Abstract
Cellular drug delivery can improve efficacy and render intracellular pathogens susceptible to compounds that cannot permeate cells. The transport of physiologically active compounds across membranes into target cells can be facilitated by cell-penetrating peptides (CPPs), such as oligoarginines. Here, we investigated whether intracellular delivery of the drug fosmidomycin can be improved by combination with the CPP octaarginine. Fosmidomycin is an antibiotic that inhibits the second reaction in the nonmevalonate pathway of isoprenoid biosynthesis, an essential pathway for many obligate intracellular pathogens, including mycobacteria and apicomplexan parasites. We observed a strict correlation between octaarginine host cell permeability and its ability to improve the efficacy of fosmidomycin. Plasmodium berghei liver-stage parasites were only partially susceptible to an octaarginine-fosmidomycin complex. Similarly, Toxoplasma gondii was only susceptible during the brief extracellular stages. In marked contrast, a salt complex of octaarginine and fosmidomycin greatly enhanced efficacy against blood-stage Plasmodium falciparum. This complex and a covalently linked conjugate of octaarginine and fosmidomycin also reverted resistance of Mycobacteria to fosmidomycin. These findings provide chemical genetic evidence for vital roles of the nonmevalonate pathway of isoprenoid biosynthesis in a number of medically relevant pathogens. Our results warrant further investigation of octaarginine as a delivery vehicle and alternative fosmidomycin formulations for malaria and tuberculosis drug development.
INTRODUCTION
Malaria and tuberculosis remain two of the most devastating diseases of mankind, causing altogether more than 1.5 million fatalities annually (1, 2). Decades of intensive research were unable to deliver vaccines that fully protect from either of the two diseases, although numerous promising candidates entered advanced clinical development (3, 4). Therefore, control of malaria and tuberculosis highly relies on access to diagnostics and treatment with potent drugs. However, noncompliance of patients and incomplete treatment schedules, among many other factors, accelerate selection for drug-resistant strains of Plasmodium falciparum and Mycobacterium tuberculosis, the intracellular pathogens causing malaria and tuberculosis, respectively. Drug resistance to antibiotics and several antimalarial drugs is now widespread around the globe (2, 5). Hence, there is an urgent and continuous need to design and develop new drugs and to reduce treatment failures by improving the efficacy of current anti-infectives.
A major limitation for antibiotics against intracellular pathogens such as P. falciparum and M. tuberculosis is the need to cross several biological membranes to access the target site. Furthermore, pathogenic mycobacteria present an intrinsically impermeable thick cell wall (6), which serves as a robust barrier to most antibiotics. Therefore, the development of novel strategies to improve the delivery and the bioavailability of existing or novel drugs is a promising avenue to significantly increase efficacy and/or reduce systemic toxicity through dose reduction. Reports have revealed the potential of short polycationic cell-penetrating peptides not only to penetrate most biological cell membranes but also to retain this desirable attribute when attached to cargos of various shapes and sizes, thereby mainly acting as a delivery vehicle of drug molecules into cells (7, 8).
Compared to the naturally existing polycationic cell-penetrating peptides, such as peptides from the human immunodeficiency virus (HIV) trans-activator of transcription (TAT) protein or the Antennapedia-homeodomain-derived antennapedia (Antp) peptide from Drosophila melanogaster, synthetic oligoarginines show excellent cell-penetrating properties across biological membranes (9). Our previous studies demonstrated that human red blood cells are impermeable to 6-carboxyfluorescein (FAM)-octaarginine amide (compound 1) (Fig. 1) but become permeable when infected with the malaria parasite Plasmodium falciparum (10), thereby offering an opportunity for antimalarial drug delivery.
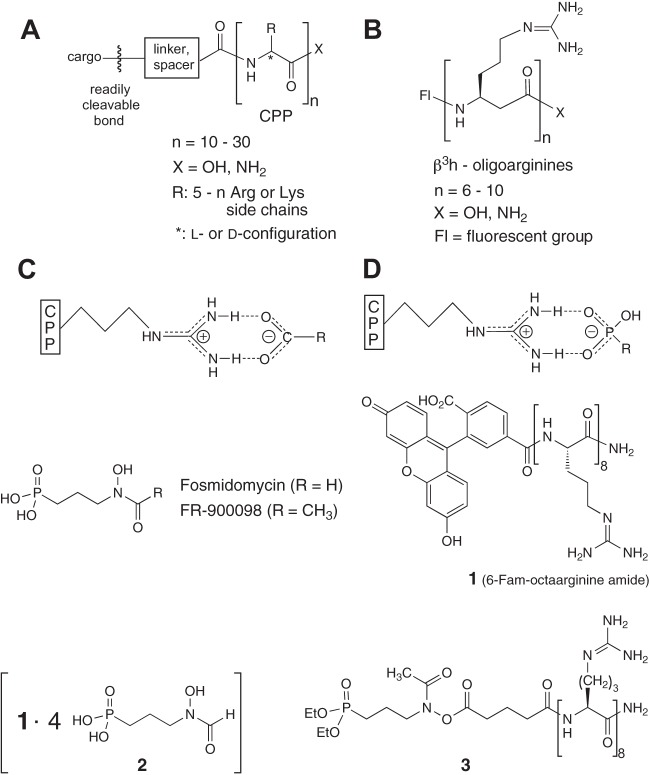
Fig 1.
Attachment of drug molecules as cargo to a carrier cell-penetrating peptide (CPP) through a readily cleavable bond for release of the drug in the target cell. For peptidolytic stability, a D-α-peptide (A) or a β-peptide (B) derivative can be employed. Instead of covalent attachment, counterion activation has been demonstrated previously for carboxylate salts (C) and was realized for the first time with a phosphonate salt (D) in the present study. Structures for fosmidomycin, FR-900098, FAM-octaarginine amide (compound 1), fosmidomycin-octaarginine salt (compound 2), and fosmidomycin-octaarginine covalent conjugate (compound 3) are shown.
