Abstract
Sleep deprivation can have deleterious effects on cognitive function and mental health. Moderate exercise training has myriad beneficial effects on cognition and mental health. However, physiological and behavioral effects of chronic moderate sleep restriction and its interaction with common activities, such as moderate exercise training, have received little investigation. The aims of this study were to examine the effects of chronic moderate sleep restriction and moderate exercise training on anxiety-related behavior, spatial memory, and neurobiological correlates in mice. Male mice were randomized to one of four 11-week treatments in a 2 [sleep restriction (~4 h loss/day) vs. ad libitum sleep] × 2 [exercise (1 h/day/6 d/wk) vs. sedentary activity] experimental design. Anxiety-related behavior was assessed with the elevated-plus maze, and spatial learning and memory were assessed with the Morris water maze. Chronic moderate sleep restriction did not alter anxiety-related behavior, but exercise training significantly attenuated anxiety-related behavior. Spatial learning and recall, hippocampal cell activity (i.e., number of c-Fos positive cells), and brain derived neurotrophic factor were significantly lower after chronic moderate sleep restriction, but higher after exercise training. Further, the benefit of exercise training for some memory variables was evident under normal sleep, but not chronic moderate sleep restriction conditions. These data indicate clear detrimental effects of chronic moderate sleep restriction on spatial memory and that the benefits of exercise training were impaired after chronic moderate sleep restriction.
Keywords: sleep restriction, moderate, anxiety, memory, BDNF, exercise training
Introduction
It is well established that sleep deprivation has negative psychological effects and impairs cognitive function [reviewed 1]. In humans, sleep deprivation induces anxiety, exacerbates anxiety disorders [2], and impairs cognitive function [3].
In rodent models, sleep loss elicits anxiety-related behavior in some, but not all studies [4, 5]. Furthermore, rodent studies consistently demonstrate sleep loss-induced impairments in cognition [6, 7, 8]. Sleep seems to be important for memory, and evidence indicates that neurons that are activated during waking are reactivated during post-training non-rapid eye movement (NREM) and rapid-eye-movement (REM) sleep [9, 10]. Consequently, it has been suggested that a reiteration of events in the form of short-term hippocampal activity transferred gradually to long-term neocortical memory stores during sleep may be involved in consolidating and encoding spatial information [11]. In rodents, prolonged sleep deprivation and severe sleep restriction over several days have been found to impair performance in the Morris water maze [6, 8, 12], a hippocampus-dependent spatial memory test widely used in rodent studies.
Nevertheless, most research in this area has involved profound and/or short-term sleep restriction [e.gs, 5–8 days (13, 14)]. It remains unclear whether similar detrimental effects might be observed with chronic moderate sleep restriction, which is likely far more common in humans. Interestingly, human experimental studies involving chronic sleep deprivation, even of rather severe amounts, has often failed to elicit negative effects neither on cognitive function nor on other health-related variables such as glucose tolerance [15, 16].
In humans, exercise training has well-established anxiolytic and cognitive benefits [17], [18], including enhanced learning and memory recall. In rodents, exercise training has [19] elicited anxiolytic effects in some, but not all studies [20, 21]. However, the benefits of exercise for cognitive function in rodents have been more consistent, including enhanced spatial memory learning and recall [22].
Whereas some evidence suggests that exercise can exacerbate psychological and cognitive consequences of severe sleep loss [23], other recent evidence indicates that prior exercise training can prevent anxiogenic effects of acute sleep loss in rodents [24]. However, to our knowledge, no prior work has addressed the interaction of daily exercise training with concomitant chronic moderate sleep loss, which commonly occurs, for example, in individuals who moderately curtail their sleep in order to exercise in the morning.
The aims of this study were to examine the effects of chronic moderate sleep restriction and moderate exercise training on anxiety-related behavior, and spatial memory. A mouse model was used to allow precise control of these variables and to explore mechanistic correlates of changes in spatial memory. These correlates included c-Fos expression, a common marker of hippocampal cell activity in relation to spatial memory [25], and hippocampal levels of brainderived neurotrophic factor (BDNF), a neurotrophin that regulates synaptic plasticity in the cortex and hippocampus and is vital to learning and memory [26], especially spatial memory. Whereas sleep deprivation has elicited impairments in c-Fos activation and BDNF levels in the hippocampus [25], exercise training has elicited enhancements in these measures [27].
Methods
Animals and care
Forty male C57BL/6J mice were obtained from breeding colonies that originated from the Jackson Laboratories (Bar Harbor, ME). Mice were group-housed (2–5 per cage) in standard caging within sound-attenuated chambers, and maintained in a neutral ambient temperature (22 ± 1 °C) and a 12:12 h light:dark cycle. Water and food were provided ad libitum. All experiments were approved by the Institutional Animal Care and Usage Committee of the University of South Carolina.
Treatment Randomization
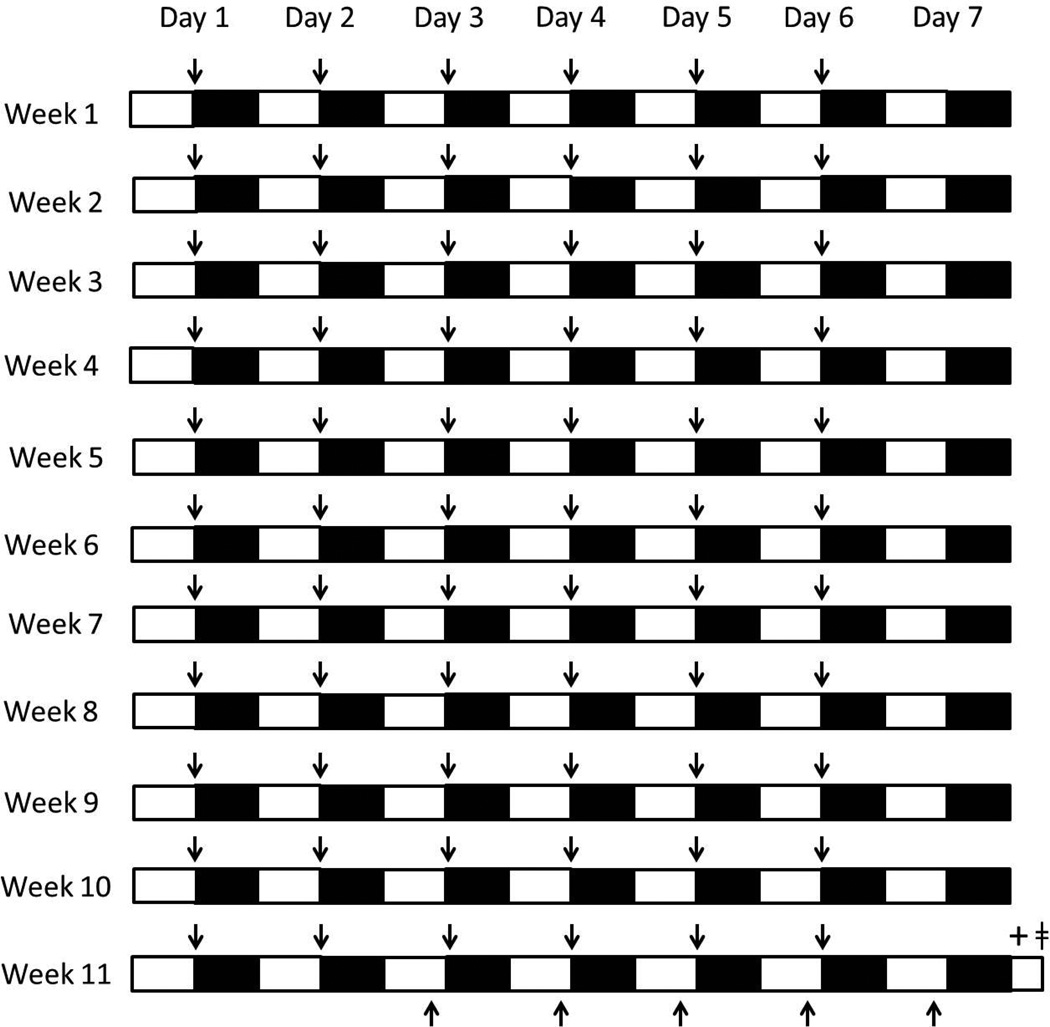
At 4 weeks of age, the mice were randomized to one of four 11-week treatment groups: normal sleep + sedentary activity (n = 10), normal sleep + exercise (n = 10), sleep restriction + sedentary activity (n = 10), and sleep restriction + exercise (n = 10). An overview of the time-course of the experimental design is described in Fig 1.
