistosomiasis mansoni. Cell Immunol. 1984;85:100–113. doi: 10.1016/0008-8749(84)90282-x. [DOI] [PubMed] [Google Scholar]
- 151.Devi SA. Kiran TR. Regional responses in antioxidant system to exercise training and dietary vitamin E in aging rat brain. Neurobiol Aging. 2004;25:501–508. doi: 10.1016/S0197-4580(03)00112-X. [DOI] [PubMed] [Google Scholar]
- 152.Dexter DT. Holley AE. Flitter WD. Slater TF. Wells FR. Daniel SE. Lees AJ. Jenner P. Marsden CD. Increased levels of lipid hydroperoxides in the parkinsonian substantia nigra: an HPLC and ESR study. Mov Disord. 1994;9:92–97. doi: 10.1002/mds.870090115. [DOI] [PubMed] [Google Scholar]
- 153.Diaz Vivancos P. Wolff T. Markovic J. Pallardó FV. Foyer CH. A nuclear glutathione cycle within the cell cycle. Biochem J. 2010;431:169–178. doi: 10.1042/BJ20100409. [DOI] [PubMed] [Google Scholar]
- 154.Ding Y. Yamada S. Wang K-Y. Shimajiri S. Guo X. Tanimoto A. Murata Y. Kitajima S. Watanabe T. Izumi H. Kohno K. Sasaguri Y. Overexpression of peroxiredoxin 4 protects against high-dose streptozotocin-induced diabetes by suppressing oxidative stress and cytokines in transgenic mice. Antioxid Redox Signal. 2010;13:1477–1490. doi: 10.1089/ars.2010.3137. [DOI] [PubMed] [Google Scholar]
- 155.Diotte NM. Xiong Y. Gao J. Chua BHL. Ho Y-S. Attenuation of doxorubicin-induced cardiac injury by mitochondrial glutaredoxin 2. Biochim Biophys Acta. 2009;1793:427–438. doi: 10.1016/j.bbamcr.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 156.Dirnagl U. Iadecola C. Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 157.Distrutti E. Hydrogen sulphide and pain. Inflamm Allergy Drug Targets. 2011;10:123–132. doi: 10.2174/187152811794776240. [DOI] [PubMed] [Google Scholar]
- 158.Dittmann LM. Danner A. Gronych J. Wolter M. Stühler K. Grzendowski M. Becker N. Bageritz J. Goidts V. Toedt G. Felsberg J. Sabel MC. Barbus S. Reifenberger G. Lichter P. Tews B. Downregulation of PRDX1 by promoter hypermethylation is frequent in 1p/19q-deleted oligodendroglial tumours and increases radio- and chemosensitivity of Hs683 glioma cells in vitro. Oncogene. 2012;31:3409–3418. doi: 10.1038/onc.2011.513. [DOI] [PubMed] [Google Scholar]
- 159.Di Domenico F. Sultana R. Tiu GF. Scheff NN. Perluigi M. Cini C. Butterfield DA. Protein levels of heat shock proteins 27, 32, 60, 70, 90 and thioredoxin-1 in amnestic mild cognitive impairment: an investigation on the role of cellular stress response in the progression of Alzheimer disease. Brain Res. 2010;1333:72–81. doi: 10.1016/j.brainres.2010.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dominy JE. Stipanuk MH. New roles for cysteine and transsulfuration enzymes: production of H2S, a neuromodulator and smooth muscle relaxant. Nutr Rev. 2004;62:348–353. doi: 10.1111/j.1753-4887.2004.tb00060.x. [DOI] [PubMed] [Google Scholar]
- 161.Drapier JC. Hirling H. Wietzerbin J. Kaldy P. Kühn LC. Biosynthesis of nitric oxide activates iron regulatory factor in macrophages. EMBO J. 1993;12:3643–3649. doi: 10.1002/j.1460-2075.1993.tb06038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Dunn KC. Aotaki-Keen AE. Putkey FR. Hjelmeland LM. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp Eye Res. 1996;62:155–169. doi: 10.1006/exer.1996.0020. [DOI] [PubMed] [Google Scholar]
- 163.Egler RA. Fernandes E. Rothermund K. Sereika S. De Souza-Pinto N. Jaruga P. Dizdaroglu M. Prochownik EV. Regulation of reactive oxygen species, DNA damage, and c-Myc function by peroxiredoxin 1. Oncogene. 2005;24:8038–8050. doi: 10.1038/sj.onc.1208821. [DOI] [PubMed] [Google Scholar]
- 164.Eismann T. Huber N. Shin T. Kuboki S. Galloway E. Wyder M. Edwards MJ. Greis KD. Shertzer HG. Fisher AB. Lentsch AB. Peroxiredoxin-6 protects against mitochondrial dysfunction and liver injury during ischemia-reperfusion in mice. Am J Physiol Gastrointest Liver Physiol. 2009;296:G266–G274. doi: 10.1152/ajpgi.90583.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Eklund H. Gleason FK. Holmgren A. Structural and functional relations among thioredoxins of different species. Proteins. 1991;11:13–28. doi: 10.1002/prot.340110103. [DOI] [PubMed] [Google Scholar]
- 166.Eltzschig HK. Eckle T. Ischemia and reperfusion—from mechanism to translation. Nat Med. 2011;17:1391–1401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Enoksson M. Fernandes AP. Prast S. Lillig CH. Holmgren A. Orrenius S. Overexpression of glutaredoxin 2 attenuates apoptosis by preventing cytochrome c release. Biochem Biophys Res Commun. 2005;327:774–779. doi: 10.1016/j.bbrc.2004.12.067. [DOI] [PubMed] [Google Scholar]
- 168.Erickson PF. Maxwell IH. Su LJ. Baumann M. Glode LM. Sequence of cDNA for rat cystathionine gamma-lyase and comparison of deduced amino acid sequence with related Escherichia coli enzymes. Biochem J. 1990;269:335–340. doi: 10.1042/bj2690335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ericson ML. Hörling J. Wendel-Hansen V. Holmgren A. Rosén A. Secretion of thioredoxin after in vitro activation of human B cells. Lymphokine Cytokine Res. 1992;11:201–207. [PubMed] [Google Scholar]
- 170.Eriksson S. Askelöf P. Axelsson K. Carlberg I. Guthenberg C. Mannervik B. Resolution of glutathione-linked enzymes in rat liver and evaluation of their contribution to disulfide reduction via thiol—disulfide interchange. Acta Chem Scand B Org Chem Biochem. 1974;28:922–930. doi: 10.3891/acta.chem.scand.28b-0922. [DOI] [PubMed] [Google Scholar]
- 171.Esposito F. Russo T. Cimino F. Generation of prooxidant conditions in intact cells to induce modifications of cell cycle regulatory proteins. Meth Enzymol. 2002;352:258–268. doi: 10.1016/s0076-6879(02)52024-3. [DOI] [PubMed] [Google Scholar]
- 172.Ezashi T. Das P. Roberts RM. Low O2 tensions and the prevention of differentiation of hES cells. Proc Natl Acad Sci U S A. 2005;102:4783–4788. doi: 10.1073/pnas.0501283102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Fan C. Zwacka RM. Engelhardt JF. Therapeutic approaches for ischemia/reperfusion injury in the liver. J Mol Med. 1999;77:577–592. doi: 10.1007/s001099900029. [DOI] [PubMed] [Google Scholar]
- 174.Fang J. Nakamura T. Cho D-H. Gu Z. Lipton SA. S-nitrosylation of peroxiredoxin 2 promotes oxidative stress-induced neuronal cell death in Parkinson's disease. Proc Natl Acad Sci U S A. 2007;104:18742–18747. doi: 10.1073/pnas.0705904104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Fang J. Zhong L. Zhao R. Holmgren A. Ebselen: a thioredoxin reductase-dependent catalyst for alpha-tocopherol quinone reduction. Toxicol Appl Pharmacol. 2005;207:103–109. doi: 10.1016/j.taap.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 176.Farout L. Friguet B. Proteasome function in aging and oxidative stress: implications in protein maintenance failure. Antioxid Redox Signal. 2006;8:205–216. doi: 10.1089/ars.2006.8.205. [DOI] [PubMed] [Google Scholar]
- 177.Fatma N. Kubo E. Sen M. Agarwal N. Thoreson WB. Camras CB. Singh DP. Peroxiredoxin 6 delivery attenuates TNF-alpha-and glutamate-induced retinal ganglion cell death by limiting ROS levels and maintaining Ca2+ homeostasis. Brain Res. 2008;1233:63–78. doi: 10.1016/j.brainres.2008.07.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Fatma N. Kubo E. Toris CB. Stamer WD. Camras CB. Singh DP. PRDX6 attenuates oxidative stress- and TGFbeta-induced abnormalities of human trabecular meshwork cells. Free Radic Res. 2009;43:783–795. doi: 10.1080/10715760903062887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Fatma N. Singh P. Chhunchha B. Kubo E. Shinohara T. Bhargavan B. Singh DP. Deficiency of Prdx6 in lens epithelial cells induces ER stress response-mediated impaired homeostasis and apoptosis. Am J Physiol Cell Physiol. 2011;301:C954–C967. doi: 10.1152/ajpcell.00061.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Feige MJ. Hendershot LM. Disulfide bonds in ER protein folding and homeostasis. Curr Opin Cell Biol. 2011;23:167–175. doi: 10.1016/j.ceb.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Feng Y. Zhong N. Rouhier N. Hase T. Kusunoki M. Jacquot J-P. Jin C. Xia B. Structural insight into poplar glutaredoxin C1 with a bridging iron-sulfur cluster at the active site. Biochemistry. 2006;45:7998–8008. doi: 10.1021/bi060444t. [DOI] [PubMed] [Google Scholar]
- 182.Fenton HJH. LXXIII.-Oxidation of tartaric acid in presence of iron. J Chem Soc Trans. 1894;65:899–910. [Google Scholar]
- 183.Fernandes AP. Capitanio A. Selenius M. Brodin O. Rundlöf A-K. Björnstedt M. Expression profiles of thioredoxin family proteins in human lung cancer tissue: correlation with proliferation and differentiation. Histopathology. 2009;55:313–320. doi: 10.1111/j.1365-2559.2009.03381.x. [DOI] [PubMed] [Google Scholar]
- 184.Fernandes AP. Holmgren A. Glutaredoxins: glutathione-dependent redox enzymes with functions far beyond a simple thioredoxin backup system. Antioxid Redox Signal. 2004;6:63–74. doi: 10.1089/152308604771978354. [DOI] [PubMed] [Google Scholar]
- 185.Fernando MR. Lechner JM. Löfgren S. Gladyshev VN. Lou MF. Mitochondrial thioltransferase (glutaredoxin 2) has GSH-dependent and thioredoxin reductase-dependent peroxidase activities in vitro and in lens epithelial cells. FASEB J. 2006;20:2645–2647. doi: 10.1096/fj.06-5919fje. [DOI] [PubMed] [Google Scholar]
- 186.Fernando MR. Nanri H. Yoshitake S. Nagata-Kuno K. Minakami S. Thioredoxin regenerates proteins inactivated by oxidative stress in endothelial cells. Eur J Biochem. 1992;209:917–922. doi: 10.1111/j.1432-1033.1992.tb17363.x. [DOI] [PubMed] [Google Scholar]
- 187.Fernando MR. Sumimoto H. Nanri H. Kawabata S. Iwanaga S. Minakami S. Fukumaki Y. Takeshige K. Cloning and sequencing of the cDNA encoding human glutaredoxin. Biochim Biophys Acta. 1994;1218:229–231. doi: 10.1016/0167-4781(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 188.Ferrante RJ. Browne SE. Shinobu LA. Bowling AC. Baik MJ. MacGarvey U. Kowall NW. Brown RH., Jr. Beal MF. Evidence of increased oxidative damage in both sporadic and familial amyotrophic lateral sclerosis. J Neurochem. 1997;69:2064–2074. doi: 10.1046/j.1471-4159.1997.69052064.x. [DOI] [PubMed] [Google Scholar]
- 189.Ferreira LF. Reid MB. Muscle-derived ROS and thiol regulation in muscle fatigue. J Appl Physiol. 2008;104:853–860. doi: 10.1152/japplphysiol.00953.2007. [DOI] [PubMed] [Google Scholar]
- 190.Ferreira NE. Omae S. Pereira A. Rodrigues MV. Miyakawa AA. Campos LCG. Santos PCJL. Dallan LA. Martinez TL. Santos RD. Mill JG. Krieger JE. Pereira AC. Thioredoxin interacting protein genetic variation is associated with diabetes and hypertension in the Brazilian general population. Atherosclerosis. 2012;221:131–136. doi: 10.1016/j.atherosclerosis.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 191.Ferri A. Fiorenzo P. Nencini M. Cozzolino M. Pesaresi MG. Valle C. Sepe S. Moreno S. Carrì MT. Glutaredoxin 2 prevents aggregation of mutant SOD1 in mitochondria and abolishes its toxicity. Hum Mol Genet. 2010;19:4529–4542. doi: 10.1093/hmg/ddq383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Figueiredo PA. Mota MP. Appell HJ. Duarte JA. The role of mitochondria in aging of skeletal muscle. Biogerontology. 2008;9:67–84. doi: 10.1007/s10522-007-9121-7. [DOI] [PubMed] [Google Scholar]
- 193.Filser M. Comini MA. Molina-Navarro MM. Dirdjaja N. Herrero E. Krauth-Siegel RL. Cloning, functional analysis, and mitochondrial localization of Trypanosoma brucei monothiol glutaredoxin-1. Biol Chem. 2008;389:21–32. doi: 10.1515/BC.2007.147. [DOI] [PubMed] [Google Scholar]
- 194.Finkel T. Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 195.Fisher AB. Peroxiredoxin 6: a bifunctional enzyme with glutathione peroxidase and phospholipase A2 activities. Antioxid Redox Signal. 2011;15:831–844. doi: 10.1089/ars.2010.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Fisher AB. Dodia C. Manevich Y. Chen JW. Feinstein SI. Phospholipid hydroperoxides are substrates for non-selenium glutathione peroxidase. J Biol Chem. 1999;274:21326–21334. doi: 10.1074/jbc.274.30.21326. [DOI] [PubMed] [Google Scholar]
- 197.Fitzgerald LA. Zhang Y. Lewis G. Van Etten JL. Characterization of a monothiol glutaredoxin encoded by Chlorella virus PBCV-1. Virus Genes. 2009;39:418–426. doi: 10.1007/s11262-009-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Fitzpatrick AM. Teague WG. Holguin F. Yeh M. Brown LAS. Airway glutathione homeostasis is altered in children with severe asthma: evidence for oxidant stress. J Allergy Clin Immunol. 2009;123:146–152.e8. doi: 10.1016/j.jaci.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Flohé L. Toppo S. Cozza G. Ursini F. A comparison of thiol peroxidase mechanisms. Antioxid Redox Signal. 2011;15:763–780. doi: 10.1089/ars.2010.3397. [DOI] [PubMed] [Google Scholar]
- 200.Forman HJ. Maiorino M. Ursini F. Signaling functions of reactive oxygen species. Biochemistry. 2010;49:835–842. doi: 10.1021/bi9020378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Forouzannia A. Richards GM. Khuntia D. Mehta MP. Motexafin gadolinium: a novel radiosensitizer for brain tumors. Expert Rev Anticancer Ther. 2007;7:785–794. doi: 10.1586/14737140.7.6.785. [DOI] [PubMed] [Google Scholar]
- 202.Foster MW. Forrester MT. Stamler JS. A protein microarray-based analysis of S-nitrosylation. Proc Natl Acad Sci U S A. 2009;106:18948–18953. doi: 10.1073/pnas.0900729106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Foster MW. Hess DT. Stamler JS. Protein S-nitrosylation in health and disease: a current perspective. Trends Mol Med. 2009;15:391–404. doi: 10.1016/j.molmed.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Francoleon NE. Carrington SJ. Fukuto JM. The reaction of H(2)S with oxidized thiols: generation of persulfides and implications to H(2)S biology. Arch Biochem Biophys. 2011;516:146–153. doi: 10.1016/j.abb.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 205.Fratelli M. Gianazza E. Ghezzi P. Redox proteomics: identification and functional role of glutathionylated proteins. Expert Rev Proteomics. 2004;1:365–376. doi: 10.1586/14789450.1.3.365. [DOI] [PubMed] [Google Scholar]
- 206.Freemerman AJ. Gallegos A. Powis G. Nuclear factor kappaB transactivation is increased but is not involved in the proliferative effects of thioredoxin overexpression in MCF-7 breast cancer cells. Cancer Res. 1999;59:4090–4094. [PubMed] [Google Scholar]
- 207.Freiesleben De Blasio B. Bak P. Pociot F. Karlsen AE. Nerup J. Onset of type 1 diabetes: a dynamical instability. Diabetes. 1999;48:1677–1685. doi: 10.2337/diabetes.48.9.1677. [DOI] [PubMed] [Google Scholar]
- 208.Fridovich I. Quantitative aspects of the production of superoxide anion radical by milk xanthine oxidase. J Biol Chem. 1970;245:4053–4057. [PubMed] [Google Scholar]
- 209.Fry M. Green DE. Cardiolipin requirement for electron transfer in complex I and III of the mitochondrial respiratory chain. J Biol Chem. 1981;256:1874–1880. [PubMed] [Google Scholar]
- 210.Fujii J. Iuchi Y. Okada F. Fundamental roles of reactive oxygen species and protective mechanisms in the female reproductive system. Reprod Biol Endocrinol. 2005;3:43. doi: 10.1186/1477-7827-3-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Fujii T. Fujii J. Taniguchi N. Augmented expression of peroxiredoxin VI in rat lung and kidney after birth implies an antioxidative role. Eur J Biochem. 2001;268:218–225. doi: 10.1046/j.1432-1033.2001.01843.x. [DOI] [PubMed] [Google Scholar]
- 212.Fukai T. Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal. 2011;15:1583–1606. doi: 10.1089/ars.2011.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Funato Y. Michiue T. Asashima M. Miki H. The thioredoxin-related redox-regulating protein nucleoredoxin inhibits Wnt-beta-catenin signalling through dishevelled. Nat Cell Biol. 2006;8:501–508. doi: 10.1038/ncb1405. [DOI] [PubMed] [Google Scholar]
- 214.Funato Y. Michiue T. Terabayashi T. Yukita A. Danno H. Asashima M. Miki H. Nucleoredoxin regulates the Wnt/planar cell polarity pathway in Xenopus. Genes Cells. 2008;13:965–975. doi: 10.1111/j.1365-2443.2008.01220.x. [DOI] [PubMed] [Google Scholar]
- 215.Funato Y. Miki H. Nucleoredoxin, a novel thioredoxin family member involved in cell growth and differentiation. Antioxid Redox Signal. 2007;9:1035–1057. doi: 10.1089/ars.2007.1550. [DOI] [PubMed] [Google Scholar]
- 216.Funato Y. Terabayashi T. Sakamoto R. Okuzaki D. Ichise H. Nojima H. Yoshida N. Miki H. Nucleoredoxin sustains Wnt/β-catenin signaling by retaining a pool of inactive dishevelled protein. Curr Biol. 2010;20:1945–1952. doi: 10.1016/j.cub.2010.09.065. [DOI] [PubMed] [Google Scholar]
- 217.Furchgott RF. Endothelium-derived relaxing factor: discovery, early studies, and identification as nitric oxide. Biosci Rep. 1999;19:235–251. doi: 10.1023/a:1020537506008. [DOI] [PubMed] [Google Scholar]
- 218.Gallogly MM. Starke DW. Leonberg AK. Ospina SME. Mieyal JJ. Kinetic and mechanistic characterization and versatile catalytic properties of mammalian glutaredoxin 2: implications for intracellular roles. Biochemistry. 2008;47:11144–11157. doi: 10.1021/bi800966v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Gao B-B. Chen X. Timothy N. Aiello LP. Feener EP. Characterization of the vitreous proteome in diabetes without diabetic retinopathy and diabetes with proliferative diabetic retinopathy. J Proteome Res. 2008;7:2516–2525. doi: 10.1021/pr800112g. [DOI] [PubMed] [Google Scholar]
- 220.García-Pardo L. Granados MD. Gaytán F. Padilla CA. Martínez-Galisteo E. Morales C. Sánchez-Criado JE. Bárcena JA. Immunolocalization of glutaredoxin in the human corpus luteum. Mol Hum Reprod. 1999;5:914–919. doi: 10.1093/molehr/5.10.914. [DOI] [PubMed] [Google Scholar]
- 221.Gardiner CS. Reed DJ. Status of glutathione during oxidant-induced oxidative stress in the preimplantation mouse embryo. Biol Reprod. 1994;51:1307–1314. doi: 10.1095/biolreprod51.6.1307. [DOI] [PubMed] [Google Scholar]
- 222.Gasdaska PY. Oblong JE. Cotgreave IA. Powis G. The predicted amino acid sequence of human thioredoxin is identical to that of the autocrine growth factor human adult T-cell derived factor (ADF): thioredoxin mRNA is elevated in some human tumors. Biochim Biophys Acta. 1994;1218:292–296. doi: 10.1016/0167-4781(94)90180-5. [DOI] [PubMed] [Google Scholar]
- 223.Geiben-Lynn R. Kursar M. Brown NV. Addo MM. Shau H. Lieberman J. Luster AD. Walker BD. HIV-1 antiviral activity of recombinant natural killer cell enhancing factors, NKEF-A and NKEF-B, members of the peroxiredoxin family. J Biol Chem. 2003;278:1569–1574. doi: 10.1074/jbc.M209964200. [DOI] [PubMed] [Google Scholar]
- 224.Gerashchenko MV. Su D. Gladyshev VN. CUG start codon generates thioredoxin/glutathione reductase isoforms in mouse testes. J Biol Chem. 2010;285:4595–4602. doi: 10.1074/jbc.M109.070532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Gertz M. Fischer F. Wolters D. Steegborn C. Activation of the lifespan regulator p66Shc through reversible disulfide bond formation. Proc Natl Acad Sci U S A. 2008;105:5705–5709. doi: 10.1073/pnas.0800691105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Gertz M. Steegborn C. The Lifespan-regulator p66Shc in mitochondria: redox enzyme or redox sensor? Antioxid Redox Signal. 2010;13:1417–1428. doi: 10.1089/ars.2010.3147. [DOI] [PubMed] [Google Scholar]
- 227.Ghezzi P. Bonetto V. Fratelli M. Thiol-disulfide balance: from the concept of oxidative stress to that of redox regulation. Antioxid Redox Signal. 2005;7:964–972. doi: 10.1089/ars.2005.7.964. [DOI] [PubMed] [Google Scholar]
- 228.Gibson KR. Winterburn TJ. Barrett F. Sharma S. MacRury SM. Megson IL. Therapeutic potential of N-acetylcysteine as an antiplatelet agent in patients with type-2 diabetes. Cardiovasc Diabetol. 2011;10:43. doi: 10.1186/1475-2840-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Giles GI. The redox regulation of thiol dependent signaling pathways in cancer. Curr Pharm Des. 2006;12:4427–4443. doi: 10.2174/138161206779010549. [DOI] [PubMed] [Google Scholar]
- 230.Gil L. Martínez G. González I. Tarinas A. Alvarez A. Giuliani A. Molina R. Tápanes R. Pérez J. León OS. Contribution to characterization of oxidative stress in HIV/AIDS patients. Pharmacol Res. 2003;47:217–224. doi: 10.1016/s1043-6618(02)00320-1. [DOI] [PubMed] [Google Scholar]
- 231.Gill R. Tsung A. Billiar T. Linking oxidative stress to inflammation: toll-like receptors. Free Radic Biol Med. 2010;48:1121–1132. doi: 10.1016/j.freeradbiomed.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Gilman AG. Nobel Lecture. G proteins and regulation of adenylyl cyclase. Biosci Rep. 1995;15:65–97. doi: 10.1007/BF01200143. [DOI] [PubMed] [Google Scholar]
- 233.Giustarini D. Milzani A. Aldini G. Carini M. Rossi R. Dalle-Donne I. S-nitrosation versus S-glutathionylation of protein sulfhydryl groups by S-nitrosoglutathione. Antioxid Redox Signal. 2005;7:930–939. doi: 10.1089/ars.2005.7.930. [DOI] [PubMed] [Google Scholar]
- 234.Gobe GC. Johnson DW. Distal tubular epithelial cells of the kidney: potential support for proximal tubular cell survival after renal injury. Int J Biochem Cell Biol. 2007;39:1551–1561. doi: 10.1016/j.biocel.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 235.Godoy JR. Funke M. Ackermann W. Haunhorst P. Oesteritz S. Capani F. Elsässer H-P. Lillig CH. Redox atlas of the mouse. Immunohistochemical detection of glutaredoxin-, peroxiredoxin-, and thioredoxin-family proteins in various tissues of the laboratory mouse. Biochim Biophys Acta. 2011;1810:2–92. doi: 10.1016/j.bbagen.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 236.Godoy JR. Oesteritz S. Hanschmann E-M. Ockenga W. Ackermann W. Lillig CH. Segment-specific overexpression of redoxins after renal ischemia and reperfusion: protective roles of glutaredoxin 2, peroxiredoxin 3, and peroxiredoxin 6. Free Radic Biol Med. 2011;51:552–561. doi: 10.1016/j.freeradbiomed.2011.04.036. [DOI] [PubMed] [Google Scholar]
- 237.González-Fernández R. Gaytán F. Martínez-Galisteo E. Porras P. Padilla CA. Sánchez Criado JE. Bárcena JA. Expression of glutaredoxin (thioltransferase) in the rat ovary during the oestrous cycle and postnatal development. J Mol Endocrinol. 2005;34:625–635. doi: 10.1677/jme.1.01715. [DOI] [PubMed] [Google Scholar]
- 238.Gougeon M-L. Apoptosis as an HIV strategy to escape immune attack. Nat Rev Immunol. 2003;3:392–404. doi: 10.1038/nri1087. [DOI] [PubMed] [Google Scholar]
- 239.Go Y-M. Jones DP. Redox compartmentalization in eukaryotic cells. Biochim Biophys Acta. 2008;1780:1273–1290. doi: 10.1016/j.bbagen.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Gravina SA. Mieyal JJ. Thioltransferase is a specific glutathionyl mixed disulfide oxidoreductase. Biochemistry. 1993;32:3368–3376. doi: 10.1021/bi00064a021. [DOI] [PubMed] [Google Scholar]
- 241.Griffith OW. Mammalian sulfur amino acid metabolism: an overview. Meth Enzymol. 1987;143:366–376. doi: 10.1016/0076-6879(87)43065-6. [DOI] [PubMed] [Google Scholar]
- 242.Grimsrud PA. Xie H. Griffin TJ. Bernlohr DA. Oxidative stress and covalent modification of protein with bioactive aldehydes. J Biol Chem. 2008;283:21837–21841. doi: 10.1074/jbc.R700019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Grogan TM. Fenoglio-Prieser C. Zeheb R. Bellamy W. Frutiger Y. Vela E. Stemmerman G. Macdonald J. Richter L. Gallegos A. Powis G. Thioredoxin, a putative oncogene product, is overexpressed in gastric carcinoma and associated with increased proliferation and increased cell survival. Hum Pathol. 2000;31:475–481. doi: 10.1053/hp.2000.6546. [DOI] [PubMed] [Google Scholar]
