Abstract
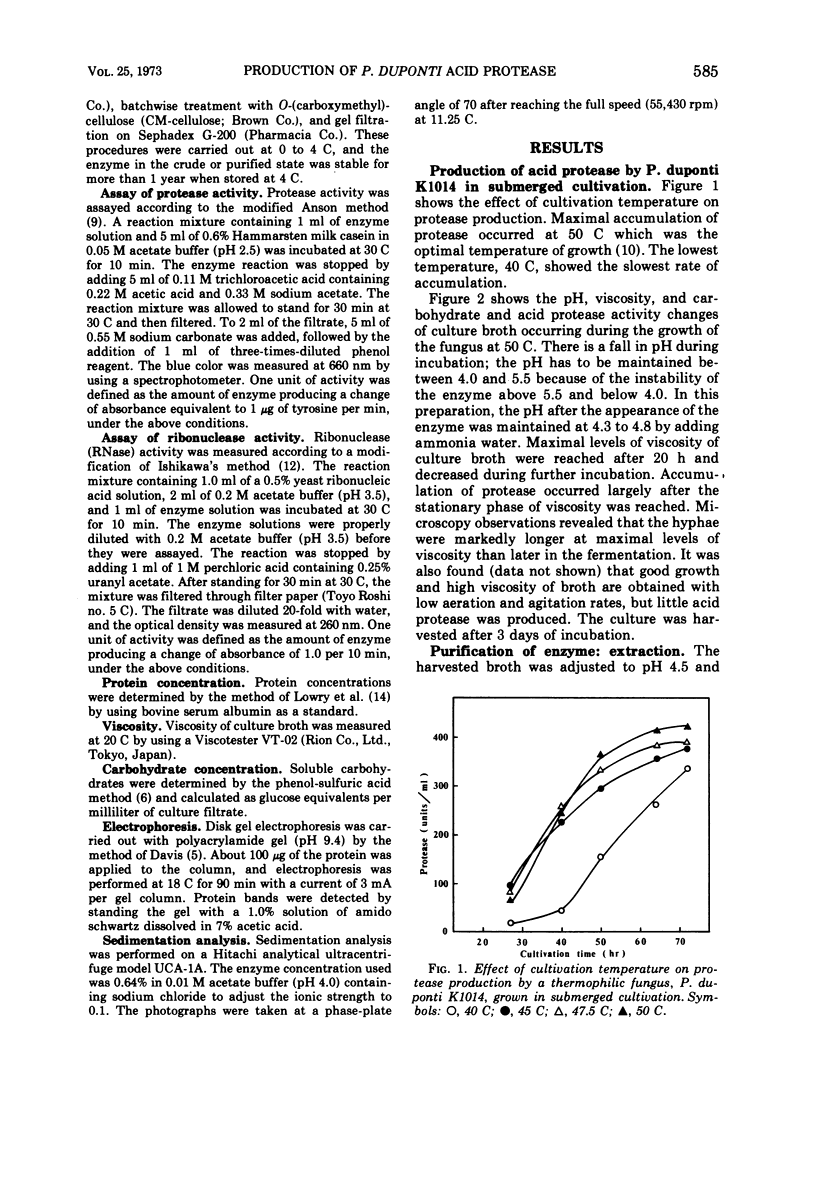
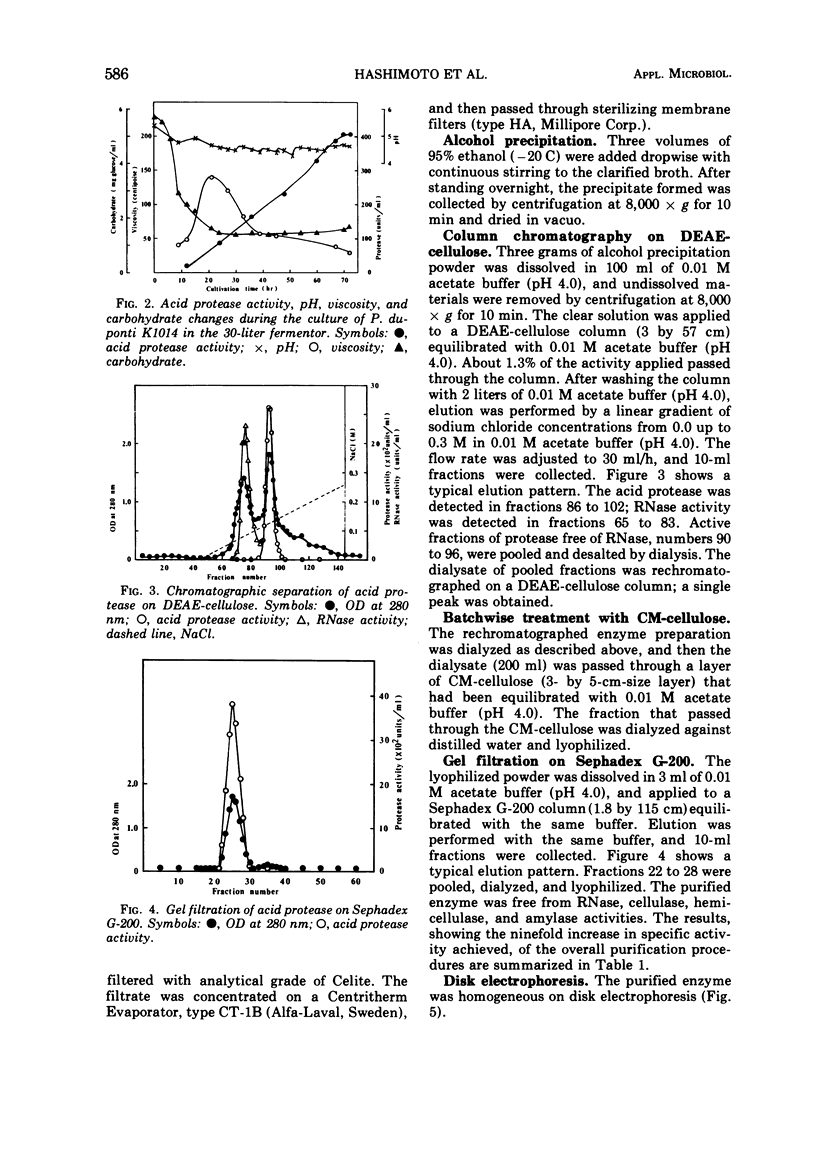
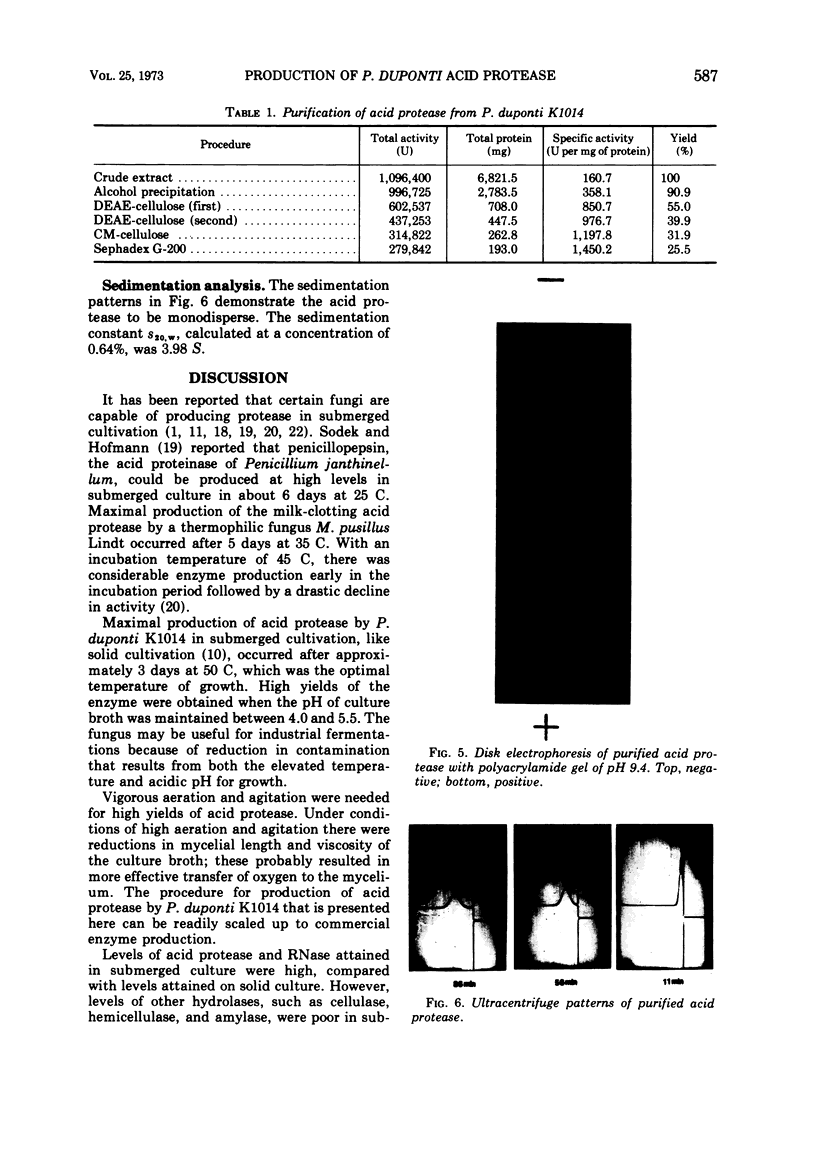
A thermophilic fungus, Penicillium duponti K1014, produced an acid protease in a submerged culture. Maximum enzyme production, in a 30-liter fermentor at a preselected optimum growth temperature of 50 C, occurred after 3 days. The productivity of the enzyme was associated with changes of viscosity and pH of culture broth during the growth. The acid protease was isolated from the culture filtrates and was purified ninefold by alcohol precipitation, column chromatography on O-(diethylaminoethyl)cellulose, batchwise treatment with O-(carboxymethyl)cellulose, and by gel filtration. The purified enzyme was homogeneous on disk electrophoresis and sedimentation analysis. The sedimentation constant s20.w, calculated at a concentration of 0.64%, was 3.98S.