SUMMARY
Lymph node development during embryogenesis involves lymphotoxin-β receptor engagement and subsequent differentiation of a poorly defined population of mesenchymal cells into lymphoid tissue organizer cells. Here, we showed that embryonic mesenchymal cells with characteristics of adipocyte precursors present in the microenvironment of lymph nodes gave rise to lymph node organizer cells. Signaling through the lymphotoxin-β receptor controlled the fate of adipocyte precursor cells by blocking adipogenesis and instead promoting lymphoid tissue stromal cell differentiation. This effect involved activation of the NF-κB2-RelB signaling pathway and inhibition of the expression of the key adipogenic factors Pparγ and Cebpα. In vivo organogenesis assays show that embryonic and adult adipocyte precursor cells can migrate into newborn lymph nodes and differentiate into a variety of lymph node stromal cells. Thus, we propose that adipose tissues act as a source of lymphoid stroma for lymph nodes and other lymphoid structures associated with fat.
INTRODUCTION
Lymph nodes (LNs) are highly organized structures distributed throughout the lymphatic vessel network at strategic sites of the body. They play a crucial role in the immune system, in that they collect antigens and antigen-presenting cells from surrounding tissues and provide an effective environment for antigen presentation to lymphocytes and for generation of memory immune responses. LN stromal cells are essential in these processes; they provide specific cues and growth factors that facilitate movement, migration to specific areas, maturation, and survival of lymphocytes (Roozendaal and Mebius, 2011). Intriguingly, most LNs are embedded in adipose tissue, but the reasons for this are unclear. Moreover, this association is found in all placental mammals, suggesting an evolutionarily conserved relationship that may reflect the need for energy and other factors during adaptive immune responses (Pond, 2003; Pond and Mattacks, 2002; Procaccini et al., 2012).
LNs are found within white adipose tissue (WAT), which is mainly involved in energy storage and is distinct from the brown adipose tissue, which specializes in energy expenditure. Peripheral LNs, such as popliteal, inguinal, or axillary LNs, are associated with subcutaneous WAT, whereas mesenteric LNs are associated with intra-abdominal WAT. Adipocyte differentiation has been extensively studied in vitro (Rosen and MacDougald, 2006), but the embryonic origin and ontogenetic mechanisms that lead to the differentiation of adipocytes in vivo are mostly unknown. Macroscopic studies on human and porcine fetuses showed that WAT starts to form before birth (Hausman et al., 1990; Wright and Hausman, 1990). Adipogenesis is generally described as a two-step process. First, mesenchymal stem cells give rise to adipocyte precursors, followed by these progenitors undergoing terminal differentiation into mature and functional adipocytes (Billon and Dani, 2012; Zeve et al., 2009).
Development of the LN anlagen starts during embryogenesis and follows a precise timing according to anatomical location: mesenteric LNs develop first, followed by the rest of the LNs along the anterior-posterior axis. Recruitment and clustering of the hematopoietic lymphoid tissue inducer (LTi) cells within the early anlagen is a crucial step of LN development. LTi cells express lymphotoxin α1β2, allowing engagement of the lymphotoxin-β receptor (LTβR), a member of the tumor necrosis factor (TNF) receptor superfamily, on immature stromal cells and their maturation in lymphoid tissue “organizer” cells (LTo). Signaling through LTβR induces activation of the NF-κB transcription factors through the classical, or canonical (NF-κB1 p50-RelA), and the alternative, or noncanonical (NF-κB2 p52-RelB), pathways (Dejardin et al., 2002; Yilmaz et al., 2003), leading to increased expression of cell adhesion molecules and cytokines necessary for continuous recruitment and retention of LTi cells and further development of the anlage (Randall et al., 2008; Ruddle and Akirav, 2009; van de Pavert and Mebius, 2010). Lack of LTβR signaling, as in Lta−/−, Ltbr−/−, and Rorc−/− mice (which lack LTi cells), results in the absence of all LNs (De Togni et al., 1994; Fütterer et al., 1998; Sun et al., 2000). Although LTβR engagement is not necessary for the initial wave of LTi cell recruitment to the LN anlage, it is required for the maturation and homeostasis of stromal cells and expression of the TNF-family ligand RANKL and the cell adhesion molecule MAdCAM-1 (Bénézech et al., 2010; Coles et al., 2006; Eberl et al., 2004; Vondenhoff et al., 2009; White et al., 2007; Yoshida et al., 2002). Retinoic acid is also involved in the expression of the chemoattractant CXCL13 by stromal cells that initiates the recruitment of LTi cells to the LN anlage (van de Pavert et al., 2009). However, the origin of the stromal cells that form the initial LN anlage remains unknown.
Here, we show in mouse embryos that a common precursor cell gave rise to both LN intrinsic organizer stromal cells and the adipocytes that reside in the adjacent fat pads. Signaling through LTβR played a key role in lineage choice in this progenitor population by inhibiting adipogenesis and instead promoting lymphoid tissue stromal cell differentiation. In vivo organogenesis experiments showed that, in the context of adult tissues, adipocyte progenitors can differentiate into a variety of LN stromal cells that include organizer cells and capsular and medullary stromal cells. Thus, we show that adipose tissues contain precursor cells with potential for both adipocytes and lymphoid tissue stromal cells, suggesting that adipose tissues may also act as a source of stromal cells for other lymphoid structures associated with fat, such as the milky spots of the omentum (Rangel-Moreno et al., 2009) or the recently described fat associated lymphoid clusters (Moro et al., 2010).
RESULTS
Adipose Tissue and LN Anlage Develop in Close Association
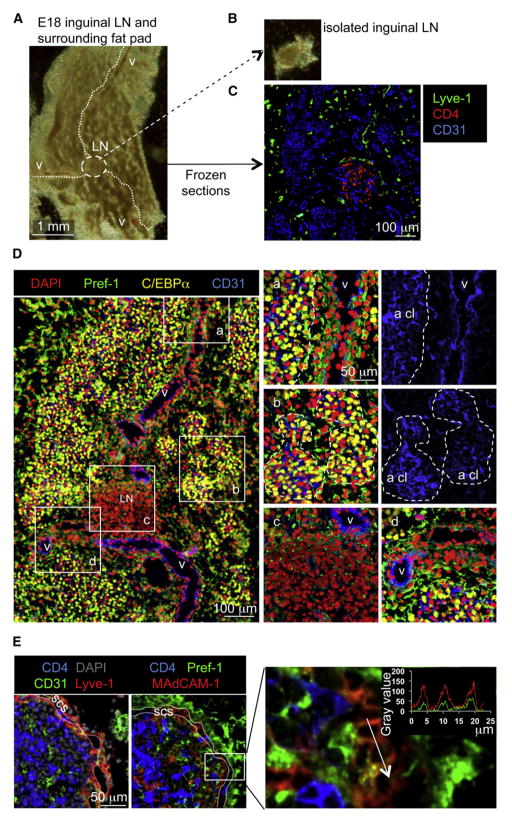
The close anatomical association between LNs and fat deposits prompted us to investigate their relationship during embryogenesis and to study LNs and their associated adipose tissues as an entity. We dissected out inguinal LNs and fat pads from embryonic day 18 (E18) mouse embryos and processed them for frozen sections and immunofluorescence staining (Figures 1A and 1B). The position of the LN anlage was highlighted by the accumulation of CD4+ LTi cells clustered within Lyve-1+ lymphatic endothelial cells at the junction of three large CD31+ blood vessels (Figures 1C and 1D). Well-defined clusters of cells expressing the adipocyte markers C/EBPα and Perilipin formed throughout the surrounding tissue around micronetworks of endothelial vessels (Figures 1Da and 1Db; Figures S1A and S1B available online). Pref-1 (also known as Dlk-1), a transmembrane protein expressed in mesenchyme and adipocyte progenitors but downregulated in adipocytes, was used for identification of adipocyte progenitors, which were interspersed throughout the whole inguinal area but accumulated preferentially around blood vessels (Figures 1Da and 1Dd). Importantly, Pref-1+ adipocyte progenitors were found lining the outer part of the developing LN and inside the anlage (Figure 1Dc). Immunostaining for MAdCAM-1, a cell adhesion molecule expressed on high endothelial venules but also on mature lymphoid tissue organizer cells in the LN anlage, revealed that a fraction of CD31−MAdCAM-1+ cells also expressed Pref-1 (Figure 1E). These observations demonstrate that in mouse embryos the initial formation of adipose tissues colocalizes with capillary networks, as shown in adult mice (Neels et al., 2004) and other mammalian species (Hausman et al., 1990; Wright and Hausman, 1990). Moreover, the widespread presence of a Pref-1+ adipocyte progenitor cell population within the developing fat pad and LN anlage suggests a link between these cells and the formation of LN stroma.
Figure 1. Identification of Embryonic Inguinal LN Anlage and Associated Fat Pad.
(A and B) Photograph of the inguinal fat pad of an E18 mouse embryo with the blood vessels (v, white dotted lines) and the LN anlage (circled area) (A) and of the LN after dissection from the fat pad (B).