Here, we investigate the potential of octaarginine derivatives as drug carriers for the antimalaria drug fosmidomycin (3-[formyl(hydroxy)amino]-propylphosphonic acid; CID 572) and its more potent analogue, FR-900098 (3-[acetyl(hydroxy)amino]propylphosphonic acid; CID 162204) (11) (Fig. 1). In contrast to mammals, most microorganisms and plants synthesize isoprenoid precursors, such as isopentenyl diphosphate (IPP), by the nonmevalonate pathway, called the 1-deoxy-d-xylulose 5-phosphate (DOXP) pathway (12). Fosmidomycin is a potent inhibitor of DOXP reductoisomerase (DXR), the enzyme catalyzing the second reaction in the DOXP pathway (11). Importantly, fosmidomycin has been tested in clinical phase II as an antimalaria drug either alone or in combination with other compounds, e.g., clindamycin and artesunate, and it showed initial promise in fosmidomycin-based combination therapies (13–18). Successful selection of cultured blood-stage P. falciparum parasites that lack an apicoplast through medium supplementation with the product IPP provided genetic proof that nonmevalonate isoprenoid biosynthesis is, at least in vitro, the only essential pathway inside the apicoplast during asexual replication in erythrocytes (19). Although fosmidomycin strongly inhibits DXR orthologues from Toxoplasma gondii and M. tuberculosis in enzyme assays, these organisms were reported to be resistant to fosmidomycin due to the poor ability of the drug to permeate the membrane (20, 21). Liver-stage Plasmodium berghei also is known to be resistant to fosmidomycin, presumably due to its inability to enter hepatocytes (22). Importantly, the choice of fosmidomycin as the cargo allowed us to extend the investigation to other pathogenic microorganisms, such as T. gondii and Toxoplasma mycobacteria, which also operate the target pathway of fosmidomycin.
MATERIALS AND METHODS
The detailed chemical synthesis of fosmidomycin-octaarginine conjugates is described in the supplemental material.
Uptake of FAM-labeled octaarginine.
FAM-labeled octaarginine (compound 1; 10 μg/ml) was added to cultured P. falciparum (strain 3D7)-infected human red blood cells, P. berghei (strain ANKA)-infected human hepatoma cells (Huh7), T. gondii (strain RH)-infected human foreskin fibroblasts (HFF), or cultured Mycobacterium smegmatis or Mycobacterium bovis BCG, followed by further incubation for 12 h. Intracellular FAM-labeled octaarginine was visualized microscopically either by direct fluorescence or in an immunofluorescence assay together with pathogen-specific antibodies. For the direct fluorescence assay, cells were fixed with 4% paraformaldehyde (PFA) for 20 min at room temperature, followed by extensive washing. 4,6-Diamidino-2-phenylindole (DAPI; 1 μg/ml) then was added and incubated for 10 min at room temperature. After extensive washing, cells were mounted using fluoromount-G (Southern Biotech). For the immunofluorescence assay, infected Huh7 cells were fixed (4% paraformaldehyde) for 20 min at room temperature and permeabilized (0.2% Triton X-100) for 15 min at room temperature. After 1 h of blocking with 1% fetal calf serum (FCS) in phosphate-buffered saline (PBS) at room temperature, cells were incubated overnight with anti-P. berghei HSP70 (PbHSP70) antibodies in PBS containing 1% FCS at 4°C. Cells were washed three times with PBS and incubated with the corresponding fluorescently labeled secondary antibody (Invitrogen) in PBS for 1 h at room temperature. Prior to microscopy, the cells were incubated with DAPI (10 μg/ml) for 10 min and washed three times with PBS.
Estimation of the partial permeability of EEFs by octaarginine.
The total number of EEFs was counted in one well of an 8-well Permanox Lab-Tek chamber slide system (Nunc). Percentages were obtained as a ratio of either FAM-octaarginine-positive EEFs to the total number or of FAM-octaarginine-negative EEFs to the total number. Mean values from three biological replicates were plotted.
Plaque assay.
For the plaque assay, HFF cells in 6-well plates were infected with T. gondii tachyzoites in the presence of the corresponding drug, cultured for 7 days, fixed with −80°C methanol, stained with crystal violet, and documented using a Zeiss microscope with a ×5 magnification objective. Experiments were performed in three independent replicates. For the tachyzoite pretreatment assay, freshly harvested T. gondii tachyzoites were preincubated with the corresponding drug for 1 h in a CO2 incubator at 37°C prior to infection of HFF cells.
Growth inhibition assay of blood-stage P. falciparum.
P. falciparum clone 3D7 was cultured at 5% hematocrit of human O+ red blood cells in RPMI 1640 supplemented with 0.05 g/liter hypoxanthine (Sigma-Aldrich), 25 mM HEPES, 0.5% Albumax II (Life Technologies), 0.25% sodium bicarbonate, and 0.01 mg/ml gentamicin (Sigma-Aldrich). All cultures were kept at 37°C under 90% nitrogen, 5% oxygen, and 5% carbon dioxide. Cultures were treated with fosmidomycin or fosmidomycin-octaarginine conjugates at the concentrations indicated. Experiments were done in triplicate for 72 h. Medium was changed daily, and blood smears were prepared every 24 h to monitor parasitemia. Briefly, samples were fixed with methanol and stained with Giemsa (Merck). For parasitemia determination, 30 microscopy fields were counted. Mean values with standard deviations from three replicates were plotted.
Growth inhibition assay of liver-stage P. berghei.
The human liver cell line Huh7 was seeded into 8-well Lab-Tek chamber slides (Nalge Nunc International Corp.) at a density of 50,000 cells/well. The next day, cells were infected with 10,000 freshly dissected P. berghei sporozoites per well. Twenty-four h after infection, either fosmidomycin or fosmidomycin-octaarginine conjugate 2 was added at various concentrations. Merosomes were isolated from cell culture supernatant 65 h after infection and counted. Mean numbers of merosomes were generated from three independent wells of an 8-well Lab-Tek Permanox chamber slide system (Nunc).
Growth inhibition assay of Mycobacterium bovis BCG.
Mycobacteria were grown in Middlebrook 7H9 broth (Becton, Dickinson) supplemented with Middlebrook ADC enrichment (Becton, Dickinson), 0.2% glycerol, and 0.5% Tween 80. Exponentially growing M. bovis BCG was diluted in fresh 7H9 complete medium to an optical density at 600 nm (OD600) of 0.005, and 200 μl per well then was incubated in a translucent 96-well flat-bottom culture plate (Nunc) in the presence of either isoniazid (INH), fosmidomycin, or fosmidomycin-octaarginine conjugates (salt 2 and conjugate 3) at various concentrations. Plates were incubated at 37°C and 80 rpm. Bacterial growth was monitored over time by OD600 measurements. Mean values with standard deviations from three replicates were plotted.
RESULTS
Chemical synthesis of fosmidomycin-octaarginine conjugates.
In recent years, cell-penetrating peptides (CPPs) with positively charged side chains, such as the natural Tat[48-60] or penetratin (AntpHD43-58) and simple oligoarginines (Rn), have been developed for carrying physiologically active compounds into target cells (23–28). In these studies, the carrier and the cargo were linked by a readily cleavable bond (23, 28) (Fig. 1A). In some cases, the carrier peptide was built of D-amino acids instead of the natural L-amino acids in order to prevent premature degradation (24, 29, 30). In other cases, proteolytically stable CPPs containing β-oligoarginines or lysines were used (31–36) (Fig. 1B).
