Abstract
Purpose
Mutations in receptor tyrosine kinase (RTK) genes can confer resistance to receptor-targeted therapies. A T798M mutation in the HER2 oncogene has been shown to confer resistance to the tyrosine kinase inhibitor (TKI) lapatinib. We studied the mechanisms of HER2-T798M-induced resistance to identify potential strategies to overcome that resistance.
Experimental Design
HER2-T798M was stably expressed in BT474 and MCF10A cells. Mutant cells and xenografts were evaluated for effects of the mutation on proliferation, signaling, and tumor growth after treatment with combinations of inhibitors targeting the EGFR-HER2-HER3-PI3K axis.
Results
A low 3% allelic frequency of the T798M mutant shifted10-fold the IC50 of lapatinib. In mutant-expressing cells, lapatinib did not block basal phosphorylation of HER2, HER3, AKT and ERK1/2. In vitro kinase assays showed increased autocatalytic activity of HER2-T798M. HER3 association with PI3K p85 was increased in mutant-expressing cells. BT474-T798M cells were also resistant to the HER2 antibody trastuzumab. These cells were sensitive to the pan-PI3K inhibitors BKM120 and XL147 and the irreversible HER2/EGFR TKI afatinib but not the MEK1/2 inhibitor CI-1040, suggesting continued dependence of the mutant cells on ErbB receptors and downstream PI3K signaling. BT474-T798M cells showed increased expression of the EGFR ligands EGF, TGFα, amphiregulin and HB-EGF. Addition of the EGFR neutralizing antibody cetuximab or lapatinib restored trastuzumab sensitivity of BT474-T798M cells and xenografts, suggesting increased EGFR ligand production was causally associated with drug resistance.
Conclusions
Simultaneous blockade of HER2 and EGFR should be an effective treatment strategy against HER2 gene-amplified breast cancer cells harboring T798M mutant alleles.
Keywords: HER2, kinase domain mutations, EGFR, lapatinib, trastuzumab, cetuximab, breast cancer
Introduction
The ErbB family of transmembrane RTKs comprises four members: EGFR (ErbB1), HER2 (ErbB2), HER3 (ErbB3) and HER4 (ErbB4) (1). Several human cancers have been associated with dysregulation of ErbB receptors. Approximately 25% of invasive breast cancers exhibit HER2 gene amplification and mRNA/protein overexpression (2). Anti-HER2 therapies such as the antibody trastuzumab are active in patients with HER2-overexpressing breast cancer (3, 4). HER2 does not have an activating ligand but can be transactivated by ligand-induced ErbB co-receptors. For example, HER2 and EGFR cooperate in the transformation of mouse fibroblasts (5). Ligand-induced EGFR forms heterodimers with HER2 (6); in turn, HER2 reduces degradation of EGFR (7) by promoting ligand binding to EGFR and inhibiting binding of EGFR to its ubiquitin ligase Cbl (8). Consistent with this mutual dependence and synergy, inhibition of EGFR can reduce the growth of HER2+ breast cancer cells both in vitro and in vivo (9-11).
The small molecule, ATP-mimetic lapatinib blocks HER2 and EGFR kinases and downstream signaling such as PI3K/AKT and MAPK (12). Lapatinib is also approved for the treatment of HER2-overexpressing breast cancer and in combination with trastuzumab is more effective than each drug given alone (13). Activation of alternate pro-survival pathways reduces the dependence of tumors on the targeted oncogenic kinase, leading to acquired drug resistance that can be overcome by combination treatments (13). Additionally, the clinical benefit of small molecule TKIs is generally limited by acquired mutations in the targeted kinase. A common causal mechanism of acquired resistance to TKIs is the development of kinase domain mutations, such as those reported in BCR-ABL (14), cKIT (15), PDGFRα (16) and EGFR (17, 18).
Mutations in the tyrosine kinase domain of HER2 have been identified in head and neck, lung, gastric and breast carcinomas (19-23). An in vitro screen using a randomly mutagenized HER2 expression library identified several kinase domain mutations associated with resistance to lapatinib (24). In this study, a T798M substitution in HER2, analogous to the gatekeeper EGFRT790M (17), ABLT315I (14) and cKITT670I (15) mutations, conferred the strongest resistance to lapatinib (24). A similar random mutagenesis approach had discovered BCR-ABL mutations that were subsequently found in patients with chronic myelogenous leukemia (CML) with acquired resistance to the ABL inhibitors imatinib and dasatinib (25). Kancha et al. cloned eight clinically observed HER2 mutations. Some were lapatinib-sensitive whereas others, including T798M, were resistant when expressed in cells without HER2 gene amplification. Interestingly, chronic exposure to lapatinib selected cancer cells with acquired L755S and T862A drug-resistant mutations (26).In The Cancer Genome Atlas (TCGA) breast cancer dataset, eight tumors harbored mutations in HER2, one of which, D769H, occurred in a tumor that was also HER2-amplified (27, 28).
To study the mechanisms by which the T798M mutation confers resistance to lapatinib and strategies to reverse such resistance in HER2 gene-amplified breast cancer, we generated BT474 cells stably expressing the mutant allele. BT474 cells stably expressing HER2T798M were resistant to eitherlapatinibor trastuzumab alone. HER2T798M exhibited increased autocatalytic activity compared to wild-type HER2.BT474-HER2T798M cells expressed higher levels of the EGFR ligands EGF, TGFα, amphiregulin, and HB-EGF. Consistent with a causal role of these ligands, the addition of the neutralizing EGFR antibody cetuximab restored sensitivity to trastuzumab in cells and xenografts expression HER2T798M. Further, inhibition of EGFR with lapatinib also synergized with trastuzumab against xenografts expressing HER2T798M, suggesting simultaneous inhibition of EGFR and HER2 abrogates the resistance induced by the gatekeeper mutation.
Methods
Generation of cells stably expressing HER2T798M
A HER2T798M expression vector was generated by subcloning the mutant sequence in the SalI/HindIII site of DNR Dual (BD Biosciences) and then recombined using Cre into the JP1520 retroviral vector. Retroviruses expressing HER2T798M were produced by transfecting Phoenix-Ampho cells using published methods (20) and then utilized to transduce BT474 and MCF10A cells. Stably transfected cells were selected in 1 mg/ml G418.
Cell culture and proliferation assays
BT474 cells were maintained in IMEM medium/10% fetal bovine serum (FBS, Gibco). MCF10A cells were maintained in DMEM/F-12 supplemented with EGF (20 ng/ml, Invitrogen/Gibco), cholera toxin (100 ng/ml, Sigma), hydrocortisone (500 ng/ml, Sigma), insulin (10 μg/ml, Invitrogen) and 5% horse serum (Hyclone). Cell proliferation was measured either by fixing and staining cells with crystal violet (29) or by using MTT (Sigma) or pre-mixed WST-1 reagent (Roche) according to the manufacturer's protocol. The following inhibitors were used for various proliferation assays: 1 μM lapatinib; 1 μM CI-1040; 1 μM BIBW2992 (all from LC Laboratories); 20 μg/ml trastuzumab; 20 μg/ml cetuximab (both from the Vanderbilt University Pharmacy); 1 μM BKM120 (Active Biochem); 6 μM XL-147 (provided by Exelixis). For MTT/WST-1 assays, 1×104 cells/well were seeded in 96-well plates. Twenty-four h after plating, cells were treated with DMSO or inhibitors. After 5 days of treatment, MTT/WST-1 assays were performed according to the manufacturer's protocol. For growth assays following HER3 knockdown, pooled HER3 and control siRNA (Dharmacon) were reverse-transfected into cells using Lipofectamine RNAi Max (Invitrogen) according to manufacturer's protocol. Briefly, cells were plated in triplicate in 24-well plates and transfected with HER3 or control siRNA (50 nM). Seven days post-transfection, cells were trypsinised and counted using a Coulter counter. Alternatively, cells were treated with the LNA oligonucleotides directed against HER3 (EZN3920) or control (EZN4455) at a final concentration of 5 μM (30) provided by Enzon Pharmaceuticals, Piscataway, NJ) for seven days.
