Abstract
Cathelicidin-related antimicrobial peptide (CRAMP) not only kills bacteria but also binds to lipopolysaccharide (LPS) to neutralize its activity. CRAMP is highly expressed in bone marrow and its expression is reported to be up-regulated by inflammatory and infectious stimuli. Here, we examined the role of CRAMP in murine osteoclastogenesis. Osteoclasts were formed in co-cultures of osteoblasts and bone marrow cells in response to 1α,25-dihydroxyvitamin D3 [1α,25(OH)2D3], prostaglandin E2 (PGE2), and Toll-like receptor (TLR) ligands such as LPS and flagellin through the induction of receptor activator of nuclear factor-κB ligand (RANKL) expression in osteoblasts. CRAMP inhibited the osteoclastogenesis in co-cultures treated with LPS and flagellin, but not in those treated with 1α,25(OH)2D3 or PGE2. Although bone marrow macrophages (BMMs) highly expressed formyl peptide receptor 2 (a receptor of CRAMP), CRAMP showed no inhibitory effect on osteoclastogenesis in BMM cultures treated with RANKL. CRAMP suppressed both LPS- and flagellin-induced RANKL expression in osteoblasts and tumour necrosis factor-α (TNF-α) expression in BMMs, suggesting that CRAMP neutralizes the actions of LPS and flagellin. LPS and flagellin enhanced the expression of CRAMP mRNA in osteoblasts. Extracellularly added CRAMP suppressed LPS- and flagellin-induced CRAMP expression. These results suggest that the production of CRAMP promoted by LPS and flagellin is inhibited by CRAMP released by osteoblasts through a feedback regulation. Even though CRAMP itself has no effect on osteoclastogenesis in mice, we propose that CRAMP is an osteoblast-derived protector in bacterial infection-induced osteoclastic bone resorption.
Keywords: cathelicidin-related antimicrobial peptide, flagellin, lipopolysaccharide, osteoclastogenesis, receptor activator of nuclear factor-κB ligand
Introduction
Mammalian immunity is composed of two systems: innate immunity and acquired immunity. Innate immunity is the first line of defence against invading pathogens and involves Toll-like receptors (TLRs) and antibacterial peptides.1 TLRs, a family of receptors expressed by host cells, enable the host recognition of pathogen-associated molecular patterns.2 Ligation of TLRs by their specific ligands enhances the production of chemokines and inflammatory cytokines. Antimicrobial peptides are conserved evolutionarily, and play important roles in innate immunity.3,4 Antimicrobial peptides have a broad antimicrobial spectrum and inactivate microorganisms by direct interaction with bacterial cell membranes.3 Beside this antimicrobial function, antimicrobial peptides play multiple roles in inflammation, cell proliferation, angiogenesis and wound healing.5
An antimicrobial peptide, cathelicidin-related antimicrobial peptide (CRAMP, encoded by the Camp gene), is a cationic peptide secreted by host cells such as neutrophils,6 epithelial cells7 and macrophages.8 CRAMP is highly expressed in bone marrow9 is induced by inflammatory or infectious stimuli, and displays direct antimicrobial activity.8,10. Mice lacking the Camp gene showed increased susceptibility to group A streptococcus11 and Escherichia coli infections.7 LL-37 is a human homologue of murine CRAMP. CRAMP/LL-37 binds to formyl peptide receptor 2 (FPR2), which acts as the receptor for CRAMP/LL-37 and mediates chemotaxis of monocytes and neutrophils.12,13 CRAMP/LL-37 is reported to enhance angiogenesis14 and wound healing.11
CRAMP/LL-37 is expressed in salivary gland and oral mucosa.15 The levels of LL-37 in serum and saliva in patients with Kostman syndrome are low because of a deficiency of neutrophils.16,17 Such patients often experience infections of periodontal ligaments together with a severe loss of alveolar bone. The concentration of LL-37 in gingival crevicular fluid was decreased in patients with severe periodontitis.18 These results suggest that CRAMP prevents the progression of periodontal diseases.
Osteoclasts are bone-resorbing multinucleated cells generated from monocyte/macrophage-lineage precursors.19 The differentiation of precursors into osteoclasts is regulated by bone-forming osteoblasts.20 Osteoblasts express two cytokines responsible for osteoclastogenesis: macrophage colony-stimulating factor (M-CSF) [also called colony-stimulating factor 1 (CSF-1)] and receptor activator of nuclear factor-κB ligand (RANKL). Osteoclast precursors express c-Fms (receptor for M-CSF) and RANK (receptor for RANKL), and differentiate into osteoclasts in response to M-CSF and RANKL.21,22 Osteoblasts constitutively express M-CSF. On the other hand, RANKL is expressed inducibly by osteoblasts in response to bone-resorption-stimulating factors such as 1α,25-dihydroxyvitamin D3 [1α,25(OH)2D3] and lipopolysaccharide (LPS).21–23 Mature osteoclasts also express RANK and TLR4. RANKL and LPS support the survival of osteoclasts and enhance their bone-resorbing activity.23
In the present study, we examined the role of CRAMP in murine osteoclastogenesis using several culture systems. CRAMP itself did not affect murine osteoclastogenesis induced by RANKL but suppressed that by LPS and flagellin through the neutralization of those TLR ligands.