Recently, Matile and his group have introduced a conceptually new mode of noncovalent attachment of the carrier CPP to a cargo (37, 38), namely, salt formation of the guanidinium cations of the CPP with carboxylate anions of a cargo (Fig. 1C). Anticipating that a similar, possibly even stronger ionic interaction would occur with a phosphonate anion, such as that in fosmidomycin and its analogues (Fig. 1D), we generated the salt (compound 2) of the FAM-labeled octaarginine amide (compound 1) with four fosmidomycin units by mixing the two compounds in water and lyophilizing the resulting solution (Fig. 1).
In another approach to carrying fosmidomycin or FR900098 into infected cells, we envisioned that acylation on oxygen of the hydroxylamine moiety would produce an active ester from which the antibiotic could be released. We expected that the phosphonate ester group would also be hydrolyzed, since both the free phosphonic acid and the free N(OH)COR group are believed to be essential for binding to the DXR enzyme target. Thus, we also generated the octaarginine amide carrying one FR900098 diethyl-ester unit (compound 3) (Fig. 1).
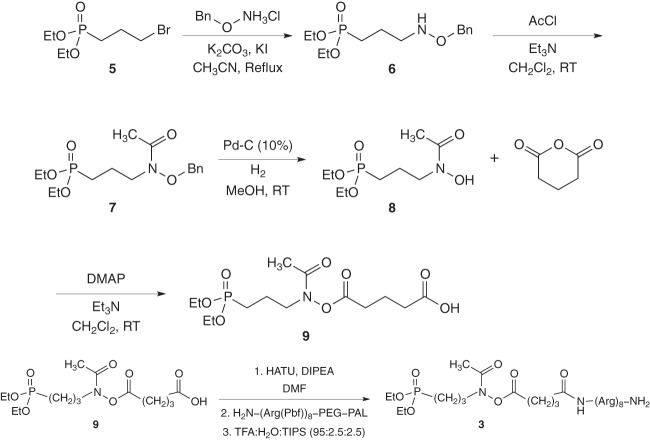
Synthesis of the targeted derivative of fosmidomycin compound 9 (Fig. 2A) was achieved by starting from the commercially available 3-bromopropylphosphonic acid diethyl-ester 5. Compound 5 was first transformed into the corresponding O-benzylhydroxylamino compound 6 by the nucleophilic substitution (SN2) reaction of the bromide group by O-benzylhydroxylamine in refluxing acetonitrile. N-acetylated compound 7 was achieved by treatment with acetyl chloride (AcCl) in the presence of triethylamine (Et3N). Subsequent hydrogenolysis in the presence of catalytic Pd-C (10%) removed the benzyl protection to give the free N-hydroxylamine compound 8.
Fig 2.
Chemical synthesis of fosmidomycin-octaarginine conjugates. The 3-bromopropylphosphosphonic acid diethyl-ester 5 was transformed into the corresponding O-benzylhydroxylamino compound 6 by a nucleophilic substitution (SN2) reaction. Subsequent N-acetylation (compound 7) and hydrogenolysis yielded the free N-hydroxylamine compound 8. Treatment with glutaric anhydride yielded the targeted derivative of the fosmidomycin compound 9. Coupling of fosmidomycin derivative 9 with the resin-bound carrier (octaarginine) and subsequent deprotection and cleavage from the resin provided the fosmidomycin-Arg8 conjugate 3 (Fig. 1). Et, ethyl; Me, methyl; Bn, benzyl; RT, room temperature; Pbf, 2,2,4,6,7-pentamethyldihydrobenzofurane. See also Results and the supplemental material for more details.
Treatment of compound 8 with glutaric acid anhydride in the presence of Et3N and 4-N,N-dimethylaminopyridine (DMAP) yielded compound 9.
Coupling of the fosmidomycin derivative 9 to the resin-bound carrier (octaarginine) was performed on a peptide amide linker-polyethylene glycol (PAL-PEG) solid support using O-(7-aza-1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HATU) as the coupling agent and diisopropyl ethyl amine (DIPEA) as the base in anhydrous dimethylformamide (DMF). Subsequent deprotection and cleavage from the resin with a mixture of trifluoroacetic acid (TFA)-H2O-triisopropylsilane (TIPS) provided the fosmidomycin-Arg8 conjugate 3 (Fig. 2B).
Uptake of FAM-octaarginine by Plasmodium-infected cells.
Previous work established that fosmidomycin is efficiently transported into P. falciparum-infected erythrocytes through the parasite-induced new permeability pathways (22). In order to estimate the potential of octaarginine to serve as a carrier for drug delivery, we first evaluated its ability to cross the multiple membrane barriers separating the parasite from the extracellular compartment. Plasmodium species are obligate intracellular pathogens that reside inside a parasitophorous vacuole (PV) in the cytoplasm of the host cell. The PV membrane (PVM), which is formed upon host cell invasion by the parasite, represents the major protective barrier between the parasite and the host cytoplasm. The PVM selectively controls the exchange of molecules between the host cell and the parasitophorous vacuole. We performed uptake assays with FAM-octaarginine (compound 1) in P. falciparum-infected human red blood cells and in Plasmodium berghei-infected human liver cells (Huh7) representing the exoerythrocytic form (EEF) of the parasite (Fig. 3A and 4A). In line with our previous findings (10), FAM-octaarginine (compound 1) efficiently penetrated infected red blood cells. After 12 h of incubation, most of the fluorescent signal was located within the PV (Fig. 3A). In contrast to P. falciparum-infected erythrocytes, where virtually every infected cell incorporated the peptide, in P. berghei-infected hepatoma cells peptide uptake was not complete, and a subset of infected cells (∼30%) did not incorporate the peptide (Fig. 4A and B). Together, the findings show that FAM-octaarginine (compound 1) is able to cross various biological membranes.
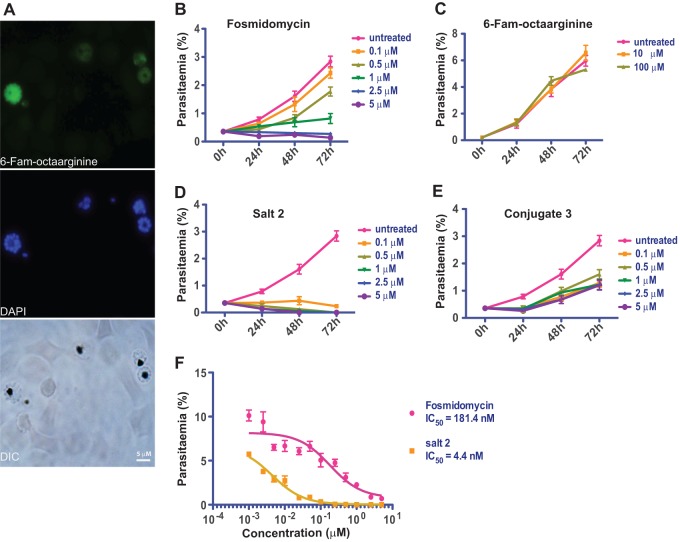
Fig 3.
