Abstract
Hemolysins produced by Vibrio anguillarum have been implicated in the development of hemorrhagic septicemia during vibriosis, a fatal fish disease. Previously, two hemolysin gene clusters responsible for the hemolysis and cytotoxicity of V. anguillarum were identified: the vah1-plp gene cluster and the rtxACHBDE gene cluster. In this study, we identified the hns gene, which encodes the H-NS protein and acts as a negative regulator of both gene clusters. The V. anguillarum H-NS protein shares strong homology with other bacterial H-NS proteins. An hns mutant exhibited increased hemolytic activity and cytotoxicity compared to the wild-type strain. Complementation of the hns mutation restored hemolytic activity and cytotoxicity levels to nearly wild-type levels. Furthermore, expression of rtxA, rtxH, rtxB, vah1, and plp increased in the hns mutant and decreased in the hns-complemented mutant strain compared to expression in the wild-type strain. Additionally, experiments using DNase I showed that purified recombinant H-NS protected multiple sites in the promoter regions of both gene clusters. The hns mutant also exhibited significantly attenuated virulence against rainbow trout. Complementation of the hns mutation restored virulence to wild-type levels, suggesting that H-NS regulates many genes that affect fitness and virulence. Previously, we showed that HlyU is a positive regulator of expression for both gene clusters. In this study, we demonstrate that upregulation by hlyU is hns dependent, suggesting that H-NS acts to repress or silence both gene clusters and HlyU acts to relieve that repression or silencing.
INTRODUCTION
Vibrio anguillarum is the causative agent of vibriosis, a fatal hemorrhagic septicemic disease. V. anguillarum infects more than 50 fresh- and saltwater fish species, including various species of economic importance to the larviculture and aquaculture industries, such as salmon, rainbow trout, turbot, sea bass, sea bream, cod, eel, and ayu (1). Infections by this bacterium have mortality rates of 30% to 100%, resulting in severe economic losses to aquaculture worldwide (2).
The ability of V. anguillarum to infect and cause disease in fish is dependent upon several virulence factors and their proper regulation (3). One of these virulence factors is hemolytic activity. In V. anguillarum M93Sm, there are two known gene clusters that encode at least three hemolysins (4, 5). Rock and Nelson (4) reported that the vah1-plp hemolysin gene cluster (Fig. 1A) contains at least two genes, vah1 and plp, that affect hemolytic activity. Vah1 (encoded by vah1) is a putative pore-forming hemolysin causing vacuolization of target cells that has strong amino acid sequence identity to Vibrio cholerae El Tor hemolysin (hlyA) and V. fluvialis hemolysin (5). Mutations in the divergently transcribed plp gene result in both increased expression of vah1 and increased hemolysis of sheep's blood, suggesting that Plp (encoded by plp) is a putative repressor of vah1 transcription (4). Restoration of plp by complementation restores the wild-type levels of vah1 transcription and hemolysis (4). Plp is a phosphatidylcholine (PC)-specific phospholipase A2 (PLA2) which causes lysis of PC-rich fish erythrocytes (L. Li et al., unpublished data). These observations suggest that Plp plays a dual role as both a repressor and a phospholipase. A second hemolysin gene cluster, rtxACHBDE (Fig. 1B), was identified in V. anguillarum (5). This gene cluster contains rtxA, which encodes a multifunctional autoprocessing repeat-in-toxin (MARTX) toxin, and specialized type 1 secretion system (T1SS) genes (rtxDBE) responsible for the secretion of RtxA. RtxA exhibits cytotoxic activity that causes Atlantic salmon kidney (ASK) cells to round and die (5). Loss of rtxA function results in avirulence (5), while mutation of vah1 causes a slight attenuation of V. anguillarum virulence (4). Strains with mutations in both vah1 and rtxA lost 98% cytotoxicity in ASK cells, suggesting that Vah1 and RtxA are the two major cytotoxins when ASK cells are treated with V. anguillarum (6). These observations strongly suggest that the RtxA hemolysin is a major virulence factor of V. anguillarum, while Vah1 plays a more minor role in virulence.
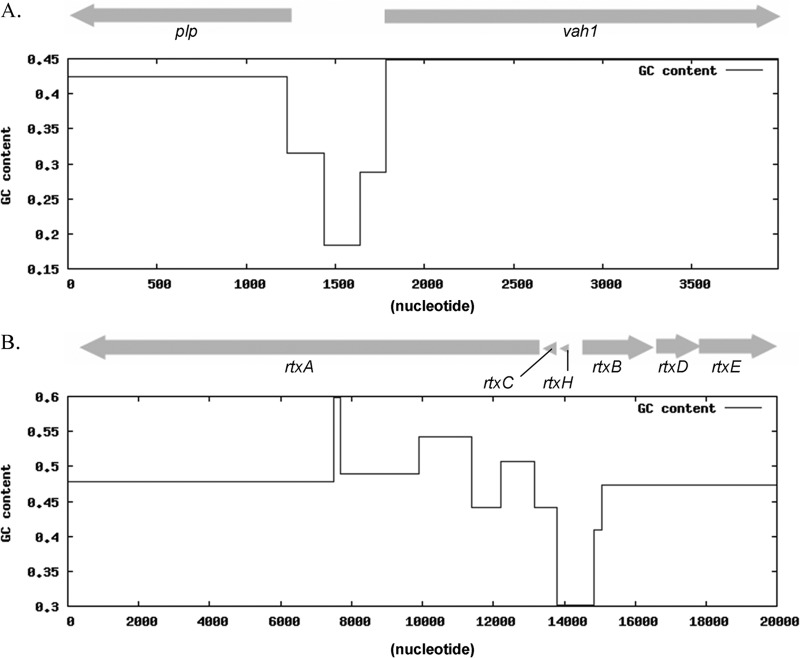
Fig 1.
V. anguillarum hemolysin genes are arranged in two gene clusters: the vah1-plp gene cluster (A) and the rtxACHBDE gene cluster (B). The GC content of each gene cluster is shown.
The histone-like nucleoid structuring protein (H-NS) is a conserved global regulator that belongs to a family of small nucleoid-associated proteins including the factor for inversion stimulation (FIS), the heat-unstable protein (HU), and the integration host factor (IHF) (7). It has been reported that H-NS function is based on self-oligomerization and binding to DNA motifs to create DNA-protein-DNA bridges that can impede the movement of RNA polymerase (8). H-NS has been shown to repress expression of several virulence genes, including the cholera toxin (ctx) (9, 10) and exopolysaccharide biosynthesis (vps) genes in V. cholerae (10, 11), the RTX toxin gene (rtxA1) in V. vulnificus (12), and T3SS1 genes in V. parahaemolyticus (13). In many bacterial species, repression by H-NS can be relieved by other regulators, and each bacterial system has developed specific approaches to attenuate the repressive action of H-NS (8). In V. vulnificus, HlyU acts as a competitor that antagonizes the binding of H-NS, resulting in derepression of rtxA1 (12). Transcriptional silencing of the V. cholerae tcpA and ctx promoters by H-NS is antagonized by the AraC-like transcriptional regulator ToxT and by IHF (10, 14, 15). While there is no report regarding H-NS in V. anguillarum, we hypothesize that H-NS is a regulator of the two hemolysin gene clusters in V. anguillarum.
In this study, we identified the sequence of an hns homologue in V. anguillarum by using the V. anguillarum M93Sm draft genome and subsequently constructed several hns mutant strains, including an hns mutant, an hns hlyU double mutant, an hns hlyU double mutant complemented with hns, and an hns hlyU double mutant complemented with hlyU. The hemolytic activity and cytotoxicity of these strains were determined. The expression levels of various hemolysin genes, including vah1, plp, rtxA, rtxH, and rtxB, were also quantified for these strains. Additionally, the H-NS binding sites in the intergenic regions in both hemolysin gene clusters were localized. Finally, the virulence of the hns mutant and hns-complemented strains was tested in rainbow trout (Oncorhynchus mykiss) and compared to the virulence of the wild-type strain.
MATERIALS AND METHODS
Identification of genes in V. anguillarum.
The V. anguillarum M93Sm draft genome (unpublished data) was annotated by the RAST (Rapid Annotation using Subsystem Technology) service (http://rast.nmpdr.org/rast.cgi), using the default settings (16).
Fish cell line, bacterial strains, plasmids, and growth conditions.
