SUMMARY
Following DNA replication, eukaryotic cells must biorient all sister chromatids prior to cohesion cleavage at anaphase. In animal cells, sister chromatids gradually biorient during prometaphase, but current models of mitosis in S. cerevisiae assume biorientation is established shortly after S-phase. This assumption is based on the observation of a bilobed distribution of yeast kinetochores early in mitosis, and suggests fundamental differences between yeast mitosis and mitosis in animal cells. By applying super-resolution imaging methods, we show that yeast and animal cells share the key property of gradual and stochastic chromosome biorientation. The characteristic bilobed distribution of yeast kinetochores, hitherto considered synonymous for biorientation, arises from kinetochores in mixed attachment states to microtubules, the length of which discriminates bioriented from syntelic attachments. Our results offer a revised view of mitotic progression in S. cerevisiae which augments the relevance of mechanistic information obtained in this powerful genetic system for mammalian mitosis.
INTRODUCTION
The elaborate dynamics of spindle assembly and checkpoint surveillance during mitosis have as their ultimate goal the proper attachment of replicated sister chromatids to kinetochore microtubules (kMTs) emanating from opposite spindle poles, a process referred to as chromosome biorientation. Failure to biorient chromatid pairs prior to dissolution of sister cohesion and mitotic exit causes aneuploidy, dramatically lowering the viability of single-cell organisms and promoting cancer and birth defects in mammals (Chandhok and Pellman, 2009; Draviam et al., 2004; Thompson et al., 2010). Understanding mitosis ultimately comes down to understanding mechanisms that promote efficient biorientation and couple cell cycle progression to acquisition of this geometry by all chromosomes.
Because of its powerful genetics and relatively simple spindle and kinetochores, the budding yeast S. cerevisiae is a good organism in which to study spindle assembly and mitotic progression. Prevailing models suggest that biorientation is established in budding yeast at the earliest stages of spindle assembly (Goshima and Yanagida, 2000). Subsequently, poleward forces exerted by kinetochore-bound microtubules pull the 16 sets of sister kinetochores and their associated pericentric DNA apart (Yeh et al., 2008). Chromosomes are postulated to remain in this bioriented configuration until the onset of anaphase (Gardner et al., 2008; Gardner et al., 2005; Pearson et al., 2004), at which point cohesion between sisters is lost allowing the two sets of sisters to separate and move towards the spindle poles. A key argument in favor of this model is that virtually all kinetochore proteins (typically visualized as GFP fusions) localize from the onset of mitosis until anaphase into two distinct lobes that lie along the spindle axis. Such a stable bilobed distribution is assumed to be synonymous with chromosome biorientation (Goshima and Yanagida, 2000; He et al., 2000; Hyland et al., 1999; Pearson et al., 2001; Zeng et al., 1999) and is consistent with electron micrographs showing that the mitotic spindle consists of ~16 short microtubules (MTs) emanating from each spindle pole body (SPB) and two sets of four interpolar MTs that interdigitate to form a connection between the poles (O'Toole et al., 1999; Winey et al., 1995). The short MTs are assumed to be bound to bioriented and separated kinetochores.
One unappealing aspect of budding yeast as a model for chromosome segregation is that it seems very different from what is observed in many other eukaryotes, including humans, in which bipolarity is established gradually over the course of a relatively long prometaphase (Kitagawa and Hieter, 2001). However, none of the studies on budding yeast actually rules out the possibility that the two bilobes contain a mixture of bioriented and syntelic kinetochores. Observing the consequential gradual resolution of syntelic attachments is expected to be difficult: rapid rates of MT growth and shrinkage (up to ~4 µm/min) (Dorn et al., 2005) combined with the small size of the yeast spindle (~1.5 µm) implies that the movement of a kinetochore from one lobe to the other would take only 10–20 s. Following such an event, the intensity of the two kinetochore lobes is expected to change by at most 10%, also making it difficult to detect the kinetochore rearrangement. Nonetheless, transient separation of sister centromeres and subsequent movement of kinetochores across the spindle midzone has been detected (He et al., 2000).
This paper attempts to distinguish directly between the early-biorientation model for yeast and a more evolutionarily plausible progressive-biorientation model accepted for higher eukaryotic cells. We combine various tagging and single-chromosome imaging strategies with statistical analysis of large numbers of chromosomes in wild-type (WT) and mutant yeast cells to provide evidence that, like human kinetochores, yeast kinetochores progressively biorient over the entire period from S-phase to anaphase onset.
RESULTS
Three assays to track kinetochore attachment and organization
We developed three assays to investigate the establishment of biorientation during yeast mitosis, each illuminating a different facet of the process: (i) Kinetochore-snapshot assay: This assay involves acquisition of 3D images of an unsynchronized population of fixed cells using a fluorescently tagged kinetochore (Ndc80-GFP) and spindle pole body (Spc42-CFP) protein. The distance between spindle poles serves as a proxy for mitotic progression, since spindle length increases monotonically from early S-phase to anaphase onset, making it possible to follow the distribution of all 16 kinetochore pairs at successive stages of mitosis (Figures 1A–1D; Figure S1A), albeit with no information on individual kinetochores. (ii) CEN IV-tracking assay: This assay involves 3D time-lapse imaging and machine-vision assisted tracking of fluorescent spots marking the motion of the centromere-proximal region of chromosome IV (CEN IV; tagged using a Tet-Operator insert and a Tet-Repressor fused to GFP) relative to the SPBs (labeled with Spc42-GFP). Such dynamic data provide a more direct view of sister chromatid attachment at different stages of mitosis and have the benefit of rapid temporal sampling (1Hz; Figures 1E–1G) but only during a short period of time (photo-bleaching limits the movies to ~200–300 s duration) and for a single kinetochore. (iii) CEN IV-snapshot assay: This assay involves imaging the position of CEN IV-proximal GFP tags at a single point in time but for many cells simultaneously. This assay is complementary to the CEN IV-tracking assay in that it determines the position of CEN IV tags relative to SPBs in hundreds of cells. Using the separation of CEN IV tags as a marker for bipolar attachment, and assuming that chromosome IV is representative of all chromosomes, we extrapolated on a statistical basis the relationship between biorientation and mitotic progression for the entire ensemble of chromosomes.
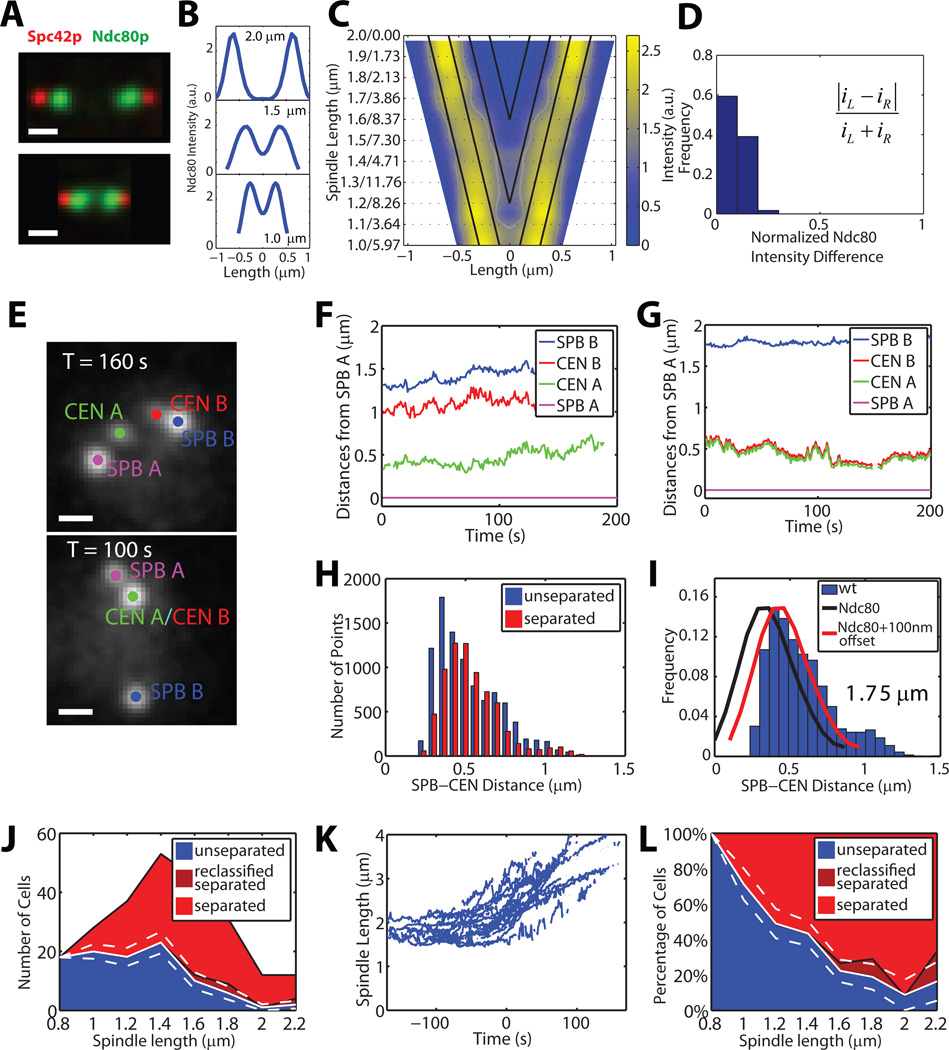
Figure 1. WT cells show bilobed distribution of kinetochores in metaphase but establish bipolarity only gradually.