Materials and methods
Animals and reagents
Seven-week-old male and newborn ddY mice were purchased from Japan SLC (Shizuoka, Japan). All experiments were conducted in accordance with the guidelines for studies with laboratory animals of the Matsumoto Dental University Experimental Animal Committee. The following reagents were used: CRAMP (ANASPEC, Fremont, CA); E. coli LPS and Staphylococcus aureus peptidoglycan (TLR2 ligand) (Sigma-Aldrich, St Louis, MO); palmitoyl-3-Cys-Ser-Lys-4 (Pam3CSK4, TLR1/2 ligand) and Salmonella Typhimurium flagellin (IMGENEX, San Diego, CA); S. aureus lipoteichoic acid (TLR2 ligand) and palmitoyl-2-Cys-Ser-Lys-4 (Pam2CSK4, TLR2/6 ligand) (Invivogen, San Diego, CA); human M-CSF (Leukoprol) (Kyowa Hakko Kirin, Tokyo, Japan); 1α,25(OH)2D3 and prostaglandin E2 (PGE2; Wako, Osaka, Japan); and human RANKL [glutathione S-transferase (GST)-RANKL] (Oriental yeast, Tokyo, Japan). All other reagents were of analytical grade.
Bone marrow macrophages
Bone marrow (BM) cells obtained from tibiae of 7-week-old mice were cultured for 16 hr in α-minimal essential medium (Sigma-Aldrich) containing 10% fetal bovine serum (SAFC Biosciences, Lenexa, KS), 100 U/ml penicillin G, 100 μg/ml streptomycin sulphate and 0·25 μg/ml amphotericin B (Invitrogen, Carlsbad, CA; Antibiotic-Antimycotic), and M-CSF (104 U/ml) in a 6-cm dish. Then, non-adherent haematopoietic cells were harvested. Subsequently, they (2 × 105 cells/well) were cultured in a 48-well plate for 3 days with M-CSF (104 U/ml) and used as bone marrow macrophages (BMMs).
Osteoclast formation assay
Primary osteoblasts were prepared from newborn mouse calvariae. In the co-culture system, osteoblasts (1 × 104 cells/well) were co-cultured with BM cells (2 × 105 cells/well) in a 48-well plate in the presence or absence of 1α,25(OH)2D3 (10−8 m), PGE2 (10−6 m), LPS (50 ng/ml) and flagellin (100 ng/ml). CRAMP (30 μg/ml) was added to some co-cultures. After 7 days in culture, cells were fixed and stained for tartrate-resistant acid phosphatase (TRAP, a marker enzyme of osteoclasts). In the BMM culture system described above, BMMs were further cultured in a 48-well plate with RANKL (200 ng/ml) and M-CSF (104 U/ml). CRAMP (30 μg/ml) was added to some BMM cultures. After 3 days, cells were fixed and stained for TRAP. TRAP-positive [TRAP(+)] multinucleated cells containing three or more nuclei were counted as osteoclasts.
Assessment of osteoclast survival and death
Osteoblasts and BM cells were co-cultured in the presence of 1α,25(OH)2D3 (10−8 m) and PGE2 (10−6 m) in a 10-cm dish pre-coated with type-I collagen gel (Nitta gelatine, Osaka, Japan). After 7 days in culture, all cells were detached from the dish by treatment with 0·2% collagenase (Wako). The suspension containing both osteoclasts and osteoblasts was re-plated in a 24-well plate. After 5 hr, osteoblasts were removed by treatment with 0·05% trypsin and 0·53 mm EDTA. Purified osteoclasts were further cultured in phenol red-free α-minimal essential medium (Wako) with or without RANKL (200 ng/ml) and LPS (50 ng/ml). CRAMP (30 μg/ml) was added to some cultures. After 24 hr, cells were fixed and stained for TRAP. TRAP(+) cells containing three or more nuclei were counted as viable osteoclasts. Meanwhile, the conditioned media were collected and subjected to quantification of lactate dehydrogenase (LDH) release. The LDH contents were measured using a commercially available kit (Wako) and a spectrophotometer (GE Healthcare Life Sciences, Little Chalfont, UK). The LDH contents extracted from purified osteoclasts using a lysis solution (0·2% Tween 20 in culture medium) was regarded as 100% release.
Real-time RT-PCR
Sub-confluent cells were used for real-time RT-PCR. For the RANKL expression assay, osteoblasts in a 12-well plate were cultured for 24 hr with or without LPS (50 ng/ml) and flagellin (100 ng/ml). CRAMP (30 μg/ml) was added to some cultures. For the Camp expression assay, osteoblasts in a 6-cm dish were cultured for 24 hr with or without 1α,25(OH)2D3 (10−8 m), PGE2 (10−6 m), LPS (50 ng/ml), flagellin (100 ng/ml), peptidoglycan (1 μg/ml), lipoteichoic acid (1 μg/ml), Pam2CSK4 (10 ng/ml), and Pam3CSK4 (10 ng/ml). BMMs in a 48-well plate were also cultured for 24 hr with or without 1α,25(OH)2D3 (10−8 m), PGE2 (10−6 m), and LPS (50 ng/ml). CRAMP (30 μg/ml) was added to some cultures. For the FPR2 expression assay, BMMs in a 48-well plate were cultured in the presence of RANKL (200 ng/ml) and M-CSF (104 U/ml), and processed for real-time RT-PCR on days 1, 2 and 3. Sub-confluent osteoblasts were also processed for this assay. Cells were lysed in TRIzol (Invitrogen). First-strand cDNA was synthesized from total RNA with an oligo(dT)12–18 primer (Invitrogen) and ReverTra Ace (ToYoBo, Osaka, Japan). Real-time RT-PCR for the quantification of cDNA was performed using the Fast SYBR Green (Applied Biosystems, Foster City, CA) and StepOnePlus system (Applied Biosystems). The following temperature profile was used: 95° for 20 seconds, followed by 40 cycles of 95° for 3 seconds and 60° for 30 seconds. The TaKaRa Bio probes used in this study were for GAPDH (TaKaRa, Shiga, Japan; MA050371), Camp (MA112322), RANKL (MA030457), and FPR2 (MA078209) of mice. GAPDH was used as an internal control for normalization. Each expression level was calculated using a relative standard curve.
