Abstract
Background
It is unclear if new co-stimulatory blockade agents, such as the CTLA-4 Ig molecule belatacept, promote or inhibit the potential for immunological tolerance in transplantation. We therefore tested the in vitro effects of BEL on human Tregs in mixed lymphocyte reactions (Treg-MLR), alone and in combination with maintenance agents used in transplant recipients.
Methods
Belatacept, mycophenolic acid (MPA) and sirolimus (SRL), either singly or in combination, were added to healthy volunteer Treg-MLR, testing:(a) 3H-TdR incorporation for inhibition of lymphoproliferation;(b) flow cytometry to analyze for newly generated CD4+CD25HighFOXP3+Tregs in CFSE labeled MLR responders. In addition, the modulatory effects of putative Tregs generated in presence of these drugs were also tested using the lymphoproliferation and flow cytometric assays.
Results
In comparison to medium controls, BEL dose-dependently inhibited both lymphoproliferation and Treg generation in HLA 2-DR matched and mismatched MLRs, either alone or in combination with MPA or SRL. However, MPA alone inhibited lymphoproliferation but significantly enhanced Treg generation at sub-therapeutic concentrations (p<0.01). In addition, purified CD4+CD127− cells generated in MLR in the presence of MPA and added as third component modulators in fresh MLRs significantly enhanced newly developed Tregs in the proliferating responder cells, compared to those generated with BEL or medium controls.
Conclusions
Belatacept alone and in combination with agents used in transplant recipients inhibits the in vitro generation of human Tregs. Belatacept might therefore be a less optimal agent for tolerance induction in human organ transplantation.
Keywords: Mixed lymphocyte reaction, Regulatory T cells, FOXP3, Belatacept, mTOR inhibition
INTRODUCTION
Lifelong immunosuppressive (IS) therapy is required in the vast majority of organ transplantation recipients. The most common agents utilized are the calcineurin inhibitors (CNI), tacrolimus (TAC) and cyclosporine, although these drugs have a significant potential for long term toxicity. Alternative oral agents, such as mycophenolic acid (MPA) and mTOR inhibitors (sirolimus, everolimus), are available to reduce CNI toxicities, although they are less optimal in preventing rejection without concurrent CNI therapy. Thus far a small percentage of recipients are completely withdrawn from CNIs because of this risk of rejection.
Belatacept (BEL) is a second-generation cytotoxic T-lymphocyte-associated antigen-4-Ig (CLTA-4 Ig) fusion protein and the first co-stimulatory blockade agent approved clinically to prevent organ transplant rejection (renal only)(1). Inhibition of the B7-CD28 co-stimulatory pathway by this new molecular construct does not appear to be associated with several side effects of CNI therapy agents, such as nephrotoxicity. However, higher rates of rejection have been seen in organ transplant recipients given BEL in a non-CNI regimen, and complications have been reported (i.e. lymphoma) (2, 3). Related to this might be the absence of an “immunoregulatory profile” involving regulatory T and dendritic cells (Tregs, DCregs), as assessed by ex vivo immunophenotyping and functional assays (4). Previous animal studies have demonstrated some differences in specific IS drugs in the promotion of regulatory cells. Calcineurin-inhibitors block T cell receptor (TCR) pathways and inhibit the expression of FOXP3, an intracellular transcription factor produced by Tregs (5–9). Anti-proliferative agents (i.e. MPA, mTOR inhibitors) and possibly co-stimulatory antagonists (i.e. BEL) do not specifically block the TCR pathway and thus might catalyze the generation of Tregs and DCregs (10–16). Alternatively, given the higher rates of rejection, BEL may inhibit the generation of protective allo-specific regulatory cells(17–19).
As the vast majority of work on the regulatory effects of co-stimulatory blockade agents has been in animal studies(17, 19), it is not clearly understood if BEL alone or in combination with other agents used with BEL in transplant recipients (MPA, SRL) impact human regulatory T cell generation in vitro or in vivo. Therefore, utilizing our in vitro human Treg-MLR assay (4, 7, 9), this study aims to clarify the regulatory properties of BEL ± MPA or SRL, analogous to IS regimens given to organ transplant recipients. Understanding these effects might be translated clinically into better understanding of which agents may or may not promote immunoregulation allowing for minimization or withdrawal of immunosuppression (tolerance), perhaps even in the absence of in vivo studies.
RESULTS
Direct effect of belatacept in inhibiting both lymphoproliferation and phenotypic Treg generation in MLR
Increasing concentrations of BEL (0 and 39–10,000 ng/mL), corresponding to doses ranging from above through therapeutic to sub-therapeutic levels during the maintenance phase (based on information provided by the drug manufacturer), were tested in MLRs using PBMC of healthy volunteers. Figure 1 shows the gating strategy used for the analyses, and Figure 2A demonstrates a dose-dependent inhibition in lymphoproliferation as measured by SI (top) and as contrasted against media controls (100%; bottom; p<0.05, n=4). Consistent with our previous observations(4), between 15–50% of CD127−CD25+CD4+ cells (thereby excluding the T effector cells) were found to express FOXP3 in MLR medium controls, depending on HLA mismatch and individual variation. BEL had a dose-dependent generalized inhibition of regulatory T cell generation in MLR (Fig. 2B and C; p<0.05). Similarly, the generation of CD4+CD127−CD25HighFOXP3+ natural Tregs was also inhibited by BEL (C). These findings were more pronounced in the DR-identical experiments as previously described (4).
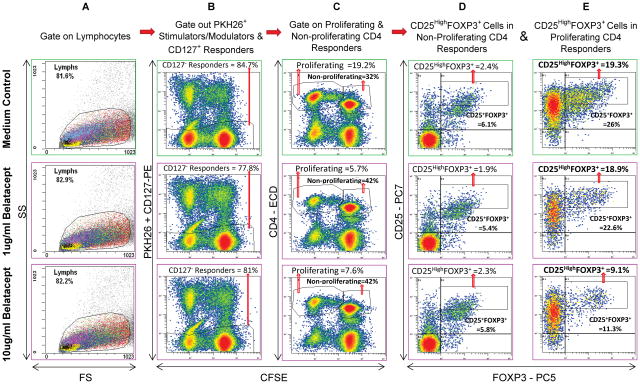
Figure 1. Scheme of flow analysis (representative 7-day experiment shown).
