Abstract
Recent work has uncovered genes for two glycosyltransferases that are thought to catalyze mannosylation of mycosaminyl sugars of polyene macrolides. These two genes are nypY from Pseudonocardia sp. strain P1 and pegA from Actinoplanes caeruleus. Here we analyze these genes by heterologous expression in various strains of Streptomyces nodosus, producer of amphotericins, and in Streptomyces albidoflavus, which produces candicidins. The NypY glycosyltransferase converted amphotericins A and B and 7-oxo-amphotericin B to disaccharide-modified forms in vivo. The enzyme did not act on amphotericin analogs lacking exocyclic carboxyl or mycosamine amino groups. Both NypY and PegA acted on candicidins. This work confirms the functions of these glycosyltransferases and provides insights into their acceptor substrate tolerance. Disaccharide-modified polyenes may have potential as less toxic antibiotics.
INTRODUCTION
Amphotericin B (Fig. 1) is used to treat life-threatening systemic mycoses and diseases caused by Leishmania parasites. It is also active against enveloped viruses and pathogenic prion proteins (1, 2). As an antifungal antibiotic, it is important because few alternatives are available and because resistance has been slow to emerge. However, the use of amphotericin B is restricted by severe side effects. Lipid formulations have reduced toxicity but are expensive (3). Chemical modification of the natural product can improve pharmacological properties, but this approach has not yet delivered a second-generation derivative that has advanced into clinical use (4). Several new analogs have been biosynthesized by genetic manipulation of the amphotericin B-producing microorganism Streptomyces nodosus. These new analogs have been obtained by reprogramming the polyketide synthase and by engineering late genes required for modification of the macrolactone core (5, 6, 7). Biosynthetic engineering has generated analogs of other important polyene macrolides, such as nystatin, candicidin, pimaricin, and rimocidin (8, 9, 10, 11).
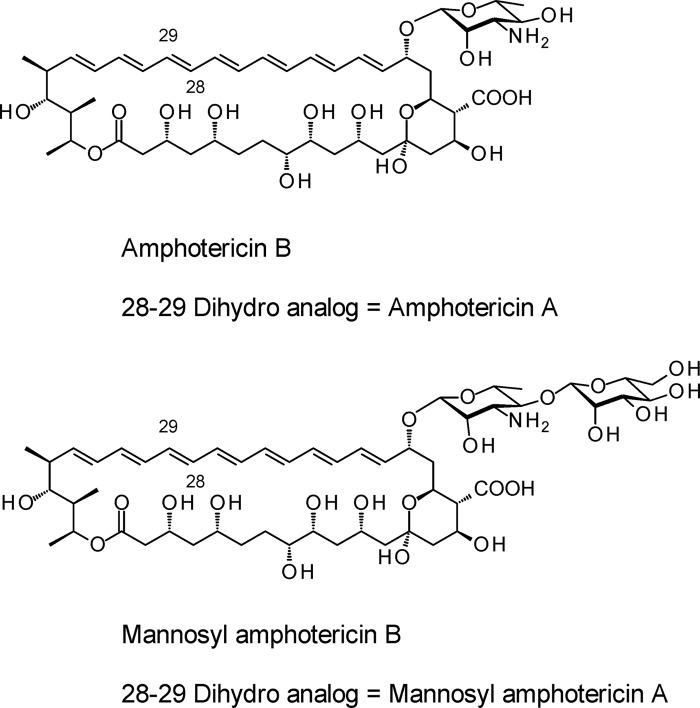
Fig 1.
Structures of amphotericins. Amphotericins B and A are cometabolites.
Chemical studies have shown that modifying the mycosamine sugar of amphotericin B can have beneficial effects. Alkylation of the amine with two 3-aminopropyl groups increases potency and reduces hemolytic activity (12). Removal of the C-2′ hydroxyl group improves selective toxicity (13). Conjugation with arabinogalactan polysaccharides converts amphotericin B to a water-soluble form (14). MFAME (N-methyl-N-d-fructosyl amphotericin B methyl ester), a semisynthetic derivative with a single additional sugar residue, has greater water solubility and reduced toxicity (15). Recent findings suggest that it may be possible to engineer bacteria to produce disaccharide-modified amphotericins and analogs. This would be important because biological production of these potentially superior compounds could be scalable and cost-effective.