(A) Representative intensity projections of 3D image stacks of fixed WT cells co-expressing Spc42-CFP (red) marking SPBs and Ndc80-GFP (green) marking kinetochores. Scale bar 0.5 µm.
(B, C) Symmetrized Ndc80p distributions of n = 59 cells. Cells were ranked according to spindle lengths, which varied between 1 µm (bottom) and 2 µm (top) in (B). V-plots show combined Ndc80-GFP intensity profiles as a function of spindle length (see Experimental Procedures). The SPB is located at the boundary of the trapezoidal intensity map. Diagonal black lines indicate loci of constant distance to the SPBs in steps of 0.2 µm.
(D) Distribution of the relative intensity difference between Ndc80 bilobes (see Experimental Procedures).
(E) Representative intensity projections of CEN IV and SPB tags in cells with separated (top) and unseparated (bottom) sisters. Scale bar 0.5µm
(F, G) Time courses of distances between SPB A and SPB B (blue), and between SPB A and proximal (CEN A, green) and distal (CEN B, red) CEN IV tag. The cell in (F) has a spindle length of ~1.4 µm with separated CEN IV tags. The cell in (G) has a spindle length of ~1.75 µm with still unseparated CEN IV tags.
(H) Histograms of SPB–CEN distances in cells with unseparated tags (blue) and separated tags (red). For cells with unseparated tags only the overall shortest SPB–CEN distance is included.
(I) Normalized histogram of SPB–CEN distances from cells with spindle lengths between 1.5 and 2 µm. Black line, Ndc80p intensity distribution in an average spindle with a length of 1.75 µm (derived from the V-plot in C). Red line, Ndc80 intensity distribution shifted by 100 nm, indicating the offset between Ndc80 and CEN IV tags.
(J) Distribution of spindle lengths in CEN IV-snapshot assay and classification into unseparated (blue), separated (light red) and reclassified as separated (dark red) CEN IV tags. White solid and dashed lines indicate mean ± bootstrapped SD of cells with syntely. See Experimental Procedures and Figure S2 for details.
(K) Time courses of SPB A–SPB B distances for cells entering anaphase. The time courses are aligned relative to one another with T = 0 s representing the frame in which the two chromosome tags start recoiling.
(L) Normalized distribution of unseparated (blue), separated (light red), and reclassified as separated (dark red) CEN IV tag as a function of spindle length (derived from (J)). White lines indicate mean ± bootstrapped SD of cells with syntely.
See also Figures S1, S2 and S3, Tables S1 and S2, and Movies S1, S2, S3, and S4.
Kinetochore distribution is bilobed and symmetric for spindles longer than one micrometer
We used the kinetochore-snapshot assay to monitor successive changes in mean kinetochore distribution as a function of SPB–SPB distance and thus, as a function of position in mitosis (Figures 1A–C, Movie S1). We found that the intensity distribution was bilobed for spindles longer than ~1.1 µm (n = 59 cells) and that the peaks of the two lobes were on average ~0.35 µm away from the spindle poles (Figure 1C). As the spindle length increased over the course of mitosis, the kMT length stayed constant and the distance between the kinetochore lobes increased.
Soon after START, budding yeast SPBs undergo a semi-conservative process of replication giving rise to distinct new and old SPBs (Jaspersen and Winey, 2004). Yeast kinetochores are known to preferentially attach to the old SPB early in mitosis (Maure et al., 2007; Tanaka et al., 2002) and we therefore asked whether such preferential attachment resulted in a difference in the peak intensities of the two lobes. We used the kinetochore-snapshot assay to observe the distribution of all kinetochores via Ndc80-GFP. For each individual cell, we calculated the asymmetry score |iL − iR| / (iL + iR), where iL and iR are the total Ndc80-GFP intensities on the left and right of the spindle midzone, respectively (Figure 1D). For all spindle lengths we observed a tight distribution of scores around 0, showing that similar numbers of kinetochores were present in each lobe (Figure 1D, Figure S1B). This suggests that even though kinetochores may initially prefer to attach to the old SPB, a mechanism must exist for symmetrizing the distribution, potentially via detachment and reattachment prior to significant SPB separation.
The bilobed distribution of kinetochores results from a tight regulation of SPB–CEN distance regardless of the type of attachment
Although bioriented attachment of chromosomes to microtubules is expected to result in a bilobed and symmetric distribution of kinetochore proteins, alternative configurations involving mixtures of syntelic, monotelic and bioriented sisters would also give rise to a bilobed pattern (Figures S1C–S1E). To investigate the actual state of kinetochore we turned to the CEN IV-tracking assay. In the tracking assay, biorientation results in separation of the two CEN tags, and this separation can be resolved visually down to the diffraction limit of the imaging system (ca. ~ 250 nm) (He et al., 2000; Pearson et al., 2001) (Figure 1E top). Replicated sisters attached in monotelic or syntelic configurations lack the tension required for centromere tag separation, and the two CEN IV tags appear as one unresolved spot (Figure 1E bottom). We imaged cells for 200–300 seconds, acquiring z-stacks of 16 slices separated by 200 nm every second, and used computational procedures to track tags in 3D with a localization precision of ~ 20 nm (Movie S2) (Thomann et al., 2003; Thomann et al., 2002). Our tracking software achieves a superresolution improvement of ~ 2-fold (relative to the diffraction limit), allowing two CEN IV tags to be resolved to distances of ≥ 150 nm (Dorn et al., 2005) (Supplementary Information).
We observed CEN IV tags with separated (Figures 1F) and unseparated (Figures 1G) CEN IV kinetochores in cells having either short or long spindles, suggesting that bipolar attachments could be established at different points in mitotic progression. We also observed CEN IV tags transitioning from unseparated to separated (Movie S3). SPB–CEN IV distances remained approximately constant throughout mitotic progression, with no major difference in presumed kMT length between separated (n=20 cells) and unseparated (n=27 cells) tags (Figure 1H). The distribution of SPB–CEN IV distances (for spindle lengths between 1.5 and 2 µm ) was very similar to the Ndc80-GFP intensity distribution as established in the kinetochore-snapshot assay but with the peak shifted ~100 nm toward larger distances (Figure 1I). This arises from a 50–90 nm positional offset between the GFP tag on the C-terminus of Ndc80 and the center of the Tet-GFP operator array (Dorn et al., 2005; Joglekar et al., 2009) as well as from the apparent reduction in Spb42-CFP to Ndc80-GFP distance when projected on the SPB–SPB axis. These observations imply that one important contributor to the bilobed distribution of kinetochore proteins is the tight regulation of SPB–CEN distances for chromatids, regardless of their monopolar or bipolar attachment.
Live-cell tracking of CEN IV tags occasionally showed frames with ‘hyper-stretched’ tags (Figure S1F), where the pericentric DNA of the chromatid attached to the closer SPB remained compacted, while the pericentric DNA of the sister chromatid attached to the more distant pole unraveled under tension. Such asymmetric stretching is consistent with a model in which both chromatids can have unseparated arms in the same lobe while a microtubule from the distant pole pulls one of the two kinetochores into the opposite lobe. The model implies that cohesion remains intact along the arms of sisters that are sterically trapped in one spindle half while the pulling force from the microtubule of the distant pole is sufficient to unravel the pericentric chromatin of one sister (Yeh et al., 2008).
Together, these CEN IV-tracking data showed that the establishment of bipolarity occurred at random times during mitotic progression, and with some cells not establishing biorientation of CEN IV until late in mitosis. However, our 47 live-cell movies were acquired with the goal of obtaining an almost equal number of cells with separated and unseparated tags (Figures S2A, S2B). Analysis of individual frames showed that separated tags were likely to be underrepresented (Figures S2C, S2D). Therefore, we turned to the CEN IV-snapshot assay for an unbiased sampling of the extent of chromatid separation.
Biorientation is established gradually up to anaphase onset
We imaged CEN IV tags relative to SPBs in an asynchronous population of n = 828 cells (see Table S1; Figures S2E–S2H). Since the sampling was random with respect to the cell cycle, the frequency of occurrence of a particular spindle length was inversely proportional to the rate of spindle elongation at this length. The observed peak in the spindle length at ~1.4 µm arises because spindle elongation slowed down at this stage of mitosis (Figure 1J). Beyond this length the distribution monotonically decreased until a spindle length ~2 µm, at which point cells entered anaphase (Figure 1K, and Movie S2). The fraction of spindles having separated CEN IV tags provided a direct measure of the probability of biorientation throughout mitosis (Figure 1L). Our analysis explicitly accounted for the possibility that spindles in which only one CEN IV spot was detected represented configurations with bioriented attachment, but that the signal arising from one tag was undetectable either because of hyper-stretching, or because the CEN IV tag was unresolvable from the SPB (Figure 1L, dark red; Figure S2; Supplementary Information). Using these approaches, we estimated that for spindle lengths around ~1– 1.2 µm, ~50% of the CEN IV tags were bioriented and this percentage increased to about 80% at 1.6 µm. Cells reached a state of complete bipolarity only at ~2 µm, shortly before anaphase onset.