Three-dimensional Matrigel culture
Cells (5×103/well) were seeded in 8-well chamber-slides. Prior to seeding, BT474 and MCF10A cells were suspended in their respective medium on growth factor reduced Matrigel (BD Biosciences) as described (31). Inhibitors were added at the time of cell seeding and replenished with fresh medium every 3 days. After 12-14 days, images were captured from at least three different fields. To quantify cell number, Matrigel was dissolved by treatment with dispase for 2 h at 37°C and acini were dissociated by pipetting. Dissociated cells were treated with trypsin and pelleted by centrifugation before being resuspended in growth medium and counted using a hemocytometer.
Immunoprecipitation and immunoblotting
Immunoprecipitations were performed with a p85 antibody (Millipore) or a HER2 antibody (Neomarkers) followed by Protein A beads (Sigma) as described (32). Immune complexes and whole cell lysates were subjected to SDS-PAGE and transferred onto nitrocellulose membranes. For immunoblot analysis, cells were lysed in 1% NP-40 buffer containing protease and phosphatase inhibitors. Samples were sonicated for 10 sec and centrifuged at 14,000 rpm for 5 min at 4°C; protein concentrations were quantitated using the BCA assay (Pierce). Primary antibodies included Y1248 P-HER2, Y1068 P-EGFR, total EGFR, Y1197 P-HER3, total AKT, S473 P-Akt, P-Erk1/2, Erk1/2, (Cell Signaling), HER3, EGFR (Santa Cruz Biotechnology), HER2 (Neomarkers), Actin (Sigma), and the 4G10 phosphotyrosine antibody (Millipore).
Cell-surface biotinylation
The Cell Surface Protein Isolation kit (Pierce) was used for biotinylation studies according to the manufacturer's protocol. After treatment with 20 μg/ml trastuzumab for 16 h at 37°C, cells were incubated with cold acid wash buffer (0.5 M NaCl, 0.2 M Na acetate, pH 3.0) for 6 min to remove bound trastuzumab. The cell monolayers were washed 3× with ice-cold PBS (pH 8.0) before the addition of freshly prepared Sulfo-NHS-Biotin reagent (2 mM; Pierce) for 30 min at 4°C. The reaction was quenched with 100 mM glycine in PBS and cells were harvested in lysis buffer (plus protease and phosphatase inhibitors) included in the kit. After sonication for 10 sec and centrifugation at 14,000 rpm, protein concentration in the supernatants was measured using the BCA assay (Pierce). Equal amounts of protein extracts (500 μg) were subjected to precipitation using immobilized Neutravidin gel (Pierce); eluates were next subjected to SDS-PAGE and HER2 immunoblot analysis.
In vitro kinase assays
Five-hundred μg of total protein from cells were precipitated with a HER2 antibody overnight at 4°C. Precipitates were subjected to an in vitro kinase assay as described (33). Briefly, precipitates were washed twice with NP-40 lysis buffer followed by two washes with kinase buffer (20 mM HEPES [pH 7.5], 10 mM MgCl2, 10 mM MnCl2, 1 mM dithiothreitol, 0.1 mM Na3VO4). The immune complexes were next divided on ice into two equal aliquots and ATP (final concentration 0.1 mM) was added to one of the kinase reactions, which were carried out for 5 min at 30°C and terminated by adding 5× loading buffer followed by boiling for 3 min. Kinase reaction products were then separated on a 7.5% SDS-PAGE gel and subjected to immunoblot analysis.
Real-time qPCR
RNA isolation and real-time qPCR was performed as described (34, 35). Primer sequences for ErbB ligands are as follows: Heregulin (HRG): forward 5′-TGGCTGACAGCAGGACTAAC-3′, reverse 5′-CTGGCCTGGATTTCTTC-3′; EGF: forward 5′-AGCTAACCCATTATGGCAACA-3′, reverse 5′-AGTTTTCACTGAGTCAGCTCCAT-3′; TGFα: forward 5′-GGACAGCACTGCCAGAGA-3′, reverse 5′-CAGGTGATTACAGGCCAAGTAG-3′; Amphiregulin (AREG): forward 5′-ATATCACATTGGAGTCACTGCCCA-3′, reverse 5′-GGGTCCATTGTCTTATGATCCAC-3′; HB-EGF: forward 5′-GAAAGACTTCCATCTAGTCACAAAGA-3′, reverse 5′-GGGAGGCCCAATCCTAGA-3′; Epiregulin (EREG): forward 5′-TGCATGCAATTTAAAGTAACTTATTTGACTA-3′, reverse 5′-ATCTTAAGGTACACAATTATCAAAGCTGA-3′; Betacellulin (BTC): forward 5′-TGCCCCAAGCAATACAAGC-3′, reverse 5′-CGTCTGCTCGGCCACC-3′
HER2T798M sequencing
Exons 19-20 of HER2 were amplified from reverse-transcribed RNA isolated from BT474T798M using the primer pair forward 5′-GTAGGATCCAGCCCACGCTC-3′ and reverse 5′-CTAGACACCACTCCACCCAG-3′. The RT-PCR product was cloned into the pCR Blunt vector (Invitrogen). A total of 96 colonies were picked to inoculate individual cultures in 96-well blocks, which were then pooled into twelve groups of 8 individual clones. These twelve pools were screened for the presence of HER2T798M by direct (Sanger) sequencing. Individual plasmids from positive pools were resequenced to determine the allelic frequency of HER2T798M.
Xenograft studies
Mouse experiments were approved by the Vanderbilt Institutional Animal Care and Use Committee. Mice were housed in the Vanderbilt Animal Care Facility. A 17β-estradiol pellet (Innovative Research of America) was injected s.c. in the dorsum of 5- to 6-week old athymic female mice (Harlan Sprague–Dawley) the day before tumor cell injection. BT474 cells (5×106) mixed 1:1 with Matrigel (BD Biosciences) were injected s.c. in the right flank of each mouse. Tumor diameters were measured with calipers twice per week and volume in mm3 calculated by the formula volume = width2 × length/2. When tumors reached a volume ≥200 mm3, mice were treated for 4-5 weeks with the following, either alone or in combination: trastuzumab 30 mg/kg twice per week i.p., lapatinib 100 mg/kg daily via orogastric gavage, and cetuximab 1 mg twice per week i.p.
Immunohistochemistry
Tumors were harvested at the end of treatment (after 4-5 weeks), fixed in formalin and paraffin-embedded. Tumor sections (5- μm) were stained with a S473 P-Akt antibody (Cell Signaling) and staining intensity was scored by an expert pathologist (M.V.E.) blinded to treatment groups. Intensity of cytoplasmic staining was scored from 0 to 3+, in 5 different fields at 400× magnification for each section. The average score from those 5 fields was then used to calculate an H-score by the following formula:3×(% of 3+ cells) + 2×(% of 2+ cells)+1×(% of 1+ cells). The mean H-scores ± S.E.M. for each treatment group were compared by ANOVA.