Fig. 1.
Experimental design. Mice in the sleep restriction treatments were deprived of sleep during the active dark period (black bars; i.e., moderate chronic sleep restriction), whereas mice in the normal sleep treatments were allowed ad libitum sleep daily for 11 consecutive weeks. All mice were allowed ad libitum sleep during the light period (white bars). Mice in the treatments involving exercise underwent exercise (↑) during the first hour of the dark period (6 days/week for 11 weeks), whereas mice in the sedentary activity treatments did not exercise. All mice were trained on the Morris water maze 2 hours prior to dark onset (↓) for five consecutive days preceding the end of the 11 week experimental treatments. Mice were subjected to the elevated plus maze followed by the Morris water maze recall test at the beginning of the light period after 11 weeks of experimental treatments (+). Mice were culled two hours later and brains were taken and processed for c-Fos and BDNF immunohistochemical analysis (□).
Sleep Restriction
Mice in treatments involving sleep restriction were restricted of sleep as previously described [28]. Mice were placed on a slowly-rotating drum (30 cm, 1 rev/min) that was encircled by water. The drum was enclosed in plexiglass with food and drinking water accessible ad libitum. Throughout the 11-week intervention, mice were placed on the rotating drum for 12 h/day for 7 days/week. Placement on the drum occurred during the 12 h dark period, whereas during the 12 h light period the mice were transferred back to their cages and allowed ad libitum sleep. One rationale for placing the mice on the rotating drum only during the dark period was to provide a simple intervention for eliciting a relatively modest amount of daily sleep restriction, since mice still sleep modest amounts during the dark period. Another rationale for limiting the sleep restriction to the dark period was to avoid potential confounds of circadian phase-shifting effects of sleep loss during the light period. As reported previously [28], the sleep restriction protocol resulted in daily moderate amounts of sleep loss of 4 h per 24 h period. The small increase in walking associated with the intervention, 274 m/day at 18 m/h, was negligible, in our view.
Mice in the treatments involving normal sleep were placed on the drum during the same time period in the dark, but the drum was locked, allowing ad libitum sleep during the dark period as well as the light period. Negligible changes in sleep occurred in this intervention.
Exercise Training
As previously described [28], mice in the treatments involving exercise training ran on a motorized treadmill for 11 weeks, 6 days/week, 60 min/day, at 18–21 m/min and a 5% grade. Exercise training for 6 days/week elicits health benefits and allowing 1 day/week of recovery reduces potential detriments from overtraining. Mice ran on the treadmill at the beginning of the dark period, a time of day when C57BL/6 mice are most active. Limited hand prodding of the mice was necessary at this moderate exercise intensity level. Mice did not exercise the day prior to tissue collection to avoid potentially confounding the effects of acute exercise with those of chronic exercise training.
Mice in the treatments involving sedentary activity were exposed to the treadmill room environment at the same time and handled in a similar manner as mice in the exercise treatments. However, these animals did not exercise.
Behavioral Testing
Elevated-Plus Maze
Mice were subjected to the elevated-plus maze at light onset immediately after the end of the last day of the 11-week treatments. Anixety-related behavior using the the elevated-plus maze has been validated in rats and mice and was used in the present study [29]. Briefly, the elevated-plus maze consisted of a maze elevated 50 cm above the floor with two open and two enclosed arms. The mice were placed in the center of the maze and videotaped for 5 minutes. The recorded video was relabeled with a random number by a separate investigator in order to blind the investigator who scored anxiety-related behaviors. The percent of open arm and closed arm time of the elevated-plus maze were measured. The animals were only considered to have entered a particular area when all four paws were contained within that area. An increased percent of time spent in the open arms of the elevated-plus maze is a behavior indicative of reduced anxiety in rats and mice. Number of fecal boli is considered to be indicative of anxiety. In order to reduce possible odors from the previous subject, the testing chambers were cleaned between animals using a weak ammonium hydroxide (15%) solution with minimal odor.
Morris Water Maze
The Morris water maze was used to access spatial memory learning and recall after the experimental treatments [30]. The Morris water maze consisted of a round white tub with a diameter of two meters. The tub was filled with tap water with white tempura paint and maintained at a temperature of aproximately 22°C. A white cylinder, placed 0.5 cm below the surface of the water, served as the escape platform. A video camera that was mounted in the center above the tub allowed assessment of the duration of time spent swimming, the swim path, and speed. Further, four quadrants (north, south, east, and west) were determined from the video recordings, and the quadrant where the submerged escape platform was placed was the designated target quadrant.
Spatial Memory Learning
The animals practiced the maze four times a day (during the two hours prior to the dark onset) for five consecutive days prior to the end of treatment week 11, in order to learn their position relative to the escape platform using available visible spatial cues. Scheduling the spatial memory acquisition at the end of the light period reduced the amount of additional sleep loss produced by this intervention, as sleep propensity in mice is greatest during the beginning of the light period [31]. Mice were given up to 90 seconds to find the submerged platform. Upon reaching the platform, mice were given 15 seconds to remain on the platform and determine their “safe” spatial location in the maze. The escape time latency (i.e., the time it took to reach the submerged platform) was measured as an indicator of learning. As learning increases, escape time latency decreases.