The quasi-exponential decay of the fraction of separated tags would be consistent with the notion that bipolarity is established in a random process throughout mitosis. Nonetheless, we were concerned that the CENIV-snapshot assay could lead to an underestimation of the fraction of sisters with separated tags because of the asymmetric location of the 11kb TetO-array on one of the chromosome arms (Figure S3A). It is conceivable that sister kinetochores separate by unraveling pericentric DNA without breaking down cohesion in the region of the array insert. To address this issue we repeated our measurements in a strain in which the tag involved symmetric insertion of two short ~6kb TetO repeats −360bp and +370bp from CEN IV (Figure S3A). The resulting spot signal displayed a compact, subresolution distribution of TetR-GFPs (Movie S4), which we verified by comparing the residuals of fitting the spot with a 3D point spread function (PSF) to background noise (Thomann et al., 2002). Contrary to the previously used 11kb TetO-array, the centroids of each of these symmetric TetO-arrays are located at 3.4 kb, which is outside the cohesive part of bioriented chromosome arms (estimated to start at 4 kb, (Pearson et al., 2001)). Accordingly, more than half (~60%) of the arrays locate in the pericentric chromatin region that follows the separating kinetochore, but occasionally may also be stretched out in a bipolar attachment. Therefore, while it is still possible with the symmetric tag design that bioriented sisters generate a single fusion spot of both tags, the probability of unresolved tag separation during biorientation is much lower, especially for longer spindles shortly before anaphase, and the fusion spot will not have the properties of a diffraction-limited signal (Figure S3B). Stretched fusion spots were identified by the PSF fitting procedure either as a signal mixture of two separated, bioriented tags or as unclassifiable (Supplementary Information). Application of our super-resolution CEN IV-tracking assay to the symmetric tag strain confirmed with high-confidence that cells with unseparated CEN IV tags were present at long spindle lengths and that in this case the tag intensity was higher than in cells with separated tags (n = 94, Figures S3C–S3F). We also reproduced the finding from the CEN IV-snapshot assay using the symmetric tag strain that bona fide unseparated tags and thus mono-orientation existed until anaphase onset (n = 3817 cells, out of which 925 were in mitosis, see Table S1, Figures S3G and S3H).
Taken together, these experiments demonstrate that biorientation occurs gradually in budding yeast during a period of prometaphase that is 20–25 min in duration, followed by a much shorter metaphase, if any, in which biorientation is complete but anaphase has not yet begun; anaphase ensues soon after that last sister chromatid is bioriented. Interestingly, the proposed ~25 min duration of prometaphase in S. cerevisiae is similar to that of prometaphase in mammalian cells but includes the majority of the ~30 min length of yeast mitosis, while in mammalian cells prometaphase covers only a fraction of the 120 min of mitosis. This suggests that mechanisms of biorientation during prometaphase are conserved between yeast and mammalian cells, whereas the roles of metaphase may differ.
Syntelic attachments in ipl1-321 mutant cells have tightly constrained SPB–CEN distances
Our hypothesis that a bilobed kinetochore distribution can arise from roughly equal partitioning of sister chromatids having syntelic attachment to the two SPBs demands that the SPB–CEN distance of syntelic attachments be tightly regulated. To determine if this is true we examined mutants of Ipl1p, a key player in the resolution of syntelic attachment (Biggins et al., 1999; Tanaka et al., 2002). In ipl1-321 cells, which carry a temperature sensitive loss-of-function mutation (Biggins et al., 1999), failure to resolve syntely at the restrictive temperature (37°C) leads to asymmetric chromosome segregation in 70–85% of cells (Biggins et al., 1999; Kim et al., 1999; Pinsky et al., 2003; Tanaka et al., 2002). Consistent with this, when we applied the kinetochore-snapshot assay to ipl1-321 cells we observed kinetochore distributions that were much more asymmetric than in wild-type (WT) cells (Figures 2A and 2B). CEN IV-tracking showed that the majority of sisters had unseparated tags (Figures 2C, Movie S5) with an SPB–CEN IV distance of 0.53 ± 0.07 µm.
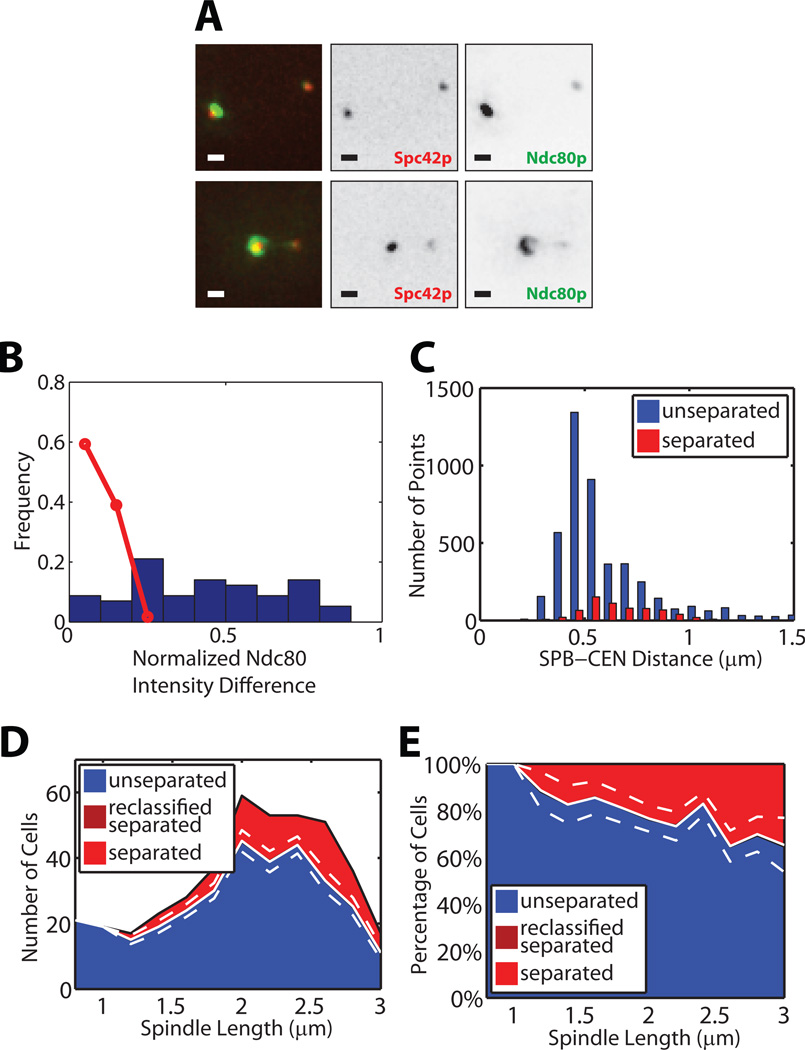
Figure 2. ipl1-321 cells show asymmetric kinetochore distributions, but have tightly regulated kinetochore-microtubule lengths.
(A) Representative intensity images (analogous to Figure 1A) of Spc42 and Ndc80 in ipl1-321 cells. Scale bar 0.5 µm.
(B) Distribution of the relative intensity difference between Ndc80 bilobes (analogous to Figure 1D for WT cells). Data from n=58 cells. Red line, distribution in WT cells.
(C) Histograms of SPB–CEN distances in cells with unseparated tags (blue) and separated tags (red) (analogous to Figure 1H for WT cells).
(D, E) Raw (D) and normalized (E) distributions of spindle lengths and classification into unseparated (blue) and separated (light red) CEN IV tags; ipl1-321 cells do not contain spindles with reclassified CEN IV tags. White solid and dashed lines indicate mean ± bootstrapped SD of syntely (see Figures 1J and 1L).
See also Tables S1 and S2, and Movie S5.
CEN IV-snapshot assays of asynchronous ipl1-321 cell populations revealed a very different distribution of spindle lengths as compared to WT cells (Figure 2D). Whereas spindle length peaked at ~1.4 µm in WT cells, it peaked at 2.0–2.5 µm in ipl1-321 cells, and fell monotonically to a maximum SPB–SPB distance of 3 µm, a length at which WT cells are well into anaphase. The differences in elongation dynamics may be related to aberrant operation of the spindle checkpoint (Pinsky and Biggins, 2005) or rapid progress to 2 µm length followed by a delay for recruitment of factors required for anaphase onset. Regardless, by anaphase onset in ipl1-321 cells, we observed that only ~30% of the initially monopolar attachments had been resolved into bipolar attachments (Figure 2E).