ASK cells (American Type Culture Collection [ATCC], Manassas, VA) were cultured at 20°C in Leibovitz-15 medium containing 100 μg/ml streptomycin and 17% fetal bovine serum (FBS) (Life Technologies, Grand Island, NY). All bacterial strains and plasmids used in this report are listed in Table 1. V. anguillarum strains were routinely grown in Luria-Bertani broth plus 2% NaCl (LB20) (17), supplemented with the appropriate antibiotic, in a shaking water bath at 27°C. Escherichia coli strains were routinely grown in Luria-Bertani broth plus 1% NaCl (LB10). Antibiotics were used at the following concentrations: streptomycin, 200 μg/ml (Sm200); ampicillin, 100 μg/ml (Ap100); chloramphenicol, 20 μg/ml (Cm20) for E. coli and 5 μg/ml (Cm5) for V. anguillarum; kanamycin, 50 μg/ml (Km50) for E. coli and 80 μg/ml (Km80) for V. anguillarum; tetracycline, 15 μg/ml (Tc15) for E. coli, 1 μg/ml (Tc1) for V. anguillarum grown in liquid medium, and 2 μg/ml (Tc2) for V. anguillarum grown in solid medium.
Table 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype or feature(s) | Reference or source |
|---|---|---|
| Strains | ||
| V. anguillarum strains | ||
| M93Sm | Spontaneous Smr mutant of M93 (serotype J-O-1); virulent | 31 |
| S305 | hlyU mutant; Smr Cmr | 6 |
| M114 | hns mutant; Smr Cmr | This study |
| M116 | hns-complemented strain; Smr Cmr Tetr | This study |
| ES114 | hns hlyU double mutant; Smr Cmr Kmr | This study |
| ES115 | hns hlyU double mutant complemented with hlyU; Smr Cmr Tetr Kmr | This study |
| ES116 | hns hlyU double mutant complemented with hns; Smr Cmr Tetr Kmr | This study |
| E. coli strains | ||
| Sm10 | thi thr leu tonA lacY supE recA RP4-2-Tc::Mu::Km (λ pir) Kmr | 39 |
| M15 | Nals Strs Rifs thi lac ara+ gal+ mtl F− recA+ uvr+ lon+ (pREP4; Kmr) | Qiagen |
| D112 | E. coli Sm10 with pDM4-hns5′-Kan-hns3′; Cmr Kmr | This study |
| Plasmids | ||
| pNQ705-1 | Cmr; suicide vector with R6K origin | 40 |
| pNQ705-hns | Used for hns insertional mutation | This study |
| pSUP202 | E. coli-V. anguillarum shuttle vector | 39 |
| pSUP202-hlyU | Used for complementation of hlyU | 6 |
| pSUP202-hns | Used for complementation of hns | This study |
| pDM4 | Cmr Kanr SacBCr; suicide vector | 18 |
| pDM4-hns5′-Kan-hns3′ | Used for hns deletion mutation | This study |
| pQE-30 UA | Expression vector with N-terminal His6 tag | Qiagen |
| pQE-30 UA/H-NS | Used for expression of rH-NS | This study |
Insertional mutagenesis.
Insertional mutations were made by using a modification of the procedure described by Milton et al. (18). Briefly, primers SD_hns(F) and SD_hns(R) (Table 2) were designed based on the target gene sequence of M93Sm. A 200- to 300-bp DNA fragment of hns was then PCR amplified and ligated into the suicide vector pNQ705 (GenBank accession no. KC795685) after digestion with SacI and XbaI. The ligation mixture was introduced into E. coli Sm10 by electroporation using a Bio-Rad Gene Pulser II apparatus (Bio-Rad, Hercules, CA). Transformants were selected on LB10 Cm20 agar plates. The construction of pNQ705-hns was confirmed by both PCR amplification and restriction analysis. The mobilizable suicide vector was transferred from E. coli Sm10 into V. anguillarum M93Sm by conjugation (18). Transconjugants were selected by utilizing the chloramphenicol resistance gene located on the suicide plasmid. The incorporation of pNQ705-hns was confirmed by PCR amplification.
Table 2.
Primers used in this study
| Primer | Sequence (5′ to 3′)a | Use and description | Reference |
|---|---|---|---|
| SD_hns(F) | GCTAGGAGCTCCAGCTTGAAGAAGCACTAGA | hns insertional mutation, forward primer, SacI site | This study |
| SD_hns(R) | GCTAGTCTAGACCAGAAAGTGCAGAAATTAA | hns insertional mutation, reverse primer, XbaI site | This study |
| hns_comp(F) | GCTAGCTGCAGTCGGCGATAAAACCTTTCAC | hns complementation, forward primer, PstI site | This study |
| hns_comp(R) | GCTAGCTGCAGGTTTACCTGAACGTGACGAC | hns complementation, reverse primer, PstI site | This study |
| Pm416 | TTAAATCTCGAATTCTTCTAGAGATTTACC | hns open reading frame, forward primer | This study |
| Pm417 | ATGTCTGAATTAACAAAAACTCTACTTAAT | hns open reading frame, reverse primer | This study |
| pr40 | CGGCTACTCGAGAGATTTACCTGCATCAAGTTG | hns 5′ region, forward primer, XhoI site | This study |
| pr41 | CGGCTATCTAGAGCACTTTCTGGTGAAACTAAG | hns 5′ region, reverse primer, XbaI site | This study |
| pr42 | CGGCTATCTAGAATTAATGCGCTTACATCAATA | hns 3′ region, forward primer, XbaI site | This study |
| pr37 | CGGCTAGAGCTCAGAAGCACTAGATAAATTAAC | hns 3′ region, reverse primer, SacI site | This study |
| pr38 | CGGCTATCTAGAGAAAAGCTTGAACACGTAGAA | Kanamycin resistance gene, forward primer, XbaI site | This study |
| Pm173 | ACTGATCTAGATCAGAAGAACTCGTCAAGAAG | Kanamycin resistance gene, reverse primer, XbaI site | This study |
| RT vah1-R1 | GACCGCCGAATCGATGATGAATC | vah1 qRT-PCR, forward primer | 4 |
| pvah1JR | GGTAGGACTGATGCCCACCTACAA | vah1 qRT-PCR, reverse primer | This study |
| plpF RT | CAGACGACCACCAGTAACCACTAA | plp qRT-PCR, forward primer | 4 |
| plpR RT | GCAATCATGATGACCCAGCAACAG | plp qRT-PCR, reverse primer | 4 |
| Pm111 | GGAAATTATTCCGCCGACGATGGA | rtxA qRT-PCR, forward primer | 5 |
| Pm112 | GCCGATACCGTATCGTTACCTGAA | rtxA qRT-PCR, reverse primer | 5 |
| Pm285 | GTGATGGTAGAAAACCTGCGG | rtxH qRT-PCR, forward primer | This study |
| Pm286 | ATGTCTGAGAAATTTGTCCAAACA | rtxH qRT-PCR, reverse primer | This study |
| iPCR rtxB R | CCGCTAACCGCATTGATATTAAGCTTGGC | rtxB qRT-PCR, forward primer | This study |
| Pm104 | TCACAATCGCCCCAACTTGCCTTG | rtxB qRT-PCR, reverse primer | This study |
| Pm297 | ACTGAGAGCTCGGTGTTGTTAAAGGCTATGGC | hlyU qRT-PCR, forward primer | 6 |
| Pm298 | ATCGATCTAGAGTATCCACTAACCCATCTCTT | hlyU qRT-PCR, reverse primer | 6 |
| Pm412 | CCGTATTTTCTGCAATCGCCATGG | vah1 promoter region (probe 1), forward primer, 5′ labeled with FAM | 6 |
| Pm324 | CACATATTGACTGATTATAATTTTATTGATATT | vah1 promoter region (probe 1), reverse primer | 6 |
| pr323a | AGGGTTTTTATAAATCCTAATTTAGATA | plp promoter region (probe 2), forward primer, 5′ labeled with FAM | This study |
| Pm320 | GAATACCCATTTTTTATTTTTTCAGACC | plp promoter region (probe 2), reverse primer | 6 |
| Pm327 | GTATTTTCTGCAATCGCCATG | vah1-plp (probe 3) intergenic region, forward primer | 6 |
| Pm413 | CACCTTTGTGGCGAATTATTAATAGATCTT | vah1-plp (probe 3) intergenic region, reverse primer, 5′ labeled with FAM | 6 |
| Pm414 | CAGTGGCTCATAAAAGCAGTTGC | rtxB-rtxH intergenic region (probe 4), forward primer, 5′ labeled with FAM | 6 |
| Pm318 | CAGCGGTAAGTAGACTGATA | rtxB-rtxH intergenic region (probe 4), reverse primer | 6 |
| Pm315 | CTCAGACATAAATAAATCACC | rtxB-rtxH intergenic region (probe 5), forward primer | 6 |
| Pm415 | CAGCGGTAAGTAGACTGATAAGCAATG | rtxB-rtxH intergenic region (probe 5), reverse primer, 5′ labeled with FAM | 6 |
Underlined sequences are engineered restriction sites.
Construction of hns hlyU double mutant.