Spatial Memory Recall
Immediately after the elevated-plus maze test, mice were subjected to the spatial memory recall portion of the Morris water maze. The submerged platform of the Morris water maze was removed and mice were allowed to swim for 90 seconds to determine spatial memory recall. The mouse swim path was overlaid on the pool's map, and the time spent in the designated target quadrant was recorded. The recorded video was relabeled with a random number by a separate investigator in order to blind the investigator who scored Morris water maze activities. The swimming speed was calculated by dividing the total swim length by the swim time at the free swim trial, which served as a measure of the locomotive ability of the animals. The time spent in the designated target quadrant was used as the main measure of spatial memory recall. More time spent in the area where the submerged platform had previously been located was indicative of greater spatial memory recall ability. Additionally, the number of times the mouse crossed the exact place where the submerged platform was previously located (i.e., annulus crossings) was measured as a supplementary indicator of spatial memory recall.
Tissue Collection
Two hours after the Morris water maze spatial memory recall test, mice were anesthetized with isofluorane and transcardially perfused with phosphate-buffered saline followed by cold 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4. Thereafter, brains were disected and placed in 4% paraformaldehyde overnight followed by being placed in a cryoprotective solution and stored at −70°C until immunohistochemical analysis procedures.
Immunohistochemistry
Following cryoprotection, 44 µm hippocampal sections were cut on a microtome and processed for c-Fos or BDNF-labeled immunihistochemistry using an immunoperoxidase method. Tissue sections were washed in tris buffered saline (TBS), followed by methanolic peroxide. Sections were washed in TBS, followed by a wash in TBS plus horse serum. For c-Fos expression, the tissue sections were incubated in rabbit anti-fos antiserum (1:10000 dilution in TBS; 48 hrs at 4°C; Calbiochem, CA). For BDNF expression, the tissue sections were incubated in rabbit anti-BDNF antiserum (1:1000 dilution in TBS; 48 hrs at 4°C; Chemicon, CA). Both c-Fos and BDNF incubated sections were rinsed in TBS after primary antibody labeling. The sections were then incubated in a biotinylated donkey anti-rabbit IgG secondary antibody (1:1000 dilution in TBS; 1.5 hr at 20–25°C; Jackson Immunoresearch, CA) and rinsed in TBS plus horse serum. Then the sections were incubated in horseradish peroxidaseconjugated streptavidin (1:1600 dilution in TBS with Triton X-100; 1 hr at 20–25°C; Jackson Immunoresearch, CA) and rinsed in TBS. c-Fos-like and BDNF immune reactivity were visualized by developing the sections in a nickel-cobalt intensified diaminobenzidine solution with hydrogen peroxide for a few minutes, which caused a blue-black visible reaction product in c-Fos-positive nuclei or BDNF postive cells. The tissue sections were then rinsed inTBS and the sections were mounted onto slides with a gelatin solution for microscopic analysis. Hippocampal CA1, CA3, and dentate gyrus (DG) cells containing c-Fos-positive nuclei and cells containing BDNF from one hemispherere were quantified under a microscope using a reticle with an area of 0.1225 mm2 magnification by an investigator blinded to the treatments.
Statistical Analysis
A 2 (SLEEP: sleep restriction vs. normal sleep) × 2 (EXERCISE: exercis vs. sedentary) ANOVA with Tukey post-hoc comparisons was used to analyze anxiety-related behavior, memory retrieval aspects of the Morris water maze, hippocampal c-Fos and BDNF positive cells. Repeated-measures SLEEP × EXERCISE × TIME ANOVA was used to analyze the time to reach the platform on repeated days during the learning trials. Associations of memory data with the numbers of c-Fos and BDNF positive cells were assessed with Pearson correlations. All statistical analyses are reported as mean ± SE, with significance set at p < 0.05.
RESULTS
Anxiety-Related Behavior
No significant main SLEEP or SLEEP × EXERCISE interaction effect was found for the percent of time spent in the open arms of the elevated-plus maze, percent of time spent in the closed arms of the elevated-plus maze, or arm entries (Table 1). Compared to sedentary activity, exercise training resulted in more time in the open arms of the maze [F(1, 36) = 4.365, p = 0.044], which is indicative of an anxiolytic effect. However, post-hoc analysis did not indicate significant effects of exercise training when restricted to either the normal sleep or chronic moderate sleep restriction conditions. No significant main EXERCISE effects were found for any of the other elevated-plus maze metrics. Further, no significant differences between treatments were found for the number of fecal boli produced.
Table 1.
Anxiety-related behaviors.
| Experimental group | Open-arm time (%) | Closed-arm time (%) | Arm entries (number) |
Fecal Bolus (number) |
|---|---|---|---|---|
| Normal sleep + sedentary | 8.70 ± 1.17 | 70.17 ± 4.11 | 12.90 ± 1.47 | 1.80 ± 0.25 |
| Normal sleep + exercise | 19.63 ± 5.14* | 66.03 ± 4.75 | 10.50 ± 0.60 | 1.90 ± 0.23 |
| Sleep restriction + sedentary | 14.07 ± 2.74 | 58.47 ± 4.67 | 10.60 ± 1.24 | 1.70 ± 0.26 |
| Sleep restriction + exercise | 17.43 ± 3.39 | 67.97 ± 2.86 | 11.40 ± 0.59 | 1.70 ± 0.21 |
Anxiety-related behavior data (Mean ± SE) occurring during the elevated-plus maze are presented after the 11-week experimental treatments. A main effect was found for exercise reducing the time spent in the open arms of the elevated plus maze. However, exercise significantly enhanced the percentage of time spent in the open arms of the elevated plus maze under normal sleep conditions but not sleep restriction conditions.
indicates differences between experimental group and normal sleeping sedentary controls. Significant differences were set at p < 0.05.