5x105 CFSE labeled responding PBMC from healthy volunteer A were cultured with 5x105 PKH26 labeled irradiated stimulator cells from laboratory volunteer B in the absence or presence of indicated concentrations of BEL. After 7 days, flow cytometric analyses were performed using monoclonal antibodies CD127-PE, CD4-ECD, CD25-PC7 and FOXP3-PC5. Viable lymphocytes were gated (column A) followed by CFSE bright and dim cells which were negative for either CD127-PE or PKH26 (column B), thus gating out CD127+ responders and any residual stimulators. This was followed by gating for CD4+ cells that were either non-proliferating (CFSE high) or proliferating (CFSE low) (column C). The cells in the non-proliferating (Column D) and proliferating (Column E) populations were analyzed by dot plots for CD25+ and FOXP3+ cells (among other subsets; not shown). Please note that when compared to the medium control (top row), fewer CD4+ cells proliferated in presence of BEL (column C). Additionally, there was a dose dependent reduction in the proportion of both total CD25+FOXP3+ Tregs and CD25highFOXP3+Tregs in the proliferating CD4+CD127− responder cells (column E). Since only the CFSE diluted proliferating fraction (as opposed to non-proliferating fraction; column D) demonstrated differences under various culture conditions, the results from only these are shown in subsequent experiments.
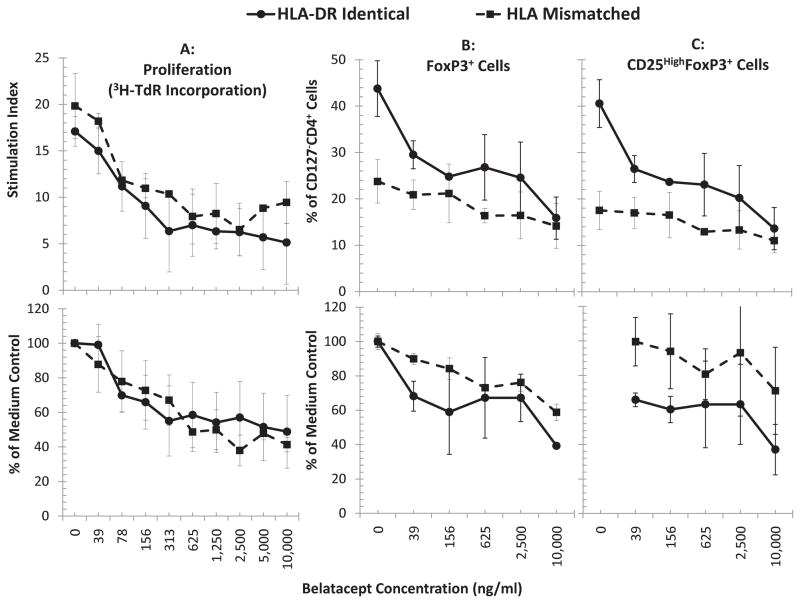
Figure 2. Effect of Belatacept on lymphoproliferation and Treg expansion in MLR (n=4):(B and C).
5x105 CFSE labeled responding PBMC from healthy volunteer A were cultured with 5x105 PKH26 labeled x-irradiated stimulator cells from HLA-DR identical or HLA-mismatched healthy volunteers (Bx or Ix respectively) in the presence of the indicated concentrations of BEL. On days 5, 7 and 9 flow cytometric analysis using the scheme shown in Figure 1 was performed and the percentage of CD4+CD127−FOXP3+ cells (total Tregs; B) or CD4+CD127−CD25HighFOXP3+ cells (natural Tregs; C) were quantitated. (A) In parallel, standard 3H thymidine incorporation assays were performed with 1x105 PBMC each of the responders and stimulators in presence of indicated concentrations of BEL. The results from peak responses on day 7 are depicted. BEL inhibited the proliferation in 3H-thymidine incorporation assay (A); similarly BEL inhibited the generation of CD4+CD127−FOXP3+ total-Tregs (B) and CD4+CD127−CD25highFOXP3+ natural-Tregs (C) in a dose dependent manner (p<0.05, n=12). The data are shown as raw values (top row); and to minimize the variations between experiments, the data were also calculated as percentage of medium control (bottom row). In subsequent figures the data are shown as percentage of medium controls. Also, since similar profile was obtained for both total and natural Tregs, the data for only natural-Tregs are shown subsequently.
Effects of Belatacept on MLRs in the presence of Mycophenolic Acid (MPA)
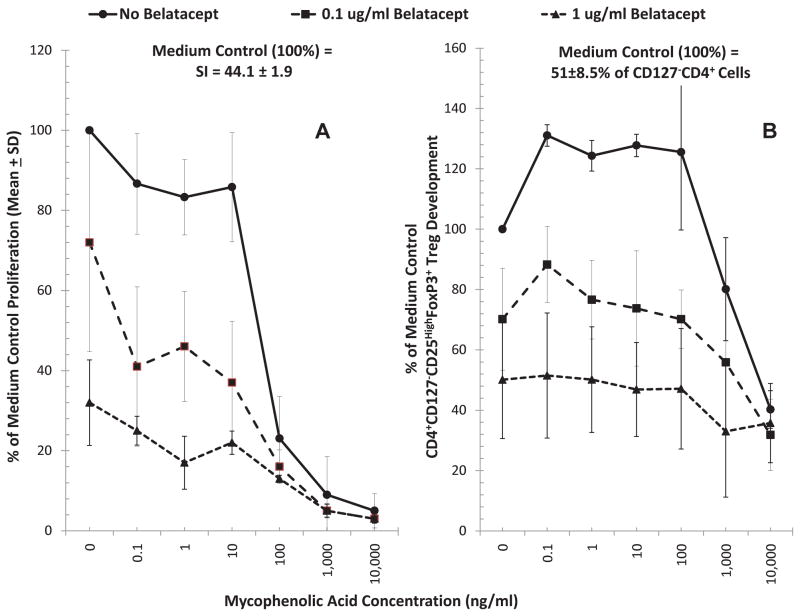
Since clinical BEL administration (at monthly intervals and hence with possible prolonged pharmacokinetic decay) is accompanied by the use of maintenance mycophenolate mofetil (MMF), we tested BEL in two concentrations (0.1 and 1μg/ml) in combination with various concentrations of mycophenolic acid (MPA), the active metabolite of MMF. As shown in Figure 3A, MPA by itself inhibited lymphoproliferation in MLR in a dose dependent manner (top blue line with no BEL). Similarly, BEL by itself inhibited proliferation (0 MPA concentration points in Figure 3A). The combinations of the two had additive or even synergistic inhibitory effect on allogeneic lymphoproliferation. The effect of these two agents on Treg generation in MLR, however, was different(Figure 3B). MPA at lower doses (0.1–100ng/ml) had a significant enhancing effect (p<0.05), although at higher doses it inhibited new generation of Tregs (top blue lines with no BEL). In contrast, BEL inhibited the generation of these subpopulations at all concentrations, either with or without MPA (Fig 3B; 2 lower lines). Thus, MPA failed to reverse the inhibition of Treg generation mediated by BEL in this Treg-MLR assay. These results are consistent with the notion that the total effect of BEL plus MPA at therapeutic concentrations negatively impacts immunoregulation.