(C) Immunofluorescence staining of an E18 inguinal fat pad, with CD4+ lymphoid tissue inducer cells (red) in the LN anlage, Lyve-1+ lymphatic endothelial cells (red), and CD31+ blood endothelial cells (blue) (10×).
(D) E18 inguinal LN and associated fat pads (left panel, 10×) and enlargements of different areas with adipocyte clusters (a cl, punctuated white lines, a–d, 40×). Cell nuclei stained with DAPI (red), Pref-1+ adipocyte precursors (green), C/EBPα+ adipocytes (yellow), and CD31+ blood vessels (blue).
(E) E18 inguinal region including the LN and the associated fat pads (40×), showing CD4+ lymphoid tissue inducer cells (blue), the CD31loLyve-1+ subcapsular sinus (scs, yellow-red) delimitated by two white lines (left panel), and Pref-1+ mesenchymal cells as well as differentiating lymphoid tissue organizer cells expressing MAdCAM-1 and Pref-1 (right panel) and its enlargement. The fluorescence intensity profile shows coexpression of MAdCAM-1 and Pref-1 at the membrane of two adjacent cells.
See also Figure S1.
Phenotypical Analysis of Adipocyte Progenitor Cells
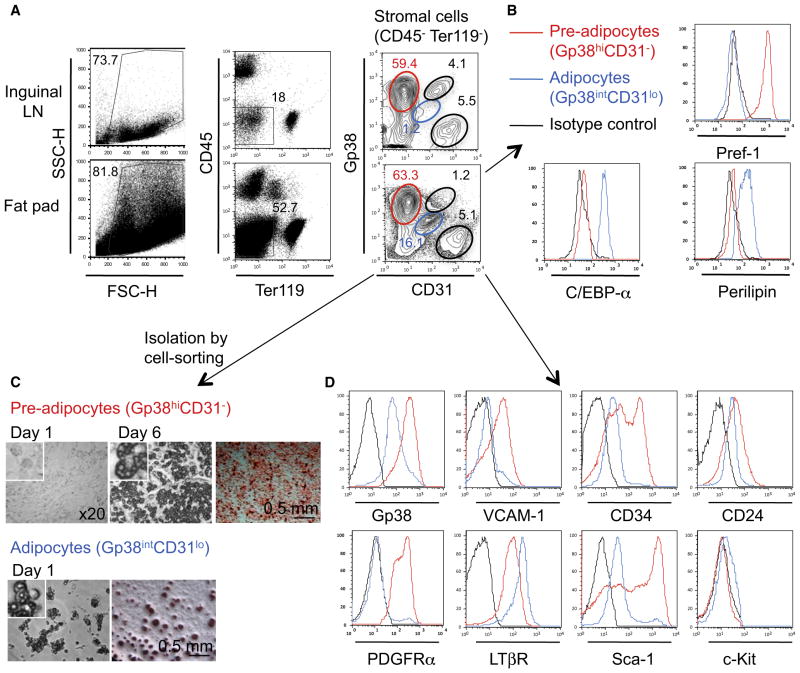
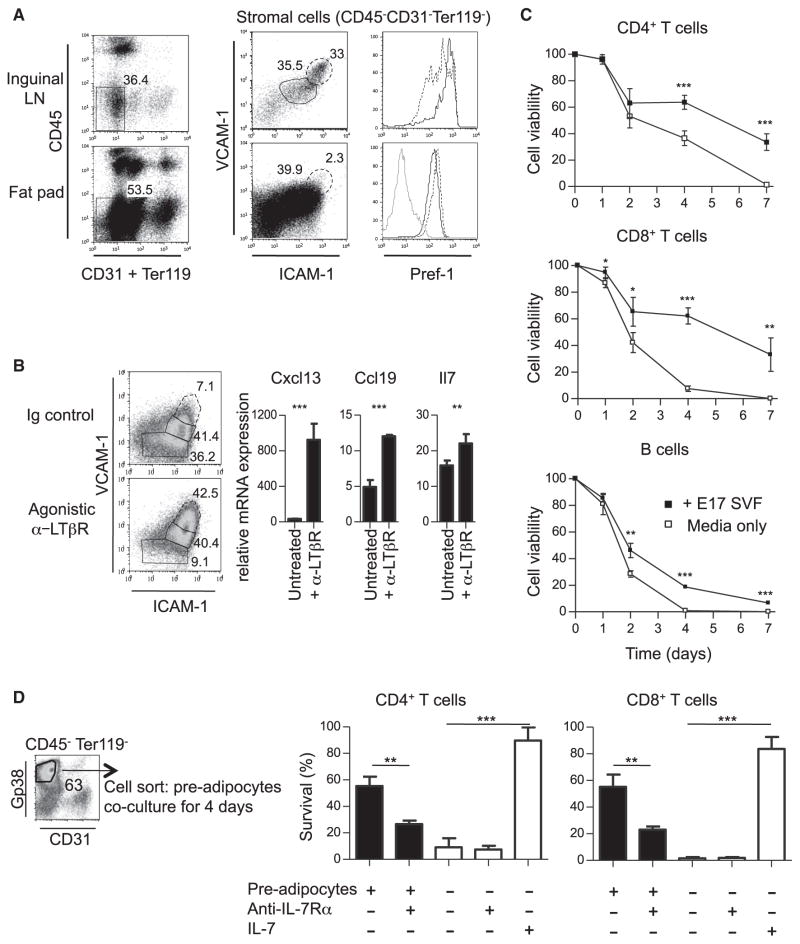
We then investigated whether Pref-1+ cells in embryonic fat pads and LNs expressed the cell surface molecules that were described to identify fibroblastic and marginal reticular cells and further compared them with adult adipocyte precursors (Link et al., 2007; Mueller and Germain, 2009). Flow-cytometric analysis of stromal cells from LN and fat pads showed a surprisingly similar profile, containing four matching populations expressing different amounts of the stromal markers Gp38 (also known as Podoplanin) and CD31: (i) Gp38−CD31− cells, (ii) Gp38−CD31hi blood endothelial cells, (iii) Gp38hiCD31int lymphatic endothelial cells, and (iv) a large population of Gp38hiCD31− cells (Figure 2A, right panels, red gate). The latter were Pref-1+ but were negative for C/EBPα and Perilipin (Figure 2B) and differentiated spontaneously into adipocytes after 6 days in culture, showing the characteristic accumulation of lipid droplets and oil red O cell staining (Figure 2C). Fat pads also contained Gp38intCD31lo cells (Figure 2A, right panels, blue gate) that were Pref-1−C/EBPα+Perilipin+ and presented with a high side scatter (SSChi) profile, suggesting that they might be committed adipocytes starting to accumulate cytoplasmic lipid droplets (Figures S2A and S2B). Indeed, Gp38intCD31lo cells also differentiated into adipocytes within 24 hr in nondifferentiating culture conditions (Figure 2C). These results identified a Gp38hiCD31− cell population with characteristics of adipocyte precursors present in developing fat pads. We hereafter refer to these cells as adipocyte precursors, whereas we refer to Gp38intCD31lo cells as adipocytes. Embryonic adipocyte precursors expressed the stem cell markers CD24 (heat-stable antigen), CD34, and Sca-1 and, consistent with their mesenchymal origin, were PDGFR-α+ and VCAM-1+, but c-Kit− (Figure 2D). These cells resembled the Lin−CD29+CD34+Sca-1+ CD24+ early adipocyte progenitor cells previously identified in adult WAT (Rodeheffer et al., 2008; Tang et al., 2008). Analysis of adult stromal vascular fraction from fat pads associated with inguinal LNs revealed that the Gp38+CD31− cell fraction also contains early adipocyte progenitor cells (Figures S2C–S2D). These results show that the phenotype of embryonic adipocyte progenitor cells resembled that of their adult counterparts (Joe et al., 2010; Rodeheffer et al., 2008; Tang et al., 2008; Uezumi et al., 2010), with the exception of the cell adhesion molecule CD24. Like their adult counterparts, the embryonic adipocyte progenitor cells might have the capacity to differentiate into other mesenchymal lineages in the presence of osteogenic- or chondrogenic-inducing media. These analyses reveal the similarity between embryonic adipocyte progenitor cells and LN stromal cell populations and prompted us to study the effect of LTβR activation during adipogenic differentiation.
Figure 2. Identification of Adipocytes and Adipocyte Precursor Cells in the Embryonic Inguinal Fat Pads.
(A) Flow-cytometric analysis of cell suspensions from E18 inguinal LNs and associated fat pads. Adipocyte precursors are gated in red and adipocytes in blue.
(B) Pref-1, C/EBPα, and Perilipin expression on adipocyte precursors (red line) and adipocytes (blue line), gated as shown in (A). Isotype control in black.
(C) Adipocyte precursors and adipocytes were cell sorted and cultured in nondifferentiating media. Microphotographs of unstained and oil red O stained cells (red). Data are representative of four independent experiments.