Results
T798M mutation in HER2 confers resistance to HER2 antagonists in HER2-amplified breast cancer cells
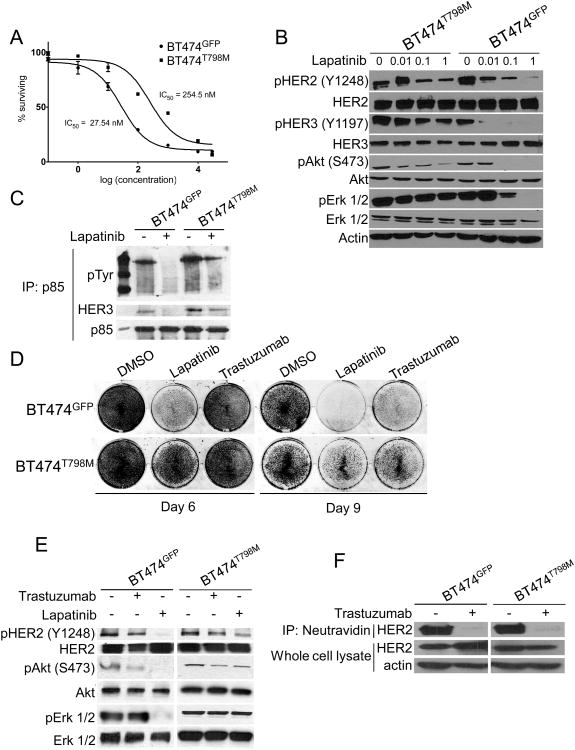
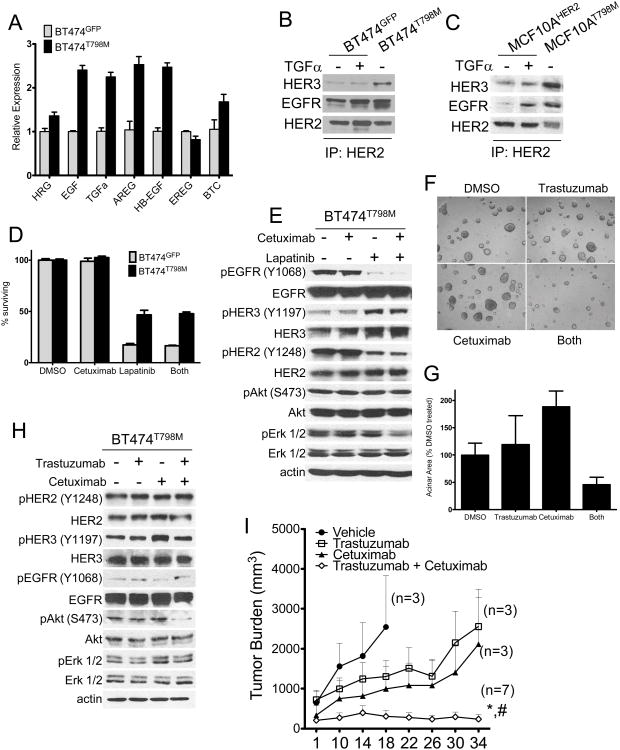
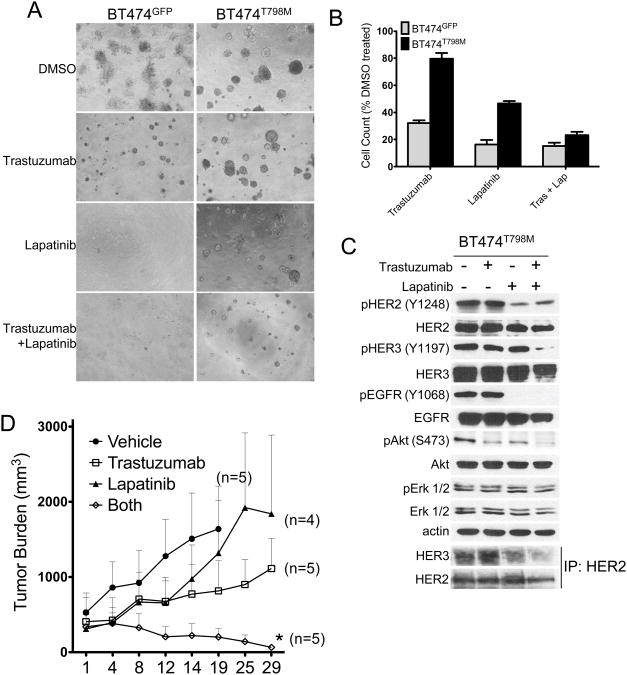
BT474 cells were stably transduced with a retroviral vector encoding either HER2T798M or GFP. Expression of the T798M mutant isoform increased the IC50 to lapatinib by 10-fold compared to GFP-expressing cells (Fig. 1A). Treatment with 1 μM lapatinib blocked phosphorylation of HER2, HER3, AKT and Erk1/2 in BT474GFP but not in BT474T798M cells (Fig. 1B). Presence of the mutation also resulted in continued association of HER3 with the p85 regulatory subunit of PI3K in the presence of lapatinib (Fig. 1C). Lapatinib or trastuzumab inhibited growth of BT474GFPcells, but BT474T798M cells were resistant to both inhibitors (Fig. 1D). Treatment with trastuzumab inhibited AKT phosphorylation in BT474GFP but not in BT474T798M cells (Fig. 1E). Trastuzumab binds to domain IV of HER2 (36) and induces receptor internalization and downregulation from the cell surface thus attenuating downstream signal transduction (37). Since trastuzumab did not inhibit downstream signaling in cells expressing T798M (Fig 1E), we investigated if this mutation would impair trastuzumab-induced receptor internalization. Cell surface biotinylation followed by precipitation of labeled proteins with neutravidin showed that trastuzumab markedly downregulated cell surface HER2 in both BT474GFP and BT474T798M cells (Fig. 1F).
Figure 1. Cells expressing HER2T798M are resistant to lapatinib and trastuzumab.
A. BT474GFP and BT474T798M cells were treated with increasing concentrations of lapatinib for 5 days. MTT assay was performed at the end of treatment and dose-inhibition curves produced by Graphpad Prizm software. IC50 values were calculated according to the formula Y=100/(1+10^(X-LogIC50)). B. BT474GFP and BT474T798M cells were treated with increasing concentrations of lapatinib for 3 h. Protein extracts were prepared and subjected to immunoblot analyses with the antibodies indicated on the left. C. Cells were treated with lapatinib for 3 h and protein extracts were subjected to immunoprecipitation with a p85 antibody. Immune complexes associated with p85 were separated by SDS-PAGE followed by immunoblot with the indicated antibodies. D. Cells were treated with either 1 μM lapatinib or 20 μg/ml trastuzumab for 9 days. Crystal violet assays were performed on days 6 and 9 and images captured using the Li-Cor Odyssey system. E. Cells were treated with 1 μM lapatinib or 20 μg/ml trastuzumab for 3 h. Protein extracts were prepared and subjected to immunoblot analyses with antibodies indicated on the left. F. Cells were treated with 20 μg/ml trastuzumab overnight and then cell surface proteins were biotinylated as described in Methods. Biotinylated proteins were captured with Neutravidin gel and analyzed by immunoblot with a HER2 antibody. Bottom panels represent HER2 and actin immunoblots of whole cell lysates to control for gel loading.
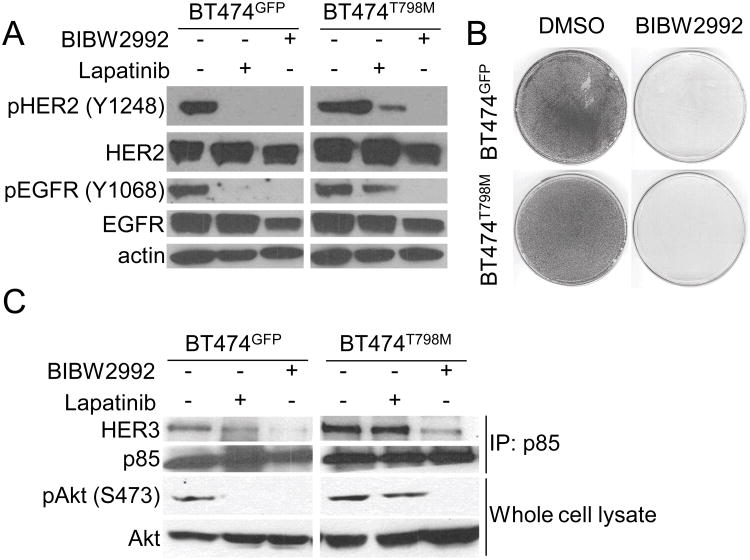
We next examined if the T798M mutant allele was required for growth and survival of mutant-expressing cells by using the covalent irreversible HER2/EGFR small molecule inhibitor BIBW2992 (afatinib). Afatinib has been shown to bind to and inhibit the EGFR T790M gatekeeper mutant (38). Treatment with BIBW2992 but not with lapatinib eliminated phosphorylation of HER2 and EGFR as measured with site-specific receptor antibodies (Fig. 2A) and potently inhibited growth of both BT474GFP and BT474T798M cells (Fig. 2B). BIBW2992 also disrupted the association of p85 with HER3 and inhibited P-AKT levels in both cell lines (Fig. 2C). Similar results were observed with treatment with the pan-ErbB irreversible inhibitor CI-1033 (39), which inhibited HER2 phosphorylation and growth of both BT474GFP and BT474T798M cells (Suppl. Fig. 1). These results suggest that HER2T798M-expressing cells rely on the mutant allele for activation of the PI3K/AKT pathway and their survival.