- 244.Gruber CW. Cemazar M. Heras B. Martin JL. Craik DJ. Protein disulfide isomerase: the structure of oxidative folding. Trends Biochem Sci. 2006;31:455–464. doi: 10.1016/j.tibs.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 245.Gruetter CA. Barry BK. McNamara DB. Gruetter DY. Kadowitz PJ. Ignarro L. Relaxation of bovine coronary artery and activation of coronary arterial guanylate cyclase by nitric oxide, nitroprusside and a carcinogenic nitrosoamine. J Cyclic Nucleotide Res. 1979;5:211–224. [PubMed] [Google Scholar]
- 246.Grunewald RW. Weber II. Kinne-Saffran E. Kinne RK. Control of sorbitol metabolism in renal inner medulla of diabetic rats: regulation by substrate, cosubstrate and products of the aldose reductase reaction. Biochim Biophys Acta. 1993;1225:39–47. doi: 10.1016/0925-4439(93)90119-l. [DOI] [PubMed] [Google Scholar]
- 247.Del Guerra S. Lupi R. Marselli L. Masini M. Bugliani M. Sbrana S. Torri S. Pollera M. Boggi U. Mosca F. Del Prato S. Marchetti P. Functional and molecular defects of pancreatic islets in human type 2 diabetes. Diabetes. 2005;54:727–735. doi: 10.2337/diabetes.54.3.727. [DOI] [PubMed] [Google Scholar]
- 248.Guo Q. Huang X. Zhang J. Luo Y. Peng Z. Li S. Downregulation of peroxiredoxin I by a novel fully human phage display recombinant antibody induces apoptosis and enhances radiation sensitization in A549 lung carcinoma cells. Cancer Biother Radiopharm. 2012;27:307–316. doi: 10.1089/cbr.2011.0989. [DOI] [PubMed] [Google Scholar]
- 249.Haber F. Wilstätter R. Unpaarigheit und Radikalketten im Reaktion-Mechanismus organischer und enzymatischer Vorgänge. Chem Ber. 1931;64:2844–2856. [Google Scholar]
- 250.Hacioglu E. Esmer I. Fomenko DE. Gladyshev VN. Koc A. The roles of thiol oxidoreductases in yeast replicative aging. Mech Ageing Dev. 2010;131:692–699. doi: 10.1016/j.mad.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Hackett NR. Heguy A. Harvey B-G. O'Connor TP. Luettich K. Flieder DB. Kaplan R. Crystal RG. Variability of antioxidant-related gene expression in the airway epithelium of cigarette smokers. Am J Respir Cell Mol Biol. 2003;29:331–343. doi: 10.1165/rcmb.2002-0321OC. [DOI] [PubMed] [Google Scholar]
- 252.Hainaut P. Milner J. Redox modulation of p53 conformation and sequence-specific DNA binding in vitro. Cancer Res. 1993;53:4469–4473. [PubMed] [Google Scholar]
- 253.Halliwell B. Gutteridge JM. The antioxidants of human extracellular fluids. Arch Biochem Biophys. 1990;280:1–8. doi: 10.1016/0003-9861(90)90510-6. [DOI] [PubMed] [Google Scholar]
- 254.Halvey PJ. Watson WH. Hansen JM. Go Y-M. Samali A. Jones DP. Compartmental oxidation of thiol-disulphide redox couples during epidermal growth factor signalling. Biochem J. 2005;386:215–219. doi: 10.1042/BJ20041829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Hamada Y. Fujii H. Kitazawa R. Yodoi J. Kitazawa S. Fukagawa M. Thioredoxin-1 overexpression in transgenic mice attenuates streptozotocin-induced diabetic osteopenia: a novel role of oxidative stress and therapeutic implications. Bone. 2009;44:936–941. doi: 10.1016/j.bone.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 256.Hamada Y. Miyata S. Nii-Kono T. Kitazawa R. Kitazawa S. Higo S. Fukunaga M. Ueyama S. Nakamura H. Yodoi J. Fukagawa M. Kasuga M. Overexpression of thioredoxin1 in transgenic mice suppresses development of diabetic nephropathy. Nephrol Dial Transplant. 2007;22:1547–1557. doi: 10.1093/ndt/gfm099. [DOI] [PubMed] [Google Scholar]
- 257.Hamnell-Pamment Y. Lind C. Palmberg C. Bergman T. Cotgreave IA. Determination of site-specificity of S-glutathionylated cellular proteins. Biochem Biophys Res Commun. 2005;332:362–369. doi: 10.1016/j.bbrc.2005.04.130. [DOI] [PubMed] [Google Scholar]
- 258.Hanschmann E-M. Lönn ME. Schütte LD. Funke M. Godoy JR. Eitner S. Hudemann C. Lillig CH. Both thioredoxin 2 and glutaredoxin 2 contribute to the reduction of the mitochondrial 2-cys peroxiredoxin prx3. J Biol Chem. 2010;285:40699–40705. doi: 10.1074/jbc.M110.185827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Hansson HA. Holmgren A. Norstedt G. Rozell B. Changes in the distribution of insulin-like growth factor I, thioredoxin, thioredoxin reductase and ribonucleotide reductase during the development of the retina. Exp Eye Res. 1989;48:411–420. doi: 10.1016/s0014-4835(89)80009-0. [DOI] [PubMed] [Google Scholar]
- 260.Hansson HA. Holmgren A. Rozell B. Täljedal IB. Immunohistochemical localization of thioredoxin and thioredoxin reductase in mouse exocrine and endocrine pancreas. Cell Tissue Res. 1986;245:189–195. doi: 10.1007/BF00218100. [DOI] [PubMed] [Google Scholar]
- 261.Harju T. Kaarteenaho-Wiik R. Soini Y. Sormunen R. Kinnula VL. Diminished immunoreactivity of gamma-glutamylcysteine synthetase in the airways of smokers' lung. Am J Respir Crit Care Med. 2002;166:754–759. doi: 10.1164/rccm.2112014. [DOI] [PubMed] [Google Scholar]
- 262.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 263.Harman D. The biologic clock: the mitochondria? J Am Geriatr Soc. 1972;20:145–147. doi: 10.1111/j.1532-5415.1972.tb00787.x. [DOI] [PubMed] [Google Scholar]
- 264.Harris AL. Hypoxia—a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 265.Harris JR. Release of a macromolecular protein component from human erythrocyte ghosts. Biochim Biophys Acta. 1968;150:534–537. doi: 10.1016/0005-2736(68)90157-0. [DOI] [PubMed] [Google Scholar]
- 266.Hasanova N. Kubo E. Kumamoto Y. Takamura Y. Akagi Y. Age-related cataracts and Prdx6: correlation between severity of lens opacity, age and the level of Prdx 6 expression. Br J Ophthalmol. 2009;93:1081–1084. doi: 10.1136/bjo.2008.152272. [DOI] [PubMed] [Google Scholar]
- 267.Hasegawa A. Suzuki S. Matsumoto Y. Okubo T. In vivo fatiguing contraction of rat diaphragm produces hydroxyl radicals. Free Radic Biol Med. 1997;22:349–354. doi: 10.1016/s0891-5849(96)00343-7. [DOI] [PubMed] [Google Scholar]
- 268.Hashemy SI. Ungerstedt JS. Zahedi Avval F. Holmgren A. Motexafin gadolinium, a tumor-selective drug targeting thioredoxin reductase and ribonucleotide reductase. J Biol Chem. 2006;281:10691–10697. doi: 10.1074/jbc.M511373200. [DOI] [PubMed] [Google Scholar]
- 269.Hattori I. Takagi Y. Nakamura H. Nozaki K. Bai J. Kondo N. Sugino T. Nishimura M. Hashimoto N. Yodoi J. Intravenous administration of thioredoxin decreases brain damage following transient focal cerebral ischemia in mice. Antioxid Redox Signal. 2004;6:81–87. doi: 10.1089/152308604771978372. [DOI] [PubMed] [Google Scholar]
- 270.Hatzopoulos AK. De Angelis MH. Wurst W. Bornkamm GW. Brielmeier M. Conrad M. Jakupoglu C. Przemeck GKH. Schneider M. Moreno SG. Mayr N. Cytoplasmic thioredoxin reductase is essential for embryogenesis but dispensable for cardiac development. Mol Cell Biol. 2005;25:1980–1988. doi: 10.1128/MCB.25.5.1980-1988.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Haunhorst P. Berndt C. Eitner S. Godoy JR. Lillig CH. Characterization of the human monothiol glutaredoxin 3 (PICOT) as iron-sulfur protein. Biochem Biophys Res Commun. 2010;394:372–376. doi: 10.1016/j.bbrc.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 272.Hayashi T. Funato Y. Terabayashi T. Morinaka A. Sakamoto R. Ichise H. Fukuda H. Yoshida N. Miki H. Nucleoredoxin negatively regulates Toll-like receptor 4 signaling via recruitment of flightless-I to myeloid differentiation primary response gene (88) J Biol Chem. 2010;285:18586–18593. doi: 10.1074/jbc.M110.106468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Hayes JD. Flanagan JU. Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- 274.Hegde A. Bhatia M. Hydrogen sulfide in inflammation: friend or foe? Inflamm Allergy Drug Targets. 2011;10:118–122. doi: 10.2174/187152811794776268. [DOI] [PubMed] [Google Scholar]
- 275.Hellberg V. Wallin I. Eriksson S. Hernlund E. Jerremalm E. Berndtsson M. Eksborg S. Arnér ESJ. Shoshan M. Ehrsson H. Laurell G. Cisplatin and oxaliplatin toxicity: importance of cochlear kinetics as a determinant for ototoxicity. J Natl Cancer Inst. 2009;101:37–47. doi: 10.1093/jnci/djn418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Hentze MW. Muckenthaler MU. Andrews NC. Balancing acts: molecular control of mammalian iron metabolism. Cell. 2004;117:285–297. doi: 10.1016/s0092-8674(04)00343-5. [DOI] [PubMed] [Google Scholar]
- 277.Herrero E. De la Torre-Ruiz MA. Monothiol glutaredoxins: a common domain for multiple functions. Cell Mol Life Sci. 2007;64:1518–1530. doi: 10.1007/s00018-007-6554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Hess DT. Matsumoto A. Kim S-O. Marshall HE. Stamler JS. Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 279.Hirota K. Matsui M. Murata M. Takashima Y. Cheng FS. Itoh T. Fukuda K. Yodoi J. Junji Y. Nucleoredoxin, glutaredoxin, and thioredoxin differentially regulate NF-kappaB, AP-1, and CREB activation in HEK293 cells. Biochem Biophys Res Commun. 2000;274:177–182. doi: 10.1006/bbrc.2000.3106. [DOI] [PubMed] [Google Scholar]
- 280.Hirota K. Murata M. Sachi Y. Nakamura H. Takeuchi J. Mori K. Yodoi J. Distinct roles of thioredoxin in the cytoplasm and in the nucleus. A two-step mechanism of redox regulation of transcription factor NF-kappaB. J Biol Chem. 1999;274:27891–27897. doi: 10.1074/jbc.274.39.27891. [DOI] [PubMed] [Google Scholar]
- 281.Hirota K. Nakamura H. Masutani H. Yodoi J. Thioredoxin superfamily and thioredoxin-inducing agents. Ann N Y Acad Sci. 2002;957:189–199. doi: 10.1111/j.1749-6632.2002.tb02916.x. [DOI] [PubMed] [Google Scholar]
- 282.Hoch FL. Cardiolipins and biomembrane function. Biochim Biophys Acta. 1992;1113:71–133. doi: 10.1016/0304-4157(92)90035-9. [DOI] [PubMed] [Google Scholar]
- 283.Hofmann B. Hecht H-J. Flohé L. Peroxiredoxins. Biol Chem. 2002;383:347–364. doi: 10.1515/BC.2002.040. [DOI] [PubMed] [Google Scholar]
- 284.Holmgren A. Thioredoxin. 6. The amino acid sequence of the protein from escherichia coli B. Eur J Biochem. 1968;6:475–484. doi: 10.1111/j.1432-1033.1968.tb00470.x. [DOI] [PubMed] [Google Scholar]
- 285.Holmgren A. Hydrogen donor system for Escherichia coli ribonucleoside-diphosphate reductase dependent upon glutathione. Proc Natl Acad Sci U S A. 1976;73:2275–2279. doi: 10.1073/pnas.73.7.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Holmgren A. Glutathione-dependent enzyme reactions of the phage T4 ribonucleotide reductase system. J Biol Chem. 1978;253:7424–7430. [PubMed] [Google Scholar]
- 287.Holmgren A. Glutathione-dependent synthesis of deoxyribonucleotides. Purification and characterization of glutaredoxin from Escherichia coli. J Biol Chem. 1979;254:3664–3671. [PubMed] [Google Scholar]
- 288.Holmgren A. Glutathione-dependent synthesis of deoxyribonucleotides. Characterization of the enzymatic mechanism of Escherichia coli glutaredoxin. J Biol Chem. 1979;254:3672–3678. [PubMed] [Google Scholar]
- 289.Holmgren A. Aslund F. Glutaredoxin. Meth Enzymol. 1995;252:283–292. doi: 10.1016/0076-6879(95)52031-7. [DOI] [PubMed] [Google Scholar]
- 290.Holmgren A. Björnstedt M. Thioredoxin and thioredoxin reductase. Meth Enzymol. 1995;252:199–208. doi: 10.1016/0076-6879(95)52023-6. [DOI] [PubMed] [Google Scholar]
- 291.Holmgren A. Buchanan BB. Wolosiuk RA. Photosynthetic regulatory protein from rabbit liver is identical with thioredoxin. FEBS Lett. 1977;82:351–354. doi: 10.1016/0014-5793(77)80619-4. [DOI] [PubMed] [Google Scholar]
- 292.Holmgren A. Luthman M. Tissue distrubution and subcellular localization of bovine thioredoxin determined by radioimmunoassay. Biochemistry. 1978;17:4071–4077. doi: 10.1021/bi00612a031. [DOI] [PubMed] [Google Scholar]
- 293.Holmgren A. Söderberg BO. Eklund H. Brändén CI. Three-dimensional structure of Escherichia coli thioredoxin-S2 to 2.8 A resolution. Proc Natl Acad Sci U S A. 1975;72:2305–2309. doi: 10.1073/pnas.72.6.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Hopkins FG. On an autoxidisable constituent of the cell. Biochem J. 1921;15:286–305. doi: 10.1042/bj0150286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Hopkins FG. On the isolation of glutathione. J Biol Chem. 1927;72:185–187. [Google Scholar]
- 296.Hopkins FG. Dixon M. On glutathione. J Biol Chem. 1922;54:527–563. [PubMed] [Google Scholar]
- 297.Hopkins FG. Harris Leslie J. On glutathione: a reinvestigation. J Biol Chem. 1929;84:269–320. With a section in the text by. [Google Scholar]
- 298.Horstkotte J. Perisic T. Schneider M. Lange P. Schroeder M. Kiermayer C. Hinkel R. Ziegler T. Mandal PK. David R. Schulz S. Schmitt S. Widder J. Sinowatz F. Becker BF. Bauersachs J. Naebauer M. Franz WM. Jeremias I. Brielmeier M. Zischka H. Conrad M. Kupatt C. Mitochondrial thioredoxin reductase is essential for early postischemic myocardial protection. Circulation. 2011;124:2892–2902. doi: 10.1161/CIRCULATIONAHA.111.059253. [DOI] [PubMed] [Google Scholar]
- 299.Hoskins ER. Hood BL. Sun M. Krivak TC. Edwards RP. Conrads TP. Proteomic analysis of ovarian cancer proximal fluids: validation of elevated peroxiredoxin 1 in patient peripheral circulation. PLoS One. 2011;6:e25056. doi: 10.1371/journal.pone.0025056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Hotta M. Tashiro F. Ikegami H. Niwa H. Ogihara T. Yodoi J. Miyazaki J. Pancreatic beta cell-specific expression of thioredoxin, an antioxidative and antiapoptotic protein, prevents autoimmune and streptozotocin-induced diabetes. J Exp Med. 1998;188:1445–1451. doi: 10.1084/jem.188.8.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Ho Y-S. Xiong Y. Ho DS. Gao J. Chua BHL. Pai H. Mieyal JJ. Targeted disruption of the glutaredoxin 1 gene does not sensitize adult mice to tissue injury induced by ischemia/reperfusion and hyperoxia. Free Radic Biol Med. 2007;43:1299–1312. doi: 10.1016/j.freeradbiomed.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Hsieh C-C. Papaconstantinou J. The effect of aging on p38 signaling pathway activity in the mouse liver and in response to ROS generated by 3-nitropropionic acid. Mech Ageing Dev. 2002;123:1423–1435. doi: 10.1016/s0047-6374(02)00084-2. [DOI] [PubMed] [Google Scholar]
- 303.Hsieh C-C. Rosenblatt JI. Papaconstantinou J. Age-associated changes in SAPK/JNK and p38 MAPK signaling in response to the generation of ROS by 3-nitropropionic acid. Mech Ageing Dev. 2003;124:733–746. doi: 10.1016/s0047-6374(03)00083-6. [DOI] [PubMed] [Google Scholar]
- 304.Hsu H-H. Hoffmann S. Di Marco GS. Endlich N. Peter-Katalinić J. Weide T. Pavenstädt H. Downregulation of the antioxidant protein peroxiredoxin 2 contributes to angiotensin II-mediated podocyte apoptosis. Kidney Int. 2011;80:959–969. doi: 10.1038/ki.2011.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Hsu M. Srinivas B. Kumar J. Subramanian R. Andersen J. Glutathione depletion resulting in selective mitochondrial complex I inhibition in dopaminergic cells is via an NO-mediated pathway not involving peroxynitrite: implications for Parkinson's disease. J Neurochem. 2005;92:1091–1103. doi: 10.1111/j.1471-4159.2004.02929.x. [DOI] [PubMed] [Google Scholar]
- 306.Huang C-F. Sun Z-J. Zhao Y-F. Chen X-M. Jia J. Zhang W-F. Increased expression of peroxiredoxin 6 and cyclophilin A in squamous cell carcinoma of the tongue. Oral Dis. 2011;17:328–334. doi: 10.1111/j.1601-0825.2010.01730.x. [DOI] [PubMed] [Google Scholar]
- 307.Huang Z. Pinto JT. Deng H. Richie JP., Jr Inhibition of caspase-3 activity and activation by protein glutathionylation. Biochem Pharmacol. 2008;75:2234–2244. doi: 10.1016/j.bcp.2008.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Huber HE. Tabor S. Richardson CC. Escherichia coli thioredoxin stabilizes complexes of bacteriophage T7 DNA polymerase and primed templates. J Biol Chem. 1987;262:16224–16232. [PubMed] [Google Scholar]
- 309.Hudemann C. Berndt C. Lillig CH. Glutaredoxine und Eisen-Schwefel-Zentren. Biospektrum. 2008;14:32–35. [Google Scholar]
- 310.Hudemann C. Lönn ME. Godoy JR. Zahedi Avval F. Capani F. Holmgren A. Lillig CH. Identification, expression pattern, and characterization of mouse glutaredoxin 2 isoforms. Antioxid Redox Signal. 2009;11:1–14. doi: 10.1089/ars.2008.2068. [DOI] [PubMed] [Google Scholar]
- 311.Hughes MN. Centelles MN. Moore KP. Making and working with hydrogen sulfide: the chemistry and generation of hydrogen sulfide in vitro and its measurement in vivo: a review. Free Radic Biol Med. 2009;47:1346–1353. doi: 10.1016/j.freeradbiomed.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 312.Hu H. Lu L. Mu W. Johnson RJ. Block ER. Patel JM. Priming donor lungs with thioredoxin-1 attenuates acute allograft injury in a rat model of lung transplantation. J Heart Lung Transplant. 2008;27:1142–1149. doi: 10.1016/j.healun.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Hunter G. Eagles BA. Non-protein sulfur compounds of the blood. J Biol Chem. 1927;72:133–146. [Google Scholar]
- 314.Hutchens MP. Dunlap J. Hurn PD. Jarnberg PO. Renal ischemia: does sex matter? Anesth Analg. 2008;107:239–249. doi: 10.1213/ane.0b013e318178ca42. [DOI] [PubMed] [Google Scholar]
- 315.Hu X. Weng Z. Chu CT. Zhang L. Cao G. Gao Y. Signore A. Zhu J. Hastings T. Greenamyre JT. Chen J. Peroxiredoxin-2 protects against 6-hydroxydopamine-induced dopaminergic neurodegeneration via attenuation of the apoptosis signal-regulating kinase (ASK1) signaling cascade. J Neurosci. 2011;31:247–261. doi: 10.1523/JNEUROSCI.4589-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Hwang IK. Yoo K-Y. Kim DW. Lee CH. Choi JH. Kwon Y-G. Kim Y-M. Choi SY. Won M-H. Changes in the expression of mitochondrial peroxiredoxin and thioredoxin in neurons and glia and their protective effects in experimental cerebral ischemic damage. Free Radic Biol Med. 2010;48:1242–1251. doi: 10.1016/j.freeradbiomed.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 317.Ichijo H. Nishida E. Irie K. Ten Dijke P. Saitoh M. Moriguchi T. Takagi M. Matsumoto K. Miyazono K. Gotoh Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- 318.Ichiki H. Hoshino T. Kinoshita T. Imaoka H. Kato S. Inoue H. Nakamura H. Yodoi J. Young HA. Aizawa H. Thioredoxin suppresses airway hyperresponsiveness and airway inflammation in asthma. Biochem Biophys Res Commun. 2005;334:1141–1148. doi: 10.1016/j.bbrc.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 319.Ikegami H. Ono M. Fujisawa T. Hiromine Y. Kawabata Y. Yamato E. Molecular scanning of the gene for thioredoxin, an antioxidative and antiapoptotic protein, and genetic susceptibility to type 1 diabetes. Ann N Y Acad Sci. 2008;1150:103–105. doi: 10.1196/annals.1447.060. [DOI] [PubMed] [Google Scholar]
- 320.Im J-Y. Lee K-W. Woo J-M. Junn E. Mouradian MM. DJ-1 induces thioredoxin 1 expression through the Nrf2 pathway. Hum Mol Genet. 2012;21:3013–3024. doi: 10.1093/hmg/dds131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Immenschuh S. Baumgart-Vogt E. Tan M. Iwahara S. Ramadori G. Fahimi HD. Differential cellular and subcellular localization of heme-binding protein 23/peroxiredoxin I and heme oxygenase-1 in rat liver. J Histochem Cytochem. 2003;51:1621–1631. doi: 10.1177/002215540305101206. [DOI] [PubMed] [Google Scholar]
- 322.Immer FF. Lippeck C. Barmettler H. Berdat PA. Eckstein FS. Kipfer B. Saner H. Schmidli J. Carrel TP. Improvement of quality of life after surgery on the thoracic aorta: effect of antegrade cerebral perfusion and short duration of deep hypothermic circulatory arrest. Circulation. 2004;110:II250–II255. doi: 10.1161/01.CIR.0000138387.61103.a0. [DOI] [PubMed] [Google Scholar]
- 323.Inomata Y. Nakamura H. Tanito M. Teratani A. Kawaji T. Kondo N. Yodoi J. Tanihara H. Thioredoxin inhibits NMDA-induced neurotoxicity in the rat retina. J Neurochem. 2006;98:372–385. doi: 10.1111/j.1471-4159.2006.03871.x. [DOI] [PubMed] [Google Scholar]
- 324.Inoue K. Takano H. Koike E. Warabi E. Yanagawa T. Yanagisawa R. Ishii T. Peroxiredoxin I is a negative regulator of Th2-dominant allergic asthma. Int Immunopharmacol. 2009;9:1281–1288. doi: 10.1016/j.intimp.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 325.Ischiropoulos H. Zhu L. Beckman JS. Peroxynitrite formation from macrophage-derived nitric oxide. Arch Biochem Biophys. 1992;298:446–451. doi: 10.1016/0003-9861(92)90433-w. [DOI] [PubMed] [Google Scholar]
- 326.Ishii T. Yamada M. Sato H. Matsue M. Taketani S. Nakayama K. Sugita Y. Bannai S. Cloning and characterization of a 23-kDa stress-induced mouse peritoneal macrophage protein. J Biol Chem. 1993;268:18633–18636. [PubMed] [Google Scholar]
- 327.Ito W. Kobayashi N. Takeda M. Ueki S. Kayaba H. Nakamura H. Yodoi J. Chihara J. Thioredoxin in allergic inflammation. Int Arch Allergy Immunol. 2011;155(Suppl 1):142–146. doi: 10.1159/000327501. [DOI] [PubMed] [Google Scholar]
- 328.Iuchi Y. Okada F. Tsunoda S. Kibe N. Shirasawa N. Ikawa M. Okabe M. Ikeda Y. Fujii J. Peroxiredoxin 4 knockout results in elevated spermatogenic cell death via oxidative stress. Biochem J. 2009;419:149–158. doi: 10.1042/BJ20081526. [DOI] [PubMed] [Google Scholar]
- 329.Ivarsson R. Quintens R. Dejonghe S. Tsukamoto K. In't Veld P. Renström E. Schuit FC. Redox control of exocytosis: regulatory role of NADPH, thioredoxin, and glutaredoxin. Diabetes. 2005;54:2132–2142. doi: 10.2337/diabetes.54.7.2132. [DOI] [PubMed] [Google Scholar]
- 330.Iverson SL. Orrenius S. The cardiolipin-cytochrome c interaction and the mitochondrial regulation of apoptosis. Arch Biochem Biophys. 2004;423:37–46. doi: 10.1016/j.abb.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 331.Jaeger T. Budde H. Flohé L. Menge U. Singh M. Trujillo M. Radi R. Multiple thioredoxin-mediated routes to detoxify hydroperoxides in Mycobacterium tuberculosis. Arch Biochem Biophys. 2004;423:182–191. doi: 10.1016/j.abb.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 332.Jakupoglu C. Przemeck GKH. Schneider M. Moreno SG. Mayr N. Hatzopoulos AK. De Angelis MH. Wurst W. Bornkamm GW. Brielmeier M. Conrad M. Cytoplasmic thioredoxin reductase is essential for embryogenesis but dispensable for cardiac development. Mol Cell Biol. 2005;25:1980–1988. doi: 10.1128/MCB.25.5.1980-1988.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Jang HH. Lee KO. Chi YH. Jung BG. Park SK. Park JH. Lee JR. Lee SS. Moon JC. Yun JW. Choi YO. Kim WY. Kang JS. Cheong G-W. Yun D-J. Rhee SG. Cho MJ. Lee SY. Two enzymes in one; two yeast peroxiredoxins display oxidative stress-dependent switching from a peroxidase to a molecular chaperone function. Cell. 2004;117:625–635. doi: 10.1016/j.cell.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 334.Jang YC. Remmen VH. The mitochondrial theory of aging: insight from transgenic and knockout mouse models. Exp Gerontol. 2009;44:256–260. doi: 10.1016/j.exger.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 335.Jekell A. Hossain A. Alehagen U. Dahlström U. Rosén A. Elevated circulating levels of thioredoxin and stress in chronic heart failure. Eur J Heart Fail. 2004;6:883–890. doi: 10.1016/j.ejheart.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 336.Jenkins MK. Johnson JG. Molecules involved in T-cell costimulation. Curr Opin Immunol. 1993;5:361–367. doi: 10.1016/0952-7915(93)90054-v. [DOI] [PubMed] [Google Scholar]
- 337.Jeong D. Cha H. Kim E. Kang M. Yang DK. Kim JM. Yoon PO. Oh JG. Bernecker OY. Sakata S. Le TT. Cui L. Lee Y-H. Kim DH. Woo S-H. Liao R. Hajjar RJ. Park WJ. PICOT inhibits cardiac hypertrophy and enhances ventricular function and cardiomyocyte contractility. Circ Res. 2006;99:307–314. doi: 10.1161/01.RES.0000234780.06115.2c. [DOI] [PubMed] [Google Scholar]
- 338.Jeong D. Kim JM. Cha H. Oh JG. Park J. Yun S-H. Ju E-S. Jeon E-S. Hajjar RJ. Park WJ. PICOT attenuates cardiac hypertrophy by disrupting calcineurin-NFAT signaling. Circ Res. 2008;102:711–719. doi: 10.1161/CIRCRESAHA.107.165985. [DOI] [PubMed] [Google Scholar]