(D) VCAM-1, CD34, CD24, PDGFRα, LTβR, Sca-1, and c-Kit expression on adipocyte precursors (red line) and adipocytes (blue line), gated as shown in (A). Isotype control in black.
See also Figure S2.
Differentiation of Adipocyte Progenitor Cells Is Inhibited by LTβR Signaling
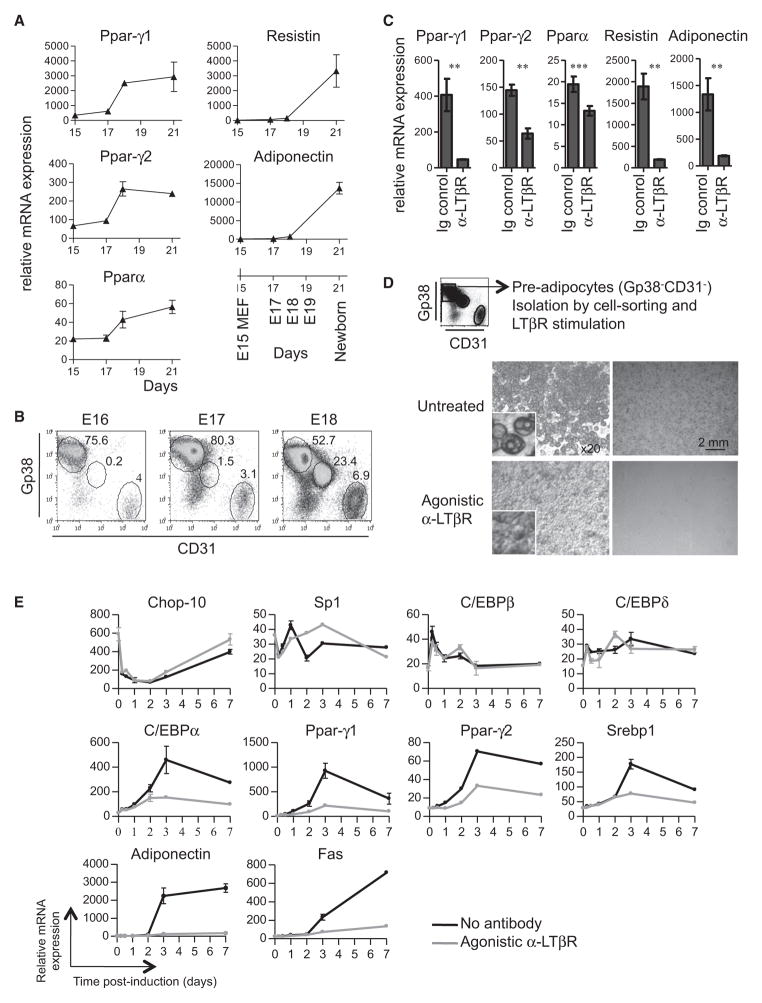
Adipocyte differentiation during embryogenesis is initiated in inguinal fat pads through induced expression of the transcription factors Pparγ and Pparα between E17 and E18, followed by expression of the WAT markers Adiponectin and Resistin (Figure 3A). Furthermore, the detection of adipocyte-specific transcription factors between E17 and E18 coincides with the appearance of the first adipocytes and the concomitant decrease in the frequency of adipocyte precursors (Figure 3B). We previously showed that treatment with agonistic LTβR antibody (Ab) of immature whole mesenteric LN anlage in an in vitro organ culture system induced maturation of the LN stroma (Bénézech et al., 2010). We examined whether signaling by LTβR in adipocyte precursors has an effect on their differentiation (Figure 2D). LTβR stimulation of E18 whole inguinal fat pads devoid of LN in organ cultures resulted in a marked decrease in expression of adipogenic markers (Figure 3C). Similarly, the spontaneous adipogenic differentiation of embryonic adipocyte precursors isolated through cell sorting was fully blocked by LTβR stimulation, with cells retaining their mesenchymal morphology and high expression of Gp38 and PDGFRα (Figure S3), demonstrating a direct inhibitory effect of this pathway on adipogenesis (Figure 3D).
Figure 3. LTβR Signaling Inhibits Adipocyte Differentiation.
(A) Real-time RT-PCR analysis in E15 MEFs and E17, E18, and newborn inguinal fat pads (devoid of LN). The means of triplicates ± SD are shown. Data are representative of three independent experiments.
(B) Flow-cytometric analysis of single-cell suspensions from E16, E17, and E18 inguinal fat pads.
(C) Real-time RT-PCR analysis of E18 inguinal fat pads cultured for 6 days with control immunoglobulin (Ig) or agonistic LTβR Ab. The means of triplicates ± SD are shown. **p < 0.01, ***p < 0.005.
(D) Adipocyte precursors were cell sorted as in Figure 2C and cultured with or without agonistic LTβR Ab. Microphotographs at day 6 of unstained and oil red O-stained cells (dark gray). Data are representative of four independent experiments.
(E) Real-time RT-PCR analysis of WT MEFs cultured with an adipogenic cocktail in the presence or absence of an agonistic LTβR Ab at the indicated times after induction of differentiation. The means of triplicates ± SD are shown. Data are representative of two independent experiments.
See also Figure S3.
We next used an ex vivo differentiation assay of mouse embryonic fibroblasts (MEFs) into lipid accumulating adipocytes upon treatment with adipogenic stimuli to analyze whether LTβR signaling affects the kinetics of gene expression associated with this process. Previous characterization of the adipogenic gene expression program showed that the initial changes within 12 hr of induction involved the downregulation of Chop-10 and Sp1, inhibitors of C/EBPβ and C/EBPδ, and the subsequent increased expression of these transcription factors (Rosen and MacDougald, 2006). The presence of an agonistic LTβR Ab in the adipogenic-inducing media did not affect the messenger RNA (mRNA) expression corresponding to Cebpb and Cebpd (Figure 3E, top panels). Following the initial step, between 12 and 72 hr, there was a marked increase in the expression of C/ebpα, Pparγ1, Pparγ2, and Srebp1α, which are induced by C/EBPβ and C/EBPδ, followed by a positive feedback loop resulting from their own actions. Interestingly, LTβR activation appears to blunt the upregulation of C/ebpα, Pparγ1, Pparγ2, and Srebp1α (Figure 3E, middle panels). The final step in adipocyte differentiation involves the expression of Adiponectin and Fas, which are induced by C/EBPα, PPARγ, and SREBP1α. LTβR signaling strongly inhibited the expression of this set of genes (Figure 3E, bottom panels), which could be the direct consequence of the impaired upregulation of Cebpα and Pparγ shown above. All together, these data demonstrate the strong effect of LTβR activation for inhibiting adipocyte differentiation through blocking of the transcriptional upregulation of Ppar-γ and C/ebpα, which are essential inducers of adipogenesis.
Activation of the Alternative NF-κB Pathway Inhibits Adipocyte Differentiation
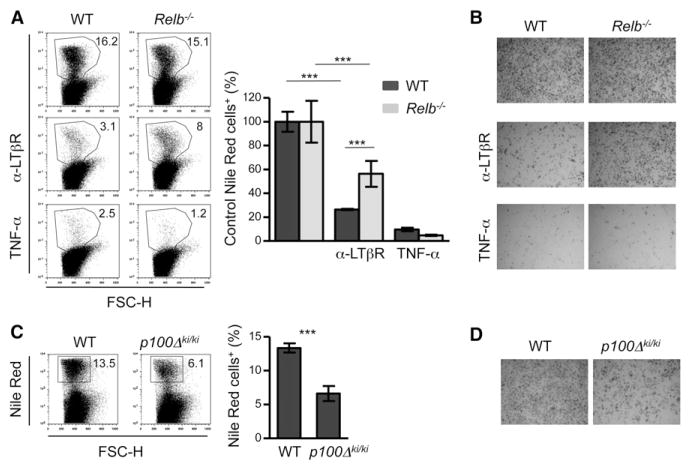
We dissected the role of the NF-κB proteins in the LTβR-induced block in adipogenic differentiation by analyzing adipogenic differentiation in MEFs deficient in different members of this family of transcription factors. In keeping with the results obtained on embryonic preadipocytes, LTβR signaling in wild-type (WT) MEFs resulted in a marked inhibition of adipogenic differentiation, as shown by the 80% decrease in lipid accumulation seen with Nile Red staining as well as reduced oil red O staining (Figures 4A and 4B). Importantly, LTβR stimulation of Relb−/− MEFs showed only a 47% reduction in adipogenic differentiation. We also tested whether RelB mediated the TNF-α-induced inhibition of adipogenic differentiation (Suzawa et al., 2003). In contrast to the effect shown by LTβR signaling, mouse TNF-α (mTNF-α) stimulation of Relb−/− and WT MEFs blocked adipogenic differentiation to the same extent (Figures 4A and 4B), indicating that the two TNF-family ligands had different mechanisms of action. Furthermore, we assessed whether the LTβR effect on adipogenesis was mediated by an autocrine loop by TNFα in MEFs. LTβR signaling blocked adipogenic differentiation to the same extent in both Tnfrsf1a−/−Tnfrsf1b−/− MEFs and their WT counterparts, whereas, as expected, mTNF-α had no effect in the double-deficient cells (Figure S4).