Figure 3. Effect of Belatacept and Mycophenolic Acid combinations on lymphoproliferation and Treg generation in MLR.
Since BEL is used clinically in combination with MPA (the in vivo active form of MMF), MLRs were also performed in presence of combinations of these drugs. Both flow 3H-TdR uptake assays and flow cytometric analysis were performed, the latter as described in Figures 1 and 2, with serial dilutions of MPA as indicated on the horizontal axis and with 0.1μg/ml or 1μg/ml concentrations of BEL. (n=3). MPA increased the generation of Tregs at sub-therapeutic concentrations, while inhibiting lymphoproliferation (top blue line with no BEL) (p<0.05). BEL, however, not only inhibited lymphoproliferation and Treg generation (points at 0 MPA) as described in Figure 2, but also abrogated the enhancement of Treg generation observed at low concentrations of MPA.
Effects of Belatacept on MLRs in the presence of Sirolimus (SRL)
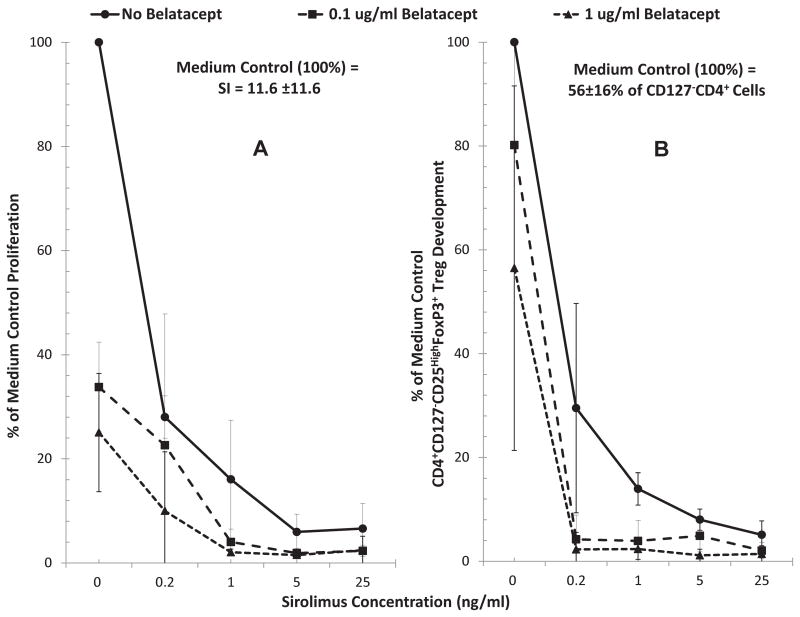
Similar assays were performed with combinations of BEL ± SRL. Both BEL and SRL inhibited lymphoproliferation (Figure 4A)and Treg generation (Figure 4B), and the combination of the two was even more significant at both BEL concentrations (0.1 and 1 ug/ml). This indicates that SRL, similar to MPA, cannot abrogate the lymphoproliferation and Treg inhibition effect of BEL.
Figure 4. Effect of Belatacept and Sirolimus combinations on lymphoproliferation and Treg generation in MLR.
Since another drug that can potentially be used with BEL is SRL (with or without MMF), MLRs were also performed in presence of combinations of these drugs. Both flow 3H-TdR uptake assays and flow cytometric analysis were performed, the latter as described in Figures 1 and 2, with serial dilutions of SRL as indicated on the horizontal axis and with 0.1μg/ml or 1μg/ml concentrations of BEL (n=3). Both BEL SRL inhibited lymphoproliferation and Treg generation and the combination of the two had summative effect (p<0.05).