- 339.Jewell UR. Kvietikova I. Scheid A. Bauer C. Wenger RH. Gassmann M. Induction of HIF-1alpha in response to hypoxia is instantaneous. FASEB J. 2001;15:1312–1314. [PubMed] [Google Scholar]
- 340.Jha N. Jurma O. Lalli G. Liu Y. Pettus EH. Greenamyre JT. Liu RM. Forman HJ. Andersen JK. Glutathione depletion in PC12 results in selective inhibition of mitochondrial complex I activity. Implications for Parkinson's disease. J Biol Chem. 2000;275:26096–26101. doi: 10.1074/jbc.M000120200. [DOI] [PubMed] [Google Scholar]
- 341.Jia L. Bonaventura C. Bonaventura J. Stamler JS. S-nitrosohaemoglobin: a dynamic activity of blood involved in vascular control. Nature. 1996;380:221–226. doi: 10.1038/380221a0. [DOI] [PubMed] [Google Scholar]
- 342.Jikimoto T. Nishikubo Y. Koshiba M. Kanagawa S. Morinobu S. Morinobu A. Saura R. Mizuno K. Kondo S. Toyokuni S. Nakamura H. Yodoi J. Kumagai S. Thioredoxin as a biomarker for oxidative stress in patients with rheumatoid arthritis. Mol Immunol. 2002;38:765–772. doi: 10.1016/s0161-5890(01)00113-4. [DOI] [PubMed] [Google Scholar]
- 343.Jiménez A. Johansson C. Ljung J. Sagemark J. Berndt KD. Ren B. Tibbelin G. Ladenstein R. Kieselbach T. Holmgren A. Gustafsson JA. Miranda-Vizuete A. Human spermatid-specific thioredoxin-1 (Sptrx-1) is a two-domain protein with oxidizing activity. FEBS Lett. 2002;530:79–84. doi: 10.1016/s0014-5793(02)03417-8. [DOI] [PubMed] [Google Scholar]
- 344.Jiménez A. Pelto-Huikko M. Gustafsson J-A. Miranda-Vizuete A. Characterization of human thioredoxin-like-1: potential involvement in the cellular response against glucose deprivation. FEBS Lett. 2006;580:960–967. doi: 10.1016/j.febslet.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 345.Jiménez A. Zu W. Rawe VY. Pelto-Huikko M. Flickinger CJ. Sutovsky P. Gustafsson J-A. Oko R. Miranda-Vizuete A. Spermatocyte/spermatid-specific thioredoxin-3, a novel Golgi apparatus-associated thioredoxin, is a specific marker of aberrant spermatogenesis. J Biol Chem. 2004;279:34971–34982. doi: 10.1074/jbc.M404192200. [DOI] [PubMed] [Google Scholar]
- 346.Jin DY. Chae HZ. Rhee SG. Jeang KT. Regulatory role for a novel human thioredoxin peroxidase in NF-kappaB activation. J Biol Chem. 1997;272:30952–30961. doi: 10.1074/jbc.272.49.30952. [DOI] [PubMed] [Google Scholar]
- 347.Jin M-H. Lee Y-H. Kim J-M. Sun H-N. Moon E-Y. Shong MH. Kim S-U. Lee SH. Lee T-H. Yu D-Y. Lee D-S. Characterization of neural cell types expressing peroxiredoxins in mouse brain. Neurosci Lett. 2005;381:252–257. doi: 10.1016/j.neulet.2005.02.048. [DOI] [PubMed] [Google Scholar]
- 348.Johansson C. Kavanagh KL. Gileadi O. Oppermann U. Reversible sequestration of active site cysteines in a 2Fe-2S-bridged dimer provides a mechanism for glutaredoxin 2 regulation in human mitochondria. J Biol Chem. 2007;282:3077–3082. doi: 10.1074/jbc.M608179200. [DOI] [PubMed] [Google Scholar]
- 349.Johansson C. Lillig CH. Holmgren A. Human mitochondrial glutaredoxin reduces S-glutathionylated proteins with high affinity accepting electrons from either glutathione or thioredoxin reductase. J Biol Chem. 2004;279:7537–7543. doi: 10.1074/jbc.M312719200. [DOI] [PubMed] [Google Scholar]
- 350.Johansson C. Roos AK. Montano SJ. Sengupta R. Filippakopoulos P. Guo K. Von Delft F. Holmgren A. Oppermann U. Kavanagh KL. The crystal structure of human GLRX5: iron-sulfur cluster co-ordination, tetrameric assembly and monomer activity. Biochem J. 2011;433:303–311. doi: 10.1042/BJ20101286. [DOI] [PubMed] [Google Scholar]
- 351.Johnston MV. Trescher WH. Ishida A. Nakajima W. Neurobiology of hypoxic-ischemic injury in the developing brain. Pediatr Res. 2001;49:735–741. doi: 10.1203/00006450-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 352.Jomova K. Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology. 2011;283:65–87. doi: 10.1016/j.tox.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 353.Jones DP. Redox potential of GSH/GSSG couple: assay and biological significance. Meth Enzymol. 2002;348:93–112. doi: 10.1016/s0076-6879(02)48630-2. [DOI] [PubMed] [Google Scholar]
- 354.Jones DP. Redefining oxidative stress. Antioxid Redox Signal. 2006;8:1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- 355.Jönsson TJ. Murray MS. Johnson LC. Lowther WT. Reduction of cysteine sulfinic acid in peroxiredoxin by sulfiredoxin proceeds directly through a sulfinic phosphoryl ester intermediate. J Biol Chem. 2008;283:23846–23851. doi: 10.1074/jbc.M803244200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Jönsson TJ. Tsang AW. Lowther WT. Furdui CM. Identification of intact protein thiosulfinate intermediate in the reduction of cysteine sulfinic acid in peroxiredoxin by human sulfiredoxin. J Biol Chem. 2008;283:22890–22894. doi: 10.1074/jbc.C800124200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Jørgensen MC. Ahnfelt-Rønne J. Hald J. Madsen OD. Serup P. Hecksher-Sørensen J. An illustrated review of early pancreas development in the mouse. Endocr Rev. 2007;28:685–705. doi: 10.1210/er.2007-0016. [DOI] [PubMed] [Google Scholar]
- 358.Joubert J. Malan SF. Novel nitric oxide synthase inhibitors: a patent review. Expert Opin Ther Pat. 2011;21:537–560. doi: 10.1517/13543776.2011.556619. [DOI] [PubMed] [Google Scholar]
- 359.Jurado J. Prieto-Alamo M-J. Madrid-Rísquez J. Pueyo C. Absolute gene expression patterns of thioredoxin and glutaredoxin redox systems in mouse. J Biol Chem. 2003;278:45546–45554. doi: 10.1074/jbc.M307866200. [DOI] [PubMed] [Google Scholar]
- 360.Kaarniranta K. Salminen A. Haapasalo A. Soininen H. Hiltunen M. Age-related macular degeneration (AMD): Alzheimer's disease in the eye? J Alzheimers Dis. 2011;24:615–631. doi: 10.3233/JAD-2011-101908. [DOI] [PubMed] [Google Scholar]
- 361.Kaarteenaho-Wiik R. Kinnula VL. Distribution of antioxidant enzymes in developing human lung, respiratory distress syndrome, and bronchopulmonary dysplasia. J Histochem Cytochem. 2004;52:1231–1240. doi: 10.1369/jhc.4A6291.2004. [DOI] [PubMed] [Google Scholar]
- 362.Kaimul AM. Nakamura H. Masutani H. Yodoi J. Thioredoxin and thioredoxin-binding protein-2 in cancer and metabolic syndrome. Free Radic Biol Med. 2007;43:861–868. doi: 10.1016/j.freeradbiomed.2007.05.032. [DOI] [PubMed] [Google Scholar]
- 363.Kakisaka Y. Nakashima T. Sumida Y. Yoh T. Nakamura H. Yodoi J. Senmaru H. Elevation of serum thioredoxin levels in patients with type 2 diabetes. Horm Metab Res. 2002;34:160–164. doi: 10.1055/s-2002-23201. [DOI] [PubMed] [Google Scholar]
- 364.Kalantari P. Narayan V. Natarajan SK. Muralidhar K. Gandhi UH. Vunta H. Henderson AJ. Prabhu KS. Thioredoxin reductase-1 negatively regulates HIV-1 transactivating protein Tat-dependent transcription in human macrophages. J Biol Chem. 2008;283:33183–33190. doi: 10.1074/jbc.M807403200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Kamimoto Y. Sugiyama T. Kihira T. Zhang L. Murabayashi N. Umekawa T. Nagao K. Ma N. Toyoda N. Yodoi J. Sagawa N. Transgenic mice overproducing human thioredoxin-1, an antioxidative and anti-apoptotic protein, prevents diabetic embryopathy. Diabetologia. 2010;53:2046–2055. doi: 10.1007/s00125-010-1784-y. [DOI] [PubMed] [Google Scholar]
- 366.Kaneto H. Fujii J. Myint T. Miyazawa N. Islam KN. Kawasaki Y. Suzuki K. Nakamura M. Tatsumi H. Yamasaki Y. Taniguchi N. Reducing sugars trigger oxidative modification and apoptosis in pancreatic beta-cells by provoking oxidative stress through the glycation reaction. Biochem J. 1996;320:855–863. doi: 10.1042/bj3200855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Kaneto H. Xu G. Song KH. Suzuma K. Bonner-Weir S. Sharma A. Weir GC. Activation of the hexosamine pathway leads to deterioration of pancreatic beta-cell function through the induction of oxidative stress. J Biol Chem. 2001;276:31099–31104. doi: 10.1074/jbc.M104115200. [DOI] [PubMed] [Google Scholar]
- 368.Kang DH. Lee DJ. Lee KW. Park YS. Lee JY. Lee S-H. Koh YJ. Koh G-Y. Choi C. Yu D-Y. Kim J. Kang SW. Peroxiredoxin II is an essential antioxidant enzyme that prevents the oxidative inactivation of VEGF receptor-2 in vascular endothelial cells. Mol Cell. 2011;44:545–558. doi: 10.1016/j.molcel.2011.08.040. [DOI] [PubMed] [Google Scholar]
- 369.Kang SW. Shin H-S. Lee K-K. Rhee SG. Yu D-Y. Lee T-H. Kim S-U. Yu S-L. Kim SH. Park DS. Moon H-B. Dho SH. Kwon K-S. Kwon HJ. Han Y-H. Jeong S. Peroxiredoxin II is essential for sustaining life span of erythrocytes in mice. Blood. 2003;101:5033–5038. doi: 10.1182/blood-2002-08-2548. [DOI] [PubMed] [Google Scholar]
- 370.Karihtala P. Soini Y. Vaskivuo L. Bloigu R. Puistola U. DNA adduct 8-hydroxydeoxyguanosine, a novel putative marker of prognostic significance in ovarian carcinoma. Int J Gynecol Cancer. 2009;19:1047–1051. doi: 10.1111/IGC.0b013e3181ad0f0d. [DOI] [PubMed] [Google Scholar]
- 371.Karunakaran S. Saeed U. Ramakrishnan S. Koumar RC. Ravindranath V. Constitutive expression and functional characterization of mitochondrial glutaredoxin (Grx2) in mouse and human brain. Brain Res. 2007;1185:8–17. doi: 10.1016/j.brainres.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 372.Kasuno K. Nakamura H. Ono T. Muso E. Yodoi J. Protective roles of thioredoxin, a redox-regulating protein, in renal ischemia/reperfusion injury. Kidney Int. 2003;64:1273–1282. doi: 10.1046/j.1523-1755.2003.00224.x. [DOI] [PubMed] [Google Scholar]
- 373.Kato N. Motohashi S. Okada T. Ozawa T. Mashima K. PICOT, protein kinase C theta-interacting protein, is a novel regulator of FcepsilonRI-mediated mast cell activation. Cell Immunol. 2008;251:62–67. doi: 10.1016/j.cellimm.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 374.Kato S. Kato M. Abe Y. Matsumura T. Nishino T. Aoki M. Itoyama Y. Asayama K. Awaya A. Hirano A. Ohama E. Redox system expression in the motor neurons in amyotrophic lateral sclerosis (ALS): immunohistochemical studies on sporadic ALS, superoxide dismutase 1 (SOD1)-mutated familial ALS, and SOD1-mutated ALS animal models. Acta Neuropathol. 2005;110:101–112. doi: 10.1007/s00401-005-1019-3. [DOI] [PubMed] [Google Scholar]
- 375.Kawahito S. Kitahata H. Oshita S. Problems associated with glucose toxicity: role of hyperglycemia-induced oxidative stress. World J Gastroenterol. 2009;15:4137–4142. doi: 10.3748/wjg.15.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Kenchappa RS. Ravindranath V. Glutaredoxin is essential for maintenance of brain mitochondrial complex I: studies with MPTP. FASEB J. 2003;17:717–719. doi: 10.1096/fj.02-0771fje. [DOI] [PubMed] [Google Scholar]
- 377.Kettenhofen NJ. Wood MJ. Formation, reactivity, and detection of protein sulfenic acids. Chem Res Toxicol. 2010;23:1633–1646. doi: 10.1021/tx100237w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Kim HS. Kang SW. Rhee SG. Clerch LB. Rat lung peroxiredoxins I and II are differentially regulated during development and by hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2001;280:L1212–L1217. doi: 10.1152/ajplung.2001.280.6.L1212. [DOI] [PubMed] [Google Scholar]
- 379.Kim H-S. Manevich Y. Feinstein SI. Pak JH. Ho YS. Fisher AB. Induction of 1-cys peroxiredoxin expression by oxidative stress in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2003;285:L363–L369. doi: 10.1152/ajplung.00078.2003. [DOI] [PubMed] [Google Scholar]
- 380.Kim K. Kim IH. Lee KY. Rhee SG. Stadtman ER. The isolation and purification of a specific “protector” protein which inhibits enzyme inactivation by a thiol/Fe(III)/O2 mixed-function oxidation system. J Biol Chem. 1988;263:4704–4711. [PubMed] [Google Scholar]
- 381.Kim SH. Fountoulakis M. Cairns N. Lubec G. Protein levels of human peroxiredoxin subtypes in brains of patients with Alzheimer's disease and Down syndrome. J Neural Transm Suppl. 2001:223–235. doi: 10.1007/978-3-7091-6262-0_18. [DOI] [PubMed] [Google Scholar]
- 382.Kimura H. Hydrogen sulfide: its production, release and functions. Amino Acids. 2011;41:113–121. doi: 10.1007/s00726-010-0510-x. [DOI] [PubMed] [Google Scholar]
- 383.Kimura H. Weisz A. Kurashima Y. Hashimoto K. Ogura T. D'Acquisto F. Addeo R. Makuuchi M. Esumi H. Hypoxia response element of the human vascular endothelial growth factor gene mediates transcriptional regulation by nitric oxide: control of hypoxia-inducible factor-1 activity by nitric oxide. Blood. 2000;95:189–197. [PubMed] [Google Scholar]
- 384.Kim YC. Masutani H. Yamaguchi Y. Itoh K. Yamamoto M. Yodoi J. Hemin-induced activation of the thioredoxin gene by Nrf2. A differential regulation of the antioxidant responsive element by a switch of its binding factors. J Biol Chem. 2001;276:18399–18406. doi: 10.1074/jbc.M100103200. [DOI] [PubMed] [Google Scholar]
- 385.Kim Y-J. Ahn J-Y. Liang P. Ip C. Zhang Y. Park Y-M. Human prx1 gene is a target of Nrf2 and is up-regulated by hypoxia/reoxygenation: implication to tumor biology. Cancer Res. 2007;67:546–554. doi: 10.1158/0008-5472.CAN-06-2401. [DOI] [PubMed] [Google Scholar]
- 386.Kim Y-J. Lee W-S. Ip C. Chae H-Z. Park E-M. Park Y-M. Prx1 suppresses radiation-induced c-Jun NH2-terminal kinase signaling in lung cancer cells through interaction with the glutathione S-transferase Pi/c-Jun NH2-terminal kinase complex. Cancer Res. 2006;66:7136–7142. doi: 10.1158/0008-5472.CAN-05-4446. [DOI] [PubMed] [Google Scholar]
- 387.Kim YS. Lee HL. Lee KB. Park JH. Chung WY. Lee KS. Sheen SS. Park KJ. Hwang SC. Nuclear factor E2-related factor 2 dependent overexpression of sulfiredoxin and peroxiredoxin III in human lung cancer. Korean J Intern Med. 2011;26:304–313. doi: 10.3904/kjim.2011.26.3.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.King GL. Loeken MR. Hyperglycemia-induced oxidative stress in diabetic complications. Histochem Cell Biol. 2004;122:333–338. doi: 10.1007/s00418-004-0678-9. [DOI] [PubMed] [Google Scholar]
- 389.Kinnula VL. Lehtonen S. Kaarteenaho-Wiik R. Lakari E. Pääkkö P. Kang SW. Rhee SG. Soini Y. Cell specific expression of peroxiredoxins in human lung and pulmonary sarcoidosis. Thorax. 2002;57:157–164. doi: 10.1136/thorax.57.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Kinoshita T. Hoshino T. Imaoka H. Ichiki H. Okamoto M. Kawayama T. Yodoi J. Kato S. Aizawa H. Thioredoxin prevents the development and progression of elastase-induced emphysema. Biochem Biophys Res Commun. 2007;354:712–719. doi: 10.1016/j.bbrc.2007.01.053. [DOI] [PubMed] [Google Scholar]
- 391.Kirby J. Halligan E. Baptista MJ. Allen S. Heath PR. Holden H. Barber SC. Loynes CA. Wood-Allum CA. Lunec J. Shaw PJ. Mutant SOD1 alters the motor neuronal transcriptome: implications for familial ALS. Brain. 2005;128:1686–1706. doi: 10.1093/brain/awh503. [DOI] [PubMed] [Google Scholar]
- 392.Kishimoto C. Shioji K. Nakamura H. Nakayama Y. Yodoi J. Sasayama S. Serum thioredoxin (TRX) levels in patients with heart failure. Jpn Circ J. 2001;65:491–494. doi: 10.1253/jcj.65.491. [DOI] [PubMed] [Google Scholar]
- 393.Kisucka J. Chauhan AK. Patten IS. Yesilaltay A. Neumann C. Van Etten RA. Krieger M. Wagner DD. Peroxiredoxin1 prevents excessive endothelial activation and early atherosclerosis. Circ Res. 2008;103:598–605. doi: 10.1161/CIRCRESAHA.108.174870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Kleemann R. Kapurniotu A. Frank RW. Gessner A. Mischke R. Flieger O. Jüttner S. Brunner H. Bernhagen J. Disulfide analysis reveals a role for macrophage migration inhibitory factor (MIF) as thiol-protein oxidoreductase. J Mol Biol. 1998;280:85–102. doi: 10.1006/jmbi.1998.1864. [DOI] [PubMed] [Google Scholar]
- 395.Knowlton KU. Badorff C. The immune system in viral myocarditis: maintaining the balance. Circ Res. 1999;85:559–561. doi: 10.1161/01.res.85.6.559. [DOI] [PubMed] [Google Scholar]
- 396.Kobayashi M. Nakamura H. Yodoi J. Shiota K. Immunohistochemical localization of thioredoxin and glutaredoxin in mouse embryos and fetuses. Antioxid Redox Signal. 2000;2:653–663. doi: 10.1089/ars.2000.2.4-653. [DOI] [PubMed] [Google Scholar]
- 397.Kobzik L. Reid MB. Bredt DS. Stamler JS. Nitric oxide in skeletal muscle. Nature. 1994;372:546–548. doi: 10.1038/372546a0. [DOI] [PubMed] [Google Scholar]
- 398.Koenen TB. Stienstra R. Van Tits LJ. De Graaf J. Stalenhoef AFH. Joosten LAB. Tack CJ. Netea MG. Hyperglycemia activates caspase-1 and TXNIP-mediated IL-1beta transcription in human adipose tissue. Diabetes. 2011;60:517–524. doi: 10.2337/db10-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Koh P-O. Proteomic analysis of focal cerebral ischemic injury in male rats. J Vet Med Sci. 2010;72:181–185. doi: 10.1292/jvms.09-0364. [DOI] [PubMed] [Google Scholar]
- 400.Koneru S. Penumathsa SV. Thirunavukkarasu M. Zhan L. Maulik N. Thioredoxin-1 gene delivery induces heme oxygenase-1 mediated myocardial preservation after chronic infarction in hypertensive rats. Am J Hypertens. 2009;22:183–190. doi: 10.1038/ajh.2008.318. [DOI] [PubMed] [Google Scholar]
- 401.Kong L. Zhou X. Li F. Yodoi J. McGinnis J. Cao W. Neuroprotective effect of overexpression of thioredoxin on photoreceptor degeneration in Tubby mice. Neurobiol Dis. 2010;38:446–455. doi: 10.1016/j.nbd.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Koreny M. Sterz F. Uray T. Schreiber W. Holzer M. Laggner A. Herkner H. Effect of cooling after human cardiac arrest on myocardial infarct size. Resuscitation. 2009;80:56–60. doi: 10.1016/j.resuscitation.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 403.Kosower NS. Kosower EM. The glutathione status of cells. Int Rev Cytol. 1978;54:109–160. doi: 10.1016/s0074-7696(08)60166-7. [DOI] [PubMed] [Google Scholar]
- 404.Kozlov AV. Szalay L. Umar F. Kropik K. Staniek K. Niedermüller H. Bahrami S. Nohl H. Skeletal muscles, heart, and lung are the main sources of oxygen radicals in old rats. Biochim Biophys Acta. 2005;1740:382–389. doi: 10.1016/j.bbadis.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 405.Kozlov G. Määttänen P. Thomas DY. Gehring K. A structural overview of the PDI family of proteins. FEBS J. 2010;277:3924–3936. doi: 10.1111/j.1742-4658.2010.07793.x. [DOI] [PubMed] [Google Scholar]
- 406.Krapfenbauer K. Engidawork E. Cairns N. Fountoulakis M. Lubec G. Aberrant expression of peroxiredoxin subtypes in neurodegenerative disorders. Brain Res. 2003;967:152–160. doi: 10.1016/s0006-8993(02)04243-9. [DOI] [PubMed] [Google Scholar]
- 407.Krauth-Siegel RL. Comini MA. Redox control in trypanosomatids, parasitic protozoa with trypanothione-based thiol metabolism. Biochim Biophys Acta. 2008;1780:1236–1248. doi: 10.1016/j.bbagen.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 408.Krimsky I. Racker E. Glutathione, a prosthetic group of glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem. 1952;198:721–729. [PubMed] [Google Scholar]
- 409.Kronschläger M. Galichanin K. Ekström J. Lou MF. Söderberg PG. Protective effect of the thioltransferase gene on in vivo UVR-300 nm-induced cataract. Invest Ophthalmol Vis Sci. 2012;53:248–252. doi: 10.1167/iovs.11-8504. [DOI] [PubMed] [Google Scholar]
- 410.Kubo E. Fatma N. Akagi Y. Beier DR. Singh SP. Singh DP. TAT-mediated PRDX6 protein transduction protects against eye lens epithelial cell death and delays lens opacity. Am J Physiol Cell Physiol. 2008;294:C842–C855. doi: 10.1152/ajpcell.00540.2007. [DOI] [PubMed] [Google Scholar]
- 411.Kubo E. Hasanova N. Tanaka Y. Fatma N. Takamura Y. Singh DP. Akagi Y. Protein expression profiling of lens epithelial cells from Prdx6-depleted mice and their vulnerability to UV radiation exposure. Am J Physiol Cell Physiol. 2010;298:C342–C354. doi: 10.1152/ajpcell.00336.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Kulisz A. Chen N. Chandel NS. Shao Z. Schumacker PT. Mitochondrial ROS initiate phosphorylation of p38 MAP kinase during hypoxia in cardiomyocytes. Am J Physiol Lung Cell Mol Physiol. 2002;282:L1324–L1329. doi: 10.1152/ajplung.00326.2001. [DOI] [PubMed] [Google Scholar]
- 413.Kumsta C. Jakob U. Redox-regulated chaperones. Biochemistry. 2009;48:4666–4676. doi: 10.1021/bi9003556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 415.Lapointe J. Hekimi S. When a theory of aging ages badly. Cell Mol Life Sci. 2010;67:1–8. doi: 10.1007/s00018-009-0138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Lappalainen Z. Lappalainen J. Oksala NKJ. Laaksonen DE. Khanna S. Sen CK. Atalay M. Diabetes impairs exercise training-associated thioredoxin response and glutathione status in rat brain. J Appl Physiol. 2009;106:461–467. doi: 10.1152/japplphysiol.91252.2008. [DOI] [PubMed] [Google Scholar]
- 417.Lappalainen Z. Lappalainen J. Oksala NKJ. Laaksonen DE. Khanna S. Sen CK. Atalay M. Diabetes impairs exercise training-associated thioredoxin response and glutathione status in rat brain. J Appl Physiol. 2009;106:461–467. doi: 10.1152/japplphysiol.91252.2008. [DOI] [PubMed] [Google Scholar]
- 418.Laurent TC. Moore EC. Reichard P. Enzymatic synthesis of deoxyribonucleotides. iv. isolation and characterization of thioredoxin, the hydrogen donor from Escherichia coli B. J Biol Chem. 1964;239:3436–3444. [PubMed] [Google Scholar]
- 419.Lee DW. Kaur D. Chinta SJ. Rajagopalan S. Andersen JK. A disruption in iron-sulfur center biogenesis via inhibition of mitochondrial dithiol glutaredoxin 2 may contribute to mitochondrial and cellular iron dysregulation in mammalian glutathione-depleted dopaminergic cells: implications for Parkinson's disease. Antioxid Redox Signal. 2009;11:2083–2094. doi: 10.1089/ars.2009.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Lee J-H. Kim K. Park E-H. Ahn K. Lim C-J. Expression, characterization and regulation of a Saccharomyces cerevisiae monothiol glutaredoxin (Grx6) gene in Schizosaccharomyces pombe. Mol Cells. 2007;24:316–322. [PubMed] [Google Scholar]
- 421.Lee SH. Kim WS. Lee SH. Oh JW. Lee HM. Jung HH. Jang JW. Jun YJ. Cho WJ. Jhun HS. Expression and distribution of thioredoxin and thioredoxin reductase in human nasal mucosa and nasal polyp. Acta Otolaryngol. 2005;125:877–882. doi: 10.1080/00016480510029293. [DOI] [PubMed] [Google Scholar]
- 422.Lee T-H. Kim S-U. Yu S-L. Kim SH. Park DS. Moon H-B. Dho SH. Kwon K-S. Kwon HJ. Han Y-H. Jeong S. Kang SW. Shin H-S. Lee K-K. Rhee SG. Yu D-Y. Peroxiredoxin II is essential for sustaining life span of erythrocytes in mice. Blood. 2003;101:5033–5038. doi: 10.1182/blood-2002-08-2548. [DOI] [PubMed] [Google Scholar]
- 423.Lee YM. Park SH. Shin D-I. Hwang J-Y. Park B. Park Y-J. Lee TH. Chae HZ. Jin BK. Oh TH. Oh YJ. Oxidative modification of peroxiredoxin is associated with drug-induced apoptotic signaling in experimental models of Parkinson disease. J Biol Chem. 2008;283:9986–9998. doi: 10.1074/jbc.M800426200. [DOI] [PubMed] [Google Scholar]
- 424.Lehtonen ST. Ohlmeier S. Kaarteenaho-Wiik R. Harju T. Pääkkö P. Soini Y. Kinnula VL. Does the oxidative stress in chronic obstructive pulmonary disease cause thioredoxin/peroxiredoxin oxidation? Antioxid Redox Signal. 2008;10:813–819. doi: 10.1089/ars.2007.1952. [DOI] [PubMed] [Google Scholar]
- 425.Lei X. Cheng W. New roles of glutathione peroxidase 1 in oxidative stress and diabetes. In: Hatfield DL, editor; Berry MJ, editor; Gladyshev VN, editor. Selenium—Its Molecular Biology and Role in Human Health. Heidelberg, Germany: Springer; 2006. pp. 173–182. [Google Scholar]
- 426.Lekli I. Mukherjee S. Ray D. Gurusamy N. Kim YH. Tosaki A. Engelman RM. Ho Y-S. Das DK. Functional recovery of diabetic mouse hearts by glutaredoxin-1 gene therapy: role of Akt-FoxO-signaling network. Gene Ther. 2010;17:478–485. doi: 10.1038/gt.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Lenaz G. Bovina C. D'Aurelio M. Fato R. Formiggini G. Genova ML. Giuliano G. Merlo Pich M. Paolucci U. Parenti Castelli G. Ventura B. Role of mitochondria in oxidative stress and aging. Ann N Y Acad Sci. 2002;959:199–213. doi: 10.1111/j.1749-6632.2002.tb02094.x. [DOI] [PubMed] [Google Scholar]