Figure 4. Activation of the Alternative NF-κB Pathway Inhibits Adipocyte Differentiation.
WT and Relb−/− MEFs were cultured with an adipogenic cocktail for 8 days in the presence or absence of an agonistic LTβR Ab or mTNF-α.
(A and B)Representative dot plots of flow-cytometric analysis of Nile Red-stained cells (A) and microphotographs of oil red O-stained cells (dark gray) (B). For Nile Red quantification, the percentage of Nile Red+ cells that differentiate after agonistic LTβR Ab or mTNF-α treatments were normalized to the control WT and Relb−/− conditions. The means of triplicates ± SD are shown. (Unpaired Student’s t test, ***p < 0.005.) Data are representative of at least three independent experiments.
(C and D) WT and p100Δki/ki MEFs from littermate embryos were cultured with an adipogenic cocktail for 8 days. Representative dot plots of flow-cytometric analysis of Nile Red-stained cells (C) and microphotographs of oil red O-stained cells (dark gray) (D). For the Nile Red quantification, the means of triplicates ± SD of % of Nile Red+ cells are shown. (Unpaired Student’s t test, ***p < 0.005.) Data are representative of at least four independent experiments.
See also Figure S4.
Consistent with an important role for the LTβR-NF-κB2-RelB pathway in blocking adipogenesis, the LTβR inhibitory effect was significantly reduced in Nfkb2−/− MEFs with respect to their WT counterparts (data not shown). To further confirm the intrinsic inhibitory effect of this pathway on adipocyte differentiation, we used MEFs from p100Δki/ki mice that carry a deletion of the carboxy-terminal region of the NF-κB2 precursor protein p100, resulting in constitutive activation of the alternative NF-κB pathway with high amounts of NF-κB2 p52-RelB dimers (Ishikawa et al., 1997). p100Δki/ki MEFs showed a 50% reduction in their adipogenic potential compared to their WT littermates (Figures 4C and 4D). These results demonstrate that the negative effect of LTβR on adipogenic differentiation is independent of TNF-α and its receptors and is mediated to a substantial degree through the alternative NF-κB pathway.
LTβR Activation of Adipocyte Precursor Cells Induces the Expression of Chemokines and Cell Adhesion Molecules
LTβR triggering on MEFs through the activation of the canonical NF-κB1-RelA and the alternative NF-κB2-RelB pathways results in the induction of the chemokines Cxcl13, Ccl21, and Ccl19 and the adhesion molecules Icam1 and Vcam1 (Dejardin et al., 2002; Lovas et al., 2008; Vondenhoff et al., 2009). Similarly, LTβR signaling and RelB are required on LN stromal cells for the expression of high amounts of the ICAM-1, VCAM-1, and chemokines that characterize their maturation to organizer cells (Bénézech et al., 2010; Cupedo et al., 2004b; Ganeff et al., 2011). Adipocyte progenitors resembled immature ICAM-1int VCAM-1int LN stromal cells (Figure 5A). LTβR stimulation of cell-sorted adipocyte precursors resulted in a marked upregulation in cell surface expression of ICAM-1 and VCAM-1 (Figure 5B, left panel). Moreover, while adipocyte precursors expressed basal amounts of Ccl19 and Il7, important factors mediating the survival of naive T cells (Link et al., 2007), LTβR triggering increased the mRNA expression for these genes and promoted a strong upregulation of Cxcl13 (Figure 5B, right panel). Collectively, these data indicate that ex vivo LTβR stimulation of adipocyte precursors is sufficient for blocking adipogenic differentiation and promoting expression of LN stromal cell markers.
Figure 5. LTβR Signaling Promoted the Expression of Lymphoid Tissue Organizer Cell Markers on Adipocyte Precursor Cells.
(A) Flow-cytometric analysis of inguinal LN (first row) and fat pad (second row) cells. The mature lymphoid tissue organizer cell population (ICAM-1hiVCAM-1hi, dashed line) and immature LN stromal cells (ICAM-1intVCAM-1int, solid line) were gated as shown on the ICAM-1 and VCAM-1 dot plots. Isotype control in gray.
(B) Flow cytometry of the cultured adipocyte precursors stained with ICAM-1 and VCAM-1 and real-time RT-PCR analysis in the adipocyte precursors at day 2. Data are representative of at least three independent experiments.
(C) Survival curves of CD62LhiCD4+ T cells, CD62LhiCD8+ T cells, and CD62LhiCD19+ B cells cultured in the presence of media alone (open squares) or cocultured with embryonic fat-pad stroma (solid squares). Representative flow-cytometric analysis is shown in Figure S5.
(D) Percentages of cell survival of CD4+ T cells and CD8+ T cells and B cells cocultured with cell-sorted embryonic adipocyte precursors in the presence of blocking IL-7Rα Ab or IL-7 over 4 days. The means of triplicates ± SD are represented; data are representative of at least three independent experiments. ns, not significant.*p < 0.05, **p < 0.01, ***p < 0.005.
See also Figure S5.
Adipocyte Precursor Cells Support T Cell Survival Ex Vivo
Based on the observation that adipocyte precursors expressed Ccl19 and Il7, we tested whether these cells were able to support lymphocyte survival ex vivo. Coculture of lymphocytes with stromal cells from embryonic fat pads significantly increased the survival of naive CD4+ and CD8+ T cells and had a moderate effect on enhancing B cell survival (Figure 5C; Figure S5). Cell-sorted adipocyte precursors were sufficient to induce a statistically significant increase in survival of naive T cells, and this effect appeared to be mediated by interleukin-7 (IL-7) and other factors, as shown previously for fibroblastic reticular cells (Link et al., 2007) (Figure 5D).
Migration of Adipocyte Precursor Cells and Differentiation into LN Stromal Cells Is LTβR Dependent
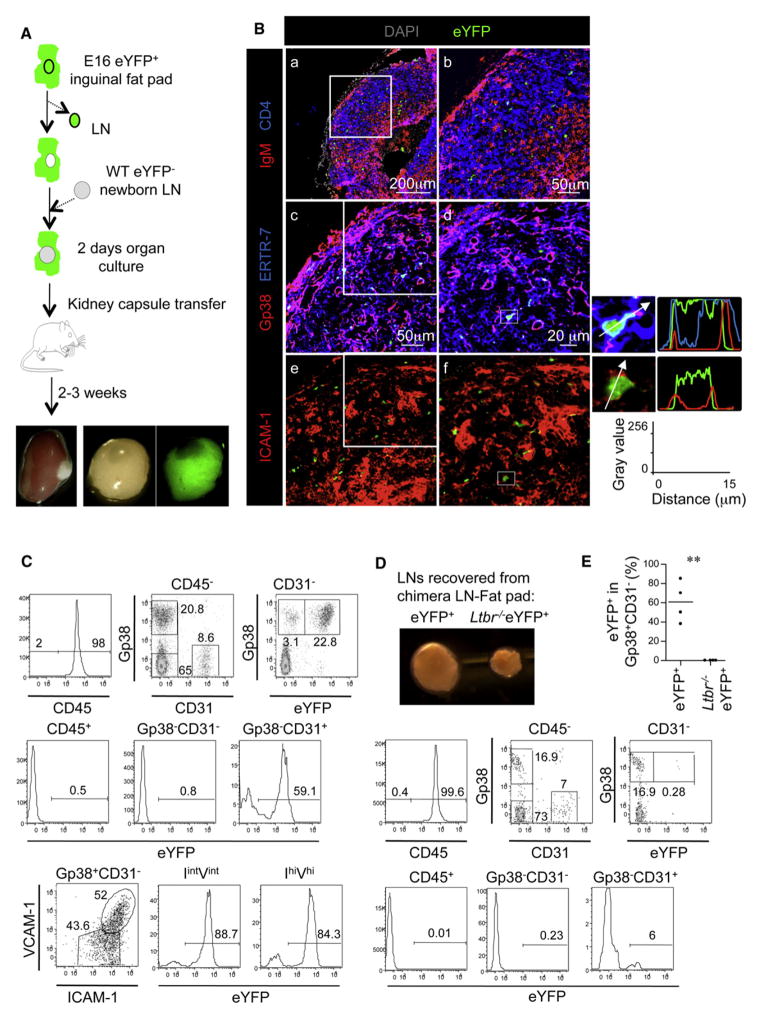
An interesting possibility is that adipocyte precursors migrate from the fat pads to the LN anlagen to become part of the stroma. To test this hypothesis, we isolated whole fat pads from Eyfp-Rosa 26+ embryos (Srinivas et al., 2001) and removed and replaced the eYFP+ LN anlage with a WT (eYFP−) newborn inguinal anlage. We then grafted this chimeric eYFP+ fat pad-eYFP− LN anlage under the kidney capsule of adult eYFP− mice (Figure 6A). Analysis of the lymphoid structures 3 weeks after grafting demonstrated that the progeny of fat pad cells that expressed eYFP in the cytoplasm had become part of the LN capsule and had contributed to networks of stromal cells in the paracortex, where they associated with stromal cell subsets expressing the markers ERTR-7, Gp38, and ICAM-1 (Figures 6Bc–6Bf). Flow-cytometric analysis showed that EYFP+ cells accounted for an average of 60% of the Gp38+ CD31− stromal cell fraction, as well as more than 50% of the Gp38−CD31+ blood endothelial cells, but were not found in the Gp38−CD31− fraction nor in the CD45+ hematopoietic compartment (Figure 6C). In the Gp38+CD31− stromal cell compartment, eYFP+ cells were found in both the ICAM-1int VCAM-1int and ICAM-1hiVCAM-1hi cell fractions, indicating that adipocyte precursors that had migrated from the fat pad underwent maturation toward a lymphoid tissue organizer cell phenotype in the LN. The high percentage of eYFP+ cells in the Gp38+CD31− and Gp38−CD31+ cell fraction demonstrated the importance of the fat pad for supporting the development of the LN stroma.