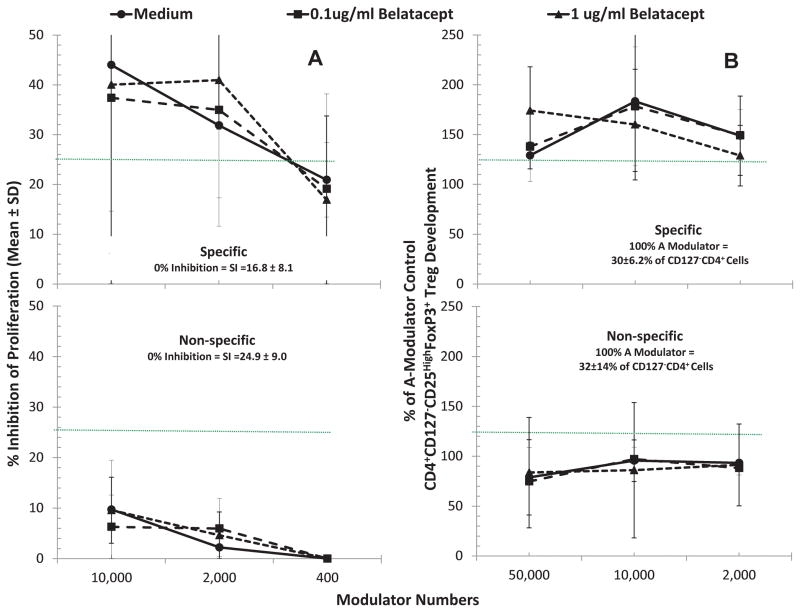
CD127−CD4+ cells derived from bulk MLRs spiked with Belatacept failed to lead to allospecific lymphoproliferative inhibition and Treg recruitment in fresh MLR readouts
Since we previously demonstrated that CD4+CD127−(FOXP3+) cells from 7-day MLRs caused immunoregulatory effects in fresh allospecific MLR readouts(7, 20), it was questioned whether similar effects would occur with cells from MLRs spiked with BEL. For this, CD127−CD4+ cells were immunoselected from bulk 7-day MLRs of responder cells from individual A stimulated with irradiated PBMC from individual B without or with BEL (shown as a flow chart in Figure 1). As depicted in Figure 5A (top), when added as third component modulators, such immunoselected CD127−CD4+ cells inhibited the lymphoproliferation in fresh MLR readouts of A-PBMC stimulated with Bx-PBMC in a dose dependent manner. This lymphoproliferation inhibition was also accompanied by the generation of CD4+CD127−CD25highFOXP3+ cells (top, Figure 5B) in the proliferating A responder cell fraction (p<0.05). This “recruitment”, however, was not different in the readout cultures in which the third component CD127−CD4+ cells were generated in the presence of BEL vs. media. However, it was observed that if the readouts were performed using non-allospecific HLA-mismatched third party stimulators, there was no significant inhibition or Treg recruitment (bottom, Figure 5A and 5B). Taken together, these results indicated an antigen-specific amplification of the regulatory effect by CD127−CD4+ cells generated in MLR, as in our prior work (7, 20). However, the magnitude of the effect of BEL-influenced modulator cells was not different from those of the non-BEL media controls, suggesting no increased immunoregulatory effect of BEL.
Figure 5. Assessment of CD4+ CD25highFOXP3+ in vitro inhibitory and recruitment functions by Tregs generated in MLR with or without Belatacept.
Bulk cultures of responder PBMC from volunteer A were stimulated with 2 DR-matched x-irradiated cells from volunteer B either in the presence of no drugs (Medium), 0.1ug/ml or 1μg/ml BEL for 7 days. Then, after depleting CD127+ cells, the CD4+ cells were enriched as described in the methods.
5A: MLR Inhibition: Standard 3H thymidine incorporation assays were performed with 1x105 each of the responders and stimulators in presence of indicated numbers of CD127−CD4+ cells from the above bulk cultures with vs. without BEL, contrasted with control fresh A-PBMC modulators. Note that there appeared to be no greater effect of CD127−CD4+ cells from “A+Bx+BEL” cultures in inhibiting 7-day 3H-TdR incorporation (not statistically significant) over that of CD127−CD4+ cells immunoselected from “A+Bx+Medium” cultures (p>0.2). Nonetheless, there was a much less marked inhibition if the stimulator cells came from a non-specific donor (Ix) (p<0.05).
5B: Treg Recruitment: In parallel, 5x105 CFSE labeled responding fresh PBMC from healthy volunteer A were cultured with 5x105 PKH26-labeled stimulator cells from the original healthy volunteer B in the presence of the indicated numbers of PKH26-labeled CD127−CD25+ modulators. On days 5, 7 and 9 flow cytometric analysis using the scheme shown in Figure 1 was performed (including the gating out of the PKH26 positive modulators and residual stimulators as well as the CD127+ responders). Data are calculated as percentage increase in CD4+CD25highFOXP3+ cell generation with the indicated modulators, over that observed with the control fresh A-PBMC modulators (100% shown by dotted line). CD127−CD4+ cells from both “A+Bx+Medium” and “A+Bx+BEL” (allospecific) cultures similarly enhanced Treg generation (p>0.2). Also, as indicated in the lower panel, using an HLA-mismatched indifferent stimulator (Ix) virtually completely abrogated the in vitro recruitment effect seen in the allospecific cultures (p=0.02).
DISCUSSION
In primary MLRs, BEL concomitantly inhibited lymphoproliferation and Treg generation (Fig. 1 and 2). But Tregs generated in bulk MLR with BEL, when added as 3rd components to new primary MLRs did not inhibit lymphoproliferation or recruit additional Tregs over non-BEL media control Tregs (Fig. 5). Moreover, BEL abrogated immunoregulatory effects mediated by low concentrations of MPA (7, 9) (Fig. 3) in vitro. These results indicated that BEL therapy may not be feasible in clinical tolerance induction protocols and/or the higher incidence of rejection seen with BEL-treated organ transplant recipients might be due to the inhibition of regulatory mechanisms.
The mechanism of action of BEL as a CTLA-4 Ig construct blocking the B7-CD28 pathway provides support that Treg inhibition can occur in human MLR. CTLA-4 is universally present on FOXP3+ Tregs. Since CD28 signaling appears essential to generating and maintaining CD4+CD25+ Tregs and co-stimulation blockade induces T-cell anergy and apoptosis (21, 22), CTLA-4 Ig therapy, therefore, might negatively affect Treg generation (23, 24). A murine liver transplant study showed that CTLA-4 blockade promoted alloreactive T cells and rejection and inhibited tolerance induction (25). In other murine models, CTLA4-Ig was deleterious to the allograft survival that was dependent on Tregs (17, 19). In fact, alternative selective inhibitors of CD28 may attenuate rejection and promote Tregs by sparing CTLA-4 (26). While preliminary, there is recent evidence that there is no apparent association between BEL therapy and Treg expansion in solid organ (renal) transplant recipients (18).
These in vitro studies provide an explanation why BEL appears to be a less potent in clinical immunosuppression as evidenced by a higher rate of acute rejection in kidney and liver transplantation when compared to CNI agents(3, 27). As signal 1 inhibitors CNI more markedly block T cell activation, while BEL blocks only costimulation and both inhibit down-stream events required for Treg generation. While still attractive as a renal sparing agent versus CNI, transplant tolerance protocols involving IS minimization or even withdrawal should perhaps avoid BEL, given its anti-tolerogenic effects. Alternative co-stimulatory blockade agents that do not affect the CD28 and CTLA4 pathways, i.e. sparing Tregs, might be more preferable.