Octaarginine improves efficacy of fosmidomycin against Plasmodium falciparum asexual blood-stage parasites. (A) FAM-octaarginine efficiently penetrates the parasitophorous vacuole of blood-stage P. falciparum. Shown are fluorescence microscopy images of FAM-octaarginine (green) in P. falciparum-infected human red blood cells. Parasite DNA was stained with DAPI (blue). DIC, differential interference contrast. (B) Time course of cultured P. falciparum parasites in the presence of fosmidomycin at the indicated concentrations (0.1 to 5 μM). (C) Control experiment with 10 or 100 μM FAM-octaarginine. (D) Inhibition of P. falciparum asexual blood-stage growth by fosmidomycin-octaarginine noncovalent salt 2. Note that inhibition is evident already at 0.1 μM. (E) Inferior inhibition by covalent conjugate 3. (F) IC50s for growth inhibition for fosmidomycin alone (red) and the fosmidomycin-octaarginine salt complex 2 (orange).
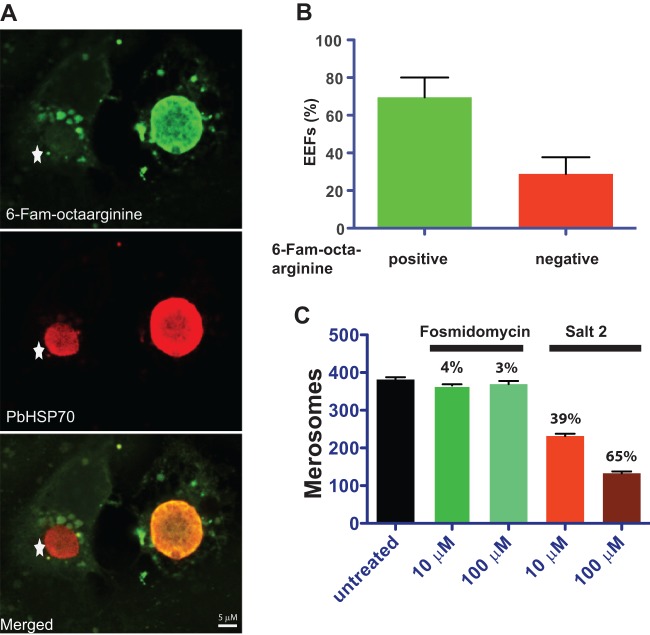
Fig 4.
Conjugation of fosmidomycin to octaarginine inhibits formation of Plasmodium berghei merosomes, the final stage of preerythrocytic development. (A) Fluorescence microscopy images of P. berghei-infected hepatoma cells. Incorporated FAM-octaarginine (green) and PbHSP70 (red) are shown. The exoerythrocytic form (EEF) on the left did not incorporate the FAM-octaarginine (star), whereas the EEF on the right did. (B) Quantification of P. berghei-infected hepatoma cells that incorporated FAM-octaarginine. Shown are mean values and standard deviations from three biological replicates. (C) Quantification of merosomes at 65 h after infection in the presence of either fosmidomycin or the fosmidomycin-octaarginine salt complex 2. Mean values with standard deviations from three independent replicates are shown. Numbers represent the percentage of inhibition of merosome formation compared to that of untreated samples.
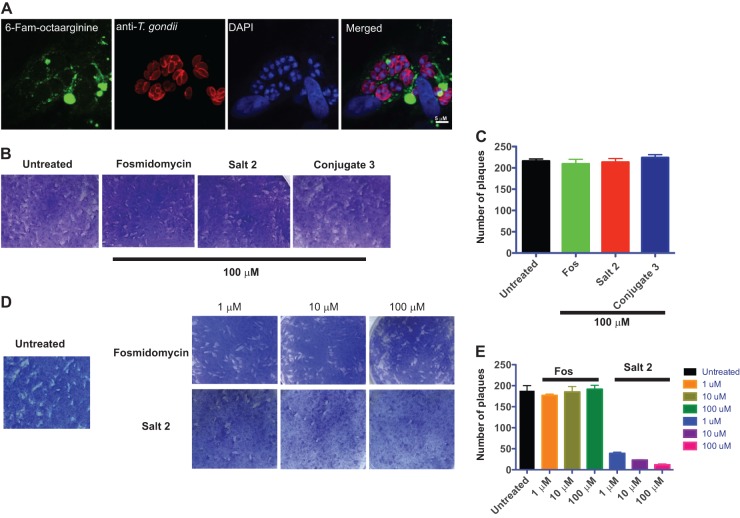
Conjugation of octaarginine to fosmidomycin improves efficacy against asexual blood-stage P. falciparum.
We next evaluated the potential of octaarginine to deliver fosmidomycin into target cells. Growth inhibition assays were performed in the presence of fosmidomycin or its octaarginine conjugate (salt 2 or conjugate 3). We first tested whether combining fosmidomycin with octaarginine could enhance the inhibitory effect of fosmidomycin on blood-stage P. falciparum (Fig. 3B). As expected, fosmidomycin showed clear inhibition of parasite growth. When the noncovalent complex of fosmidomycin and octaarginine (salt 2) was used, a striking increase in the activity was observed. Complete inhibition of P. falciparum growth already occurred at a concentration of 0.1 μM (Fig. 3D). This result indicates that oligoarginine is an efficient delivery vehicle for fosmidomycin to the parasite inside red blood cells.
When the covalently attached FR-900098-octaarginine conjugate 3 was tested, the inhibitory effect on parasite growth was weaker than that of the salt complex or fosmidomycin (Fig. 3E), suggesting that FR-900098 was, at least in this experimental setup, only partially released from the covalent derivative (conjugate 3). The observed significant activity at lower concentrations (0.1 μM and 0.5 μM) indicated that the active antibiotic must have become available. In order to quantify how octaarginine improves the efficacy of fosmidomycin, we calculated the 50% inhibitory concentrations (IC50s) of salt complex 2 and compared them to those of fosmidomycin (Fig. 3F). Growth inhibition experiments with either fosmidomycin or salt conjugate 2 were performed over a concentration range of 1 nM to 5 μM. The IC50 of salt complex 2 was 4.4 nM, whereas it was 181 nM for free fosmidomycin (Fig. 3F). Hence, the ∼40-fold increase in activity could be attributed directly to the addition of octaarginine.