- 428.Léveillard T. Mohand-Saïd S. Lorentz O. Hicks D. Fintz A-C. Clérin E. Simonutti M. Forster V. Cavusoglu N. Chalmel F. Dollé P. Poch O. Lambrou G. Sahel J-A. Identification and characterization of rod-derived cone viability factor. Nat Genet. 2004;36:755–759. doi: 10.1038/ng1386. [DOI] [PubMed] [Google Scholar]
- 429.Liang H. Li X. Li S. Zheng M-Q. Rozanski GJ. Oxidoreductase regulation of Kv currents in rat ventricle. J Mol Cell Cardiol. 2008;44:1062–1071. doi: 10.1016/j.yjmcc.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Liang M. Pietrusz JL. Thiol-related genes in diabetic complications: a novel protective role for endogenous thioredoxin 2. Arterioscler Thromb Vasc Biol. 2007;27:77–83. doi: 10.1161/01.ATV.0000251006.54632.bb. [DOI] [PubMed] [Google Scholar]
- 431.Li F. Sonveaux P. Rabbani ZN. Liu S. Yan B. Huang Q. Vujaskovic Z. Dewhirst MW. Li C-Y. Regulation of HIF-1alpha stability through S-nitrosylation. Mol Cell. 2007;26:63–74. doi: 10.1016/j.molcel.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Lillig CH. Berndt C. Holmgren A. Glutaredoxin systems. Biochim Biophys Acta. 2008;1780:1304–1317. doi: 10.1016/j.bbagen.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 433.Lillig CH. Berndt C. Vergnolle O. Lönn ME. Hudemann C. Bill E. Holmgren A. Characterization of human glutaredoxin 2 as iron-sulfur protein: a possible role as redox sensor. Proc Natl Acad Sci U S A. 2005;102:8168–8173. doi: 10.1073/pnas.0500735102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Lillig CH. Holmgren A. Thioredoxin and related molecules—from biology to health and disease. Antioxid Redox Signal. 2007;9:25–47. doi: 10.1089/ars.2007.9.25. [DOI] [PubMed] [Google Scholar]
- 435.Lillig CH. Lönn ME. Enoksson M. Fernandes AP. Holmgren A. Short interfering RNA-mediated silencing of glutaredoxin 2 increases the sensitivity of HeLa cells toward doxorubicin and phenylarsine oxide. Proc Natl Acad Sci U S A. 2004;101:13227–13232. doi: 10.1073/pnas.0401896101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Lill R. Kispal G. Maturation of cellular Fe-S proteins: an essential function of mitochondria. Trends Biochem Sci. 2000;25:352–356. doi: 10.1016/s0968-0004(00)01589-9. [DOI] [PubMed] [Google Scholar]
- 437.Lill R. Mühlenhoff U. Iron-sulfur protein biogenesis in eukaryotes: components and mechanisms. Annu Rev Cell Dev Biol. 2006;22:457–486. doi: 10.1146/annurev.cellbio.22.010305.104538. [DOI] [PubMed] [Google Scholar]
- 438.Li L. Shoji W. Takano H. Nishimura N. Aoki Y. Takahashi R. Goto S. Kaifu T. Takai T. Obinata M. Increased susceptibility of MER5 (peroxiredoxin III) knockout mice to LPS-induced oxidative stress. Biochem Biophys Res Commun. 2007;355:715–721. doi: 10.1016/j.bbrc.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 439.Lim J. Luderer U. Oxidative damage increases and antioxidant gene expression decreases with aging in the mouse ovary. Biol Reprod. 2011;84:775–782. doi: 10.1095/biolreprod.110.088583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Li N. Oberley TD. Modulation of antioxidant enzymes, reactive oxygen species, and glutathione levels in manganese superoxide dismutase-overexpressing NIH/3T3 fibroblasts during the cell cycle. J Cell Physiol. 1998;177:148–160. doi: 10.1002/(SICI)1097-4652(199810)177:1<148::AID-JCP16>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 441.Lippoldt A. Padilla CA. Gerst H. Andbjer B. Richter E. Holmgren A. Fuxe K. Localization of thioredoxin in the rat brain and functional implications. J Neurosci. 1995;15:6747–6756. doi: 10.1523/JNEUROSCI.15-10-06747.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Liu W. Nakamura H. Shioji K. Tanito M. Oka S. Ahsan MK. Son A. Ishii Y. Kishimoto C. Yodoi J. Thioredoxin-1 ameliorates myosin-induced autoimmune myocarditis by suppressing chemokine expressions and leukocyte chemotaxis in mice. Circulation. 2004;110:1276–1283. doi: 10.1161/01.CIR.0000141803.41217.B6. [DOI] [PubMed] [Google Scholar]
- 443.Liu Y. Min W. Thioredoxin promotes ASK1 ubiquitination and degradation to inhibit ASK1-mediated apoptosis in a redox activity-independent manner. Circ Res. 2002;90:1259–1266. doi: 10.1161/01.res.0000022160.64355.62. [DOI] [PubMed] [Google Scholar]
- 444.Li X. Tang K. Xie B. Li S. Rozanski GJ. Regulation of Kv4 channel expression in failing rat heart by the thioredoxin system. Am J Physiol Heart Circ Physiol. 2008;295:H416–H424. doi: 10.1152/ajpheart.91446.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Löfgren S. Fernando MR. Xing K-Y. Wang Y. Kuszynski CA. Ho Y-S. Lou MF. Effect of thioltransferase (glutaredoxin) deletion on cellular sensitivity to oxidative stress and cell proliferation in lens epithelial cells of thioltransferase knockout mouse. Invest Ophthalmol Vis Sci. 2008;49:4497–4505. doi: 10.1167/iovs.07-1404. [DOI] [PubMed] [Google Scholar]
- 446.Longo VD. Gralla EB. Valentine JS. Superoxide dismutase activity is essential for stationary phase survival in Saccharomyces cerevisiae. Mitochondrial production of toxic oxygen species in vivo. J Biol Chem. 1996;271:12275–12280. doi: 10.1074/jbc.271.21.12275. [DOI] [PubMed] [Google Scholar]
- 447.Lönn ME. Hudemann C. Berndt C. Cherkasov V. Capani F. Holmgren A. Lillig CH. Expression pattern of human glutaredoxin 2 isoforms: identification and characterization of two testis/cancer cell-specific isoforms. Antioxid Redox Signal. 2008;10:547–557. doi: 10.1089/ars.2007.1821. [DOI] [PubMed] [Google Scholar]
- 448.Lopes de Faria JB. Silva KC. Lopes de Faria JM. The contribution of hypertension to diabetic nephropathy and retinopathy: the role of inflammation and oxidative stress. Hypertens Res. 2011;34:413–422. doi: 10.1038/hr.2010.263. [DOI] [PubMed] [Google Scholar]
- 449.Loschen G. Flohé L. Chance B. Respiratory chain linked H(2)O(2) production in pigeon heart mitochondria. FEBS Lett. 1971;18:261–264. doi: 10.1016/0014-5793(71)80459-3. [DOI] [PubMed] [Google Scholar]
- 450.Lovell MA. Xie C. Gabbita SP. Markesbery WR. Decreased thioredoxin and increased thioredoxin reductase levels in Alzheimer's disease brain. Free Radic Biol Med. 2000;28:418–427. doi: 10.1016/s0891-5849(99)00258-0. [DOI] [PubMed] [Google Scholar]
- 451.Lüdemann H. Dormeyer M. Sticherling C. Stallmann D. Follmann H. Krauth-Siegel RL. Trypanosoma brucei tryparedoxin, a thioredoxin-like protein in African trypanosomes. FEBS Lett. 1998;431:381–385. doi: 10.1016/s0014-5793(98)00793-5. [DOI] [PubMed] [Google Scholar]
- 452.Lu J. Berndt C. Holmgren A. Metabolism of selenium compounds catalyzed by the mammalian selenoprotein thioredoxin reductase. Biochim Biophys Acta. 2009;1790:1513–1519. doi: 10.1016/j.bbagen.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 453.Lundberg M. Fernandes AP. Kumar S. Holmgren A. Cellular and plasma levels of human glutaredoxin 1 and 2 detected by sensitive ELISA systems. Biochem Biophys Res Commun. 2004;319:801–809. doi: 10.1016/j.bbrc.2004.04.199. [DOI] [PubMed] [Google Scholar]
- 454.Lundström J. Holmgren A. Protein disulfide-isomerase is a substrate for thioredoxin reductase and has thioredoxin-like activity. J Biol Chem. 1990;265:9114–9120. [PubMed] [Google Scholar]
- 455.Luthman M. Eriksson S. Holmgren A. Thelander L. Glutathione-dependent hydrogen donor system for calf thymus ribonucleoside-diphosphate reductase. Proc Natl Acad Sci U S A. 1979;76:2158–2162. doi: 10.1073/pnas.76.5.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Luthman M. Holmgren A. Glutaredoxin from calf thymus. Purification to homogeneity. J Biol Chem. 1982;257:6686–6690. [PubMed] [Google Scholar]
- 457.Lysell J. Stjernholm Vladic Y. Ciarlo N. Holmgren A. Sahlin L. Immunohistochemical determination of thioredoxin and glutaredoxin distribution in the human cervix, and possible relation to cervical ripening. Gynecol Endocrinol. 2003;17:303–310. doi: 10.1080/gye.17.4.303.310. [DOI] [PubMed] [Google Scholar]
- 458.Madian AG. Myracle AD. Diaz-Maldonado N. Rochelle NS. Janle EM. Regnier FE. Differential carbonylation of proteins as a function of in vivo oxidative stress. J Proteome Res. 2011;10:3959–3972. doi: 10.1021/pr200140x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Ma D. Warabi E. Yanagawa T. Kimura S. Harada H. Yamagata K. Ishii T. Peroxiredoxin I plays a protective role against cisplatin cytotoxicity through mitogen activated kinase signals. Oral Oncol. 2009;45:1037–1043. doi: 10.1016/j.oraloncology.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 460.Magaki S. Raghavan R. Mueller C. Oberg KC. Vinters HV. Kirsch WM. Iron, copper, and iron regulatory protein 2 in Alzheimer's disease and related dementias. Neurosci Lett. 2007;418:72–76. doi: 10.1016/j.neulet.2007.02.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Magnusson CG. Björnstedt M. Holmgren A. Human IgG is substrate for the thioredoxin system: differential cleavage pattern of interchain disulfide bridges in IgG subclasses. Mol Immunol. 1997;34:709–717. doi: 10.1016/s0161-5890(97)00092-8. [DOI] [PubMed] [Google Scholar]
- 462.Malaspina A. Kaushik N. De Belleroche J. Differential expression of 14 genes in amyotrophic lateral sclerosis spinal cord detected using gridded cDNA arrays. J Neurochem. 2001;77:132–145. doi: 10.1046/j.1471-4159.2001.t01-1-00231.x. [DOI] [PubMed] [Google Scholar]
- 463.Malik G. Gorbounov N. Das S. Gurusamy N. Otani H. Maulik N. Goswami S. Das DK. Ischemic preconditioning triggers nuclear translocation of thioredoxin and its interaction with Ref-1 potentiating a survival signal through the PI-3-kinase-Akt pathway. Antioxid Redox Signal. 2006;8:2101–2109. doi: 10.1089/ars.2006.8.2101. [DOI] [PubMed] [Google Scholar]
- 464.Malik G. Nagy N. Ho Y-S. Maulik N. Das DK. Role of glutaredoxin-1 in cardioprotection: an insight with Glrx1 transgenic and knockout animals. J Mol Cell Cardiol. 2008;44:261–269. doi: 10.1016/j.yjmcc.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 465.Mannick JB. Regulation of apoptosis by protein S-nitrosylation. Amino Acids. 2007;32:523–526. doi: 10.1007/s00726-006-0427-6. [DOI] [PubMed] [Google Scholar]
- 466.Mansfield KD. Guzy RD. Pan Y. Young RM. Cash TP. Schumacker PT. Simon MC. Mitochondrial dysfunction resulting from loss of cytochrome c impairs cellular oxygen sensing and hypoxic HIF-alpha activation. Cell Metab. 2005;1:393–399. doi: 10.1016/j.cmet.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Marino SM. Gladyshev VN. Structural analysis of cysteine S-nitrosylation: a modified acid-based motif and the emerging role of trans-nitrosylation. J Mol Biol. 2010;395:844–859. doi: 10.1016/j.jmb.2009.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Markovic J. García-Gimenez JL. Gimeno A. Viña J. Pallardó FV. Role of glutathione in cell nucleus. Free Radic Res. 2010;44:721–733. doi: 10.3109/10715762.2010.485989. [DOI] [PubMed] [Google Scholar]
- 469.Marquez VE. Arias DG. Piattoni CV. Robello C. Iglesias AA. Guerrero SA. Cloning, expression, and characterization of a dithiol glutaredoxin from Trypanosoma cruzi. Antioxid Redox Signal. 2010;12:787–792. doi: 10.1089/ars.2009.2907. [DOI] [PubMed] [Google Scholar]
- 470.Martinez-Vicente M. Sovak G. Cuervo AM. Protein degradation and aging. Exp Gerontol. 2005;40:622–633. doi: 10.1016/j.exger.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 471.Martin JL. Thioredoxin—a fold for all reasons. Structure. 1995;3:245–250. doi: 10.1016/s0969-2126(01)00154-x. [DOI] [PubMed] [Google Scholar]
- 472.Masoro EJ. Caloric restriction and aging: an update. Exp Gerontol. 2000;35:299–305. doi: 10.1016/s0531-5565(00)00084-x. [DOI] [PubMed] [Google Scholar]
- 473.Matsue H. Edelbaum D. Shalhevet D. Mizumoto N. Yang C. Mummert ME. Oeda J. Masayasu H. Takashima A. Generation and function of reactive oxygen species in dendritic cells during antigen presentation. J Immunol. 2003;171:3010–3018. doi: 10.4049/jimmunol.171.6.3010. [DOI] [PubMed] [Google Scholar]
- 474.Matsui M. Oshima M. Oshima H. Takaku K. Maruyama T. Yodoi J. Taketo MM. Early embryonic lethality caused by targeted disruption of the mouse thioredoxin gene. Dev Biol. 1996;178:179–815. doi: 10.1006/dbio.1996.0208. [DOI] [PubMed] [Google Scholar]
- 475.Matsuoka T. Kajimoto Y. Watada H. Kaneto H. Kishimoto M. Umayahara Y. Fujitani Y. Kamada T. Kawamori R. Yamasaki Y. Glycation-dependent, reactive oxygen species-mediated suppression of the insulin gene promoter activity in HIT cells. J Clin Invest. 1997;99:144–150. doi: 10.1172/JCI119126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Matsushima S. Ide T. Yamato M. Matsusaka H. Hattori F. Ikeuchi M. Kubota T. Sunagawa K. Hasegawa Y. Kurihara T. Oikawa S. Kinugawa S. Tsutsui H. Overexpression of mitochondrial peroxiredoxin-3 prevents left ventricular remodeling and failure after myocardial infarction in mice. Circulation. 2006;113:1779–1786. doi: 10.1161/CIRCULATIONAHA.105.582239. [DOI] [PubMed] [Google Scholar]
- 477.Matsushima S. Zablocki D. Sadoshima J. Application of recombinant thioredoxin1 for treatment of heart disease. J Mol Cell Cardiol. 2011;51:570–573. doi: 10.1016/j.yjmcc.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Matsushima Y. Nanri H. Nara S. Okufuji T. Ohta M. Hachisuka K. Ikeda M. Hindlimb unloading decreases thioredoxin-related antioxidant proteins and increases thioredoxin-binding protein-2 in rat skeletal muscle. Free Radic Res. 2006;40:715–722. doi: 10.1080/10715760600580488. [DOI] [PubMed] [Google Scholar]
- 479.Matsuzawa A. Ichijo H. Redox control of cell fate by MAP kinase: physiological roles of ASK1-MAP kinase pathway in stress signaling. Biochim Biophys Acta. 2008;1780:1325–1336. doi: 10.1016/j.bbagen.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 480.Matthews JR. Wakasugi N. Virelizier JL. Yodoi J. Hay RT. Thioredoxin regulates the DNA binding activity of NF-kappa B by reduction of a disulphide bond involving cysteine 62. Nucleic Acids Res. 1992;20:3821–3830. doi: 10.1093/nar/20.15.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Maurice MM. Nakamura H. Gringhuis S. Okamoto T. Yoshida S. Kullmann F. Lechner S. Van der Voort EA. Leow A. Versendaal J. Muller-Ladner U. Yodoi J. Tak PP. Breedveld FC. Verweij CL. Expression of the thioredoxin-thioredoxin reductase system in the inflamed joints of patients with rheumatoid arthritis. Arthritis Rheum. 1999;42:2430–2439. doi: 10.1002/1529-0131(199911)42:11<2430::AID-ANR22>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 482.May JM. Mendiratta S. Hill KE. Burk RF. Reduction of dehydroascorbate to ascorbate by the selenoenzyme thioredoxin reductase. J Biol Chem. 1997;272:22607–22610. doi: 10.1074/jbc.272.36.22607. [DOI] [PubMed] [Google Scholar]
- 483.Ma ZA. Zhao Z. Turk J. Mitochondrial dysfunction and β-cell failure in type 2 diabetes mellitus. Exp Diabetes Res. 2012;2012:703538. doi: 10.1155/2012/703538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.McCord JM. Fridovich I. The utility of superoxide dismutase in studying free radical reactions. I. Radicals generated by the interaction of sulfite, dimethyl sulfoxide, and oxygen. J Biol Chem. 1969;244:6056–6063. [PubMed] [Google Scholar]
- 485.McCord JM. Fridovich I. The utility of superoxide dismutase in studying free radical reactions. II. The mechanism of the mediation of cytochrome c reduction by a variety of electron carriers. J Biol Chem. 1970;245:1374–1377. [PubMed] [Google Scholar]
- 486.McDonagh B. Padilla CA. Pedrajas JR. Bárcena JA. Biosynthetic and iron metabolism is regulated by thiol proteome changes dependent on glutaredoxin-2 and mitochondrial peroxiredoxin-1 in Saccharomyces cerevisiae. J Biol Chem. 2011;286:15565–15576. doi: 10.1074/jbc.M110.193102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.McIntyre LA. Fergusson DA. Hébert PC. Moher D. Hutchison JS. Prolonged therapeutic hypothermia after traumatic brain injury in adults: a systematic review. JAMA. 2003;289:2992–2999. doi: 10.1001/jama.289.22.2992. [DOI] [PubMed] [Google Scholar]
- 488.Meister A. Biosynthesis and functions of glutathione, an essential biofactor. J Nutr Sci Vitaminol Spec. 1992;No:1–6. doi: 10.3177/jnsv.38.special_1. [DOI] [PubMed] [Google Scholar]
- 489.Meister A. Anderson ME. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 490.Mesecke N. Mittler S. Eckers E. Herrmann JM. Deponte M. Two novel monothiol glutaredoxins from Saccharomyces cerevisiae provide further insight into iron-sulfur cluster binding, oligomerization, and enzymatic activity of glutaredoxins. Biochemistry. 2008;47:1452–1463. doi: 10.1021/bi7017865. [DOI] [PubMed] [Google Scholar]
- 491.Messina JP. Lawrence DA. Cell cycle progression of glutathione-depleted human peripheral blood mononuclear cells is inhibited at S phase. J Immunol. 1989;143:1974–1981. [PubMed] [Google Scholar]
- 492.Metzen E. Zhou J. Jelkmann W. Fandrey J. Brüne B. Nitric oxide impairs normoxic degradation of HIF-1alpha by inhibition of prolyl hydroxylases. Mol Biol Cell. 2003;14:3470–3481. doi: 10.1091/mbc.E02-12-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Meyer LM. Löfgren S. Ho Y-S. Lou M. Wegener A. Holz F. Söderberg P. Absence of glutaredoxin1 increases lens susceptibility to oxidative stress induced by UVR-B. Exp Eye Res. 2009;89:833–839. doi: 10.1016/j.exer.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 494.Meyer M. Pahl HL. Baeuerle PA. Regulation of the transcription factors NF-kappa B and AP-1 by redox changes. Chem Biol Interact. 1994;91:91–100. doi: 10.1016/0009-2797(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 495.Mieyal JJ. Gallogly MM. Qanungo S. Sabens EA. Shelton MD. Molecular mechanisms and clinical implications of reversible protein S-glutathionylation. Antioxid Redox Signal. 2008;10:1941–1988. doi: 10.1089/ars.2008.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 496.Miller JA., Jr. Miller FS. Westin B. Hypothermia in the treatment of asphyxia neonatorum. Biol Neonat. 1964;6:148–163. doi: 10.1159/000239893. [DOI] [PubMed] [Google Scholar]
- 497.Miranda-Vizuete A. Gustafsson JA. Spyrou G. Molecular cloning and expression of a cDNA encoding a human thioredoxin-like protein. Biochem Biophys Res Commun. 1998;243:284–288. doi: 10.1006/bbrc.1997.8003. [DOI] [PubMed] [Google Scholar]
- 498.Miranda-Vizuete A. Ljung J. Damdimopoulos AE. Gustafsson JA. Oko R. Pelto-Huikko M. Spyrou G. Characterization of Sptrx, a novel member of the thioredoxin family specifically expressed in human spermatozoa. J Biol Chem. 2001;276:31567–31574. doi: 10.1074/jbc.M101760200. [DOI] [PubMed] [Google Scholar]
- 499.Miseta A. Csutora P. Relationship between the occurrence of cysteine in proteins and the complexity of organisms. Mol Biol Evol. 2000;17:1232–1239. doi: 10.1093/oxfordjournals.molbev.a026406. [DOI] [PubMed] [Google Scholar]
- 500.Misra MK. Sarwat M. Bhakuni P. Tuteja R. Tuteja N. Oxidative stress and ischemic myocardial syndromes. Med Sci Monit. 2009;15:RA209–RA219. [PubMed] [Google Scholar]
- 501.Missé D. Yssel H. Trabattoni D. Oblet C. Lo Caputo S. Mazzotta F. Pène J. Gonzalez J-P. Clerici M. Veas F. IL-22 participates in an innate anti-HIV-1 host-resistance network through acute-phase protein induction. J Immunol. 2007;178:407–415. doi: 10.4049/jimmunol.178.1.407. [DOI] [PubMed] [Google Scholar]
- 502.Mitchell DA. Marletta MA. Thioredoxin catalyzes the S-nitrosation of the caspase-3 active site cysteine. Nat Chem Biol. 2005;1:154–158. doi: 10.1038/nchembio720. [DOI] [PubMed] [Google Scholar]
- 503.Mitchell J. Morris A. De Belleroche J. Thioredoxin reductase 1 haplotypes modify familial amyotrophic lateral sclerosis onset. Free Radic Biol Med. 2009;46:202–211. doi: 10.1016/j.freeradbiomed.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 504.Mitsui A. Hamuro J. Nakamura H. Kondo N. Hirabayashi Y. Ishizaki-Koizumi S. Hirakawa T. Inoue T. Yodoi J. Overexpression of human thioredoxin in transgenic mice controls oxidative stress and life span. Antioxid Redox Signal. 2002;4:693–696. doi: 10.1089/15230860260220201. [DOI] [PubMed] [Google Scholar]
- 505.Miyamoto M. Kishimoto C. Shioji K. Nakamura H. Toyokuni S. Nakayama Y. Kita M. Yodoi J. Sasayama S. Difference in thioredoxin expression in viral myocarditis in inbred strains of mice. Jpn Circ J. 2001;65:561–564. doi: 10.1253/jcj.65.561. [DOI] [PubMed] [Google Scholar]
- 506.Miyamoto N. Izumi H. Miyamoto R. Kubota T. Tawara A. Sasaguri Y. Kohno K. Nipradilol and timolol induce Foxo3a and peroxiredoxin 2 expression and protect trabecular meshwork cells from oxidative stress. Invest Ophthalmol Vis Sci. 2009;50:2777–2784. doi: 10.1167/iovs.08-3061. [DOI] [PubMed] [Google Scholar]
- 507.Miyamoto S. Kawano H. Hokamaki J. Soejima H. Kojima S. Kudoh T. Nagayoshi Y. Sugiyama S. Sakamoto T. Yoshimura M. Nakamura H. Yodoi J. Ogawa H. Increased plasma levels of thioredoxin in patients with glucose intolerance. Intern Med. 2005;44:1127–1132. doi: 10.2169/internalmedicine.44.1127. [DOI] [PubMed] [Google Scholar]
- 508.Mizusawa H. Ishii T. Bannai S. Peroxiredoxin I (macrophage 23 kDa stress protein) is highly and widely expressed in the rat nervous system. Neurosci Lett. 2000;283:57–60. doi: 10.1016/s0304-3940(00)00910-1. [DOI] [PubMed] [Google Scholar]
- 509.Mochizuki M. Kwon Y-W. Yodoi J. Masutani H. Thioredoxin regulates cell cycle via the ERK1/2-cyclin D1 pathway. Antioxid Redox Signal. 2009;11:2957–2971. doi: 10.1089/ars.2009.2623. [DOI] [PubMed] [Google Scholar]
- 510.Moghaddam DA. Heber A. Capin D. Kreutz T. Opitz D. Lenzen E. Bloch W. Brixius K. Brinkmann C. Training increases peroxiredoxin 2 contents in the erythrocytes of overweight/obese men suffering from type 2 diabetes. Wien Med Wochenschr. 2011;161:511–518. doi: 10.1007/s10354-011-0037-0. [DOI] [PubMed] [Google Scholar]
- 511.Molitoris BA. Marrs J. The role of cell adhesion molecules in ischemic acute renal failure. Am J Med. 1999;106:583–592. doi: 10.1016/s0002-9343(99)00061-3. [DOI] [PubMed] [Google Scholar]
- 512.Moon E-Y. Kang JS. Han SH. Yang K-H. Pyo S. Lee M-Y. Lee H-K. Yu D-Y. Differential role of peroxiredoxin II (PrxII) on the expression of toll-like receptor 4 (TLR4) and B-cell activating factor (BAFF) in ovalbumin (OVA)-induced mouse asthma. Int Immunopharmacol. 2008;8:935–944. doi: 10.1016/j.intimp.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 513.Moos PJ. Edes K. Cassidy P. Massuda E. Fitzpatrick FA. Electrophilic prostaglandins and lipid aldehydes repress redox-sensitive transcription factors p53 and hypoxia-inducible factor by impairing the selenoprotein thioredoxin reductase. J Biol Chem. 2003;278:745–750. doi: 10.1074/jbc.M211134200. [DOI] [PubMed] [Google Scholar]
- 514.Moreno MC. Campanelli J. Sande P. Sánez DA. Keller Sarmiento MI. Rosenstein RE. Retinal oxidative stress induced by high intraocular pressure. Free Radic Biol Med. 2004;37:803–812. doi: 10.1016/j.freeradbiomed.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 515.Moreno SG. Laux G. Brielmeier M. Bornkamm GW. Conrad M. Testis-specific expression of the nuclear form of phospholipid hydroperoxide glutathione peroxidase (PHGPx) Biol Chem. 2003;384:635–643. doi: 10.1515/BC.2003.070. [DOI] [PubMed] [Google Scholar]
- 516.Morihira M. Hasebe N. Baljinnyam E. Sumitomo K. Matsusaka T. Izawa K. Fujino T. Fukuzawa J. Kikuchi K. Ischemic preconditioning enhances scavenging activity of reactive oxygen species and diminishes transmural difference of infarct size. Am J Physiol Heart Circ Physiol. 2006;290:H577–H583. doi: 10.1152/ajpheart.00817.2004. [DOI] [PubMed] [Google Scholar]