Figure 6. Embryonic Adipocyte Precursor Cells Are Able to Migrate into Newborn LNs and Differentiate into LN Stromal Cells.
(A) Chimeric E16 eYFP+ fat pads-eYFP− WT newborn LNs were generated and cultured during 2 days before transplantation under the kidney capsule of adult WT mice.
(B) Immunofluorescence staining of whole LN (a, 10× tile scan) and enlargement of the highlighted section showing the progeny of the eYFP+ cells (b, 25×) or consecutive sections (c and e, 25×). Enlargements of (c) and (e) are shown in (d) and (f) (40×). The fluorescence intensity profiles show cytoplasmic expression of eYFP and membrane expression of ERTR7, Gp38, and ICAM-1.
(C) Flow-cytometric analysis of the chimeric LN cell suspensions. The CD45+ hematopoietic fraction and CD45− stromal fractions containing Gp38−CD31− cells and Gp38−CD31+ blood endothelial cells were gated as shown (top row), and the percentages of eYFP+ cells in these fractions are shown (middle row). The percentages of eYFP+ cells in the CD45−CD31− stromal fraction is shown (top row). The Gp38+CD31−ICAM-1intVCAM-1int immature LN stromal (IintVint) and Gp38+CD31−ICAM-1hiVCAM-1hi mature lymphoid tissue organizer (IhiVhi) cell populations were gated as shown (bottom row), and percentages of eYFP+ cells in these fractions are shown (bottom row).
(D) Microphotograph of the LNs isolated from the chimeras with eYFP+Ltbr+/+ (left) and eYFP+Ltbr−/− (right) fat pads and flow-cytometric analysis, as in (C), of LNs from chimeras with eYFP+Ltbr−/− fat pads. Data are representative of four independent experiments.
(E) Quantification of the percentage of eYFP+ cells in the Gp38+CD31− LN cell fraction. **p < 0.01.
See also Figure S6.
We then tested the role of LTβR in this process by generating chimeric eYFP+Ltbr−/− fat pad-eYFP− LN anlage. Grafted LNs that were surrounded by eYFP+Ltbr−/− fat pads showed a marked reduction in their size compared to the LNs associated with eYFP+Ltbr+/+ fat pads (Figure 6D). Flow-cytometric analysis of the resulting LNs revealed that eYFP+Ltbr−/− cells were absent from the Gp38+CD31− stromal cell compartment and accounted for a small fraction of the Gp38−CD31+ cell compartment (Figures 6D and 6E), demonstrating the crucial role of LTβR signaling in the differentiation of LN stromal cells from the fat pad and the sustained growth of the LN.
Similar to their embryonic counterparts, adult adipocyte precursors retain their ability to migrate into LNs and differentiate into different stromal cell populations (Figure S6A). These results indicate that adipocyte progenitors are capable of migrating into LNs and, upon LTβR engagement, contribute to their stromal cell content, whereas blood vessels from the fat pad vascularize the grafts.
Differentiation of Adipocyte Precursor Cells into LN Stromal Cells
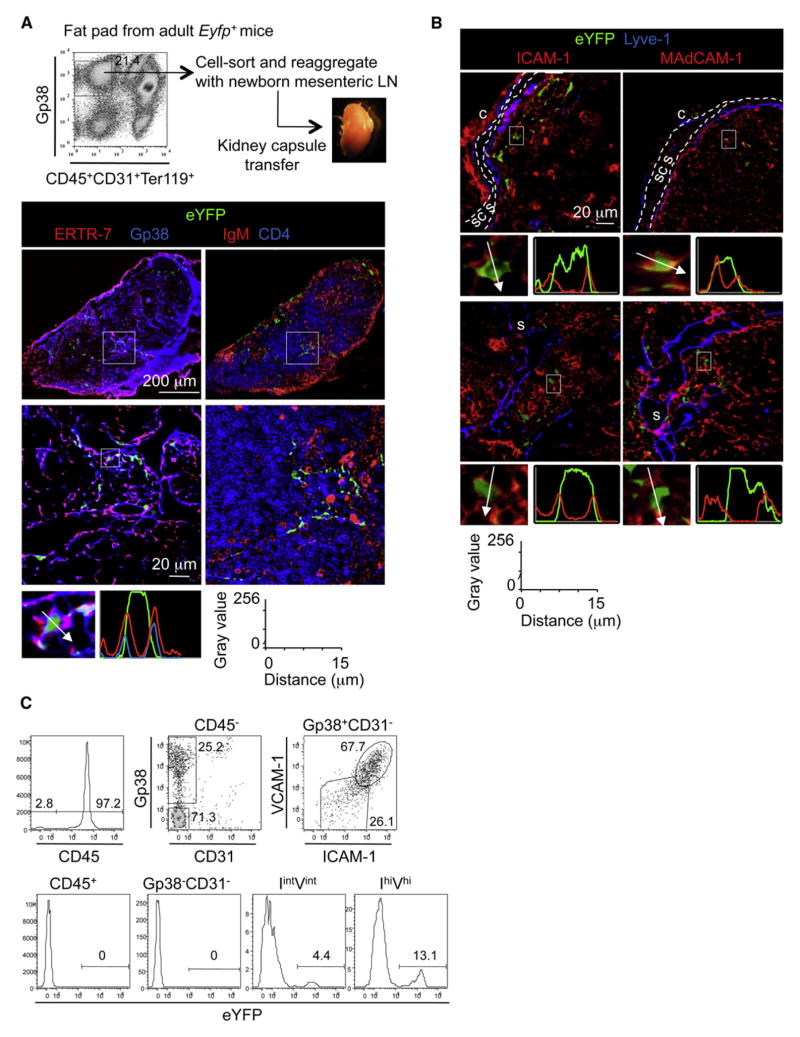
We next tested the capacity of adult adipocyte precursors to give rise to LN stroma in vivo. Due to the absence of preadipocyte reporter mice for direct testing of the fate of these cells, we performed organogenesis assays. Reaggregated lymphoid organs were generated from a mixture of WT newborn mesenteric LN “carrier” cells and Gp38+CD31−eYFP+ “spike” adipocyte precursors from inguinal fat pads of Eyfp-Rosa 26+ mice and grafted under the kidney capsule of WT adult mice (Cupedo et al., 2004a; White et al., 2007). Three weeks after grafting, the reaggregate organs presented with the structure of lymphoid tissues, with a fibrous capsule, subcapsular sinus, and lymphatic sinus, and contained high endothelial venules and segregated B and T cell areas. The progeny of donor eYFP+ adipocyte precursors formed part of the stromal cell networks in the para-cortex, expressing the markers ERTR-7 and Gp38 (Figure 7A).
Figure 7. Adult Adipocyte Precursor Cells Can Differentiate into LN Stromal Cells.
(A) Strategy to generate chimeric reaggregate tissues. Immunofluorescence stainings of reaggregate organs with tile scans of whole organs on the upper row (10×) and enlargements on the bottom row (40×).The fluorescence intensity profile shows cytoplasmic expression of eYFP and membrane expression of ERTR7 andGp38.
(B) Immunofluorescence stainings of reaggregate organs (40×) with subcapsular sinus (scs) delimitated by two white lines (s, sinus; c, capsule). Data are representative of three grafts in three independent experiments. The fluorescence intensity profile shows cytoplasmic expression of eYFP and membrane expression of ICAM-1 and MAdCAM-1.
(C) Flow-cytometric analysis of the reaggregate organs. The CD45+ hematopoietic fraction and CD45− stromal fractions containing Gp38−CD31−, immature LN stromal (IintVint), and mature lymphoid tissue organizer (IhiVhi) cell populations were gated as shown (top row). Percentages of eYFP+ cells in these different fractions are shown (bottom row). Data are representative of at least two independent experiments.
See also Figure S6.