Spatial Memory Acquisition
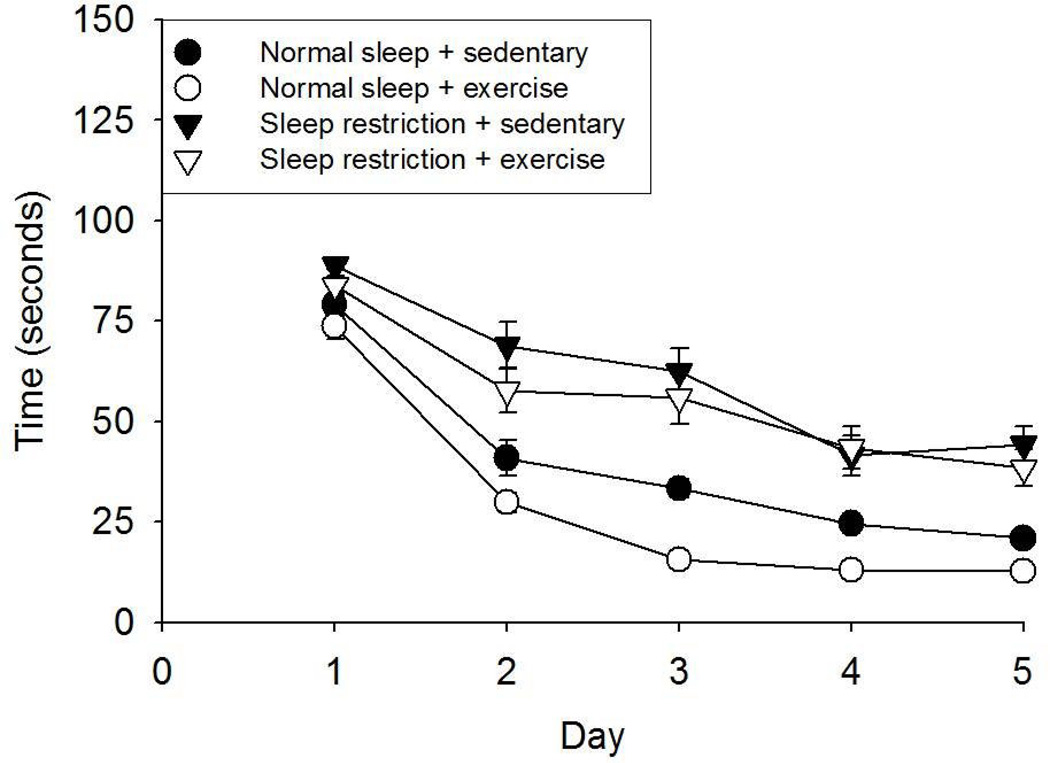
Daily practice reduced the time to reach the hidden platform during the acquisition phase of the Morris water maze for all treatments [TIME: F(1, 36) = 120.652, p < 0.001] (Fig 2). Time to reach the platform was longer in the sleep restriction vs. normal sleep conditions [F(1, 36) = 174.602, p < 0.001], and this difference increased over time [F(1, 36) = 5.476, p = 0.025]. During the acquisition phase, the time to reach the platform was shorter following exercise training compared with sedentary activity [F(1, 36) = 19.352, p < 0.001]. However, no significant SLEEP × EXERCISE or TIME × EXERCISE interactions was found for this variable.
Fig 2.
Morris water maze data (Mean ± SE) from the memory acquisition portion of the test occurring after the 11-week experimental treatments. All experimental groups learned to find the hidden platform as indicated by reductions in time to reach the platform over the 5 day memory acquisition. Sleep restricted mice took significantly more time to find the hidden platform over the learning period than normal sleeping mice. Further, mice that exercised took significantly less time to find the hidden platform over time than sedentary mice.
Spatial Memory Recall
Morris water maze spatial memory recall results are presented in Table 2. A SLEEP × EXERCISE interaction was found for time spent in the targeted quadrant [F(1, 36) = 4.171, p = 0.048]. Compared with normal sleep, sleep restriction resulted in less percentage of time spent in the targeted quadrant of the Morris water maze [F(1, 36) = 25.900, p < 0.001], and this effect was more apparent under exercise training conditions vs. sedentary conditions. Exercise training elicited a small non-significant increase in time spent in the targeted quadrant under sleep restriction conditions and an increase in the time spent in the targeted quadrant under normal sleep conditions (p = 0.015), resulting in a main EXERCISE effect [F(1, 36) = 6.086, p = 0.019].
Table 2.
Morris water maze memory recall.
| Experimental group | Distance swam (cm) |
Speed swam (cm/s) |
Time in designated quadrant (%) |
Annulus crossings (number) |
|---|---|---|---|---|
| Normal sleep + sedentary | 1418.8 ± 37.4 | 15.8 ± 0.4 | 45.0 ± 2.4 | 3.3 ± 0.5 |
| Normal sleep + exercise | 1411.5 ± 51.7 | 15.7 ± 0.6 | 57.8 ± 3.5* | 6.1 ± 0.6* |
| Sleep restriction + sedentary | 1413.5 ± 36.7 | 15.7 ± 0.4 | 36.4 ± 2.6 | 2.8 ± 0.6 |
| Sleep restriction + exercise | 1424.2 ± 33.6 | 15.8 ± 0.4 | 37.6 ± 2.7 | 2.2 ± 0.6 |
Morris water maze data (Mean ± SE) from the memory recall portion of the test occurring after the 11-week experimental treatments. A significant SLEEP × EXERCISE interaction was found for percentage of time spent in the designated quadrant and number of annulus crossings. A significant main effect of sleep restriction attenuating the time spent in the designated quadrant and number of annuls crossings was found. A significant main effect of exercise enhancing the time spent in the designated quadrant and the number of annulus crossings was found. However the benefit of exercise on enhancing these measures was found during normal sleep conditions but not sleep restricted conditions.
indicates differences between experimental group and normal sleeping sedentary controls. Significant differences were set at p < 0.05.
A SLEEP × EXERCISE interaction was observed for the number of times the animals crossed the location in which the platform had previously been located (i.e., annulus crossings) [F(1, 36) = 9.616, p = 0.004) (Table 2). Sleep restriction decreased the number of annulus crossings compared to normal sleep [F(1, 36) = 16.104, p < 0.001]. This effect was significant under exercise training conditions but not sedentary conditions (p = 0.005). Exercise training elicited an increase in annulus crossings under normal sleep conditions (p = 0.005), but exercise training actually resulted in a slight non-significant decrease in annulus crossings under sleep restriction conditions. However, no significant main EXERCISE effect was found for annulus crossings. Annulus crossings were found to be correlated with the percentage of time spent in the targeted quadrant of the Morris water maze (r = 0.468, p = 0.002). All treatment groups exhibited similar distances swam and swimming speeds during the spatial memory recall portion of the Morris water maze suggesting that recall data were not confounded by differences in locomotor ability between treatments.