Figure 2. Cells expressing HER2T798M remain dependent on the ErbB pathway to activate PI3K/AKT.
A. Cells were treated with 1 μM lapatinib or 1 μM BIBW2992 for 3 h. Protein extracts were then prepared and subjected to immunoblot analyses with the indicated antibodies. B. Cells were treated with 1 μM BIBW2992. Monolayers were fixed and stained with crystal violet when DMSO-treated cells reached confluence (day 6). C. Cells were treated with 1 μM lapatinib or 1 μM BIBW2992 for 3 h; protein extracts were precipitated with a p85 antibody. Antibody pulldowns were next washed, separated by SDS-PAGE and subjected to immunoblot analysis as described in Methods using the indicated antibodies (upper two panels). Immunoblot analyses on whole cell extracts were performed using the antibodies indicated on the left (lower two panels).
HER2T798M has increased autocatalytic activity compared to wild-type HER2
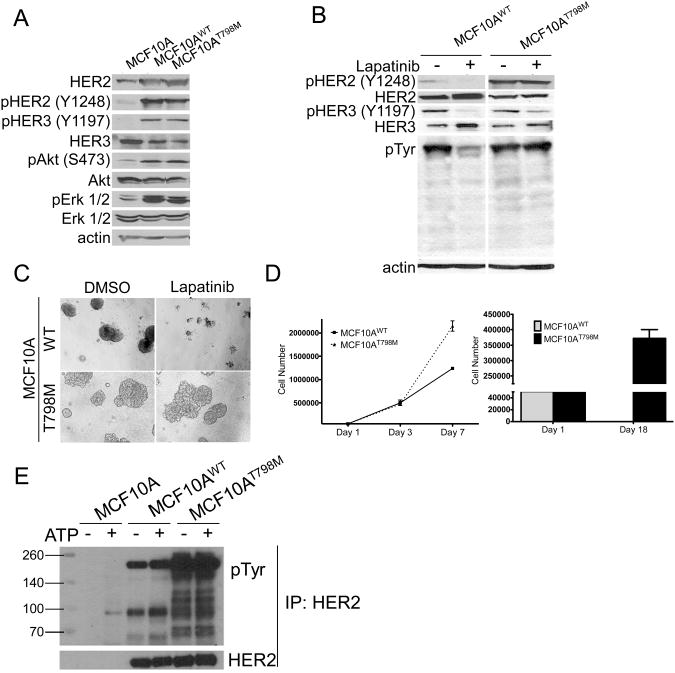
Expression of T798M resulted in increased HER3 phosphorylation and its association with the p85 subunit of PI3K (Suppl. Fig. 2), suggesting that the mutant HER2 might be catalytically more active than wild-type HER2. To examine this possible gain-of-function, we stably expressed HER2WT or HER2T798M in MCF10A human mammary epithelial cells that normally express low levels of HER2. Expression of both HER2WT and HER2T798M resulted in increased phosphorylation of HER2, HER3, AKT and ERK1/2 compared to controls (Fig. 3A). Treatment with lapatinib inhibited pHER2 and pHER3 as well as growth in 3D matrigel of MCF10AWT but not MCF10AT798M cells (Fig. 3B, C). In addition, MCF10AT798M acini were larger and more invasive than MCF10AWT acini and proliferated faster compared to MCF10AWT cells (Fig. 3D) in both full-serum and serum-free conditions, further supporting a gain of HER2 function conferred by the mutant allele. Finally, to test HER2 kinase activity, HER2WT and HER2T798M were immunoprecipitated from both cell types and the pull-downs were tested in an in vitro kinase reaction (Fig. 3E). Immunoprecipitates from parental MCF10A were used as controls. HER2T798M showed markedly higher tyrosine phosphorylation than HER2WT, suggesting that the mutant allele has higher catalytic activity (Fig. 3E).
Figure 3. HER2T798M exhibits a gain of function compared to HER2WT.
A. Retroviral infection was used to produce MCF10A cells stably expressing either HER2WT or HER2T798M. Whole cell lysates were subjected to immunoblot analyses with antibodies indicated on the left. B. Cells were treated with 1 μM lapatinib for 3 h and whole lysates were subjected to immunoblot analyses with the indicated antibodies. C. Cells were plated in Matrigel and treated with DMSO or 1 μM lapatinib. Cells were plated in triplicates and images captured on day 14. Fresh medium and inhibitor were replenished every 3 days. D. MCF10AWT or MCF10AT798M (5×104/well) were plated in triplicates in 6-well plates in absence or presence of 10% serum. For growth assay in serum-containing media, cells were counted on days 1, 3 and 7. For growth assays in serum-free condition, cells were counted on day 1 and 18. E. HER2 was immunoprecipitated from 500 μg of protein extract from MCF10A cells with a C-terminus HER2 antibody where indicated. Immobilized HER2 was used for in vitro kinase assays as described in Methods [26]. Kinase reactions were eluted from beads and subjected to SDS-PAGE and immunoblot analysis with HER2 and anti-phosphotyrosine antibodies.
HER2T798M expressed at low frequency is sufficient to confer resistance
It has been shown that a very low allele frequency of the EGFRT790M gatekeeper mutation is enough to confer resistance to the EGFR TKI gefitinib (40). Using dilutional cloning, we found that approximately 3.1% of HER2 alleles contain T798M in lapatinib-resistant BT474T798M cells (Suppl. Fig. 3). This is consistent with earlier reports which demonstrated that T790M mutation in EGFR can render cells resistant to gefitinib with an allele frequency of 3.3% (40). To support further that this low frequency of expression was sufficient to confer resistance, we performed co-culture experiments wherein varying proportions of BT474GFP (green) and BT474T798M cells (unlabeled) were mixed and then subjected to selection with lapatinib. We found that lapatinib-resistant acini emerge when as low as 5% of the total cell population is BT474T798M (Suppl. Fig. 4). In this experiment, expansion of the mutant population of cells was indicated by loss of the GFP fluorescence from the resistant acini.
HER2T798M-expressing cells rely on HER3-PI3K for survival
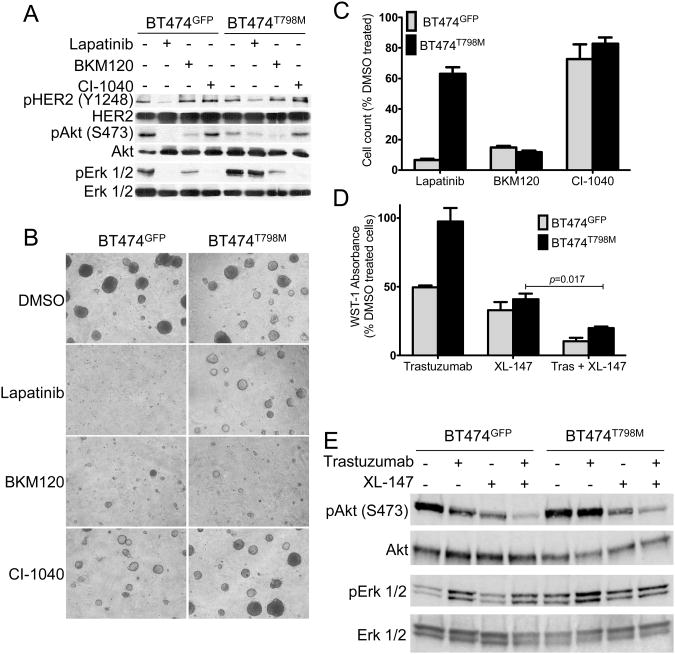
In HER2 gene-amplified breast cancer cells, HER2 potently activates the PI3K pro-survival pathway mainly by dimerizing with and phosphorylating the HER3 co-receptor (41, 42). To determine if cells bearing the T798M mutant remain dependent on PI3K/AKT, we treated BT474GFP and BT474T798M cells with the pan-PI3K inhibitor BKM120 (43). Treatment with BKM120 blocked phosphorylation of AKT in S473 (Fig. 4A) and inhibited growth of both BT474GFP and BT474T798M cells (Fig. 4B, C). Amplified HER2 signaling also hyperactivates the RAS/MEK/ERK pathway (44). Treatment with the MEK1/2 inhibitor CI-1040 (45) blocked phosphorylation of ERK1/2 in both cell types (Fig. 4A) but did not inhibit their growth (Fig. 4B, C). These data suggest that expression of HER2T798M does not dispense with the dependence of HER2-overexpressing cells on PI3K. We next examined if treatment with a second pan-PI3K inhibitor, XL-147 (46), would overcome resistance to trastuzumab of cells expressing HER2T798M. The combination of trastuzumab and XL-147 was more potent than either drug alone at inhibiting BT474T798M cell growth (Fig. 4D). Immunoblot analyses confirmed that XL-147 inhibited S473 P-AKT in both BT474GFP and BT474T798M cells (Fig. 4E). Because HER3 is the major activator of PI3K in HER2-dependent cells, we next tested the effects of HER3 inhibition. RNAi-induced knockdown of HER3 resulted in growth inhibition of both BT474GFP and BT474T798M cells (Suppl. Fig. 5A). Further, knockdown of HER3 using a HER3-specific locked nucleic acid (LNA) antisense oligonucleotide (30) markedly reduced S473 P-AKT in BT474T798M cells (Suppl. Fig. 5B). These results suggest BT474T798M cells remain dependent on the HER3-PI3K axis for survival.