The hns hlyU double mutant was constructed by allelic exchange of hns followed by insertional mutation of hlyU. The allelic exchange mutation was made by using a modification of the procedure described by Milton et al. (18). Briefly, the plasmid pDM4 (GenBank accession no. KC795686) was used to construct an hns::Km allelic exchange mutant as described previously (18). The 5′ region of hns was amplified using the primer pair pr40 and pr41 (Table 2), digested with XhoI and XbaI, and then cloned into the region between the XhoI and XbaI sites on pDM4. The 3′ region of hns was amplified using the primer pair pr42 and pr37 (Table 2), digested with XbaI and SacI, and then cloned into the region between the XbaI and SacI sites on the derivative pDM4 plasmid containing the 5′ region of hns. Finally, the kanamycin resistance gene was amplified from the TOPO2.1 vector (Life Technologies) with the primer pair pr38 and pm173 (Table 2), digested with XbaI, and inserted into the XbaI site between the 5′ and 3′ hns regions on the derivative pDM4 plasmid. The resulting plasmid, pDM4-hns::Km, was transformed into E. coli Sm10 to produce the transformant strain D112, which was mated with V. anguillarum M93Sm. Single-crossover transconjugants were selected with LB20 Kan80 Sm200 Cm5 plates, and subsequently, double-crossover transconjugants were selected with LB20 Kan80 Sm200 plates containing 5% sucrose. The resulting V. anguillarum mutants were checked for the desired allelic exchange by PCR amplification and then were subjected to insertional mutation of hlyU as described above.
Complementation of the mutants.
The various mutants were complemented by cloning the appropriate target gene fragment into the shuttle vector pSUP202 (GenBank accession no. AY428809) as described by Rock and Nelson (4). Briefly, primers hns_comp(F) and hns_comp(R) (Table 2) were designed with a PstI site added at the 5′ end of each primer. The primer pair was then used to amplify the entire target gene plus ∼500 bp of the 5′-flanking region and ∼200 bp of the 3′-flanking region from genomic DNA of V. anguillarum M93Sm. The DNA fragment was then ligated into pSUP202 after digestion with EcoRI and AgeI, and the ligation mixture was introduced into E. coli Sm10 by electroporation using a Bio-Rad Gene Pulser II apparatus. Transformants were selected on LB10 Tc15Ap100 agar plates. The complementing plasmid was transferred from E. coli Sm10 into the V. anguillarum mutant by conjugation (18). Transconjugants were selected by utilizing the tetracycline resistance gene located on the plasmid. The transconjugants were then confirmed by PCR amplification and restriction digestion.
Hemolytic activity assay.
The blood agar hemolysis assay was carried out using the method described by Rock and Nelson (4). Briefly, V. anguillarum colonies were transferred onto blood agar plates, and hemolytic activity was determined by measuring the diameter of beta-hemolysis on plates containing Trypticase soy agar (TSA) plus either 5% sheep blood (Northeast Laboratories Service, Waterville, ME) or 5% trout blood after 24 h at 27°C. Trout blood was taken from live, healthy, farm-raised rainbow trout (Oncorhynchus mykiss) by use of a 3-ml sterile syringe supplemented with 10 μl 0.5 M disodium EDTA (Sigma-Aldrich). The blood was then stored on ice and used in casting plates within 6 h.
Cytotoxicity assay.
The cytotoxicity assay was carried out using a modification of the method described by Li et al. (6). Cytotoxic activity of V. anguillarum strains was determined by measurement of released lactate dehydrogenase (LDH). ASK cells (20,000 cells/well for assays using V. anguillarum supernatants and 10,000 cells/well for assays using washed V. anguillarum cells) were seeded into a 96-well tissue culture plate and incubated in Leibovitz-15 medium supplemented with 17% FBS at 20°C for 24 h to allow cells to attach. V. anguillarum cultures grown for 18 h were centrifuged (9,000 × g, 5 min, 4°C). The resulting culture supernatant was harvested and filter sterilized using 22-μm filters (Millipore Corp., Billerica, MA). The bacterial pellet was washed twice in nine-salts solution (NSS) (19) and resuspended in fresh NSS (at ∼2 × 109 cells ml−1). V. anguillarum culture supernatant (50 μl) was added to wells containing ASK cells plus 50 μl phosphate-buffered saline (PBS) (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.4 mM KH2PO4; pH 7.4) and incubated at 20°C for 6 h. Washed bacterial cells were added to ASK cells at a multiplicity of infection (MOI) of 200 and incubated at 20°C for 4 h. To determine the release of LDH, a CytoTox-ONE homogeneous membrane integrity assay kit (Promega, Madison, WI) was used following the manufacturer's instructions. The assay measures the generation of the fluorescent resorufin product, which is proportional to the amount of LDH, using an excitation wavelength of 560 nm and an emission wavelength of 590 nm. Fluorescence was read by a Stratagene MX3005P QPCR system at an excitation wavelength of 550 nm and an emission wavelength of 570 nm.
RNA isolation.
Exponential-phase cells (∼0.5 × 108 CFU ml−1) and stationary-phase cells (2 × 109 CFU ml−1) of various V. anguillarum strains were treated with RNAprotect bacterial reagent (Qiagen, Valencia, CA) following the manufacturer's instructions. Total RNA was isolated using an RNeasy kit and QIAcube (Qiagen) following the manufacturer's instructions. All purified RNA samples were quantified spectrophotometrically by measuring absorption at 260 nm and 280 nm, using a Nanodrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Pittsburgh, PA), and were stored at −75°C for future use.
Real-time qRT-PCR.
Quantitative reverse transcriptase PCR (qRT-PCR) was used to quantify various mRNAs by use of an Mx3005 multiplex quantitative PCR system and Brilliant II SYBR green single-step qRT-PCR master mix (Agilent Technologies, Wilmington, DE) with 10 ng of total RNA in 25-μl reaction mixtures. The thermal profile was 50°C for 30 min, 95°C for 15 min, and then 40 cycles of 95°C for 30 s and 55°C for 30 s. Fluorescence was measured at the end of the 55°C step during every cycle. Samples were run in triplicate along with no-RT and no-template controls. All experiments were repeated at least twice.
Overexpression and purification of the V. anguillarum H-NS protein.
The DNA fragment encoding H-NS was PCR amplified by using primers Pm416 and Pm417 (Table 2) and then cloned into a six-His-tag expression plasmid, pQE30-UA (Qiagen), generating the plasmid pQE-30 UA/H-NS (Table 1), which encodes H-NS with an N-terminal fusion tag. The correct recombinant clone (rH-NS; confirmed by sequencing) was used for expression of the His-tagged H-NS protein in E. coli M15. Expression and purification of rH-NS were carried out using a modification of the procedures described by Li et al. (6). Briefly, 10 ml of an overnight bacterial culture growing at 37°C in Luria broth supplemented with 50 μg/ml kanamycin and 100 μg/ml ampicillin was added to 250 ml of the same fresh medium. When the optical density at 600 nm (OD600) reached 0.6, 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) was added to induce the expression of the H-NS protein. After bacteria were grown for an additional 5 h at 37°C, the cells were collected and lysed by sonication under nondenaturing conditions. The soluble supernatant containing rH-NS was then purified from this fraction by affinity chromatography using Ni-nitrilotriacetic acid resin columns (Qiagen) according to the manufacturer's instructions. The concentration of the purified rH-NS protein was determined by measuring the absorbance at 280 nm, using a Nanodrop ND-1000 spectrophotometer (Thermo Scientific). The purity of rH-NS was assessed by SDS-polyacrylamide gel electrophoresis, with only a single band visible following staining with Coomassie blue.
DNase I protection assay.
DNA probes for the intergenic region of each of the hemolysin gene clusters were amplified from V. anguillarum genomic DNA by PCR (Table 2). Probes were labeled with 6-carboxyfluorescein (FAM) at the 5′ end (see Fig. S1 in the supplemental material). The assay was carried out using a method modified from the work of Li et al. (6). Briefly, 7.5 × 1011 copies of DNA probe and various amounts of rH-NS (up to 3 μM) were incubated for 1 h at 27°C in a total volume of 20 μl containing 4 μl of 5× binding buffer, 1 μg poly-l-lysine, and 1 μg poly(dI-dC) (DIG gel shift kit, 2nd generation; Roche Applied Science, Indianapolis, IN). The DNA-protein complex was then digested by adding 0.005 U RQ1 RNase-free DNase (New England BioLabs, Ipswich, MA) in a total volume of 25 μl containing 2.5 μl of 10× concentrated reaction buffer at 37°C for 15 min. The reaction was stopped by heating at 95°C for 10 min. The DNA was purified using a QIAquick PCR purification kit (Qiagen) and a QIAcube according to the standard protocol, except that the elution volume was adjusted to 30 μl. The DNA in the eluate (3 μl) was added to 9 μl Hi-Di formamide containing 1 μl GeneScan 600 LIZ size standard (Applied Biosystems), and the mixture was submitted to capillary electrophoresis fragment analysis (Rhode Island Genomics and Sequencing Center).