Pseudonocardia autotrophica has been found to synthesize tetraene NPP (nystatin-like Pseudonocardia polyene), a nystatin with an N-acetyl-glucosaminyl residue linked to the mycosaminyl sugar. This compound is 300 times more water soluble than nystatin A1 but only half as active. Engineering of the NPP polyketide synthase gave a heptaene analog that has improved activity (16). The extending glycosyltransferase gene was not present in the main NPP biosynthesis cluster but may be located nearby (17). Another new nystatin obtained from Pseudonocardia sp. strain P1 was found to contain a hexosyl residue attached to the mycosaminyl sugar. The NypY glycosyltransferase that adds the extra sugar was identified after genome sequencing (18).
Actinoplanes caeruleus synthesizes 67-121C, an aromatic heptaene modified with a mannosyl residue β-1,4 linked to the mycosaminyl sugar (19). Recently, we carried out draft genome sequencing of this organism and identified 67-121 late biosynthetic genes. The pegA gene for the extending glycosyltransferase was expressed in Streptomyces nodosus, but only trace amounts of mannosyl-amphotericins were produced (20). It was not possible to determine whether this was due to inefficient recognition of amphotericins by PegA, low intracellular levels of GDP–d-mannose, rapid export of amphotericins before mannosylation could occur, or other unknown limiting factors.
PegA and NypY show 51% sequence identity (20), suggesting that both enzymes use GDP–α-d-mannose as the activated sugar donor. In this study, we investigated whether NypY could recognize amphotericin B or its analogs in vivo. The activities of PegA and NypY toward aromatic heptaene acceptors were also assessed by expressing the genes in a candicidin producer.
MATERIALS AND METHODS
Streptomyces nodosus, S. nodosus NM (ΔamphNM), S. nodosus DII (ΔamphDII), S. nodosus DII-NM (ΔamphDII-NM), and S. nodosus KR16 were from our laboratory collection. Streptomyces albidoflavus DSM40624 (formerly Streptomyces griseus IMRU 3570) was obtained from DSMZ, Braunschweig, Germany.
The pIJ10257-nypY plasmid was a kind gift of Ryan Seipke and Matt Hutchings, University of East Anglia, United Kingdom. The nypY gene was amplified from this template using primers NypF2 5′-CTAGAGATCTTCTAGAACAGGAGGCCCCATATG-3′ and NypR2 5′-GATCAAGCTTCAGGGGGTGGCCGGGTGACGGTTC-3′. PCR was carried out using Phusion high fidelity DNA polymerase. Cloning of the gene into the pIAGO plasmid (21) and transformation of Streptomyces protoplasts were conducted as described previously (5).
For polyene production, transformants were grown on fructose-dextrin-soya medium containing thiostrepton (50 μg/ml), and extractions were performed as detailed previously (5, 7). High-performance liquid chromatography (HPLC) was conducted using a reverse-phase C18 column (4.6 by 150 mm). Solvent A was 0.1% (vol/vol) formic acid in water, and solvent B was methanol containing 0.1% (vol/vol) formic acid. Polyenes were separated using a gradient of 50 to 100% solvent B over 30 min at a flow rate of 1 ml/min. Heptaenes and tetraenes were detected by monitoring A405 and A315, respectively.
Liquid chromatography coupled to mass spectrometry (LC-MS) was carried out using a Xevo QTof mass spectrometer coupled to an Acquity LC system with an Acquity UPLC BEH C18 column (2.1 by 50 mm) (Waters Corporation, Massachusetts, USA). Solvent A was 0.1% (vol/vol) formic acid in water, solvent B was 0.1% (vol/vol) formic acid in acetonitrile. The flow rate was 0.6 ml/min, and the gradient was as follows: 95% solvent A with solvent 5% B for 0.5 min, followed by a linear gradient to 100% solvent B over the next 2.1 min. After 1 min at 100% solvent B, the gradient was returned to 95% solvent A and 5% solvent B over 0.2 min. Polyene separation was comparable to that obtained by HPLC. The electrospray ionization (ESI) capillary voltage was 3 kV, the cone voltage was 30 V, and the collision energy was 4 eV. The MS acquisition rate was 10 spectra per second and m/z data ranging from 50 to 2,000 Da was collected. Mass accuracy was achieved using a reference lock mass scan, once every 10 s.