Figure 4. Cells expressing HER2T798M are dependent on PI3K for survival.
A. Cells were treated with 1 μM lapatinib, 1 μM BKM120 or 1 μM CI-1040 for 3 h. Protein extracts were prepared and subjected to immunoblot analyses with the indicated antibodies. B. Cells in 3D Matrigel growth assays were treated with DMSO, 1 μM lapatinib, 1 μM BKM120 and 1 μM CI-1040. Cells were plated in triplicates and images captured on day 14. Media and inhibitors were replenished every 3 days. C. Quantification of 3D growth assays was performed by dissolution of the Matrigel and mechanical disruption of acini into single cell suspensions for cell counting as described in Methods. The bars represent the mean of 3 wells ±S.E.M. D. Cells (104/well) were seeded in 96-well plates and 24 h after plating treatment with 20 μg/ml trastuzumab, 6 μM XL-147 or the combination was begun. WST-1 absorbance was measured on day 5. Fresh medium and inhibitors were replenished after 3 days. The bars represent the mean of 4 wells ± S.E.M. E. Cells were treated with 20 μg/ml trastuzumab, 6 μM XL-147 or the combination for 3 h. Protein extracts prepared were prepared and subjected to immunoblot analyses using antibodies indicated on the left.
BT474T798M cells overexpress EGFR ligands and combined blockade of EGFR and HER2 inhibits their growth
We speculated that another explanation for both the increased intracellular signaling generated by HER2T798M (Fig. 1C, 3B; Suppl. Fig. 2) and the acquired resistance to trastuzumab would be enhanced activation of HER2 by ligand-induced ErbB co-receptors. Therefore, we performed quantitative PCR with mRNA isolated from BT474GFP and BT474T798M cells using primers specific for the ErbB receptor ligands heregulin, EGF, TGFα, amphiregulin, HB-EGF, epiregulin, and betacellulin. BT474T798M cells expressed >2-fold higher levels of the EGFR ligands EGF, TGFα, amphiregulin and HB-EGF compared to BT474GFP cells (Fig. 5A). Consistent with enhanced ErbB ligand production, HER2 antibody pulldowns contained more HER3 and EGFR in BT474T798M than in BT474GFPcells in the absence of exogenous TGFα (Fig. 5B,C). If overexpression of EGFR ligands is causal to the resistance to HER2 inhibitors, we proposed that blockade of ligand binding with the EGFR antibody cetuximab should overcome resistance to lapatinib and trastuzumab. However, treatment of BT474T798M cells with cetuximab did not add to the modest growth inhibition by lapatinib, although EGFR phosphorylation was inhibited. In addition, P-HER2, P-HER3, P-AKT and P-Erk1/2 were maintained following treatment with this combination (Fig. 5D,E). We speculate that this activation reflects EGFR-independent HER2 signaling, which is maintained because of the inability of lapatinib to bind HER2T798M with high affinity and inhibit its kinase activity.
Figure 5. HER2T798M cells express higher levels of EGFR ligands and HER2-containing heterodimers.
A. RT qPCR analysis for ErbB receptor ligand expression was performed using RNA template from BT474GFP and BT474T798M cells, normalized to expression in GFP cells for each ligand. B. Lysates prepared from BT474GFP (± 40 ng/ml TGFα) and BT474T798M were immunoprecipitated with a HER2 C-terminal antibody followed by immunoblot analyses with the indicated antibodies. C. Lysates prepared from MCF10AWT (± 40 ng/ml TGFα) and MCF10AT798M were analyzed as in B. D. BT474T798M cells were treated with 20 μg/ml cetuximab and 1 μM lapatinib, either alone or in combination for 5 days. MTT assays were performed at the end of treatment. E. Cells were treated with 20 μg/ml cetuximab and/or 1 μM lapatinib for 3 h. Lysates were prepared and subjected to immunoblot analyses with the indicated antibodies. F. For 3D Matrigel growth assays, cells were treated with 20 μg/ml trastuzumab, 20 μg/ml cetuximab or the combination. Cells were plated in duplicates and images captured on day 14. Fresh medium and inhibitors were replenished every 3 days. G. Average acinar area was quantified with the ImageJ software. Each bar represents the mean acinar area ± S.E.M (n=2). H. Cells were treated with 20 μg/ml cetuximab and/or and 20 μg/ml trastuzumab and lysates analyzed as in E. I. BT474T798M xenografts were established in female athymic mice as indicated in Methods. Once tumors reached at least ≥200 mm3 in volume, mice were treated with trastuzumab (30 mg/kg i.p. twice per week), cetuximab (1 mg i.p. twice per week) or both antibodies. Each data point represents the mean tumor volume ± SEM. *, p<0.005 vs. trastuzumab; #, p<0.05 vs. cetuximab. The number of mice in each treatment arm is indicated next to each curve.
In 3D Matrigel, the HER2 T798M mutant-expressing cells were resistant to single agent cetuximab or trastuzumab. However, the combination of trastuzumab and cetuximab blocked BT474T798M cell growth (Fig. 5F,G). Consistent with the growth inhibitory effect of the combination, immunoblot analysis showed that treatment with both antibodies markedly inhibited S473 P-AKT (Fig. 5H). To test the effect of the combination in vivo, we injected BT474T798M cells into athymic nude mice and treated established tumors with trastuzumab, cetuximab, or the combination for five weeks. Trastuzumab or cetuximab alone had no effect on tumor growth. However, treatment with both antibodies resulted in complete response in 3/7 (43%) mice (Fig. 5I). Immunohistochemical analysis of tumors after ∼5 weeks of treatment showed that cetuximab alone or in combination with trastuzumab resulted in a slight decrease in cytoplasmic P-Akt intensity (Suppl. Fig 6). These observations suggest that dual inhibition of HER2 and EGFR limits the growth of trastuzumab-resistant cells in tumors bearing HER2T798M.
Finally, we hypothesized that since lapatinib also inhibits the EGFR tyrosine kinase, BT474T798M cells should be sensitive to dual HER2 blockade with trastuzumab and lapatinib. In this combination, trastuzumab should partially downregulate mutant HER2 and lapatinib should inhibit transactivation of HER2 (and HER3) by ligand-induced EGFR. Indeed, the combination of trastuzumab and lapatinib inhibited BT474T798M cell growth in 3D matrigel even though the cells were resistant to each drug alone (Fig. 6A,B). Treatment with the combination also inhibited Y1068-pEGFR and Y1197-pHER3. Treatment with trastuzumab had no effect whereas lapatinib modestly inhibited HER2/HER3 heterodimers. However, treatment with both inhibitors markedly reduced HER2/HER3 heterodimers potentially explaining the inhibition of S473 P-AKT (Fig. 6C). Further, treatment with both inhibitors but not each drug alone induced regression of BT474T798M xenografts established in athymic mice (Fig. 6D). There was a modest reduction in S473 P-Akt levels as measured by IHC in tumors in all treatment arms at the end of 4 weeks of therapy (Suppl. Fig. 7). These results suggest that even though HER2T798M confers resistance to lapatinib or trastuzumab when used as single agents, simultaneous blockade of HER2 and EGFR with both of these inhibitors is effective against HER2-overexpressing breast cancer cells bearing HER2T798M alleles.