Tumour necrosis factor-α measurements by ELISA
Confluent BMMs in a 96-well plate were cultured for 24 hr with or without LPS (50 ng/ml) and flagellin (100 ng/ml) in the presence of M-CSF (104 U/ml). CRAMP (30 μg/ml) was added to some BMM cultures. RAW 264 cells, a murine monocytic cell line, were obtained from Riken (Saitama, Japan; RCB 0535). Confluent RAW 264 cells in a 96-well plate were treated for 24 hr with flagellin (1 μg/ml) in the presence or absence of CRAMP (30 μg/ml). The conditioned medium was collected for tumour necrosis factor-α (TNF-α) ELISA (Quantikine ELISA kit; R&D Systems, Minneapolis, MN).
Statistics
Statistical analyses were performed using the two-tailed Student's t-test, as appropriate. P < 0·05 was considered statistically significant.
Results
We first examined the effects of CRAMP on osteoclast formation induced by 1α,25(OH)2D3 and PGE2 in co-cultures of osteoblasts and BM cells (Fig. 1a). 1α,25(OH)2D3 and PGE2 stimulated osteoclastogenesis in the co-cultures. CRAMP showed no effects on osteoclastogenesis induced by 1α,25(OH)2D3 and PGE2. Then we examined the effects of CRAMP on osteoclast formation in BMM cultures treated with RANKL and M-CSF (Fig. 1b). Osteoclastogenesis induced by RANKL and M-CSF in BMM cultures was not inhibited by CRAMP.
Figure 1.
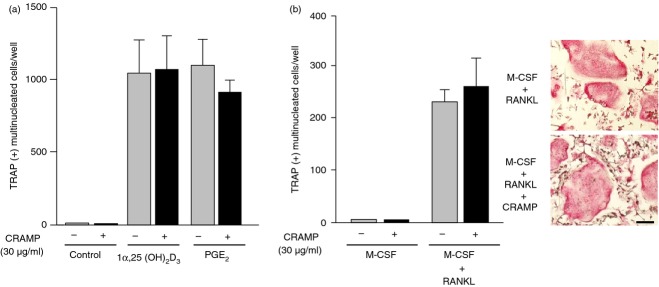
Effect of cathelicidin-related antimicrobial peptide (CRAMP) on osteoclast formation induced by receptor activator of nuclear factor-κB ligand (RANKL). (a) Effects of CRAMP on osteoclast formation induced by 1α,25(OH)2D3 and prostaglandin E2 (PGE2) in co-cultures. Osteoblasts and bone marrow (BM) cells were co-cultured with or without 1α,25(OH)2D3 (10−8 m) and PGE2 (10−6 m). CRAMP (30 μg/ml) was added to some co-cultures. After 7 days, the number of tartrate-resistant acid phosphatase-positive [TRAP(+)] multinucleated cells was counted. (b) Effects of CRAMP on osteoclast formation induced by RANKL in BM macrophage (BMM) cultures. BMMs were cultured with macrophage colony-stimulating factor (M-CSF; 104 U/ml) or M-CSF plus RANKL (200 ng/ml). CRAMP (30 μg/ml) was added to some BMM cultures. After 6 days, cells were fixed and stained for TRAP (right). The number of TRAP(+) multinucleated cells was counted (left). Data are expressed as the mean ± SD (n = 4). Bar, 100 μm.