A previous report described the broad-spectrum activity of TP10, a fragment of the cell-penetrating peptide transportan against P. falciparum and the kinetoplastid protozoan parasite Trypanosoma brucei (39). To test whether the observed improved efficacy of fosmidomycin in combination with octaarginine was a result of a potential synergistic growth-inhibitory property of octaarginine, we included control experiments with FAM-octaarginine alone (Fig. 3C). We found that the use of up to 100 μM FAM-octaarginine has no effect on the growth of P. falciparum. This result supports the notion that octaarginine acts as a delivery vehicle only. Our results also indicate that the inhibitory activity of TP10 on parasite growth is not a general property of cell-penetrating peptides but rather is a distinct characteristic of transportan.
Collectively, our results show that the efficacy of the antibiotic fosmidomycin against blood-stage P. falciparum can be substantially increased when complexed with cell-penetrating peptides.
Fosmidomycin-octaarginine complex displays activity against P. berghei preerythrocytic stages.
We next investigated the effect of fosmidomycin-octaarginine salt complex 2 on liver-stage parasites. There have been contradictory reports on the ability of fosmidomycin to inhibit the growth of liver-stage P. berghei. While two studies claimed a complete lack of activity on liver-stage parasites, presumably due to poor drug uptake (22, 40), another study reported significant inhibition of apicoplast biogenesis in liver-stage parasites; as a consequence, merosome formation occurred at 10 mM fosmidomycin (21).
In order to test enhanced causal prophylactic efficacy of fosmidomycin conjugation to octaarginine on liver-stage parasites, we monitored P. berghei liver-stage development in the presence of fosmidomycin or its octaarginine salt complex 2 (Fig. 4). We assessed liver-stage size and morphology and the number of merosomes for the final stage of preerythrocytic development. We did not observe any apparent changes in the morphology of liver stages in the presence of either fosmidomycin or the salt complex (data not shown). Enumeration of merosomes 65 h after infection revealed a moderate but significant reduction when either 10 or 100 μM fosmidomycin-octaarginine salt complex 2 was applied (Fig. 4C). In marked contrast, addition of fosmidomycin alone had no effect (Fig. 4C). Of note, the inhibitory activity of the fosmidomycin-octaarginine salt complex 2 on liver-stage parasites remained incomplete, and we failed to achieve full inhibition of merosome release even at higher concentrations (data not shown). This incapacity to fully inhibit Plasmodium liver-stage growth might be at least partially explained by the observed incomplete uptake of FAM-octaarginine into Plasmodium-infected hepatoma cells (Fig. 4B). In conclusion, these findings provide chemical genetic evidence for an important function of the nonmevalonate isoprenoid biosynthesis pathway in preerythrocytic development of the malaria parasite.
Inhibition of extracellular Toxoplasma gondii tachyzoites by the fosmidomycin-octaarginine complex.
To independently confirm the carrier function of octaarginine in antiparasitic drug delivery, we decided to extend our investigation to Toxoplasma gondii, another apicomplexan parasite and the causative agent of toxoplasmosis. Recent data demonstrated that T. gondii DXR is required for parasite growth (21). We first tested whether FAM-octaarginine can enter T. gondii-infected HFFs (Fig. 5A). When T. gondii-infected HFFs were grown in the presence of FAM-octaarginine, the fluorescently labeled peptide was excluded from the parasitophorous vacuole, although it was clearly visible in the cytosol of the host cell. These data indicate that only the first membrane barrier, i.e., the host plasma membrane, can be overcome by the octaarginine carrier. We next investigated the ability of fosmidomycin-octaarginine complex 2 to inhibit the intracellular growth of T. gondii (Fig. 5B and C). Neither fosmidomycin nor the salt conjugate had a significant effect on parasite growth. This result is probably a direct consequence of the inability of FAM-octaarginine to penetrate the PV of T. gondii (Fig. 5A).
Fig 5.
Fosmidomycin-octaarginine salt complex inhibits extracellular T. gondii tachyzoites but does not affect intracellular replication. (A) Fluorescent micrographs of T. gondii-infected human foreskin fibroblasts (HFFs) showing incorporated FAM-octaarginine (green), anti-T. gondii antibody (red), and DAPI-stained DNA (blue). Plaque assay (B) and corresponding quantification (C) after treatment of T. gondii-infected HFFs with either 100 μM fosmidomycin, salt 2, or conjugate 3 are shown. Also shown are plaque assay (D) and corresponding quantification (E) after pretreatment of T. gondii tachyzoites with either fosmidomycin or fosmidomycin-octaarginine salt complex 2 prior to infecting new HFFs.
In order to test this hypothesis, we bypassed the PV and preincubated free T. gondii tachyzoites in the presence of fosmidomycin or fosmidomycin-octaarginine salt complex 2 for 1 h prior to infection of fresh HFFs (Fig. 5D and E). Pretreatment of tachyzoites with up to 100 μM fosmidomycin had no effect on parasite growth, confirming that fosmidomycin does not cross the parasite plasma membrane. In marked contrast, pretreatment with fosmidomycin-octaarginine salt complex 2 led to a concentration-dependent inhibition of parasite growth with a pronounced inhibition already at 1 μM. This result shows that the PV membrane, but not the parasite membrane, constitutes the major barrier to the fosmidomycin-octaarginine complex in intracellular T. gondii.
Octaarginine derivatives render fosmidomycin effective against mycobacteria.
Reversal of refractoriness to fosmidomycin of T. gondii tachyzoites and P. berghei liver-stage parasites by the octaarginine salt complex 2 prompted us to extend our study to a different domain of organisms, bacteria. The genus Mycobacterium contains the causative agents of tuberculosis and leprosy. The respective pathogens, M. tuberculosis and M. leprae, are protected by a cell wall of highly complex structure and exceptional thickness that ranks as one of the most robust among bacteria. The presence of complex glycoconjugates, such as arabinogalactan and lipids of long-chain mycolic acids, contributes to the substantial rigidity and reduced permeability of the mycobacterial cell wall to lipophilic and hydrophilic molecules. The high intrinsic resistance of mycobacteria to most antibiotics is partly attributed to their unique cell envelope.