Figure 6. Simultaneous blockade of EGFR and HER2 inhibits HER2T798M expressing tumors.
A. Cells in 3D Matrigel growth assays were treated with DMSO, 20 μg/ml trastuzumab, 1 μM lapatinib or both drugs. Cells were plated in duplicates and images captured on day 14. Media and inhibitors were replenished every 3 days. B. Quantification of 3D growth assays was performed as described earlier and in Methods. C. Cells were treated with similar concentrations of trastuzumab, lapatinib or the combination for 3 h; whole cell lysates were prepared and subjected to SDS-PAGE followed by immunoblot analyses with the indicated antibodies. Lowest two panels represent immunoprecipitation with a HER2 antibody followed by immunoblot analyses of the antibody pulldowns with HER2 and HER3 antibodies. D. BT474T798M xenografts were established in female athymic mice as indicated in Methods. Once xenografts reached ≥200 mm3 in volume, mice were treated with trastuzumab (30 mg/kg i.p. twice per week), lapatinib (100 mg/kg/day) or the combination. Each data point represents the mean tumor volume ± SEM. *p<0.05 vs. trastuzumab. The number of mice in each treatment arm is indicated next to the curves.
Discussion
In this report, we studied cellular and biochemical effects of the gatekeeper T798M mutation in the HER2kinase domain in breast cancer cells. Expression of T798M conferred resistance to both lapatinib and trastuzumab. Since the T790M gatekeeper mutation in EGFR has increased affinity for ATP (47), we speculate the corresponding T798M mutation in HER2 may similarly stabilize HER2 in an active conformation (26).Consistent with this, we observed that HER2T798M has increased catalytic activity compared to HER2WT. In addition, structural modeling of T798M suggests that the methionine substitution at T798 sterically in hibits drug binding and/or further destabilizes the inactive conformation of HER2. Since lapatinib binds preferentially the inactive confirmation of HER2, this further contributes to drug resistance (24). This may also explain the marked shift in the IC50 of lapatinib against HER2T798M cells.
Trastuzumab is unable to block transactivation of HER2 by ligand-induced ErbB co-receptors (48). Exogenous and endogenous ligands of EGFR and HER3 have been shown to rescue from the growth inhibitory effect of the antibody (35, 49, 50). Further, HER2+ xenografts selected in vivo for acquired resistance to trastuzumab exhibit higher levels of P-EGFR and EGFR/HER2 heterodimers as well as overexpression of EGFR, TGFα, HB-EGF, and heregulin RNAs compared to parental trastuzumab-sensitive tumors (51). Finally, expression of drug-resistant mutants of EGFR (38) and HER2 (33, 52) also result in overproduction of ErbB receptor ligands. Thus, we examined expression of ErbB receptor ligands in cells expressing the HER2 mutant. BT474T798M cells overexpressed EGF, TGFα, amphiregulin and HB-EGF mRNAs, and exhibited higher levels of HER2-containing heterodimers compared to BT474GFP cells. Addition of cetuximab, an antibody that blocks ligand binding to EGFR, overcame the resistance to trastuzumab (Fig. 5), suggesting ligand overexpression was causal to this resistance. These data suggest that although HER2T798M is intrinsically sensitive to trastuzumab-induced downregulation similar to HER2WT (Fig. 1F), its expression increases ligand-induced HER2-containing heterodimers (Fig. 5B,C), potentially explaining how T798M contributes to trastuzumab resistance. Further studies are required to determine the mechanisms by which amplification of HER2 signaling results in enhanced production of ErbB receptor ligands.
The HER2T798M kinase was exquisitely sensitive to submicromolar concentrations of BIBW2992 (afatinib). This is an irreversible small molecule inhibitor that covalently binds Cys805 and Cys773 in the ATP pocket of HER2 and EGFR, respectively, and potently inhibits the receptors' kinase activity (53). Afatinib is also effective against the analogous drug-resistant T790M mutation in EGFR and has shown clinical activity in lung cancers harboring this mutation (54).Cells expressing HER2T798M were also sensitive to HER3 knockdown and to two pan-PI3K inhibitors currently in clinical development. These results suggest that cells expressing the mutant continue to rely on the HER2-HER3-PI3K axis for growth and survival. Therefore, afatinib and other irreversible HER2 TKIs, HER3 antibodies, PI3K inhibitors and drugs that disrupt ligand-induced HER2-HER3 dimers (i.e., pertuzumab) represent clinical approaches and combinations currently used in patients with HER2-overexpressing cancers that may prevent the acquisition or the effects of HER2T798M.
Mutations in HER2 are present in breast tumors but, to our knowledge, with very few exceptions they have all been reported in breast cancers without HER2 gene amplification (23, 27, 53). One possibility is that allelic dilution may render it difficult to identify this drug resistant mutant by conventional direct sequencing in cancers with HER2 gene-amplification. It is also possible that this mutant may require enrichment after treatment of a clinically-sensitive tumor with lapatinib, analogous to the enrichment of imatinib-resistant gatekeeper mutants in BCR-ABL in patients with CML (14). To detect low-frequency mutations, direct sequencing will need to be combined with methodologies which are more sensitive to detect rare mutations, especially in highly amplified genes such as HER2. This has been observed in lung cancer, where use of SURVEYOR but not Sanger sequencing (40) detected the EGFR T790M mutation in gefitinib-resistant primary lung tumors. This suggests next-generation massively parallel sequencing that can provide deep gene coverage may be required to detect HER2 mutations in patients with HER2 gene amplified breast cancer. This is also consistent with our findings that a low frequency of expression of the mutant allele (∼3%) was sufficient to confer drug resistance. Finally, sequencing efforts to date have focused on pre-treatment samples. We speculate many resistance-associated mutations are acquired (or enriched from a small and undetectable pre-existing population). As such, they may only be detected following treatment and progression of clinically resistant disease.
Supplementary Material
Translational Relevance.
Despite the effectiveness of HER2-targeted therapies such as trastuzumab and lapatinib, most patients with metastatic HER2 gene-amplified breast cancer treated with these drugs eventually progress. Mutations in RTK genes have been demonstrated as possible mechanisms of resistance to small molecule tyrosine kinase inhibitors. The advent of next generation sequencing approaches has allowed for the detection of low frequency mutant alleles in HER2 gene-amplified breast cancers. This work investigated the effects of the ‘gatekeeper’ (T798M) mutation in HER2. Results show that cells expressing this lapatinib- and trastuzumab-resistant mutant overexpress EGFR ligands. Simultaneous blockade of HER2 and EGFR by the combined use of trastuzumab and lapatinib or by the addition of the EGFR antibody cetuximab to trastuzumab reversed drug resistance, thus identifying combinations that may prevent the acquisition of T798M mutations in patients with HER2+ breast cancer.
Acknowledgments
This work was supported by the following: R01 grant CA80195 (CLA), ACS Clinical Research Professorship Grant CRP-07-234, The Lee Jeans Translational Breast Cancer Research Program, Breast Cancer Specialized Program of Research Excellence (SPORE) P50 CA98131, Vanderbilt-Ingram Cancer Center Support Grant P30 CA68485, Susan G. Komen for the Cure Foundation Grant SAC100013; DOD Breast Cancer Research Program post-doctoral award W81XWH-09-1-0474 (RG);DOD Breast Cancer Research Program post-doctoral award BC087465 (BNR) and NCI K08 CA143153 (BNR).