RESULTS
The nypY gene was amplified from plasmid pIJ10257-nypY using primers NypF2 and NypR2. The PCR product was digested with BglII and HindIII and cloned between the BamHI and HindIII sites of the expression vector pIAGO. The resulting plasmid pIAGO-nypY was transformed into S. nodosus, and polyenes were extracted and analyzed. HPLC analysis revealed new tetraene and heptaene species in addition to the more abundant amphotericins B and A (Fig. 2). Further analysis by LC-MS revealed that the new compounds were mannosyl-amphotericins B ([M + H]+ = 1,086.6) and A ([M + H]+ = 1,088.6) (Fig. 1). High-resolution mass spectrometry revealed exact masses of 1,086.5464 and 1,088.5640 for protonated forms of mannosyl-amphotericin B (calculated for C53H84NO22 = 1,086.5485) and mannosyl-amphotericin A (calculated for C53H86NO22 = 1,088.5641) (see Fig. S1 to S4 in the supplemental material). Only about 5% of the total polyene was converted to disaccharide-modified forms.
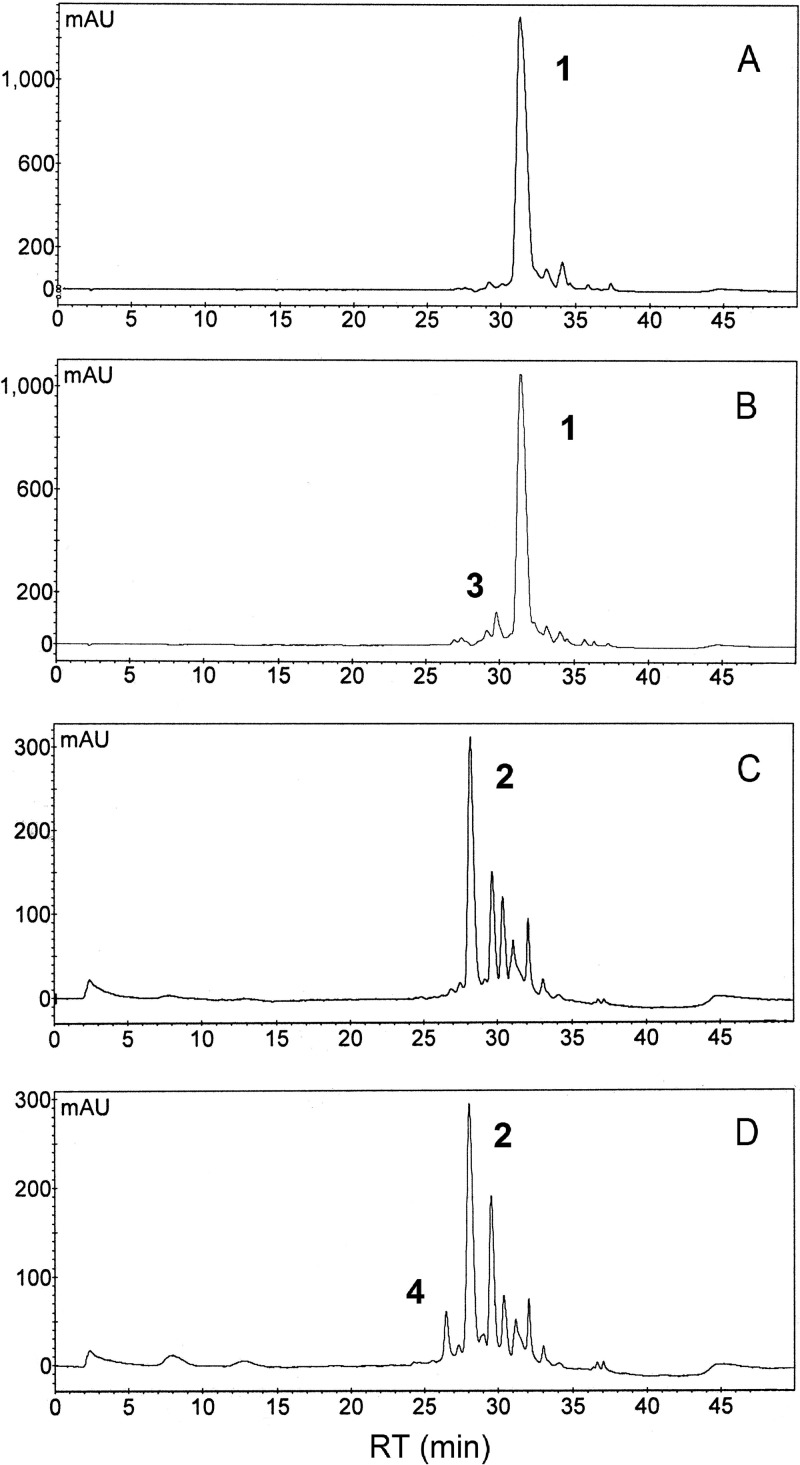
Fig 2.