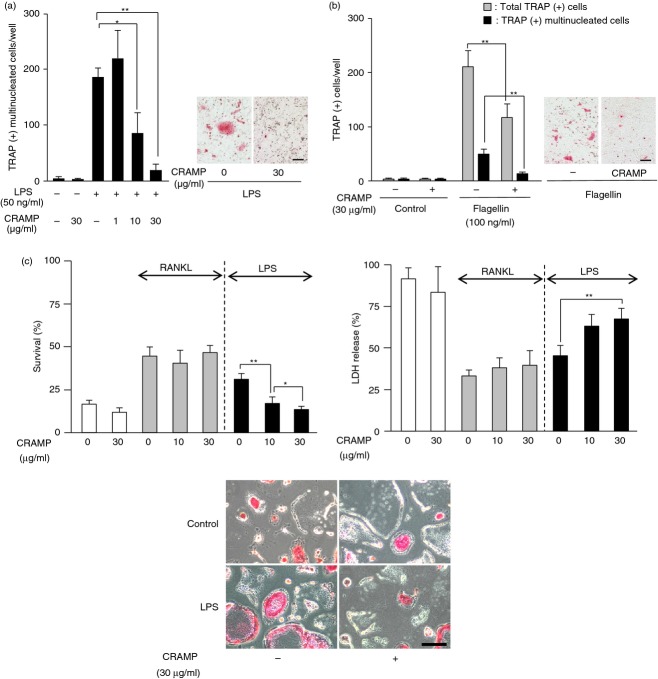
CRAMP is reported to bind to LPS (TLR4 ligand) and neutralizes its activity.24 We next examined the effects of CRAMP on LPS-induced osteoclastogenesis in the co-cultures (Fig. 2a). LPS stimulated the formation of osteoclasts. The LPS-induced osteoclastogenesis was dose-dependently suppressed by CRAMP. We also examined the effects of CRAMP on osteoclastogenesis induced by other TLR ligands. We found that CRAMP suppressed flagellin (TLR5 ligand) -induced osteoclastogenesis in the co-cultures (Fig. 2b). Flagellin modestly stimulated osteoclast formation. Therefore, in addition to TRAP(+) multinucleated cells, the number of total TRAP(+) cells including mononuclear and binuclear cells was also counted in this experiment (Fig. 2b). Other TLR ligands, such as peptidoglycan (TLR2 ligand), lipoteichoic acid (TLR2 ligand), Pam2CSK4 (TLR2/6 ligand) and Pam3CSK4 (TLR1/2 ligand), also stimulated osteoclastogenesis in the co-cultures, which is consistent with previous reports.25–27 However, CRAMP showed no inhibitory effects on the osteoclastogenesis induced by those TLR ligands (data not shown).
Figure 2.
Effects of cathelicidin-related antimicrobial peptide (CRAMP) on osteoclast formation promoted by lipopolysaccharide (LPS) and flagellin. (a) Effects of CRAMP on LPS-induced osteoclast formation in co-cultures. Osteoblasts and bone marrow (BM) cells were co-cultured in the presence or absence of LPS (50 ng/ml) with increasing concentrations of CRAMP (1, 10 and 30 μg/ml). After 7 days, cells were fixed and stained for tartrate-resistant acid phosphatase (TRAP) (right). The number of TRAP(+) multinucleated cells was counted (left). (b) Effects of CRAMP on flagellin-induced osteoclast formation in co-cultures. Osteoblasts and BM cells were co-cultured in the presence or absence of flagellin (100 ng/ml). CRAMP (30 μg/ml) was added to some co-cultures. After 7 days, cells were fixed and stained for TRAP (right). The numbers of TRAP(+) multinucleated cells and of total TRAP(+) cells including mononuclear and binuclear cells were counted (left). (c) Effects of CRAMP on the survival of osteoclasts. Purified osteoclasts (728 ± 75 cells/well) were cultured in the presence or absence of receptor activator of nuclear factor-κB ligand (RANKL) (200 ng/ml) or LPS (50 ng/ml). CRAMP (10 and 30 μg/ml) was added to some osteoclast cultures. After 24 hr, cells were fixed and stained for TRAP (lower panels). The number of TRAP(+) multinucleated cells was counted (upper left). The conditioned media were collected to measure lactate dehydrogenase (LDH) activity (upper right). Data are expressed as the mean ± SD (n = 4 to n = 6). *P < 0·05; **P < 0·01. Bar, 100 μm.
We reported that LPS as well as RANKL promoted the survival of osteoclasts.23 Purified osteoclasts in culture disappeared spontaneously within 48 hr (Fig. 2c). The survival (%) of osteoclasts was increased by RANKL and LPS. CRAMP reduced the survival (%) of osteoclasts promoted by LPS, but not by RANKL (Fig. 2c, upper left). LDH is released into the culture medium from cells following either apoptosis or necrosis. The LDH release (%) was decreased by RANKL and LPS. CRAMP reversed the reduction of the LDH release by LPS but not RANKL in a dose-dependent manner (Fig. 2c, upper right).
Next, we examined the effects of CRAMP on RANKL and Camp mRNA expression in osteoblasts and TNF-α secretion in BMMs treated with LPS and flagellin (Fig. 3). CRAMP is encoded by the Camp gene. Both LPS and flagellin stimulated RANKL and Camp mRNA expression in osteoblasts in culture (Fig. 3a,b). CRAMP suppressed the LPS- and flagellin-induced expression of RANKL and Camp mRNA in osteoblasts. LPS stimulated TNF-α secretion in BMMs in culture, which was also suppressed by CRAMP (Fig. 3c). Flagellin did not promote the secretion of TNF-α in BMMs, probably because of the low level of TLR5 expression in BMMs (Fig. 3c). We found that RAW 264 cells secreted TNF-α in response to flagellin (Fig. 3d). Flagellin-induced TNF-α secretion by RAW 264 cells was suppressed by CRAMP.
Figure 3.