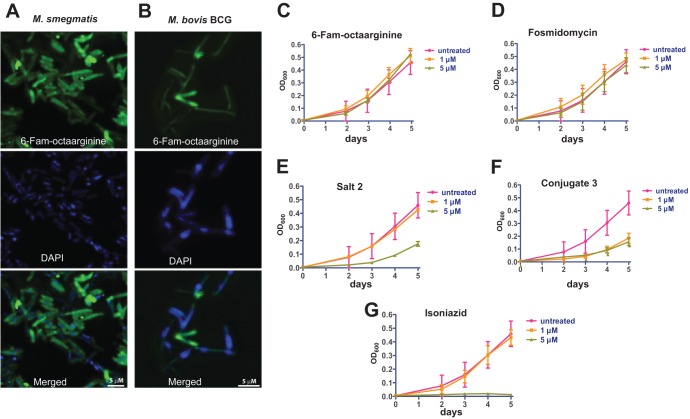
We first assessed whether FAM-octaarginine (compound 1) is able to enter M. smegmatis and M. bovis BCG, two common nonpathogenic experimental surrogates of pathogenic mycobacteria (Fig. 6A and B). Exponentially growing bacilli of both strains were incubated with 10 μg/ml of FAM-octaarginine (compound 1) for 12 h at 37°C. Microscopic analysis demonstrated that FAM-octaarginine efficiently penetrates bacteria of both strains. We next sought to investigate whether the combination of fosmidomycin and octaarginine (salt 2 and conjugate 3) could inhibit mycobacterial growth in vitro. The gene dxr, which encodes the enzyme DXR, is essential for M. tuberculosis growth (20). In addition, recombinant DXR is clearly sensitive to fosmidomycin in enzyme assays (41). However, growth of the pathogen is not affected by fosmidomycin, presumably due to the poor uptake of the drug by bacteria (20).
Fig 6.
Efficacy of fosmidomycin either complexed with or conjugated to octaarginine against Mycobacterium species. (A and B) Fluorescent micrographs of Mycobacterium smegmatis (A) and Mycobacteria bovis BCG (B) showing incorporation of FAM-octaarginine (green). DAPI (blue) is shown as a reference for bacterial DNA. (C) Growth curves of cultured M. bovis BCG in the presence of FAM-octaarginine (C), fosmidomycin (D), the fosmidomycin-octaarginine salt complex 2 (E), the covalent conjugate 3 (F), and isoniazid, a first-line drug for tuberculosis therapy (G).
Based on our observation that mycobacteria are highly permeable to FAM-octaarginine (Fig. 6A and B), we hypothesized that combination of fosmidomycin and octaarginine would improve drug uptake and thereby increase susceptibility of mycobacteria. We monitored the growth of nonpathogenic M. bovis BCG in the presence of fosmidomycin alone or in combination with octaarginine (salt 2 and conjugate 3) and used isoniazid as a positive control (Fig. 6). Mycobacterial growth was reduced when fosmidomycin was combined with octaarginine in either noncovalent (Fig. 6E) or covalent form (Fig. 6F). When octaarginine alone or unmodified fosmidomycin was used, mycobacterial growth remained unaffected (Fig. 6C and D). In contrast to our findings in apicomplexan parasites, the covalently linked fosmidomycin-octaarginine conjugate 3 showed significant inhibition in mycobacteria (Fig. 6F).
DISCUSSION
Plasmodium berghei liver-stage parasites, Toxoplasma gondii, and mycobacteria all are known to be resistant to the antibiotic fosmidomycin, despite an essential role of DRX, the molecular target of fosmidomycin, in these pathogens (20, 21, 22, 24). Accordingly, the failure of fosmidomycin to inhibit growth of these pathogens was attributed to poor drug uptake. In the present study, we have overcome the restriction of fosmidomycin by carrier-mediated delivery to intracellular pathogens. We evaluated the potential of octaarginine, a polycationic cell-penetrating peptide, as a delivery vehicle for fosmidomycin to improve efficacy. We hypothesized that octaarginine would improve the delivery of fosmidomycin across the multiple biological membranes separating the target compartment of intracellular pathogens from the extracellular space. Our investigations show that the combination of octaarginine and fosmidomycin dramatically increases its efficacy toward blood-stage P. falciparum. In addition, the fosmidomycin-octaarginine combination has a significant inhibitory effect on maturation of liver-stage P. berghei, whereas up to 100 μM fosmidomycin alone had no effect. We note that these results are in opposition to a previous report by Nair et al., which claimed a significant effect on merosome formation of P. berghei at 10 μM fosmidomycin (21), but are in full support of studies that show no efficacy against P. berghei liver stages (22, 40).
We observed a strict correlation between the permeability of a given cell type to octaarginine and its ability to improve the efficacy of fosmidomycin in these cells. When octaarginine was unable to cross a biological membrane, the pathogen beyond this barrier remained unaffected by fosmidomycin-octaarginine conjugates. This was the case for intracellular T. gondii, for example. In these cells, FAM-octaarginine did not penetrate the PV, which correlates with resistance to the fosmidomycin-octaarginine conjugate. This view is further supported by the observation that free T. gondii tachyzoites, which are completely permeable to FAM-octaarginine, show a concentration-dependent sensitivity to the fosmidomycin-octaarginine complex. We speculate that the limited activity of the fosmidomycin-octaarginine complex against liver-stage P. berghei is also a consequence of the incomplete uptake of the peptide. These results suggest that both the PVM and the parasite plasma membrane are critical barriers to fosmidomycin in liver-stage P. berghei parasites and, most importantly, in T. gondii-infected host cells. The carrier peptide FAM-octaarginine used in this study has a molecular weight of 1.6 kDa, which is well above the proposed cut off 1.2 kDa for the PVM of T. gondii-infected cells (42), probably explaining the inability of this molecule to penetrate the PV. Strikingly, the observation that the PVM of both blood-stage and liver-stage Plasmodium organisms are permeable to FAM-octaarginine hints at a PVM-resident transport protein operating in Plasmodium parasites. Patch clamp experiments identified a solute pore in blood-stage parasites that is permeable to molecules up to a size of 1.4 kDa, which is less than the size of FAM-octaarginine (43, 44). The apparent different permeability of the Plasmodium PVM compared to that of the T. gondii PVM is best explained by a distinct carrier in the Plasmodium PVM, which is known to be particularly rich in species-specific proteins (45). Although a previous study has reported a successful application of oligoarginine for the delivery of triclosan against both extracellular and intracellular forms of T. gondii (24), we did not observe permeability of the T. gondii PVM for octaarginine.
The application of octaarginine as a versatile delivery vehicle for fosmidomycin was further broadened by our finding that mycobacterial fosmidomycin resistance could be reverted by combining the drug with octaarginine. Interestingly, both the noncovalent salt complex (salt 2) and the covalently linked fosmidomycin-octaarginine conjugate (conjugate 3) showed promising activity against M. bovis BCG. These results open an attractive opportunity for the use of fosmidomycin in combination with octaarginine as a candidate antituberculosis agent. Further investigations, including efficacy testing of fosmidomycin derivatives in animal models of tuberculosis, are beyond the scope of the current study but will be part of our future work. Very recent data demonstrated that M. tuberculosis changes its cell wall/membrane permeability to antibiotics depending on the physiological state (46). Dormant bacilli represent a phenotypic drug-resistant subpopulation that contributes to the prolonged chemotherapy required to cure tuberculosis. Whether octaarginine-antibiotic conjugates have the potential to shorten tuberculosis treatment by improved eradication of the difficult-to-treat dormant M. tuberculosis population remains to be determined.