Detection of mannosyl-amphotericins A and B by HPLC. Heptaenes and tetraenes were detected by monitoring at A405 and A315, respectively. (A and C) Heptaenes (A) and tetraenes (C) from S. nodosus pIAGO. (B and D) Heptaenes (B) and tetraenes (D) from S. nodosus carrying pIAGO-nypY. The peaks are numbered as follows: 1, amphotericin B; 2, amphotericin A; 3, mannosyl-amphotericin B; 4, mannosyl-amphotericin A. Abbreviations: mAU, milli absorbance units; RT, retention time.
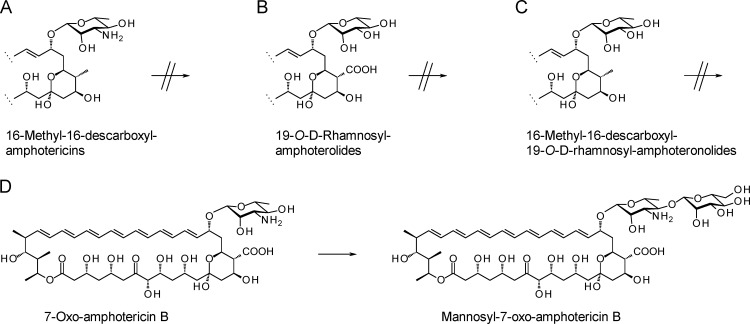
We were keen to assess the prospects for enzymatic addition of mannose residues to other monoglycosylated amphotericin analogs. A number of mutants that synthesize these compounds are available. These strains were used as hosts to assess the acceptor substrate tolerance of NypY in vivo. Analogs lacking exocyclic carboxyl groups are of interest because of their reduced toxicity (22). S. nodosus strain NM synthesizes 16-descarboxyl-16-methyl-amphotericin B and 8-deoxy-16-descarboxyl-16-methyl-amphotericin A (22). Strain DII lacks the AmphDII GDP–d-mycosamine synthase and synthesizes analogs modified with a neutral sugar, 19-O-d-rhamnosyl-amphoteronolides A and B (5). A double mutant, strain DII-NM, synthesizes analogs lacking the exocyclic carboxyl and sugar amino groups (19-O-d-rhamnosyl-16-descarboxyl-16-methyl-amphoteronolides A and B) (5). The pIAGO-nypY plasmid was transformed into each strain. HPLC and LC-MS analyses revealed no new polyenes. In each case, the range of products was identical to that synthesized by control strains containing the empty vector (not shown). The exocyclic carboxyl and sugar amino groups appear to be essential for efficient recognition by NypY (Fig. 3).
Fig 3.
Activity of NypY against amphotericin analogs produced by S. nodosus NM (A), S. nodosus DII (B), S. nodosus DII-NM (C), and S. nodosus KR16 (D). 7-Oxo-amphotericin A was not detected in extracts from KR16 transformants.