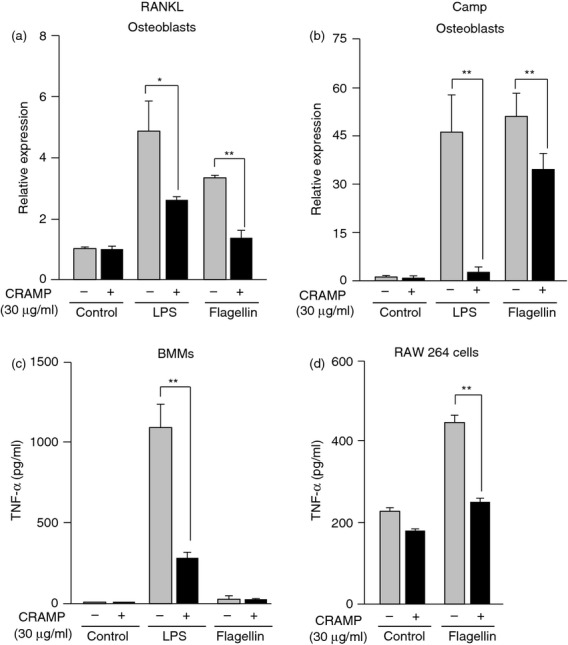
Effects of cathelicidin-related antimicrobial peptide (CRAMP) on receptor activator of nuclear factor-κB ligand (RANKL) mRNA expression in osteoblasts and tumour necrosis factor-α (TNF-α) secretion in bone marrow macrophages (BMMs). (a) Effects of CRAMP on RANKL mRNA expression in osteoblasts. Osteoblasts were cultured with or without lipopolysaccharide (LPS; 50 ng/ml) and flagellin (100 ng/ml). CRAMP (30 μg/ml) was added to some cultures. After 24 hr, cells were processed for real-time RT-PCR. (b) Effects of CRAMP on cathelicidin antimicrobial peptide (Camp) mRNA expression in osteoblasts. Osteoblasts were cultured with or without LPS (50 ng/ml) and flagellin (100 ng/ml). CRAMP (30 μg/ml) was added to some cultures. After 24 hr, cells were processed for real-time RT-PCR. (c) Effects of CRAMP on TNF-α secretion by BMMs. The BMMs were cultured with or without LPS (50 ng/ml) and flagellin (100 ng/ml). CRAMP (30 μg/ml) was added to some cultures. After 24 hr, the conditioned medium was collected for ELISA. (d) Effects of CRAMP on TNF-α secretion by RAW 264 cells. RAW 264 cells were cultured with or without flagellin (1 μg/ml). CRAMP (30 μg/ml) was added to some cultures. After 24 hr, the conditioned medium was collected for ELISA. Data are expressed as the mean ± SD (n = 3, or n = 4). *P < 0·05; **P < 0·01.
We next examined the expression of mRNA for CRAMP and its receptor FPR2 in osteoblasts and BMMs (Fig. 4). The level of Camp mRNA was higher in BMMs than osteoblasts (Fig. 4a). Neither 1α,25(OH)2D3 nor PGE2 enhanced the expression in BMMs or osteoblasts. Interestingly, LPS greatly enhanced Camp expression in osteoblasts but not in BMMs (Fig. 4a). Other TLR ligands, such as flagellin, peptidoglycan, lipoteichoic acid, Pam2CSK4 and Pam3CSK4, also enhanced the expression in osteoblasts (Fig. 4b). We finally examined the expression of FPR2 mRNA in BMMs and osteoblasts (Fig. 4c). FPR2 mRNA was highly expressed by BMMs but not in osteoblasts. When BMMs were treated with RANKL in the presence of M-CSF, osteoclasts appeared on day three (Fig. 4c, inset). FPR2 mRNA expression in BMMs decreased sharply during their differentiation into osteoclasts.
Figure 4.
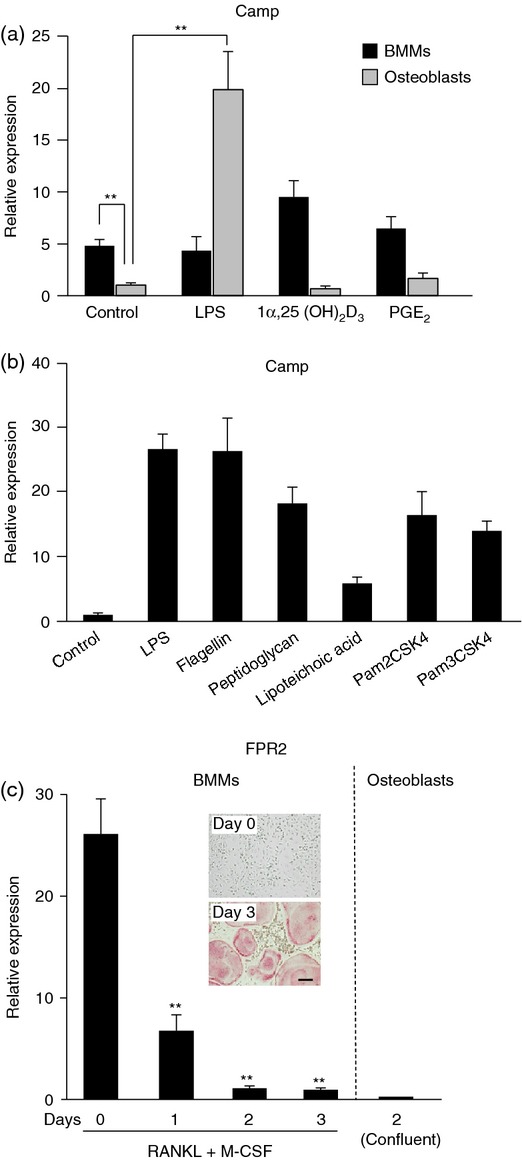
Expression of mRNAs for cathelicidin antimicrobial peptide (Camp) and its receptor formyl peptide receptor 2 (FPR2) in osteoblasts and bone marrow macrophages (BMMs). (a) Effects of lipopolysaccharide (LPS), 1α,25(OH)2D3, and prostaglandin E2 (PGE2) on Camp mRNA expression in BMMs and osteoblasts. BMMs and osteoblasts were treated with LPS (50 ng/ml), 1α,25(OH)2D3 (10−8 m), or PGE2 (10−6 m) for 24 hr. Cells were then processed for real-time RT-PCR. Data are expressed as the mean ± SD (n = 4). **P < 0·01. (b) Effects of Toll-like receptor (TLR) ligands on Camp mRNA expression in osteoblasts. Osteoblasts were treated with LPS (50 ng/ml), flagellin (100 ng/ml), peptidoglycan (1 μg/ml), lipoteichoic acid (1 μg/ml), Pam2CSK4 (10 ng/ml), or Pam3CSK4 (10 ng/ml) for 24 hr. Cells were then processed for real-time RT-PCR. Data are expressed as the mean ± SD (n = 4). (c) Expression of FPR2 mRNA in BMMs (left) and osteoblasts (right). BMMs were treated with macrophage colony-stimulating factor (M-CSF; 104 U/ml) and receptor activator of nuclear factor-κB ligand (RANKL; 200 ng/ml) for the indicated time periods, and then processed for real-time RT-PCR. Osteoblasts were also processed for real-time RT-PCR. Data are expressed as the mean ± SD (n = 4). **P < 0·01, compared with Day 0.