- 517.Morinaka A. Funato Y. Uesugi K. Miki H. Oligomeric peroxiredoxin-I is an essential intermediate for p53 to activate MST1 kinase and apoptosis. Oncogene. 2011;30:4208–4218. doi: 10.1038/onc.2011.139. [DOI] [PubMed] [Google Scholar]
- 518.Morrison SJ. Csete M. Groves AK. Melega W. Wold B. Anderson DJ. Culture in reduced levels of oxygen promotes clonogenic sympathoadrenal differentiation by isolated neural crest stem cells. J Neurosci. 2000;20:7370–7376. doi: 10.1523/JNEUROSCI.20-19-07370.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519.Moskovitz J. Roles of methionine suldfoxide reductases in antioxidant defense, protein regulation and survival. Curr Pharm Des. 2005;11:1451–1457. doi: 10.2174/1381612053507846. [DOI] [PubMed] [Google Scholar]
- 520.Moskovitz J. Methionine sulfoxide reductases: ubiquitous enzymes involved in antioxidant defense, protein regulation, and prevention of aging-associated diseases. Biochim Biophys Acta. 2005;1703:213–219. doi: 10.1016/j.bbapap.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 521.Mühlenhoff U. Gerber J. Richhardt N. Lill R. Components involved in assembly and dislocation of iron-sulfur clusters on the scaffold protein Isu1p. EMBO J. 2003;22:4815–4825. doi: 10.1093/emboj/cdg446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Mühlenhoff U. Molik S. Godoy JR. Uzarska MA. Richter N. Seubert A. Zhang Y. Stubbe J. Pierrel F. Herrero E. Lillig CH. Lill R. Cytosolic monothiol glutaredoxins function in intracellular iron sensing and trafficking via their bound iron-sulfur cluster. Cell Metab. 2010;12:373–385. doi: 10.1016/j.cmet.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Mukherjee A. Martin SG. The thioredoxin system: a key target in tumour and endothelial cells. Br J Radiol. 2008;81:S57–S68. doi: 10.1259/bjr/34180435. [DOI] [PubMed] [Google Scholar]
- 524.Müller BAL. Dhalla NS. Mechanisms of the beneficial actions of ischemic preconditioning on subcellular remodeling in ischemic-reperfused heart. Curr Cardiol Rev. 2010;6:255–264. doi: 10.2174/157340310793566118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Multhaup G. Ruppert T. Schlicksupp A. Hesse L. Beher D. Masters CL. Beyreuther K. Reactive oxygen species and Alzheimer's disease. Biochem Pharmacol. 1997;54:533–539. doi: 10.1016/s0006-2952(97)00062-2. [DOI] [PubMed] [Google Scholar]
- 526.Munemasa Y. Ahn JH. Kwong JMK. Caprioli J. Piri N. Redox proteins thioredoxin 1 and thioredoxin 2 support retinal ganglion cell survival in experimental glaucoma. Gene Ther. 2009;16:17–25. doi: 10.1038/gt.2008.126. [DOI] [PubMed] [Google Scholar]
- 527.Munemasa Y. Kitaoka Y. Kuribayashi J. Ueno S. Modulation of mitochondria in the axon and soma of retinal ganglion cells in a rat glaucoma model. J Neurochem. 2010;115:1508–1519. doi: 10.1111/j.1471-4159.2010.07057.x. [DOI] [PubMed] [Google Scholar]
- 528.Munemasa Y. Kwong JMK. Kim SH. Ahn JH. Caprioli J. Piri N. Thioredoxins 1 and 2 protect retinal ganglion cells from pharmacologically induced oxidative stress, optic nerve transection and ocular hypertension. Adv Exp Med Biol. 2010;664:355–363. doi: 10.1007/978-1-4419-1399-9_41. [DOI] [PubMed] [Google Scholar]
- 529.Murata H. Ihara Y. Nakamura H. Yodoi J. Sumikawa K. Kondo T. Glutaredoxin exerts an antiapoptotic effect by regulating the redox state of Akt. J Biol Chem. 2003;278:50226–50233. doi: 10.1074/jbc.M310171200. [DOI] [PubMed] [Google Scholar]
- 530.Murry CE. Jennings RB. Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 531.Mu ZM. Yin XY. Prochownik EV. Pag, a putative tumor suppressor, interacts with the Myc Box II domain of c-Myc and selectively alters its biological function and target gene expression. J Biol Chem. 2002;277:43175–43184. doi: 10.1074/jbc.M206066200. [DOI] [PubMed] [Google Scholar]
- 532.Nadeem A. Chhabra SK. Masood A. Raj HG. Increased oxidative stress and altered levels of antioxidants in asthma. J Allergy Clin Immunol. 2003;111:72–78. doi: 10.1067/mai.2003.17. [DOI] [PubMed] [Google Scholar]
- 533.Nagaoka Y. Iuchi Y. Ikeda Y. Fujii J. Glutathione reductase is expressed at high levels in pancreatic islet cells. Redox Rep. 2004;9:321–324. doi: 10.1179/135100004225006812. [DOI] [PubMed] [Google Scholar]
- 534.Nagy N. Malik G. Fisher AB. Das DK. Targeted disruption of peroxiredoxin 6 gene renders the heart vulnerable to ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2006;291:H2636–H2640. doi: 10.1152/ajpheart.00399.2006. [DOI] [PubMed] [Google Scholar]
- 535.Nagy N. Malik G. Tosaki A. Ho Y-S. Maulik N. Das DK. Overexpression of glutaredoxin-2 reduces myocardial cell death by preventing both apoptosis and necrosis. J Mol Cell Cardiol. 2008;44:252–260. doi: 10.1016/j.yjmcc.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 536.Naidoo N. ER and aging-Protein folding and the ER stress response. Ageing Res Rev. 2009;8:150–159. doi: 10.1016/j.arr.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 537.Nakamura H. Extracellular functions of thioredoxin. Novartis Found Symp. 2008;291:184–192. doi: 10.1002/9780470754030.ch14. discussion 192–195, 221–224. [DOI] [PubMed] [Google Scholar]
- 538.Nakamura H. Herzenberg LA. Bai J. Araya S. Kondo N. Nishinaka Y. Herzenberg LA. Yodoi J. Circulating thioredoxin suppresses lipopolysaccharide-induced neutrophil chemotaxis. Proc Natl Acad Sci U S A. 2001;98:15143–15148. doi: 10.1073/pnas.191498798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Nakamura H. Hoshino Y. Okuyama H. Matsuo Y. Yodoi J. Thioredoxin 1 delivery as new therapeutics. Adv Drug Deliv Rev. 2009;61:303–309. doi: 10.1016/j.addr.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 540.Nakamura H. Masutani H. Tagaya Y. Yamauchi A. Inamoto T. Nanbu Y. Fujii S. Ozawa K. Yodoi J. Expression and growth-promoting effect of adult T-cell leukemia-derived factor. A human thioredoxin homologue in hepatocellular carcinoma. Cancer. 1992;69:2091–2097. doi: 10.1002/1097-0142(19920415)69:8<2091::aid-cncr2820690814>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 541.Nakamura H. Masutani H. Yodoi J. Extracellular thioredoxin and thioredoxin-binding protein 2 in control of cancer. Semin Cancer Biol. 2006;16:444–451. doi: 10.1016/j.semcancer.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 542.Nakamura H. De Rosa SC. Yodoi J. Holmgren A. Ghezzi P. Herzenberg LA. Herzenberg LA. Chronic elevation of plasma thioredoxin: inhibition of chemotaxis and curtailment of life expectancy in AIDS. Proc Natl Acad Sci U S A. 2001;98:2688–2693. doi: 10.1073/pnas.041624998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.Nakamura H. De Rosa S. Roederer M. Anderson MT. Dubs JG. Yodoi J. Holmgren A. Herzenberg LA. Herzenberg LA. Elevation of plasma thioredoxin levels in HIV-infected individuals. Int Immunol. 1996;8:603–611. doi: 10.1093/intimm/8.4.603. [DOI] [PubMed] [Google Scholar]
- 544.Neumann CA. Fang Q. Are peroxiredoxins tumor suppressors? Curr Opin Pharmacol. 2007;7:375–380. doi: 10.1016/j.coph.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 545.Neumann CA. Krause DS. Carman CV. Das S. Dubey DP. Abraham JL. Bronson RT. Fujiwara Y. Orkin SH. Van Etten RA. Essential role for the peroxiredoxin Prdx1 in erythrocyte antioxidant defence and tumour suppression. Nature. 2003;424:561–565. doi: 10.1038/nature01819. [DOI] [PubMed] [Google Scholar]
- 546.Nimata M. Kishimoto C. Shioji K. Ishizaki K. Kitaguchi S. Hashimoto T. Nagata N. Kawai C. Upregulation of redox-regulating protein, thioredoxin, in endomyocardial biopsy samples of patients with myocarditis and cardiomyopathies. Mol Cell Biochem. 2003;248:193–196. doi: 10.1023/a:1024156923322. [DOI] [PubMed] [Google Scholar]
- 547.Nishino T. Okamoto K. Eger BT. Pai EF. Nishino T. Mammalian xanthine oxidoreductase—mechanism of transition from xanthine dehydrogenase to xanthine oxidase. FEBS J. 2008;275:3278–3289. doi: 10.1111/j.1742-4658.2008.06489.x. [DOI] [PubMed] [Google Scholar]
- 548.Nishiyama A. Matsui M. Iwata S. Hirota K. Masutani H. Nakamura H. Takagi Y. Sono H. Gon Y. Yodoi J. Identification of thioredoxin-binding protein-2/vitamin D(3) up-regulated protein 1 as a negative regulator of thioredoxin function and expression. J Biol Chem. 1999;274:21645–21650. doi: 10.1074/jbc.274.31.21645. [DOI] [PubMed] [Google Scholar]
- 549.Noblanc A. Kocer A. Chabory E. Vernet P. Saez F. Cadet R. Conrad M. Drevet JR. Glutathione peroxidases at work on epididymal spermatozoa: an example of the dual effect of reactive oxygen species on mammalian male fertilizing ability. J Androl. 2011;32:641–650. doi: 10.2164/jandrol.110.012823. [DOI] [PubMed] [Google Scholar]
- 550.Nogoceke E. Gommel DU. Kiess M. Kalisz HM. Flohé L. A unique cascade of oxidoreductases catalyses trypanothione-mediated peroxide metabolism in Crithidia fasciculata. Biol Chem. 1997;378:827–836. doi: 10.1515/bchm.1997.378.8.827. [DOI] [PubMed] [Google Scholar]
- 551.Nonn L. Berggren M. Powis G. Increased expression of mitochondrial peroxiredoxin-3 (thioredoxin peroxidase-2) protects cancer cells against hypoxia and drug-induced hydrogen peroxide-dependent apoptosis. Mol Cancer Res. 2003;1:682–689. [PubMed] [Google Scholar]
- 552.Nonn L. Williams RR. Erickson RP. Powis G. The absence of mitochondrial thioredoxin 2 causes massive apoptosis, exencephaly, and early embryonic lethality in homozygous mice. Mol Cell Biol. 2003;23:916–922. doi: 10.1128/MCB.23.3.916-922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Nony PA. Schnellmann RG. Mechanisms of renal cell repair and regeneration after acute renal failure. J Pharmacol Exp Ther. 2003;304:905–912. doi: 10.1124/jpet.102.035022. [DOI] [PubMed] [Google Scholar]
- 554.Nordberg J. Arnér ES. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med. 2001;31:1287–1312. doi: 10.1016/s0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- 555.Oblong JE. Berggren M. Gasdaska PY. Powis G. Site-directed mutagenesis of active site cysteines in human thioredoxin produces competitive inhibitors of human thioredoxin reductase and elimination of mitogenic properties of thioredoxin. J Biol Chem. 1994;269:11714–11720. [PubMed] [Google Scholar]
- 556.Ock J. Han HS. Hong SH. Lee SY. Han Y-M. Kwon B-M. Suk K. Obovatol attenuates microglia-mediated neuroinflammation by modulating redox regulation. Br J Pharmacol. 2010;159:1646–1662. doi: 10.1111/j.1476-5381.2010.00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Odeh H. Hunker KL. Belyantseva IA. Azaiez H. Avenarius MR. Zheng L. Peters LM. Gagnon LH. Hagiwara N. Skynner MJ. Brilliant MH. Allen ND. Riazuddin S. Johnson KR. Raphael Y. Najmabadi H. Friedman TB. Bartles JR. Smith RJH. Kohrman DC. Mutations in Grxcr1 are the basis for inner ear dysfunction in the pirouette mouse. Am J Hum Genet. 2010;86:148–160. doi: 10.1016/j.ajhg.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558.Okado-Matsumoto A. Matsumoto A. Fujii J. Taniguchi N. Peroxiredoxin IV is a secretable protein with heparin-binding properties under reduced conditions. J Biochem. 2000;127:493–501. doi: 10.1093/oxfordjournals.jbchem.a022632. [DOI] [PubMed] [Google Scholar]
- 559.Oliveira L. Bouton C. Drapier JC. Thioredoxin activation of iron regulatory proteins. Redox regulation of RNA binding after exposure to nitric oxide. J Biol Chem. 1999;274:516–521. doi: 10.1074/jbc.274.1.516. [DOI] [PubMed] [Google Scholar]
- 560.Orme-Johnson WH. Beinert H. On the formation of the superoxide anion radical during the reaction of reduced iron-sulfur proteins with oxygen. Biochem Biophys Res Commun. 1969;36:905–911. doi: 10.1016/0006-291x(69)90289-7. [DOI] [PubMed] [Google Scholar]
- 561.Ott M. Gogvadze V. Orrenius S. Zhivotovsky B. Mitochondria, oxidative stress and cell death. Apoptosis. 2007;12:913–922. doi: 10.1007/s10495-007-0756-2. [DOI] [PubMed] [Google Scholar]
- 562.Packer L. Fuehr K. Low oxygen concentration extends the lifespan of cultured human diploid cells. Nature. 1977;267:423–425. doi: 10.1038/267423a0. [DOI] [PubMed] [Google Scholar]
- 563.Padilla CA. Martínez-Galisteo E. López-Barea J. Holmgren A. Bárcena JA. Immunolocalization of thioredoxin and glutaredoxin in mammalian hypophysis. Mol Cell Endocrinol. 1992;85:1–12. doi: 10.1016/0303-7207(92)90119-q. [DOI] [PubMed] [Google Scholar]
- 564.Pagliei S. Ghezzi P. Bizzarri C. Sabbatini V. Frascaroli G. Sozzani S. Caselli G. Bertini R. Thioredoxin specifically cross-desensitizes monocytes to MCP-1. Eur Cytokine Netw. 2002;13:261–267. [PubMed] [Google Scholar]
- 565.Pai HV. Starke DW. Lesnefsky EJ. Hoppel CL. Mieyal JJ. What is the functional significance of the unique location of glutaredoxin 1 (GRx1) in the intermembrane space of mitochondria? Antioxid Redox Signal. 2007;9:2027–2033. doi: 10.1089/ars.2007.1642. [DOI] [PubMed] [Google Scholar]
- 566.Pak JH. Choi WH. Lee HM. Joo W-D. Kim J-H. Kim Y-T. Kim Y-M. Nam J-H. Peroxiredoxin 6 overexpression attenuates cisplatin-induced apoptosis in human ovarian cancer cells. Cancer Invest. 2011;29:21–28. doi: 10.3109/07357907.2010.535056. [DOI] [PubMed] [Google Scholar]
- 567.Pak JH. Kim T. Joon Kim M. Yong Kim J. Choi H. Kim SA. Tchah H. Reduced expression of 1-cys peroxiredoxin in oxidative stress-induced cataracts. Exp Eye Res. 2006;82:899–906. doi: 10.1016/j.exer.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 568.Palande KK. Beekman R. Van der Meeren LE. Beverloo HB. Valk PJM. Touw IP. The antioxidant protein peroxiredoxin 4 is epigenetically down regulated in acute promyelocytic leukemia. PLoS One. 2011;6:e16340. doi: 10.1371/journal.pone.0016340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 569.Palmer LA. Gaston B. Johns RA. Normoxic stabilization of hypoxia-inducible factor-1 expression and activity: redox-dependent effect of nitrogen oxides. Mol Pharmacol. 2000;58:1197–1203. doi: 10.1124/mol.58.6.1197. [DOI] [PubMed] [Google Scholar]
- 570.Pandolfo M. Iron and Friedreich ataxia. J Neural Transm Suppl. 2006;70:143–146. doi: 10.1007/978-3-211-45295-0_22. [DOI] [PubMed] [Google Scholar]
- 571.Pan S. Berk BC. Glutathiolation regulates tumor necrosis factor-alpha-induced caspase-3 cleavage and apoptosis: key role for glutaredoxin in the death pathway. Circ Res. 2007;100:213–219. doi: 10.1161/01.RES.0000256089.30318.20. [DOI] [PubMed] [Google Scholar]
- 572.Pantopoulos K. Iron metabolism and the IRE/IRP regulatory system: an update. Ann N Y Acad Sci. 2004;1012:1–13. doi: 10.1196/annals.1306.001. [DOI] [PubMed] [Google Scholar]
- 573.Parikh H. Carlsson E. Chutkow WA. Johansson LE. Storgaard H. Poulsen P. Saxena R. Ladd C. Schulze PC. Mazzini MJ. Jensen CB. Krook A. Björnholm M. Tornqvist H. Zierath JR. Ridderstråle M. Altshuler D. Lee RT. Vaag A. Groop LC. Mootha VK. TXNIP regulates peripheral glucose metabolism in humans. PLoS Med. 2007;4:e158. doi: 10.1371/journal.pmed.0040158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 574.Park CB. Larsson N-G. Mitochondrial DNA mutations in disease and aging. J Cell Biol. 2011;193:809–818. doi: 10.1083/jcb.201010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575.Park J-G. Yoo J-Y. Jeong S-J. Choi J-H. Lee M-R. Lee M-N. Hwa Lee J. Kim HC. Jo H. Yu D-Y. Kang SW. Rhee SG. Lee M-H. Oh GT. Peroxiredoxin 2 deficiency exacerbates atherosclerosis in apolipoprotein E-deficient mice. Circ Res. 2011;109:739–749. doi: 10.1161/CIRCRESAHA.111.245530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576.Park JW. Mieyal JJ. Rhee SG. Chock PB. Deglutathionylation of 2-Cys peroxiredoxin is specifically catalyzed by sulfiredoxin. J Biol Chem. 2009;284:23364–23374. doi: 10.1074/jbc.M109.021394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 577.Parks D. Bolinger R. Mann K. Redox state regulates binding of p53 to sequence-specific DNA, but not to non-specific or mismatched DNA. Nucleic Acids Res. 1997;25:1289–1295. doi: 10.1093/nar/25.6.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 578.Park S-Y. Yu X. Ip C. Mohler JL. Bogner PN. Park Y-M. Peroxiredoxin 1 interacts with androgen receptor and enhances its transactivation. Cancer Res. 2007;67:9294–9303. doi: 10.1158/0008-5472.CAN-07-0651. [DOI] [PubMed] [Google Scholar]
- 579.Parnham M. Sies H. Ebselen: prospective therapy for cerebral ischaemia. Expert Opin Investig Drugs. 2000;9:607–619. doi: 10.1517/13543784.9.3.607. [DOI] [PubMed] [Google Scholar]
- 580.Patel JM. Hu H. Lu L. Deem A. Akindipe O. Brantly M. Block ER. Antony VB. Baz MA. Thioredoxin as a biomarker for graft rejection in lung transplant recipients. Biomarkers. 2008;13:486–495. doi: 10.1080/13547500802061822. [DOI] [PubMed] [Google Scholar]
- 581.Pearlstein DP. Ali MH. Mungai PT. Hynes KL. Gewertz BL. Schumacker PT. Role of mitochondrial oxidant generation in endothelial cell responses to hypoxia. Arterioscler Thromb Vasc Biol. 2002;22:566–573. doi: 10.1161/01.atv.0000012262.76205.6a. [DOI] [PubMed] [Google Scholar]
- 582.Pearson GD. Merrill GF. Deletion of the Saccharomyces cerevisiae TRR1 gene encoding thioredoxin reductase inhibits p53-dependent reporter gene expression. J Biol Chem. 1998;273:5431–5434. doi: 10.1074/jbc.273.10.5431. [DOI] [PubMed] [Google Scholar]
- 583.Pedrajas JR. Padilla CA. McDonagh B. Bárcena JA. Glutaredoxin participates in the reduction of peroxides by the mitochondrial 1-CYS peroxiredoxin in Saccharomyces cerevisiae. Antioxid Redox Signal. 2010;13:249–258. doi: 10.1089/ars.2009.2950. [DOI] [PubMed] [Google Scholar]
- 584.Pekkari K. Avila-Cariño J. Bengtsson A. Gurunath R. Scheynius A. Holmgren A. Truncated thioredoxin (Trx80) induces production of interleukin-12 and enhances CD14 expression in human monocytes. Blood. 2001;97:3184–3190. doi: 10.1182/blood.v97.10.3184. [DOI] [PubMed] [Google Scholar]
- 585.Pekkari K. Goodarzi MT. Scheynius A. Holmgren A. Avila-Cariño J. Truncated thioredoxin (Trx80) induces differentiation of human CD14+ monocytes into a novel cell type (TAMs) via activation of the MAP kinases p38, ERK, and JNK. Blood. 2005;105:1598–1605. doi: 10.1182/blood-2004-04-1577. [DOI] [PubMed] [Google Scholar]
- 586.Pekkari K. Gurunath R. Arner ES. Holmgren A. Truncated thioredoxin is a mitogenic cytokine for resting human peripheral blood mononuclear cells and is present in human plasma. J Biol Chem. 2000;275:37474–37480. doi: 10.1074/jbc.M001012200. [DOI] [PubMed] [Google Scholar]
- 587.Pekkari K. Holmgren A. Truncated thioredoxin: physiological functions and mechanism. Antioxid Redox Signal. 2004;6:53–61. doi: 10.1089/152308604771978345. [DOI] [PubMed] [Google Scholar]
- 588.Peltoniemi MJ. Rytilä PH. Harju TH. Soini YM. Salmenkivi KM. Ruddock LW. Kinnula VL. Modulation of glutaredoxin in the lung and sputum of cigarette smokers and chronic obstructive pulmonary disease. Respir Res. 2006;7:133. doi: 10.1186/1465-9921-7-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 589.Peltoniemi M. Kaarteenaho-Wiik R. Säily M. Sormunen R. Pääkkö P. Holmgren A. Soini Y. Kinnula VL. Expression of glutaredoxin is highly cell specific in human lung and is decreased by transforming growth factor-beta in vitro and in interstitial lung diseases in vivo. Hum Pathol. 2004;35:1000–1007. doi: 10.1016/j.humpath.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 590.Peng Y. Yang P-H. Guo Y. Ng SSM. Liu J. Fung PCW. Tay D. Ge J. He M-L. Kung H-F. Lin MC. Catalase and peroxiredoxin 5 protect Xenopus embryos against alcohol-induced ocular anomalies. Invest Ophthalmol Vis Sci. 2004;45:23–29. doi: 10.1167/iovs.03-0550. [DOI] [PubMed] [Google Scholar]
- 591.Pennington JD. Jacobs KM. Sun L. Bar-Sela G. Mishra M. Gius D. Thioredoxin and thioredoxin reductase as redox-sensitive molecular targets for cancer therapy. Curr Pharm Des. 2007;13:3368–3377. [PubMed] [Google Scholar]
- 592.Pennisi G. Cornelius C. Cavallaro MM. Salinaro AT. Cambria MT. Pennisi M. Bella R. Milone P. Ventimiglia B. Migliore MR. Di Renzo L. De Lorenzo A. Calabrese V. Redox regulation of cellular stress response in multiple sclerosis. Biochem Pharmacol. 2011;82:1490–1499. doi: 10.1016/j.bcp.2011.07.092. [DOI] [PubMed] [Google Scholar]
- 593.Pérez VI. Bokov A. Van Remmen H. Mele J. Ran Q. Ikeno Y. Richardson A. Is the oxidative stress theory of aging dead? Biochim Biophys Acta. 2009;1790:1005–1014. doi: 10.1016/j.bbagen.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.Pérez VI. Cortez LA. Lew CM. Rodriguez M. Webb CR. Van Remmen H. Chaudhuri A. Qi W. Lee S. Bokov A. Fok W. Jones D. Richardson A. Yodoi J. Zhang Y. Tominaga K. Hubbard GB. Ikeno Y. Thioredoxin 1 overexpression extends mainly the earlier part of life span in mice. J Gerontol A Biol Sci Med Sci. 2011;66:1286–1299. doi: 10.1093/gerona/glr125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 595.Perrone L. Devi TS. Hosoya K-I. Terasaki T. Singh LP. Inhibition of TXNIP expression in vivo blocks early pathologies of diabetic retinopathy. Cell Death Dis. 2010;1:e65. doi: 10.1038/cddis.2010.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 596.Perrone L. Devi TS. Hosoya K. Terasaki T. Singh LP. Thioredoxin interacting protein (TXNIP) induces inflammation through chromatin modification in retinal capillary endothelial cells under diabetic conditions. J Cell Physiol. 2009;221:262–272. doi: 10.1002/jcp.21852. [DOI] [PubMed] [Google Scholar]
- 597.Perry TL. Godin DV. Hansen S. Parkinson's disease: a disorder due to nigral glutathione deficiency? Neurosci Lett. 1982;33:305–310. doi: 10.1016/0304-3940(82)90390-1. [DOI] [PubMed] [Google Scholar]
- 598.Perry TL. Yong VW. Idiopathic Parkinson's disease, progressive supranuclear palsy and glutathione metabolism in the substantia nigra of patients. Neurosci Lett. 1986;67:269–274. doi: 10.1016/0304-3940(86)90320-4. [DOI] [PubMed] [Google Scholar]
- 599.Peterson JD. Herzenberg LA. Vasquez K. Waltenbaugh C. Glutathione levels in antigen-presenting cells modulate Th1 versus Th2 response patterns. Proc Natl Acad Sci U S A. 1998;95:3071–3076. doi: 10.1073/pnas.95.6.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 600.Petropoulos I. Friguet B. Protein maintenance in aging and replicative senescence: a role for the peptide methionine sulfoxide reductases. Biochim Biophys Acta. 2005;1703:261–266. doi: 10.1016/j.bbapap.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 601.Phelan SA. Wang X. Wallbrandt P. Forsman-Semb K. Paigen B. Overexpression of Prdx6 reduces H2O2 but does not prevent diet-induced atherosclerosis in the aortic root. Free Radic Biol Med. 2003;35:1110–1120. doi: 10.1016/s0891-5849(03)00462-3. [DOI] [PubMed] [Google Scholar]
- 602.Phillips PG. Birnby LM. Narendran A. Hypoxia induces capillary network formation in cultured bovine pulmonary microvessel endothelial cells. Am J Physiol. 1995;268:L789–L800. doi: 10.1152/ajplung.1995.268.5.L789. [DOI] [PubMed] [Google Scholar]
- 603.Picciocchi A. Saguez C. Boussac A. Cassier-Chauvat C. Chauvat F. CGFS-type monothiol glutaredoxins from the cyanobacterium Synechocystis PCC6803 and other evolutionary distant model organisms possess a glutathione-ligated [2Fe-2S] cluster. Biochemistry. 2007;46:15018–15026. doi: 10.1021/bi7013272. [DOI] [PubMed] [Google Scholar]
- 604.Pierrou S. Broberg P. O'Donnell RA. Pawłowski K. Virtala R. Lindqvist E. Richter A. Wilson SJ. Angco G. Möller S. Bergstrand H. Koopmann W. Wieslander E. Strömstedt P-E. Holgate ST. Davies DE. Lund J. Djukanovic R. Expression of genes involved in oxidative stress responses in airway epithelial cells of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175:577–586. doi: 10.1164/rccm.200607-931OC. [DOI] [PubMed] [Google Scholar]
- 605.Pineda-Molina E. Klatt P. Vázquez J. Marina A. García de Lacoba M. Pérez-Sala D. Lamas S. Glutathionylation of the p50 subunit of NF-kappaB: a mechanism for redox-induced inhibition of DNA binding. Biochemistry. 2001;40:14134–14142. doi: 10.1021/bi011459o. [DOI] [PubMed] [Google Scholar]