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
References
- 1.Yarden Y, Sliwkowski M. Untangling the ErbB signaling network. Nat Rev Mol Cell Biol. 2001;2(2):127–37. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 2.Ross JS, Fletcher JA. The HER-2/neu Oncogene in Breast Cancer: Prognostic Factor, Predictive Factor, and Target for Therapy. Stem Cells. 1998;16:413–28. doi: 10.1002/stem.160413. [DOI] [PubMed] [Google Scholar]
- 3.Vogel C, Cobleigh M, Tripathy D, Gutheil J, Harris L, Fehrenbacher L, et al. Efficacy and safety of trastuzuamb as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20(3):719–26. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 4.Slamon D, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpress HER2. New Eng J Med. 2001;344:783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 5.Kokai Y, Myers JN, Wada T, Brown VI, LeVea CM, Davis JG, et al. Synergistic interaction of p185c-neu and the EGF receptor leads to transformation of rodent fibroblasts. Cell. 1989;58(2):287–92. doi: 10.1016/0092-8674(89)90843-x. [DOI] [PubMed] [Google Scholar]
- 6.Wada T, Qian X, Greene MI. Intermolecular association of the p185neu protein and EGF receptor modulates EGF receptor function. Cell. 1990;61(7):1339–47. doi: 10.1016/0092-8674(90)90697-d. [DOI] [PubMed] [Google Scholar]
- 7.Worthylake R, Opresko LK, Wiley HS. ErbB-2 amplification inhibits down-regulation and induces constitutive activation of both ErbB-2 and epidermal growth factor receptors. J Biol Chem. 1999;274(13):8865–74. doi: 10.1074/jbc.274.13.8865. [DOI] [PubMed] [Google Scholar]
- 8.Karunagaran D, Tzahar E, Beerli R, Chen X, Graus-Porta D, Ratzkin B, et al. ErbB-2 is a common auxiliary subunit of NDF and EGF receptors: implications for breast cancer. EMBO J. 1996;15(2):254–64. [PMC free article] [PubMed] [Google Scholar]
- 9.Moulder SL, Yakes FM, Muthuswamy SK, Bianco R, Simpson JF, Arteaga CL. Epidermal Growth Factor Receptor (HER1) Tyrosine Kinase Inhibitor ZD1839 (Iressa) Inhibits HER2/neu (erbB2)-overexpressing Breast Cancer Cells in Vitro and in Vivo. Cancer Res. 2001;61(24):8887–95. [PubMed] [Google Scholar]
- 10.Moasser MM, Basso A, Averbuch SD, Rosen N. The Tyrosine Kinase Inhibitor ZD1839 (Iressa) Inhibits HER2-driven Signaling and Suppresses the Growth of HER2-overexpressing Tumor Cells. Cancer Res. 2001;61(19):7184–8. [PubMed] [Google Scholar]
- 11.Lenferink AEG, Simpson JF, Shawver LK, Coffey RJ, Forbes JT, Arteaga CL. Blockade of the epidermal growth factor receptor tyrosine kinase suppresses tumorigenesis in MMTV/Neu + MMTV/TGF-beta bigenic mice. Proc Natl Acad Sci U S A. 2000;97(17):9609–14. doi: 10.1073/pnas.160564197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xia W, Mullin RJ, Keith BR, Liu LH, Ma H, Rusnak DW, et al. Anti-tumor activity of GW572016: a dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT pathways. Oncogene. 2002;21(41):6255–63. doi: 10.1038/sj.onc.1205794. [DOI] [PubMed] [Google Scholar]
- 13.Garrett JT, Olivares Ma G, Rinehart C, Granja-Ingram ND, Sanchez V, Chakrabarty A, et al. Transcriptional and posttranslational up-regulation of HER3 (ErbB3) compensates for inhibition of the HER2 tyrosine kinase. Proc Natl Acad Sci U S A. 2011;108(12):5021–6. doi: 10.1073/pnas.1016140108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah NP, Nicoll JM, Nagar B, Gorre ME, Paquette RL, Kuriyan J, et al. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell. 2002;2(2):117–25. doi: 10.1016/s1535-6108(02)00096-x. [DOI] [PubMed] [Google Scholar]
- 15.Antonescu CR, Besmer P, Guo T, Arkun K, Hom G, Koryotowski B, et al. Acquired Resistance to Imatinib in Gastrointestinal Stromal Tumor Occurs Through Secondary Gene Mutation. Clin Cancer Res. 2005;11(11):4182–90. doi: 10.1158/1078-0432.CCR-04-2245. [DOI] [PubMed] [Google Scholar]
- 16.Corless CL, Schroeder A, Griffith D, Town A, McGreevey L, Harrell P, et al. PDGFRA Mutations in Gastrointestinal Stromal Tumors: Frequency, Spectrum and In Vitro Sensitivity to Imatinib. J Clin Oncol. 2005;23(23):5357–64. doi: 10.1200/JCO.2005.14.068. [DOI] [PubMed] [Google Scholar]
- 17.Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, Zakowski MF, et al. Acquired Resistance of Lung Adenocarcinomas to Gefitinib or Erlotinib Is Associated with a Second Mutation in the EGFR Kinase Domain. PLoS Med. 2005;2(3):e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balak MN, Gong Y, Riely GJ, Somwar R, Li AR, Zakowski MF, et al. Novel D761Y and Common Secondary T790M Mutations in Epidermal Growth Factor Receptor-Mutant Lung Adenocarcinomas with Acquired Resistance to Kinase Inhibitors. Clin Cancer Res. 2006;12(21):6494–501. doi: 10.1158/1078-0432.CCR-06-1570. [DOI] [PubMed] [Google Scholar]
- 19.Cohen EEW, Lingen MW, Martin LE, Harris PL, Brannigan BW, Haserlat SM, et al. Response of Some Head and Neck Cancers to Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors May Be Linked to Mutation of ERBB2 rather than EGFR. Clin Cancer Res. 2005;11(22):8105–8. doi: 10.1158/1078-0432.CCR-05-0926. [DOI] [PubMed] [Google Scholar]
- 20.Lee JW, Soung YH, Kim SY, Nam SW, Park WS, Wang YP, et al. ERBB2 kinase domain mutation in the lung squamous cell carcinoma. Cancer Lett. 2006;237(1):89–94. doi: 10.1016/j.canlet.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 21.Shigematsu H, Takahashi T, Nomura M, Majmudar K, Suzuki M, Lee H, et al. Somatic Mutations of the HER2 Kinase Domain in Lung Adenocarcinomas. Cancer Res. 2005;65(5):1642–6. doi: 10.1158/0008-5472.CAN-04-4235. [DOI] [PubMed] [Google Scholar]
- 22.Stephens P, Hunter C, Bignell G, Edkins S, Davies H, Teague J, et al. Lung cancer: intragenic ERBB2 kinase mutations in tumours. Nature. 2004;431(7008):525–6. doi: 10.1038/431525b. Epub 2004/10/01. [DOI] [PubMed] [Google Scholar]
- 23.Lee JW, Soung YH, Seo SH, Kim SY, Park CH, Wang YP, et al. Somatic Mutations of ERBB2 Kinase Domain in Gastric, Colorectal, and Breast Carcinomas. Clin Cancer Res. 2006;12(1):57–61. doi: 10.1158/1078-0432.CCR-05-0976. [DOI] [PubMed] [Google Scholar]
- 24.Trowe T, Boukouvala S, Calkins K, Cutler RE, Fong R, Funke R, et al. EXEL-7647 Inhibits Mutant Forms of ErbB2 Associated with Lapatinib Resistance and Neoplastic Transformation. Clin Cancer Res. 2008;14(8):2465–75. doi: 10.1158/1078-0432.CCR-07-4367. [DOI] [PubMed] [Google Scholar]
- 25.Azam M, Latek RR, Daley GQ. Mechanisms of Autoinhibition and STI-571/Imatinib Resistance Revealed by Mutagenesis of BCR-ABL. Cell. 2003;112(6):831–43. doi: 10.1016/s0092-8674(03)00190-9. [DOI] [PubMed] [Google Scholar]