Despite their predominant syntely (Tanaka et al., 2002), the SPB–CEN IV distance of ipl1-321 cells was tightly regulated, as evidenced by a sharp peak in the length distribution at 0.5 µm (Figure 2C). Remarkably, the position centers of unseparated and separated CEN IV tags perfectly colocalized with the position centers of unseparated and separated tags in WT cells at 37°C (Table S2; the difference in SPB–CEN distance between room temperature and 37°C arises from differences in microtubule dynamics at elevated temperatures (Dorn et al., 2005; Jaqaman et al., 2006)). This provides evidence that the bilobed intensity distribution results from the tight regulation of SPB–CEN distances, rather than from sister chromatid separation due to biorientation.
stu2-277 mutant cells fail to establish bipolar attachments yet exhibit a bilobed kinetochore distribution
An alternative way to probe the connection between bipolarity and bilobed kinetochore distributions is to examine mutations in which defects in kMT dynamics interfere with kinetochore-microtubule capture. We studied this in cells carrying a mutation in the microtubule associated protein XMAP215/Stu2p (Brouhard et al., 2008; Vasquez et al., 1994; Wang and Huffaker, 1997). While stu2-279 or stu2-277 cells establish bilobed kinetochore distributions at the restrictive temperature (Gillett et al., 2004), they arrest in a checkpoint-dependent fashion (He et al., 2001; Severin et al., 2001), which arises from a defect in kMT dynamics (Pearson et al., 2003). We confirmed that stu2-277 cells at 37°C exhibited bilobed kinetochore distributions (Figure 3A). Regardless of point in mitosis kinetochore lobes were ~ 0.4 µm from the SPBs (Figure 3B). Analysis of 55 cells showed that stu2-277 cells often had kinetochore lobes that spread perpendicular to the spindle axis (Figure 3A) and that were less symmetric across the spindle midzone than those in WT cells (Figure 3C), although more symmetric than in ipl1-321 cells. CEN IV-tracking in live cells (Movie S6) showed that in stu2-277 cells the SPB–CEN IV distance was tightly controlled with a mean value of 0.51 ± 0.06 µm (a value similar to WT and ipl1-321 cells; Figure 3D; Table S2). The majority of cells (38 of 50) had unseparated tags (Figure 3E), suggesting that in stu2-277 cells a nearly symmetric bilobed distribution of kinetochores can arise early in mitosis even in the absence of sister separation and bipolarity. A minority of cells (6 of 50) contained separated CEN IV tags (Movie S6), while a further set of 6 cells contained CEN IV tags that lay well away from the spindle axis in a position that is characteristic of detached chromosomes (Movie S6). Consistent with our data on WT and ipl1-321 cells, the SPB–CEN IV distance distributions for unseparated and separated tags in stu2-277 cells strongly overlapped (Figure 3D), supporting the notion that each of the two lobes of Ndc80-GFP intensity contains a mixture of mono- and bioriented kinetochores.
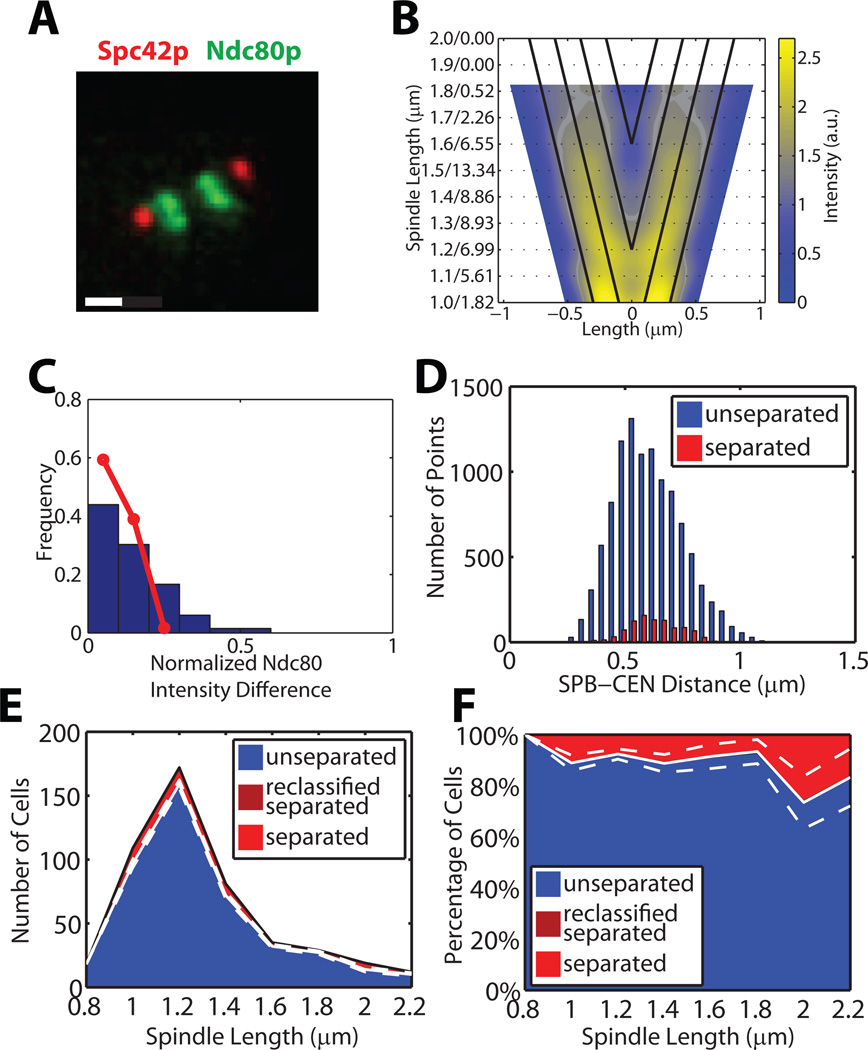
Figure 3. stu2-277 cells show symmetric kinetochore distributions and have regulated kinetochore-microtubule length, but establish few bipolar attachments.
(A) Representative intensity images (analogous to Figure 1A) of Spc42 and Ndc80 in stu2-277 cells. Scale bar 0.5 µm.
(B) Symmetrized Ndc80p distributions of n = 55 stu2-277 cells (analogous to Figure 1C for WT cells).
(C) Distribution of the relative intensity difference between Ndc80p bilobes (analogous to Figure 1D for WT cells). Red line, distribution in WT cells.
(D) Histograms of SPB–CEN distances in cells with unseparated tags (blue) and separated tags (red) (analogous to Figure 1H for WT cells).
(E, F) Raw (E) and normalized (F) distributions of spindle lengths and classification into unseparated (blue) and separated (light red) CEN IV tags; cells do not contain spindles with reclassified CEN IV tags. White solid and dashed lines indicate mean ± bootstrapped SD of cells with syntely (see Figures 1J and 1L).
See also Figure S4, Tables S1 and S2, and Movie S6.
The CEN IV-snapshot assay of asynchronous stu2-277 cell populations showed that spindle elongation slows at 1.3 to 1.4 µm length (Figure 3E). Compared to WT, the peak is narrower and higher, suggesting that stu2-277 cells spend more time at this stage of mitosis. About 90% of the spindles had unseparated tags throughout mitosis (Figure 3F, Table S1), confirming that in these cells the Ndc80-GFP bilobes consist primarily of mono-oriented kinetochores.
To further elucidate the impact that functional impairment of a MT-associated protein can have on spindle organization, we analyzed the tubulin distribution along the SPB–SPB axis (Figure S4). Both WT and stu2-277 cells showed intensity maxima 0.25–0.35 µm from the SPBs for all spindle lengths, consistent with a model in which most spindle microtubules correspond to kMTs ending at the kinetochore bilobes, while a few interpolar microtubules cross the spindle midzone and stretch between the poles. In WT cells, tubulin intensity maxima colocalized with the kinetochore lobes and were about twice as high as the midzone intensity for spindles in the length range 1.1–1.9 µm. This is consistent with the idea that each lobe is associated with 16 kMTs and the midzone with 8 microtubules. In contrast, stu2-277 cells had a maxima-to-midzone intensity ratio of ~1.25 for spindles up to 1.6 µm in length, suggesting that only ~10 kMTs end in each of the bilobes. Together with our finding that most kinetochores in one lobe belonged to unseparated sisters with a monopolar attachment, this implies that the majority of these attachments are monotelic. Thus, although defects in STU2 function have little effect on the length regulation of kMTs, they may prevent MTs from growing to a length where they can reach from one SPB across the midzone to capture kinetochores in a distant lobe. Consequently, correction of syntely is dramatically impaired.
cin8Δ mutant cells exhibit a weaker bilobed distribution of kinetochores due to less-regulated SPB–CEN distance
Our data thus far suggest that the bilobed distribution of kinetochores is the result of tight kMT length regulation, regardless of whether sister chromatids have achieved bipolar attachment. To test the consequences of disrupting this regulation we deleted the kinesin-5 motor protein Cin8p, which has been shown to control kMT length (Gardner et al., 2008) and the processes that shape the bilobed kinetochore distribution (Gardner et al., 2008; Tytell and Sorger, 2006).