GC content plotting.
The GC contents of the two gene clusters (Fig. 1) were plotted using the GC-Profile program (http://tubic.tju.edu.cn/GC-Profile) (20) with the following settings: halting parameter of 1 and minimum length to segment of 100 bp.
Fish infection studies.
Various V. anguillarum strains were tested for virulence in rainbow trout (Oncorhynchus mykiss) by intraperitoneal (i.p.) injection. Briefly, V. anguillarum cells grown in LB20 supplemented with appropriate antibiotics for 22 h at 27°C were harvested by centrifugation (9,000 × g, 5 min, 4°C), washed twice in NSS, and resuspended in NSS (∼2 × 109 cells ml−1). Initial cell density was estimated by measurement of the OD600. The actual cell density of NSS suspensions was determined by serial dilution and spot plating. All fish were examined prior to the start of each experiment to determine that they were free of disease or injury. It should be noted that all the negative-control fish survived. Fish were anesthetized with tricaine methanesulfonate (Western Chemical, Ferndale, WA) at 100 mg/liter for induction and 52.5 mg/liter for maintenance. V. anguillarum strains were injected i.p. into fish in 100 μl NSS vehicle. Fish that were between 15 and 25 cm long were injected with bacteria diluted with NSS at a dose of ∼4 × 105 CFU/fish, or with NSS only as a negative control. Ten fish were used for each experimental group. Fish inoculated with different bacterial strains were maintained in separate 10-gallon tanks with constant water flow (200 ml/min) at 19 ± 1°C. The tanks were separated to prevent possible cross-contamination. Death due to vibriosis was determined by the observation of gross clinical signs and was confirmed by the recovery and isolation of V. anguillarum cells resistant to the appropriate antibiotics from the head kidneys of dead fish. Observations were made for 14 days. All fish used in this research project were obtained from the URI East Farm Aquaculture Center. All fish infection protocols were approved by the URI IACUC.
Statistical analysis.
Two-tailed Student's t tests assuming unequal variances were used for statistical analyses for all experiments except the fish infection experiment (P values of <0.05 were considered statistically significant). For fish infection experiments, a Kaplan-Meier survival analysis with the log rank significance test was performed on fish survival percentages (P values of <0.05 were considered statistically significant).
RESULTS
Identification of hns in V. anguillarum.
The V. anguillarum hns gene (GenBank accession number KC795684) was found in the RAST annotation of the V. anguillarum M93Sm draft genome (unpublished data). It encodes a predicted 137-amino-acid protein with a molecular mass of 15,299 Da and has strong homology to H-NS proteins found in a variety of Vibrio species, including Vibrio harveyi (92% similarity and 84% identity), Vibrio coralliilyticus (84% similarity and 76% identity), V. cholerae (90% similarity and 82% identity), V. parahaemolyticus (90% similarity and 85% identity), and V. vulnificus (93% similarity and 87% identity).
Mutation of hns increases hemolytic activity.
It was previously shown that V. anguillarum wild-type cells exhibit beta-hemolysis on 5% TSA-sheep blood agar (5). When the hemolytic activity of the hns mutant (M114) was tested on 5% TSA-sheep blood agar, it was found that mutation of hns resulted in increased hemolysis compared to that of the wild type (M93Sm) (Fig. 2). Furthermore, when the hns mutation was complemented (M116; hns+), hemolysis was reduced to levels below those of the wild type (Fig. 2), suggesting that H-NS is a negative regulator of at least one of the two hemolysins (RtxA and Vah1). The lower hemolytic activity was probably due to the overexpression of H-NS, since pSUP202 is a multicopy plasmid.
Fig 2.
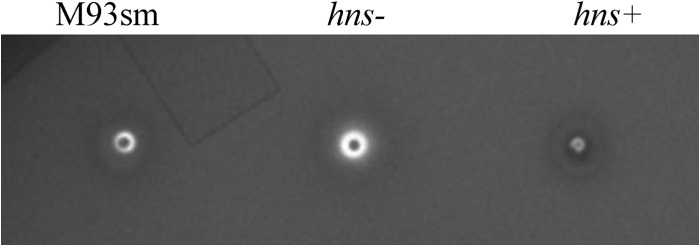
Hemolytic activity of V. anguillarum wild-type (M93Sm), hns mutant (hns−) and hns-complemented mutant (hns+) strains on TSA-5% sheep blood agar after 24 h at 27°C.
Mutation of hns increases cytotoxicity against ASK cells.
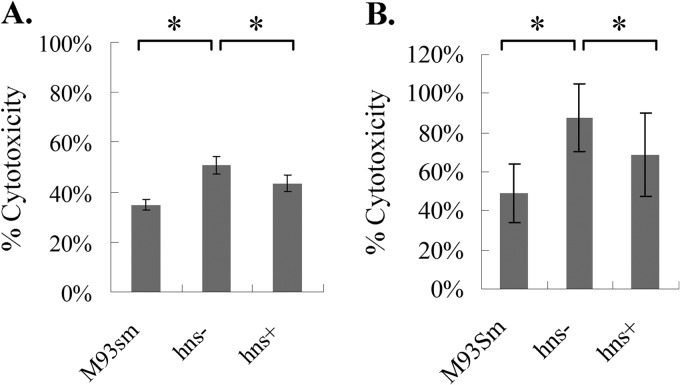
It was previously demonstrated that both vah1 and rtxA contribute to the cytotoxicity of V. anguillarum cells against ASK cells (5). In order to determine whether hns acts to regulate cytotoxic activity, we tested the cytotoxic activities of both culture supernatants and washed cells of the hns mutant and the hns-complemented mutant (hns+) and compared them against those of culture supernatant and washed cells of the wild type (M93Sm). The results showed that mutation of hns significantly increased the cytotoxicity of both V. anguillarum culture supernatant (>40%) and cells (MOI of 200) (∼80%) against ASK cells compared to the cytotoxicity of wild-type V. anguillarum M93Sm. Complementation of the hns mutation significantly decreased cytotoxicity of both culture supernatant and cells against ASK cells compared to the cytotoxic activity of the hns mutant (Fig. 3A and B). These data support the suggestion that hns negatively regulates at least one of the two hemolytic/cytotoxic activities encoded by rtxA and vah1.
Fig 3.
(A) Cytotoxicity of culture supernatants from V. anguillarum wild-type (M93Sm), hns mutant (hns−), and hns-complemented mutant (hns+) strains against ASK cells after 6 h at 20°C. (B) Cytotoxicity of washed cells of V. anguillarum wild-type (M93Sm), hns mutant (hns−), and hns-complemented mutant (hns+) strains against ASK cells after 4 h at 20°C (MOI = 200). Asterisks represent P values of <0.05 between two bracketed strains. Error bars represent 1 standard deviation.
H-NS negatively regulates hemolysin genes at the transcriptional level.
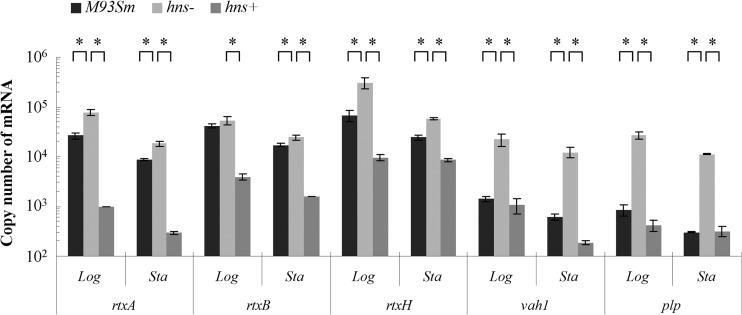
Since the two hemolysin/cytotoxin gene clusters are each organized into two divergent transcriptional units (Fig. 1), with intergenic regions shown to bind HlyU (6), we wanted to investigate the effects of H-NS upon the expression of the various genes within the gene clusters. Real-time qRT-PCR was performed to quantify expression of members of the hemolysin gene clusters, including vah1, plp, rtxA, rtxH, and rtxB, in the wild-type strain (M93Sm), the hns mutant, and the hns-complemented mutant (hns+) during both the exponential and stationary growth phases. Real-time qRT-PCR data revealed that for the hns mutant compared to the wild type during the exponential and stationary growth phases, expression of rtxA increased 2.91- and 2.14-fold, respectively; expression of rtxB increased 1.28- and 1.43-fold, respectively; expression of rtxH increased 4.56- and 2.39-fold, respectively; expression of vah1 increased 16.21- and 20.01-fold, respectively; and expression of plp increased 31.27- and 36.88-fold, respectively (Fig. 4; see Table S1 in the supplemental material). Furthermore, complementation of the hns mutation downregulated the expression of these genes back to or below wild-type levels. The data strongly suggest that H-NS is a negative regulator of gene expression from both the rtxACHBDE and vah1-plp gene clusters.