Altogether, our results provide a valuable basis for alternative formulation of antimicrobial agents using cell-penetrating peptides. Efficacy was observed with the noncovalent drug conjugation to octaarginine that was previously reported by others (33, 34, 47), and this was confirmed in this study for the antibiotic fosmidomycin against diverse pathogens, warranting further investigation of octaarginine as a drug delivery vehicle.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Steffen Bormann and Felista Tansi for critical reading of the manuscript.
Footnotes
Published ahead of print 15 July 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00427-13.
REFERENCES
- 1.Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. 2005. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature 434:214–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization 2012. Global tuberculosis report. WHO, Geneva, Switzerland [Google Scholar]
- 3.Vannice KS, Brown GV, Akanmori BD, Moorthy VS. 2012. MALVAC 2012 scientific forum: accelerating development of second-generation malaria vaccines. Malar. J. 9:372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaufmann SHE, Gengenbacher M. 2012. Recombinant live vaccine candidates against tuberculosis. Curr. Opin. Biotechnol. 23:900–907 [DOI] [PubMed] [Google Scholar]
- 5.Hyde JE. 2007. Drug-resistant malaria–an insight. FEBS J. 274:4688–4698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gengenbacher M, Kaufmann SHE. 2012. Mycobacterium tuberculosis: success through dormancy. FEMS Microbiol. Rev. 36:514–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Langel U. 2002. Cell-penetrating peptides. Processes and application CRC Press, Boca Raton, FL [Google Scholar]
- 8.Pujals S, Fernández-Carneado J, López-Iglesias C, Bogan MJ, Giralt E. 2006. Mechanistic aspects of CPP-mediated intracellular drug delivery: relevance of CPP self-assembly. BBA Biomembr. 1758:264–279 [DOI] [PubMed] [Google Scholar]
- 9.Lindgren M, Hällbrink M, Prochiantz A, Langel U. 2000. Cell-penetrating peptides. Trends Pharmacol. Sci. 21:99–103 [DOI] [PubMed] [Google Scholar]
- 10.Kamena F, Monnanda B, Makou D, Capone S, Patora-Komisarska Seebach KD. 2011. On the mechanism of eukaryotic-cell penetration of α- and β-oligoarginines: targeting infected erythrocytes. Chem. Biodivers. 8:1–12 [DOI] [PubMed] [Google Scholar]
- 11.Jomaa H, Wiesner J, Sandenbrand S, Altincicek B, Weidemeyer C, Hintz M, Türbachova I, Eberl M, Zeidler J, Lichtenhalder HK, Soldati D, Beck E. 1999. Inhibitors of the nonmevalonate pathway of isoprenoid biosynthesis as antimalarial drugs. Science 285:1573–1576 [DOI] [PubMed] [Google Scholar]
- 12.Wanka M, Skorupinska-Tudek K, Swiezewska E. 2001. Isoprenoid biosynthesis via 1-deoxy-D-xylulose 5-phosphate/2-C-methyl-D-erythritol 4-phosphate (DOXP/MEP) pathway. Acta Biochim. Pol. 48:663–672 [PubMed] [Google Scholar]
- 13.Missinou MA, Borrmann S, Schindler A, Issifou S, Adegnika AA, Matsiegui PB, Binder R, Lell B, Wiesner J, Baranek T, Jomaa H, Kremsner PG. 2002. Fosmidomycin for malaria. Lancet 360:1941–1942 [DOI] [PubMed] [Google Scholar]
- 14.Wiesner J, Borrmann S, Jomaa H. 2003. Fosmidomycin for the treatment of malaria. Parasitol. Res. 90:71–76 [DOI] [PubMed] [Google Scholar]
- 15.Borrmann S, Adegnika AA, Matsiegui PB, Issifou S, Schindler A, Mawili-Mboumba DP, Baranek T, Wiesner J, Jomaa H, Kremsner PG. 2004. Fosmidomycin-clindamycin for Plasmodium falciparum infections in African children. J. Infect. Dis. 189:901–908 [DOI] [PubMed] [Google Scholar]
- 16.Borrmann S, Issifou S, Esser G, Adegnika AA, Ramharter M, Matsiegui PB, Oyakhirome S, Mawili-Mboumba DP, Missinou MA, Kun JF, Jomaa H, Kremsner PG. 2004. Fosmidomycin-clindamycin for the treatment of Plasmodium falciparum malaria. J. Infect. Dis. 190:1534–1540 [DOI] [PubMed] [Google Scholar]
- 17.Borrmann S, Adegnika AA, Moussavou F, Oyakhirome S, Esser G, Matsiegui PB, Ramharter M, Lundgren I, Kombila M, Issifou S, Hutchinson D, Wiesner J, Jomaa H, Kremsner PG. 2005. Short-course regimens of artesunate-fosmidomycin in treatment of uncomplicated Plasmodium falciparum malaria. Antimicrob. Agents Chemother. 49:3749–3754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borrmann S, Lundgren I, Oyakhirome S, Impouma B, Matsiegui PB, Adegnika AA, Issifou S, Kun JF, Hutchinson D, Wiesner J, Jomaa H, Kremsner PG. 2006. Fosmidomycin plus clindamycin for treatment of pediatric patients aged 1 to 14 years with Plasmodium falciparum malaria. Antimicrob. Agents Chemother. 50:2713–2718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeh E, Derisi JL. 2011. Chemical rescue of malaria parasites lacking an apicoplast defines organelle function in blood-stage Plasmodium falciparum. PLoS Biol. 9:e1001138. 10.1371/journal.pbio.1001138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown AC, Parish T. 2008. DRX is essential in Mycobacterium tuberculosis and fosmidomycin resistance is due to lack of uptake. BMC Microbiol. 8:78. 10.1186/1471-2180-8-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nair SC, Brooks CF, Goodman CD, Sturm A, McFadden GI, Sundriyal S, Anglin JL, Song Y, Moreno SN, Striepen B. 2011. Apicoplast isoprenoid precursor synthesis and the molecular basis of fosmidomycin resistance in Toxoplasma gondii. J. Exp. Med. 208:1547–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baumeister S, Wiesner J, Reichenberg A, Hintz M, Bietz S, Harb OS, Roos DS, Kordes M, Friesen J, Matuschewski K, Lingelbach K, Jomaa H, Seeber F. 2011. Fosmidomycin uptake into Plasmodium- and Babesia-infected erythrocytes is facilitated by parasite-induced new permeability pathways. PLoS One 6:e19334. 10.1371/journal.pone.0019334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rothbard JB, Garlington S, Lin Q, Kirschberg T, Kreider E, McGrane PL, Wender PA, Khavari PA. 2000. Conjugation of arginine oligomers to cyclosporin A facilitates topical delivery and inhibition of inflammation. Nat. Med. 6:1253–1257 [DOI] [PubMed] [Google Scholar]