- 606.Poole LB. Formation and functions of protein sulfenic acids. Curr Protoc Toxicol Chapter. 2004;17:Unit17.1. doi: 10.1002/0471140856.tx1701s18. [DOI] [PubMed] [Google Scholar]
- 607.Potter LR. Guanylyl cyclase structure, function and regulation. Cell Signal. 2011;23:1921–1926. doi: 10.1016/j.cellsig.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 608.Power JHT. Asad S. Chataway TK. Chegini F. Manavis J. Temlett JA. Jensen PH. Blumbergs PC. Gai W-P. Peroxiredoxin 6 in human brain: molecular forms, cellular distribution and association with Alzheimer's disease pathology. Acta Neuropathol. 2008;115:611–622. doi: 10.1007/s00401-008-0373-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 609.Powis G. Gasdaska JR. Gasdaska PY. Berggren M. Kirkpatrick DL. Engman L. Cotgreave IA. Angulo M. Baker A. Selenium and the thioredoxin redox system: effects on cell growth and death. Oncol Res. 1997;9:303–312. [PubMed] [Google Scholar]
- 610.Powis G. Kirkpatrick DL. Thioredoxin signaling as a target for cancer therapy. Curr Opin Pharmacol. 2007;7:392–397. doi: 10.1016/j.coph.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 611.Prigge JR. Eriksson S. Iverson SV. Meade TA. Capecchi MR. Arnér ESJ. Schmidt EE. Hepatocyte DNA replication in growing liver requires either glutathione or a single allele of txnrd1. Free Radic Biol Med. 2012;52:803–810. doi: 10.1016/j.freeradbiomed.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 612.Prospéri MT. Ferbus D. Karczinski I. Goubin G. A human cDNA corresponding to a gene overexpressed during cell proliferation encodes a product sharing homology with amoebic and bacterial proteins. J Biol Chem. 1993;268:11050–11056. [PubMed] [Google Scholar]
- 613.Qanungo S. Starke DW. Pai HV. Mieyal JJ. Nieminen A-L. Glutathione supplementation potentiates hypoxic apoptosis by S-glutathionylation of p65-NFkappaB. J Biol Chem. 2007;282:18427–18436. doi: 10.1074/jbc.M610934200. [DOI] [PubMed] [Google Scholar]
- 614.Qi Y. Grishin NV. Structural classification of thioredoxin-like fold proteins. Proteins. 2005;58:376–388. doi: 10.1002/prot.20329. [DOI] [PubMed] [Google Scholar]
- 615.Qu D. Rashidian J. Mount MP. Aleyasin H. Parsanejad M. Lira A. Haque E. Zhang Y. Callaghan S. Daigle M. Rousseaux MWC. Slack RS. Albert PR. Vincent I. Woulfe JM. Park DS. Role of Cdk5-mediated phosphorylation of Prx2 in MPTP toxicity and Parkinson's disease. Neuron. 2007;55:37–52. doi: 10.1016/j.neuron.2007.05.033. [DOI] [PubMed] [Google Scholar]
- 616.Rahlfs S. Schirmer RH. Becker K. The thioredoxin system of Plasmodium falciparum and other parasites. Cell Mol Life Sci. 2002;59:1024–1041. doi: 10.1007/s00018-002-8484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 617.Rahman I. Morrison D. Donaldson K. MacNee W. Systemic oxidative stress in asthma, COPD, and smokers. Am J Respir Crit Care Med. 1996;154:1055–1060. doi: 10.1164/ajrccm.154.4.8887607. [DOI] [PubMed] [Google Scholar]
- 618.Rahman I. Van Schadewijk AA. Hiemstra PS. Stolk J. Van Krieken JH. MacNee W. De Boer WI. Localization of gamma-glutamylcysteine synthetase messenger rna expression in lungs of smokers and patients with chronic obstructive pulmonary disease. Free Radic Biol Med. 2000;28:920–925. doi: 10.1016/s0891-5849(00)00179-9. [DOI] [PubMed] [Google Scholar]
- 619.Rajagopal I. Ahn BY. Moss B. Mathews CK. Roles of vaccinia virus ribonucleotide reductase and glutaredoxin in DNA precursor biosynthesis. J Biol Chem. 1995;270:27415–27418. doi: 10.1074/jbc.270.46.27415. [DOI] [PubMed] [Google Scholar]
- 620.Ramalingam M. Kim S-J. Reactive oxygen/nitrogen species and their functional correlations in neurodegenerative diseases. J Neural Transm. 2012;119:891–910. doi: 10.1007/s00702-011-0758-7. [DOI] [PubMed] [Google Scholar]
- 621.Rani S. Mehta JP. Barron N. Doolan P. Jeppesen PB. Clynes M. O'Driscoll L. Decreasing Txnip mRNA and protein levels in pancreatic MIN6 cells reduces reactive oxygen species and restores glucose regulated insulin secretion. Cell Physiol Biochem. 2010;25:667–674. doi: 10.1159/000315086. [DOI] [PubMed] [Google Scholar]
- 622.Rao RSP. Møller IM. Pattern of occurrence and occupancy of carbonylation sites in proteins. Proteomics. 2011;11:4166–4173. doi: 10.1002/pmic.201100223. [DOI] [PubMed] [Google Scholar]
- 623.Rasche A. Al-Hasani H. Herwig R. Meta-analysis approach identifies candidate genes and associated molecular networks for type-2 diabetes mellitus. BMC Genomics. 2008;9:310. doi: 10.1186/1471-2164-9-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 624.Rashidian J. Rousseaux MW. Venderova K. Qu D. Callaghan SM. Phillips M. Bland RJ. During MJ. Mao Z. Slack RS. Park DS. Essential role of cytoplasmic cdk5 and Prx2 in multiple ischemic injury models, in vivo. J Neurosci. 2009;29:12497–12505. doi: 10.1523/JNEUROSCI.3892-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 625.Ratts R. Zeng H. Berg EA. Blue C. McComb ME. Costello CE. vanderSpek JC. Murphy JR. The cytosolic entry of diphtheria toxin catalytic domain requires a host cell cytosolic translocation factor complex. J Cell Biol. 2003;160:1139–1150. doi: 10.1083/jcb.200210028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 626.Ray S. Watkins DN. Misso NLA. Thompson PJ. Oxidant stress induces gamma-glutamylcysteine synthetase and glutathione synthesis in human bronchial epithelial NCI-H292 cells. Clin Exp Allergy. 2002;32:571–577. doi: 10.1046/j.0954-7894.2002.01294.x. [DOI] [PubMed] [Google Scholar]
- 627.Raza H. Dual localization of glutathione S-transferase in the cytosol and mitochondria: implications in oxidative stress, toxicity and disease. FEBS J. 2011;278:4243–4251. doi: 10.1111/j.1742-4658.2011.08358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 628.Rebrin I. Bayne A-CV. Mockett RJ. Orr WC. Sohal RS. Free aminothiols, glutathione redox state and protein mixed disulphides in aging Drosophila melanogaster. Biochem J. 2004;382:131–136. doi: 10.1042/BJ20040506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 629.Reckenfelderbäumer N. Krauth-Siegel RL. Catalytic properties, thiol pK value, and redox potential of Trypanosoma brucei tryparedoxin. J Biol Chem. 2002;277:17548–17555. doi: 10.1074/jbc.M112115200. [DOI] [PubMed] [Google Scholar]
- 630.Reddy PG. Bhuyan DK. Bhuyan KC. Lens-specific regulation of the thioredoxin-1 gene, but not thioredoxin-2, upon in vivo photochemical oxidative stress in the Emory mouse. Biochem Biophys Res Commun. 1999;265:345–349. doi: 10.1006/bbrc.1999.1691. [DOI] [PubMed] [Google Scholar]
- 631.Reich E. Tamary A. Sionov RV. Melloul D. Involvement of thioredoxin-interacting protein (TXNIP) in glucocorticoid-mediated beta cell death. Diabetologia. 2012;55:1048–1057. doi: 10.1007/s00125-011-2422-z. [DOI] [PubMed] [Google Scholar]
- 632.Reinbothe TM. Ivarsson R. Li D-Q. Niazi O. Jing X. Zhang E. Stenson L. Bryborn U. Renström E. Glutaredoxin-1 mediates NADPH-dependent stimulation of calcium-dependent insulin secretion. Mol Endocrinol. 2009;23:893–900. doi: 10.1210/me.2008-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 633.Reiser K. François KO. Schols D. Bergman T. Jörnvall H. Balzarini J. Karlsson A. Lundberg M. Thioredoxin-1 and protein disulfide isomerase catalyze the reduction of similar disulfides in HIV gp120. Int J Biochem Cell Biol. 2012;44:556–562. doi: 10.1016/j.biocel.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 634.Van Remmen H. Ikeno Y. Hamilton M. Pahlavani M. Wolf N. Thorpe SR. Alderson NL. Baynes JW. Epstein CJ. Huang T-T. Nelson J. Strong R. Richardson A. Life-long reduction in MnSOD activity results in increased DNA damage and higher incidence of cancer but does not accelerate aging. Physiol Genomics. 2003;16:29–37. doi: 10.1152/physiolgenomics.00122.2003. [DOI] [PubMed] [Google Scholar]
- 635.Van Remmen H. Richardson A. Oxidative damage to mitochondria and aging. Exp Gerontol. 2001;36:957–968. doi: 10.1016/s0531-5565(01)00093-6. [DOI] [PubMed] [Google Scholar]
- 636.Reynaert NL. Wouters EFM. Janssen-Heininger YMW. Modulation of glutaredoxin-1 expression in a mouse model of allergic airway disease. Am J Respir Cell Mol Biol. 2007;36:147–151. doi: 10.1165/rcmb.2006-0259RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 637.De Rey-Pailhade J. Nouvelle recherches physiologique sur la substance organique hydrogénant le soufre à froid. C R Acad Sci. 1888;107:43–44. [Google Scholar]
- 638.De Rey-Pailhade J. Sur un corps d'origine organique hydrogénant le soufre 1 à froid. C R Acad Sci. 1888;106:1683–1684. [Google Scholar]
- 639.De Rey-Pailhade J. Sur le role physiologique du soufre. essai 15. Bull Soc Hist Nat Toulouse 1888 [Google Scholar]
- 640.Rhee SG. Jeong W. Chang T-S. Woo HA. Sulfiredoxin, the cysteine sulfinic acid reductase specific to 2-Cys peroxiredoxin: its discovery, mechanism of action, and biological significance. Kidney Int Suppl. 2007:S3–S8. doi: 10.1038/sj.ki.5002380. [DOI] [PubMed] [Google Scholar]
- 641.Rhee SG. Kang SW. Chang TS. Jeong W. Kim K. Peroxiredoxin, a novel family of peroxidases. IUBMB Life. 2001;52:35–41. doi: 10.1080/15216540252774748. [DOI] [PubMed] [Google Scholar]
- 642.Rhee SG. Kang SW. Netto LE. Seo MS. Stadtman ER. A family of novel peroxidases, peroxiredoxins. Biofactors. 1999;10:207–209. doi: 10.1002/biof.5520100218. [DOI] [PubMed] [Google Scholar]
- 643.Rhee SG. Woo HA. Multiple functions of peroxiredoxins: peroxidases, sensors and regulators of the intracellular messenger H2O2, and protein chaperones. Antioxid Redox Signal. 2011;15:781–794. doi: 10.1089/ars.2010.3393. [DOI] [PubMed] [Google Scholar]
- 644.Rietveld A. Sijens P. Verkleij AJ. de Kruijff B. Interaction of cytochrome c and its precursor apocytochrome c with various phospholipids. EMBO J. 1983;2:907–913. doi: 10.1002/j.1460-2075.1983.tb01520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 645.Robinson NC. Functional binding of cardiolipin to cytochrome c oxidase. J Bioenerg Biomembr. 1993;25:153–163. doi: 10.1007/BF00762857. [DOI] [PubMed] [Google Scholar]
- 646.Rodbell M. Nobel lecture. Signal transduction: evolution of an idea. Biosci Rep. 1995;15:117–133. doi: 10.1007/BF01207453. [DOI] [PubMed] [Google Scholar]
- 647.Rodríguez-Manzaneque MT. Tamarit J. Bellí G. Ros J. Herrero E. Grx5 is a mitochondrial glutaredoxin required for the activity of iron/sulfur enzymes. Mol Biol Cell. 2002;13:1109–1121. doi: 10.1091/mbc.01-10-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 648.Rohrbach S. Gruenler S. Teschner M. Holtz J. The thioredoxin system in aging muscle: key role of mitochondrial thioredoxin reductase in the protective effects of caloric restriction? Am J Physiol Regul Integr Comp Physiol. 2006;291:R927–R935. doi: 10.1152/ajpregu.00890.2005. [DOI] [PubMed] [Google Scholar]
- 649.Rollins MF. Van der Heide DM. Weisend CM. Kundert JA. Comstock KM. Suvorova ES. Capecchi MR. Merrill GF. Schmidt EE. Hepatocytes lacking thioredoxin reductase 1 have normal replicative potential during development and regeneration. J Cell Sci. 2010;123:2402–2412. doi: 10.1242/jcs.068106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 650.Rong W. Kimura H. Grundy D. The neurophysiology of hydrogen sulfide. Inflamm Allergy Drug Targets. 2011;10:109–117. doi: 10.2174/187152811794776295. [DOI] [PubMed] [Google Scholar]
- 651.Rothfork JM. Timmins GS. Harris MN. Chen X. Lusis AJ. Otto M. Cheung AL. Gresham HD. Inactivation of a bacterial virulence pheromone by phagocyte-derived oxidants: new role for the NADPH oxidase in host defense. Proc Natl Acad Sci U S A. 2004;101:13867–13872. doi: 10.1073/pnas.0402996101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 652.Rouault TA. The role of iron regulatory proteins in mammalian iron homeostasis and disease. Nat Chem Biol. 2006;2:406–414. doi: 10.1038/nchembio807. [DOI] [PubMed] [Google Scholar]
- 653.Rozell B. Bárcena JA. Martínez-Galisteo E. Padilla CA. Holmgren A. Immunochemical characterization and tissue distribution of glutaredoxin (thioltransferase) from calf. Eur J Cell Biol. 1993;62:314–323. [PubMed] [Google Scholar]
- 654.Rozell B. Hansson HA. Luthman M. Holmgren A. Immunohistochemical localization of thioredoxin and thioredoxin reductase in adult rats. Eur J Cell Biol. 1985;38:79–86. [PubMed] [Google Scholar]
- 655.Rubartelli A. Bajetto A. Allavena G. Wollman E. Sitia R. Secretion of thioredoxin by normal and neoplastic cells through a leaderless secretory pathway. J Biol Chem. 1992;267:24161–24164. [PubMed] [Google Scholar]
- 656.Rubartelli A. Bonifaci N. Sitia R. High rates of thioredoxin secretion correlate with growth arrest in hepatoma cells. Cancer Res. 1995;55:675–680. [PubMed] [Google Scholar]
- 657.Rundlöf A-K. Janard M. Miranda-Vizuete A. Arnér ESJ. Evidence for intriguingly complex transcription of human thioredoxin reductase 1. Free Radic Biol Med. 2004;36:641–656. doi: 10.1016/j.freeradbiomed.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 658.Ruoppolo M. Lundström-Ljung J. Talamo F. Pucci P. Marino G. Effect of glutaredoxin and protein disulfide isomerase on the glutathione-dependent folding of ribonuclease A. Biochemistry. 1997;36:12259–12267. doi: 10.1021/bi970851s. [DOI] [PubMed] [Google Scholar]
- 659.Russel M. Filamentous phage assembly. Mol Microbiol. 1991;5:1607–1613. doi: 10.1111/j.1365-2958.1991.tb01907.x. [DOI] [PubMed] [Google Scholar]
- 660.Rutledge BJ. Rayburn H. Rosenberg R. North RJ. Gladue RP. Corless CL. Rollins BJ. High level monocyte chemoattractant protein-1 expression in transgenic mice increases their susceptibility to intracellular pathogens. J Immunol. 1995;155:4838–4843. [PubMed] [Google Scholar]
- 661.Sabens Liedhegner EA. Gao X-H. Mieyal JJ. Mechanisms of altered redox regulation in neurodegenerative diseases-focus on s-glutathionylation. Antioxid Redox Signal. 2012;16:543–566. doi: 10.1089/ars.2011.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 662.Sackesen C. Ercan H. Dizdar E. Soyer O. Gumus P. Tosun BN. Büyüktuncer Z. Karabulut E. Besler T. Kalayci O. A comprehensive evaluation of the enzymatic and nonenzymatic antioxidant systems in childhood asthma. J Allergy Clin Immunol. 2008;122:78–85. doi: 10.1016/j.jaci.2008.03.035. [DOI] [PubMed] [Google Scholar]
- 663.Sadek CM. Damdimopoulos AE. Pelto-Huikko M. Gustafsson JA. Spyrou G. Miranda-Vizuete A. Sptrx-2, a fusion protein composed of one thioredoxin and three tandemly repeated NDP-kinase domains is expressed in human testis germ cells. Genes Cells. 2001;6:1077–1090. doi: 10.1046/j.1365-2443.2001.00484.x. [DOI] [PubMed] [Google Scholar]
- 664.Sadek CM. Jiménez A. Damdimopoulos AE. Kieselbach T. Nord M. Gustafsson J-A. Spyrou G. Davis EC. Oko R. Van der Hoorn FA. Miranda-Vizuete A. Characterization of human thioredoxin-like 2. A novel microtubule-binding thioredoxin expressed predominantly in the cilia of lung airway epithelium and spermatid manchette and axoneme. J Biol Chem. 2003;278:13133–13142. doi: 10.1074/jbc.M300369200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 665.Saeed U. Ray A. Valli RK. Kumar AMR. Ravindranath V. DJ-1 loss by glutaredoxin but not glutathione depletion triggers Daxx translocation and cell death. Antioxid Redox Signal. 2010;13:127–144. doi: 10.1089/ars.2009.2832. [DOI] [PubMed] [Google Scholar]
- 666.Sahaf B. Rosén A. Secretion of 10-kDa and 12-kDa thioredoxin species from blood monocytes and transformed leukocytes. Antioxid Redox Signal. 2000;2:717–726. doi: 10.1089/ars.2000.2.4-717. [DOI] [PubMed] [Google Scholar]
- 667.Sahaf B. Söderberg A. Spyrou G. Barral AM. Pekkari K. Holmgren A. Rosén A. Thioredoxin expression and localization in human cell lines: detection of full-length and truncated species. Exp Cell Res. 1997;236:181–192. doi: 10.1006/excr.1997.3699. [DOI] [PubMed] [Google Scholar]
- 668.Sahlin L. Wang H. Stjernholm Y. Lundberg M. Ekman G. Holmgren A. Eriksson H. The expression of glutaredoxin is increased in the human cervix in term pregnancy and immediately post-partum, particularly after prostaglandin-induced delivery. Mol Hum Reprod. 2000;6:1147–1153. doi: 10.1093/molehr/6.12.1147. [DOI] [PubMed] [Google Scholar]
- 669.Saini HK. Machackova J. Dhalla NS. Role of reactive oxygen species in ischemic preconditioning of subcellular organelles in the heart. Antioxid Redox Signal. 2004;6:393–404. doi: 10.1089/152308604322899468. [DOI] [PubMed] [Google Scholar]
- 670.Saitoh M. Nishitoh H. Fujii M. Takeda K. Tobiume K. Sawada Y. Kawabata M. Miyazono K. Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 671.Sakai K. Matsumoto K. Nishikawa T. Suefuji M. Nakamaru K. Hirashima Y. Kawashima J. Shirotani T. Ichinose K. Brownlee M. Araki E. Mitochondrial reactive oxygen species reduce insulin secretion by pancreatic beta-cells. Biochem Biophys Res Commun. 2003;300:216–222. doi: 10.1016/s0006-291x(02)02832-2. [DOI] [PubMed] [Google Scholar]
- 672.Saleh D. Ernst P. Lim S. Barnes PJ. Giaid A. Increased formation of the potent oxidant peroxynitrite in the airways of asthmatic patients is associated with induction of nitric oxide synthase: effect of inhaled glucocorticoid. FASEB J. 1998;12:929–937. [PubMed] [Google Scholar]
- 673.Salinas AE. Wong MG. Glutathione S-transferases—a review. Curr Med Chem. 1999;6:279–309. [PubMed] [Google Scholar]
- 674.Samiec PS. Drews-Botsch C. Flagg EW. Kurtz JC. Sternberg P., Jr. Reed RL. Jones DP. Glutathione in human plasma: decline in association with aging, age-related macular degeneration, and diabetes. Free Radic Biol Med. 1998;24:699–704. doi: 10.1016/s0891-5849(97)00286-4. [DOI] [PubMed] [Google Scholar]
- 675.Samuel SM. Thirunavukkarasu M. Penumathsa SV. Koneru S. Zhan L. Maulik G. Sudhakaran PR. Maulik N. Thioredoxin-1 gene therapy enhances angiogenic signaling and reduces ventricular remodeling in infarcted myocardium of diabetic rats. Circulation. 2010;121:1244–1255. doi: 10.1161/CIRCULATIONAHA.109.872481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 676.Sandau KB. Fandrey J. Brüne B. Accumulation of HIF-1alpha under the influence of nitric oxide. Blood. 2001;97:1009–1015. doi: 10.1182/blood.v97.4.1009. [DOI] [PubMed] [Google Scholar]
- 677.Sanjuán-Pla A. Cervera AM. Apostolova N. Garcia-Bou R. Víctor VM. Murphy MP. McCreath KJ. A targeted antioxidant reveals the importance of mitochondrial reactive oxygen species in the hypoxic signaling of HIF-1alpha. FEBS Lett. 2005;579:2669–2674. doi: 10.1016/j.febslet.2005.03.088. [DOI] [PubMed] [Google Scholar]
- 678.Sarafian TA. Verity MA. Vinters HV. Shih CC. Shi L. Ji XD. Dong L. Shau H. Differential expression of peroxiredoxin subtypes in human brain cell types. J Neurosci Res. 1999;56:206–212. [PubMed] [Google Scholar]
- 679.Sasagawa I. Matsuki S. Suzuki Y. Iuchi Y. Tohya K. Kimura M. Nakada T. Fujii J. Possible involvement of the membrane-bound form of peroxiredoxin 4 in acrosome formation during spermiogenesis of rats. Eur J Biochem. 2001;268:3053–3061. doi: 10.1046/j.1432-1327.2001.02200.x. [DOI] [PubMed] [Google Scholar]
- 680.Sastre J. Pallardó FV. Viña J. The role of mitochondrial oxidative stress in aging. Free Radic Biol Med. 2003;35:1–8. doi: 10.1016/s0891-5849(03)00184-9. [DOI] [PubMed] [Google Scholar]
- 681.Sato A. Hara T. Nakamura H. Kato N. Hoshino Y. Kondo N. Mishima M. Yodoi J. Thioredoxin-1 suppresses systemic inflammatory responses against cigarette smoking. Antioxid Redox Signal. 2006;8:1891–1896. doi: 10.1089/ars.2006.8.1891. [DOI] [PubMed] [Google Scholar]
- 682.Sauri H. Ashjian PH. Kim AT. Shau H. Recombinant natural killer enhancing factor augments natural killer cytotoxicity. J Leukoc Biol. 1996;59:925–931. doi: 10.1002/jlb.59.6.925. [DOI] [PubMed] [Google Scholar]
- 683.Sauri H. Butterfield L. Kim A. Shau H. Antioxidant function of recombinant human natural killer enhancing factor. Biochem Biophys Res Commun. 1995;208:964–969. doi: 10.1006/bbrc.1995.1428. [DOI] [PubMed] [Google Scholar]
- 684.Schapira AH. Jenner P. Etiology and pathogenesis of Parkinson's disease. Mov Disord. 2011;26:1049–1055. doi: 10.1002/mds.23732. [DOI] [PubMed] [Google Scholar]
- 685.Schenk H. Vogt M. Dröge W. Schulze-Osthoff K. Thioredoxin as a potent costimulus of cytokine expression. J Immunol. 1996;156:765–771. [PubMed] [Google Scholar]
- 686.Schraders M. Lee K. Oostrik J. Huygen PLM. Ali G. Hoefsloot LH. Veltman JA. Cremers FPM. Basit S. Ansar M. Cremers CWRJ. Kunst HPM. Ahmad W. Admiraal RJC. Leal SM. Kremer H. Homozygosity mapping reveals mutations of GRXCR1 as a cause of autosomal-recessive nonsyndromic hearing impairment. Am J Hum Genet. 2010;86:138–147. doi: 10.1016/j.ajhg.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 687.Schreck R. Rieber P. Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991;10:2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 688.Schroeder BO. Wu Z. Nuding S. Groscurth S. Marcinowski M. Beisner J. Buchner J. Schaller M. Stange EF. Wehkamp J. Reduction of disulphide bonds unmasks potent antimicrobial activity of human β-defensin 1. Nature. 2011;469:419–423. doi: 10.1038/nature09674. [DOI] [PubMed] [Google Scholar]
- 689.Schulze PC. De Keulenaer GW. Yoshioka J. Kassik KA. Lee RT. Vitamin D3-upregulated protein-1 (VDUP-1) regulates redox-dependent vascular smooth muscle cell proliferation through interaction with thioredoxin. Circ Res. 2002;91:689–695. doi: 10.1161/01.res.0000037982.55074.f6. [DOI] [PubMed] [Google Scholar]
- 690.Schulze PC. Yoshioka J. Takahashi T. He Z. King GL. Lee RT. Hyperglycemia promotes oxidative stress through inhibition of thioredoxin function by thioredoxin-interacting protein. J Biol Chem. 2004;279:30369–30374. doi: 10.1074/jbc.M400549200. [DOI] [PubMed] [Google Scholar]
- 691.Seghieri G. Di Simplicio P. De Giorgio LA. Anichini R. Alberti L. Franconi F. Relationship between metabolic glycaemic control and platelet content of glutathione and its related enzymes, in insulin-dependent diabetes mellitus. Clin Chim Acta. 2000;299:109–117. doi: 10.1016/s0009-8981(00)00283-7. [DOI] [PubMed] [Google Scholar]
- 692.Sekhar RV. McKay SV. Patel SG. Guthikonda AP. Reddy VT. Balasubramanyam A. Jahoor F. Glutathione synthesis is diminished in patients with uncontrolled diabetes and restored by dietary supplementation with cysteine and glycine. Diabetes Care. 2011;34:162–167. doi: 10.2337/dc10-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 693.Selenius M. Rundlöf A-K. Olm E. Fernandes AP. Björnstedt M. Selenium and the selenoprotein thioredoxin reductase in the prevention, treatment and diagnostics of cancer. Antioxid Redox Signal. 2010;12:867–880. doi: 10.1089/ars.2009.2884. [DOI] [PubMed] [Google Scholar]
- 694.Semenza GL. Hypoxia-inducible factor 1: oxygen homeostasis and disease pathophysiology. Trends Mol Med. 2001;7:345–350. doi: 10.1016/s1471-4914(01)02090-1. [DOI] [PubMed] [Google Scholar]
- 695.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 696.Semenza GL. Pulmonary vascular responses to chronic hypoxia mediated by hypoxia-inducible factor 1. Proc Am Thorac Soc. 2005;2:68–70. doi: 10.1513/pats.200404-029MS. [DOI] [PubMed] [Google Scholar]
- 697.Sengupta R. Holmgren A. The role of thioredoxin in the regulation of cellular processes by S-nitrosylation. Biochim Biophys Acta. 2011;1820:589–700. doi: 10.1016/j.bbagen.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 698.Seo MS. Kang SW. Kim K. Baines IC. Lee TH. Rhee SG. Identification of a new type of mammalian peroxiredoxin that forms an intramolecular disulfide as a reaction intermediate. J Biol Chem. 2000;275:20346–20354. doi: 10.1074/jbc.M001943200. [DOI] [PubMed] [Google Scholar]