Discussion
CRAMP failed to inhibit RANKL-induced osteoclastogenesis in murine BMM cultures. However, it suppressed osteoclastogenesis induced by LPS and flagellin in co-cultures with calvarial osteoblasts. CRAMP inhibited RANKL expression in osteoblasts treated with LPS and flagellin but not 1α,25(OH)2D3 or PGE2.
Is there cross-talk among the downstream cascades of CRAMP, 1α,25(OH)2D3 and LPS/flagellin for RANKL expression in osteoblasts? 1α,25(OH)2D3 activates RANKL gene expression through the vitamin D receptor.28 On the other hand, LPS induces RANKL expression through the adaptor molecule, MyD88.23 1α,25(OH)2D3 but not LPS induced RANKL expression in MyD88-deficient osteoblasts.23 Our experiment showed that CRAMP failed to affect osteoclast formation in co-culture treated with 1α,25(OH)2D3. These results suggest that intracellular signalling cascades induced by CRAMP, 1α,25(OH)2D3 and LPS/flagellin in osteoblasts may be independent of each other for RANKL induction.
Lipopolysaccharide and flagellin bind to TLRs in a manner different from that of other TLR ligands. The binding of LPS to TLR4 involves two interactions: hydrophobic interaction between fatty acid chain R2 of LPS and phenylalanine and leucine residues of TLR4, and hydrophilic interaction between negatively charged phosphate groups of LPS and lysine and arginine residues of TLR4.29 Similarly, the binding of flagellin to TLR5 requires hydrophilic interaction between the negatively charged domain of flagellin and lysine and arginine residues of TLR5.30 In contrast, lipoteichoic acid, Pam2CSK4 and Pam3CSK4 bind to their TLRs through hydrophobic interaction between lipid moieties of the ligands and hydrophobic amino acids of TLRs.31 Therefore, it is suggested that the positively charged portion of CRAMP binds to the negatively charged portion of LPS and flagellin, and inhibits the interaction of LPS and flagellin with their TLRs.
Although Camp mRNA was expressed at higher levels in BMMs than in osteoblasts, its expression was greatly enhanced in osteoblasts but not BMMs in response to LPS. Neither 1α,25(OH)2D3 nor PGE2 had a stimulatory effect on Camp expression. Other TLR ligands also induced the expression of Camp mRNA in osteoblasts. These results suggest that osteoblasts as well as epithelial cells and neutrophils play a part in defence against bacterial infections through Camp expression. In addition, we found that extracellularly added CRAMP suppressed the expression of Camp mRNA induced by LPS and flagellin. These results suggest that the transcription of the Camp gene promoted by LPS and flagellin is inhibited by CRAMP released by the target cells through a feedback regulation.
BMMs but not osteoblasts strongly expressed the mRNA of FPR2, a receptor of CRAMP. Nevertheless, CRAMP showed no effect on RANKL-induced osteoclastogenesis from BMMs. BMM-derived osteoclasts failed to express FPR2. CRAMP is shown to activate extracellular signal-regulated kinase (ERK) through FPR2.13 We found that CRAMP modestly increased ERK phosphorylation in BMMs (see Supplementary material, Fig. S1). Therefore, CRAMP failed to affect the proliferation of BMMs and their differentiation into osteoclasts probably because of the modest activation of ERK. CRAMP itself had no effect on the survival of osteoclasts, but inhibited the pro-survival effect of LPS. These results suggest that CRAMP itself has no effect on the osteoclastogenesis induced by RANKL in mice but suppresses that induced by LPS and flagellin through the neutralization of LPS and flagellin.