Currently, a number of induction and maintenance IS agents appear to have the tolerogenic effect by both blocking immune activation but also preserving regulatory cells. Induction with alemtuzumab, an anti-CD52 monoclonal as well as the polyclonal anti-thymocyte globulins appear to have such effects (8, 28, 29), as does SRL maintenance therapy in our previous work(9). However, IL-2 receptor inhibitors as induction agents are likely anti-tolerogenic as they directly block CD25 expression important in Treg generation and maintenance.
As an inhibitor of downstream IL-2 signaling after T cell activation, the mTOR inhibitor SRL, blocks alloreactive T cell proliferation but appears to promote Tregs (CD4+CD25highFOXP3+), regulatory cytokines (TGF-β1), and tolerogenic dendritic cells (DC2, ILT3+/4+) (30–36). In contrast, CNI therapy inhibits IL-2 transcription and thus negatively affects Treg generation (5, 6, 37, 38). We have also demonstrated robust donor-specific regulatory effects of SRL in MLR cultures (7). Clinically, direct conversion from tacrolimus to sirolimus increases systemic (blood, allograft, marrow) Tregs, blood DCregs and regulatory proteogenomic signatures in liver transplant recipients(8, 9). Although clinical data for MPA-induced immunoregulation are less robust(39–41), our observation in this paper that low concentrations of MPA had the most pronounced effect on Treg generation in MLR (Fig 3, top lines) needs to be further explored. It is not clear, however, if the positive Treg effects of both MPA and mTOR inhibitors are due to the actual agents themselves or primarily the withdrawal of CNI therapy.
A caveat of our experimental design is that the Treg-MLR assay may not simulate the in vivo effects of the agents in transplant patients and drug concentrations may fluctuate in vivo vs. our controlled culture environments. However, we believe our in vitro studies present novel findings and confirm what other studies have shown in vivo. First, we are able to test immunophenotypic and allospecific immunoregulation of immunosuppressive drugs, such as belatacept and others (4, 7, 28), in combination with other agents used clinically in patients. This is particularly relevant for belatacept as it is only indicated in kidney recipients in combination with other agents, such as mycophenolate mofetil(42). In addition, the ability to test allospecific Treg inhibition, generation, and self-recruitment is a novel aspect of the assay that may correlate with donor-specific immunoregulation (4, 7, 28), although this needs further confirmation. For belatacept, the next step would be to use this assay in transplant recipients treated with belatacept and other agents to determine if similar effects are seen in vivo as well.
In summary, although BEL is a renal sparing immunosuppressive agent, it per se inhibits Treg generation in vitro (current study) and does not promote Tregs expansion in vivo in renal transplant patients(15, 18). This lack of regulatory activity likely translates to a higher risk of rejection and difficulty in using this agent alone in transplant recipients. Alternative forms of co-stimulatory blockade molecules are needed to allow for a more favorable balance of Tregs to effector cells when considering minimization or withdrawal of IS therapy.
MATERIALS AND METHODS
Human Subjects
Peripheral blood mononuclear cells (PBMC) were obtained from healthy laboratory volunteers. HLA typing was performed by the Northwestern HLA laboratory using standard molecular methods (reverse sequence specific oligonucleotide probe hybridization), and HLA 2 DR matched or mismatched were selected as responders and stimulators. The research was conducted with the approval of our Institutional Review Board.
Immunosuppressive Drugs (IS)
Belatacept was provided by Bristol-Myers Squibb®. Sirolimus manufactured by Pfizer® (New York, NY) was purchased through the Northwestern Memorial Hospital pharmacy and mycophenolic acid was purchased from Sigma-Aldrich® (St. Louis, MO). These immunosuppressive drugs were used at therapeutic and sub-therapeutic concentrations as indicated, mirroring the 5 mg/kg belatacept maintenance phase (3–5μg/ml trough levels) after 16 weeks post-transplantation (manufacturer’s information).
Effect of Immunosuppressive Drugs on Primary Treg-MLR
Responding PBMC from healthy volunteer (A) were cultured with irradiated (3000 rads) stimulator cells from HLA-2 DR matched (Bx) or HLA mismatched (Ix) laboratory volunteers in NAB-CM, as previously described (7, 20). To these cultures indicated concentrations of the immunosuppressive drugs were added. On days 5, 7 and 9, the proliferative responses or the development of new Tregs were monitored as described below.
a. Assessment of Proliferation
1x105 responding A-PBMC were stimulated with 1x105 irradiated (3000 rads) Bx or Ix in 96-well U-bottom culture plates in triplicate, in the absence or presence of immunosuppressive drugs. On days 4, 6 and 8, 1μCi 3H-TdR was added to each well and after another 18 hours these were processed using a Tomtec cell harvester (Hamden, CT). Radioactive incorporation was measured using a Packard-Beta counter (Meriden, CT). The Stimulation Index (SI) was calculated using the formula:
The data were also calculated as percentage of medium control using the formula:
b. Flow cytometric analysis
0.5x106 CFSE labeled responder cells were cultured with 0.5x106 PKH26 labeled and irradiated stimulator cells in the absence or presence of the various immunosuppressive drugs at indicated concentrations. These MLR cultures were tested at 5, 7 ± 9 days for the surface markers CD127, CD4 and CD25 and for intracellular FOXP3 by incubating with the following monoclonal antibodies: anti- CD127-phycoerythrin, CD4-Electron-coupled-dye plus CD25-phycoerythrin-cyanin-7 (all from Beckman-Coulter, Miami, FL), and after permeabilization and fixation by CD25-phycoerythrin-cyanin-5 conjugated anti-FOXP3 (eBiosciences, San Diego, CA, as per manufacturer’s instructions). The data were acquired on a 5-color FC500 flow cytometer (Beckman-Coulter, Miami, FL) for 1x105 cellular events. Isotype controls were used to determine background fluorescence. The PKH26 labeled residual stimulators and positive CD127+ (phycoerythrin positive) responders were gated out and then CFSE bright (non-proliferating) and CFSE dim (proliferating) populations among the viable CD4 cells were analyzed for CD25 and FOXP3 expression as shown in Figure 1.