- 699.Seth D. Stamler JS. The SNO-proteome: causation and classifications. Curr Opin Chem Biol. 2011;15:129–136. doi: 10.1016/j.cbpa.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 700.Shaked M. Ketzinel-Gilad M. Ariav Y. Cerasi E. Kaiser N. Leibowitz G. Insulin counteracts glucotoxic effects by suppressing thioredoxin-interacting protein production in INS-1E beta cells and in Psammomys obesus pancreatic islets. Diabetologia. 2009;52:636–644. doi: 10.1007/s00125-009-1274-2. [DOI] [PubMed] [Google Scholar]
- 701.Shau H. Butterfield LH. Chiu R. Kim A. Cloning and sequence analysis of candidate human natural killer-enhancing factor genes. Immunogenetics. 1994;40:129–134. doi: 10.1007/BF00188176. [DOI] [PubMed] [Google Scholar]
- 702.Shau H. Merino A. Chen L. Shih CC. Colquhoun SD. Induction of peroxiredoxins in transplanted livers and demonstration of their in vitro cytoprotection activity. Antioxid Redox Signal. 2000;2:347–354. doi: 10.1089/ars.2000.2.2-347. [DOI] [PubMed] [Google Scholar]
- 703.Shelton MD. Chock PB. Mieyal JJ. Glutaredoxin: role in Reversible Protein S-Glutathionylation and Regulation of Redox Signal Transduction and Protein Translocation. Antioxid Redox Signal. 2005;7:348–366. doi: 10.1089/ars.2005.7.348. [DOI] [PubMed] [Google Scholar]
- 704.Shelton MD. Distler AM. Kern TS. Mieyal JJ. Glutaredoxin regulates autocrine and paracrine proinflammatory responses in retinal glial (muller) cells. J Biol Chem. 2009;284:4760–4766. doi: 10.1074/jbc.M805464200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 705.Shelton MD. Kern TS. Mieyal JJ. Glutaredoxin regulates nuclear factor kappa-B and intercellular adhesion molecule in Müller cells: model of diabetic retinopathy. J Biol Chem. 2007;282:12467–12474. doi: 10.1074/jbc.M610863200. [DOI] [PubMed] [Google Scholar]
- 706.Shibuya N. Tanaka M. Yoshida M. Ogasawara Y. Togawa T. Ishii K. Kimura H. 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid Redox Signal. 2009;11:703–714. doi: 10.1089/ars.2008.2253. [DOI] [PubMed] [Google Scholar]
- 707.Shioji K. Kishimoto C. Nakamura H. Masutani H. Yuan Z. Oka S. Yodoi J. Overexpression of Thioredoxin-1 in Transgenic Mice Attenuates Adriamycin-Induced Cardiotoxicity. Circulation. 2002;106:1403–1409. doi: 10.1161/01.cir.0000027817.55925.b4. [DOI] [PubMed] [Google Scholar]
- 708.Shioji K. Kishimoto C. Nakamura H. Toyokuni S. Nakayama Y. Yodoi J. Sasayama S. Upregulation of thioredoxin (TRX) expression in giant cell myocarditis in rats. FEBS Lett. 2000;472:109–113. doi: 10.1016/s0014-5793(00)01446-0. [DOI] [PubMed] [Google Scholar]
- 709.Shiota M. Yokomizo A. Kashiwagi E. Takeuchi A. Fujimoto N. Uchiumi T. Naito S. Peroxiredoxin 2 in the nucleus and cytoplasm distinctly regulates androgen receptor activity in prostate cancer cells. Free Radic Biol Med. 2011;51:78–87. doi: 10.1016/j.freeradbiomed.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 710.Shi Y. Ren Y. Zhao L. Du C. Wang Y. Zhang Y. Li Y. Zhao S. Duan H. Knockdown of thioredoxin interacting protein attenuates high glucose-induced apoptosis and activation of ASK1 in mouse mesangial cells. FEBS Lett. 2011;585:1789–1795. doi: 10.1016/j.febslet.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 711.Shi ZZ. Osei-Frimpong J. Kala G. Kala SV. Barrios RJ. Habib GM. Lukin DJ. Danney CM. Matzuk MM. Lieberman MW. Glutathione synthesis is essential for mouse development but not for cell growth in culture. Proc Natl Acad Sci U S A. 2000;97:5101–5106. doi: 10.1073/pnas.97.10.5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 712.Shoelson SE. Lee J. Yuan M. Inflammation and the IKK beta/I kappa B/NF-kappa B axis in obesity- and diet-induced insulin resistance. Int J Obes Relat Metab Disord. 2003;27(Suppl 3):S49–S52. doi: 10.1038/sj.ijo.0802501. [DOI] [PubMed] [Google Scholar]
- 713.Sibbing D. Pfeufer A. Perisic T. Mannes AM. Fritz-Wolf K. Unwin S. Sinner MF. Gieger C. Gloeckner CJ. Wichmann H-E. Kremmer E. Schäfer Z. Walch A. Hinterseer M. Näbauer M. Kääb S. Kastrati A. Schömig A. Meitinger T. Bornkamm GW. Conrad M. Von Beckerath N. Mutations in the mitochondrial thioredoxin reductase gene TXNRD2 cause dilated cardiomyopathy. Eur Heart J. 2011;32:1121–1133. doi: 10.1093/eurheartj/ehq507. [DOI] [PubMed] [Google Scholar]
- 714.Sies H. Oxidative stress: from basic research to clinical application. Am J Med. 1991;91:31S–38S. doi: 10.1016/0002-9343(91)90281-2. [DOI] [PubMed] [Google Scholar]
- 715.Sies H. Cadenas E. Oxidative stress: damage to intact cells and organs. Philos Trans R Soc Lond B Biol Sci. 1985;311:617–631. doi: 10.1098/rstb.1985.0168. [DOI] [PubMed] [Google Scholar]
- 716.Silberstein DS. Ali MH. Baker SL. David JR. Human eosinophil cytotoxicity-enhancing factor. Purification, physical characteristics, and partial amino acid sequence of an active polypeptide. J Immunol. 1989;143:979–983. [PubMed] [Google Scholar]
- 717.Siliprandi R. Canella R. Carmignoto G. Schiavo N. Zanellato A. Zanoni R. Vantini G. N-methyl-D-aspartate-induced neurotoxicity in the adult rat retina. Vis Neurosci. 1992;8:567–573. doi: 10.1017/s0952523800005666. [DOI] [PubMed] [Google Scholar]
- 718.Simonet WS. Hughes TM. Nguyen HQ. Trebasky LD. Danilenko DM. Medlock ES. Long-term impaired neutrophil migration in mice overexpressing human interleukin-8. J Clin Invest. 1994;94:1310–1319. doi: 10.1172/JCI117450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 719.Di Simplicio P. De Giorgio LA. Cardaioli E. Lecis R. Miceli M. Rossi R. Anichini R. Mian M. Seghieri G. Franconi F. Glutathione, glutathione utilizing enzymes and thioltransferase in platelets of insulin-dependent diabetic patients: relation with platelet aggregation and with microangiopatic complications. Eur J Clin Invest. 1995;25:665–669. doi: 10.1111/j.1365-2362.1995.tb01983.x. [DOI] [PubMed] [Google Scholar]
- 720.Singh S. Banerjee R. PLP-dependent H(2)S biogenesis. Biochim Biophys Acta. 2011;1814:1518–1527. doi: 10.1016/j.bbapap.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 721.Skelly AH. Type 2 diabetes mellitus. Nurs Clin North Am. 2006;41:531–547. doi: 10.1016/j.cnur.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 722.Söderberg A. Sahaf B. Rosén A. Thioredoxin reductase, a redox-active selenoprotein, is secreted by normal and neoplastic cells: presence in human plasma. Cancer Res. 2000;60:2281–2289. [PubMed] [Google Scholar]
- 723.Soderberg BO. Holmgren A. Branden CI. Structure of oxidized thioredoxin to 4 with 5 A resolution. J Mol Biol. 1974;90:143–152. doi: 10.1016/0022-2836(74)90262-9. [DOI] [PubMed] [Google Scholar]
- 724.Soerensen J. Jakupoglu C. Beck H. Förster H. Schmidt J. Schmahl W. Schweizer U. Conrad M. Brielmeier M. The role of thioredoxin reductases in brain development. PLoS One. 2008;3:e1813. doi: 10.1371/journal.pone.0001813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 725.Sogawa K. Numayama-Tsuruta K. Ema M. Abe M. Abe H. Fujii-Kuriyama Y. Inhibition of hypoxia-inducible factor 1 activity by nitric oxide donors in hypoxia. Proc Natl Acad Sci U S A. 1998;95:7368–7373. doi: 10.1073/pnas.95.13.7368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 726.Sohal RS. Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 727.Sohlman R. The Legacy of Alfred Nobel. London, United Kingdom: The Bodley Head Ltd.; 1983. [Google Scholar]
- 728.Soini Y. Kallio JP. Hirvikoski P. Helin H. Kellokumpu-Lehtinen P. Kang SW. Tammela TLJ. Peltoniemi M. Martikainen PM. Kinnula VL. Oxidative/nitrosative stress and peroxiredoxin 2 are associated with grade and prognosis of human renal carcinoma. APMIS. 2006;114:329–337. doi: 10.1111/j.1600-0463.2006.apm_315.x. [DOI] [PubMed] [Google Scholar]
- 729.Somani SM. Husain K. Exercise training alters kinetics of antioxidant enzymes in rat tissues. Biochem Mol Biol Int. 1996;38:587–595. [PubMed] [Google Scholar]
- 730.Son A. Kato N. Horibe T. Matsuo Y. Mochizuki M. Mitsui A. Kawakami K. Nakamura H. Yodoi J. Direct association of thioredoxin-1 (TRX) with macrophage migration inhibitory factor (MIF): regulatory role of TRX on MIF internalization and signaling. Antioxid Redox Signal. 2009;11:2595–2605. doi: 10.1089/ars.2009.2522. [DOI] [PubMed] [Google Scholar]
- 731.Song JJ. Lee YJ. Differential role of glutaredoxin and thioredoxin in metabolic oxidative stress-induced activation of apoptosis signal-regulating kinase 1. Biochem J. 2003;373:845–853. doi: 10.1042/BJ20030275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 732.Song JJ. Rhee JG. Suntharalingam M. Walsh SA. Spitz DR. Lee YJ. Role of glutaredoxin in metabolic oxidative stress. Glutaredoxin as a sensor of oxidative stress mediated by H2O2. J Biol Chem. 2002;277:46566–45675. doi: 10.1074/jbc.M206826200. [DOI] [PubMed] [Google Scholar]
- 733.Song JS. Cho HH. Lee B-J. Bae YC. Jung JS. Role of thioredoxin 1 and thioredoxin 2 on proliferation of human adipose tissue-derived mesenchymal stem cells. Stem Cells Dev. 2011;20:1529–1537. doi: 10.1089/scd.2010.0364. [DOI] [PubMed] [Google Scholar]
- 734.Sorokina EM. Feinstein SI. Milovanova TN. Fisher AB. Identification of the amino acid sequence that targets peroxiredoxin 6 to lysosome-like structures of lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2009;297:L871–L880. doi: 10.1152/ajplung.00052.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 735.Sorokina EM. Feinstein SI. Zhou S. Fisher AB. Intracellular targeting of peroxiredoxin 6 to lysosomal organelles requires MAPK activity and binding to 14-3-3ɛ. Am J Physiol Cell Physiol. 2011;300:C1430–C1441. doi: 10.1152/ajpcell.00285.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 736.Spyrou G. Enmark E. Miranda-Vizuete A. Gustafsson J. Cloning and expression of a novel mammalian thioredoxin. J Biol Chem. 1997;272:2936–2941. doi: 10.1074/jbc.272.5.2936. [DOI] [PubMed] [Google Scholar]
- 737.Staal FJ. Roederer M. Herzenberg LA. Herzenberg LA. Intracellular thiols regulate activation of nuclear factor kappa B and transcription of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1990;87:9943–9947. doi: 10.1073/pnas.87.24.9943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 738.Stadtman ER. Levine RL. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids. 2003;25:207–218. doi: 10.1007/s00726-003-0011-2. [DOI] [PubMed] [Google Scholar]
- 739.Stadtman ER. Moskovitz J. Levine RL. Oxidation of methionine residues of proteins: biological consequences. Antioxid Redox Signal. 2003;5:577–582. doi: 10.1089/152308603770310239. [DOI] [PubMed] [Google Scholar]
- 740.Stipanuk MH. Beck PW. Characterization of the enzymic capacity for cysteine desulphhydration in liver and kidney of the rat. Biochem J. 1982;206:267–277. doi: 10.1042/bj2060267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 741.Strey CW. Spellman D. Stieber A. Gonatas JO. Wang X. Lambris JD. Gonatas NK. Dysregulation of stathmin, a microtubule-destabilizing protein, and up-regulation of Hsp25, Hsp27, and the antioxidant peroxiredoxin 6 in a mouse model of familial amyotrophic lateral sclerosis. Am J Pathol. 2004;165:1701–1718. doi: 10.1016/S0002-9440(10)63426-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 742.Studer L. Csete M. Lee SH. Kabbani N. Walikonis J. Wold B. McKay R. Enhanced proliferation, survival, and dopaminergic differentiation of CNS precursors in lowered oxygen. J Neurosci. 2000;20:7377–7383. doi: 10.1523/JNEUROSCI.20-19-07377.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 743.Su D. Berndt C. Fomenko DE. Holmgren A. Gladyshev VN. A conserved cis-proline precludes metal binding by the active site thiolates in members of the thioredoxin family of proteins. Biochemistry. 2007;46:6903–6910. doi: 10.1021/bi700152b. [DOI] [PubMed] [Google Scholar]
- 744.Su D. Novoselov SV. Sun Q-A. Moustafa ME. Zhou Y. Oko R. Hatfield DL. Gladyshev VN. Mammalian selenoprotein thioredoxin-glutathione reductase. Roles in disulfide bond formation and sperm maturation. J Biol Chem. 2005;280:26491–26498. doi: 10.1074/jbc.M503638200. [DOI] [PubMed] [Google Scholar]
- 745.Sultana R. Boyd-Kimball D. Cai J. Pierce WM. Klein JB. Merchant M. Butterfield DA. Proteomics analysis of the Alzheimer's disease hippocampal proteome. J Alzheimers Dis. 2007;11:153–164. doi: 10.3233/jad-2007-11203. [DOI] [PubMed] [Google Scholar]
- 746.Sultana R. Boyd-Kimball D. Cai J. Pierce WM. Klein JB. Merchant M. Butterfield DA. Proteomics analysis of the Alzheimer's disease hippocampal proteome. J Alzheimers Dis. 2007;11:153–164. doi: 10.3233/jad-2007-11203. [DOI] [PubMed] [Google Scholar]
- 747.Sutcliffe EL. Rao S. Duplicity of protein kinase C-θ: novel insights into human T-cell biology. Transcription. 2011;2:189–192. doi: 10.4161/trns.2.4.16565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 748.Svensson MJ. Larsson J. Thioredoxin-2 affects lifespan and oxidative stress in Drosophila. Hereditas. 2007;144:25–32. doi: 10.1111/j.2007.0018-0661.01990.x. [DOI] [PubMed] [Google Scholar]
- 749.Swaroop M. Bradley K. Ohura T. Tahara T. Roper MD. Rosenberg LE. Kraus JP. Rat cystathionine beta-synthase. Gene organization and alternative splicing. J Biol Chem. 1992;267:11455–11461. [PubMed] [Google Scholar]
- 750.Sylvie J. Ellen C. Kris V. The role of Wnt in cell signaling and cell adhesion during early vertebrate development. Front Biosci. 2012;17:2352–2366. doi: 10.2741/3858. [DOI] [PubMed] [Google Scholar]
- 751.Tagaya Y. Maeda Y. Mitsui A. Kondo N. Matsui H. Hamuro J. Brown N. Arai K. Yokota T. Wakasugi H. ATL-derived factor (ADF), an IL-2 receptor/Tac inducer homologous to thioredoxin; possible involvement of dithiol-reduction in the IL-2 receptor induction. EMBO J. 1989;8:757–764. doi: 10.1002/j.1460-2075.1989.tb03436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 752.Takagi Y. Mitsui A. Nishiyama A. Nozaki K. Sono H. Gon Y. Hashimoto N. Yodoi J. Overexpression of thioredoxin in transgenic mice attenuates focal ischemic brain damage. Proc Natl Acad Sci U S A. 1999;96:4131–4136. doi: 10.1073/pnas.96.7.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 753. This reference has been deleted.
- 754.Takahashi K-I. Chin K. Nakamura H. Morita S. Sumi K. Oga T. Matsumoto H. Niimi A. Fukuhara S. Yodoi J. Mishima M. Plasma thioredoxin, a novel oxidative stress marker, in patients with obstructive sleep apnea before and after nasal continuous positive airway pressure. Antioxid Redox Signal. 2008;10:715–726. doi: 10.1089/ars.2007.1949. [DOI] [PubMed] [Google Scholar]
- 755.Takashima Y. Hirota K. Nakamura H. Nakamura T. Akiyama K. Cheng FS. Maeda M. Yodoi J. Differential expression of glutaredoxin and thioredoxin during monocytic differentiation. Immunol Lett. 1999;68:397–401. doi: 10.1016/s0165-2478(99)00087-5. [DOI] [PubMed] [Google Scholar]
- 756.Taketani Y. Kinugasa K. Furukawa S. Nakamura H. Otsuki R. Yasuda H. Fujita T. Kanzaki K. Masutani H. Yodoi J. Yeast thioredoxin-enriched extracts for mitigating the allergenicity of foods. Biosci Biotechnol Biochem. 2011;75:1872–1879. doi: 10.1271/bbb.100734. [DOI] [PubMed] [Google Scholar]
- 757.Takeuchi J. Hirota K. Itoh T. Shinkura R. Kitada K. Yodoi J. Namba T. Fukuda K. Thioredoxin inhibits tumor necrosis factor- or interleukin-1-induced NF-kappaB activation at a level upstream of NF-kappaB-inducing kinase. Antioxid Redox Signal. 2000;2:83–92. doi: 10.1089/ars.2000.2.1-83. [DOI] [PubMed] [Google Scholar]
- 758.Tamaki H. Nakamura H. Nishio A. Nakase H. Ueno S. Uza N. Kido M. Inoue S. Mikami S. Asada M. Kiriya K. Kitamura H. Ohashi S. Fukui T. Kawasaki K. Matsuura M. Ishii Y. Okazaki K. Yodoi J. Chiba T. Human thioredoxin-1 ameliorates experimental murine colitis in association with suppressed macrophage inhibitory factor production. Gastroenterology. 2006;131:1110–1121. doi: 10.1053/j.gastro.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 759.Tanaka T. Hosoi F. Yamaguchi-Iwai Y. Nakamura H. Masutani H. Ueda S. Nishiyama A. Takeda S. Wada H. Spyrou G. Yodoi J. Thioredoxin-2 (TRX-2) is an essential gene regulating mitochondria-dependent apoptosis. EMBO J. 2002;21:1695–1703. doi: 10.1093/emboj/21.7.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 760.Tang K. Li X. Zheng M-Q. Rozanski GJ. Role of apoptosis signal-regulating kinase-1-c-Jun NH2-terminal kinase-p38 signaling in voltage-gated K+ channel remodeling of the failing heart: regulation by thioredoxin. Antioxid Redox Signal. 2011;14:25–35. doi: 10.1089/ars.2010.3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 761.Taniguchi Y. Taniguchi-Ueda Y. Mori K. Yodoi J. A novel promoter sequence is involved in the oxidative stress-induced expression of the adult T-cell leukemia-derived factor (ADF)/human thioredoxin (Trx) gene. Nucleic Acids Res. 1996;24:2746–2752. doi: 10.1093/nar/24.14.2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 762.Tanito M. Agbaga M-P. Anderson RE. Upregulation of thioredoxin system via Nrf2-antioxidant responsive element pathway in adaptive-retinal neuroprotection in vivo and in vitro. Free Radic Biol Med. 2007;42:1838–1850. doi: 10.1016/j.freeradbiomed.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 763.Tanito M. Masutani H. Nakamura H. Ohira A. Yodoi J. Cytoprotective effect of thioredoxin against retinal photic injury in mice. Invest Ophthalmol Vis Sci. 2002;43:1162–1167. [PubMed] [Google Scholar]
- 764.Tanito M. Nishiyama A. Tanaka T. Masutani H. Nakamura H. Yodoi J. Ohira A. Change of redox status and modulation by thiol replenishment in retinal photooxidative damage. Invest Ophthalmol Vis Sci. 2002;43:2392–2400. [PubMed] [Google Scholar]
- 765.Tannenbaum SR. White FM. Regulation and specificity of S-nitrosylation and denitrosylation. ACS Chem Biol. 2006;1:615–618. doi: 10.1021/cb600439h. [DOI] [PubMed] [Google Scholar]
- 766.Tan SM. Zhang Y. Cox AJ. Kelly DJ. Qi W. Tranilast attenuates the up-regulation of thioredoxin-interacting protein and oxidative stress in an experimental model of diabetic nephropathy. Nephrol Dial Transplant. 2011;26:100–110. doi: 10.1093/ndt/gfq355. [DOI] [PubMed] [Google Scholar]
- 767.Tao L. Gao E. Bryan NS. Qu Y. Liu H-R. Hu A. Christopher TA. Lopez BL. Yodoi J. Koch WJ. Feelisch M. Ma XL. Cardioprotective effects of thioredoxin in myocardial ischemia and reperfusion: role of S-nitrosation [corrected] Proc Natl Acad Sci U S A. 2004;101:11471–11476. doi: 10.1073/pnas.0402941101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 768.Tao L. Gao E. Hu A. Coletti C. Wang Y. Christopher TA. Lopez BL. Koch W. Ma XL. Thioredoxin reduces post-ischemic myocardial apoptosis by reducing oxidative/nitrative stress. Br J Pharmacol. 2006;149:311–318. doi: 10.1038/sj.bjp.0706853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 769.Tao L. Jiao X. Gao E. Lau WB. Yuan Y. Lopez B. Christopher T. RamachandraRao SP. Williams W. Southan G. Sharma K. Koch W. Ma XL. Nitrative inactivation of thioredoxin-1 and its role in postischemic myocardial apoptosis. Circulation. 2006;114:1395–1402. doi: 10.1161/CIRCULATIONAHA.106.625061. [DOI] [PubMed] [Google Scholar]
- 770.Taub J. Lau JF. Ma C. Hahn JH. Hoque R. Rothblatt J. Chalfie M. A cytosolic catalase is needed to extend adult lifespan in C. elegans daf-C and clk-1 mutants. Nature. 1999;399:162–166. doi: 10.1038/20208. [DOI] [PubMed] [Google Scholar]
- 771.Tezel G. The immune response in glaucoma: a perspective on the roles of oxidative stress. Exp Eye Res. 2011;93:178–186. doi: 10.1016/j.exer.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 772.Thénard LJ. Observations sur des nouvelles combinaisons entre l'oxigène et divers acides. Ann Chim Phys. 1818;8:306–312. [Google Scholar]
- 773.Thiel UJE. Feltens R. Adryan B. Gieringer R. Brochhausen C. Schuon R. Fillies T. Grus F. Mann WJ. Brieger J. Analysis of differentially expressed proteins in oral squamous cell carcinoma by MALDI-TOF MS. J Oral Pathol Med. 2011;40:369–379. doi: 10.1111/j.1600-0714.2010.00982.x. [DOI] [PubMed] [Google Scholar]
- 774.Tiitto L. Kaarteenaho-Wiik R. Sormunen R. Holmgren A. Pääkkö P. Soini Y. Kinnula VL. Expression of the thioredoxin system in interstitial lung disease. J Pathol. 2003;201:363–370. doi: 10.1002/path.1435. [DOI] [PubMed] [Google Scholar]
- 775.Timmermann B. Jarolim S. Russmayer H. Kerick M. Michel S. Krüger A. Bluemlein K. Laun P. Grillari J. Lehrach H. Breitenbach M. Ralser M. A new dominant peroxiredoxin allele identified by whole-genome re-sequencing of random mutagenized yeast causes oxidant-resistance and premature aging. Aging (Albany NY) 2010;2:475–486. doi: 10.18632/aging.100187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 776.Todoroki Y. Tsukahara H. Ohshima Y. Shukunami K-I. Nishijima K. Kotsuji F. Hata A. Kasuga K. Sekine K. Nakamura H. Yodoi J. Mayumi M. Concentrations of thioredoxin, a redox-regulating protein, in umbilical cord blood and breast milk. Free Radic Res. 2005;39:291–297. doi: 10.1080/10715760500053578. [DOI] [PubMed] [Google Scholar]
- 777.Tonissen KF. Di Trapani G. Thioredoxin system inhibitors as mediators of apoptosis for cancer therapy. Mol Nutr Food Res. 2009;53:87–103. doi: 10.1002/mnfr.200700492. [DOI] [PubMed] [Google Scholar]
- 778.Toppo S. Flohé L. Ursini F. Vanin S. Maiorino M. Catalytic mechanisms and specificities of glutathione peroxidases: variations of a basic scheme. Biochim Biophys Acta. 2009;1790:1486–1500. doi: 10.1016/j.bbagen.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 779.Torii M. Wang L. Ma N. Saito K. Hori T. Sato-Ueshima M. Koyama Y. Nishikawa H. Katayama N. Mizoguchi A. Shiku H. Yodoi J. Kuribayashi K. Kato T. Thioredoxin suppresses airway inflammation independently of systemic Th1/Th2 immune modulation. Eur J Immunol. 2010;40:787–796. doi: 10.1002/eji.200939724. [DOI] [PubMed] [Google Scholar]
- 780.Townsend DM. Tew KD. Tapiero H. The importance of glutathione in human disease. Biomed Pharmacother. 2003;57:145–155. doi: 10.1016/s0753-3322(03)00043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 781.Trifunovic A. Hansson A. Wredenberg A. Rovio AT. Dufour E. Khvorostov I. Spelbrink JN. Wibom R. Jacobs HT. Larsson N-G. Somatic mtDNA mutations cause aging phenotypes without affecting reactive oxygen species production. Proc Natl Acad Sci U S A. 2005;102:17993–17998. doi: 10.1073/pnas.0508886102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 782.Truscott RJW. Age-related nuclear cataract-oxidation is the key. Exp Eye Res. 2005;80:709–725. doi: 10.1016/j.exer.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 783.Tse HM. Milton MJ. Schreiner S. Profozich JL. Trucco M. Piganelli JD. Disruption of innate-mediated proinflammatory cytokine and reactive oxygen species third signal leads to antigen-specific hyporesponsiveness. J Immunol. 2007;178:908–917. doi: 10.4049/jimmunol.178.2.908. [DOI] [PubMed] [Google Scholar]
- 784.Tulsawani R. Kelly LS. Fatma N. Chhunchha B. Kubo E. Kumar A. Singh DP. Neuroprotective effect of peroxiredoxin 6 against hypoxia-induced retinal ganglion cell damage. BMC Neurosci. 2010;11:125. doi: 10.1186/1471-2202-11-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 785.Turanov AA. Su D. Gladyshev VN. Characterization of alternative cytosolic forms and cellular targets of mouse mitochondrial thioredoxin reductase. J Biol Chem. 2006;281:22953–22963. doi: 10.1074/jbc.M604326200. [DOI] [PubMed] [Google Scholar]
- 786.Turoczi T. Chang VW-H. Engelman RM. Maulik N. Ho YS. Das DK. Thioredoxin redox signaling in the ischemic heart: an insight with transgenic mice overexpressing Trx1. J Mol Cell Cardiol. 2003;35:695–704. doi: 10.1016/s0022-2828(03)00117-2. [DOI] [PubMed] [Google Scholar]
- 787.Turunen N. Karihtala P. Mantyniemi A. Sormunen R. Holmgren A. Kinnula VL. Soini Y. Thioredoxin is associated with proliferation, p53 expression and negative estrogen and progesterone receptor status in breast carcinoma. APMIS. 2004;112:123–132. doi: 10.1111/j.1600-0463.2004.apm1120207.x. [DOI] [PubMed] [Google Scholar]
