Abstract
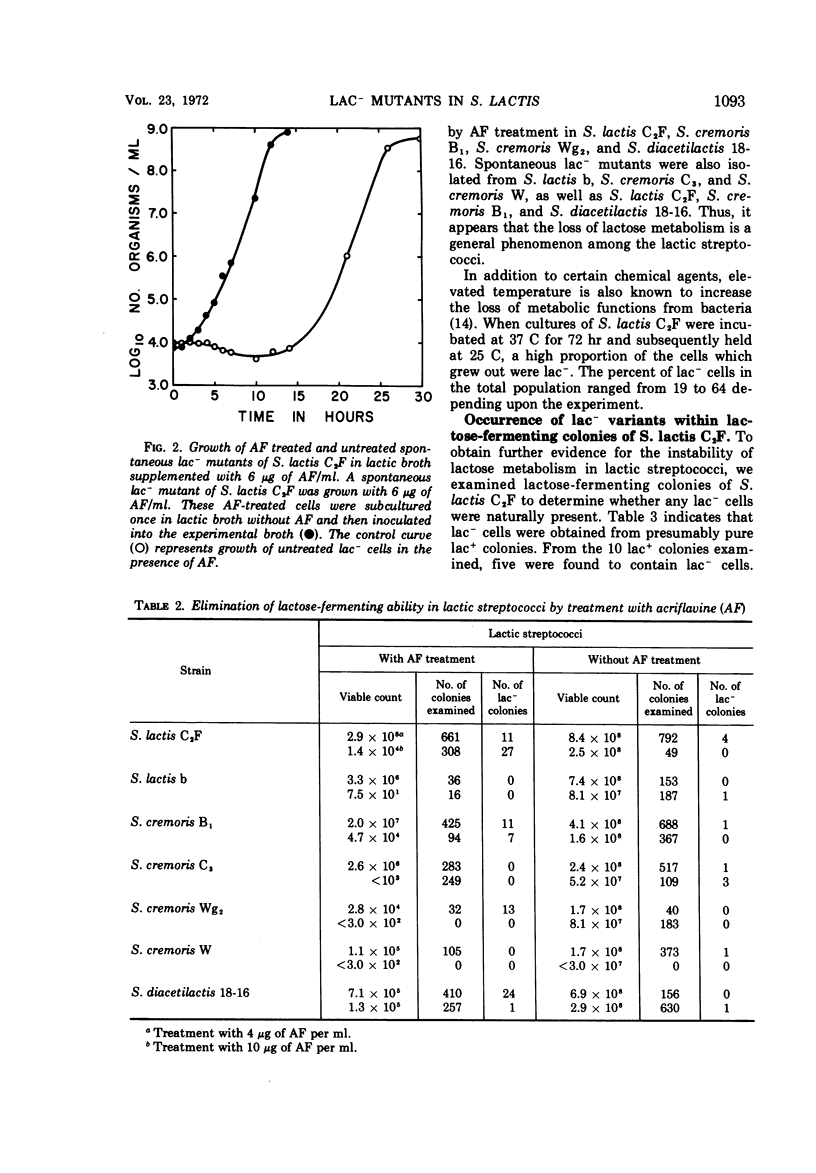
Lactose-negative mutants occurred spontaneously in broth cultures of Streptococcus lactis C2F. Instability of lactose metabolism was noted in other strains of S. lactis, in strains of S. cremoris, and in S. diacetilactis. Colonies of S. lactis C2F grown with lactose as the carbohydrate source also possessed lac- cells. Treatment of lactic streptococci with the mutagen acriflavine (AF) increased the number of non-lactose-fermenting variants. The effect of AF on growth and on loss of lactose-fermenting ability in S. lactis C2F was consequently further examined. The presence of AF appears to favor competitively the growth of spontaneously occurring lactose-negative cells and appears to act in the conversion of lactose-positive to non-lactose-fermenting cells. The lactose-negative mutants partially revert to lactose-positive variants which remain defective in lactose metabolism and remain unable to coagulate milk. The lactose-negative cells become dominant in continuous culture growth and provide evidence that alterations in the characteristics of starter strains can be produced by continuous culture, in this case, the complete loss in ability to ferment lactose.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barksdale L. I. : Lysogenic Conversions in Bacteria. Bacteriol Rev. 1959 Dec;23(4):202–212. doi: 10.1128/br.23.4.202-212.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easterling S. B., Johnson E. M., Wohlhieter J. A., Baron L. S. Nature of lactose-fermenting Salmonella strains obtained from clinical sources. J Bacteriol. 1969 Oct;100(1):35–41. doi: 10.1128/jb.100.1.35-41.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRSCH A. Growth and nisin production of a strain of Streptococcus lactis. J Gen Microbiol. 1951 Feb;5(1):208–221. doi: 10.1099/00221287-5-1-208. [DOI] [PubMed] [Google Scholar]
- Hirota Y. THE EFFECT OF ACRIDINE DYES ON MATING TYPE FACTORS IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1960 Jan;46(1):57–64. doi: 10.1073/pnas.46.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LWOFF A. Lysogeny. Bacteriol Rev. 1953 Dec;17(4):269–337. doi: 10.1128/br.17.4.269-337.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Walter L. A., Sandine W. E., Elliker P. R. Involvement of phosphoenolpyruvate in lactose utilization by group N streptococci. J Bacteriol. 1969 Aug;99(2):603–610. doi: 10.1128/jb.99.2.603-610.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L., Miller A., 3rd, Sandine W. E., Elliker P. R. Mechanisms of lactose utilization by lactic acid streptococci: enzymatic and genetic analyses. J Bacteriol. 1970 Jun;102(3):804–809. doi: 10.1128/jb.102.3.804-809.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse M. L. Reversion instability of an extreme polar mutant of the galactose operon. Genetics. 1967 Jun;56(2):331–340. doi: 10.1093/genetics/56.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P. Extrachromosomal inheritance in bacteria. Bacteriol Rev. 1969 Jun;33(2):210–263. doi: 10.1128/br.33.2.210-263.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Lerner S. A., Lin E. C. Replacement of a phosphoenolpyruvate-dependent phosphotransferase by a nicotinamide adenine dinucleotide-linked dehydrogenase for the utilization of mannitol. J Bacteriol. 1967 Feb;93(2):642–648. doi: 10.1128/jb.93.2.642-648.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakil J. R., Shahani K. M. Carbohydrate metabolism of lactic acid cultures. V. Lactobionate and gluconate metabolism of Streptococcus lactis UN. J Dairy Sci. 1969 Dec;52(12):1928–1934. doi: 10.3168/jds.S0022-0302(69)86876-1. [DOI] [PubMed] [Google Scholar]
- WOHLHIETER J. A., FALKOW S., CITARELLA R. V., BARON L. S. CHARACTERIZATION OF DNA FROM A PROTEUS STRAIN HARBORING AN EPISOME. J Mol Biol. 1964 Aug;9:576–588. doi: 10.1016/s0022-2836(64)80228-x. [DOI] [PubMed] [Google Scholar]