Assessment of Regulatory Functions
To evaluate the immunoregulatory capabilities of cells that are produced in presence of IS, bulk MLR cultures were prepared in the absence or presence of the drugs, the putative regulatory cells were purified and functionally tested in a second freshly set MLR as described below in detail.
a. Bulk Culture Generation of “Regulatory Cells” in Presence of IS
40x106 responder cells (A) were stimulated with 40x106 irradiated stimulator cells (Bx) in multiple T-75 flasks at 1x106 cells/ml concentrations in the absence or presence of indicated concentrations of IS (i.e., BEL or MPA). After 7 days the CD127+ cells were depleted and CD4+ were purified using respective microbeads and AutoMACS (Miltenyi Biotech), using the manufacture’s protocols. The resulting cells were >98% CD127−CD4+ and were >50% CD25HighFOXP3+. These cells were added as (third component) modulator cells at indicated numbers to freshly prepared MLRs to measure specific inhibition and the generation of new Tregs (Please see below).
b. MLR Inhibition by “Regulatory Cells” generated in Presence of IS
1x105 A-responder cells were stimulated with 1x105 irradiated stimulator cells from the original B individual (Bx) or a third party individual who did not have common HLA with the A or B individuals (Ix) in presence 1x104, 2x103 and 400 CD127−CD4+ modulator cells generated as above. Similar numbers of fresh x-irradiated or non-irradiated A-responder cells were added as comparative modulator controls. After an additional 7 days, standard 3H-TdR incorporation assays were performed. The back-responses by un-irradiated modulators stimulated with Bx or Ix from experimental combinations to get the delta CPM. The delta CPM is used for calculating stimulation indices, as well as the percentage inhibition by the third component Tregs using the formula: 1 - [Delta CPM in presence of Treg modulators / Delta CPM in presence of additional (fresh) A-Modulators] x 100.
c. “Infectious” Treg-MLR recruitment by “Regulatory Cells” generated in Presence of IS
5x105 CFSE-labeled A-responder cells were stimulated with 5x105 irradiated PKH26-lablled stimulator cells from the original B individual (Bx) or a third party individual who did not have common HLA with the A or B individuals (Ix) in presence of 5x104, 1x104 and 2x103 PKH26-labeled CD127−CD4+ modulator cells generated as above. The control cultures received similar numbers of PKH26-labeled A-modulator cells. On days 5, 7 and 9, flow cytometric analysis using the scheme shown in Figure 1 was performed (including the gating out of the PKH26 positive modulators and residual stimulators as well as the CD127+ responders). Data are calculated as percentage increase in CD4+CD25highFOXP3+ cells newly generation with the indicated modulators, over that observed with the control fresh A-PBMC modulators.
Statistical Methods
Data were analyzed using univariate and graphical methods wherever applicable. Nonparametric (Wilcoxon signed rank test) statistical methods were used to compare paired groups. Statistical significance was established at a two-sided alpha level of 0.05 using SAS 9.1 statistical software (SAS Inc., Cary, NC).
Acknowledgments
This study was primarily supported by a Bristol-Myers Squibb® investigator-initiated grant (Levitsky, Miller and Mathew). Partial funding was also obtained from NIH Grant #2R01DK25243-25A2 and a VA Merit Review Award (Miller and Mathew).
ABBREVIATIONS
- BEL
Belatacept
- 3H-TdR
Tritiated Thymidine
- CFSE
Carboxyfluorescein succinimidyl ester
- CPM
Counts per minute
- FOXP3
Forkhead/winged helix transcription factor P3
- HLA
Human leukocyte antigen
- HSC
Hematopoietic stem cells
- IS
Immunosuppression
- MHC
Major Histocompatibility Complex
- MLR
Mixed Lymphocyte Reaction
- MPA
Mycophenolic acid
- PBMC
Peripheral blood mononuclear cells
- SI
Stimulation Index
- SRL
Sirolimus
- TAC
Tacrolimus
- Treg
T-regulatory cell
Footnotes
AUTHORSHIP:
Josh Levitsky MD MS: Participated in research design, data analysis and the writing of the paper. Dr. Levitsky is a speaker for Bristol-Meyer Squibb® for the drug Baraclude® (not Nulojix®) Address: Division of Gastroenterology & Hepatology, Comprehensive Transplant Center, Department of Medicine, Northwestern University Feinberg School of Medicine, 676 N St. Clair St., 19th Floor, Chicago, IL 60611.
Joshua Miller MD: Participated in research design, research performance, data analysis and the writing of the paper. No conflict of interest. Address: Comprehensive Transplant Center, Northwestern University Feinberg School of Medicine, 300 E. Superior St., Tarry 11th Floor, Chicago, IL 60611.
Xuemei Huang MD: Participated in research performance and data analysis. No conflict of interest. Address: Comprehensive Transplant Center, Northwestern University Feinberg School of Medicine, 300 E. Superior St., Tarry 11th Floor, Chicago, IL 60611.
Li Chen PhD: Participated in research performance and data analysis. No conflict of interest. Address: Comprehensive Transplant Center, Northwestern University Feinberg School of Medicine, 300 E. Superior St., Tarry 11th Floor, Chicago, IL 60611.
Dhivya Chandrasekaran MS: Participated in research performance and data analysis. No conflict of interest. Address: Comprehensive Transplant Center, Northwestern University Feinberg School of Medicine, 300 E. Superior St., Tarry 11th Floor, Chicago, IL 60611.
James M. Mathew PhD: Participated in research design, research performance, data analysis and the writing of the paper. No conflict of interest. Address: Comprehensive Transplant Center, Northwestern University Feinberg School of Medicine, 300 E. Superior St., Tarry 11th Floor, Chicago, IL 60611.