Recently, Supanchart al.32 reported that LL-37 suppressed RANKL-induced osteoclastogenesis in human peripheral blood mononuclear cell (PBMC) cultures. CRAMP and LL-37 share 47% identity and 67% similarity in their amino acid sequences. Therefore, these observations raised the question of whether LL-37 and CRAMP have similar activities or have distinct activities, which might account for the different results obtained by us and by Supanchart et al.32 A candidate target for different actions of CRAMP/LL-37 was the P2X7 purinergic receptor. LL-37 enhanced ATP-induced Ca2+ influx mediated by the human P2X7 receptor,33 while CRAMP inhibited it mediated by the mouse P2X7 receptor.34 Therefore, we examined the effects of CRAMP and LL-37 on human and mouse osteoclastogenesis (see Supplementary material, Fig. S2). CRAMP as well as LL-37 suppressed osteoclastogenesis in human PBMC cultures but not in mouse BMM cultures (Fig. S2). In addition, Supanchart et al.32 showed that the neutralizing antibody against the human P2X7 receptor failed to reverse the effect of LL-37 in human PBMC cultures. These results suggest that the P2X7 receptor-mediated signalling is not involved in osteoclastogenesis in humans and mice. The neutralization of LPS and flagellin by LL-37/CRAMP would work commonly in mice and humans. These results suggest that LL-37/CRAMP produced by osteoblasts in response to LPS acts as a protector of bone resorption induced by bacterial infection.
Acknowledgments
This work was supported by Grants-in-aid from the Japan Society for the Promotion of Science [23792135 (YN), 24593112 (MN), 24659833 (NT) and 24390417 (NU)].
Glossary
- 1α,25(OH)2D3
1α,25-dihydroxyvitamin D3
- BM
bone marrow
- BMM
bone marrow macrophage
- Camp
cathelicidin antimicrobial peptide
- CRAMP
cathelicidin-related antimicrobial peptide
- ERK
extracellular signal-regulated kinase
- FPR2
formyl peptide receptor 2
- LDH
lactate dehydrogenase
- LPS
lipopolysaccharide
- M-CSF
macrophage colony-stimulating factor
- Pam2CSK4
palmitoyl-2-Cys-Ser-Lys-4
- Pam3CSK4
palmitoyl-3-Cys-Ser-Lys-4
- PBMC
peripheral blood mononuclear cell
- PGE2
prostaglandin E2
- RANKL
receptor activator of nuclear factor-κB ligand
- TNF
tumour necrosis factor
- TLR
Toll-like receptor
- TRAP
tartrate-resistant acid phosphatase
Disclosures
The authors declare no potential conflicts of interest with respect to the authorship and publication of this article.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Effects of cathelicidin-related antimicrobial peptide (CRAMP) on extracellular signal-regulated kinase (ERK) phosphorylation in mouse bone marrow macrophages (BMMs).
Figure S2. Effects of cathelicidin-related antimicrobial peptide (CRAMP) and LL-37 on human and mouse osteoclast formation induced by receptor activator of nuclear factor-κB ligand (RANKL).
References
- 1.Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11:519–31. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 2.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–84. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 3.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–95. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 4.Tomasinsig L, Zanetti M. The cathelicidins – structure, function and evolution. Curr Protein Pept Sci. 2005;6:23–34. doi: 10.2174/1389203053027520. [DOI] [PubMed] [Google Scholar]
- 5.Hancock RE, Diamond G. The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol. 2000;8:402–10. doi: 10.1016/s0966-842x(00)01823-0. [DOI] [PubMed] [Google Scholar]
- 6.Soehnlein O, Wantha S, Simsekyilmaz S, et al. Neutrophil-derived cathelicidin protects from neointimal hyperplasia. Sci Transl Med. 2011;3:103ra98. doi: 10.1126/scitranslmed.3002531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chromek M, Slamová Z, Bergman P, et al. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat Med. 2006;12:636–41. doi: 10.1038/nm1407. [DOI] [PubMed] [Google Scholar]
- 8.Rosenberger CM, Gallo RL, Finlay BB. Interplay between antibacterial effectors: a macrophage antimicrobial peptide impairs intracellular Salmonella replication. Proc Natl Acad Sci USA. 2004;101:2422–7. doi: 10.1073/pnas.0304455101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallo RL, Kim KJ, Bernfield M, Kozak CA, Zanetti M, Merluzzi L, Gennaro R. Identification of CRAMP, a cathelin-related antimicrobial peptide expressed in the embryonic and adult mouse. J Biol Chem. 1997;272:13088–93. doi: 10.1074/jbc.272.20.13088. [DOI] [PubMed] [Google Scholar]
- 10.Kovach MA, Ballinger MN, Newstead MW, et al. Cathelicidin-related antimicrobial peptide is required for effective lung mucosal immunity in Gram-negative bacterial pneumonia. J Immunol. 2012;189:304–11. doi: 10.4049/jimmunol.1103196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nizet V, Ohtake T, Lauth X, et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454–7. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- 12.Yang D, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, Oppenheim JJ, Chertov O. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192:1069–74. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurosaka K, Chen Q, Yarovinsky F, Oppenheim JJ, Yang D. Mouse cathelin-related antimicrobial peptide chemoattracts leukocytes using formyl peptide receptor-like 1/mouse formyl peptide receptor-like 2 as the receptor and acts as an immune adjuvant. J Immunol. 2005;174:6257–65. doi: 10.4049/jimmunol.174.10.6257. [DOI] [PubMed] [Google Scholar]