Fig 4.
Expression of rtxA, rtxB, rtxH, plp, and vah1 determined by qRT-PCR analysis of V. anguillarum wild-type (M93Sm), hns mutant (hns−), and hns-complemented mutant (hns+) strains during logarithmic (Log)- and stationary (Sta)-phase growth. The data presented are representative of two independent experiments. Each value is the average for three replicates. Asterisks represent P values of <0.05 between two bracketed strains. Error bars represent 1 standard deviation.
Mutation of hns does not affect the expression of hlyU.
Since HlyU was previously shown to bind to the intergenic regions of both hemolysin gene clusters to increase their transcription (6), we wanted to determine whether mutation of hns would affect hlyU transcription. Real-time qRT-PCR was performed to measure the expression of hlyU in the wild-type strain (M93Sm), the hns mutant, and the hns-complemented mutant (hns+) during the exponential and stationary growth phases (see Fig. S2A in the supplemental material). No statistically significant difference in expression of hlyU was found between M93Sm and both the hns mutant and the hns-complemented mutant (hns+) in either the log phase or stationary phase (see Fig. S2A). These results rule out the possibility that H-NS regulates hemolysin gene expression by regulating the expression of hlyU.
Upregulation of hemolysin genes by hlyU is hns dependent.
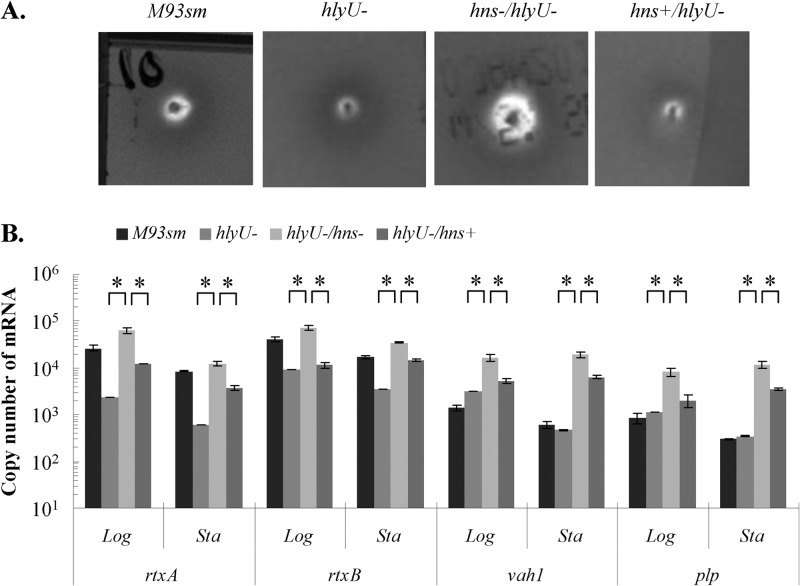
As noted above, Li et al. (6) showed that an hlyU mutant (S305) had decreased hemolytic activity on sheep blood agar compared to that of the wild type and that complementation of hlyU (S307) resulted in increased activity compared to that of the wild type. In an effort to determine the roles of hns and hlyU in the regulation of hemolysin gene transcription, we examined the hemolytic activity and measured the transcription of hemolysin genes (vah1, plp, rtxA, and rtxB) in each hemolysin transcriptional unit. The first set of these determinations was carried out with cells lacking a functional hlyU gene: the hlyU mutant, the hns hlyU double mutant (ES114), and the hns hlyU double mutant complemented with hns (ES116; hns+) (Fig. 5). Determinations of hemolytic activity and hemolysin gene expression were also done for wild-type M93Sm. Hemolytic activity in the hlyU mutant decreased compared to that of wild-type M93Sm, as previously reported by Li et al. (6). In contrast, hemolytic activity in the hlyU hns double mutant increased over that in M93Sm, and when hns was used to complement the double mutant, hemolysis decreased to the levels seen in the hlyU mutant (compare Fig. 5A with Fig. 2). Changes in transcription of rtxA, rtxB, vah1, and plp corresponded with the changes in hemolysis (Fig. 5B; see Table S1 in the supplemental material). Specifically, transcription of each gene (rtxA, rtxB, vah1, and plp) increased in the absence of a functional hns gene and decreased in the presence of a functional hns gene.
Fig 5.
(A) Hemolytic activity of V. anguillarum wild-type (M93Sm), hlyU mutant (hlyU−), hns hlyU double mutant (hns−/hlyU−), and hns-complemented hns hlyU double mutant (hns+/hlyU−) strains on TSA-5% sheep blood agar after 24 h at 27°C. (B) Expression of rtxA, rtxB, plp, and vah1 determined by qRT-PCR analysis of V. anguillarum wild-type (M93Sm), hlyU mutant (hlyU−), hns hlyU double mutant (hlyU−/hns−), and hns-complemented hns hlyU double mutant (hlyU−/hns+) strains during logarithmic (Log)- and stationary (Sta)-phase growth. The data presented are representative of two independent experiments. Each value is the average for three replicates. Asterisks represent P values of <0.05 between two bracketed strains. Error bars represent 1 standard deviation.
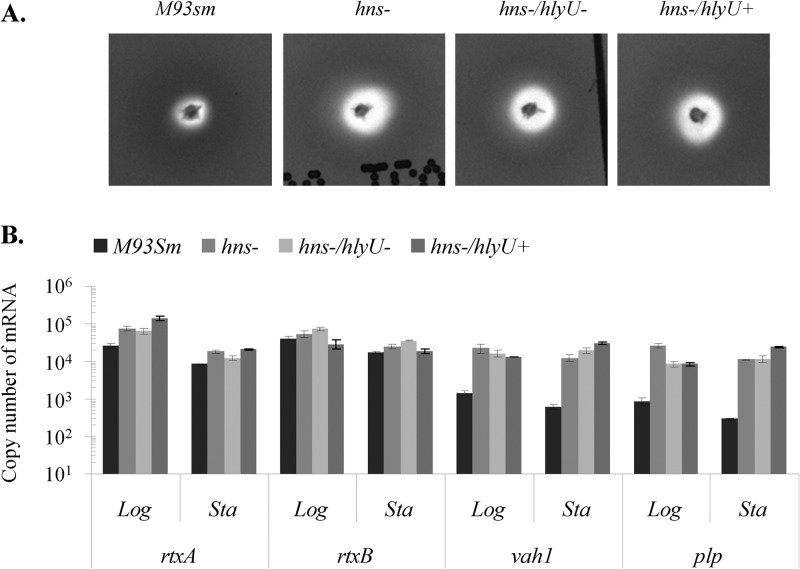
The second set of determinations was carried out with cells lacking a functional hns gene: the hns mutant, the hns hlyU double mutant, and the hns hlyU double mutant complemented with hlyU (ES115; hlyU+) (Fig. 6). Determinations of hemolytic activity and hemolysin gene expression were also done for wild-type M93Sm. In the absence of a functional hns gene, hemolytic activity increased regardless of the presence or absence of hlyU (Fig. 2 and 6A). Determinations of hemolysin transcription by qRT-PCR corresponded with the results of the hemolysis assay (Fig. 6B). In the absence of hns, rtxA and rtxB expression increased over the levels in wild-type cells. For rtxA, all increases were >2-fold and were significant (P < 0.05). For rtxB, increases were small (generally <2-fold) and generally not significant. The presence or absence of hlyU had little or no effect (<2-fold) on rtxA and rtxB gene expression (Fig. 6B; see Table S1 in the supplemental material). Similarly, in the absence of a functional hns gene, expression of both vah1 and plp increased >9-fold in both exponential- and stationary-phase cells, regardless of the presence or absence of a functional hlyU gene (Fig. 6B; see Table S1). As with the rtxACHBDE gene cluster, our data show that in the absence of hns, complementation with hlyU gave only minimal changes in expression of both vah1 and plp (around 2-fold) over that in the hns mutant strain, with almost no change in vah1 and plp expression between the two strains (Fig. 6B; see Table S1). These data indicate that upregulation of hemolysin genes by hlyU is hns dependent.
Fig 6.
(A) Hemolytic activity of V. anguillarum wild-type (M93Sm), hns mutant (hns−), hns hlyU double mutant (hns−/hlyU−), and hlyU-complemented hns hlyU double mutant (hns−/hlyU+) strains on TSA-5% sheep blood agar after 24 h at 27°C. (B) Expression of rtxA, rtxB, plp, and vah1 determined by qRT-PCR analysis of V. anguillarum wild-type (M93Sm), hns mutant (hns−), hns hlyU double mutant (hns−/hlyU−), and hlyU-complemented hns hlyU double mutant (hns−/hlyU+) strains during logarithmic (Log)- and stationary (Sta)-phase growth. The data presented are representative of two independent experiments. Each value is the average for three replicates. Error bars represent 1 standard deviation.