Hippocampal Activation
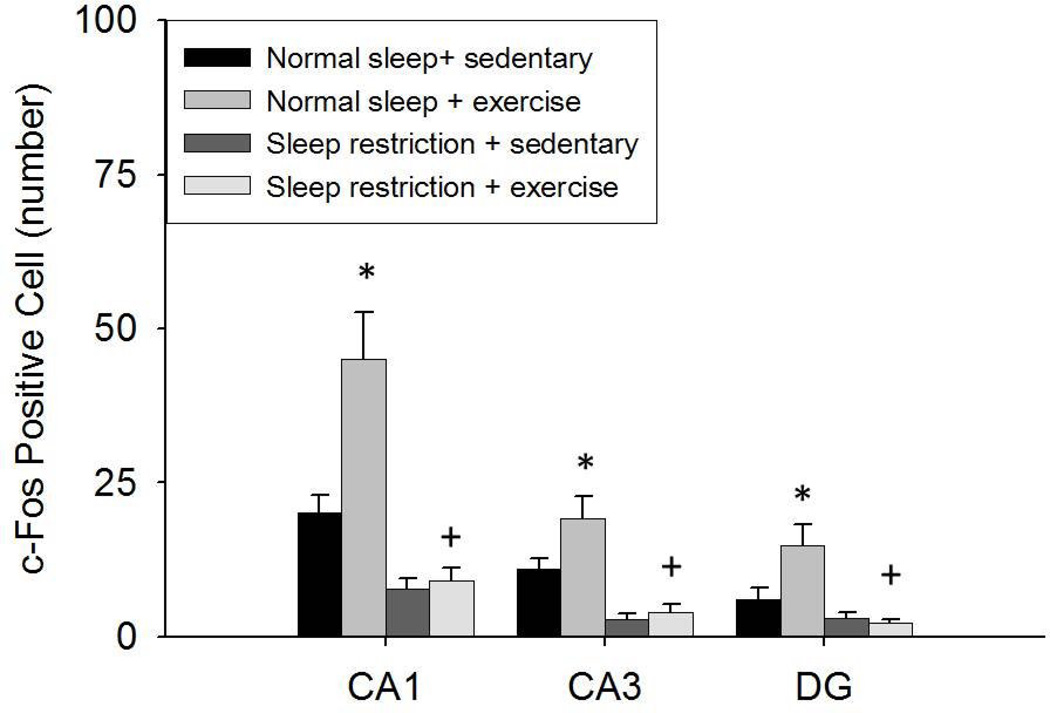
Hippocampal c-Fos positive cell data are presented in Fig. 3 and Fig. 4. Fewer c-Fos positive cells were found in the CA1, CA3, and DG areas after sleep restriction vs. normal sleep [F(1, 36) = 30.494, p < 0.001; F(1, 36) = 30.330, p < 0.001; F(1, 36) = 15.587, p < 0.001, respectively], although post-hoc analysis determined that the effect of sleep restriction was not significant when restricted to either the sedentary or exercise conditions. Exercise training increased the number of c-Fos positive cells in the CA1, CA3, and DG areas [F(1, 36) = 9.026, p = 0.005; F(1, 36) = 4.822, p = 0.035; F(1, 36) = 4.252, p = 0.046, respectively]. However, this effect was apparent only under normal sleep conditions, in which exercise elicited a significantly greater increase in c-Fos positive cell in all of these areas compared with sedentary activity. Under sleep restriction conditions, the effect of exercise vs. sedentary activity was negligible. This pattern of results represented SLEEP × EXERCISE interactions c-Fos positive cells in CA1 and DG areas [F(1, 36) = 7.203, p = 0.011; F(1, 36) = 5.587, p = 0.024, respectively], but not the CA3 area of the hippocampus.
Fig 3.
The number (Mean ± SE) of c-Fos positive cells in the CA1, CA3, and DG areas of the hippocampus after the 11-week treatments. A main effect was found for sleep restriction attenuating c-Fos positive cells was found during both sedentary and exercise conditions. A main effect was found for exercise enhancing c-Fos positive cells. However, exercise enhanced c-Fos positive cells under normal sleeping conditions but not sleep restriction conditions. (*) indicates differences between experimental group and normal sleeping sedentary controls. (+) indicates differences between normal sleeping exercise and sleep restriction exercise groups. Significant differences were set at p < 0.05.
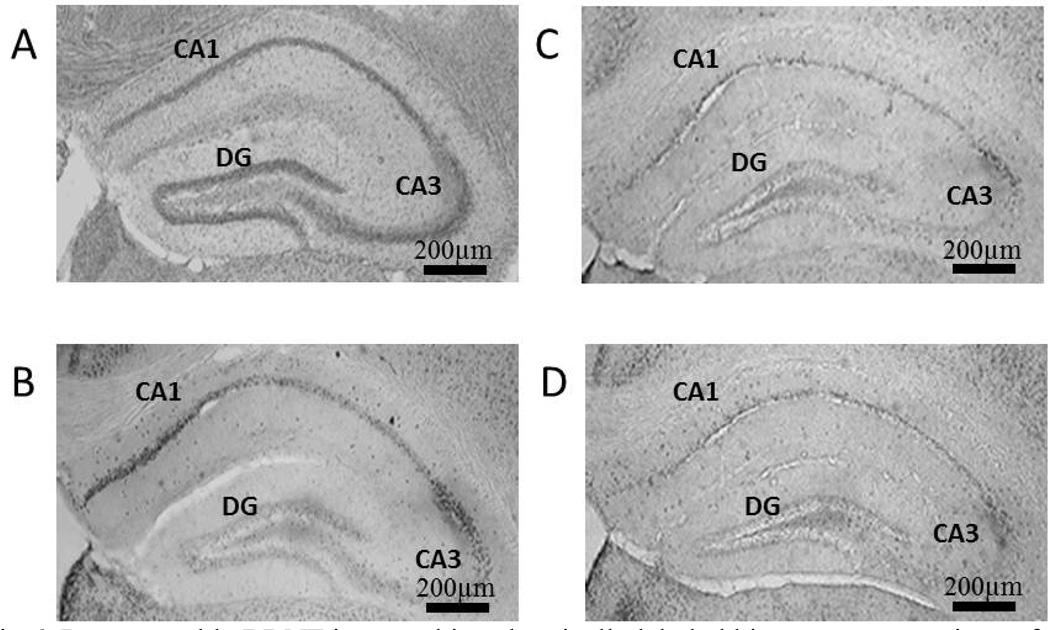
Fig 4.
Representable c-Fos immunohistochemically labeled hippocampus sections of mice that experienced the Morris water maze: (A) normal sleep + sedentary, (B) normal sleep + exercise, (C) sleep restriction + sedentary and (D) sleep restriction + exercise.
Significant positive correlations were found between the number of c-Fos positive cells in the CA1, CA3, and DG areas of the hippocampus and the percentage of time spent in the designated quadrant of the memory recall portion of the Morris water maze (r = 0.621, p < 0.001; r = 0.579, p < 0.001; r = 0.474, p = 0.002, respectively).