Lymphoid tissue organizer cells, as well as their adult counterpart, the marginal reticular cells that reside along the subcapsular sinus, can be identified by expression of MAdCAM-1 (Buettner et al., 2010; Katakai et al., 2008; Mueller and Germain, 2009). Importantly, although adipocyte precursors from the fat pads do not express MAdCAM-1, the progeny of these cells within lymphoid tissue microenvironments of reaggregate lymphoid organs became MAdCAM-1+ (Figure S2D). EYFP+ cells coexpressing ICAM-1 and MAdCAM-1 were found close to the subcapsular sinus (Figure 7B, upper panel) and the lymphatic sinus (Figure 7B, lower panel) of the reaggregates. Flow-cytometric analysis showed that the eYFP+ adipocyte precursors gave rise to Gp38+CD31−ICAM-1intVCAM-1int and Gp38+CD31−ICAM-1hiVCAM-1hi stromal cells, but neither to Gp38−CD31− cells (Figure 7C) nor to blood and lymphatic endothelial cells (data not shown), demonstrating that adipocyte precursors differentiated in vivo into LN organizer cells and marginal reticular cells. Adipocyte precursors isolated form eYFP+Ltbr−/− mice failed to survive and differentiate when reaggregated with WT newborn mesenteric LN carrier cells, demonstrating the critical role of LTβR in the differentiation of adipocyte precursors into LN stromal cells (Figure S6B).
DISCUSSION
The widespread distribution of LNs throughout the body represents an important factor in the effective initiation of immune responses to infection and the generation of immune memory. Although the importance of hematopoietic-stromal cell crosstalk mechanisms—notably LTi cell-stromal organizer cell interactions—are becoming increasingly well described, relatively little is known about the initiation and regulation of earlier stages of LN stromal cell development, including the origin of LN organizer cells. Through experiments that enable direct precursor-product relationships to be analyzed and defined in vivo, we show that adipocyte precursors have the potential to give rise to both adipose and lymphoid tissue stromal cells and that the LTβR represents a key molecular switch in the choice between the two cell lineages, with LTβR ligation by LTα1β2-expressing hematopoietic cells blocking adipogenic differentiation and inducing maturation toward LN stromal cell types. Our results put forward a new model of LN development, wherein these organs and their surrounding fat pads are intimately linked in terms of developmental origin yet can be distinguished by their dependency upon LTβR signaling.
In ontogenetic analysis, we found that embryonic adipocyte precursors were localized around vasculature in the developing fat pads and were also found surrounding and residing within the LN stromal anlage. The perivascular location of these cells, coupled with their expression of LTβR, suggests that they are appropriately positioned and equipped to interact with and respond to LTα1β2+ LTi cells during LN anlage formation. However, the specific route of adipocyte precursor cell migration into either embryonic LN anlage or adult LNs (through the LN capsule or entrance through blood or lymphatic flow) remains to be investigated. We also found that adipocyte progenitors express Il7 and Ccl19, similar to what has been previously shown for T zone reticular cells (Link et al., 2007), which may act as survival signals for LTi cells and T cells (Chappaz and Finke, 2010; Coles et al., 2006; Meier et al., 2007).
In functional experiments, we found that LTβR stimulation had a dramatic effect on the differentiation of adipocyte progenitors. Previous reports have shown that the TNFα-TNF-R1 pathway also has an inhibitory effect on adipocyte differentiation (Cawthorn et al., 2007; Chae and Kwak, 2003; Suzawa et al., 2003). Interestingly, however, although LTβR and TNF-R1 activate a gene expression program through the classical, or canonical, NF-κB pathway, only LTβR activates the alternative, or noncanonical, NF-κB pathway through NF-κB2 p52-RelB heterodimers (Dejardin et al., 2002; Yilmaz et al., 2003), and several reports have shown that the NF-κB-inducing kinase exerts multiple effects that either decrease translation of the PPARγ protein or inhibit its binding to DNA (Guntur et al., 2010; Suzawa et al., 2003; Tang et al., 2006). In contrast, we show here a mechanism by which the negative effect of LTβR on adipocyte differentiation is exerted by the alternative NF-κB pathway through NF-κB2 and RelB, resulting in a block at the transcriptional level of the upregulation of Pparγ and Cebpα genes as well as their downstream targets. These results are in agreement with a gene array study showing that LTβR stimulation of MEFs results in downregulation of Pparγ expression that is dependent on RelB but independent of RelA (Lovas et al., 2008). Whether the p52-RelB complexes act as transcriptional repressors by directly binding to the regulatory region of the Pparγ and Cebpα genes or through an indirect mechanism remains to be elucidated.
Concomitant with the inhibition of adipogenesis, LTβR signaling induced a marked increase in expression of ICAM-1 and VCAM-1 as well as mRNA levels of Cxcl13, the latter characteristic of the maturation of stromal cells toward lymphoid tissue organizer cells. Perhaps most importantly, experiments involving the generation of chimeric fat pad-LN anlage and subsequent monitoring of their development in vivo allowed us to demonstrate directly the LN stromal cell potential of adipocyte progenitors and the central function of LTβR in this process. Notably, we also showed that this developmental potential is not restricted to embryonic cells, because adult adipocyte precursors also differentiated into LN stromal cell populations in vivo. We suggest that the abundance and accessibility of adipose tissue in the body makes adipocyte precursors an ideal potential source of stromal cells for the bioengineering of artificial lymphoid tissues.
Our study highlighting the developmental link between adipose tissue and LN stromal environments is of interest in relation to other reports. For example, in adults, T lymphocytes are found in adipose tissues, their phenotype varying according to location and metabolic status (Kaminski and Randall, 2010; Ouchi et al., 2011). Visceral fat is known to contain larger numbers of lymphocytes in obese subjects compared to lean subjects, but regulatory T cells are enriched in the visceral fat compared to subcutaneous fat and spleen of lean subjects (Feuerer et al., 2009). The inflammatory status of adipose tissues is probably influenced by the presence of activated lymphocytes expressing the LTβR ligands, in that both adipocyte precursors and adipocytes are LTβR+. Interestingly, a study of patients with Sjögren’s syndrome has shown that fat deposition in salivary glands is an indicator of the severity of the disease, suggesting that recruitment of adipocyte precursors might correlate with the formation of specific tertiary lymphoid tissues (Izumi et al., 1997). Further study will be necessary to test whether secondary lymphoid tissues and their associated fat respond as a whole during immune responses and inflammation.
Finally, LTβR-mediated control of the fate of adipocyte progenitor cells underlines the plasticity of these cells and provides an explanation for the simultaneous emergence of the LTβR gene and LN structures in mammalian species. The association of fat deposits and lymphoid structures is evolutionarily conserved, as indicated by studies on monotremes that revealed the presence of “lymphoid nodules” in fatty tissues at sites where LNs are present in placental mammals (Diener and Ealey, 1965). Moreover, this association is not limited to LN, as shown by the presence of lymphoid clusters in other adipose tissues, such as the omentum and the fat associated with the mesenteric vessels. Thus, we propose that differentiation of adipocyte precursors into lymphoid stromal cells in the presence of proinflammatory signals is a common phenomenon that could also contribute to the remodeling of lymphoid tissues during immune responses, the regeneration of these organs following viral infections that affect the stromal structure, and/or the formation of ectopic lymphoid tissues in chronic inflammatory diseases. Interestingly, a role for retinoic acid has recently been described in the early stage of the LN stroma maturation and appears important for the recruitment of LTi cells before LTβR can be triggered on stromal cells. Given that retinoic acid is also required transiently during the initial maturation of adipocyte precursors (Rosen and MacDougald, 2006), these observations lead to the attractive hypothesis that retinoic acid release at the site of the LN node-fat pad formation during embryogenesis may be a common first step in their differentiation and that subsequent LTβR activation enables lymphoid tissue stromal cell maturation and LN development.
EXPERIMENTAL PROCEDURES
Mice
BALB/c (H-2d), C57BL/6 (H-2b), Ltbr−/−, and Eyfp-Rosa 26 (Srinivas et al., 2001) (C57BL/6 background) mice were bred and maintained under specific pathogen-free conditions in the Biomedical Services Unit at the University of Birmingham. All experiments in this report were performed according to Home Office and local ethics committee regulations.
Isolation of Cells, Ex Vivo Culture, and Embryonic Organ Culture
Embryonic inguinal LNs and fat pads were isolated and disaggregated as previously described (Bénézech et al., 2010). Adult fat pads were digested for 1 hr in 2.5 mg/ml Collagenase D (Roche), 100 μg/ml DNase I (Sigma-Aldrich) in RF10 media with 2% fetal calf serum at 37°C with constant shaking (120 rpm). In some experiments, freshly isolated fat pads were explanted in fetal organ culture prepared as described (Bénézech et al., 2010). Primary MEFs were derived from E15.5 embryos and differentiated into adipocytes after 2 days postconfluency by treatment for 48 hr with the adipocyte differentiation cocktail (85 nmol/l insulin, 0.5 mM methylisobutylxanthine, and 1 μM dexamethasone). The medium with insulin only was then replaced every 2 days for 6 days before analysis by oil red O and Nile Red (Sigma-Aldrich) stainings. Agonistic LTβR Ab (clone 4H8, 2 μg/ml) (Banks et al., 2005; Dejardin et al., 2002) or mTNF-α (10 ng/ml) were added to the cultures as indicated.