H-NS binds to the intergenic regions of both hemolysin gene clusters.
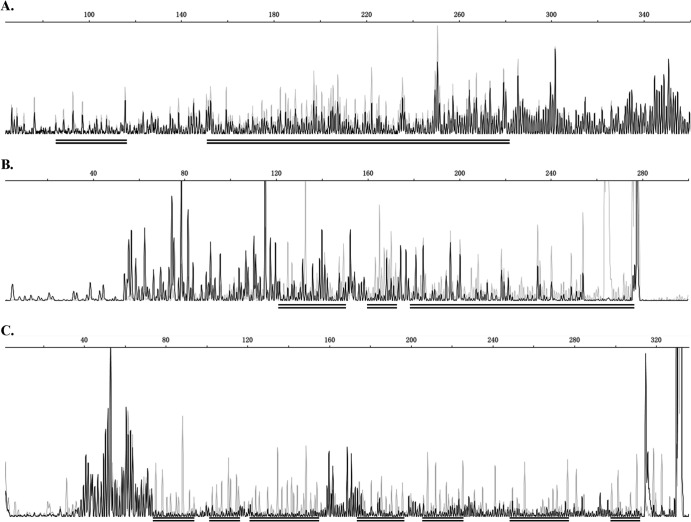
Previously, Li et al. (6) demonstrated that HlyU binds to the intergenic regions between the divergently transcribed genes of each of the two hemolysin gene clusters to upregulate gene expression. In an effort to determine whether H-NS acted in a similar fashion to help regulate expression of the hemolysin gene clusters in V. anguillarum, we carried out DNase I protection assays as described in Materials and Methods. The results of these experiments revealed that rH-NS protected multiple regions in both the rtxB-rtxH and vah1-plp intergenic regions (Fig. 7). These regions are AT-rich (72 to 74% AT) and correspond to other H-NS binding sites described for other bacteria (21–27) (Fig. 8). The H-NS binding sites cover the promoter regions of all four genes (rtxB, rtxH, plp, and vah1), with little or no overlap with the HlyU binding site in each of the intergenic regions (Fig. 8) (6). In the vah1-plp intergenic region, rH-NS bound to five sites, covering the −10 and −35 regions of both the plp and vah1 promoters, but did not cover the HlyU binding site. In the rtxB-rtxH intergenic region, rH-NS bound to six sites, covering the −35 regions of both the rtxB and rtxH promoters. In addition, rH-NS also bound to a seventh site, just within the rtxB coding sequence. rH-NS was also found to protect the three rtxB-proximal bases of the HlyU binding site (Fig. 8).
Fig 7.
Capillary electrophoresis of FAM-labeled DNA probe 1 (A) and probe 2 (B), specific for the vah1-plp intergenic region, and probe 4 (C), specific for the rtxB-rtxH intergenic region, from DNase protection assays in the presence (black lines) and absence (gray lines) of rH-NS, demonstrating that H-NS binds to specific sequences in the vah1-plp and rtxB-rtxH intergenic regions and protects against DNase I digestion. DNA probes were prepared and labeled with FAM, incubated with rH-NS (0 or 3 μM) followed by DNase I, and then analyzed by DNA fragment analysis as described in Materials and Methods. The binding region sequences are indicated by double black underlining. The underlined DNA fragments indicate those that are more common in the presence of rH-NS (black lines) than in its absence (gray lines). The location of each probe is shown in Fig. S1 in the supplemental material.
Fig 8.
Intergenic regions of the vah1-plp (A) and rtxACHBDE (B) gene clusters. The transcriptional start sites are shown as bold, italicized sequences and labeled “+1.” The −10 and −35 promoter sequences are shown as bold, italicized sequences and labeled “−10” and “−35.” Ribosomal binding sites are shown as bold, italicized sequences and labeled “RBS.” Sequences protected by HlyU are shown in bold italics and labeled “HlyU protection.” Sequences protected by H-NS are underlined.
The vah1-plp and rtxACHBDE gene clusters are unlikely to have been acquired horizontally.
Recently, it was reported that a major role of H-NS proteins is to silence horizontally acquired genetic elements distinguished by AT-rich sequences (25–27). This raised the question of whether either or both of the hemolysin gene clusters (rtxACHBDE and vah1-plp) might be xenogeneic in origin. We examined the GC contents of the two gene clusters and compared them to the average GC content of the whole genome of V. anguillarum. The results of this examination revealed that the GC content of the rtxACHBDE gene cluster is 47.3% and the GC content of the vah1-plp gene cluster is 42.5%. Both values are very similar to the average GC content of the whole genome (44.51%) of V. anguillarum (28). The intergenic regions do have low GC/elevated AT percentages, with the plp-vah1 intergenic region having 26% GC and the rtxB-rtxH region having 28% GC (Fig. 1). Additionally, examination of the published V. anguillarum 775 genome (GenBank assembly ID GCA_000217675.1) revealed that the placement of the hemolysin genes in relation to the surrounding genes, within 7.5 kbp to 10 kbp of DNA flanking each gene cluster, is the same as that found in strain M93Sm. Furthermore, we saw no evidence of any tRNA genes, transposase genes, interrupted genes, or pseudogenes in these surrounding regions. Finally, when we examined the codon usage patterns of the hemolysin genes and compared them to those for the chromosomes in which they are found (chromosome I for the rtx genes and chromosome II for the plp and vah1 genes), no significant differences were observed. These observations suggest that while the two hemolysin gene clusters are negatively regulated by H-NS, they were not acquired horizontally.
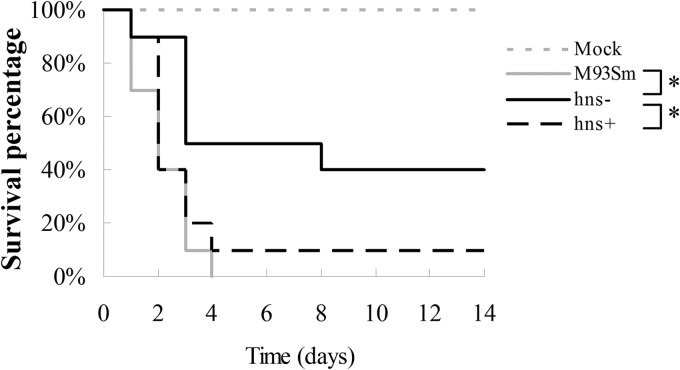
The hns mutant has attenuated virulence against rainbow trout.
Since the expression of both hemolysin gene clusters is affected by H-NS, we tested the virulence of M93Sm, the hns mutant, and the hns-complemented mutant (hns+). Groups of 10 rainbow trout were infected by i.p. injection as described in Materials and Methods, using wild-type M93Sm, the hns mutant, or the hns-complemented mutant of V. anguillarum in NSS at a dose of ∼4 × 105 CFU/fish, or using NSS only as a negative control. All M93Sm-infected trout died by day 4, while 60% of hns mutant-infected trout died by day 14. These results (Fig. 9) show that there is a significant difference (P = 0.005) in virulence between the M93Sm wild type and the hns mutant. Complementation of hns restored virulence back to wild-type levels, with 90% mortality by day 4. Thus, there was a significant difference (P = 0.029) in the virulence of the hns+ and hns mutant strains and no significant difference (P = 0.413) between the wild-type and hns+ strains.
Fig 9.
Survival percentages for rainbow trout injected i.p. with NSS only (mock; gray dashed line) or with ∼4 × 105 CFU/fish of wild-type M93Sm (gray solid line), the hns mutant (hns−; black solid line), or the hns-complemented mutant (hns+; black dashed line). Each experimental group had 10 fish. V. anguillarum cells were suspended in NSS. Asterisks represent P values of <0.05 between two bracketed strains.
At first glance, the decline in virulence for the hns mutant appears to be counterintuitive, since both hemolysin gene clusters are upregulated in the hns mutant. However, hns is considered important for bacterial fitness by properly regulating virulence and other genes during growth (26, 27). To determine whether the loss of hns affected the growth of V. anguillarum, we tested the growth of M93Sm, the hns mutant, and the hns-complemented mutant (hns+) in LB20 (see Fig. S3 in the supplemental material). While the three strains grew to nearly identical cell densities (OD600 = 1.04 for M93Sm, 0.97 for the hns mutant, and 0.97 for the hns-complemented mutant) at stationary phase, the hns mutant had a longer generation time than the wild type (58 min versus 48 min; P < 0.05). However, complementing the hns mutation did not result in a shorter generation time than that of the hns mutant (60 min versus 58 min), suggesting that there is no correlation between virulence in fish and fitness in LB20 for these three strains.