- 26.Kancha RK, von Bubnoff N, Bartosch N, Peschel C, Engh RA, Duyster J. Differential sensitivity of ERBB2 kinase domain mutations towards lapatinib. PloS one. 2011;6(10):e26760. doi: 10.1371/journal.pone.0026760. Epub 2011/11/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bose R, Kavuri SM, Searleman AC, Shen W, Shen D, Koboldt DC, et al. Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov. 2013;3(2):224–37. doi: 10.1158/2159-8290.CD-12-0349. Epub 2012/12/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller TW, Hennessy BT, Gonzalez-Angulo AM, Fox EM, Mills GB, Chen H, et al. Hyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in estrogen receptor-positive human breast cancer. J Clin Invest. 2010;120(7):2406–13. doi: 10.1172/JCI41680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cook RS, Garrett JT, Sanchez V, Stanford JC, Young C, Chakrabarty A, et al. ErbB3 Ablation Impairs PI3K/Akt-Dependent Mammary Tumorigenesis. Cancer Res. 2011;71(11):3941–51. doi: 10.1158/0008-5472.CAN-10-3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30(3):256–68. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- 32.Engelman JA, Jänne PA, Mermel C, Pearlberg J, Mukohara T, Fleet C, et al. ErbB-3 mediates phosphoinositide 3-kinase activity in gefitinib-sensitive non-small cell lung cancer cell lines. Proc Natl Acad Sci U S A. 2005;102(10):3788–93. doi: 10.1073/pnas.0409773102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ryan BM, Konecny GE, Kahlert S, Wang HJ, Untch M, Meng G, et al. Survivin expression in breast cancer predicts clinical outcome and is associated with HER2, VEGF, urokinase plasminogen activator and PAI-1. Ann Oncol. 2006;17(4):597–604. doi: 10.1093/annonc/mdj121. Epub 2006/01/13. [DOI] [PubMed] [Google Scholar]
- 34.Chakrabarty A, Rexer BN, Wang SE, Cook RS, Engelman JA, Arteaga CL. H1047R phosphatidylinositol 3-kinase mutant enhances HER2-mediated transformation by heregulin production and activation of HER3. Oncogene. 2010;29(37):5193–203. doi: 10.1038/onc.2010.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang SE, Xiang B, Guix M, Olivares MG, Parker J, Chung CH, et al. Transforming growth factor beta engages TACE and ErbB3 to activate phosphatidylinositol-3 kinase/Akt in ErbB2-overexpressing breast cancer and desensitizes cells to trastuzumab. Mol Cell Biol. 2008;28(18):5605–20. doi: 10.1128/MCB.00787-08. Epub 2008/07/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khoury T, Mojica W, Hicks D, Starostik P, Ademuyiwa F, Janarthanan B, et al. ERBB2 juxtamembrane domain (trastuzumab binding site) gene mutation is a rare event in invasive breast cancers overexpressing the ERBB2 gene. Mod Pathol. 2011;24(8):1055–9. doi: 10.1038/modpathol.2011.64. [DOI] [PubMed] [Google Scholar]
- 37.Drebin JA, Link VC, Stern DF, Weinberg RA, Greene MI. Down-modulation of an oncogene protein product and reversion of the transformed phenotype by monoclonal antibodies. Cell. 1985;41(3):695–706. doi: 10.1016/s0092-8674(85)80050-7. [DOI] [PubMed] [Google Scholar]
- 38.Regales L, Gong Y, Shen R, de Stanchina E, Vivanco I, Goel A, et al. Dual targeting of EGFR can overcome a major drug resistance mutation in mouse models of EGFR mutant lung cancer. J Clin Invest. 2009;119(10):3000–10. doi: 10.1172/JCI38746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ocaña A, Amir E. Irreversible pan-ErbB tyrosine kinase inhibitors and breast cancer: Current status and future directions. Cancer Treat Rev. 2009;35(8):685–91. doi: 10.1016/j.ctrv.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 40.Engelman JA, Mukohara T, Zejnullahu K, Lifshits E, Borras AM, Gale C-M, et al. Allelic dilution obscures detection of a biologically significant resistance mutation in EGFR-amplified lung cancer. J Clin Invest. 2006;116(10):2695–706. doi: 10.1172/JCI28656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brachmann SM, Hofmann I, Schnell C, Fritsch C, Wee S, Lane H, et al. Specific apoptosis induction by the dual PI3K/mTor inhibitor NVP-BEZ235 in HER2 amplified and PIK3CA mutant breast cancer cells. Proc Natl Acad Sci U S A. 2009;106(52):22299–304. doi: 10.1073/pnas.0905152106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holbro T, Beerli RR, Maurer F, Koziczak M, Barbas CF, Hynes NE. The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proc Natl Acad Sci U S A. 2003;100(15):8933–8. doi: 10.1073/pnas.1537685100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maira S-M, Pecchi S, Huang A, Burger M, Knapp M, Sterker D, et al. Identification and characterization of NVP-BKM120, an orally available pan class I PI3-Kinase inhibitor. Mol Cancer Ther. 2012;11(2):317–28. doi: 10.1158/1535-7163.MCT-11-0474. [DOI] [PubMed] [Google Scholar]
- 44.Dankort D, Maslikowski B, Warner N, Kanno N, Kim H, Wang Z, et al. Grb2 and Shc adapter proteins play distinct roles in Neu (ErbB-2)-induced mammary tumorigenesis: implications for human breast cancer. Mol Cell Biol. 2001;21(5):1540–51. doi: 10.1128/MCB.21.5.1540-1551.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.LoRusso PM, Adjei AA, Varterasian M, Gadgeel S, Reid J, Mitchell DY, et al. Phase I and Pharmacodynamic Study of the Oral MEK Inhibitor CI-1040 in Patients With Advanced Malignancies. J Clin Oncol. 2005;23(23):5281–93. doi: 10.1200/JCO.2005.14.415. [DOI] [PubMed] [Google Scholar]
- 46.Chakrabarty A, Sánchez V, Kuba Ma G, Rinehart C, Arteaga CL. Feedback upregulation of HER3 (ErbB3) expression and activity attenuates antitumor effect of PI3K inhibitors. Proc Natl Acad Sci U S A. 2012;109(8):2718–23. doi: 10.1073/pnas.1018001108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yun CH, Mengwasser KE, Toms AV, Woo MS, Greulich H, Wong KK, et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci U S A. 2008;105(6):2070–5. doi: 10.1073/pnas.0709662105. Epub 2008/01/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Junttila TT, Akita RW, Parsons K, Fields C, Lewis Phillips GD, Friedman LS, et al. Ligand-Independent HER2/HER3/PI3K Complex Is Disrupted by Trastuzumab and Is Effectively Inhibited by the PI3K Inhibitor GDC-0941. Cancer Cell. 2009;15(5):429–40. doi: 10.1016/j.ccr.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 49.Motoyama AB, Hynes NE, Lane HA. The efficacy of ErbB receptor-targeted anticancer therapeutics is influenced by the availability of epidermal growth factor-related peptides. Cancer Res. 2002;62(11):3151–8. [PubMed] [Google Scholar]
- 50.Moulder SL, Yakes FM, Muthuswamy SK, Bianco R, Simpson JF, Arteaga CL. Epidermal growth factor receptor (HER1) tyrosine kinase inhibitor ZD1839 (Iressa) inhibits HER2/neu (erbB2)-overexpressing breast cancer cells in vitro and in vivo. Cancer Res. 2001;61(24):8887–95. [PubMed] [Google Scholar]
- 51.Ritter CA, Perez-Torres M, Rinehart C, Guix M, Dugger T, Engelman JA, et al. Human breast cancer cells selected for resistance to trastuzumab in vivo overexpress epidermal growth factor receptor and ErbB ligands and remain dependent on the ErbB receptor network. Clin Cancer Res. 2007;13(16):4909–19. doi: 10.1158/1078-0432.CCR-07-0701. [DOI] [PubMed] [Google Scholar]
- 52.Wang SE, Yu Y, Criswell TL, Debusk LM, Lin PC, Zent R, et al. Oncogenic mutations regulate tumor microenvironment through induction of growth factors and angiogenic mediators. Oncogene. 2010;29(23):3335–48. doi: 10.1038/onc.2010.112. Epub 2010/04/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bean J, Riely GJ, Balak M, Marks JL, Ladanyi M, Miller VA, et al. Acquired Resistance to Epidermal Growth Factor Receptor Kinase Inhibitors Associated with a Novel T854A Mutation in a Patient with EGFR-Mutant Lung Adenocarcinoma. Clin Cancer Res. 2008;14(22):7519–25. doi: 10.1158/1078-0432.CCR-08-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miller VA, Hirsh V, Cadranel J, Chen YM, Park K, Kim SW, et al. Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): a phase 2b/3 randomised trial. Lancet Oncol. 2012;13(5):528–38. doi: 10.1016/S1470-2045(12)70087-6. Epub 2012/03/29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.