Antibodies and Flow Cytometry
Abs used for flow cytometry are listed in Tables S1 and S2. Fluorescence-activated cell sorting (FACS) analysis was performed with FACSCalibur and LSRFortessa (BD Biosciences). Data were analyzed with FlowJo software (TreeStar).
Cell Sorting, Cell Culture, and LN Reaggregate Organs
Isolation of cells from embryonic and adult fat pads was performed via MoFlow (Dako Cytomation) cell sorting from disaggregated cell suspensions. LN reaggregates were obtained by mixing mesenteric LN cells with eYFP+ CD45−Ter119−CD31−Gp38+ sorted cells (10:1) and grafted under the kidney capsule of C57Bl6 mice as described (White et al., 2007).
Fat Pad and LN Chimeras
LNs from Eyfp-Rosa 26 E16–E18 embryos were dissected from their corresponding fat pads and replaced with WT E16–E18 or newborn LNs. The resulting chimeras were prepared for organ cultures for 2 days and grafted under the kidney capsule of adult mice. Small pieces of adipose tissue from adult inguinal fat pads from Eyfp-Rosa 26 mice were associated with WT newborn LNs.
RT-PCR and real-time RT-PCR
cDNA and real-time PCRs were performed as previously described (Bénézech et al., 2010). The means of triplicates ± SD (multiplied 1,000 fold) of the ratio of the gene of interest to Hprt are shown. Primer sequences are shown in Table S3.
Lymphocyte Survival Assay
Single-cell suspensions of digested E17 inguinal fat pads (without LNs) were plated in a 24-well plate at a density of 250,000 cells per well and were incubated in complete RPMI 1640 medium (containing 10% fetal bovine serum). Sorted adipocyte precursors from E17 inguinal fat pads were plated in a 48-well plate at a density of 150,000 cells per well. After 24 hr, nonadherent cells were removed and fresh medium was added. Cells were grown for another 48 hr before addition of lymphocytes from disaggregated adult LNs at a ratio of 4:1. After various incubation times, nonadherent lymphocytes were harvested, the number of live cells was determined by trypan blue dye exclusion, and cells were stained with CD4, CD8, CD62L, CD19, and the fixable viability dye eFluor 780 (eBioscience). The percentage of surviving cells was calculated based on the total number of viable naive CD62hiCD4+ and CD62LhiCD8+ T cells and CD62LhiCD19+ B cells before and after culture.
Immunofluorescence Staining
For tissue-section staining, tissues were treated as described (Bénézech et al., 2010). Abs used are listed in Tables S1 and S2. Confocal images were acquired using a Zeiss LSM 510 laser scanning confocal head with a Zeiss Axio Imager Z1 microscope.
Statistical Analysis
Statistical significance was determined for all analysis with an unpaired two-tailed Student’s t test.
Supplementary Material
Acknowledgments
We are grateful to the personnel of the Biomedical Services Unit of the University of Birmingham for taking care of our animal colonies and to R. Bird for cell sorting. We are indebted to K. Pfeffer, D. Kioussis, S. Srinivas, M. Pasparakis, and R. Reed for providing mouse strains, tissues, and reagents. We want to thank E. Jenkinson for continuous support. We are thankful to P. Lane, A. Rot, V. Bekiaris, A. Brendolan, M. Coles, T. Cupedo, J. Girdlestone, R. Golub, K. Serre, and S. van de Pavert for comments on the manuscript. We are grateful to F. Weih for discussing data before publication. This work was supported by a Biotechnology and Biological Sciences Research Council project grant to J.H.C. and G.A., the European Union FP7 integrated project Inflammation and Cancer Research in Europe (INFLA-CARE) to J.C., and the College of Medical and Dental Sciences, University of Birmingham. The work in C.F.W.’s laboratory was supported by National Institutes of Health grant AI33068. E.M. was the recipient of an MRC doctoral training account postdoctoral studentship.
Footnotes
Supplemental Information includes six figures and three tables and can be found with this article online at http://dx.doi.org/10.1016/j.immuni.2012.06.010.
Author contributions are as follows: C.B., G.A., and J.H.C. designed and performed the research and collected and analyzed the data. E.M., G.D., M.K., K.N., A.W., and C.F.W. facilitated the research. C.B., G.A., and J.H.C. wrote the manuscript.
References
- Banks TA, Rickert S, Benedict CA, Ma L, Ko M, Meier J, Ha W, Schneider K, Granger SW, Turovskaya O, et al. A lymphotoxin-IFN-beta axis essential for lymphocyte survival revealed during cytomegalovirus infection. J Immunol. 2005;174:7217–7225. doi: 10.4049/jimmunol.174.11.7217. [DOI] [PubMed] [Google Scholar]
- Bénézech C, White A, Mader E, Serre K, Parnell S, Pfeffer K, Ware CF, Anderson G, Caamaño JH. Ontogeny of stromal organizer cells during lymph node development. J Immunol. 2010;184:4521–4530. doi: 10.4049/jimmunol.0903113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billon N, Dani C. Developmental origins of the adipocyte lineage: new insights from genetics and genomics studies. Stem Cell Rev. 2012;8:55–66. doi: 10.1007/s12015-011-9242-x. [DOI] [PubMed] [Google Scholar]
- Buettner M, Pabst R, Bode U. Stromal cell heterogeneity in lymphoid organs. Trends Immunol. 2010;31:80–86. doi: 10.1016/j.it.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Cawthorn WP, Heyd F, Hegyi K, Sethi JK. Tumour necrosis factor-alpha inhibits adipogenesis via a beta-catenin/TCF4(TCF7L2)-dependent pathway. Cell Death Differ. 2007;14:1361–1373. doi: 10.1038/sj.cdd.4402127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae GN, Kwak SJ. NF-kappaB is involved in the TNF-alpha induced inhibition of the differentiation of 3T3-L1 cells by reducing PPARgamma expression. Exp Mol Med. 2003;35:431–437. doi: 10.1038/emm.2003.56. [DOI] [PubMed] [Google Scholar]
- Chappaz S, Finke D. The IL-7 signaling pathway regulates lymph node development independent of peripheral lymphocytes. J Immunol. 2010;184:3562–3569. doi: 10.4049/jimmunol.0901647. [DOI] [PubMed] [Google Scholar]
- Coles MC, Veiga-Fernandes H, Foster KE, Norton T, Pagakis SN, Seddon B, Kioussis D. Role of T and NK cells and IL7/IL7r interactions during neonatal maturation of lymph nodes. Proc Natl Acad Sci USA. 2006;103:13457–13462. doi: 10.1073/pnas.0604183103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupedo T, Jansen W, Kraal G, Mebius RE. Induction of secondary and tertiary lymphoid structures in the skin. Immunity. 2004a;21:655–667. doi: 10.1016/j.immuni.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Cupedo T, Vondenhoff MF, Heeregrave EJ, De Weerd AE, Jansen W, Jackson DG, Kraal G, Mebius RE. Presumptive lymph node organizers are differentially represented in developing mesenteric and peripheral nodes. J Immunol. 2004b;173:2968–2975. doi: 10.4049/jimmunol.173.5.2968. [DOI] [PubMed] [Google Scholar]
- De Togni P, Goellner J, Ruddle NH, Streeter PR, Fick A, Mariathasan S, Smith SC, Carlson R, Shornick LP, Strauss-Schoenberger J, et al. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science. 1994;264:703–707. doi: 10.1126/science.8171322. [DOI] [PubMed] [Google Scholar]
- Dejardin E, Droin NM, Delhase M, Haas E, Cao Y, Makris C, Li ZW, Karin M, Ware CF, Green DR. The lymphotoxin-beta receptor induces different patterns of gene expression via two NF-kappaB pathways. Immunity. 2002;17:525–535. doi: 10.1016/s1074-7613(02)00423-5. [DOI] [PubMed] [Google Scholar]
- Diener E, Ealey EH. Immune system in a monotreme: studies on the Australian echidna (Tachyglossus aculeatus) Nature. 1965;208:950–953. doi: 10.1038/208950a0. [DOI] [PubMed] [Google Scholar]
- Eberl G, Marmon S, Sunshine MJ, Rennert PD, Choi Y, Littman DR. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004;5:64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, Mathis D. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fütterer A, Mink K, Luz A, Kosco-Vilbois MH, Pfeffer K. The lymphotoxin beta receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity. 1998;9:59–70. doi: 10.1016/s1074-7613(00)80588-9. [DOI] [PubMed] [Google Scholar]