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coetzee J. N. Transduction in Proteus morganii. Nature. 1966 Apr 9;210(5032):220–220. doi: 10.1038/210220a0. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Hashimoto H., Iwaasa T., Yokotsuka T. Thermostable acid protease produced by Penicillium duponti K1014, a true thermophilic fungus newly isolated from compost. Appl Microbiol. 1972 Dec;24(6):986–992. doi: 10.1128/am.24.6.986-992.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T., Toh-E A., Uno I., Hasunuma K. Isolation and characterization of nuclease mutants in Neurospora crassa. Genetics. 1969 Sep;63(1):75–92. doi: 10.1093/genetics/63.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ottesen M., Rickert W. The isolation and partial characterization of an acid protease produced by Mucor miehei. C R Trav Lab Carlsberg. 1970;37(14):301–325. [PubMed] [Google Scholar]
- Rickert W. The degradation of the B-chain of oxidized insulin by Mucor miehei protease. C R Trav Lab Carlsberg. 1970;38(1):1–17. [PubMed] [Google Scholar]
- Sodek J., Hofmann T. Large-scale preparation and some properties of penicillopepsin, the acid proteinase of Penicillium janthinellum. Can J Biochem. 1970 Apr;48(4):425–431. doi: 10.1139/o70-069. [DOI] [PubMed] [Google Scholar]
- Somkuti G. A., Babel F. J. Conditions influencing the synthesis of acid protease by Mucor pusillus Lindt. Appl Microbiol. 1967 Nov;15(6):1309–1312. doi: 10.1128/am.15.6.1309-1312.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somkuti G. A., Babel F. J. Purification and properties of Mucor pusillus acid protease. J Bacteriol. 1968 Apr;95(4):1407–1414. doi: 10.1128/jb.95.4.1407-1414.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]