Hippocampal Brain-Derived Neurotropic Factor
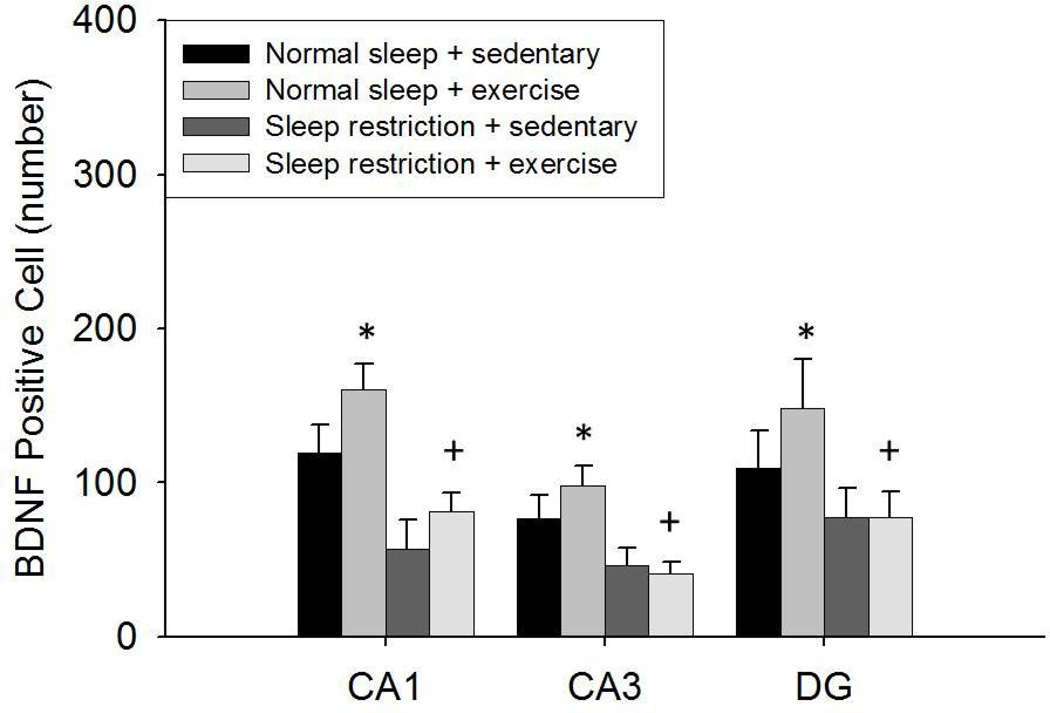
Hipppocampal BDNF positive cell data are shown in Fig. 5 and Fig. 6. BDNF positive cells in the CA1, CA3, and DG areas of the hippocampus were lower after sleep restriction compared with that after normal sleep [F(1, 36) = 17.079, p < 0.001; F(1, 36) = 13.047, p = 0.001; F(1, 36) = 4.599, p = 0.039, respectively]. However, no significant main EXERCISE or SLEEP × EXERCISE interaction was found for BDNF positive cells in any of these areas of the hippocampus.
Fig 5.
The number (Mean ± SE) of BDNF positive cells in the CA1, CA3, and DG areas of the hippocampus after the 11-week treatments. A main effect was found for sleep restriction attenuating BDNF positive cells during both sedentary and exercise conditions. BDNF positive cells were greater in the exercised conditions compared to the sedentary conditions in normal sleeping but not sleep restricted mice. (*) indicates differences between experimental group and normal sleeping sedentary controls. (+) indicates differences between normal sleeping exercise and sleep restricted exercise groups. Significant differences were set at p < 0.05.
Fig 6.
Representable BDNF immunohistochemically labeled hippocampus sections of mice that experienced the Morris water maze (A) normal sleep + sedentary, (B) normal sleep + exercise, (C) sleep restriction + sedentary and (D) sleep restriction + exercise. Highlights Chronic moderate sleep restriction impaired spatial memory in mice. Exercise training elicited anxiolytic effects and improved spatial memory. The effects of exercise training on memory were attenuated under conditions of sleep restriction.
Significant positive correlations were observed between BDNF positive cells in the CA1, CA3, and DG areas of the hippocampus with the percentage of time spent in the designated quadrant of maze after the memory recall portion of the Morris water maze (r = 0.425, p = 0.006; r = 0.363, p = 0.021; r = 0.352, p = 0.026, respectively).
Discussion
Chronic moderate sleep restriction did not significantly influence anxiety-behavior compared with normal sleep, whereas moderate exercise training had a significant anxiolytic effect compared with the sedentary activity. Chronic moderate sleep restriction impaired spatial memory acquisition and recall, with corresponding reductions in hippocampal c-Fos activation and BDNF activity. Significant beneficial effects of exercise on spatial memory were observed under normal sleep conditions, but not under chronic moderate sleep restriction conditions. Moreover, exercise training enhanced c-Fos levels in the hippocampus. Changes in BDNF after exercise training showed a similar, but nonsignificant pattern. Our findings suggest that chronic moderate sleep restriction attenuated the benefits of exercise training for spatial memory.
The lack of change in anxiety behavior after sleep restriction in the present study is consistent with the results of some studies involving sleep restriction in rats [4], but not with the results of other studies [5]. However, these studies have tended to involve profound amounts of sleep restriction over short durations. The results from the present study resemble those of Novati et al. (2011) who found that chronic moderate sleep restriction (4 hours per day for 1 month) did not affect anxiety-related behaviors [4]. Together with the present findings, the results suggest that more modest and/or chronic sleep restriction interventions may not elicit increases in anxiety in rodents.
Exercise training elicited a significant increase in time spent in the open arms of the maze, a result that is indicative of reduced anxiety. These data are consistent with the results of some other rodent studies indicating enhanced time spent in the open arms of the elevated-plus maze following both forced and volitional exercise training [26, 32, 33]. However, other animal studies have not found anxiolytic effects in the elevated plus maze after exercise training [e.g., 34]. The discrepancy in findings might be attributed to several factors, including differences between species or in the mode, frequency, or intensity of the exercise.
The results showing impairment in spatial memory following moderate sleep restriction are consistent with other animal studies showing impaired memory in the Morris water maze after severe amounts of sleep deprivation elicited either prior to or during the learning trials [7, 8]. The results of the present study suggest that even moderate amounts of chronic sleep loss might be detrimental to memory. Further research in humans is needed to establish whether chronic moderate sleep restriction also impairs memory similar to the effects of more severe sleep restriction [35].
The findings of enhanced spatial memory acquisition and recall after exercise training are consistent with the results of many other rodent studies that have found that both forced treadmill exercise and voluntary wheel running resulted in enhanced measures of memory in the Morris water maze [23, 36]. The findings are also consistent with epidemiologic and experimental research studies showing benefits of physical activity and exercise training on learning in humans [18].
That the enhancements in spatial memory recall following exercise training were attenuated by chronic moderate sleep restriction compared to normal sleep suggests that adequate daily sleep amounts may be necessary for cognitive benefits of exercise training to be realized in mice. However, from another standpoint, exercise training neither reversed nor exacerbated the detrimental effects of sleep restriction on spatial memory. A recent study found that prior exercise training over four weeks prevented impairment in memory (radial arm water maze) following REM sleep loss [37]. However, that study cannot be directly compared with the present study that in which exercise training occurred concomitantly with sleep restriction. Whether the findings of the present study have any implications for humans will require research. It is noteworthy that extreme levels of exercise can exacerbate cognitive deficits produced by severe sleep loss in humans [23]. However, it will be important to establish how the common conditions of moderate sleep loss and moderate exercise interact in humans.
The c-Fos and BDNF data were generally consistent with the behavioral data. For example, chronic moderate sleep restriction attenuated hippocampal c-Fos expression, which is consistent with other evidence that 10 h of acute sleep deprivation reduced c-Fos neuron activation in the CA1 and DG areas of the hippocampus [25]. In rodents, acute sleep deprivation can impair neurogenesis within the hippocampus [38]. Even mild sleep restriction (i.e., 6 h) after learning results has resulted in reduced neurogenesis in the DG area of the hippocampus in rats [6]. Thus, it is plausible that sleep restriction might have reduced hippocampal cell activation because there were fewer numbers of neurons available.