Strain KR16 synthesizes 7-oxo-amphotericins A and B, which contain an additional ketone group at C-7 (6). In this case, the structural alteration in the macrolactone ring is more distant from the glycosylation site. In S. nodosus KR16, NypY mannosylated about 5% of the 7-oxo-amphotericin B. The exact mass of the mannosyl-7-oxo-amphotericin B analog ([M + H]+) was 1,100.5271 (calculated for C53H82NO23 = 1,100.5278) (Fig. 3; see Fig. S5 to S7 in the supplemental material). No form of the 7-oxo-amphotericin A tetraene analog was detected in extracts of S. nodosus KR16 carrying pIAGO-nypY.
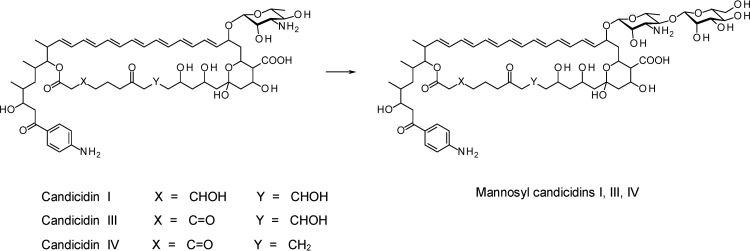
We also investigated whether the NypY and PegA polyene-extending glycosyltransferases could act on aromatic heptaene acceptors. The candicidin producer S. albidoflavus DSM40624 was transformed with the pIAGO-pegA1 (20) and pIAGO-nypY plasmids. The transformants were cultured, and polyenes were extracted into methanol by the same procedures used for amphotericins. The S. albidoflavus pIAGO control strain produced heptaenes with masses appropriate for the components of the candicidin complex (9). These heptaenes were candicidin I ([M + H]+ = 1,111.5912), candicidin III ([M + H]+ = 1,109.5789), and candicidin IV ([M + H]+ = 1093.5835) (Fig. 4; see Fig. S8 to S11 in the supplemental material). The S. albidoflavus pIAGO-pegA1 transformant yielded these heptaenes and mannosylated forms ([M + H]+ = 1,273.6360, [M + H]+ = 1,271.6313, and [M + H]+ = 1,255.6376) (see Fig. S10). The S. albidoflavus pIAGO-nypY transformant gave the same mannosylated forms (see Fig. S11). HPLC separation of mannosylated and unmodified candicidins was poor, and further work will be required to isolate the new compounds.
Fig 4.
Activity of NypY and PegA toward candicidins. Candicidin II is an isomer of III in which the hemiketal is opened.
DISCUSSION
Enzymatic methods to extend polyene macrolide glycosylation may eventually have biotechnological importance. NypY recognizes amphotericins A and B, 7-oxo-amphotericin B, and all components of the candicidin complex. PegA shows very little activity toward amphotericins A and B (20) but did mannosylate candicidins, analogs of its natural substrate, 67-121A. Unlike 67-121 aromatic heptaenes, the various candicidins differ in the polyol chain region as a result of variable β-ketone processing during polyketide synthesis (9). PegA is able to tolerate this structural variation in acceptor substrates. With both NypY and PegA, the yields of disaccharide-modified polyenes from heterologous hosts were low. Protein engineering should increase yields and allow mannosylation of analogs lacking exocyclic carboxyl groups or sugar amino groups.
Since no dimycosaminylated polyenes were detected, NypY and PegA appear to discriminate between GDP–d-mycosamine and GDP–d-mannose. Future work will attempt to engineer the extending glycosyltransferases to add mycosaminyl rather than mannosyl sugars and to assess and further redesign sugar substrate specificity.
Polyene macrolide glycosyltransferases are not closely related to enzymes that glycosylate other natural products, some of which have been crystallized and redesigned to use alternative substrates (23, 24). However, Lei and coworkers have already identified amino acid residues in the FscMI mycosaminyltransferase that are critical for donor and acceptor substrate specificity (25). This should assist future efforts to improve yields of disaccharide-modified polyenes, which will then allow purification, complete structural characterization, and assessment of biological activities.