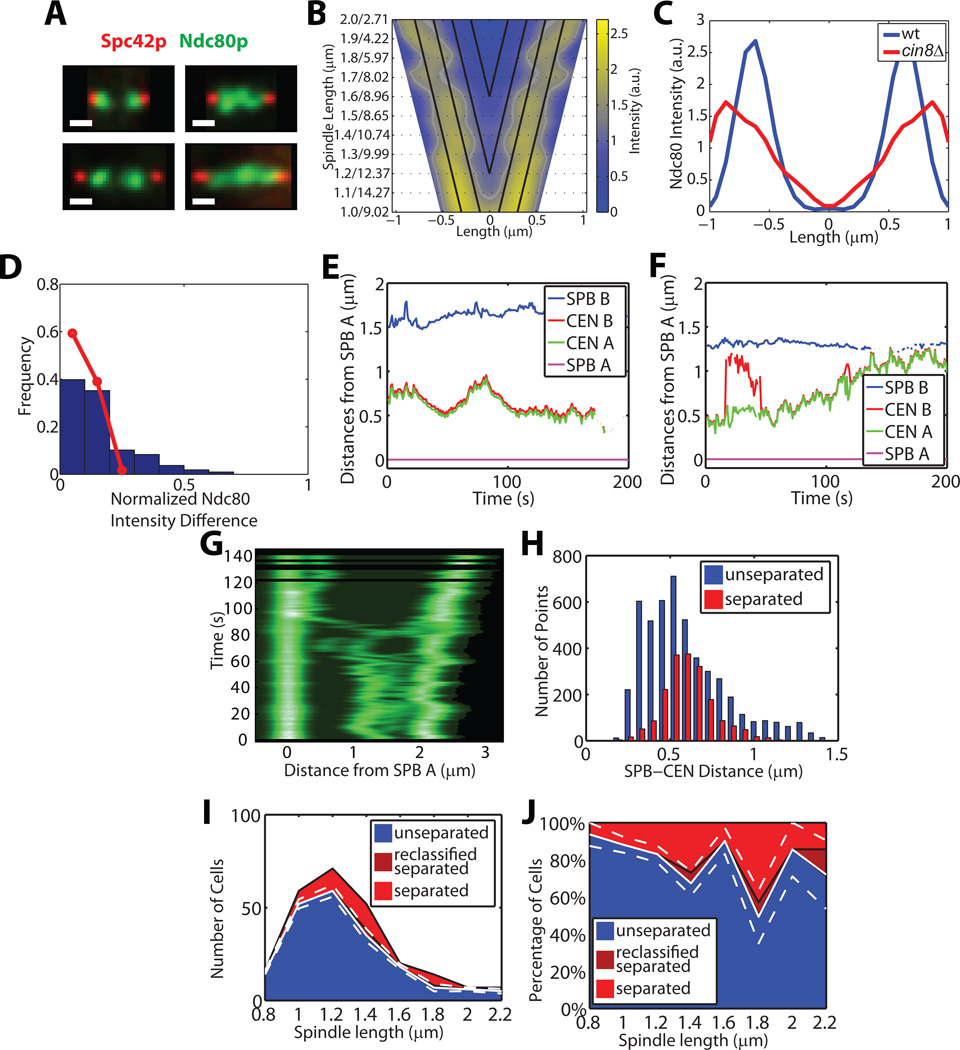
Consistent with previous reports, our assay revealed a looser and less clearly defined kinetochore distribution in cin8Δ than in WT cells (Figures 4A–4C, n = 104 cells), although a bilobed distribution was visible in some cells (Figure 4A) with Ndc80-GFP intensity peaking ~0.30 µm from the SPBs (Figure 4B). The maxima were less well-defined than in WT cells, especially for spindles with a length close to ~2.0 µm (Figure 4C), However, cin8Δ cells had nearly equal numbers of kinetochores attached to each SPB (Figure 4D). In agreement with these data, live-cell trajectories displayed increased fluctuations in the SPB–CEN IV distances in cin8Δ cells (n = 25 cells) (Figures 4E–4G) and higher growth and shrinkage speeds than in WT cells (Table S2). The larger CEN IV tag displacements in cin8Δ cells were accompanied by frequent spindle midzone crossings(60% in cin8Δ cells compared to 11% in WT; Figures 4E and 4F; Movie S7; Table S2), and resulted in a longer tail in the SPB–CEN IV distance distribution (Figure 4H). We also observed more transient separation and re-joining of CEN IV tags before anaphase (Figure 4G and Movie S7). A substantial portion of the tags remained unseparated until anaphase in cin8Δ cells (Figures 4I and 4J). Since cin8Δ cells have a low rate of chromosome loss (Hoyt et al., 1992), it cannot be that all sister chromatids with unseparated tags have monotelic or syntelic attachments. Instead, in the absence of Cin8p, tension across chromatids with bipolar attachments is reduced and the length of kMTs is aberrant. The presence of tensionless bipolar attachments implies that kinetochore sisters in the same lobe have one long kMT emanating from the opposite SPB. In agreement with this, the tubulin distribution revealed higher intensities in the spindle midzone (Figure S4C).
Figure 4. cin8Δ cells show symmetric kinetochore distributions and have less regulated kinetochore-microtubule lengths, leading to transient fusion of bioriented sister kinetochores.
(A) Representative intensity images (analogous to Figure 1A) of Spc42 and Ndc80 in cin8Δ cells. Scale bar 0.5 µm.
(B) Symmetrized Ndc80p distributions of n = 104 cin8Δ cells (analogous to Figure 1C for WT cells).
(C) Comparison of the WT (blue) and cin8Δ (red) Ndc80p distributions at 2 µm spindle length.
(D) Distribution of the relative intensity difference between Ndc80p bilobes (analogous to Figure 1D for WT cells). Red line, distribution in WT cells.
(E, F) Time courses of distances from SPB A to other tags (analogous to Figure 1F) for two cells with spindle midzone crossings by CEN IV tags. The example (F) shows a cell with transient separation and fusion of sister CEN IV tags, suggesting that the sisters have established biorientation but the kinetochore-microtubule length is deregulated.
(G) Kymograph aligned with respect to the position of SPB A of a cin8Δ cell entering anaphase at time ~80 s. At T = 60 s the CEN IV tags separate but both cross the spindle midzone, then fuse again and cross back on the other side (Time = 76 s), before they separate permanently.
(H) Histograms of SPB–CEN distances in cells with unseparated tags (blue) and separated tags (red) (analogous to Figure 1H for WT cells).
(I, J) Raw (I) and normalized (J) distributions of spindle lengths and their classification into spindles with unseparated (blue), separated (light red), and reclassified as separated (dark red) CEN IV tags. White solid and dashed lines indicate mean ± bootstrapped SD of cells with syntely (see Figures 1J and 1L). See also Tables S1 and S2, and Movie S7.
DISCUSSION
In this paper we provide evidence that biorientation of S. cerevisiae chromosomes is achieved gradually over an extended period of the cell cycle from S-phase to anaphase onset and thus that the fundamental features of progressive biorientation are conserved between yeast and man. Pairs of sister kinetochores enter S. cerevisiae mitosis with a syntelic rather than a monopolar orientation as in mammalian cells, but in both cases we propose that biorientation requires kinetochore-microtubule capture, the imposition of pulling forces and separation of centromere-proximal but not distal chromatin. These findings augment the relevance of mechanistic information obtained from yeast, which offers powerful genetics and a much simpler spindle geometry for the analysis of the molecular regulation of mitotic processes.
We arrived at this alternate model of yeast mitosis by combining live-cell tracking of single kinetochore pairs with unbiased statistical assays of single kinetochore localization and kinetochore population distributions in large numbers of wild-type and ipl1-321, stu2-277, and cin8Δ mutant cells. Our model implies that the characteristic bilobed distribution of kinetochore proteins visible throughout mitosis does not arise from two sets of separated sister chromatids as commonly assumed; instead, each lobe contains a mixture of bioriented and syntelic pairs until just prior to anaphase, at which point all pairs achieve biorientation.
We also find that the conserved feature of the bilobed kinetochore geometry is not the distance between the lobes (which increases over the course of mitosis) but rather the distance between each lobe and the spindle pole it is associated with, which remains constant at ~300–400 nm from S-phase to anaphase. Our data suggest that this arises from tight regulation of the length of kMTs. We have previously shown that in G1, SPB–CEN distances also measure ~400 nm (Dorn et al., 2005) implying that kMT length may be controlled by a universal mechanism that is independent of the cell cycle phase and the geometry of chromosome-MT attachment. Recent studies in vivo and in vitro have identified the kinesins CIN8 and KIP3 as plausible candidates for length-sensitive regulators of kMT dynamics (Gardner et al., 2008; Su et al., 2011; Varga et al., 2009) and our experiments with cin8Δ mutants indeed show that the maintenance of the characteristic kinetochore bilobes requires correct regulation of kMT dynamics at a set length of 300–400 nm.
Implications for the role of Ipl1/Aurora B in yeast mitosis
The model in which yeast kinetochores biorient early in mitosis posed several puzzles: 1) If all kinetochores are bioriented immediately after S-phase, why does it take ~90 minutes until anaphase onset? 2) What, if not monitoring a gradual biorientation, is the function of the many components of the spindle assembly checkpoint that are conserved between yeast and man? 3) Why are kinetochore proteins and pathways that serve the resolution of syntely in mammalian cells highly conserved in yeast? This applies in particular to the Ipl1/Aurora B kinase, which in mammalian cells phosphorylates components of tension-free sister kinetochores to promote release of erroneous microtubule attachments (Cimini et al., 2006; Knowlton et al., 2006). Loss of function mutants of Ipl1p dramatically increase aneuploidy in S. cerevisiae (Chan and Botstein, 1993), and prevent meiosis progression (Meyer et al., 2013), suggesting that error-correction is required also in yeast to ensure proper segregation of sister chromatids. Whether the function of IPL1 is restricted to only the earliest moments of mitosis as implied by the early-biorientation model, or is essential throughout, had yet to be determined.