In the present study, exercise training enhanced hippocampal neuron activation in the CA1, CA3, and DG areas. These data are consistent with other data indicating that exercise training enhances hippocampus-dependent tasks [39]. Potential mechanisms by which exercise training might enhance spatial memory include increased hippocampal neurogenesis [40], hippocampus size [39], and BDNF levels [26]. Consistent with the behavioral data, the results suggest that adequate sleep is needed for the benefits of exercise training on hippocampal neuron activation to be realized.
In the present study, chronic moderate sleep restriction reduced the number of BDNF positive cells in the CA1, CA3, and DG areas of the hippocampus. These findings, combined with previous research showing reductions in BDNF protein and BDNF mRNA after acute sleep deprivation, suggest that impaired BDNF regulation might be involved with sleep loss-induced detriments in cognition [41].
We found non-significant trends for exercise-induced increases in BDNF levels compared to sedentary activity. Nonetheless, in post-hoc analysis we found that under normal sleep conditions, exercise elicited significant increases in BDNF positive cells compared with sedentary conditions. These data are consistent with other studies, which have indicated that exercise training can increase hippocampal BDNF protein levels and mRNA expression [36, 42]. However, our present findings suggest that the detriments of chronic sleep restriction may supersede the benefits of exercise training on a neuro-molecular level.
Notwithstanding the results, there were several limitations to the present study. First, it is unclear the extent to which impaired memory recall can be explained by sleep loss that occurred prior to the initial learning trials or between exposure to the maze and testing of memory recall. Prior studies of rats suggest that the timing of sleep after learning might be important for spatial memory consolidation [6]. Second, scheduling the memory recall immediately after the last period of nighttime sleep restriction did not allow a delineation of the effects of chronic vs. acute sleep restriction, though the amount of sleep restriction for a single day (4 hr) was modest.
Third, although 4 h of daily sleep restriction was relatively mild compared with that induced in other studies, the sleep restriction constituted a decrease of 24 h sleep duration of approximately 33%, which likely exceeds that which most humans experience on a chronic basis. Further research should explore milder amounts of chronic sleep restriction.
Fourth, although there were logistical and theoretical rationales for limiting the sleep restriction to the dark cycle, this protocol disrupted the natural 24 h sleep pattern of mice. This could have unknown consequences.
Fifth, the timing of the period of spatial memory acquisition (end of light period) did not correspond with the timing of memory recall testing (beginning of light period). This could have influenced the memory recall results, as studies have shown some specificity of time-of-day for cognition [43]. Sixth, the novelty/anxiety associated with the elevated plus-maze could have influenced performance in the Morris water maze.
In conclusion, although detrimental effects of dramatic short-term sleep restriction on memory have been established, this study demonstrated significant impairment after chronic and relatively mild sleep restriction. Corresponding reductions in hippocampal c-Fos and BDNF positive cells were found after sleep restriction. Consistent with previous work, exercise training elicited reductions on anxiety-related behavior. Exercise also promoted increased memory recall with corresponding increased c-Fos positive cells. However, the benefits of exercise for spatial memory were observed only under normal sleep conditions. These data suggest that chronic moderate sleep deprivation can attenuate some of the cognitive benefits of exercise training in mice.
Acknowledgements
This research was supported by the National Institutes of Health (HL 71560 and HL095799 to SDY). We thank Matthew Davis, David Elliott, and J. Larry Durstine for their contributions to this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McCoy JG, Strecker RE. The cognitive cost of sleep lost. Neurobiol Learn Mem. 2011;96:564–582. doi: 10.1016/j.nlm.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roy-Byrne PP, Uhde TW, Post RM. Effects of one night’s sleep deprivation on mood and behavior in panic disorder. Patients with panic disorder compared with depressed patients and normal controls. Arch Gen Psychiatry. 1986;43:895–899. doi: 10.1001/archpsyc.1986.01800090085011. [DOI] [PubMed] [Google Scholar]
- 3.Killgore WD. Effects of sleep deprivation on cognition. Prog Brain Res. 2010;185:105–129. doi: 10.1016/B978-0-444-53702-7.00007-5. [DOI] [PubMed] [Google Scholar]
- 4.Novati A, Hulshof HJ, Kookhaas JM, Lucassen PJ, Meerlo P. Chronic sleep restriction causes a decrease in hippocampal volume in adolescent rats, which is not explained by changes in glucocorticoid levels or neurogenesis. Neuroscience. 2011;190:145–155. doi: 10.1016/j.neuroscience.2011.06.027. [DOI] [PubMed] [Google Scholar]
- 5.Suchecki D, Tiba PA, Tufik S. Hormonal and behavioral responses of paradoxical sleep-deprived rats to the elevated plus maze. J Neuroendocrinol. 2002;14:549–554. doi: 10.1046/j.1365-2826.2002.00812.x. [DOI] [PubMed] [Google Scholar]
- 6.Hairston IS, Little MT, Scanlon MD, Barakat MT, Palmer TD, Sapolsky RM, Heller HC. Sleep restriction suppresses neurogenesis induced by hippocampus-dependent learning. J Neurophysiol. 2005;94:4224–4233. doi: 10.1152/jn.00218.2005. [DOI] [PubMed] [Google Scholar]
- 7.Yang S, Sun H, Huang Z, Yao M, Qu W. Repeated sleep restriction inn adolescent rats altered sleep patterns and impaired spatial learning/memory ability. Sleep. 2012;35:849–859. doi: 10.5665/sleep.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan Z, Peng X, Fang J. Sleep deprivation impairs spatial memory and decreases extracellular signal-regulated kinase phosphorylation in the hippocampus. Brain Res. 2004;1018:38–47. doi: 10.1016/j.brainres.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 9.Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- 10.Poe GR, Nitz DA, McNaughton BL, Barnes CA. Experience dependent phase-reversal of hippocampal neuron firing during REM sleep. Brain Res. 2000;855:176–180. doi: 10.1016/s0006-8993(99)02310-0. [DOI] [PubMed] [Google Scholar]
- 11.Sutherland GR, McNaughton B. Memory trace reactivation in hippocampal and neocortical neuronal ensembles. Curr Opin Neurobiol. 2000;10:180–186. doi: 10.1016/s0959-4388(00)00079-9. [DOI] [PubMed] [Google Scholar]
- 12.McCoy JG, Christie MA, Kim Y, Brennan R, Poeta DL, McCarley RW, Strecker RE. Chronic sleep restriction impairs spatial memory in rats. Neuroreport. 2013;24:91–95. doi: 10.1097/WNR.0b013e32835cd97a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roman V, Walstra I, Luiten PG, Meerlo P. Too little sleep gradually desensitizes the serotonin 1A receptor system. Sleep. 2005;28:1505–1510. [PubMed] [Google Scholar]