DISCUSSION
Vibriosis caused by V. anguillarum has been recognized as a major problem for salmonid culture due to the significant economic losses it causes (29). While this bacterium uses a variety of virulence factors, including iron transport/siderophore systems (30), the EmpA metalloprotease (31, 32), motility (33, 34), lipopolysaccharides (LPS) (33, 34), and exopolysaccharides (EPS) (35), it is the hemolysins/cytotoxins that directly kill host cells (4, 5) and are thought to be the major contributors to the hemorrhagic septicemia that is characteristic of vibriosis (2). Previously, we identified and described three hemolysin/cytotoxin genes of V. anguillarum M93Sm: vah1 (4, 36), rtxA (5), and plp (4; L. Li, X. Mou, and D. R. Nelson, unpublished data). The three hemolysin genes (and associated transport genes) are organized into two gene clusters (Fig. 1). Additionally, both hemolytic activity and expression of the three hemolysin genes (vah1, plp, and rtxA) are higher in log phase than in stationary phase (6). Recently, we reported that HlyU positively regulates the expression of both hemolysin gene clusters by specifically binding to the vah1-plp and rtxB-rtxH intergenic regions (6).
In this study, we examined the role of H-NS in the regulation of hemolysin activity and gene expression in V. anguillarum M93Sm. Initially, the hns homologue in V. anguillarum was identified using the V. anguillarum M93Sm draft genome, and an hns mutant and hns-complemented strain were constructed. Mutation of hns resulted in increased hemolytic activity on 5% TSA-sheep blood agar, while complementation of the hns mutation reduced hemolysis to levels below those of the wild type (Fig. 2). Mutation of hns also increased the cytotoxicity of both V. anguillarum culture supernatant (diluted 1:1 with PBS) and V. anguillarum cells (at an MOI of 200) against ASK cells, while complementation of the hns mutation reduced cytotoxic activity (Fig. 3). Transcription of the three hemolysin genes (and related rtx genes) in the presence and absence of hns corresponded with hemolysin and cytotoxin activity, with increased transcription in the hns mutant and decreased transcription in the hns-complemented mutant (Fig. 4; see Table S1 in the supplemental material). These data show that H-NS is a negative regulator of hemolytic and cytotoxic activity by acting as a repressor of hemolysin gene expression.
The results presented here correspond with the well-documented role of H-NS as a repressor and silencer of many genes in Gram-negative bacteria, especially in the repression of virulence genes (8). Recently, Liu et al. (12) demonstrated that expression of rtxA1 in Vibrio vulnificus is repressed by H-NS and that HlyU acts as an antirepressor by interfering with H-NS binding to the upstream regulatory region of rtxA1. Specifically, data from competitive gel mobility shift assays between HlyU and H-NS showed that HlyU could displace H-NS from the promoter, with binding at a low concentration, and that H-NS needs a much higher concentration to displace the bound HlyU protein (12). Similarly, our data show that H-NS represses expression from both hemolysin gene clusters in V. anguillarum, regardless of the presence of hlyU, and that upregulation of hemolysin gene expression by HlyU is dependent upon the presence of hns (Fig. 5 and 6; see Table S1). These observations strongly suggest that in V. anguillarum, H-NS functions to repress transcription of both hemolysin gene clusters and that HlyU acts as an antirepressor. Additionally, both gene clusters are arranged as divergently transcribed genes, with one HlyU binding site in the center of each intergenic region (Fig. 8) (also see Fig. 7 in the work of Li et al. [6]) flanked by 2 to 4 H-NS binding sites that extend toward the promoter sites (Fig. 8). The sites protected by rH-NS (Fig. 8) are AT-rich. The five H-NS binding sites in the vah1-plp intergenic region have A+T% values that range from 64.5% to 84.3%, while the seven H-NS-protected sites in the rtxB-rtxH intergenic region have A+T% values that range from 47.4% to 81.25%. In contrast, the flanking structural genes have much lower A+T% values. The A+T% for plp is 57%, that for vah1 is 55.7%, that for rtxACH is 51.6%, and that for rtxBDE is 54.5% (Fig. 1). This reveals an interesting discontinuity between the structural genes and the intergenic regulatory regions. A similar discontinuity is also seen between the rtx structural genes and the intergenic regions in V. vulnificus and V. cholerae. Additionally, the fact that the structural genes have A+T% values nearly identical to that for the whole genome of V. anguillarum (55.49%) suggests that these virulence genes were not acquired horizontally. This is further supported by the observations detailed above showing that there is no evidence of any tRNA genes, transposase genes, interrupted genes, or pseudogenes in the 7.5 to 10 kbp of DNA flanking the hemolysin gene clusters. Furthermore, codon usage in the hemolysin genes is not significantly different from that in the chromosomes in which the gene clusters reside.
It has been suggested that self-oligomerization and binding of H-NS to AT-rich DNA to form DNA-protein-DNA bridges impede the movement of RNA polymerase, thus repressing gene expression (8). H-NS repression may be reversed by different mechanisms (8). In V. cholerae, the binding site of the transcriptional activator ToxT overlaps H-NS binding sites. ToxT displaces H-NS and directly activates transcription (15). In contrast, the activation of pagC and ugtL transcription from H-NS-mediated repression in Salmonella enterica requires both SlyA and PhoP, with SlyA displacing H-NS and PhoP acting as a transcriptional activator (37). There are several SlyA and H-NS binding sites in the pagC promoter region, with little or no overlap between the sites (37). In V. vulnificus, the binding site of the antirepressor HlyU is far upstream of the transcription start site (positions −376 to −417) for the rtxA1 operon and overlaps two H-NS binding sites (12). Binding of HlyU to DNA relieves the H-NS repression at all H-NS binding sites of the rtxA1 promoter region (12). Our results demonstrate that in V. anguillarum, HlyU relieves H-NS repression but does not act to directly activate transcription. We have not yet identified a transcriptional activator of hemolytic activity. However, our data do indicate that transcription from both hemolysin gene clusters is higher during exponential-phase growth than during stationary-phase growth. Additionally, the binding sites for HlyU and H-NS in the V. anguillarum rtxH-rtxB intergenic region are much closer to the +1 transcription start sites than is the case in V. vulnificus CMCP6. In V. vulnificus, the five H-NS binding sites are at positions −289 to −459 relative to the transcription start site of rtxH (vv20481) (12), while the H-NS binding sites in V. anguillarum are at positions −23 to −100 for rtxH-proximal sites and positions +42 to −70 for rtxB-proximal sites (Fig. 8). These differences probably reflect the relative sizes of the intergenic regions for each organism. In V. vulnificus CMCP6, the intergenic region between rtxH and rtxB is 1,028 nucleotides (nt), while in V. anguillarum M93Sm, the intergenic region is only 325 nt. It would be interesting to examine the H-NS and HlyU binding sites in the rtx intergenic region of V. vulnificus YJ016, which is only 362 nt.
Although mutation of hns resulted in increased expression and activities of the three hemolysins (RtxA, Vah1, and Plp), the overall virulence of the hns mutant was slightly attenuated in rainbow trout. Similar results were observed in uropathogenic Escherichia coli. Mice injected intravenously with 108 CFU of the hns mutant had a higher survival rate than those injected with the wild type, although the mutant showed a higher level of alpha-hemolysin expression and activity (38). Our data suggest that there is no correlation between virulence in fish and fitness in LB20, but it should be noted that growth in LB20 is very different from growth in fish. It is likely that removal of H-NS-mediated repression/gene silencing results in an unfavorable alteration of virulence gene expression and a reduction in fitness in the host environment.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Research Initiative of the USDA Cooperative State Research, Education, and Extension Service (grant 2008-35204-04605, awarded to D.R.N.).
This research was based in part upon work conducted using the Rhode Island Genomics Sequencing Center, which is supported in part by the National Science Foundation (EPSCoR grant 0554548).
We thank Terence Bradley and Ian Jaffe for their generous help and for supplying the rainbow trout used in this research. We thank Shelby Hillman and Marta Gomez-Chiarri for their assistance with the fish infection experiment. We also thank Ling Li and Paul Johnson for the tutorial on operating equipment used in this study.
Footnotes
Published ahead of print 8 July 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00506-13.