- Ganeff C, Remouchamps C, Boutaffala L, Benezech C, Galopin G, Vandepaer S, Bouillenne F, Ormenese S, Chariot A, Schneider P, et al. Induction of the alternative NF-κB pathway by lymphotoxin αβ (LTαβ) relies on internalization of LTβ receptor. Mol Cell Biol. 2011;31:4319–4334. doi: 10.1128/MCB.05033-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guntur KV, Guilherme A, Xue L, Chawla A, Czech MP. Map4k4 negatively regulates peroxisome proliferator-activated receptor (PPAR) gamma protein translation by suppressing the mammalian target of rapamycin (mTOR) signaling pathway in cultured adipocytes. J Biol Chem. 2010;285:6595–6603. doi: 10.1074/jbc.M109.068502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausman GJ, Wright JT, Jewell DE, Ramsay TG. Fetal adipose tissue development. Int J Obes. 1990;14(Suppl 3):177–185. [PubMed] [Google Scholar]
- Ishikawa H, Carrasco D, Claudio E, Ryseck RP, Bravo R. Gastric hyperplasia and increased proliferative responses of lymphocytes in mice lacking the COOH-terminal ankyrin domain of NF-kappaB2. J Exp Med. 1997;186:999–1014. doi: 10.1084/jem.186.7.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi M, Eguchi K, Nakamura H, Nagataki S, Nakamura T. Premature fat deposition in the salivary glands associated with Sjögren syndrome: MR and CT evidence. AJNR Am J Neuroradiol. 1997;18:951–958. [PMC free article] [PubMed] [Google Scholar]
- Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FM. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski DA, Randall TD. Adaptive immunity and adipose tissue biology. Trends Immunol. 2010;31:384–390. doi: 10.1016/j.it.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katakai T, Suto H, Sugai M, Gonda H, Togawa A, Suematsu S, Ebisuno Y, Katagiri K, Kinashi T, Shimizu A. Organizer-like reticular stromal cell layer common to adult secondary lymphoid organs. J Immunol. 2008;181:6189–6200. doi: 10.4049/jimmunol.181.9.6189. [DOI] [PubMed] [Google Scholar]
- Link A, Vogt TK, Favre S, Britschgi MR, Acha-Orbea H, Hinz B, Cyster JG, Luther SA. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol. 2007;8:1255–1265. doi: 10.1038/ni1513. [DOI] [PubMed] [Google Scholar]
- Lovas A, Radke D, Albrecht D, Yilmaz ZB, Möller U, Habenicht AJ, Weih F. Differential RelA- and RelB-dependent gene transcription in LTbetaR-stimulated mouse embryonic fibroblasts. BMC Genomics. 2008;9:606. doi: 10.1186/1471-2164-9-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier D, Bornmann C, Chappaz S, Schmutz S, Otten LA, Ceredig R, Acha-Orbea H, Finke D. Ectopic lymphoid-organ development occurs through interleukin 7-mediated enhanced survival of lymphoid-tissue-inducer cells. Immunity. 2007;26:643–654. doi: 10.1016/j.immuni.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- Mueller SN, Germain RN. Stromal cell contributions to the homeostasis and functionality of the immune system. Nat Rev Immunol. 2009;9:618–629. doi: 10.1038/nri2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neels JG, Thinnes T, Loskutoff DJ. Angiogenesis in an in vivo model of adipose tissue development. FASEB J. 2004;18:983–985. doi: 10.1096/fj.03-1101fje. [DOI] [PubMed] [Google Scholar]
- Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pond CM. Paracrine relationships between adipose and lymphoid tissues: implications for the mechanism of HIV-associated adipose redistribution syndrome. Trends Immunol. 2003;24:13–18. doi: 10.1016/s1471-4906(02)00004-2. [DOI] [PubMed] [Google Scholar]
- Pond CM, Mattacks CA. The activation of the adipose tissue associated with lymph nodes during the early stages of an immune response. Cytokine. 2002;17:131–139. doi: 10.1006/cyto.2001.0999. [DOI] [PubMed] [Google Scholar]
- Procaccini C, Jirillo E, Matarese G. Leptin as an immunomodulator. Mol Aspects Med. 2012;33:35–45. doi: 10.1016/j.mam.2011.10.012. [DOI] [PubMed] [Google Scholar]
- Randall TD, Carragher DM, Rangel-Moreno J. Development of secondary lymphoid organs. Annu Rev Immunol. 2008;26:627–650. doi: 10.1146/annurev.immunol.26.021607.090257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel-Moreno J, Moyron-Quiroz JE, Carragher DM, Kusser K, Hartson L, Moquin A, Randall TD. Omental milky spots develop in the absence of lymphoid tissue-inducer cells and support B and T cell responses to peritoneal antigens. Immunity. 2009;30:731–743. doi: 10.1016/j.immuni.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell. 2008;135:240–249. doi: 10.1016/j.cell.2008.09.036. [DOI] [PubMed] [Google Scholar]
- Roozendaal R, Mebius RE. Stromal cell-immune cell interactions. Annu Rev Immunol. 2011;29:23–43. doi: 10.1146/annurev-immunol-031210-101357. [DOI] [PubMed] [Google Scholar]
- Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7:885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- Ruddle NH, Akirav EM. Secondary lymphoid organs: responding to genetic and environmental cues in ontogeny and the immune response. J Immunol. 2009;183:2205–2212. doi: 10.4049/jimmunol.0804324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Unutmaz D, Zou YR, Sunshine MJ, Pierani A, Brenner-Morton S, Mebius RE, Littman DR. Requirement for RORgamma in thymocyte survival and lymphoid organ development. Science. 2000;288:2369–2373. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- Suzawa M, Takada I, Yanagisawa J, Ohtake F, Ogawa S, Yamauchi T, Kadowaki T, Takeuchi Y, Shibuya H, Gotoh Y, et al. Cytokines suppress adipogenesis and PPAR-gamma function through the TAK1/TAB1/NIK cascade. Nat Cell Biol. 2003;5:224–230. doi: 10.1038/ncb942. [DOI] [PubMed] [Google Scholar]
- Tang X, Guilherme A, Chakladar A, Powelka AM, Konda S, Virbasius JV, Nicoloro SM, Straubhaar J, Czech MP. An RNA interference-based screen identifies MAP4K4/NIK as a negative regulator of PPARgamma, adipogenesis, and insulin-responsive hexose transport. Proc Natl Acad Sci USA. 2006;103:2087–2092. doi: 10.1073/pnas.0507660103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, Tallquist MD, Graff JM. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322:583–586. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uezumi A, Fukada S, Yamamoto N, Takeda S, Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol. 2010;12:143–152. doi: 10.1038/ncb2014. [DOI] [PubMed] [Google Scholar]
- van de Pavert SA, Mebius RE. New insights into the development of lymphoid tissues. Nat Rev Immunol. 2010;10:664–674. doi: 10.1038/nri2832. [DOI] [PubMed] [Google Scholar]
- van de Pavert SA, Olivier BJ, Goverse G, Vondenhoff MF, Greuter M, Beke P, Kusser K, Höpken UE, Lipp M, Niederreither K, et al. Chemokine CXCL13 is essential for lymph node initiation and is induced by retinoic acid and neuronal stimulation. Nat Immunol. 2009;10:1193–1199. doi: 10.1038/ni.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vondenhoff MF, Greuter M, Goverse G, Elewaut D, Dewint P, Ware CF, Hoorweg K, Kraal G, Mebius RE. LTbetaR signaling induces cytokine expression and up-regulates lymphangiogenic factors in lymph node anlagen. J Immunol. 2009;182:5439–5445. doi: 10.4049/jimmunol.0801165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White A, Carragher D, Parnell S, Msaki A, Perkins N, Lane P, Jenkinson E, Anderson G, Caamaño JH. Lymphotoxin a-dependent and -independent signals regulate stromal organizer cell homeostasis during lymph node organogenesis. Blood. 2007;110:1950–1959. doi: 10.1182/blood-2007-01-070003. [DOI] [PubMed] [Google Scholar]
- Wright JT, Hausman GJ. Adipose tissue development in the fetal pig examined using monoclonal antibodies. J Anim Sci. 1990;68:1170–1175. doi: 10.2527/1990.6841170x. [DOI] [PubMed] [Google Scholar]
- Yilmaz ZB, Weih DS, Sivakumar V, Weih F. RelB is required for Peyer’s patch development: differential regulation of p52-RelB by lymphotoxin and TNF. EMBO J. 2003;22:121–130. doi: 10.1093/emboj/cdg004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H, Naito A, Inoue J, Satoh M, Santee-Cooper SM, Ware CF, Togawa A, Nishikawa S, Nishikawa S. Different cytokines induce surface lymphotoxin-alphabeta on IL-7 receptor-alpha cells that differentially engender lymph nodes and Peyer’s patches. Immunity. 2002;17:823–833. doi: 10.1016/s1074-7613(02)00479-x. [DOI] [PubMed] [Google Scholar]
- Zeve D, Tang W, Graff J. Fighting fat with fat: the expanding field of adipose stem cells. Cell Stem Cell. 2009;5:472–481. doi: 10.1016/j.stem.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.