- 14.Kim Y, Laposky AD, Bergmann BM, Turek FW. Repeated sleep restriction in rats leads to homeostatic and allostatic responses during recovery sleep. Proc Natl Acad Sci U S A. 2007;104:10697–10702. doi: 10.1073/pnas.0610351104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Youngstedt SD, Kline CE, Zielinski MR, Kripke DF, Devlin TM, Bogan RK, Wilcox S, Hardin JW. Tolerance of chronic 90-minute time-in-bed restriction in older long sleepers. Sleep. 2009;32:1467–1479. doi: 10.1093/sleep/32.11.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zielinski MR, Kline CE, Kripke DF, Bogan RK, Youngstedt SD. No effect of 8-week time in bed restriction on glucose tolerance in older long sleepers. J Sleep Res. 2008;17:412–419. doi: 10.1111/j.1365-2869.2008.00673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strohle A. Physical activity, exercise, depression and anxiety disorders. J Neural Transm. 2009;116:777–784. doi: 10.1007/s00702-008-0092-x. [DOI] [PubMed] [Google Scholar]
- 18.Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- 19.Erickson KI, Kramer AF. Aerobic exercise effects on cognitive and neural plasticity in older adults. Br J Sports Med. 2009;43:22–24. doi: 10.1136/bjsm.2008.052498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia-Capdevila S, Portell-Cortes I, Torras-Garcia M, Coll-Andreu M, Costa-Miserachs D. Effects of long-term voluntary exercise on learning and memory processes: dependency of the task and level of exercise. Behav Brain Res. 2009;202:162–170. doi: 10.1016/j.bbr.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 21.Lalanza JF, Sanchez-Roige S, Gagliano H, Fuentes S, Bayod S, Camins A, Pallas M, Armario A, Escorihuela RM. Physiological and behavioural consequences of long-term moderate treadmill exercise. Psychoneuroendocrinology. 2012 doi: 10.1016/j.psyneuen.2012.03.008. (In Press) [DOI] [PubMed] [Google Scholar]
- 22.Alaei H, Moloudi R, Sarkaki AR. Effects of treadmill running on mid-term memory and swim speed in the rat with Morris water maze test. J Bodyw Mov Ther. 2008;12:72–75. doi: 10.1016/j.jbmt.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Scott JP, McNaughton LR, Polman RC. Effects of sleep deprivation and exercise on cognitive, motor performance and mood. Physiol Behav. 2006;87:396–408. doi: 10.1016/j.physbeh.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 24.Vollert C, Zagaar M, Hovatta I, Taneja M, Vu A, Dao A, Levine A, Alkadhi K, Salim S. Exercise prevents sleep deprivation-associated anxiety-like behavior in rats: potential role of oxidative stress mechanisms. Behav Brain Res. 2011;224:233–240. doi: 10.1016/j.bbr.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 25.Pierard C, Liscia P, Phillippin JN, Mons N, Lafon T, Chauveau F, Van Beers P, Drouet I, Serra A, Jouanin JC, Beracochea D. Modafinil restores memory performance and neural activity impaired by sleep deprivation in mice. Pharmacol Biochem Behav. 2007;88:55–63. doi: 10.1016/j.pbb.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 26.Gorski JA, Zeiler SR, Tamowski S, Jones KR. Brain-derived neurotrophic factor is required for the maintenance of cortical dendrites. J Neurosci. 2003;23:6856–6865. doi: 10.1523/JNEUROSCI.23-17-06856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neeper SA, Gomez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995;373:109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- 28.Zielinski MR, Davis JM, Fadel JR, Youngstedt SD. Influence of chronic moderate sleep restriction and exercise on inflammation and carcinogenesis in mice. Brain Behav Immun. 2012;26:672–679. doi: 10.1016/j.bbi.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2:322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D’Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- 31.Zielinski MR, Taishi P, Clinton JM, Krueger JM. 5’-Ectonucleotidase-knockout mice lack non-REM sleep responses to sleep deprivation. Eur J Neurosci. 2012;35:1789–1798. doi: 10.1111/j.1460-9568.2012.08112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fulk LJ, Stock HS, Lynn A, Marshall J, Wilson MA, Hand GA. Chronic physical exercise reduces anxiety-like behavior in rats. Int J Sports Med. 2004;25:78–82. doi: 10.1055/s-2003-45235. [DOI] [PubMed] [Google Scholar]
- 33.Pietrelli A, Lopez-Costa JJ, Goni R, Lopez EM, Brusco A, Basso N. Effects of moderate and chronic exercise on the nitrergic system and behavioral parameters in rats. Brain Res. 2011;1389:71–82. doi: 10.1016/j.brainres.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 34.Lalanza JF, Sanchez-Roige S, Gagliano H, Fuentes S, Bayod S, Camins A, Pallas M, Armario A, Escorihuela RM. Physiological and behavioural consequences of long-term moderate treadmill exercise. Psychoneuroendocrinology. 2012;37:1745–1754. doi: 10.1016/j.psyneuen.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 35.Jiang F, VanDyke RD, Zhang J, Li F, Gozal D, Shen X. Effect of chronic sleep restriction on sleepiness and working memory in adolescents and young adults. J Clin Exp Neuropsychol. 2011;33:892–900. doi: 10.1080/13803395.2011.570252. [DOI] [PubMed] [Google Scholar]
- 36.Fordyce DE, Wehner JM. Physical activity enhances spatial learning performance with an associated alteration in hippocampal protein kinase C activity in C57BL/6 and DBA/2 mice. Brain Res. 1993;619:111–119. doi: 10.1016/0006-8993(93)91602-o. [DOI] [PubMed] [Google Scholar]
- 37.Zagaar M, Alhaider I, Dao A, Levine A, Alkarawi A, Alzubaidy M, Alkadhi K. The beneficial effect of regular exercise on cognition in REM sleep deprivation: behavioral, electrophysiolgical and molecular evidence. Neurobiol Dis. 2012;45:1153–1162. doi: 10.1016/j.nbd.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 38.McDermott CM, Hardy MN, Bazan NG, Magee JC. Sleep deprivation-induced alterations in excitatory synaptic transmission in the CA1 region of the rat hippocampus. J Physiol. 2006;570:553–565. doi: 10.1113/jphysiol.2005.093781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.VanPraag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guzman-Marin R, Ying Z, Suntsova N, Methippara M, Bashir T, Szymusiak R, Gomez-Pinilla F, McGinty D. Suppression of hippocampal plasticity-related gene expression by sleep deprivation in rats. J Physiol. 2006;575:807–819. doi: 10.1113/jphysiol.2006.115287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berchtold NC, Kesslak JP, Cotman CW. Hippocampal brain derived neurotrphic factor gene regulation by exercise and the medial septum. J Neurosci Res. 2002;68:511–521. doi: 10.1002/jnr.10256. [DOI] [PubMed] [Google Scholar]
- 43.Schmidt C, Collette F, Cajochen C, Peigneux P. A time to think: circadian rhythms in human cognition. Cogn Neuropsychol. 2007;24:755–789. doi: 10.1080/02643290701754158. [DOI] [PubMed] [Google Scholar]