References
- 1.Larsen CP, Pearson TC, Adams AB, et al. Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. Am J Transplant. 2005;5 (3):443. doi: 10.1111/j.1600-6143.2005.00749.x. [DOI] [PubMed] [Google Scholar]
- 2.Durrbach A, Pestana JM, Pearson T, et al. A phase III study of belatacept versus cyclosporine in kidney transplants from extended criteria donors (BENEFIT-EXT study) Am J Transplant. 2010;10 (3):547. doi: 10.1111/j.1600-6143.2010.03016.x. [DOI] [PubMed] [Google Scholar]
- 3.Vincenti F, Charpentier B, Vanrenterghem Y, et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study) Am J Transplant. 2010;10 (3):535. doi: 10.1111/j.1600-6143.2009.03005.x. [DOI] [PubMed] [Google Scholar]
- 4.Levitsky J, Mathew JM, Flaa CW, Rosen A, Tambur AR, Miller J. The human “Treg MLR”: Immune monitoring for FOXP3+ T Regulatory Cell Generation. Transplantation. 2009 Dec;88(11):1303. doi: 10.1097/TP.0b013e3181bbee98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Szabo G, Gavala C, Mandrekar P. Tacrolimus and cyclosporine A inhibit allostimulatory capacity and cytokine production of human myeloid dendritic cells. Journal of Investigative Medicine. 2001;49 (5):442. doi: 10.2310/6650.2001.33789. [DOI] [PubMed] [Google Scholar]
- 6.Woltman AM, de Fijter JW, Kamerling SW, Paul LC, Daha MR, van Kooten C. The effect of calcineurin inhibitors and corticosteroids on the differentiation of human dendritic cells. European Journal of Immunology. 2000;30 (7):1807. doi: 10.1002/1521-4141(200007)30:7<1807::AID-IMMU1807>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 7.Levitsky J, Gallon L, Miller J, et al. Allospecific regulatory effects of sirolimus and tacrolimus in the human mixed lymphocyte reaction. Transplantation. 2011;91 (2):199. doi: 10.1097/TP.0b013e318200e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levitsky J, Miller J, Wang E, et al. Immunoregulatory profiles in liver transplant recipients on different immunosuppressive agents. Hum Immunol. 2009;70 (3):146. doi: 10.1016/j.humimm.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levitsky J, Mathew JM, Abecassis M, Tambur A, Leventhal J, Chandresekaran D, Herrera N, Al-Saden P, Gallon L, Abdul-Nabi A, Yang GY, Kurian S, Salomon D, Miller J. Systemic immunoregulatory and proteogenomic effects of tacrolimus to sirolimus conversion in liver transplant recipients. Hepatology. 2012 Jan 11; doi: 10.1002/hep.25579. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seveno C, Coulon F, Haspot F, et al. Induction of regulatory cells and control of cellular but not vascular rejection by costimulation blockade in hamster-to-rat heart xenotransplantation. Xenotransplantation. 2007;14 (1):25. doi: 10.1111/j.1399-3089.2006.00361.x. [DOI] [PubMed] [Google Scholar]
- 11.Lan YY, Wang Z, Raimondi G, et al. “Alternatively activated” dendritic cells preferentially secrete IL-10, expand Foxp3+CD4+ T cells, and induce long-term organ allograft survival in combination with CTLA4-Ig. J Immunol. 2006;177 (9):5868. doi: 10.4049/jimmunol.177.9.5868. [DOI] [PubMed] [Google Scholar]
- 12.Pilat N, Wekerle T. Belatacept and Tregs: friends or foes? Immunotherapy. 2012;4 (4):351. doi: 10.2217/imt.12.13. [DOI] [PubMed] [Google Scholar]
- 13.Furuzawa-Carballeda J, Lima G, Uribe-Uribe N, et al. High levels of IDO-expressing CD16+ peripheral cells, and Tregs in graft biopsies from kidney transplant recipients under belatacept treatment. Transplant Proc. 2010;42 (9):3489. doi: 10.1016/j.transproceed.2010.08.037. [DOI] [PubMed] [Google Scholar]
- 14.Ko HJ, Cho ML, Lee SY, et al. CTLA4-Ig modifies dendritic cells from mice with collagen-induced arthritis to increase the CD4+CD25+Foxp3+ regulatory T cell population. J Autoimmun. 2010;34 (2):111. doi: 10.1016/j.jaut.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Bluestone JA, Liu W, Yabu JM, et al. The effect of costimulatory and interleukin 2 receptor blockade on regulatory T cells in renal transplantation. Am J Transplant. 2008;8 (10):2086. doi: 10.1111/j.1600-6143.2008.02377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davies JK, Barbon CM, Voskertchian A, Nadler LM, Guinan EC. Ex vivo alloanergization with belatacept: a strategy to selectively modulate alloresponses after transplantation. Cell Transplant. 2012;21 (9):2047. doi: 10.3727/096368912X637479. [DOI] [PubMed] [Google Scholar]
- 17.Riella LV, Liu T, Yang J, et al. Deleterious effect of CTLA4-Ig on a Treg-dependent transplant model. Am J Transplant. 2012;12 (4):846. doi: 10.1111/j.1600-6143.2011.03929.x. [DOI] [PubMed] [Google Scholar]
- 18.Chavez H, Beaudreuil S, Abbed K, et al. Absence of CD4CD25 regulatory T cell expansion in renal transplanted patients treated in vivo with Belatacept mediated CD28-CD80/86 blockade. Transpl Immunol. 2007;17 (4):243. doi: 10.1016/j.trim.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Charbonnier LM, Vokaer B, Lemaitre PH, Field KA, Leo O, Le Moine A. CTLA4-Ig Restores Rejection of MHC Class-II Mismatched Allografts by Disabling IL-2-Expanded Regulatory T Cells. Am J Transplant. 2012;12 (9):2313. doi: 10.1111/j.1600-6143.2012.04184.x. [DOI] [PubMed] [Google Scholar]
- 20.Levitsky J, Miller J, Leventhal J, et al. The human “Treg MLR”: Immune monitoring for FOXP3+ T Regulatory Cell Generation. Transplantation. 2009;88 (11):1303. doi: 10.1097/TP.0b013e3181bbee98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang Q, Henriksen KJ, Boden EK, et al. Cutting edge: CD28 controls peripheral homeostasis of CD4+CD25+ regulatory T cells. J Immunol. 2003;171 (7):3348. doi: 10.4049/jimmunol.171.7.3348. [DOI] [PubMed] [Google Scholar]