- 788.Tyther R. Ahmeda A. Johns E. Sheehan D. Proteomic identification of tyrosine nitration targets in kidney of spontaneously hypertensive rats. Proteomics. 2007;7:4555–4564. doi: 10.1002/pmic.200700503. [DOI] [PubMed] [Google Scholar]
- 789.Ueno M. Masutani H. Arai RJ. Yamauchi A. Hirota K. Sakai T. Inamoto T. Yamaoka Y. Yodoi J. Nikaido T. Thioredoxin-dependent redox regulation of p53-mediated p21 activation. J Biol Chem. 1999;274:35809–35815. doi: 10.1074/jbc.274.50.35809. [DOI] [PubMed] [Google Scholar]
- 790.Ugarte N. Petropoulos I. Friguet B. Oxidized mitochondrial protein degradation and repair in aging and oxidative stress. Antioxid Redox Signal. 2010;13:539–549. doi: 10.1089/ars.2009.2998. [DOI] [PubMed] [Google Scholar]
- 791.Ullrich V. Kissner R. Redox signaling: bioinorganic chemistry at its best. J Inorg Biochem. 2006;100:2079–2086. doi: 10.1016/j.jinorgbio.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 792.Umar S. Van der Laarse A. Nitric oxide and nitric oxide synthase isoforms in the normal, hypertrophic, and failing heart. Mol Cell Biochem. 2010;333:191–201. doi: 10.1007/s11010-009-0219-x. [DOI] [PubMed] [Google Scholar]
- 793.Urig S. Becker K. On the potential of thioredoxin reductase inhibitors for cancer therapy. Semin Cancer Biol. 2006;16:452–465. doi: 10.1016/j.semcancer.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 794.Ursini F. Heim S. Kiess M. Maiorino M. Roveri A. Wissing J. Flohé L. Dual function of the selenoprotein PHGPx during sperm maturation. Science. 1999;285:1393–1396. doi: 10.1126/science.285.5432.1393. [DOI] [PubMed] [Google Scholar]
- 795.Valdman A. Häggarth L. Cheng L. Lopez-Beltran A. Montironi R. Ekman P. Egevad L. Expression of redox pathway enzymes in human prostatic tissue. Anal Quant Cytol Histol. 2009;31:367–374. [PubMed] [Google Scholar]
- 796.Del Val G. Yee BC. Lozano RM. Buchanan BB. Ermel RW. Lee YM. Frick OL. Thioredoxin treatment increases digestibility and lowers allergenicity of milk. J Allergy Clin Immunol. 1999;103:690–697. doi: 10.1016/s0091-6749(99)70244-7. [DOI] [PubMed] [Google Scholar]
- 797.Vinten-Johansen J. Zhao Z-Q. Jiang R. Zatta AJ. Dobson GP. Preconditioning and postconditioning: innate cardioprotection from ischemia-reperfusion injury. J Appl Physiol. 2007;103:1441–1448. doi: 10.1152/japplphysiol.00642.2007. [DOI] [PubMed] [Google Scholar]
- 798.Wakasugi N. Tagaya Y. Wakasugi H. Mitsui A. Maeda M. Yodoi J. Tursz T. Adult T-cell leukemia-derived factor/thioredoxin, produced by both human T-lymphotropic virus type I- and Epstein-Barr virus-transformed lymphocytes, acts as an autocrine growth factor and synergizes with interleukin 1 and interleukin 2. Proc Natl Acad Sci U S A. 1990;87:8282–8286. doi: 10.1073/pnas.87.21.8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 799.Wang J. Silva JP. Gustafsson CM. Rustin P. Larsson NG. Increased in vivo apoptosis in cells lacking mitochondrial DNA gene expression. Proc Natl Acad Sci U S A. 2001;98:4038–4043. doi: 10.1073/pnas.061038798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 800.Wang MX. Wei A. Yuan J. Trickett A. Knoops B. Murrell GAC. Expression and regulation of peroxiredoxin 5 in human osteoarthritis. FEBS Lett. 2002;531:359–362. doi: 10.1016/s0014-5793(02)03511-1. [DOI] [PubMed] [Google Scholar]
- 801.Wang R. Signaling pathways for the vascular effects of hydrogen sulfide. Curr Opin Nephrol Hypertens. 2011;20:107–112. doi: 10.1097/MNH.0b013e3283430651. [DOI] [PubMed] [Google Scholar]
- 802.Wang X. Phelan SA. Forsman-Semb K. Taylor EF. Petros C. Brown A. Lerner CP. Paigen B. Mice with targeted mutation of peroxiredoxin 6 develop normally but are susceptible to oxidative stress. J Biol Chem. 2003;278:25179–25190. doi: 10.1074/jbc.M302706200. [DOI] [PubMed] [Google Scholar]
- 803.Wang X. Phelan SA. Petros C. Taylor EF. Ledinski G. Jürgens G. Forsman-Semb K. Paigen B. Peroxiredoxin 6 deficiency and atherosclerosis susceptibility in mice: significance of genetic background for assessing atherosclerosis. Atherosclerosis. 2004;177:61–70. doi: 10.1016/j.atherosclerosis.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 804.Wang Y. Phelan SA. Manevich Y. Feinstein SI. Fisher AB. Transgenic mice overexpressing peroxiredoxin 6 show increased resistance to lung injury in hyperoxia. Am J Respir Cell Mol Biol. 2006;34:481–486. doi: 10.1165/rcmb.2005-0333OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 805.Watabe S. Hiroi T. Yamamoto Y. Fujioka Y. Hasegawa H. Yago N. Takahashi SY. SP-22 is a thioredoxin-dependent peroxide reductase in mitochondria. Eur J Biochem. 1997;249:52–60. doi: 10.1111/j.1432-1033.1997.t01-1-00052.x. [DOI] [PubMed] [Google Scholar]
- 806.Webster CM. Kelly S. Koike MA. Chock VY. Giffard RG. Yenari MA. Inflammation and NFkappaB activation is decreased by hypothermia following global cerebral ischemia. Neurobiol Dis. 2009;33:301–312. doi: 10.1016/j.nbd.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 807.Wei Q. Jiang H. Xiao Z. Baker A. Young MR. Veenstra TD. Colburn NH. Sulfiredoxin-Peroxiredoxin IV axis promotes human lung cancer progression through modulation of specific phosphokinase signaling. Proc Natl Acad Sci U S A. 2011;108:7004–7009. doi: 10.1073/pnas.1013012108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 808.Weiss G. Goossen B. Doppler W. Fuchs D. Pantopoulos K. Werner-Felmayer G. Wachter H. Hentze MW. Translational regulation via iron-responsive elements by the nitric oxide/NO-synthase pathway. EMBO J. 1993;12:3651–3657. doi: 10.1002/j.1460-2075.1993.tb06039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 809.Weiss S. Asthma: epidemiology. In: Fishman J, editor; Elias J, editor; Fishman M, editor; Grippi L, editor; Kaiser L, editor; Senior R, editor. Fishman's Pulmonary Diseases and Disorders. New-York: McGraw-Hill; 1998. pp. 735–743. [Google Scholar]
- 810.Wen ST. Van Etten RA. The PAG gene product, a stress-induced protein with antioxidant properties, is an Abl SH3-binding protein and a physiological inhibitor of c-Abl tyrosine kinase activity. Genes Dev. 1997;11:2456–2467. doi: 10.1101/gad.11.19.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 811.White CL. Senkevich TG. Moss B. Vaccinia virus G4L glutaredoxin is an essential intermediate of a cytoplasmic disulfide bond pathway required for virion assembly. J Virol. 2002;76:467–472. doi: 10.1128/JVI.76.2.467-472.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 812.White CL. Weisberg AS. Moss B. A glutaredoxin, encoded by the G4L gene of vaccinia virus, is essential for virion morphogenesis. J Virol. 2000;74:9175–9183. doi: 10.1128/jvi.74.19.9175-9183.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 813.Whiteman M. Moore PK. Hydrogen sulfide and the vasculature: a novel vasculoprotective entity and regulator of nitric oxide bioavailability? J Cell Mol Med. 2009;13:488–507. doi: 10.1111/j.1582-4934.2009.00645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 814.Widder JD. Fraccarollo D. Galuppo P. Hansen JM. Jones DP. Ertl G. Bauersachs J. Attenuation of angiotensin II-induced vascular dysfunction and hypertension by overexpression of Thioredoxin 2. Hypertension. 2009;54:338–344. doi: 10.1161/HYPERTENSIONAHA.108.127928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 815.Wiekowski MT. Chen SC. Zalamea P. Wilburn BP. Kinsley DJ. Sharif WW. Jensen KK. Hedrick JA. Manfra D. Lira SA. Disruption of neutrophil migration in a conditional transgenic model: evidence for CXCR2 desensitization in vivo. J Immunol. 2001;167:7102–7110. doi: 10.4049/jimmunol.167.12.7102. [DOI] [PubMed] [Google Scholar]
- 816.Wilson RB. Iron dysregulation in Friedreich ataxia. Semin Pediatr Neurol. 2006;13:166–175. doi: 10.1016/j.spen.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 817.Wingert RA. Galloway JL. Barut B. Foott H. Fraenkel P. Axe JL. Weber GJ. Dooley K. Davidson AJ. Schmid B. Schmidt B. Paw BH. Shaw GC. Kingsley P. Palis J. Schubert H. Chen O. Kaplan J. Zon LI. Deficiency of glutaredoxin 5 reveals Fe-S clusters are required for vertebrate haem synthesis. Nature. 2005;436:1035–1039. doi: 10.1038/nature03887. [DOI] [PubMed] [Google Scholar]
- 818.Winkler A. Njålsson R. Carlsson K. Elgadi A. Rozell B. Abraham L. Ercal N. Shi Z-Z. Lieberman MW. Larsson A. Norgren S. Glutathione is essential for early embryogenesis—analysis of a glutathione synthetase knockout mouse. Biochem Biophys Res Commun. 2011;412:121–126. doi: 10.1016/j.bbrc.2011.07.056. [DOI] [PubMed] [Google Scholar]
- 819.Witte S. Villalba M. Bi K. Liu Y. Isakov N. Altman A. Inhibition of the c-Jun N-terminal kinase/AP-1 and NF-kappaB pathways by PICOT, a novel protein kinase C-interacting protein with a thioredoxin homology domain. J Biol Chem. 2000;275:1902–1909. doi: 10.1074/jbc.275.3.1902. [DOI] [PubMed] [Google Scholar]
- 820.Wolf G. Aumann N. Michalska M. Bast A. Sonnemann J. Beck JF. Lendeckel U. Newsholme P. Walther R. Peroxiredoxin III protects pancreatic ß cells from apoptosis. J Endocrinol. 2010;207:163–175. doi: 10.1677/JOE-09-0455. [DOI] [PubMed] [Google Scholar]
- 821.Wolin MS. Ahmad M. Gupte SA. The sources of oxidative stress in the vessel wall. Kidney Int. 2005;67:1659–1661. doi: 10.1111/j.1523-1755.2005.00257.x. [DOI] [PubMed] [Google Scholar]
- 822.Wood ZA. Schröder E. Robin Harris J. Poole LB. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem Sci. 2003;28:32–40. doi: 10.1016/s0968-0004(02)00003-8. [DOI] [PubMed] [Google Scholar]
- 823.Woo H-J. Bae CH. Song S-Y. Kim Y-W. Lee H-M. Kim Y-D. Expression of glutaredoxin-1 in nasal polyps and airway epithelial cells. Am J Rhinol Allergy. 2009;23:288–293. doi: 10.2500/ajra.2009.23.3318. [DOI] [PubMed] [Google Scholar]
- 824.Woolston CM. Deen S. Al-Attar A. Shehata M. Chan SY. Martin SG. Redox protein expression predicts progression-free and overall survival in ovarian cancer patients treated with platinum-based chemotherapy. Free Radic Biol Med. 2010;49:1263–1272. doi: 10.1016/j.freeradbiomed.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 825.Woolston CM. Storr SJ. Ellis IO. Morgan DAL. Martin SG. Expression of thioredoxin system and related peroxiredoxin proteins is associated with clinical outcome in radiotherapy treated early stage breast cancer. Radiother Oncol. 2011;100:308–313. doi: 10.1016/j.radonc.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 826.Wu H. Lin L. Giblin F. Ho Y-S. Lou MF. Glutaredoxin 2 knockout increases sensitivity to oxidative stress in mouse lens epithelial cells. Free Radic Biol Med. 2011;51:2108–2117. doi: 10.1016/j.freeradbiomed.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 827.Wu H. Xing K. Lou MF. Glutaredoxin 2 prevents H(2)O(2)-induced cell apoptosis by protecting complex I activity in the mitochondria. Biochim Biophys Acta. 2010;1797:1705–1715. doi: 10.1016/j.bbabio.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 828.Wu J. Tang Q. Shen J. Yao A. Wang F. Pu L. Yu Y. Li X. Li G. Zhang F. Sun B. Kong L. Li D. Zhang Y. Guo X. Wang X. Comparative proteome profile during the early period of small-for-size liver transplantation in rats revealed the protective role of Prdx5. J Hepatol. 2010;53:73–83. doi: 10.1016/j.jhep.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 829.Wu X. Teng Z. Jiang L. Fan Y. Zhang Y. Li X. Zhang Y. Human thioredoxin exerts cardioprotective effect and attenuates reperfusion injury in rats partially via inhibiting apoptosis. Chin Med J. 2008;121:819–826. [PubMed] [Google Scholar]
- 830.Wu XY. Fu ZX. Wang XH. Peroxiredoxins in colorectal neoplasms. Histol Histopathol. 2010;25:1297–1303. doi: 10.14670/HH-25.1297. [DOI] [PubMed] [Google Scholar]
- 831.Xia TH. Bushweller JH. Sodano P. Billeter M. Björnberg O. Holmgren A. Wüthrich K. NMR structure of oxidized Escherichia coli glutaredoxin: comparison with reduced E. coli glutaredoxin and functionally related proteins. Protein Sci. 1992;1:310–321. doi: 10.1002/pro.5560010302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 832.Xing K-Y. Lou MF. Effect of age on the thioltransferase (glutaredoxin) and thioredoxin systems in the human lens. Invest Ophthalmol Vis Sci. 2010;51:6598–6604. doi: 10.1167/iovs.10-5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 833.Xiong Y. Uys JD. Tew KD. Townsend DM. S-glutathionylation: from molecular mechanisms to health outcomes. Antioxid Redox Signal. 2011;15:233–270. doi: 10.1089/ars.2010.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 834.Yakes FM. Van Houten B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc Natl Acad Sci U S A. 1997;94:514–519. doi: 10.1073/pnas.94.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 835.Yamada Y. Nakamura H. Adachi T. Sannohe S. Oyamada H. Kayaba H. Yodoi J. Chihara J. Elevated serum levels of thioredoxin in patients with acute exacerbation of asthma. Immunol Lett. 2003;86:199–205. doi: 10.1016/s0165-2478(03)00006-3. [DOI] [PubMed] [Google Scholar]
- 836.Yamaguchi T. Sano K. Takakura K. Saito I. Shinohara Y. Asano T. Yasuhara H. Ebselen in acute ischemic stroke: a placebo-controlled, double-blind clinical trial. Ebselen Study Group. Stroke. 1998;29:12–17. doi: 10.1161/01.str.29.1.12. [DOI] [PubMed] [Google Scholar]
- 837.Yamamoto M. Yamato E. Toyoda S-I. Tashiro F. Ikegami H. Yodoi J. Miyazaki J-I. Transgenic expression of antioxidant protein thioredoxin in pancreatic beta cells prevents progression of type 2 diabetes mellitus. Antioxid Redox Signal. 2008;10:43–49. doi: 10.1089/ars.2007.1586. [DOI] [PubMed] [Google Scholar]
- 838.Yamamoto M. Yang G. Hong C. Liu J. Holle E. Yu X. Wagner T. Vatner SF. Sadoshima J. Inhibition of endogenous thioredoxin in the heart increases oxidative stress and cardiac hypertrophy. J Clin Invest. 2003;112:1395–1406. doi: 10.1172/JCI17700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 839.Yamanaka H. Maehira F. Oshiro M. Asato T. Yanagawa Y. Takei H. Nakashima Y. A possible interaction of thioredoxin with VDUP1 in HeLa cells detected in a yeast two-hybrid system. Biochem Biophys Res Commun. 2000;271:796–800. doi: 10.1006/bbrc.2000.2699. [DOI] [PubMed] [Google Scholar]
- 840.Yanagawa T. Ishikawa T. Ishii T. Tabuchi K. Iwasa S. Bannai S. Omura K. Suzuki H. Yoshida H. Peroxiredoxin I expression in human thyroid tumors. Cancer Lett. 1999;145:127–132. doi: 10.1016/s0304-3835(99)00243-8. [DOI] [PubMed] [Google Scholar]
- 841.Yanagawa T. Iwasa S. Ishii T. Tabuchi K. Yusa H. Onizawa K. Omura K. Harada H. Suzuki H. Yoshida H. Peroxiredoxin I expression in oral cancer: a potential new tumor marker. Cancer Lett. 2000;156:27–35. doi: 10.1016/s0304-3835(00)00434-1. [DOI] [PubMed] [Google Scholar]
- 842.Yanagawa T. Omura K. Harada H. Ishii T. Uwayama J. Nakaso K. Iwasa S. Koyama Y. Onizawa K. Yusa H. Yoshida H. Peroxiredoxin I expression in tongue squamous cell carcinomas as involved in tumor recurrence. Int J Oral Maxillofac Surg. 2005;34:915–920. doi: 10.1016/j.ijom.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 843.Yang H-Y. Kwon J. Cho E-J. Choi H-I. Park C. Park H-R. Park S-H. Chung K-J. Ryoo ZY. Cho K-O. Lee T-H. Proteomic analysis of protein expression affected by peroxiredoxin V knock-down in hypoxic kidney. J Proteome Res. 2010;9:4003–4015. doi: 10.1021/pr100190b. [DOI] [PubMed] [Google Scholar]
- 844.Yang S. Luo A. Hao X. Lai Z. Ding T. Ma X. Mayinuer M. Shen W. Wang X. Lu Y. Ma D. Wang S. Peroxiredoxin 2 inhibits granulosa cell apoptosis during follicle atresia through the NFKB pathway in mice. Biol Reprod. 2011;84:1182–1189. doi: 10.1095/biolreprod.110.087569. [DOI] [PubMed] [Google Scholar]
- 845.Yang Y. Ago T. Zhai P. Abdellatif M. Sadoshima J. Thioredoxin 1 negatively regulates angiotensin II-induced cardiac hypertrophy through upregulation of miR-98/let-7. Circ Res. 2011;108:305–313. doi: 10.1161/CIRCRESAHA.110.228437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 846.Yan Y. Sabharwal P. Rao M. Sockanathan S. The antioxidant enzyme Prdx1 controls neuronal differentiation by thiol-redox-dependent activation of GDE2. Cell. 2009;138:1209–1221. doi: 10.1016/j.cell.2009.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 847.Yao J. Taylor M. Davey F. Ren Y. Aiton J. Coote P. Fang F. Chen JX. Yan SD. Gunn-Moore FJ. Interaction of amyloid binding alcohol dehydrogenase/Abeta mediates up-regulation of peroxiredoxin II in the brains of Alzheimer's disease patients and a transgenic Alzheimer's disease mouse model. Mol Cell Neurosci. 2007;35:377–382. doi: 10.1016/j.mcn.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 848.Yegorova S. Yegorov O. Lou MF. Thioredoxin induced antioxidant gene expressions in human lens epithelial cells. Exp Eye Res. 2006;83:783–792. doi: 10.1016/j.exer.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 849.Yin T. Hou R. Liu S. Lau WB. Wang H. Tao L. Nitrative inactivation of thioredoxin-1 increases vulnerability of diabetic hearts to ischemia/reperfusion injury. J Mol Cell Cardiol. 2010;49:354–361. doi: 10.1016/j.yjmcc.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 850.Yki-Järvinen H. Glucose toxicity. Endocr Rev. 1992;13:415–431. doi: 10.1210/edrv-13-3-415. [DOI] [PubMed] [Google Scholar]
- 851.Yoon Y. Galloway CA. Jhun BS. Yu T. Mitochondrial dynamics in diabetes. Antioxid Redox Signal. 2011;14:439–457. doi: 10.1089/ars.2010.3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 852.Yoshida T. Oka S. Masutani H. Nakamura H. Yodoi J. The role of thioredoxin in the aging process: involvement of oxidative stress. Antioxid Redox Signal. 2003;5:563–570. doi: 10.1089/152308603770310211. [DOI] [PubMed] [Google Scholar]
- 853.Yoshida Y. Yoshikawa A. Kinumi T. Ogawa Y. Saito Y. Ohara K. Yamamoto H. Imai Y. Niki E. Hydroxyoctadecadienoic acid and oxidatively modified peroxiredoxins in the blood of Alzheimer's disease patients and their potential as biomarkers. Neurobiol Aging. 2009;30:174–185. doi: 10.1016/j.neurobiolaging.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 854.Yoshihara E. Fujimoto S. Inagaki N. Okawa K. Masaki S. Yodoi J. Masutani H. Disruption of TBP-2 ameliorates insulin sensitivity and secretion without affecting obesity. Nat Commun. 2010;1:127. doi: 10.1038/ncomms1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 855.Yoshioka J. Chutkow WA. Lee S. Kim JB. Yan J. Tian R. Lindsey ML. Feener EP. Seidman CE. Seidman JG. Lee RT. Deletion of thioredoxin-interacting protein in mice impairs mitochondrial function but protects the myocardium from ischemia-reperfusion injury. J Clin Invest. 2012;122:267–279. doi: 10.1172/JCI44927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 856.Yoshioka J. Imahashi K. Gabel SA. Chutkow WA. Burds AA. Gannon J. Schulze PC. MacGillivray C. London RE. Murphy E. Lee RT. Targeted deletion of thioredoxin-interacting protein regulates cardiac dysfunction in response to pressure overload. Circ Res. 2007;101:1328–1338. doi: 10.1161/CIRCRESAHA.106.160515. [DOI] [PubMed] [Google Scholar]
- 857.Yoshioka J. Schulze PC. Cupesi M. Sylvan JD. MacGillivray C. Gannon J. Huang H. Lee RT. Thioredoxin-interacting protein controls cardiac hypertrophy through regulation of thioredoxin activity. Circulation. 2004;109:2581–2586. doi: 10.1161/01.CIR.0000129771.32215.44. [DOI] [PubMed] [Google Scholar]
- 858.Yuan Z. Kishimoto C. Shioji K. Nakamura H. Yodoi J. Sasayama S. Temocapril treatment ameliorates autoimmune myocarditis associated with enhanced cardiomyocyte thioredoxin expression. Mol Cell Biochem. 2003;248:185–192. doi: 10.1023/a:1024104906484. [DOI] [PubMed] [Google Scholar]
- 859.Zelko IN. Mariani TJ. Folz RJ. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med. 2002;33:337–349. doi: 10.1016/s0891-5849(02)00905-x. [DOI] [PubMed] [Google Scholar]
- 860.Zeller T. Klug G. Thioredoxins in bacteria: functions in oxidative stress response and regulation of thioredoxin genes. Naturwissenschaften. 2006;93:259–266. doi: 10.1007/s00114-006-0106-1. [DOI] [PubMed] [Google Scholar]
- 861.Von Zglinicki T. Saretzki G. Döcke W. Lotze C. Mild hyperoxia shortens telomeres and inhibits proliferation of fibroblasts: a model for senescence? Exp Cell Res. 1995;220:186–193. doi: 10.1006/excr.1995.1305. [DOI] [PubMed] [Google Scholar]
- 862.Zhang B. Wang Y. Su Y. Peroxiredoxins, a novel target in cancer radiotherapy. Cancer Lett. 2009;286:154–160. doi: 10.1016/j.canlet.2009.04.043. [DOI] [PubMed] [Google Scholar]
- 863.Zhang H. Luo Y. Zhang W. He Y. Dai S. Zhang R. Huang Y. Bernatchez P. Giordano FJ. Shadel G. Sessa WC. Min W. Endothelial-specific expression of mitochondrial thioredoxin improves endothelial cell function and reduces atherosclerotic lesions. Am J Pathol. 2007;170:1108–1120. doi: 10.2353/ajpath.2007.060960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 864.Zhang H. Tao L. Jiao X. Gao E. Lopez BL. Christopher TA. Koch W. Ma XL. Nitrative thioredoxin inactivation as a cause of enhanced myocardial ischemia/reperfusion injury in the aging heart. Free Radic Biol Med. 2007;43:39–47. doi: 10.1016/j.freeradbiomed.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 865.Zhang SX. Garcia-Gras E. Wycuff DR. Marriot SJ. Kadeer N. Yu W. Olson EN. Garry DJ. Parmacek MS. Schwartz RJ. Identification of direct serum-response factor gene targets during Me2SO-induced P19 cardiac cell differentiation. J Biol Chem. 2005;280:19115–19126. doi: 10.1074/jbc.M413793200. [DOI] [PubMed] [Google Scholar]
- 866.Zhang Y. Ikeno Y. Qi W. Chaudhuri A. Li Y. Bokov A. Thorpe SR. Baynes JW. Epstein C. Richardson A. Van Remmen H. Mice deficient in both Mn superoxide dismutase and glutathione peroxidase-1 have increased oxidative damage and a greater incidence of pathology but no reduction in longevity. J Gerontol A Biol Sci Med Sci. 2009;64:1212–1220. doi: 10.1093/gerona/glp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 867.Zhao R. Holmgren A. A novel antioxidant mechanism of ebselen involving ebselen diselenide, a substrate of mammalian thioredoxin and thioredoxin reductase. J Biol Chem. 2002;277:39456–39462. doi: 10.1074/jbc.M206452200. [DOI] [PubMed] [Google Scholar]
- 868.Zhou F. Gomi M. Fujimoto M. Hayase M. Marumo T. Masutani H. Yodoi J. Hashimoto N. Nozaki K. Takagi Y. Attenuation of neuronal degeneration in thioredoxin-1 overexpressing mice after mild focal ischemia. Brain Res. 2009;1272:62–70. doi: 10.1016/j.brainres.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 869.Zhou J. Damdimopoulos AE. Spyrou G. Brüne B. Thioredoxin 1 and thioredoxin 2 have opposed regulatory functions on hypoxia-inducible factor-1alpha. J Biol Chem. 2007;282:7482–7490. doi: 10.1074/jbc.M608289200. [DOI] [PubMed] [Google Scholar]
- 870.Zhou Y. Kok KH. Chun AC. Wong CM. Wu HW. Lin MC. Fung PC. Kung H. Jin DY. Mouse peroxiredoxin V is a thioredoxin peroxidase that inhibits p53-induced apoptosis. Biochem Biophys Res Commun. 2000;268:921–927. doi: 10.1006/bbrc.2000.2231. [DOI] [PubMed] [Google Scholar]
- 871.Zhu X. Huang C. Peng B. Overexpression of thioredoxin system proteins predicts poor prognosis in patients with squamous cell carcinoma of the tongue. Oral Oncol. 2011;47:609–614. doi: 10.1016/j.oraloncology.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 872.Zieger MA. Gupta MP. Wang M. Proteomic analysis of endothelial cold-adaptation. BMC Genomics. 2011;12:630. doi: 10.1186/1471-2164-12-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 873.Zucchi R. Danesi R. Cardiac toxicity of antineoplastic anthracyclines. Curr Med Chem Anticancer Agents. 2003;3:151–171. doi: 10.2174/1568011033353434. [DOI] [PubMed] [Google Scholar]