- 14.Koczulla R, von Degenfeld G, Kupatt C, et al. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest. 2003;111:1665–72. doi: 10.1172/JCI17545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murakami M, Ohtake T, Dorschner RA, Gallo RL. Cathelicidin antimicrobial peptides are expressed in salivary glands and saliva. J Dent Res. 2002;81:845–50. doi: 10.1177/154405910208101210. [DOI] [PubMed] [Google Scholar]
- 16.Pütsep K, Carlsson G, Boman HG, Andersson M. Deficiency of antibacterial peptides in patients with morbus Kostmann: an observation study. Lancet. 2002;360:1144–9. doi: 10.1016/S0140-6736(02)11201-3. [DOI] [PubMed] [Google Scholar]
- 17.Carlsson G, Wahlin YB, Johansson A, Olsson A, Eriksson T, Claesson R, Hänström L, Henter JI. Periodontal disease in patients from the original Kostmann family with severe congenital neutropenia. J Periodontol. 2006;77:744–51. doi: 10.1902/jop.2006.050191. [DOI] [PubMed] [Google Scholar]
- 18.Puklo M, Guentsch A, Hiemstra PS, Eick S, Potempa J. Analysis of neutrophil-derived antimicrobial peptides in gingival crevicular fluid suggests importance of cathelicidin LL-37 in the innate immune response against periodontogenic bacteria. Oral Microbiol Immunol. 2008;23:328–35. doi: 10.1111/j.1399-302X.2008.00433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Udagawa N, Takahashi N, Akatsu T, et al. Origin of osteoclasts: mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. Proc Natl Acad Sci USA. 1990;87:7260–4. doi: 10.1073/pnas.87.18.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahashi N, Akatsu T, Udagawa N, Sasaki T, Yamaguchi A, Moseley JM, Martin TJ, Suda T. Osteoblastic cells are involved in osteoclast formation. Endocrinology. 1988;123:2600–2. doi: 10.1210/endo-123-5-2600. [DOI] [PubMed] [Google Scholar]
- 21.Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev. 1999;20:345–57. doi: 10.1210/edrv.20.3.0367. [DOI] [PubMed] [Google Scholar]
- 22.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–42. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 23.Sato N, Takahashi N, Suda K, et al. MyD88 but not TRIF is essential for osteoclastogenesis induced by lipopolysaccharide, diacyl lipopeptide, and IL-1α. J Exp Med. 2004;200:601–11. doi: 10.1084/jem.20040689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagaoka I, Hirota S, Niyonsaba F, Hirata M, Adachi Y, Tamura H, Heumann D. Cathelicidin family of antibacterial peptides CAP18 and CAP11 inhibit the expression of TNF-α by blocking the binding of LPS to CD14+ cells. J Immunol. 2001;167:3329–38. doi: 10.4049/jimmunol.167.6.3329. [DOI] [PubMed] [Google Scholar]
- 25.Ha H, Lee JH, Kim HN, et al. Stimulation by TLR5 modulates osteoclast differentiation through STAT1/IFN-β. J Immunol. 2008;180:1382–9. doi: 10.4049/jimmunol.180.3.1382. [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto C, Oda T, Yokoyama S, Tominari T, Hirata M, Miyaura C, Inada M. Toll-like receptor 2 heterodimers, TLR2/6 and TLR2/1 induce prostaglandin E production by osteoblasts, osteoclast formation and inflammatory periodontitis. Biochem Biophys Res Commun. 2012;428:110–5. doi: 10.1016/j.bbrc.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 27.Sato T, Watanabe K, Kumada H, Toyama T, Tani-Ishii N, Hamada N. Peptidoglycan of Actinomyces naeslundii induces inflammatory cytokine production and stimulates osteoclastogenesis in alveolar bone resorption. Arch Oral Biol. 2012;57:1522–8. doi: 10.1016/j.archoralbio.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 28.Pike JW, Meyer MB, Martowicz ML. New techniques in transcription research extend our understanding of the molecular actions of the vitamin D hormone. IBMS Bonekey. 2009;6:169–80. [Google Scholar]
- 29.Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature. 2009;458:1191–5. doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]
- 30.Yoon SI, Kurnasov O, Natarajan V, Hong M, Gudkov AV, Osterman AL, Wilson IA. Structural basis of TLR5-flagellin recognition and signaling. Science. 2012;335:859–64. doi: 10.1126/science.1215584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang JY, Nan X, Jin MS, et al. Recognition of lipopeptide patterns by Toll-like receptor 2-Toll-like receptor 6 heterodimer. Immunity. 2009;31:873–84. doi: 10.1016/j.immuni.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 32.Supanchart C, Thawanaphong S, Makeudom A, Bolscher JG, Nazmi K, Kornak U, Krisanaprakornkit S. The antimicrobial peptide, LL-37, inhibits in vitro osteoclastogenesis. J Dent Res. 2012;91:1071–7. doi: 10.1177/0022034512460402. [DOI] [PubMed] [Google Scholar]
- 33.Tomasinsig L, Pizzirani C, Skerlavaj B, et al. The human cathelicidin LL-37 modulates the activities of the P2X7 receptor in a structure-dependent manner. J Biol Chem. 2008;283:30471–81. doi: 10.1074/jbc.M802185200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seil M, Kabre E, Nagant C, Vandenbranden M, Fontanils U, Marino A, Pochet S, Dehaye JP. Regulation by CRAMP of the responses of murine peritoneal macrophages to extracellular ATP. Biochim Biophys Acta. 2010;1798:569–78. doi: 10.1016/j.bbamem.2009.11.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.