- 22.Lio CW, Dodson LF, Deppong CM, Hsieh CS, Green JM. CD28 facilitates the generation of Foxp3(-) cytokine responsive regulatory T cell precursors. J Immunol. 2010;184 (11):6007. doi: 10.4049/jimmunol.1000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barnes MJ, Griseri T, Johnson AM, Young W, Powrie F, Izcue A. CTLA-4 promotes Foxp3 induction and regulatory T cell accumulation in the intestinal lamina propria. Mucosal Immunol. 2012 doi: 10.1038/mi.2012.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karman J, Jiang JL, Gumlaw N, et al. Ligation of cytotoxic T lymphocyte antigen-4 to T cell receptor inhibits T cell activation and directs differentiation into Foxp3+ regulatory T cells. J Biol Chem. 2012;287 (14):11098. doi: 10.1074/jbc.M111.283705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muthukumar T, Dadhania D, Ding R, et al. Messenger RNA for FOXP3 in the urine of renal-allograft recipients. New England Journal of Medicine. 2005;353 (22):2342. doi: 10.1056/NEJMoa051907. [DOI] [PubMed] [Google Scholar]
- 26.Zhang T, Fresnay S, Welty E, et al. Selective CD28 blockade attenuates acute and chronic rejection of murine cardiac allografts in a CTLA-4-dependent manner. Am J Transplant. 2011;11 (8):1599. doi: 10.1111/j.1600-6143.2011.03624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klintmalm GB, Feng S, Lake J, Vargas H, Wekerle T, Meadows-Shropshire S, Agarwal M, Garcia-Valdecassis JC. Belatacept-based immunosupression in de novo liver transplant recipients: 1-year experience from a phase II study. Am J Transplant. 2011;11 (Suppl 2):137. doi: 10.1111/ajt.12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levitsky J, Leventhal JR, Miller J, et al. Favorable effects of alemtuzumab on allospecific regulatory T-cell generation. Hum Immunol. 2012;73 (2):141. doi: 10.1016/j.humimm.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Macedo C, Walters JT, Orkis EA, et al. Long-term effects of alemtuzumab on regulatory and memory T-cell subsets in kidney transplantation. Transplantation. 2012;93 (8):813. doi: 10.1097/TP.0b013e318247a717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Battaglia M, Stabilini A, Draghici E, et al. Rapamycin and interleukin-10 treatment induces T regulatory type 1 cells that mediate antigen-specific transplantation tolerance. Diabetes. 2006;55 (1):40. [PubMed] [Google Scholar]
- 31.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105 (12):4743. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 32.Nikolaeva N, Bemelman FJ, Yong SL, van Lier RA, ten Berge IJ. Rapamycin does not induce anergy but inhibits expansion and differentiation of alloreactive human T cells. Transplantation. 2006;81 (3):445. doi: 10.1097/01.tp.0000194860.21533.b9. [DOI] [PubMed] [Google Scholar]
- 33.Dodge IL, Demirci G, Strom TB, Li XC. Rapamycin induces transforming growth factor-beta production by lymphocytes. Transplantation. 2000;70 (7):1104. doi: 10.1097/00007890-200010150-00020. [DOI] [PubMed] [Google Scholar]
- 34.Fischer R, Turnquist HR, Taner T, Thomson AW. Use of rapamycin in the induction of tolerogenic dendritic cells. Handb Exp Pharmacol. 2009;(188):215. doi: 10.1007/978-3-540-71029-5_10. [DOI] [PubMed] [Google Scholar]
- 35.Turnquist HR, Raimondi G, Zahorchak AF, Fischer RT, Wang Z, Thomson AW. Rapamycin-conditioned dendritic cells are poor stimulators of allogeneic CD4+ T cells, but enrich for antigen-specific Foxp3+ T regulatory cells and promote organ transplant tolerance. Journal of Immunology. 2007;178 (11):7018. doi: 10.4049/jimmunol.178.11.7018. [DOI] [PubMed] [Google Scholar]
- 36.Valmori D, Tosello V, Souleimanian NE, et al. Rapamycin-mediated enrichment of T cells with regulatory activity in stimulated CD4+ T cell cultures is not due to the selective expansion of naturally occurring regulatory T cells but to the induction of regulatory functions in conventional CD4+ T cells. J Immunol. 2006;177 (2):944. doi: 10.4049/jimmunol.177.2.944. [DOI] [PubMed] [Google Scholar]
- 37.Baan CC, van der Mast BJ, Klepper M, et al. Differential effect of calcineurin inhibitors, anti-CD25 antibodies and rapamycin on the induction of FOXP3 in human T cells. Transplantation. 2005;80 (1):110. doi: 10.1097/01.tp.0000164142.98167.4b. [DOI] [PubMed] [Google Scholar]
- 38.Gao W, Lu Y, El Essawy B, Oukka M, Kuchroo VK, Strom TB. Contrasting effects of cyclosporine and rapamycin in de novo generation of alloantigen-specific regulatory T cells. Am J Transplant. 2007;7 (7):1722. doi: 10.1111/j.1600-6143.2007.01842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fourtounas C, Dousdampanis P, Sakellaraki P, et al. Different immunosuppressive combinations on T-cell regulation in renal transplant recipients. Am J Nephrol. 2010;32 (1):1. doi: 10.1159/000313940. [DOI] [PubMed] [Google Scholar]
- 40.Abadja F, Videcoq C, Alamartine E, Berthoux F, Mariat C. Differential effect of cyclosporine and mycophenolic acid on the human regulatory T cells and TH-17 cells balance. Transplant Proc. 2009;41 (8):3367. doi: 10.1016/j.transproceed.2009.08.031. [DOI] [PubMed] [Google Scholar]
- 41.Demirkiran A, Sewgobind VD, van der Weijde J, et al. Conversion from calcineurin inhibitor to mycophenolate mofetil-based immunosuppression changes the frequency and phenotype of CD4+FOXP3+ regulatory T cells. Transplantation. 2009;87 (7):1062. doi: 10.1097/TP.0b013e31819d2032. [DOI] [PubMed] [Google Scholar]
- 42.Nulojix® - Bristol-Myers Squibb. packageinserts.bms.com/pi/pi_nulojix.pdf.