No tension can be exerted on bipolar attachments until the spindle length is more than twice the kMT length. This implies that a tension-sensitive, Ipl1p-dependent error correction mechanism would continuously promote detachment and recapture of kMTs regardless of attachment status until the spindle is ≥1 µm long (Figure 5A). A priori, such repeated rounds of random detachment and reattachment would isomerize the attachment geometry, resulting in spindles with an equal probability for each of the 32 kinetochores to get linked to either of the two SPBs (50% bioriented, 50% syntelic divided equally among the two SPBs). Consistent with this hypothesis, for WT spindles ~1.2 µm in length, we observed a 50%:50% split between unseparated (syntelic) and separated (bioriented) CEN IV tags (Figure 1L) and a symmetric distribution of Ndc80-GFP intensity into two lobes (Figure S1B). In this model Ipl1p is essential for the isomerization of attachments (Figure 5B). In agreement with this, ipl1-321 cells displayed highly asymmetric Ndc80-GFP intensity distributions (Figures 2A and 2B) and nearly 100% of the ~1 µm spindles contained unseparated tags (Figure 2E). Remarkably, these cells also maintained SPB–CEN distances at ~0.4 µm (Table S2 and Figures 2C and 5B) showing that Ipl1p is not critically implicated in microtubule length control.
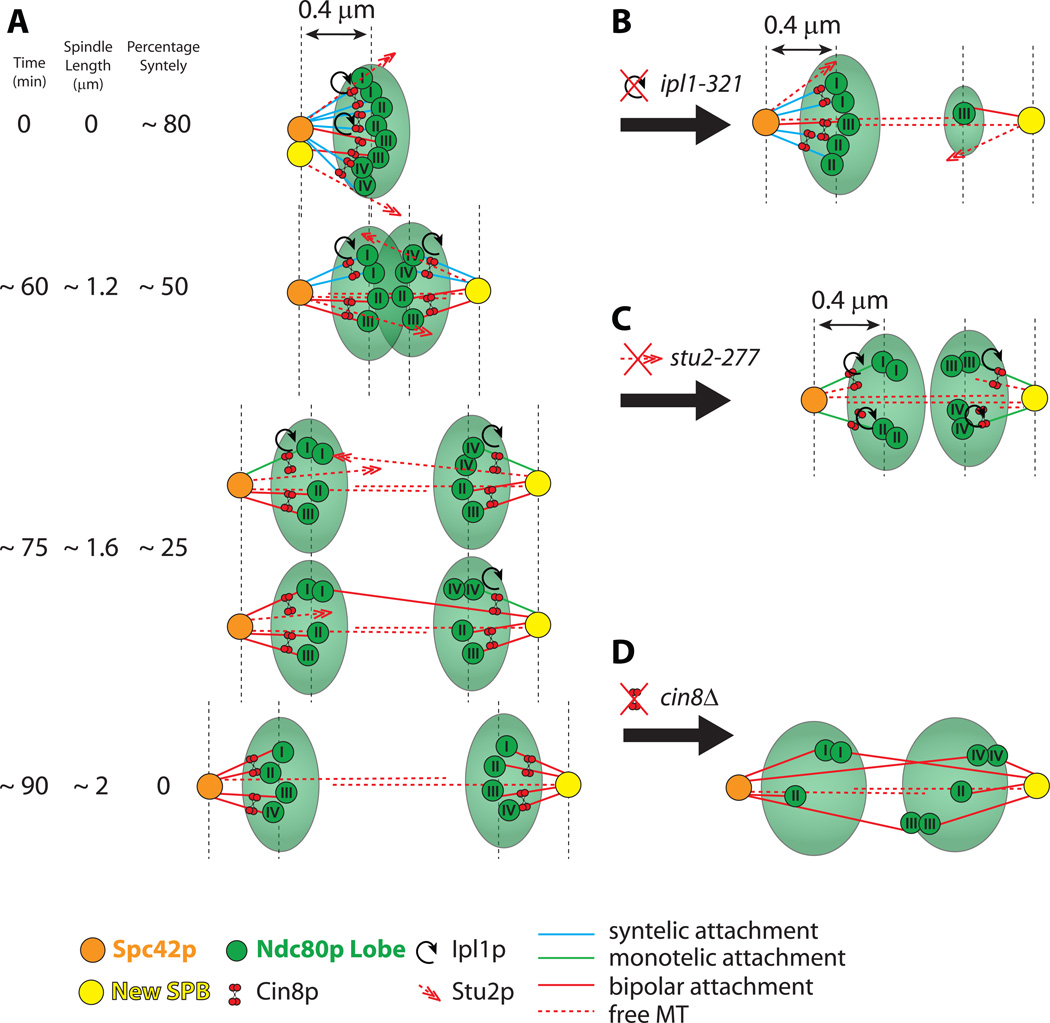
Figure 5. Model for mitosis progression and bipolarity establishment in budding yeast.
(A) Schematic mitotic progression (from top to bottom) and bipolarity establishment in WT cells budding yeast.
(B) Model changes in ipl1-321 cells. Cells have a large percentage of syntelic attachments to one SPB, and as a result have an asymmetric Ndc80p distribution.
(C) Model changes in stu2-277 cells. Cells have equal numbers of attachments to both SPBs, but have a substantial fraction of monotelic attachments. The overall Ndc80p distribution is bilobed.
(D) Model changes in cin8Δ cells. Cells establish bipolar attachments but do not constrain kinetochore microtubule lengths. This results in wider Ndc80p bilobes and frequent crossings of the spindle midzone by centromere tags.
See also Figure S5
Given that kMT lengths are regulated at ~400 nm, sisters with bipolar attachment begin to sense tension in spindles >1 µm in length. It is well established that in this configuration each of the sister kinetochores pulls centromere-flanking DNA into a C-loop while the sister-chromatid arms remain paired by cohesion (Pearson et al., 2001; Yeh et al., 2008). As a result of spindle elongation sister kinetochores are no longer exposed to Ipl1p activity, which remains associated with the cohesive portion of the chromatin (Tanaka et al., 2002). Bioriented sisters therefore have more stable attachments than syntelic attachments, which continue to isomerize in a stochastic process under the influence of Ipl1p. Consistent with the notion of a purely random syntely resolution without the need for any decision-making by “smart kinetochores” (Indjeian and Murray, 2007), the fraction of unseparated CENIV tags decreased over time in a exponential decay curve with first order kinetics (Figure 1L). After converting spindle elongation as the scale of mitotic progression into time (Supplementary Information) we found that the characteristic time for the resolution of one syntelic attachment is ~800 s (Figure S5A). This time scale increased by one order of magnitude to ~8000 s in ipl1-321strains (Figure S5B), indicating that Ipl1p plays a prominent role during all of mitotic progression. In this model deregulation of the SPB–CEN distance, as we observed in cin8Δ strains, would be expected to compromise the discrimination between bioriented and syntelic chromatids. Indeed, while cin8Δ alone has a fairly mild phenotype, the synthetic lethality of cin8Δ with deletion of the checkpoint component Mad2 (Geiser et al., 1997) supports such a defect.
Implications for recapture of monotelic attachments
Our model implies that the later syntely is corrected, the longer the MTs must be that project from an SPB to the more distant kinetochore lobe. Consistent with this idea, our data show that establishment of spindle bipolarity is substantially compromised in stu2-277 mutants, which have fewer long microtubules than WT cells (Figure 5C). We also found that the ratio between kMTs and interpolar microtubules is much lower in stu2-277 than in WT cells (Figure S4B), suggesting that a significant number of the mono-oriented attachments are not syntelic but monotelic. We surmise that STU2 mutations lower the probability for formation and capture of long kMTs from the distant pole and for binding to kMTs from the proximal pole. Consistent with this proposal, we and others have noted an increase in the number of detached sister pairs in stu2-277 cells; the kinetochores on these detached chromosomes (but curiously not those lying within the kinetochore lobes) bind Mad2p and provoke checkpoint-dependent mitotic arrest (Gillett et al., 2004). Based on our data it seems that kMTs are unable to reach across the spindle midzone and capture distant kinetochores, leaving most chromatid pairs mono-oriented.
Implications for spindle organization
Our model predicts that kMTs must transiently project from one SPB across the spindle midzone to the kinetochore cluster near the opposite SPB. We know that there cannot be too many of these kMTs at any one time, because EM reveals very few microtubules at the midzone beyond the two sets of four that run from pole to pole (Winey et al., 1995). However, given the fast MT turnover relative to the size of the nucleus (Table S2), long “projecting” MTs do not last for more than a few seconds and are unlikely to be captured by the EM snapshot of the MT configuration. The model also suggests an alternative explanation for the observed recovery of kinetochore protein fluorescence following photobleaching of one of the two lobes (Pearson et al., 2004). This was originally interpreted as reflecting midzone crossing by kinetochores having bipolar attachments but we propose that it actually arises when kinetochores transition from syntelic to bipolar attachment and thus one of them changes spindle side. Indeed, predicted recovery rates based on our direct analysis of syntely resolution agree quantitatively with the measurements of fluorescent recovery after photobleaching (FRAP) published by (Pearson et al., 2004) (see Supplemental Experimental Procedures and Figure S3J).