- 24.Samuel BU, Hearn B, Mack D, Wender P, Rothbard J, Kirisits MJ, Mui E, Wernimont S, Roberts CW, Muench SP, Rice DW, Prigge ST, Law AB, McLeod R. 2003. Delivery of antimicrobials into parasites. Proc. Natl. Acad. Sci. U. S. A. 100:14281–14286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goun EA, Pillow TH, Jones LR, Rothbard JB, Wender PA. 2006. Molecular transporters: synthesis of oligoguanidinium transporters and their application to drug delivery and real-time imaging. ChemBioChem 7:1497–1515 [DOI] [PubMed] [Google Scholar]
- 26.Wender PA, Galliher WC, Goun EA, Jones LR, Pillow TH. 2008. The design of guanidinium-rich transporters and their internalization mechanisms. Adv. Drug Deliv. Rev. 60:452–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wender PA, Verma VA, Paxton TJ, Pillow TH. 2008. Function-oriented synthesis, step economy, and drug design. Acc. Chem. Res. 41:40–49 [DOI] [PubMed] [Google Scholar]
- 28.Dubikovskaya EA, Thorne SH, Pillow TH, Contag CH, Wender PA. 2008. Overcoming resistance of small-molecule therapeutics through conjugation with releasable octaarginine transporters. Proc. Natl. Acad. Sci. U. S. A. 105:12128–12133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wittenhagen LM, Carreon JR, Prestwich EG, Kelley SO. 2005. Phototoxicity of peptidoconjugates modulated by a single amino acid. Angew. Chem. 117:2598–2602 [DOI] [PubMed] [Google Scholar]
- 30.Tünnemann G, Ter-avetisyan G, Martin RM, Stockl M, Hermann A, Cardoso C. 2008. Live-cell imaging of cell penetration ability and toxicity of oligoarginines. J. Pept. Sci. 14:469–476 [DOI] [PubMed] [Google Scholar]
- 31.Umezawa N, Gelman MA, Haigis MC, Raines RT, Gellman SH. 2002. Translocation of a b-peptide across cell membranes. J. Am. Chem. Soc. 124:368–369 [DOI] [PubMed] [Google Scholar]
- 32.Rueping M, Mahajan YR, Sauer M, Seebach D. 2002. Cellular uptake studies with b-peptides. ChemBioChem 3:257–259 [DOI] [PubMed] [Google Scholar]
- 33.Seebach D, Namamoto K, Mahajan JR, Bindschädler P, Sustmann R, Kirsch M, Ryder NS, Weiss M, Sauer M, Roth C, Werner S, Beer HD, Munding C, Walde P, Voser M. 2004. Chemical and biological investigations of β-oligoarginines. Chem. Biodivers. 1:65–97 [DOI] [PubMed] [Google Scholar]
- 34.Geueke B, Namamoto K, Agarkova I, Perriard JC, Kohler HP, Seebach D. 2005. Bacterial cell penetration by β3-oligoarginines: indication for passive transfer through the lipid bilayer. ChemBioChem 6:982–985 [DOI] [PubMed] [Google Scholar]
- 35.Weiss HM, Wirz B, Schweitzer A, Amstutz R, Rodriguez-Perez MI, Andres H, Metz Y, Gardiner J, Seebach D. 2007. ADME investigations of unnatural peptides: distribution of a 14C-labeled β3-octaarginine in rats. Chem. Biodivers. 4:1413–1437 [DOI] [PubMed] [Google Scholar]
- 36.Seebach D, Gardiner J. 2008. β-Peptidic peptidomimetics. Acc. Chem. Res. 41:1366–1375 [DOI] [PubMed] [Google Scholar]
- 37.Nishihara M, Perret F, Takeuchi T, Futaki S, Lazar AN, Coleman AW, Sakai N, Matile S. 2005. Arginine magic with new counterions up the sleeve. Org. Biomol. Chem. 3:1569–1669 [DOI] [PubMed] [Google Scholar]
- 38.Montenegro J, Gehin C, Bang EK, Fin A, Doval DA, Riezman H, Sakai N, Matile S. 2011. Conceptually new entries into cells. Chimia (Aarau) 65:853–858 [DOI] [PubMed] [Google Scholar]
- 39.Arrighi RB, Ebikeme C, Jiang Y, Ranford-Cartwright L, Barrett MP, Langel U, Faye I. 2008. Cell-penetrating peptide TP10 shows broad-spectrum activity against both Plasmodium falciparum and Trypanosoma brucei brucei. Antimicrob. Agents Chemother. 52:3414–3417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haussig JM, Matuschweski K, Kooij TWA. 2013. Experimental genetics of Plasmodium berghei NFU in the apicoplast iron-sulfur cluster biogenesis pathways. PLoS One 8:e67269. 10.1371/journal.pone.0067269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dhiman RK, Schaeffer ML, Bailey AM, Testa CA, Scherman H, Crick DC. 2005. 1-Deoxy-D-xylulose 5-phosphate reductoisomerase (IspC) from Mycobacterium tuberculosis: towards understanding mycobacterial resistance to fosmidomycin. J. Bacteriol. 187:8395–8402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwab JC, Beckers CJ, Joiner KA. 1994. The parasitophorous vacuole membrane surrounding intracellular Toxoplasma gondii functions as a molecular sieve. Proc. Natl. Acad. Sci. U. S. A. 18:509–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Desai SA, Krogstad DJ, McCleskey EW. 1993. A nutrient-permeable channel on the intraerythrocytic malaria parasite. Nature 362:643–646 [DOI] [PubMed] [Google Scholar]
- 44.Desai SA, Rosenberg RL. 1997. Pore size of the malaria parasite's nutrient channel. Proc. Natl. Acad. Sci. U. S. A. 94:2045–2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spielmann T, Montagna GN, Hecht L, Matuschweski K. 2012. Molecular make-up of the Plasmodium parasitophorous vacuolar membrane. Int. J. Med. Microbiol. 302:179–186 [DOI] [PubMed] [Google Scholar]
- 46.Sarathy J, Dartois V, Dick T, Gengenbacher M. 2013. Reduced drug uptake in phenotypically resistant nutrient-starved non-replicating Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 57:1648–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kamei N, Morishita M, Takayama K. 2009. Importance of intermolecular interaction on the improvement of intestinal therapeutic peptide/protein absorption using cell-penetrating peptides. J. Control Release 136:179–186 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.