In the biosynthesis of landomycins, regulatory mechanisms ensure that a hexasaccharide side chain is fully assembled before the antibiotic is exported from the producing cell. The transport proteins are induced only at a late stage by partially glycosylated forms; this prevents premature secretion of intermediates (26). Another challenge will be to investigate whether similar mechanisms are important in biosynthesis of disaccharide-modified polyenes. The identification of NypY by Barke and coworkers (18) will help several groups to extend their work (8, 9, 10, 11, 16) on engineered biosynthesis of new polyene macrolides.
Supplementary Material
ACKNOWLEDGMENT
This work was conducted with the financial support of Science Foundation Ireland under grant 09/RFP/GEN2132.
Footnotes
Published ahead of print 2 August 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02197-13.
REFERENCES
- 1.Georgopapadakou NH, Walsh TJ. 1996. Antifungal agents: chemotherapeutic targets and immunologic strategies. Antimicrob. Agents Chemother. 40:279–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abu-Salah KM. Amphotericin B: an update. Br. J. Biomed. Sci. 52:122–133 [PubMed] [Google Scholar]
- 3.Lemke A, Kiderlen AF, Kayser O. 2005. Amphotericin B. Appl. Microbiol. Biotechnol. 68:151–162 [DOI] [PubMed] [Google Scholar]
- 4.Volmer A, Szpilman AM, Carreira EM. 2010. Synthesis and biological evaluation of amphotericin B derivatives. Nat. Prod. Rep. 27:1329–1349 [DOI] [PubMed] [Google Scholar]
- 5.Hutchinson E, Murphy B, Dunne T, Breen C, Rawlings B, Caffrey P. 2010. Redesign of polyene macrolide glycosylation: engineered biosynthesis of 19-(O)-perosaminyl-amphoteronolide B. Chem. Biol. 17:174–182 [DOI] [PubMed] [Google Scholar]
- 6.Power P, Dunne T, Murphy B, Nic Lochlainn L, Rai D, Borissow C, Rawlings B, Caffrey P. 2008. Engineered synthesis of 7-oxo- and 15-deoxy-15-oxo-amphotericins: insights into structure-activity relationships in polyene antibiotics. Chem. Biol. 15:78–86 [DOI] [PubMed] [Google Scholar]
- 7.Stephens N, Rawlings B, Caffrey P. 2012. Streptomyces nodosus host strains optimised for polyene glycosylation engineering. Biosci. Biochem. Biotechnol. 76:384–387 [DOI] [PubMed] [Google Scholar]
- 8.Brautaset T, Sletta H, Nedal A, Borgos SEF, Degnes KF, Bakke I, Volokhan O, Sekurova ON, Treshalin ID, Mirchink EP, Dikiy A, Ellingsen TE, Zotchev SB. 2008. Improved antifungal polyene macrolides via engineering of the nystatin biosynthetic genes in Streptomyces noursei. Chem. Biol. 15:1198–1206 [DOI] [PubMed] [Google Scholar]
- 9.Zhou Y, Li J, Zhu J, Chen S, Bai L, Zhou X, Wu H, Deng Z. 2008. Incomplete β-ketone processing as a mechanism for polyene structural variation in the FR-008/candicidin complex. Chem. Biol. 15:629–638 [DOI] [PubMed] [Google Scholar]
- 10.Mendes MV, Recio E, Fouces R, Luiten R, Martin JF, Aparicio JF. 2001. Engineered biosynthesis of novel polyenes: a pimaricin derivative produced by targeted gene disruption in Streptomyces natalensis. Chem. Biol. 8:635–644 [DOI] [PubMed] [Google Scholar]
- 11.Seco EM, Cuesta T, Fotso S, Laatsch H, Malpartida F. 2005. Two polyene amides produced by genetically modified Streptomyces diastaticus var. 108. Chem. Biol. 12:1093–1101 [DOI] [PubMed] [Google Scholar]
- 12.Paquet V, Carreira EM. 2006. Significant improvement of antifungal activity of polyene macrolides by bisalkylation of the mycosamine. Org. Lett. 8:1807–1809 [DOI] [PubMed] [Google Scholar]