Our model further predicts that the lower rates of FRAP observed in STU2 mutant cells (Kosco et al., 2001) do not originate from lower kMT dynamics, but from the lack of the long projecting microtubules necessary to convert monopolar to bipolar attachments. Consistent with this, measurements of chromosomal directional instability show very similar switching between growth and shrinkage and vice versa for WT and stu2-277 cells (Table S2). Hence loss of function of Stu2p does not primarily affect the dynamics of attached kMTs, but specifically lowers the efficiency of growth of unattached spindle MTs.
Relation between kinetochore attachment and aneuploidy
Cancer cells characterized by persistent aneuploidy have hyperstable kinetochore-kMT attachments (Bakhoum et al., 2009). Our model provides a possible explanation for this curious behavior as it demonstrates that the resolution of syntely is sensitive to the rate of kinetochore detachment. Studies of tetraploid cells in yeast (Mayer and Aguilera, 1990; Storchova et al., 2006) and mice (Fujiwara et al., 2005) have shown that increases in ploidy compromise the fidelity of chromosome segregation. Our model offers a possible explanation also for this observation. The greater the number of chromosomes in a cell, the greater the number of syntelic attachments, and the longer it takes to convert them into bipolar attachments. This increase in the time required for bipolarity establishment, accompanied by gradual adaptation of the spindle checkpoint (Rudner and Murray, 1996) would result in increased rates of missegregation. Thus, the current analysis of mitotic progression and chromosome biorientation in yeast unifies our understanding of mitosis in simple and complex eukaryotes and suggests several testable hypotheses about the origins of chromosome missegregation and aneuploidy in general.
EXPERIMENTAL PROCEDURES
Yeast strains and growth conditions
Yeast strains were grown and prepared for microscopy using standard conditions (Rines et al., 2004). See Extended Experimental Procedures for details.
Microscopy
All images were acquired using DeltaVision microscopes with a 100× lens and a Photometrics CoolSnap HQ camera. See Extended Experimental Procedures for details.
Image and data analysis
Kinetochore-snapshot assay
Our goal was to measure and visualize the distribution of kinetochores along the spindle as a function of spindle length. An analogous procedure was followed to analyze tubulin. In general terms, we detected SPBs and extracted Ndc80-GFP or Tub1-GFP intensities as in (Sprague et al., 2003). However, intensities were extracted from 3D stacks to capture all of the signal (see Extended Experimental Procedures). We visualized the distribution of kinetochores or tubulin as a function of spindle length using V-plots, in which the fluorescence for a given spindle length is plotted along a horizontal strip, with color encoding the intensity (see Extended Experimental Procedures).
CEN IV-tracking assay
Our goal was to track the dynamic behavior of an individual chromosome for 100–300 s in mitosis. SPBs and CEN IV tag positions were determined in 3D using a modified version of the super-resolution spot detection approach in (Dorn et al., 2005), allowing us to quantify the dynamics of chromosomal directional instability (see Extended Experimental Procedures). We considered that a CEN crossed the midzone if it was observed for at least 5 frames on each side of the spindle equator, defined as the middle 20% of the spindle length.
CEN IV-snapshot assay
To map out the progression of establishing bipolar attachments, we detected the presence or absence of sister separation in chromosome IV in large cell populations and related the state to the SPB–SPB distances, which served as a surrogate for mitotic progression. To measure separation of CEN IV tags, as well as SPB–CEN distances it was critical to use super-resolution methods (see Extended Experimental Procedures). We applied the algorithm described in (Thomann et al., 2002) to separate overlapping point spread functions. Since the resolution gain by this method increases with higher signal-to-noise ratio, we imaged snapshots of many spindles at one time point with higher exposure times for additional photon collection. Tension across bioriented sisters sometimes unraveled the Tet-operator sequence in one of the two sisters, which would cause misclassifications of sisters as still unseparated. To correct such errors we developed an algorithm for reclassification of configurations with only three detectable spots based on the relative intensities between CEN and SPB tags (see Extended Experimental Procedures).
Supplementary Material
HIGHLIGHTS.
S. cerevisiae chromosomes biorient in a stochastic process until anaphase onset
Microtubule length control discriminates bioriented from syntelic attachments
Bilobed kinetochore distribution is not synonymous with biorientation
ACKNOWLEDGEMENTS
We thank T. Warsi, E. Gillett, M.C. Hou, M. Niepel, S. Ventz, A.W. Murray, D. Needleman and L. Serrano for discussions. E.M. gratefully acknowledges Guo-Cheng Yuan for his encouragement. This work was funded by the grant NIH R01 GM068956 to P.K.S. and G.D. E. M. was supported in part by a long-term fellowship from the Human Frontier Science Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bakhoum SF, Genovese G, Compton DA. Deviant kinetochore microtubule dynamics underlie chromosomal instability. Current biology : CB. 2009;19:1937–1942. doi: 10.1016/j.cub.2009.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggins S, Severin FF, Bhalla N, Sassoon I, Hyman AA, Murray AW. The conserved protein kinase Ipl1 regulates microtubule binding to kinetochores in budding yeast. Genes & development. 1999;13:532–544. doi: 10.1101/gad.13.5.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouhard GJ, Stear JH, Noetzel TL, Al-Bassam J, Kinoshita K, Harrison SC, Howard J, Hyman AA. XMAP215 is a processive microtubule polymerase. Cell. 2008;132:79–88. doi: 10.1016/j.cell.2007.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CS, Botstein D. Isolation and characterization of chromosome-gain and increase-in-ploidy mutants in yeast. Genetics. 1993;135:677–691. doi: 10.1093/genetics/135.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandhok NS, Pellman D. A little CIN may cost a lot: revisiting aneuploidy and cancer. Curr Opin Genet Dev. 2009;19:74–81. doi: 10.1016/j.gde.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Cimini D, Wan X, Hirel CB, Salmon ED. Aurora kinase promotes turnover of kinetochore microtubules to reduce chromosome segregation errors. Current biology : CB. 2006;16:1711–1718. doi: 10.1016/j.cub.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Dorn JF, Jaqaman K, Rines DR, Jelson GS, Sorger PK, Danuser G. Yeast kinetochore microtubule dynamics analyzed by high-resolution three-dimensional microscopy. Biophys J. 2005;89:2835–2854. doi: 10.1529/biophysj.104.058461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draviam VM, Xie S, Sorger PK. Chromosome segregation and genomic stability. Curr Opin Genet Dev. 2004;14:120–125. doi: 10.1016/j.gde.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Fujiwara T, Bandi M, Nitta M, Ivanova EV, Bronson RT, Pellman D. Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature. 2005;437:1043–1047. doi: 10.1038/nature04217. [DOI] [PubMed] [Google Scholar]
- Gardner MK, Bouck DC, Paliulis LV, Meehl JB, O'Toole ET, Haase J, Soubry A, Joglekar AP, Winey M, Salmon ED, et al. Chromosome congression by Kinesin-5 motor-mediated disassembly of longer kinetochore microtubules. Cell. 2008;135:894–906. doi: 10.1016/j.cell.2008.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner MK, Pearson CG, Sprague BL, Zarzar TR, Bloom K, Salmon ED, Odde DJ. Tension-dependent regulation of microtubule dynamics at kinetochores can explain metaphase congression in yeast. Mol Biol Cell. 2005;16:3764–3775. doi: 10.1091/mbc.E05-04-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiser JR, Schott EJ, Kingsbury TJ, Cole NB, Totis LJ, Bhattacharyya G, He L, Hoyt MA. Saccharomyces cerevisiae genes required in the absence of the CIN8-encoded spindle motor act in functionally diverse mitotic pathways. Mol Biol Cell. 1997;8:1035–1050. doi: 10.1091/mbc.8.6.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillett ES, Espelin CW, Sorger PK. Spindle checkpoint proteins and chromosome-microtubule attachment in budding yeast. J Cell Biol. 2004;164:535–546. doi: 10.1083/jcb.200308100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshima G, Yanagida M. Establishing biorientation occurs with precocious separation of the sister kinetochores, but not the arms, in the early spindle of budding yeast. Cell. 2000;100:619–633. doi: 10.1016/s0092-8674(00)80699-6. [DOI] [PubMed] [Google Scholar]
- He X, Asthana S, Sorger PK. Transient sister chromatid separation and elastic deformation of chromosomes during mitosis in budding yeast. Cell. 2000;101:763–775. doi: 10.1016/s0092-8674(00)80888-0. [DOI] [PubMed] [Google Scholar]