REFERENCES
- 1.Frans I, Michiels CW, Bossier P, Willems K, Lievens B, Rediers H. 2011. Vibrio anguillarum as a fish pathogen: virulence factors, diagnosis and prevention. J. Fish Dis. 34:643–661 [DOI] [PubMed] [Google Scholar]
- 2.Austin B, Austin DA. 2012. Bacterial fish pathogens: disease of farmed and wild fish, 5th ed. Springer, New York, NY [Google Scholar]
- 3.O'Toole R, Milton DL, Hörstedt P, Wolf-Watz H. 1997. RpoN of the fish pathogen Vibrio (Listonella) anguillarum is essential for flagellum production and virulence by the water-borne but not intraperitoneal route of inoculation. Microbiology 143:3849–3859 [DOI] [PubMed] [Google Scholar]
- 4.Rock JL, Nelson DR. 2006. Identification and characterization of a hemolysin gene cluster in Vibrio anguillarum. Infect. Immun. 74:2777–2786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li L, Rock JL, Nelson DR. 2008. Identification and characterization of a repeat-in-toxin gene cluster in Vibrio anguillarum. Infect. Immun. 76:2620–2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li L, Mou X, Nelson DR. 2011. HlyU is a positive regulator of hemolysin expression in Vibrio anguillarum. J. Bacteriol. 193:4779–4789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorman CJ. 2004. H-NS: a universal regulator for a dynamic genome. Nat. Rev. Microbiol. 2:391–400 [DOI] [PubMed] [Google Scholar]
- 8.Stoebel DM, Free A, Dorman CJ. 2008. Anti-silencing: overcoming H-NS-mediated repression of transcription in Gram-negative enteric bacteria. Microbiology 154:2533–2545 [DOI] [PubMed] [Google Scholar]
- 9.Nye MB, Pfau JD, Skorupski K, Taylor RK. 2000. Vibrio cholerae H-NS silences virulence gene expression at multiple steps in the ToxR regulatory cascade. J. Bacteriol. 182:4295–4303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stonehouse EA, Hulbert RR, Nye MB, Skorupski K, Taylor RK. 2011. H-NS binding and repression of the ctx promoter in Vibrio cholerae. J. Bacteriol. 193:979–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang H, Ayala JC, Silva AJ, Benitez JA. 2012. The histone-like nucleoid structuring protein (H-NS) is a repressor of Vibrio cholerae exopolysaccharide biosynthesis (vps) genes. Appl. Environ. Microbiol. 78:2482–2488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu M, Naka H, Crosa JH. 2009. HlyU acts as an H-NS antirepressor in the regulation of the RTX toxin gene essential for the virulence of the human pathogen Vibrio vulnificus CMCP6. Mol. Microbiol. 72:491–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kodama T, Yamazaki C, Park KS, Akeda Y, Iida T, Honda T. 2010. Transcription of Vibrio parahaemolyticus T3SS1 genes is regulated by a dual regulation system consisting of the ExsACDE regulatory cascade and H-NS. FEMS Microbiol. Lett. 311:10–17 [DOI] [PubMed] [Google Scholar]
- 14.Stonehouse E, Kovacikova G, Taylor RK, Skorupski K. 2008. Integration host factor positively regulates virulence gene expression in Vibrio cholerae. J. Bacteriol. 190:4736–4748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu RR, DiRita VJ. 2002. Regulation of gene expression in Vibrio cholerae by ToxT involves both antirepression and RNA polymerase stimulation. Mol. Microbiol. 43:119–134 [DOI] [PubMed] [Google Scholar]
- 16.Aziz R, Bartels D, Best A, DeJongh M, Disz T, Edwards R, Formsma K, Gerdes S, Glass E, Kubal M. 2008. The RAST Server: rapid annotations using subsystems technology. BMC Genomics 9:75. 10.1186/1471-2164-9-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaatanen P. 1976. Microbiological studies in coastal waters of the northern Baltic Sea. I. Distribution and abundance of bacteria and yeasts in the Tvarminne area. Walter Andre Nottback Found. Sci. Rep. 1:1–58 [Google Scholar]
- 18.Milton DL, O'Toole R, Horstedt P, Wolf-Watz H. 1996. Flagellin A is essential for the virulence of Vibrio anguillarum. J. Bacteriol. 178:1310–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mårdén P, Tunlid A, Malmcrona-Friberg K, Odham G, Kjelleberg S. 1985. Physiological and morphological changes during short term starvation of marine bacterial isolates. Arch. Microbiol. 142:326–332 [Google Scholar]
- 20.Gao F, Zhang C-T. 2006. GC-Profile: a Web-based tool for visualizing and analyzing the variation of GC content in genomic sequences. Nucleic Acids Res. 34:W686–W691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grainger DC, Hurd D, Goldberg MD, Busby SJW. 2006. Association of nucleoid proteins with coding and non-coding segments of the Escherichia coli genome. Nucleic Acids Res. 34:4642–4652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Owen-Hughes TA, Pavitt GD, Santos DS, Sidebotham JM, Hulton CSJ, Hinton JCD, Higgins CF. 1992. The chromatin-associated protein H-NS interacts with curved DNA to influence DNA topology and gene expression. Cell 71:255–265 [DOI] [PubMed] [Google Scholar]
- 23.Lang B, Blot N, Bouffartigues E, Buckle M, Geertz M, Gualerzi CO, Mavathur R, Muskhelishvili G, Pon CL, Rimsky S. 2007. High-affinity DNA binding sites for H-NS provide a molecular basis for selective silencing within proteobacterial genomes. Nucleic Acids Res. 35:6330–6337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamada H, Yoshida T, Tanaka K, Sasakawa C, Mizuno T. 1991. Molecular analysis of the Escherichia coli hns gene encoding a DNA-binding protein, which preferentially recognizes curved DNA sequences. Mol. Gen. Genet. 230:332–336 [DOI] [PubMed] [Google Scholar]
- 25.Lucchini S, Rowley G, Goldberg MD, Hurd D, Harrison M, Hinton JCD. 2006. H-NS mediates the silencing of laterally acquired genes in bacteria. PLoS Pathog. 2:e81. 10.1371/journal.ppat.0020081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oshima T, Ishikawa S, Kurokawa K, Aiba H, Ogasawara N. 2006. Escherichia coli histone-like protein H-NS preferentially binds to horizontally acquired DNA in association with RNA polymerase. DNA Res. 13:141–153 [DOI] [PubMed] [Google Scholar]
- 27.Navarre WW, Porwollik S, Wang Y, McClelland M, Rosen H, Libby SJ, Fang FC. 2006. Selective silencing of foreign DNA with low GC content by the H-NS protein in Salmonella. Science 313:236–238 [DOI] [PubMed] [Google Scholar]
- 28.Naka H, Dias GM, Thompson CC, Dubay C, Thompson FL, Crosa JH. 2011. Complete genome sequence of the marine fish pathogen Vibrio anguillarum harboring the pJM1 virulence plasmid and genomic comparison with other virulent strains of V. anguillarum and V. ordalii. Infect. Immun. 79:2889–2900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naka H, Crosa JH. 2011. Genetic determinants of virulence in the marine fish pathogen Vibrio anguillarum. Fish Pathol. 46:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crosa JH. 1980. A plasmid associated with virulence in the marine fish pathogen Vibrio anguillarum specifies an iron-sequestering system. Nature 284:566–568 [DOI] [PubMed] [Google Scholar]
- 31.Denkin SM, Nelson DR. 2004. Regulation of Vibrio anguillarum empA metalloprotease expression and its role in virulence. Appl. Environ. Microbiol. 70:4193–4204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Milton DL, Norqvist A, Wolf-Watz H. 1992. Cloning of a metalloprotease gene involved in the virulence mechanism of Vibrio anguillarum. J. Bacteriol. 174:7235–7244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Norqvist A, Wolf-Watz H. 1993. Characterization of a novel chromosomal virulence locus involved in expression of a major surface flagellar sheath antigen of the fish pathogen Vibrio anguillarum. Infect. Immun. 61:2434–2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chart H. 1983. Multiflagellate variants of Vibrio anguillarum. J. Gen. Microbiol. 129:2193–2197 [DOI] [PubMed] [Google Scholar]
- 35.Weber B, Chen C, Milton DL. 2010. Colonization of fish skin is vital for Vibrio anguillarum to cause disease. Environ. Microbiol. Rep. 2:133–139 [DOI] [PubMed] [Google Scholar]
- 36.Hirono I, Masuda T, Aoki T. 1996. Cloning and detection of the hemolysin gene of Vibrio anguillarum. Microb. Pathog. 21:173–182 [DOI] [PubMed] [Google Scholar]
- 37.Perez JC, Latifi T, Groisman EA. 2008. Overcoming H-NS-mediated transcriptional silencing of horizontally acquired genes by the PhoP and SlyA proteins in Salmonella enterica. J. Biol. Chem. 283:10773–10783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Müller CM, Dobrindt U, Nagy G, Emödy L, Uhlin BE, Hacker J. 2006. Role of histone-like proteins H-NS and StpA in expression of virulence determinants of uropathogenic Escherichia coli. J. Bacteriol. 188:5428–5438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simon R, Priefer U, Pühler A. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Nat. Biotechnol. 1:784–791 [Google Scholar]
- 40.McGee K, Hörstedt P, Milton DL. 1996. Identification and characterization of additional flagellin genes from Vibrio anguillarum. J. Bacteriol. 178:5188–5198 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.