- 13.Wilcock BC, Endo MM, Uno BE, Burke MD. 2013. C2′-OH of amphotericin B plays an important role in binding the primary sterol of human cells but not yeast cells. J. Am. Chem. Soc. 135:8488–8491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ehrenfreund-Kleinman T, Azzam T, Falk R, Polacheck L, Golenser J, Domb AJ. 2002. Synthesis and characterization of novel water-soluble amphotericin B-arabinogalactan conjugates. Biomaterials 23:1327–1335 [DOI] [PubMed] [Google Scholar]
- 15.Szlinder-Richert J, Mazerski J, Cybulska B, Grybowska J, Borowski E. 2001. MFAME, N-methyl-N-d-fructosyl amphotericin B methyl ester, a new amphotericin B derivative of low toxicity: relationship between self-association and effects on red blood cells. Biochim. Biophys. Acta 1528:15–24 [DOI] [PubMed] [Google Scholar]
- 16.Lee MJ, Kong D, Han K, Sherman DH, Bai L, Deng Z, Lin S, Kim ES. 2012. Structural analysis and biosynthetic engineering of a solubility-improved and less-hemolytic nystatin-like polyene in Pseudonocardia autotrophica. Appl. Microbiol. Biotechnol. 95:157–168 [DOI] [PubMed] [Google Scholar]
- 17.Kong D, Lee MJ, Lin S, Kim ES. 2013. Biosynthesis and pathway engineering of antifungal polyene macrolides in actinomycetes. J. Ind. Microbiol. Biotechnol. 40:529–543 [DOI] [PubMed] [Google Scholar]
- 18.Barke J, Seipke RF, Gruschow S, Heavens D, Drou N, Bibb MJ, Goss RJM, Yu DW, Hutchings MI. 2010. A mixed community of actinomycetes produce multiple antibiotics for the fungus farming ant Acromyrmex octospinosus. BMC Biol. 8:109–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wright JJ, Greeves D, Mallams AK, Picker DH. 1977. Structural elucidation of heptaene macrolide antibiotics 67-121A and 67-121C. J. Chem. Soc. Chem. Commun. 1977:710–712 [Google Scholar]
- 20.Stephens N, Rawlings B, Caffrey P. 2013. Versatility of enzymes catalyzing late steps in polyene 67-121C biosynthesis. Biosci. Biochem. Biotechnol. 77:880–883 [DOI] [PubMed] [Google Scholar]
- 21.Aguirrezabalaga I, Olano C, Allende N, Rodriguez L, Brana AF, Mendez C, Salas JA. 2000. Identification and expression of genes involved in biosynthesis of l-oleandrose and its intermediate l-olivose in the oleandomycin producer Streptomyces antibioticus. Antimicrob. Agents Chemother. 44:1266–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carmody M, Murphy B, Byrne B, Power P, Rai D, Rawlings B, Caffrey P. 2005. Biosynthesis of amphotericin derivatives lacking exocyclic carboxyl groups. J. Biol. Chem. 280:34420–34426 [DOI] [PubMed] [Google Scholar]
- 23.Suna Y, Zeng F, Zhang W, Qiao J. 2012. Structure-based phylogeny of polyene macrolide antibiotic glycosyltransferases. Gene 499:288–296 [DOI] [PubMed] [Google Scholar]
- 24.Chang A, Singh S, Phillips GN, Thorson JS. 2011. Glycosyltransferase structural biology and its role in the design of catalysts for glycosylation. Curr. Opin. Biotechnol. 22:800–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lei X, Kong L, Zhang C, Liu Q, Yao F, Zhang W, Deng Z, You D. 2013. In vivo investigation of the substrate recognition capability and activity affecting amino acid residues of glycosyltransferase FscMI in the biosynthesis of candicidin. Mol. Biosyst. 9:422–430 [DOI] [PubMed] [Google Scholar]
- 26.Ostash I, Ostash B, Luzhetskyy A, Bechthold A, Walker S, Fedorenko V. 2008. Coordination of export and glycosylation of landomycins in Streptomyces cyanogenus S136. FEMS Microbiol. Lett. 285:195–202 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.