- He X, Rines DR, Espelin CW, Sorger PK. Molecular analysis of kinetochore-microtubule attachment in budding yeast. Cell. 2001;106:195–206. doi: 10.1016/s0092-8674(01)00438-x. [DOI] [PubMed] [Google Scholar]
- Hoyt MA, He L, Loo KK, Saunders WS. Two Saccharomyces cerevisiae kinesin-related gene products required for mitotic spindle assembly. J Cell Biol. 1992;118:109–120. doi: 10.1083/jcb.118.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland KM, Kingsbury J, Koshland D, Hieter P. Ctf19p: A novel kinetochore protein in Saccharomyces cerevisiae and a potential link between the kinetochore and mitotic spindle. J Cell Biol. 1999;145:15–28. doi: 10.1083/jcb.145.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indjeian VB, Murray AW. Budding yeast mitotic chromosomes have an intrinsic bias to biorient on the spindle. Current biology : CB. 2007;17:1837–1846. doi: 10.1016/j.cub.2007.09.056. [DOI] [PubMed] [Google Scholar]
- Jaqaman K, Dorn JF, Jelson GS, Tytell JD, Sorger PK, Danuser G. Comparative autoregressive moving average analysis of kinetochore microtubule dynamics in yeast. Biophys J. 2006;91:2312–2325. doi: 10.1529/biophysj.106.080333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaspersen SL, Winey M. The budding yeast spindle pole body: structure, duplication, and function. Annual review of cell and developmental biology. 2004;20:1–28. doi: 10.1146/annurev.cellbio.20.022003.114106. [DOI] [PubMed] [Google Scholar]
- Joglekar AP, Bloom K, Salmon ED. In vivo protein architecture of the eukaryotic kinetochore with nanometer scale accuracy. Current biology : CB. 2009;19:694–699. doi: 10.1016/j.cub.2009.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Kang JS, Chan CS. Sli15 associates with the ipl1 protein kinase to promote proper chromosome segregation in Saccharomyces cerevisiae. J Cell Biol. 1999;145:1381–1394. doi: 10.1083/jcb.145.7.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa K, Hieter P. Evolutionary conservation between budding yeast and human kinetochores. Nature reviews Molecular cell biology. 2001;2:678–687. doi: 10.1038/35089568. [DOI] [PubMed] [Google Scholar]
- Knowlton AL, Lan W, Stukenberg PT. Aurora B is enriched at merotelic attachment sites, where it regulates MCAK. Current biology : CB. 2006;16:1705–1710. doi: 10.1016/j.cub.2006.07.057. [DOI] [PubMed] [Google Scholar]
- Kosco KA, Pearson CG, Maddox PS, Wang PJ, Adams IR, Salmon ED, Bloom K, Huffaker TC. Control of microtubule dynamics by Stu2p is essential for spindle orientation and metaphase chromosome alignment in yeast. Mol Biol Cell. 2001;12:2870–2880. doi: 10.1091/mbc.12.9.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maure JF, Kitamura E, Tanaka TU. Mps1 kinase promotes sister-kinetochore biorientation by a tension-dependent mechanism. Current biology : CB. 2007;17:2175–2182. doi: 10.1016/j.cub.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer VW, Aguilera A. High levels of chromosome instability in polyploids of Saccharomyces cerevisiae. Mutation research. 1990;231:177–186. doi: 10.1016/0027-5107(90)90024-x. [DOI] [PubMed] [Google Scholar]
- Meyer RE, Kim S, Obeso D, Straight PD, Winey M, Dawson DS. Mps1 and Ipl1/Aurora B act sequentially to correctly orient chromosomes on the meiotic spindle of budding yeast. Science. 2013;339:1071–1074. doi: 10.1126/science.1232518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Toole ET, Winey M, McIntosh JR. High-voltage electron tomography of spindle pole bodies and early mitotic spindles in the yeast Saccharomyces cerevisiae. Mol Biol Cell. 1999;10:2017–2031. doi: 10.1091/mbc.10.6.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson CG, Maddox PS, Salmon ED, Bloom K. Budding yeast chromosome structure and dynamics during mitosis. J Cell Biol. 2001;152:1255–1266. doi: 10.1083/jcb.152.6.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson CG, Maddox PS, Zarzar TR, Salmon ED, Bloom K. Yeast kinetochores do not stabilize Stu2p-dependent spindle microtubule dynamics. Mol Biol Cell. 2003;14:4181–4195. doi: 10.1091/mbc.E03-03-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson CG, Yeh E, Gardner M, Odde D, Salmon ED, Bloom K. Stable kinetochore-microtubule attachment constrains centromere positioning in metaphase. Current biology : CB. 2004;14:1962–1967. doi: 10.1016/j.cub.2004.09.086. [DOI] [PubMed] [Google Scholar]
- Pinsky BA, Biggins S. The spindle checkpoint: tension versus attachment. Trends in cell biology. 2005;15:486–493. doi: 10.1016/j.tcb.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Pinsky BA, Tatsutani SY, Collins KA, Biggins S. An Mtw1 complex promotes kinetochore biorientation that is monitored by the Ipl1/Aurora protein kinase. Dev Cell. 2003;5:735–745. doi: 10.1016/s1534-5807(03)00322-8. [DOI] [PubMed] [Google Scholar]
- Rines D, Thomann D, Dorn J, Goodwin P, Sorger PK. Live cell imaging of yeast. In: Goldman RD, Spector DL, editors. Live Cell Imaging: A Laboratory Manual. Woodbury, NY: Cold Spring Harbor Laboratory Press; 2004. p. 631. [Google Scholar]
- Rudner AD, Murray AW. The spindle assembly checkpoint. Current opinion in cell biology. 1996;8:773–780. doi: 10.1016/s0955-0674(96)80077-9. [DOI] [PubMed] [Google Scholar]
- Severin F, Habermann B, Huffaker T, Hyman T. Stu2 promotes mitotic spindle elongation in anaphase. J Cell Biol. 2001;153:435–442. doi: 10.1083/jcb.153.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague BL, Pearson CG, Maddox PS, Bloom KS, Salmon ED, Odde DJ. Mechanisms of microtubule-based kinetochore positioning in the yeast metaphase spindle. Biophys J. 2003;84:3529–3546. doi: 10.1016/S0006-3495(03)75087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storchova Z, Breneman A, Cande J, Dunn J, Burbank K, O'Toole E, Pellman D. Genome-wide genetic analysis of polyploidy in yeast. Nature. 2006;443:541–547. doi: 10.1038/nature05178. [DOI] [PubMed] [Google Scholar]
- Su X, Qiu W, Gupta ML, Jr, Pereira-Leal JB, Reck-Peterson SL, Pellman D. Mechanisms underlying the dual-mode regulation of microtubule dynamics by Kip3/kinesin-8. Molecular cell. 2011;43:751–763. doi: 10.1016/j.molcel.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka TU, Rachidi N, Janke C, Pereira G, Galova M, Schiebel E, Stark MJ, Nasmyth K. Evidence that the Ipl1-Sli15 (Aurora kinase-INCENP) complex promotes chromosome biorientation by altering kinetochore-spindle pole connections. Cell. 2002;108:317–329. doi: 10.1016/s0092-8674(02)00633-5. [DOI] [PubMed] [Google Scholar]
- Thomann D, Dorn J, Sorger PK, Danuser G. Automatic fluorescent tag localization II: Improvement in super-resolution by relative tracking. J Microsc. 2003;211:230–248. doi: 10.1046/j.1365-2818.2003.01223.x. [DOI] [PubMed] [Google Scholar]
- Thomann D, Rines DR, Sorger PK, Danuser G. Automatic fluorescent tag detection in 3D with super-resolution: application to the analysis of chromosome movement. J Microsc. 2002;208:49–64. doi: 10.1046/j.1365-2818.2002.01066.x. [DOI] [PubMed] [Google Scholar]
- Thompson SL, Bakhoum SF, Compton DA. Mechanisms of chromosomal instability. Current biology : CB. 2010;20:R285–R295. doi: 10.1016/j.cub.2010.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tytell JD, Sorger PK. Analysis of kinesin motor function at budding yeast kinetochores. J Cell Biol. 2006;172:861–874. doi: 10.1083/jcb.200509101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga V, Leduc C, Bormuth V, Diez S, Howard J. Kinesin-8 motors act cooperatively to mediate length-dependent microtubule depolymerization. Cell. 2009;138:1174–1183. doi: 10.1016/j.cell.2009.07.032. [DOI] [PubMed] [Google Scholar]
- Vasquez RJ, Gard DL, Cassimeris L. XMAP from Xenopus eggs promotes rapid plus end assembly of microtubules and rapid microtubule polymer turnover. J Cell Biol. 1994;127:985–993. doi: 10.1083/jcb.127.4.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang PJ, Huffaker TC. Stu2p: A microtubule-binding protein that is an essential component of the yeast spindle pole body. J Cell Biol. 1997;139:1271–1280. doi: 10.1083/jcb.139.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winey M, Mamay CL, O'Toole ET, Mastronarde DN, Giddings TH, Jr, McDonald KL, McIntosh JR. Three-dimensional ultrastructural analysis of the Saccharomyces cerevisiae mitotic spindle. J Cell Biol. 1995;129:1601–1615. doi: 10.1083/jcb.129.6.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh E, Haase J, Paliulis LV, Joglekar A, Bond L, Bouck D, Salmon ED, Bloom KS. Pericentric chromatin is organized into an intramolecular loop in mitosis. Current biology : CB. 2008;18:81–90. doi: 10.1016/j.cub.2007.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Kahana JA, Silver PA, Morphew MK, McIntosh JR, Fitch IT, Carbon J, Saunders WS. Slk19p is a centromere protein that functions to stabilize mitotic spindles. J Cell Biol. 1999;146:415–425. doi: 10.1083/jcb.146.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.